-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Genetic Dissection of the Female Head Transcriptome Reveals Widespread Allelic Heterogeneity
For traits with complex genetic inheritance it has generally proven very difficult to identify the majority of the specific causative variants involved. A range of hypotheses have been put forward to explain this so-called “missing heritability”. One idea—allelic heterogeneity, where genes each harbor multiple different causative variants—has received little attention, because it is difficult to detect with most genetic mapping designs. Here we make use of a panel of Drosophila melanogaster lines derived from multiple founders, allowing us to directly test for the presence of multiple alleles at a large set of genetic loci influencing gene expression. We find that the vast majority of loci harbor more than two functional alleles, demonstrating extensive allelic heterogeneity at the level of gene expression and suggesting that such heterogeneity is an important factor determining the genetic basis of complex trait variation in general.
Published in the journal: Genetic Dissection of the Female Head Transcriptome Reveals Widespread Allelic Heterogeneity. PLoS Genet 10(5): e32767. doi:10.1371/journal.pgen.1004322
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004322Summary
For traits with complex genetic inheritance it has generally proven very difficult to identify the majority of the specific causative variants involved. A range of hypotheses have been put forward to explain this so-called “missing heritability”. One idea—allelic heterogeneity, where genes each harbor multiple different causative variants—has received little attention, because it is difficult to detect with most genetic mapping designs. Here we make use of a panel of Drosophila melanogaster lines derived from multiple founders, allowing us to directly test for the presence of multiple alleles at a large set of genetic loci influencing gene expression. We find that the vast majority of loci harbor more than two functional alleles, demonstrating extensive allelic heterogeneity at the level of gene expression and suggesting that such heterogeneity is an important factor determining the genetic basis of complex trait variation in general.
Introduction
Uncovering the genetic basis of quantitative phenotypes is a central, yet unresolved problem in biology. There is a major discrepancy between the heritability estimates of most quantitative traits and the amount of heritable variation accounted for by all variants localized to a causative site. This phenomenon is often referred to as the “missing heritability” problem. Several hypotheses have been offered as possible explanations, including widespread epistasis [1], the infinitesimal model (many, very small effect loci influencing the phenotype of interest that are difficult to detect statistically) [2]–[4], rare alleles of large effect, that are also statistically difficult to detect [5]–[7], and widespread allelic heterogeneity (many independent effects segregating at each causative locus) [7]. This quest to understand the genetic basis of complex traits has given rise to a community-based strategy of creating freely-available genetic resource populations in model organisms such as mice [8]–[10], Arabidopsis thaliana [11], [12], maize [13]–[16], and Drosophila melanogaster [17]–[20]. Those organisms with the greatest genetic resources and with a community of researchers focused on a single system provide a logical starting point toward finding the missing heritability associated with quantitative phenotypes. In addition, the experimental designs of some of these resources are well suited to test different hypotheses for the sources of missing heritability. For example, Bloom et al. [21] used a large segregant pool from a two line yeast cross to demonstrate that epistasis is not a major contributor to the heritability of most traits. In particular, resources that have a well-defined multi-haplotype structure can be used to identify the extent of allelic heterogeneity [22] owing to the ability to estimate trait means for each haplotype at each mapped QTL. By focusing effort on these community resources, the hope is that we will gain a better understanding of the causes of missing heritability problem.
Much of the genetic variation underlying whole organism phenotypes is thought to be due to regulatory variation, i.e., variants influencing gene expression [23]–[26]. Causative loci are linked to whole organism phenotypes through the transcriptome, an interrelated network of transcripts whose abundances influence the resulting phenotype. The transcript abundances of most genes are quantitative traits themselves and have heritabilities comparable to typical whole-organism phenotypes [24], [26], [27]. Increasingly, expression quantitative trait locus (eQTL) mapping is being used to identify the source of genetic variation in transcript abundances with the ultimate goal of linking variation at the nucleotide level to variation in gene expression and to variation in visible phenotypes. Expression QTL studies have shown that most genes have local (cis) eQTL that tend to be located near the transcription start site and to be of fairly large effect. Distant regulatory effects (trans-eQTL) are more difficult to identify, likely because they are more numerous and are of smaller average effect, leaving a great deal of variation in transcript abundance unexplained [23], [24], [26], [27]. There is a growing movement toward identifying the causative quantitative trait nucleotides (QTN) underlying cis-eQTL, often with the assumption there is a single causative site [28]–[30]. However, if most eQTL harbor allelic heterogeneity [31], identifying a single causative variant will cause researchers to miss a significant portion of the genetic variation [7].
