-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Ebola Virus Entry: A Curious and Complex Series of Events
article has not abstract
Published in the journal: Ebola Virus Entry: A Curious and Complex Series of Events. PLoS Pathog 11(4): e32767. doi:10.1371/journal.ppat.1004731
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004731Summary
article has not abstract
Introduction
Ebola virus (EBOV) belongs to the Filoviridae family of negative-sense RNA viruses. Since its identification in 1976, sporadic outbreaks have occurred in Central Africa. The 2014 outbreak in West Africa provides evidence that EBOV is emerging into new geographic regions. While previous outbreaks have been confined to small areas, the most recent outbreak is atypically widespread with over 25,000 people infected. Evidence from the limited-sequence studies that have been published suggests that the outbreak resulted from a single reservoir-to-human transmission event and subsequent human-to-human spread [1]. As neither an EBOV vaccine nor antivirals are currently available, this outbreak highlights the critical need for the development of effective vaccines and therapeutics.
Infection is initiated by virions entering dendritic cells, macrophages, and, perhaps, hepatocytes [2]. Virus replication in these cells is thought to be critical for initiation of systemic infection, leading to virus spread to new sites with infection of additional cell populations. Thus, a better understanding of virus entry will not only provide insight into both host cell and virus biology, but also elucidate therapeutic targets. Here we provide a brief overview of the current understanding of EBOV entry and identify important questions that remain unanswered in the field.
Filovirus Particles
The uniquely shaped filamentous particles made by filoviruses are surrounded by an envelope acquired during virion budding from the plasma membrane (Fig 1). Recent studies provide evidence that the outer leaflet of the viral envelope contains phosphatidylserine (PtdSer), which serves as an important attachment factor during entry [3,4]. Inside the envelope, the viral matrix proteins VP40 and VP24 line the inner leaflet and provide structural support. Surrounded by this protective layer of lipids and matrix proteins, the RNA genome is associated with several viral proteins, forming the ribonucleoprotein (RNP) complex. A single viral glycoprotein (GP), encoded by the virus, embeds in the viral envelope and is required for virion/cellular membrane fusion. The mature GP is composed of two subunits, GP1 (~140 kDa) and GP2 (~26 kDa), that heterodimerize through disulfide bonds and associate to form trimers (Fig 2). The crystal structure of the EBOV GP reveals that this trimer forms a chalice-like shape, with the GP2 forming the base and GP1 forming the cup [5]. Surrounding and protecting this chalice is the N-glycan-containing cap region and a heavily N - and O-glycosylated mucin-like domain (MLD) of GP1. Glycans on these regions are important for shielding the GP from neutralizing antibodies [6].
