-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Battling Phages: How Bacteria Defend against Viral Attack
article has not abstract
Published in the journal: Battling Phages: How Bacteria Defend against Viral Attack. PLoS Pathog 11(6): e32767. doi:10.1371/journal.ppat.1004847
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004847Summary
article has not abstract
Introduction
Bacteriophages (phages) are accomplished, bacteria-specific, viral predators with far-reaching impact: from the food and biotechnology industries [1] to global nutrient cycling [2] to human health and disease [3]; wherever bacteria thrive, it seems, so do predatory phages. In order to survive the constant onslaught of phage, bacteria have evolved mechanistically diverse defense strategies that act at every stage of the phage life cycle (Fig 1) [4,5]. Phages rapidly co-evolve to overcome these barriers, resulting in a constant, and often surprising, molecular arms race [6]. In this review, I highlight the spectrum of “innate” strategies used by bacteria to evade phage predation, with particular attention paid to more recent findings in the field. For a discussion of the CRISPR-Cas adaptive immune system, readers are directed to several recent reviews [4–6].
Fig. 1. An overview of bacterial defense systems against phage. 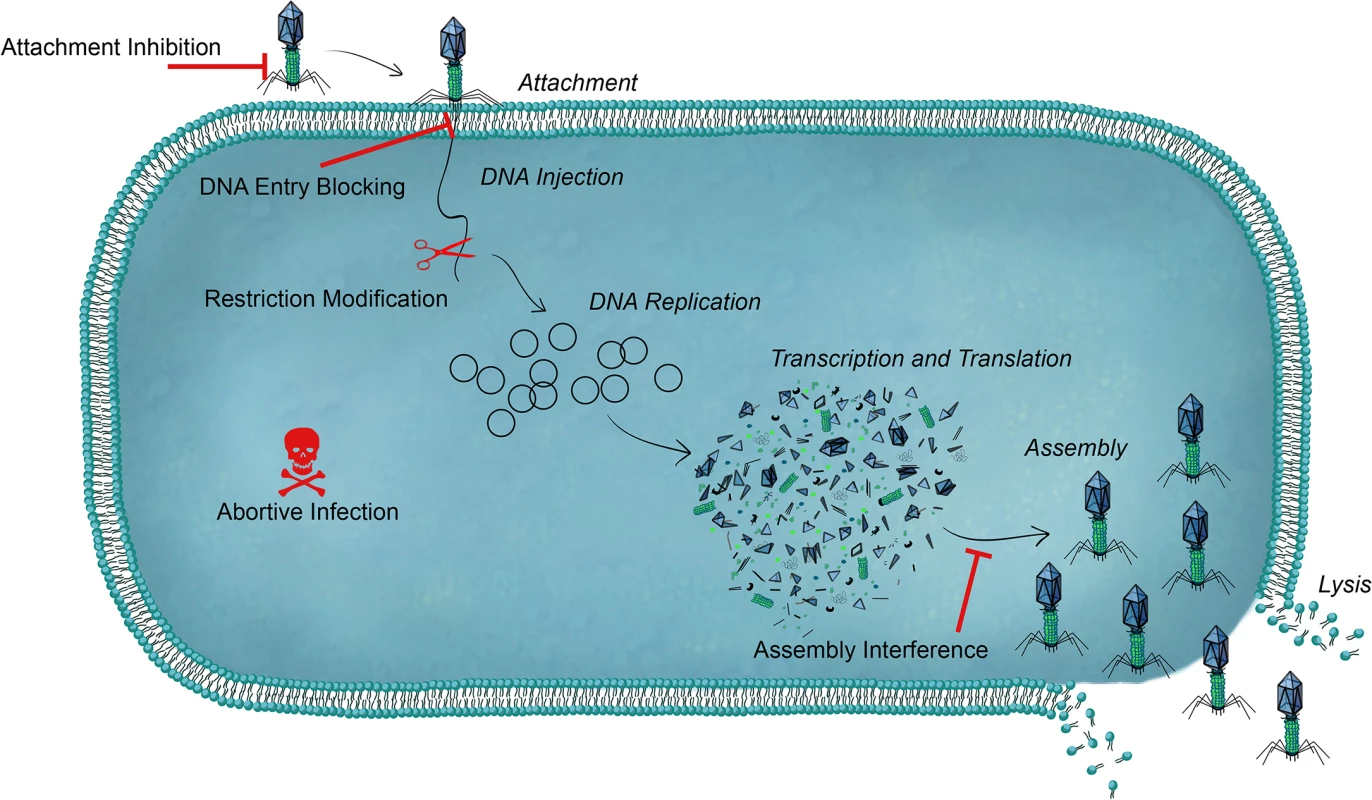
Each step of the phage lytic replication cycle is shown in italics. For simplicity, the cell wall and outer membrane (for gram-negative bacteria) is not shown. Bacteria can use a range of antiphage systems that can target all stages of the phage life cycle. Preventing Phage Attachment
A successful phage infection starts with adsorption of the virus to a specific bacterial surface receptor. Phage receptors, typically protein, polysaccharide, or lipopolysaccharide (LPS), must not only be present on the surface of the cell, but must be accessible and in a permissive spatial distribution. Therefore, strategies to prevent phage adsorption include modifying receptor structure through mutation and concealing receptors with an additional physical barrier [4,5]. A decrease in receptor availability can be mediated by phase variation in which receptor expression is subject to heritable, reversible switching, allowing for population heterogeneity in an effort to ensure survival. Bordetella bronchiseptica varies between the Bvg+ phase, which is required for pulmonary colonization, and the Bvg- phase. In the Bvg+ phase, the bacteria express numerous virulence and colonization factors, including the adhesin pertactin. Phages that use pertactin as a receptor have been identified [7]. Not surprisingly, these are temperate phages associated with clinical isolates of B. bronchiseptica. Similarly, Vibrio cholerae O1 serogroup strains rely on expression of the LPS O1 antigen for efficient colonization of the intestinal tract. Prevalent phages associated with V. cholerae in clinical samples, although in a virulent, not