-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Loss and Gain of Natural Killer Cell Receptor Function in an African Hunter-Gatherer Population
The genes that control the response of the human immune system vary enormously between individuals. Understanding the evolution of these genetic differences and how they individualize immune responses is central to understanding how the immune system works in health and disease. In this regard, the KhoeSan of southern Africa are particularly informative because they are genetically diverse, divergent from other modern human populations and have been subject to unique demographic history. In the KhoeSan population, we studied variable genes that control natural killer cell function. We identified two recently evolved, novel gene variants that have unusual function; one completely changed its ligand specificity and the other lost its capacity for signal transduction.
Published in the journal: Loss and Gain of Natural Killer Cell Receptor Function in an African Hunter-Gatherer Population. PLoS Genet 11(8): e32767. doi:10.1371/journal.pgen.1005439
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005439Summary
The genes that control the response of the human immune system vary enormously between individuals. Understanding the evolution of these genetic differences and how they individualize immune responses is central to understanding how the immune system works in health and disease. In this regard, the KhoeSan of southern Africa are particularly informative because they are genetically diverse, divergent from other modern human populations and have been subject to unique demographic history. In the KhoeSan population, we studied variable genes that control natural killer cell function. We identified two recently evolved, novel gene variants that have unusual function; one completely changed its ligand specificity and the other lost its capacity for signal transduction.
Introduction
Natural killer (NK) cells are versatile lymphocytes that contribute to reproduction and immune defense [1,2]. Modulating the activities of human NK-cells are the killer-cell immunoglobulin-like receptors (KIR). These receptors engage the HLA class I ligands (HLA-A, -B and -C) expressed on the surface of most human cells. Such interactions direct NK cells to kill virus-infected cells and tumor cells; they also induce the secretion of cytokines that activate other leukocytes or guide fetal trophoblast cells to invade the uterus during pregnancy. In human populations, both receptors and ligands are highly polymorphic. Their combinatorial diversity contributes to the resistance of individuals to infection, and their susceptibility to autoimmunity and pregnancy syndromes [1,3]. A minority of HLA-A and -B allotypes are ligands for KIR, whereas all HLA-C allotypes fulfill this role. HLA-C arose more recently than HLA-A and -B and has evolved to become the predominant polymorphic KIR ligand [4]. In reproduction it is the only polymorphic ligand, because HLA-C is expressed by fetal trophoblast cells whereas HLA-A and -B are not [1].
KIR engage the upward face of the HLA class I molecule formed by the α1 domain, the α2 domain and the bound peptide antigen [5]. αβ T cell receptors engage the same face, in an overlapping but different way [6]. Dimorphism at position 80 in the α1 domain of HLA-C defines two mutually exclusive epitopes, C1 (asparagine 80) and C2 (lysine 80), recognized by different KIR [7]. All the numerous (>1,700) HLA-C allotypes have either the C1 or C2 epitope. Human KIR are comprised of four phylogenetic lineages, of which the KIR that recognize HLA-C are all lineage III [4]. They have two extracellular immunoglobulin-like domains (D1 and D2), which together form the site that binds HLA-C [5]. Within the binding site, dimorphism at position 44 in the D1 domain determines if a KIR is specific for C1 (lysine 44) or C2 (methionine 44). KIR2DL1 and KIR2DS1 encode inhibitory and activating C2 receptors, respectively. KIR2DL2/3 encodes inhibitory C1 receptors. (There is no activating C1 receptor). The inhibitory receptors are highly polymorphic, with 25 KIR2DL1 and 36 KIR2DL2/3 variants being defined, whereas KIR2DS1 with seven variants is relatively conserved.
Among individuals and populations, KIR are further diversified by gene content variation [8]. Whereas KIR2DL2/3 is present on almost every human KIR haplotype described, neither KIR2DL1 nor KIR2DS1 are present on every haplotype. Represented in every human population are two distinctive KIR haplotype groups: A and B [1]. KIR A haplotypes encode high avidity inhibitory receptors for HLA class I and have one activating receptor gene; B haplotypes encode low avidity inhibitory receptors for HLA class I and have several activating receptor genes. This bipartite system of functionally distinctive KIR haplotypes appears unique to humans because it is not present in chimpanzees or any other species investigated [9].
For reasons of practicality, the functional properties of KIR2DL1 and KIR2DL2/3 have been studied mainly in the context of allotypic variants that combine high avidity, high specificity and high frequency in Europeans [1]. In contrast, for sub-Saharan African populations, which have the highest genetic diversity [10–12] and among the highest mortality from infectious disease and pregnancy complications [13], KIR investigation is in its infancy and has so far focused on West African and Bantu-speaking populations [14]. Within sub-Saharan Africa, some indigenous populations are as different from each other as they are from Europeans [10,12,15]. Notably, the KhoeSan who reside across southern Africa descend from the deepest human population divergence and have among the greatest genetic diversity of any population [10,16,17]. During the last 2,000 years there has been admixture between the KhoeSan and Bantu-speaking agriculturalists who expanded southwards [11,18]. More recently, the arrival of European colonists over the past 500 years has introduced novel infectious diseases including smallpox and tuberculosis [19]. Here we describe high-resolution genetic and functional studies on the HLA-C specific KIR of the KhoeSan and their comparison to other populations.
Results
Two unusual KIR2DL1 alleles evolved in the KhoeSan after their divergence from other modern humans
From analysis of 61 KhoeSan we identified ten KIR2DL1 alleles (Fig 1A and S1A Fig). Of these, 2DL1*022 and 2DL1*026 are new discoveries that have frequencies in the KhoeSan of 17.2% and 4.2%, respectively. Being absent from all previously studied populations [14,20–24], suggested that 2DL1*022 and 2DL1*026 are specific to the KhoeSan. To test this hypothesis we examined additional populations for the presence of these alleles. We first examined data from the 1000 Genomes project dataset [25] by probing for sequence-specific reads that correspond to the 2DL1*022 and 2DL1*026 alleles. KIR3DL3, a framework gene present on all KIR haplotypes, served as the positive control. All 2,496 individuals sampled had reads corresponding to KIR3DL3, as did the eight KhoeSan who were analyzed similarly by whole-exome sequencing [26]. The eight KhoeSan individuals also gave allele-specific KIR2DL1 reads consistent with their high-resolution KIR2DL1 genotype (S2A Fig). In this context it is striking that none of the 2,496 individuals, representing 26 different populations worldwide (S2B Fig), was found to have either 2DL1*022 or 2DL1*026 (S2C Fig). Because the 1000 Genome dataset represents a limited subset of sub-Saharan African population diversity [12,27], we expanded our search to include four further groups.
Fig. 1. A variant of KIR2DL1 originating in the KhoeSan is a C1-specific receptor and not a C2-specific receptor like other KIR2DL1. 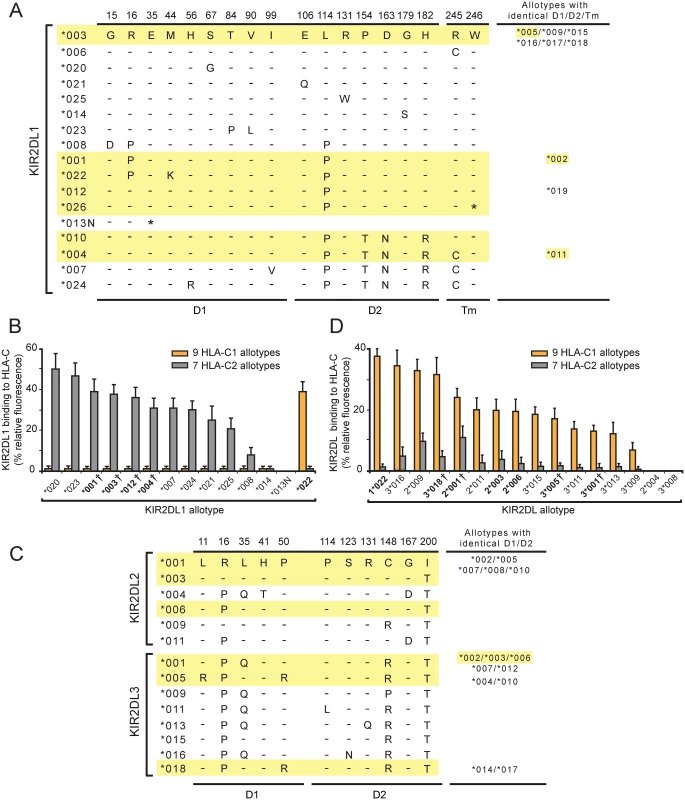
(A) This alignment of KIR2DL1 sequence differences shows the sites of polymorphism in the D1 domain (D1), the D2 domain (D2) and the transmembrane region (Tm). Dashes denote identity with the KIR2DL1*003 sequence, an asterisk denotes a termination codon. Sequences of the KhoeSan KIR2DL1 allotypes are highlighted in yellow. The names of allotypes with D1, D2 and Tm identical to an aligned sequence are listed in the column at the right. (B) Binding of KIR2DL1-Fc fusion proteins to microbeads coated with C1-bearing and seven C2-bearing HLA-C allotypes. Each binding value was normalized to that of the W6/32 antibody and these normalized values were averaged for the C1 (N = 9) and C2 (N = 7) allotype groups. The names of allotypes present in the KhoeSan are boldened. A dagger following the listed allotype indicates that the allotype represents a group of two or more alleles that encode identical ligand binding domains (see Panel A). (C) This alignment of KIR2DL2/3 sequence differences shows the sites of polymorphism in the D1 and D2 domains. Dashes denote identity with the KIR2DL2*001 sequence Sequences of the KhoeSan KIR2DL2/3 allotypes are highlighted in yellow. The names of allotypes with D1 and D2 identical to an aligned sequence are listed in the column at the right. (D) Binding of KIR2DL2/3-Fc fusion proteins to microbeads coated with C1-bearing and C2-bearing HLA-C allotypes. Each binding value was normalized to that of the W6/32 antibody and these normalized values were averaged for the C1 (N = 9) and C2 (N = 7) groups. The names of allotypes present in the KhoeSan are boldened. Groups of allotypes with identical D1 and D2 domains, and which are represented by a single KIR2DL2/3-Fc, are as shown in the column on the right of Panel A. A dagger following the listed allotype indicates that the allotype represents a group of two or more alleles that encode identical ligand binding domains (see Panel A). To determine if 2DL1*022 or 2DL1*026 are present in African hunter-gatherer populations other than the KhoeSan we examined three groups: the Hadza who are an isolated click-speaking population that live in northern Tanzania [10] and the central African Mbuti and Baka Pygmies. Together with the KhoeSan, these hunter-gatherer groups may have formed a larger proto-KhoeSan-Pygmy population prior to their divergence 50,000–100,000 years ago [12,28,29]. Neither 2DL1*022 nor 2DL1*026 was detected in any Hadza or Pygmy individual (S2C Fig). Despite the relatively low number of individuals sampled (52 Hadza and 40 Pygmies) this result indicates that 2DL1*022 and 2DL1*026 are not present at any appreciable frequency in these groups.
