-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Adequate Th2-Type Response Associates with Restricted Bacterial Growth in Latent Mycobacterial Infection of Zebrafish
Tuberculosis is a common and potentially lethal lung disease spread worldwide. One third of the world's population is estimated to be infected with Mycobacterium tuberculosis, yet most individuals develop a latent disease which has the potential to reactivate. Some are thought to be able to clear the infection. The current vaccine does not give adequate protection against the disease, and due to incorrect use of antibiotics, resistance to treatment has substantially increased. There is an urgent need for novel treatment approaches, such as modulation of the host's immune response. However, the ideal immune response against tuberculosis is unknown. In addition, more accurate diagnostic tools are needed for distinguishing the high risk individuals among latent patients so that treatment could be given to those that are most likely to benefit from it. In this study, we used the Mycobacterium marinum-zebrafish model to study the T cell responses in mycobacterial infection. Utilizing the natural heterogeneity of the zebrafish population, we found associations between the disease severity (bacterial load) and the type and magnitude of T cell responses. Our results on typical T cell signatures are useful as diagnostic biomarkers as well as provide new understanding needed for therapeutic approaches based on immunomodulation.
Published in the journal: Adequate Th2-Type Response Associates with Restricted Bacterial Growth in Latent Mycobacterial Infection of Zebrafish. PLoS Pathog 10(6): e32767. doi:10.1371/journal.ppat.1004190
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004190Summary
Tuberculosis is a common and potentially lethal lung disease spread worldwide. One third of the world's population is estimated to be infected with Mycobacterium tuberculosis, yet most individuals develop a latent disease which has the potential to reactivate. Some are thought to be able to clear the infection. The current vaccine does not give adequate protection against the disease, and due to incorrect use of antibiotics, resistance to treatment has substantially increased. There is an urgent need for novel treatment approaches, such as modulation of the host's immune response. However, the ideal immune response against tuberculosis is unknown. In addition, more accurate diagnostic tools are needed for distinguishing the high risk individuals among latent patients so that treatment could be given to those that are most likely to benefit from it. In this study, we used the Mycobacterium marinum-zebrafish model to study the T cell responses in mycobacterial infection. Utilizing the natural heterogeneity of the zebrafish population, we found associations between the disease severity (bacterial load) and the type and magnitude of T cell responses. Our results on typical T cell signatures are useful as diagnostic biomarkers as well as provide new understanding needed for therapeutic approaches based on immunomodulation.
Introduction
Tuberculosis (TB) is a pulmonary disease spread worldwide. It is caused by an infection with Mycobacterium tuberculosis. Only 5–10% of infected individuals develop a primary active disease while the most common outcome of infection is a latent or subclinical disease with no evident symptoms. This latent disease has the inherent ability to reactivate after years or even decades of latency and is therefore a major global threat. The existing vaccine, the Bacille Calmette-Guérin (BCG), is not entirely safe and does not confer protection against latent or reactivated TB. Current antibiotic regimens have started losing their efficacy due to the spread of antibiotic resistance genes [1]. In total, the primary active infections and reactivated infections cause 1–2 million deaths yearly, which makes M. tuberculosis the deadliest bacterium for humans [2].
The dichotomy to a latent and active tuberculosis is an over-simplification, as the infection can actually lead to a wide spectrum of disease states ranging from a well-controlled (or even cleared) latent disease to fulminant, severe forms of TB. Within the latent population, a “sub-spectrum” exists leading to differences in the risk of reactivation [3]. The pathogenesis of tuberculosis has been widely studied for decades, but as it seems that TB is not a single disease but a spectrum of different outcomes, it remains poorly understood. Better understanding on the factors that contribute to the type of TB disease is crucial for the development future treatment strategies.
The TB spectrum is likely to arise from genetic variation both in the host and in different pathogen strains as well as from environmental factors. It is known that adaptive immunity and especially T helper (Th) cells are required for controlling the disease. HIV-infected individuals are more susceptible to active and reactivated tuberculosis due to the defective T lymphocyte response [4]. Mice lacking T helper responses are hypersusceptible to TB [5]. Based on the observations that IL-12 or IFN-γ deficient mice are unable to restrict mycobacterial infection, it was initially concluded that Th1 cells are the predominant mediators of protective immunity to M. tuberculosis [6]–[8]. In mice, observations of an early Th1 response (2–3 weeks post infection, wpi) followed by a Th2 response simultaneously with the onset of a chronic phase, have led to a presumption that Th2 response is detrimental to the host by leading to a failure of Th1 response to clear the infection [9], [10]. Subsequently, it has also been shown that the lack of Th2 responses in IL-4, IL-13 or Stat-6 deficient mice does not lead to better resistance to M. tuberculosis infection but, instead, to increased bacterial burdens at later stages of infection [11]. Although the role of humoral immunity in response to mycobacterial infection is still unclear, there is evidence that Th2 responses are needed as well for optimal protective immunity [1], [12]. Despite the emerging understanding of the roles of different Th subtypes in TB immunity, it is still not known what type of Th profiles are needed at different phases of infection to provide optimal protection. In part, this is due to the lack of suitable animal models for studying the full spectrum of disease outcomes, including latency and reactivation.
Several animal models have been developed with the aim of understanding the complex pathogenesis of tuberculosis. The murine model of pulmonary TB is well standardized and has made many valuable contributions to the knowledge of the disease pathomechanisms, especially on the role of T cells as mediators of protective immunity [13]. A major constraint of the model is that mice do not develop spontaneous latency although they can restrict the bacterial growth to chronic progressive infection. One of the rare animal models developing true latency is the Cynomolgus macaque. In the macaque, a low-dose M. tuberculosis infection leads to active primary disease in 50% and latent disease in 50% of individuals [14]. In the rabbit model of latent TB, the lung bacterial burdens start declining at 4 wpi following a primary phase with limited bacterial growth. In the rabbit TB model, different outcomes of infection can be induced by using mycobacterial strains with different virulence properties [15], [16].
In addition to the mammalian models of TB, we have previously shown that infection of adult zebrafish with their natural pathogen, Mycobacterium marinum, can be used to model latent TB [17]. M. marinum is a close genetic relative of M. tuberculosis, and typically infects cold-blooded hosts, such as frogs and various freshwater and saltwater fish species [18]. M. marinum infection of zebrafish embryos has been established as an elegant model to dissect the innate mechanisms of protective host responses in active mycobacterial infection [19]–[21]. However, the full spectrum of mycobacterial disease outcomes can be observed only in the adult zebrafish, due to the full maturation of adaptive immune system after the first four weeks post fertilization [21], [22]. In the adult zebrafish model, the injection of a low dose of M. marinum (ATCC 927 type strain) into the abdominal cavity leads to a systemic infection, characterized by an initial 3–4 week phase with rapid bacterial growth, followed in most individuals by a latent phase with stable bacterial burdens. In the latently infected fish, the majority of the mycobacterial population passes into a non-replicative state, dormancy, but can be experimentally reactivated by immunosuppression [17].
The wide disease spectrum typical of mycobacterial disease results from various host - and pathogen-associated factors. It is known that genetic determinants lead to an inherent, stable preference towards either T helper 1 or T helper 2 response that varies between human individuals [23], [24]. As a starting point for our study, we hypothesized that the differences that control the T helper response might be associated with the establishment of the wide spectrum seen in TB patients and that differences in T cell polarity might be related to the progression of the disease. Taking advantage of the heterogeneity of the zebrafish population and the wide spectrum of mycobacterial disease outcomes in the zebrafish model, we set out to look for differences in T helper responses involved in regulating protective response. Finding such differences would 1) allow the use of T helper markers among latently infected individuals to distinguish between those at high or low risk of reactivation and 2) provide understanding on what type of T cell response gives the optimal protection against mycobacterial infection and allow development of novel kinds of therapeutic or preventive approaches.
