-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
Mutations in VAPB/ALS8 are associated with amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA), two motor neuron diseases that often include alterations in energy metabolism. We have shown that C. elegans and Drosophila neurons secrete a cleavage product of VAPB, the N-terminal major sperm protein domain (vMSP). Secreted vMSPs signal through Roundabout and Lar-like receptors expressed on striated muscle. The muscle signaling pathway localizes mitochondria to myofilaments, alters their fission/fusion balance, and promotes energy production. Here, we show that neuronal loss of the C. elegans VAPB homolog triggers metabolic alterations that appear to compensate for muscle mitochondrial dysfunction. When vMSP levels drop, cytoskeletal or mitochondrial abnormalities in muscle induce elevated DAF-16, the Forkhead Box O (FoxO) homolog, transcription factor activity. DAF-16 promotes muscle triacylglycerol accumulation, increases ATP levels in adults, and extends lifespan, despite reduced muscle mitochondria electron transport chain activity. Finally, Vapb knock-out mice exhibit abnormal muscular triacylglycerol levels and FoxO target gene transcriptional responses to fasting and refeeding. Our data indicate that impaired vMSP signaling to striated muscle alters FoxO activity, which affects energy metabolism. Abnormalities in energy metabolism of ALS patients may thus constitute a compensatory mechanism counterbalancing skeletal muscle mitochondrial dysfunction.
Published in the journal: VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in. PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003738
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003738Summary
Mutations in VAPB/ALS8 are associated with amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA), two motor neuron diseases that often include alterations in energy metabolism. We have shown that C. elegans and Drosophila neurons secrete a cleavage product of VAPB, the N-terminal major sperm protein domain (vMSP). Secreted vMSPs signal through Roundabout and Lar-like receptors expressed on striated muscle. The muscle signaling pathway localizes mitochondria to myofilaments, alters their fission/fusion balance, and promotes energy production. Here, we show that neuronal loss of the C. elegans VAPB homolog triggers metabolic alterations that appear to compensate for muscle mitochondrial dysfunction. When vMSP levels drop, cytoskeletal or mitochondrial abnormalities in muscle induce elevated DAF-16, the Forkhead Box O (FoxO) homolog, transcription factor activity. DAF-16 promotes muscle triacylglycerol accumulation, increases ATP levels in adults, and extends lifespan, despite reduced muscle mitochondria electron transport chain activity. Finally, Vapb knock-out mice exhibit abnormal muscular triacylglycerol levels and FoxO target gene transcriptional responses to fasting and refeeding. Our data indicate that impaired vMSP signaling to striated muscle alters FoxO activity, which affects energy metabolism. Abnormalities in energy metabolism of ALS patients may thus constitute a compensatory mechanism counterbalancing skeletal muscle mitochondrial dysfunction.
Introduction
ALS is a lethal neurodegenerative disease characterized by the combined degeneration of lower and upper motor neurons [1]. Most ALS cases occur sporadically, but about 10% are familial. These genetic cases are caused by mutations in multiple genes, including in the Vapb (VAMP/synaptobrevin-associated protein B) gene. Mutations in Vapb lead to ALS8 that manifests as ALS or late-onset SMA, a motor neuron disease restricted to lower motor neurons [2]–[4]. While Vapb mutations are rare, reduced VAPB mRNA or protein levels have been reported in sporadic ALS patients, a mSOD1 ALS mouse model, and ALS8 patient motor neurons derived from induced pluripotent stem cells [5]–[7]. Hence, a loss of VAPB might be relevant in non-ALS8 patients.
VAPB, and its paralog VAPA, are broadly expressed type II membrane proteins that are evolutionarily conserved. These VAPs have been implicated in regulating lipid transport and homeostasis at intracellular organelle contact sites, endoplasmic reticulum (ER) dynamics, and membrane trafficking [8]–[12]. In addition to these cell autonomous functions, the VAP vMSP is cleaved from the transmembrane domain in the cytoplasm and secreted in a cell-type specific fashion [13]–[15]. Secreted vMSPs antagonize Eph receptor signaling through a direct interaction with the extracellular domain [13]. More recently, we have shown in C. elegans and Drosophila that neurons secrete vMSPs to regulate mitochondrial localization and function in striated muscle [15]. vMSPs interact with muscle SAX-3 Roundabout and CLR-1 Lar-like protein-tyrosine phosphatase receptors to down-regulate CLR-1 signaling. VAP loss causes uncontrolled CLR-1 Lar-like receptor activation in body wall muscle. CLR-1 stimulates actin filament assembly in the muscle belly that requires the actin-related protein 2/3 (Arp2/3) complex. These ectopic actin filaments displace mitochondria from I-bands, cause aberrant fission and fusion balance, and impair respiratory chain activity. Hence, vMSPs secreted by neurons promote muscle mitochondrial localization and function, perhaps in an effort to modulate energy homeostasis.
vMSP signaling to muscle mitochondria might be relevant for the energy balance in ALS8 disease. Out of five ALS8 patients studied, five had increased cholesterol levels, four had reduced HDL, three had elevated triacylglycerol levels, and one was diabetic [16]. More generally, ALS is associated with a spectrum of abnormalities in energy metabolism, including mitochondrial defects in neurons and skeletal muscle, insulin resistance, dyslipidemia, and hypermetabolism [17]. These metabolic abnormalities are positively correlated with survival. For instance, increased prediagnostic body fat is associated with decreased risk of ALS mortality [18] and in some patient populations, higher LDL/HDL ratios correlate with increased survival time [19],[20]. However, the cause(s) of the metabolic defects and their relationship to each other are not well understood.
Here we show in C. elegans that loss of the VAP homolog VPR-1 causes triacylglycerol (TAG) accumulation in striated body wall muscle. Mosaic analysis and tissue-specific expression studies provide compelling evidence that VPR-1 acts in neurons, not muscles to regulate fat levels. Multiple lines of evidence support the model that impaired vMSP signaling from neurons to muscle increases TAG levels in muscle. We propose that this fat metabolism alteration is part of a compensatory response mediated by the DAF-16/FoxO transcription factor. FoxO promotes muscle fat accumulation, maintains ATP levels during aging, and extends lifespan without influencing muscle mitochondrial morphology, localization, or function. Finally, we provide evidence that skeletal muscle metabolism is abnormal in Vapb mutant mice. Our results support the model that disrupting vMSP signaling to muscle triggers a compensatory response involving FoxO transcription factors.
Results
vpr-1/vap loss increases fat levels in adult body wall muscle
In our studies of vpr-1(tm1411) null mutant hermaphrodites, we noticed that body wall muscles often contain large lipid-like droplets not observed in wild-type controls. These apparent lipid-like droplets were visible in young adults (1–3 days post L4 stage) by differential interference contrast (DIC) microscopy (Figure 1A). In transgenic vpr-1 mutants expressing mitochondrial matrix-targeted GFP (mitoGFP) in muscle, droplets are observed in the muscle belly surrounded by mitochondria (Figure S1). The vast majority of visible droplets in peripheral tissues are found in muscle. Transmission electron microscopy (TEM) of vpr-1(tm1411) mutant muscle shows an expanded muscle belly filled with mitochondria, as previously reported [15], and large droplets (Figures 1B and S2). The droplets are often found in close proximity to mitochondria and ER. Large muscle droplets were not observed in young adult wild-type muscle (Figures 1B and S2). However, muscle lipid droplets and abnormal mitochondria are observed in very old (18 day) wild-type adults [21], [22]. In these old worms, large lipid droplets accumulate in the muscle, intestine, and epidermis. We did not detect abnormally large droplets in young vpr-1 mutant intestinal and epidermal tissues by TEM. Instead, intestinal and epidermal tissues looked similar to wild-type controls, although it is difficult to assess minor differences (Figure S2). Hence, muscle droplets accumulate in aging vpr-1 mutant worms.
Fig. 1. Fat levels in body wall muscle of wild-type and vpr-1 mutant worms. 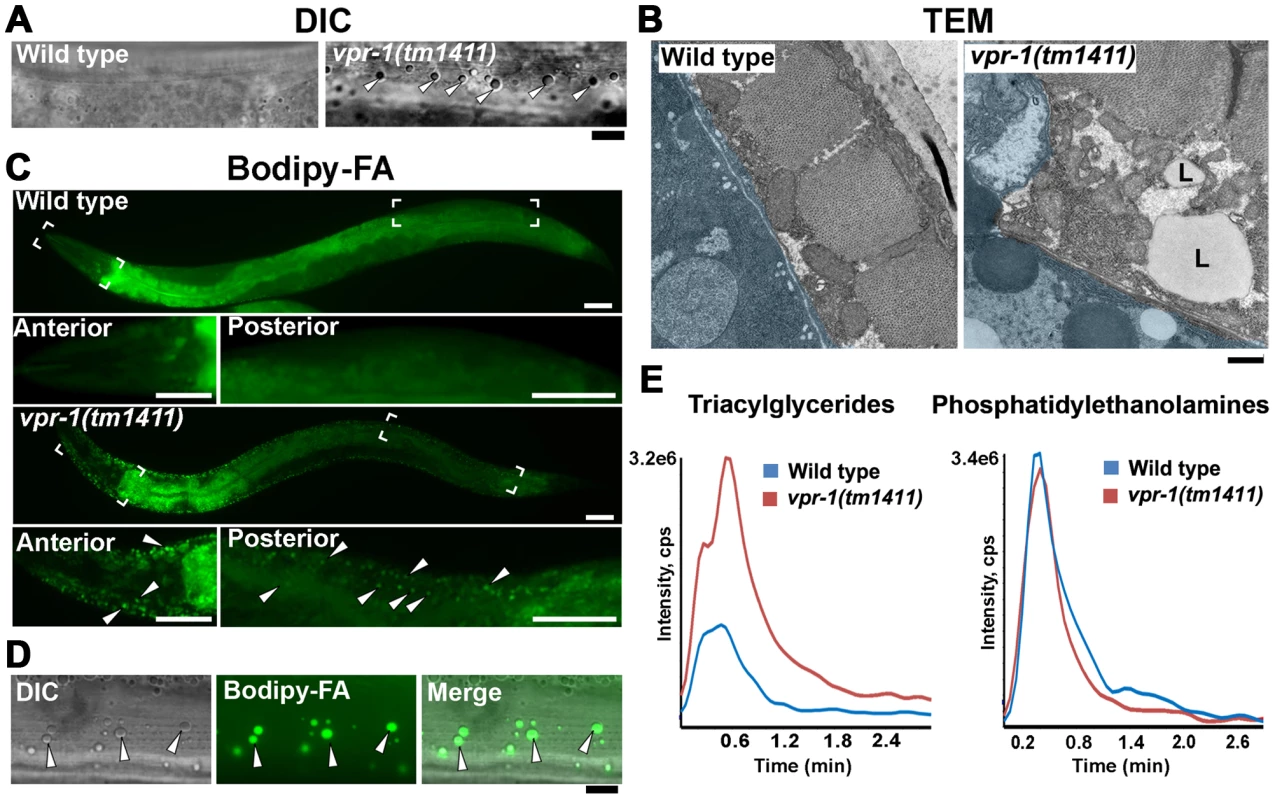
(A) DIC images of muscle in live adult hermaphrodites. Arrowheads indicate lipid-like droplets. Bar, 5 µm. (B) Transmission electron micrographs of body wall muscle cytoplasm in wild-type and vpr-1(tm1411) mutant hermaphrodites. Light blue color demarcates muscle boundary. L, Lipid-like droplet. Bar, 0.5 µm. (C) Fluorescent images of muscle in live adult hermaphrodites fed Bodipy-FAs. Close-up images of boxed areas are shown below. Arrowheads indicate examples of Bodipy-FA-stained droplets. Anterior is to the left in all panels. Bars, 50 µm. (D) High magnification images of muscle showing Bodipy-FA-stained fluorescent droplets and droplets observed by DIC microscopy. Bar, 5 µm. (E) Comparison of total ion chromatograms of wild-type and vpr-1(tm1411) mutant adults extracts for 18∶0 TAG (Neutral Loss 284) and phosphatidylethanolamine (Neutral Loss 141). To directly test whether these droplets contain lipid, we fed vpr-1 mutant worms E. coli incubated with Bodipy-conjugated fatty acids (Bodipy-FAs). These fluorescent compounds can be used to directly visualize fat stores in live tissue [23], [24]. In wild-type hermaphrodite controls, dietary Bodipy-FAs were observed primarily in the intestine with a few small droplets present in muscle. In contrast, muscles of vpr-1(tm1411) null mutants contained numerous large Bodipy-FA-stained droplets (Figure 1C). The fluorescent droplets fully overlapped with those observed in muscle by DIC microscopy (Figure 1D). Similar results are observed with Sudan Black B, which darkly stains neutral TAGs in fixed opaque worms (Figure S3). Bodipy-FAs are continuously transported from the diet, to the worm's intestinal cells, and then to the muscle, where they are tightly packed in membrane-bound vesicles. Bodipy-FAs are also incorporated into yolk lipoprotein complexes [23], which are specifically endocytosed by oocytes [25]. Although yolk accumulates in the pseudocoelom of vpr-1 mutants (due to defective oogenesis), it is not up-taken by muscle (Figure S4). Both Bodipy-FA and Sudan Black staining show a mild increase in intestinal fat content in vpr-1 mutants. Whether this apparent increase is due to fat accumulation or increased fat synthesis is not clear.
