-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Positive Selection and Multiple Losses of the LINE-1-Derived Gene in Mammals Suggest a Dual Role in Genome Defense and Pluripotency
Transposable elements comprise major portions of most animal genomes and are selfish genetic elements that may encode proteins needed for their own spread to new genomic locations. Though often considered genomic parasites, these elements also occasionally create novel genes that prove beneficial to the host, a process called 'domestication'. Here, we describe the evolution of a gene, L1TD1, which is derived from the protein-coding regions of the L1 mobile element family. We show that L1TD1 was born in the common ancestor of placental mammals. L1TD1 expression in stem cells and its requirement to maintain the pluripotent state of human embryonic stem cells suggested it might have been originally domesticated for such a pluripotency role. We find that L1TD1's evolution does not fit with the predictions of this model; in fact, L1TD1 has rapidly evolved in primates and mice and has been lost several times in mammals. We suggest an alternate model that L1TD1 was born as a means to defend genomes against transposable elements, perhaps L1 itself. We propose that following this initial domestication, L1TD1 later became incorporated into pluripotency programs in some mammalian lineages.
Published in the journal: Positive Selection and Multiple Losses of the LINE-1-Derived Gene in Mammals Suggest a Dual Role in Genome Defense and Pluripotency. PLoS Genet 10(9): e32767. doi:10.1371/journal.pgen.1004531
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004531Summary
Transposable elements comprise major portions of most animal genomes and are selfish genetic elements that may encode proteins needed for their own spread to new genomic locations. Though often considered genomic parasites, these elements also occasionally create novel genes that prove beneficial to the host, a process called 'domestication'. Here, we describe the evolution of a gene, L1TD1, which is derived from the protein-coding regions of the L1 mobile element family. We show that L1TD1 was born in the common ancestor of placental mammals. L1TD1 expression in stem cells and its requirement to maintain the pluripotent state of human embryonic stem cells suggested it might have been originally domesticated for such a pluripotency role. We find that L1TD1's evolution does not fit with the predictions of this model; in fact, L1TD1 has rapidly evolved in primates and mice and has been lost several times in mammals. We suggest an alternate model that L1TD1 was born as a means to defend genomes against transposable elements, perhaps L1 itself. We propose that following this initial domestication, L1TD1 later became incorporated into pluripotency programs in some mammalian lineages.
Introduction
Retroelements have profoundly shaped mammalian genomes over millions of years. Insertion of these selfish elements can lead to gene inactivation or changes in transcriptional profiles of neighboring genes. Moreover, the presence of large stretches of almost identical sequence distributed across the genome poses recombinational hazards, leading to chromosomal rearrangements, often with pathological consequences. With a few celebrated exceptions [1], the retrotransposition activity of retroelements is rarely beneficial [2]. However, individual transposition events can occasionally drive dramatic episodes of adaptation through the generation of genetic novelty [3]. Indeed, repetitive elements have often been co-opted as transcriptional regulatory elements like promoters, enhancers, and insulators [4].
One particularly striking class of such genetic novelty results from the ‘domestication’ or ‘capture' of retroelement - and retrovirus-derived protein-coding genes by host genomes, a process by which these domesticated coding regions are exapted for a new function that is beneficial to the host [5], [6]. Such instances are often recognized by the preservation of individual protein-coding frames despite the mutational decay of the rest of the parental retroelement. This extinction of the replication-competent retroelement but not its protein-coding gene implies that selection for the benefit of the host genome must have prevented the mutational attrition of that particular gene.
Retroelements and retroviruses must integrate into the host genome as part of their replication cycle, presenting the host with a source of potentially advantageous protein-coding sequences. The syncytin genes of eutherian mammals represent some of the best characterized examples of such domestication events in which envelope genes from ancient retroviruses have been preserved for their membrane-fusing and/or immunosuppressive activities in the syncytiotrophoblast, the layer of the placenta which mediates maternal-fetal nutritional transfer [7]–[9]. In fact, loss of syncytin-A in mice is embryonic lethal, consistent with its indispensable role in placental function [10]. Syncytin genes thus represent a dramatic example of the maintenance and possibly invention of an essential function, placentation, via retroviral gene domestication. Importantly, syncytin is not unique in this regard; similar domestications of sushi-ichi LTR retrotransposon protein-coding regions have also created multiple host genes (e.g., Peg10) involved in genomic imprinting and placentation [11], [12].
Though syncytin represents a dramatic and beneficial genetic innovation, not all domesticated retroviral genes serve conserved, essential functions. The Fv1 gene in mice represents a domesticated gag (viral capsid and nucleocapsid encoding) gene, which can actively interfere with the uncoating of incoming retroviral capsids [13], [14]. As expected for this genome defense role, Fv1 is under strong diversifying selection, presumably as a result of constant innovation required to recognize and block different retroviruses [15]. As a result of this diversifying selection to chase the sequence of incoming capsids, orthologous genes can have dramatically different antiviral specificities [16]–[19]. Fv1 has also been lost or pseudogenized at least twice in the Mus genus [15], perhaps because retention of Fv1 may depend on persistence of selection from incoming viruses.
Mammalian genomes acquired both syncytin and Fv1 genes as a result of the insertion of a retrovirus into the germline. Though such endogenous retroviruses have markedly impacted the mammalian genome, these elements have spent relatively little time coevolving with their host compared to the non-LTR retroposons, which date back at least to the origin of Metazoa [20]. The LINE-1 (Long INterspersed Element-1, L1) non-LTR retroposons make up a significant fraction of the human genome. Given their ancient history of coevolution with mammalian genomes, it is not surprising that there are numerous examples of exaptation of non-LTR retroelements into non-coding RNAs, as promoter or other regulatory elements, or as small portions of a coding region [4], . Many coding region exaptations are "exonizations", where cryptic splice sites within an intronic repetitive element are utilized so that a portion of the retroposon is incorporated as a novel exon of a host gene transcript, often as a minor splice isoform of the gene [25]–[28]. For example, a portion of an RTE non-LTR element was exapted as an additional coding exon of the existing ruminant bucentaur gene [29]. In contrast, only a single instance of a novel protein coding gene formed entirely anew from the domestication of a non-LTR retroposon has been described – L1TD1, or LINE-1 type Transposase Domain-containing 1 (L1TD1 has no known enzymatic activity despite its designation as a ‘transposase’ domain, see below). Though originally identified among a set of genes specifically expressed in murine embryonic stem cells, hence its original name ECAT11 (Embryonic Stem Cell Associated Transcript 11) [30], L1TD1's origins and evolution remain poorly characterized.
In both human and mouse, L1TD1 expression is high in undifferentiated stem cells and decreases precipitously upon differentiation [31]. In humans, L1TD1 is intricately linked with the cellular program for pluripotency maintenance, where it functions in a regulatory network with the core stem factors NANOG, SOX2, and OCT4. L1TD1 expression appears to be tightly controlled by these pluripotency factors, which all bind the L1TD1 promoter [32]. Depletion of L1TD1 in human ES cells results in an immediate downregulation of OCT4 (also known as POU5F1) and NANOG, and is sufficient to induce differentiation. In contrast, L1TD1 in mice is completely dispensable for the maintenance and induction of pluripotency [30], [33]. These studies suggest that despite similarities in stem-cell specific expression, the function of L1TD1 in pluripotency maintenance may have changed since the common ancestor of rodents and primates.
We wished to understand how a retroelement-derived gene could become incorporated into such an important cellular process in mammalian cells. To this end, we traced the evolutionary origins, history, and selective pressures of the L1TD1 gene in mammals. In contrast to the expectation that L1TD1 would be an essential gene if it had been domesticated for its function in mammalian pluripotency, we find several cases where L1TD1 has been lost as well as several cases of selection for genetic innovations in L1TD1. This leads us to propose an evolutionary transition model wherein L1TD1 was first retained for a role in genome defense in stem cells and germline (similar to Fv1). Subsequently, L1TD1 may have been exapted for an essential function (similar to syncytin) in pluripotency maintenance in a subset of eutherian mammals.
Results
L1TD1 originated in the common ancestor of placental mammals
To gain insight into the cellular function of L1TD1, we decided to date its evolutionary origin and examine how its gene structure has changed since its birth. Since the only published analysis examined five mammalian genomes for its presence [33], we decided to search for the L1TD1 gene in a much larger sample of diverse mammalian genomes. In the human and mouse genomes, L1TD1 is found between the single-copy INADL and KANK4 genes (Figure 1A). We found that all mammals and even bird genomes encode INADL and KANK4 in close proximity to each other. We therefore used these flanking genes to identify the syntenic locus in other mammalian genomes, and used the sequence from this locus to identify L1TD1 coding sequences where present. We also extended our search to the rest of the genome, using repeat-masked and unmasked human and mouse L1TD1 sequences as BLAST search queries. We were never able to identify an intact copy of L1TD1 outside the INADL-KANK4 syntenic locus, although we did find some pseudogenes with obvious inactivating mutations in other locations. Although the previously published analysis [33] suggested that dog L1TD1 lacks a portion of coding exon 1, in fact we find that L1TD1 is complete and intact in the dog genome (Table S2).
Fig. 1. The mammalian L1TD1 gene was born from a tandem insertion of L1 ORF1. 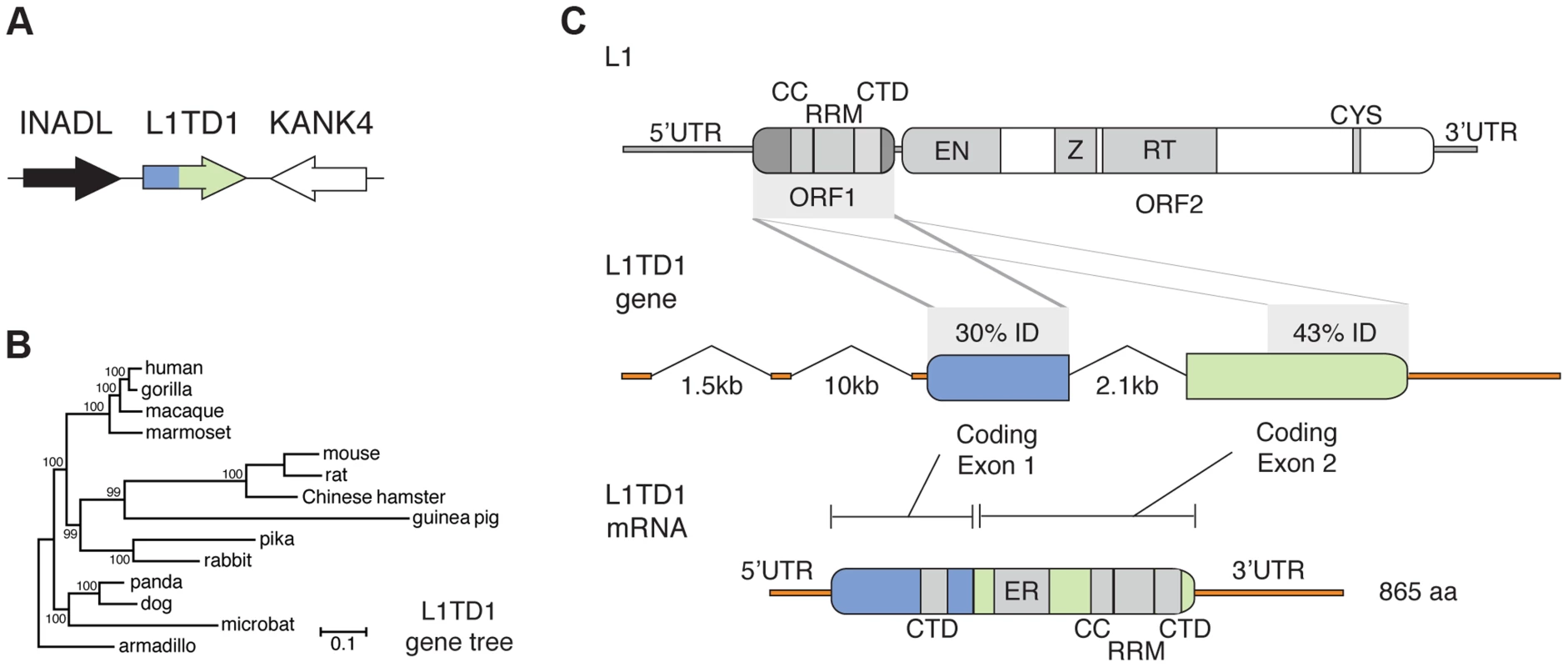
A. Genomic context of L1TD1. Single-copy genes INADL and KANK4 flank L1TD1 in human, mouse and dog genomes; this shared syntenic arrangement helped us identify L1TD1 orthologs in other mammalian genomes. B. L1TD1 evolved according to the accepted species tree. We aligned L1TD1 nucleotide sequences and generated a maximum-likelihood phylogeny (see Materials and Methods). Bootstrap values show the percentage of 1000 replicates in which descendent taxa cluster together, and the scale bar shows substitutions per site according to the GTR+I+G evolutionary model. C. L1TD1 comprises two L1-ORF1p-like regions. L1 elements encode an approximately 6.5 kb transcript containing two open reading frames. L1 ORF1 encodes a protein, ORF1p, with RNA-binding and chaperone activity. ORF1p contains a coiled-coil motif (CC), a RNA-recognition motif (RRM), and a C-terminal domain (CTD). ORF2 encodes a protein with endonuclease and reverse transcription enzymatic functions. Sequence identity demonstrates that L1TD1 was formed from the domestication of two copies of ORF1 from L1. The two copies may derive from independent insertions or from duplication after a single insertion. Human coding exon 1 and coding exon 2 share 30% and 43% amino acid identity, respectively, with ORF1p of human L1.3. In coding exon 1, only the CTD is conserved, while in coding exon 2, the CC, RRM, and CTD are all conserved. Coding exon 2 also contains a variable length glutamic acid-rich region (ER). After splicing, the human L1TD1 transcript is 3849 nucleotides in length and encodes a single 865 amino acid protein product. We found L1TD1 in many diverse placental mammalian genomes (Table S2, Dataset S1). However, L1TD1 is absent from both marsupial and platypus genomes. This species distribution implies that the L1TD1 gene was born in the common ancestor of placental mammals, after the split from marsupials. A phylogenetic tree using L1TD1 nucleotide sequences follows the expected species tree (Figure 1B). Together with the observed shared syntenic location, this tree demonstrates that the sequences we identified represent truly orthologous genes rather than several independent L1 domestication events. This dates the origin of L1TD1 to at least 106 million years ago, making it more ancient than the well-known domesticated retroelement genes syncytin and Fv1 [9], [15].