Fig. 1. Ebola Virus Particle. 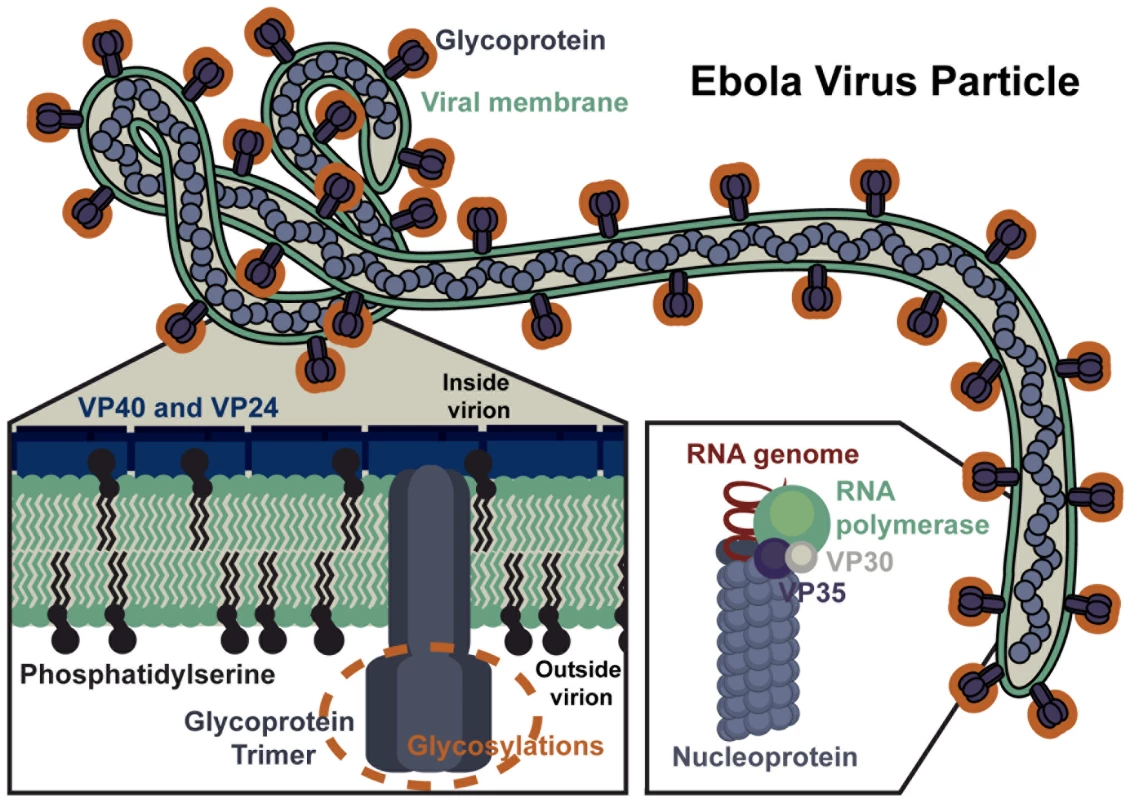
An EBOV particle is shown with key viral proteins highlighted, including: the viral glycoprotein, matrix proteins (VP40, VP24), and viral ribonucleoprotein complex (RNA-dependent RNA polymerase, VP30, VP35, nucleoprotein, and RNA). Fig. 2. Structure of the EBOV glycoprotein (GP). 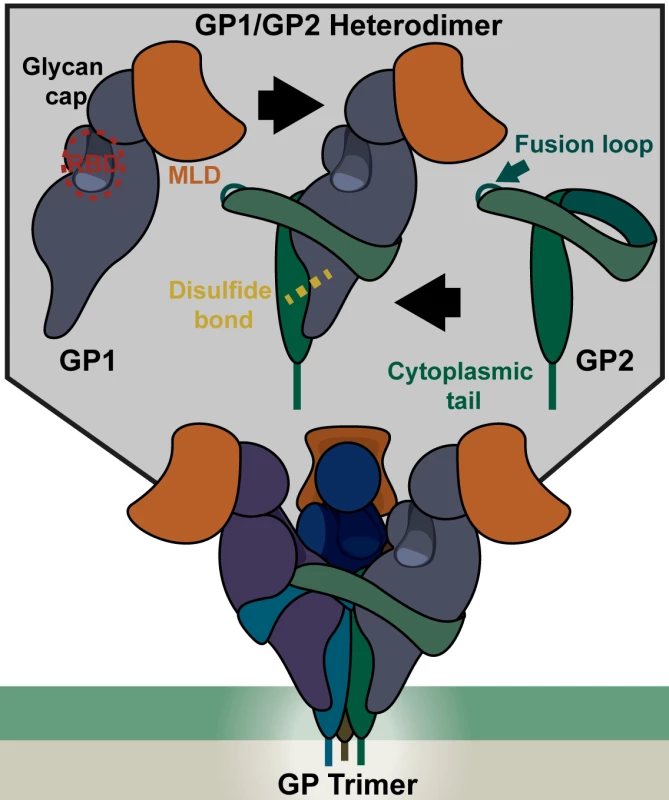
(Top) Each monomer of GP consists of a GP1 and GP2 heterodimer associated via a disulfide bond. GP1 contains the receptor binding domain (RBD), protected by a glycan cap and a mucin like domain (MLD) at the apex of the structure. The RBD interacts with the endosomal receptor Niemann-Pick C1 (NPC1) upon proteolytic processing of GP1 that removes the glycan cap and MLD. The fusion loop, which imbeds in the target membrane during fusion, is part of the GP2 monomer, but remains hidden in the mature pre-fusion GP trimer. (Bottom)The GP trimer consists of three GP1/2 heterodimers (shown in different shades) that associate through several GP1/GP2 and GP2/GP2 interactions. Adherence and Internalization
The cell surface interactions of filoviruses differ from other characterized enveloped virus/cell surface receptor interactions in that amino acid residues of EBOV GP are not thought to interact with a cell surface receptor. Instead, these viruses bind to target cells through two types of relatively non-specific receptors: C-type lectins (CLECs) that interact with glycans on EBOV GP and PtdSer receptors that interact with the viral envelope PtdSer (Fig 3A). CLECs (LSECTin, DC-SIGN [dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin], L-SIGN [liver/lymph node-specific ICAM-3 grabbing nonintegrin], mannose-binding lectin, and hMGL [human macrophage galactose - and N-acetylgalactosamine-specific C-type lectin]) bind N - and O-linked glycans on EBOV GP, leading to enhanced EBOV entry, although the details of how these interactions lead to virion internalization have yet to be studied. Cells lacking CLEC expression remain permissive for EBOV infection, providing evidence that CLEC-independent uptake mechanisms also occur. More recently appreciated is the role of cellular receptors that bind to PtdSer present in the viral envelope (reviewed in [7]). These PtdSer receptors include