-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
A 21st Century Perspective of Poliovirus Replication
article has not abstract
Published in the journal: A 21st Century Perspective of Poliovirus Replication. PLoS Pathog 11(6): e32767. doi:10.1371/journal.ppat.1004825
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004825Summary
article has not abstract
Why Poliovirus Replication Has Been Studied for More Than 50 Years
Poliovirus is the etiologic agent of poliomyelitis, an acute flaccid paralysis affecting 1%–2% of infected patients and, on rare occasions, causing death by paralyzing muscles that control the throat or breathing. A striking feature of infection is lifelong disabilities that may affect survivors of the acute disease. Transmitted by the fecal—oral and oral—oral route, this virus (three serotypes) was one of the most feared pathogens in industrialized countries during the 20th century affecting hundreds of thousands of children every year, via outbreaks during warm summer months. Although there are highly effective vaccines to control poliomyelitis, it remains endemic in a few countries, from which spread and outbreaks continue to occur throughout the world. Since its discovery in 1908, poliovirus has been intensively studied to better understand and control this formidable pathogen. The history of poliovirus is not, however, limited to the fight against the disease. Poliovirus replication studies also have played important roles in the development of modern virology since poliovirologists and, more generally, picornavirologists have been pioneers in many domains of molecular virology. Poliovirus was, for example, the first animal RNA virus to have its complete genome sequence determined, the first RNA animal virus for which an infectious clone was constructed, and, along with the related rhinovirus, the first human virus that had its three-dimensional structure solved by X-ray crystallography. Indeed, the history of over half a century of poliovirus replication studies is marked by major discoveries, many of which are summarized here and illustrated in Fig 1.
Fig. 1. Replication cycle of poliovirus, annotated with references to key findings during the past 65 years. 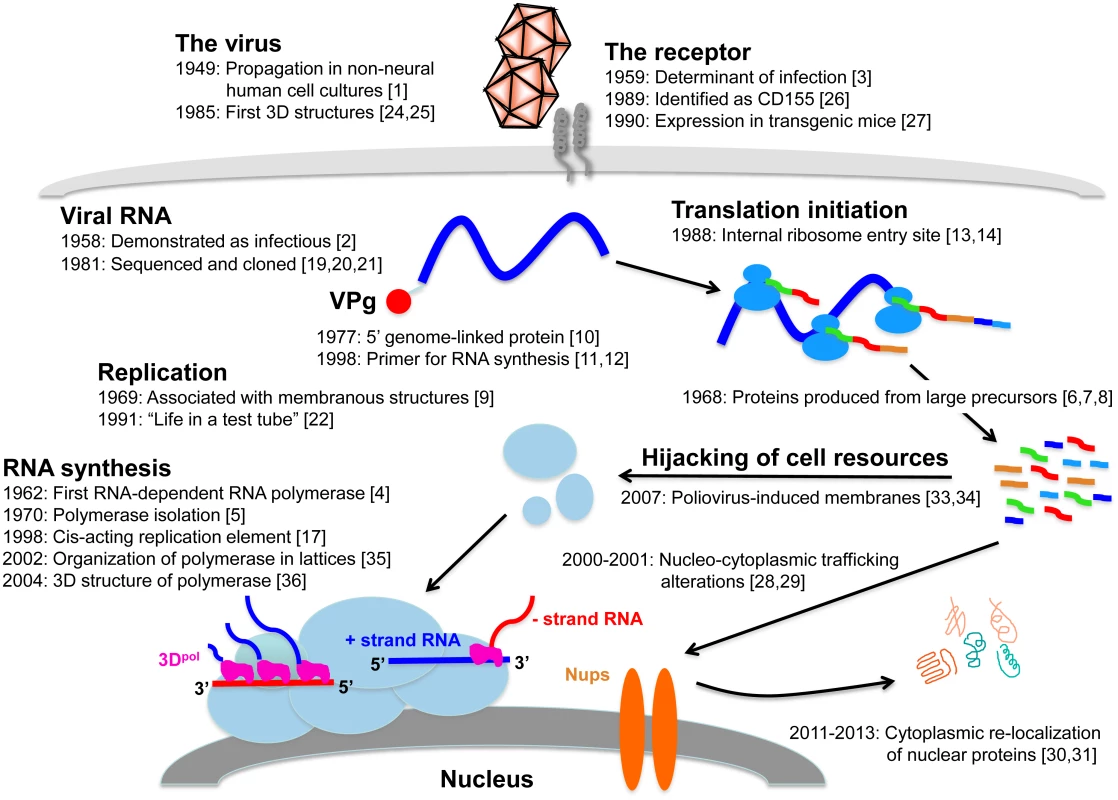
Abbreviations and symbols: 3D, three-dimensional; receptor proteins (grey cylinders spanning plasma membrane); RNA, ribonucleic acid; VPg, viral protein, genome-linked (red oval); ribosomes (medium blue oval shapes bound to viral RNA); + strand RNA, positive-sense genomic RNA (blue horizontal or wavy line);—strand RNA, negative-sense antigenomic RNA (red horizontal or wavy line); 3Dpol, viral RNA-dependent RNA polymerase (pink shapes); membranous vesicles (light blue oval shapes); Nups, nucleoporins (orange, oblong oval shapes). 1950–1970: The Early Years
In 1949, John Enders, Thomas Weller, and Frederick Robbins performed a landmark study showing that poliovirus could be propagated in cultured, non-neural human cells that did not correspond to the tissues infected during the disease [1]. Not only did these Nobel Prize-winning studies pave the way for the development of highly effective vaccines against poliovirus, but they also opened the door for virologists to study the molecular mechanisms of poliovirus replication in cultured cells that were much more readily manipulated than neural tissue. Isolated poliovirus genomic RNA was then shown to be infectious for susceptible HeLa cells in monolayers, demonstrating that the viral genome itself is the carrier of the biological activity responsible for infection [2]. John Holland and coworkers then reproduced this experiment with normally nonsusceptible cells, demonstrating that the block to poliovirus growth in nonpermissive, nonprimate cells was due to the absence of specific receptors, defining cell determinants of poliovirus infection [3].