Two L1 ORF1-homologous regions constitute the L1TD1 gene
Human L1TD1 (RefSeq NM_001164835.1) comprises two protein-coding exons (of four total exons), which together encode an 865 amino acid protein. Each of these two exons is homologous to the first open reading frame (ORF1) of L1 (Figure 1C), whose protein product (ORF1p) functions as an RNA-binding protein that greatly enhances L1 retrotransposition [34]. ORF1p appears to be important in ensuring L1 ‘cis-preference’ - the preference for the ORF2 protein (ORF2p) to act upon the same RNA from which it was translated, so that an L1's machinery is less often ‘hijacked’ by other elements or other L1s [34]–[36]. L1TD1 has no discernible homology to the other open reading frame of L1, ORF2, which encodes the enzymatic activities of L1. The second coding exon of L1TD1 has higher conservation with ORF1p, showing 43% amino acid identity (58% amino acid similarity) with human L1 (L1.3), and apparent preservation of the coiled-coil, RNA-recognition, and C-terminal motifs of L1-ORF1p (Figure 1C). In contrast, the first coding exon retains only 30% amino acid identity (36% similarity) with ORF1p (L1.3), preserving the C-terminal domain but not the coiled-coil or RNA-recognition motif. This greater sequence identity in the second coding exon could either reflect a more recent origin from L1-ORF1 or greater constraint to preserve ancestral L1-ORF1p like functions. Coding exon 2 also encodes a ∼300 amino acid low-complexity glutamic acid-rich region that separates the two ORF1p-like regions. The annotated mouse L1TD1 gene (RefSeq NM_001081202.1) is similar to human L1TD1 but includes an extra intron that removes ∼200 nucleotides encoding ∼70 amino acids of this low-complexity region. However, we find that the reading frame is maintained through this mouse intron, and EST data suggest that it is often retained in the mature mouse L1TD1 transcript. Overall, we find that L1TD1 has maintained conservation with ORF1p of L1 despite its independent evolution for tens of millions of years; this suggests some aspect of L1 ORF1p function may still be utilized in L1TD1.
To discern the order of the L1 domestication events that led to the origin of L1TD1 and to confirm our dating of its birth, we generated a phylogeny of the two L1TD1 ORF1p-like amino acid sequences together with representative L1 ORF1p sequences from diverse mammalian genomes (Figure 2). We find that L1TD1 N-terminal regions (the protein product of coding exon 1) group together with strong bootstrap values (node A), as do L1TD1 C-terminal regions (the protein product of coding exon 2) (node B), demonstrating that the double ORF1p structure arose just once since the divergence of placental mammals and has not been subject to gene conversion between the two exons since. Furthermore, the L1TD1 N-terminal and C-terminal clades branch off from placental mammal L1-ORF1p sequences (node C) after marsupial and placental mammal L1-ORF1p sequences diverged (node D). This supports our conclusion that L1TD1 was born via L1 domestication in the ancestral eutherian mammal. Our phylogeny cannot distinguish whether the two homologous regions of L1TD1 derived from two independent L1 insertions in proximity to each other, or whether a single L1 inserted and subsequently experienced tandem duplication. Examination of the relative splice acceptor positions in coding exons 1 and 2 also does not help to distinguish these two possibilities. Nevertheless, it does appear that both coding exons were born in close temporal and physical proximity to each other, giving rise to the ancestral L1TD1 gene. Our analyses further show that this bipartite double-ORF1p organization of L1TD1 has been conserved since its birth.
Fig. 2. Phylogenetic tree of representative L1-ORF1p sequences and mammalian orthologs of the two L1TD1 ORF1p-like regions. 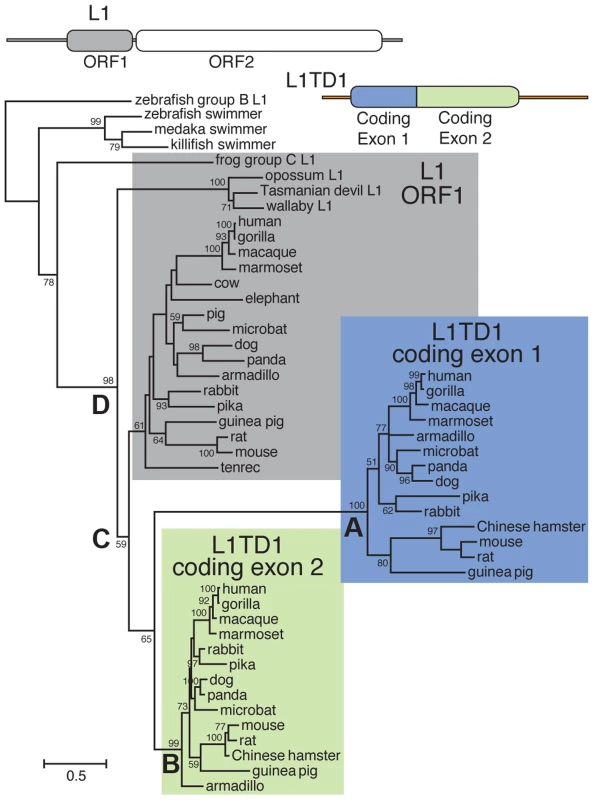
Predicted protein sequences of L1 ORF1p and the ORF1p-like regions encoded by each L1TD1 exon were aligned and this alignment was used to generate a maximum-likelihood tree (see Materials and Methods). Bootstrap values show percentage of 1000 replicate trees in which the descendent taxa clustered together (only values >50% shown). The scale shows the number of substitutions per site. The tree was rooted using fish swimmer elements as outgroups. The N-terminal L1TD1 ORF1p-like sequences cluster together with a high bootstrap value (node A), as do the C-terminal L1TD1 ORF1p-like sequences (node B), confirming that we have identified true L1TD1 orthologs and that the double ORF1p structure arose just once since the divergence of placental mammals and has not been subject to gene conversion between the two exons since. The L1TD1 N-terminal and C-terminal clades branch off from placental mammal L1-ORF1p sequences (node C) after marsupial and placental mammal L1-ORF1p sequences diverged (node D). The tree does not help to distinguish whether L1 ORF1 was independently domesticated twice, or just once with a subsequent genomic tandem duplication. Extensive sequence divergence between paralogous ORF1p-like sequence means that deep nodes of the tree are poorly resolved. Nonetheless, the tree supports our model in which both L1TD1 exons were born after marsupials and placental mammals diverged. Multiple, independent losses of L1TD1 in mammals
Despite its widespread conservation, our genome-wide searches nevertheless revealed that L1TD1 has been lost on at least three separate occasions during the evolution of placental mammals (Figure 3). First, we find that L1TD1 is missing in all three Afrotherian genomes we surveyed – elephant, hyrax and tenrec. These three species constitute an approximately 85 million-year-old subclade. However, we find L1TD1 is intact and present in the armadillo genome, which diverged from the three sequenced members of Afrotheria nearly 100 million years ago. Using parsimony, we infer that L1TD1 was lost once in the ancestor of elephant, hyrax and tenrec between 84 and 100 million years ago.
Fig. 3. L1TD1 has been lost multiple times in eutherian mammals. 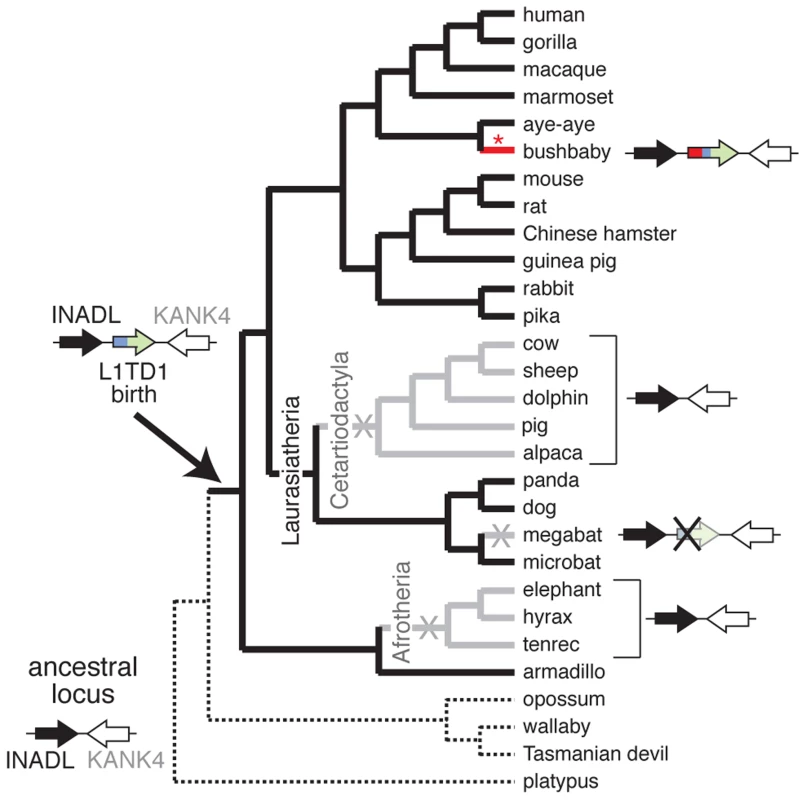
A species tree shows the presence or absence of L1TD1 across mammals. Arrows depict L1TD1's genomic locus; black (INADL) and white (KANK4) arrows depict the flanking genes we used to identify syntenic regions, and the blue/green arrow depicts L1TD1. L1TD1's presence in the armadillo genome but not in platypus, opossum, wallaby or Tasmanian devil indicates it was most likely born before the divergence of placental mammals, but after divergence from marsupials (solid branches). L1TD1 function was lost in three lineages (X's, and gray branches); it is present as a pseudogene in megabat and entirely missing from Afrotherian and Cetartiodactylan genomes. The bushbaby L1TD1 gene acquired a novel N-terminal region (depicted in red) through a more recent L1 ORF1p domestication event (red asterisk) that occurred after bushbaby diverged from lemurs (Figure 4B). Second, we find that L1TD1 is missing from the genomes of all Cetartiodactyla. This clade of mammals originated 64 million years ago and comprises even-toed ungulates, whales, and dolphins. All sequenced genomes of this clade (cow, sheep, dolphin, pig and alpaca, Figure 3) lack L1TD1. In contrast to its loss in Cetartiodactyla, L1TD1 is present in nearly all other members of the 80 million year old Laurasiatheria clade, which includes Cetartiodactyla. The most parsimonious explanation is that the common ancestor of Cetartiodactyla lost L1TD1 between 64 and 80 million years ago.
