-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Host Delivery of Favorite Meals for Intracellular Pathogens
article has not abstract
Published in the journal: Host Delivery of Favorite Meals for Intracellular Pathogens. PLoS Pathog 11(6): e32767. doi:10.1371/journal.ppat.1004866
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004866Summary
article has not abstract
Introduction
Pathogens grow and cause disease by exploiting the host as a rich and diverse source of food. However, it is not always an easy task to tap these food resources since the host innate immune response restricts pathogen access to crucial nutrients (“nutritional immunity”) [1]. Pathogens have acquired various mechanisms to evade host nutritional innate immunity and to trigger the host to generate additional preferable sources of carbon, nitrogen, and energy.
Pathogens utilize various nutrients at vastly different rates. Some nutrients such as metal ions, cofactors, and monomeric components of proteins, lipids, and carbohydrates are directly incorporated into biomass. In addition, pathogens need to degrade substantial amounts of nutrients to small excreted waste products in order to obtain the energy they require for assembling biomass components and maintaining homeostasis (such as counteracting dissipation of membrane gradients). Uptake and metabolism of such energy sources is generally much faster compared to nutrients that are directly incorporated into new biomass.
While extracellular pathogens can often exploit rich energy sources delivered to them by the host circulation, intracellular bacterial pathogens depend on their surrounding host cells for supply of energy sources at sufficiently high rates. This extensive metabolic interplay between host cells and the pathogens that they nurture is likely full of fascinating, rich biology. However, these major fluxes remain poorly characterized since common methods to study pathogen metabolism such as tracking incorporation of isotope-labelled carbon/nitrogen into biomass are not informative on nutrients converted into excreted waste products.
On the other hand, new approaches start to unravel how intracellular pathogens acquire energy sources at sufficiently high rates for growth and disease—in particular, intravacuolar pathogens that must import nutrients across the vacuolar membrane. This Pearl article will highlight acquisition of energy sources by intravacuolar pathogens and its role in disease. For other aspects of microbial nutrition in vivo and host mechanisms for nutrient restriction, the reader is referred to various recent reviews [2–5].
The Hunger for Energy
Bacterial proliferation requires high amounts of energy. For bacteria such as Escherichia coli, generating a daughter cell requires hydrolysis of some 8 x 109 adenosine triphosphate (ATP) molecules to assemble biomass and support essential maintenance requirements, even if all monomeric components (amino acids, nucleosides, sugars, etc.) are freely available [6]. Even in minimal media with a single carbon source such as glucose where the bacterium has to synthesize all biomass components itself, energy production is the single most important metabolic activity of E. coli (about 37% of glucose is used for ATP generation) [6]). Pathogens thus must access a suitable host energy source to cause disease. Relevant energy sources can be identified based on consumption and waste product profiles as measured by metabolomics or from major growth defects of strains defective for certain nutrient utilization pathways.
For extracellular pathogens with direct access to blood or interstitial fluid, host glucose and glutamine provide rich energy sources that are rapidly replenished by the host circulation. In contrast, intracellular pathogens access diverse host cell metabolites, but these nutrients are quickly exhausted if not actively replenished by the host cell. As an example, ten Shigella cells that rapidly grow in the cytosol of a human epithelial cell would completely consume the most abundant host metabolites within just a few minutes [6,7]). Therefore, a robust continuous host nutrient supply pipeline within viable host cells is essential to meet the energy demands of intravacuolar pathogens that also face the challenge of importing across the pathogen-containing vacuole.
What nutrients can be delivered by host cells at sufficiently high rates to meet the energy demands of intracellular pathogens? The host cells mostly depend on abundant blood metabolites, in particular glucose and glutamine, but also lactate in areas with limited oxygenation (Fig 1). Host cells possess high-rate uptake systems for these metabolites, and during inflammation, glucose transport is even further enhanced.
