-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting Polypeptide
Currently 15 million people worldwide are infected by hepatitis D virus (HDV). HDV is the smallest virus known to infect human. With co-infection of its helper hepatitis B virus (HBV), viral hepatitis D is considered as the most severe form of viral hepatitis. No specific anti-HDV drugs are available; antivirals against HBV do not ameliorate hepatitis D. We report mice expressing a human bile acids transporter sodium taurocholate co-transporting polypeptide (NTCP) in the liver support HDV infection, providing a useful model for studying antivirals against HDV and understanding how the simplest virus interacts with a host in vivo. Our transcriptome analyses of livers of infected mice have unveiled interaction landscape of HDV and the hosts, laying a foundation for further studies.
Published in the journal: Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting Polypeptide. PLoS Pathog 11(4): e32767. doi:10.1371/journal.ppat.1004840
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004840Summary
Currently 15 million people worldwide are infected by hepatitis D virus (HDV). HDV is the smallest virus known to infect human. With co-infection of its helper hepatitis B virus (HBV), viral hepatitis D is considered as the most severe form of viral hepatitis. No specific anti-HDV drugs are available; antivirals against HBV do not ameliorate hepatitis D. We report mice expressing a human bile acids transporter sodium taurocholate co-transporting polypeptide (NTCP) in the liver support HDV infection, providing a useful model for studying antivirals against HDV and understanding how the simplest virus interacts with a host in vivo. Our transcriptome analyses of livers of infected mice have unveiled interaction landscape of HDV and the hosts, laying a foundation for further studies.
Introduction
Hepatitis D virus (HDV) is the smallest virus known to infect humans with a single—stranded, circular RNA genome of about 1.7 kilobases in length. It is enveloped by surface proteins from its helper hepatitis B virus (HBV) and undergoes a unique replication cycle via an intermediate, antigenomic RNA [1,2]. Prevalence of HDV infection remains high in many areas around the world despite the implementation of vaccine programs against HBV. Currently 15 million people are infected by HDV among the 240 million chronic HBV carriers. Viral hepatitis D is considered as one of the most severe forms of human viral hepatitis. However, there are no specific antivirals available for clinical treatment of the infection and antiviral therapies against HBV do not ameliorate hepatitis D. The mechanisms that determine whether an individual clears HDV spontaneously or becomes chronically infected are unclear [3,4]. Understanding HDV infection and developing antivirals against HDV have been hampered by the lack of reliable cell culture systems and convenient small animal models susceptible for efficient HDV infection.
Sodium taurocholate co—transporting polypeptide (NTCP), a multiple transmembrane transporter predominantly expressed in the liver [5], was recently identified as a common entry receptor for HDV and HBV [6]. This finding has enabled cell culture systems that support HDV and HBV infection in vitro. For instance, exogenous expression of human NTCP (hNTCP) rendered HDV infection of multiple mammalian cell lines, while successful HBV infection has only been achieved in hNTCP—expressing human hepatoma cells [6–9]. It is reasonable to speculate that additional human hepatocyte—specific factors are required for HBV infection of mice, however, transgenic expression of hNTCP may confer susceptibility of mouse hepatocytes to de novo HDV infection, which may provide a much—needed convenient small animal model for investigation of HDV pathogenesis and evaluation of antiviral drugs against HDV in vivo. In addition, as no other virus is simpler than HDV yet still can infect mammals, studying HDV infection in a susceptible mouse model may also help to illuminate how an animal reacts to the invading of the smallest pathogen.
We report herein that transgenic mice expressing hNTCP in hepatocytes, designated as hNTCP—Tg, support de novo HDV infection. Active HDV genome replication in the livers of infected mice was demonstrated by the presence of antigenomic RNA and edited RNA species. Infection kinetic studies revealed that HDV infection of hNTCP-Tg mice was acute and age—dependent. The infection was efficiently blocked by monoclonal antibodies specifically recognizing the critical regions of HBV envelope proteins. In our efforts toward unraveling the mechanism underlying the resolution of HDV infection in the hNTCP-Tg mice, we obtained evidence suggesting that adaptive immunity was not required for the clearance of HDV infection in the mouse model. Instead, HDV infection of hNTCP-Tg mice induced a type-I interferon (IFN) response that might have contributed to the suppression of HDV replication. Intriguingly, HDV infection could also be efficiently cleared in hNTCP-Tg type I interferon receptor 1 (IFNα/βR1) null mice. RNA—seq analyses of liver transcriptome of the HDV infected hNTCP-Tg and hNTCP-Tg/IFNα/βR1-/- mice revealed that, in addition to known interferon—stimulated genes (ISGs), HDV infection was also associated with up—regulation of novel cellular genes yet uncharacterized for antiviral activity.
Results
Human NTCP transgenic C57BL/6 mice
We and others previously demonstrated that HDV infection is restricted by murine Ntcp in cell cultures, various mammalian cells complemented with human — but not mouse NTCP supported HDV infection [7,8]. To generate a mouse model for HDV infection, we created C57BL/6 mouse lines expressing hNTCP with a C—terminal tag (C9), driven by a mouse albumin enhancer/promoter (Fig 1A). Mice were screened for the transgene by real—time PCR with primers specific for the human NTCP, and the expression levels of hNTCP mRNA in the liver were examined by real—time PCR after reverse transcription (qRT-PCR). One hNTCP transgenic (hNTCP-Tg) C57BL/6 mouse line that was confirmed for germline transmission of the hNTCP transgene by Southern blot analysis (Fig 1B) and expression of high level human NTCP mRNA in liver (Fig 1C—left) was selected for breeding and subsequent experiments. The expression level of hNTCP transgene was similar to that of the endogenous murine Ntcp (Fig 1C-right). There was no significant difference in the expression of hNTCP transgene between the homozygotes and heterozygotes at both mRNA level quantified by qRT-PCR (Fig 1C—right) and protein level examined by Western blot using antibodies against the C9 tag (Fig 1D). Immunofluorescent staining of liver sections with a C9—tag specific antibody showed hNTCP only expressed in the hepatocytes of hNTCP transgenic mice (Fig 1E).