The first evidence for an RNA-dependent RNA polymerase used by the virus for RNA genome replication was reported in 1962 by Baltimore and Franklin [4]. Until then, it was believed that the genome of RNA viruses was replicated in the cell nucleus by a cellular DNA-dependent RNA polymerase. Studies on another picornavirus, mengovirus, revealed the existence of an actinomycin D-resistant replication activity in the cytoplasm of infected cells that was later also identified in poliovirus-infected cells [4]. This virus-specific enzyme was isolated eight years later [5].
In the late 1960s, Summers and Maizel [6], and others [7,8], showed that the genomic RNA of poliovirus is translated to produce very large polypeptides that are then specifically cleaved into smaller functional proteins. This discovery was the first demonstration that picornavirus proteins were produced from large precursors proteolytically processed in infected cells [6]. At the same time, cellular fractionation studies revealed that poliovirus RNAs are synthesized in replication complexes bound to distinct membranous structures in the cytoplasm of infected cells [9].
1970–2000: The Late 20th Century
This period corresponds to several major advances in understanding the mechanisms of translation and replication of poliovirus genomic RNA. In 1977, a small protein of 22 amino acids, called VPg, was discovered to be covalently linked to the 5' end of poliovirus RNA [10]. Its absence when the virus genome is translated on polysomes indicated that VPg is removed prior to or during translation, whereas its presence at the 5’ end of the newly synthesized positive - and negative-strand RNAs suggested that VPg might be involved in the initiation of RNA synthesis [11]. This hypothesis was confirmed later by Paul et al., who demonstrated that VPg linked by the poliovirus RNA polymerase 3Dpol to the 5'-terminal uridylic acid of the virus genome is the key player in protein-primed initiation of poliovirus RNA synthesis [12]. Experiments with dicistronic vectors subsequently led to the discovery of a novel mechanism of initiation of translation through ribosome binding to RNA secondary structures within the long 5' nontranslated region of the virus genome. This internal ribosome entry site (or IRES) allows recognition by the host translation apparatus in the absence of a 5’ cap structure on viral mRNAs [13,14]. Since infection by poliovirus and many other picornaviruses leads to the shut off of cap-dependent translation [15,16], the IRES allows the virus to effectively compete for the cellular translation machinery via cap-independent mechanisms. Finally, a cis-acting replication element (or cre) was identified in picornavirus genomic RNAs. Cre sequences are RNA stem-loop structures almost exclusively located within the coding region, and they are required for viral RNA replication. These elements bind viral proteins involved in RNA replication complex formation, allowing specific recognition of viral RNAs in the cytoplasm of infected cells among a myriad of poly(A)-containing host cellular mRNAs. Cre sequences promote uridylylation of VPg, the primer for initiation of viral RNA synthesis, by 3Dpol. Their functions appear to be strand-specific, since the cre is required for positive-strand RNA synthesis but may not be essential for negative-strand RNA synthesis. A cre was first found in the capsid coding region of human rhinovirus (HRV) RNA and later in the 2C coding region of poliovirus RNA [17,18]. Since then, similar types of internal recognition elements have been detected for other positive-strand RNA viruses.