Fig. 1. Schematic overview of host nutrient supply for intracellular pathogens (blue, cytosolic pathogen; green, vacuolar pathogen; AA, amino acids; Glc, glucose; Glyc, glycerol). 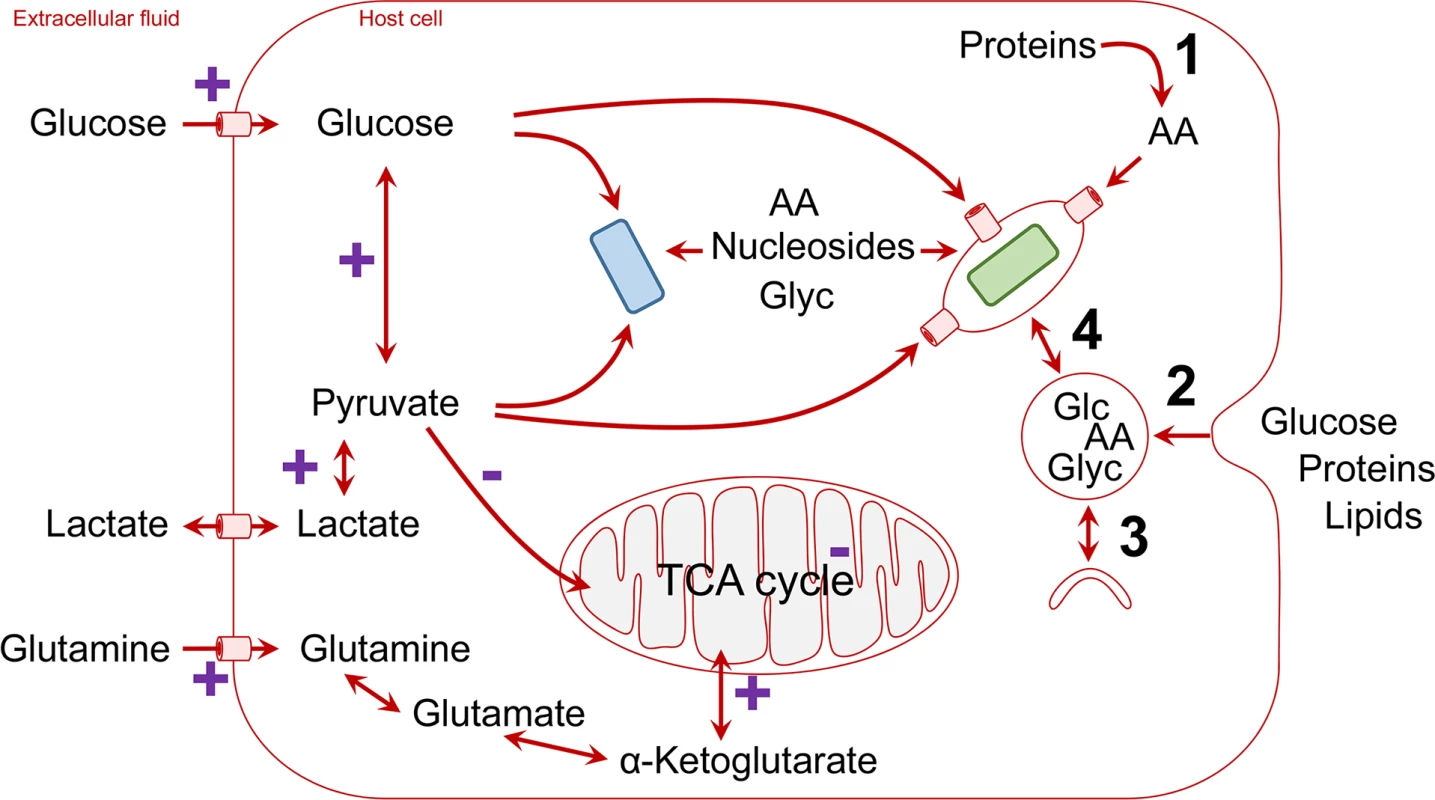
Cellular mechanisms that convert polymeric nutrients into small building blocks and deliver them to vacuolar pathogens are shown on the right (1, degradation of proteins to amino acids by proteasomes; 2, endocytosis and degradation in lysosomes; 3, autophagosome formation and delivery to lysosomes; 4, vesicle trafficking and fusion/luminal exchange with pathogen-containing vacuole). Pathways that are stimulated (+) or repressed during hypoxic conditions within inflammatory foci are labelled in purple. Pathogens utilize diverse sources of host energy (Table 1). Cytosolic pathogens such as enteroinvasive E. coli (EIEC) can directly use incoming glucose (Fig 1) [2], whereas intravacuolar pathogens can access host cell glucose when using pathogen-encoded or host cell glucose transporters in the vacuolar membrane (Fig 1) [8]. As an alternative pathway, Salmonella-containing vacuoles have extensive exchange with endocytic vesicles [9,10], which may enhance acquisition of glucose from the extracellular environment [2,11]. Interestingly, activation of peroxisome proliferator-activated receptor γ (PPAR γ) or PPARδ enhances glucose availability for intravacuolar pathogens in the M2 subset of host macrophages and promotes pathogen persistence, which has been shown for Salmonella and Brucella [12,13]. However, during acute infection, glucose in infected tissues seems to play a moderate role for Salmonella nutrition [14].These studies show a clear dynamic interaction between the host metabolism and metabolism of intravacuolar pathogens.