Fig. 1. Human NTCP transgenic (hNTCP-Tg) C57BL/6 mice. 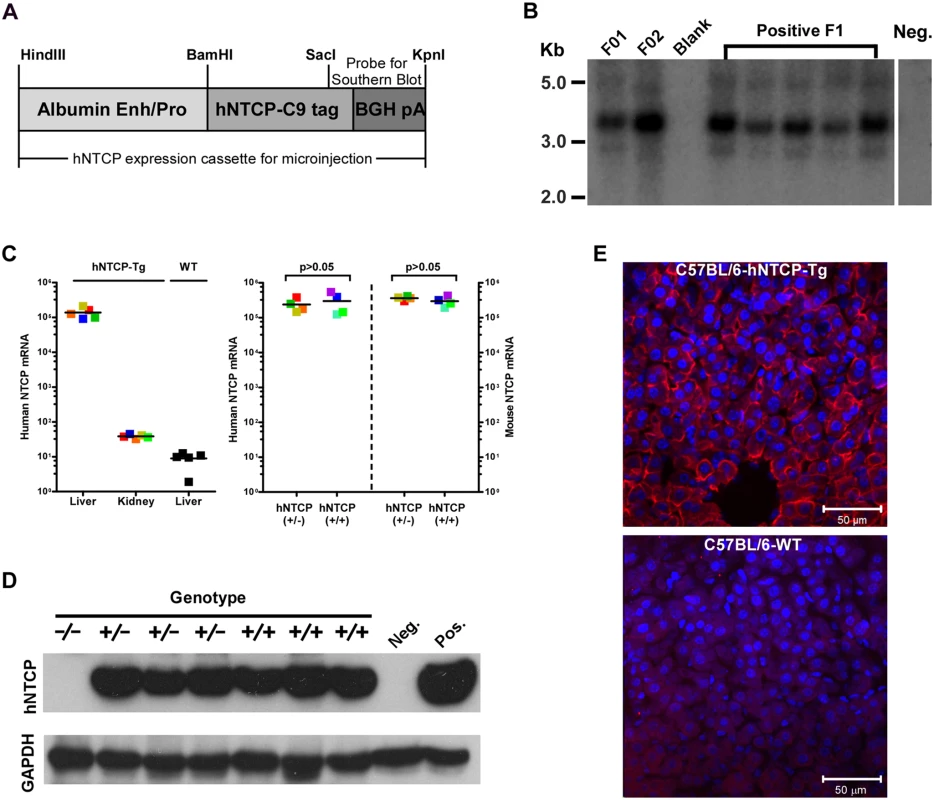
(A) Schematic diagram of human NTCP transgene introduced into mouse genome. Expression of hNTCP was controlled by a mouse albumin enhancer/promoter with a Bovine Growth Hormone (BGH) polyadenylation signal. The human NTCP gene was fused with a tag (C9) at its C—terminus (hNTCP-C9). The position of the probe for Southern blot analysis of human NTCP transgene was shown. (B) Southern blot analysis of the hNTCP transgene. Genomic DNA from the hNTCP-Tg or a wild—type (WT) mouse was digested with Bam HI. 5 μg of digested genomic DNA was separated by agarose gel electrophoresis and analyzed by Southern blot hybridization with a [α-32P] dCTP—labeled probe containing a 428bp chimeric fragment from 3’ hNTCP and BGH poly A region. The F1 offspring positive in PCR screening were examined for germline transmission of hNTCP, indicated by the presence of a 3.67 kb band, the F1 mice were from Founder 1 (F01). (C–E) Expression of human NTCP in the hNTCP-Tg mice. (C) Human NTCP mRNA level was assessed with quantitative realtime PCR after reverse transcription (qRT-PCR) in the transgenic mice (n = 5) or wild—type mice (n = 5) (left). mRNA level of human NTCP or mouse Ntcp in the hNTCP-Tg homozygotes (n = 4) or heterozygotes (n = 4) mice was assessed by qRT-PCR (right). The mRNA copy numbers per 20 ng total liver RNA were presented, the detecting limit was ~10 copies per 20 ng liver RNA. The mRNA level of mNtcp and hNTCP was comparable in hNTCP-Tg mice. Squares with the same color represent data—points from the same mouse; bars indicate the median of each group. Statistical significance was calculated by Mann—Whitney—Wilcoxon Test. (D) Western blot analysis of hNTCP-C9 transgene in total 30 μg liver lysate. Expression of hNTCP transgene was detected by a monoclonal antibody (1D4) that specifically recognizes the C—terminal tag (C9) of human NTCP transgene. Mouse GAPDH was used as a loading control. (E) Immunofluorescence staining of liver from an hNTCP-Tg mouse or a wild—type littermate. Expression of the hNTCP transgene was detected by staining the C9 tag in red; nuclei were stained with DAPI in blue. HDV infects human NTCP transgenic mice in vivo
Homozygous and heterozygous hNTCP-Tg C57BL/6 mice were both tested for their susceptibility to HDV infection. The viral infection efficiency in hNTCP-Tg mice of 9–10 days old (n = 10) correlated with the dose of inoculating virus and was independent of the hNTCP transgene homozygosity or gender of the transgenic mice (Fig 2A). At the highest HDV dose (5×1010 genome equivalents, GEq) tested, no infection was observed in the wild—type littermates (n = 6) that shared the same genetic background, microbiota and environment with the hNTCP-Tg mice. In the hNTCP-Tg mice, approximately 3% hepatocyte was being infected as examined by immunofluorescent staining positive for HDV delta antigens and the infection occurred in randomly scattered hepatocytes (Fig 2B). No significant liver histopathological changes were observed in the infected mice. There was only modest apoptosis, which was about 0.8% of the total cells, in the liver of HDV infected mice (S1 Fig). The HDV infection of hNTCP-Tg mice could be efficiently blocked by monoclonal antibody 2D3 specifically recognizing the pre-S1 domain of HBV large envelope protein (n = 6) [6], but not a control antibody (1C10) recognizing the core protein of HBV (n = 6) (Fig 2C). Similarly, a monoclonal antibody 17B9 targeting the S domain of the HBV envelope [6] that presumably attaches with the heparan sulfate proteoglycan on hepatocytes [10] also greatly reduced HDV infection in the mice (n = 5) (Fig 2D). These results show that HDV infection of hNTCP-Tg mice depends on both the pre-S1 and the S region of HBV envelop proteins, and suggest these animals are useful for evaluating inhibitors against HDV entry. HDV undergoes a unique replication cycle via an intermediate, antigenomic RNA [11]. In the hNTCP-Tg but not wild—type littermates, both genomic and antigenomic HDV RNA were readily detectable by Northern blot analysis (Fig 2E), indicating HDV effectively replicated in the hNTCP-Tg mice.
Fig. 2. HDV infects human NTCP transgenic mice in vivo. 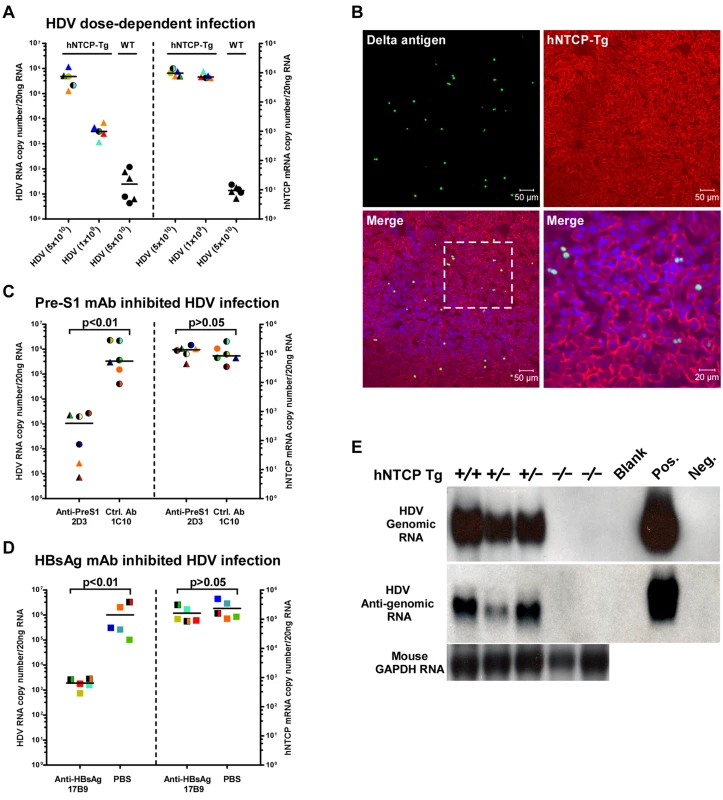
The hNTCP transgenic C57BL/6 mice were inoculated with HDV by intraperitoneal (i.p.) injection on day 9 after birth. (A) hNTCP-Tg or wild—type littermates were inoculated with indicated genome equivalents (GEq) HDV. Mice were sacrificed 6 days after viral inoculation. RNA levels of HDV and human NTCP was determined by qRT-PCR. (B) Immunofluorescence staining of HDV delta antigens in liver. Photos taken from liver sections of a mouse challenged with 3.3×1010 GEq HDV were shown. The human NTCP transgene was stained by the C9 tag in red; HDV delta antigens in green; nuclei in blue. (C) qRT-PCR analysis of HDV RNA levels in the hNTCP-Tg mice challenged with 6.7×1010 GEq HDV. A mouse monoclonal antibodies (mAb) 2D3 targeting to the pre-S1 region of HBV L protein was injected i.p. (10mg/kg) one hour prior to the viral challenge, a mcAb 1C10 (10mg/kg) recognizing the core protein of HBV was used as a control. RNA copy numbers of HDV and human NTCP were determined on day 6 post infection. 