members of the T-cell immunoglobulin and mucin domain (TIM) family, TIM-1 and TIM-4, and protein complexes composed of Gas6 or Protein S and the TAM family of receptor tyrosine kinases, Tyro3, Axl, and Mer. Given that PtdSer is believed to be present on the surface of most, if not all, viral envelopes, it is not surprising that virion entry via virion-associated PtdSer/host PtdSer receptors interactions is not limited to filoviruses, but has recently been observed to mediate entry of a variety of enveloped viruses including flaviviruses, alphaviruses, and baculoviruses. While evidence suggests that TIM-1 can mediate virion internalization without cytoplasmic tail signaling [3,8], mechanistic details of TIM-1-dependent virion uptake are not currently established. In contrast, virus/TAM family receptor interactions trigger a signaling cascade that dampens the cell’s innate immune response, increasing the target cell permissivity [9]. Interestingly, enveloped virus interactions with CLECs may also immunomodulate immune responses of virus-infected DCs [10]. The relative importance of these various cell surface interactions for EBOV entry and pathogenesis, regardless of whether they bind GP glycans or envelope lipids, remains to be characterized in vivo.
Fig. 3. Steps of Ebola Virus Entry. 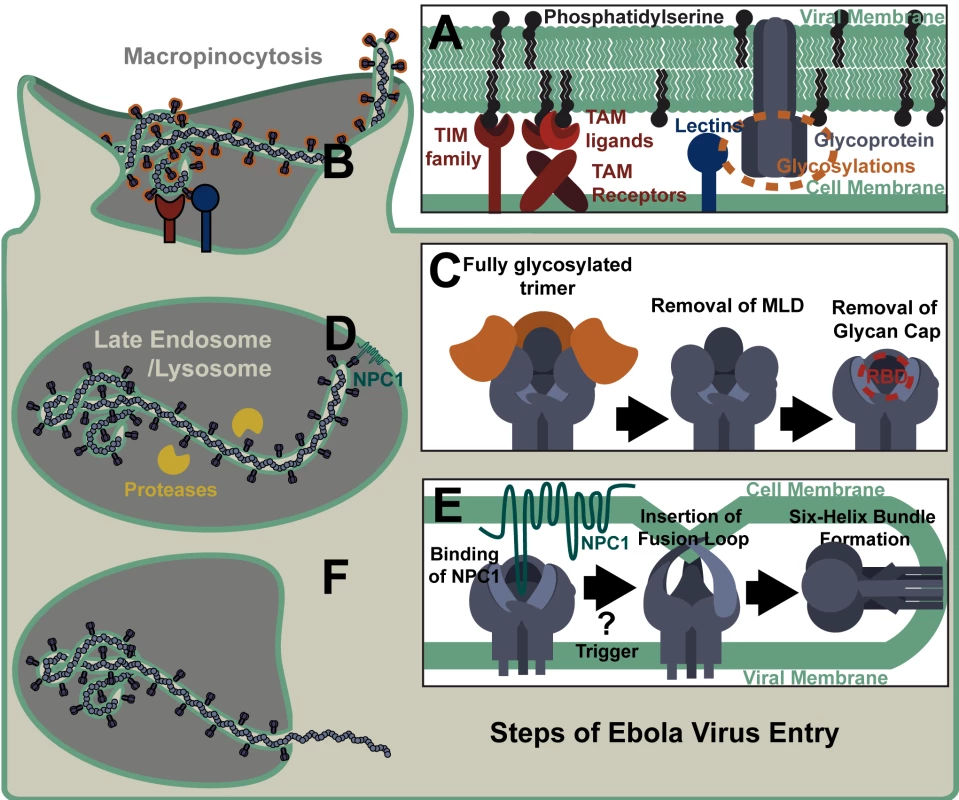
(A) Cell surface receptors bind EBOV particles through interactions with either virion-associated phosphatidylserine or viral glycoprotein glycans. (B) Virus is internalized through ruffling of the plasma membrane and macropinocytosis. (C) During trafficking through endosomes, the EBOV glycoprotein is cleaved by proteases that remove the mucin-like domain (MLD) and glycan cap, exposing the receptor binding domain (RBD). Shown is a stepwise removal of those sequences, although in the cell these cleavage events may occur concurrently. (D) The RBD interacts with NPC1 in the late endosome/lysosome. (E) Binding of the NPC1 C-loop by the glycoprotein is followed by one or more triggers that release the fusion loop, allowing for its insertion into the target membrane. Subsequent transition of the EBOV GP into a six helix bundle results in the host and viral membranes being brought together, leading to fusion. (F) Release of the viral nucleoprotein into the cytoplasm prior to the initiation of virus replication. Ebola virions are thought to be internalized primarily through macropinocytosis (Fig 3B), although other routes of uptake have also been reported [11–14]. The mechanism triggering EBOV uptake remains unknown; however, we and others have shown that PtdSer receptor-dependent internalization of viral particles does not