-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
RAB-10-Dependent Membrane Transport Is Required for Dendrite Arborization
Dendrites are cellular extensions from neurons that gather information from other neurons or cues from the external environment to convey to the nervous system of an organism. Dendrites are often extensively branched, raising the question of how neurons supply plasma membrane and dendrite specific proteins from the source of synthesis inside the cell to developing dendrites. We have examined membrane trafficking in the PVD neuron in the nematode worm C. elegans to investigate how new membrane and dendrite proteins are trafficked. The PVD neuron is easy to visualize and has remarkably long and widely branched dendrites positioned along the skin of the worm, which transmits information about harsh touch and cold temperature to the nervous system. We have discovered that a key organizer of vesicle trafficking, the RAB-10 protein, localizes to membrane vesicles and is required to traffic these vesicles that contain plasma membrane and dendrite proteins to the growing PVD dendrite. Further, our work revealed that a complex of proteins, termed the exocyst, that helps fuse membrane vesicles at the plasma membrane, localizes with RAB-10 and is required for dendrite branching. Together, our work has revealed a novel mechanism for how neurons build dendrites that could be used to help repair damaged neurons in human diseases and during aging.
Published in the journal: RAB-10-Dependent Membrane Transport Is Required for Dendrite Arborization. PLoS Genet 11(9): e32767. doi:10.1371/journal.pgen.1005484
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005484Summary
Dendrites are cellular extensions from neurons that gather information from other neurons or cues from the external environment to convey to the nervous system of an organism. Dendrites are often extensively branched, raising the question of how neurons supply plasma membrane and dendrite specific proteins from the source of synthesis inside the cell to developing dendrites. We have examined membrane trafficking in the PVD neuron in the nematode worm C. elegans to investigate how new membrane and dendrite proteins are trafficked. The PVD neuron is easy to visualize and has remarkably long and widely branched dendrites positioned along the skin of the worm, which transmits information about harsh touch and cold temperature to the nervous system. We have discovered that a key organizer of vesicle trafficking, the RAB-10 protein, localizes to membrane vesicles and is required to traffic these vesicles that contain plasma membrane and dendrite proteins to the growing PVD dendrite. Further, our work revealed that a complex of proteins, termed the exocyst, that helps fuse membrane vesicles at the plasma membrane, localizes with RAB-10 and is required for dendrite branching. Together, our work has revealed a novel mechanism for how neurons build dendrites that could be used to help repair damaged neurons in human diseases and during aging.
Introduction
Dendrites and axons are two distinct functional and morphological domains of neurons. Due to the complexity and heterogeneity in the morphology of dendrites, it has been challenging to study the development of dendrites in comparison with the more uniform, simply structured thread-like axons. Previous studies have shown that dendritic growth relies heavily on the secretory pathway and endosomal function[1,2]. In Drosophila, loss-of-function mutations in genes encoding the small GTPases Rab1 and Sar1, which are key regulators of ER-to-Golgi vesicular transport [3], severely reduce the growth of dendrites. Notably, loss of Rab1 and Sar1 do not diminish axon outgrowth, suggesting that the mechanisms underlying the extension of dendrites and axon are distinct [1]. Furthermore, Golgi outposts, which are primarily found in dendrites but not axons, play an important role in supplying membranes for dendritic branching and growth [1]. These experiments suggest that membrane components generated in the ER and trafficked to the Golgi are essential for dendritic growth. In addition, Rab5 and Rab11-dependent endocytic membrane trafficking has also been implicated in dendrite morphogenesis [2,4]. The molecular mechanisms that deliver membranes from the Golgi, Golgi-outposts and endosomes to the dendritic plasma membrane, however, are unclear.
To identify the membrane trafficking mechanisms that support dendrite branching and growth, we use the C. elegans multi-dendritic PVD neurons as a model. The PVD neurons exist as a pair, PVDL and PVDR, and they function to detect harsh mechanical forces and cold temperatures [5–7]. Each PVD neuron sits on one side of the animal and has a single axon that extends to the ventral nerve cord, as well as a highly branched dendritic arbor that covers most of the body, except for the neck and head [8]. Recently, the transmembrane leucine-rich repeat protein DMA-1 was identified as a PVD dendritic receptor. DMA-1 recognizes skin-derived pre-patterned cues that promote dendrite stabilization and branching [9–11]. In addition, the claudin-like transmembrane protein HPO-30 also promotes dendrite stabilization [12]. Both DMA-1 and HPO-30 are dendrite specific proteins that are rarely observed in axons. The mechanisms that regulate their sorting and trafficking to the dendritic membranes are still unknown [9,12].
The small GTPase Rab10, which is an ortholog to the yeast Sec4p protein that controls post-Golgi vesicle trafficking, has been shown to mediate polarized membrane addition during axonal growth in mammals. In axons Rab10 is activated by the mammalian ortholog of the Drosophila gene Lethal giant larvae, Lgl1. The Lgl1 protein dissociates the Rab10-GDI complex [13]. Activated Rab10 then interacts with multiple effector proteins to direct distinct steps of axonal membrane addition. These include an initial interaction with myosin Vb (MYO5B), which controls the biogenesis of post-Golgi Rab10 carriers [14]. Rab10 then binds with c-Jun N-terminal kinase-interacting protein 1 (JIP1) to facilitate anterograde transport of Rab10 cargos [15]. Finally, Rab10 binds myristoylated alanine-rich C-kinase substrate (MARCKS). The Rab10-MARCKS interaction allows the docking and fusion of Rab10 vesicles with the axonal plasma membrane [16]. Unlike extensive studies on the role of Rab10 in axonal growth, it is unclear whether Rab10 is required for dendrite arborization during development.
Several studies have shown association between the conserved exocyst complex and Rab10 GTPases [17]. The exocyst complex is composed of eight subunits. In C. elegans these are encoded by the genes sec-3, sec-5, sec-6, sec-8, sec-10, sec-15, exoc-7 and exoc-8 [18]. The exocyst complex functions as the effector of the yeast Rab10 ortholog Sec4p, and facilitates tethering, docking and fusion of secretory vesicles during bud formation [17]. The exocyst complex also associates with Rab10 in renal epithelial cells and may mediate membrane transport to the primary cilium [19]. Both Rab10 and the exocyst complex are further required for the exocytic transport of the glucose transporter Glut4 [20,21]. However, whether Rab10 and the exocyst complex function together during other processes, such as dendritic growth and branching, is not clear.
Here, we report that loss of the C. elegans rab-10 gene reduces dendritic arborization of the PVD neuron. We show that RAB-10 functions cell-autonomously, and localizes to the Golgi and the early endosomes in the PVD neurons. Further, we find that deficiencies in rab-10 and the exocyst subunits cause accumulation of the dendritic membrane proteins DMA-1 and HPO-30 within intracellular vesicles. We also show that Rab10 and the exocyst complex are required for dendrite arborization in Drosophila, and dendritic spine formation in mammalian neurons. Together, these data suggest that Rab10 and the exocyst complex play a conserved role in controlling Golgi-to-plasma membrane and/or endosome-to plasma membrane trafficking required for dendrite morphogenesis.
Results
Loss of rab-10 affects PVD dendritic arborization
To identify potential regulators of membrane trafficking during dendritic branching and growth of the multi-dendritic PVD neuron, a candidate-based genetic screen was performed. We crossed animals harboring mutations in genes encoding proteins important for various membrane trafficking pathways with a PVD neuron-specific fluorescent marker (F49H12.4>gfp or ser2prom3>gfp). The morphology of PVD dendritic arbors was then examined. We found that two putative null alleles of rab-10 presented severely abnormal PVD dendrite morphology (Table 1 and Fig 1A–1E). In the proximal region (including the middle and tail areas), rab-10 (ok1494) and rab-10 (dx2) mutant animals contained far fewer dendritic branches compared to wild-type. For example, a count of secondary dendrites within a 100μm long region along the primary dendrite anterior to the PVD cell body in rab-10 (ok1494) and rab-10 (dx2) animals revealed an average of 1.1±0.3 and 2.8±0.7 secondary dendrites. In comparison, wild-type animals within this region contained 11.1±0.5 secondary dendrites (Fig 1F and 1G). Tertiary and quaternary branches were even more affected and were essentially absent in the proximal region of rab-10 (ok1494) and rab-10 (dx2) animals (Fig 1F, 1H and 1I). Interestingly, in the distal area of the PVD, the dendritic branching and growth were minimally affected by loss of rab-10, indicating that a rab-10-independent mechanism mediates distal dendritic branching (Fig 1A–1E, 1G–1I). The growth of the primary dendrite and axon of the PVD appeared normal in rab-10 deficient animals (Fig 1A–1E, and S1 Fig). rab-10 was also required for the dendritic arborization of the FLP neuron, which covers the head region and has a similar morphology and function as the PVD neuron (S2 Fig) [8]. However, rab-10 was dispensable for the growth of unbranched dendrites of OLL, AWB, and AWC neurons, suggesting a specific role of rab-10 in mediating the growth of dendritic branches in multi-dendritic neurons (S2 Fig). Together, these data indicate that RAB-10 is required for the elaboration of branched dendrites in C. elegans.