Tab. 1. Main energy sources of intracellular pathogens. 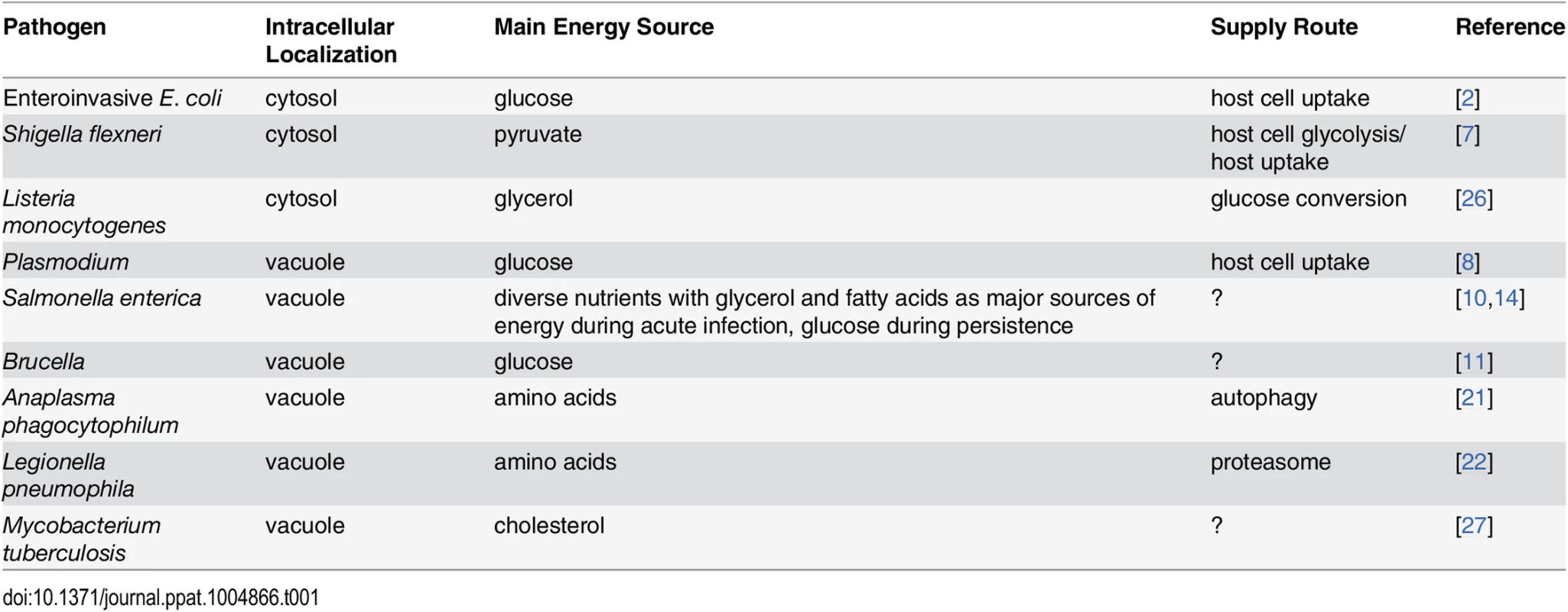
Lactate is excreted by cells that ferment glucose and can be reimported by other cells. Fermentation occurs in tissues with limited oxygen supply [15], a condition often encountered in infected tissues. In addition, activated macrophages also switch from respiration to fermentation, even in the presence of ample oxygen [16]. As a result, lactate levels are elevated in many infected host microenvironments. Extracellular lactate can be rapidly imported by cells and is immediately converted in the cytosol into pyruvate if enough oxygen is present to consume released reduction equivalents (Fig 1). An alternative route to pyruvate is host cell glucose uptake and metabolism through glycolysis. Being one of the major high-flux pathways in mammalian cells, glycolysis can continuously provide pyruvate at very high rates that easily meet even voracious demands for fast-growing intracellular Shigella (Fig 1) [7]. Various other intracellular pathogens, such as Legionella, can also effectively utilize pyruvate, but its mechanism of import into the pathogen-containing vacuoles and whether it plays a role in nutritional virulence are not yet established [7,17–19].
It is important to note that in primary cells with sufficient oxygenation, host cell mitochondria also take up pyruvate for fueling the tricarboxylic acid (TCA) cycle. Pathogens will thus compete with the mitochondria for the cytoplasmic pyruvate pool (Fig 1). This important aspect might be underestimated in common cell culture infection models that employ cell lines with minor mitochondrial pyruvate uptake even in presence of oxygen (aerobic glycolysis, the Warburg effect; see below). On the other hand, even fully functional mitochondria have a pyruvate transporter with only moderate affinity (KM in the range of 0.5 mM) and low transport rate [20], compared to bacteria such as E. coli, which has at least two high-affinity pyruvate transporters (KM in the range of 10 μM) [21]. Pathogens might thus effectively compete with mitochondria even in primary cells with active respiration.
In addition to exploiting host cell nutrient uptake, or host metabolism, some intravacuolar pathogens employ diverse sophisticated mechanisms to exploit valuable nutrients released by host cell degradation of polymeric biomass components, in particular proteins (Fig 1). Coxiella resides in phagolysosomes, where it resists the highly adverse conditions and captures amino acids released from proteins as part of the normal host cell protein turnover [22]. Anaplasma phagocytophilum uses the type IV-translocated effector 1 (Ats-1) to promote the host autophagy degradation pathway and gain access to amino acids [23]. In contrast, Legionella pneumophila also use amino acids (or amino acid-derived pyruvate) as the main intracellular energy sources that are metabolized in their TCA cycle [24]. To mobilize sufficient levels of host cell amino acids, Legionella injects into the host cell the type IV-translocated Ankyrin B (AnkB) effector, which functions on the pathogen-containing vacuole (PCV) as a platform for the assembly of polyubiquitinated proteins, which are targeted for proteasomal degradation [17]. Inhibition of AnkB-dependent proteasomal degradation blocks Legionella growth within the PCV, and this growth defect is totally bypassed upon supplementation of cysteine (Cys), serine (Ser), alanine (Ala), pyruvate, or citrate, all of which feed the TCA cycle [17].