In both the Afrotheria and Cetartiodactyla, we find no trace of the L1TD1 gene anywhere in the genome, perhaps indicating loss of L1TD1 via genomic deletions of the whole gene. However, given that the Afrotheria and Cetartiodactyla loss events likely occurred at least 64 and 84 million years ago, it is equally likely that after pseudogenization the L1TD1 sequence has simply degenerated beyond recognition in these lineages.
The third instance of loss of L1TD1 occurred in the genome of the megabat Pteropus vampyrus (Figure 3). The microbat Myotis lucifugus still encodes an intact L1TD1, but P. vampyrus contains a pseudogenized version of L1TD1 with multiple frameshifts and stop codons. In contrast to the complete lack of a recognizable L1TD1 in the Afrotheria and Cetartiodactyla, L1TD1 is still discernible in the megabat, suggesting this loss may be more recent.
These three loss events in mammals strongly challenge the possibility that L1TD1 was born into a role of pluripotency maintenance in the common ancestor of placental mammals, as we would expect such a gene to be essential and not subject to subsequent loss. It is formally possible that these lineages independently acquired another L1TD1-like activity that allowed the loss of the originally acquired L1TD1. However, it is more parsimonious that L1TD1's role in pluripotency maintenance was acquired much later than its birth.
The loss of L1TD1 in mammals parallels the multiple losses of the Fv1 restriction factor in the Mus genus [15]. We therefore considered whether, like Fv1, L1TD1 may have rapidly evolved under positive selection due to some role in a genetic conflict.
Genetic innovation of primate and mouse L1TD1 supports a ‘genetic conflict’ hypothesis
If L1TD1 were indeed playing a role in genome defense against some pathogen, one hallmark of the ensuing conflict might be the rapid evolution of L1TD1 coding sequence, a signature commonly seen at many host-virus interaction interfaces [37]. Such rapid change would be expected if the coevolving pathogen constantly evolved to evade L1TD1 recognition, in which case L1TD1 would be expected to rapidly evolve to ‘re-establish’ recognition of the rogue element. Indeed, it has been found that primate L1 ORF1p experienced an episode of adaptive evolution [38], consistent with the idea that L1 could be evolving to escape some sort of genome defense factor.
Due to the finding of positive selection on primate L1 ORF1p [38] and the availability of multiple primate genome assemblies and DNA samples, we first focused on characterizing signatures of evolutionary selection in L1TD1 of primates. Through database searches and PCR-based sequencing of genomic DNA samples, we assembled 18 L1TD1 sequences spanning the simian primate phylogeny, representing more than 40 million years of evolution (Figure 4A). Consistent with L1TD1's important role in pluripotency maintenance in human ES cells, we found no instances of L1TD1 loss or pseudogenization in primates. Overall, we found a high degree of conservation in L1TD1 in primates, with average dN/dS of ∼0.5 over the primate phylogeny. dN/dS is a normalized ratio indicating whether amino acid-altering evolutionary changes occur more often than expected given the rate of neutral mutations, with values <1 indicating overall conservation, and values >1 indicating overall positive selection.
Fig. 4. Novelty in L1TD1 of primates and mice. 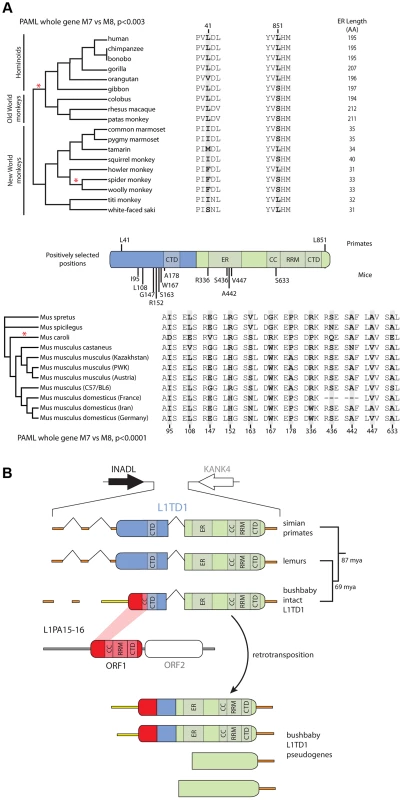
A. Site-specific PAML analyses reveal a signature of positive selection in L1TD1. The labels on the annotated schematic indicate positions that are highly likely to be evolving under positive selection (P>90%) according to PAML NSsites (Table 1) in primates and mice (above and below the gene diagram, respectively). A species tree of primates or an L1TD1 gene tree of mice, shows branches with statistically significant episodic diversifying selection (p<0.05) according to HyPhy's Branch-site REL (marked with a red asterisk). To the right of each tree, the amino acids found at each positively selected position are shown, along with the length of the glutamic acid-rich region in each primate. Position numberings are based upon the human and M. musculus (C57/BL6) sequences. B. The L1TD1 gene of bushbaby has acquired a novel 5′ end of coding exon 1 through the insertion of a portion of a L1 element from the L1PA15-16 class (shown in red). The gene retains high sequence conservation with L1TD1 of lemurs and simian primates across the latter half of its first coding exon and all of its second coding exon (shown in blue and green). This insertion is unique amongst all the species we have examined, and is not evident in lemur genomes (mouse lemur or aye-aye). Elsewhere in the genome, bushbaby contains at least two complete and two partial processed L1TD1 pseudogenes that allowed us to infer the structure of the active L1TD1 gene. Though we observe an overall signature of purifying selection in L1TD1, there could nonetheless be signatures of diversifying selection occurring on just a few domains or residues. To assess whether positive selection has acted on primate L1TD1, we used maximum likelihood methods (NSsites models in the PAML suite [39]) to test for positive selection at individual codons. We found that a model permitting positive selection in the alignment fit the primate data significantly better than models that disallow positive selection (M8 vs. M7, M8 vs. M8a, p<0.01). Both coding exons show evidence of positive selection, suggesting that both domesticated ORF1p-like regions have been recurrently selected for functional novelty. Further, the positive selection is remarkably localized. Only a few L1TD1 codons (∼1%) show a signature of positive selection, but these codons have a high average dN/dS (>8, Table 1). Such highly localized signatures of recurrent positive selection often represent direct contacts with antagonistic entities (discussed below). We note there is no overlap between the positively selected sites in primate L1TD1 and the regions previously found in L1 ORF1p, which are largely in the coiled-coil domain [38], [40]. FUBAR, carried out in the HyPhy suite of programs confirmed our finding of highly localized positive selection (Materials and Methods, Table 1) (Murrell, 2013 #).
Tab. 1. Primate and mouse L1TD1 are evolving under positive selection. 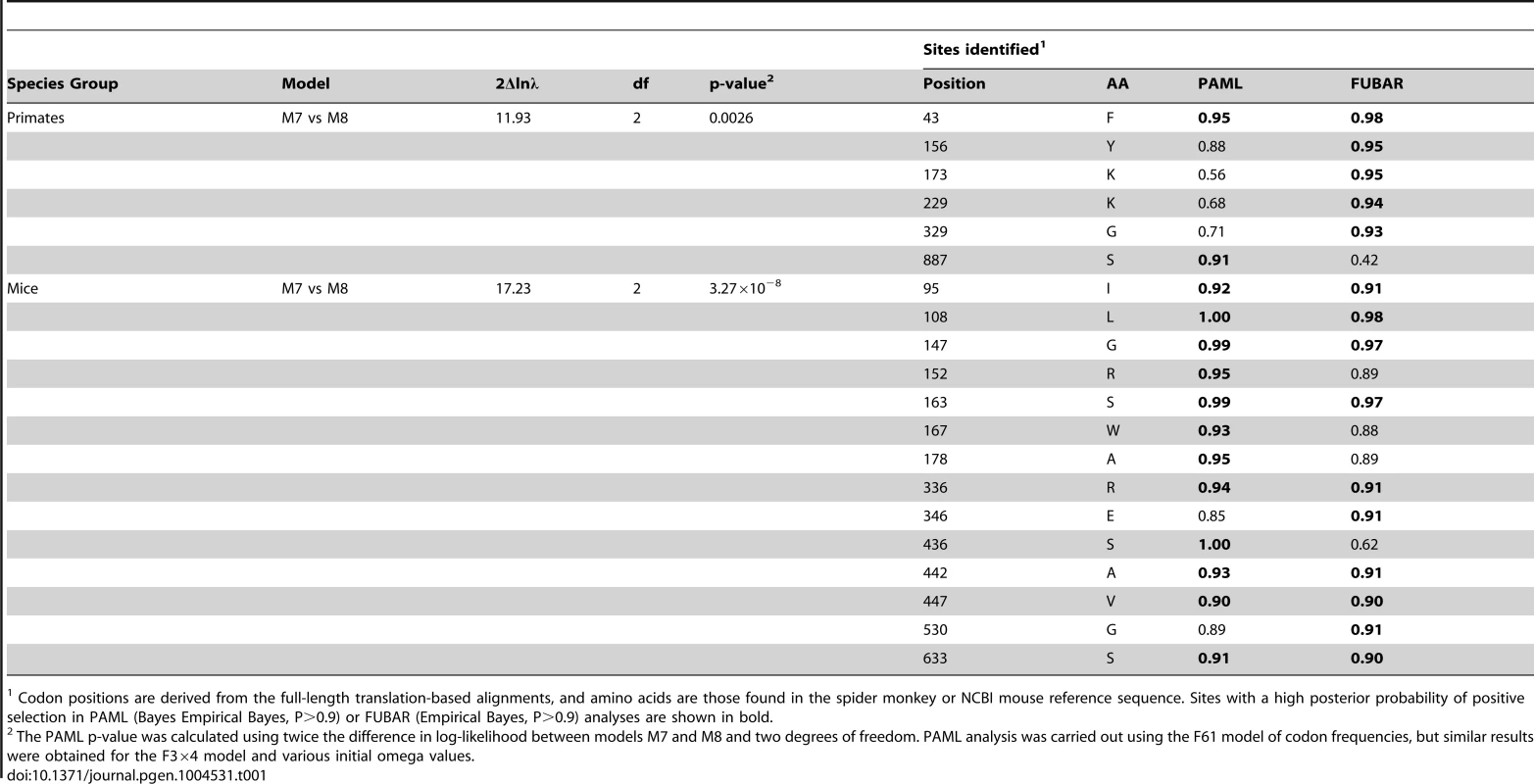
Codon positions are derived from the full-length translation-based alignments, and amino acids are those found in the spider monkey or NCBI mouse reference sequence. Sites with a high posterior probability of positive selection in PAML (Bayes Empirical Bayes, P>0.9) or FUBAR (Empirical Bayes, P>0.9) analyses are shown in bold. Since we hypothesize that the ancestral function of L1TD1 may have been genome defense, we expect that L1TD1 should have evolved under positive selection within many branches of the mammalian phylogeny. To generalize our finding of pervasive positive selection in the primates, we analyzed the evolution of L1TD1 within the genus Mus. We assembled 10 complete L1TD1 sequences from databases, PCR-based sequencing, and RNA-seq data. Again, all species contained an intact coding sequence with a high degree of conservation (average dN/dS = 0.46). However, similar to the primate analysis, PAML NSsites found a small proportion of sites with a high average dN/dS (∼3%, dN/dS >12), as well as a highly significant gene-wide signature of positive selection (Figure 4A; M8 vs. M7, M8 vs. M8a, p<0.01). We also found statistical support using FUBAR for 8/12 positions identified as positively selected by PAML (Table 1; PAML M7 vs M8 BEB, P>0.9; FUBAR P>0.9). To eliminate any false positive selection signals that could arise from phylogenetic discordance, we used the HyPhy program GARD to identify potential recombination breakpoints in the Mus L1TD1 alignment [41]. The alternative phylogenies given by GARD attempt to correct for any recombination that may have occurred and given rise to a scenario where no single tree accurately fits the entire L1TD1 sequence. While GARD found no statistically significant breakpoints according to the KH test, we nonetheless tested whether Mus L1TD1 retained a statistically significant signature of positive selection integrating the generated alternative phylogenies (Figure S1). Using the GARD-generated trees, we still observed a strong signature of gene-wide positive selection according to PAML (p<0.01) and FUBAR still identified 7 positively selected positions.