2D3 blocked more than 95% HDV infection in hNTCP-Tg mice; the hNTCP mRNA level was similar between the two groups after the infection. (D) qRT-PCR analysis of the levels of HDV RNA in the mice inoculated with 4.3×1010 GEq HDV. A monoclonal antibody17B9 targeting to the S region of HBV envelop proteins was injected i.p. (10mg/kg) one hour before the viral challenge, PBS was used as a control. RNA copy numbers of HDV and human NTCP were determined on day 6 post infection. (E) Northern blot analysis of HDV genome and antigenome RNA. Result from individual mouse inoculated with 5×1010 GEq HDV was shown. Mouse GAPDH RNA was used as RNA loading control. RNA from HepG2-NTCP cells infected or not by HDV was used as positive or negative control. For panels (A, C, D), data—points from individual mouse were shown in the same color and pattern. The detecting limit for HDV RNA was 10–100 copies per 20 ng liver total RNA in this and other HDV in vivo infection experiments. hNTCP-Tg homozygotes in mono—color, heterozygotes in two—color; male in triangle, female in circle, gender not determined in square; bars indicate the median of each group. Statistical significance was calculated by Mann—Whitney—Wilcoxon Test. We next tested the susceptibility of the hNTCP-Tg mice to HDV infection at different age. Interestingly, while challenge of hNTCP-Tg mice younger than 17 days by intraperitoneal (i.p.) injection resulted in marked HDV infection, as indicated by the presence of approximately 1000 copies of HDV RNAs per cell (~106 copies/20ng liver total RNA) at 9 days post infection in the livers of mice (S2A Fig), challenge of the transgenic mice older than 4 weeks with HDV failed to establish effective infection (S2B and S2C Fig), although these mice efficiently expressed hNTCP in the livers regardless of their genotype of being homozygote or heterozygote of the transgene. Together these results demonstrate that hNTCP transgenic mice support HDV entry and RNA replication in hepatocytes in vivo in an age—dependent manner.
Kinetics of HDV infection in hNTCP-Tg, or hNTCP-Tg with Prkdc mutation (SCID) mice
Because HDV infection of hNTCP-Tg mice should only result in a single—round infection of hepatocytes, it is interesting to know how the host immune system responds to the virus infection. In addressing this question, we first determined the kinetics of HDV replication in the liver of transgenic mice. hNTCP-Tg mice were infected by about 1×1010 GEq of HDV at 9 day after birth and sacrificed on 2, 6, 10, 14, and 18 days post infection. Intrahepatic HDV RNA reached peak level around 6 day post infection and then declined from the peak by approximately 1000 fold within next 12 days (Fig 3A). These observations suggest that HDV infection of the hNTCP-Tg mice is transient.
Fig. 3. Clearance of HDV in hNTCP-Tg mice or hNTCP-Tg with Prkdc mutation (SCID) mice. 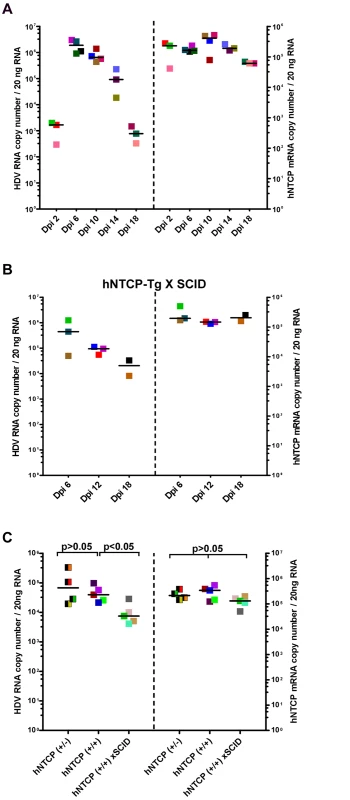
(A) hNTCP-Tg homozygotes were i.p. inoculated with 1×1010 GEq of HDV 9 days after birth and sacrificed on indicated days post infection (Dpi). RNA levels of HDV and hNTCP transgene expression were determined by qRT-PCR. (B) hNTCP-Tg homozygotes with homozygous Prkdc mutation (SCID) were i.p. inoculated with 5.3×1010 GEq of HDV 9 days after birth, the mice were sacrificed on indicated days post infection. RNA levels of HDV and hNTCP transgene were determined by qRT-PCR. (C) Side—by—side experiment for direct comparison of HDV infection in the mice bearing human NTCP. Heterozygous or homozygous hNTCP-Tg mice, or hNTCP-Tg homozygotes with homozygous Prkdc mutation (SCID) as indicated, were i.p. inoculated with 1.3×109 GEq of HDV 9 days after birth, the mice were sacrificed on day 6 post infection. RNA levels of HDV and hNTCP transgene expression were determined by qRT-PCR. For panels (A–C), results from three individual mice were shown in the same color and pattern; bars indicate the median of each group. Statistical significance was calculated by Mann—Whitney—Wilcoxon Test. To explore host factors responsible for controlling the HDV infection in vivo, we first investigated the role of adaptive immunity. Specifically, a hNTCP-Tg scid mouse line was established by crossing hNTCP-Tg with adaptive immunity deficient Prkdcscid mice which bear a premature stop codon in the Prkdc gene whereby the differentiation of lymphoid cells was disrupted in these mice [12]. Similar to that observed in hNTCP-Tg mice, HDV infection was cleared rapidly in hNTCP-Tg mice with a homozygous Prkdc gene mutation (hNTCP / Prkdcscid) (n = 8) (Fig 3B), indicating that adaptive immunity is dispensable for viral clearance in these mice. Interestingly, on day 6 after viral inoculation, intrahepatic HDV RNA level in the hNTCP+/+/Prkdcscid mice was significantly lower than that of the hNTCP+/+ mice (Fig 3C); perhaps a higher level of innate immunity activity in the Prkdcscid mice may have contributed to the rapid HDV clearance at early time [13,14].