We also performed mass spectrometry of lipid extracts to determine the lipid composition of wild-type and vpr-1 mutant adult hermaphrodites. Lipids were analyzed by electrospray ionization tandem mass spectrometry (ESI-MS/MS). ESI-MS/MS analysis of the extracts detected a robust increase in TAGs in vpr-1 mutant extracts, but not in the membrane phospholipids phosphatidylethanolamine and phosphatidylcholine (Figure 1E and data not shown). These data indicate that loss of vpr-1 causes TAG accumulation in muscle of adult hermaphrodite worms.
Increased ER stress does not cause muscle TAG accumulation in vpr-1/vap mutants
VAP homologs have been implicated in ER stress pathways [13], [26], [27], which can modulate lipid metabolism and homeostasis [28]. Furthermore, mitochondrial dysfunction is sometimes associated with ER stress. We considered the possibility that increased ER stress might cause the high muscle fat levels in vpr-1 mutants. Three lines of evidence argue against this possibility. First, an integrated hsp-4/BiPp::gfp ER stress reporter [29] did not show elevated stress levels in vpr-1 mutants (Figure S5A). Second, vpr-1 mutants are not more sensitive than wild type to tunicamycin treatment, which induces ER stress (Figure S5B). Third, RNA-mediated interference (RNAi) of xbp-1, an ER stress-responsive transcription factor, in vpr-1 mutants had no effect on muscle fat levels in 3-day old adults (18.0±3.6 droplets/mm2 for vpr-1(tm1411) [n = 12] versus 17.3±3.6 droplets/mm2 for vpr-1(tm1411) xbp-1 RNAi [n = 10]; P = 0.28). These data indicate that increased ER stress does not cause the muscle TAG defect in vpr-1 mutants.
vpr-1/vap acts cell nonautonomously to regulate fat accumulation
vpr-1 is ubiquitously expressed and its homologs have been implicated in regulating lipid dynamics via a cell autonomous mechanism [10],[30]–[32]. To determine in which cell type(s) VPR-1 functions to regulate muscle fat, we first used genetic mosaic analysis. Transgenic vpr-1(tm1411) mutant hermaphrodites were generated containing the vpr-1 genomic locus and the lineage marker sur-5::GFP expressed from an extrachromosomal array [33]. In C. elegans, extrachromosomal arrays are spontaneously lost at low frequency during cell division, thereby generating mosaic worms. When these events occur early in development, mosaic worms can be generated with losses in neurons, body wall muscles, intestinal cells, and the germ line.
Expressing the vpr-1 genomic locus in vpr-1(tm1411) null worms rescued the fat metabolism defect in muscle (Figure 2), as well as the muscle mitochondrial defects, sterility, slow growth, and other phenotypes. Body wall muscles are generated from multiple cell lineages, including the EMS lineage. Transgene array loss in the EMS lineage generates mosaic worms that have a subset of muscles lacking vpr-1 expression. These muscle cells exhibited low fat levels, identical to muscle cells that express vpr-1 (Figure 2). Therefore, VPR-1 is not required in body wall muscle for fat accumulation. Mosaic worms lacking vpr-1 in the E lineage, which generates the intestine, also did not exhibit elevated muscle fat droplets, indicating that vpr-1 is not required in the intestine. In contrast to muscle and intestine loss, vpr-1 loss in the AB lineage, which generates the neurons, did cause increased fat droplets in muscles (Figure 2). Unexpectedly, we also found that vpr-1 loss in the germ cell lineage causes muscle fat accumulation (Figure 2). These results indicate that VPR-1 acts cell nonautonomously in neurons and germ cells (or their differentiation products) to modulate fat levels in muscle.
Fig. 2. vpr-1 mosaic analysis. 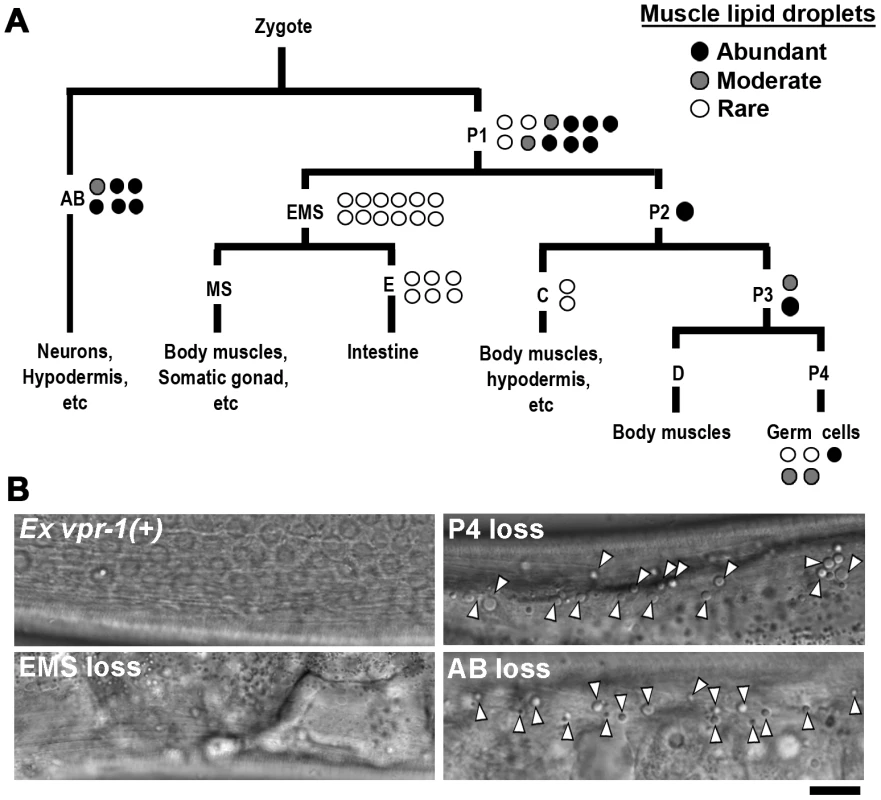
(A) Analysis of vpr-1 genetic mosaics showing the lineages of major tissues. Each circle indicates one genetic mosaic worm. Points at which the genomic copy of vpr-1(+) was lost and the resulting phenotype are shown. (B) Representative DIC images of muscle in vpr-1(tm1411) mutant mosaic worms. Ex vpr-1(+) indicates expression of the vpr-1 genomic locus via an extrachromosomal array. Arrowheads indicate fat droplets. Bar, 5 µm. vpr-1 null mutants are sterile, due to a failure of germ cells to differentiate into sperm and oocytes. Sperm secrete signaling molecules, such as MSPs that may influence fat metabolism [14]. To test whether sperm affect fat levels, we mated sterile 1-day-old adult vpr-1(tm1411) hermaphrodites to wild-type males. Supplying sperm to the reproductive tract reduces muscle fat levels in vpr-1(tm1411) mutants, as visualized with Bodipy-FAs (Figure S6A). Sperm did not rescue the sterility or muscle mitochondrial defects of vpr-1 mutants (data not shown). However, preventing spermatogenesis in wild-type hermaphrodites using the fog-3(q443) null mutation causes mild muscle fat accumulation, as well as mild mitochondrial morphology defects (Figure S6B), without affecting oxygen consumption [34]. These data indicate that the spermatogenesis defects in vpr-1 mutants contribute to muscle fat levels and perhaps mitochondrial defects. Two mechanisms appear to affect muscle fat levels, one mechanism involving neuronal vpr-1 and a second mechanism involving sperm, which can modify specific vpr-1-dependent pathways. Here, we focus on the neuronal mechanism.
Genetic mosaics assess the effect of vpr-1 loss from cells within an otherwise vpr-1(+) background. To test whether VPR-1 expression is sufficient in neurons, we expressed VPR-1 under the control of tissue-specific promoters in vpr-1 null mutants. Consistent with genetic mosaic analysis, VPR-1 expression using the myo-3 muscle-specific promoter or the ges-1 intestine-specific promoter did not influence muscle fat levels. In contrast, over-expressing the vpr-1 cDNA with the unc-119 pan-neuronal promoter completely rescued the muscle fat levels in approximately 30–40% of transgenic mutant worms (Figures 3A and 3B). These rescued transgenic mutants were still sterile. The incomplete rescue appears to be due to the germ line defects (i.e. lack of sperm) and missing vpr-1 introns or 3′UTR in the transgene (P. Cottee and M. Miller, unpublished). Consistent with these observations, driving neuronal expression of the vpr-1 genomic locus instead of the cDNA rescued several vpr-1 mutant phenotypes with increased efficiency. These results indicate that VPR-1 acts cell nonautonomously in neurons to regulate muscle fat levels.