In addition to phylogeny-wide selection at a few specific codons, branch-specific analyses of dN/dS ratios highlighted episodic positive selection along a few specific branches of the primate phylogeny including the branch leading to the common ancestor of spider monkey and titi monkey, as well as the branch leading to the OWMs and hominoids (Figure 4A, red asterisks, Branch-site REL, p<0.05) [41]. Using PAML, the branch leading to the common ancestor of gorilla, human and chimpanzees (after the split from orangutan) showed a whole gene dN/dS of 2.87 (1 synonymous change and 11 nonsynonymous changes); the branch preceding it shows a whole gene dN/dS of 1.671 (1 synonymous change, 5 nonsynonymous changes). While neither of these ratios is significantly greater than one, it is notable that these two branches span a time window ∼9–20 Mya, overlapping the ∼40–12 Mya time period in the lineage leading to humans shown to exhibit positive selection in L1-ORF1 and of particularly intense L1 activity [38].
Our finding that L1TD1 has evolved under positive selection in primates and mice would be unexpected if its sole function was in pluripotency; we would expect such genes to be highly conserved. We excluded an intriguing alternative possibility that genes involved in pluripotency might not be evolving as slowly as one would intuitively assume. In a genome-wide analysis of dN/dS values calculated from trios of human-chimpanzee-macaque orthologs [42], genes identified in a screen for determinants of ES cell identity [43] are indeed evolving more slowly than control genes (Wilcoxon p = 0.008; Figure S2). Thus, our finding of positive selection in L1TD1 is indeed unexpected if its only role were in pluripotency maintenance.
We cannot evaluate the extent to which positive selection has shaped protein regions that have experienced length-changing insertion-deletion changes, because they are unsuitable for codon-based analyses of positive selection. However, we note significant divergence in the glutamic-acid rich region of L1TD1 that separates the two L1 ORF1p homology regions. For instance, New World monkeys have a deletion of ∼140 amino acids at the beginning of L1TD1 coding exon 2 relative to the Old World monkeys and hominoids. The functional significance as well as the selective pressures that might have driven these changes is unknown; it is quite possible that these changes are completely neutral and have little impact on L1TD1 function.
We also discovered a substantial restructuring of the L1TD1 gene of bushbaby (Otolemur garnettii) that may have a significant impact on its function. In the bushbaby genome, we found that the latter two-thirds of the L1TD1 coding region are completely typical and align well with orthologous sequences from other primates and mammals, with no stop codons or frameshifts. However, the first one-third of bushbaby L1TD1 is not orthologous to any other L1TD1 (Figure 4B). Instead, it appears the bushbaby L1TD1 has acquired an entirely novel 5' end. Two processed L1TD1 pseudogenes in the bushbaby genome also include this novel 5' end. Since processed pseudogenes lack introns, they provide independent confirmation of the transcript structure of L1TD1. On closer examination, the novel 1400 nucleotides at the beginning of the bushbaby L1TD1 includes ∼360 nucleotides of protein-coding sequence from ORF1p of a L1 element of the L1PA15-16 class, a primate-specific L1 element. The remaining ∼1000-nucleotide 5′ UTR region comprises a patchwork of several other repetitive elements, and likely arose by a series of nested insertion events. Although the repetitive element portions of this 5' UTR are found in several other loci in the bushbaby genome, the only places they are found in this particular combination are at the L1TD1 syntenic locus and in the L1TD1 processed pseudogenes (Figure 4B). Thus, it appears that bushbaby L1TD1 has undergone a recent remodeling, replenishing its N-terminal region with a more current version of a L1 element than the version captured in the original domestication event ∼100 million years ago. The newly replenished version of bushbaby L1TD1 has evolved under purifying selection (Figure S3), ruling out the alternate possibility that this L1TD1 rearrangement is a pseudogenization event. This novel L1TD1 structure is not evident in the genomes of two lemur species, the gray mouse lemur (Microcebus murinus) and the aye-aye (Daubentonia madagascariensis), which both encode a more typical L1TD1, and we have not observed evidence of bushbaby-like L1TD1 restructuring in the any other mammalian genomes examined (Figure 3). This implies that the bushbaby L1TD1 remodeling occurred after the split between the lemur and bushbaby lineages, nearly 60 million years ago. Closer examination of other prosimian lineages will allow a more precise dating of this event.
From these forms of variation in the primate and mouse lineages, we conclude that genetic innovation has been adaptively selected for in L1TD1 through a number of mechanisms including mutation of individual amino acids and perhaps expansions and contractions of the glutamic acid-rich region. Importantly, the fixation of a new gene structure in the bushbaby derived from a recently active L1 suggests selection for functional novelty through yet another L1-derived sequence. The signature of positive selection in both mice and primates suggests L1TD1 may be coevolving with some pathogen. Intriguingly, a strong signature of positive selection, indicative of such ‘arms-races’, has been previously seen in primate L1 evolution, with an especially striking signature in ORF1p [38], [40]. Combined with its origin as a domesticated L1 gene and the novelty in bushbaby (which further emphasizes the selection pressure to maintain the L1-like character of L1TD1), we considered whether L1TD1 could be engaged in a genetic conflict with L1 or some other retroelement. This function could parallel the known role of another domesticated retroelement gene, Fv1, in defense against elements similar to its progenitor [13], [14]. To more clearly understand this hypothetical relationship, we reasoned that genes that function solely in L1 restriction might be lost in species where active L1s have previously been shown to have gone extinct – the megabats and the rice rats.
L1TD1 and the loss of L1 activity in megabat genomes
Previous studies have demonstrated that a number of megabat species lack active L1 elements. Indeed, it is likely that L1s experienced an extinction event in the megabat ancestor between 24 and 58 million years ago [44]. We decided to explore the potentially antagonistic relationship between L1TD1 and L1 by looking in bat species where the most detailed studies of L1 extinction have been completed. In addition to identifying L1TD1 sequences from seven available chiropteran genome sequences, we amplified and sequenced the L1TD1 coding exons from six additional bat species (Figure 5). We were able to unambiguously determine the complete coding sequence of L1TD1 for twelve bat species; in the thirteenth species, Rhinolophus eloquens, we were only able to obtain part of coding exon 1 of L1TD1.
Fig. 5. Loss of L1TD1 in megabats appears to follow the loss of L1 activity. 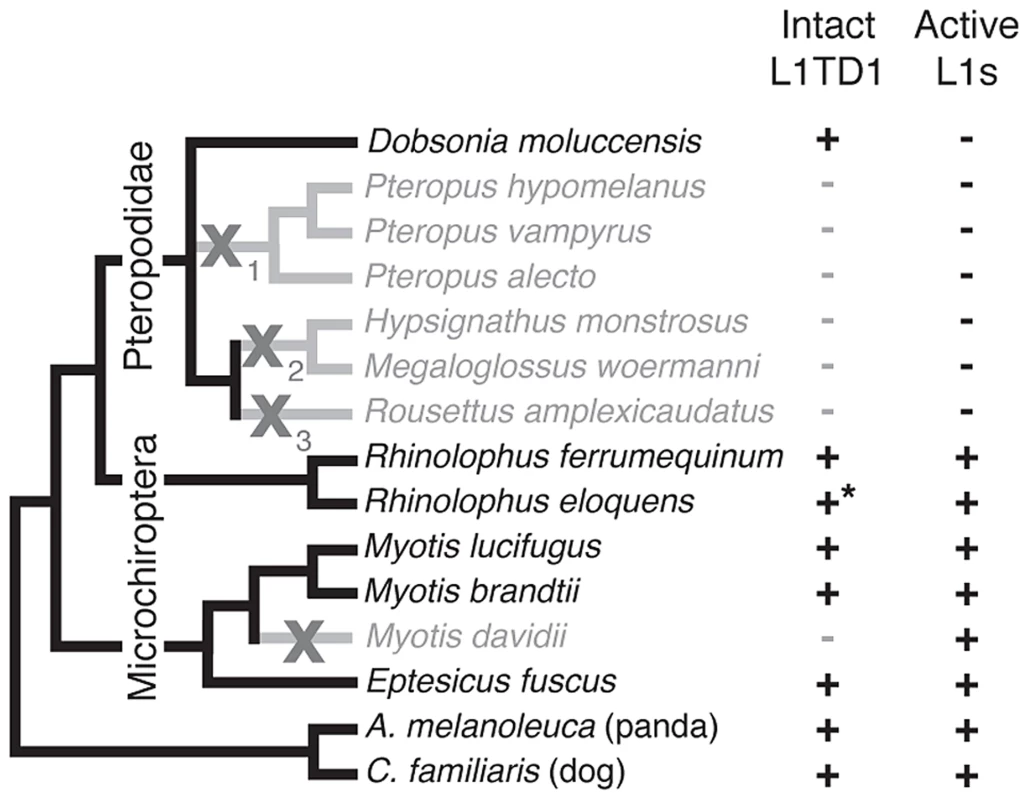
We obtained L1TD1 sequences from thirteen bat species (Materials and Methods). We show a species tree partly based upon a published megabat phylogeny [82], inferring placement of additional taxa using species from the same genus. In addition, we used our L1TD1 sequences to resolve relationships in the Pteropus and Myotis/Eptesicus clades. Species in which L1TD1 appears intact are shown in black, and those in which L1TD1 harbors inactivating mutations (stop codons, frameshifting insertions/deletions) are shown in gray. Some species share the same inactivating mutation(s) represented by the subscripts of the gray X symbols, suggesting L1TD1 was lost three independent times in the megabats. For one species, R. eloquens (starred), we were only able to obtain part of coding exon 1 of L1TD1, but this region is intact. Presence or absence of active L1s is based upon previous data [44] and analysis of the M. davidii genome assembly (Yang and Wichman, unpublished data). Bats (Chiroptera) are divided into the monophyletic megabat clade Megachiroptera and a polyphyletic microbat clade, the Microchiroptera. Among the Microchiroptera, our survey revealed yet another case of an independent L1TD1 loss, in the genome of the microbat Myotis davidii (Figure 5). In this genome, our bioinformatics analysis (Yang and Wichman, unpublished data) shows that the most recently inserted L1s have ∼99.5% identity to each other and retain their open reading frame; further, these highly similar sequences maintain conservation of amino acids known to be completely conserved across young mammalian L1s [45], suggesting L1s are still active in the M. davidii lineage. This situation parallels that of Cetartiodactyla and Afrotheria, which have both lost L1TD1 but likely maintain active L1s.