The complete sequence of poliovirus genomic RNA was reported in 1981 by two different groups [19,20]. Poliovirus RNA was shown to be more than 7,400 nucleotides long, polyadenylated at the 3' terminus, and composed of an open reading frame (ORF) of 2,207 consecutive triplets, spanning over 89% of the total nucleotide sequence. This single ORF encodes the viral polyprotein that is ultimately cleaved into more than 15 intermediate and mature viral polypeptides. Poliovirus was also the first RNA animal virus for which an infectious, cloned complementary DNA copy of the RNA genome was constructed. Transfection of this clone into mammalian cells produced infectious poliovirus and, via genetic manipulation, led to new insights about the functions of viral proteins and RNA sequences and their roles in the picornavirus intracellular replication cycle [21].
Transcribed viral RNA from a plasmid-derived cDNA was also used in vitro for the de novo synthesis of infectious poliovirus, a first for an animal RNA virus. Published in 1991 by Eckard Wimmer’s group, "Life in a Test Tube" resulted from the addition of viral RNA to a crude cytoplasmic extract of uninfected HeLa cells. These extracts contain all essential elements for poliovirus replication, including cytoplasmic membranes and components required for virion assembly [22]. Implementation of this system allowed the use of “in vitro genetics” with defined templates and fractionated extracts to understand many aspects of the virus life cycle, especially the mechanism of initiation of RNA synthesis to yield VPg-linked progeny RNAs [23].
Based upon X-ray crystallography studies, poliovirus and its relative, HRV, were the first animal viruses for which the three-dimensional virus structure was solved in 1985 by the groups of Hogle and Rossmann [24,25]. And in 1989, Racaniello and coworkers identified CD155, a member of the immunoglobulin superfamily, as the poliovirus receptor [26]. This finding was followed by the generation of mice carrying CD155 as a transgene, allowing studies of poliovirus infection and pathogenesis in vivo in a nonprimate model [27].
2000–2015: The 21st Century
Numerous studies carried out during this period uncovered the ability of poliovirus to usurp cellular components and structures for its own benefit, reflecting the necessity for this virus with a very limited coding capacity to hijack cell resources during infection. Relocalization of nuclear proteins in the cytoplasm during poliovirus infection was first reported in 2000–2001. Cleavage of nuclear pore complex components such as Nup153 and p62 by the viral 2A proteinase led to the accumulation in the cytoplasm of virus-infected cells of a number of proteins normally present in the nucleus [28,29]. These relocalized proteins often manifest RNA binding capabilities and function in host cell RNA metabolism steps. For example, the nuclear protein SRp20, a cellular splicing factor, is relocalized to the cytoplasm of poliovirus-infected human cells. It was identified as an important IRES trans-acting factor (ITAF) for poliovirus translation [30]. Another cellular protein that is redistributed in the cytoplasm during poliovirus infection is 5'-tyrosyl-DNA phosphodiesterase-2 (TDP2), a DNA repair enzyme identified as the source of VPg unlinkase activity that cleaves the protein-RNA covalent linkage of VPg at the 5’ end of virion RNA [31,32]. VPg unlinkase/TDP2 activity may be essential to provide a balance between the translation, replication, and packaging functions of viral RNA.