require the presence of a viral glycoprotein on the particle [4,15]. Further, virion binding to CLECs or PtdSer receptors has not been shown to directly trigger macropinocytosis, so elucidation of the mechanism eliciting filovirion macropinocytosis is still needed. Another piece of the virion internalization puzzle that remains unsolved is how vesicular stomatitis virus (VSV) pseudovirions that are pseudotyped with EBOV GP internalize via macropinocytosis, whereas VSV containing its native G glycoprotein enters cells through clathrin-coated pits [16,17]. One possible explanation for this apparent disparity is that VSV displaying its native G glycoprotein interacts directly with its recently identified cellular receptor, the ubiquitous LDL receptor [18]. In contrast, the EBOV GP on pseudotyped VSV does not strongly interact with any cell surface receptors and EBOV instead utilizes less specific, lower affinity internalization mechanisms, such as PtdSer/PtdSer receptor and/or glycan/lectin interactions. Potentially, it is through these latter interactions that EBOV macropinocytosis occurs.
Processing and Trafficking
Ebola virions internalize into early endosomes and subsequently traffic to the late endosome/lysosome in a Rab5 and Rab7 GTPase-dependent manner [19]. Within the endosome, low-pH-dependent proteases remove the heavily glycosylated MLD and glycan cap from GP1, resulting in a 17 - to 19-kDa protein (Fig 3C) [20,21]. Cathepsins L and B were initially identified as the proteases essential for EBOV GP processing and their cleavage sites within EBOV GP have been mapped [22,23]. However, bacterial thermolysin and proteases present in Vero E6 cells and mouse embryonic fibroblasts can effectively substitute for these cathepsins [20,24]. Proteolytic processing of GP is necessary for exposure of the GP1 receptor binding domain (RBD), but is insufficient to initiate virus fusion at 37°C [5,25]. However, at higher temperatures under low pH and/or mild reducing conditions, the 19-kDa form of the GP binds to liposomes, suggesting that this version of the trimer is in a fusion-ready state [26].
Endolysosomal Receptor Binding
The exposed RBD of the proteolytically primed GP1 binds to the late endosomal/lysosomal protein NPC1 (Fig 3D) [27,28]. This novel endosomal interaction is essential for subsequent filovirus/cell membrane fusion. Whether processed GP interaction with NPC1 directly triggers fusion or subsequent steps are needed remains unclear. Several groups have shown that additional endosomal proteolysis and/or reduction are required for EBOV fusion, but the chronology and endosomal location of these events have yet to be clarified [20,21,26,29].