Results
Expansion of T lymphocytes is associated with limited mycobacterial growth in the zebrafish
Our previous work [17] provided evidence that functional lymphocyte response is a prerequisite for latency and mycobacterial dormancy in the M. marinum infection of zebrafish. To further demonstrate the significance of lymphocyte responses in the immune defence against mycobacteria in zebrafish, we carried out adoptive transfer experiments on low-dose (21±7 cfu) M. marinum-infected rag1 (−/−) fish. Spleen and kidney marrow cells were transferred from WT or rag1 (−/−) zebrafish immunized with heat-killed M. marinum to rag1 (−/−) recipients at 2 wpi. At 4 wpi, bacterial burdens were significantly lower in the fish that received transplants from immunized WT donors, compared to the fish that received transplants from rag1 (−/−) donors (3.2×105 vs. 1.8×106). This indicates that heat-killed M. marinum-induced lymphocytes, rather than NK cells or other innate immune cells, transferred additional immune protection against M. marinum infection to rag1-deficient zebrafish. (Figure 1A).
Fig. 1. T cell numbers are higher in individuals with low bacterial loads. 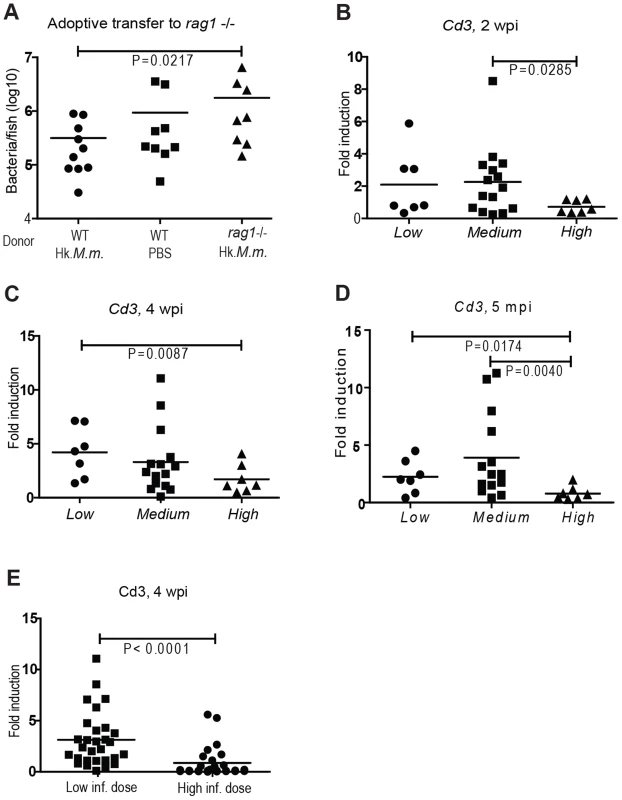
(A) In an adoptive transfer experiment, we transferred spleen and kidney cells to low-dose infected rag1 (−/−) mutant fish 12 dpi. The donors were WT immunocompetent fish treated with heat-killed M. marinum (Hk.M.m.) or PBS and rag1 (−/−) fish treated with Hk.M.m. 10 d prior to the adoptive transfer. The bacterial loads of the recipient fish were measured 4 wpi by q-PCR, n = 8–10/group. (B–D) At all time points of this study, zebrafish infected with a low dose of M. marinum (21±7 cfu) were divided in subpopulations according to the bacterial load into upper quarter (High) (n = 7), lower quarter (Low) (n = 7) and a Medium group (n = 15). The changes in the total T cell numbers were assessed in the different subpopulations of low-dose infected WT fish by measuring cd3 transcription by q-RT-PCR during a primary (2 and 4 weeks) or a late stage (5 months) mycobacterial infection (E) As a control experiment for assessing the effect of initially high bacterial load on cd3, WT zebrafish were infected with a high dose (2691±520 cfu) and the cd3 levels of this group (n = 25) were compared to those of the group (n = 30) infected with a low dose (21±7 cfu) at 4 wpi. In our current study of ∼150 individuals, a total of 10% of zebrafish were able to clear the bacterial number below the detection limit of M. marinum q-PCR-analysis (∼100 bacteria) (Figure S1B–D). These individuals capable of clearance were not detected in the groups that were collected at 2 wpi, suggesting that the clearance is likely to occur after the activation of adaptive responses. These results attest the significance of adaptive responses in the immune protection against zebrafish mycobacteriosis, and support the view of similarity of human and zebrafish anti-tuberculosis immunity.
In the current study, we aimed at further elucidating the details of adaptive immune response leading to a variety of infection outcomes. Unlike many other commonly used laboratory animals, zebrafish populations are genetically heterogeneous. This characteristic causes large variations and standard deviations in most studies utilizing this model, including our studies on mycobacterial infection. On the positive side, the zebrafish population provides starting material for studying the natural differences between individuals. To be able to elucidate the host factors affecting the outcome of infection, the variation in environmental and bacterial factors was first minimized: the bacterial strain, bacterial growth conditions, infection procedure, infection dose and housing of infected fish (water quality and temperature, feeding etc.) were carefully standardized.
WT adult zebrafish were infected with a low dose (21±7 cfu) of M. marinum, collected at various time points and divided into three subpopulations, based on their bacterial burdens. The subpopulations were named Low (25% of the population, individuals with the lowest bacterial burdens), Medium (50% of individuals) and High (25% of the population, individuals with the highest bacterial burdens, including the primary-progressive). The Low, Medium and High subpopulations were analyzed at various stages of mycobacterial disease: at 2 wpi (primary active disease), 4 wpi (the onset of latency in the majority of zebrafish) and 5 months post infection (mpi; late stage at which most individuals maintain latency). The reactivation risk of latent mycobacterial infection is thought to increase with increasing bacterial load [25]. The bacterial burdens in the subgroups at different time points are shown in Figure S1.
To dissect the differences in the total T lymphocyte numbers between the Low, Medium and High subpopulations, we quantified the cluster of differentiation (cd) 3 levels from internal organs of the zebrafish by q-RT-PCR. Zebrafish cd3 has been shown to be an ortholog of the mammalian T cell marker cd3 [26]. Here, the cd3 expression as a marker for T cell numbers in zebrafish was further validated as described in Figure S2. Induction of T cell expansion was similar in the Low and Medium groups, seen as a 2-fold induction in cd3 expression level already at 2 wpi (Figure 1B) and peaking to 4-fold around 4 wpi (Figure 1C), compared to the cd3 expression levels in non-infected zebrafish. The High group differed from the rest of the population by showing a modest T cell expansion (max. 1.7±1.3), which was only seen at 4 wpi (Figure 1C). A similar pattern remained at a late stage of the infection (5 months, Figure 1D). To assess whether the limited T cell expansion is the cause or the consequence of enhanced bacterial growth in the High group, the low-dose-infected fish were compared with a group infected with a high dose of M. marinum (2691±520 cfu). Based on our previous work [17] a high initial dose causes the bacterial load to be significantly higher than with a low initial dose during the first 2 weeks of infection and this difference will even out by 4 wpi. At 4 wpi, cd3 expression levels were significantly lower in the high-dose group (Figure 1E), suggesting that the reduced T cell numbers in the High subpopulation may at least partly be affected by the rapid bacterial growth.
Taken together, these results indicate that an early T cell expansion associates with protective response against mycobacterial infection, as the fish with highest cd3 expression levels were always found in the Low and Medium subgroups. However, individuals with modest lymphoproliferative response were found equally in all the three subgroups, suggesting that other factors besides efficient T cell expansion are required for mounting a protective response against mycobacterial infection.
Controlled mycobacterial infection is characterized by sufficient induction of Th2-type responses
As Th cells are potent orchestrators of immune responses during infection, it is reasonable to assume that in addition to total lymphocyte numbers, variation in Th response types may be an important factor underlying the wide spectrum of outcomes in mycobacterial infections. For zebrafish, antibody markers or reporter lines for FACS (fluorescence-activated cell sorting) analysis of different T lymphocyte populations are not available. To assess the Th1/Th2 balance of individuals with different infection outcomes, we measured the levels of master regulator transcription factors for Th1/Th2 lineage development, T-box transcription factor 21 (tbx21) and gata3, from the internal organs of infected zebrafish. Tbx21 is a Th1 cell transcription factor important for Th1 lineage commitment and gata3 is a well-known regulator of Th2 cell differentiation also playing a role in endothelial cell biology [27]. The central T cell transcription factors tbx21, gata3 and foxp3 have been identified in the fish [28]–[30]. The enrichment of tbx21 and gata3 in the zebrafish T cell population was validated as described in Figure S2. During infection, the alterations in the transcript levels of these transcription factors reflect the changes in the numbers of the corresponding T helper cells. The ratio of Th2/Th1 markers was used to assess the balance of T helper cell response. In addition, the induction of a Th2-type cytokine IL-4 (IL4b) [31] and a Th1-type cytokine IFN-γ (ifnγ1-2) was measured and the ratio was calculated.