Transcriptome analysis of HDV infected livers from hNTCP-Tg and interferon α/βreceptor 1 (IFNα/βR1 -/-) deficient hNTCP-Tg mice
We further examined HDV infection in hNTCP-Tg mice with deficiency in IFNα/βR1 (hNTCP-Tg/ IFNα/βR1 -/-), which were established by crossing hNTCP-Tg with IFNα/βR1 null mice. It’s known that IFNα/βR1 is essential for type I IFN-mediated signal transduction and its deficiency greatly reduces host antiviral activities in general [15,16]. Consistent with this notion, the efficiency of HDV infection in hNTCP-Tg/IFNα/βR1 -/- mice was significantly higher than that in the normal hNTCP-Tg mice examined by qPCR (Fig 4A) and immunofluorescent staining of HDV delta antigen (S3A Fig),with about 10 folds increase of HDV RNA copies and 3–5 folds of more delta antigen positive cells, respectively. However, to our surprise, HDV infection was also efficiently cleared within 2 weeks in the hNTCP-Tg/IFNα/βR1 -/- mice (n = 14) (Fig 4B), suggesting that clearance of HDV infection in the transgenic mice may also be achieved via type I IFN-independent mechanism(s). In order to further investigate the mechanisms of HDV clearance in the infected mice, we performed RNA—seq analysis to capture the transcriptomic landscapes in the liver of different hNTCP-Tg mouse lines upon HDV infection (n = 3 in each group). The cellular factors that mediate the type I interferon antiviral response are ISGs [17]. We first compiled a list of 583 mouse ISGs based on the previously reported datasets [18,19], and analyzed their expression fold changes in the infected mice (S1 Table). Comparing to the mock—infected hNTCP-Tg mice, hNTCP-Tg mice infected by HDV exhibited an elevated level of ISGs in the liver sample (S3B Fig). A dot plot for ISG expression fold change is shown in Fig 4C. Ifit1, Ifi44, Rsad2, Ccl7, Slfn1, Isg15, Mx1, Tgtp1, Gbp3, Ifit3, Ifit2, Ddx60, Oasl1, Zbp1, Oasl2, Cxcl10 and Irf7 were among the most significantly up—regulated ISGs in the hNTCP-Tg mice comparing to the wild—type mice similarly inoculated with HDV (Fig 4C, left). In marked contrast, no ISGs were significantly induced in HDV—infected hNTCP-Tg/IFNα/βR1 -/- mice, although the virus infection was also efficiently cleared in those mice (Fig 4C, right). Together these results indicate that although HDV infection of hNTCP-Tg mice induces a type I IFN response, which may subsequently suppress the replication of the virus, other cellular factors may mediate IFN—independent clearance of HDV infection.
Fig. 4. Human NTCP transgenic mice homozygous for IFNα/βR1-/- cleared HDV infection. 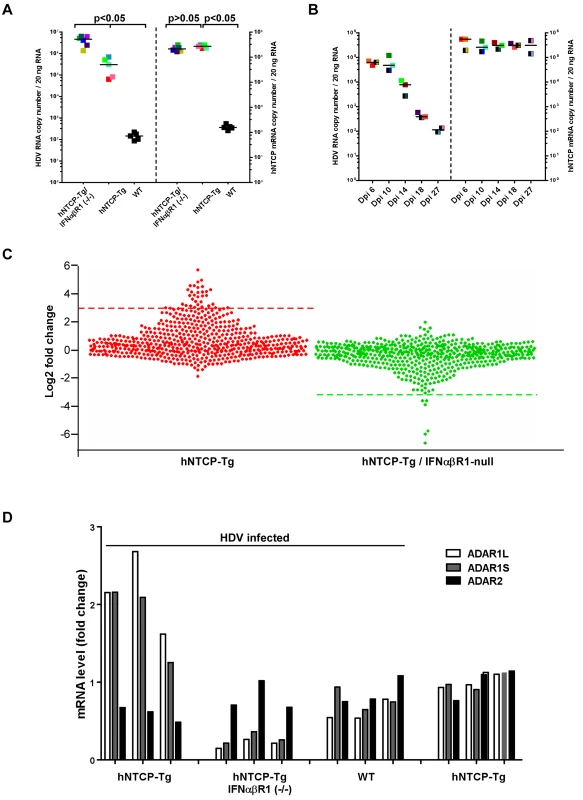
(A) hNTCP-Tg mice, hNTCP-Tg IFNα/βR1 -/- mice or wild—type C57BL/6 mice were i.p. inoculated with 4.8×1010 GEq HDV 9 days after birth, the mice were sacrificed on day 6 post infection. RNA levels of HDV and hNTCP transgene were determined by qRT-PCR. Results from individual mouse were shown in the same color and pattern; All hNTCP-Tg mice were homozygotes of hNTCP transgene and were in mono—color; bars indicate the median of each group. Statistical significance was calculated by Mann—Whitney—Wilcoxon Test. (B) IFNα/βR1 -/- mice with hNTCP transgene were i.p. inoculated with 7.9×109 GEq HDV 9 days after birth, the mice were sacrificed at indicated days after infection. RNA levels of HDV and hNTCP transgene were determined by qRT-PCR. Results from individual mouse were shown in the same color and pattern. All mice were null of IFNα/βR1 (IFNα/βR1 -/-); hNTCP-Tg homozygotes in one—color, heterozygotes in two—color; bars indicates the median of each group. (C) Dot plots of ISGs expression fold change in hNTCP-Tg, or hNTCP-Tg/IFNα/βR1 -/- mice in comparison to wild—type mice. All mice were inoculated with 1.6×1010 GEq HDV on day 9 after birth and sacrificed at day 6 post infection. Dots above the red line (left) or below the green line (right) are genes with the most significant change (log 2 fold change >3). left, from top down: Ifit1, 2010002M12Rik, Ifi44, Rsad2, Ccl7, Slfn1, Slfn4, Isg15, Mx1, Tgtp1, Gbp3, Ifit3, Ifit2, Ms4a4c, Cmpk2, Ddx60,Gm14446, Pydc4, Oas3,Oasl1, Zbp1, Oasl2,Xaf1, Apol9b, Gbp2b, Cxcl10, Irf7, BC094916, Trim30b, Pyhin1, Herc6, Ddx4, Gbp6, Slfn8; right, from bottom up: Oas1a, Pydc4, Oas2, Oas3, Clec4e, Oasl2. The data were calculated from average of multiple duplicate (n = 3) of mice gene expression values in each group, the gene expression data of different samples is normalized to upper quartile of fragments before calculation. Significant up—regulation (log 2 fold change >2) were also found in additional 51 genes, including Mx2, Oas1a,Oas1b, Trim30d, Stat1,Trim5 and Samhd1. See also S1 Table for details. (D) Fold changes of expression levels of ADARs in HDV inoculated hNTCP-Tg, hNTCP-Tg/IFNα/βR1-/- or WT C57/BL6 mice in comparison to that of mock—inoculated hNTCP-Tg mice. Mice were inoculated with about 5×1010 GEq of HDV or not, as indicated, on day 9 after birth and were sacrificed on day 6 after the inoculation. The relative mRNA levels of ADARs were determined by qRT-PCR and cellular GAPDH was used as an internal control. The average mRNA level of ADAR1L,ADAR1S, ADAR2 from three mock—inoculated hNTCP-Tg mice was set as 1.0. Results from individual mouse were shown. To identify cellular factors mediating IFN—independent innate immune response against HDV, a systematic investigation using hierarchical clustering analysis of total 7802 genes expressed in the livers of infected mice was performed. The transcriptome analysis revealed multiple genes were up—regulated in HDV inoculated hNTCP-Tg/IFNα/βR1 -/- mice. Among them, 22 genes (Gm26130, mt-Ts2, Mgst2, Cyp7a1, Vps45, etc.) were clustered as a hot block next to a block containing Irf7 gene that is the master regulator for the induction of type I interferon during viral infection (Honda et al., 2005) (S4 Fig). Interestingly, most of these 22 genes apparently do not have a known function in the host anti—pathogen process or innate immunity. Together, these results unveiled the interaction landscape of HDV and the hosts, and they also indicate that multiple ISGs in hNTCP-Tg and additional novel cellular factors identified in hNTCP-Tg/ IFNα/βR1 null mice may be relevant to the clearance of HDV infection in the animals.