temperate, relationship, depend on wild-type levels of O1 antigen expression. The O1 antigen is subject to phase variation, and these phase variants are protected from phage infection and attenuated for virulence [8]. Although the classical view of phase variation is often that its purpose is to facilitate evasion of the host immune system [9], phages also apply powerful selective forces on bacterial surface molecules, and the high levels of variation observed with many of these molecules may be driven by either, or both, forces. The expression of surface receptors can also be modulated by competing phages. The availability of the Pseudomonas aeruginosa type IV pilus (TFP), which is important in pathogenesis and biofilm formation, can be modulated by lysogenic conversion. Phage D3112 encodes a protein called Tip that binds to a TFP ATPase and prevents its localization, resulting in a loss of surface piliation and protection from other phages that depend on TFP for infection [10].
In some cases, phage receptors may be hidden behind a physical barrier, such as a capsule or other extracellular polymer. The K1 capsule of Escherichia coli has been shown to directly interfere with phage T7 attachment to its LPS receptor [11]. In addition to hiding receptors to prevent phage attachment, bacteria may produce decoys. Phage T4 levels can be reduced by the presence of outer membrane vesicles (OMVs), leading to the suggestion that shedding of OMVs into the environment may act as a decoy to prevent phage adsorption that would otherwise lead to a productive infection [12].
Blocking DNA Entry
Following attachment to a suitable surface receptor, superinfection exclusion (Sie) systems can act to block phage DNA injection into host cells. Sie systems are typically phage encoded and act to protect a lysogenized host from infection by other, often closely related, phages. The Sie systems that have been described mechanistically are membrane-anchored or membrane-associated proteins. The Streptococcus thermophilus phage TP-J34 produces the LtpTP-J34 membrane-localized lipoprotein, which is thought to interact with the tape measure protein of other phages [13]. Since the tape measure protein in Siphoviridae is involved in channel formation for DNA passage, LtpTP-J34 blocks the injection process and renders the incoming phage non-infectious. The E. coli phage HK97 produces gp15, a predicted transmembrane protein that inhibits DNA entry of HK97 and the closely related phage HK75 [14]. Although several injection-blocking Sie systems have been identified, there are still many details yet to be elucidated regarding the mechanistic basis for their activity. These systems likely provide a strong selective advantage to the bacterium because, unlike the receptor blocking strategies, Sie systems conceivably protect not only the specific cell confronting phage superinfection but also the surrounding population, as the infecting phage is rendered non-infectious following DNA ejection.