Tab. 1. PVD dendrite morphogenesis is defective in rab-10, rab-1 and exocyst mutants. 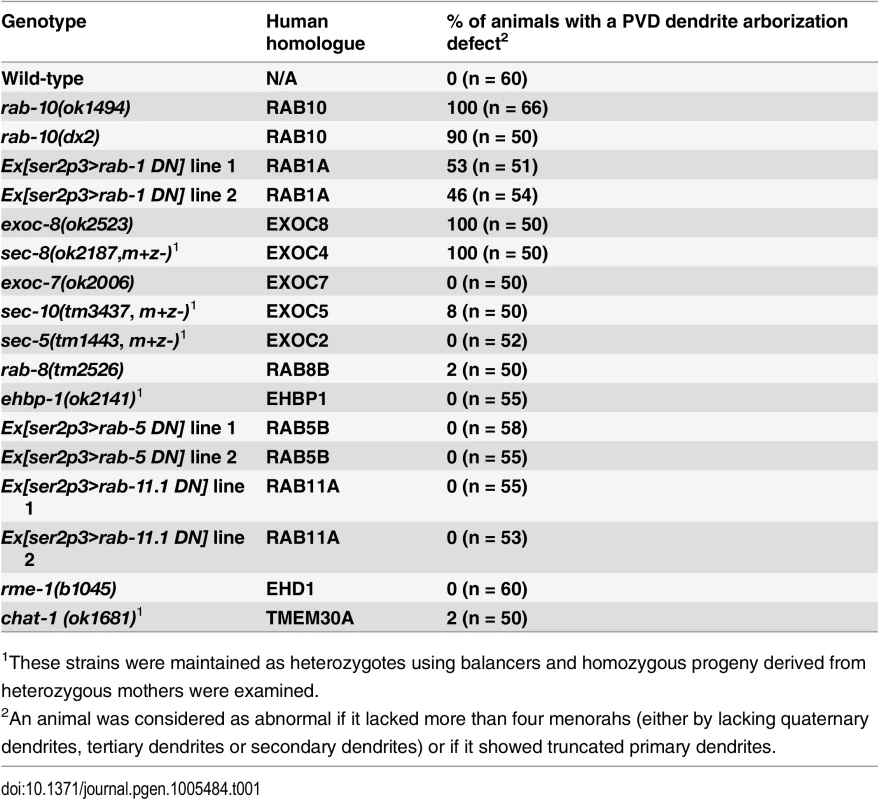
1These strains were maintained as heterozygotes using balancers and homozygous progeny derived from heterozygous mothers were examined. Fig. 1. RAB-10 promotes formation of proximal PVD dendritic arbors. 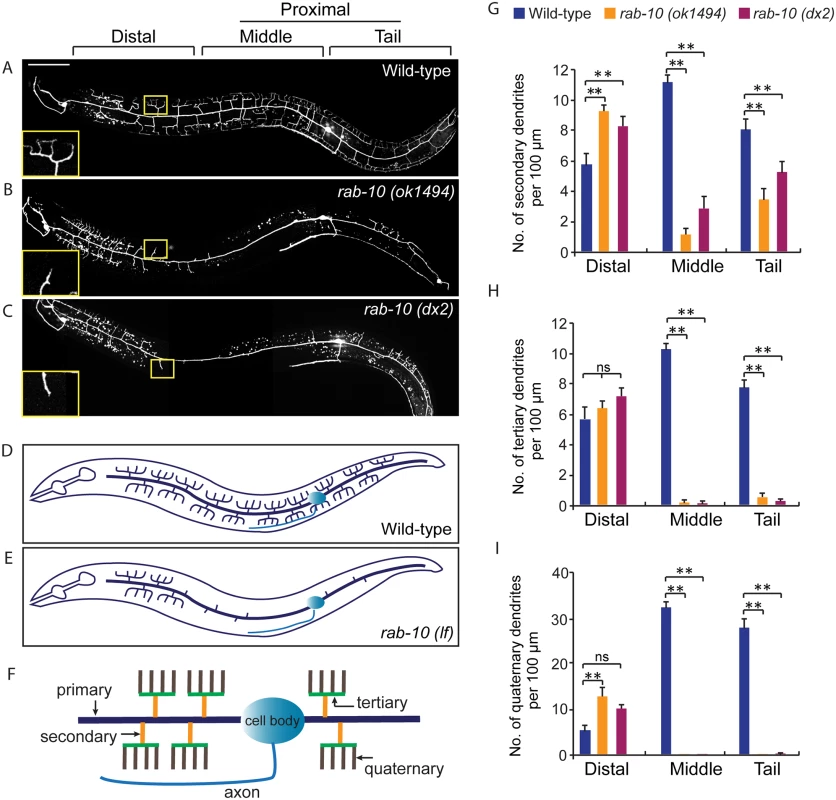
(A-C) Images show PVD morphology of (A) wild-type, (B) rab-10 (ok1494), and (C) rab-10 (dx2) mutant worms at the L4 stage. PVD morphology was visualized using a PVD>GFP reporter line wdIs51 (F49H12.4>gfp). The inset images are enlarged views (2.5 fold) of the regions indicated by the boxes. All images are maximum intensity projections of z-stacks of images acquired using a spinning disk confocal microscope. (D, E) A schematic image of PVD morphology in (D) wild-type and (E) rab-10 loss-of-function mutant worms. (F) A schematic image of menorahs in the PVD neuron. (G, H) Quantification of number of (G) secondary dendrites, (H) tertiary dendrites and (I) quaternary dendrites in three 100 μm x 100 μm areas from the distal, middle and tail region, respectively. 10 animals at the L4 stage were randomly selected and quantified for each genotype. Scale bar, 50 μm. A one-way ANOVA followed by post-hoc comparisons using the Dunnett’s test was used to compare wild-type and mutant animals. ns: not significant; **: P<0.01. Error bars report ±SEM. RAB-10 functions cell-autonomously in the PVD neuron
To determine if RAB-10 activity is required within the PVD, we tagged full length RAB-10 at its N-terminus with GFP and expressed this construct under a PVD-specific promoter (ser2prom3) in rab-10(ok194) animals. GFP::RAB-10 expressed from multicopy extrachromosomal arrays in the PVD neuron fully rescued the morphology of the PVD dendritic arbor in most animals in two independent lines (Fig 2A–2C), indicating that RAB-10 functions within the PVD to promote proximal dendritic arborization. As a GTPase, RAB-10 cycles between the GDP-bound inactive form and GTP-bound active form. To test whether its function in promoting dendrite branching and growth requires GTPase activity, we expressed both GDP-locked (T23N) and GTP-locked (Q68L) forms of RAB-10 and examined their rescuing ability. Dominant-negative RAB-10 (T23N) not only failed to rescue the proximal PVD defects in rab-10 (ok1494) mutants, but also disrupted the distal dendrite arbor in wild-type animals (Fig 2C, S3 Fig). In contrast, constitutively active RAB-10 (Q68L) fully rescued the PVD dendrite morphogenesis defects in rab-10 (ok1494) mutant animals (Fig 2C). Over-expressing constitutively active RAB-10 (Q68L) did not cause over-growth of dendrites in PVD neuron (S4 Fig), suggesting that other factors limit dendrite growth and patterning. We conclude that RAB-10 functions cell autonomously in the PVD neuron and requires GTPase activity to promote PVD dendrite morphogenesis.
Fig. 2. RAB-10 functions cell-autonomously in the PVD neuron. 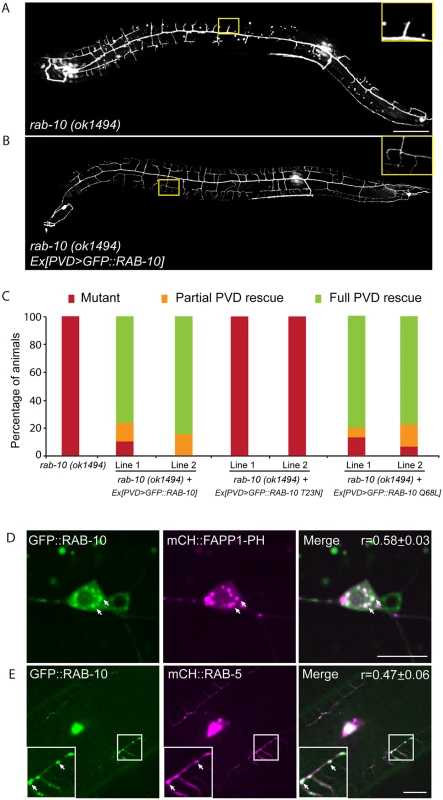
(A, B) The dendritic morphogenesis defect in rab-10 (ok1494) mutant animals was rescued by PVD-specific expression of GFP::RAB-10 driven from a multicopy extrachromosomal array. PVD morphology was visualized using the PVD>gfp marker strain wdIs51. The inset images are enlarged views (2.5 fold) of the regions indicated by the boxes. Both images are maximum intensity projections of z-stacks. Scale bar, 50 μm. (C) Quantification of the cell-autonomous rescue of dendritic morphogenesis defect by cell-specific expression of wild type, GDP-locked, and GTP-locked forms of RAB-10. For each construct, at least two rab-10 (ok1494) lines carrying arrays expressing ser2prom3> gfp::rab-10 WT, GDP-locked (T23N), and GTP-locked (Q68L) forms were generated. Animals were categorized based on their PVD dendrite morphology. At least 29 animals at the L4 or young adult stage were scored for each line. Partial PVD rescued animals contained one or more full menorahs in the proximal region. Full PVD rescued animals contained PVD dendritic arbors that were indistinguishable from wild type animals. (D) Colocalization of GFP::RAB-10 and mCHERRY::FAPP1-PH in the PVD. Magenta, mCHERRY::FAPP1-PH; green, GFP::RAB-10. (E) Colocalization between GFP::RAB-10 and mCHERRY::RAB-5 in PVD. Magenta, mCHERRY::RAB-5; green, GFP::RAB-10. r-value reports mean Pearson’s correlation coefficient ± SEM for colocalization of GFP::RAB-10 and mCHERRY::FAPP1-PH, and GFP::RAB-10 and mCHERRY::RAB-5. At least 15 animals at the L4 or young adult stage were quantified for each group of colocalization analysis. The inset images are enlarged views (2 fold) of the regions indicated by the boxes. Arrows indicate vesicles co-labeled by both GFP and mCherry reporters. Error bars report ±SEM. Scale bar, 10μm. RAB-10 colocalizes with the Golgi and endosomes in the PVD neuron
RAB-10 orthologs are known to regulate Golgi to plasma membrane vesicle trafficking as well as endocytic recycling events [22–26]. To determine if RAB-10 localizes with either Golgi or endosomes in the PVD neuron we tagged full length RAB-10 with GFPnovo2, a mutant form of GFP that is brighter. We generated a single copy insertion line using miniMos method to minimize potential ectopic localization of RAB-10 protein induced by over-expression [27,28]. Similar to the multicopy line, we crossed this line into the rab-10 null mutant and found that it fully rescued the PVD dendrite arborization defect (S5 Fig). Many intracellular vesicles were labeled by GFP::RAB-10 in the PVD dendrites. Notably, there was a strong correlation between GFP::RAB-10 localization and mCherry::FAPP1-PH (Golgi reporter) and a strong correlation of GFP::RAB-10 and mCherry::RAB-5 (early endosome reporter) (Fig 2D and 2E) [29]. These observations are consistent with previous studies showing RAB-10 localization to the Golgi and early endosomes in the C. elegans intestinal cells [24]. Supporting a role in mediating vesicular trafficking, time-lapse recordings revealed that GFP::RAB-10 labeled vesicles moved bi-directionally along dendrites, consistent with localization to transport vesicles (S1 Movie). Together, these data suggest that RAB-10 might mediate PVD dendrite outgrowth by regulating Golgi-to-membrane trafficking or endosomal membrane recycling events.
To attempt to determine whether the PVD dendrite morphogenesis defect in rab-10 mutants might be due to a role for RAB-10 in Golgi-to-plasma membrane trafficking, endocytic recycling, or both, we perturbed each pathway and tested whether it altered PVD dendritic arborization. Consistent with previous studies in Drosophila and rat hippocampus neurons implicating ER-to-Golgi trafficking in dendritic growth, over-expressing dominant-negative RAB-1 (a key regulator of ER-to-Golgi trafficking) caused dramatically reduced branching in the PVD (Table 1, and S6 Fig) [1]. In contrast, genetic loss of rme-1 or chat-1, and over-expression of dominant-negative RAB-5 or RAB-11.1 (all key regulators of endocytic recycling) [30–33], had no effect of PVD dendrite morphology (Table 1). Together, these data suggest a role for RAB-10 in mediating Golgi-to-plasma membrane trafficking that is important for dendrite morphogenesis. Notably, however, we cannot rule the possibility that RAB-10 regulates endosomal recycling independent of rme-1, rab-5 or rab-11.1. Thus, RAB-10 might also regulate an endosome-to-plasma membrane transport pathway independent of RME-1, RAB-5, RAB-11.1 function in the PVD neuron that is required for dendritic arborization.