In contrast, among the megabats, we found that most species that have lost active L1s have also lost L1TD1. The only exception was the megabat species Dobsonia moluccensis, which has lost active L1s but possesses an apparently intact L1TD1 that has evolved under purifying selection for at least some of the time since it diverged from other megabats (Figure S4). Our observation of unique inactivating coding mutations among subsets of the megabat species suggests there were at least three independent losses of L1TD1 (Figure 5, X's with subscripts). However, due to the poor resolution of the phylogenetic relationships of the other six megabat species with Dobsonia moluccensis (Figure 5), we cannot formally rule out the alternate possibility that the initial loss of L1TD1 function occurred once, via a non-coding mutation. Under both scenarios, we infer that the extinction of L1s preceded loss of L1TD1 in the megabats [44].
Thus, it appears that loss of L1 may have led to loss of L1TD1 in megabats except in Dobsonia moluccensis (see Discussion). This evolutionary relationship between L1 elements and L1TD1 is consistent with the possibility that L1TD1 was domesticated by mammalian genomes to antagonize L1s, similar to the domestication of Fv1 for defense against retroviruses. Under this model, loss of active L1s would relieve the selective pressure to maintain L1TD1 in megabat genomes.
L1TD1 and the loss of L1 activity in rice rat genomes
In addition to the loss of active L1s in the megabats, a group of rice rats in the subfamily Sigmodontinae are the only other group of mammals in which a loss of active L1s has been well characterized [45]. To investigate whether these species might exhibit a correlation similar to that of the megabats, in which L1 loss and L1TD1 loss co-occur, we sequenced the L1TD1 gene from ten L1-less Sigmodontinae species (5 complete sequences, 4 coding exon 1s, 1 coding exon 2), as well as two Sigmodontinae species that retain active L1s (Oecomys bicolor and Reithrodontomys fulvescens). In all cases, we found an intact coding sequence, suggesting these species have retained a functional copy of the L1TD1 gene (Dataset S1).
We suggest two hypotheses for the apparent retention of L1TD1 in these species, despite the absence of active L1s. First, it is possible that not enough time has passed for inactivating mutations to appear in the coding sequence of L1TD1 in these species; we note that the loss of active L1s in these species is much more recent than the loss of L1s in megabats (7.2–12.3 million years ago in the rodents vs 24–58 million years ago in the megabats). While we cannot definitively rule out the possibility that L1TD1 is neutrally evolving within these species, overall the tree is better fit by a single purifying dN/dS than by a dN/dS fixed at 1 (p<0.001). Further, most of the Sigmodontinae sequences exhibit pairwise dN/dS ratios that suggest purifying selection within the clade (Table S1). We therefore favor the second possibility that retention of a functional L1TD1 in these species results from selection pressures independent of L1s. For example, L1TD1 could play an essential role in pluripotency (despite the dispensable role of the Mus L1TD1). Intriguingly, despite having an overall tree length similar to our Mus alignment, we do not find any evidence of positive selection in the L1TD1 genes from Sigmodontinae using PAML NSsites on either full sequences (M7 vs M8, p = 0.33), coding exon 1 alone (p = 0.53), or coding exon 2 alone (p = 0.84). Alternatively, L1TD1 in sigmodonts could play a role in genome defense against another element active in these genomes. Indeed, the sigmodontine rodents have a highly active family of ERVs, the mysTR elements [46]. Expansion of this family preceded the death of L1s, but these elements are very active, with 3500 to 7000 species-specific insertions in the L1-extinct species examined [47]. This recent ERV amplification in Sigmodontinae contrasts with the megabats (where L1TD1 has been lost in many species); there are apparently no highly active DNA or RNA elements in megabats [48]. If L1TD1 can suppress retroelements other than L1s, this could explain why the gene is retained in sigmodontine rodents but not in megabats.
While these results do not provide additional support for our proposed correlation between L1 loss and L1TD1 loss, they do provide another example of the potentially stochastic co-option of L1TD1 into essential cellular processes that has driven the variable gain and loss of this gene throughout mammalian evolution.
Discussion
Domesticated genes such as L1TD1 provide a window into the opportunistic means by which host genomes can exapt new genetic functions from their resident mobile elements. L1 elements and non-autonomous SINE elements that rely on L1 for mobility comprise a substantial fraction of the human genome (at least 17 and 30 percent, respectively [49]). This abundance, together with a relatively long history of vertical transmission has provided ample opportunities for host genome domestication of L1 elements. Indeed, we see evidence of this exaptation for enhancer functions, in exonization of Alu elements and small L1 fragments, and even in long non-coding RNAs [4], [6], [21]–[23], [25]–[28]. However, L1TD1 is unique in being the only known host protein-coding gene whose protein coding sequence is derived almost entirely from the coding sequence of an L1. In addition, its exclusive expression in stem cells and the germline and the observation that L1TD1 appears to be required for pluripotency maintenance in human ES cells [31], make it a powerful example of retroelement domestication for an essential function. Exaptation for an important function such as pluripotency maintenance draws immediate parallels to other retroelement domestications such as the retroviral envelope-derived syncytin genes and the sushi-retroelement derived Peg10-related genes, both of which serve essential roles in mammalian placentation [7], [11], [12]. Indeed, at first glance, our estimate of the evolutionary age of L1TD1 (only slightly younger than Peg10 and older than any identified syncytin gene) does suggest long-term preservation for some important function.
Nonetheless, our elucidation of L1TD1's evolutionary history challenges these parallels. For instance, we found multiple instances of L1TD1 loss or pseudogenization at different stages of mammalian evolution, in contrast to Peg10, which is intact in all examined mammals [11], [50], [51]. Furthermore, Peg10 is subject to strong purifying selection (unpublished data), presumably due to the constraint imposed by its essential functions in placentation and genomic imprinting. In contrast, we have found evidence of diversifying selection acting on L1TD1 in primates and mice, along with genetic innovation in bushbaby via a partial replacement of L1TD1 coding sequence with the ORF1 of a younger L1 element. This pattern of genetic innovation and sporadic loss is more reminiscent of another class of domesticated retroelement genes, exemplified by the Fv1 retroviral gag-derived gene in Mus species, which serves as a form of host defense against a broad array of incoming retroviral capsids [15], [18].
It is noteworthy that germline and early embryonic tissues represent the primary battleground for the conflict between hosts and L1 elements. In order to propagate new copies to future generations, L1s must successfully retrotranspose in these cells. However, these are the very compartments where a host could incur the greatest fitness cost from L1 replication. The highly specific expression pattern of L1TD1 suggests that its target could be genomic retroelements that mobilize during germline development and early embryonic stages.
Three findings lead us to consider the intriguing speculation that L1TD1 may target L1 elements themselves (although other functions for L1TD1 are quite possible). First, we find that extinction of L1 activity appears to have been followed by L1TD1 loss in several megabat lineages. This relationship between L1 loss and L1TD1 loss is far from perfect. For example, in the microbat Myotis davidii and other mammalian genomes (Afrotheria and Cetartiodactyla), L1TD1 loss is not accompanied by L1 extinction. This is easily explained, because a number of different restriction factors exist to defend against L1 in mammalian genomes [52], and in particular genomes, the previously beneficial function of L1TD1 may become redundant and dispensable. Also, if a rapidly evolving L1 were to escape restriction by L1TD1, control over this rogue element could be regained through adaptive mutations in restriction factors besides L1TD1, thereby relaxing selective pressure to maintain L1TD1. Alternatively, L1 retrotransposition rates are known to have varied over evolutionary time: periods where L1 retroposition rates are low could also lead to relaxed selective pressures on L1TD1 retention. A similar relaxation of selective pressure has been invoked to explain the idiosyncratic loss of Fv1 in Mus species [15]. Second, we found that bushbaby L1TD1 underwent a partial replacement with a more current version of L1 ORF1. Although such ‘replenishment’ could represent a neutral event unique to the bushbaby, the fact that it has occurred suggests a model in which the ‘newer’ version of L1TD1 was fixed in the bushbaby genome because it conferred a selective advantage over the pre-existing version, which itself formed from a much more ancient L1 domestication. Third, our observation that L1TD1 has experienced positive selection in primates (and mice) is consistent with previous findings that L1 ORF1 has evolved under positive selection during primate evolution [38], [40]. Like Fv1 action against distantly homologous retroviral capsids, the observed positive selection suggests the possibility that L1TD1 may be in direct conflict with L1 ORF1p from active L1 elements. Since L1 elements also provide the machinery for non-autonomous elements like SINEs [53] and likely HAL1s [54], it is also possible that these elements represent the true targets of L1TD1, rather than L1 itself.
L1TD1 could function as a L1 restriction factor through a number of possible mechanisms. First, analogously to Fv1, L1TD1 might interfere with the homotrimerization of L1-ORF1p that is necessary for L1 retrotransposition. This trimerization normally happens through ORF1p's coiled-coil domain [55]. We note that L1TD1 coding exon 2 has preserved its coiled-coil domain, which may be necessary for this interaction. Under this model, L1TD1 could act as a dominant negative version of ORF1p. Its binding to ORF1p could lead to L1 restriction, which could then drive episodes of diversifying selection in L1 ORF1p to escape L1TD1 restriction [40]. In response to these escape mutations in L1, we might expect L1TD1 to rapidly evolve to restrict the variant L1 – a situation consistent with the positive selection we observe in L1TD1. Another possibility is that L1TD1 could outcompete L1 ORF1p for binding to the ORF2p protein, the likely rate-limiting component for retrotransposition. Alternatively, L1TD1's RNA-binding activity could be the source of its restrictive abilities. In this model, L1TD1 could act as a competitive inhibitor of L1 ORF1p, binding the RNA of transcribed retroelements and blocking the downstream pathway that would normally create new copies. This could function through simple titration or through a more complex mechanism whereby L1TD1 localizes L1 RNA to P-bodies [32] for sequestration or decapping and subsequent degradation. The previously reported association of L1TD1 with the TRIM28 (KAP1) complex [56] suggests yet another possible mechanism of restriction. TRIM28 is known to function in the silencing of exogenous and endogenous retroviral integrations by recruitment of heterochromatin proteins to some retroviral elements [57]. Under this epigenetic silencing model, L1TD1 may function in an analogous role to the zinc finger adaptor proteins that help target TRIM28 and consequently the silencing complex to particular genomic sequences. Thus, constant adaptation to bind either DNA or RNA at L1 integration sites could drive diversifying selection of L1TD1. It is intriguing to imagine a model wherein the silencing machinery could target any genomic parasite through a modular adapter protein that recognizes the newly integrated sequence (although it must be noted that such activities have not been ascribed to L1 ORF1p from which L1TD1 derived). Further characterization will shed light into whether L1TD1 encodes any or all of these biochemical activities.
When we examined the genome sequences of bats, we found that many species that have lost active L1s have also lost L1TD1. However, Dobsonia moluccensis still maintains L1TD1 as an intact protein-coding gene, despite the fact that this species lost active L1s many million years previously. Similarly, we found L1TD1 intact in the subset of the Sigmodontinae rodents previously shown to have lost active L1s [45]. This implies that the proposed selection for restricting active L1 elements cannot be the only constraint that dictates retention of L1TD1. We hypothesize that idiosyncratic, highly divergent L1 insertion patterns in different mammalian genomes could provide an explanation of both the retention of L1TD1 in mammals without an active L1 and the essential role in pluripotency maintenance that L1TD1 plays in humans. Under this model, L1TD1's role as either important or dispensable for pluripotency maintenance depends on the pattern of where L1s have inserted in the genome of each species. In various contexts, transposable elements have been shown to affect the expression of genes in their vicinity [58], [59], and some elements are included in the untranslated regions of host mRNAs [60], [61]. If L1TD1 transcriptionally or post-transcriptionally silences L1 elements, this silencing could alter the expression of host genes near L1s, or with L1s in their UTRs. Under this model, in some mammalian genomes, L1 insertions would not be in proximity to genes that impact the pluripotency program (Figure S5). In these species, extinction of L1 would result in the loss of constraint on L1TD1 (e.g., megabat P. vampyrus), and experimental knock-down of L1TD1 would have no effect on pluripotency maintenance (e.g., M. musculus [33]). In contrast, in other mammalian genomes where L1 insertions occurred near genes whose repression is important for pluripotency maintenance, loss of L1TD1 would lead to loss of pluripotency (e.g., H. sapiens [31]). In fact, repression could have been incorporated into any number of functionally important pathways in the cells where L1TD1 is expressed, simply depending on which genes L1s landed next to during evolution. We hypothesize that in such species, extinction of L1 activity would not relax the selective pressures to maintain L1TD1 (e.g., megabat D. moluccensis, Sigmodontine rice rats), which would now be required to recognize ‘dead’ L1 copies in order to maintain these repressive programs.
Although we present the hypothesis that L1TD1 was originally domesticated as an anti-L1 restriction factor and was subsequently recruited for pluripotency regulation in humans by virtue of direct repression of L1 elements near functionally relevant genes, this idea is at present highly speculative. L1TD1 has experienced a long evolutionary history functioning in the context of other factors expressed in pluripotent cells, and it is possible that L1TD1 could have been coopted by these factors. For instance, L1TD1 could be involved in regulating RNA involved in pluripotency via its ancestral chaperone function, or in regulating important protein complexes via protein-protein interactions. We look forward to experimental investigation of all of these hypotheses; none of them can completely explain all of our evolutionary observations (L1TD1 loss and retention in various lineages, positive selection and remodeling in bushbaby), perhaps suggesting that L1TD1's function has changed multiple times over the course of mammalian evolution.
In summary, we posit that L1TD1's original function could have been in genome defense (similar to Fv1), and that it still has defense functions in many mammalian species where it is retained (including humans). We note that our evidence for L1TD1 diversifying selection (and inferred genome defense) comes in part from the primate lineage, which is also where its role in pluripotency maintenance is best established. Later in evolution, by virtue of either its transcriptional or post-transcriptional silencing of L1, L1TD1 may have become intricately enmeshed into the transcriptional program of L1-proximal genes in some species, meaning that L1TD1 also acquired an absolutely essential function (similar to Peg10 and syncytin). An interesting feature of this model is that L1TD1's essential function (or lack thereof) is thus a consequence of stochastic L1 insertion patterns in different mammalian genomes. This stochasticity could provide an explanation for why L1TD1 has been lost in some species, and why it is responsible for pluripotency maintenance in only some mammalian genomes.
Materials and Methods
Ethics statement
The animals used in this study are wild-derived laboratory animals of the species Mus musculus, Mus spretus, Mus spicilegus, Mus mattheyi and Apodemus uralensis. None of these species are protected. Permits for catching the founding members of each line were not required at the time they were caught. Some specimens were caught on the properties of private landowners, with their oral permission to enter the property and catch mice. All animal work was carried out by experienced personnel at the Max Planck Institute for Evolutionary Biology, following the legal requirements in accordance with German animal welfare law (Tierschutzgesetz) and FELASA guidelines. Permits for keeping mice were obtained from the local veterinary office “Veterinäramt Kreis Plön” (permit number: 1401-144/PLÖ-004697). The dissection of animals and organ extractions were performed according to the German Animal Welfare Act § 8a Abs. 1 Nr. 3b TierSchG; ‘Organ-/Gewebsentnahme zu wissenschaftlichen/diagnostischen Zwecken TierSchG' V 312-72241.123-34.
Biological materials
Sigmodontine rodent liver tissue was obtained on loan from the Museum at Texas Tech University (Lubbock, TX).
For Mus samples, mice of different ages were sacrificed by CO2 asphyxiation followed by cervical dislocation. Mice were then dissected and tissues were snap-frozen within 5 minutes post-mortem. Liver (front view: front left lobe), both testes, and whole brain including brain stem were collected. For the outbred populations, Iran (AH), France (M), and Germany (CB) for Mus musculus domesticus, and Austria (WI) and Kazakhstan (KH) for Mus musculus musculus, eight individuals each were sampled. For inbred groups, Mus musculus castaneus (TA), Mus spretus (SP), Mus spicilegus (SC), Mus mattheyi (MA) and Apodemus uralensis (AP), four individuals each were sampled. All mice were obtained from the mouse collection at the Max Planck Institute for Evolutionary Biology.
Mus transcriptome sequencing, processing, and mapping/assembly
The sampled tissues of each Mus group were used for RNA extraction with the RNAeasy Kit (Qiagen) and pooled at equimolar concentrations. Quality of the RNA was measured with BioAnalyzer chips (Agilent), for the individual samples and pools, and samples with RIN values below 7.5 were discarded. The pools were subsequently submitted to the Cologne Center for Genomics (CCG) for further processing and sequencing. The sequencing of the samples was performed using a polyA tail purification step, followed by cDNA synthesis, Illumina library preparation, and sequencing with an Illumina HiSeq 2000 sequencer. Each transcriptome sample was sequenced in approximately one third of a HiSeq2000 flow-cell lane (one flow-cell lane per taxon).
All raw data files were trimmed for adaptors and quality using Trimmomatic [62]. The quality trimming was performed base-wise, removing bases below quality score of 20 (Q20), and keeping reads whose average quality was of at least Q30. Reads whose trimmed length was shorter than 40 bases were excluded from further analyses, and pairs missing one member because of poor quality were also removed from any further analyses.
Quality-filtered transcriptome reads were aligned against the mm10 version of the mouse reference genome from UCSC [63] using NextGenMap [64]. Reads which were ambiguously or poorly mapped (MAPQ <20) were removed from the analyses. Quality-filtered transcriptome reads for each taxon were merged into a single input file, discarding tissue information, and assembled de novo with the Trinity platform [65] using default parameters.
Sequence collection
L1TD1 sequences were obtained from publicly available primate genome databases using PSI-BLAST [66] against the NR database or TBLASTN [66] against the HTGS database, with human L1TD1 as a search seed (Table S2). For sequencing of other primate, Sigmodontinae, and Mus species (Table S2), exon 1 and exon 2 were PCR amplified individually from genomic DNA using oligonucleotides designed against intronic regions. PCR primers were designed against intronic regions neighboring each exon based upon an alignment of mouse, rat, and Chinese hamster for rodents or human, rhesus, and squirrel monkey for primates (Primates Exon1 Sense: CAGAATCCAGTCTTGACAACATATCC; Primates Exon1 Antisense: CAGGAGAATCACTTGAACCTGGG; Primates Exon2 Sense: GTCAGAATGGAAGCCATATTAAAATTAGTG; Primates Exon2 Antisense: GCTATTAGCTGTCCATCCTTCTGG; Rodent Exon1 Sense: GYAAGWAMAYTTTCATTTGYTTATAKTTC; Rodent Exon1 Antisense: CCYATCARTYTCTRGAACYCCYRTCAARC; Rodent Exon2 Sense: GGMAAGYATACTAAATTYAGAGGGTRAAATAG; Rodent Exon2 Antisense: AASTCAACCAACMYKCAGRKAGTK). PCR products were sequenced using standard Sanger sequencing.
For Mus sequences, informative reads were obtained with samtools [67] from alignments overlapping with the L1TD1 annotation. Known sequences from the coding sequences of L1TD1 were used to identify the most similar assembled contigs in each taxon using nucleotide-nucleotide blast [68] (e-value <1e-10).
L1TD1 sequences from primate, Sigmodontinae, and Mus species have been deposited in Genbank under accession numbers KJ994281-KJ994329.
Alignments and positive selection analysis
Primate and Mus nucleotide sequences were aligned using the ClustalW ‘translation align’ function in Geneious Pro (Biomatters Ltd.). Alignments were refined manually, including truncation of the poorly aligned glutamic-acid rich region, and this alignment and an established primate phylogeny [69] or Mus L1TD1 gene tree were input into the CODEML sites model of PAML [70] to detect positive selection at individual sites. Positively selected sites were classified as those sites with a M8 Bayes empirical Bayes posterior probability >90%. FUBAR was performed using the web-based implementation of HyPhy (www.datamonkey.org) [71], [72]. To test for signatures of positive selection along individual branches of the primate phylogeny, we used Branch-site REL in HyPhy [41] or the branch model of PAML. For PAML, the statistical significance of any branch that showed dN/dS >1 in the free ratio model (model = 1) was tested using a two-ratio tree (model = 2) by specifying the branch of interest as a foreground branch and all other branches as background branches. We then compared the likelihood of a model where the foreground branch had a freely estimated dN/dS with the likelihood of a model where that branch had dN/dS fixed at the neutral value of 1 [39].
For the analysis shown in Figure S2, we obtained dN/dS values from a genome-wide analysis of trios of human-chimpanzee-macaque orthologs [42], made available via Adam Siepel's website (http://compgen.bscb.cornell.edu/orthologs). To be conservative, we filtered out dN/dS values that might be artificially high for technical reasons: we eliminated values calculated from alignments of fewer than 100 codons, eliminated alignments that contained fewer than 5 evolutionary changes of any type, and included only RefSeq genes and not other genes derived from less confident annotation sets. In this dataset, alignments with no synonymous changes had apparent dN/dS values of 999; again, to be conservative, we replaced dN/dS values for these genes with a more conservative estimate of dN/dS = 2. We converted sequence identifiers to gene symbols using Bioconductor [73], allowing us to cross-reference dN/dS values with genes identified in a screen for ES cell determinants [43]. For the plot shown in Figure S2, we selected a list of 127 ES cell determinants validated by secondary screening using three markers of stem cell identity [43]; other overlapping lists of validated hits from this screen show similar evolutionary patterns.
Mammalian sequence collection and alignment
To identify previously unannotated copies of L1TD1 in sequenced genomes, the genomic sequence between flanking genes INADL and KANK4 was extracted and compared with the corresponding human genomic region (INADL-L1TD1-KANK4) using Dotter [74]. Visual inspection of Dotter output enabled extraction of L1TD1 protein-coding sequences. Multispecies L1TD1 alignments were generated using CLUSTALW [75] with manual adjustment. For all mammals mentioned in the text, genome sequences were searched for non-syntenic and/or pseudogene copies of L1TD1 using TBLASTN with a RepeatMasked version [48] of human and mouse L1TD1 sequences, and in some cases L1TD1 sequence from a more closely related species.
To identify representative L1 ORF1p sequences (Dataset S2) used to build the tree shown in Figure 2, we first selected a small number of L1 ORF1p consensus sequences from RepBase [76] and obtained the sequence of an active human L1 from Genbank (LRE2, accession AAB60344.1). We then used TBLASTN [66] against whole genome assemblies (Table S2) to identify a single intact L1-ORF1p sequence from the genome assemblies of each species shown in Figure 2, selecting a copy arbitrarily from among the blast hits that did not show inactivating mutations in ORF1p.
Phylogenetic inference
To construct the tree shown in Figure 1B, we used our alignment of L1TD1 nucleotide sequences and the jModelTest 2 program [77] to determine that the best-fitting evolutionary model for this alignment is the GTR model with invariant sites and gamma distributed rates (GTR+I+G). We then generated a maximum-likelihood phylogeny using PhyML [78] and the GTR+I+G evolutionary model with four site categories for the gamma distribution. 1000 replicate trees were constructed, and the tree with the highest log-likelihood was chosen and displayed using MEGA5 [79].
To construct the tree shown in Figure 2, predicted protein sequences were aligned by hand (Dataset S2). We used ProtTest 3 [80] to determine that the best-fitting evolutionary model for this alignment is the JTT model with gamma-distributed rates (JTT+G). We then used PHYML to generate maximum-likelihood trees with the JTT+G model (four site categories for the gamma distribution). 1000 replicate trees were constructed, and the tree with the highest log-likelihood was chosen and displayed using MEGA5 [79]. Bootstrap values represent the percentage of trees in which the descendent taxa cluster together.
Dates of divergence mentioned in the text were generated using the TimeTree web service [81].
Bat sequencing and database sequence collection
We obtained L1TD1 sequences for Pteropus alecto, Pteropus vampyrus, Rhinolophus ferrumequinum, Myotis lucifugus, Myotis davidii, Myotis brandtii and Eptesicus fuscus from publicly available genome sequences. For other bat species, genomic DNA samples were obtained from tissues from The Museum, Texas Tech University. Sample accession numbers were previously published [44]. Degenerate PCR primers were designed against intronic regions neighboring each exon based upon an alignment of P. vampyrus and M. lucifugus sequences (Exon1 Sense: TTTCAGATGATTTTKTAAAWWGAKTTTRGGGG; Exon1 Antisense: TYYTMWYWAWTWAAMARSTGTTAASYYWTTSTTC; Exon2 Sense: TGGGGWTCCMAGCCTTYAAGAAMAAATC; Exon2 Antisense: CATCMCCAAGTATACTGTTAGCTGTCCATC). For Rousettus amplexicaudatus and Rhinolophus eloquens we designed a second set of primers based upon the new sequences we generated to amplify Exon1 (Sense: AAATATCACCCACATGGAAAGAATTAG; Antisense: TTCTCTTGAATCCCATATCTTCTTCC). PCR products were sequenced by Sanger sequencing. For Nyctimene albiventer, Megaerops niphanae and Cynopterus sphinx, we were unable to amplify or sequence any product from either exon with multiple PCR optimizations of annealing temperature, amount of genomic DNA template and concentration of magnesium ion. For two bat species, it appeared that more than one closely related L1TD1 sequence was present. Upon cloning and sequencing multiple PCR products, we found that M. woermanni had 4 sequence variants and R. amplexicaudatus had 2 variants. For each species, at least one inactivating mutation was shared by all variants.
Supporting Information
Zdroje
1. PardueML, DeBaryshePG (2011) Retrotransposons that maintain chromosome ends. Proc Natl Acad Sci USA 108 : 20317–20324.
2. CordauxR, BatzerMA (2009) The impact of retrotransposons on human genome evolution. Nat Rev Genet 10 : 691–703.
3. BrosiusJ, GouldSJ (1992) On "genomenclature": a comprehensive (and respectful) taxonomy for pseudogenes and other "junk DNA". Proc Natl Acad Sci U S A 89 : 10706–10710.
4. FeschotteC (2008) Transposable elements and the evolution of regulatory networks. Nat Rev Genet 9 : 397–405.
5. SmitAF (1999) Interspersed repeats and other mementos of transposable elements in mammalian genomes. Curr Opin Genet Dev 9 : 657–663.
6. SinzelleL, IzsvakZ, IvicsZ (2009) Molecular domestication of transposable elements: from detrimental parasites to useful host genes. Cell Mol Life Sci 66 : 1073–1093.
7. MiS, LeeX, LiX, VeldmanGM, FinnertyH, et al. (2000) Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403 : 785–789.
8. BlackSG, ArnaudF, PalmariniM, SpencerTE (2010) Endogenous retroviruses in trophoblast differentiation and placental development. Am J Reprod Immunol 64 : 255–264.
9. DupressoirA, LavialleC, HeidmannT (2012) From ancestral infectious retroviruses to bona fide cellular genes: role of the captured syncytins in placentation. Placenta 33 : 663–671.
10. DupressoirA, VernochetC, BawaO, HarperF, PierronG, et al. (2009) Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc Natl Acad Sci USA 106 : 12127–12132.
11. OnoR, NakamuraK, InoueK, NaruseM, UsamiT, et al. (2006) Deletion of Peg10, an imprinted gene acquired from a retrotransposon, causes early embryonic lethality. Nat Genet 38 : 101–106.
12. SuzukiS, OnoR, NaritaT, PaskAJ, ShawG, et al. (2007) Retrotransposon silencing by DNA methylation can drive mammalian genomic imprinting. PLoS Genet 3: e55.
13. BenitL, De ParsevalN, CasellaJF, CallebautI, CordonnierA, et al. (1997) Cloning of a new murine endogenous retrovirus, MuERV-L, with strong similarity to the human HERV-L element and with a gag coding sequence closely related to the Fv1 restriction gene. J Virol 71 : 5652–5657.
14. BestS, Le TissierP, TowersG, StoyeJP (1996) Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 382 : 826–829.
15. YanY, Buckler-WhiteA, WollenbergK, KozakCA (2009) Origin, antiviral function and evidence for positive selection of the gammaretrovirus restriction gene Fv1 in the genus Mus. Proc Natl Acad Sci USA 106 : 3259–3263.
16. JungYT, KozakCA (2000) A single amino acid change in the murine leukemia virus capsid gene responsible for the Fv1(nr) phenotype. J Virol 74 : 5385–5387.
17. KozakCA, ChakrabortiA (1996) Single amino acid changes in the murine leukemia virus capsid protein gene define the target of Fv1 resistance. Virology 225 : 300–305.
18. StevensA, BockM, EllisS, LeTissierP, BishopKN, et al. (2004) Retroviral capsid determinants of Fv1 NB and NR tropism. J Virol 78 : 9592–9598.
19. KozakCA (1985) Analysis of wild-derived mice for Fv-1 and Fv-2 murine leukemia virus restriction loci: a novel wild mouse Fv-1 allele responsible for lack of host range restriction. J Virol 55 : 281–285.
20. MalikHS, BurkeWD, EickbushTH (1999) The age and evolution of non-LTR retrotransposable elements. Mol Biol Evol 16 : 793–805.
21. BrosiusJ (1999) RNAs from all categories generate retrosequences that may be exapted as novel genes or regulatory elements. Gene 238 : 115–134.
22. FeschotteC, PrithamEJ (2007) DNA transposons and the evolution of eukaryotic genomes. Annu Rev Genet 41 : 331–368.
23. VolffJN (2006) Turning junk into gold: domestication of transposable elements and the creation of new genes in eukaryotes. Bioessays 28 : 913–922.
24. KuwabaraT, HsiehJ, MuotriA, YeoG, WarashinaM, et al. (2009) Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci 12 : 1097–1105.
25. Lev-MaorG, SorekR, ShomronN, AstG (2003) The birth of an alternatively spliced exon: 3' splice-site selection in Alu exons. Science 300 : 1288–1291.
26. SorekR, AstG, GraurD (2002) Alu-containing exons are alternatively spliced. Genome Res 12 : 1060–1067.
27. LorencA, MakalowskiW (2003) Transposable elements and vertebrate protein diversity. Genetica 118 : 183–191.
28. SelaN, KimE, AstG (2010) The role of transposable elements in the evolution of non-mammalian vertebrates and invertebrates. Genome Biol 11: R59.
29. IwashitaS, UenoS, NakashimaK, SongSY, OhshimaK, et al. (2006) A tandem gene duplication followed by recruitment of a retrotransposon created the paralogous bucentaur gene (bcntp97) in the ancestral ruminant. Mol Biol Evol 23 : 798–806.
30. MitsuiK, TokuzawaY, ItohH, SegawaK, MurakamiM, et al. (2003) The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113 : 631–642.
31. WongRC, IbrahimA, FongH, ThompsonN, LockLF, et al. (2011) L1TD1 is a marker for undifferentiated human embryonic stem cells. PLoS ONE 6: e19355.
32. NarvaE, RahkonenN, EmaniMR, LundR, PursiheimoJP, et al. (2012) RNA-binding protein L1TD1 interacts with LIN28 via RNA and is required for human embryonic stem cell self-renewal and cancer cell proliferation. Stem Cells 30 : 452–460.
33. IwabuchiKA, YamakawaT, SatoY, IchisakaT, TakahashiK, et al. (2011) ECAT11/L1td1 is enriched in ESCs and rapidly activated during iPSC generation, but it is dispensable for the maintenance and induction of pluripotency. PLoS ONE 6: e20461.
34. MartinSL (2006) The ORF1 protein encoded by LINE-1: structure and function during L1 retrotransposition. Journal of biomedicine & biotechnology 2006 : 45621.
35. WeiW, GilbertN, OoiSL, LawlerJF, OstertagEM, et al. (2001) Human L1 retrotransposition: cis preference versus trans complementation. Mol Cell Biol 21 : 1429–1439.
36. KulpaDA, MoranJV (2006) Cis-preferential LINE-1 reverse transcriptase activity in ribonucleoprotein particles. Nat Struct Mol Biol 13 : 655–660.
37. DaughertyMD, MalikHS (2012) Rules of engagement: molecular insights from host-virus arms races. Annu Rev Genet 46 : 677–700.
38. KhanH, SmitA, BoissinotS (2006) Molecular evolution and tempo of amplification of human LINE-1 retrotransposons since the origin of primates. Genome Res 16 : 78–87.
39. NielsenR, YangZ (1998) Likelihood models for detecting positively selected amino acid sites and applications to the HIV-1 envelope gene. Genetics 148 : 929–936.
40. BoissinotS, FuranoAV (2001) Adaptive evolution in LINE-1 retrotransposons. Mol Biol Evol 18 : 2186–2194.
41. Kosakovsky PondSL, PosadaD, GravenorMB, WoelkCH, FrostSD (2006) Automated phylogenetic detection of recombination using a genetic algorithm. Mol Biol Evol 23 : 1891–1901.
42. GibbsRA, RogersJ, KatzeMG, BumgarnerR, WeinstockGM, et al. (2007) Evolutionary and biomedical insights from the rhesus macaque genome. Science 316 : 222–234.
43. ChiaNY, ChanYS, FengB, LuX, OrlovYL, et al. (2010) A genome-wide RNAi screen reveals determinants of human embryonic stem cell identity. Nature 468 : 316–320.
44. CantrellMA, ScottL, BrownCJ, MartinezAR, WichmanHA (2008) Loss of LINE-1 activity in the megabats. Genetics 178 : 393–404.
45. GrahnRA, RinehartTA, CantrellMA, WichmanHA (2005) Extinction of LINE-1 activity coincident with a major mammalian radiation in rodents. Cytogenet Genome Res 110 : 407–415.
46. CantrellMA, EdererMM, EricksonIK, SwierVJ, BakerRJ, et al. (2005) MysTR: an endogenous retrovirus family in mammals that is undergoing recent amplifications to unprecedented copy numbers. J Virol 79 : 14698–14707.
47. EricksonIK, CantrellMA, ScottL, WichmanHA (2011) Retrofitting the genome: L1 extinction follows endogenous retroviral expansion in a group of muroid rodents. J Virol 85 : 12315–12323.
48. Smit AF, Hubley R, Green P (1996–2004) RepeatMasker Open-3.0. http://www.repeatmasker.org.
49. LanderES, LintonLM, BirrenB, NusbaumC, ZodyMC, et al. (2001) Initial sequencing and analysis of the human genome. Nature 409 : 860–921.
50. OnoR, ShiuraH, AburataniH, KohdaT, Kaneko-IshinoT, et al. (2003) Identification of a large novel imprinted gene cluster on mouse proximal chromosome 6. Genome Res 13 : 1696–1705.
51. EsnaultC, CornelisG, HeidmannO, HeidmannT (2013) Differential evolutionary fate of an ancestral primate endogenous retrovirus envelope gene, the EnvV syncytin, captured for a function in placentation. PLoS Genet 9: e1003400.
52. ZamudioN, Bourc'hisD (2010) Transposable elements in the mammalian germline: a comfortable niche or a deadly trap? Heredity (Edinb) 105 : 92–104.