Restriction-Modification Systems
If a phage successfully adsorbs and injects its DNA into a bacterium, several lines of intracellular innate defenses may be in place to prevent phage replication and release. One such barrier is restriction-modification (R-M) systems that can destroy invading DNA. Classically, R-M systems are composed of a restriction endonuclease (REase) and a cognate methyltransferase (MTase) [15]. The MTase normally methylates self-DNA at specific recognition sites, whereas foreign DNA may be unmodified. The R-M REases recognize this unmodified DNA and cleave it into harmless fragments. R-M systems are widely distributed and rather diverse: they are classified into four types according to their subunit composition, recognition site, and mechanism of action [16]. Phages can incorporate modified bases to resist classical R-M systems [6]; however, some bacteria have modification-dependent REases (e.g., MrcBC in E. coli [17]) that act only on modified DNA.
Abortive Infection
The phage resistance strategies described thus far all result in survival of the bacterial cell facing viral challenge. In contrast, abortive infection (Abi) systems lead to death of the infected cell as a sacrifice to protect the surrounding clonal population from predation. Abi systems are often encoded by mobile genetic elements, including prophages and plasmids [6]. These systems are mechanistically diverse and can act at any stage of phage development to decrease or eliminate the production of progeny viruses. The RexAB system in phage lambda protects lysogenized cells from infection by many other coliphages by inducing a loss of membrane potential, leading to decreased ATP levels [18]. Over 20 Abis, designated AbiA to AbiZ, have been found in Lactococcus lactis, a bacterium that faces phage attack during its extensive use in cheese-making fermentation processes [19]. AbiP acts early in the phage replication cycle to disrupt both phage DNA replication and the temporal switch from early to late gene expression [20]. AbiZ induces premature lysis of infected cells, ensuring that viral assembly is incomplete and infectious virions are not released [21]. Toxin-antitoxin (TA) systems have recently been shown to mediate Abi [22]. For example, the widespread AbiE system induces bacteriostasis [23] to prevent phage proliferation.
Assembly Interference
The phage-inducible chromosomal islands (PICIs) of gram-positive bacteria are phage parasites that have the capacity to interfere with the reproduction of certain phages [24]. The best-studied members of this growing family of PICIs are the Staphylococcus aureus pathogenicity islands (SaPIs), which carry and disseminate critical virulence factors [25]. SaPIs reside stably in the bacterial chromosome but are induced to excise, replicate, and package themselves upon infection by specific “helper” phages. All SaPIs described thus far affect helper phage particle assembly and DNA packaging, but in contrast to other phage-resistance mechanisms, SaPIs must permit the intracellular phage program to progress so as to allow for the production of mature phage particles loaded with SaPI DNA rather than phage DNA [26]. As with the Abi systems, the infected cell dies as a consequence of phage infection, but phage reproduction is limited and SaPIs are spread to neighboring cells. SaPIs use several unique strategies to interfere with phage reproduction. They can remodel the phage capsid proteins to generate small capsids that are tailored to the smaller SaPI genome and exclude the larger helper phage genome [24,27]. SaPIs encode phage packaging interference (Ppi) proteins, which are thought to block the phage terminase small subunit (required for recognition of phage DNA and initiation of packaging), permitting the SaPI terminase small subunit to bind the phage-encoded large subunit to cleave SaPI DNA for packaging [24]. A third interference mechanism involves interrupting phage late gene activation, which is essential for phage packaging and cell lysis [26]. A PICI-like element in V. cholerae was recently shown to inhibit a virulent phage [28], although the mechanistic basis for this activity is not yet known.
Conclusions
The strong selective pressure exerted by phages plays a key role in controlling the number and composition of bacterial populations in most, if not all, ecosystems. Conversely, bacterial strategies to resist phage attack function by controlling phage numbers and composition, thus helping to establish a predator–prey dynamic equilibrium. Many phage resistance strategies depend on the use of horizontally acquired, “selfish” elements (plasmids and prophages) that can provide efficient barriers to phage infection but that do not compromise the physiological integrity of their host cell. Thus, many of the phage resistance strategies outlined here represent competitive advances between mobile parasitic elements that depend equally on their bacterial host for long-term survival. Regardless of the origin of these systems, the consequences of the interplay between bacteria and phages necessitate molecular characterization of the many antiphage systems that are not fully understood.