To determine whether 2DL1*022 and 2DL1*026 are present in other southern African populations, we examined 100 Zulu individuals whose genomes were sequenced as part of the African Genome Variation Project [27]. With the same approach used to probe the 1000 Genomes dataset, we identified three Zulus having 2DL1*022 and two having 2DL1*026 (S2C Fig). As these five individuals were all 2DL1 heterozygous, we estimate that the frequencies of 2DL1*022 and 2DL1*026 in the Zulu population are approximately 1.5% and 1%, respectively. Examination of the centromeric half of the KIR haplotypes, the location of the KIR2DL1 gene, showed that each Zulu allele is likely present on the identical haplotype background to that found in the KhoeSan (S3 Fig). Together with their low frequencies, this suggests that these alleles were introduced into the Zulu population as a result of admixture with KhoeSan hunter-gatherers. This interpretation is supported by studies that have demonstrated recent KhoeSan admixture with the Zulus [10,11,18,27] and by the absence of both 2DL1*022 and 2DL1*026 from a Bantu-speaking population in east Africa (Kenyan Luhya from the 1000 Genomes dataset). These data support an evolutionary model in which 2DL1*022 and 2DL1*026 rose in frequency in the KhoeSan populations sometime after their divergence from the other groups and thus within the past 100,000 years [12,28,29].
KIR2DL1*022 differs from 2DL1*001, also present in the KhoeSan, by a single non-synonymous substitution in codon 44 (Fig 1A). Thus 2DL1*022 likely evolved from 2DL1*001 by a point mutation that caused methionine to be replaced by lysine at position 44 (Fig 1A). Position 44 dimorphism determines whether a given KIR2DL has specificity for the C1 or C2 epitope of HLA-C [7]. Prior to investigation of the KhoeSan, all the known KIR2DL1 allotypes (n = 23) had methionine 44 and were predicted to be C2-specific. Conversely, and in complementary fashion, the known KIR2DL2/3 allotypes (n = 36) all had lysine 44 and were predicted to be C1 specific. In this context, 2DL1*022 appears an extraordinary KIR2DL1 allotype, being predicted to be a C1 receptor and not a C2 receptor like other KIR2DL1 allotypes. Thus, the mutation that created 2DL1*022 had two important functional effects: loss of C2 recognition and gain of C1 recognition.
KIR2DL1*026, the other KhoeSan-specific KIR2DL1 allele, differs from 2DL1*012, also present in the KhoeSan, by one nucleotide substitution. Thus KIR2DL1*026 likely arose from 2DL1*012 by point mutation. This substitution converted the tryptophan codon at position 246 to a termination codon (Fig 1A). Position 246 is situated at the boundary between the transmembrane domain and the cytoplasmic tail. Consequently, 2DL1*026 lacks the immunoreceptor tyrosine-based inhibitory motifs of the cytoplasmic tail that mediate inhibitory signaling function [30]. Less obvious is the effect that absence of a cytoplasmic tail could have on the association of 2DL1*026 with cellular membranes. Thus, the mutation that created 2DL1*026 clearly has a major effect in abrogating inhibitory signaling function, but it also has potential to alter the amount of receptor that reaches the NK cell-surface.
KhoeSan specific 2DL1*022 is an unusually strong and specific C1 receptor
To determine the avidity and specificity of 2DL1*022 for HLA class I, and also to compare its binding reactivity with other KIR2DL1 allotypes, we made a panel of 14 KIR2DL1-Fc fusion proteins that covers the allotypic range of KIR2DL1 binding sites (Fig 1A). Each KIR-Fc was tested for binding to a panel of 97 microbeads in which each bead is coated with one of 31 HLA-A, 50 HLA-B and 16 HLA-C allotypes. Our previous work has shown that the results obtained with this cell-free bead-binding assay correlate well with those derived from in vitro functional assays of NK cell cytotoxicity [31,32].
Assessment of the pairwise interactions between 14 KIR2DL1-Fc and 16 HLA-C allotypes shows that 2DL1*022 binds to all nine C1-bearing HLA-C allotypes but to none of the seven C2-bearing HLA-C allotypes in the test panel (Fig 1B). KIR2DL1*022 also binds HLA-B*46 : 01 and HLA-B*73 : 01, two exceptional C1-bearing HLA-B allotypes but to no other HLA-B allotype, or any HLA-A allotype. Eleven HLA-C1 bearing allotypes are present in the KhoeSan (S1C Fig). Neither HLA-B*46 : 01 or HLA-B*73 : 01 are present in the KhoeSan, their distributions being focused on Southeast Asia (B*46 : 01) or West Asia (B*73 : 01) [9,33,34]. As we predicted, 2DL1*022 functions as a C1-specific receptor and not a C2-specific receptor like eleven of the 13 other KIR2DL1-Fc. These eleven KIR2DL1-Fc molecules varied in their avidity for C2 by half an order of magnitude. In contrast, 2DL1*013N-Fc and 2DL1*014-Fc bound to no HLA class I allotype (Fig 1B). For 2DL1*013N this result was anticipated, because the protein is a fragment that terminates prematurely at residue 34 in the D1 domain. On the other hand, 2DL1*014 was expected to bind HLA class I, because it differs from 2DL1*003 only by substitution of glycine for serine at position 179 in the D2 domain (Fig 1A). Neither the 2DL1*013N nor the 2DL1*014 allotype is present in the KhoeSan. Overall, these results vividly illustrate how the natural polymorphism of KIR2DL1 modulates the avidity, specificity and functionality of this NK cell receptor in human populations.
In the KhoeSan, mutation of 2DL1*001, a strong C2 receptor, produced the C1 receptor, 2DL1*022. We therefore examined how the properties of 2DL1*022 compare to the prototypical C1 receptors encoded by the KIR2DL2/3 gene. (This gene has two distinctive allelic lineages, 2DL2 and 2DL3, hence the KIR2DL2/3 name). KIR-Fc proteins were made from six 2DL2 and nine 2DL3 allotypes (Fig 1C) and their binding to HLA class I coated beads was compared to 2DL1*022 (Fig 1D). As a group, the KIR2DL2/3 allotypes are not as specific for C1 as the KIR2DL1 allotypes are for C2. KIR2DL2/3 exhibit a range of avidity for C1, but increasing avidity for C1 is accompanied by increased cross-reactivity with C2 (Fig 1D). This is, however, not the case for 2DL1*022, which has a higher avidity for C1 than any of the KIR2DL2/3 allotypes, but no significant C2 cross-reactivity. KIR2DL1*022 has completely lost recognition of C2 while gaining a stronger, more specific, recognition of C1 than any KIR2DL2/3 allotype. Thus KIR2DL1*022 is seen to have unique functional properties, ones that will clearly have a profound functional impact on the KhoeSan and Zulu individuals who carry this allele.