Poliovirus replication sites on cellular membranes (first described in 1969) were also shown to be the result of viral hijacking of components of cellular membrane metabolic pathways, leading to intracellular membrane remodeling and generation of specialized sites distinct in protein and lipid composition from that of the host cell. Belov, Altan-Bonnet, and colleagues demonstrated that viral proteins promoted membrane-binding of members of the Arf family of small guanosine triphosphatases (GTPases), leading to the formation of phosphatidylinositol-4-phosphate lipid-enriched organelles that are essential for poliovirus RNA replication [33, 34]. Indeed, these poliovirus-induced membranes contain viral RNA-dependent RNA polymerases organized in lattices of hundreds of molecules observed for the first time in 2002 by electron microscopy [35]. The two-dimensional planar and tubular oligomeric arrays of poliovirus polymerase display cooperative RNA binding and elongation. Understanding the nature of polymerase interface domains, polymerase activation, and template RNA/rNTP interactions has been greatly facilitated by the complete structure of the poliovirus RNA polymerase, which was reported in 2004 [36]. Combining our knowledge of polymerase structure, function, and genetics has also revealed novel insights into how poliovirus polymerase fidelity contributes to the quasi-species nature of RNA virus populations [37,38].
Why Poliovirus Replication Must Still Be Studied
Despite a dramatic reduction in the number of cases of poliomyelitis (350,000 cases in 1988 versus 416 cases in 2013), as well as of polio endemic countries (125 countries in 1988 versus 3 countries in 2014), poliovirus is not yet eradicated from our planet and is still the target of a massive worldwide vaccination campaign (http://www.polioeradication.org/). Moreover, vaccine-associated paralytic poliomyelitis resulting from the use of a live, attenuated vaccine complicates any poliovirus endgame strategy [39]. Finally, enteroviruses other than poliovirus have emerged as serious threats to public health. These include enterovirus 71, responsible in infants and young children for hand, foot, and mouth disease with the potential for severe central nervous system complications, and enterovirus D68, detected in children hospitalized with severe lower respiratory symptoms and asthma. In this context, there is an absolute necessity to identify potential new targets for novel antienteroviral drugs and develop new, safe, and effective vaccines. Given the plethora of cellular, molecular, and genetic tools produced during the study of poliovirus replication for more than half a century, it is paramount to continue to use these invaluable research tools to expand our knowledge of the replication and virulence determinants of emerging enteroviruses. In addition, there is still much to be learned about the pathogenic mechanisms of enteroviruses as well as the mechanistic details of poliovirus replication, particularly how the virus weaves the functions of host cell activities into a complex web of intracellular events in its reproductive cycle. Such information may be required to implement the final push that could ultimately result in the eradication of this legendary human pathogen.
Zdroje
1. Enders J. F., Weller T. H., and Robbins F. C.. 1949. Cultivation of the Lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science 109 : 85–87. 17794160
2. Alexander H. E., Koch G., Mountain I. M., and Van Damme O.. 1958. Infectivity of ribonucleic acid from poliovirus in human cell monolayers. J. Exp. Med. 108 : 493–506. 13575680
3. Holland J. J., M. L. C., and Syverton J. T.. 1959. Mammalian cell-virus relationship. III. Poliovirus production by non-primate cells exposed to poliovirus ribonucleic acid. Proc. Soc. Exp. Biol. Med. 100 : 843–845. 13645739
4. Baltimore D., and Franklin R. M.. 1962. Preliminary data on a virus-specific enzyme system responsible for the synthesis of viral RNA. Biochem. Biophys. Res. Commun. 9 : 388–392. 13966258
5. Ehrenfeld E., Maizel J. V., and Summers D. F.. 1970. Soluble RNA polymerase complex from poliovirus-infected HeLa cells. Virology 40 : 840–846. 4317360
6. Summers D. F., and Maizel J. V.. 1968. Evidence for large precursor proteins in poliovirus synthesis. Proc. Natl. Acad. Sci. U.S.A. 59 : 966–971. 4296044
7. Holland J. J., and Kiehn E. D.. 1968. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc. Natl. Acad. Sci. U.S.A. 60 : 1015–1022. 4299264
8. Jacobson M. F., and Baltimore D.. 1968. Polypeptide cleavages in the formation of poliovirus proteins. Proc. Natl. Acad. Sci. U.S.A. 61 : 77–84. 4301595
9. Caliguiri L. A., and Tamm I.. 1969. Membranous structures associated with translation and transcription of poliovirus RNA. Science 166 : 885–886. 4310189
10. Lee Y. F., Nomoto A., Detjen B. M., and Wimmer E.. 1977. A protein covalently linked to poliovirus genome RNA. Proc. Natl. Acad. Sci. U.S.A. 74 : 59–63. 189316
11. Nomoto A., Detjen B., Pozzatti R., and Wimmer E.. 1977. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature 268 : 208–213. 196204
12. Paul A. V., van Boom J. H., Filippov D., and Wimmer E.. 1998. Protein-primed RNA synthesis by purified poliovirus RNA polymerase. Nature 393 : 280–284. 9607767
13. Pelletier J., and Sonenberg N.. 1988. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 334 : 320–325. 2839775
14. Jang S. K., Krausslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., and Wimmer E.. 1988. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J. Virol. 62 : 2636–2643. 2839690
15. Fernandez-Munoz R., and Darnell J. E.. 1976. Structural difference between the 5' termini of viral and cellular mRNA in poliovirus-infected cells: possible basis for the inhibition of host protein synthesis. J. Virol. 18 : 719–726. 178904
16. Ehrenfeld E., and Lund H.. 1977. Untranslated vesicular stomatitis virus messenger RNA after poliovirus infection. Virology 80 : 297–308. 196392
17. McKnight K. L., and Lemon S. M.. 1998. The rhinovirus type 14 genome contains an internally located RNA structure that is required for viral replication. RNA. 4 : 1569–1584. 9848654
18. Goodfellow I., Chaudhry Y., Richardson A., Meredith J., Almond J. W., Barclay W., and Evans D. J.. 2000. Identification of a cis-acting replication element within the poliovirus coding region. J. Virol. 74 : 4590–4600. 10775595
19. Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S., Anderson C. W., and Wimmer E.. 1981. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature 291 : 547–553. 6264310
20. Racaniello V. R., and Baltimore D.. 1981. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc. Natl. Acad. Sci. U.S.A. 78 : 4887–4891. 6272282
21. Racaniello V. R., and Baltimore D.. 1981. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science 214 : 916–919. 6272391
22. Molla A., Paul A. V., and Wimmer E.. 1991. Cell-free, de novo synthesis of poliovirus. Science 254 : 1647–1651. 1661029
23. Barton D. J., and Flanegan J. B.. 1993. Coupled translation and replication of poliovirus RNA in vitro: synthesis of functional 3D polymerase and infectious virus. J. Virol. 67 : 822–831. 8380467
24. Hogle J. M., Chow M., and Filman D. J.. 1985. Three-dimensional structure of poliovirus at 2.9 A resolution. Science 229 : 1358–1365. 2994218
25. Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G., and et al. 1985. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature 317 : 145–153. 2993920
26. Mendelsohn C. L., Wimmer E., and Racaniello V. R.. 1989. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell 56 : 855–865. 2538245
27. Ren R. B., Costantini F., Gorgacz E. J., Lee J. J., and Racaniello V. R.. 1990. Transgenic mice expressing a human poliovirus receptor: a new model for poliomyelitis. Cell 63 : 353–362. 2170026
28. Belov G. A., Evstafieva A. G., Rubtsov Y. P., Mikitas O. V., Vartapetian A. B., and Agol V. I.. 2000. Early alteration of nucleocytoplasmic traffic induced by some RNA viruses. Virology 275 : 244–248. 10998323
29. Gustin K. E., and Sarnow P.. 2001. Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition. EMBO J. 20 : 240–249. 11226174
30. Fitzgerald K. D., and Semler B. L.. 2011. Re-localization of cellular protein SRp20 during poliovirus infection: bridging a viral IRES to the host cell translation apparatus. PLoS Pathog. 7:e1002127. doi: 10.1371/journal.ppat.1002127 21779168
31. Virgen-Slane R., Rozovics J. M., Fitzgerald K. D., Ngo T., Chou W., van der Heden van Noort G. J., Filippov D. V., Gershon P. D., and Semler B. L.. 2012. An RNA virus hijacks an incognito function of a DNA repair enzyme. Proc. Natl. Acad. Sci. U.S.A. 109 : 14634–14639. doi: 10.1073/pnas.1208096109 22908287
32. Ambros V., Pettersson R. F., and Baltimore D.. 1978. An enzymatic activity in uninfected cells that cleaves the linkage between poliovirion RNA and the 5' terminal protein. Cell 15 : 1439–1446. 215328