Importantly, diverse sources of nutrients are most likely captured within a specific tissue as major sources of carbon and energy (Table 1). Salmonella access many diverse host nutrients in infected mouse spleen [14]. Major nutrients include glycerol and fatty acids that are presumably released by lipid degradation. In addition, Salmonella obtains carbohydrates such as N-acetylglucosamine [14], which is usually part of macromolecules, suggesting again host cell degradation as part of the nutrient supply pipeline. Together, data for Salmonella suggest that instead of one major energy source, the host–pathogen metabolic interface can be much more complex, with a diversified portfolio of energy sources. Interestingly, glycerol generated from lipid degradation and/or glycolytic intermediates can also be a major energy source for intracellular pathogens (Table 1) [3,25,26]. The intravacuolar pathogen Mycobacterium tuberculosis seems to be peculiar, as it mainly consumes host lipids such as cholesterol as sources of energy [27] but also remodels some of these lipids to generate its own essential lipids, including mycolic acids [28]. We speculate that it is more likely that many intravacuolar pathogens have evolved to utilize a diverse portfolio of host energy sources that are imported into the PCV lumen, instead of relying on one nutrient that may become scarce under certain conditions (Table 1).
Pathogen Sources of Energy as Essential Host Metabolites
There is an emerging theme that many sources of energy, and amino acids in particular, are essential for intracellular pathogens as well as their host cells. Human cells are auxotrophic for nine amino acids (leucine [Leu], isoleucine [Ile], methionine [Met], valine [Val], threonine [Thr], phenylalanine [Phe], tryptophan [Trp], histidine [His], and lysine [Lys]), while Cys is semiessential and is the most limiting amino acid in human cells. Therefore, intracellular pathogens have evolved with nutritional strategies to enhance the level of these essential sources of energy. The cytosolic pathogen, Francisella, is auxotrophic for six amino acids (His, Lys, Met, Cys, arginine [Arg], and tyrosine [Tyr]). Interestingly, Francisella boosts the levels of free Cys in the host cell cytosol using its γ-glutamyl transpeptidase (Ggt) enzyme to cleave host glutathione (GSH) (L-γ-L-glutamyl-L-Cysteinyl-glycine) [29]. Similarly, the intravacuolar pathogen Legionella is auxotrophic for several amino acids (Leu, Ile, Met, Val, Thr, Cys, and Arg), five of which are essential for human cells [30]. We speculate that access of intracellular pathogens to host energy sources has been a major factor in nutritional evolution and adaptation of pathogens to the intravacuolar environment. Future studies should determine the role of host auxotrophy in the nutritional and metabolic evolution of intracellular pathogens [30,31].
Importing Nutrients across the PCV Membrane
Intravacuolar pathogens are faced with the additional challenge of importing nutrients across the vacuolar membrane. However, there is very limited knowledge of how intravacuolar pathogens import nutrients from the host cell cytosol across the vacuolar membrane and into the lumen of the pathogen-containing vacuole (Fig 1). In addition to the transporters/pores employed by Plasmodium and Toxoplasma (see above), evidence for bacterial pathogens suggests participation of host solute-carrier (SLC) transporters, the second largest superfamily (~400 putative transporters) of membrane proteins in humans [32]. The SLCs include passive transporters, Na+ - or H+-coupled symporters, and antiporters, located in cellular and organelle membranes. About 25% of all SLCs are members of seven SLC families that transport amino acids, but other substrates such as glucose, lipids, and drugs are also transported by specific SLCs.
In particular, it has been shown that the host cationic amino acid transporter SLC7A1 is acquired by the PCV-harboring Salmonella and Mycobacterium within macrophages, where it imports Arg across the pathogen-containing vacuolar membrane [33]. SLC1A5, which imports neutral amino acids, is essential for intravacuolar proliferation of Legionella [34], but it remains to be determined whether the transporter is localized to the membrane of the PCV (Fig 1). High-throughput proteomic analyses of the Legionella PCV within human macrophages indicated the presence of a few SLCs that transport various amino acids [35], and transcriptome analysis has shown up-regulation of many SLCs during infection [36]. In addition, some pathogens might translocate their own nutrient transporters to be incorporated into the vacuolar membrane (as proposed for the Toxoplasma pore). Finally, PCVs might extensively communicate with other cellular vesicles, exchanging luminal contents. This has been documented for Salmonella, which acquires extracellular nutrients through stealing cargo of normal host cell endocytosis [9,10].
Modulation of Host Cell Metabolism during Infection In Vivo—Challenges
It is clear that energy supply is one of the most crucial aspects determining pathogen growth and virulence. However, still only a small minority of pathogen metabolism studies are focused on this important issue (Table 1). The classical focus on auxotrophic strains is not informative for energy production, and sophisticated metabolomic studies that determine carbon and/or nitrogen label incorporation in biomass can yield only indirect evidence for energy. The most direct approach to unravel pathogen energy production is quantitative analysis of metabolic waste products in combination with various labeling and mutagenesis strategies [7]. We can expect more insights from similar approaches for various pathogen–host interactions.
