-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Intersection of the Extrinsic Hedgehog and WNT/Wingless Signals with the Intrinsic Hox Code Underpins Branching Pattern and Tube Shape Diversity in the Airways
Tubes are common structural elements of many internal organs,
facilitating fluid flow and material exchange. To meet the local needs of diverse tissues, the branching patterns and tube shapes vary regionally. Diametric tapering and specialized branch targeting to the brain represent two common examples of variations with organismal benefits in the Drosophila airways and our vascular system. Several extrinsic signals instruct tube diversifications but the impact of intrinsic factors remains underexplored. Here, we show that the local, tube-intrinsic Hox code instructs the pattern and shape of the dorsal trunk (DT), the main Drosophila airway. In the cephalic part (DT1), where Bithorax Complex (BX-C) Hox genes are not expressed, the extrinsic Hedgehog signal is epistatic to WNT/Wingless signals. Hedgehog instructs anterior DT1 cells to take a long and narrow tube fate targeting the brain. In more posterior metameres, BX-C genes make the extrinsic WNT/Wingless signals epistatic over Hedgehog. There, WNT/Wingless instruct all DT cells to take the thick and short tube fate. Moreover, BX-C genes modulate the outputs of WNT/wingless signaling, making the DT tubes thicker in more posterior metameres. We provide a model for how intrinsic factors modify extrinsic signaling to control regional tube morphologies in a network.
Published in the journal: The Intersection of the Extrinsic Hedgehog and WNT/Wingless Signals with the Intrinsic Hox Code Underpins Branching Pattern and Tube Shape Diversity in the Airways. PLoS Genet 11(1): e32767. doi:10.1371/journal.pgen.1004929
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004929Summary
Tubes are common structural elements of many internal organs,
facilitating fluid flow and material exchange. To meet the local needs of diverse tissues, the branching patterns and tube shapes vary regionally. Diametric tapering and specialized branch targeting to the brain represent two common examples of variations with organismal benefits in the Drosophila airways and our vascular system. Several extrinsic signals instruct tube diversifications but the impact of intrinsic factors remains underexplored. Here, we show that the local, tube-intrinsic Hox code instructs the pattern and shape of the dorsal trunk (DT), the main Drosophila airway. In the cephalic part (DT1), where Bithorax Complex (BX-C) Hox genes are not expressed, the extrinsic Hedgehog signal is epistatic to WNT/Wingless signals. Hedgehog instructs anterior DT1 cells to take a long and narrow tube fate targeting the brain. In more posterior metameres, BX-C genes make the extrinsic WNT/Wingless signals epistatic over Hedgehog. There, WNT/Wingless instruct all DT cells to take the thick and short tube fate. Moreover, BX-C genes modulate the outputs of WNT/wingless signaling, making the DT tubes thicker in more posterior metameres. We provide a model for how intrinsic factors modify extrinsic signaling to control regional tube morphologies in a network.Introduction
Branched tubular networks, like our vasculature transport and exchange vital gases and nutrients along entire organisms. The branching patterns, tube structures and dimensions in these networks show considerable regional variations to meet the different needs of target organs and ensure optimal organ function and animal fitness [1–4]. Adaptations of branch morphologies to the tissue environments can be achieved by changing the local availability of extrinsic factors like guidance molecules and/or by intrinsic regional differences in tube cell competence to respond and modify signaling outcomes. Although the prominent roles of variations in extrinsic signals in organotypic branching become widely established [3,5,6], the tube intrinsic mechanisms determining the differential responses of tube cells to signaling remain to be explored [7–10].
Despite the huge evolutionary distance of insects and mammals, the formation and maturation of the respiratory tube network in Drosophila melanogaster has served as a fruitful model system of branching morphogenesis [11,12]. Here, we use this system to evaluate the contribution of tube intrinsic, regionally differential competence in diversification of tube morphology.