At 2 wpi, the induction of both gata3 and tbx21 was significantly higher in Low and Medium than in the High group (Figure S3A&D). At this time point, there were no significant differences in the gata3/tbx21 ratio between the three groups (Figure 2A). At 4 wpi, gata3 was still significantly more induced in Low and Medium groups compared to High group (Figure S3B). However, the tbx21 levels were similar in all groups (Figure S3E). As determined by the gata3/tbx21 ratio, the Low group had developed a significantly more Th2-biased response than the High group by 4 wpi (Figure 2B), suggesting that insufficiency of Th2 cells is a differentiating factor between the Low and High individuals. The il4/ifnγ ratios generally followed a similar pattern (Figure 2D–E). Also at 2 wpi, the il4/ifnγ ratio was significantly higher in the Low group compared the other two, although there were no significant differences in the gata3/tbx21 ratio at this time point. At 2 weeks, it is likely that the adaptive Th response is in the process of maturation conducted by the cytokines excreted by innate immune cells. Similar patterns were observed at the late time point 5 months post infection (Figure S3C&F, Figure 2C&F).
Fig. 2. Controlled mycobacterial infection is characterized by Th2-type response from 4 weeks post infection. 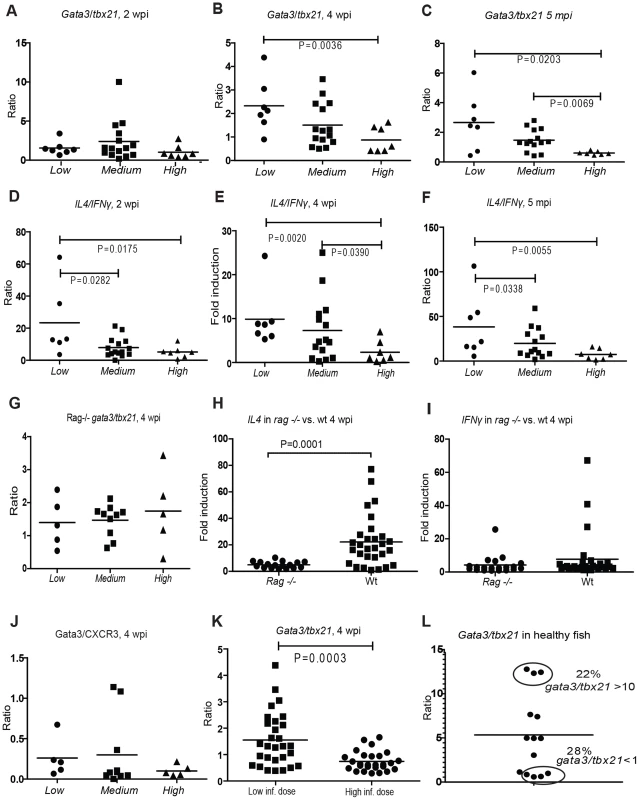
(A–C) Tbx21 (t-bet) and gata3 induction was measured in the different subpopulations at 2, 4 wpi and 5 mpi (months post infection). The gata3/tbx21 ratio was calculated to determine the dominant Th type. (D–F) The induction of selected type cytokines for Th1 (IFNγ1-2) and Th2 response (IL4b) was measured in the different subpopulations. The il4/IFNγ ratio of induction was calculated. (G–I) Rag1 (−/−) mutant zebrafish (n = 20) were infected with 35±18 cfu of M. marinum and analyzed at 4 wpi. (G) Grouping of mutant fish was carried out according to bacterial load similarly to wt fish (See Figure S1E). The association of gata3/tbx21 with the bacterial load was assessed. (H) The induction of il4 at 4 wpi was compared between wt and rag1 (−/−) fish. (I) The induction of ifnγ at 4 wpi was compared between wt and rag1 (−/−) fish. (J) Semi-quantitative western blots were carried out at 4 wpi from a population of 20 fish. Gata3 antibody was used as the Th2 marker, and CXCR3 as the Th1 marker. The bacterial loads were measured from the corresponding DNA samples to allow grouping to subpopulations. (K) As a control experiment for assessing the effect of initially high bacterial load on gata3/tbx21 ratio, WT zebrafish were infected with a high dose (2691±520 cfu) and the gata3/tbx21 ratio of this group (n = 25) was compared to those of the group (n = 30) infected with a low dose (21±7 cfu) at 4 wpi. (L) To assess the natural polarization pattern of T cells with regard to gata3/tbx21, WT zebrafish (n = 14) were stimulated by an intraperitoneal injection of heat-killed M. marinum. Gata3/tbx21 ratio was determined 10 days post injection. At 4 wpi, the Th2/Th1 balance was also assessed at the protein level by semi-quantitative Western blot analysis of gata3 and CXCR3 (a CXC chemokine receptor preferentially expressed on Th1 cells) from individuals in Low, Medium and High groups (Figure 2J, Figure S4). The results showed a similar trend as seen with q-RT-PCR analyses.
To assess the importance of functional, specific lymphocytes for the changes in the levels of the markers used in this study, we also carried out similar infection experiments in rag1 (−/−) mutants. The fish were infected with a low dose (35±18 cfu) and collected 4 wpi. We found that there was some induction of gata3, tbx21, il4 and ifnγ in the infected rag1 (−/−) mutants (Figures S2H&I and 2H&I) showing the proportion of lymphocyte-independent induction of these markers. However, the induction of gata3, tbx21 and il4 was significantly higher in the WT fish (Figures S2H&I and 2H) than in rag1 (−/−) fish at 4 wpi. This clearly demonstrates the major contribution of functional lymphocytes in the changes seen in these markers during mycobacterial infection. The rag1 (−/−) fish were grouped according to bacterial load (Figure S1E) as previously described for the WT fish, and association of the gata3/tbx21 ratio and bacterial load was assessed. In the absence of functional lymphocytes no association was detected, implying that the differences in this ratio relevant to the course of mycobacterial infection seen in WT fish are indeed derived from lymphocytes. However, the expression levels of ifnγ were similar in rag1 (−/−) and WT fish showing that the induction of this Th1-type cytokine in mycobacterial infection might not be as dependent on functional lymphocytes as the other markers used.
To assess whether the Th2/Th1 balance is directly influenced by the bacterial burden in the beginning of the infection, low-dose (21±7 cfu) infected fish were compared to fish infected with a high mycobacterial dose (2691±520 cfu) at 4 wpi. Average of gata3/tbx21 ratio was found to be lower in the high-dose infected group (Figure 2K), suggesting that rapid bacterial growth can lead to changes in this ratio. To investigate whether the differences in the disease outcome could result from genetically defined Th1/Th2 preferences, we stimulated healthy WT zebrafish by an i.p. injection of heat-killed M. marinum and 10 days later, analyzed the gata3 and tbx21 transcript levels. The spectrum of individual Th2/Th1 responses was broad, similarly to that seen in humans [23]. The gata3/tbx21 ratio varied from 0.6 to 12.8 within a group of 14 zebrafish. 22% of the individuals were substantially Th2-biased (gata3/tbx21>10), whereas 28% had a bias towards Th1 (gata3/tbx21<1) (Figure 2L). This observation of inherent Th1/Th2 phenotypes in healthy fish suggests that genetic Th1/Th2 preferences may in part lead to the development of wide disease spectrum in mycobacterial infections. Based on these results, it seems plausible that both the bacterium and the host can affect the gata3/tbx21 ratio during mycobacterial infection.
At any of the time points of the study, the average gata3/tbx21 ratio was never >1.0 in the progressive High group, whereas Th2 dominant response (average gata3/tbx21 1.5–2.7) was seen in the Low group in the primary infection. Altogether, these results show that induction of Th2-type responses during the first four weeks of mycobacterial infection are associated with controlling the bacterial growth.