Zdroje
1. Samson JE, Moineau S (2013) Bacteriophages in Food Fermentations: New Frontiers in a Continuous Arms Race. Annu Rev Food Sci Technol 4 : 347–368. doi: 10.1146/annurev-food-030212-182541 23244395
2. Suttle CA (2007) Marine viruses—major players in the global ecosystem. Nat Rev Micro 5 : 801–812.
3. De Paepe M, Leclerc M, Tinsley CR, Petit M - A (2014) Bacteriophages: an underestimated role in human and animal health? Front Cell Infect Microbiol 4 : 39. doi: 10.3389/fcimb.2014.00039 24734220
4. Labrie SJ, Samson JE, Moineau S (2010) Bacteriophage resistance mechanisms. Nat Rev Micro 8 : 317–327. doi: 10.1038/nrmicro2315 20348932
5. Dy RL, Richter C, Salmond GPC, Fineran PC (2014) Remarkable Mechanisms in Microbes to Resist Phage Infections. Annu Rev Virol 1 : 307–331.
6. Samson JE, Magadán AH, Sabri M, Moineau S (2013) Revenge of the phages: defeating bacterial defences. Nat Rev Micro: 11 : 675–687. doi: 10.1038/nrmicro3096 23979432
7. Liu M, Deora R, Doulatov SR, Gingery M, Eiserling FA, et al. (2002) Reverse transcriptase-mediated tropism switching in Bordetella bacteriophage. Science 295 : 2091–2094. 11896279
8. Seed KD, Faruque SM, Mekalanos JJ, Calderwood SB, Qadri F, et al. (2012) Phase variable O antigen biosynthetic genes control expression of the major protective antigen and bacteriophage receptor in Vibrio cholerae O1. PLoS Pathog 8: e1002917. doi: 10.1371/journal.ppat.1002917 23028317
9. van der Woude MW, Bäumler AJ (2004) Phase and antigenic variation in bacteria. Clin Microbiol Rev 17 : 581–611. 15258095
10. Chung I-Y, Jang H-J, Bae H-W, Cho Y-H (2014) A phage protein that inhibits the bacterial ATPase required for type IV pilus assembly. Proc Natl Acad Sci USA 111 : 11503–11508. doi: 10.1073/pnas.1403537111 25049409
11. Scholl D, Adhya S, Merril C (2005) Escherichia coli K1's capsule is a barrier to bacteriophage T7. Appl Environ Microbiol 71 : 4872–4874. 16085886
12. Manning AJ, Kuehn MJ (2011) Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol 11 : 258. doi: 10.1186/1471-2180-11-258 22133164
13. Bebeacua C, Lorenzo Fajardo JC, Blangy S, Spinelli S, Bollmann S, et al. (2013) X-ray structure of a superinfection exclusion lipoprotein from phage TP-J34 and identification of the tape measure protein as its target. Mol Microbiol 89 : 152–165. doi: 10.1111/mmi.12267 23692331
14. Cumby N, Edwards AM, Davidson AR, Maxwell KL (2012) The bacteriophage HK97 gp15 moron element encodes a novel superinfection exclusion protein. J Bacteriol 194 : 5012–5019. doi: 10.1128/JB.00843-12 22797755
15. Tock MR, Dryden DT (2005) The biology of restriction and anti-restriction. Curr Opin Microbiol 8 : 466–472. 15979932
16. Roberts RJ, Belfort M, Bestor T, Bhagwat AS, Bickle TA, et al. (2003) A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes. Nucleic Acids Res 31 : 1805–1812. 12654995
17. Stewart FJ, Panne D, Bickle TA, Raleigh EA (2000) Methyl-specific DNA binding by McrBC, a modification-dependent restriction enzyme. J Mol Biol 298 : 611–622. 10788324
18. Snyder L (1995) Phage-exclusion enzymes: a bonanza of biochemical and cell biology reagents? Mol Microbiol 15 : 415–420. 7540246
19. Chopin M-C, Chopin A, Bidnenko E (2005) Phage abortive infection in lactococci: variations on a theme. Curr Opin Microbiol 8 : 473–479. 15979388
20. Domingues S, Chopin A, Ehrlich SD, Chopin M-C (2004) The Lactococcal abortive phage infection system AbiP prevents both phage DNA replication and temporal transcription switch. J Bacteriol 186 : 713–721. 14729697
21. Durmaz E, Klaenhammer TR (2007) Abortive phage resistance mechanism AbiZ speeds the lysis clock to cause premature lysis of phage-infected Lactococcus lactis. J Bacteriol 189 : 1417–1425. 17012400
22. Fineran PC, Blower TR, Foulds IJ, Humphreys DP, Lilley KS, et al. (2009) The phage abortive infection system, ToxIN, functions as a protein-RNA toxin-antitoxin pair. Proc Natl Acad Sci USA 106 : 894–899. doi: 10.1073/pnas.0808832106 19124776
23. Dy RL, Przybilski R, Semeijn K, Salmond GPC, Fineran PC (2014) A widespread bacteriophage abortive infection system functions through a Type IV toxin-antitoxin mechanism. Nucleic Acids Res 42 : 4590–4605. doi: 10.1093/nar/gkt1419 24465005
24. Ram G, Chen J, Kumar K, Ross HF, Ubeda C, et al. (2012) Staphylococcal pathogenicity island interference with helper phage reproduction is a paradigm of molecular parasitism. Proc Natl Acad Sci USA 109 : 16300–16305. doi: 10.1073/pnas.1204615109 22991467
25. Novick RP, Christie GE, Penadés JR (2010) The phage-related chromosomal islands of Gram-positive bacteria. Nat Rev Micro 8 : 541–551.