HDV RNA editing occurs in infected mice
HDV RNA editing is a crucial step late in theHDV lifecycle for switching from viral RNA replication to packaging. HDV RNA editing was detected in mouse liver injected with copies of HDV DNA genome using hydrodynamics—based transfection in vivo [20]. It has been shown by in vitro studies that HDV RNA editing is controlled by ADAR1 which is also an ISG [21] [22]. Using the novel mouse models, we examined the levels of ADAR1 and HDV editing upon the viral infection in vivo. The expression level of both ADAR1 variants, ADAR1S and ADAR1L, was induced in hNTCP-Tg mice upon the infection (Fig 4D). Consistent with ADAR2 not being an ISG, HDV infection did not induce ADAR2 level in hNTCP-Tg mice. Intriguingly, the degree of HDV RNA editing was comparable in hNTCP-Tg/IFNα/βR1-/- and hNTCP-Tg mice (S5A and S5B Fig), hence it is tempting to speculate that the baseline expression of ADAR1 in these mice may be sufficient for HDV RNA editing. We further examined the degree of viral RNA editing as a function of time upon de novo HDV infection of hNTCP-Tg mice. The result showed the degree of viral RNA editing in the animals increased from day 6 to day 18 after infection; nonetheless, during the entire experiment period, only a small fraction of viral RNA was edited with the highest ratio of 4.6% on day 18 after viral infection (S5C Fig).
Discussion
HDV is enveloped by glycoproteins derived from HBV [23], and it shares species restriction with its helper virus HBV at entry level. Mice are not a natural host for both HDV and HBV and do not support de novo infection by either virus. In this study, we demonstrated that hNTCP-Tg C57BL/6 mice can be infected by HDV. Unlike HDV, HBV infection may be restricted by unknown host factors in mice as successful HBV infection has only been achieved in hNTCP expressing hepatoma cells from human but not mouse [7,8]. It is speculated that in addition to hNTCP that facilitates HBV entry, other human hepatocyte—specific factors may be required to enable the mouse hepatocytes to support efficient formation of HBV cccDNA, which is essential for establishment of HBV infection [24,25]. Nevertheless, our work reported herein provides strong genetic evidence suggesting that hNTCP is a functional receptor for HDV infection in vivo.
Both human — and mouse NTCP can co—transport bile salts from circulation into hepatocytes with sodium [26]. In agreement with the results from cell—based studies suggesting that mouse Ntcp is not a functional receptor for HDV, the wild—type neonatal C57BL/6 mice which express mouse Ntcp at a level similar to that of hNTCP in the hNTCP-Tg littermates, did not support HDV infection. In contrast, mice bearing hNTCP, irrespective of the sex or the homozygosis of hNTCP transgene, were readily susceptible to HDV infection. Intriguingly, the receptor binding pre-S1 lipopeptide was shown to be able to bind to mouse hepatocyte in vitro [27] and in vivo [28], and to mouse NTCP albeit at a lower efficiency as elucidated by us [7]. The mRNA level of hNTCP was comparable to that of endogenous mNTCP in the hNTCP-Tg mice. It is unclear from current study whether the endogenous mNTCP competes for the pre-S1 domain mediated HDV interaction with hNTCP and thereby negatively affects the viral infection. Apparently mNTCP did not exert a trans—dominant negative effect on hNTCP in the transgenic mice. It will be interesting to compare HDV infection efficiency between hNTCP-Tg mice and mice with their endogenous NTCP genes replaced by hNTCP. Nonetheless, HDV effectively replicated in the liver of hNTCP transgenic mice, Northern blot analysis readily detected antigenomic RNA that is the intermediate and a diagnostic marker of HDV replication. Moreover, although quantifying the degree of RNA editing was limited by the resolution of the RNA editing assays, the study showed that HDV underwent evident RNA editing, an event essential for production of large delta antigen and switch to viral assembly, in the hNTCP-Tg mice during the in vivo infection. Importantly, the HDV infection of hNTCP-Tg mice could be effectively blocked by monoclonal antibodies recognizing either pre-S1 or S domain of HBV envelope proteins, suggesting that interactions between pre-S1 and hNTCP as well as S and heparan sulfate proteoglycans on hepatocytes are essential for HDV infection in vivo.
In contrast to immune—deficient uPA/SCID mice implanted with human hepatocytes, which have been used for studying HDV infection and drug candidate evaluation [29,30], hNTCP transgenic mice and their derivatives are heritable, easier to handle and more consistent among individual animals. They can serve as valuable and convenient models for evaluating antivirals, in particular HDV entry inhibitors. Because entry of HBV and HDV are both mediated by envelope proteins of HBV, hNTCP-Tg mice thus may also be used for evaluating HBV entry inhibitors using HDV as a surrogate. Moreover, by crossing with other mice bearing well—defined mutation(s) of various immunodeficiency and large—scale analysis of liver transcriptome, they created an unprecedented opportunity for in—depth studies of HDV viral infection and the host immune defense against HDV infection in vivo. In fact, our work reported herein has already revealed several unique characteristics of HDV infection in the transgenic mice.
First, we showed that neonatal but not adult hNTCP-Tg C57BL/6 mice supported readily detectable HDV infection in the liver. It is known that significant differences exist between adults and neonates in innate as well as adaptive immunity. For example, the neonatal innate immune system is biased against the production of pro—inflammatory cytokines [31] and dendritic cells (DC) may be immature until about 5 weeks of age [32]. In addition, age—dependent susceptibility of mice to virus infection has been reported for many different viruses and frequently the infection efficiency is related to the mouse genetic background [33–35]. It remains to be tested whether the HDV infection efficiency differs by age in other mouse strain(s). In addition, effects of intrahepatic immunity maturation and other age—dependent physiological changes on the susceptibility of HDV infection can also not be ruled out [36,37].
Second, concerning the infection efficiency of HDV infection in mice in vivo, we showed that inoculating hNTCP-Tg C57BL/6 mice at 9 days after birth with 3.3X1010 mge of HDV resulted in about 3% cells infected by the virus as indicated by the immunofluorescent staining of the delta antigen. It was reported that passage of HDV to woodchucks chronically infected by WHV could infect 10 to 40% hepatocyte, depending on whether the inoculated virus was first or second passage of HDV in woodchucks [38]. However, as there was no HBV infection in hNTCP-Tg mice, HDV only underwent single round infection in the mouse model reported here. The observed infection rate in the animals may also be affected by other factors, such as the route of inoculation and variations among HDV preparations. Direct intravenous injection of the virus may increase the infection rate in hNTCP-Tg mice, but it was not feasible for the 9-days animals.
Third, we observed that HDV infection of hNTCP-Tg mice was transient, irrespective to the status of their immune competency (with Prkdcscid or IFNα/βR1-/-). Previous studies reported that no significant liver histopathological changes were found in HDV transgenic mice [39,40] or in experimental HDV infected chimpanzees [41]. Reports on experimental HDV inoculation into neonatal or SCID mice with WHV enveloped HDV, which took advantage of HDV ribonucleoprotein’s compatibility with WHV envelops thereby bypassing the species restriction at entry level of HDV, showed a transient infection in the livers of infected animals [42]. We showed herein that the receptor mediated, de novo infection of HDV was cleared in about two weeks in the hNTCP-Tg mice upon viral inoculation with no obvious liver pathological changes. Interestingly, only few cells positive for HDV delta antigen were found to be TUNEL positive in the liver samples of infected mice. More studies, ideally using hNTCP-Tg mice deficient in hepatic apoptosis or necrosis, are needed to clarify whether HDV infection results in the death of the hepatocytes in the mice. Surprisingly, intrahepatic HDV RNA was cleared after infection at comparable kinetics among normal, homozygous Prdkcscid and IFNα/βR1-/- hNTCP-Tg mice; this suggested that the clearance of HDV infection in hNTCP-Tg mice was either due to the activation of innate immune response or accumulation of large delta antigen that suppressed HDV RNA replication. However, the latter hypothesis is not supported by a recent finding that HDV mono—infection of immune deficient (SCID/beige) mice transplanted with human hepatocytes persisted intrahepatically for more than 6 weeks [29]. It will be interesting to further investigate the underlying mechanisms controlling the apparently different outcome of the HDV infection in the hNTCP transgenic versus the human hepatocyte—transplanted mouse models, for example whether the difference is due to the activity of NK cells presented in the hNTCP-Tg/Prdkcscid but not in the xenotransplanted SCID/beige mice. Another possible explanation of the discrepancy between the two models is that HDV infection may induce production of cytokines or other soluble factors by non—parenchymal cells that species—specifically inhibit HDV replication in hepatocytes of the infected mice.