In order to further confirm the reliability of our markers and to characterize the response in the different subgroups, we measured a wider selection of Th2 and Th1 signature genes at 4 wpi: Th2: interleukins il13 and il4, immunoglobulin M (IgM) constant region, V-maf musculoaponeurotic fibrosarcoma oncogene (cmaf), STAT6, St2 (Figure 3A–F) Th1: il12, interferon gamma 1-2 (IFNγ1-2), nitric oxide synthase 2b (Nos2b), tumor necrosis factor alpha (TNFα);(Figure 3G–J). It has been previously shown that the expression levels of the selected Th2 marker genes are upregulated in zebrafish in response to recombinant IL-4 treatment, thus representing a Th2-type response in the zebrafish [31]. In line with gata3 and il4 markers, there was a significantly higher induction of Th2 signature cytokines and other Th2 related genes in the Low group than in the High group (Figure 3A–F). At the same time, Th1 markers showed a somewhat higher induction in the High group (Figure 3G–J). However, the differences in Th2 markers were more indicative of the bacterial burden and the disease state than those seen in Th1 markers. The Low group showed substantial IgM induction at 4 wpi differing significantly from the rest of the population (Figure 3C). Only three antibody classes exist in zebrafish, namely IgM, IgD, and fish-specific IgZ, whereas IgG, IgA and IgE are absent [32]. Secreted tetrameric IgM is the most abundant zebrafish serum immunoglobulin [32], and is induced by Th2-mediated IL-4 signaling [31]. Taken together, these data suggest that Th2-type cytokines participate in the control of bacterial growth during the first four weeks of primary mycobacterial infection.
Fig. 3. Controlled mycobacterial infection is distinguished from progressive infection by higher induction of Th2 markers. 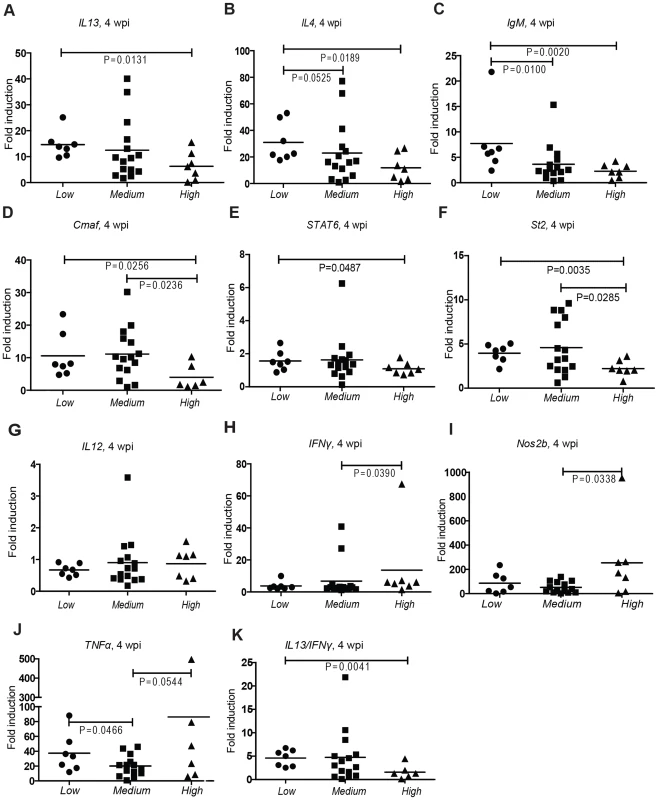
The groups with different bacterial loads were analyzed for typical Th2 (A–F) and Th1 (G–J) markers by q-RT-PCR at 4 wpi. (K) The ratio of the inductions of il13 to ifnγ was also calculated in the different subgroups at 4 wpi. Th2 dominance is beneficial for the maintenance of long-term latent mycobacterial infection
As zebrafish with higher T cell numbers and a Th2-biased response were able to control the infection most efficiently, we next wanted to study whether these protective features were relevant for the ability of maintaining long-term latency. Even though the individuals with a primary progressive disease had already been removed from the experiment, large variations in the bacterial numbers (Figure S1D) and dormancy (Figure 4A) were seen at a late time point, when the population was again divided into subgroups according to their bacterial loads at 5 mpi. The individuals with highest levels of dormancy-associated mycobacterial citrate synthase I (GltA1) expression were found in the Low group, whereas in the High group GltA1 expression levels were significantly lower than in the other groups (Figure 4A). This shows that there is a spectrum even within the “latent” population that has survived the primary infection.
Fig. 4. Low Th2/Th1 and high Treg are associated with activity of disease at late-stage mycobacterial infection. 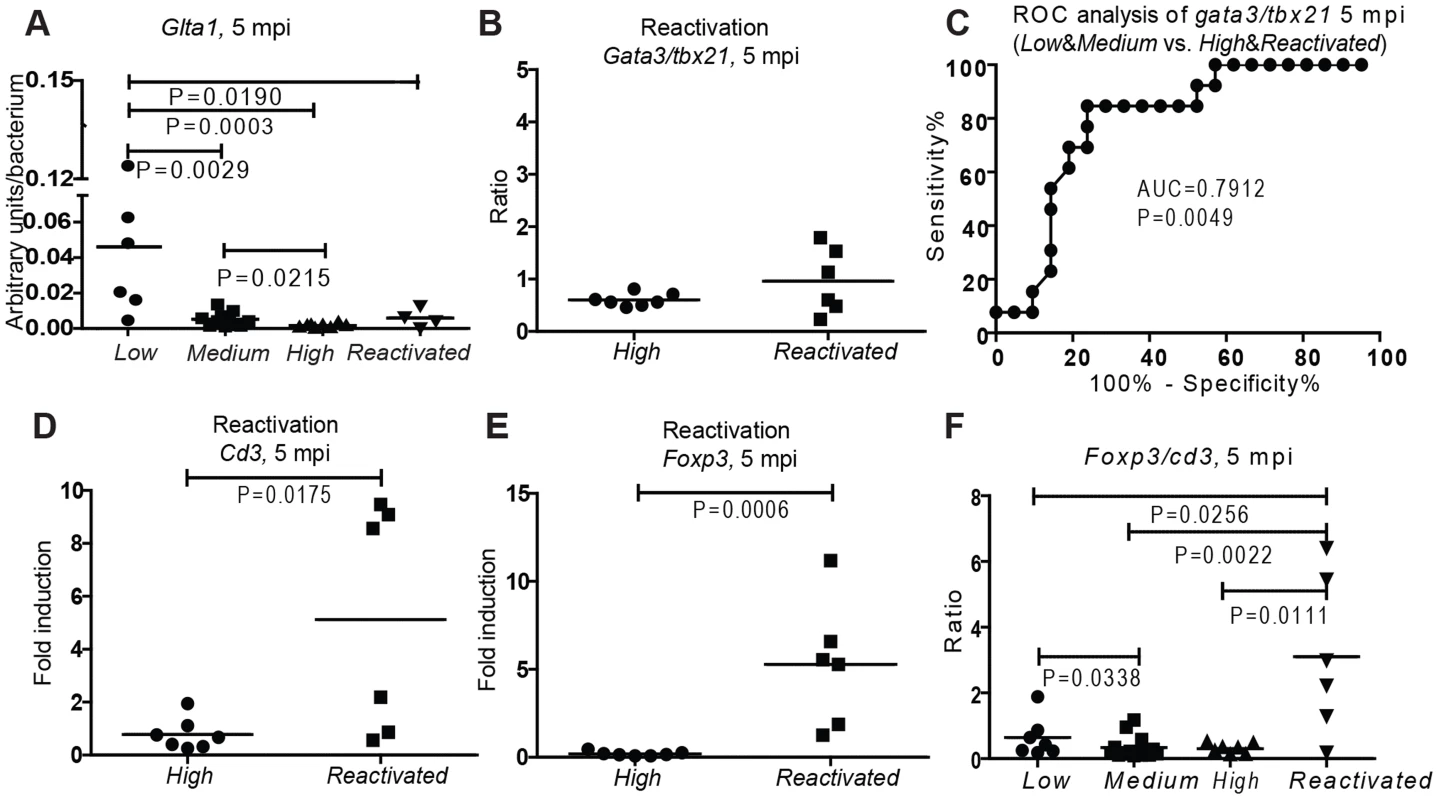
(A) The proportion of dormant bacteria in each non-stimulated subgroup was assessed by measuring the expression of a mycobacterial dormancy-associated gene GltA1. (B, D&E) The T cell inductions of Reactivated group (fish showing symptoms between 8 and 20 weeks after an initial controlled phase) and High group at 5 mpi. (C) ROC analysis of the gata3/tbx21 ratio as a biomarker to distinguish individuals with a high bacterial burden from the individuals with a lower bacterial load (AUC = area under the curve). (F) Induction of foxp3 normalized to cd3 induction in the subgroups at 5 mpi and fish showing symptoms between 2 and 5 mpi. The total T cell number still seemed to play a role at this late time point so that the High group had significantly less T cells than the other groups (Figure 1D). However, there was no significant difference in the total T cell number between the Low and Medium group. The protective role of Th2-type responses associated with lower bacterial numbers remained even at this late time point (Figures 2C&F). These results indicate the importance of high T cell numbers and a tendency to Th2-biased responses in determining how well the infection is controlled. It is plausible that the ideal response for inducing stable latency is similar to that required for maintaining long-term latency.