However, separation of host and pathogen metabolites to directly determine participation of host and pathogen pathways remains challenging because of the very short turnover time, which can result in substantially altered metabolomes during pathogen purification attempts. New techniques that can follow metabolites in a spatially resolved manner would offer fascinating opportunities to overcome these difficulties. In the meantime, specific perturbation of host and/or pathogen enzymes can provide important insights on relevant pathways and their localization in the host or pathogen cells. Once major energy sources have been unraveled, host pathways that supply them at sufficiently high rates can be investigated. In many cases, such pipelines are manipulated by the pathogen as a major part of their molecular virulence mechanisms.
Our knowledge of metabolic host responses to bacterial pathogens during infection is still limited because of the major experimental challenges of the infection model and the complicated analytical tools and methods. Many studies have utilized metabolic flux analysis revealing host cell metabolic alterations and carbon fluxes during infection by various pathogens (see [37] for a recent review). In general, some common themes have been observed, but otherwise there are major differences between the pathogens in terms of the host metabolic modulation during infection. Some of these modulations can be caused by direct manipulation of the host metabolism by the pathogen, while others are indirect host cell responses to infection. However, the in vitro tissue culture systems used to determine the host cell metabolic response are difficult to extrapolate to the in vivo conditions in infected tissues.
We focused this Pearl article on the diverse strategies utilized by various intracellular pathogens to acquire preferable energy sources at sufficiently high rates within the host cell. However, the two main carbon and nitrogen sources for mammalian cells are glucose (Glu) and glutamine (Gln), which are imported by SLC transporters [32]. A two-enzymatic step converts Gln into Glu and then to α-ketogluterate, which feeds the TCA cycle (Fig 1). In most differentiated cells, there is a balanced carbon flux through various catabolic pathways, while oxidative phosphorylation from the TCA cycle is the main route to generate ATP [37]. However, most transformed cells utilize glycolysis as the main catabolic pathway for generation of ATP, which has been designated as aerobic glycolysis or the Warburg effect [37]. In addition to enhanced glycolysis in transformed cells, glutaminolysis is enhanced, which provides TCA intermediates (Fig 1) [37]. Tissue culture studies using primary or transformed cell lines utilize media containing high levels of glucose and amino acids (especially glutamine) and growth factors, which alter cellular regulation of nutrient transporters and metabolic pathways. These in vitro nutritional environments are rarely encountered by bacterial pathogens in vivo. Therefore, the metabolic responses observed in tissue culture may vary considerably from the in vivo environment. Moreover, in response to hypoxia encountered within inflammatory foci, the cells respond to it through up-regulation of the hypoxia inducible factor (HIF-1) [38,39], which activates hundreds of genes required for adaption to hypoxia, including the glucose transporter and glycolytic enzymes as well as lactate dehydrogenase [5,40]. In addition, part of the inflammatory response is triggering nuclear factor kappa B (NF-κB), which is a major regulator of various nutrient transporters and metabolic pathways [5,40]. However, since macrophages in hypoxic inflammatory foci undergo metabolic shift to aerobic glycolysis and enhanced glutaminolysis, the generation of high levels of lactate and pyruvate and TCA intermediates [5,37,40] may provide a major source of carbon and energy for various intracellular pathogens in vivo (Fig 1). However, in vitro modeling of the dynamic hypoxic inflammatory foci that contain various host cells would be very challenging with the current technologies available. It is clear that major advances in the field will depend on the development of innovative tools and technologies to model the infection in vitro and to overcome the challenges in deciphering host-microbe nutritional and metabolic cross talk in vivo.
Zdroje
1. Rohmer L, Hocquet D, Miller SI. Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends Microbiol. 2011;19(7):341–8. doi: 10.1016/j.tim.2011.04.003 21600774
2. Eisenreich W, Dandekar T, Heesemann J, Goebel W. Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nat Rev Microbiol. 2010;8(6):401–12. doi: 10.1038/nrmicro2351 20453875
3. Fuchs TM, Eisenreich W, Heesemann J, Goebel W. Metabolic adaptation of human pathogenic and related nonpathogenic bacteria to extra - and intracellular habitats. FEMS microbiology reviews. 2012;36(2):435–62. doi: 10.1111/j.1574-6976.2011.00301.x 22092350
4. Abu Kwaik Y, Bumann D. Microbial quest for food in vivo: 'Nutritional virulence' as an emerging paradigm. Cell Microbiol. 2013;15(6):882–90. doi: 10.1111/cmi.12138 23490329
5. Tsalikis J, Croitoru DO, Philpott DJ, Girardin SE. Nutrient sensing and metabolic stress pathways in innate immunity. Cell Microbiol. 2013;15(10):1632–41. doi: 10.1111/cmi.12165 23834352
6. Orth JD, Conrad TM, Na J, Lerman JA, Nam H, Feist AM, et al. A comprehensive genome-scale reconstruction of Escherichia coli metabolism—2011. Molecular systems biology. 2011;7 : 535. doi: 10.1038/msb.2011.65 21988831
7. Kentner D, Martano G, Callon M, Chiquet P, Brodmann M, Burton O, et al. Shigella reroutes host cell central metabolism to obtain high-flux nutrient supply for vigorous intracellular growth. Proc Natl Acad Sci U S A. 2014;111(27):9929–34. doi: 10.1073/pnas.1406694111 24958876
8. Kirk K, Lehane AM. Membrane transport in the malaria parasite and its host erythrocyte. The Biochemical journal. 2014;457(1):1–18. doi: 10.1042/BJ20131007 24325549
9. Drecktrah D, Knodler LA, Howe D, Steele-Mortimer O. Salmonella trafficking is defined by continuous dynamic interactions with the endolysosomal system. Traffic. 2007;8(3):212–25. 17233756
10. Krieger V, Liebl D, Zhang Y, Rajashekar R, Chlanda P, Giesker K, et al. Reorganization of the endosomal system in Salmonella-infected cells: the ultrastructure of Salmonella-induced tubular compartments. PLoS Pathog. 2014;10(9):e1004374. doi: 10.1371/journal.ppat.1004374 25254663
11. Bowden SD, Rowley G, Hinton JC, Thompson A. Glucose and glycolysis are required for the successful infection of macrophages and mice by Salmonella enterica serovar typhimurium. Infect Immun. 2009;77(7):3117–26. doi: 10.1128/IAI.00093-09 19380470
12. Eisele NA, Ruby T, Jacobson A, Manzanillo PS, Cox JS, Lam L, et al. Salmonella require the fatty acid regulator PPARdelta for the establishment of a metabolic environment essential for long-term persistence. Cell Host Microbe. 2013;14(2):171–82. doi: 10.1016/j.chom.2013.07.010 23954156
13. Xavier MN, Winter MG, Spees AM, den Hartigh AB, Nguyen K, Roux CM, et al. PPARgamma-mediated increase in glucose availability sustains chronic Brucella abortus infection in alternatively activated macrophages. Cell Host Microbe. 2013;14(2):159–70. doi: 10.1016/j.chom.2013.07.009 23954155
14. Steeb B, Claudi B, Burton NA, Tienz P, Schmidt A, Farhan H, et al. Parallel exploitation of diverse host nutrients enhances Salmonella virulence. PLoS Pathog. 2013;9(4):e1003301. doi: 10.1371/journal.ppat.1003301 23633950
15. Goda N, Kanai M. Hypoxia-inducible factors and their roles in energy metabolism. International journal of hematology. 2012;95(5):457–63. doi: 10.1007/s12185-012-1069-y 22535382.
16. Galvan-Pena S, O'Neill LA. Metabolic reprograming in macrophage polarization. Frontiers in immunology. 2014;5 : 420. doi: 10.3389/fimmu.2014.00420 25228902
17. Price CT, Al-Quadan T, Santic M, Rosenshine I, Abu Kwaik Y. Host proteasomal degradation generates amino acids essential for intracellular bacterial growth. Science. 2011;334(6062):1553–7. doi: 10.1126/science.1212868 22096100
18. Dieppedale J, Gesbert G, Ramond E, Chhuon C, Dubail I, Dupuis M, et al. Possible links between stress defense and the tricarboxylic acid (TCA) cycle in Francisella pathogenesis. Molecular & cellular proteomics: MCP. 2013;12(8):2278–92.
19. Weinstein I, Guss ML, Altenbern RA. Pyruvate oxidation by Pasteurella tularensis strains of graded virulence. J Bacteriol. 1962;83 : 1010–6. 14005758
20. Schell JC, Rutter J. The long and winding road to the mitochondrial pyruvate carrier. Cancer & metabolism. 2013;1(1):6.
21. Kreth J, Lengeler JW, Jahreis K. Characterization of pyruvate uptake in Escherichia coli K-12. PLoS One. 2013;8(6):e67125. doi: 10.1371/journal.pone.0067125 23818977
22. Omsland A, Hackstadt T, Heinzen RA. Bringing culture to the uncultured: Coxiella burnetii and lessons for obligate intracellular bacterial pathogens. PLoS Pathog. 2013;9(9):e1003540. doi: 10.1371/journal.ppat.1003540 24039571
23. Niu H, Xiong Q, Yamamoto A, Hayashi-Nishino M, Rikihisa Y. Autophagosomes induced by a bacterial Beclin 1 binding protein facilitate obligatory intracellular infection. Proc Natl Acad Sci U S A. 2012;109 : 20800–7. doi: 10.1073/pnas.1218674109 23197835
24. Fonseca MV, Swanson MS. Nutrient salvaging and metabolism by the intracellular pathogen Legionella pneumophila. Frontiers in cellular and infection microbiology. 2014;4 : 12. doi: 10.3389/fcimb.2014.00012 24575391
25. Gillmaier N, Götz A, Schulz A, Eisenreich W, Goebel W. Metabolic Responses of Primary and Transformed Cells to Intracellular Listeria monocytogenes. PLoS ONE. 2012;7(12):e52378. doi: 10.1371/journal.pone.0052378 23285016
26. Grubmuller S, Schauer K, Goebel W, Fuchs TM, Eisenreich W. Analysis of carbon substrates used by Listeria monocytogenes during growth in J774A.1 macrophages suggests a bipartite intracellular metabolism. Frontiers in cellular and infection microbiology. 2014;4 : 156. doi: 10.3389/fcimb.2014.00156 25405102
27. Pandey AK, Sassetti CM. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci U S A. 2008;105(11):4376–80. doi: 10.1073/pnas.0711159105 18334639
28. Smith PL, Chiossone DC, McCafferty GP. Characterization of LTC4 effects on rabbit ileal mucosa in vitro. Naunyn-Schmiedeberg's archives of pharmacology. 1990;341(1–2):94–100.
29. Alkhuder K, Meibom KL, Dubail I, Dupuis M, Charbit A. Glutathione provides a source of cysteine essential for intracellular multiplication of Francisella tularensis. PLoS Pathog. 2009;5(1):e1000284. doi: 10.1371/journal.ppat.1000284 19158962
30. Price CT, Richards AM, Von Dwingelo JE, Samara HA, Abu Kwaik Y. Amoeba host-Legionella synchronization of amino acid auxotrophy and its role in bacterial adaptation and pathogenic evolution. Environ Microbiol. 2014;16(2):350–8. doi: 10.1111/1462-2920.12290 24112119
31. Abu Kwaik Y. Nutrition-based evolution of intracellular pathogens. Environ Microbiol Rep. 2015;7(1):2–3. doi: 10.1111/1758-2229.12236 25721587
32. Rask-Andersen M, Masuram S, Fredriksson R, Schioth HB. Solute carriers as drug targets: current use, clinical trials and prospective. Molecular aspects of medicine. 2013;34(2–3):702–10.
33. Das P, Lahiri A, Lahiri A, Sen M, Iyer N, Kapoor N, et al. Cationic amino acid transporters and Salmonella Typhimurium ArgT collectively regulate arginine availability towards intracellular Salmonella growth. PLoS One. 2010;5(12):e15466. doi: 10.1371/journal.pone.0015466 21151933
34. Wieland H, Ullrich S, Lang F, Neumeister B. Intracellular multiplication of Legionella pneumophila depends on host cell amino acid transporter SLC1A5. Mol Microbiol. 2005;55(5):1528–37. 15720558
35. Bruckert WM, Abu Kwaik Y. The complete and ubiquitinated proteome of the Legionella-containing vacuole within human macrophages. Journal of proteome research. 2015;14(1):236–248. doi: 10.1021/pr500765x 25369898
36. Price CT, Abu Kwaik Y. The transcriptome of Legionella pneumophila-infected human monocyte-derived macrophages. PLoS One. 2014; 9(12): e114914. doi: 10.1371/journal.pone.0114914 25485627
37. Eisenreich W, Heesemann J, Rudel T, Goebel W. Metabolic host responses to infection by intracellular bacterial pathogens. Frontiers in cellular and infection microbiology. 2013;3 : 24. doi: 10.3389/fcimb.2013.00024 23847769
38. Movafagh S, Crook S, Vo K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: new developments in an old debate. Journal of cellular biochemistry. 2015;116(5):696–703. doi: 10.1002/jcb.25074 25546605
39. Vyas P. Targeting HIF function: the debate continues. Blood. 2014;124(24):3510–1. doi: 10.1182/blood-2014-10-605055 25477482
40. Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nature reviews Immunology. 2009;9(9):609–17. doi: 10.1038/nri2607 19704417
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage TraffickingČlánek An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived NeuronsČlánek Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPsČlánek Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African TrypanosomiasisČlánek Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin ReleaseČlánek HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T CellsČlánek Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 6- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Introducing “Research Matters”
- Exploring Host–Pathogen Interactions through Biological Control
- Analysis of Bottlenecks in Experimental Models of Infection
- Expected and Unexpected Features of the Newly Discovered Bat Influenza A-like Viruses
- Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage Trafficking
- Recombinant Murine Gamma Herpesvirus 68 Carrying KSHV G Protein-Coupled Receptor Induces Angiogenic Lesions in Mice
- TRIM30α Is a Negative-Feedback Regulator of the Intracellular DNA and DNA Virus-Triggered Response by Targeting STING
- Targeting Human Transmission Biology for Malaria Elimination
- Two Cdc2 Kinase Genes with Distinct Functions in Vegetative and Infectious Hyphae in
- An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived Neurons
- Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPs
- Virulence Factors of Induce Both the Unfolded Protein and Integrated Stress Responses in Airway Epithelial Cells
- Peptide-MHC-I from Endogenous Antigen Outnumber Those from Exogenous Antigen, Irrespective of APC Phenotype or Activation
- Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis
- An Ultrasensitive Mechanism Regulates Influenza Virus-Induced Inflammation
- The Role of Human Transportation Networks in Mediating the Genetic Structure of Seasonal Influenza in the United States
- Host Delivery of Favorite Meals for Intracellular Pathogens
- Complement-Opsonized HIV-1 Overcomes Restriction in Dendritic Cells
- Inter-Seasonal Influenza is Characterized by Extended Virus Transmission and Persistence
- A Critical Role for CLSP2 in the Modulation of Antifungal Immune Response in Mosquitoes
- Twilight, a Novel Circadian-Regulated Gene, Integrates Phototropism with Nutrient and Redox Homeostasis during Fungal Development
- Surface-Associated Lipoproteins Link Virulence to Colitogenic Activity in IL-10-Deficient Mice Independent of Their Expression Levels
- Latent Membrane Protein LMP2A Impairs Recognition of EBV-Infected Cells by CD8+ T Cells
- Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains
- Neuronal Subtype and Satellite Cell Tropism Are Determinants of Varicella-Zoster Virus Virulence in Human Dorsal Root Ganglia Xenografts
- Molecular Basis for the Selective Inhibition of Respiratory Syncytial Virus RNA Polymerase by 2'-Fluoro-4'-Chloromethyl-Cytidine Triphosphate
- Structure of the Virulence Factor, SidC Reveals a Unique PI(4)P-Specific Binding Domain Essential for Its Targeting to the Bacterial Phagosome
- Activated Brain Endothelial Cells Cross-Present Malaria Antigen
- Fungal Morphology, Iron Homeostasis, and Lipid Metabolism Regulated by a GATA Transcription Factor in
- Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin Release
- A Macrophage Subversion Factor Is Shared by Intracellular and Extracellular Pathogens
- A Novel AT-Rich DNA Recognition Mechanism for Bacterial Xenogeneic Silencer MvaT
- Reovirus FAST Proteins Drive Pore Formation and Syncytiogenesis Using a Novel Helix-Loop-Helix Fusion-Inducing Lipid Packing Sensor
- The Role of ExoS in Dissemination of during Pneumonia
- IRF-5-Mediated Inflammation Limits CD8 T Cell Expansion by Inducing HIF-1α and Impairing Dendritic Cell Functions during Infection
- Discordant Impact of HLA on Viral Replicative Capacity and Disease Progression in Pediatric and Adult HIV Infection
- Crystal Structure of USP7 Ubiquitin-like Domains with an ICP0 Peptide Reveals a Novel Mechanism Used by Viral and Cellular Proteins to Target USP7
- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- HPV16 Down-Regulates the Insulin-Like Growth Factor Binding Protein 2 to Promote Epithelial Invasion in Organotypic Cultures
- The νSaα Specific Lipoprotein Like Cluster () of . USA300 Contributes to Immune Stimulation and Invasion in Human Cells
- RSV-Induced H3K4 Demethylase KDM5B Leads to Regulation of Dendritic Cell-Derived Innate Cytokines and Exacerbates Pathogenesis
- Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular
- Border Patrol Gone Awry: Lung NKT Cell Activation by Exacerbates Tularemia-Like Disease
- The Curious Road from Basic Pathogen Research to Clinical Translation
- From Cell and Organismal Biology to Drugs
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
- A 21st Century Perspective of Poliovirus Replication
- Is Development of a Vaccine against Feasible?
- Waterborne Viruses: A Barrier to Safe Drinking Water
- Battling Phages: How Bacteria Defend against Viral Attack
- Archaea in and on the Human Body: Health Implications and Future Directions
- Degradation of Human PDZ-Proteins by Human Alphapapillomaviruses Represents an Evolutionary Adaptation to a Novel Cellular Niche
- Natural Variants of the KPC-2 Carbapenemase have Evolved Increased Catalytic Efficiency for Ceftazidime Hydrolysis at the Cost of Enzyme Stability
- Potent Cell-Intrinsic Immune Responses in Dendritic Cells Facilitate HIV-1-Specific T Cell Immunity in HIV-1 Elite Controllers
- The Mammalian Cell Cycle Regulates Parvovirus Nuclear Capsid Assembly
- Host Reticulocytes Provide Metabolic Reservoirs That Can Be Exploited by Malaria Parasites
- The Proteome of the Isolated Containing Vacuole Reveals a Complex Trafficking Platform Enriched for Retromer Components
- NK-, NKT- and CD8-Derived IFNγ Drives Myeloid Cell Activation and Erythrophagocytosis, Resulting in Trypanosomosis-Associated Acute Anemia
- Successes and Challenges on the Road to Cure Hepatitis C
- BRCA1 Regulates IFI16 Mediated Nuclear Innate Sensing of Herpes Viral DNA and Subsequent Induction of the Innate Inflammasome and Interferon-β Responses
- A Structural and Functional Comparison Between Infectious and Non-Infectious Autocatalytic Recombinant PrP Conformers
- Phosphorylation of the Peptidoglycan Synthase PonA1 Governs the Rate of Polar Elongation in Mycobacteria
- Human Immunodeficiency Virus Type 1 Nef Inhibits Autophagy through Transcription Factor EB Sequestration
- Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
- EBV BART MicroRNAs Target Multiple Pro-apoptotic Cellular Genes to Promote Epithelial Cell Survival
- Single-Cell and Single-Cycle Analysis of HIV-1 Replication
- TRIM32 Senses and Restricts Influenza A Virus by Ubiquitination of PB1 Polymerase
- The Herpes Simplex Virus Protein pUL31 Escorts Nucleocapsids to Sites of Nuclear Egress, a Process Coordinated by Its N-Terminal Domain
- Host Transcriptional Response to Influenza and Other Acute Respiratory Viral Infections – A Prospective Cohort Study
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- Battling Phages: How Bacteria Defend against Viral Attack
- A 21st Century Perspective of Poliovirus Replication
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