Here we describe transcriptome-wide mapping in female head tissue in the Drosophila Synthetic Population Resource (DSPR) [17], [18], one of the major genetic reference panels in the Drosophila model system. Our goals are two-fold. First, we aim to provide a comprehensive map of cis - and trans-eQTL for female head tissue in the DSPR. A key advantage of genetic reference panels is the potential to integrate phenotypes measured at multiple levels on genetically identical individuals. Incorporating eQTL data with visible phenotype data can increase mapping power and help users identify candidate genes [9], [23], [25], [32]. Second, we use the large set of discovered eQTL to quantify the number of alleles segregating at each causative locus, providing an evaluation of the degree of allelic heterogeneity at both cis - and trans-eQTL. The hypothesis that allelic heterogeneity is prevalent in quantitative traits has not been tested directly, in part because it is difficult to do so using a genome-wide association (GWAS) framework. Within loci, linkage disequilibrium makes it very difficult to distinguish between two SNPs tagging two independent causative sites versus a single causative site. In addition, the step-wise regression approaches used, for example [2], [33], to identify multiple SNPs in a gene region associated with a phenotype lack power. The result is that the majority of GWAS that have identified multiple SNPs at a single locus using conditional analysis rarely identify more than two such SNPs despite very large sample sizes e.g. [2] but see [33]. In contrast, mapping in the DSPR and other multi-parental advanced generation intercross mapping panels take a haplotype based approach, providing a natural way to distinguish between multiple alleles at each QTL and a way to ascertain the potential contribution of allelic heterogeneity to the missing heritability problem.
Results and Discussion
We mapped genome-wide expression variation using trans-heterozygote F1 individuals from 596 crosses between DSPR population A (pA) females and population B (pB) males, thus avoiding mapping variation for inbreeding depression. Gene expression was assayed using Nimblegen 12×135 K arrays, and we analyzed the resulting data using a custom data analysis pipeline (see methods) to identify all significant eQTL.
The female head eQTL map
We identified a total of 7922 eQTLs corresponding to 7850 transcripts out of a total of 11064 transcripts tested (Figure 1). Details for all eQTLs are in Table S1. Of these, 7704 transcripts were associated with a single cis-eQTL, 71 were associated with both cis - and trans-eQTL, and 75 were associated with only trans-eQTL. A small percentage of eQTLs (∼7%; Table 1) were associated with only a single recombinant inbred line (RIL) population (pA or pB; see methods), but for most eQTL fitting both pA and pB was necessary to explain the eQTL signal, indicating that causative variants were present in both populations.
Fig. 1. The locations of all mapped eQTL. 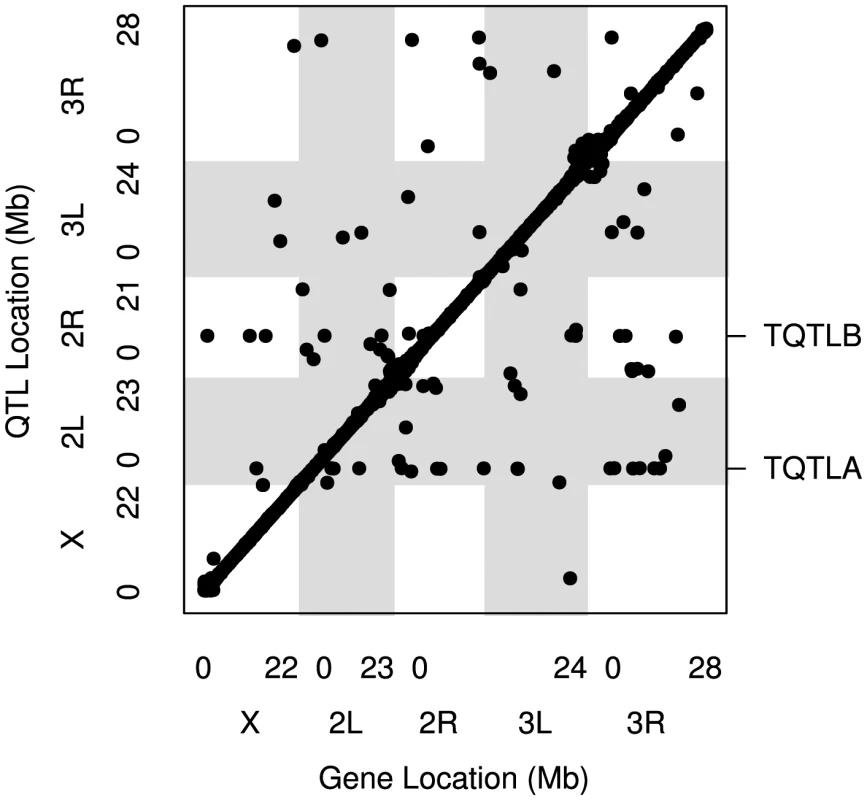
The location of the transcripts whose expression measures are mapped are along the x axis and the location of the corresponding eQTL peaks are along the y axis. Points falling along the diagonal indicate eQTL mapping to the same location as transcripts (cis) while those off the diagonal map to different locations (trans). The clusters of points near each centromere are within 1.5 cM of the target gene (cis) but are further away in physical distance due to the low recombination rate and lower mapping resolution in this region. Grey and white shading denote the different chromosome arms. The two trans hotspots we identified are labeled on the right axis (See Figure 4). Tab. 1. Numbers of <i>cis</i>- and <i>trans</i>-eQTL mapped for different models. 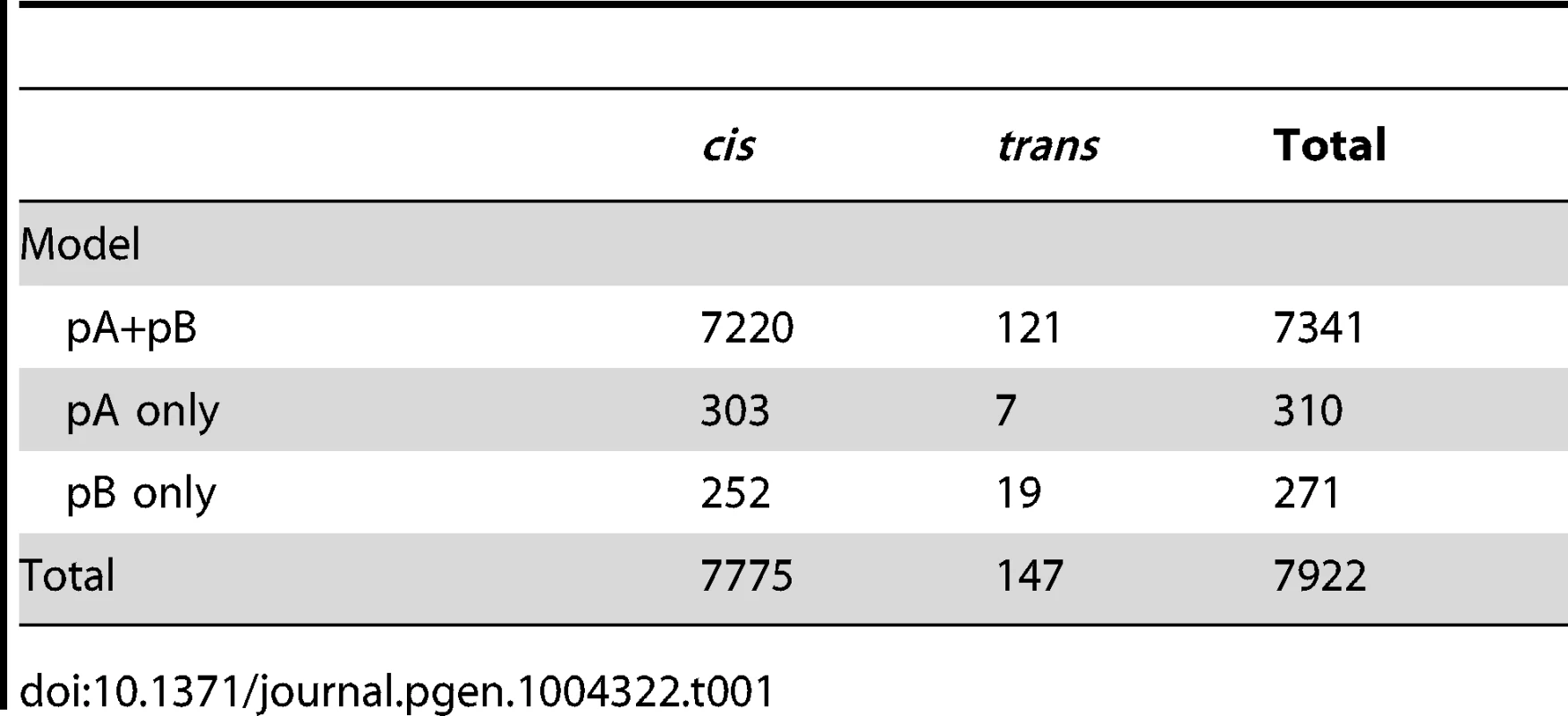
The amount of variation explained by our mapped eQTLs was high (Figure 2), though our stringent, experiment-wise permutation-based correction for multiple tests severely limits our ability to detect QTL of small effect. Not surprisingly, the variance explained by cis-eQTLs was higher than trans-eQTLs [24]. Our cis-eQTLs explained a median of 24% of the phenotypic variance, and 855 eQTL explained more than 50% of the phenotypic variance. Using our heritability estimates for each transcript abundance, we estimated the percentage of the heritability each eQTL explained. The median for the percent heritability explained by each eQTL was 73%. Our trans-eQTLs explained lower levels of variance, the median phenotypic variance explained was 15%, and the median percent heritability explained was 38%. However, if heritability values are underestimated, and/or we overestimate the effects of eQTLs (which is likely due to the Beavis effect [34]), these values will be inflated. This effect is obvious for the set of eQTL estimated to explain greater than 100% of the heritability (Figure 2A).
Fig. 2. The distributions of the percentage of the genetic variation (A) and phenotypic variation (B) explained for cis- (black line) and trans- (blue line) eQTL. 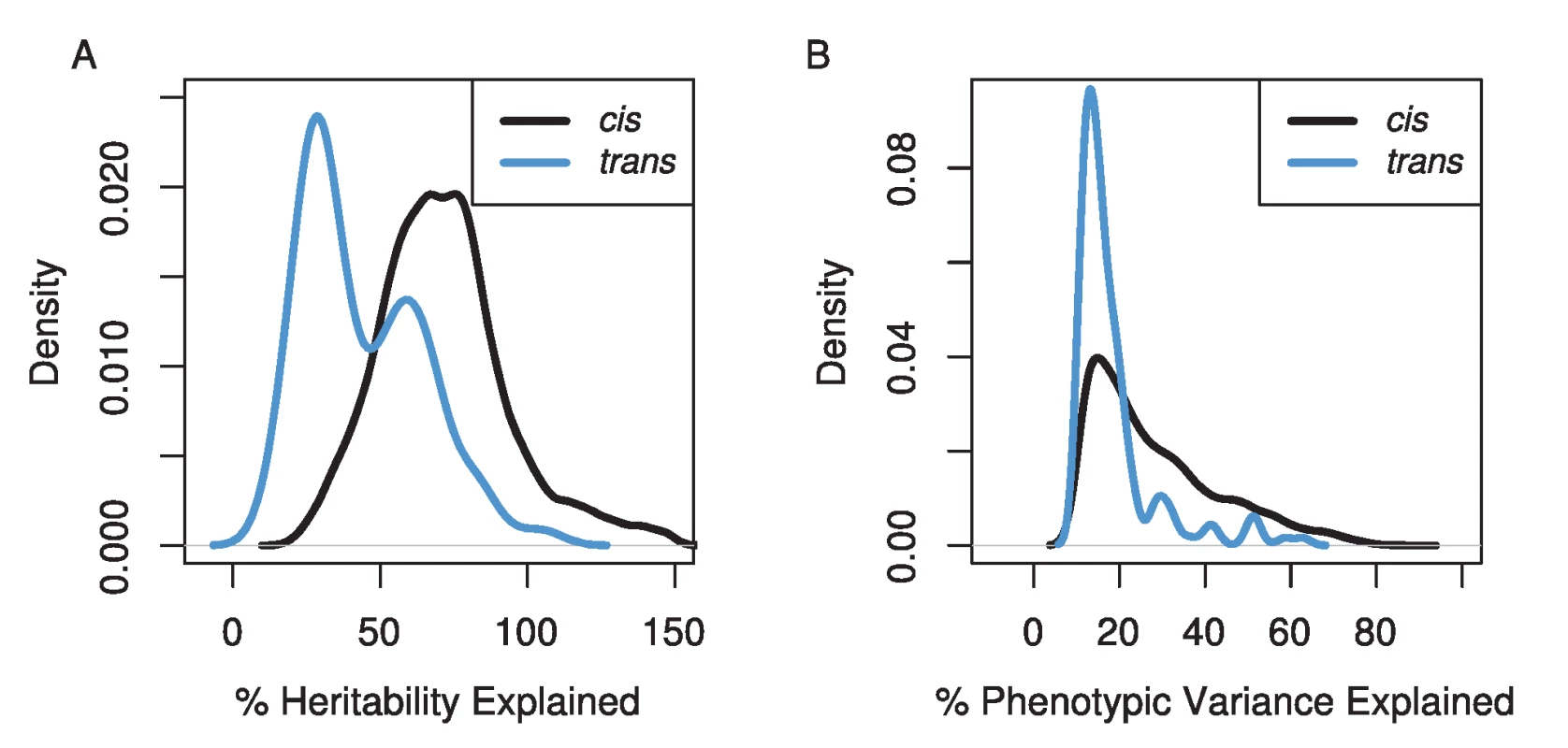
Estimates of the percentage of genetic variance explained can be greater than 100% due to underestimates of heritability for transcripts and/or overestimates of effect sizes of eQTL. Our mapping resolution was high (Figure 3). We used two methods for estimating confidence intervals, a 3 LOD drop and the Bayesian credible interval. We excluded confidence intervals that spanned centromeres or occurred near telomeres, because these tend to cover very large physical distances (7% of eQTLs). The Bayesian credible intervals tended to be narrower than 3 LOD drops (median BCI = 110 kb, 0.25 cM; median 3 LOD drop = 240 kb, 0.51 cM), but the range was larger for BCIs (BCI: 0–4.5 Mb, 0–6.5 cM; 3 LOD drop: 20 kb–4.0 Mb, 0.001–3.9 cM). The median number of genes within cis-eQTL CIs was 32 (range 1–551), and within trans-eQTL CIs, the median was 44 (range: 5–479).