Fig. 3. Effect of tissue-specific vpr-1 expression on fat levels. 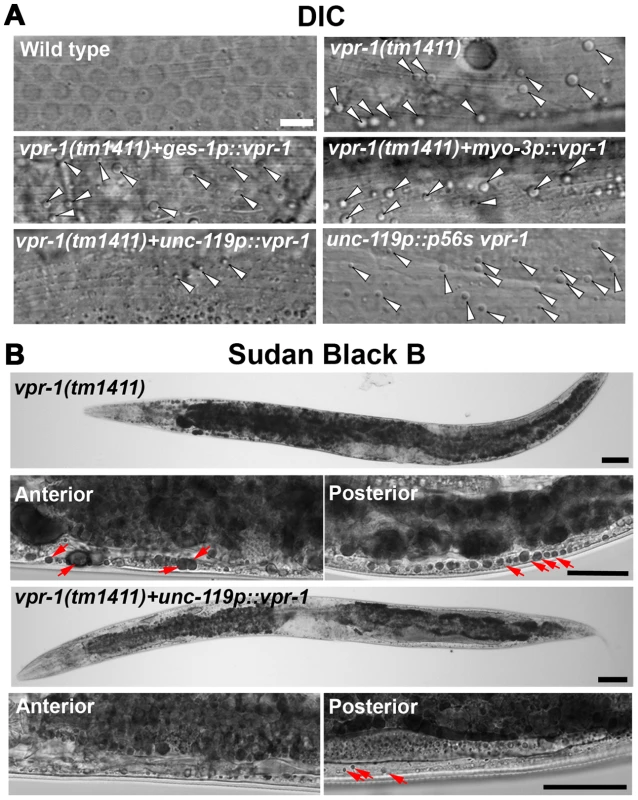
(A) DIC images of muscle in live wild-type and vpr-1(tm1411) mutant hermaphrodites expressing wild-type VPR-1 or VPR-1(P56S) under indicated tissue-specific promoters. Arrowheads indicate lipid-like droplets. Bar, 5 µm. (B) Sudan Black B staining images of vpr-1 mutants expressing vpr-1 under the unc-119 pan-neuronal promoter. Arrows indicate muscle fat droplets. Anterior is to the left in all panels. Wild-type controls (Figure S3) are similar to transgenic vpr-1(tm1411) mutants expressing unc119p::vpr-1. Low magnification bars, 50 µm; high magnification bars, 25 µm. vMSP signaling to muscle regulates muscle fat levels
The VAPB P56S mutation acts as a dominant negative by inhibiting secretion of the wild-type and mutant vMSPs [13], [15]. To test whether neuronal vMSP secretion affects muscle fat levels, we generated transgenic worms expressing P56S VPR-1 under the unc-119 neuronal promoter. P56S VPR-1 overexpression in wild-type worms causes increased muscle lipid droplets in most worms (Figure 3A), suggesting that vMSP secretion from neurons influences muscle fat accumulation.
vMSP signaling to muscle is transduced via muscle SAX-3 Robo and CLR-1 Lar-like receptors [15]. sax-3 mutations cause incompletely penetrant and variably expressed defects in muscle mitochondrial morphology [15]. Similarly, we observed incompletely penetrant defects in muscle fat accumulation by TEM and DIC microscopy (Figure S2; 11.1±13.2 fat droplets/mm2 for sax-3(ky123) [n = 13] versus 0.9±1.8 droplets/mm2 for wild type [n = 8]). Impaired vMSP signaling causes uncontrolled CLR-1 Lar receptor activity and ectopic Arp2/3-dependent actin filaments in muscle. A reduction of clr-1 or arx-2, which encodes Arp2, rescues the muscle mitochondrial defects, but not the sterility in vpr-1 mutants [15]. To test whether excess CLR-1 Lar and Arp2/3 activities cause muscle lipid accumulation, we used RNAi to down-regulate their functions in vpr-1 mutants. clr-1 or arx-2 RNAi restored mitochondria to I-bands, as previously reported [15], and reduced muscle fat droplets in vpr-1(tm1411) mutants when compared to the mutant control (Figure 4; 18.0±3.6 droplets/mm2 for vpr-1(tm1411) [n = 12] versus 0.6±1.3 droplets/mm2 for vpr-1(tm1411) clr-1 RNAi [n = 9, P<0.001] and 3.1±2.0 droplets/mm2 for vpr-1(tm1411) arx-2 RNAi [n = 6, P<0.001]). Similar results were observed using TEM [15]. We also found that overexpressing arx-2/arp2 specifically in wild-type muscle causes mild mitochondrial morphology and fat accumulation defects (Figure S7). Taken together, the data strongly support the hypothesis that impaired vMSP signaling from neurons to muscle causes elevated fat levels in muscle.
Fig. 4. Effect of Arp2/3 inactivation on muscle fat levels. 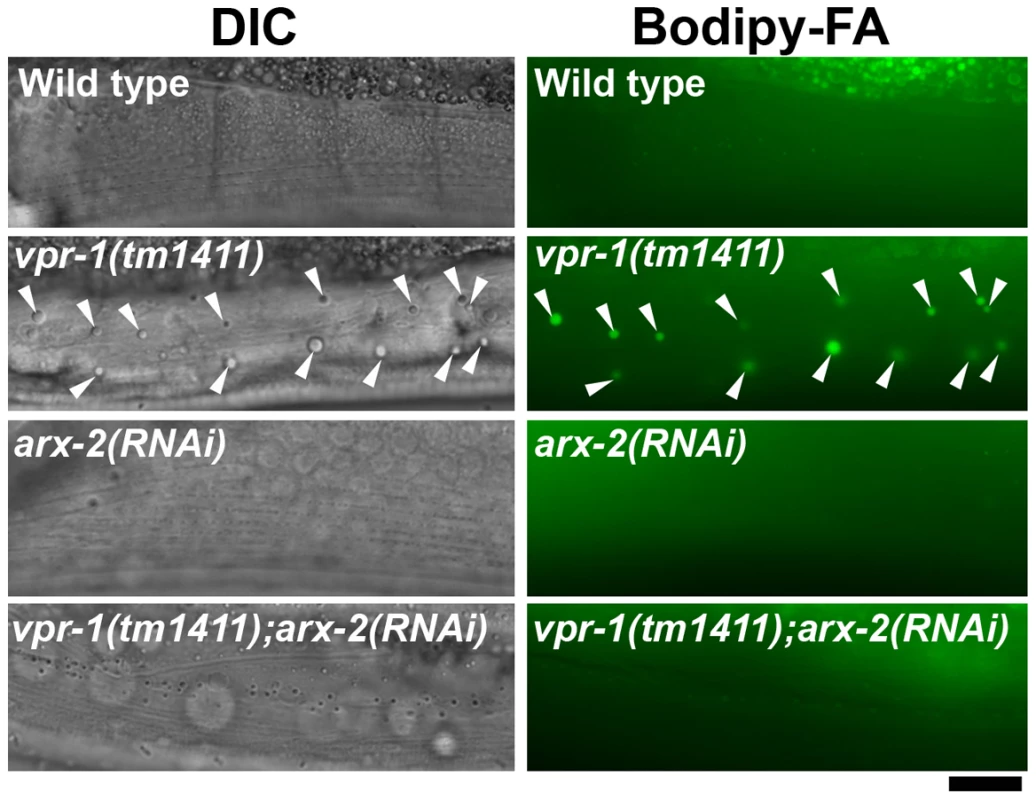
DIC and fluorescent images of muscle in live 3-day-old hermaphrodite worms fed Bodipy-FAs. arx-2 encodes the Arp2 component of the Arp2/3 complex. Arrowheads indicate Bodipy-FA-stained fat droplets. Bar, 5 µm. DAF-16/FoxO is required for fat accumulation in vpr-1/vap mutants
The elevated TAGs in vpr-1 mutants and continuous accumulation of dietary Bodipy-FAs in muscle suggested that fat metabolism and transport pathways are altered. Reduced energy production triggers enhanced activity of the DAF-16/FoxO transcription factor, which controls expression of genes involved in fat synthesis, fat transport, β-oxidation, and stress resistance [35]–[39]. We hypothesized that the muscle cytoskeletal or mitochondrial defects trigger elevated FoxO activity. To investigate if DAF-16 affects fat metabolism in vpr-1 mutants, we generated vpr-1(tm1411) daf-16(mu86) double mutants. Muscles of daf-16(mu86) null mutants contain few Bodipy-FA-stained droplets, similar to muscles of wild-type controls. However, muscle fat levels in the double mutants are also low, and strongly reduced when compared to those in vpr-1(tm1411) mutants alone (Figure 5A). daf-16 loss did not affect food intake, assessed by measuring pharyngeal pumping rates (Figure 5B; P>0.05), muscle mitochondria (see below), or sterility of vpr-1(tm1411) mutants. We conclude that the elevated fat levels in vpr-1 null mutants require DAF-16/FoxO activity.
Fig. 5. DAF-16 activity in vpr-1 mutants. 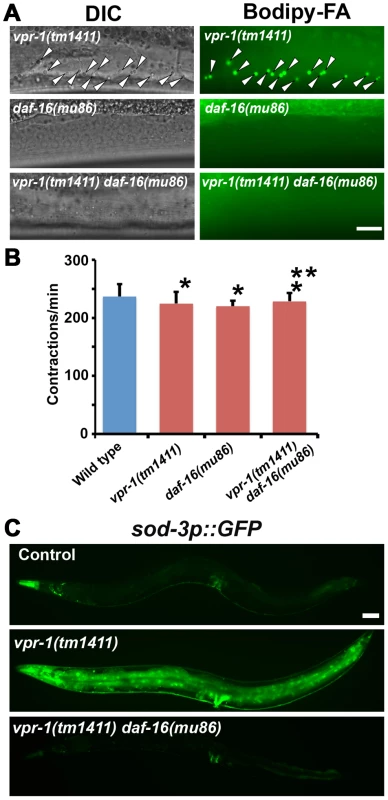
(A) DIC and fluorescent images of muscle in live 3-day-old hermaphrodite worms fed Bodipy-FAs. Arrowheads indicate Bodipy-FA-stained droplets. Wild-type controls (not shown) are similar to daf-16(mu86) mutants (See figures 1C and 4). Bar, 5 µm. (B) Pharyngeal pumping rates of 1-day-old adult hermaphrodites. Wild type (236.7±21.1 [n = 11]) and vpr-1(tm1411) mutants (220.1±9.6 [n = 11]) have similar pharyngeal pumping rate. Error bars represent SD. *P>0.05 compared to wild type. **P>0.05 compared to vpr-1(tm1411) mutant. (C) Transgenic worms expressing GFP under control of the sod-3 promoter, a direct DAF-16/FoxO target. Anterior is to the left in all panels. Bar, 50 µm. We next examined DAF-16/FoxO transcriptional activity using an integrated transgenic line that expresses GFP under the sod-3 promoter (sod-3p::GFP), a direct DAF-16 target [35], [40]. When worms were cultured under normal growth conditions, about 40–50% of 1-day-old adult vpr-1(tm1411) transgenic worms showed increased GFP expression relative to control transgenic animals (Figure 5C). By day three of adulthood, most vpr-1(tm1411) mutants show broad GFP expression throughout the body, including the intestine, neurons, vulva muscles, and body wall muscles. The elevated GFP expression is due to DAF-16 because GFP expression is suppressed in transgenic vpr-1(tm1411) daf-16(mu86) double mutants (Figure 5C). These data indicate that vpr-1 loss causes elevated DAF-16 activity in muscles and other cell types.
To investigate the mechanism(s) by which VPR-1 controls DAF-16/FoxO, we analyzed DAF-16 subcellular localization in vpr-1(tm1411) mutants. An integrated and rescuing transgenic line was used to express DAF-16::GFP under its endogenous promoter. DAF-16::GFP translocates from cytoplasm to nucleus upon loss of insulin signaling, although other mechanisms exist that regulate nuclear DAF-16 activity independent of translocation [41], [42]. Under normal growth conditions at 20°C, DAF-16::GFP in vpr-1 mutant and control transgenic strains was distributed throughout the cytoplasm and nucleus with no significant difference between the two strains (Figures 6A and 6B). However, vpr-1 mutants appear more sensitive to higher temperatures that require increased metabolic activity (Figures 6A and 6B). We conclude that VPR-1 does not have a strong effect on DAF-16 nuclear translocation under standard conditions.
Fig. 6. DAF-16 localization and activity in wild-type and mutant worms. 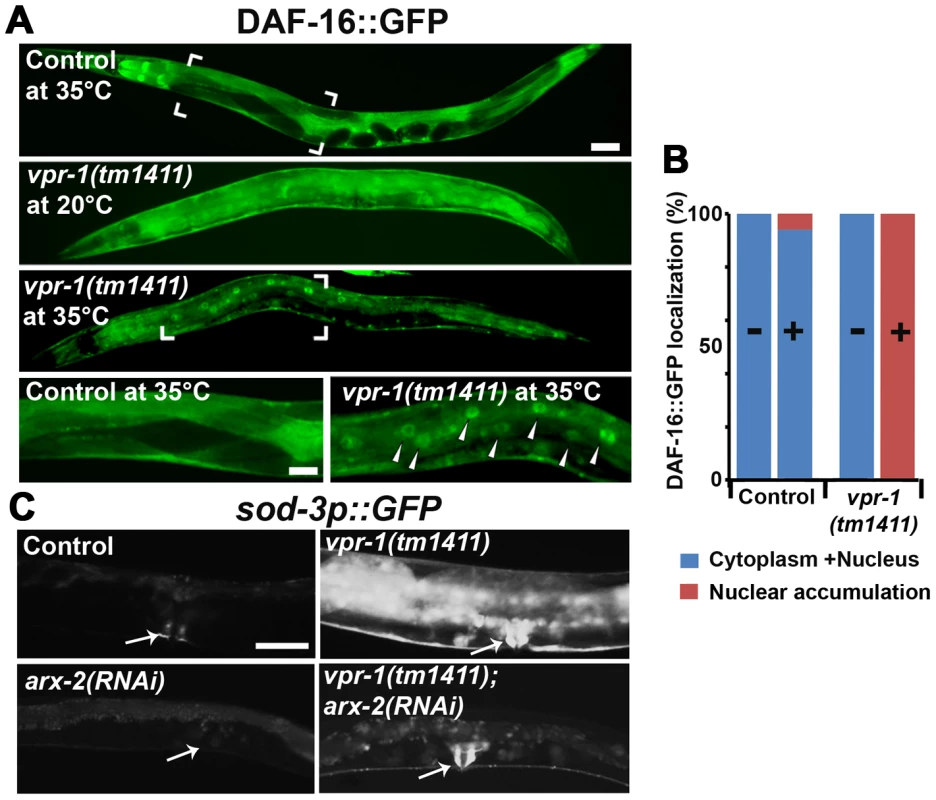
(A) Transgenic strains expressing DAF-16::GFP under its endogenous promoter. Transgenic controls raised at 20°C are similar to those raised at 20°C then shifted to 35°C for 30 minutes (see panel B for quantification). Close up images of boxed areas are shown. Anterior is to the left in all panels. Low magnification bar, 50 µm; high magnification bar, 25 µm. (B) Quantification of DAF-16::GFP localization in control (n = 157) and vpr-1(tm1411) mutants (n = 49). (−), incubation under normal growth condition; (+), incubation at 35°C for 30 minutes. (C) Magnified images showing transgenic lines expressing GFP under the sod-3 promoter. arx-2 encodes Arp2. Arrows indicate vulva muscle region. Anterior is to the left in all panels. Bar, 50 µm. DAF-16/FoxO likely acts downstream of the Arp2/3 complex
The results thus far strongly support the model that impaired vMSP signaling to muscle triggers DAF-16-dependent muscle fat accumulation. We hypothesized that cytoskeletal or mitochondrial abnormalities in vpr-1 mutant muscles induce elevated DAF-16 transcriptional activity. If this idea is correct, then inactivating the Arp2/3 complex in vpr-1 mutants should attenuate DAF-16 activity. To assess DAF-16 transcriptional activity, we used the integrated sod-3p::GFP transgenic reporter. arx-2/arp2 RNAi in vpr-1(tm1411) mutants causes a strong reduction in sod-3p::GFP expression in body wall muscle, the intestine, and other cells (Figure 6C). arx-2 RNAi in wild-type worms has little effect on GFP expression. Therefore, the elevated DAF-16 activity in vpr-1 mutants is at least partially dependent on the Arp2/3 complex.
One possibility is that DAF-16 causes the mitochondrial abnormalities in vpr-1 mutants. To test this model, we first evaluated mitochondria using the mitoGFP transgene expressed in body wall muscle. As previously documented [15], wild-type muscles contain linear mitochondrial tubules positioned along I-bands. In contrast, vpr-1(tm1411) mutants contain disorganized and interconnected mitochondrial networks in the muscle belly (Figure 7A). Loss of daf-16 in vpr-1(tm1411) mutants did not affect muscle mitochondrial morphology or localization (Figure 7A). Next, we examined mitochondrial functional status using MitoTracker CMXRos, which accumulates in the mitochondrial matrix depending on membrane potential, and oxygen consumption of whole worms. DAF-16 loss did not affect the reduced MitoTracker CMXRos accumulation (Figure 7B) or the low oxygen consumption rates of vpr-1 mutants (Figures 7C and 7D). We conclude that DAF-16 does not affect the muscle mitochondrial defects in vpr-1 mutants and likely acts downstream of Arp2/3.
Fig. 7. Effect of DAF-16 inactivation on muscle mitochondria. 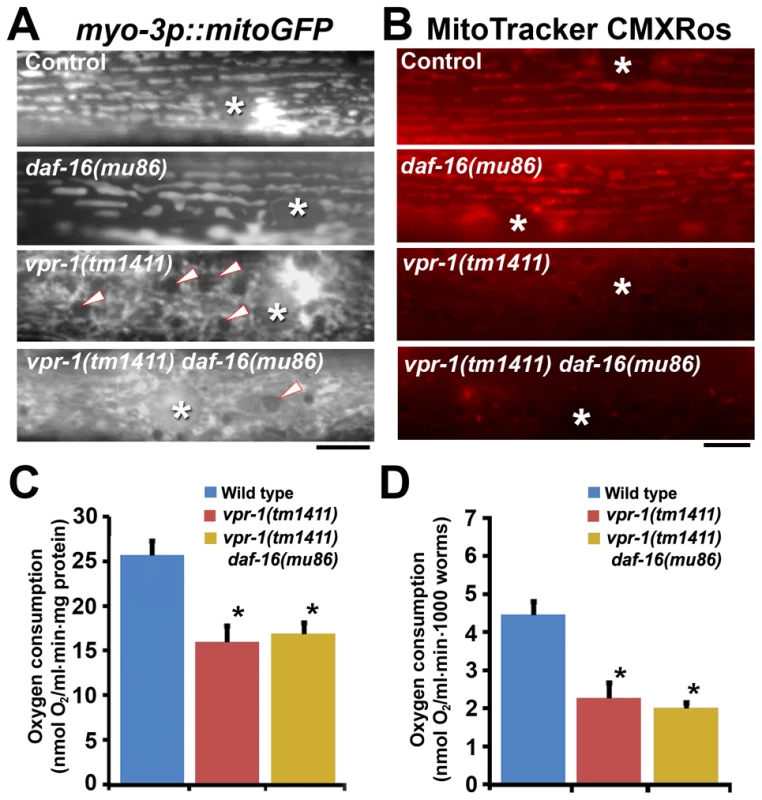
(A) Muscle mitochondrial tubules in indicated genotypes visualized using mitoGFP. Arrowheads indicate fat droplets. Asterisks indicate nucleus. Bar, 5 µm. (B) MitoTracker CMXRos staining of wild-type and mutant muscle. Asterisks indicate nucleus. Bar, 5 µm. (C and D) Oxygen consumption rates of wild-type and mutant hermaphrodites. Measured consumption rates were normalized by protein content (C) or number of worms (D). Error bars represent SD. *, P<0.001 compared to wild type. Oxygen consumption rate of wild-type and vpr-1(tm1411) mutants includes published data [15] measured together with vpr-1(tm1411) daf-16(mu86) mutants. DAF-16/FoxO increases ATP levels and extends lifespan of vpr-1/vap mutants
As the intestine and epidermis are fat storage sites in C. elegans, we hypothesized that the increase in muscle fat is an attempt to provide fuel for energy production. Our previous studies showed that 1-day-old adult vpr-1(tm1411) mutants have reduced ATP levels when compared to controls [15]. However, the ATP levels in vpr-1 mutants did not decrease over the next two days, as observed in the wild type (Figure 8A). 3-day-old adult vpr-1(tm1411) mutants had higher ATP levels than wild-type controls at the same age (Figure 8A). Similar ATP dynamics have been observed in aging worms with mutations in the daf-2 insulin receptor or clk-1, a mitochondrial protein involved in ubiquinone biosynthesis [43], [44]. Hence, DAF-16 may help maintain ATP levels in these aging worms. To test whether DAF-16 affects the energy balance of vpr-1 mutants, we measured ATP levels in single and double mutant extracts. daf-16 loss did not influence ATP levels in 1-day-old adult vpr-1(tm1411) mutants (Figure 8A). However, daf-16 is required for the high ATP concentration in 3-day old mutant adults (Figure 8A; P<0.001). ATP levels in daf-16 mutants are similar to wild-type controls (data not shown), as previously shown [43], [44]. These data indicate that DAF-16/FoxO helps vpr-1 mutants maintain ATP levels during aging.
Fig. 8. Effect of DAF-16 inactivation on ATP level and lifespan. 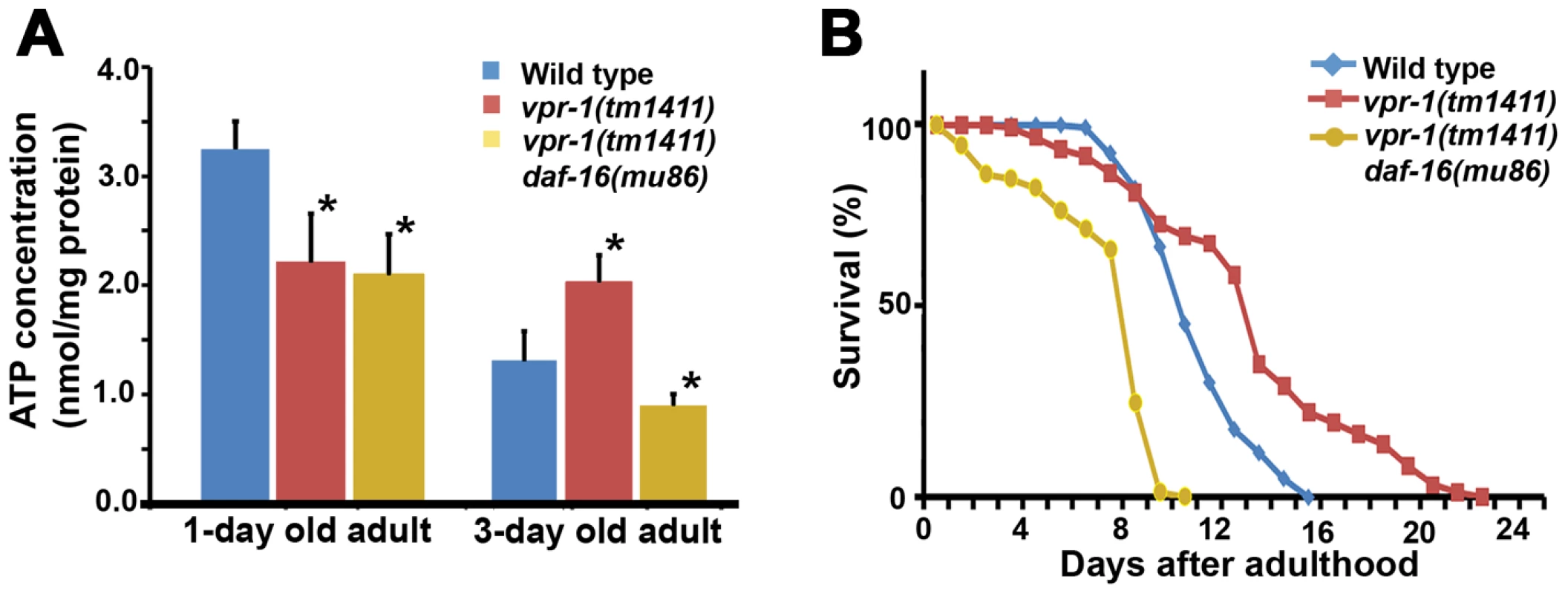
(A) ATP concentration in wild-type and vpr-1(tm1411) mutant adult extracts. *, P<0.001 compared to wild type. Error bars represent SD. ATP concentration of wild-type and vpr-1(tm1411) mutants at 1-day-old adults include published data [15] measured together with vpr-1(tm1411) daf-16(mu86) mutants. (B) Lifespan measurements of indicated genotypes. The lifespan of daf-16(mu86) mutants (not shown) was similar to the wild type, as previously shown. Based on the abnormalities in energy metabolism, we tested whether DAF-16 influences lifespan in vpr-1 mutants. Similar to other worm mutants with mild or tissue-specific reduction in mitochondrial function, vpr-1(tm1411) mutants have slightly extended adult lifespan compared to wild-type worms (Figure 8B; mean adult lifespan ± SD of 12.9±4.4 days [n = 154] for vpr-1(tm1411) versus 10.5±2.1 days [n = 159] for wild type, P<0.001). daf-16 loss in vpr-1(tm1411) mutants causes a strong reduction in lifespan relative to vpr-1 mutants and wild-type controls (Figure 8B; 6.9±2.5 days for vpr-1(tm1411) daf-16(mu86) [n = 250]; P<0.001). The lifespan of daf-16 single mutants was similar to wild type (data not shown), as previously shown [38], [45]. These data indicate that DAF-16/FoxO activity extends survival of vpr-1 mutants.
Vapb knockout mice exhibit signs of abnormal skeletal muscle energy metabolism
The data thus far indicate that VPR-1 loss causes profound defects in muscle energy metabolism. We hypothesized that the regulatory function of vMSPs on energy metabolism was conserved in mammals, and studied energy metabolism of Vapb −/ − mice [4]. In basal conditions, Vapb −/− mice do not exhibit overt defects in energy metabolism. In particular, body weight and glycemia appear normal with age (L. Dupuis, unpublished results). However, an energy metabolism defect of Vapb deficient mice might be unmasked by modifying insulin supply through feeding and fasting paradigms. In worms and mice, fasting reduces insulin signaling and increases FoxO activity, resulting in altered metabolic gene expression. We used Vapb −/ − mice of 2–6 months of age to avoid any confounding effect of the motor dysfunction observed at 18 months [4]. Mice were either fasted for 24 hours (fasted group) or fasted for 16 hours and refed for 8 hours to synchronize meals (fed group). In +/+ mice, fasting decreased the TAG levels in the gastrocnemius (GA) muscle (Figure 9A; P<0.05). In contrast, TAG levels remained unchanged upon fasting in Vapb −/− GA and tibialis anterior (TA) muscles (Figure 9A and data not shown). In liver, TAG levels were unchanged upon fasting and feeding in either +/+ or −/ − mice (Figure 9A). Thus, Vapb ablation increases the resistance of muscle lipid stores to fasting induced mobilization.
Fig. 9. Effect of Vapb ablation on fasting/refeeding energy metabolism in mice. 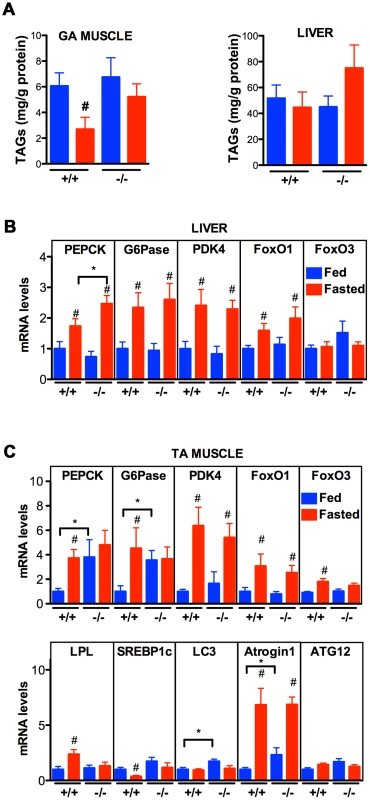
(A) TAG concentration in GA muscle and liver of wild-type (+/+) and Vapb knock-out (−/−) mice after 24-hour fasting (red) or 24 hours fasting followed by 6 hours of refeeding (blue). (B and C) Quantitative RT-PCR of indicated genes in liver (B) and TA muscle (C) of wild-type (+/+) and Vapb knock-out (−/−) mice after 24-hour fasting (red) or 24 hours fasting followed by 6 hours of refeeding (blue). Relative mRNA levels are shown on the Y-axis. #, P<0.05 compared to fed mice of the same genotype. *, P<0.05 compared to +/+ under the same condition. We next looked at mRNA levels of metabolic genes by quantitative RT-PCR. In liver, Vapb ablation potentiated induction of the direct FoxO1 target gene phosphoenolpyruvate carboxykinase (PEPCK) in response to fasting, but had no effect on fasting induction of other FoxO1 targets such as glucose 6-phosphatase (G6Pase) and pyruvate dehydrogenase kinase (PDK4) (Figure 9B). FoxO1 and FoxO3 mRNA and proteins were similar in +/+ and −/ − livers, and FoxO1 up-regulation by fasting appeared normal in −/ − liver (Figure 9B).
We also examined putative FoxO1 and FoxO3 target genes in +/+ and mutant TA muscle. Feeding decreased expression of PEPCK, G6Pase, and lipoprotein lipase (LPL), and increased expression of the lipogenic transcription factor SREBP1c (Figure 9C). This regulation was lost in Vapb −/ − muscles, as feeding did not modify expression of these four genes. Vapb genotype did not affect levels of PDK4 mRNA. FoxO1 and FoxO3 expression was down-regulated upon feeding in control TA muscles, but FoxO3 regulation was lost in −/ − muscles (Figure 9C). The expression of muscle FoxO3 targets LC3 and Atrogin1 was up-regulated in fed −/ − mice, while another FoxO3 target, ATG12, was unchanged. These results indicate that muscles of Vapb −/ − mice are partially insensitive to fasting/feeding alterations in lipid mobilization and FoxO target gene expression. Hence, Vapb mutant worms and mice appear to have muscle energy metabolism alterations, at least in part involving FoxO targets. Whether the putative metabolic changes in mouse muscle are due to secreted vMSPs is not yet clear.
Discussion
Results from Drosophila and C. elegans support the model that VAP MSP domains are secreted neurogenic factors that promote striated muscle oxidative metabolism [15]. In C. elegans, neurons cleave the vMSP and secrete it into the surrounding environment. Secreted vMSPs signal through SAX-3 Roundabout and CLR-1 Lar-like receptors expressed in muscle, down-regulating Lar signaling to the Arp2/3 complex. This signaling pathway restricts actin filament formation to I-bands of the myofilaments, thereby localizing mitochondria to I-bands and promoting mitochondrial function [13], [15]. Here we show that impaired vMSP signaling to muscle triggers increased DAF-16/FoxO transcription factor activity. FoxO promotes TAG accumulation in muscle, helps maintain ATP levels during aging, and extends lifespan. We propose that reduced vMSP signaling puts animals in an energy deficit, which triggers an altered metabolic response involving FoxO. Evidence for this model and implications for ALS are discussed below.
A VAPB cell nonautonomous mechanism for regulating muscle TAGs
VAPs physically interact with multiple proteins involved in lipid binding and transport, such as oxysterol binding protein and ceramide-transfer protein [10], [11], [30], [46]. Although the biological role of these interactions is not well understood, VAPs have been proposed to act in macromolecular complexes for transporting lipids between organelles at membrane contact sites. This mechanism depends on VAP function in the same cell in which lipid dynamics occur (i.e. a cell autonomous function). Here we show in C. elegans that vpr-1/vap loss triggers a robust increase in striated muscle TAG levels. Unexpectedly, this function does not require VPR-1 in muscle. Genetic mosaic and cell-type specific expression studies demonstrate that VPR-1 acts in neurons, consistent with the signaling function. Indeed, muscle vMSP receptors and the downstream Arp2/3 complex mediate this lipid metabolism response. We also found that sperm presence can modulate striated muscle TAG metabolism. Neurons and sperm are two cell types capable of secreting MSP domains [15], [47]. Our data do not exclude cell autonomous roles for VPR-1 in regulating lipid dynamics. Nevertheless, they highlight the importance of testing VAP autonomy when evaluating biological mechanism.
The connection between VAPB and FoxO
We show that vpr-1/vap loss triggers elevated DAF-16/FoxO activity, resulting in muscle TAG accumulation. Inactivating the Arp2/3 complex largely suppresses these metabolic alterations, as well as the muscle mitochondrial defects. These data support the model that impaired vMSP signaling to muscle triggers elevated FoxO activity. Consistent with this model, over-expressing Arp2 specifically in wild-type muscle causes TAG accumulation and mitochondrial defects. Although we cannot eliminate the possibility that Arp2/3 acts in other tissues, it appears to be a muscle-specific suppressor of vpr-1 mutants. How might the Arp2/3 complex regulate FoxO? One possibility is that vpr-1 mutants go into energy deficit as they age, as mitochondrial dysfunction is thought to increase FoxO activity [48]–[51]. An alternative possibility is that FoxO acts downstream of Arp2/3, but in parallel to mitochondria. In either case, reduced insulin signaling could be involved. A strong reduction in insulin causes increased FoxO nuclear translocation, which is not observed in vpr-1 mutants under standard conditions. However, subtle changes can be more difficult to detect.
Additional mechanisms could also modulate FoxO in vpr-1 mutants. The vMSP/ephrin receptor VAB-1 directly interacts with DAF-18/PTEN (phosphatase and tensin homolog deleted on chromosome ten), which regulates FoxO activity [52]. VAB-1 is expressed throughout the adult nervous system and in the gonad [53], [54]. Previous studies have shown that sperm presence can modulate DAF-16/FoxO translocation and transcriptional activity [55], perhaps through secreted MSPs. Whether sperm act via the Arp2/3 complex is not clear. An interesting possibility is that global MSP signals from neurons and sperm are sensed through distinct mechanisms. These mechanisms might converge on muscle metabolic output to meet changes in energy requirements.
In mammals, FoxO transcription factors are critical regulators of energy metabolism, particularly under fasting conditions. We show that Vapb ablation in mice renders muscle lipid stores resistant to fasting, a situation analogous to lipid accumulation in vpr-1 mutant worm muscles. Dysregulated lipid stores in mutant mice is associated with alterations in muscle gene expression consistent with abnormal FoxO1 and FoxO3 activity [56]. For instance, FoxO1 target gene mRNAs for PEPCK and G6Pase are clearly up-regulated in muscle of young Vapb −/ − mice in the fed state (i.e. in the presence of insulin that decreases FoxO1 activity). Similar results are observed for FoxO3 target genes LC3 and Atrogin-1. These data suggest that FoxO1/3 are less sensitive to insulin inhibition in Vapb −/ − mice.
Not all FoxO target genes studied are sensitive to Vapb ablation. For instance, VAPB does not appear to influence PDK4 and ATG12 mRNAs. Additionally, some of the mRNAs studied showed uncoupling from circulating insulin levels, consistent with an insensitivity of FoxO1 to insulin. SREBP1c mRNA, which is negatively regulated by FoxO1 [57], was increased by feeding in +/+ mice, but not in −/ − mice. A similar, albeit mirror situation was observed for LPL, a gene positively regulated by FoxO1 [58]. Hence, FoxO1/3 might participate in the abnormal lipid mobilization in Vapb −/ − mice, but other mechanisms are likely at work to avoid the major consequences of chronic muscle FoxO activation, such as muscle atrophy [59]. In summary, our findings show that VAPB is involved in modulating mouse muscle energy metabolism upon fasting and refeeding, possibly via altered FoxO activity. Whether this occurs through a cell autonomous or a cell nonautonomous mechanism, like in C. elegans and Drosophila, remains to be determined.
FoxO is protective in vap mutants
A key finding in worms is that DAF-16/FoxO activity prolongs the adult lifespan of vpr-1 mutants from 6.9±2.5 to 12.9±4.4 days. This lifespan increase may be due to metabolic alterations that compensate for mitochondrial dysfunction. Consistent with this idea, FoxO extends the lifespan of C. elegans with reduced mitochondrial function [48], [49], [60]. The FoxO-dependent fat accumulation in vpr-1 mutant muscle may reflect an effort to increase energy production. We show that DAF-16 helps vpr-1 mutants maintain ATP levels in 3-day old adults. Among the numerous DAF-16 metabolic genes are those involved in fat synthesis and transport, β-oxidation, the glyoxylate cycle, and gluconeogenesis [37]. However, additional DAF-16 targets may also be involved, such as stress resistance enzymes [37], [38], [61]. vpr-1 mutants are more resistant than the wild type to reactive oxygen species and ER stress. Based on identified DAF-16 targets and vpr-1 mutant phenotypes, DAF-16 might increase energy substrate availability in muscle, stimulate anaerobic metabolism, increase oxidative metabolism in non-muscle cells, or decrease ATP consumption. Further studies are necessary to distinguish among these possibilities, as well as other models.
Implications for ALS
Metabolic alterations in ALS patients and mouse models are hypothesized to compensate for mitochondrial dysfunction, particularly in skeletal muscle [17], [19], [62], [63]. Differentially expressed gene networks involved in oxidative metabolism and the cytoskeleton, including up-regulated FoxO1 and FoxO3 mRNAs have been found in ALS patient skeletal muscles [64], [65]. Our studies of VAPB in worms, flies, and mice are consistent with impaired vMSP signaling to muscle causing some of these alterations. Importantly, vpr-1 loss in worms, Vapb depletion in zebrafish, or Vapb loss in mice does not cause motor neuron degeneration [4], [15], providing strong evidence that mitochondrial and metabolic defects are not secondary consequences of neurodegeneration. These data contrast with a recent Drosophila study suggesting that Vapb loss causes neurodegeneration via increased phosphoinositides [66]. In humans, metabolic alterations caused by reduced VAPB function may not be sufficient to induce motor neuron degeneration, although they could strongly predispose to ALS. Redundancy could be an important consideration in the different models. The worm genome encodes a single vap homolog, but many genes with MSP domains. Vertebrate genomes typically encode VAPA and VAPB, which are approximately 60% identical. Vap mutant flies have the most severe developmental defects and the fewest MSP genes in the genome.
In summary, our results support the model that striated muscle mitochondrial dysfunction alters FoxO activity, which in turn affects energy metabolism and promotes survival. It is possible that reduced vMSP signaling causes some of the mitochondrial and metabolic alterations in ALS patients. Perhaps vMSPs might protect against ALS via effects on skeletal muscle energy metabolism.
Materials and Methods
C. elegans genetics, strains, and RNA-mediated interference
C. elegans Bristol N2 is the wild-type strain. Worms were grown on NGM plates with NA22 bacteria as the food source [67]. Strain construction and marker scoring were done as previously described [15], [54]. The strains and genetic markers used or generated were as follows: CF1553 muIs84[pAD76(sod-3::GFP)], CF1038 daf-16(mu86) I, vpr-1(tm1411)/hT2[bli-4(e937) let-?(q782) qIs48] I;III, SJ4005 zcIs4[hsp-4::GFP], TJ356 zIs356[daf-16p::daf-16::GFP; rol-6] IV, fog-3(q443) I/hT2[bli-4(e937) let-?(q782) qIs48] I;III, CX3198 sax-3(ky123) X, and XM1004 vpr-1(tm1411) daf-16(mu86)/hT2[bli-4(e937) let-?(q782) qIs48] I;III. Transgenics expressing vit-2p::vit-2::gfp were generated by crossing into the pwIs23 integrated line. RNAi was performed using the feeding method starting at the L1 stage, as previously described [15]. arx-2, clr-1, and xbp-1 RNAi clones are from the genome-wide library [68]. Each clone was sequenced for confirmation.
Transgenics
To generate transgenic C. elegans, the marker plasmids pRF4 [rol-6] (60 ng/µl) or myo-3p::mito::GFP (30–60 ng/µl) were mixed with myo-3p::vpr-1 (60 ng/µl), ges-1p::vpr-1(60 ng/µl), unc-119p::vpr-1(60 ng/µl), unc-119p::vpr-1 P56S (60 ng/µl), or myo-3p::arx-2::mCherry (60 ng/µl) and microinjected into the gonads of young adult hermaphrodites. Injected worms were incubated for 24 hours, transferred to new NGM plates, and screened for transgenic progeny. Transgenic lines were selected based on the roller phenotype or GFP expression. Multiple independent transgenic lines were generated for all strains. To conduct genetic mosaic analysis, 10 ng/µl WRM06B28 fosmid DNA containing the vpr-1 genomic locus was mixed with 10 ng/µl pTG96 (sur-5p::GFP) plasmid and microinjected into the gonads of vpr-1(tm1411)/hT2 hermaphrodites. Transgenic lines were selected based on GFP expression. Transgenic lines were maintained as vpr-1(tm1411) homozygotes, as the fosmid rescued the sterility, mitochondria, fat metabolism, slow growth, and embryonic defects. For lineage scoring, approximately 15,000 worms were screened. Transgene loss in the AB lineage was scored by GFP loss in head and tail neurons, the nerve cords, and the excretory gland. Transgene loss in the P1 lineage was scored by GFP loss in the intestine, muscle, somatic gonad, and hyp11. The P2 lineage was scored by GFP loss in numerous body wall muscle cells and hyp11, the P3 lineage was scored by GFP loss in body wall muscle, and the P4 lineage was inferred by a sterile phenotype without GFP loss. Transgene loss in the EMS lineage was scored by GFP loss in the intestine and somatic gonad, while loss in the E lineage was score by exclusive GFP loss in the intestine.
Transmission electron microscopy
TEM was performed as previously described [15].
Bodipy-FA and Sudan Black B staining
For the Bodipy-FA experiments, a 5 mM Bodipy-FA (Molecular probe, U.S.A) stock solution was prepared in DMSO and kept at −20°C. A 200 µM working solution diluted in distilled water was dropped onto seeded plates and allowed to dry. L4 stage worms were placed on the plates and incubated in the dark for 24 hours at 20°C. Bodipy-FAs can get trapped in intestinal gut granules that are not present in muscle.
Sudan Black B staining was conducted as described in previous studies [15]. Briefly, synchronized 1-day-old adult worms were collected into microfuge tubes containing M9 solution. Worms were washed five times, incubated for 40 minutes at 20°C to remove intestinal bacteria, and fixed in 1% paraformaldehyde. The fixed worms were washed three times in cold M9 solution and dehydrated through a 25%, 50%, and 70% ethanol series. Sudan Black B solution was added to the worms and incubated for 1 hour. To remove excess stain, worms were washed five times with 70% ethanol. To normalize for staining variability among experiments, wild type and vpr-1(tm1411) mutants were processed in the same tube and identified based on gonad morphology.
Lipid analysis by ESI-MS/MS
For the lipid analysis by ESI-MS/MS, lipids from equal masses of wild type and vpr-1(tm1411) mutant adults were extracted by chloroform-methanol following a modified Bligh/Dyer extraction [69]. A mixture of internal standards including T17 : 1 TAG was added to the chloroform-methanol phase before extraction. The extracted samples were concentrated to dryness under a nitrogen stream, reconstituted with methanol∶chloroform (1∶1 v/v) and transferred to HPLC auto samplers. Lipids were analyzed by ESI-MS/MS using an API 4000 (Applied Biosystems/MDS Sciex, Concord, Ontario, Canada) triple quadrupole mass spectrometer. Extracted lipid samples (5 ml) were infused into the mass spectrometer with a solvent mixture of chloroform-methanol (1∶2, v/v) containing 0.1% formic acid using a Shimadzu Prominence HPLC with a refrigerated auto sampler (Shimadzu Scientific Instruments, Inc. Columbia, MD). Lipids were analyzed in positive ion mode using an API 4000 (Applied Biosystems/MDS Sciex, Concord, Ontario, Canada) triple quadruple mass spectrometer. Samples (5 µl) were directly infused into the electrospray source using a Shimadzu Prominence HPLC with a refrigerated auto sampler (Shimadzu Scientific Instruments, Inc. Columbia, MD). Neutral loss (NL) scanning (228, 254, 256, 268, 278, 280, 284, and 304) of naturally occurring aliphatic chains (i.e. building block of TAG molecular species) were utilized to determine the identities of each molecular species. NL scanning of 141 was used for profiling phosphatidylethanolamine. The following analysis parameters were used: ion spray voltage 5000 V, de-clustering potential 40 V, temperature 300°C (for TAG), collision energy 35 V, and collisionally activated dissociation 5.
Mitochondrial staining
To assess mitochondrial transmembrane potential, worms were stained using the MitoTracker CMXRos dye (Molecular Probes, U.S.A), as previously described [15]. This lipophilic cationic fluorescent dye accumulates in mitochondria in a membrane potential-dependent manner [70]. L4 larval stage worms were placed on dried plates containing a 100 µM MitoTracker CMXRos dye solution (dropped on bacteria). After 24 hours incubation in the dark, worms were transferred to a new NGM plate and incubated in the dark for 20 minutes to remove intestinal background. Worms were mounted on dried 2% agarose pads without anesthetic. Wild-type and vpr-1(tm1411) mutant hermaphrodites were cultured on the same plates.
ATP concentration measurement
ATP concentration was measured as described previously, with slight modification [15]. Briefly, 150 worms were individually picked and placed into tubes containing M9 buffer, washed four times, and incubated at 20°C for 40 minutes to remove intestinal bacteria. These worms were then washed four times with TE solution (100 mM Tris–Cl, pH 7.6, 4 mM EDTA) and placed into microfuge tubes containing 300 µl TE solution. Worm extracts were prepared by a series of cycles including freezing, thawing, and sonicating. These extracts were boiled for 10 minutes to release ATP and block ATPase activity. Carcasses and insoluble material were pelleted in a microcentrifuge at 20,000×g for 10 minutes. The soluble extracts were diluted in a 1∶10 ratio using TE solution. ATP concentration in 60 µl of diluted extracts was measured using the ENLITEN ATP Assay System (Promega, U.S.A), according to the manufacturer's instructions. A luminometer (Berthold, Germany) was used for quantification. Protein concentration was determined using the BCA protein assay (Pierce, U.S.A). ATP measurements were repeated at least three times for each strain.
Oxygen consumption
Oxygen consumption rates were measured as previously described using the oxygraph system (Hansatech, UK) with minor modifications [15]. Worms were cultured at 20°C and synchronized to the 1-day-old adult stage. For each test, 1000 worms were individually picked and placed into a glass tube with 1 ml M9 buffer at 20°C. Collected worms were incubated for 40 min at 20°C to remove intestinal bacteria, carefully washed five times, and placed into 1 ml M9 buffer. The worm solution was loaded into the chamber equipped with a S1 Clark type polarographic oxygen electrode disc maintained at 20°C. Oxygen concentration was measured for 10 minutes. For normalization, worms were carefully collected from the chamber and protein content was measured using the BCA test kit (Pierce, U.S.A.). Rates were normalized to either total protein content or number of worms. We performed at least three independent measurements per strain.
Feeding rate and lifespan assays
To measure feeding rates, worms were cultured at 20°C and 1-day-old adult worms were placed on new NGM plates. Feeding behavior was recorded using a Zeiss Lumar stereomicroscope with AxioCam MRM digital camera. Measurements were conducted during a 30 second period at room temperature (22°C). The rhythmic contractions of the pharyngeal bulb were counted. For each strain, over 20 worms were counted. To determine lifespan of worms, L4 larval stage worms were placed on new NGM plates seeded with NA22 bacteria and cultured at 20°C. The L4 stage was used because a small percentage of vpr-1 mutants die during L1–L4 stages and vpr-1 mutants develop slowly. Worms were monitored every day and transferred to flesh NGM plates. Death was scored by failure to respond to touching with a platinum wire. Wild-type worms fed NA22 bacteria have slightly shorter lifespan than worms fed OP50 bacteria.
Tunicamycin resistance
To analyze ER homeostasis, worms were cultured on plates with tunicamycin (Sigma, U.S.A) from the embryonic stage to adulthood. NGM plates with 0.1% DMSO and 0 or 5.0 µg/ml tunicamycin were prepared. About 30 adult worms were placed on each tunicamycin plate and allowed to lay embryos for 30 minutes. Adult worms were then removed. Twelve hours later the number of hatched embryos was counted and compared with the number of worms that reached the adult stage within 96 hours. We performed at least three independent measurements for each strain.
Mouse experiments
Mouse experiments were performed using the Institutional European Guidelines, under the supervision of an authorized investigator (LD), and approved by the local ethical committee for animal experiments (CREMEAS, agreement N° AL/01/08/02/13). Vapb −/ − mice were used and genotyped as described [4]. Mice (8–10 per group) were either fasted for 24 hours from 5PM (fasted group), or fasted from 5PM to 9AM and refed until sacrifice at 5PM. GA and TA muscle and liver tissues were collected, and rapidly frozen in liquid nitrogen for subsequent analyses of gene expression and TAG levels. The tissues were stored at −80°C until the time of analysis.
For RT-qPCR, frozen liver and muscle tissues were placed into tubes containing 5 mm stainless steel beads (Qiagen, Courtaboeuf, France) and 1 ml of Trizol reagent (Invitrogen, Paisley, UK) and homogenized using a TissueLyser (Qiagen). RNA was prepared from tissue homogenates following Trizol manufacturer's instructions. RNA reverse transcription and SYBR Green real-time PCR assays were performed using the Bio-Rad (Biorad, Marnes la Coquette, France) iCycler kits and protocols. PCR conditions were 3 min at 94°C, followed by 40 cycles of 45 s at 94°C and 10 s at 60°C. Primers are shown in Table S1. For western blotting, liver and TA muscles were incubated in Lysis buffer containing complete protease and phosphatase inhibitor cocktails. Protein concentration was measured using BCA Protein Assay. Equal amount of protein (50 µg) were separated by SDS-PAGE 10% and blotted onto nitrocellulose membrane. Membranes were saturated with 10% milk and then incubated with the primary antibodies FoxO1 (Proteintech; 18592-1-AP), FoxO3a (Cell signaling; #2497), VAPB [4] and Histone H3 (Cell signaling; #9715), all diluted (1∶1000) followed by anti-rabbit secondary antibody, diluted 1∶5000.
For TAG analysis, tissue powder was homogenized in lysis buffer (250 mM Sucrose solution, 1 mM EDTA, 2% SDT, 1 mM DTT, 10 mM Tris HCl pH 7.4) containing protease inhibitors (Sigma P8340) and phosphatase inhibitors (Sigma 8345), centrifuged at 12000×rpm for 15 minutes at room temperature. TAG concentration was determined in duplicate for each sample in 5 µl of supernatant, using the enzymatic method of analysis (Randox Triglyceride Colorimetric Assay Kit, Randox Laboratories Limited, UK) as described by the manufacturer. Lipid values were normalized to protein concentration.
Supporting Information
Zdroje
1. KiernanMC, VucicS, CheahBC, TurnerMR, EisenA, et al. (2011) Amyotrophic lateral sclerosis. Lancet 377 : 942–955.
2. NishimuraAL, Mitne-NetoM, SilvaHC, Richieri-CostaA, MiddletonS, et al. (2004) A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet 75 : 822–831.
3. ChenHJ, AnagnostouG, ChaiA, WithersJ, MorrisA, et al. (2010) Characterization of the properties of a novel mutation in VAPB in familial amyotrophic lateral sclerosis. J Biol Chem 285 : 40266–40281.
4. KabashiE, El OussiniH, BercierV, Gros-LouisF, ValdmanisPN, et al. (2013) Investigating the contribution of VAPB/ALS8 loss of function in amyotrophic lateral sclerosis. Hum Mol Genet 22 : 2350–2360.
5. AnagnostouG, AkbarMT, PaulP, AngelinettaC, SteinerTJ, et al. (2010) Vesicle associated membrane protein B (VAPB) is decreased in ALS spinal cord. Neurobiol Aging 31 : 969–985.
6. TeulingE, AhmedS, HaasdijkE, DemmersJ, SteinmetzMO, et al. (2007) Motor neuron disease-associated mutant vesicle-associated membrane protein-associated protein (VAP) B recruits wild-type VAPs into endoplasmic reticulum-derived tubular aggregates. J Neurosci 27 : 9801–9815.
7. Mitne-NetoM, Machado-CostaM, MarchettoMC, BengtsonMH, JoazeiroCA, et al. (2011) Downregulation of VAPB expression in motor neurons derived from induced pluripotent stem cells of ALS8 patients. Hum Mol Genet 20 : 3642–3652.
8. LoewenCJ, LevineTP (2005) A highly conserved binding site in vesicle-associated membrane protein-associated protein (VAP) for the FFAT motif of lipid-binding proteins. J Biol Chem 280 : 14097–14104.
9. AmarilioR, RamachandranS, SabanayH, LevS (2005) Differential regulation of endoplasmic reticulum structure through VAP-Nir protein interaction. J Biol Chem 280 : 5934–5944.