As we followed the large population of fish infected with a low initial dose, we saw that from 8 wpi to 5 mpi, there was a 17% overall morbidity in the population (data not shown). Following an eight-week period of asymptomatic infection, until the end of the 5 month follow-up, these spontaneously reactivated individuals were collected as they started showing symptoms of mycobacterial disease. These individuals had a slightly higher bacterial load than the fish in the High group (Figure S1D) but did not differ in terms of dormancy (Figure 4A). Thus, it is likely that the disease is active in the fish in the High group as well, although the fish did not yet show external signs of disease. Based on the gata3/tbx21 ratios, the Th2/Th1 balance was similar in the High and Reactivated groups (Figure 4B), differing significantly from the Low and Medium groups. As direct assessment of the bacterial load within a population of asymptomatic humans is difficult, measuring the Th2/Th1 balance from a blood sample could be used as a clinical biomarker for estimating the activity of the disease. We carried out a ROC-analysis of gata3/tbx21 ratio and found that gata3/tbx21 ratio had a high sensitivity and specificity as a marker in distinguishing the high load individuals (High&Reactivated) from the well-controlling population (Low&Medium)(Figure 4C).
Spontaneous reactivation is associated with pronounced foxp3 induction
We next wanted to study whether there were some differences in the T cell responses between the progressive High and Reactivated groups. In the Reactivated group, half of the individuals showed high induction of cd3. This was surprising, as within all the other groups, high cd3 was generally associated with better disease control. A possibly detrimental role of the induced T cell response in the Reactivated group could be explained by a non-ideal polarization of these cells. The most striking difference between the High and Reactivated group was the significantly higher induction of foxp3 in the Reactivated group (Figure 4E), indicating a pronounced regulatory T helper (Treg) proliferation. When the expression levels of gata3, tbx21 and foxp3 were analyzed relative to cd3 in the Reactivated, only foxp3 was significantly different between the Reactivated and High group (P = 0.0006, 10-fold higher proportion than in the High group, Figure 4E). In fact, the induction of foxp3 was higher in the Reactivated group compared to any of the asymptomatic groups (Figure 4F). This implies that the increase in foxp3 expression is higher than the increase in the total T cell number and thus, the increased proportion of foxp3 cells could be used for distinguishing Reactivated individuals from the rest of the population at a late stage of infection. The role of foxp3 positive cells in reactivation of tuberculosis and its applicability in diagnostics warrants further investigation.
Discussion
It is known that T cell responses are essential in restricting mycobacterial growth in human tuberculosis as well as in various mammalian models for studying tuberculosis. The zebrafish is a newcomer in the field of immunology, and the components of its adaptive immune system have only recently been elucidated in more detail. It has been shown [33] that there are professional antigen presenting cells capable of inducing antigen-specific T cell responses in zebrafish. In our recent publication [34], we have demonstrated the presence of specific and protective immune responses against M. marinum infection in zebrafish. We have shown that vaccination of zebrafish with the Bacillus Calmette-Guérin (BCG) increases survival of adult zebrafish from infection with M. marinum. Furthermore, vaccination of zebrafish with plasmid DNA encoding mycobacterial antigens increases survival, reduces the spreading of bacteria as well as the number of granulomas in M. marinum infection, compared to vaccination with a control pDNA lacking the antigen-encoding sequence. Rag1 (−/−) zebrafish lacking functional adaptive lymphocytes are not protected by the antigen-pDNA vaccine. In the antigen-pDNA -vaccinated zebrafish, interferon gamma expression levels are significantly higher during infection than in the control pDNA vaccinated fish, demonstrating the specificity of the anti-mycobacterial immune response induced in zebrafish.
For studies of zebrafish lymphocyte populations, antibody markers for FACS analysis are not available. To characterize the T cell responses of individuals with different infection outcomes, we measured expression levels of marker genes reflecting the total number of T cells and the Th profile from the internal organs of infected zebrafish. We also used the transgenic lck:GFP zebrafish as a tool to isolate T cells and to validate the T cell markers that were used in the study (Figure S2). We showed that the Th marker genes are enriched in sorted lck+ T cells and demonstrated a correlation between the cd3 transcript level and the total number of lck+ cells from lck:GFP reporter fish. These assessments could only be done from uninfected zebrafish, because it is likely that M. marinum infection influences the expression of the GFP reporter gene driven by the lck promoter. It has been previously shown for other pathogenic mycobacteria that one of the mycobacterial virulence strategies is to inhibit host T cell receptor signaling by interfering with the expression and phosphorylation of lck [35]–[37]. Thus, the use of the lck:GFP reporter zebrafish line as a tool to study T cell responses in M. marinum infections would first require careful assessment of the effects of the bacterium on zebrafish lck expression. Instead, we have here relied on the use of several parallel markers reflecting the different Th profiles. In the future, development of new research tools is needed for a more detailed characterization of Th responses and their role in the pathogenesis of mycobacterial infection in the zebrafish model.
The results of the current study support the view that both Th1 and Th2 responses are induced in an optimal anti-TB-response. In the well-controlling zebrafish individuals, both Th1 and Th2-type responses were efficiently induced and showed temporal and quantitative differences compared to the Th responses of progressive individuals. The individuals that are not capable of restricting bacterial growth and, subsequently, are likely to develop a progressive disease, maintain a more Th1-biased response at all stages of infection, not reflecting an excessive Th1-type response but, instead, a lack of Th2-type induction.
Our study shows that a low-dose M. marinum infection elicits different types of responses in different individuals. It is known that environmental factors, such as nutritional status or infections, influence the differentiation of T helper cells. In addition to environmental factors, it has recently been demonstrated in mice and humans that each mouse strain as well as each human individual has a genetically defined Th1/Th2 bias, and that the characteristic Th phenotype is sustained over the time [23]. Generally, genetic variation in the associated transcription factors, cytokines or cytokine receptors may define the inherent individual Th bias. It is known that there are various single nucleotide polymorphisms in the enhancer regions of human Th differentiation genes, and that these polymorphisms are related to the susceptibility to various disease states [24]. In the studied heterogeneous zebrafish population, the individual outcome of mycobacterial infection can be assumed to be affected by host genetic factors, including the inherent bias in T helper phenotype, as the variation in environmental and bacterial factors is minimal. Based on the results of this study, a Th1-type response is induced equally efficiently in both progressive and well-controlling individuals, but the lack of a Th2-type response causes the disease to progress in the (genetically) susceptible population. However, in a control experiment, in which zebrafish were infected with a high initial dose of M. marinum, we saw that the rapid growth of bacteria may also alter the Th2/Th1 balance tilting it towards Th1. Also, we saw that the high initial dose caused the total cd3 expression levels to remain low suggesting bacterium-induced T cell inhibition. In humans, it is known that M. tuberculosis can cause apoptosis of specific T cells [38] and delayed activation of CD4-positive T cells [39]. It is likely that both the bacteria and the genetic determinants of the host are capable of affecting the T cell responses in mycobacterial infection, and it is challenging to distinguish the contribution of either alone. Also, additional host factors alongside with those related T cell responses are likely to affect the disease outcome.