The interactions of KIR2DL with HLA-C are not only diversified by KIR2DL1 and KIR2DL2/3 polymorphism, but also by polymorphism within the subsets of C1-bearing and C2-bearing HLA-C allotypes. Binding to C2 by the 11 KIR2DL1 allotypes varied over half an order of magnitude and with a similar hierarchy for each of the KIR allotypes (Fig 2A). Thus HLA-C*15 : 02 is always the strongest ligand for KIR2DL1 and HLA-C*04 : 01 the weakest. Analogous patterns were observed for the binding of 2DL1*022 and the 15 KIR2DL2/3 allotypes to C1-bearing HLA-B and -C allotypes (Fig 2B). Here, HLA-B*73 : 01 is the strongest ligand for 2DL1*022 and KIR2DL2/3 and HLA-C*16 : 01 the weakest. The basis for these hierarchies within the C1 - and C2-bearing allotypes arise from either the differing peptide repertoires presented by specific HLA-C or by polymorphism at sites other than position 80 that defines the C1 and C2 epitopes.
Fig. 2. KIR polymorphism modulates the avidity and specificity for HLA-C, as well as KIR abundance at the cell surface. 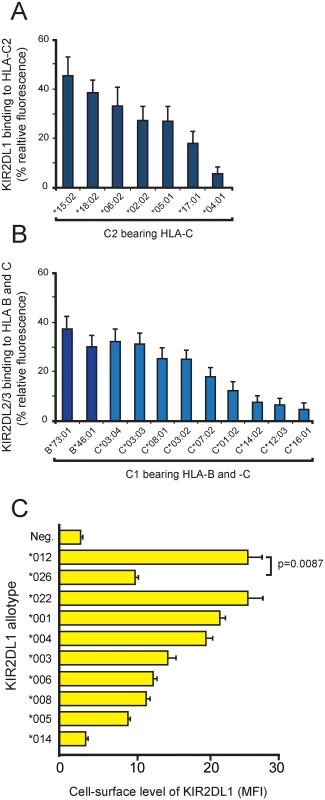
(A) Binding of KIR2DL1-Fc fusion proteins to C2-bearing HLA-C allotypes. For each C2-bearing HLA-C allotype, the KIR2DL1 binding is the mean of the values obtained with 11 different KIR2DL1-Fc (2DL1*001, *003, *004, *007, *008, *012, *020, *021, *023 *024, *025). Each individual binding value was normalized to the binding of the W6/32 antibody before calculating the average. (B) Binding of KIR2DL1*022-Fc and KIR2DL2/3-Fc fusion proteins to C1-bearing HLA-B and -C allotypes. For each C1-bearing HLA-B and HLA-C allotype, the KIR2DL2/3 binding is the mean of the values obtained with 16 KIR2DL-Fc fusion proteins (2DL1*022; 2DL2*001,*003, *004, *006, 009 *011; and 2DL3*001, *005, *008, *009, *011, *013, *015, *016, *018). The proteins were tested against microbeads coated with one of nine C1 HLA-C or two C1 HLA-B allotypes. Each individual binding value was normalized to the binding to that of the W6/32 antibody before calculating the average. (C) Variable cell-surface expression of KIR2DL1. FLAG-tagged KIR2DL1 allotypes were transfected into HeLa cells. Cell-surface expression was detected using FLAG-specific antibody and analysis by flow cytometry. MFI = median fluorescence intensity. The experiment was performed in triplicate, error bars give the standard deviation. The difference between 2DL1*012 and 2DL1*026 is statistically significant as assessed by a two-tailed t-test. KhoeSan specific 2DL1*026 lacks a cytoplasmic tail but is cell-surface expressed
KIR2DL1*026 and 2DL1*012 encode identical extracellular domains that bind C2 with high avidity and specificity (Fig 1B). To determine if 2DL1*026, which lacks a cytoplasmic tail, reaches the cell-surface, we examined the expression of FLAG-tagged 2DL1*026 and 2DL1*012 in transiently transfected HeLa cells. For comparison, eight other KIR2DL1 allotypes were included in the analysis (Fig 2C). KIR2DL1*026 is cell-surface expressed at a significantly lower level than 2DL1*012 (p = 0.0087), but within the range observed for other KIR2DL1 allotypes. Although KIR2DL1*026 cannot mediate NK cell inhibition directly, because it lacks a cytoplasmic domain, it could have indirect effects, either by preventing C2 from binding to other receptors or by contributing to the adhesive interactions of NK cells with target cells. That 2DL1*014 is not cell-surface expressed and cannot bind HLA class I suggests that its defining residue, serine 179, prevents proper protein folding. Other KIR allotypes with impaired folding that causes intracellular retention have been described [35–37].
Characterizing the KhoeSan population is a high frequency of weak C2 receptors
Unlike some other populations, there is no single 2DL1 allele that is present at high frequency in the KhoeSan (Fig 3 and S1A Fig). The ten KhoeSan 2DL1 alleles vary in frequency from 1.1–21.3%. In addition, 18% of KhoeSan KIR haplotypes lack the KIR2DL1 gene, constituting an eleventh allele: the 'blank'. The frequency of 2DL1*022, (17.2%) is more than double that of 2DL1*001 (7.0%), the parental allele from which it evolved. Likewise, 2DL1*026 (4.2%) has a higher frequency than 2DL1*012 (1.1%), the parental allele from which it evolved. The impact of both 2DL1*022 and 2DL1*026 has been to reduce the capacity of KIR2DL1 to function as an inhibitory C2 receptor in the KhoeSan.
Fig. 3. The KhoeSan have high KIR2DL1 diversity compared to other human populations. 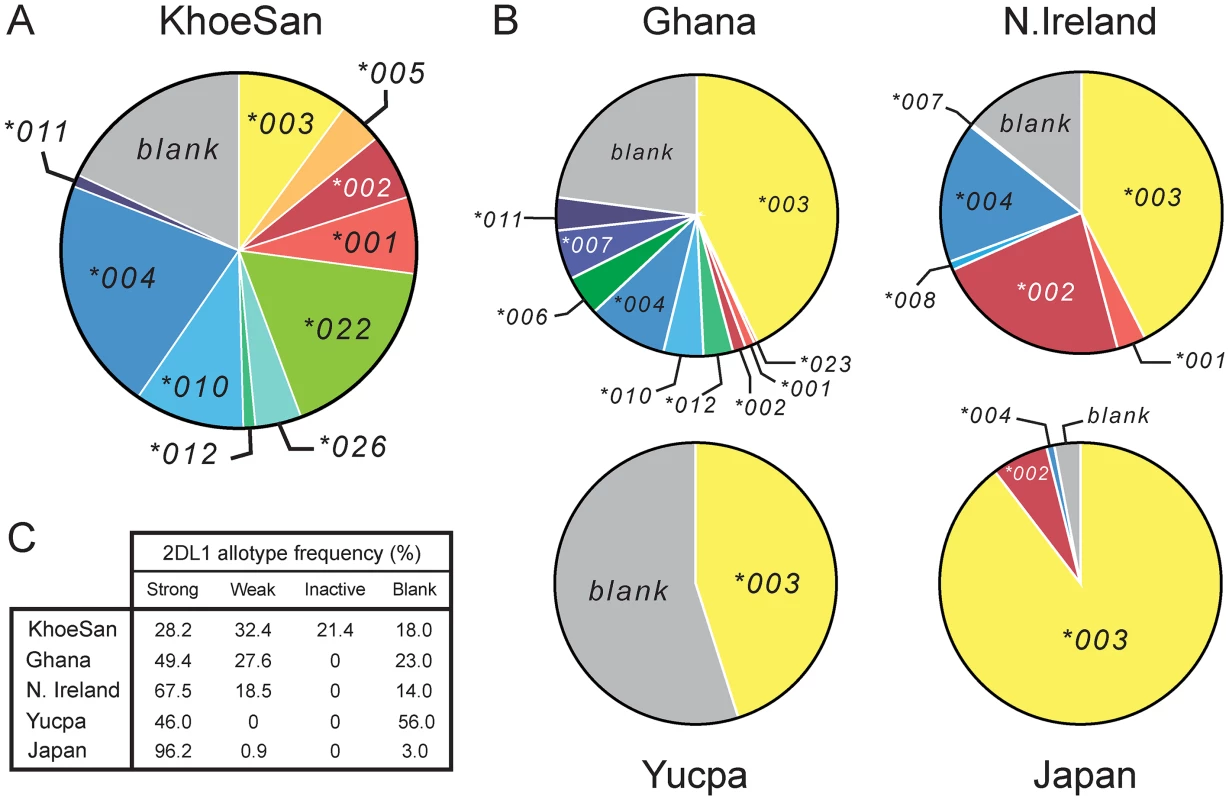
(A and B) The pie charts show the number and relative frequencies of KIR2DL1 alleles in the KhoeSan of Southern Africa (A), and four other populations representing four continents (B): the Ga-Adangbe from Ghana in Western Africa [14], Northern Ireland Caucasians from Europe [21], Japanese from East Asia [24] and Yucpa Amerindians from South America [20]. The 'blank' is the frequency of KIR haplotypes that lack the KIR2DL1 gene. (C) Also compared in the five populations are the frequencies of strong KIR2DL1, weak KIR2DL1, KIR2DL1 that are not inhibitory C2 receptors (inactive) and the absence of KIR2DL1 (blank). The definition and designation of these KIR2DL1 categories are given in S4 Fig. This effect of the KhoeSan-specific KIR2DL1 alleles is reinforced by the relatively low frequency in the KhoeSan of other alleles encoding strong inhibitory C2 receptors (2DL1*001, *002, *003 and *005) and relatively high frequency of alleles encoding weaker inhibitory C2 receptors. Included in the latter are the ‘blank’, the 2DL1*004, 2DL1*010 and 2DL1*011 receptors that have reduced avidity for C2 (Fig 1B and S4 Fig) and the 2DL1*004 and 2DL1*011 allotypes that have reduced signaling capacity caused by the cysteine residue at position 245 [38] (Fig 1A). In sum, the frequency of weak or inactive 2DL1 allotypes in the KhoeSan is 71.8%, whereas the 28.2% frequency of strong 2DL1 allotypes in the KhoeSan is much lower than that of other populations (Fig 3C).