53. DewannieuxM, EsnaultC, HeidmannT (2003) LINE-mediated retrotransposition of marked Alu sequences. Nat Genet 35 : 41–48.
54. BaoW, JurkaJ (2010) Origin and evolution of LINE-1 derived "half-L1" retrotransposons (HAL1). Gene 465 : 9–16.
55. KhazinaE, TruffaultV, ButtnerR, SchmidtS, ColesM, et al. (2011) Trimeric structure and flexibility of the L1ORF1 protein in human L1 retrotransposition. Nat Struct Mol Biol 18 : 1006–1014.
56. WolfD, GoffSP (2009) Embryonic stem cells use ZFP809 to silence retroviral DNAs. Nature 458 : 1201–1204.
57. RoweHM, TronoD (2011) Dynamic control of endogenous retroviruses during development. Virology 411 : 273–287.
58. RebolloR, FarivarS, MagerDL (2012) C-GATE - catalogue of genes affected by transposable elements. Mobile DNA 3 : 9.
59. RebolloR, RomanishMT, MagerDL (2012) Transposable elements: an abundant and natural source of regulatory sequences for host genes. Annu Rev Genet 46 : 21–42.
60. JordanIK, RogozinIB, GlazkoGV, KooninEV (2003) Origin of a substantial fraction of human regulatory sequences from transposable elements. Trends Genet 19 : 68–72.
61. SzakST, PickeralOK, MakalowskiW, BoguskiMS, LandsmanD, et al. (2002) Molecular archeology of L1 insertions in the human genome. Genome Biol 3: R52.
62. LohseM, BolgerAM, NagelA, FernieAR, LunnJE, et al. (2012) RobiNA: a user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Res 40: W622–627.
63. FujitaPA, RheadB, ZweigAS, HinrichsAS, KarolchikD, et al. (2011) The UCSC Genome Browser database: update 2011. Nucleic Acids Res 39: D876–882.
64. SedlazeckFJ, ReschenederP, von HaeselerA (2013) NextGenMap: fast and accurate read mapping in highly polymorphic genomes. Bioinformatics 29 : 2790–2791.
65. HaasBJ, PapanicolaouA, YassourM, GrabherrM, BloodPD, et al. (2013) De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8 : 1494–1512.
66. AltschulSF, MaddenTL, SchafferAA, ZhangJ, ZhangZ, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 : 3389–3402.
67. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079.
68. McGinnisS, MaddenTL (2004) BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res 32: W20–25.
69. PerelmanP, JohnsonWE, RoosC, SeuanezHN, HorvathJE, et al. (2011) A molecular phylogeny of living primates. PLoS Genet 7: e1001342.
70. YangZ (1997) PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci 13 : 555–556.
71. Kosakovsky PondSL, FrostSDW (2005) Not So Different After All: A Comparison of Methods for Detecting Amino Acid Sites Under Selection. Mol Biol Evol 22 : 1208–1222.
72. MurrellB, MoolaS, MabonaA, WeighillT, ShewardD, et al. (2013) FUBAR: a fast, unconstrained bayesian approximation for inferring selection. Mol Biol Evol 30 : 1196–1205.
73. GentlemanRC, CareyVJ, BatesDM, BolstadB, DettlingM, et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80.
74. SonnhammerEL, DurbinR (1995) A dot-matrix program with dynamic threshold control suited for genomic DNA and protein sequence analysis. Gene 167: GC1–10.
75. LarkinMA, BlackshieldsG, BrownNP, ChennaR, McGettiganPA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23 : 2947–2948.
76. JurkaJ, KapitonovVV, PavlicekA, KlonowskiP, KohanyO, et al. (2005) Repbase Update, a database of eukaryotic repetitive elements. Cytogenet Genome Res 110 : 462–467.
77. DarribaD, TaboadaGL, DoalloR, PosadaD (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9 : 772.
78. GuindonS, GascuelO (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52 : 696–704.
79. TamuraK, PetersonD, PetersonN, StecherG, NeiM, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28 : 2731–2739.
80. DarribaD, TaboadaGL, DoalloR, PosadaD (2011) ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27 : 1164–1165.
81. KumarS, HedgesSB (2011) TimeTree2: species divergence times on the iPhone. Bioinformatics 27 : 2023–2024.
82. AlmeidaFC, GianniniNP, DeSalleR, SimmonsNB (2011) Evolutionary relationships of the old world fruit bats (Chiroptera, Pteropodidae): another star phylogeny? BMC Evol Biol 11 : 281.
Štítky
Genetika Reprodukčná medicína
Článek An Evolutionarily Conserved Role for the Aryl Hydrocarbon Receptor in the Regulation of MovementČlánek Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced ActivityČlánek Co-regulated Transcripts Associated to Cooperating eSNPs Define Bi-fan Motifs in Human Gene NetworksČlánek Identification of a Regulatory Variant That Binds FOXA1 and FOXA2 at the Type 2 Diabetes GWAS LocusČlánek tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa CellsČlánek Derlin-1 Regulates Mutant VCP-Linked Pathogenesis and Endoplasmic Reticulum Stress-Induced ApoptosisČlánek A Genetic Assay for Transcription Errors Reveals Multilayer Control of RNA Polymerase II FidelityČlánek The Proprotein Convertase KPC-1/Furin Controls Branching and Self-avoidance of Sensory Dendrites inČlánek Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell SenescenceČlánek BMPs Regulate Gene Expression in the Dorsal Neuroectoderm of and Vertebrates by Distinct MechanismsČlánek Unkempt Is Negatively Regulated by mTOR and Uncouples Neuronal Differentiation from Growth Control
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Translational Regulation of the Post-Translational Circadian Mechanism
- An Evolutionarily Conserved Role for the Aryl Hydrocarbon Receptor in the Regulation of Movement
- Eliminating Both Canonical and Short-Patch Mismatch Repair in Suggests a New Meiotic Recombination Model
- Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced Activity
- Co-regulated Transcripts Associated to Cooperating eSNPs Define Bi-fan Motifs in Human Gene Networks
- Targeted H3R26 Deimination Specifically Facilitates Estrogen Receptor Binding by Modifying Nucleosome Structure
- Role for Circadian Clock Genes in Seasonal Timing: Testing the Bünning Hypothesis
- The Tandem Repeats Enabling Reversible Switching between the Two Phases of β-Lactamase Substrate Spectrum
- The Association of the Vanin-1 N131S Variant with Blood Pressure Is Mediated by Endoplasmic Reticulum-Associated Degradation and Loss of Function
- Identification of a Regulatory Variant That Binds FOXA1 and FOXA2 at the Type 2 Diabetes GWAS Locus
- Regulation of Flowering by the Histone Mark Readers MRG1/2 via Interaction with CONSTANS to Modulate Expression
- The Actomyosin Machinery Is Required for Retinal Lumen Formation
- Plays a Conserved Role in Assembly of the Ciliary Motile Apparatus
- Hidden Diversity in Honey Bee Gut Symbionts Detected by Single-Cell Genomics
- Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria
- tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa Cells
- Causal Variation in Yeast Sporulation Tends to Reside in a Pathway Bottleneck
- Tissue-Specific RNA Expression Marks Distant-Acting Developmental Enhancers
- WC-1 Recruits SWI/SNF to Remodel and Initiate a Circadian Cycle
- Clonal Expansion of Early to Mid-Life Mitochondrial DNA Point Mutations Drives Mitochondrial Dysfunction during Human Ageing
- Methylation QTLs Are Associated with Coordinated Changes in Transcription Factor Binding, Histone Modifications, and Gene Expression Levels
- Differential Management of the Replication Terminus Regions of the Two Chromosomes during Cell Division
- Obesity-Linked Homologues and Establish Meal Frequency in
- Derlin-1 Regulates Mutant VCP-Linked Pathogenesis and Endoplasmic Reticulum Stress-Induced Apoptosis
- Stress-Induced Nuclear RNA Degradation Pathways Regulate Yeast Bromodomain Factor 2 to Promote Cell Survival
- The MAPK p38c Regulates Oxidative Stress and Lipid Homeostasis in the Intestine
- Widespread Genome Reorganization of an Obligate Virus Mutualist
- Trans-kingdom Cross-Talk: Small RNAs on the Move
- The Vip1 Inositol Polyphosphate Kinase Family Regulates Polarized Growth and Modulates the Microtubule Cytoskeleton in Fungi
- Myosin Vb Mediated Plasma Membrane Homeostasis Regulates Peridermal Cell Size and Maintains Tissue Homeostasis in the Zebrafish Epidermis
- GLD-4-Mediated Translational Activation Regulates the Size of the Proliferative Germ Cell Pool in the Adult Germ Line
- Genome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
- Translational Regulation of the DOUBLETIME/CKIδ/ε Kinase by LARK Contributes to Circadian Period Modulation
- Positive Selection and Multiple Losses of the LINE-1-Derived Gene in Mammals Suggest a Dual Role in Genome Defense and Pluripotency
- Out of Balance: R-loops in Human Disease
- A Genetic Assay for Transcription Errors Reveals Multilayer Control of RNA Polymerase II Fidelity
- Altered Behavioral Performance and Live Imaging of Circuit-Specific Neural Deficiencies in a Zebrafish Model for Psychomotor Retardation
- Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development
- Meta-analysis of Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments
- The Proprotein Convertase KPC-1/Furin Controls Branching and Self-avoidance of Sensory Dendrites in
- Hydroxymethylated Cytosines Are Associated with Elevated C to G Transversion Rates
- Memory and Fitness Optimization of Bacteria under Fluctuating Environments
- Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell Senescence
- Interspecific Tests of Allelism Reveal the Evolutionary Timing and Pattern of Accumulation of Reproductive Isolation Mutations
- PRO40 Is a Scaffold Protein of the Cell Wall Integrity Pathway, Linking the MAP Kinase Module to the Upstream Activator Protein Kinase C
- Low Levels of p53 Protein and Chromatin Silencing of p53 Target Genes Repress Apoptosis in Endocycling Cells
- SPDEF Inhibits Prostate Carcinogenesis by Disrupting a Positive Feedback Loop in Regulation of the Foxm1 Oncogene
- RRP6L1 and RRP6L2 Function in Silencing Regulation of Antisense RNA Synthesis
- BMPs Regulate Gene Expression in the Dorsal Neuroectoderm of and Vertebrates by Distinct Mechanisms
- Unkempt Is Negatively Regulated by mTOR and Uncouples Neuronal Differentiation from Growth Control
- Atkinesin-13A Modulates Cell-Wall Synthesis and Cell Expansion in via the THESEUS1 Pathway
- Dopamine Signaling Leads to Loss of Polycomb Repression and Aberrant Gene Activation in Experimental Parkinsonism
- Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation
- Bipartite Recognition of DNA by TCF/Pangolin Is Remarkably Flexible and Contributes to Transcriptional Responsiveness and Tissue Specificity of Wingless Signaling
- The Olfactory Transcriptomes of Mice
- Muscular Dystrophy-Associated and Variants Disrupt Nuclear-Cytoskeletal Connections and Myonuclear Organization
- Interplay of dFOXO and Two ETS-Family Transcription Factors Determines Lifespan in
- Evidence for Widespread Positive and Negative Selection in Coding and Conserved Noncoding Regions of
- Genome-Wide Association Meta-analysis of Neuropathologic Features of Alzheimer's Disease and Related Dementias
- Rejuvenation of Meiotic Cohesion in Oocytes during Prophase I Is Required for Chiasma Maintenance and Accurate Chromosome Segregation
- Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals
- Local Effect of Enhancer of Zeste-Like Reveals Cooperation of Epigenetic and -Acting Determinants for Zygotic Genome Rearrangements
- Differential Responses to Wnt and PCP Disruption Predict Expression and Developmental Function of Conserved and Novel Genes in a Cnidarian
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals
- Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development
- Genome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
- Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