Fig. 3. Distributions of the width of our confidence intervals for eQTL in A & C) physical distance (kb) and B & D) genetic distance (cM) using either a 3 LOD drop (A & B) or the Bayesian credible interval (C & D). 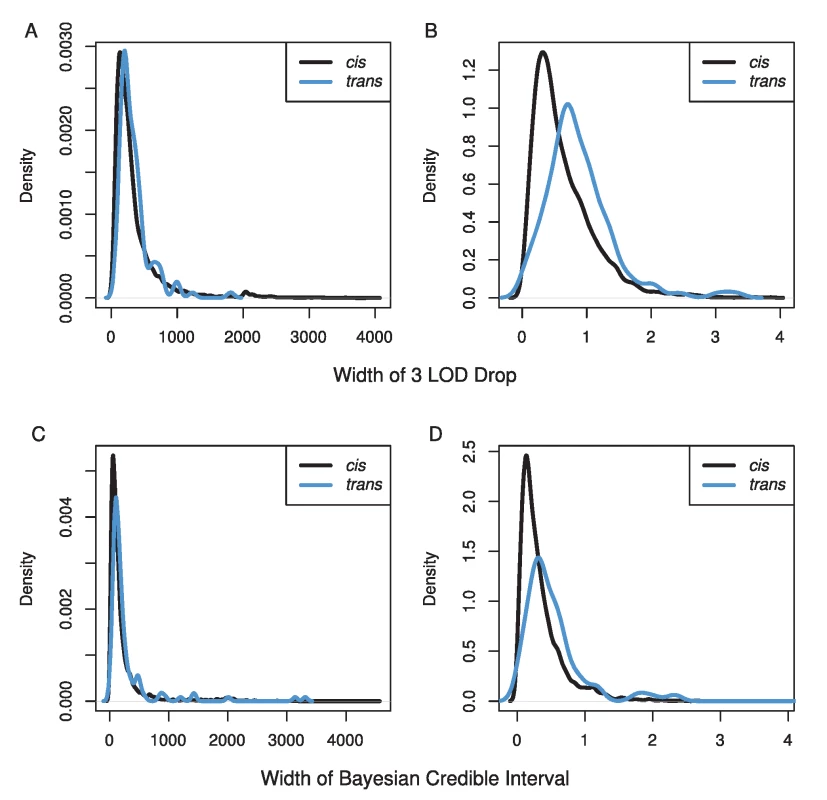
Black lines show CIs for cis-eQTL while blue lines show CIs for trans-eQTL. We have provided a comprehensive map of eQTLs for female head tissue in the Drosophila model system within the constraints of our statistical power. There is little doubt many smaller effect eQTLs exist that we were not able to identify given our conservative statistical threshold. Our use of trans-heterozygote individuals means that we not only avoid the effects of inbreeding depression, but we have also obtained estimates for all eQTL for both pA and pB DSPR populations. Overall, our results confirm what many other researchers have observed, widespread large effect cis-eQTLs and smaller effect trans-eQTLs [23], [24], [26], [27]. One of the major advantages of a stable genetic panel is the ability to measure multiple traits at multiple levels on genetically identical individuals, which allows for the potential to combine these sources of data to identify causative genes [9], [23], [25], [32]. We expect this dataset to be very useful to DSPR users, particularly those interrogating phenotypes measured in females with relevance to neuroanatomy or behavior. All of the raw and analyzed data are freely available at http://FlyRILs.org/Data. The data have also been deposited in NCBI's Gene Expression Omnibus [35] and are accessible through GEO Series accession number GSE52076.
Trans-eQTL hotspots
We identified regions of the genome associated with a high trans-eQTL density to identify eQTL regulating the expression of several other genes (trans hotspots). There were two regions of high trans-eQTL density, TQTLA and TQTLB (Figure 4; Table 2). These clusters regulate several genes distributed throughout the genome, as is apparent in Figure 1. We used a gene ontology term finder [36] to determine whether the sets of genes regulated by these trans-eQTL were related to a common process. The set of 16 genes regulated by TQTLA showed enrichment for circadian rhythm of gene expression (2 of the 16 genes regulated by TQTLA; P = 0.0007). We used principal components analysis on the set of 16 genes to create a composite variable. All 16 genes load fairly evenly on the first principal component (absolute value range: 0.08–0.20). We then correlated this composite variable with expression measures for each gene in the TQTLA region to identify possible candidate genes. The gene timeless (tim) was highly correlated with the TQTLA composite variable (r = 0.90), and it does have a significant cis-eQTL. All other genes in the interval had a correlation with an absolute value of less than 0.5. Additionally, after correlating the expression of each of the 16 transcripts regulated by TQTLA with the expression of all genes in the TQTLA region, timeless showed the maximum pairwise correlation in all 16 cases (absolute value of correlation range:0.35–0.84). The estimated haplotype means follow this pattern and are correlated with the estimated effects for the timeless cis-eQTL in most cases (average absolute value correlation: 0.65; min: 0.03; max: 0.99). The gene timeless (tim) is expressed in the adult central nervous system [37] and is involved in transcriptional regulation of circadian rhythm [38].
Fig. 4. The density of trans-eQTLs along the genome. 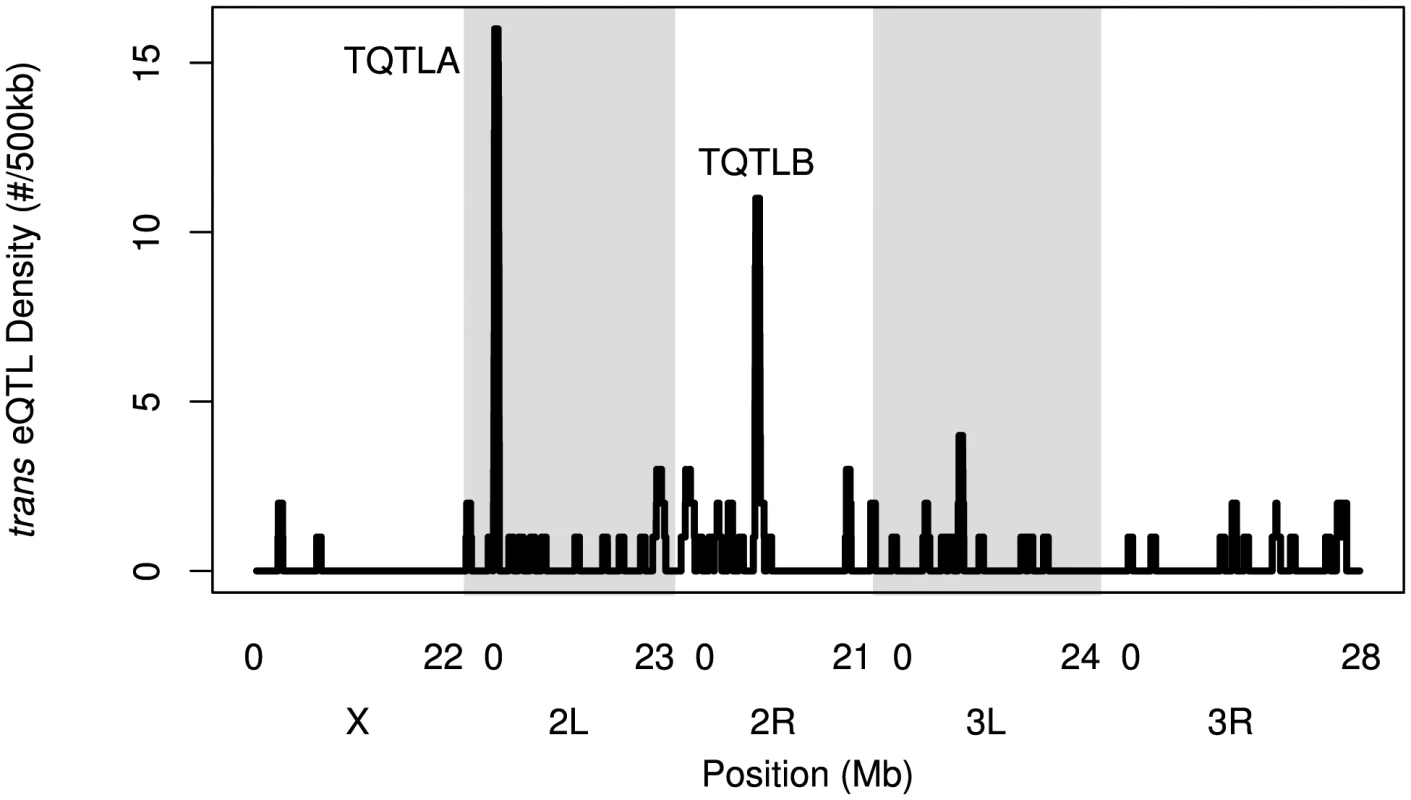
Density is the number of genes (not transcripts) with a trans-eQTL per 500 kilobases. Grey and white shading denote the different chromosome arms. Potential hotspots (regions with a density greater than 5) are noted. Tab. 2. <i>trans</i>-eQTL regulating multiple genes. 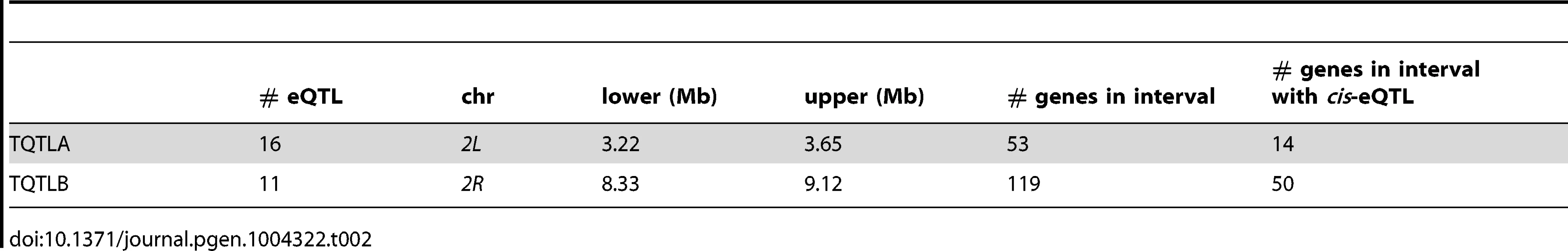
Not all genes in the TQTLA interval are included in our expression set. For example, some genes may have been dropped due to the presence of SNPs in probes, or were not included in the Nimblegen probe set to begin with. For TQTLA, 23 genes in the interval are not represented in the expression set. However, none of these genes are associated with any terms involving circadian rhythm, regulation of gene expression, or transcription (http://FlyBase.org) [39], and we therefore do not consider any of these likely candidate genes.
The genes associated with TQTLB are enriched for several GO terms including compound eye pigmentation (2/11 genes; P = 0.005), the umbrella term: single-organism metabolic process (6/11 genes; P = 0.007), and several specific metabolic process terms: tryptophan metabolic process (2/11 genes; P = 0.008), indolalkylamine metabolic process (2/11 genes; P = 0.0008), indole-containing compound metabolic process (2/11 genes; P = 0.002), aromatic amino acid family metabolic process (2/11 genes; P = 0.006). Once again we performed PCA to create a composite variable. sugarbabe (sug) was the gene most highly correlated with the TQTLB composite variable (r = −0.63) and does have a significant cis-eQTL. All other genes in the interval had a correlation with an absolute value of less than 0.4. Loadings were again fairly even (absolute value range for all other genes: 0.08–0.39). Pairwise correlations between the transcripts associated with TQTLB and the expression measures in the interval showed sugarbabe to be most highly correlated in all cases except two: gene CG5321 and gene CG6834 (absolute value of correlation range for all other genes: 0.40–0.52). These two genes were also the two with the lowest loading values on the composite variable. The correlation between the estimated haplotype effects for the cis-eQTL for sugarbabe, and the effects for the trans-eQTLs were moderate (mean absolute value correlation: 0.24; min: 0.005; max: 0.44). The gene sugarbabe (sug) is expressed in the adult head [37], is involved in regulation of transcription [40], is involved in regulation of response to starvation [41], and is part of the insulin-like growth factor signaling pathway [41]. The 21 genes not included in the interval are not associated with any terms involving metabolism, regulation of gene expression, or transcription (http://FlyBase.org) [39].