26. Ram G, Chen J, Ross HF, Novick RP (2014) Precisely modulated pathogenicity island interference with late phage gene transcription. Proc Natl Acad Sci USA: 111 : 14536–14541. doi: 10.1073/pnas.1406749111 25246539
27. Ruzin A, Lindsay J, Novick RP (2001) Molecular genetics of SaPI1—a mobile pathogenicity island in Staphylococcus aureus. Mol Microbiol 41 : 365–377. 11489124
28. Seed KD, Lazinski DW, Calderwood SB, Camilli A (2013) A bacteriophage encodes its own CRISPR/Cas adaptive response to evade host innate immunity. Nature 494 : 489–491. doi: 10.1038/nature11927 23446421
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage TraffickingČlánek An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived NeuronsČlánek Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPsČlánek Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African TrypanosomiasisČlánek Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin ReleaseČlánek HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T CellsČlánek Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 6- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Introducing “Research Matters”
- Exploring Host–Pathogen Interactions through Biological Control
- Analysis of Bottlenecks in Experimental Models of Infection
- Expected and Unexpected Features of the Newly Discovered Bat Influenza A-like Viruses
- Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage Trafficking
- Recombinant Murine Gamma Herpesvirus 68 Carrying KSHV G Protein-Coupled Receptor Induces Angiogenic Lesions in Mice
- TRIM30α Is a Negative-Feedback Regulator of the Intracellular DNA and DNA Virus-Triggered Response by Targeting STING
- Targeting Human Transmission Biology for Malaria Elimination
- Two Cdc2 Kinase Genes with Distinct Functions in Vegetative and Infectious Hyphae in
- An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived Neurons
- Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPs
- Virulence Factors of Induce Both the Unfolded Protein and Integrated Stress Responses in Airway Epithelial Cells
- Peptide-MHC-I from Endogenous Antigen Outnumber Those from Exogenous Antigen, Irrespective of APC Phenotype or Activation
- Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis
- An Ultrasensitive Mechanism Regulates Influenza Virus-Induced Inflammation
- The Role of Human Transportation Networks in Mediating the Genetic Structure of Seasonal Influenza in the United States
- Host Delivery of Favorite Meals for Intracellular Pathogens
- Complement-Opsonized HIV-1 Overcomes Restriction in Dendritic Cells
- Inter-Seasonal Influenza is Characterized by Extended Virus Transmission and Persistence
- A Critical Role for CLSP2 in the Modulation of Antifungal Immune Response in Mosquitoes
- Twilight, a Novel Circadian-Regulated Gene, Integrates Phototropism with Nutrient and Redox Homeostasis during Fungal Development
- Surface-Associated Lipoproteins Link Virulence to Colitogenic Activity in IL-10-Deficient Mice Independent of Their Expression Levels
- Latent Membrane Protein LMP2A Impairs Recognition of EBV-Infected Cells by CD8+ T Cells
- Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains
- Neuronal Subtype and Satellite Cell Tropism Are Determinants of Varicella-Zoster Virus Virulence in Human Dorsal Root Ganglia Xenografts
- Molecular Basis for the Selective Inhibition of Respiratory Syncytial Virus RNA Polymerase by 2'-Fluoro-4'-Chloromethyl-Cytidine Triphosphate
- Structure of the Virulence Factor, SidC Reveals a Unique PI(4)P-Specific Binding Domain Essential for Its Targeting to the Bacterial Phagosome
- Activated Brain Endothelial Cells Cross-Present Malaria Antigen
- Fungal Morphology, Iron Homeostasis, and Lipid Metabolism Regulated by a GATA Transcription Factor in
- Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin Release
- A Macrophage Subversion Factor Is Shared by Intracellular and Extracellular Pathogens