As latent tuberculosis exists in a major part of the human population, its spontaneous reactivation is a serious global threat. Latent tuberculosis, when not initially caused by a resistant strain, can be treated with a 9-month isoniazid monotherapy that reduces the risk of reactivation by 60–90%. However, poor treatment compliance is a common problem in treating this asymptomatic disease, as only half of the patients complete therapy [40]. The poor compliance, in turn, affects the increased antibiotic resistance to isoniazid complicating the treatment of both latent and active tuberculosis. Therefore, it would be of paramount importance to be able to recognize the small population of latently infected individuals with a higher bacterial load and to allocate the treatment to only those who are most likely to benefit from it. At a late time point, at 5 months post infection, there was a zebrafish subpopulation present with a clearly more active disease, as determined by the total bacterial load and mycobacterial dormancy gene (GltA1) expression. The fish with a higher bacterial load had a lower gata3/tbx21 ratio. Based on our results, analysis of the Th1/Th2 ratio from peripheral blood mononuclear cells could provide a correlate of activity of disease among the carriers of latent M. tuberculosis infection. The risk of reactivation is also thought to increase with increasing bacterial loads [25], and thus the Th1/Th2 ratio could have predictive value in evaluating the risk of reactivation of a latent infection. The potential of the Th1/Th2 ratio as a biomarker in the human population warrants further investigation.
The high induction of foxp3 expression in spontaneously reactivated individuals is in line with a previous human study showing that quantification of Foxp3 from antigen-induced peripheral blood mononuclear cells can be used to discriminate between latent and active TB [41]. During infection, regulatory T cells (Treg) have an important role in controlling excessive inflammation to prevent tissue damage, but at the same time, their immunosuppressive function can prevent bacterial clearance [42]. The role of Treg cells has been investigated during the early response to TB infection, and there is evidence that M. tuberculosis induces the expansion of antigen-specific Treg cells thus delaying the priming of effector T cells in the lymph nodes and the subsequent arrival of T cells to the infection site [43]. As M. tuberculosis is capable of such exploitation of the Treg response as part of its virulence strategy during the early TB infection, it is plausible that similar pathogen-driven expansion of antigen-specific Treg cells could also play a role in the reactivation of latent TB and the subsequent transmission of the disease. On the whole, the role of Treg cells in reactivation of latent TB is highly interesting and calls for further characterization.
The existence of individuals that are able to clear mycobacterial infection illustrates that the optimal immune response to fight TB has already developed during the evolution. Adaptive mechanisms underlying mycobacterial clearance have so far remained enigmatic, and their better understanding will undoubtedly provide valuable knowledge for drug and vaccine development against tuberculosis. The zebrafish model is uniquely suitable for dissecting the natural spectrum of mycobacterial infection in large scale population studies. Analysis of the protective immunity leading to the eradication of bacteria in zebrafish can provide valuable knowledge for the development of new innovative approaches to prevention and treatment of tuberculosis.
The importance of Th1-type response in controlling mycobacterial infection is generally recognized because mycobacteria are (facultative) intracellular pathogens. The general – and simplified – paradigm of the reciprocal regulation between Th1 and Th2 responses has led to the idea that Th2 response in tuberculosis might inhibit the bacterial clearance by Th1 immunity. Therefore most tuberculosis vaccines currently under development aim at promoting an efficient Th1 response and inhibiting the induction of a Th2 response [44]. In the studied zebrafish population, 10% of the individuals were able to clear the infection after the activation of adaptive responses (>2 wpi). These clearers had a similar, Th2-biased response as the other individuals in the well-controlling Low and Medium subgroups. On the other hand, inability to induce Th2 responses seems to be a trait that is associated with progressive mycobacterial infection in the zebrafish. Our finding argues against the paradigm of Th2 response not being useful for controlling tuberculosis. If this holds true in human TB, the current therapeutic and preventive approaches promoting Th1 and inhibiting Th2-type response need to be thoroughly reconsidered.
Materials and Methods
Zebrafish lines and maintenance
For most experiments, adult (5–8 month-old) wild-type AB zebrafish were used. In addition, adult, rag1 (−/−) hu1999 mutant fish and lck:GFP transgenic fish (both from ZIRC) were used. Fish were kept in a flow-through system with a light/dark cycle of 14 h/10 h and were fed with SDS 400 food twice daily.
Ethics statement
All experiments have been accepted by the Animal Experiment Board in Finland (under the Regional State Administrative Agency for Southern Finland) and were carried out in accordance with the EU-directive 2010/63/EU on the protection of animals used for scientific purposes and with the Finnish Act on Animal Experimentation (62/2006). Permit for the zebrafish facility: LSLH-2007-7254/Ym-23, Permit for experiments: ESAVI/6407/04.10.03/2012, PH1267A and ESAVI/733/04.10.07/2013.
Experimental infection
M. marinum (ATCC 927) was cultured as described in [17]. In brief, bacteria were grown at 29°C in standard mycobacterium medium 7H9 (BD) with standard additives to an OD600 of 0.495–0.680 Anesthetized fish were intraperitoneally (i.p.) injected with 5 µl of bacteria suspended in sterile PBS using an Omnican 100 30 G insulin needle (Braun, Melsungen, Germany). The bacterial dose was verified by plating on 7H10 (BD) with the standard additives. The low infection dose was 21±7 cfu and the high dose 1783±364 cfu.
Injections with heat-killed M. marinum
M. marinum (ATCC 927) was transferred from 7H10 plate into 10 ml of liquid 7H9 medium with standard additives and cultured for 3–4 days at 29°C to an OD600 of 0.490. Pelleted bacteria were resuspended in PBS corresponding to half of the original culture volume. The bacteria were heat-killed at 100°C for 20 min and thereafter homogenized for 4 min with 4000 rpm using homogenization tubes from Mobio (California, USA) and Mobio PowerLyzer24 bead beater. Samples were plated on 7H10 and LB to verify proper killing. Heat-killed bacteria were injected in a volume of 5 µl i.p using Omnican 100 30 G insulin needles (Braun).
Transplantation of lymphatic cells from AB to rag1 (−/−) fish
Kidney and spleen were collected from a euthanized AB fish in 20 µl of sterile PBS. The organs were gently homogenized by pipetting up and down ∼20 times. 10 µl of this suspension was injected i.p. into an anesthetized recipient rag1 (−/−) fish.
FACS sorting
For lymphocyte sorting experiments, lck:GFP fish were euthanized and their internal organs collected in ice-cold HBSS supplemented with 2% FBS (both from Life technologies, CA, USA). The tissue was mechanically disrupted by pipetting and passed through a 50 µm cell strainer to prepare single cell suspensions. Cells were washed twice with cold HBSS (+FBS), pelleted at 4°C, 300 g for 5 min and resuspended in 1 ml of the same buffer. 1 ml of Histopaque-1077 (Sigma-Aldrich, MO, USA) was then added under the cell suspension and lymphocytes and other mononuclear cells were enriched by centrifugation at room temperature for 20 min, 400 g. After centrifugation, the middle phase containing the target cells was transferred into a new tube, washed once and resuspended in HBSS (+FBS). Lck+ lymphocytes were sorted with FACSAria I (BD) (purity ≥95% based on GFP expression), collected by centrifugation and RNA was extracted using TRI reagent as described in [17].
q-PCR
The samples for gene expression analysis and mycobacterial quantitation were prepared using TRI reagent for DNA-RNA co-extraction (MRC, OH, USA) as previously described in [17]. RNA samples were treated with DNAse (Fermentas) according to the manufacturer's protocol. Bacterial loads were measured by q-PCR from DNA samples using SENSIFAST NO-ROX SYBR kit with M. marinum-specific primers as described in [17]. A dilution series of DNA extracted from mycobacterial culture was included in each run to allow absolute quantification. Gene expression was measured by q-RT-PCR using Bio-Rad iScript One-Step RT-PCR Kit with SYBR Green with various primers. Host genes were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or to elongation factor 1 alpha (Ef1a), and the mycobacterial dormancy gene GltA1 was normalized to the total bacterial load. q-RT-PCR results were analyzed using the ΔCt method. The induction of host genes was compared to a baseline RNA sample extracted from a pool of healthy, non-infected zebrafish and shown as fold induction compared to average normal levels. GltA1 levels are shown in arbitrary units. Gene accession numbers and primer sequences can be found in Text S1.