We have identified two trans hotspots, and, in both cases, we were able to use our expression dataset to narrow the causative gene to a single likely candidate gene. Previous eQTL studies have identified many more trans hotspots that regulate many more genes (hundreds or thousands) than our two identified hotspots (TQTLA: 16 genes; TQTLB: 11 genes; e.g. [27], [42], reviewed in [24], [26]). However, while some of these global regulators of gene expression have been confirmed as true signals, most notably in yeast [43], [44], Kang et al. [43] show how hotspots can result from confounding factors such as batch effects. In our dataset, we employed PCA to correct for possible batch effects [45]. This method has been shown to increase power to detect eQTL [29], [45], [46], however, it makes identifying even true trans global regulators impossible. The signal that results from a global regulator is statistically indistinguishable from an unmeasured batch effect. In addition, even true global regulators can confound the detection of other true eQTLs, and correcting for these true global regulators increases the power to detect these other associations [43], [45]. It is possible to distinguish true trans hotspots from batch effects using biological replicates [43], but for our study we chose to maximize the number of RILs rather than increase replication to maximize our statistical power to map eQTL. As a result, we are unable to detect many trans hotspots in this study. However, our stringent statistical correction does give us increased confidence that the eQTL we do identify are indeed true signals.
Most eQTLs are multiallelic
The vast majority of our eQTLs appear to be multiallelic (Figure S1). In 95% of cases, the number of alleles estimated at cis-eQTL was 3 or greater. For trans-eQTL this percentage was somewhat lower, at 78%. Figure 5 shows an example of an eQTL where the best model is a two allele model and of an eQTL where the full haplotype model is the best model. In cases where we estimated multiple alleles, we were able to explain additional phenotypic variance compared to the best two allele model (Figure S2), sometimes as much as an additional 27%. We investigated our ability to accurately estimate the number of alleles by performing a simulation designed to provide the highest power to distinguish between different alleles (see methods). Our simulation revealed that our estimator underestimates the number of alleles in 63% of cases, correctly estimates the true number of alleles in 26% of cases, and overestimates the number of alleles in 10% of cases (Figure 6). This bias toward underestimating the number of alleles gets increasingly severe as the true number of alleles increases. Our simulations with a lower effect size (5%) and normally distributed allelic effects both resulted in an even stronger bias toward underestimating the true number of alleles. Our allele number distribution for cis-eQTLs is no doubt composed of a mixture of eQTLs of varied numbers of true alleles. Overall, it is closest to the distribution we obtain for a simulation of ∼5 alleles. So while most of our estimates for cis-eQTL are for 3–4 alleles, many may be determined by many more alleles.
Fig. 5. Standardized estimated means for each founder genotype for single observed cis-eQTL where A) the full model is the best model, and B) a two allele model is the best model. 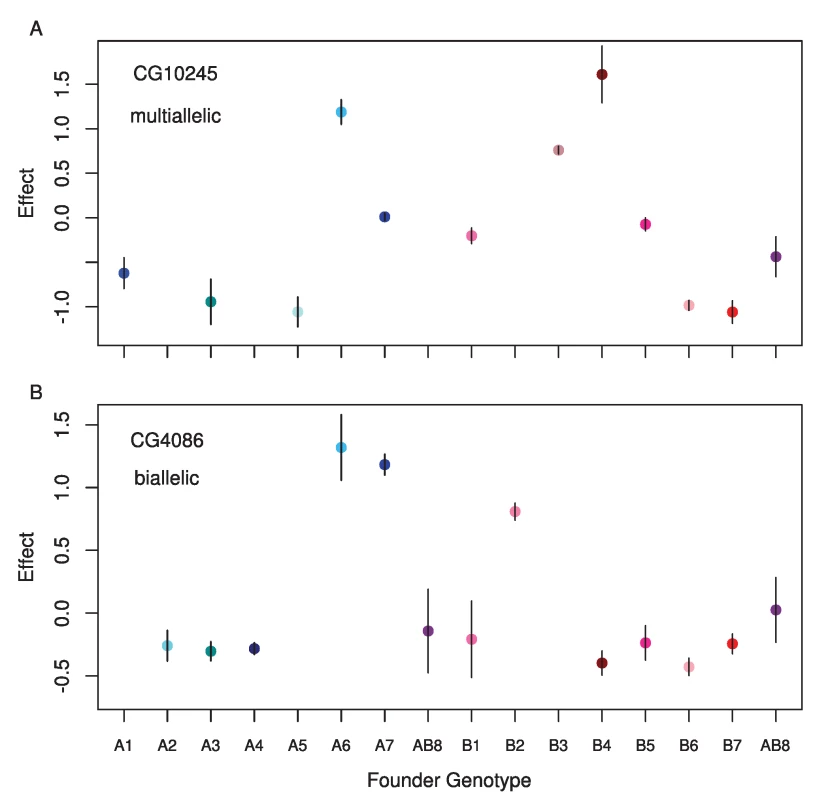
The gene name for each is displayed in the upper left corner. Fig. 6. Estimated number of alleles for simulated (grey circles) and observed data (blue circles). 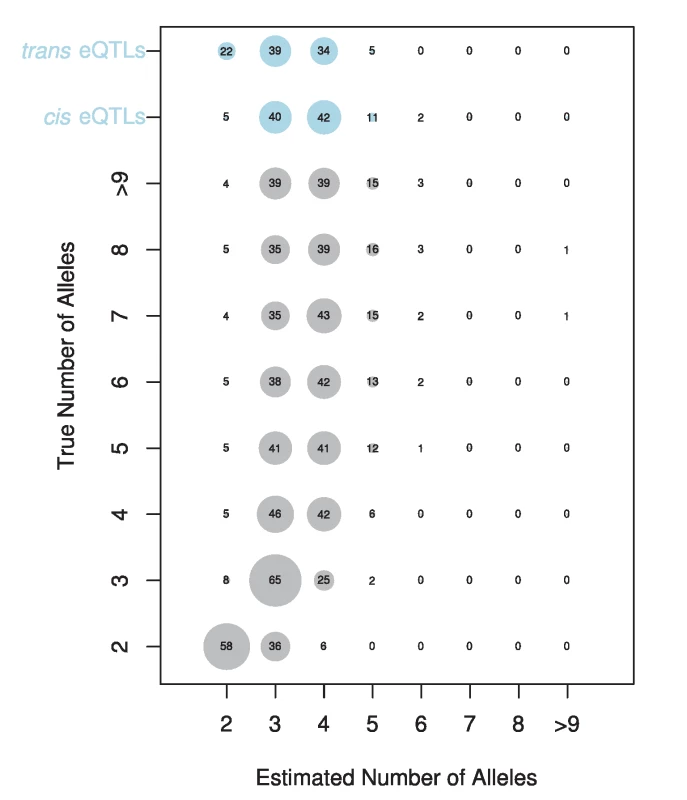
The true number of alleles versus the estimated number of alleles is displayed for simulated data. The size of each circle and the number displayed denotes the percentage of times each number of alleles is estimated for a given true number of alleles. The estimated number of alleles for our cis- and trans-eQTL are shown at the top of the plot. Our results indicate widespread allelic heterogeneity for both cis - and trans-eQTLs. The focus of mapping studies is often to identify the single causative variant underlying a significant signal, the implicit assumption being that the causative loci are biallelic. cis-eQTL in particular, with their large effects, are thought to be more likely than other traits to have a simple genetic architecture and be biallelic [22], [28]–[30]. Baud et al. [22] found some support for this idea when comparing a two allele model to the full haplotype model in hippocampus eQTLs in the heterogeneous stock mouse resource [32]. They found that in 97% of cases, the two allele model was superior for cis-eQTLs while trans-eQTLs were more likely to be multiallelic [22]. However, in contrast to these findings, cis-eQTLs have been found to be multiallelic in Drosophila [47], Arabidopsis [42], and humans [31], [48]. Our results strongly confirm the result of multiallelism in Drosophila with 95% of cis-eQTLs estimated to be due to 3 or more alleles. This result indicates that in Drosophila, widespread allelic heterogeneity exists at one of the most basic levels of genetic variation: cis-regulatory variation.
Widespread allelic heterogeneity is one potential explanation for the missing heritability problem in the study of complex traits. Allelic heterogeneity presents a statistical challenge for GWAS [7]. GWAS utilize natural populations and interrogate each SNP (or other specific variant) for association with the phenotype of interest. At the single gene level, it is difficult to distinguish between simple linkage disequilibrium between a single causative variant and other, nearby neutral SNPs, and multiple independent causative SNPs. If GWAS focus only on the strongest association at a locus, in the presence of allelic heterogeneity that individual variant will account for less of the variation than the entire gene, causing the effect of the locus to be underestimated [7]. In this respect, haplotype-based mapping approaches, such as the one described here, have an advantage because entire haplotypes (and thus an entire set of causative variants associated with a single gene) are tested together. The effect size associated with the causative gene will tend to be larger and easier to detect in this framework. This effect, combined with the more favorable frequencies of alleles in linkage based panels could explain why these studies tend to explain very large proportions of the heritable variation [9], [21], [49], while GWAS grapple with large amounts of missing heritability. However, one drawback of current haplotype-based methods is that they do not have single gene resolution and therefore identifying the causative gene within the QTL interval can be a significant challenge. Furthermore, while identifying the causative loci under allelic heterogeneity is easier with haplotype based methods, the subsequent identification of the causative SNPs within the loci is made much more complicated by heterogeneity [17], [18], [50].
Allelic heterogeneity is typical for Mendelian diseases (http://www.omim.org/) and it has been suggested as the likely model for quantitative traits [51]. There is a growing body of empirical [2], [17], [22], [31], [42], [47], [50] and theoretical [7] support for this idea. For example, one of the largest GWA studies found support for allelic heterogeneity for human height by identifying several cases of multiple SNPs likely associated with the same gene [2]. Even age related macular degeneration, the first successful GWA study [52], has subsequently been shown to harbor multiple functional alleles [53]–[56]. Our results should therefore not be surprising. However, they do suggest the community should focus on developing experimental designs and analytical methods, e.g., [7], that function well under a model of allelic heterogeneity.