EBOV membrane fusion events are thought to be similar to those described for other viral glycoproteins [30]. A hydrophobic fusion loop of GP2, normally buried beneath a neighboring GP1 monomer [5], becomes exposed by the fusion trigger (Fig 2 and Fig 3E). Low pH conditions are necessary for conformational changes within the fusion loop that promote fusion [31]. In the form of a fist-like structure, hydrophobic GP2 residues present at the tip of the fusion loop insert into the target membrane [32]. The GP2 trimer unwinds and refolds into a six-helix bundle in which the fusion loop and GP transmembrane domain meet [33]. The resulting fusion pore allows for release of the RNP into the cytoplasm and the start of virus replication (Fig 3F).
Lessons from EBOV Entry Studies and Outstanding Questions
To date, investigation of EBOV entry has led to three paradigm-shifting insights. First, the discovery of PtdSer receptor-mediated entry for not only filoviruses but also for a variety of other enveloped viruses has helped to mechanistically elucidate the broad tropism of some enveloped viruses. Second, recognition of the low pH-dependent endosomal proteolytic processing of EBOV GP identified a novel low-pH-dependent mechanism that has now been shown to be required for a number of enveloped viruses. Third, identifying an endosomal receptor for filoviruses altered the understanding of potential locations of virus/receptor interactions. While this latter observation was initially made for filoviruses, recent studies have shown that lysosomal-associated membrane protein 1 (LAMP1) is a lysosomal receptor for Lassa virus, suggesting this endosomal mechanism of fusion control may be broader than previously appreciated [34].
Despite the progress over the past five years in understanding EBOV entry, many critical questions remain unanswered. For instance, how does PtdSer become enriched on the outer leaflet of enveloped virus membranes? Do virion interactions with CLECs, TIM proteins, or TAM/Gas6 complexes mediate direct internalization of virions into endosomes? If TIM proteins do directly mediate virus internalization, mechanistically, how is this accomplished since some TIM molecules are not thought to signal? Alternatively, do these receptors solely accumulate virions on the surface of cells? If so, is there a yet unidentified cell-surface receptor that is required for EBOV internalization? If PtdSer receptors do directly mediate EBOV internalization, do the different receptors mediate internalization through the same endosomal pathway and into the same endosomal compartment? For those filoviruses that do not require Cathepsin B and L, what endosomal proteases are responsible for their GP processing? What is the role of NPC1? Does NPC1 binding directly lead to EBOV fusion? If so, why are additional proteolysis events required and what protease(s) in which vesicular compartment mediate this second processing step? Finally, in terms of the big picture, what selective advantages are there for enveloped viruses such as filoviruses to use relatively non-specific, low-affinity mechanisms for internalization? Certainly, a growing body of evidence suggests that there must be advantages. These might include a breadth of tropism that would otherwise not be available to enveloped viruses. Further, using these entry mechanisms protects critical RBD and fusion residues from neutralizing antibodies by limiting extracellular exposure of these GP elements; in the current entry model for filoviruses, these sequences are solely exposed late within the endosomal/lysosomal compartments. A better understanding of these paradigm-shifting findings could provide additional drug targets to complement the current repertoire of Ebola virus antivirals in development. No doubt, the continued pursuit of answers to these questions by a number of groups will provide significant insights into both EBOV and, more broadly, virus biology.
Zdroje
1. Gire SK, Goba A, Andersen KG, Sealfon RS, Park DJ, Kanneh L, et al. (2014) Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 345 : 1369–1372. doi: 10.1126/science.1259657 25214632
2. Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, Geisbert JB, et al. (2003) Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am J Pathol 163 : 2347–2370. 14633608
3. Meertens L, Carnec X, Lecoin MP, Ramdasi R, Guivel-Benhassine F, Lew E, et al. (2012) The TIM and TAM Families of Phosphatidylserine Receptors Mediate Dengue Virus Entry. Cell host & microbe 12 : 544–557.
4. Moller-Tank S, Kondratowicz AS, Davey RA, Rennert PD, Maury W (2013) Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J Virol 87 : 8327–8341. doi: 10.1128/JVI.01025-13 23698310
5. Lee JE, Fusco ML, Hessell AJ, Oswald WB, Burton DR, Saphire EO. (2008) Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature 454 : 177–182. doi: 10.1038/nature07082 18615077
6. Lennemann NJ, Rhein BA, Ndungo E, Chandran K, Qiu X, Maury W. (2014) Comprehensive functional analysis of N-linked glycans on Ebola virus GP1. MBio 5: e00862–00813. doi: 10.1128/mBio.00862-13 24473128
7. Moller-Tank S, Maury W (2014) Phosphatidylserine receptors: Enhancers of enveloped virus entry and infection. Virology 468-470C: 565–580.