Forth, two lines of independent evidence presented in this study strongly suggest that HDV infection of hNTCP-Tg mice induces a type I IFN response suppressing HDV replication in hepatocytes. Firstly, intrahepatic HDV RNA in IFNα/βR1 null hNTCP-Tg mice is about 10-fold higher than that in normal hNTCP-Tg mice. In addition, dozens ISGs, among which some have been characterized for their antiviral activities against various other viral infections, for example Mx1, Ifit1, Isg15, Ifi44, Ddx60, Oasl (Liu et al., 2012; Schoggins et al., 2011) and Irf7 were up-regulated upon HDV inoculation in hNTCP-Tg mice. This is the first report demonstrating that HDV infection induces an early activation of IFN response in vivo. Of note, Hartwig et al. showed that treatment of cultured cells with IFNα increased both ADAR expression levels and RNA editing [43] and enhanced HDV RNA editing was shown to increase the expression level of large delta antigen which could further restrict the HDV replication in cells [44]. It will be interesting to further investigate how the type I IFN response restricts HDV replication in hepatocytes in vivo.
Finally, liver RNA-seq analyses of HDV-infected normal and IFNα/βR1 null hNTCP-Tg mice also revealed that expression of additional cellular genes was associated with HDV infection. The majority of these genes have not been characterized for activity in immune response or antiviral infection. Interestingly, 22 genes including Gm26130, a snoRNA gene, and Cyp7a1, the rate-limiting enzyme in the synthesis of bile acid from cholesterol via the classic pathway, and several mitochondrial tRNAs are clustered in the analysis of 7802 liver genes of the infected mice. Although further experiments are needed to dissect the possible antiviral roles of these molecules, it is tempting to speculate that at least some of them may function in parallel or in addition to the known ISGs, and be relevant in HDV viral clearance. Of note, as the smallest virus known to infect humans, HDV encodes only one protein (delta antigen), which modulates viral replication through interaction with cellular DNA-dependent RNA polymerases and other host factors [1,2]. Studying of HDV infection in hNTCP-Tg mouse model thus opens a unique door for understanding how an animal reacts to invasion by the smallest viral pathogen.
In summary, our studies of HDV infection in hNTCP-Tg mice not only proved that NTCP is a functional receptor for HDV infection in vivo and hNTCP-Tg mouse is a useful model for studying antivirals against the infection, and they also shed new light on the interaction between HDV and host immunity, and laid a foundation for future investigation toward better understanding the pathogenesis of HDV infection.
Materials and Methods
Ethics statement
All animals were housed in the animal facility of the National Institute of Biological Sciences (NIBS), Beijing. Animal experiments were conducted following the National Guidelines for Housing and Care of Laboratory Animals and performed in accordance with NIBS institutional regulations after approved by the institution's Institutional Animal Care and Use Committee (IACUC). The protocol number is NIBS-0012.
Production of hNTCP transgenic mice
The human NTCP gene with a C9 tag [6] was cloned into a vector with an expression cassette driven by mouse albumin enhancer/promoter. The recombinant plasmid was linearized and introduced into the pronuclei of C57BL/6NCrlVr mouse zygotes. PCR primers for identifying hNTCP transgene are hNTCP-F (5′ - GGATAGGGATCCGCCACCATGGAGGCCCACAACGCG-3′) and hNTCP-BGH-R (5′-ATTTCCCTCGA GCCATAGAGCCCACCGCAT-3′).
Mutant mice with severe combined immune deficiency or interferon (alpha and beta) receptor 1 knockout
Fox Chase SCID® (CB17/Icr-Prkdcscid/IcrlcoCrlVr, homozygous for the severe combined immune deficiency spontaneous mutation Prkdc) mice were from the Vital River, Beijing, China; Interferon (alpha and beta) receptor 1 knockout (B6.129S2-Ifnar1tm1Agt/Mmjax) mice [15] backcrossed to C57BL/6 for at least 5 generations were from the Jackson Laboratory, Maine, USA. hNTCP transgenic mice homogeneous for Prkdc mutation or null for IFNα/βR1 were obtained by cross breed hNTCP transgenic mice with the corresponding immune deficient mice, respectively. The genotypes of the mice were determined by PCR with DNA isolated from mouse tail. Animals were hosted in an SPF mouse facility and all animal experiments were conducted following the national guidelines for housing and care of laboratory animals and performed in accordance with institutional regulations after approval by the IACUC at National Institute of Biological Sciences, Beijing.
Expression analysis of the hNTCP transgene
RNA from mouse liver or kidney was extracted using TRIzol® Reagent (Invitrogen). The total RNA was reverse transcribed into cDNA with PrimeScriptTM RT-PCR Kit (Takara), cDNA obtained from 20ng RNA was used for real time PCR assay. See S1 Text. Supplemental experimental procedures for details. Western blot analysis for the expression of hNTCP was performed by using liver samples collected from hNTCP transgenic or wild-type mice, total 30 μg protein was loaded and 10μg/ml 1D4 antibody (Santa Cruz Biotech) was used for detecting the C9 tag fused at the C-terminus of the hNTCP transgene. Mouse GAPDH was used as a loading control.
HDV production, mouse viral inoculation, quantification of HDV RNA and ADAR mRNAs in mouse liver samples
HDV was produced as previously described by using two plasmids transfection in Huh-7 cells [6]. Mice were inoculated with purified HDV by intraperitoneal (i.p.) injection. To minimize the influence of variables, in each experiment (usually presented as one panel of a figure in the manuscript), mouse littermates were injected with HDV from same viral preparation. Mouse liver samples were homogenized in liquid nitrogen immediately after collection, and then lysed by TRIzol® reagent. The total RNA was reverse transcribed into cDNA with PrimeScriptTM RT-PCR Kit (Takara), cDNA from 20ng RNA was used for real time PCR assay. See S1 Text. Supplemental experimental procedures for details.
Analysis of HDV genome and antigenome by Northern blot and HDV editing efficiency by RT-PCR amplification combined with Nco I digestion
Liver tissue RNA was extracted using TRIzol® reagent. 2μg total RNA was electrophoresed through formaldehyde-containing 1% agarose gels, blotted onto a nitrocellulose membrane (Hybond-C Extra, Amersham), and hybridized with digoxigenin (DIG)-labeled RNA probes for HDV genome, HDV antigenome, or mouse GAPDH, respectively. For detecting HDV RNA editing, Nco I restriction digestion of PCR-amplified cDNA derived from HDV RNA was used. 300 ng total RNA was reverse transcribed using random hexamers, followed by PCR using primers specific to HDV cDNAs. PCR products were purified and subjected to overnight digestion with restriction enzyme Nco I (NEB). The total digestion products were separated by 4% polyacrylamide gel electrophoresis (PAGE), and the gel was stained with silver nitrate. In independent experiments, HDV RNA editing was also examined using the [32P] dCTP labeling method as reported by Casey et al [45] or quantified by microcapillary electrophoresis analysis using an Agilent 2100 bioanalyzer. See S1 Text. Supplemental experimental procedures for details.