Methods
Mapping population
We used RILs from the DSPR (http://FlyRILs.org) to map genome-wide expression variation. The DSPR has been described in detail previously. Complete details of the development of the DSPR, founder whole genome re-sequencing, and RIL genotyping are described in [17]. The development of the hidden Markov model to infer the mosaic structure of the RILs and the power and mapping resolution of the DSPR for QTL mapping are described in [18]. Briefly, the DSPR is a multi-founder advanced intercross panel consisting of a set of over 1700 RILs of Drosophila melanogaster. Two 8-way synthetic populations (pA and pB) were created from two independent sets of 7 inbred founder lines (A1–A7 or B1–B7) with one additional line (AB8) shared by both populations. Each synthetic population was maintained as two independent replicate subpopulations (pA1 and pA2 or pB1 and pB2), kept at a large population size, and allowed to freely recombine for 50 generations. At generation 50, each subpopulation gave rise to ∼500 RILs via 25 generations of full-sib mating. The genomes of the original fifteen inbred founder lines have been completely re-sequenced, and the complete underlying founder haplotype structure of all RILs in the panel has been determined via Restriction-Associated DNA (RAD) sequencing along with a hidden Markov model (HMM).
In order to avoid potentially mapping QTL for inbreeding depression, we phenotyped trans-heterozygote F1 individuals from crosses between pA females and pB males. The crosses were done to maintain the subpopulation structure by crossing pA1 to pB2 and pA2 to pB1. In both cases, we arbitrarily crossed pA and pB RILs with the same line number (i.e., pA11*pB21, …, pA1n*pB2n, pA21*pB11, …, pA2n*pB1n). For each of 596 crosses, we generated 4–6 replicate cross vials containing 10 virgin pA females and 10 pB males and cleared the adults after 24–48 hours to maintain roughly equal larval density across experimental vials. Both the inbred RIL parents and the experimental trans-heterozygous cross progeny were raised on standard cornmeal-yeast-molasses media at 25°C, 50% relative humidity, and on a 12∶12 light∶dark regime.
RNA isolation and arrays
Progeny from each cross vial were allowed to emerge and mate in the source vial for 2–4 days. Then 250–300 females were harvested over CO2 from the multiple replicate vials. Since we did not isolate virgin females on eclosion, females are very likely mated. These experimental females were kept for 24 hours in fresh vials to minimize any effects of the anesthesia before the heads were isolated (3–5 days old). Heads were removed by transferring the females without anesthesia to a 50 ml conical bottom centrifuge tube, freezing in liquid nitrogen, vigorously vortexing, and sieving using dry ice-chilled brass analytical sieves (mesh sizes 0.0165 and 0.0278 inches), separating heads from bodies and from legs and wings. Head samples were stored at −80°C until RNA isolation.
We did not have any technical or biological replicates aside from the effect of pooling 250–300 individuals, collected from multiple source vials, for each sample. This was intentional because we are mainly interested in the variance among RILs. There were two exceptions to this lack of replication. Crosses A1.299×B2.299 and A1.350×B2.350 were prepared independently twice.
RNA was isolated using TRIzol Reagent (Life Technologies), cleaned up using RNeasy Mini spin columns (Qiagen), concentrated—if necessary—using a vacuum centrifuge, and shipped to the Carver Center for Genomics Microarray Center at the University of Iowa for cDNA synthesis and array hybridization. We used Nimblegen 12×135 K arrays to assay genome-wide gene expression. These arrays assay 16,637 transcripts with eight 60 bp probes per transcript. Each array holds 12 different crosses.
Data analysis pipeline
All data analysis was performed in R [57]. Initially, we performed standard quantile normalization and corrected for background effects using the normalize and backgroundCorrect functions in the oligo package to correct for any overall array effects [58]–[61]. We then created a custom probe-to-transcript map using the most recent version of the CDS file available at FlyBase (v. 5.48). We blasted all probe sequences against the CDS, requiring an exact match [62], [63]. We eliminated any probe sequences without an exact 60 bp match to a transcript (6842 probes). We did not require a unique match given many transcripts from the same gene share portions of their sequences. Thus a single probe can correspond to multiple transcripts.
Single nucleotide polymorphisms in probe sequences are known to affect array hybridization and thus expression measurement [64]–[68]. We took advantage of the availability of full genome sequences for all 15 founder lines to identify SNPs within probe sequences. We first updated the alignment and SNP calling for the founder re-sequencing data using the Burrows-Wheeler Aligner (BWA) [69] with the following switches: -m 50000000 -R 5000, followed by the SAMtools [70] mpileup command (the initial alignment used Mosaik and a custom SNP caller, see [17]) to obtain an accurate, comprehensive list of SNPs in the founder lines (http://FlyRILs.org/Data, Release 3). We also applied the following filters: 1) at least one founder was fixed for the minor allele and at least three founders were fixed for the major allele (given a coverage of 10×), 2) minimum overall coverage of 90 (5 per sample), and 3) maximum overall coverage of 3600. A large proportion of our probe sequences contained SNPs segregating in the set of DSPR founder lines. Because we have the full genome sequences in silico of all RILs in the panel, we were able to identify all positions in probes that are SNPs in our RIL panel and test for the effect of each SNP on the expression measurement. We discarded any probes containing multiple SNPs (22018 probes). For probes containing a single SNP, we used the haplotype probabilities from the hidden Markov model to infer the probability each RIL harbored the minor allele and assigned a genotype value to each cross by adding the paternal and maternal probabilities. In the case of perfect certainty, genotype values are: 2 = AA, 1 = Aa, and 0 = aa. We then tested for the effect of the SNP on the expression measurement by fitting the following model:
where y is the expression measurement, S is subpopulation, M is the cross genotype at the marker, and βs and βm are the corresponding effect estimates. We then eliminated all probes with a p-value less than 0.05 (21141 probes).Following re-mapping of probes and elimination of probes with SNPs affecting expression, transcripts were associated with a variable number of probes instead of each transcript being associated with exactly 8 probes as in the original NimbleGen array design. We eliminated any transcript associated with fewer than four probes. Next, we performed standard RMA using the basicRMA function in the oligo package [61] to combine probe-specific data and generate a single expression measure per transcript. Many genes are associated with multiple transcripts. Whether the expression of different transcripts can be independently assessed is dependent on how many probes uniquely map to each transcript. We calculated pairwise correlations between each transcript in each set of transcripts associated with a single gene. If all of the pairwise correlations between the set of transcripts were > = 0.95, we used the average expression for the gene. Otherwise, we mapped each transcript separately. We will refer to all expression measures (including those averaged across transcripts for a single gene) simply as transcripts for clarity.
We followed the methods of [29], [46] and used principal components analysis (PCA) to minimize batch effects [45] and increase our power to detect QTL. Following quantile normalization of each transcript to coerce each transcript distribution to be normal, we performed PCA on the entire set of transcripts. We selected the first 10 principal components to correct our expression measurements. The percentage of the variance explained by each remaining principal component was below 1% (Figure S3). We then fit the following model
where yi is the ith expression measurement, S is subpopulation, xj is the jth principal component, and βs,i and βj are the corresponding effect estimates. We used the resulting residuals for the remaining analyses. We performed an additional round of quantile normalization on these residuals to ensure normality.We estimated the narrow-sense heritabilities for all transcripts by fitting a linear mixed model using the polygenic function in the GenABEL package [71]. Briefly, the model includes a random effect polygenic term whose variance is determined by the kinship matrix between RIL crosses. We calculated the kinship matrix using the genome-wide haplotype assignments resulting from the HMM. At each position spaced every 0.025 cM, we calculated the probability of identity by decent and averaged these across the genome to obtain the relationship coefficient. Our kinship matrix is thus estimated over genetic distance. We then used the polygenic function to calculate heritabilities for each transcript [71].
To map eQTLs, we first selected transcripts expressed above background levels. We utilized the two replicated samples, A1.299×B2.299 and A1.350×B2.350, to identify the point where measurements were less repeatable and excluded all transcripts with expression levels below this point (Figure S4). This cutoff excluded approximately 23% of transcripts. For all included transcripts, we performed haplotype-based genome scans by fitting the following model at regularly spaced positions every 10 KB across the genome (11768 positions; http://FlyRILs.org/Data, Release 3).
where yr,i is the ith transcript, μ is the grand mean, GA,j are the genotype probabilities for the jth paternal RIL, GB,j are the genotype probabilities for the jth maternal RIL, and βA,j, and βB,j are the corresponding effect estimates. Because we assayed only females, the model for the X chromosome is the same as for the autosomes. At each position, we calculated the F-statistic for the overall effect of genotype and obtained LOD scores.To identify the statistical significance threshold, we performed 1000 permutations of the expression measures [72]. The entire set of expression measures was permuted together to maintain the correlation structure in the dataset. We used these permutations to determine a conservative genome-wide, experiment-wise 5% significance threshold (threshold = 14.99). We also determined a separate threshold for cis-eQTL. We defined cis-eQTL as QTL occurring within 1.5 cM of the transcription start [18] site for each transcript (1.5 cM is our typical confidence interval width). To define a cis-only threshold, we only included the LOD scores for the positions within 1.5 cM of the transcription start for each gene (threshold = 14.4).
We identified all peaks with LOD scores exceeding the above-defined thresholds. When multiple nearby peaks were identified, we determined whether their 3 LOD drop intervals overlapped, and, if so, only the peak with the highest LOD score was retained. We expect 3 LOD drops to be a conservative estimate of the 95% confidence interval. Standard 2 LOD drops have been shown to be overly narrow for pA×pB cross designs [18]. It should be noted however, that confidence intervals on QTL locations are not true 95% confidence intervals and effect size, sample size, and the number of haplotypes in the model affect the degree of coverage. We also calculated Bayes credible intervals, for which 95% coverage tends to be more consistent [73], [74].
In a pA×pB cross, a mapped QTL may be due to genomic variation at that position in only one population or in both. We identified peaks associated with only a single population using Akaike's Information Criterion (AIC). We calculated the AIC for three models: pA alone, pB alone, and pA & pB. The smallest AIC indicates the model with the best fit. Thus any cases in which the lowest AIC resulted from a reduced model, the QTL peak was concluded to be due to variation in a single population.