8. Moller-Tank S, Albritton LM, Rennert PD, Maury W (2014) Characterizing Functional Domains for TIM-Mediated Enveloped Virus Entry. J Virol 88 : 6702–6713. doi: 10.1128/JVI.00300-14 24696470
9. Bhattacharyya S, Zagórska A, Lew ED, Shrestha B, Rothlin CV, Naughton J, et al. (2013) Enveloped Viruses Disable Innate Immune Responses in Dendritic Cells by Direct Activation of TAM Receptors. Cell Host & Microbe 14 : 136–147.
10. Mesman AW, Zijlstra-Willems EM, Kaptein TM, de Swart RL, Davis ME, Ludlow M, et al. (2014) Measles Virus Suppresses RIG-I-like Receptor Activation in Dendritic Cells via DC-SIGN-Mediated Inhibition of PP1 Phosphatases. Cell Host Microbe 16 : 31–42. doi: 10.1016/j.chom.2014.06.008 25011106
11. Bhattacharyya S, Hope TJ, Young JA (2011) Differential requirements for clathrin endocytic pathway components in cellular entry by Ebola and Marburg glycoprotein pseudovirions. Virology 419 : 1–9. doi: 10.1016/j.virol.2011.07.018 21855102
12. Hunt CL, Kolokoltsov AA, Davey RA, Maury W (2011) The tyro3 receptor kinase axl enhances macropinocytosis of zaire ebolavirus. J Virol 85 : 334–347. doi: 10.1128/JVI.01278-09 21047970
13. Saeed MF, Kolokoltsov AA, Albrecht T, Davey RA (2010) Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog 6(9):e1001110. doi: 10.1371/journal.ppat.1001110 20862315
14. Nanbo A, Imai M, Watanabe S, Noda T, Takahashi K, Neumann G, et al. (2010) Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS Pathog 6(9):e1001121. doi: 10.1371/journal.ppat.1001121 20886108
15. Jemielity S, Wang JJ, Chan YK, Ahmed AA, Li W, Monahan S, et al. (2013) TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog 9: e1003232. doi: 10.1371/journal.ppat.1003232 23555248
16. Cureton DK, Massol RH, Saffarian S, Kirchhausen TL, Whelan SP (2009) Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog 5: e1000394. doi: 10.1371/journal.ppat.1000394 19390604
17. Piccinotti S, Kirchhausen T, Whelan SP (2013) Uptake of rabies virus into epithelial cells by clathrin-mediated endocytosis depends upon actin. J Virol 87 : 11637–11647. doi: 10.1128/JVI.01648-13 23966407
18. Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M (2013) LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci U S A 110 : 7306–7311. doi: 10.1073/pnas.1214441110 23589850
19. Saeed MF, Kolokoltsov AA, Albrecht T, Davey RA (2010) Cellular Entry of Ebola Virus Involves Uptake by a Macropinocytosis-Like Mechanism and Subsequent Trafficking through Early and Late Endosomes. PLoS Pathog 6: e1001110. doi: 10.1371/journal.ppat.1001110 20862315
20. Schornberg K, Matsuyama S, Kabsch K, Delos S, Bouton A, White J. (2006) Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein. J Virol 80 : 4174–4178. 16571833
21. Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM (2005) Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308 : 1643–1645. 15831716
22. Dube D, Brecher MB, Delos SE, Rose SC, Park EW, Schornberg KL, et al. (2009) The primed ebolavirus glycoprotein (19-kilodalton GP1,2): sequence and residues critical for host cell binding. J Virol 83 : 2883–2891. doi: 10.1128/JVI.01956-08 19144707
23. Hood CL, Abraham J, Boyington JC, Leung K, Kwong PD, Nabel GJ. (2010) Biochemical and structural characterization of cathepsin L-processed Ebola virus glycoprotein: implications for viral entry and immunogenicity. J Virol 84 : 2972–2982. doi: 10.1128/JVI.02151-09 20053739
24. Marzi A, Reinheckel T, Feldmann H (2012) Cathepsin B & L are not required for ebola virus replication. PLoS Negl Trop Dis 6: e1923. doi: 10.1371/journal.pntd.0001923 23236527
25. Bale S, Liu T, Li S, Wang Y, Abelson D, Fusco M, et al. (2011) Ebola virus glycoprotein needs an additional trigger, beyond proteolytic priming for membrane fusion. PLoS Negl Trop Dis 5: e1395. doi: 10.1371/journal.pntd.0001395 22102923
26. Brecher M, Schornberg KL, Delos SE, Fusco ML, Saphire EO, White JM. (2012) Cathepsin cleavage potentiates the Ebola virus glycoprotein to undergo a subsequent fusion-relevant conformational change. Journal of virology 86 : 364–372. doi: 10.1128/JVI.05708-11 22031933
27. Miller EH, Obernosterer G, Raaben M, Herbert AS, Deffieu MS, Krishnan A, et al. (2012) Ebola virus entry requires the host-programmed recognition of an intracellular receptor. The EMBO journal 31 : 1947–1960. doi: 10.1038/emboj.2012.53 22395071
28. Cote M, Misasi J, Ren T, Bruchez A, Lee K, Filone CM, et al. (2011) Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature 477 : 344–348. doi: 10.1038/nature10380 21866101
29. Wong AC, Sandesara RG, Mulherkar N, Whelan SP, Chandran K (2010) A forward genetic strategy reveals destabilizing mutations in the Ebolavirus glycoprotein that alter its protease dependence during cell entry. Journal of virology 84 : 163–175. doi: 10.1128/JVI.01832-09 19846533
30. White JM, Delos SE, Brecher M, Schornberg K (2008) Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit Rev Biochem Mol Biol 43 : 189–219. doi: 10.1080/10409230802058320 18568847
31. Gregory SM, Harada E, Liang B, Delos SE, White JM, Tamm LK. (2011) Structure and function of the complete internal fusion loop from Ebolavirus glycoprotein 2. Proceedings of the National Academy of Sciences of the United States of America 108 : 11211–11216. doi: 10.1073/pnas.1104760108 21690393
32. Gregory SM, Larsson P, Nelson EA, Kasson PM, White JM, Tamm LK. (2014) Ebolavirus entry requires a compact hydrophobic fist at the tip of the fusion loop. J Virol 88 : 6636–6649. doi: 10.1128/JVI.00396-14 24696482
33. Weissenhorn W, Carfí A, Lee K - H, Skehel JJ, Wiley DC (1998) Crystal Structure of the Ebola Virus Membrane Fusion Subunit, GP2, from the Envelope Glycoprotein Ectodomain. Molecular Cell 2 : 605–616. 9844633
34. Jae LT, Raaben M, Herbert AS, Kuehne AI, Wirchnianski AS, Soh TK, et al. (2014) Virus entry. Lassa virus entry requires a trigger-induced receptor switch. Science 344 : 1506–1510. doi: 10.1126/science.1252480 24970085
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human SkinČlánek Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?Článek The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious DiseasesČlánek Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting PolypeptideČlánek A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 4- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Pathogens as Biological Weapons of Invasive Species
- Selection and Spread of Artemisinin-Resistant Alleles in Thailand Prior to the Global Artemisinin Resistance Containment Campaign
- Endopeptidase-Mediated Beta Lactam Tolerance
- Prospective Large-Scale Field Study Generates Predictive Model Identifying Major Contributors to Colony Losses
- Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human Skin
- Structural Determinants of Phenotypic Diversity and Replication Rate of Human Prions
- Sigma Factor SigB Is Crucial to Mediate Adaptation during Chronic Infections
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Heterologous Expression in Remodeled . : A Platform for Monoaminergic Agonist Identification and Anthelmintic Screening
- Novel Disease Susceptibility Factors for Fungal Necrotrophic Pathogens in Arabidopsis
- Interleukin 21 Signaling in B Cells Is Required for Efficient Establishment of Murine Gammaherpesvirus Latency
- Phosphorylation at the Homotypic Interface Regulates Nucleoprotein Oligomerization and Assembly of the Influenza Virus Replication Machinery
- Human Papillomaviruses Activate and Recruit SMC1 Cohesin Proteins for the Differentiation-Dependent Life Cycle through Association with CTCF Insulators
- Ubiquitous Promoter-Localization of Essential Virulence Regulators in
- TGF-β Suppression of HBV RNA through AID-Dependent Recruitment of an RNA Exosome Complex
- The Immune Adaptor ADAP Regulates Reciprocal TGF-β1-Integrin Crosstalk to Protect from Influenza Virus Infection
- Antagonism of miR-328 Increases the Antimicrobial Function of Macrophages and Neutrophils and Rapid Clearance of Non-typeable (NTHi) from Infected Lung
- The Epigenetic Regulator G9a Mediates Tolerance to RNA Virus Infection in
- Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?