KIR2DL1*022 and 2DL1*026 are present on conserved haplotypes that are recently evolved
To examine the genetic background of KIR2DL1*022 and KIR2DL1*026, we determined structures for the KIR2DL1-containing centromeric region of KhoeSan KIR haplotypes. Extensive diversity was observed, there being 70 different haplotypes among a total of 110 haplotypes characterized from 55 unrelated individuals. For each KIR2DL1 allele we determined how many different haplotypes have the allele and what their frequencies are in the KhoeSan. Because the linkage disequilibrium (LD) between nine of the eleven KhoeSan 2DL1 alleles and other genes of the centromeric region is low, there is a strong positive correlation (r2 = 0.96) between an allele's frequency and the number of different haplotypes on which it occurs (Fig 4A). For example, a total of seven haplotypes have 2DL1*001 and they are all different in their linked KIR alleles and genes (Fig 4A). That we find numerous different haplotypes reflects the high diversity of KhoeSan genomes [10,16]. Dramatic exceptions to this pattern are the haplotypes containing the KhoeSan specific KIR2DL1 alleles, which are in complete LD with the other centromeric KIR genes and alleles. Among the 23 haplotypes containing 2DL1*022 only two are unique, and they differ only in KIR3DL3 at the centromeric end of the KIR locus (Fig 4B). The six haplotypes containing 2DL1*026 are all identical (Fig 4B). The high LD across these haplotypes shows that they have not been broken and mixed by meiotic recombination, which is consistent with their recent evolution [39] (Fig 4C).
Fig. 4. KIR2DL1*022 and 2DL1*026 are of recent origin compared to other KhoeSan KIR2DL1 alleles. 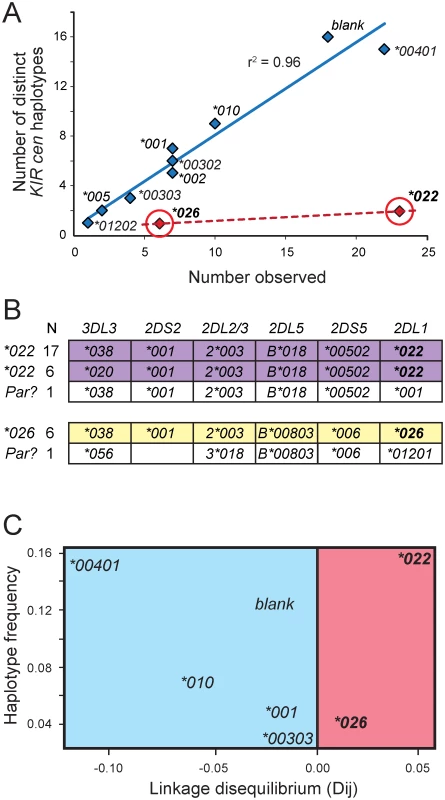
(A) For each KhoeSan KIR2DL1 allele, the number of centromeric KIR haplotypes on which the allele is present in the KhoeSan (number observed) is plotted against the number of different (distinct) haplotypes on which the allele is present. In total, 110 haplotypes were analyzed. Haplotypes that lack KIR2DL1 are denoted ‘blank’. The r2 was calculated from Pearson correlation of the alleles shown in blue. This analysis excluded 2DL1*022 and *026 (shown in red). (B) Shows the allele content of centromeric KIR haplotypes containing either 2DL1*022 (purple) or 2DL1*026 (yellow). The observed number of each haplotype is given on the left. Also shown (in white) are the KhoeSan haplotypes that are the putative parents (Par?) of the derived 2DL*022-containing and 2DL1*026-containing haplotypes. The putative parents are the haplotypes that differ from the derived haplotypes by the least number of nucleotide substitutions. (C) Plot of haplotype frequency against linkage disequilibrium (LD). The analysis was conditioned so that 2DL2*003-bearing haplotypes were analyzed. The figure illustrates the high level of linkage disequilibrium observed for haplotypes containing 2DL1*022 and 2DL1*026 suggesting they appeared more recently in the KhoeSan population than other KIR2DL1 alleles. Discussion
Our study shows how KIR2DL1 polymorphism has given rise to NK cell receptors that vary substantially in their capacity to recognize HLA-C and propagate intracellular signals. Emphasizing the value of defining structural and functional KIR variation at high resolution is our discovery in the KhoeSan of two unusual allotypes of KIR2DL1, the inhibitory NK cell receptor for the C2 epitope of HLA-C. The alleles encoding these allotypes were derived by point mutation from older, more widespread KIR2DL1 alleles that encode strong, inhibitory C2 receptors. In stark contrast to the parental allotypes, neither progeny is a strong, inhibitory C2 receptor. KIR2DL1*026 has no capacity for signal transduction and 2DL1*022 recognizes C1 with specificity and avidity that exceeds that of any KIR2DL2/3 allotype, the archetypal C1 receptor. The methionine to lysine substitution at position 44 that defines KIR2DL1*022 occurs within the HLA-C binding site of the KIR [5]. Here, residue 44 in the D1 domain of the KIR interacts with residue 80 of the α1 domain of HLA-C. For KIR2DL1*001, the parent allele of KIR2DL1*022, methionine 44 binds to lysine 80 of the C2 epitope of HLA-C [5,7]. In contrast, lysine 44 in KIR2DL1*022 binds to asparagine 80 of the C1 epitope of HLA-C.
KIR2DL1*022 is the most vivid example of how genetic polymorphism can change KIR specificity for HLA class I. For other allotypes of KIR2DL1 and KIR2DL2/3, the effects of their defining substitutions can act to alter different functional properties: receptor avidity [31,32,40], stability, cell-surface abundance and signal transduction [38]. Throughout the KIR molecule are sites where natural substitutions affect receptor functions. Many of these are away from the HLA-C binding site and likely involve conformational changes, including ones that affect the relative orientation of the extracellular D1 and D2 domains that combine to form the binding site [31,40]. That KIR2DL1*022 and 2DL1*026 have lost their parents’ capacity to function as inhibitory C2 receptors, exemplifies a more widespread trend in the KhoeSan. That is an accumulation of KIR2DL1 allotypes with low avidity for HLA-C2 or weakened signaling function, as well as KIR B haplotypes lacking the KIR2DL1 gene (Fig 3C).
In human populations worldwide there is an inverse correlation between the frequency of HLA-C allotypes carrying the C2 epitope and the frequency of the KIR A haplotypes encoding strong KIR2DL1 allotypes. This correlation reflects the increased risk of spontaneous abortion, preeclampsia, and low birth-weight that is associated with pregnancies in which a KIR A homozygous mother who lacks the C2 epitope is carrying a fetus that expresses a C2 epitope of paternal origin [41,42]. In these pregnancies, the interaction of paternal C2 on extravillous trophoblast cells with maternal uterine NK cells expressing the strong KIR2DL1 encoded by KIR A haplotypes can lead to incomplete placentation. In general, Africans have a higher frequency of the C2 epitope than other populations and the C2 frequency of the KhoeSan is particularly high (63.4%; Fig 5). The reasons for the high C2 frequency are unknown, but may include protection against specific diseases though interaction of C2-expressing HLA-C with NK cells or CD8 T cells. Thus the emergence of 2DL1*022 and 2DL1*026, as well as the general increase of weaker inhibitory KIR2DL1 allotypes, in the KhoeSan could have acted to reduce the incidence of preeclampsia. In this manner, the KhoeSan retained the ability to both fight infection and reproduce efficiently.
Fig. 5. The C2 frequency in the KhoeSan is unusually high. 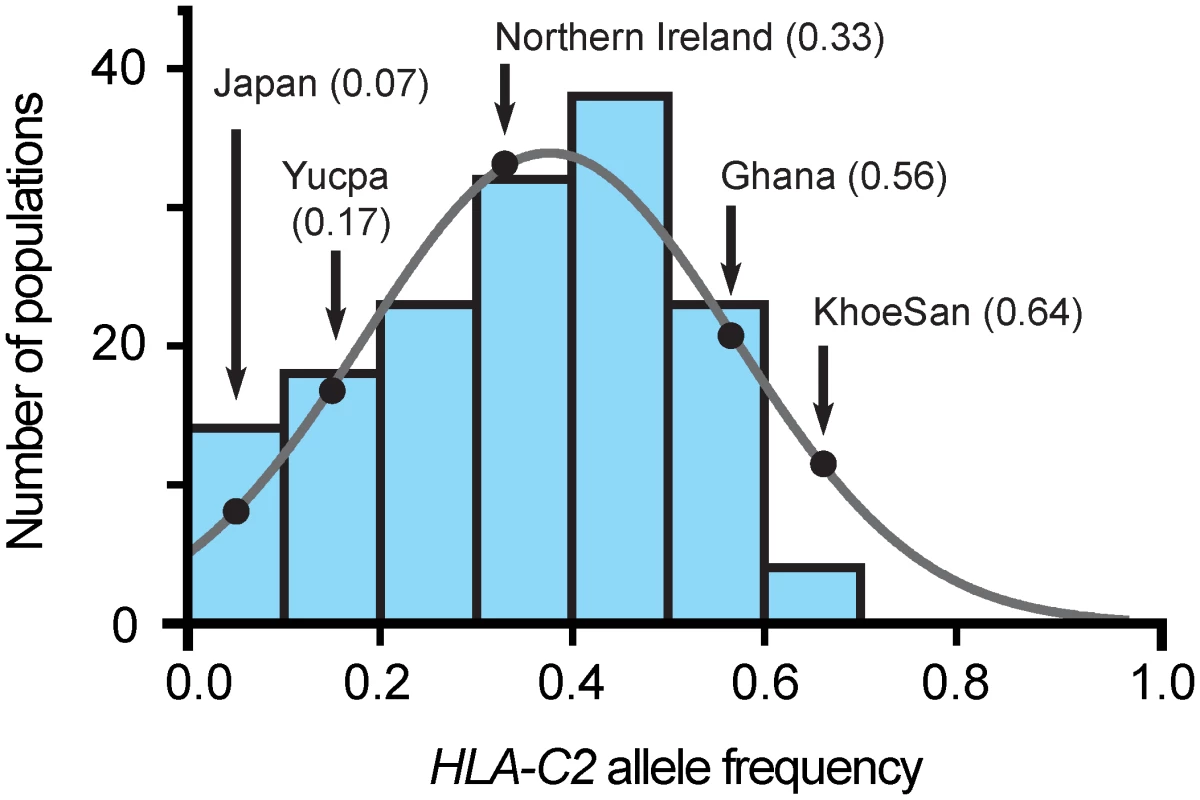
Each of the seven blue-shaded vertical bars gives the number of populations, of 140 considered [34], that have a C2 frequency within the range covered by the bar, given on the horizontal axis. The frequency data are not significantly different from a normal distribution (grey line). The black-shaded dots on the curve give the frequencies for the KhoeSan and the four other populations for which KIR2DL1 allele frequencies are given in Fig 3. In assessing the effect of a high C2 frequency on the KhoeSan, it is informative to consider the Yucpa, an indigenous South American population that has a low frequency of C2 and a high frequency of C1 (82.7%) [20]. Accompanying the abundance of C1 are two Yucpa-specific KIR2DL3 alleles, both arising by point mutation of the older, widespread 2DL3*001. KIR2DL3*009 has lower C1 avidity than 2DL3*001 and 2DL3*008N is non-functional. These Yucpa specific 2DL3 have a frequency of 41.8% compared to 8.2% for their 2DL3*001 parent. In the Yucpa, the high C1 frequency combines with a much-reduced frequency of strong inhibitory C1 receptors, whereas in KhoeSan, the high C2 frequency combines with a much-reduced frequency of strong inhibitory C2 receptors. These analogous behaviors at the two extremes of the frequency spectrum appear to reflect a buffering mechanism that maintains a balance between C1, C2 and their inhibitory receptors in human populations.
One possibility is that 2DL1*022 and 2DL1*026 increased in frequency as a consequence of genetic drift. Thus, they would represent two of the many private alleles that are present in the KhoeSan because of their unique demographic history [12]. Unlike other African hunter-gatherer groups, the KhoeSan have maintained a large effective population size and high levels of genetic diversity [10,43]. These characteristics argue against the KhoeSan having been subject to a classic bottleneck of the type experienced by other African hunter-gatherer populations, such as the Tanzanian Hadza [10], or migrant modern humans who left Africa and populated other continents [44]. An alternative interpretation is that 2DL1*022 and 2DL1*026 rose in frequency in the KhoeSan under positive selection. Supporting this model are the distinctive functional properties of the 2DL1*022 and 2DL1*026 proteins, the evidence for balancing selection at the KIR locus [20,24,45,46] and the evidence for diversifying selection at position 44, where lysine determines the unique functionality of KIR2DL1*022 [9]. To establish if drift or selection is responsible for emergence of the new, variant KIR, will require extensive demographic simulations and the development of appropriate programs that simulate co-evolution between unlinked, highly polymorphic loci.
Materials and Methods
Ethics statement
Sampling of the ≠Khomani San in Upington, South Africa and neighboring villages occurred in 2006. Institution Review Board (IRB) approval was obtained from Stanford University [Protocol 13829] for assessment of genetic diversity and ancestry inference. Individuals who were still living in 2011 were re-consented (IRB approved from Stanford University and Stellenbosch University, South Africa). ≠Khomani N|u-speaking individuals, local community leaders, traditional leaders, non-profit organizations and a legal counselor were all consulted regarding the aims of this research, prior to collection of DNA. All individuals consented orally to participation, with a second, local native speaker witnessing and were re-consented with written consent. DNA was collected via saliva and all individuals were as described in previous studies [10,26].
Study populations
Genomic DNA samples were isolated from saliva samples donated by 61 KhoeSan individuals of the ≠Khomani San population as described by Henn et al. [10]. KIR2DL1, KIR2DL2/3 and HLA-C allele frequencies were determined for 55 unrelated individuals. The additional six individuals comprised five additional family members of two of the 55 unrelated individuals, and a sibling of another. The sequences and frequencies of KhoeSan KIR and HLA-C alleles were compared to those of Ghanaians [14], Northern Irish [21] Japanese [24] and South Amerindians [20], and also to three non-KhoeSan hunter-gatherer populations. These comprised 20 Mbuti and 20 Baka Pygmies from The Democratic Republic of Congo and Cameroon, and 52 Hadza from northern Tanzania [10]. We also analyzed the KIR sequence data of 100 Zulus from South Africa [27]. Allele frequencies for the C1 and C2 epitopes of HLA-C were determined using data deposited at www.allelefrequencies.net [34]. The 140 populations analyzed were chosen for being anthropologically well characterized, for having minimal admixture with other populations, and for having a size of 40 individuals or more. This panel of populations is described in Abi Rached et al. [33].
High-resolution KIR2DL1 and KIR2DL2/3 genotyping
Nucleotide sequences were determined for all exons of KIR2DL1 and KIR2DL2/3 genes from sixteen randomly selected unrelated KhoeSan individuals as well as the seven-member family. Sequences for two previously unknown alleles, KIR2DL1*022 (GU323355) and KIR2DL1*026 (JX523630) were confirmed by re-amplification, cloning and sequencing, as described [26]. A pyrosequencing-based method for allele-level KIR2DL1 and KIR2DL2/3 genotyping [14], was expanded to include detection of the new KhoeSan variants (S5 Fig). This method provides a semi-quantitative measure of SNP genotypes (the peak-height ratio) that determines both allele identity and copy-number genotype [14]. Centromeric KIR haplotypes were characterized as described [14], with modification to accommodate the newly-discovered 3DL3*038 and 2DL5B*018 alleles [26]. Pyrosequencing and standard Sanger sequencing were used to determine the 2DL1 alleles present in the Pygmy and Hadza populations.
High-resolution HLA genotyping
The 61 KhoeSan individuals were HLA-C genotyped at allele-level resolution using bead-based SSOP hybridization (One Lambda) and detection by a Luminex-100 instrument (Luminex corp. Austin, TX).
KIR nomenclature
KIR genes and alleles were named by the KIR nomenclature committee [47] formed from members of the WHO Nomenclature Committee for factors of the HLA system, and the HUGO Genome Nomenclature Committee. A curated database is available at http://www.ebi.ac.uk/ipd/kir/ [47].
Searching for KIR2DL1*022 and KIR2DL1*026 alleles in the 1000 Genomes and African Genome Variation Project datasets
The high-coverage exome data from the May 2013 release of the 1000 Genomes project [25] were used to determine the frequency of KIR2DL1*022 and KIR2DL1*026 in populations worldwide. All read-pairs that map to the KIR regions (Build Hg19: chr19 : 55,228,188–55,383,188 and GL000209.1) were extracted using SAMtools 0.1.18 [48]. For 39 individuals the data have insufficient coverage and were excluded from the analysis, which was performed on data from 2,496 individuals representing 26 populations (S2B Fig). Individual fastq files were probed using locus-specific and allele-specific sequence-string searches. Where required, individual fastq files were filtered for locus-specificity using Bowtie (version 0.12.7) [49], aligned to references and the SNP genotypes inspected manually. As controls we included data from eight KhoeSan individuals who had previously been sequenced using Illumina whole-exome paired end technology [26] and, independently, KIR genotyped to allele-level resolution by pyrosequencing [14]. Three of the eight individuals have KIR2DL1*022, and one other has KIR2DL1*026.
We used the same method to determine the frequencies of 2DL1*022 and 2DL1*026 in 100 Zulus whose genomes were sequenced as part of the African Genome Variation project [27]. Zulus are a Bantu-speaking population from southern Africa, who show evidence for recent admixture with the KhoeSan [11,18]. For each Zulu individual having either 2DL1*022 or 2DL1*026 we used manual inspection of sequence reads mapped to each KIR gene to infer the likely centromeric KIR haplotype structure.
Binding assay of KIR-Fc fusion proteins to beads coated with HLA class I
KIR-Fc fusion proteins were generated from insect cells (cabbage looper moth Hi5 cells, kindly provided by Prof. K.C. Garcia, Stanford University) infected with baculovirus as described [50]. The KIR-Fc fusion protein corresponding to each 2DL1, 2DL2 and 2DL3 allotype was tested for binding to a panel of microbeads, each of which is coated with one of 31 HLA-A, 50 HLA-B and 16 HLA-C allotypes (LabScreen Single-Antigen Beads, One Lambda, lot #8). To account for differences in the amount of HLA class I protein coating each bead, the binding of each KIR-Fc fusion protein was normalized to the binding of W6/32, a monoclonal antibody detecting a common epitope of HLA class I. Binding values were calculated using the formula (specific binding—bead background fluorescence)/(W6/32 binding—bead background fluorescence).
Cell-surface expression of KIR2DL1 in transiently transfected HeLa
Recombinant cDNA encoding the extracellular, stem, transmembrane and cytoplasmic domain (amino acids 1–336) of KIR2DL1*003 with an N-terminal 3X FLAG-tag was manufactured by Genscript (Piscataway, NJ) and cloned into the pcDNA3.1+ expression vector. Site-directed mutagenesis was performed with the QuikChange Kit (Stratagene), according to the manufacturer’s instructions, to generate nine further KIR2DL1 variants. HeLa cells (ATCC Cell Lines, VA) were plated in 15.6mm wells at 5 x 104 cells/well in 500μl DMEMc for 24hrs and then transfected with a pcDNA3.1+ vector encoding FLAG-tagged KIR2DL1 allotypes using the Fugene transfection reagent (Promega). After 36h, adherent cells were dissociated from the wells using 200μl 0.05% trypsin EDTA solution and stained with 25μl mouse polyclonal FITC-conjugated FLAG-specific antibody (Sigma-Aldrich) at a final concentration of 3μg/ml. Cells expressing FLAG-tagged KIR2DL1 allotypes were detected by flow cytometry (Accuri C6 cytometer, BD Biosciences). Dead cells were identified by staining with propidium iodide and excluded from the analysis. The median fluorescence intensity (MFI) of FITC-conjugated anti-FLAG antibody bound to each positively staining cell was used as a measure of the cell-surface expression of KIR2DL1. At least 50,000 such cells were analyzed in each experiment. Three independent transfections were performed for each allotype.
Population and molecular genetic analysis
The KIR locus has extensive structural and allelic polymorphism [8,51], as well as recombination hotspots that flank the centromeric KIR region [52,53]. These characteristics preclude the use of methods that use SNP analysis and the identification of regions of extended haplotype homozygosity as evidence for selection [54–56]. We examined the patterns of LD associated with specific alleles, using a method designed for analysis of a polymorphic multigene family [39,57]. This approach was applied to the analysis of the haplotypes in centromeric region of the KhoeSan KIR locus, the regions containing the KIR2DL1 gene.
Supporting Information
Zdroje
1. Parham P, Moffett A (2013) Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol 13 : 133–144. doi: 10.1038/nri3370 23334245
2. Cooper MA, Colonna M, Yokoyama WM (2009) Hidden talents of natural killers: NK cells in innate and adaptive immunity. EMBO Rep 10 : 1103–1110. doi: 10.1038/embor.2009.203 19730434
3. Bashirova AA, Martin MP, McVicar DW, Carrington M (2006) The killer immunoglobulin-like receptor gene cluster: tuning the genome for defense. Annu Rev Genomics Hum Genet 7 : 277–300. 16824023
4. Parham P, Norman PJ, Abi-Rached L, Guethlein LA (2012) Human-specific evolution of killer cell immunoglobulin-like receptor recognition of major histocompatibility complex class I molecules. Philos Trans R Soc Lond B Biol Sci 367 : 800–811. doi: 10.1098/rstb.2011.0266 22312047
5. Boyington JC, Motyka SA, Schuck P, Brooks AG, Sun PD (2000) Crystal structure of an NK cell immunoglobulin-like receptor in complex with its class I MHC ligand. Nature 405 : 537–543. 10850706
6. Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, et al. (1987) The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature 329 : 512–518. 2443855
7. Winter CC, Long EO (1997) A single amino acid in the p58 killer cell inhibitory receptor controls the ability of natural killer cells to discriminate between the two groups of HLA-C allotypes. J Immunol 158 : 4026–4028. 9126959
8. Uhrberg M, Valiante NM, Shum BP, Shilling HG, Lienert-Weidenbach K, et al. (1997) Human diversity in killer cell inhibitory receptor genes. Immunity 7 : 753–763. 9430221
9. Abi-Rached L, Moesta AK, Rajalingam R, Guethlein LA, Parham P (2010) Human-specific evolution and adaptation led to major qualitative differences in the variable receptors of human and chimpanzee natural killer cells. PLoS Genet 6: e1001192. doi: 10.1371/journal.pgen.1001192 21079681
10. Henn BM, Gignoux CR, Jobin M, Granka JM, Macpherson JM, et al. (2011) Hunter-gatherer genomic diversity suggests a southern African origin for modern humans. Proc Natl Acad Sci U S A 108 : 5154–5162. doi: 10.1073/pnas.1017511108 21383195
11. Petersen DC, Libiger O, Tindall EA, Hardie RA, Hannick LI, et al. (2013) Complex patterns of genomic admixture within southern Africa. PLoS Genet 9: e1003309. doi: 10.1371/journal.pgen.1003309 23516368
12. Tishkoff SA, Reed FA, Friedlaender FR, Ehret C, Ranciaro A, et al. (2009) The genetic structure and history of Africans and African Americans. Science 324 : 1035–1044. doi: 10.1126/science.1172257 19407144
13. Nakimuli A, Chazara O, Byamugisha J, Elliott AM, Kaleebu P, et al. (2014) Pregnancy, parturition and preeclampsia in women of African ancestry. Am J Obstet Gynecol 210 : 510–520 e511. doi: 10.1016/j.ajog.2013.10.879 24184340
14. Norman PJ, Hollenbach JA, Nemat-Gorgani N, Guethlein LA, Hilton HG, et al. (2013) Co-evolution of human leukocyte antigen (HLA) class I ligands with killer-cell immunoglobulin-like receptors (KIR) in a genetically diverse population of sub-Saharan Africans. PLoS Genet 9: e1003938. doi: 10.1371/journal.pgen.1003938 24204327
15. Bryc K, Auton A, Nelson MR, Oksenberg JR, Hauser SL, et al. (2010) Genome-wide patterns of population structure and admixture in West Africans and African Americans. Proc Natl Acad Sci U S A 107 : 786–791. doi: 10.1073/pnas.0909559107 20080753
16. Schuster SC, Miller W, Ratan A, Tomsho LP, Giardine B, et al. (2010) Complete Khoisan and Bantu genomes from southern Africa. Nature 463 : 943–947. doi: 10.1038/nature08795 20164927
17. Lachance J, Vernot B, Elbers CC, Ferwerda B, Froment A, et al. (2012) Evolutionary history and adaptation from high-coverage whole-genome sequences of diverse African hunter-gatherers. Cell 150 : 457–469. doi: 10.1016/j.cell.2012.07.009 22840920
18. Chimusa ER, Meintjies A, Tchanga M, Mulder N, Seioghe C, et al. (2015) A genomic portrait of haplotype diversity and signatures of selection in indigenous southern African populations. PLoS Genet 11: e1005052. doi: 10.1371/journal.pgen.1005052 25811879
19. Howell N (2007) Demography of the Dobe! Kung. New Brunswick, N.J.: AldineTransaction.
20. Gendzekhadze K, Norman PJ, Abi-Rached L, Graef T, Moesta AK, et al. (2009) Co-evolution of KIR2DL3 with HLA-C in a human population retaining minimal essential diversity of KIR and HLA class I ligands. Proc Natl Acad Sci U S A 106 : 18692–18697. doi: 10.1073/pnas.0906051106 19837691
21. Middleton D, Meenagh A, Gourraud PA (2007) KIR haplotype content at the allele level in 77 Northern Irish families. Immunogenetics 59 : 145–158. 17200871
22. Nemat-Gorgani N, Edinur HA, Hollenbach JA, Traherne JA, Dunn PP, et al. (2014) KIR diversity in Maori and Polynesians: populations in which HLA-B is not a significant KIR ligand. Immunogenetics 66 : 597–611. doi: 10.1007/s00251-014-0794-1 25139336
23. Vierra-Green C, Roe D, Hou L, Hurley CK, Rajalingam R, et al. (2012) Allele-level haplotype frequencies and pairwise linkage disequilibrium for 14 KIR loci in 506 European-American individuals. PLoS One 7: e47491. doi: 10.1371/journal.pone.0047491 23139747
24. Yawata M, Yawata N, Draghi M, Little AM, Partheniou F, et al. (2006) Roles for HLA and KIR polymorphisms in natural killer cell repertoire selection and modulation of effector function. J Exp Med 203 : 633–645. 16533882
25. Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, et al. (2012) An integrated map of genetic variation from 1,092 human genomes. Nature 491 : 56–65. doi: 10.1038/nature11632 23128226
26. Kidd JM, Sharpton TJ, Bobo D, Norman PJ, Martin AR, et al. (2014) Exome capture from saliva produces high quality genomic and metagenomic data. BMC Genomics 15 : 262. doi: 10.1186/1471-2164-15-262 24708091
27. Gurdasani D, Carstensen T, Tekola-Ayele F, Pagani L, Tachmazidou I, et al. (2015) The African Genome Variation Project shapes medical genetics in Africa. Nature 517 : 327–332. doi: 10.1038/nature13997 25470054
28. Gronau I, Hubisz MJ, Gulko B, Danko CG, Siepel A (2011) Bayesian inference of ancient human demography from individual genome sequences. Nat Genet 43 : 1031–1034. doi: 10.1038/ng.937 21926973
29. Schlebusch CM, Skoglund P, Sjodin P, Gattepaille LM, Hernandez D, et al. (2012) Genomic variation in seven Khoe-San groups reveals adaptation and complex African history. Science 338 : 374–379. doi: 10.1126/science.1227721 22997136
30. Burshtyn DN, Scharenberg AM, Wagtmann N, Rajagopalan S, Berrada K, et al. (1996) Recruitment of tyrosine phosphatase HCP by the killer cell inhibitor receptor. Immunity 4 : 77–85. 8574854
31. Moesta AK, Norman PJ, Yawata M, Yawata N, Gleimer M, et al. (2008) Synergistic polymorphism at two positions distal to the ligand-binding site makes KIR2DL2 a stronger receptor for HLA-C than KIR2DL3. J Immunol 180 : 3969–3979. 18322206
32. Hilton HG, Vago L, Older Aguilar AM, Moesta AK, Graef T, et al. (2012) Mutation at positively selected positions in the binding site for HLA-C shows that KIR2DL1 is a more refined but less adaptable NK cell receptor than KIR2DL3. J Immunol 189 : 1418–1430. doi: 10.4049/jimmunol.1100431 22772445
33. Abi-Rached L, Jobin MJ, Kulkarni S, McWhinnie A, Dalva K, et al. (2011) The shaping of modern human immune systems by multiregional admixture with archaic humans. Science 334 : 89–94. doi: 10.1126/science.1209202 21868630
34. Gonzalez-Galarza FF, Christmas S, Middleton D, Jones AR (2011) Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res 39: D913–919. doi: 10.1093/nar/gkq1128 21062830
35. VandenBussche CJ, Mulrooney TJ, Frazier WR, Dakshanamurthy S, Hurley CK (2009) Dramatically reduced surface expression of NK cell receptor KIR2DS3 is attributed to multiple residues throughout the molecule. Genes Immun 10 : 162–173. doi: 10.1038/gene.2008.91 19005473
36. Pando MJ, Gardiner CM, Gleimer M, McQueen KL, Parham P (2003) The protein made from a common allele of KIR3DL1 (3DL1*004) is poorly expressed at cell surfaces due to substitution at positions 86 in Ig domain 0 and 182 in Ig domain 1. J Immunol 171 : 6640–6649. 14662867
37. Thomas R, Yamada E, Alter G, Martin MP, Bashirova AA, et al. (2008) Novel KIR3DL1 alleles and their expression levels on NK cells: convergent evolution of KIR3DL1 phenotype variation? J Immunol 180 : 6743–6750. 18453594
38. Bari R, Bell T, Leung WH, Vong QP, Chan WK, et al. (2009) Significant functional heterogeneity among KIR2DL1 alleles and a pivotal role of arginine 245. Blood 114 : 5182–5190. doi: 10.1182/blood-2009-07-231977 19828694
39. Klitz W, Thomson G (1987) Disequilibrium pattern analysis. II. Application to Danish HLA A and B locus data. Genetics 116 : 633–643. 3476350
40. Frazier WR, Steiner N, Hou L, Dakshanamurthy S, Hurley CK (2013) Allelic Variation in KIR2DL3 Generates a KIR2DL2-like Receptor with Increased Binding to its HLA-C Ligand. J Immunol.
41. Hiby SE, Walker JJ, O'Shaughnessy K M, Redman CW, Carrington M, et al. (2004) Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med 200 : 957–965. 15477349
42. Nakimuli A, Chazara O, Hiby SE, Farrell L, Tukwasibwe S, et al. (2015) A KIR B centromeric region present in Africans but not Europeans protects pregnant women from pre-eclampsia. Proc Natl Acad Sci U S A 112 : 845–850. doi: 10.1073/pnas.1413453112 25561558
43. Kim HL, Ratan A, Perry GH, Montenegro A, Miller W, et al. (2014) Khoisan hunter-gatherers have been the largest population throughout most of modern-human demographic history. Nat Commun 5 : 5692. doi: 10.1038/ncomms6692 25471224
44. Oppenheimer S (2012) Out-of-Africa, the peopling of continents and islands: tracing uniparental gene trees across the map. Philos Trans R Soc Lond B Biol Sci 367 : 770–784. doi: 10.1098/rstb.2011.0306 22312044
45. Hollenbach JA, Nocedal I, Ladner MB, Single RM, Trachtenberg EA (2012) Killer cell immunoglobulin-like receptor (KIR) gene content variation in the HGDP-CEPH populations. Immunogenetics 64 : 719–737. doi: 10.1007/s00251-012-0629-x 22752190
46. Norman PJ, Abi-Rached L, Gendzekhadze K, Korbel D, Gleimer M, et al. (2007) Unusual selection on the KIR3DL1/S1 natural killer cell receptor in Africans. Nat Genet 39 : 1092–1099. 17694054
47. Robinson J, Halliwell JA, McWilliam H, Lopez R, Marsh SG (2013) IPD—the Immuno Polymorphism Database. Nucleic Acids Res 41: D1234–1240. doi: 10.1093/nar/gks1140 23180793
48. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079. doi: 10.1093/bioinformatics/btp352 19505943
49. Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. doi: 10.1186/gb-2009-10-3-r25 19261174
50. Hilton HG, Moesta AK, Guethlein LA, Blokhuis J, Parham P, et al. (2015) The production of KIR-Fc fusion proteins and their use in a multiplex HLA class I binding assay. Journal of Immunological Methods doi: 10.1016/j.jim.2015.06.012
51. Shilling HG, Guethlein LA, Cheng NW, Gardiner CM, Rodriguez R, et al. (2002) Allelic polymorphism synergizes with variable gene content to individualize human KIR genotype. J Immunol 168 : 2307–2315. 11859120
52. Wilson MJ, Torkar M, Haude A, Milne S, Jones T, et al. (2000) Plasticity in the organization and sequences of human KIR/ILT gene families. Proc Natl Acad Sci U S A 97 : 4778–4783. 10781084
53. Norman PJ, Cook MA, Carey BS, Carrington CV, Verity DH, et al. (2004) SNP haplotypes and allele frequencies show evidence for disruptive and balancing selection in the human leukocyte receptor complex. Immunogenetics 56 : 225–237. 15185041
54. Hancock AM, Rienzo AD (2008) Detecting the Genetic Signature of Natural Selection in Human Populations: Models, Methods, and Data. Annu Rev Anthropol 37 : 197–217. 20622977
55. Nielsen R (2005) Molecular signatures of natural selection. Annu Rev Genet 39 : 197–218. 16285858
56. Oleksyk TK, Smith MW, O'Brien SJ (2010) Genome-wide scans for footprints of natural selection. Philos Trans R Soc Lond B Biol Sci 365 : 185–205. doi: 10.1098/rstb.2009.0219 20008396
57. Thomson G, Klitz W (1987) Disequilibrium pattern analysis. I. Theory. Genetics 116 : 623–632. 3623083
Štítky
Genetika Reprodukčná medicína
Článek Let-7 Represses Carcinogenesis and a Stem Cell Phenotype in the Intestine via Regulation of Hmga2Článek Binding of Multiple Rap1 Proteins Stimulates Chromosome Breakage Induction during DNA ReplicationČlánek SLIRP Regulates the Rate of Mitochondrial Protein Synthesis and Protects LRPPRC from DegradationČlánek Protein Composition of Infectious Spores Reveals Novel Sexual Development and Germination Factors inČlánek The Formin Diaphanous Regulates Myoblast Fusion through Actin Polymerization and Arp2/3 RegulationČlánek Runx1 Transcription Factor Is Required for Myoblasts Proliferation during Muscle Regeneration
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 8- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Putting the Brakes on Huntington Disease in a Mouse Experimental Model
- Identification of Driving Fusion Genes and Genomic Landscape of Medullary Thyroid Cancer
- Evidence for Retromutagenesis as a Mechanism for Adaptive Mutation in
- TSPO, a Mitochondrial Outer Membrane Protein, Controls Ethanol-Related Behaviors in
- Evidence for Lysosome Depletion and Impaired Autophagic Clearance in Hereditary Spastic Paraplegia Type SPG11
- Loss and Gain of Natural Killer Cell Receptor Function in an African Hunter-Gatherer Population
- Trans-Reactivation: A New Epigenetic Phenomenon Underlying Transcriptional Reactivation of Silenced Genes
- Early Developmental and Evolutionary Origins of Gene Body DNA Methylation Patterns in Mammalian Placentas
- Strong Selective Sweeps on the X Chromosome in the Human-Chimpanzee Ancestor Explain Its Low Divergence
- Dominance of Deleterious Alleles Controls the Response to a Population Bottleneck
- Transient 1a Induction Defines the Wound Epidermis during Zebrafish Fin Regeneration
- Systems Genetics Reveals the Functional Context of PCOS Loci and Identifies Genetic and Molecular Mechanisms of Disease Heterogeneity
- A Genome Scale Screen for Mutants with Delayed Exit from Mitosis: Ire1-Independent Induction of Autophagy Integrates ER Homeostasis into Mitotic Lifespan
- Non-synonymous FGD3 Variant as Positional Candidate for Disproportional Tall Stature Accounting for a Carcass Weight QTL () and Skeletal Dysplasia in Japanese Black Cattle
- The Relationship between Gene Network Structure and Expression Variation among Individuals and Species
- Calmodulin Methyltransferase Is Required for Growth, Muscle Strength, Somatosensory Development and Brain Function
- The Wnt Frizzled Receptor MOM-5 Regulates the UNC-5 Netrin Receptor through Small GTPase-Dependent Signaling to Determine the Polarity of Migrating Cells
- Nbs1 ChIP-Seq Identifies Off-Target DNA Double-Strand Breaks Induced by AID in Activated Splenic B Cells
- CCNYL1, but Not CCNY, Cooperates with CDK16 to Regulate Spermatogenesis in Mouse
- Evidence for a Common Origin of Blacksmiths and Cultivators in the Ethiopian Ari within the Last 4500 Years: Lessons for Clustering-Based Inference
- Of Fighting Flies, Mice, and Men: Are Some of the Molecular and Neuronal Mechanisms of Aggression Universal in the Animal Kingdom?
- Hypoxia and Temperature Regulated Morphogenesis in
- The Homeodomain Iroquois Proteins Control Cell Cycle Progression and Regulate the Size of Developmental Fields
- Evolution and Design Governing Signal Precision and Amplification in a Bacterial Chemosensory Pathway
- Rac1 Regulates Endometrial Secretory Function to Control Placental Development
- Let-7 Represses Carcinogenesis and a Stem Cell Phenotype in the Intestine via Regulation of Hmga2
- Functions as a Positive Regulator of Growth and Metabolism in
- The Nucleosome Acidic Patch Regulates the H2B K123 Monoubiquitylation Cascade and Transcription Elongation in
- Rhoptry Proteins ROP5 and ROP18 Are Major Murine Virulence Factors in Genetically Divergent South American Strains of
- Exon 7 Contributes to the Stable Localization of Xist RNA on the Inactive X-Chromosome
- Regulates Refractive Error and Myopia Development in Mice and Humans
- mTORC1 Prevents Preosteoblast Differentiation through the Notch Signaling Pathway
- Regulation of Gene Expression Patterns in Mosquito Reproduction
- Molecular Basis of Gene-Gene Interaction: Cyclic Cross-Regulation of Gene Expression and Post-GWAS Gene-Gene Interaction Involved in Atrial Fibrillation
- The Spalt Transcription Factors Generate the Transcriptional Landscape of the Wing Pouch Central Region
- Binding of Multiple Rap1 Proteins Stimulates Chromosome Breakage Induction during DNA Replication
- Functional Divergence in the Role of N-Linked Glycosylation in Smoothened Signaling
- YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers
- Coordinated Evolution of Influenza A Surface Proteins
- The Evolutionary Potential of Phenotypic Mutations
- Genome-Wide Association and Trans-ethnic Meta-Analysis for Advanced Diabetic Kidney Disease: Family Investigation of Nephropathy and Diabetes (FIND)
- New Routes to Phylogeography: A Bayesian Structured Coalescent Approximation
- SLIRP Regulates the Rate of Mitochondrial Protein Synthesis and Protects LRPPRC from Degradation
- Satellite DNA Modulates Gene Expression in the Beetle after Heat Stress
- SHOEBOX Modulates Root Meristem Size in Rice through Dose-Dependent Effects of Gibberellins on Cell Elongation and Proliferation
- Reduced Crossover Interference and Increased ZMM-Independent Recombination in the Absence of Tel1/ATM
- Suppression of Somatic Expansion Delays the Onset of Pathophysiology in a Mouse Model of Huntington’s Disease
- Protein Composition of Infectious Spores Reveals Novel Sexual Development and Germination Factors in
- The Evolutionarily Conserved LIM Homeodomain Protein LIM-4/LHX6 Specifies the Terminal Identity of a Cholinergic and Peptidergic . Sensory/Inter/Motor Neuron-Type
- SmD1 Modulates the miRNA Pathway Independently of Its Pre-mRNA Splicing Function
- piRNAs Are Associated with Diverse Transgenerational Effects on Gene and Transposon Expression in a Hybrid Dysgenic Syndrome of .
- Retinoic Acid Signaling Regulates Differential Expression of the Tandemly-Duplicated Long Wavelength-Sensitive Cone Opsin Genes in Zebrafish
- The Formin Diaphanous Regulates Myoblast Fusion through Actin Polymerization and Arp2/3 Regulation
- Genome-Wide Analysis of PAPS1-Dependent Polyadenylation Identifies Novel Roles for Functionally Specialized Poly(A) Polymerases in
- Runx1 Transcription Factor Is Required for Myoblasts Proliferation during Muscle Regeneration
- Regulation of Mutagenic DNA Polymerase V Activation in Space and Time
- Variability of Gene Expression Identifies Transcriptional Regulators of Early Human Embryonic Development
- The Drosophila Gene Interacts Genetically with and Shows Female-Specific Effects of Divergence
- Functional Activation of the Flagellar Type III Secretion Export Apparatus
- Retrohoming of a Mobile Group II Intron in Human Cells Suggests How Eukaryotes Limit Group II Intron Proliferation
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Exon 7 Contributes to the Stable Localization of Xist RNA on the Inactive X-Chromosome
- YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers
- SmD1 Modulates the miRNA Pathway Independently of Its Pre-mRNA Splicing Function
- Molecular Basis of Gene-Gene Interaction: Cyclic Cross-Regulation of Gene Expression and Post-GWAS Gene-Gene Interaction Involved in Atrial Fibrillation
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