We identified trans-eQTLs influencing multiple transcripts by estimating the trans-eQTL density across the genome using a 500 kb sliding window with a step size of 1 kb. Our estimate of density included only unique genes, not transcripts to avoid counting multiple transcripts associated with a single gene as independent events. If trans-eQTL density in a window exceeded the density expected by chance under a Poisson distribution, we concluded it was a significant trans hotspot. This threshold for a Poisson distribution given the total number of trans-eQTLs (147), the window size (500 kb), the size of the genome tested (118 Mb) and the Bonferonni corrected P-value threshold (117,741 tests; P = 4.2×10−7) is a trans-eQTL density greater than 6. We delineated the size of these hotspot regions as the lowermost and uppermost confidence interval bound for any trans-eQTL peak included in a window exceeding a density of 6.
Our initial scan identified 3 trans hotspots but upon further investigation, we determined one to be a false signal resulting from a single gene family. All of the eQTL peaks associated with this hotspot represent 13 members of a single gene family located on the X chromosome: Stellate (Ste). In addition, members of this family also occur at an unlocalized region in the heterochromatin on the X chromosome. The “trans-” eQTL we map regulating this family is located at the very tip of the X chromosome, making it very likely we are tagging this heterochromatic location of Stellate members, and it is in fact an additional cis effect. In fact, all thirteen members show two peaks, one cis peak and a second “trans” peak at the tip of the X, indicating most of our probes for these genes are tagging multiple members of this gene family. In addition, Stellate is expressed in adult males and involved in spermatogenesis (http://FlyBase.org) [39]. It is likely we are seeing high expression due to large numbers of copies of gene family members (∼200 copies) [75]. We therefore excluded this trans hotspot.
Estimating the number of alleles at eQTLs
We estimated the number of alleles at each eQTL using a model comparison technique similar to the method employed by Yalcin et al. [76] and Baud et al. [22] The major difference in our approach is that we consider models with more than 2 alleles and do not restrict our analysis to specific SNPs in the QTL interval. The merge analysis employed by Baud et al. [22] considered all two allele models associated with a single SNP within the QTL interval. We simply assign different alleles to different haplotypes without those necessarily corresponding to SNPs in the interval. This method also allows us to consider models with several alleles. For each eQTL, at the peak position, we fit all possible models for different numbers of alleles, fitting a maximum of 11337 models at each eQTL. We first estimated the haplotype means at the peak, sorted these means, and then fit all possible models that did not change the order of the haplotype means for 2, 3, 4, 5, 6, 7, 8, and 16 (the full model allowing different estimates for AB8 in pA RILs and AB8 in pB RILs) alleles (Figure S5). We only included haplotypes at the peak that occurred at least 5 times (at a probability of greater than 95%) in our set of crosses. Haplotypes at lower frequencies lead to inaccurate estimates of haplotype means with large standard errors. For each possible allele grouping, individual founder haplotype probabilities in each allele group were summed to obtain a probability each RIL harbored each allele group. For example, if haplotypes A3 and A5 are grouped as a single allele named allele 1, and the probabilities a given RIL cross harbors the A3 or A5 haplotype are 0.90 and 0.03 respectively, then the probability that RIL cross harbors allele 1 is 0.93 (i.e., the probability the RIL cross harbors either A3 OR A5 and thus allele 1). Alleles were only combined within pA and within pB given that the pA and pB sets of probabilities are independent. The model fit was as follows:
where yr,i is the ith transcript, μ is the grand mean, na is the number of pA allele groupings, nb is the number of pB allele groupings, GA,c are the genotype probabilities for the cth paternal allele group, GB,d are the genotype probabilities for the dth maternal allele group, and βA,c, and βB,d are the corresponding effect estimates. The model with the lowest P-value was chosen as the best model and the number of alleles associated with this model was recorded. We also explored using Akaike's information criterion (AIC) to choose the best model, however simulations revealed a higher error rate using AIC (see below). Table S3 provides hard coded genotype assignments for all RIL crosses at all significant eQTL.Simulation
To test our method of estimating the number of alleles associated with QTL, we simulated QTL stemming from between 2 and 15 different alleles and subsequently estimated the number of alleles using the model comparison methodology described above. We intentionally set up this simulation to make distinguishing different alleles as easy as possible. We performed 1000 iterations for each of 2, 3, 4, 5, 6, 7, 8 and 15 alleles (the full model assuming the same effect for AB8 in the pA and pB panels). For each iteration, we randomly selected 600 pA RILs and 600 pB RILs from the DSPR panel and randomly paired them to create pA-pB crosses. We then simulated a QTL in this set of RIL crosses at a randomly selected position in the genome with the chosen number of alleles. We assigned the different alleles equal effects, because we found equal effects gave higher power to distinguish different alleles compared to pulling effects from a normal distribution (Figure S6). For example, for a four allele model each founder haplotype was randomly assigned an effect of 1, 2, 3, or, 4. We assumed an additive model to calculate a genetic effect for each cross. We generated a set of random normal deviates N(μ = 0, ) to correspond to environmental variance where z = the percent of the phenotypic variance explained by the QTL and is the genetic variance at the QTL. The percent of the total phenotypic variance explained by the QTL was randomly chosen from our observed distribution of phenotypic variance explained by cis-eQTLs. These effects tend to be quite large, however, we found large effects lead to higher power to distinguish different alleles (Figure S7). We then estimated the number of alleles at our simulated QTL as described above. We used two methods to determine the best model: 1) the model with the lowest P-value, and 2) the model with the lowest AIC. Our results showed the method using P-values had a greater accuracy (P-value method: 26% accuracy; AIC method: 19% accuracy). More importantly, the AIC method overestimates the true number of alleles more often, estimating more than two alleles in 83% of cases when the true number of alleles is two (Table S2). We prefer the method that is more conservative, meaning it has a greater tendency to underestimate rather than overestimate the number of alleles, and we therefore use the P-value method in all subsequent analysis (Figure S8). Complete sensitivity information for the different methods and the different simulation models can be seen in Figures S5, S6, S7 and in Table S2.
Supporting Information
Zdroje
1. ZukO, HechterE, SunyaevSR, LanderES (2012) The mystery of missing heritability: Genetic interactions create phantom heritability. Proceedings of the National Academy of Sciences 109 : 1193–1198 doi:10.1073/pnas.1119675109
2. Lango AllenH, EstradaK, LettreG, BerndtSI, WeedonMN, et al. (2010) Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467 : 832–838 doi:10.1038/nature09410
3. YangJ, BenyaminB, McevoyBP, GordonS, HendersAK, et al. (2010) Common SNPs explain a large proportion of the heritability for human height. Nat Genet 42 : 565–569 doi:10.1038/ng.608
4. RockmanMV (2012) The QTN program and the alleles that matter for evolution: all that's gold does not glitter. Evolution 66 : 1–17 doi:10.1111/j.1558-5646.2011.01486.x
5. PritchardJ (2001) Are rare variants responsible for susceptibility to complex diseases? Am J Hum Genet 69 : 124–137.
6. BansalV, LibigerO, TorkamaniA, SchorkNJ (2010) Statistical analysis strategies for association studies involving rare variants. Nat Rev Genet 11 : 773–785 doi:10.1038/nrg2867
7. ThorntonKR, ForanAJ, LongAD (2013) Properties and Modeling of GWAS when Complex Disease Risk Is Due to Non-Complementing, Deleterious Mutations in Genes of Large Effect. PLoS Genet 9: e1003258 doi:10.1371/journal.pgen.1003258.s011
8. ChurchillGA, AireyDC, AllayeeH, AngelJM, AttieAD, et al. (2004) The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat Genet 36 : 1133–1137 doi:10.1038/ng1104-1133
9. AylorDL, ValdarW, Foulds-MathesW, BuusRJ, VerdugoRA, et al. (2011) Genetic analysis of complex traits in the emerging Collaborative Cross. Genome Res 21 : 1213–1222 doi:10.1101/gr.111310.110
10. PhilipVM, SokoloffG, Ackert-BicknellCL, StrizM, BranstetterL, et al. (2011) Genetic analysis in the Collaborative Cross breeding population. Genome Res 21 : 1223–1238 doi:10.1101/gr.113886.110
11. KoverPX, ValdarW, TrakaloJ, ScarcelliN, EhrenreichIM, et al. (2009) A Multiparent Advanced Generation Inter-Cross to Fine-Map Quantitative Traits in Arabidopsis thaliana. PLoS Genet 5: e1000551 doi:10.1371/journal.pgen.1000551
12. HuangX, PauloM-J, BoerM, EffgenS, KeizerP, et al. (2011) Analysis of natural allelic variation in Arabidopsis using a multiparent recombinant inbred line population. P Natl Acad Sci Usa 108 : 4488–4493 doi:10.1073/pnas.1100465108
13. YuJ, HollandJB, McMullenMD, BucklerES (2008) Genetic design and statistical power of nested association mapping in maize. Genetics 178 : 539–551 doi:10.1534/genetics.107.074245
14. BucklerES, HollandJB, BradburyPJ, AcharyaCB, BrownPJ, et al. (2009) The Genetic Architecture of Maize Flowering Time. Science 325 : 714–718 doi:10.1126/science.1174276
15. McMullenMD, KresovichS, Sanchez VilledaH, BradburyP, LiH, et al. (2009) Genetic Properties of the Maize Nested Association Mapping Population. Science 325 : 737–740 doi:10.1126/science.1174320