Protein extraction and western blotting
For Western blotting, fish were collected 4 weeks after infection with 21±7 cfu. The peritoneal cavity of the euthanized fish was emptied and the collected organs were homogenized in 1.5 ml of TRI reagent (MRC, OH, USA) using the PowerLyzer24 bead beater. RNA-DNA co-extraction was carried out as described in [17]. After DNA extraction, the remaining interphase and organic phase were used for protein extraction according to the TRI reagent manufacturer's protocol. In brief, proteins were precipitated by adding 3 volumes of acetone and pelleted at 12,000 g for 10 min at 4°C. The protein pellet was washed three times with 0.8 ml of 0.3 M guanidine hydrochloride in 95% ethanol supplemented with 2.5% glycerol (v:v) and once with 1 ml of ethanol containing 2.5% glycerol (v:v). For solubilization of the protein pellet, 0.1 ml of 1% SDS per 10 mg of tissue sample was used. For Western blotting, 40 µg of total protein was resolved on a 10% SDS-PAGE gel and blotted onto Amersham Hybond ECL nitrocellulose membrane (GE Healthcare, Little Chalfont, UK). The following primary antibodies were used: anti-Gata-3 (IN) Z-Fish (AnaSpec, California, USA), anti-CXCR-3.2 (IN) Z-Fish (AnaSpec), anti-GFP antibody NB600-303 (Novus biological, Colorado, USA). In addition, actin was detected from all the membranes with anti-actin (MAB1501) antibody (Millipore, Temecula, USA) for sample normalization. IRDye infrared secondary antibodies (LI-COR Biosciences, Nebraska, USA) and Odyssey CLx (LI-COR) were used for target protein detection and Image Studio software (LI-COR) was used for protein quantitation. A representative image of the blots showing 10 individuals can be found in the Supplementary material (Figure S4).
Statistical analysis
Statistical analysis was carried out using the GraphPad Prism software (5.02). For determination of statistical significance of differences between the different groups, a non-parametric one-tailed Mann-Whitney test was used, if not stated otherwise. P-values<0.05 were considered significant. For estimating the predictive value of gata3/tbx21 and foxp3 expression for activity of the disease a ROC analysis was carried out with a confidence interval of 95%. AUC (area under curve) value of 0.5 indicates no connection and 1.0 indicates a perfect marker.
Supporting Information
Zdroje
1. AbebeF, BjuneG (2009) The protective role of antibody responses during mycobacterium tuberculosis infection. Clinical & Experimental Immunology 157 (2) 235–243.
2. World Health Organization. (2013) Global tuberculosis report 2013. http://www.who.int/tb/publications/global_report/en/
3. BarryCE, BoshoffHI, DartoisV, DickT, EhrtS, et al. (2009) The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nature Reviews Microbiology 7 (12) 845–855.
4. HavlirDV, BarnesPF (1999) Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med 340 (5) 367–373.
5. MoguesT, GoodrichME, RyanL, LaCourseR, NorthRJ (2001) The relative importance of T cell subsets in immunity and immunopathology of airborne mycobacterium tuberculosis infection in mice. J Exp Med 193 (3) 271–280.
6. FlynnJL, ChanJ, TrieboldKJ, DaltonDK, StewartTA, et al. (1993) An essential role for interferon gamma in resistance to mycobacterium tuberculosis infection. J Exp Med 178 (6) 2249.
7. CooperAM, DaltonDK, StewartTA, GriffinJP, RussellDG, et al. (1993) Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med 178 (6) 2243–2247.
8. CooperAM, MagramJ, FerranteJ, OrmeIM (1997) Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J Exp Med 186 (1) 39–45.
9. OrmeIM, RobertsAD, GriffinJP, AbramsJS (1993) Cytokine secretion by CD4 T lymphocytes acquired in response to mycobacterium tuberculosis infection. J Immunol 151 (1) 518–525.
10. Hernandez-PandoR, OrozcoeH, SampieriA, PavonL, VelasquilloC, et al. (1996) Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology 89 (1) 26–33.
11. JungYJ, LaCourseR, RyanL, NorthRJ (2002) Evidence inconsistent with a negative influence of T helper 2 cells on protection afforded by a dominant T helper 1 response against mycobacterium tuberculosis lung infection in mice. Infect Immun 70 (11) 6436–6443.
12. Kozakiewicz L, Phuah J, Flynn J, Chan J. (2013) The role of B cells and humoral immunity in mycobacterium tuberculosis infection. In: Anonymous The New Paradigm of Immunity to Tuberculosis. Springer. pp. 225–250.
13. GuptaU, KatochV (2005) Animal models of tuberculosis. Tuberculosis 85 (5) 277–293.
14. LinPL, RodgersM, SmithL, BigbeeM, MyersA, et al. (2009) Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun 77 (10) 4631.
15. SubbianS, O'BrienP, KushnerNL, YangG, TsenovaL, et al. (2013) Molecular immunologic correlates of spontaneous latency in a rabbit model of pulmonary tuberculosis. Cell Commun Signal 11 (1) 16–811X-11-16.
16. SubbianS, TsenovaL, O'BrienP, YangG, KushnerNL, et al. (2012) Spontaneous latency in a rabbit model of pulmonary tuberculosis. The American Journal of Pathology 181 (5) 1711–1724.
17. ParikkaM, HammarénMM, HarjulaSE, HalfpennyNJ, OksanenKE, et al. (2012) Mycobacterium marinum causes a latent infection that can be reactivated by gamma irradiation in adult zebrafish. PLoS Pathogens 8 (9) e1002944.
18. KaattariI, RhodesM, KaattariS, ShottsE (2006) The evolving story of mycobacterium tuberculosis clade members detected in fish. J Fish Dis 29 (9) 509–520.
19. TakakiK, CosmaCL, TrollMA, RamakrishnanL (2012) An in vivo platform for rapid high-throughput antitubercular drug discovery. Cell Reports 2 (1) 175–84.
20. BergRD, RamakrishnanL (2012) Insights into tuberculosis from the zebrafish model. Trends Mol Med 18 (12) 689–90.
21. MeijerAH, SpainkHP (2011) Host-pathogen interactions made transparent with the zebrafish model. Curr Drug Targets 12 (7) 1000.
22. RenshawSA, TredeNS (2012) A model 450 million years in the making: Zebrafish and vertebrate immunity. Disease Models & Mechanisms 5 (1) 38–47.
23. OlsonNC, SallamR, DoyleMF, TracyRP, HuberSA (2013) T helper cell polarization in healthy people: Implications for cardiovascular disease. Journal of Cardiovascular Translational Research 6 (5) 772–786.
24. HawkinsRD, LarjoA, TripathiSK, WagnerU, LuuY, et al. (2013) Global chromatin state analysis reveals lineage-specific enhancers during the initiation of human T helper 1 and T helper 2 cell polarization. Immunity 38 (6) 1271–1284.
25. LinPL, FlynnJAL (2010) Understanding latent tuberculosis: A moving target. The Journal of Immunology 185 (1) 15.
26. YoderJA, OrcuttTM, TraverD, LitmanGW (2007) Structural characteristics of zebrafish orthologs of adaptor molecules that associate with transmembrane immune receptors. Gene 401 (1) 154–164.
27. KanhereA, HertweckA, BhatiaU, GökmenMR, PeruchaE, et al. (2012) T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nature Communications 3 : 1268.
28. WangL, ShangN, FengH, GuoQ, DaiH (2013) Molecular cloning of grass carp (ctenopharyngodon idellus) T-bet and GATA-3, and their expression profiles with IFN-γ in response to grass carp reovirus (GCRV) infection. Fish Physiol Biochem 39 (4) 793–805.
29. MitraS, AlnabulsiA, SecombesCJ, BirdS (2010) Identification and characterization of the transcription factors involved in T-cell development, t-bet, stat6 and foxp3, within the zebrafish, danio rerio. FEBS Journal 277 (1) 128–147.
30. KumariJ, BogwaldJ, DalmoRA (2009) Transcription factor GATA-3 in atlantic salmon (salmo salar): Molecular characterization, promoter activity and expression analysis. Mol Immunol 46 (15) 3099–3107.