- A Novel AT-Rich DNA Recognition Mechanism for Bacterial Xenogeneic Silencer MvaT
- Reovirus FAST Proteins Drive Pore Formation and Syncytiogenesis Using a Novel Helix-Loop-Helix Fusion-Inducing Lipid Packing Sensor
- The Role of ExoS in Dissemination of during Pneumonia
- IRF-5-Mediated Inflammation Limits CD8 T Cell Expansion by Inducing HIF-1α and Impairing Dendritic Cell Functions during Infection
- Discordant Impact of HLA on Viral Replicative Capacity and Disease Progression in Pediatric and Adult HIV Infection
- Crystal Structure of USP7 Ubiquitin-like Domains with an ICP0 Peptide Reveals a Novel Mechanism Used by Viral and Cellular Proteins to Target USP7
- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- HPV16 Down-Regulates the Insulin-Like Growth Factor Binding Protein 2 to Promote Epithelial Invasion in Organotypic Cultures
- The νSaα Specific Lipoprotein Like Cluster () of . USA300 Contributes to Immune Stimulation and Invasion in Human Cells
- RSV-Induced H3K4 Demethylase KDM5B Leads to Regulation of Dendritic Cell-Derived Innate Cytokines and Exacerbates Pathogenesis
- Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular
- Border Patrol Gone Awry: Lung NKT Cell Activation by Exacerbates Tularemia-Like Disease
- The Curious Road from Basic Pathogen Research to Clinical Translation
- From Cell and Organismal Biology to Drugs
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
- A 21st Century Perspective of Poliovirus Replication
- Is Development of a Vaccine against Feasible?
- Waterborne Viruses: A Barrier to Safe Drinking Water
- Battling Phages: How Bacteria Defend against Viral Attack
- Archaea in and on the Human Body: Health Implications and Future Directions
- Degradation of Human PDZ-Proteins by Human Alphapapillomaviruses Represents an Evolutionary Adaptation to a Novel Cellular Niche
- Natural Variants of the KPC-2 Carbapenemase have Evolved Increased Catalytic Efficiency for Ceftazidime Hydrolysis at the Cost of Enzyme Stability
- Potent Cell-Intrinsic Immune Responses in Dendritic Cells Facilitate HIV-1-Specific T Cell Immunity in HIV-1 Elite Controllers
- The Mammalian Cell Cycle Regulates Parvovirus Nuclear Capsid Assembly
- Host Reticulocytes Provide Metabolic Reservoirs That Can Be Exploited by Malaria Parasites
- The Proteome of the Isolated Containing Vacuole Reveals a Complex Trafficking Platform Enriched for Retromer Components
- NK-, NKT- and CD8-Derived IFNγ Drives Myeloid Cell Activation and Erythrophagocytosis, Resulting in Trypanosomosis-Associated Acute Anemia
- Successes and Challenges on the Road to Cure Hepatitis C
- BRCA1 Regulates IFI16 Mediated Nuclear Innate Sensing of Herpes Viral DNA and Subsequent Induction of the Innate Inflammasome and Interferon-β Responses
- A Structural and Functional Comparison Between Infectious and Non-Infectious Autocatalytic Recombinant PrP Conformers
- Phosphorylation of the Peptidoglycan Synthase PonA1 Governs the Rate of Polar Elongation in Mycobacteria
- Human Immunodeficiency Virus Type 1 Nef Inhibits Autophagy through Transcription Factor EB Sequestration
- Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
- EBV BART MicroRNAs Target Multiple Pro-apoptotic Cellular Genes to Promote Epithelial Cell Survival
- Single-Cell and Single-Cycle Analysis of HIV-1 Replication
- TRIM32 Senses and Restricts Influenza A Virus by Ubiquitination of PB1 Polymerase
- The Herpes Simplex Virus Protein pUL31 Escorts Nucleocapsids to Sites of Nuclear Egress, a Process Coordinated by Its N-Terminal Domain
- Host Transcriptional Response to Influenza and Other Acute Respiratory Viral Infections – A Prospective Cohort Study
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- Battling Phages: How Bacteria Defend against Viral Attack
- A 21st Century Perspective of Poliovirus Replication
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