The fly respiratory network, also called the tracheal system ramifies extensively to deliver oxygen to each cell in the body (Fig. 1A) [13,14]. It derives from 10 primordial cell clusters specified in ectodermal para-segments (PS) 4–13 on each side of the body [15]. At stage 11, the metameric cell clusters invaginate and begin to extend 6 primary branches. The tracheal metameres (Tr1-10) interconnect into a network through the activities of 5 to 6 specialized fusion cells in each branching unit [16–18]. These cells find and adhere to their ipsilateral or contralateral counterparts in neighboring metameres to form continuous tubes.
Fig. 1. Developmental and genetic distinction between DTa1 and the rest of DT. 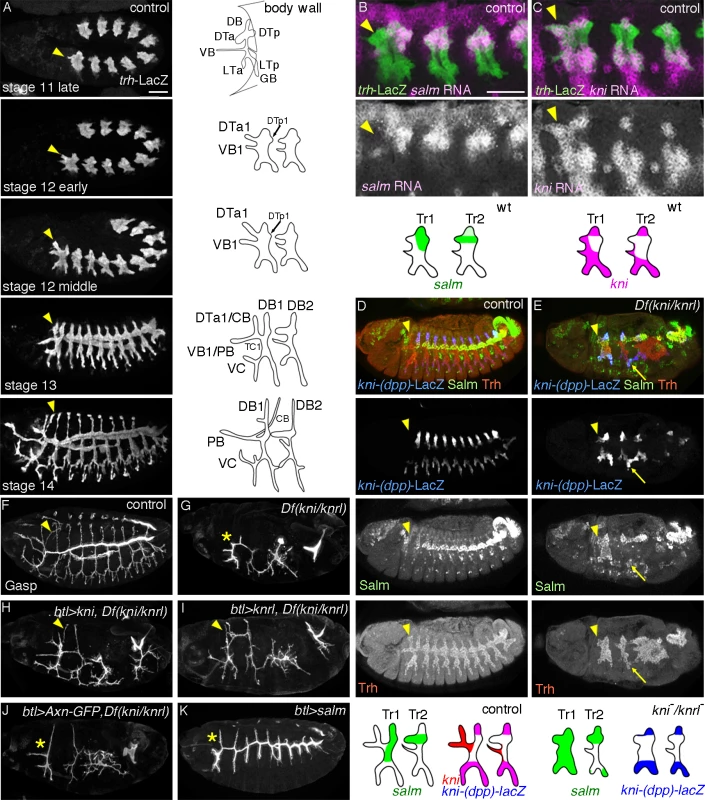
Embryos are aligned with their anterior left and dorsal up unless otherwise indicated. Tracheal branching morphogenesis is controlled by two conceptually different groups of extrinsic signals [6,19]. One is required in all primary branches while the other class specifies unique sets of primary branches. FGF/Branchless (Bnl) [20] is dynamically expressed in the surrounding tissues and serves as a general extrinsic branching signal by activating FGFR/Breathless (Btl) on the airway cells [21–23]. Btl activation initiates oriented cell migration and enhances branch elongation while it also organizes cell fate specification along each primary branch. The gradual specification of distinct fusion and terminal cell fates at the branch tips is essential for both branch fusion and further ramification branching [14,20].
The second class of signals includes BMP, Wnts and Hh proteins. BMP/Decapentaplegic (Dpp) is expressed in dorsal and lateral stripes along the length of the embryo [24]. Dpp signaling specifies and licenses dorsally (dorsal branch, DB) and ventrally migrating primary branches (lateral trunk, LT and ganglionic branch, GB) by inducing the expression of the TFs kni and knirps-related (knrl) in airway cells [24–26]. Wg is expressed in a repetitive pattern of transverse ectodermal stripes and together with other WNT signaling molecules specifies dorsal trunk (DT) identity [27–29]. It upregulates the expression of the TF salm [30] in the major airways of the network. salm promotes short and thick tubes by suppressing intercalation of the tube constituent cells [31,32]. On the other hand, kni/knrl can repress salm expression in DB [25] and promotes cell intercalation in the long and narrow tubes of dorsal (DB) and ventral branches [31]. Hh is also segmentally expressed in ectodermal stripes and modulates the airway branching, both indirectly through positive regulation of bnl expression [33] and directly by acting on terminal cell specification or extension [34,35]. The functions of hh in primary branching remain to be thoroughly studied [34,36,37]. Collectively, the differential primary branch identities established by the second class of extrinsic signals are intimately linked with the distinctive branching patterns and dimensions of individual primary branches [38].