33. Belov G. A., Altan-Bonnet N., Kovtunovych G., Jackson C. L., Lippincott-Schwartz J., and Ehrenfeld E.. 2007. Hijacking components of the cellular secretory pathway for replication of poliovirus RNA. J. Virol. 81 : 558–567. 17079330
34. Hsu N. Y., Ilnytska O., Belov G., Santiana M., Chen Y. H., Takvorian P. M., Pau C., van der Schaar H., Kaushik-Basu N., Balla T., Cameron C. E., Ehrenfeld E., van Kuppeveld F. J., and Altan-Bonnet N.. 2010. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell 141 : 799–811. doi: 10.1016/j.cell.2010.03.050 20510927
35. Lyle J. M., Bullitt E., Bienz K., and Kirkegaard K.. 2002. Visualization and functional analysis of RNA-dependent RNA polymerase lattices. Science 296 : 2218–2222. 12077417
36. Thompson A. A., and Peersen O. B.. 2004. Structural basis for proteolysis-dependent activation of the poliovirus RNA-dependent RNA polymerase. EMBO J. 23 : 3462–3471. 15306852
37. Pfeiffer J. K., and Kirkegaard K.. 2003. A single mutation in poliovirus RNA-dependent RNA polymerase confers resistance to mutagenic nucleotide analogs via increased fidelity. Proc. Natl. Acad. Sci. U.S.A. 100 : 7289–7294. 12754380
38. Vignuzzi M., Stone J. K., Arnold J. J., Cameron C. E., and Andino R.. 2006. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature 439 : 344–348. 16327776
39. Orenstein W. A. 2015. Eradicating polio: how the world's pediatricians can help stop this crippling illness forever. Pediatrics 135 : 196–202. doi: 10.1542/peds.2014-3163 25548328
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage TraffickingČlánek An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived NeuronsČlánek Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPsČlánek Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African TrypanosomiasisČlánek Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin ReleaseČlánek HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T CellsČlánek Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 6- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Introducing “Research Matters”
- Exploring Host–Pathogen Interactions through Biological Control
- Analysis of Bottlenecks in Experimental Models of Infection
- Expected and Unexpected Features of the Newly Discovered Bat Influenza A-like Viruses
- Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage Trafficking
- Recombinant Murine Gamma Herpesvirus 68 Carrying KSHV G Protein-Coupled Receptor Induces Angiogenic Lesions in Mice
- TRIM30α Is a Negative-Feedback Regulator of the Intracellular DNA and DNA Virus-Triggered Response by Targeting STING
- Targeting Human Transmission Biology for Malaria Elimination
- Two Cdc2 Kinase Genes with Distinct Functions in Vegetative and Infectious Hyphae in
- An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived Neurons
- Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPs
- Virulence Factors of Induce Both the Unfolded Protein and Integrated Stress Responses in Airway Epithelial Cells
- Peptide-MHC-I from Endogenous Antigen Outnumber Those from Exogenous Antigen, Irrespective of APC Phenotype or Activation
- Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis
- An Ultrasensitive Mechanism Regulates Influenza Virus-Induced Inflammation
- The Role of Human Transportation Networks in Mediating the Genetic Structure of Seasonal Influenza in the United States
- Host Delivery of Favorite Meals for Intracellular Pathogens
- Complement-Opsonized HIV-1 Overcomes Restriction in Dendritic Cells
- Inter-Seasonal Influenza is Characterized by Extended Virus Transmission and Persistence
- A Critical Role for CLSP2 in the Modulation of Antifungal Immune Response in Mosquitoes
- Twilight, a Novel Circadian-Regulated Gene, Integrates Phototropism with Nutrient and Redox Homeostasis during Fungal Development
- Surface-Associated Lipoproteins Link Virulence to Colitogenic Activity in IL-10-Deficient Mice Independent of Their Expression Levels
- Latent Membrane Protein LMP2A Impairs Recognition of EBV-Infected Cells by CD8+ T Cells
- Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains
- Neuronal Subtype and Satellite Cell Tropism Are Determinants of Varicella-Zoster Virus Virulence in Human Dorsal Root Ganglia Xenografts
- Molecular Basis for the Selective Inhibition of Respiratory Syncytial Virus RNA Polymerase by 2'-Fluoro-4'-Chloromethyl-Cytidine Triphosphate