31. ZhuLY, PanPP, FangW, ShaoJZ, XiangLX (2012) Essential role of IL-4 and IL-4Ralpha interaction in adaptive immunity of zebrafish: Insight into the origin of Th2-like regulatory mechanism in ancient vertebrates. J Immunol 188 (11) 5571–5584.
32. FillatreauS, SixA, MagadanS, CastroR, SunyerJO, et al. (2013) The astonishing diversity of ig classes and B cell repertoires in teleost fish. Frontiers in Immunology 4 : 28.
33. Lugo-VillarinoG, BallaKM, StachuraDL, BañuelosK, WerneckMBF, et al. (2010) Identification of dendritic antigen-presenting cells in the zebrafish. Proceedings of the National Academy of Sciences 107 (36) 15850.
34. OksanenKE, HalfpennyNJ, SherwoodE, HarjulaSE, HammarénMM, et al. (2013) An adult zebrafish model for preclinical tuberculosis vaccine development. Vaccine 31 (45) 5202–5209.
35. ZeaAH, OchoaMT, GhoshP, LongoDL, AlvordWG, et al. (1998) Changes in expression of signal transduction proteins in T lymphocytes of patients with leprosy. Infect Immun Feb;66 (2) 499–504.
36. KumarS, NaqviRA, KhannaN, RaoDN (2011) Disruption of HLA-DR raft, deregulations of Lck-ZAP-70-Cbl-b cross-talk and miR181a towards T cell hyporesponsiveness in leprosy. Mol Immunol 48 (9–10) 1178–90.
37. MahonRN, SandeOJ, RojasRE, LevineAD, HardingCV, et al. (2012) Mycobacterium tuberculosis ManLAM inhibits T-cell-receptor signaling by interference with ZAP-70, Lck and LAT phosphorylation. Cell Immunol 275 (1–2) 98–105.
38. HirschCS, JohnsonJL, OkweraA, KanostRA, WuM, et al. (2005) Mechanisms of apoptosis of T-cells in human tuberculosis. J Clin Immunol 25 (4) 353–364.
39. UrdahlK, ShafianiS, ErnstJ (2011) Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunology 4 (3) 288–293.
40. SmithBM, MenziesD (2011) Treatment of latent TB: First do no harm. Expert Review of Anti-Infective Therapy 9 (5) 491–493.
41. WuB, HuangC, Kato-MaedaM, HopewellPC, DaleyCL, et al. (2007) Messenger RNA expression of IL-8, FOXP3, and IL-12beta differentiates latent tuberculosis infection from disease. J Immunol 178 (6) 3688–3694.
42. BelkaidY (2008) Role of Foxp3-positive regulatory T cells during infection. Eur J Immunol 38 (4) 918–921.
43. ShafianiS, Tucker-HeardG, KariyoneA, TakatsuK, UrdahlKB (2010) Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med 207 (7) 1409–1420.
44. KaufmannSH (2013) Tuberculosis vaccines: Time to think about the next generation. 25 (2) 172–181.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA SplicingČlánek Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance GenesČlánek The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 6- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- BdlA, DipA and Induced Dispersion Contribute to Acute Virulence and Chronic Persistence of
- Morphotype Transition and Sexual Reproduction Are Genetically Associated in a Ubiquitous Environmental Pathogen
- A Nucleic-Acid Hydrolyzing Single Chain Antibody Confers Resistance to DNA Virus Infection in HeLa Cells and C57BL/6 Mice
- HopW1 from Disrupts the Actin Cytoskeleton to Promote Virulence in Arabidopsis
- Ly6C Monocytes Become Alternatively Activated Macrophages in Schistosome Granulomas with Help from CD4+ Cells
- Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA Splicing
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- Antibody Responses to : Role in Pathogenesis and Diagnosis of Encephalitis?
- Discovery of a Novel Compound with Anti-Venezuelan Equine Encephalitis Virus Activity That Targets the Nonstructural Protein 2
- Activation of Focal Adhesion Kinase by Suppresses Autophagy via an Akt/mTOR Signaling Pathway and Promotes Bacterial Survival in Macrophages
- Crossing the Interspecies Barrier: Opening the Door to Zoonotic Pathogens
- Catching Fire: , Macrophages, and Pyroptosis
- IscR Is Essential for Type III Secretion and Virulence
- Selective Chemical Inhibition of Quorum Sensing in Promotes Host Defense with Minimal Impact on Resistance
- The Glycosylated Rv1860 Protein of Inhibits Dendritic Cell Mediated TH1 and TH17 Polarization of T Cells and Abrogates Protective Immunity Conferred by BCG
- A Genome-Wide Tethering Screen Reveals Novel Potential Post-Transcriptional Regulators in
- Structural Insights into SraP-Mediated Adhesion to Host Cells
- Human IGF1 Regulates Midgut Oxidative Stress and Epithelial Homeostasis to Balance Lifespan and resistance in
- Cycling Empirical Antibiotic Therapy in Hospitals: Meta-Analysis and Models
- Rab11 Regulates Trafficking of -sialidase to the Plasma Membrane through the Contractile Vacuole Complex of
- Mitogen and Stress Activated Kinases Act Co-operatively with CREB during the Induction of Human Cytomegalovirus Immediate-Early Gene Expression from Latency
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- A Central Role for Carbon-Overflow Pathways in the Modulation of Bacterial Cell Death
- An Invertebrate Warburg Effect: A Shrimp Virus Achieves Successful Replication by Altering the Host Metabolome via the PI3K-Akt-mTOR Pathway
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
- A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees () Prevails after -Mediated, or , Transmission
- Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance Genes
- Ubiquitin-Mediated Response to Microsporidia and Virus Infection in
- Preclinical Detection of Variant CJD and BSE Prions in Blood
- Toll-Like Receptor 8 Agonist and Bacteria Trigger Potent Activation of Innate Immune Cells in Human Liver
- Progressive Proximal-to-Distal Reduction in Expression of the Tight Junction Complex in Colonic Epithelium of Virally-Suppressed HIV+ Individuals
- The Triggering Receptor Expressed on Myeloid Cells 2 Inhibits Complement Component 1q Effector Mechanisms and Exerts Detrimental Effects during Pneumococcal Pneumonia
- Differential Activation of Acid Sphingomyelinase and Ceramide Release Determines Invasiveness of into Brain Endothelial Cells
- Forward Genetic Screening Identifies a Small Molecule That Blocks Growth by Inhibiting Both Host- and Parasite-Encoded Kinases
- Defining Immune Engagement Thresholds for Control of Virus-Driven Lymphoproliferation
- Growth Factor and Th2 Cytokine Signaling Pathways Converge at STAT6 to Promote Arginase Expression in Progressive Experimental Visceral Leishmaniasis
- Multimeric Assembly of Host-Pathogen Adhesion Complexes Involved in Apicomplexan Invasion
- Biogenesis of Influenza A Virus Hemagglutinin Cross-Protective Stem Epitopes
- Adequate Th2-Type Response Associates with Restricted Bacterial Growth in Latent Mycobacterial Infection of Zebrafish
- Protective Efficacy of Passive Immunization with Monoclonal Antibodies in Animal Models of H5N1 Highly Pathogenic Avian Influenza Virus Infection
- Fructose-Asparagine Is a Primary Nutrient during Growth of in the Inflamed Intestine
- The Calcium-Dependent Protein Kinase 3 of Influences Basal Calcium Levels and Functions beyond Egress as Revealed by Quantitative Phosphoproteome Analysis
- A Translocated Effector Required for Dissemination from Derma to Blood Safeguards Migratory Host Cells from Damage by Co-translocated Effectors
- Functional Characterization of a Novel Family of Acetylcholine-Gated Chloride Channels in
- Both α2,3- and α2,6-Linked Sialic Acids on O-Linked Glycoproteins Act as Functional Receptors for Porcine Sapovirus
- The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
- MicroRNA-146a Provides Feedback Regulation of Lyme Arthritis but Not Carditis during Infection with
- Recombination in Enteroviruses Is a Biphasic Replicative Process Involving the Generation of Greater-than Genome Length ‘Imprecise’ Intermediates
- Cytoplasmic Viral RNA-Dependent RNA Polymerase Disrupts the Intracellular Splicing Machinery by Entering the Nucleus and Interfering with Prp8
- and Are Associated with Murine Susceptibility to Infection and Human Sepsis
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