RNA-seq and data analysis
RNA-seq analysis was conducted using the total RNA of livers from 3 mock-inoculated hNTCP transgenic (hNTCP+/-) mice, and HDV-inoculated mice including three hNTCP+/-, three hNTCP+/-/IFNα/βR-/-, and three wild-type mice. Mice were inoculated on day 9 after birth, and sacrificed 6 days after the inoculation. RNA-seq was performed using Illumina Genome Analyzer IIx system. Sequence data was deposited at Sequence Read Archive (SRA) of the NCBI under BioProject PRJNA236433. See S1 Text. Supplemental experimental procedures for details.
Supporting Information
Zdroje
1. Lai MM (1995) The molecular biology of hepatitis delta virus. Annu Rev Biochem 64 : 259–286. 7574482
2. Taylor JM (2006) Hepatitis delta virus. Virology 344 : 71–76. 16364738
3. Ciancio A, Rizzetto M (2014) Chronic hepatitis D at a standstill: where do we go from here? Nat Rev Gastroenterol Hepatol 11 : 68–71. doi: 10.1038/nrgastro.2013.164 24019153
4. Wedemeyer H, Manns MP (2010) Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol 7 : 31–40. doi: 10.1038/nrgastro.2009.205 20051970
5. Hagenbuch B, Meier PJ (1994) Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Invest 93 : 1326–1331. 8132774
6. Yan H, Zhong G, Xu G, He W, Jing Z, et al. (2012) Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 1: e00049. doi: 10.7554/eLife.00049 23150796
7. Yan H, Peng B, He W, Zhong G, Qi Y, et al. (2013) Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide. J Virol 87 : 7977–7991. doi: 10.1128/JVI.03540-12 23678176
8. Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, et al. (2013) Hepatitis B and D Viruses Exploit Sodium Taurocholate Co-transporting Polypeptide for Species-specific Entry into Hepatocytes. Gastroenterology 146 : 1070–1083. doi: 10.1053/j.gastro.2013.12.024 24361467
9. Li H, Zhuang Q, Wang Y, Zhang T, Zhao J, et al. (2014) HBV life cycle is restricted in mouse hepatocytes expressing human NTCP. Cell Mol Immunol 11 : 175–183. doi: 10.1038/cmi.2013.66 24509445
10. Sureau C, Salisse J (2013) A conformational heparan sulfate binding site essential to infectivity overlaps with the conserved hepatitis B virus a-determinant. Hepatology 57 : 985–994. doi: 10.1002/hep.26125 23161433
11. Chen PJ, Kalpana G, Goldberg J, Mason W, Werner B, et al. (1986) Structure and replication of the genome of the hepatitis delta virus. Proc Natl Acad Sci U S A 83 : 8774–8778. 2430299
12. Custer RP, Bosma GC, Bosma MJ (1985) Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol 120 : 464–477. 2412448
13. Christianson SW, Greiner DL, Schweitzer IB, Gott B, Beamer GL, et al. (1996) Role of natural killer cells on engraftment of human lymphoid cells and on metastasis of human T-lymphoblastoid leukemia cells in C57BL/6J-scid mice and in C57BL/6J-scid bg mice. Cell Immunol 171 : 186–199. 8806787
14. Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, et al. (1995) Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol 154 : 180–191. 7995938
15. Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, et al. (1994) Functional role of type I and type II interferons in antiviral defense. Science 264 : 1918–1921. 8009221
16. Stetson DB, Medzhitov R (2006) Type I interferons in host defense. Immunity 25 : 373–381. 16979569
17. Sen GC (2001) Viruses and interferons. Annu Rev Microbiol 55 : 255–281. 11544356
18. Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, et al. (2011) A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472 : 481–485. doi: 10.1038/nature09907 21478870
19. Liu SY, Sanchez DJ, Aliyari R, Lu S, Cheng G (2012) Systematic identification of type I and type II interferon-induced antiviral factors. Proc Natl Acad Sci U S A 109 : 4239–4244. doi: 10.1073/pnas.1114981109 22371602
20. Chang J, Sigal LJ, Lerro A, Taylor J (2001) Replication of the human hepatitis delta virus genome Is initiated in mouse hepatocytes following intravenous injection of naked DNA or RNA sequences. J Virol 75 : 3469–3473. 11238873
21. George CX, Samuel CE (1999) Human RNA-specific adenosine deaminase ADAR1 transcripts possess alternative exon 1 structures that initiate from different promoters, one constitutively active and the other interferon inducible. Proc Natl Acad Sci U S A 96 : 4621–4626. 10200312
22. Casey JL (2012) Control of ADAR1 editing of hepatitis delta virus RNAs. Curr Top Microbiol Immunol 353 : 123–143. doi: 10.1007/82_2011_146 21732238
23. Bonino F, Heermann KH, Rizzetto M, Gerlich WH (1986) Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J Virol 58 : 945–950. 3701932
24. Guidotti LG, Matzke B, Schaller H, Chisari FV (1995) High-level hepatitis B virus replication in transgenic mice. J Virol 69 : 6158–6169. 7666518
25. Raney AK, Eggers CM, Kline EF, Guidotti LG, Pontoglio M, et al. (2001) Nuclear covalently closed circular viral genomic DNA in the liver of hepatocyte nuclear factor 1 alpha-null hepatitis B virus transgenic mice. J Virol 75 : 2900–2911. 11222715
26. Stieger B (2011) The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol: 205–259. doi: 10.1007/978-3-642-14541-4_5 21103971
27. Meier A, Mehrle S, Weiss TS, Mier W, Urban S (2012) The myristoylated preS1-domain of the hepatitis B virus L-protein mediates specific binding todifferentiated hepatocytes. Hepatology 58 : 31–42.
28. Schieck A, Schulze A, Gahler C, Muller T, Haberkorn U, et al. (2013) Hepatitis B virus hepatotropism is mediated by specific receptor recognition in the liver and not restricted to susceptible hosts. Hepatology 58 : 43–53. doi: 10.1002/hep.26211 23292963
29. Giersch K, Helbig M, Volz T, Allweiss L, Mancke LV, et al. (2013) Persistent hepatitis D virus mono-infection in humanized mice is efficiently converted by hepatitis B virus to a productive co-infection. J Hepatol 60 : 538–544. doi: 10.1016/j.jhep.2013.11.010 24280293
30. Lutgehetmann M, Mancke LV, Volz T, Helbig M, Allweiss L, et al. (2012) Humanized chimeric uPA mouse model for the study of hepatitis B and D virus interactions and preclinical drug evaluation. Hepatology 55 : 685–694. doi: 10.1002/hep.24758 22031488
31. Levy O (2007) Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 7 : 379–390. 17457344
32. Dakic A, Shao QX, D'Amico A, O'Keeffe M, Chen WF, et al. (2004) Development of the dendritic cell system during mouse ontogeny. J Immunol 172 : 1018–1027. 14707075
33. Sigel MM (1952) Influence of age on susceptibility to virus infections with particular reference to laboratory animals. Annu Rev Microbiol 6 : 247–280. 13008397
34. Tregoning JS, Yamaguchi Y, Wang B, Mihm D, Harker JA, et al. (2010) Genetic susceptibility to the delayed sequelae of neonatal respiratory syncytial virus infection is MHC dependent. J Immunol 185 : 5384–5391. doi: 10.4049/jimmunol.1001594 20921522
35. Sellers RS (2012) The gene or not the gene—that is the question: understanding the genetically engineered mouse phenotype. Vet Pathol 49 : 5–15. doi: 10.1177/0300985811421324 21971987
36. Publicover J, Gaggar A, Nishimura S, Van Horn CM, Goodsell A, et al. (2013) Age-dependent hepatic lymphoid organization directs successful immunity to hepatitis B. J Clin Invest 123 : 3728–3739. doi: 10.1172/JCI68182 23925290
37. Publicover J, Goodsell A, Nishimura S, Vilarinho S, Wang ZE, et al. (2011) IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J Clin Invest 121 : 1154–1162. doi: 10.1172/JCI44198 21393863
38. Ponzetto A, Cote PJ, Popper H, Hoyer BH, London WT, et al. (1984) Transmission of the hepatitis B virus-associated delta agent to the eastern woodchuck. Proc Natl Acad Sci U S A 81 : 2208–2212. 6585793
39. Guilhot S, Huang SN, Xia YP, La Monica N, Lai MM, et al. (1994) Expression of the hepatitis delta virus large and small antigens in transgenic mice. J Virol 68 : 1052–1058. 8289334
40. Polo JM, Jeng KS, Lim B, Govindarajan S, Hofman F, et al. (1995) Transgenic mice support replication of hepatitis delta virus RNA in multiple tissues, particularly in skeletal muscle. J Virol 69 : 4880–4887. 7609056
41. Ponzetto A, Hoyer BH, Popper H, Engle R, Purcell RH, et al. (1987) Titration of the infectivity of hepatitis D virus in chimpanzees. J Infect Dis 155 : 72–78. 3794405
42. Netter HJ, Kajino K, Taylor JM (1993) Experimental transmission of human hepatitis delta virus to the laboratory mouse. J Virol 67 : 3357–3362. 8497056
43. Hartwig D, Schutte C, Warnecke J, Dorn I, Hennig H, et al. (2006) The large form of ADAR 1 is responsible for enhanced hepatitis delta virus RNA editing in interferon-alpha-stimulated host cells. J Viral Hepat 13 : 150–157. 16475990
44. Chao M, Hsieh SY, Taylor J (1990) Role of two forms of hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J Virol 64 : 5066–5069. 2398535
45. Casey JL, Gerin JL (1995) Hepatitis D virus RNA editing: specific modification of adenosine in the antigenomic RNA. J Virol 69 : 7593–7600. 7494266
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human SkinČlánek Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?Článek The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious DiseasesČlánek A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 4- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Pathogens as Biological Weapons of Invasive Species
- Selection and Spread of Artemisinin-Resistant Alleles in Thailand Prior to the Global Artemisinin Resistance Containment Campaign
- Endopeptidase-Mediated Beta Lactam Tolerance
- Prospective Large-Scale Field Study Generates Predictive Model Identifying Major Contributors to Colony Losses
- Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human Skin
- Structural Determinants of Phenotypic Diversity and Replication Rate of Human Prions
- Sigma Factor SigB Is Crucial to Mediate Adaptation during Chronic Infections
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Heterologous Expression in Remodeled . : A Platform for Monoaminergic Agonist Identification and Anthelmintic Screening
- Novel Disease Susceptibility Factors for Fungal Necrotrophic Pathogens in Arabidopsis
- Interleukin 21 Signaling in B Cells Is Required for Efficient Establishment of Murine Gammaherpesvirus Latency
- Phosphorylation at the Homotypic Interface Regulates Nucleoprotein Oligomerization and Assembly of the Influenza Virus Replication Machinery
- Human Papillomaviruses Activate and Recruit SMC1 Cohesin Proteins for the Differentiation-Dependent Life Cycle through Association with CTCF Insulators
- Ubiquitous Promoter-Localization of Essential Virulence Regulators in
- TGF-β Suppression of HBV RNA through AID-Dependent Recruitment of an RNA Exosome Complex
- The Immune Adaptor ADAP Regulates Reciprocal TGF-β1-Integrin Crosstalk to Protect from Influenza Virus Infection
- Antagonism of miR-328 Increases the Antimicrobial Function of Macrophages and Neutrophils and Rapid Clearance of Non-typeable (NTHi) from Infected Lung
- The Epigenetic Regulator G9a Mediates Tolerance to RNA Virus Infection in
- Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?
- Hantaan Virus Infection Induces Both Th1 and ThGranzyme B+ Cell Immune Responses That Associated with Viral Control and Clinical Outcome in Humans
- Viral Inhibition of the Transporter Associated with Antigen Processing (TAP): A Striking Example of Functional Convergent Evolution
- Plasma Membrane Profiling Defines an Expanded Class of Cell Surface Proteins Selectively Targeted for Degradation by HCMV US2 in Cooperation with UL141
- Optineurin Regulates the Interferon Response in a Cell Cycle-Dependent Manner
- IFIT1 Differentially Interferes with Translation and Replication of Alphavirus Genomes and Promotes Induction of Type I Interferon
- The EBNA3 Family of Epstein-Barr Virus Nuclear Proteins Associates with the USP46/USP12 Deubiquitination Complexes to Regulate Lymphoblastoid Cell Line Growth
- Hepatitis C Virus RNA Replication Depends on Specific and -Acting Activities of Viral Nonstructural Proteins
- A Neuron-Specific Antiviral Mechanism Prevents Lethal Flaviviral Infection of Mosquitoes
- The Aspartate-Less Receiver (ALR) Domains: Distribution, Structure and Function
- Global Genome and Transcriptome Analyses of Epidemic Isolate 98-06 Uncover Novel Effectors and Pathogenicity-Related Genes, Revealing Gene Gain and Lose Dynamics in Genome Evolution
- The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious Diseases
- Ebola Virus Entry: A Curious and Complex Series of Events
- Conserved Spirosomes Suggest a Single Type of Transformation Pilus in Competence
- Spatial Structure, Transmission Modes and the Evolution of Viral Exploitation Strategies
- Bacterial Cooperation Causes Systematic Errors in Pathogen Risk Assessment due to the Failure of the Independent Action Hypothesis
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Cerebrospinal Fluid Cytokine Profiles Predict Risk of Early Mortality and Immune Reconstitution Inflammatory Syndrome in HIV-Associated Cryptococcal Meningitis
- Utilize Host Actin for Efficient Maternal Transmission in
- Borna Disease Virus Phosphoprotein Impairs the Developmental Program Controlling Neurogenesis and Reduces Human GABAergic Neurogenesis
- An Effector Peptide Family Required for Toll-Mediated Immunity
- Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting Polypeptide
- A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
- Quadruple Quorum-Sensing Inputs Control Virulence and Maintain System Robustness
- Leukocyte-Derived IFN-α/β and Epithelial IFN-λ Constitute a Compartmentalized Mucosal Defense System that Restricts Enteric Virus Infections
- A Strategy for O-Glycoproteomics of Enveloped Viruses—the O-Glycoproteome of Herpes Simplex Virus Type 1
- Macrocyclic Lactones Differ in Interaction with Recombinant P-Glycoprotein 9 of the Parasitic Nematode and Ketoconazole in a Yeast Growth Assay
- Neofunctionalization of the α1,2fucosyltransferase Paralogue in Leporids Contributes to Glycan Polymorphism and Resistance to Rabbit Hemorrhagic Disease Virus
- The Extracytoplasmic Linker Peptide of the Sensor Protein SaeS Tunes the Kinase Activity Required for Staphylococcal Virulence in Response to Host Signals
- Murine CMV-Induced Hearing Loss Is Associated with Inner Ear Inflammation and Loss of Spiral Ganglia Neurons
- Dual miRNA Targeting Restricts Host Range and Attenuates Neurovirulence of Flaviviruses
- GATA-Dependent Glutaminolysis Drives Appressorium Formation in by Suppressing TOR Inhibition of cAMP/PKA Signaling
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- Genetic Analysis Using an Isogenic Mating Pair of Identifies Azole Resistance Genes and Lack of Locus’s Role in Virulence
- A Temporal Gate for Viral Enhancers to Co-opt Toll-Like-Receptor Transcriptional Activation Pathways upon Acute Infection
- Neutrophil Recruitment to Lymph Nodes Limits Local Humoral Response to
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