16. LiH, BradburyP, ErsozE, BucklerES, WangJ (2011) Joint QTL Linkage Mapping for Multiple-Cross Mating Design Sharing One Common Parent. PLOS ONE 6: e17573 doi:10.1371/journal.pone.0017573
17. KingEG, MerkesCM, McNeilCL, HooferSR, SenS, et al. (2012) Genetic dissection of a model complex trait using the Drosophila Synthetic Population Resource. Genome Res 22 : 1558–1566 doi:10.1101/gr.134031.111
18. KingEG, MacdonaldSJ, LongAD (2012) Properties and power of the Drosophila Synthetic Population Resource for the routine dissection of complex traits. Genetics 191 : 935–949 doi:10.1534/genetics.112.138537
19. Mackay TFC, Richards S, Gibbs RA (2008) Proposal to Sequence a Drosophila Genetic Reference Panel: A Community Resource for the Study of Genotypic and Phenotypic Variation: 1–32. Available at https://www.genome.gov/Pages/Research/Sequencing/SeqProposals/DrosophilaSeq.pdf
20. MackayTFC, RichardsS, StoneEA, BarbadillaA, AyrolesJF, et al. (2012) The Drosophila melanogaster Genetic Reference Panel. Nature 482 : 173–178 doi:10.1038/nature10811
21. BloomJS, EhrenreichIM, LooWT, LiteT-LV, KruglyakL (2013) Finding the sources of missing heritability in a yeast cross. Nature 494 : 234–237 doi:10.1038/nature11867
22. BaudA, HermsenR, GuryevV, StridhP, GrahamD, et al. (2013) Combined sequence-based and genetic mapping analysis of complex traits in outbred rats. Nat Genet 45 : 767–775 doi:10.1038/ng.2644
23. CooksonW, LiangL, AbecasisGR, MoffattM, LathropM (2009) Mapping complex disease traits with global gene expression. Nat Rev Genet 10 : 184–194 doi:10.1038/nrg2537
24. GibsonG, WeirB (2005) The quantitative genetics of transcription. Trends Genet 21 : 616–623 doi:10.1016/j.tig.2005.08.010
25. SunG, SchliekelmanP (2011) A Genetical Genomics Approach to Genome Scans Increases Power for QTL Mapping. Genetics 187 : 939–953 doi:10.1534/genetics.110.123968
26. GiladY, RifkinSA, PritchardJK (2008) Revealing the architecture of gene regulation: the promise of eQTL studies. Trends Genet 24 : 408–415 doi:10.1016/j.tig.2008.06.001
27. EhrenreichIM, GerkeJP, KruglyakL (2010) Genetic Dissection of Complex Traits in Yeast: Insights from Studies of Gene Expression and Other Phenotypes in the BYxRM Cross. Cold Spring Harbor Symposia on Quantitative Biology 74 : 145–153 doi:10.1101/sqb.2009.74.013
28. VeyrierasJ-B, KudaravalliS, KimSY, DermitzakisET, GiladY, et al. (2008) High-Resolution Mapping of Expression-QTLs Yields Insight into Human Gene Regulation. PLoS Genet 4: e1000214 doi:10.1371/journal.pgen.1000214.s023
29. GaffneyDJ, VeyrierasJ-B, DegnerJF, Pique-RegiR, PaiAA, et al. (2012) Dissecting the regulatory architecture of gene expression QTLs. Genome Biol 13: R7 doi:10.1186/gb-2012-13-1-r7
30. DegnerJF, PaiAA, Pique-RegiR, VeyrierasJ-B, GaffneyDJ, et al. (2012) DNase I sensitivity QTLs are a major determinant of human expression variation. Nature 482 : 390–394 doi:10.1038/nature10808
31. LappalainenT, SammethM, FriedländerMR, HoenPACT, MonlongJ, et al. (2013) Transcriptome and genome sequencing uncovers functional variation in humans. Nature 501 : 506–511 doi:10.1038/nature12531
32. HuangG-J, ShifmanS, ValdarW, JohannessonM, YalcinB, et al. (2009) High resolution mapping of expression QTLs in heterogeneous stock mice in multiple tissues. Genome Res 19 : 1133–1140 doi:10.1101/gr.088120.108
33. YangJ, FerreiraT, MorrisAP, MedlandSE, MaddenPAF, et al. (2012) Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies addtional variants influencing comlex traits. Nat Genet 44 : 369–375 doi:10.1038/ng.2213
34. XuS (2003) Theoretical basis of the Beavis effect. Genetics 165 : 2259–2268.
35. EdgarR, DomrachevM, LashAE (2002) Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Research 30 : 207–210.
36. BoyleEI, WengS, GollubJ, JinH, BOTSTEIND, et al. (2004) GO::TermFinder–open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics 20 : 3710–3715 doi:10.1093/bioinformatics/bth456
37. RobinsonSW, HerzykP, DowJAT, LeaderDP (2012) FlyAtlas: database of gene expression in the tissues of Drosophila melanogaster. Nucleic Acids Research 41: D744–D750 doi:10.1093/nar/gks1141
38. KohK, EvansJM, HendricksJC, SehgalA (2006) A Drosophila model for age-associated changes in sleep:wake cycles. P Natl Acad Sci Usa 103 : 13843–13847 doi:10.1073/pnas.0605903103
39. MarygoldSJ, LeylandPC, SealRL, GoodmanJL, ThurmondJ, et al. (2012) FlyBase: improvements to the bibliography. Nucleic Acids Research 41: D751–D757 doi:10.1093/nar/gks1024
40. ZinkeI, SchützCS, KatzenbergerJD, BauerM, PankratzMJ (2002) Nutrient control of gene expression in Drosophila: microarray analysis of starvation and sugar-dependent response. EMBO J 21 : 6162–6173.
41. VargheseJ, LimSF, CohenSM (2010) Drosophila miR-14 regulates insulin production and metabolism through its target, sugarbabe. Genes & Development 24 : 2748–2753 doi:10.1101/gad.1995910
42. ZhangX, CalAJ, BorevitzJO (2011) Genetic architecture of regulatory variation in Arabidopsis thaliana. Genome Res 21 : 725–733 doi:10.1101/gr.115337.110
43. KangHM, YeC, EskinE (2008) Accurate Discovery of Expression Quantitative Trait Loci Under Confounding From Spurious and Genuine Regulatory Hotspots. Genetics 180 : 1909–1925 doi:10.1534/genetics.108.094201
44. SmithEN, KruglyakL (2008) Gene–Environment Interaction in Yeast Gene Expression. PLoS Biol 6: e83 doi:10.1371/journal.pbio.0060083.st004
45. LeekJT, StoreyJD (2007) Capturing heterogeneity in gene expression studies by surrogate variable analysis. PLoS Genet 3 : 1724–1735 doi:10.1371/journal.pgen.0030161
46. PickrellJK, MarioniJC, PaiAA, DegnerJF, EngelhardtBE, et al. (2010) Understanding mechanisms underlying human gene expression variation with RNA sequencing. Nature 464 : 768–772 doi:10.1038/nature08872
47. GruberJD, LongAD (2008) Cis-regulatory Variation Is Typically Polyallelic in Drosophila. Genetics 181 : 661–670 Available: http://www.genetics.org/cgi/doi/10.1534/genetics.108.098459.
48. PowellJE, HendersAK, McRaeAF, KimJ, HemaniG, et al. (2013) Congruence of Additive and Non-Additive Effects on Gene Expression Estimated from Pedigree and SNP Data. PLoS Genet 9: e1003502 doi:10.1371/journal.pgen.1003502.s016
49. NuzhdinSV, PasyukovaE, DildaC, ZengZ, MackayTFC (1997) Sex-specific quantitative trait loci affecting longevity in Drosophila melanogaster. P Natl Acad Sci Usa 94 : 9734–9739.
50. KislukhinG, KingEG, WaltersKN, MacdonaldSJ, LongAD (2013) The Genetic Architecture of Methotrexate Toxicity Is Similar in Drosophila melanogaster and Humans. G3 (Bethesda) 3 : 1301–1310 doi:10.1534/g3.113.006619
51. McClellanJ, KingM-C (2010) Genetic Heterogeneity in Human Disease. Cell 141 : 210–217 doi:10.1016/j.cell.2010.03.032
52. KleinRJ (2005) Complement Factor H Polymorphism in Age-Related Macular Degeneration. Science 308 : 385–389 doi:10.1126/science.1109557
53. HughesAE, OrrN, EsfandiaryH, Diaz-TorresM, GoodshipT, et al. (2006) A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat Genet 38 : 1173–1177 doi:10.1038/ng1890
54. LiM, Atmaca-SonmezP, OthmanM, BranhamKEH, KhannaR, et al. (2006) CFH haplotypes without the Y402H coding variant show strong association with susceptibility to age-related macular degeneration. Nat Genet 38 : 1049–1054 doi:10.1038/ng1871
55. MallerJ, GeorgeS, PurcellS, FagernessJ, AltshulerD, et al. (2006) Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet 38 : 1055–1059 doi:10.1038/ng1873
56. SwaroopA, BranhamKE, ChenW, AbecasisG (2007) Genetic susceptibility to age-related macular degeneration: a paradigm for dissecting complex disease traits. Human Molecular Genetics 16: R174–R182 doi:10.1093/hmg/ddm212
57. R Core Team (2013) R: a language and environment for statistical computing. Available: http://www.R-project.org/.
58. BolstadBM, IrizarryRA, AstrandM, SpeedTP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19 : 185–193.
59. IrizarryRA, BolstadBM, CollinF, CopeLM, HobbsB, et al. (2003) Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Research 31: e15.
60. IrizarryRA, HobbsB, CollinF, Beazer-BarclayYD, AntonellisKJ, et al. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4 : 249–264 doi:10.1093/biostatistics/4.2.249
61. CarvalhoBS, IrizarryRA (2010) A framework for oligonucleotide microarray preprocessing. Bioinformatics 26 : 2363–2367 doi:10.1093/bioinformatics/btq431
62. AltschulSF, GishW, MillerW, MyersEW, LipmanDJ (1990) Basic local alignment search tool. J Mol Biol 215 : 403–410 doi:10.1016/S0022-2836(05)80360-2
63. CamachoC, CoulourisG, AvagyanV, MaN, PapadopoulosJ, et al. (2009) BLAST+: architecture and applications. BMC Bioinformatics 10 : 421 doi:10.1186/1471-2105-10-421
64. AlbertsR, TerpstraP, LiY, BreitlingR, NapJ-P, et al. (2007) Sequence polymorphisms cause many false cis eQTLs. PLOS ONE 2: e622 doi:10.1371/journal.pone.0000622
65. BenovoyD, KwanT, MajewskiJ (2008) Effect of polymorphisms within probe-target sequences on olignonucleotide microarray experiments. Nucleic Acids Research 36 : 4417–4423 doi:10.1093/nar/gkn409
66. ChenL, PageGP, MehtaT, FengR, CuiX (2009) Single nucleotide polymorphisms affect both cis - and trans-eQTLs. Genomics 93 : 501–508 doi:10.1016/j.ygeno.2009.01.011
67. CiobanuDC, LuL, MozhuiK, WangX, JagalurM, et al. (2010) Detection, validation, and downstream analysis of allelic variation in gene expression. Genetics 184 : 119–128 doi:10.1534/genetics.109.107474
68. RamasamyA, TrabzuniD, GibbsJR, DillmanA, HernandezDG, et al. (2013) Resolving the polymorphism-in-probe problem is critical for correct interpretation of expression QTL studies. Nucleic Acids Research 41: e88 doi:10.1093/nar/gkt069
69. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760 doi:10.1093/bioinformatics/btp324
70. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079 doi:10.1093/bioinformatics/btp352
71. AulchenkoYS, de KoningD-J, HaleyC (2007) Genomewide rapid association using mixed model and regression: A fast and simple method for genomewide pedigree-based quantitative trait loci association analysis. Genetics 177 : 577–585 doi:10.1534/genetics.107.075614
72. ChurchillGA, DoergeRW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138 : 963–971.
73. ManichaikulA, DupuisJ, SenS, BromanKW (2006) Poor performance of bootstrap confidence intervals for the location of a quantitative trait locus. Genetics 174 : 481–489 Available: http://www.genetics.org/cgi/doi/10.1534/genetics.106.061549.