10. LevS, Ben HalevyD, PerettiD, DahanN (2008) The VAP protein family: from cellular functions to motor neuron disease. Trends Cell Biol 18 : 282–290.
11. PerettiD, DahanN, ShimoniE, HirschbergK, LevS (2008) Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol Biol Cell 19 : 3871–3884.
12. De VosKJ, MorotzGM, StoicaR, TudorEL, LauKF, et al. (2012) VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum Mol Genet 21 : 1299–1311.
13. TsudaH, HanSM, YangY, TongC, LinYQ, et al. (2008) The amyotrophic lateral sclerosis 8 protein VAPB is cleaved, secreted, and acts as a ligand for Eph receptors. Cell 133 : 963–977.
14. HanSM, CotteePA, MillerMA (2010) Sperm and oocyte communication mechanisms controlling C. elegans fertility. Dev Dyn 239 : 1265–1281.
15. HanSM, TsudaH, YangY, VibbertJ, CotteeP, et al. (2012) Secreted VAPB/ALS8 Major Sperm Protein Domains Modulate Mitochondrial Localization and Morphology via Growth Cone Guidance Receptors. Dev Cell 22 : 348–362.
16. MarquesVD, BarreiraAA, DavisMB, Abou-SleimanPM, SilvaWAJr, et al. (2006) Expanding the phenotypes of the Pro56Ser VAPB mutation: proximal SMA with dysautonomia. Muscle Nerve 34 : 731–739.
17. DupuisL, PradatPF, LudolphAC, LoefflerJP (2011) Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol 10 : 75–82.
18. GalloV, WarkPA, JenabM, PearceN, BrayneC, et al. (2013) Prediagnostic body fat and risk of death from amyotrophic lateral sclerosis: The EPIC cohort. Neurology 80 : 829–838.
19. DupuisL, CorciaP, FerganiA, Gonzalez De AguilarJL, Bonnefont-RousselotD, et al. (2008) Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 70 : 1004–1009.
20. DorstJ, KuhnleinP, HendrichC, KassubekJ, SperfeldAD, et al. (2011) Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J Neurol 258 : 613–617.
21. HerndonLA, SchmeissnerPJ, DudaronekJM, BrownPA, ListnerKM, et al. (2002) Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419 : 808–814.
22. McGeeMD, WeberD, DayN, VitelliC, CrippenD, et al. (2011) Loss of intestinal nuclei and intestinal integrity in aging C. elegans. Aging cell 10 : 699–710.
23. KubagawaHM, WattsJL, CorriganC, EdmondsJW, SztulE, et al. (2006) Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat Cell Biol 8 : 1143–1148.
24. KlapperM, EhmkeM, PalgunowD, BohmeM, MatthausC, et al. (2011) Fluorescence-based fixative and vital staining of lipid droplets in Caenorhabditis elegans reveal fat stores using microscopy and flow cytometry approaches. J Lipid Res 52 : 1281–1293.
25. GrantB, HirshD (1999) Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell 10 : 4311–4326.
26. MoumenA, VirardI, RaoulC (2011) Accumulation of wildtype and ALS-linked mutated VAPB impairs activity of the proteasome. PloS One 6: e26066.
27. GkogkasC, MiddletonS, KremerAM, WardropeC, HannahM, et al. (2008) VAPB interacts with and modulates the activity of ATF6. Hum Mol Genet 17 : 1517–1526.
28. BasseriS, AustinRC (2012) Endoplasmic reticulum stress and lipid metabolism: mechanisms and therapeutic potential. Biochem Res Int 2012 : 841362.
29. UranoF, CalfonM, YonedaT, YunC, KiralyM, et al. (2002) A survival pathway for Caenorhabditis elegans with a blocked unfolded protein response. J Cell Biol 158 : 639–646.
30. WylesJP, McMasterCR, RidgwayND (2002) VAMP-associated protein-A (VAP-A) interacts with the oxysterol binding protein (OSBP) to modify export from the endoplasmic reticulum. J Biol Chem 277 : 29908–29918.
31. JansenM, OhsakiY, Rita RegaL, BittmanR, OlkkonenVM, et al. (2011) Role of ORPs in sterol transport from plasma membrane to ER and lipid droplets in mammalian cells. Traffic 12 : 218–231.
32. PerettiD, DahanN, ShimoniE, HirschbergK, LevS (2008) Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol Biol Cell 19 : 3871–3884.
33. YochemJ, HermanRK (2003) Investigating C. elegans development through mosaic analysis. Development 130 : 4761–4768.
34. YangY, HanSM, MillerMA (2010) MSP hormonal control of the oocyte MAP kinase cascade and reactive oxygen species signaling. Dev Biol 342 : 96–107.
35. OhSW, MukhopadhyayA, DixitBL, RahaT, GreenMR, et al. (2006) Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat Genet 38 : 251–257.
36. Halaschek-WienerJ, KhattraJS, McKayS, PouzyrevA, StottJM, et al. (2005) Analysis of long-lived C. elegans daf-2 mutants using serial analysis of gene expression. Genome Res 15 : 603–615.
37. MurphyCT (2006) The search for DAF-16/FOXO transcriptional targets: approaches and discoveries. Exp Gerontol 41 : 910–921.
38. MurphyCT, McCarrollSA, BargmannCI, FraserA, KamathRS, et al. (2003) Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424 : 277–283.
39. McElweeJ, BubbK, ThomasJH (2003) Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell 2 : 111–121.
40. HendersonST, BonafeM, JohnsonTE (2006) daf-16 protects the nematode Caenorhabditis elegans during food deprivation. J Gerontol A Biol Sci Med Sci 61 : 444–460.
41. LandisJN, MurphyCT (2010) Integration of diverse inputs in the regulation of Caenorhabditis elegans DAF-16/FOXO. Dev Dyn 239 : 1405–1412.
42. WilliamsTW, DumasKJ, HuPJ (2010) EAK proteins: novel conserved regulators of C. elegans lifespan. Aging 2 : 742–747.
43. HouthoofdK, BraeckmanBP, LenaertsI, BrysK, MatthijssensF, et al. (2005) DAF-2 pathway mutations and food restriction in aging Caenorhabditis elegans differentially affect metabolism. Neurobiol Aging 26 : 689–696.
44. BraeckmanBP, HouthoofdK, De VreeseA, VanfleterenJR (2002) Assaying metabolic activity in ageing Caenorhabditis elegans. Mech Ageing Dev 123 : 105–119.
45. LinK, HsinH, LibinaN, KenyonC (2001) Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet 28 : 139–145.
46. StefanCJ, ManfordAG, BairdD, Yamada-HanffJ, MaoY, et al. (2011) Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell 144 : 389–401.
47. KosinskiM, McDonaldK, SchwartzJ, YamamotoI, GreensteinD (2005) C. elegans sperm bud vesicles to deliver a meiotic maturation signal to distant oocytes. Development 132 : 3357–3369.
48. DillinA, HsuAL, Arantes-OliveiraN, Lehrer-GraiwerJ, HsinH, et al. (2002) Rates of behavior and aging specified by mitochondrial function during development. Science 298 : 2398–2401.
49. LeeSS, LeeRY, FraserAG, KamathRS, AhringerJ, et al. (2003) A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet 33 : 40–48.
50. BillingO, KaoG, NarediP (2011) Mitochondrial function is required for secretion of DAF-28/insulin in C. elegans. PloS One 6: e14507.
51. GreerEL, BankoMR, BrunetA (2009) AMP-activated protein kinase and FoxO transcription factors in dietary restriction-induced longevity. Ann N Y Acad Sci 1170 : 688–692.
52. BrisbinS, LiuJ, BoudreauJ, PengJ, EvangelistaM, et al. (2009) A role for C. elegans Eph RTK signaling in PTEN regulation. Dev Cell 17 : 459–469.
53. GeorgeSE, SimokatK, HardinJ, ChisholmAD (1998) The VAB-1 Eph receptor tyrosine kinase functions in neural and epithelial morphogenesis in C. elegans. Cell 92 : 633–643.
54. MillerMA, RuestPJ, KosinskiM, HanksSK, GreensteinD (2003) An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev 17 : 187–200.
55. MiyataS, BegunJ, TroemelER, AusubelFM (2008) DAF-16-dependent suppression of immunity during reproduction in Caenorhabditis elegans. Genetics 178 : 903–918.
56. ChengZ, WhiteMF (2011) Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid Redox Signal 14 : 649–661.
57. KameiY, MiuraS, SuganamiT, AkaikeF, KanaiS, et al. (2008) Regulation of SREBP1c gene expression in skeletal muscle: role of retinoid X receptor/liver X receptor and forkhead-O1 transcription factor. Endocrinology 149 : 2293–2305.
58. KameiY, OhizumiH, FujitaniY, NemotoT, TanakaT, et al. (2003) PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci U S A 100 : 12378–12383.
59. KameiY, MiuraS, SuzukiM, KaiY, MizukamiJ, et al. (2004) Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem 279 : 41114–41123.
60. ReaSL, VenturaN, JohnsonTE (2007) Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol 5: e259.
61. HondaY, HondaS (1999) The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J 13 : 1385–1393.
62. CrugnolaV, LampertiC, LucchiniV, RonchiD, PeverelliL, et al. (2010) Mitochondrial respiratory chain dysfunction in muscle from patients with amyotrophic lateral sclerosis. Arch Neurol 67 : 849–854.
63. ZhouJ, YiJ, FuR, LiuE, SiddiqueT, et al. (2010) Hyperactive intracellular calcium signaling associated with localized mitochondrial defects in skeletal muscle of an animal model of amyotrophic lateral sclerosis. J Biol Chem 285 : 705–712.
64. BernardiniC, CensiF, LattanziW, BarbaM, CalcagniniG, et al. (2013) Mitochondrial network genes in the skeletal muscle of amyotrophic lateral sclerosis patients. PloS One 8: e57739.
65. LegerB, VerganiL, SoraruG, HespelP, DeraveW, et al. (2006) Human skeletal muscle atrophy in amyotrophic lateral sclerosis reveals a reduction in Akt and an increase in atrogin-1. FASEB J 20 : 583–585.
66. ForrestS, ChaiA, SanhuezaM, MarescottiM, ParryK, et al. (2013) Increased levels of phosphoinositides cause neurodegeneration in a Drosophila model of amyotrophic lateral sclerosis. Hum Mol Genet 22 : 2689–2704.
67. BrennerS (1974) The genetics of Caenorhabditis elegans. Genetics 77 : 71–94.
68. KamathRS, AhringerJ (2003) Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30 : 313–321.
69. BlighEG, DyerWJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37 : 911–917.
70. GilmoreK, WilsonM (1999) The use of chloromethyl-X-rosamine (Mitotracker red) to measure loss of mitochondrial membrane potential in apoptotic cells is incompatible with cell fixation. Cytometry 36 : 355–358.
Štítky
Genetika Reprodukčná medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