- Hantaan Virus Infection Induces Both Th1 and ThGranzyme B+ Cell Immune Responses That Associated with Viral Control and Clinical Outcome in Humans
- Viral Inhibition of the Transporter Associated with Antigen Processing (TAP): A Striking Example of Functional Convergent Evolution
- Plasma Membrane Profiling Defines an Expanded Class of Cell Surface Proteins Selectively Targeted for Degradation by HCMV US2 in Cooperation with UL141
- Optineurin Regulates the Interferon Response in a Cell Cycle-Dependent Manner
- IFIT1 Differentially Interferes with Translation and Replication of Alphavirus Genomes and Promotes Induction of Type I Interferon
- The EBNA3 Family of Epstein-Barr Virus Nuclear Proteins Associates with the USP46/USP12 Deubiquitination Complexes to Regulate Lymphoblastoid Cell Line Growth
- Hepatitis C Virus RNA Replication Depends on Specific and -Acting Activities of Viral Nonstructural Proteins
- A Neuron-Specific Antiviral Mechanism Prevents Lethal Flaviviral Infection of Mosquitoes
- The Aspartate-Less Receiver (ALR) Domains: Distribution, Structure and Function
- Global Genome and Transcriptome Analyses of Epidemic Isolate 98-06 Uncover Novel Effectors and Pathogenicity-Related Genes, Revealing Gene Gain and Lose Dynamics in Genome Evolution
- The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious Diseases
- Ebola Virus Entry: A Curious and Complex Series of Events
- Conserved Spirosomes Suggest a Single Type of Transformation Pilus in Competence
- Spatial Structure, Transmission Modes and the Evolution of Viral Exploitation Strategies
- Bacterial Cooperation Causes Systematic Errors in Pathogen Risk Assessment due to the Failure of the Independent Action Hypothesis
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Cerebrospinal Fluid Cytokine Profiles Predict Risk of Early Mortality and Immune Reconstitution Inflammatory Syndrome in HIV-Associated Cryptococcal Meningitis
- Utilize Host Actin for Efficient Maternal Transmission in
- Borna Disease Virus Phosphoprotein Impairs the Developmental Program Controlling Neurogenesis and Reduces Human GABAergic Neurogenesis
- An Effector Peptide Family Required for Toll-Mediated Immunity
- Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting Polypeptide
- A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
- Quadruple Quorum-Sensing Inputs Control Virulence and Maintain System Robustness
- Leukocyte-Derived IFN-α/β and Epithelial IFN-λ Constitute a Compartmentalized Mucosal Defense System that Restricts Enteric Virus Infections
- A Strategy for O-Glycoproteomics of Enveloped Viruses—the O-Glycoproteome of Herpes Simplex Virus Type 1
- Macrocyclic Lactones Differ in Interaction with Recombinant P-Glycoprotein 9 of the Parasitic Nematode and Ketoconazole in a Yeast Growth Assay
- Neofunctionalization of the α1,2fucosyltransferase Paralogue in Leporids Contributes to Glycan Polymorphism and Resistance to Rabbit Hemorrhagic Disease Virus
- The Extracytoplasmic Linker Peptide of the Sensor Protein SaeS Tunes the Kinase Activity Required for Staphylococcal Virulence in Response to Host Signals
- Murine CMV-Induced Hearing Loss Is Associated with Inner Ear Inflammation and Loss of Spiral Ganglia Neurons
- Dual miRNA Targeting Restricts Host Range and Attenuates Neurovirulence of Flaviviruses
- GATA-Dependent Glutaminolysis Drives Appressorium Formation in by Suppressing TOR Inhibition of cAMP/PKA Signaling
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- Genetic Analysis Using an Isogenic Mating Pair of Identifies Azole Resistance Genes and Lack of Locus’s Role in Virulence
- A Temporal Gate for Viral Enhancers to Co-opt Toll-Like-Receptor Transcriptional Activation Pathways upon Acute Infection
- Neutrophil Recruitment to Lymph Nodes Limits Local Humoral Response to
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