- Structure of the Virulence Factor, SidC Reveals a Unique PI(4)P-Specific Binding Domain Essential for Its Targeting to the Bacterial Phagosome
- Activated Brain Endothelial Cells Cross-Present Malaria Antigen
- Fungal Morphology, Iron Homeostasis, and Lipid Metabolism Regulated by a GATA Transcription Factor in
- Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin Release
- A Macrophage Subversion Factor Is Shared by Intracellular and Extracellular Pathogens
- A Novel AT-Rich DNA Recognition Mechanism for Bacterial Xenogeneic Silencer MvaT
- Reovirus FAST Proteins Drive Pore Formation and Syncytiogenesis Using a Novel Helix-Loop-Helix Fusion-Inducing Lipid Packing Sensor
- The Role of ExoS in Dissemination of during Pneumonia
- IRF-5-Mediated Inflammation Limits CD8 T Cell Expansion by Inducing HIF-1α and Impairing Dendritic Cell Functions during Infection
- Discordant Impact of HLA on Viral Replicative Capacity and Disease Progression in Pediatric and Adult HIV Infection
- Crystal Structure of USP7 Ubiquitin-like Domains with an ICP0 Peptide Reveals a Novel Mechanism Used by Viral and Cellular Proteins to Target USP7
- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- HPV16 Down-Regulates the Insulin-Like Growth Factor Binding Protein 2 to Promote Epithelial Invasion in Organotypic Cultures
- The νSaα Specific Lipoprotein Like Cluster () of . USA300 Contributes to Immune Stimulation and Invasion in Human Cells
- RSV-Induced H3K4 Demethylase KDM5B Leads to Regulation of Dendritic Cell-Derived Innate Cytokines and Exacerbates Pathogenesis
- Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular
- Border Patrol Gone Awry: Lung NKT Cell Activation by Exacerbates Tularemia-Like Disease
- The Curious Road from Basic Pathogen Research to Clinical Translation
- From Cell and Organismal Biology to Drugs
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
- A 21st Century Perspective of Poliovirus Replication
- Is Development of a Vaccine against Feasible?
- Waterborne Viruses: A Barrier to Safe Drinking Water
- Battling Phages: How Bacteria Defend against Viral Attack
- Archaea in and on the Human Body: Health Implications and Future Directions
- Degradation of Human PDZ-Proteins by Human Alphapapillomaviruses Represents an Evolutionary Adaptation to a Novel Cellular Niche
- Natural Variants of the KPC-2 Carbapenemase have Evolved Increased Catalytic Efficiency for Ceftazidime Hydrolysis at the Cost of Enzyme Stability
- Potent Cell-Intrinsic Immune Responses in Dendritic Cells Facilitate HIV-1-Specific T Cell Immunity in HIV-1 Elite Controllers
- The Mammalian Cell Cycle Regulates Parvovirus Nuclear Capsid Assembly
- Host Reticulocytes Provide Metabolic Reservoirs That Can Be Exploited by Malaria Parasites
- The Proteome of the Isolated Containing Vacuole Reveals a Complex Trafficking Platform Enriched for Retromer Components
- NK-, NKT- and CD8-Derived IFNγ Drives Myeloid Cell Activation and Erythrophagocytosis, Resulting in Trypanosomosis-Associated Acute Anemia
- Successes and Challenges on the Road to Cure Hepatitis C
- BRCA1 Regulates IFI16 Mediated Nuclear Innate Sensing of Herpes Viral DNA and Subsequent Induction of the Innate Inflammasome and Interferon-β Responses
- A Structural and Functional Comparison Between Infectious and Non-Infectious Autocatalytic Recombinant PrP Conformers
- Phosphorylation of the Peptidoglycan Synthase PonA1 Governs the Rate of Polar Elongation in Mycobacteria
- Human Immunodeficiency Virus Type 1 Nef Inhibits Autophagy through Transcription Factor EB Sequestration
- Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
- EBV BART MicroRNAs Target Multiple Pro-apoptotic Cellular Genes to Promote Epithelial Cell Survival
- Single-Cell and Single-Cycle Analysis of HIV-1 Replication
- TRIM32 Senses and Restricts Influenza A Virus by Ubiquitination of PB1 Polymerase
- The Herpes Simplex Virus Protein pUL31 Escorts Nucleocapsids to Sites of Nuclear Egress, a Process Coordinated by Its N-Terminal Domain
- Host Transcriptional Response to Influenza and Other Acute Respiratory Viral Infections – A Prospective Cohort Study
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- Battling Phages: How Bacteria Defend against Viral Attack
- A 21st Century Perspective of Poliovirus Replication
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