74. Broman KW, Sen S (2009) A Guide to QTL Mapping with R/qtl. Springer New York.
75. LyckegaardEM, ClarkAG (1989) Ribosomal DNA and Stellate gene copy number variation on the Y chromosome of Drosophila melanogaster. P Natl Acad Sci Usa 86 : 1944–1948.
76. YalcinB (2005) Using Progenitor Strain Information to Identify Quantitative Trait Nucleotides in Outbred Mice. Genetics 171 : 673–681 doi:10.1534/genetics.104.028902
Štítky
Genetika Reprodukčná medicína
Článek Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin PathwayČlánek Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human DiseasesČlánek G×G×E for Lifespan in : Mitochondrial, Nuclear, and Dietary Interactions that Modify LongevityČlánek PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial MatrixČlánek Rapid Evolution of PARP Genes Suggests a Broad Role for ADP-Ribosylation in Host-Virus ConflictsČlánek The Impact of Population Demography and Selection on the Genetic Architecture of Complex TraitsČlánek Lifespan Extension by Methionine Restriction Requires Autophagy-Dependent Vacuolar AcidificationČlánek The Case for Junk DNA
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 5- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Genetic Interactions Involving Five or More Genes Contribute to a Complex Trait in Yeast
- A Mutation in the Gene in Dogs with Hereditary Footpad Hyperkeratosis (HFH)
- Loss of Function Mutation in the Palmitoyl-Transferase HHAT Leads to Syndromic 46,XY Disorder of Sex Development by Impeding Hedgehog Protein Palmitoylation and Signaling
- Heterogeneity in the Frequency and Characteristics of Homologous Recombination in Pneumococcal Evolution
- Genome-Wide Nucleosome Positioning Is Orchestrated by Genomic Regions Associated with DNase I Hypersensitivity in Rice
- Null Mutation in PGAP1 Impairing Gpi-Anchor Maturation in Patients with Intellectual Disability and Encephalopathy
- Single Nucleotide Variants in Transcription Factors Associate More Tightly with Phenotype than with Gene Expression
- Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin Pathway
- Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human Diseases
- Epistatically Interacting Substitutions Are Enriched during Adaptive Protein Evolution
- Meiotic Drive Impacts Expression and Evolution of X-Linked Genes in Stalk-Eyed Flies
- G×G×E for Lifespan in : Mitochondrial, Nuclear, and Dietary Interactions that Modify Longevity
- Population Genomic Analysis of Ancient and Modern Genomes Yields New Insights into the Genetic Ancestry of the Tyrolean Iceman and the Genetic Structure of Europe
- p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
- Whole Exome Re-Sequencing Implicates and Cilia Structure and Function in Resistance to Smoking Related Airflow Obstruction
- Allelic Expression of Deleterious Protein-Coding Variants across Human Tissues
- R-loops Associated with Triplet Repeat Expansions Promote Gene Silencing in Friedreich Ataxia and Fragile X Syndrome
- PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial Matrix
- The Impairment of MAGMAS Function in Human Is Responsible for a Severe Skeletal Dysplasia
- Octopamine Neuromodulation Regulates Gr32a-Linked Aggression and Courtship Pathways in Males
- Mlh2 Is an Accessory Factor for DNA Mismatch Repair in
- Activating Transcription Factor 6 Is Necessary and Sufficient for Alcoholic Fatty Liver Disease in Zebrafish
- The Spatiotemporal Program of DNA Replication Is Associated with Specific Combinations of Chromatin Marks in Human Cells
- Rapid Evolution of PARP Genes Suggests a Broad Role for ADP-Ribosylation in Host-Virus Conflicts
- Genome-Wide Inference of Ancestral Recombination Graphs
- Mutations in Four Glycosyl Hydrolases Reveal a Highly Coordinated Pathway for Rhodopsin Biosynthesis and N-Glycan Trimming in
- SHP2 Regulates Chondrocyte Terminal Differentiation, Growth Plate Architecture and Skeletal Cell Fates
- The Impact of Population Demography and Selection on the Genetic Architecture of Complex Traits
- Retinoid-X-Receptors (α/β) in Melanocytes Modulate Innate Immune Responses and Differentially Regulate Cell Survival following UV Irradiation
- Genetic Dissection of the Female Head Transcriptome Reveals Widespread Allelic Heterogeneity
- Genome Sequencing and Comparative Genomics of the Broad Host-Range Pathogen AG8
- Copy Number Variation Is a Fundamental Aspect of the Placental Genome
- GOLPH3 Is Essential for Contractile Ring Formation and Rab11 Localization to the Cleavage Site during Cytokinesis in
- Hox Transcription Factors Access the RNA Polymerase II Machinery through Direct Homeodomain Binding to a Conserved Motif of Mediator Subunit Med19
- Drosha Promotes Splicing of a Pre-microRNA-like Alternative Exon
- Predicting the Minimal Translation Apparatus: Lessons from the Reductive Evolution of
- PAX6 Regulates Melanogenesis in the Retinal Pigmented Epithelium through Feed-Forward Regulatory Interactions with MITF
- Enhanced Interaction between Pseudokinase and Kinase Domains in Gcn2 stimulates eIF2α Phosphorylation in Starved Cells
- A HECT Ubiquitin-Protein Ligase as a Novel Candidate Gene for Altered Quinine and Quinidine Responses in
- dGTP Starvation in Provides New Insights into the Thymineless-Death Phenomenon
- Phosphorylation Modulates Clearance of Alpha-Synuclein Inclusions in a Yeast Model of Parkinson's Disease
- RPM-1 Uses Both Ubiquitin Ligase and Phosphatase-Based Mechanisms to Regulate DLK-1 during Neuronal Development
- More of a Good Thing or Less of a Bad Thing: Gene Copy Number Variation in Polyploid Cells of the Placenta
- More of a Good Thing or Less of a Bad Thing: Gene Copy Number Variation in Polyploid Cells of the Placenta
- Heritable Transmission of Stress Resistance by High Dietary Glucose in
- Revertant Mutation Releases Confined Lethal Mutation, Opening Pandora's Box: A Novel Genetic Pathogenesis
- Lifespan Extension by Methionine Restriction Requires Autophagy-Dependent Vacuolar Acidification
- A Genome-Wide Assessment of the Role of Untagged Copy Number Variants in Type 1 Diabetes
- Selectivity in Genetic Association with Sub-classified Migraine in Women
- A Lack of Parasitic Reduction in the Obligate Parasitic Green Alga
- The Proper Splicing of RNAi Factors Is Critical for Pericentric Heterochromatin Assembly in Fission Yeast
- Discovery and Functional Annotation of SIX6 Variants in Primary Open-Angle Glaucoma
- Six Homeoproteins and a linc-RNA at the Fast MYH Locus Lock Fast Myofiber Terminal Phenotype
- EDR1 Physically Interacts with MKK4/MKK5 and Negatively Regulates a MAP Kinase Cascade to Modulate Plant Innate Immunity
- Genes That Bias Mendelian Segregation
- The Case for Junk DNA
- An In Vivo EGF Receptor Localization Screen in Identifies the Ezrin Homolog ERM-1 as a Temporal Regulator of Signaling
- Mosaic Epigenetic Dysregulation of Ectodermal Cells in Autism Spectrum Disorder
- Hyperactivated Wnt Signaling Induces Synthetic Lethal Interaction with Rb Inactivation by Elevating TORC1 Activities
- Mutations in the Cholesterol Transporter Gene Are Associated with Excessive Hair Overgrowth
- Scribble Modulates the MAPK/Fra1 Pathway to Disrupt Luminal and Ductal Integrity and Suppress Tumour Formation in the Mammary Gland
- A Novel CH Transcription Factor that Regulates Expression Interdependently with GliZ in
- Phosphorylation of a WRKY Transcription Factor by MAPKs Is Required for Pollen Development and Function in
- Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics
- Spermatid Cyst Polarization in Depends upon and the CPEB Family Translational Regulator
- Insights into the Genetic Structure and Diversity of 38 South Asian Indians from Deep Whole-Genome Sequencing
- Intron Retention in the 5′UTR of the Novel ZIF2 Transporter Enhances Translation to Promote Zinc Tolerance in
- A Dominant-Negative Mutation of Mouse Causes Glaucoma and Is Semi-lethal via LBD1-Mediated Dimerisation
- Biased, Non-equivalent Gene-Proximal and -Distal Binding Motifs of Orphan Nuclear Receptor TR4 in Primary Human Erythroid Cells
- Ras-Mediated Deregulation of the Circadian Clock in Cancer
- Retinoic Acid-Related Orphan Receptor γ (RORγ): A Novel Participant in the Diurnal Regulation of Hepatic Gluconeogenesis and Insulin Sensitivity
- Extensive Diversity of Prion Strains Is Defined by Differential Chaperone Interactions and Distinct Amyloidogenic Regions
- Fine Tuning of the UPR by the Ubiquitin Ligases Siah1/2
- Paternal Poly (ADP-ribose) Metabolism Modulates Retention of Inheritable Sperm Histones and Early Embryonic Gene Expression
- Allele-Specific Genome-wide Profiling in Human Primary Erythroblasts Reveal Replication Program Organization
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial Matrix
- Null Mutation in PGAP1 Impairing Gpi-Anchor Maturation in Patients with Intellectual Disability and Encephalopathy
- Phosphorylation of a WRKY Transcription Factor by MAPKs Is Required for Pollen Development and Function in
- p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy







