-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis
Drug resistance is complicating the treatment of parasitic diseases including African trypanosomiasis, a fatal disease if left untreated. Development of a vaccine is unlikely due to parasite antigenic variation. Current chemotherapy relies primarily on four drugs. Three of these drugs access the cell’s interior through surface transporters and resistance mechanisms are largely associated with loss-of-function mutations in the involved surface drug transporters. We reasoned that using an alternative drug entrance would circumvent parasite resistance due to mutation in a surface transporter. We have developed a drug nanocarrier that consists of polymeric nanoparticles coated with a single domain antibody that targets the trypanosome surface. This new formulation reduces the minimal curative dose and, most importantly, circumvents drug resistance in a resistant cell line as a result of mutations in the surface transporter that mediate drug uptake. This study presents a proof-of-concept of a novel technology for reversing transporter-related drug resistance with applications to other infectious diseases.
Published in the journal: Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis. PLoS Pathog 11(6): e32767. doi:10.1371/journal.ppat.1004942
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004942Summary
Drug resistance is complicating the treatment of parasitic diseases including African trypanosomiasis, a fatal disease if left untreated. Development of a vaccine is unlikely due to parasite antigenic variation. Current chemotherapy relies primarily on four drugs. Three of these drugs access the cell’s interior through surface transporters and resistance mechanisms are largely associated with loss-of-function mutations in the involved surface drug transporters. We reasoned that using an alternative drug entrance would circumvent parasite resistance due to mutation in a surface transporter. We have developed a drug nanocarrier that consists of polymeric nanoparticles coated with a single domain antibody that targets the trypanosome surface. This new formulation reduces the minimal curative dose and, most importantly, circumvents drug resistance in a resistant cell line as a result of mutations in the surface transporter that mediate drug uptake. This study presents a proof-of-concept of a novel technology for reversing transporter-related drug resistance with applications to other infectious diseases.
Introduction
Human African trypanosomiasis, also known as sleeping sickness, is caused by the flagellated protozoa T. b. gambiense and T. b. rhodesiense, which are transmitted by tsetse flies of the genus Glossina from human and/or animal reservoirs [1–2]. Trypanosomes evade their hosts’ humoral immune response through continuous variation of the variant surface glycoprotein through a process called antigenic variation, hampering the generation of conventional vaccines [3]. Therefore, treatment of African trypanosomiasis with chemotherapy is the only viable control option. HAT chemotherapy relies primarily on four drugs: pentamidine, suramin, melarsoprol and, most recently, eflornithine/nifurtimox combination therapy (NECT) [4]. All of them have limitations, ranging from problems with poor efficacy and acute toxicity to drug resistance [5].One of the most promising new therapeutic approaches for improved chemotherapy focuses on the design of polymeric nanostructures as drug delivery systems. Chitosan is a biodegradable and biocompatible compound obtained by partial deacetylation of the natural polymer chitin. Chitosan may be prepared as nanoparticle (NP) drug carriers functionalized with agents such as polyethylene glycol (PEG). Targeted delivery of nanoparticles enhances the effectiveness of the treatment, minimizes toxicity and prevents drug metabolism and elimination [6]. Active targeting and delivery can be achieved by coupling ligands or antibodies onto the surface of the NPs. For example, the single-domain antibodies (called nanobodies) are small antibodies fragments, derived from camelids heavy chain antibodies through recombinant gene technology, with unique antigen recognition properties; they can be used to target biological structures or specific cell types [7], including African trypanosomes [8–9].
Here we have developed a new polyvalent drug delivery system for the treatment of African trypanosomiasis based on PEGylated chitosan nanoparticles coated with a nanobody that specifically recognizes conserved cryptic epitopes on the parasite surface [8]. Nanoparticles were loaded with the trypanocidal drug pentamidine and its effectiveness was assayed in vitro and in vivo against T. brucei and a pentamidine resistant cell line.
Results and Discussion
Generation and characterization of the drug delivery system
We designed a nanocarrier for drugs, consisting of pentamidine-loaded functionalized PEGylated-chitosan nanoparticles, coated by a single-domain antibody (nanobody) derived from camel heavy-chain antibodies, which targets the surface of T. brucei [8–9]. More precisely, this nanobody, known as NbAn33, specifically recognizes a conserved N-linked high mannose oligosaccharide present in most VSGs [8–10]. The nanobody epitope is located close the parasite surface membrane, inaccessible for large molecules such as conventional antibodies. Heterofunctional PEG chains were employed to link NbAn33 to chitosan NPs. The molecular weight of the PEG used, 3 kDa, was an important parameter in the design of the nanocarrier. As previously reported [11], its chain length (26 nm) allows the nanobody linked to the NPs to reach its recognition epitope concealed within the densely packed VSG surface coat (about 10–15 nm thick) acting as an anchor rope for the nanoparticle.
Pentamidine-loaded functionalized PEGylated-chitosan nanoparticles coated by NbAn33, NbAn33-pentamidine-chNPs, were generated by a coacervation method [12] which allowed the generation of well-stabilized spherical NPs with an average size of ~ 135 nm in diameter [13–15] (S1 Table). A Zeta (ζ) potential value analysis showed no substantial differences between the surface charge properties of pentamidine loaded NPs and empty NPs, indicating that pentamidine was trapped inside NPs rather than just absorbed at the surface (S1 Table). PEGylation of chitosan NPs was qualitatively confirmed by nuclear magnetic resonance. The maximum pentamidine concentration loaded into NPs, expressed as entrapment efficiency and drug loading capacity, was 67% and 23%, respectively. The in vitro characterization of pentamidine release showed a biphasic profile at physiological pH: 40% of the encapsulated pentamidine was rapidly released within the first 12 h, while the remaining 60% was released at a constant rate during the following ~5 days (S1 Fig). Interestingly, the NPs showed a pH-responsive drug release (S1 Fig), likely due to swelling/degradation of the NP matrix at acid pH. This behaviour may be advantageous for the intracellular delivery of pentamidine in acidic compartments. Finally, results from blood compatibility studies indicated a broad in vivo safety margin for all the nanoparticulate formulations (S2 Table).
In vitro trypanotoxicity studies
The-inhibitory concentration (IC50) value of free pentamidine for bloodstream trypanosomes was 9.6 ± 0.3 nM (Fig 1A). The trypanolytic effect of pentamidine was significantly improved when it was loaded into NbAn33-pentamidine-chNPs. The IC50 value was 0.69 nM, which represents an approximately 14-fold reduction in drug concentration relative to free pentamidine (P<0.0001) (Fig 1A). To evaluate the contribution of NbAn33 to nanoparticles efficacy, the effect of pentamidine-loaded PEGylated chitosan nanoparticles (pentamidine-chNPs) that had not been coated by the NbAn33 was tested in parallel. The trypanocidal activity of non-coated NPs was still higher than free pentamidine (IC50 value of 1.94 nM; P<0.0001, versus free pentamidine) but lower than NbAn33-pentamidine-chNPs (P<0.0001) (Fig 1A). One factor in the relative effectiveness of the uncoated NPs compared to free pentamidine may be the electrostatic interactions between positively charged nanoparticles with the slightly negatively charged surface of the bloodstream forms. As expected, neither unloaded PEGylated chitosan nanoparticles (chNPs-empty) nor empty nanobody-coated PEGylated-chitosan nanoparticles (NbAn33-chNPs) had any effect on parasite viability (S2 Fig).
Fig. 1. Sensitive profile of T. brucei bloodstream forms. 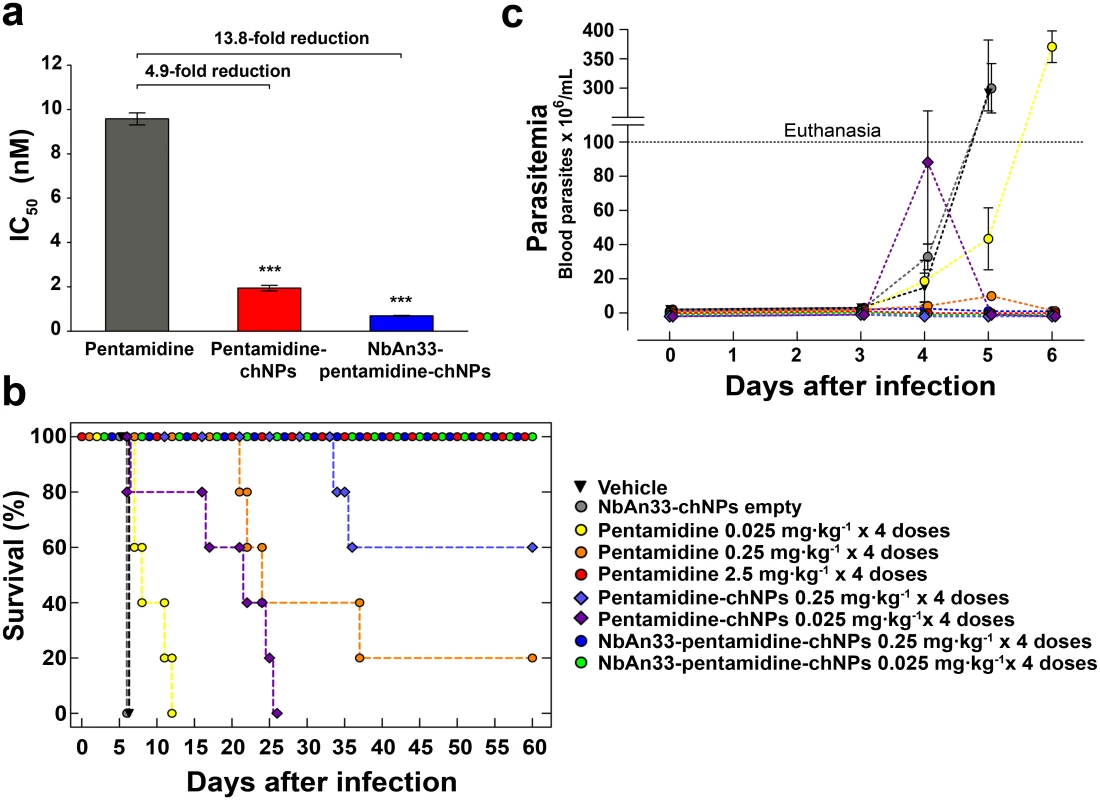
(a) IC50 analysis. Pentamidine (grey column); pentamidine-loaded PEGylated chitosan nanoparticles (pentamidine-chNPs, red column) and nanobody-coated pentamidine-loaded PEGylated chitosan nanoparticles (NbAn33-pentamidine-chNPs, blue column). Errors bars indicate S.D. from 3–9 independent experiments. Statistical significance was ***, p<0.001. (b) Therapeutic effect in T. brucei acute infection mouse model. Survival (Kaplan-Meier plot) of female C57BL/6J mice infected with T. brucei AnT1.1 (1 x 104 parasites). The treatment started once the parasites were detected in blood, at the 3rd day after inoculation and consisted in a daily dose in four consecutive days. Treatment with pentamidine, pentamidine-chNPs, NbAn33-pentamidine-chNPs, NbAn33-chNPs empty (nanobody-coated non pentamidine-loaded PEGylated chitosan nanoparticles) and vehicle (physiological saline solution). (c) Parasitemia in T. brucei acute infection mouse model. Treatment with vehicle (physiological saline solution), NbAn33-chNPs empty (nanobody-coated PEGylated-chitosan nanoparticles), free pentamidine, pentamidine-chNPs (pentamidine-loaded PEGylated chitosan nanoparticles), NbAn33-pentamidine-chNPs (nanobody-coated pentamidine-loaded PEGylated chitosan nanoparticles). In vivo therapeutic studies
The minimal full curative dose of pentamidine in a mouse model of acute infection of T. brucei was previously established as four doses of 2.5 mg·kg-1 administrated daily by intraperitoneal injection in four consecutive days, starting upon detection of parasites in blood (day 3 after infection) [16–17] and this was also curative in our model of infection (Fig 1B). Mice were intraperitoneally infected with 104 parasites. In the group of mice treated with a 10-fold lower dose of free pentamidine (4×0.25 mg·kg-1) the parasites disappeared from the peripheral blood after the third dose (Fig 1C). However, the infection relapsed and the mice began to die at day 22 after infection, curing only 20% of the treated animals (Fig 1B).
Having established the suboptimal pentamidine curative dose (4×0.25 mg·kg-1), we treated mice with an equal dose of pentamidine loaded into NbAn33-chNPs. Clearance of parasites from this group was complete after the first dose and the treatment successfully cured 100% of the animals (Fig 1B and 1C). Infected mice were also treated with the same pentamidine dose loaded into chNPs that were not coated with the nanobody. In this group parasites disappeared from the blood after the first dose, however 40% of the treated mice succumbed to the infection (Fig 1B and 1C). Next, a dose that was 100-fold lower than the minimal curative dose of pentamidine was tested. At that low dosage (4×0.025 mg·kg-1), free pentamidine did not cure any of the mice from trypanosome infection. Although the parasitemia levels increased less rapidly than in vehicle treated mice the parasites never disappeared from the blood and all mice died after 7–12 days of infection (Fig 1B and 1C). As observed in vitro, pentamidine loaded into non coated NPs was more effective than free pentamidine probably as a consequence of a sustainable drug release from the nanoparticles. Remarkably, treatment with 4 doses of NbAn33-pentamidine-chNPs at 0.025 mg·kg-1 was able to eliminate the parasitemia after the second dose, curing all treated mice (Fig 1B and 1C). However, the same low dose of pentamidine loaded into chNPs non-coated by the NbAn33, despite clearing the parasites from the blood after the third dose, did not cure mice, with 100% of the treated animals dying from the infection (median survival time 22 days).
In order to investigate the circulation kinetics of NbAn33-chNPs injected intraperitoneally, we compared the percentage of the dose of NbAn33-chNPs in peripheral blood when administered intraperitoneally versus intravenously. Thus, NbAn33-chNPs labelled with the infrared fluorophore (DY-649) were administered intravenously or intraperitoneally and fluorescence in blood was measured at various time points. Nanoparticles concentration in blood at 15 min after intravenous injection was taken as reference value (100%). About 90% of the NbAn33-chNPs intraperitoneally administered was detectable in blood after 60 min post injection (Fig 2). These results demonstrated that the intraperitoneal route was suitable for NbAn33-chNPs administration.
Fig. 2. Circulation kinetics of fluorescent chitosan nanoparticles in mouse model. 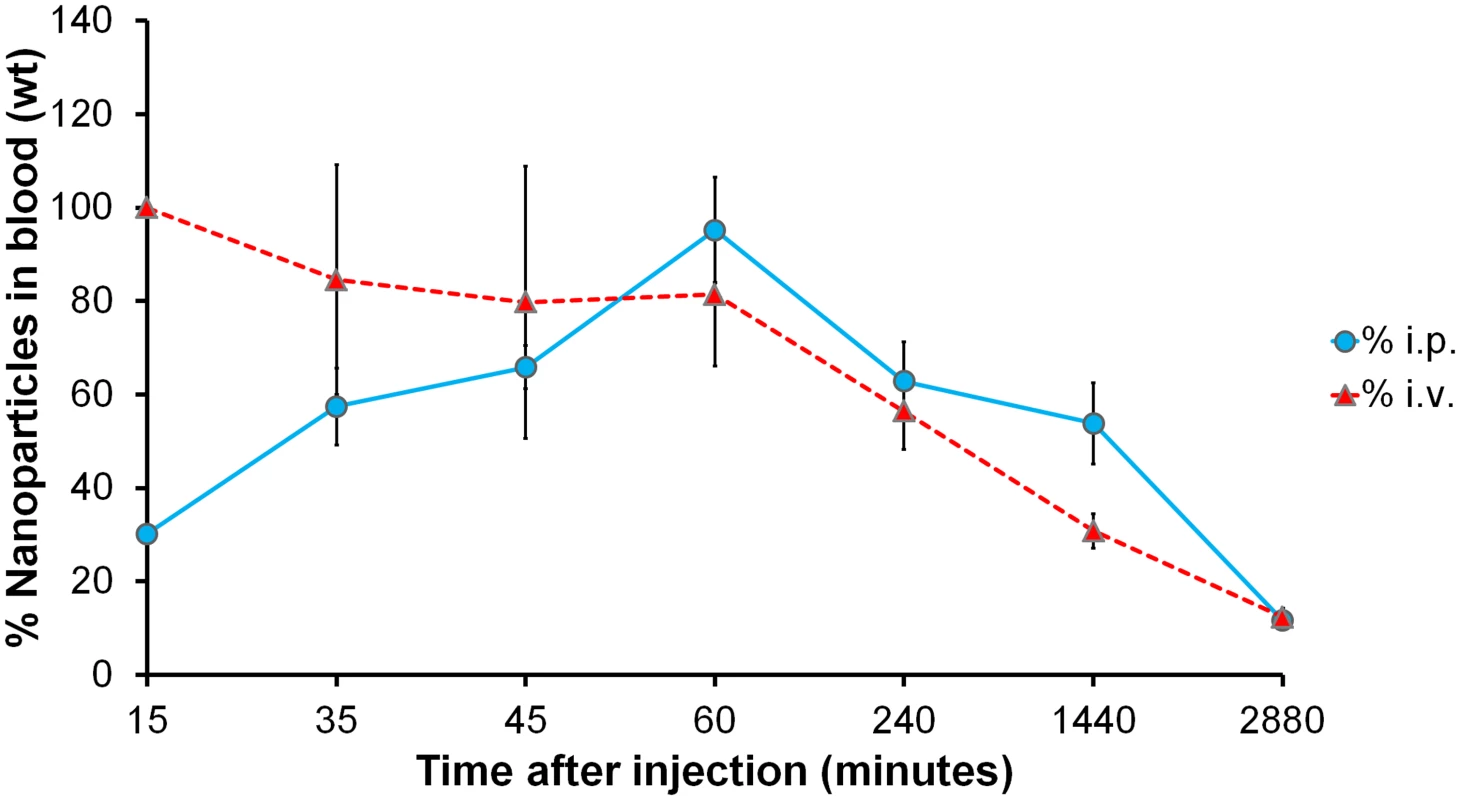
Percentage of the initial dose in peripheral blood when injected via intravenous (i.v.) and intraperitoneal (i.p.) vs. time. Error bars represent the S.D. from 5 mice. Uptake of NbAn33-coated NPs in bloodstream trypanosomes
Three previous studies have shown that NbAn33 is internalized by endocytosis in bloodstream trypanosomes [18–20]. Therefore, one would anticipate that nanoparticles coated by the nanobody NbAn33 would enter the cell via this route. Nanoparticles uptake was monitored by fluorescence after incubating bloodstream trypanosomes with Alexa-labelled-NbAn33-chNPs at 37°C. As expected, the fluorescence signal concentrates rapidly in the flagellar pocket (FP) (Fig 3A) and after in the endocytic pathway colocalizing with fluorescent tomato lectin, a marker of this route in bloodstream African trypanosomes [21] (Fig 3A). Moreover, a comparative study of the trypanocidal actvity of NbAn33-pentamidine-chNPs at 4°C and 37°C showed that at 37°C trypanotoxicity was time dependent (20% and 65% of cell death after 2 and 4 hour of incubation in the presence of pentamidine (30 μM) (Fig 3B). However, no activity was observed at 4°C under the conditions of the experiment. Together, these results indicate that NbAn33-chNPs internalization depends on the endocytic process.
Fig. 3. Endocytosis of NbAn33-chNPs. 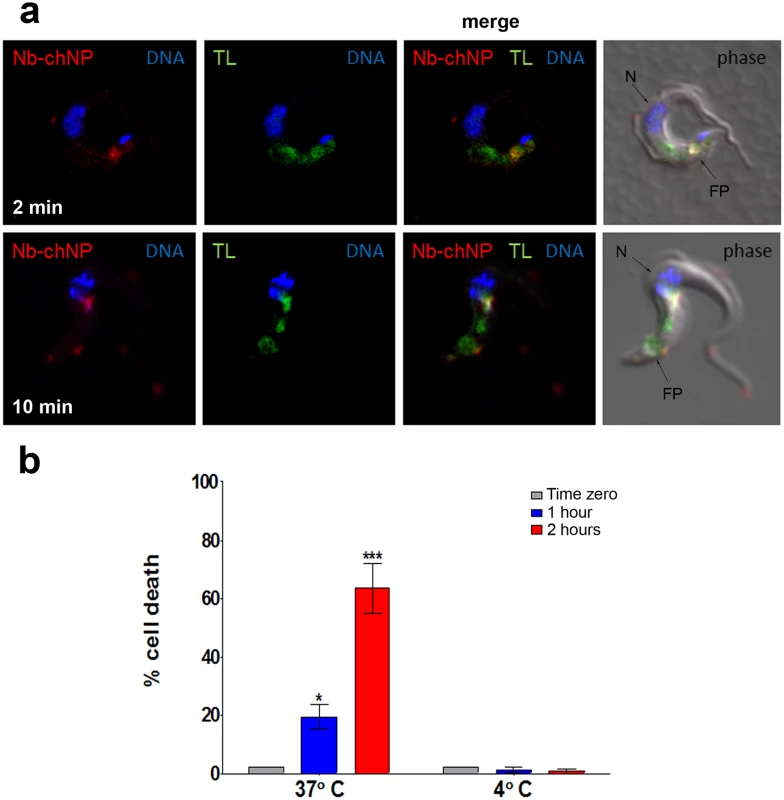
(a) Bloodstream trypanosomes observed by fluorescence microscopy after incubation with NbAn33-chNPs-Alexa Fluor 594 (red) and tomato lectin-FITC (TL, green) as described in Materials and Methods. Samples were taken after 2 minutes (bottom panel) and 10 minutes (top panel) of incubation. DNA is stained with DAPI (blue). Regions of colocalization appear yellow in merged images. (b) Parasite viability after incubation with NbAn33-pentamidine-chNPs at 37° and 4°C for 2 h. Cell death was estimated by propidium iodide staining and FACS analysis at three time points. Error bars represent the S.D. from three independent experiments. Statistical significance was *, p<0.05; ***, p<0.001. Effectiveness of pentamidine loaded NbAn33-chNPs in a pentamidine resistant cell line
Resistance to pentamidine in T. brucei is associated with mutations in cell surface transport proteins, specifically in the TbAT1/P2-adenosine transporter [22–24] and in the aquaglyceroporin 2 (AQP2) channel [25–28]. However, pentamidine loaded into nanoparticles coated by the nanobody NbAn33 is internalized by the endocytic route (Fig 3). We reasoned that this alternative drug entrance would circumvent parasite resistance to the drug. To test the hypothesis that the trypanocidal action of encapsulated pentamidine was not dependent on cell surface transporters, a T. brucei pentamidine-resistant cell line was selected after in vitro exposure to increasing concentrations of the drug (up to 50 nM). The resistant clonal cell line, designated TbR25, was genetically and functionally characterized to determine whether resistance was due to a mutation in one of the known pentamidine transporters in the plasma membrane. The TbAT1/P2-adenosine transporter gene was amplified from wild type and TbR25 genomic DNA. No differences in length or sequence were observed between both PCR products. Moreover, no significant change in TbAT1/P2 expression levels was noted by real-time qRT-PCR analysis between wild type and resistant cell line (Fig 4A). Finally, TbR25 and wild type trypanosomes were equally sensitive to diminazene aceturate (Fig 4B), another trypanocidal aromatic diamidine where uptake is almost exclusively mediated by the TbAT1/P2 adenosine transporter [29–31] Together, these data demonstrated that the adenosine transporter was not involved in the resistance to pentamidine in the TbR25 cell line.
Fig. 4. Characterization of the pentamidine resistant strain TbR25. 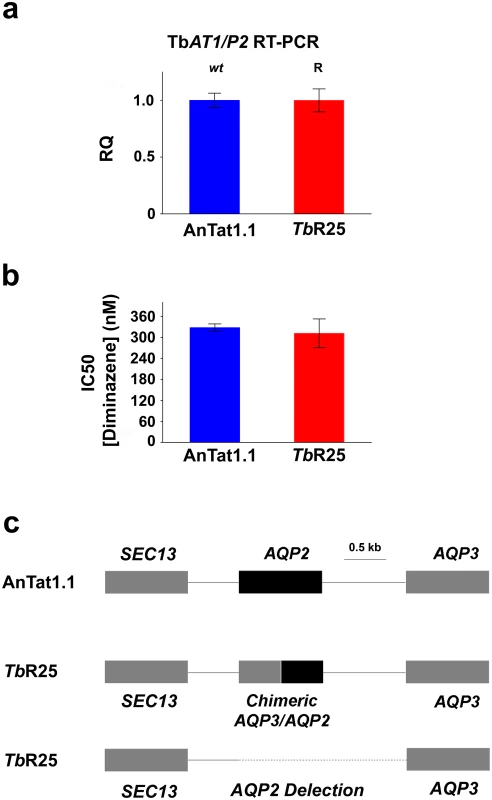
(a) Relative quantification (RQ) of TbAT1/P2 expression in wild type AnTat 1.1 and TbR25 strains estimated by qRT-PCR. (b) IC50 value for diminazene aceturate in the same strains. Error bars indicate S.D. from 3 replicates. (c) Schematic illustration of the AQP2/AQP3 locus showing the heterozygote character of TbR25 strain with a chimeric gene in one allele and the complete deletion of AQP2 gene and the intergenic region in the other (deletion from position 3441867 to 3443663 in chromosome 10, -Tb927_10_v5). We next searched for mutations in the genomic locus encoding the closely related AQP2 and AQP3 aquaglyceroporins. Interestingly, genomic PCR analysis of the AQP2/AQP3 locus showed the deletion of AQP2 gene in one allele and an AQP3/AQP2 chimeric gene in the other allele (Fig 4C and S3 Fig). In the chimera AQP3/AQP2 the first 453 nucleotides were from AQP3 and the remaining 462 nucleotides, from AQP2. In contrast, the AQP3 sequence was intact in both alleles. Rearrangements of the AQP2/AQP3 locus resulting in the loss of the wild-type AQP2 gene loss also occur in the field, where this genotype is associated with melarsoprol treatment failure [27]. This result was in agreement with the previous observation that loss of AQP2 renders T. brucei much less sensitive to pentamidine [25, 27–28]. Our results thus suggest that the mechanism of acquired resistance to pentamidine in TbR25 cell line was due to the deletion of AQP2 in one allele and the presence of the chimera AQP3/AQP2 in the other allele (Fig 4D and S3 Fig). Although a number of chimeric rearrangements in the AQP2/AQP3 have been reported [27–28] this is the first rearrangement where it is the amino terminal end is of AQP3 and the carboxy-terminal sequence is from AQP2 rather than the other way around. Recently, it has been published a model of pentamidine permeation through TbAQP2 [32], proposing that three bulky amino acids, present in the TbAQP3 pore but not in the TbAQP2 pore, help explain the difference in pentamidine permeation between these otherwise closely-related proteins. According to this model, the TbR25 rearrangement introduces at least one bulky amino acid, tryptophan 102 of AQP3, into the pore, restricting the passage of the relatively large pentamidine molecule.
Having established that the resistance mechanism was due to the absence of the wild-type coding sequence of TbAQP2, we decided to test whether NbAn33-pentamidine-chNPs were able to circumvent this resistant mechanism by avoiding the classical uptake through transporters. The IC50 value for pentamidine in the pentamidine-resistant strain TbR25 was 115 ± 5 nM (Fig 5A). However, NbAn33-pentamidine-chNPs reduced the IC50 to 9.9 ± 0.7 nM, similar to the IC50 of the wild type strain to free pentamidine (9.6 ± 0.3 nM). Uncoated pentamidine-chNPs were also able to reduce the IC50 of TbR25, although only 2.1-fold relative to free pentamidine (P<0.001) (Figs 1A and 5A).
Fig. 5. Sensitive profile of the pentamidine resistant strain TbR25. 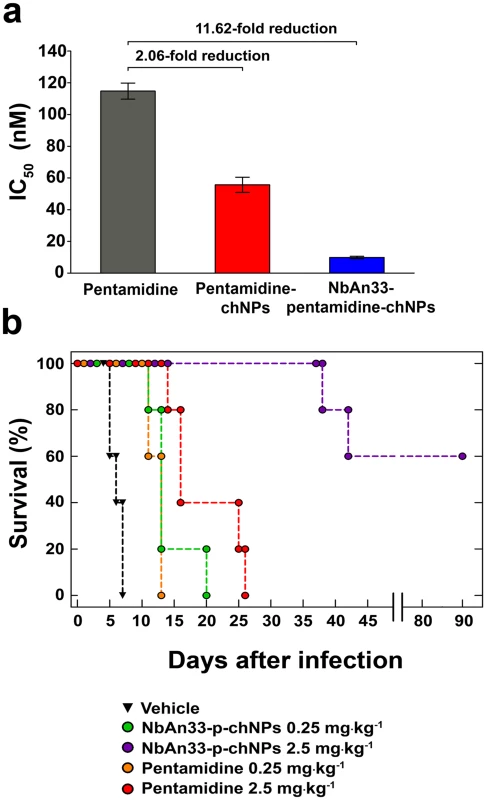
(a) IC50 analysis. Free pentamidine (grey column); pentamidine-chNPs (red column) and NbAn33-pentamidine-chNPs (blue column). Errors bars indicate SEM from 3–9 independent experiments. Fold reductions are indicated in the graph. (b) Therapeutic effect in TbR25 acute infection mouse model. Survival (Kaplan-Meier plot) of female C57BL/6J mice infected with TbR25 (inoculum 2.5 x 106 parasites). Next, we tested whether the new delivery system was able to overcome pentamidine resistance in vivo. As observed for other pentamidine resistant trypanosomes [24, 26], the TbR25 cell line was significantly less virulent than the wild type. In order to establish infection the mice were subjected to immunosuppression and inoculated with a parasite load 250-fold higher than required with our standard strain AnTat 1.1. Once infected, the animals were treated with the curative dose for wild type trypanosomes (four daily doses of 2.5 mg·kg-1) of free pentamidine and its equivalent loaded within the chNPS coated with NbAn33. All mice treated with free pentamidine died after infection, whereas 60% of the mice survived following treatment with NbAn33-pentamidine-chNPs, and the mice that did succumb to the infection lived substantially longer than those treated with free pentamidine (Fig 5B). The difference observed in NbAn33-pentamidine-chNPs effectiveness in TbR25 resistant cell line compared to wild type might be related to the loss of virulence and the fact that mice were immunosuppressed before TbR25 infection with 250 times higher parasite load. The cause of this loss of virulence is not known but is independent of mutations in the AQP2 genes since such mutations are present in field isolates [27]. Therefore, the virulence attenuation phenotype of TbR25 is likely to be associated to laboratory-selected T. brucei mutants.
For the continued treatability of African trypanosomiasis, as for many other infectious diseases, the emergence and spread of drug resistance is the major concern together with the absence of vaccines. Most of trypanocidal drugs do not diffuse freely across parasite cell membrane, but several transporters are responsible for their uptake, thereby making a crucial contribution to their selectivity [33–34]. In African trypanosomes, resistance mechanisms have generally been associated with mutations that reduce drug import, through loss of function or changes in substrate selectivity. Notably, there is currently little evidence for the involvement of drug export transporters such as multi-drug resistance-associated carriers in acquired drug resistance, as observed for other protozoa such as Plasmodium and Leishmania spp [35–36]. A recent genomic-scale screening has linked most of the current HAT drugs to specific genes encoding surface proteins involved in their uptake [22], confirming some previous results. Eflornithine resistance was associated with loss of amino acid transporter family member AAT6 [22, 37–38], suramin was linked to invariant surface glycoprotein ISG75, melarsoprol to adenosine transporter TbAT1/P2 [38–41], pentamidine to P-type H+-ATPases that maintain the proton-motive force across the plasma membrane, and pentamidine/melarsoprol cross-resistance to aquaglyceroporins, specifically to aquaglyceroporin 2, which encodes the High Affinity Pentamidine Transporter (HAPT1) and controls the susceptibility to both drugs [25, 27–28].
The use of chitosan nanoparticles-based therapy allows drug release to be tailored to the specific target site through the choice of various polymer and copolymer combinations and formulation procedures [42–43]. When the nanocarrier was designed, all possible parameters that could influence the grade of success of our nanodevice were taken into account. One of the most important was the pathway that the nanocarrier has to follow to deliver the drug. As shown in Fig 3A, once the nanoparticles reach the surface of the parasite they are taken up by endocytosis in the flagellar pocket. Along the endocytic pathway the pH is decreasing, reaching the lowest value (~pH 5) in the lysosome. Previous reports comparing different polymeric NPs have concluded that drug release from chitosan NPs is pH dependent [44], making chitosan a suitable polymer for this specific nanocarrier.
Pentamidine-loaded poly (D,L-lactide) and polymethacrylate nanoparticles have been previously used against Leishmania (L. infantum and L. major) [45–47]. Pentamidine-loaded nanoparticles were between 3.3 and 6 times more effective than free pentamidine against Leishmania in a murine model vivo. By contrast, NbAn33-pentamidine-chNPs were 100 times more effective than free pentamidine against T. brucei in vivo. However, these results are not directly comparable because Leishmania is an intracellular parasite while T. brucei is extracellular. Another crucial difference is the use of the nanobody NbAn33 for active targeting which notably increases the effectiveness of the formulation as shown in this study.
In summary, the development of chitosan nanoparticles loaded with current trypanocidal drugs coated by a specific nanobody against trypanosomes can reduce the minimal curative dose of these drugs, enhancing their efficacy, minimizing the toxicity and circumventing resistance mechanisms associated to mutation in surface transporters. The significance of the report is not limited to a single nanobody used to demonstrate the technology, nor to a single drug, nor indeed to trypanosomiasis. Due to its versatility, the possibilities that offer this targeted nanobody-system are enormous as it can be adapted to encapsulate any substance with a reported biological action. This opens up a plethora of new possible therapies to treat different infectious diseases. The most urgent issue in the treatment of infectious disease today is that resistance to drugs is spreading much faster than new drugs are being developed and approved. The use of encapsulated, nanobody-targeted drugs as described here has the potential to reverse resistance to many first-line treatments.
Materials and Methods
Ethics statement
All experiments complied with the with the guidelines of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (CETS n° 123) and were approved by the Ethics Committee of the Spanish National Research Council (CSIC, file UEA2011/JAGS/1).
Parasites
Bloodstream forms of monomorphic T. brucei AnTat 1.1 strain (Institute of Tropical Medicine, Belgium) were grown in axenic culture at 37°C and 5% CO2 in HMI-9 medium supplemented with 20% heat-inactivated foetal bovine serum (Gibco) [48].
Nanobody NbAn33
The nanobody NbAn33 used in this study was selected as previously described [8]. NbAn33 recognizes a glycosylated (Man9–5GlcNAc2) epitope on T. brucei VSGs, as indicated by its binding to synthetic Man9 and Man7 and by its competition for binding with concanavalin A [8]. Accordingly, NbAn33 binds equally well to several VSGs, such as the MiTat 1.1, MiTat 1.4, MiTat 1.5 and ETat 1.2 VSGs, which represent different VSG classes and share the conserved N-linked Man5–9 carbohydrate [8–9].
Preparation of nanobody-coated pentamidine-loaded functionalized PEGylated-chitosan nanoparticles
For the synthesis of copolymer chitosan-graft-PEG (chitosan-g-PEG), chitosan hydrochloride (80 mg) was dissolved in 11.5 mL of filtered deionized water. To which MeO-PEG-CH2CO2H (14.2 mg) and N-hydroxysuccinimide (NHS, 1.6 mg) were added. 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC, 21.7 mg) was then added gradually and the resulting solution was stirred at room temperature for 22 h. The solution was ultrafiltered (0.22 μm pore size) and lyophilized.
PEGylated chitosan nanoparticles were prepared by a coacervation method avoiding the use of toxic organic solvents [13–15]. Briefly, the copolymer chitosan-g-PEG (1%, w/v) was dissolved in 10 mL of an aqueous solution of acetic acid (2%, v/v). Next, pluronic F-68 and pentamidine (isethionate salt, Sigma) were added at increasing concentrations up to 2% (w/v) and 0.01 M, respectively. Approximately 2.5 mL of a solution of sodium sulphate (20%, w/v) was added drop wise (0.5 mL·min-1) to the chitosan solution under mechanical stirring (2,000 rpm). The stirring was continued for 1 h to ensure the formation of pentamidine loaded functionalized PEGylated-chitosan nanoparticles (pentamidine-chNPs). The newly formed pentamidine-chNPs were cleaned by 3 consecutive cycles of centrifugation for 30 min at 11,000 rpm in a Centrikon T-124 high-speed centrifuge (Kontron) and re-dispersion in double-distilled water until the conductivity of the supernatant was ≤ 10 μS·cm-1, measured in a Crison micro pH 2001 conductometer (Crison). Finally, nanobody NbAn33at 1 mg·mL-1 was added to the pentamidine-NPs suspension (10 : 1 weight ratio) in 10 mL of phosphate buffered saline (PBS, pH 7.4) containing EDC and NHS. The reaction was left for 3 h at 25°C under mechanical stirring (200 rpm). The resulting NbAn33-coated pentamidine-chNPs (NbAn33-pentamidine-chNPs) were submitted to a single wash cycle by centrifugation for 30 min at 11,000 rpm in a Centrikon T-124 high-speed centrifuge (Kontron) and suspended in 10 mL of physiological saline solution (0.09%w/v NaCl). Non-pentamidine loaded nanoparticles (NbAn33-chNPs) were prepared in parallel.
Nanoparticles characterization
Mean particle diameter (± standard deviation, S.D.) was determined by photon correlation spectroscopy (PCS) using a Malvern 4700 analyzer (Malvern). The scattering angle was set at 60° and the measurement was made after suitable dilution of the aqueous nanoparticle suspensions (0.1%, w/v). The stability of the formulations was evaluated by measuring the size of the particles after 1 month of storage at 4°C in water.
The electrophoretic mobility measurements can qualitatively distinguish the mode of pentamidine association with the NPs: encapsulation within the NP matrix or adsorption on the NP surface. Briefly, the measurements were performed in 0.1% (w/v) aqueous suspensions of NbAn33-chNPs and NbAn33-pentamidine-chNPs in 1 mM KNO3 pH 6 using a Malvern Zetasizer 2000 electrophoresis device (Malvern). Measurements were performed after 24 h of contact of NPs in water under mechanical stirring (50 rpm) at 25°C. The experimental uncertainty of the measurements was below 5%. The electrophoretic mobility was converted into zeta potential (ζ mV) values as described by O’Brien and White [13].
Quantification of pentamidine loaded into the chitosan nanoparticles
Ultraviolet-visible spectrophotometry (UV—Vis) was used to quantify the amount of pentamidine loaded into NPs at a wavelength of 261 nm in a 8500 UV-Vis Dinko spectrophotometer (Dinko). The spectrophotometric method employed was validated by ultra-high-pressure liquid chromatography and mass spectrometry (UPLC-MS) in an Acquity UPLC/QTOF Synapt G2 (Waters). After NbAn33-pentamidine-chNPs synthesis, the supernatant was obtained by double centrifugation for 30 min at 11,000 rpm and 25°C in centrifuge machine Centrikon T-124 high-speed centrifuge (Kontron) and the amount of pentamidine was measured in triplicate. Drug incorporation to NPs was expressed in terms of pentamidine entrapment efficiency (%) (encapsulated drug [mg]/total drug in the NP suspension [mg] × 100) and pentamidine loading (%) (encapsulated drug [mg]/carrier [mg] × 100).
In vitro release studies of pentamidine from chitosan nanoparticles
The study of pentamidine release was performed using the NbAn33-pentamidine-NPs with the maximal loading and entrapment efficiency reached (~23% and ~ 66%, respectively, S1 Table). The assay was performed at 37°C in triplicate following the dialysis bag method using Spectra/Por 6 dialysis membrane tubing (Spectrumlabs). The release medium for neutral and acidic conditions was PBS at pH 7.4 and pH 5 respectively. The dialysis bag with pore size 2000 Da retained the NPs, but allowed the free pentamidine to diffuse through the membrane into the release medium. Briefly, 1 mL of nanoparticle suspension (containing 0.35 mg·mL-1 of pentamidine) was pipetted into the bags, and the two ends fixed by clamps. The bags were then placed in a conical flask filled with 100 mL of the receiving phase, and were stirred at 200 rpm. Samples of 1 mL were taken at different times (0.5 h, 1 h, 3 h, 6 h, 9 h and 24 h, and 2, 3, 4 and 5 days) and analyzed for pentamidine content by UV—Vis spectrometry at 261 nm. To maintain dialysis conditions an equal volume of the release medium at 37°C was added after taking each sample.
Blood compatibility and cytotoxicity
The interaction of control (pentamidine unloaded) PEGylated chitosan nanoparticles, NbAn33-chNPs, pentamidine-chNPs, and NbAn33-pentamidine-chNPs, with blood components was investigated in triplicate following a well-defined procedure [49]. The in vitro effect of the nanoparticulate formulations on erythrocyte lysis was evaluated using PBS as negative control (0% lysis) and the non-ionic surfactant Triton X-100 (1%, w/v) as positive control (100% lysis) of hemolysis. Briefly, EDTA-anticoagulated blood was centrifuged at 1,500 rpm for 20 min to remove the plasma fraction. The original volume was then replaced with 150 mM NaCl. Upon repetition of this step, the final suspension was diluted 1 : 10 with 100 mM PBS. About 100 red blood cells·mL-1 were incubated at 37°C for 2 h with the nanoparticles (0.05%, w/v) under mechanical stirring (200 rpm), and centrifuged at 1,500 rpm for 20 min. Hemoglobin release was evaluated by measuring the UV absorbance of the supernatant at 545 nm. Hemolysis (%) was determined as ([absorbance of test sample—absorbance of control] / highest absorbance of positive control) × 100.
Interaction of platelets with nanoparticulate systems may determine their activation (and aggregation) which generates thrombotic complications and blood incompatibilities. This process is quantified by enzyme-linked immunosorbent assay (ELISA) in terms of soluble P-selectin (sP-selectin) release after incubating the nanoparticles with blood. Blood samples were centrifuged at 1,000 rpm for 20 min to remove platelet-rich supernatant. Then, blood was again centrifuged and mixed with previously extracted plasma to obtain platelet-rich plasma (PRP). Platelet-poor plasma (PPP) was obtained by centrifuging the remaining blood at 3,000 rpm for10 min. The PRP was then diluted 1 : 100 with 1% ammonium oxalate, and adjusted to a final platelet concentration of 1·108 mL-1. A sample of 0.1 mL of PRP was incubated for 1 h with 50 mg of nanoparticles at 37°C. The supernatant was then centrifuged 10 min at 5,000 rpm. Soluble P-selectin concentration in the plasma was determined using ELISA kit (eBioscence,) following to the manufacturer’s indications.
Opsonization of nanoparticles with complement components (i.e., C3a and C5a) leads to blood clearance by the reticuloendothelial system (RES). Complement activation was quantified by measuring C3a release upon incubation of the nanoparticles with pooled citrated plasma obtained by blood centrifugation. C3 cleavage was monitored by measuring the formation of its activated peptide (C3a desArg) with Complement C3a des Arg Human ELISA kit (Enzo).
Plasma clotting time was determined following the Howell’s method that evaluates plasma recalcification time (PRT). T1/2 max was quantified as the time at which half the saturate (maximum) absorbance value was reached. PBS was used as negative control. Each blood sample was centrifuged at 3,000 rpm, 25 min at 8°C to obtain the PPP. Samples of 0.1 mL of PPP were incubated with 50 μg of nanoparticles in PBS for 5 min at 37°C in a 96 well plate. Finally, 0.1 mL of 25 mM CaCl2 solution was added and the plasma solution was monitored for clotting by manually dipping a silicone-coated stainless-steel wire hook into the solution to detect fibrin threads. Clotting times were estimated as the time at which the first fibrin strand was formed on the hook.
Trypanotoxicity assay
Trypanotoxicity was determined using an adapted version of the resazurin sodium salt method [50]. Briefly, exponentially growing parasites (monomorphic T. brucei AnTat 1.1 and pentamidine resistant clonal cell line TbR25) were harvested and prepared at an initial density of 2·105 trypanosomes·mL-1. 50 μL of this trypanosome suspension was added to each well of a flat-bottom 96-well plate containing doubling dilutions of the drugs (50 μL), excepting for two rows which received only media. Eleven dilution points were tested, ranging from 680 nM to 165 pM in final pentamidine concentration. In the case of diminazene aceturate the range was between 2 μM and 2 nM. Cultured plates were incubated at 37°C in an atmosphere of 5% CO2 for 20 h before the addition of 20 μL of the colorimetric viability indicator resazurin sodium salt (Sigma) at 0.5 mM. After a further 4 h of incubation, the reaction was stopped by the addition of 50 μL of 3% SDS in water. The plates were read on a Tecan Infinite F200 reader (Tecan Austria GmbH) using an excitation wavelength of 535 nm and an emission wavelength of 590 nm. The experiment was performed with six replicates per concentration and repeated on at least four independent occasions. The results of fluorescence were normalized to 100% of the no-drug control. The IC50 was defined as the concentration of drug required to reduce the fluorescence output by 50% and its value was determined by plotting to an equation for a sigmoid curve with a variable slope, of log (test compound concentration) versus the normalized fluorescence, using Prism 5 (GraphPad Software). Statistical significance was determined by unpaired student’s t-test.
For temperature dependent trypanotoxicity assays parasites growing in logarithmic phase (5×105 cell·mL-1) were treated with NbAn33-pentamidine-chNPs (30 μM of final pentamidine) at 37°C and 4°C respectively. Samples (5×105 cells) taken at three different set time points were incubated on ice for at least 5 min, washed with phosphate-buffered saline (PBS) and incubated with propidium iodide (PI) staining solution (PBS containing 40 μg·mL-1 PI and 100 μg·mL-1 ribonuclease A) for at least 20 min on ice. The experiment was performed in parallel with untreated parasites as a control. The analysis was performed with a BD FACScanto II flow cytometer (BD Biosciences) and FlowJo software. The experiment was performed in triplicate and repeated at least three times.
In vivo therapy experiments
Animal experimental protocols were approved by the Ethics Committee of the Spanish Council of Scientific Research (CSIC). The drug delivery system NbAn33-pentamidine-chNPs was tested in vivo against the monomorphic AnTat1.1 strain using a modification of the approach previously described [16–17]. Briefly, five female C57BL/6J mice (8-week-old; Jackson Laboratories) per group were intraperitoneally infected with 104 parasites each. Once parasites were detected in the blood, at day 3 after infection, the mice were treated daily on four consecutive days with i.p. injections of pentamidine in physiological saline solution. The dosages used for each group were pentamidine 2.5 mg·kg-1; pentamidine 0.25 mg·kg-1; pentamidine 0.025 mg·kg-1; NbAn33-pentamidine-chNPs 0.25 mg·kg-1; NbAn33-pentamidine-chNPs 0.025 mg·kg-1; pentamidine-chNPs 0.25 mg·kg-1 and pentamidine-chNPs 0.025 mg·kg-1 (drug concentrations mg·kg-1 were calculated considering the molecular weight of the isethionate salt). Two control mice groups were either left untreated (injected with the same volume of physiological saline solution) or received pentamidine-free NbAn33-chNPs. We followed the parasitemia by counting the number of trypanosomes in tail-vein blood with an optical microscope with a Neubauer chamber every day during the first week, and afterwards, once per week until 60 days post-infection. Parasite survival was monitored and recorded every day until 60 days post-infection. Mice were considered cured when there was no parasitemia relapse detected in the 60 days period. Animals were humanely sacrificed when they showed severe clinical signs or when the parasitemia reached 108 parasites·mL-1.
Circulation kinetics of nanoparticles
Fluorescent NbAn33-chNPs were generated as previously described [51] with some modifications. Briefly, 0.5 mL of NbAn33-chNPs (1 mg·mL-1) were fluorescently labeled with 0.18 mM (0.2 mg) of Dy649-NHS (NbAn33-chNPs-Dy649) (Dyomics GmbH) in phosphate buffered saline (PBS) at RT for 45 min. Labeled NP were washed three times by centrifugation at 14,000 g for 30 min and resuspended in PBS at a final concentration of 1 mg·mL-1.
Mice were administered with 0.1 mg of NbAn33-chNPs-Dy649 (1 mg·mL-1) intravenously and intraperitoneally. At various time points (15 min, 30 min, 45 min, 60 min, 4 h, 24 h and 48 h), 5 μL of blood was collected from the tail and immediately diluted in 200 μL Hanks’ Balanced Salt solution (pH 7.4). The fluorescence of blood samples was measured in a plate reader (excitation 655 nm, emission 676 nm) and correlated with a standard curve of NbAn33-chNPs-Dy649 in whole blood.
In vitro generation of T. brucei resistant cell line
T. brucei AnTat 1.1 bloodstream forms (5×104 cells·mL-1) were growth in a 24-well plate in the presence of increasing concentrations of pentamidine, ranging from 1 nM to 10 nM. The highest concentration of pentamidine at which growth was detected was 1 nM. Parasites growing at this concentration were diluted to 5×104 cells·mL-1 and sub-cultured into two new wells with fresh medium containing one and half and double the drug concentration, respectively. When cells were growing at 50 nM at a rate comparable to the original (wild type) strain they were diluted to an average density of 0.3 cells·mL-1 and cultured in a 24 wells plate. Ten clones grew and five were selected for further DNA and RNA content analysis.
In vivo therapy experiments in a pentamidine resistant cell line
Mice were first immunosuppressed with 200 mg·kg-1 of cyclophosphamide (Sigma) and infected the next day with 2.5×106 parasites of TbR25. After ten passages in mice to adapt the resistant cell line to the host, 25 female C57BL/6J mice (6-week-old; Jackson Laboratories) were intraperitoneally injected with cyclophosphamide and infected with 2.5×106 parasites the next day. Once parasites were detected in blood samples, at day 1 post infection, mice were treated daily on four consecutive days. The dosages used for each group were pentamidine 2.5 mg·kg-1; pentamidine 0.25 mg·kg-1; NbAn33-pentamidine-chNPs 2.5 mg·kg-1 and NbAn33-pentamidine-chNPs 0.25 mg·kg-1. Control mice were left untreated (physiological saline injections). Parasitemia and survival were monitored as described for infection with wild type AnTat1.1. Animals were humanely sacrificed when showing severe clinical signs or when the parasitemia reached 108 parasites·mL-1.
Nanoparticles uptake
NbAn33-chNPs (1 mL at 1 mg·mL-1) were labelled using the Alexa Fluor 594 Protein labeling kit (Invitrogen), in 5 mM KCl, 80 mM NaCl, 1 mM MgSO4, 20 mM Na2HPO4, 2 mM NaH2PO4, pH 7.4) at room temperature for 1 h. Labelled NP were washed three times by centrifugation at 14,000 g for 30 min and resuspended in 5 mM KCl, 80 mM NaCl, 1 mM MgSO4, 20 mM Na2HPO4, 2 mM NaH2PO4, 20 mM glucose, pH 7.4, at a final concentration of 1 mg·mL-1. Live bloodstream forms (106 cells·mL-1) were incubated in 5 mM KCl, 80 mM NaCl, 1 mM MgSO4, 20 mM Na2HPO4, 2 mM NaH2PO4, 20 mM glucose, pH 7.4 (TDB) with Alexa 594 labelled NbAn33-chNPs (50 μg·mL-1) for 10 min at 37°C. NPs excess was removed by centrifugation at 4°C. Parasites were resuspended in TDB with tomato lectin-FITC conjugate (Sigma) at 20 μg·mL-1, incubated for either 2 or 10 min at 37°C and then fixed in 4% paraformaldehyde in PBS for 1 h at 4°C. Finally, trypanosomes were washed with PBS three times, spread on poly-L-lysine-coated slides, and mounted in DAPI-containing Vectashield medium (Vector Laboratories). For fluorescence microscopy analysis image acquisition was performed with a LSH 710 Confocal Microscope (Zeiss) and image analysis with ZEN 2012 (Zeiss) software.
Genotyping
Genomic DNA was extracted using DNAzol reagent (Invitrogen), according to the manufacturer’s protocol. The TbAT1/P2 complete open reading frame (TriTrypDB Tb927.5.286b) was amplified by PCR using the specific primers AT1F (5’ ATG CTC GGG TTT GAC TCA GC 3’) and AT1R (5’ CTA CTT GGG AAG CCC CTC AT 3’) [52]. The PCR was performed with AccuTherm DNA polymerase (Genecraft Germany) with the followings parameters: 1 cycle of 95°C for 2 min and 35 cycles of (95°C, 50 s; 50°C, 50 s; 72°C, 2.5 min). PCR products were run on a 1% agarose gel and purified on a silica membrane column (Nucleospin gel and PCR clean up, Macherey Nagel). The purified PCR products were directly sequenced with the same primers as used for PCR amplification.
The AQP2 (TriTrypDB Tb927.10.14170) and AQP3 complete genomic sequences (TriTrypDB Tb927.10.14160) were amplified with AccuTherm DNA polymerase using the forward primer AQP2/3F (5' GCT CCA GAA AAT CAG AAT GC 3') and the reverse primers AQP2R (5' GCG AAG GGT ATT GAC GGT TA 3') and AQP3R (5' GTG CCA CAC TAA TCT GCA TG 3'), respectively. The PCR conditions were: 1 cycle of 95°C for 2 min and 35 cycles of (95°C, 50 s; 47°C, 50 s; 72°C, 2.5 min). The PCR products were purified and sequenced. Two internal forward primers were designed to confirm the chimeric AQP2/AQP3 sequence; AQP2Fi (5' GAG CGG TGG GAT GCA GAT G 3') and AQP3Fi (5' CGC CAC GGT TAT CAT TGA TGG G 3'). TbAQP3/TbAQP2 sequence was submitted to GenBank; accession number KR059026. The complete AQP2-AQP3 locus was amplified using the forward primer SEC13 (5’ CAAAATCAGCGGGTTCACTG 3’) located at the end of the SEC13 gene (TriTrypDB Tb927.10.14180) and the reverse primer AQP3R. The PCR was performed with AccuTherm DNA polymerase with the followings parameters: 1 cycle of 95°C for 2 min and 35 cycles of (95°C, 50 s; 50°C, 50 s; 72°C, 6.5 min). PCR products were run on a 0.8% agarose gel and purified on a silica membrane column (Nucleospin gel and PCR clean up, Macherey Nagel). The purified bands were directly sequenced with different combinations of the above primers to cover the complete sequence of the locus.
Real time quantitative reverse transcription PCR (real-time qRT-PCR)
Trypanosomes were harvested in 1 mL TRIzol reagent (Invitrogen) and total RNA was isolated following manufacturer’s protocol. First strand cDNA synthesis was performed using SuperScript III Reverse Transcriptase (Invitrogen) and Oligo dT20 as primer. Quantitative PCR amplification was performed using SYBR Green Master Mix (Bio Rad). The levels of TbAT1 mRNA were normalized against actin mRNA and the relative quantification was calculated by the ΔΔCT method. Primers for TbAT1 were AT1_863F (5’ CGA CTT CGC AGC AGA TGT TAA TG 3’) and AT1_956R (5’ CGG CAG GGT AGA CGA GAA ATG 3’), For actin the primer used were ACT206F (5’ AAT GAG CAA GCG ATG ATG GG 3’) and ACT348R (5’ GCA ACT CGT TAT AGA AGG TAT GG 3’) [53]. Thermal cycling was carried out as follows: 1 cycle of 95°C for 5 min and 40 cycles of (95°C, 15 s; 60°C, 60 s).
Supporting Information
Zdroje
1. Barrett MP, Burchmore RJ, Stich A, Lazzari JO, Frasch AC, Cazzulo JJ, et al. The trypanosomiases. Lancet. 2003;362(9394):1469–80. Epub 2003/11/07. S0140-6736(03)14694-6 [pii]; doi: 10.1016/S0140-6736(03)14694-6 14602444.
2. Brun R, Blum J. Human african trypanosomiasis. Infect Dis Clin North Am. 2012;26(2):261–73. Epub 2012/05/29. S0891-5520(12)00012-8 [pii] doi: 10.1016/j.idc.2012.03.003 22632638.
3. Glover L, Hutchinson S, Alsford S, McCulloch R, Field MC, Horn D. Antigenic variation in African trypanosomes: the importance of chromosomal and nuclear context in VSG expression control. Cell Microbiol. 2013. Epub 2013/09/21. doi: 10.1111/cmi.12215 24047558.
4. Priotto G, Kasparian S, Mutombo W, Ngouama D, Ghorashian S, Arnold U, et al. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet. 2009;374(9683):56–64. Epub 2009/06/30. S0140-6736(09)61117-X [pii] doi: 10.1016/S0140-6736(09)61117-X 19559476.
5. Delespaux V, de Koning HP. Drugs and drug resistance in African trypanosomiasis. Drug Resist Updat. 2007;10(1–2):30–50. Epub 2007/04/06. S1368-7646(07)00021-0 [pii] doi: 10.1016/j.drup.2007.02.004 17409013.
6. Arias JL. Novel strategies to improve the anticancer action of 5-fluorouracil by using drug delivery systems. Molecules. 2008;13(10):2340–69. Epub 2008/10/03. 13102340 [pii]. 18830159.
7. Unciti-Broceta JD, Del Castillo T, Soriano M, Magez S, Garcia-Salcedo JA. Novel therapy based on camelid nanobodies. Therapeutic delivery. 2013;4(10):1321–36. Epub 2013/10/15. doi: 10.4155/tde.13.87 24116915.
8. Stijlemans B, Conrath K, Cortez-Retamozo V, Van Xong H, Wyns L, Senter P, et al. Efficient targeting of conserved cryptic epitopes of infectious agents by single domain antibodies. African trypanosomes as paradigm. J Biol Chem. 2004;279(2):1256–61. Epub 2003/10/07. doi: 10.1074/jbc.M307341200 M307341200 [pii]. 14527957.
9. Baral TN, Magez S, Stijlemans B, Conrath K, Vanhollebeke B, Pays E, et al. Experimental therapy of African trypanosomiasis with a nanobody-conjugated human trypanolytic factor. Nat Med. 2006;12(5):580–4. Epub 2006/04/11. nm1395 [pii] doi: 10.1038/nm1395 16604085.
10. Magez S, Radwanska M, Stijlemans B, Xong HV, Pays E, De Baetselier P. A conserved flagellar pocket exposed high mannose moiety is used by African trypanosomes as a host cytokine binding molecule. J Biol Chem. 2001;276(36):33458–64. Epub 2001/06/19. doi: 10.1074/jbc.M103412200 M103412200 [pii]. 11404356.
11. Arias JL, Unciti-Broceta JD, Maceira J, Del Castillo T, Hernandez-Quero J, Magez S, et al. Nanobody conjugated PLGA nanoparticles for active targeting of African Trypanosomiasis. J Control Release. 2015;197 : 190–8. Epub 2014/12/03. S0168-3659(14)00739-1 [pii] doi: 10.1016/j.jconrel.2014.11.002 25445702.
12. Arias JL. Advanced methodologies to formulate nanotheragnostic agents for combined drug delivery and imaging. Expert Opin Drug Deliv. 2011;8(12):1589–608. Epub 2011/11/22. doi: 10.1517/17425247.2012.634794 22097904.
13. Arias JL, Reddy LH, Couvreur P. Superior preclinical efficacy of gemcitabine developed as chitosan nanoparticulate system. Biomacromolecules. 2011;12(1):97–104. Epub 2010/12/02. doi: 10.1021/bm101044h 21117615.
14. Malhotra M, Tomaro-Duchesneau C, Prakash S. Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases. Biomaterials. 2013;34(4):1270–80. doi: 10.1016/j.biomaterials.2012.10.013 23140978
15. Torrecilla D, Lozano MV, Lallana E, Neissa JI, Novoa-Carballal R, Vidal A, et al. Anti-tumor efficacy of chitosan-g-poly(ethylene glycol) nanocapsules containing docetaxel: Anti-TMEFF-2 functionalized nanocapsules vs. non-functionalized nanocapsules. European Journal of Pharmaceutics and Biopharmaceutics. 2013;83(3):330–7. doi: 10.1016/j.ejpb.2012.10.017 23262164
16. Thuita JK, Karanja SM, Wenzler T, Mdachi RE, Ngotho JM, Kagira JM, et al. Efficacy of the diamidine DB75 and its prodrug DB289, against murine models of human African trypanosomiasis. Acta Trop. 2008;108(1):6–10. Epub 2008/08/30. S0001-706X(08)00210-6 [pii] doi: 10.1016/j.actatropica.2008.07.006 18722336.
17. Frearson JA, Brand S, McElroy SP, Cleghorn LA, Smid O, Stojanovski L, et al. N-myristoyltransferase inhibitors as new leads to treat sleeping sickness. Nature. 2010;464(7289):728–32. Epub 2010/04/03. nature08893 [pii] doi: 10.1038/nature08893 20360736; PubMed Central PMCID: PMC2917743.
18. Stijlemans B, Caljon G, Natesan SK, Saerens D, Conrath K, Perez-Morga D, et al. High affinity nanobodies against the Trypanosome brucei VSG are potent trypanolytic agents that block endocytosis. PLoS Pathog. 2011;7(6):e1002072. Epub 2011/06/24. doi: 10.1371/journal.ppat.1002072 10-PLPA-RA-4101 [pii]. 21698216; PubMed Central PMCID: PMC3116811.
19. Caljon G, Stijlemans B, Saerens D, Van Den Abbeele J, Muyldermans S, Magez S, et al. Affinity is an important determinant of the anti-trypanosome activity of nanobodies. PLoS Negl Trop Dis. 2012;6(11):e1902. Epub 2012/11/21. doi: 10.1371/journal.pntd.0001902 PNTD-D-12-00780 [pii]. 23166849; PubMed Central PMCID: PMC3499403.
20. De Vooght L, Caljon G, Stijlemans B, De Baetselier P, Coosemans M, Van den Abbeele J. Expression and extracellular release of a functional anti-trypanosome Nanobody(R) in Sodalis glossinidius, a bacterial symbiont of the tsetse fly. Microb Cell Fact. 2012;11 : 23. Epub 2012/02/18. 1475-2859-11-23 [pii] doi: 10.1186/1475-2859-11-23 22335892; PubMed Central PMCID: PMC3311065.
21. Nolan DP, Geuskens M, Pays E. N-linked glycans containing linear poly-N-acetyllactosamine as sorting signals in endocytosis in Trypanosoma brucei. Curr Biol. 1999;9(20):1169–72. Epub 1999/10/26. S0960-9822(00)80018-4 [pii] doi: 10.1016/S0960-9822(00)80018-4 10531030.
22. Alsford S, Eckert S, Baker N, Glover L, Sanchez-Flores A, Leung KF, et al. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature. 2012;482(7384):232–6. Epub 2012/01/27. nature10771 [pii] doi: 10.1038/nature10771 22278056; PubMed Central PMCID: PMC3303116.
23. Bernhard SC, Nerima B, Maser P, Brun R. Melarsoprol - and pentamidine-resistant Trypanosoma brucei rhodesiense populations and their cross-resistance. Int J Parasitol. 2007;37(13):1443–8. Epub 2007/07/03. S0020-7519(07)00180-4 [pii] doi: 10.1016/j.ijpara.2007.05.007 17602691.
24. Carter NS, Berger BJ, Fairlamb AH. Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and-resistant Trypanosoma brucei brucei. J Biol Chem. 1995;270(47):28153–7. Epub 1995/11/24. 7499305.
25. Baker N, Glover L, Munday JC, Aguinaga Andres D, Barrett MP, de Koning HP, et al. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc Natl Acad Sci U S A. 2012. Epub 2012/06/20. 1202885109 [pii] doi: 10.1073/pnas.1202885109 22711816.
26. Bridges DJ, Gould MK, Nerima B, Maser P, Burchmore RJ, de Koning HP. Loss of the high-affinity pentamidine transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol Pharmacol. 2007;71(4):1098–108. Epub 2007/01/20. mol.106.031351 [pii] doi: 10.1124/mol.106.031351 17234896.
27. Graf FE, Ludin P, Wenzler T, Kaiser M, Brun R, Pyana PP, et al. Aquaporin 2 Mutations in Trypanosoma brucei gambiense Field Isolates Correlate with Decreased Susceptibility to Pentamidine and Melarsoprol. PLoS Negl Trop Dis. 2013;7(10):e2475. doi: 10.1371/journal.pntd.0002475 24130910
28. Munday JC, Eze AA, Baker N, Glover L, Clucas C, Aguinaga Andres D, et al. Trypanosoma brucei aquaglyceroporin 2 is a high-affinity transporter for pentamidine and melaminophenyl arsenic drugs and the main genetic determinant of resistance to these drugs. The Journal of antimicrobial chemotherapy. 2013. Epub 2013/11/16. doi: 10.1093/jac/dkt442 24235095.
29. de Koning HP, Anderson LF, Stewart M, Burchmore RJ, Wallace LJ, Barrett MP. The trypanocide diminazene aceturate is accumulated predominantly through the TbAT1 purine transporter: additional insights on diamidine resistance in african trypanosomes. Antimicrob Agents Chemother. 2004;48(5):1515–9. Epub 2004/04/24. 15105099; PubMed Central PMCID: PMC400564.
30. Munday JC, Rojas Lopez KE, Eze AA, Delespaux V, Van Den Abbeele J, Rowan T, et al. Functional expression of TcoAT1 reveals it to be a P1-type nucleoside transporter with no capacity for diminazene uptake. Int J Parasitol Drugs Drug Resist. 2013;3 : 69–76. Epub 2014/02/18. doi: 10.1016/j.ijpddr.2013.01.004 S2211-3207(13)00005-5 [pii]. 24533295; PubMed Central PMCID: PMC3862423.
31. Munday JC, Tagoe DN, Eze AA, Krezdorn JA, Rojas Lopez KE, Alkhaldi AA, et al. Functional analysis of drug resistance-associated mutations in the Trypanosoma brucei adenosine transporter 1 (TbAT1) and the proposal of a structural model for the protein. Mol Microbiol. 2015. Epub 2015/02/25. doi: 10.1111/mmi.12979 25708978.
32. Munday JC, Settimo L, de Koning HP. Transport proteins determine drug sensitivity and resistance in a protozoan parasite, Trypanosoma brucei. Front Pharmacol. 2015;6 : 32. Epub 2015/03/31. doi: 10.3389/fphar.2015.00032 25814953; PubMed Central PMCID: PMC4356943.
33. Barrett MP, Vincent IM, Burchmore RJS, Kazibwe AJN, Matovu E. Drug resistance in human African trypanosomiasis. Future Microbiology. 2011;6(9):1037–47. doi: 10.2217/fmb.11.88 21958143
34. Garcia-Salcedo J, Munday J, Unciti-Broceta J, Koning H. Progress Towards New Treatments for Human African Trypanosomiasis. In: Magez S, Radwanska M, editors. Trypanosomes and Trypanosomiasis: Springer Vienna; 2014. p. 217–38.
35. Mäser P, Wittlin S, Rottmann M, Wenzler T, Kaiser M, Brun R. Antiparasitic agents: new drugs on the horizon. Current Opinion in Pharmacology. 2012;12(5):562–6. doi: 10.1016/j.coph.2012.05.001 22652215
36. Vanaerschot M, Huijben S, Van den Broeck F, Dujardin JC. Drug resistance in vectorborne parasites: multiple actors and scenarios for an evolutionary arms race. FEMS Microbiol Rev. 2013. Epub 2013/07/03. doi: 10.1111/1574-6976.12032 23815683.
37. Vincent IM, Creek D, Watson DG, Kamleh MA, Woods DJ, Wong PE, et al. A molecular mechanism for eflornithine resistance in African trypanosomes. PLoS Pathog. 2010;6(11):e1001204. Epub 2010/12/03. doi: 10.1371/journal.ppat.1001204 21124824; PubMed Central PMCID: PMC2991269.
38. Schumann Burkard G, Jutzi P, Roditi I. Genome-wide RNAi screens in bloodstream form trypanosomes identify drug transporters. Mol Biochem Parasitol. 2011;175(1):91–4. Epub 2010/09/21. S0166-6851(10)00233-1 [pii] doi: 10.1016/j.molbiopara.2010.09.002 20851719.
39. Carter NS, Fairlamb AH. Arsenical-resistant trypanosomes lack an unusual adenosine transporter. Nature. 1993;361(6408):173–6. Epub 1993/01/14. doi: 10.1038/361173a0 8421523.
40. Maser P, Sutterlin C, Kralli A, Kaminsky R. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science. 1999;285(5425):242–4. Epub 1999/07/10. 7645 [pii]. 10398598.
41. Matovu E, Stewart ML, Geiser F, Brun R, Maser P, Wallace LJ, et al. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot Cell. 2003;2(5):1003–8. Epub 2003/10/14. 14555482; PubMed Central PMCID: PMC219364.
42. Park JH, Saravanakumar G, Kim K, Kwon IC. Targeted delivery of low molecular drugs using chitosan and its derivatives. Advanced Drug Delivery Reviews. 2010;62(1):28–41. doi: 10.1016/j.addr.2009.10.003 19874862
43. Sinha VR, Singla AK, Wadhawan S, Kaushik R, Kumria R, Bansal K, et al. Chitosan microspheres as a potential carrier for drugs. International Journal of Pharmaceutics. 2004;274(1–2):1–33. doi: 10.1016/j.ijpharm.2003.12.026 15072800
44. Manca ML, Loy G, Zaru M, Fadda AM, Antimisiaris SG. Release of rifampicin from chitosan, PLGA and chitosan-coated PLGA microparticles. Colloids and Surfaces B: Biointerfaces. 2008;67(2):166–70. doi: 10.1016/j.colsurfb.2008.08.010 18835764
45. Durand R, Paul M, Rivollet D, Houin R, Astier A, Deniau M. Activity of pentamidine-loaded methacrylate nanoparticles against Leishmania infantum in a mouse model. International Journal for Parasitology. 1997;27(11):1361–7. doi: 10.1016/S0020-7519(97)00124-0 9421724
46. Fusai T, Deniau M, Durand R, Bories C, Paul M, Rivollet D, et al. Action of pentamidine-bound nanoparticles against Leishmania on an in vivo model. Parasite (Paris, France). 1994;1(4):319–24. 9140499
47. Durand R, Paul M, Rivollet D, Fessi H, Houin R, Astier A, et al. Activity of pentamidine-loaded poly (D,L-lactide) nanoparticles against Leishmania infantum in a murine model. Parasite. 1997;4(4):331–6. 9587601
48. Hirumi H, Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. The Journal of parasitology. 1989;75(6):985–9. Epub 1989/12/01. 2614608.
49. Dash BC, Réthoré G, Monaghan M, Fitzgerald K, Gallagher W, Pandit A. The influence of size and charge of chitosan/polyglutamic acid hollow spheres on cellular internalization, viability and blood compatibility. Biomaterials. 2010;31(32):8188–97. doi: 10.1016/j.biomaterials.2010.07.067 20701967
50. Unciti-Broceta JD, Maceira J, Morales S, Garcia-Perez A, Munoz-Torres ME, Garcia-Salcedo JA. Nicotinamide inhibits the lysosomal cathepsin b-like protease and kills African trypanosomes. J Biol Chem. 2013. Epub 2013/02/28. M112.449207 [pii] doi: 10.1074/jbc.M112.449207 23443665.
51. Kourtis IC, Hirosue S, de Titta A, Kontos S, Stegmann T, Hubbell JA, et al. Peripherally Administered Nanoparticles Target Monocytic Myeloid Cells, Secondary Lymphoid Organs and Tumors in Mice. PLoS ONE. 2013;8(4):e61646. doi: 10.1371/journal.pone.0061646 23626707
52. Stewart ML, Burchmore RJ, Clucas C, Hertz-Fowler C, Brooks K, Tait A, et al. Multiple genetic mechanisms lead to loss of functional TbAT1 expression in drug-resistant trypanosomes. Eukaryot Cell. 2010;9(2):336–43. Epub 2009/12/08. EC.00200-09 [pii] doi: 10.1128/EC.00200-09 19966032; PubMed Central PMCID: PMC2823006.
53. Spitznagel D, Ebikeme C, Biran M, Nic a' Bhaird N, Bringaud F, Henehan GT, et al. Alanine aminotransferase of Trypanosoma brucei—a key role in proline metabolism in procyclic life forms. FEBS J. 2009;276(23):7187–99. Epub 2009/11/10. EJB7432 [pii] doi: 10.1111/j.1742-4658.2009.07432.x 19895576.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage TraffickingČlánek An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived NeuronsČlánek Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPsČlánek Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin ReleaseČlánek HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T CellsČlánek Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 6- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Introducing “Research Matters”
- Exploring Host–Pathogen Interactions through Biological Control
- Analysis of Bottlenecks in Experimental Models of Infection
- Expected and Unexpected Features of the Newly Discovered Bat Influenza A-like Viruses
- Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage Trafficking
- Recombinant Murine Gamma Herpesvirus 68 Carrying KSHV G Protein-Coupled Receptor Induces Angiogenic Lesions in Mice
- TRIM30α Is a Negative-Feedback Regulator of the Intracellular DNA and DNA Virus-Triggered Response by Targeting STING
- Targeting Human Transmission Biology for Malaria Elimination
- Two Cdc2 Kinase Genes with Distinct Functions in Vegetative and Infectious Hyphae in
- An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived Neurons
- Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPs
- Virulence Factors of Induce Both the Unfolded Protein and Integrated Stress Responses in Airway Epithelial Cells
- Peptide-MHC-I from Endogenous Antigen Outnumber Those from Exogenous Antigen, Irrespective of APC Phenotype or Activation
- Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis
- An Ultrasensitive Mechanism Regulates Influenza Virus-Induced Inflammation
- The Role of Human Transportation Networks in Mediating the Genetic Structure of Seasonal Influenza in the United States
- Host Delivery of Favorite Meals for Intracellular Pathogens
- Complement-Opsonized HIV-1 Overcomes Restriction in Dendritic Cells
- Inter-Seasonal Influenza is Characterized by Extended Virus Transmission and Persistence
- A Critical Role for CLSP2 in the Modulation of Antifungal Immune Response in Mosquitoes
- Twilight, a Novel Circadian-Regulated Gene, Integrates Phototropism with Nutrient and Redox Homeostasis during Fungal Development
- Surface-Associated Lipoproteins Link Virulence to Colitogenic Activity in IL-10-Deficient Mice Independent of Their Expression Levels
- Latent Membrane Protein LMP2A Impairs Recognition of EBV-Infected Cells by CD8+ T Cells
- Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains
- Neuronal Subtype and Satellite Cell Tropism Are Determinants of Varicella-Zoster Virus Virulence in Human Dorsal Root Ganglia Xenografts
- Molecular Basis for the Selective Inhibition of Respiratory Syncytial Virus RNA Polymerase by 2'-Fluoro-4'-Chloromethyl-Cytidine Triphosphate
- Structure of the Virulence Factor, SidC Reveals a Unique PI(4)P-Specific Binding Domain Essential for Its Targeting to the Bacterial Phagosome
- Activated Brain Endothelial Cells Cross-Present Malaria Antigen
- Fungal Morphology, Iron Homeostasis, and Lipid Metabolism Regulated by a GATA Transcription Factor in
- Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin Release
- A Macrophage Subversion Factor Is Shared by Intracellular and Extracellular Pathogens
- A Novel AT-Rich DNA Recognition Mechanism for Bacterial Xenogeneic Silencer MvaT
- Reovirus FAST Proteins Drive Pore Formation and Syncytiogenesis Using a Novel Helix-Loop-Helix Fusion-Inducing Lipid Packing Sensor
- The Role of ExoS in Dissemination of during Pneumonia
- IRF-5-Mediated Inflammation Limits CD8 T Cell Expansion by Inducing HIF-1α and Impairing Dendritic Cell Functions during Infection
- Discordant Impact of HLA on Viral Replicative Capacity and Disease Progression in Pediatric and Adult HIV Infection
- Crystal Structure of USP7 Ubiquitin-like Domains with an ICP0 Peptide Reveals a Novel Mechanism Used by Viral and Cellular Proteins to Target USP7
- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- HPV16 Down-Regulates the Insulin-Like Growth Factor Binding Protein 2 to Promote Epithelial Invasion in Organotypic Cultures
- The νSaα Specific Lipoprotein Like Cluster () of . USA300 Contributes to Immune Stimulation and Invasion in Human Cells
- RSV-Induced H3K4 Demethylase KDM5B Leads to Regulation of Dendritic Cell-Derived Innate Cytokines and Exacerbates Pathogenesis
- Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular
- Border Patrol Gone Awry: Lung NKT Cell Activation by Exacerbates Tularemia-Like Disease
- The Curious Road from Basic Pathogen Research to Clinical Translation
- From Cell and Organismal Biology to Drugs
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
- A 21st Century Perspective of Poliovirus Replication
- Is Development of a Vaccine against Feasible?
- Waterborne Viruses: A Barrier to Safe Drinking Water
- Battling Phages: How Bacteria Defend against Viral Attack
- Archaea in and on the Human Body: Health Implications and Future Directions
- Degradation of Human PDZ-Proteins by Human Alphapapillomaviruses Represents an Evolutionary Adaptation to a Novel Cellular Niche
- Natural Variants of the KPC-2 Carbapenemase have Evolved Increased Catalytic Efficiency for Ceftazidime Hydrolysis at the Cost of Enzyme Stability
- Potent Cell-Intrinsic Immune Responses in Dendritic Cells Facilitate HIV-1-Specific T Cell Immunity in HIV-1 Elite Controllers
- The Mammalian Cell Cycle Regulates Parvovirus Nuclear Capsid Assembly
- Host Reticulocytes Provide Metabolic Reservoirs That Can Be Exploited by Malaria Parasites
- The Proteome of the Isolated Containing Vacuole Reveals a Complex Trafficking Platform Enriched for Retromer Components
- NK-, NKT- and CD8-Derived IFNγ Drives Myeloid Cell Activation and Erythrophagocytosis, Resulting in Trypanosomosis-Associated Acute Anemia
- Successes and Challenges on the Road to Cure Hepatitis C
- BRCA1 Regulates IFI16 Mediated Nuclear Innate Sensing of Herpes Viral DNA and Subsequent Induction of the Innate Inflammasome and Interferon-β Responses
- A Structural and Functional Comparison Between Infectious and Non-Infectious Autocatalytic Recombinant PrP Conformers
- Phosphorylation of the Peptidoglycan Synthase PonA1 Governs the Rate of Polar Elongation in Mycobacteria
- Human Immunodeficiency Virus Type 1 Nef Inhibits Autophagy through Transcription Factor EB Sequestration
- Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
- EBV BART MicroRNAs Target Multiple Pro-apoptotic Cellular Genes to Promote Epithelial Cell Survival
- Single-Cell and Single-Cycle Analysis of HIV-1 Replication
- TRIM32 Senses and Restricts Influenza A Virus by Ubiquitination of PB1 Polymerase
- The Herpes Simplex Virus Protein pUL31 Escorts Nucleocapsids to Sites of Nuclear Egress, a Process Coordinated by Its N-Terminal Domain
- Host Transcriptional Response to Influenza and Other Acute Respiratory Viral Infections – A Prospective Cohort Study
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- Battling Phages: How Bacteria Defend against Viral Attack
- A 21st Century Perspective of Poliovirus Replication
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