Loss of exocyst complex components cause defects in PVD dendritic morphogenesis
Rab GTPases are molecular switches that exert their functions by recruiting and releasing specific effectors [34]. We next sought to identify possible effectors that function with RAB-10 in mediating the branching and growth of PVD dendrites. We first examined EHBP-1, an Eps 15 domain binding protein that acts as an effector of RAB-10 in endocytic recycling and secretory pathways [26]. Mutant animals of ehbp-1, however, had normal PVD dendritic arbors (Table 1). Importantly, we cannot fully exclude the possibility that the PVD dendrite development was rescued by maternally loaded ehbp-1 mRNA or EHBP-1 protein, since we can only examine ehbp-1 homozygous animals derived from heterozygous mothers. We next tested members of the exocyst complex, an established effector of yeast Sec4p with which RAB-10 shares high homology [35]. Notably, loss of the exocyst subunit exoc-8 in viable null mutant animals or loss of sec-8 in the mutant progeny of heterozygous sec-8 mutant mothers, caused a PVD dendrite morphogenesis defect in the proximal but not in the distal region (Table 1, and Fig 3A–3F)[36]. Further, the growth of the primary dendrite and the axon was normal (Fig 3A–3C, S1 Fig). This phenotype was similar to rab-10 mutants, although the growth of secondary dendrites was only mildly affected in exoc-8 and sec-8 mutant worms (Fig 3A–3F). Expression of exoc-8 cDNA under the PVD-specific promoter (ser2prom3) fully rescued the dendritic arborization defect in exoc-8 (ok2523) mutant worms, indicating that the exocyst complex functions in the PVD to promote dendritic arborization (Fig 3G).
Fig. 3. Mutations in exoc-8 and sec-8 reduce PVD dendritic arborization. 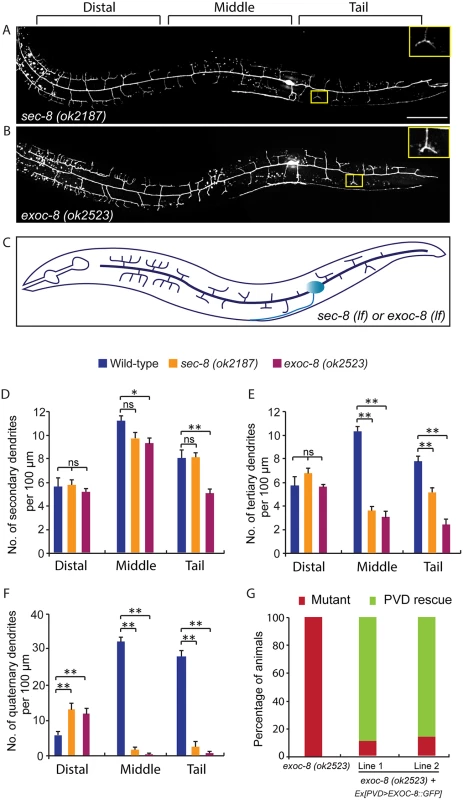
(A, B) Images show PVD morphology of (A) sec-8 (ok2187) and (B) exoc-8 (ok2523) mutant animals at the L4 stage. PVD morphology was visualized using the wdIs51 strain. The inset images are enlarged views (2.5 fold) of the regions indicated by the boxes. Both images are maximum intensity projections of z-stacks. Scale bar, 50 μm. (C) A schematic of PVD morphology of sec-8 (ok2187) and exoc-8 (ok2523) mutant animals. (D-F) Quantification of the number of (D) secondary dendrites (E) tertiary dendrites and (F) quaternary dendrites in three 100 μm x 100 μm areas from the distal, middle and tail regions, respectively. 10 animals at the L4 stage were quantified for each genotype. A one-way ANOVA followed by post-hoc comparisons using the Dunnett’s test was used to compare wild-type and mutant animals. ns: not significant; *: P<0.05; **: P<0.01. (G) Quantification of cell-autonomous rescue of dendritic morphogenesis defect by cell-specific expression of exoc-8::gfp construct. Results of two independent lines are shown. At least 38 animals at the L4 or young adult stage were scored for each line. Homozygous mutants of two other exocyst components, sec-5 and sec-10, derived from the heterozygous mothers did not show any obvious PVD dendrite defect (Table 1). To test whether this was due to maternal rescue, we used a newly developed targeted protein degradation system to specifically remove the SEC-5 protein from the PVD [37]. This system takes advantage of cell type specific expression of the E3 ubiquitin ligase substrate-recognition subunit ZIF-1, which recognizes proteins tagged with the 36 amino acid ZF1 zinc-finger domain. To selectively remove SEC-5 in the PVD neuron, we drove ZIF-1 in PVD using the ser2prom3 promoter in a sec-5::zf1::yfp knock-in strain (sec-5(xn51))—a strain where both copies of the endogenous sec-5 genes are tagged with zf1 (Fig 4A) [37]. Confirming a role for the exocyst complex in promoting dendritic arborization, depleting ZF1::YFP tagged SEC-5 in PVD resulted in PVD dendrite arborization defects (Fig 4C–4E). In some of these animals, both distal and proximal regions lacked menorah structures, suggesting that sec-5 might be important for the growth of both proximal and distal dendrites (Fig 4D). Control animals expressing ser2prom3>ZIF-1 transgene alone and control animals expressing ZF1::YFP tagged SEC-5 alone had normal PVD dendrite morphology (Fig 4B and 4E). We conclude that the exocyst complex plays a cell-autonomous role in promoting dendritic arborization and that maternal contributions of some of exocyst components contribute to this function. Further, based on the more severe PVD phenotype after ZF-1 tag-directed loss of sec-5 versus the exoc-8 null mutant, our results also suggest that exocyst components have different requirements (perhaps reflecting their differential necessity for exocyst activity) during PVD dendrite morphogenesis.
Fig. 4. ZIF-1-mediated degradation of SEC-5 in the PVD reduces dendrite arborization. 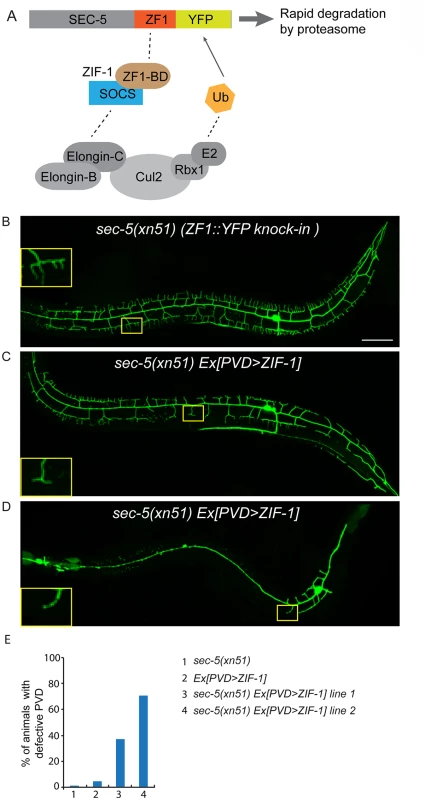
(A) A cartoon showing how ZIF-1 recruits the SEC-5::ZF1::YFP fusion protein to an ECS E3 ligase complex for ubiquitylation (Ub) and subsequent rapid degradation by the proteasome. (B-D) Fluorescence images show the morphology of the PVD neuron using the PVD>myr-gfp marker strain wyIs592 in (A) sec-5(xn51) (expresses SEC-5 tagged with YFP and the ZF1 zinc-finger domain), (B and C) sec-5(xn51) with a transgene expressing ser2prom3>zif-1 (which encodes a E3 ubiquitin ligase substrate-recognition subunit). The inset images are enlarged views (2.5 fold) of the regions indicated by the boxes. All images are maximum intensity projections of z-stacks. L4 or young adult stage animals were examined. Scale bar, 50 μm. (E) Quantification of percentage of animals showing PVD dendrite arborization defect. At least 50 animals at the L4 or young adult stage were quantified for each genotype. RAB-10 and EXOC-8 mediate transport of the dendritic membrane proteins DMA-1 and HPO-30
Yeast Sec4p and the exocyst complex function together to promote docking and possible fusion of post-Golgi vesicles [17]. Thus, we hypothesized that the PVD dendritic arborization defects in rab-10, exoc-8 and sec-8 mutants might in part be due to the failure of docking of post-Golgi vesicles. To test this, we built fluorescent reporters for the dendritic membrane proteins, DMA-1 and HPO-30, transmembrane proteins expressed in PVD neurons that function to mediate dendritic branching and stabilization [9–12]. Consistent with previous studies, DMA-1::GFP and HPO-30::GFP were localized in the dendritic membranes, and in some intracellular vesicles in wild-type animals (Fig 5A–5C, and S7 Fig) [9,12]. These two transgene reporters likely represent endogenous protein localization, as the transgenes rescued the PVD dendrite arborization defects of dma-1(tm5159) and hpo-30(ok2147) mutants (S5 Fig).
Fig. 5. Loss of rab-10 and exoc-8 perturbs DMA-1 transport to dendritic membranes. 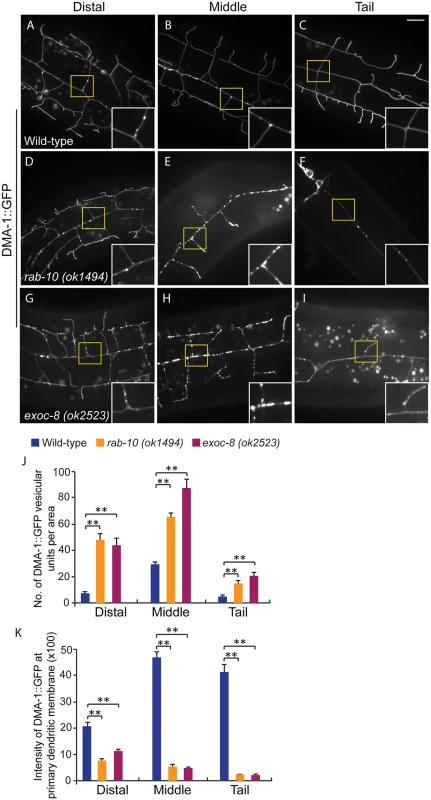
(A-I) Images show subcellular localization of DMA-1::GFP in (A-C) wild-type, (D-F) rab-10 (ok1494) and (G-I) exoc-8 (ok2523) L4 stage animals in the distal, middle and tail region, respectively. All images are maximum intensity projections of z-stacks. Insets are 2.5 fold enlarged views. Scale bars, 10 μm. (J) Quantification of number of vesicles or vesicle clusters per area (76.8μm x 76.8μm) in wild-type, rab-10 (ok1494) and exoc-8 (ok2523) mutant worms in the distal, middle, and tail regions, respectively. At least 8 animals were quantified for each genotype. (K) Quantification of the intensity of DMA-1::GFP at the surface of the primary dendrites in wild-type, rab-10 (ok1494) and exoc-8 (ok2523) mutant worms in the distal, middle, and tail regions, respectively. 10 animals were quantified for each genotype. A one-way ANOVA followed by post-hoc comparisons using the Dunnett’s test was used to compare wild-type and mutant animals. **: P<0.01. Error bars report ±SEM. Consistent with a role in docking and fusion of vesicles, loss of rab-10 and exoc-8 caused severe accumulation of DMA-1::GFP containing vesicles within the growing PVD dendrites (Fig 5D–5I). We quantified the number of vesicular units (which might be a single vesicle or a vesicle cluster) in three areas—the distal, middle and proximal regions. We found that wild-type animals contained on average 29.6±1.9 vesicular units in a 76.8μm x 76.8 μm area from the middle region of the PVD. In contrast rab-10 (ok1494) and exoc-8 (ok2523) mutant worms contained a dramatic two-to-three fold increase in vesicular units—65.6±2.8 and 87.8±6.8, respectively (Fig 5J). Furthermore, in wild-type animals the DMA-1::GFP containing vesicles mainly localized to the primary dendrites, and rarely appeared in the higher-order dendrites (including the secondary, tertiary and quaternary dendrites; Fig 5A–5C). In contrast, in rab-10 (ok1494) and exoc-8 (ok2523) mutant worms, numerous vesicles appeared in the higher-order dendrites (Fig 5D–5I and S8 Fig).
The presence of numerous DMA-1::GFP intracellular vesicles in rab-10 and exoc-8 mutants suggested that delivery of DMA-1 to the membrane might be reduced. To test this idea, the fluorescence intensity of DMA-1::GFP at the surface (which we presume is predominantly plasma membrane localization) of the primary dendrites was determined. In the distal region of the dendritic arbor, the intensity of DMA-1::GFP at the primary dendrite in rab-10 (ok1494) and exoc-8 (ok2523) mutant worms was decreased by 63.9% and 47.8% compared with wild-type animals (Fig 5K). In the rab-10 mutants, reduction of dma-1 by RNAi-mediated knock-down further suppressed distal dendritic arborization, suggesting that the reduced levels of DMA-1 on the surface of distal dendrites is sufficient to promote dendritic stabilization and branching (S9 Fig). In the proximal region, the decrease in DMA-1 was more dramatic. The intensity of DMA-1::GFP at the surface of the primary dendrite in rab-10 (ok1494) and exoc-8 (ok2523) mutant worms was decreased by 89.5% and 90.3% in the middle regions, and 94.4% and 94.8% in the tail regions compared to wild-type animals (Fig 5K). HPO-30::GFP showed similar vesicular accumulation and decreased dendrite surface localization in rab-10 (ok1494) and exoc-8 (ok2523) mutant animals (S7 Fig). These results offer compelling evidence that rab-10 and exocyst activity are required for the vesicular delivery of DMA-1 and HPO-3 to the dendritic membrane.
EXOC-8 and SEC-8 promote fusion of RAB-10 vesicles
The similar phenotypes after loss of exocyst components and rab-10, as well as functions of these molecules in yeast and vertebrates in delivering vesicles to the plasma membrane [17,19,35,38,39] led us to examine whether RAB-10 and exocyst components coexist in vesicles in the PVD dendrites. EXOC-8::GFP and mCherry::RAB-10 strongly colocalized on intracellular vesicles (Fig 6A), indicating that the exocyst might function together with rab-10 to mediate vesicle delivery. Next, we examined the subcellular localization of RAB-10 in animals harboring mutations in the exocyst components exoc-8 and sec-8. Loss of these exocyst components caused a dramatic accumulation of RAB-10 labeled vesicles in the PVD dendrites (Fig 6B). Wild-type animals contained 39.8±2.6 GFP::RAB-10 vesicular units in a 88.1μm x 88.1 μm area from the middle region of the PVD neuron. Worms with mutations in exoc-8 (ok2523) and sec-8 (ok2187) contained over three fold more GFP::RAB-10 vesicular unites—129.7±7.5 and 127.6±2.7, respectively (Fig 6C). To determine whether the accumulated vesicles containing GFP::RAB-10 were the same population observed in exoc-8(ok2523) mutants carrying DMA-1::GFP or HPO-30::GFP, we expressed DMA-1::GFP or HPO-30::GFP and mCherry::RAB-10 in exoc-8(ok2523) mutant animals. Confirming these are the same vesicle population, most of the mCherry::RAB-10 labeled vesicles also contained DMA-1::GFP or HPO-30::GFP (Fig 6D and 6E). To test whether RAB-10 functions to recruit EXOC-8 onto vesicles, the EXOC-8::GFP reporter was crossed into the rab-10 loss-of-function mutant. EXOC-8::GFP, however still localized to vesicles in PVD neurons in rab-10 mutants (S10 Fig). Taken together, these results suggest the exocyst complex promotes fusion of RAB-10 carriers within the PVD neuron to facilitate dendritic growth and stabilization, but that the exocyst complex is recruited to these vesicles in a RAB-10 independent manner.
Fig. 6. exoc-8 and sec-8 are required for fusion of RAB-10 vesicles. 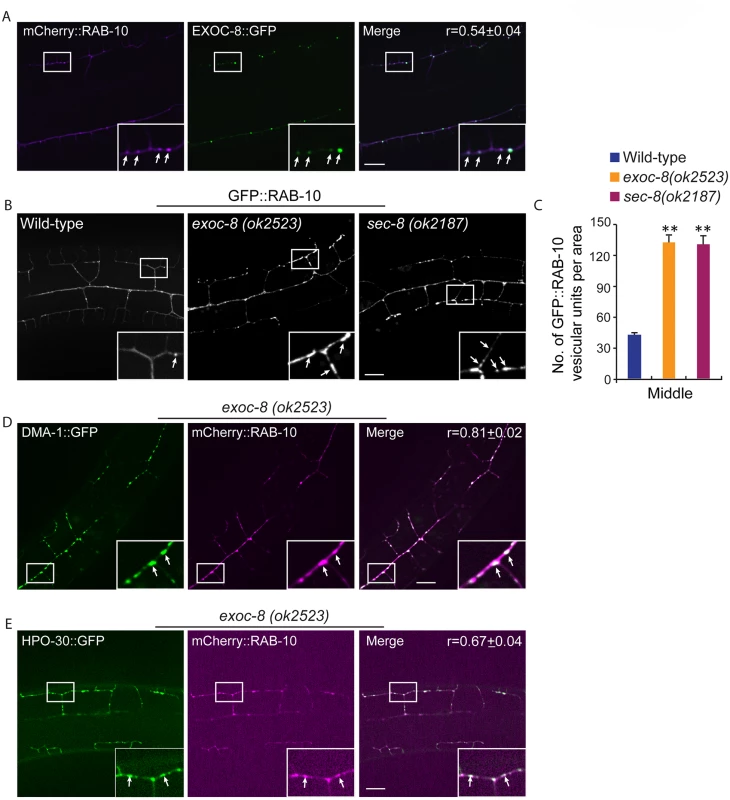
(A) mCherry::RAB-10 colocalizes with EXOC-8::GFP. r-value reports the mean Pearson’s correlation coefficient ± SEM for colocalization of RAB-10 and EXOC-8. 15 animals at the L4 stage were quantified for each genotype. Insets are 2.5 fold enlarged views. Arrows indicate vesicles co-labeled by both EXOC-8::GFP and mCherry::RAB-10. Scale bar, 10 μm. (B) Images showing GFP::RAB-10 reporter in wild-type, exoc-8(ok2523) and sec-8(ok2187) animals. All images are maximum intensity projections of z-stacks. Insets are 2.5 fold enlarged views. Arrows indicate vesicles labeled by GFP::RAB-10. Scale bar, 10 μm. (C) Quantification of the number of GFP::RAB-10 vesicles or vesicle clusters per area (88.1μm x 88.1μm) in wild-type, exoc-8(ok2523) and sec-8(ok2187) animals. 15 animals at the L4 stage were quantified for each genotype. A one-way ANOVA followed by post-hoc comparisons using the Dunnett’s test was used to compare wild-type and mutant animals. **: P<0.01. (D) mCherry::RAB-10 colocalizes with DMA-1::GFP in exoc-8(ok2523) animals. (E) mCherry::RAB-10 colocalizes with HPO-30::GFP in exoc-8(ok2523) animals. r-value reports the mean Pearson’s correlation coefficient ± SEM for colocalization of RAB-10 and DMA-1 or HPO-30. 15 animals at the L4 to young adult stage were quantified for each genotype. Insets are 2.5 fold enlarged views. Arrows indicate vesicles co-labeled by both mCherry::RAB-10 and DMA-1::GFP or HPO-30::GFP. Error bars report ±SEM. Scale bar, 10 μm. Drosophila and mammalian Rab10 and exocyst subunits mediate dendritic morphogenesis and dendritic spine formation, respectively
To investigate whether Rab10 and exocyst complex-mediated dendritic membrane transport is required for the dendrite morphogenesis in other organisms, we examined Drosophila class IV dendritic arborization neurons and cultured rat hippocampal neurons after loss of rab10 and exocyst components. In Drosophila, RNAi mediated knock-down of rab10 and exo84 significantly reduced dendrite branching and growth (S11 Fig). Compared to animals treated with control RNAi, rab10 and exo84 RNAi targeted loss resulted in a 20% decrease in both the number of total end points and the total dendritic arbor (S11 Fig). ShRNA mediated knock-down of rab10, sec8 and exoc84 did not alter the total dendrite length in cultured rat embryonic hippocampal neurons, but did lead to a dramatic 67% reduction in the density of dendritic spines at 21 days in vitro (DIV) compared to control shRNA treated neurons (S12 Fig). Collectively, we conclude that rab10 and the exocyst complex have important and likely conserved roles during dendrite morphogenesis.
RAB-8 functions redundantly with RAB-10 to promote dendritic branching
Since some higher-order dendrites in the PVD still formed in rab-10 null alleles, we hypothesized that another Rab protein might regulate dendritic branching. To test this idea, we examined the function of the RAB-10 related GTPase RAB-8 [40]. Notably, homozygous viable rab-8(tm2526) null mutant animals showed normal PVD morphology (Fig 7A). This suggested that if RAB-8 functions in the PVD, it might act redundantly with RAB-10. To test this idea, we first attempted to create animals with null mutations in both rab-8 and rab-10. The rab-8 and rab-10 double mutant animals, however, were sterile, which made it challenging to determine PVD morphology [26]. Interestingly, we found that expression of a dominant-negative RAB-8 (T22N) in the PVD neuron caused a severe dendrite morphogenesis defect (S3 Fig). In 15% (n = 40) and 12% (n = 50) of transgenic animals (two independent lines) harboring the transgene of dominant negative RAB-8, the dendritic growth in both distal and proximal regions was dramatically reduced (S3 Fig). These results suggest that the dominant negative RAB-8 might act to block the function of both the RAB-8 and RAB-10 proteins (perhaps through inhibition of a common guanine nucleotide exchange factor) and that RAB-8 may function redundantly with RAB-10 to promote PVD morphogenesis.
Fig. 7. Deficiency in rab-8 enhances the dendritic arborization defect of rab-10 mutant. 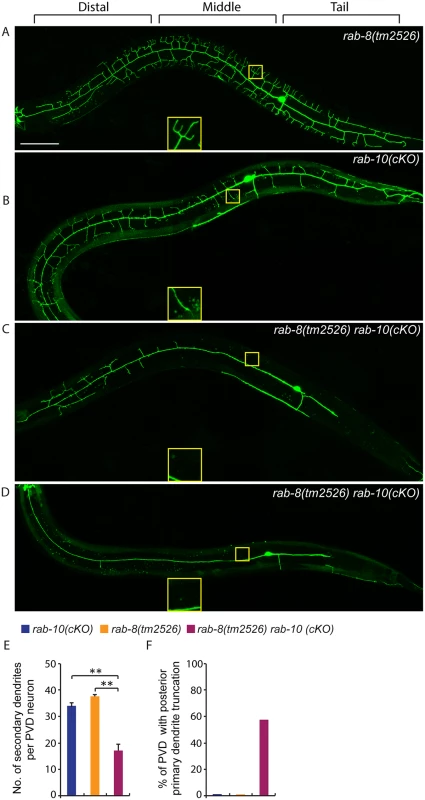
(A-D) Fluorescence images show the morphology of PVD neuron using the PVD>myr-gfp marker strain wyIs592 in (A) rab-8(tm2526), (B) rab-10 conditional knock-out (cKO), and (C-D) rab-10(cKO) rab-8(tm2526). Insets are 2.5 fold enlarged views. All images are maximum intensity projections of z-stacks. L4 or young adult stage animals were examined. Scale bar, 50 μm. (E) Quantification of number of secondary dendrites per PVD neuron in the above three genotypes (n = 35, 25 and 27, respectively). A one-way ANOVA followed by post-hoc comparisons using the Dunnett’s test was used to compare single and double mutants. **: P<0.01. Error bars report ±SEM. (F) Quantification of percentage of animals showing truncated posterior primary dendrite. To more directly test the idea that RAB-10 and RAB-8 function together in regulating PVD morphogenesis, we took advantage of a newly developed CRIPSR/Cas9-mediated conditional knock-out method and specifically disrupted the function of the rab-10 gene in the PVD neuron and other descendants of the seam cell lineage by restricting Cas9 endonuclease expression using nhr-81 promoter (Pnhr-81>Cas9)[41,42]. In three separate lines, approximately 5–10% of animals (11.6% (35/303), 5.1% (4/78) and 4.9% (3/61)) targeted with conditional PVD knock-out of rab-10 (which we refer to as rab-10(cKO)) generated a PVD phenotype. Animals displaying a PVD dendrite arborization defect, showed a similar phenotype as that of rab-10 null mutants (Fig 7B). These results suggest that the CRIPSR/Cas9-mediated conditional knock-out only disrupts the rab-10 gene in the PVD lineage in a small percentage of transgenic animals, but that when it does target rab-10, it completely perturbs rab-10 function. We crossed the most penetrant rab-10(cKO) line into the rab-8 deletion mutant. We hypothesized that if rab-8 functions redundantly with rab-10, it should enhance the rab-10(cKO) PVD phenotype. From 302 animals that carried the Pnhr-81>Cas9 and PU6>rab-10-sgRNAs transgene, 27 animals (8.9%) showed severely defective PVD dendritic arbors. We measured the total number of secondary dendrites, and found that the rab-10(cKO) rab-8(tm2526) animals had fewer secondary dendrites than rab-10(cKO) alone (Fig 7E). Further, we observed that 55.6% (15/27) of rab-10(cKO) rab-8(tm2526) animals had truncated posterior primary dendrites, which was rarely observed in rab-10(cKO) or rab-8(tm2526) strains (2.8% (1/35) and 0% (0/25), respectively) (Fig 7F). Taken together these results suggest that the related GTPases RAB-8 and RAB-10 function redundantly to promote dendritic morphogenesis in the PVD neuron.
Discussion
In this study, we used the two multi-dendritic PVD neurons (PVDL and PVDR) in C. elegans, as a model system for studying dendritic arborization. We show that the small GTPase RAB-10 is required for the growth and branching of higher-order dendrites in PVD neurons. RAB-10 localizes to Golgi and early endosomes, and its loss resulted in severe dendrite arborization defects in the proximal region of PVD neurons. Furthermore, we found that mutations in several exocyst complex components, resulted in a similar dendrite morphogenesis defect. We propose that the exocyst complex is an effector of RAB-10 and promotes docking and fusion of secretory vesicles and/or recycling endosomes, which is crucial for dendritic growth and branching (Fig 8).
Fig. 8. A summary model of how RAB-10 and exocyst complex function during dendrite arborization. 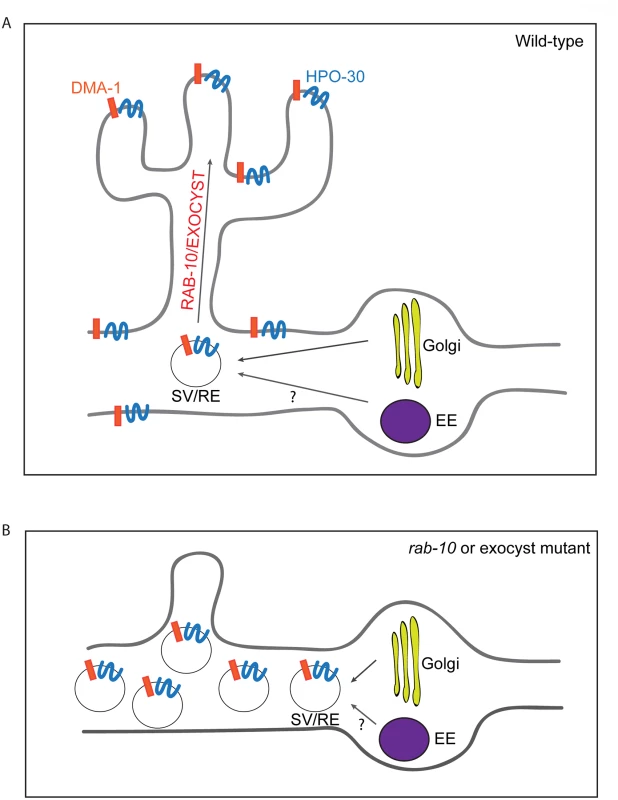
(A) In wild-type animals DMA-1 and HPO-30 are transported in vesicles from the Golgi, and the trafficking of these vesicles to the proximal dendritic plasma membraneare is mediated by RAB-10 and the exocyst complex. The RAB-10 and exocyst complex might also mediate the trafficking of recycling endosome vesicles that are important for dendrite branching and stabilization. (B) In rab-10 or exocyst mutants vesicles containing DMA-1 and HPO-30 do not traffic to the plasma membrane and accumulate in the proximal PVD dendrites. SV: secretory vesicle; RE: recycling endosome; EE: early endosome. rab-10 is a novel regulator of dendritic growth and branching
Previous work in Drosophila dendritic arborization neurons identified rab1, sec23 and sar1, three genes that mediate ER-to-Golgi traffic, as essential regulators of membrane addition during dendritic growth [1]. This study also demonstrated that laser ablation of Golgi-outposts within the dendrites reduced dendrite arborization. Together this work strongly implicated ER-to-Golgi trafficking as well as Golgi outposts as essential for dendrite morphogenesis. These findings left open the question, however, of how membrane trafficking from the Golgi to the dendritic plasma membrane is regulated. Through a candidate-based genetic screen and analysis of the C. elegans multidendritic PVD neuron we have identified the small GTPase Rab10 as a post-Golgi regulator of vesicle trafficking required for dendrite morphogenesis.
Rab10 is known to regulate both Golgi-to-plasma membrane vesicle trafficking and endocytic recycling [13,22,24–26,43–45]. Our data suggest that the C. elegans RAB-10 protein primarily promotes post-Golgi trafficking in the PVD neuron to facilitate dendritic growth and branching. We did not observe any obvious PVD dendrite morphogenesis defects after inhibiting numerous important mediators of endocytic recycling. This includes rme-1 and chat-1 mutant animals and animals carrying transgenes for dominant-negative rab-5 or rab-11.1 GTPases [30,31]. In contrast, animals expressing a dominant-negative rab-1, which regulates ER-to-Golgi trafficking, showed dramatically reduced PVD dendrite branching and growth. Furthermore, animals harboring exoc-8 and sec-8 mutations, which encode two subunits of the exocyst complex that function to dock secretory vesicles onto fusion sites on the plasma membrane, led to PVD dendrite morphogenesis defects similar to rab-10 [17]. These data strongly implicate a function for the RAB-10 protein in mediating membrane trafficking from the secretory pathway that is crucial for dendritic branching in the PVD. Since we cannot rule out the possibility that RAB-10 might also regulate recycling of endosomes in a RME-1/RAB-5/ RAB-11.1-independent manner, we suggest it is possible that RAB-10 might also contribute to PVD morphogenesis by regulating endocytic trafficking (see Fig 8).
Higher-order dendrites are sensitive to loss of RAB-10
Although loss of rab-10 resulted in a dramatic reduction in dendrite arborization in the PVD and FLP neurons, the absence of rab-10 function did not affect the growth of the PVD primary dendrites or the unbranched dendrites of the OLL, AWB and AWC neuron. This might reflect a heavy reliance on post-Golgi trafficking in the PVD and FLP neurons to supply the dramatic expansion in membrane required to form highly branched dendrites. In support of this idea, a recent study in Drosophila revealed that Rab10 and exocyst-mediated membrane trafficking is crucial for the elaborate branching of tracheal terminal cells [44]. It is also possible that rab-10 may transport cargoes that are necessary for the branching and growth of higher-order dendrites. Consistent with this notion, loss of rab-10 severely affected the dendritic transport of DMA-1 and HPO-30, two dendritic specific transmembrane proteins that are required for the branching and stabilization of higher-order dendrites.
The exocyst complex promotes fusion of RAB-10 carrier vesicles and likely functions as an effector of RAB-10
The exocyst complex is a well-characterized effector of yeast Sec4p, which shares high sequence homology with RAB-10 [26]. In yeast the exocyst complex functions to target Sec4p secretory vesicles to sites of exocytosis [35]. Suggesting a similar mechanism in the PVD dendrite, we found that loss of the exocyst components exoc-8 and sec-8 caused a dendrite morphogenesis defect that was similar to rab-10 mutants. In animals carrying mutations in these genes, GFP tagged DMA-1 and HPO-30 were sequestered in intracellular vesicles and the dendrite surface expression was greatly reduced. The similarity of the phenotypes suggests that the exocyst complex functions as an effector of RAB-10 to mediate trafficking of secretory vesicles to the dendritic plasma membrane. This idea is further supported by the colocalization of RAB-10 and EXOC-8 on intracellular vesicles in the PVD dendrites. Notably, compared to the PVD dendrite morphogenesis defects observed in the rab-10 mutants, the dendrite phenotypes in exoc-8 and sec-8 mutants were not as severe. This could indicate that another mechanism mediates tethering, docking or fusion of RAB-10 cargo vesicles to the dendritic plasma membrane. However, it is likely that the exoc-8 and sec-8 mutant animals examined were not nulls for exocyst function. While the deletion allele of exoc-8(ok2523) is thought to completely remove exoc-8 function [36], it does not lead to lethality, which is associated with loss of all other exocyst subunits. These observations suggest that loss of exoc-8 does not completely eliminate exocyst function [46]. Further, the sec-8(ok2187) homozygotes were obtained from heterozygous mothers that likely provided maternally loaded sec-8 mRNA or SEC-8 protein. Consistent with this idea, the removal of the exocyst component SEC-5 through ZF-1 tag-mediated degradation often resulted in a complete loss of dendritic branching. We thus suggest that the exocyst complex is the primary effector of RAB-10. Further, given that loss of SEC-5 lead to a more severe dendrite arborization defect than loss of RAB-10, it is likely the exocyst complex has functions outside of regulating RAB-10 vesicle trafficking (possibly RAB-8 vesicle trafficking, see below).
Distal dendrite arborization likely requires both rab-8 and rab-10
In rab-10 mutants, the growth and branching of more distal dendrites was minimally affected, suggesting that a rab-10 independent mechanism exists. Notably, expressing dominant negative RAB-10 in PVD caused both distal and proximal dendrite morphogenesis defects. A possible explanation is that dominant negative RAB-10 competes for RAB activators, such as a guanine nucleotide exchange factor (GEF), that interferes with another RAB protein that is essential for distal dendrites. A strong candidate for this other RAB protein is RAB-8. Although loss of rab-8 had no obvious effect on PVD dendrite morphogenesis, expression of a dominant negative RAB-8 in the PVD caused similar dendritic phenotype to the dominant negative RAB-10. rab-8 is related to rab-10, and rab-8 and rab-10 function redundantly in mediating secretion in C. elegans germ cells [26]. Using a newly developed CRIPSR/Cas9-mediated conditional knock-out method, we found that conditional knockout of rab-10 in a rab-8 null mutant showed stronger dendrite arborization defect than loss of rab-10 alone. These observations offer compelling evidence that RAB-10 and RAB-8 function redundantly to regulate PVD dendritic arborization.
RAB-10 and the exocyst complex are also required for dendrite development in flies and mammals
RAB-10 and the exocyst complex are evolutionarily conserved from C. elegans to humans. We observed that knock-down of rab10 or exo84 caused a significant reduction of the dendrite arbor in the Drosophila class IV dendritic arborization neuron. Knock-down of rab10, exoc84 or sec8 had no effect on the total length of the dendrites in rat cultured hippocampal neurons, but the number of dendritic spines was greatly reduced. Compared to the PVD dendrite phenotypes of C. elegans rab-10 mutants, knock-down of rab10 in Drosophila and rat neurons showed weaker dendrite morphogenesis defects. This might be due to the use of null alleles of rab-10 in our studies of C. elegans compared to the RNAi strategy that led to reductions but not complete loss of rab10 function in Drosophila and rat. Consistent with this, knock-out of sec5 in Drosophila neurons causes more severe neurite outgrowth defect [47]. Nevertheless, our results imply that rab-10 and exocyst-dependent membrane transport is a conserved mechanism used to build dendritic arbors and dendritic spines during neural development.
Dendrite morphogenesis defects are associated with many neurological and neurodevelopment disorders, including Autism spectrum disorders, Alzheimer’s disease and Parkinson’s disease [48,49]. Recovery from these diseases will rely on efficient dendrite regrowth and branching to form functional neuronal circuits. Here we identified RAB-10 and the exocyst complex as critical and conserved players during dendrite morphogenesis. Our findings reveal an evolutionarily conserved membrane transport mechanism that can efficiently supply membranes and newly synthesized transmembrane proteins to support rapid dendrite growth and morphogenesis. These findings may help in the development of new therapeutic strategies to help repair damaged neurons in human diseases and during aging.
Materials and Methods
Ethics statement
All animals were euthanized in accordance with the recommendations of the American Veterinary Medical Association and the UCSF Institutional Animal Care and Use Committee. Rats were euthanized with CO2 prior to dissection. After treatment with CO2, bilateral thoracotomy was performed to ensure the death of the animal. All experimental procedures used comply with regulations adopted by UCSF authorities with support from the Guidelines on Euthanasia of the American Veterinary Medical Association.
C. elegans genetics
C. elegans was grown on OP50 Escherichia coli-seeded nematode growth medium agar plates at 20℃ unless otherwise noted. Alleles used are as follows: Linkage group I (LGI): rab-10 (ok1494), rab-10 (dx2), exoc-8 (ok2523), sec-8 (ok2187), exoc-7 (ok2006), rab-8 (tm2526) and dma-1(tm5159). LGII: sec-5 (tm1413), sec-5(xn51), and rrf-3(pk1426). LGIV: sec-10 (tm3437), and chat-1 (ok1681). LGV: ehbp-1 (ok2140), rme-1 (b1045) and hpo-30(ok2047). Primer sequences for genotyping are available on request. RNAi experiments were performed as previously described [50], except that the rrf-3(pk1246) mutation was used to enhance the RNAi efficacy in the PVD neuron. The dma-1 RNAi clone was obtained from Vidal RNAi feeding library and verified by DNA sequencing [51].
Transgenes and molecular biology
Standard germ line transformation by gonadal micro-injection was used to generate transgenic lines. Plasmid DNAs and fusion PCR products were used at 1–20 ng/ μl, and co-injection marker unc-119 (+) or Pmyo-2>mcherry or Pmyo-3>mcherry or Punc-122>rfp or Podr-1>gfp was used at 1–30 ng/ μl. Chromosome integrated stable lines were generated by following gamma ray irradiation protocol [52]. Single copy transgene was generated by following a miniMos-based protocol [28]. Transgenes used are listed in S1 Table.
All the plasmids were constructed using standard molecular cloning methods. pPD49.26 and pPD95.75 were used as vectors. Coding regions of rab-1, rab-8 and rab-11.1 with dominant-negative mutations were amplified from a RAB toolkit [53]. Fusion PCR was performed as previously described [54]. Plasmids and fusion PCR product used are listed in S2 Table. Primers used are listed in S3 Table.
CRIPSR/Cas9-mediated conditional knock-out of rab-10
pWZ243 Pnhr-81>Cas9 (20ng/ul), pWZ170 PU6>rab-10-sgRNA #1 (target DNA sequence was 5’GAAGAGCATGTCATACGGT3’) (20ng/ul), pWZ171 PU6>rab-10-sgRNA #2 (target DNA sequence was 5’GCAATTTGAAGAGCATGTCATA3’) (20ng/ul), Pmyo-2>mcherry (1ng/ul) and Pmyo-3>mcherry (5ng/ul) were injected into wyIs592 (ser2prom3>myr-gfp) worms. Transgenic lines were identified and maintained based on fluorescence of the mCherry co-injection markers expressed in the pharyngeal muscles and body wall muscles. L4 and young adult stage transgenic animals were quantified for PVD dendrite arborization defects.
Quantification of dendritic arborization defect
To quantify the percentage of animals with abnormal PVD dendritic morphology (Table 1), mid-L4 to young adult stage hermaphrodite animals were anesthetized using 1mg/ml levamisole in M9 buffer, mounted on 2% agar pads and examined for PVD morphology using a compound fluorescence microscope (Carl Zeiss) with a 63X/1.4NA objective lens. Wild-type animals develop many menorah-like structures with higher-order dendrites. An animal was considered abnormal if it lacked more than four menorahs (either by lacking quaternary dendrites, tertiary dendrites or secondary dendrites) or if it had truncated primary dendrites.
To quantify PVD dendrite and axon morphology, z-stack images of PVD neurons were taken of mid - or late L4 stage animals using either a spinning disk confocal microscope (Axio Imager; Carl Zeiss) with a 40x objective lens (1.4 NA) equipped with an EM charge-coupled device (CCD) camera (Hamamatsu Photonics) and a spinning disc confocal scan head (CSU-10; Yokogawa Electric Corporation) controlled by Micro-Manager software with 488 and 561 laser lines (for images showed in Figs 1A–1C, 2A–2B, 3A–3B, and S2A–S2D and S3A–S3B), or using a Zeiss LSM710 confocal microscope (Carl Zeiss) with a Plan-Apochromat 40X/1.3NA objective (for images showed in Figs 4B–4D, 7A–7D, S2E–S2F, S3C–S3D, S4A–S4B, S5A–S5F, S6A–S6C and S9A–S9B). The numbers of secondary, tertiary and quaternary dendrites were quantified using maximum intensity projections generated from z-stack images using ImageJ.
Time-lapse imaging of GFP::RAB-10
Mid-L4 stage hermaphrodite animals were anesthetized using 1mg/ml levamisole in M9 buffer, mounted on 2% agar pads, and then single focus plane images for GFP::RAB-10 labeled vesicles in the primary dendrites were captured using a spinning disk confocal microscope with a 100x objective lens (1.4 NA). Images were taken 1 frame per 0.8 second for 1.6 minutes (120 frames). Movies were made using ImageJ.
Colocalization analysis
Mid-L4 stage transgenic animals were anesthetized using 1mg/ml levamisole in M9 buffer, mounted on 2% agar pads and dual color images were collected using a spinning disk confocal microscope (Axio Imager; Carl Zeiss) with either a 63x (for images showed in Figs 2D–2E and 6D–6E) or 100x (for images showed in Fig 6A) objective lens (1.4 NA). Colocalization between RAB-10 and RAB-5, FAPP1-PH and EXOC-8 was quantified using Coloc 2, a Fiji’s plugin for colocalization analysis (http://fiji.sc/Coloc_2). The Pearson correlation coefficient index is shown for each group.
Quantification of intensity of DMA-1::GFP and HPO-30::GFP
Z-stacks of ser2prom3>DMA-1::GFP and ser2prom3>HPO-30::GFP fluorescence in distal, middle and tail region of mid-L4 stage worms were taken using a spinning disk confocal microscope (Axio Imager; Carl Zeiss) with a 100x Plan-Aprochromat objective lens (1.4 NA). Fluorescence across the primary dendrites was quantified using the “measure” function of ImageJ software from single focal plane images. Background subtraction levels were determined from regions outside of the worms, lacking any GFP signal.
Quantification of DMA-1::GFP, HPO-30::GFP and GFP::RAB-10 vesicular units
Z-stacks of ser2prom3>DMA-1::GFP and ser2prom3>HPO-30::GFP fluorescence in distal, middle and tail region of mid-L4 stage worms were taken using a spinning disk confocal microscope (Axio Imager; Carl Zeiss) with a 100x Plan-Aprochromat objective lens (1.4 NA). Number of vesicular units (either single vesicles or vesicle clusters) was quantified from images of maximum intensity projections generated from z-stack images using ImageJ. The size of each image was 76.8μm x 76.8 μm.
Z-stacks of ser2prom3>GFP::RAB-10 fluorescence in middle region of mid-L4 stage worms were taken using a spinning disk confocal microscope (Axio Imager; Carl Zeiss) with a 63x Plan-Aprochromat objective lens (1.4 NA). Number of vesicular units (either single vesicles or vesicle clusters) was quantified from maximum intensity projections generated from z-stacks using ImageJ. The size of each image was 88.1μm x 88.1 μm.
Drosophila strains and quantification of the dendritic arbor of class IV neurons
The UAS-Gal4 system was used to express RNAi in the class IV neurons of Drosophila larvae. UAS-RNAi lines are available from Bloomington Stock Center: y[1] v[1]; P{y[+t7.7] v[+t1.8] = TRiP.JF02058}attP2 (BL 26289), y[1] v[1]; P{y[+t7.7] v[+t1.8] = TRiP.JF03139}attP2 (BL 28712) and from the Vienna Drosophila Resource Center: w[1118]; P{GD16778}v46791/CyO; (v46791), w[1118]; P{GD11816}v30112/TM3; (v30112). UAS - RNAi lines were crossed to ppktdGFP; ppkgal4, UAS-dcr2 virgins. Larvae resulting from this cross were imaged at third larval instar. The dendritic arbor was quantified as described [55].
shRNA-mediated knockdown in rat cultured hippocampal neurons
Hippocampal neurons cultured from E19 Long-Evans rats (Charles River Lab) were plated at a density of 200,000 neurons per 18 mm acid treated glass coverslips (Fisher) coated with 0.06 mg/ml poly-D-lysine (Sigma) and 2.5 ug/ml Laminin (Sigma). Neurons were plated using plating media (Modified Eagle Medium + 10% Fetal Bovine Serum (Hyclone), 0.45% dextrose, 0.11 mg/ml sodium pyruvate, 2mM glutamine, Reagents from UCSF cell culture facility). Cultures were transferred to maintenance media (Neurobasal Media, Invitrogen+ 0.5mM Glutamine+1X B27, Invitrogen and Penicillin/Streptomycin) 4 hours post plating. Half of the media was replaced with fresh media every 4 days.
All shRNA constructs were made in the pLentilox3.7 vector backbone using TTCAAGAGA as the loop sequence. Sequences used for constructing the shRNAs are: 1) Rab10 (NM_017359.2 Start position: 2373) “TTGACTCTATCATTGTTTA” 2) Exoc84 (NM_139043.1 Start position: 310) “CGCAGAACCTGAAGCGCAA” 3) Sec8 (NM_053875.1 Start position: 887) “CCGTTAAAGCCATTAAAGA”. ShRNA transfections were performed using Lipofectamine-2000 (Invitrogen) following manufacturer’s guidelines. Transfections were done at DIV7 and DIV14 and fixed 48 hours post transfection at DIV9 and DIV16, respectively using 4% PFA+4% sucrose at room temperature for 15 min. We did not observe toxicity or neuronal death in any of the shRNA treatment conditions. Neurons were blocked in blocking buffer (10% normal donkey serum+ 0.2M glycine+ 0.1% triton-x100 in Phosphate buffer saline) for an hour followed by overnight incubation with primary antibodies (mouse anti GFP, from Roche and rabbit anti-MAP2 from Chemicon). Coverslips were washed thrice in PBS and incubated for 2 hours with secondary antibodies (mouse Alexa 488 and Rabbit Alexa 568, from Jackson ImmunoResearch) followed by three washes in PBS before mounting onto slides for analysis using Fluromont mounting media from EMS).
Neuronal morphology quantification in rat cultured neurons
Imaging of rat hippocampal neurons was performed on Leica Sp5 scanning confocal microscope with Laser lines 488 and 561 using a 40X NA 1.25 objective with zoom 0 for dendrite length and zoom 3.0 for dendritic spine imaging. Confocal image stacks were acquired at 1024X1024 pixels with 0.4micron z spacing between two frames such that the entire depth of the neuron/dendrite was imaged. Analysis was performed on maximum projected image stacks using ImageJ. ShRNA transfected cells were identified by GFP expression. Neurite length was calculated using the ‘Measure’ function of ImageJ and dendrite density was calculated by manually counting the number of spines per 100 microns of dendrite length.
Statistical analysis
One-way ANOVA followed by post-hoc comparisons using the Dunnett’s test was used for all the figures, except S11 Fig (one-way ANOVA followed by post-hoc comparisons using the Holm-Sidak test was used in this figure). Error bar means standard error of mean (SEM) for all the figures except S11 Fig (error bar means standard deviation in this figure).
Supporting Information
Zdroje
1. Ye B, Zhang Y, Song W, Younger SH, Jan LY, et al. (2007) Growing dendrites and axons differ in their reliance on the secretory pathway. Cell 130 : 717–729. 17719548
2. Satoh D, Sato D, Tsuyama T, Saito M, Ohkura H, et al. (2008) Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat Cell Biol 10 : 1164–1171. doi: 10.1038/ncb1776 18758452
3. Lee MC, Miller EA, Goldberg J, Orci L, Schekman R (2004) Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol 20 : 87–123. 15473836
4. Lazo OM, Gonzalez A, Ascano M, Kuruvilla R, Couve A, et al. (2013) BDNF regulates Rab11-mediated recycling endosome dynamics to induce dendritic branching. J Neurosci 33 : 6112–6122. doi: 10.1523/JNEUROSCI.4630-12.2013 23554492
5. Way JC, Chalfie M (1989) The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes Dev 3 : 1823–1833. 2576011
6. Chatzigeorgiou M, Yoo S, Watson JD, Lee WH, Spencer WC, et al. (2010) Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat Neurosci 13 : 861–868. doi: 10.1038/nn.2581 20512132
7. Li W, Kang L, Piggott BJ, Feng Z, Xu XZ (2011) The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans. Nat Commun 2 : 315. doi: 10.1038/ncomms1308 21587232
8. Smith CJ, Watson JD, Spencer WC, O'Brien T, Cha B, et al. (2010) Time-lapse imaging and cell-specific expression profiling reveal dynamic branching and molecular determinants of a multi-dendritic nociceptor in C. elegans. Dev Biol 345 : 18–33. doi: 10.1016/j.ydbio.2010.05.502 20537990
9. Liu OW, Shen K (2012) The transmembrane LRR protein DMA-1 promotes dendrite branching and growth in C. elegans. Nat Neurosci 15 : 57–63.
10. Dong X, Liu OW, Howell AS, Shen K (2013) An extracellular adhesion molecule complex patterns dendritic branching and morphogenesis. Cell 155 : 296–307. doi: 10.1016/j.cell.2013.08.059 24120131
11. Salzberg Y, Diaz-Balzac CA, Ramirez-Suarez NJ, Attreed M, Tecle E, et al. (2013) Skin-derived cues control arborization of sensory dendrites in Caenorhabditis elegans. Cell 155 : 308–320. doi: 10.1016/j.cell.2013.08.058 24120132
12. Smith CJ, O'Brien T, Chatzigeorgiou M, Spencer WC, Feingold-Link E, et al. (2013) Sensory neuron fates are distinguished by a transcriptional switch that regulates dendrite branch stabilization. Neuron 79 : 266–280. doi: 10.1016/j.neuron.2013.05.009 23889932
13. Wang T, Liu Y, Xu XH, Deng CY, Wu KY, et al. (2011) Lgl1 activation of rab10 promotes axonal membrane trafficking underlying neuronal polarization. Dev Cell 21 : 431–444. doi: 10.1016/j.devcel.2011.07.007 21856246
14. Liu Y, Xu XH, Chen Q, Wang T, Deng CY, et al. (2013) Myosin Vb controls biogenesis of post-Golgi Rab10 carriers during axon development. Nat Commun 4 : 2005. doi: 10.1038/ncomms3005 23770993
15. Deng CY, Lei WL, Xu XH, Ju XC, Liu Y, et al. (2014) JIP1 mediates anterograde transport of Rab10 cargos during neuronal polarization. J Neurosci 34 : 1710–1723. doi: 10.1523/JNEUROSCI.4496-13.2014 24478353
16. Xu XH, Deng CY, Liu Y, He M, Peng J, et al. (2014) MARCKS regulates membrane targeting of Rab10 vesicles to promote axon development. Cell Res 24 : 576–594. doi: 10.1038/cr.2014.33 24662485
17. He B, Guo W (2009) The exocyst complex in polarized exocytosis. Curr Opin Cell Biol 21 : 537–542. doi: 10.1016/j.ceb.2009.04.007 19473826
18. Frische EW, Pellis-van Berkel W, van Haaften G, Cuppen E, Plasterk RH, et al. (2007) RAP-1 and the RAL-1/exocyst pathway coordinate hypodermal cell organization in Caenorhabditis elegans. EMBO J 26 : 5083–5092. 17989692
19. Babbey CM, Bacallao RL, Dunn KW (2010) Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am J Physiol Renal Physiol 299: F495–506. doi: 10.1152/ajprenal.00198.2010 20576682
20. Sano H, Eguez L, Teruel MN, Fukuda M, Chuang TD, et al. (2007) Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab 5 : 293–303. 17403373
21. Inoue M, Chang L, Hwang J, Chiang SH, Saltiel AR (2003) The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature 422 : 629–633. 12687004
22. Babbey CM, Ahktar N, Wang E, Chen CC, Grant BD, et al. (2006) Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell 17 : 3156–3175. 16641372
23. Schuck S, Gerl MJ, Ang A, Manninen A, Keller P, et al. (2007) Rab10 is involved in basolateral transport in polarized Madin-Darby canine kidney cells. Traffic 8 : 47–60. 17132146
24. Chen CC, Schweinsberg PJ, Vashist S, Mareiniss DP, Lambie EJ, et al. (2006) RAB-10 is required for endocytic recycling in the Caenorhabditis elegans intestine. Mol Biol Cell 17 : 1286–1297. 16394106
25. Glodowski DR, Chen CC, Schaefer H, Grant BD, Rongo C (2007) RAB-10 regulates glutamate receptor recycling in a cholesterol-dependent endocytosis pathway. Mol Biol Cell 18 : 4387–4396. 17761527
26. Shi A, Chen CC, Banerjee R, Glodowski D, Audhya A, et al. (2010) EHBP-1 functions with RAB-10 during endocytic recycling in Caenorhabditis elegans. Mol Biol Cell 21 : 2930–2943. doi: 10.1091/mbc.E10-02-0149 20573983
27. Arakawa H, Kudo H, Batrak V, Caldwell RB, Rieger MA, et al. (2008) Protein evolution by hypermutation and selection in the B cell line DT40. Nucleic Acids Res 36: e1. 18073192
28. Frokjaer-Jensen C, Davis MW, Sarov M, Taylor J, Flibotte S, et al. (2014) Random and targeted transgene insertion in Caenorhabditis elegans using a modified Mos1 transposon. Nat Methods 11 : 529–534. doi: 10.1038/nmeth.2889 24820376
29. Guo P, Hu T, Zhang J, Jiang S, Wang X (2010) Sequential action of Caenorhabditis elegans Rab GTPases regulates phagolysosome formation during apoptotic cell degradation. Proc Natl Acad Sci U S A 107 : 18016–18021. doi: 10.1073/pnas.1008946107 20921409
30. Grant B, Zhang Y, Paupard MC, Lin SX, Hall DH, et al. (2001) Evidence that RME-1, a conserved C. elegans EH-domain protein, functions in endocytic recycling. Nat Cell Biol 3 : 573–579. 11389442
31. Chen B, Jiang Y, Zeng S, Yan J, Li X, et al. (2010) Endocytic sorting and recycling require membrane phosphatidylserine asymmetry maintained by TAT-1/CHAT-1. PLoS Genet 6: e1001235. doi: 10.1371/journal.pgen.1001235 21170358
32. Woodman PG (2000) Biogenesis of the sorting endosome: the role of Rab5. Traffic 1 : 695–701. 11208157
33. Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG (1996) Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol 135 : 913–924. 8922376
34. Grosshans BL, Ortiz D, Novick P (2006) Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A 103 : 11821–11827. 16882731
35. Guo W, Roth D, Walch-Solimena C, Novick P (1999) The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J 18 : 1071–1080. 10022848
36. Jiu Y, Jin C, Liu Y, Holmberg CI, Jantti J (2012) Exocyst subunits Exo70 and Exo84 cooperate with small GTPases to regulate behavior and endocytic trafficking in C. elegans. PLoS One 7: e32077. doi: 10.1371/journal.pone.0032077 22389680
37. Armenti ST, Lohmer LL, Sherwood DR, Nance J (2014) Repurposing an endogenous degradation system for rapid and targeted depletion of C. elegans proteins. Development 141 : 4640–4647. doi: 10.1242/dev.115048 25377555
38. Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, et al. (1998) Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 93 : 731–740. 9630218
39. Tsuboi T, Ravier MA, Xie H, Ewart MA, Gould GW, et al. (2005) Mammalian exocyst complex is required for the docking step of insulin vesicle exocytosis. J Biol Chem 280 : 25565–25570. 15878854
40. Pereira-Leal JB, Seabra MC (2000) The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J Mol Biol 301 : 1077–1087. 10966806
41. Shen Z, Zhang X, Chai Y, Zhu Z, Yi P, et al. (2014) Conditional knockouts generated by engineered CRISPR-Cas9 endonuclease reveal the roles of coronin in C. elegans neural development. Dev Cell 30 : 625–636. doi: 10.1016/j.devcel.2014.07.017 25155554
42. Miyabayashi T, Palfreyman MT, Sluder AE, Slack F, Sengupta P (1999) Expression and function of members of a divergent nuclear receptor family in Caenorhabditis elegans. Dev Biol 215 : 314–331. 10545240
43. Lerner DW, McCoy D, Isabella AJ, Mahowald AP, Gerlach GF, et al. (2013) A Rab10-dependent mechanism for polarized basement membrane secretion during organ morphogenesis. Dev Cell 24 : 159–168. doi: 10.1016/j.devcel.2012.12.005 23369713
44. Jones TA, Nikolova LS, Schjelderup A, Metzstein MM (2014) Exocyst-mediated membrane trafficking is required for branch outgrowth in Drosophila tracheal terminal cells. Dev Biol 390 : 41–50. doi: 10.1016/j.ydbio.2014.02.021 24607370
45. Chen S, Li L, Li J, Liu B, Zhu X, et al. (2014) SEC-10 and RAB-10 coordinate basolateral recycling of clathrin-independent cargo through endosomal tubules in Caenorhabditis elegans. Proc Natl Acad Sci U S A 111 : 15432–15437. doi: 10.1073/pnas.1408327111 25301900
46. Jiu Y, Hasygar K, Tang L, Liu Y, Holmberg CI, et al. (2014) par-1, atypical pkc, and PP2A/B55 sur-6 are implicated in the regulation of exocyst-mediated membrane trafficking in Caenorhabditis elegans. G3 (Bethesda) 4 : 173–183.
47. Murthy M, Teodoro RO, Miller TP, Schwarz TL (2010) Sec5, a member of the exocyst complex, mediates Drosophila embryo cellularization. Development 137 : 2773–2783. doi: 10.1242/dev.048330 20630948
48. Einstein G, Buranosky R, Crain BJ (1994) Dendritic pathology of granule cells in Alzheimer's disease is unrelated to neuritic plaques. J Neurosci 14 : 5077–5088. 8046469
49. McNeill TH, Brown SA, Rafols JA, Shoulson I (1988) Atrophy of medium spiny I striatal dendrites in advanced Parkinson's disease. Brain Res 455 : 148–152. 3416180
50. Aguirre-Chen C, Bulow HE, Kaprielian Z (2011) C. elegans bicd-1, homolog of the Drosophila dynein accessory factor Bicaudal D, regulates the branching of PVD sensory neuron dendrites. Development 138 : 507–518. doi: 10.1242/dev.060939 21205795
51. Rual JF, Ceron J, Koreth J, Hao T, Nicot AS, et al. (2004) Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res 14 : 2162–2168. 15489339
52. Inoue T, Sherwood DR, Aspock G, Butler JA, Gupta BP, et al. (2002) Gene expression markers for Caenorhabditis elegans vulval cells. Mech Dev 119 Suppl 1: S203–209. 14516686
53. Gallegos ME, Balakrishnan S, Chandramouli P, Arora S, Azameera A, et al. (2012) The C. elegans rab family: identification, classification and toolkit construction. PLoS One 7: e49387. doi: 10.1371/journal.pone.0049387 23185324
54. Hobert O (2002) PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32 : 728–730. 11962590
55. Iyer EP, Iyer SC, Sullivan L, Wang D, Meduri R, et al. (2013) Functional genomic analyses of two morphologically distinct classes of Drosophila sensory neurons: post-mitotic roles of transcription factors in dendritic patterning. PLoS One 8: e72434. doi: 10.1371/journal.pone.0072434 23977298
Štítky
Genetika Reprodukčná medicína
Článek The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis inČlánek Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Retraction: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Signaling from Within: Endocytic Trafficking of the Robo Receptor Is Required for Midline Axon Repulsion
- A Splice Region Variant in Lowers Non-high Density Lipoprotein Cholesterol and Protects against Coronary Artery Disease
- The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis in
- A NIMA-Related Kinase Suppresses the Flagellar Instability Associated with the Loss of Multiple Axonemal Structures
- Slit-Dependent Endocytic Trafficking of the Robo Receptor Is Required for Son of Sevenless Recruitment and Midline Axon Repulsion
- Expression of Concern: Protein Under-Wrapping Causes Dosage Sensitivity and Decreases Gene Duplicability
- Mutagenesis by AID: Being in the Right Place at the Right Time
- Identification of as a Genetic Modifier That Regulates the Global Orientation of Mammalian Hair Follicles
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- Evaluating the Performance of Fine-Mapping Strategies at Common Variant GWAS Loci
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- Differential Expression of Ecdysone Receptor Leads to Variation in Phenotypic Plasticity across Serial Homologs
- Receptor Polymorphism and Genomic Structure Interact to Shape Bitter Taste Perception
- Cognitive Function Related to the Gene Acquired from an LTR Retrotransposon in Eutherians
- Critical Function of γH2A in S-Phase
- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
- Integration of Genome-Wide SNP Data and Gene-Expression Profiles Reveals Six Novel Loci and Regulatory Mechanisms for Amino Acids and Acylcarnitines in Whole Blood
- A Genome-Wide Association Study of a Biomarker of Nicotine Metabolism
- Cell Cycle Regulates Nuclear Stability of AID and Determines the Cellular Response to AID
- A Genome-Wide Association Analysis Reveals Epistatic Cancellation of Additive Genetic Variance for Root Length in
- Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
- RAB-10-Dependent Membrane Transport Is Required for Dendrite Arborization
- Basolateral Endocytic Recycling Requires RAB-10 and AMPH-1 Mediated Recruitment of RAB-5 GAP TBC-2 to Endosomes
- Dynamic Contacts of U2, RES, Cwc25, Prp8 and Prp45 Proteins with the Pre-mRNA Branch-Site and 3' Splice Site during Catalytic Activation and Step 1 Catalysis in Yeast Spliceosomes
- ARID1A Is Essential for Endometrial Function during Early Pregnancy
- Predicting Carriers of Ongoing Selective Sweeps without Knowledge of the Favored Allele
- An Interaction between RRP6 and SU(VAR)3-9 Targets RRP6 to Heterochromatin and Contributes to Heterochromatin Maintenance in
- Photoreceptor Specificity in the Light-Induced and COP1-Mediated Rapid Degradation of the Repressor of Photomorphogenesis SPA2 in Arabidopsis
- Autophosphorylation of the Bacterial Tyrosine-Kinase CpsD Connects Capsule Synthesis with the Cell Cycle in
- Multimer Formation Explains Allelic Suppression of PRDM9 Recombination Hotspots
- Rescheduling Behavioral Subunits of a Fixed Action Pattern by Genetic Manipulation of Peptidergic Signaling
- A Gene Regulatory Program for Meiotic Prophase in the Fetal Ovary
- Cell-Autonomous Gβ Signaling Defines Neuron-Specific Steady State Serotonin Synthesis in
- Discovering Genetic Interactions in Large-Scale Association Studies by Stage-wise Likelihood Ratio Tests
- The RCC1 Family Protein TCF1 Regulates Freezing Tolerance and Cold Acclimation through Modulating Lignin Biosynthesis
- The AMPK, Snf1, Negatively Regulates the Hog1 MAPK Pathway in ER Stress Response
- The Parkinson’s Disease-Associated Protein Kinase LRRK2 Modulates Notch Signaling through the Endosomal Pathway
- Multicopy Single-Stranded DNA Directs Intestinal Colonization of Enteric Pathogens
- Recurrent Domestication by Lepidoptera of Genes from Their Parasites Mediated by Bracoviruses
- Three Different Pathways Prevent Chromosome Segregation in the Presence of DNA Damage or Replication Stress in Budding Yeast
- Identification of Four Mouse Diabetes Candidate Genes Altering β-Cell Proliferation
- The Intolerance of Regulatory Sequence to Genetic Variation Predicts Gene Dosage Sensitivity
- Synergistic and Dose-Controlled Regulation of Cellulase Gene Expression in
- Genome Sequence and Transcriptome Analyses of : Metabolic Tools for Enhanced Algal Fitness in the Prominent Order Prymnesiales (Haptophyceae)
- Ty3 Retrotransposon Hijacks Mating Yeast RNA Processing Bodies to Infect New Genomes
- FUS Interacts with HSP60 to Promote Mitochondrial Damage
- Point Mutations in Centromeric Histone Induce Post-zygotic Incompatibility and Uniparental Inheritance
- Genome-Wide Association Study with Targeted and Non-targeted NMR Metabolomics Identifies 15 Novel Loci of Urinary Human Metabolic Individuality
- Outer Hair Cell Lateral Wall Structure Constrains the Mobility of Plasma Membrane Proteins
- A Large-Scale Functional Analysis of Putative Target Genes of Mating-Type Loci Provides Insight into the Regulation of Sexual Development of the Cereal Pathogen
- A Genetic Selection for Mutants Reveals an Interaction between DNA Polymerase IV and the Replicative Polymerase That Is Required for Translesion Synthesis
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