The region-specific modification of serially homologous organs and appendices is a general theme in animal development [39–42]. The evolutionary conserved Hox gene complexes are key selector genes of tissue identities along the anterior posterior (A-P) axis of animals [39,41,43–45] [46–50]. In Drosophila, 2 groups of Hox genes, the Antennapedia complex (ANTP-C) and Bithorax complex (BX-C) confer regional differences to the body plan by graded expression of their products in register with para-segmental units [39,51]. The 3 protein-coding genes of the BX-C [52] are expressed in distinct and partially overlapping domains along the A-P embryonic axis. Ultrabithorax (Ubx) expression initiates in the cells of PS5, abdominal-A (abdA) expression starts from PS7 and Abdominal-B (AbdB) from PS10 [44,53]. A connection between regional modification of airway morphology and Hox genes has been established already in the early studies of Hox-gene mutants. Upon loss of all BX-C genes the tracheal metameres Tr2-Tr10 become transformed to Tr1 [39,54]. However, the genetic and molecular mechanisms establishing the different branch morphologies along the airway tubes have been largely unexplored.
Here, we focus on the regulation of 2 distinct morphological modifications along the DT major airways. We first analyze how the most anterior part of the DT diverges its branching pattern and tube size to generate long and narrow tubes targeting the head. We show that these regional modifications in cell behaviors are controlled by a combination of hh signaling and the airway intrinsic Hox code. We further investigate the mechanism of tube tapering in the central domain of the DT airways. We find that BX-C genes modulate the anterior-to-posterior gradation of DT tube diameter partly through regulating the expression of level of salm, a target of WNT/wg signaling. Our work highlights that the intrinsic Hox code locally modifies the outcomes of extrinsic signals to establish regionally different branching patterns and to coordinate tube shapes in register with the embryo axis.
Results and Discussion
Specialized branching patterns and tube shapes in Tr1
The DT is a continuous tube running along the A-P axis of the embryo. It connects with the exterior through the narrow tube of the spiracular chamber in the posterior spiracle (PSP) [55]. The DT is constructed by the fusion of an anterior (DTa) and a posterior (DTp) branch from each tracheal metamere (Tr1-Tr10) [13,17]. The DT airway encompasses several regional variations that provide a suitable system for the study of the interplay of external signaling with intrinsic factors during tube morphogenesis [13]. First, the most anterior end of the DT extends several specialized branches (see below). Second, it shows a pronounced posterior to anterior diameter tapering contrasting the largely cylindrical shape of other primary branches in the network. Third, its most anterior metameric unit (Tr1) lacks a DTa fusion cell, whereas the most posterior one (Tr10) does not generate a fusion cell in its posterior branch (DTp).
More generally, Tr1 is distinct from the rest of the tracheal metameres because it encompasses more cells and branches to oxygenate the specialized organs of the head and thorax. The specialized branches of Tr1 include the cerebral branch (CB) targeting the brain, the pharyngeal branch (PB) to the anterior intestine, the ventral cephalic branch (VC) extending to epidermis and muscles and the ganglionic branch GB0, which penetrates the ventral nerve cord. Among these, the CB and VC are directly linked to the anterior end of the DT airways.
Despite the pronounced differences in the final branching patterns, branching in Tr1 is comparable to the common stereotypic primary branching of Tr2-Tr9 during stage 11 (Fig. 1A). At stage 12 however, DTa1, elongates further than other DTa branches and shifts dorsally. By stage 13, visceral branch 1 (VB1) and DTa1 co-segregate from the transverse connectives (TC). Later, DTa1 extends dorsally and posteriorly and turns towards the brain, forming CB [13]. DTa1/CB develops very narrow and long tubes compared to the thick and short DT branches in posterior metameres. VB1 extends more anteriorly, forming the pharyngeal branch (PB) (Fig. 1A).
kni expression in DTa1/CB is essential for CB formation
The gradual morphological diversification of CB/DTa1 compared to DTa braches in other metameres prompted us to examine the expression of branch identity TFs, salm and kni. In a “typical”, central metamere, salm expression is upregulated by wg/WNT signaling [27–29] and is detected in DB and DT at stage 11 [30]. Later, kni is induced in DBs by dpp, where it represses salm expression [24–26]. kni and salm are co-expressed in DB1/10 (see below). In contrast to the DTa of other metameres, we found that kni expression is strongly upregulated in DTa1 from late stage 11 (Fig. 1B). Concomitantly, salm is not detectable in DTa1 (Fig. 1C) although it becomes upregulated in DTp1 as in other metameres (Fig. 1D) [30].
To test the significance of the differential kni expression in DTa1 we analyzed mutants lacking both kni and its paralog knrl. In these chromosomal deficiency mutants, the abdominal segments are missing due to the early gap gene function of kni, while trunk development is rather normal [56]. In the trunk region of Df(kni/knrl) mutants, the formation of kni positive primary branches [24,25] is variably affected, ranging from complete absence to branch stalling [25], while the salm-positive DT branches can form and fuse (see below) [57]. We noticed that in kni/knrl mutants, airway cells initiate branch outgrowth in the dorsal and ventral directions and respond to dpp by inducing the dpp-responsive kni reporter, kni-(dpp)-lacZ (Fig. 1E). In these embryos, salm is ectopically detected in kni-(dpp)-lacZ positive cells in either dorsal or ventral cells near the wg stripe (Fig. 1E). This suggests that dpp mediated kni/knrl induction suppresses salm induction by wg in both the dorsal [25,26] and ventral branches. However, in the first and the last metameres of Df(kni/knrl) mutants, salm expression additionally expands to nearly cover the entire metamere, including the putative CB/DTa1 (Fig. 1E). This suggests that kni functions in DTa1 to repress salm. Additionally, the competence of tracheal cells to induce salm is differentially modulated in the terminal Tr1 and Tr10 metameres compared to the central metameres.
Consistent with the notion that generalized reduction of wg/WNT signaling can bypass the requirement of dpp/BMP signaling during DB extension in Tr2-Tr10 [31], btl-Gal4 [58] mediated overexpression of GFP fused to Axin (Axn) [59–61], a negative regulator of wg signaling moderately rescues dorsal extension of the residual DBs (DB1 and DB2) of Df(kni/knrl) mutants but does not appreciably rescue the extension of DTa1/CB (Fig. 1J). In sharp contrast, btl-gal4 driven UAS-kni or UAS-knrl in Df(kni/knrl) mutants restores CB formation (Fig. 1F-I). Thus, we conclude that kni induction and the resultant salm repression in DTa1/CB are essential for its formation and extension. In support for this, btl-gal4 driven UAS-salm in wild-type background significantly suppresses DTa1/CB formation but has little effect on the extension of DTa in Tr2-Tr10 [28,62], which endogenously expresses salm (Fig. 1K). Collectively, the results suggest that kni induction and the resultant salm repression in DTa1/CB are essential for its formation and extension.
hh is necessary for DTa1 patterning
The diversified expression of kni in DTa1 compared to the remaining DTa branches could be regulated by differential expression of exogenous guidance factors around DTa1 and/or by intrinsic differences of competence among the DT1 cells. Firstly, to investigate which extrinsic factors are upstream of kni induction in DTa1, we analyzed the expression or function of known, secreted airway branching regulators.
At stage 11, Tr1 like the rest of the tracheal metameres is surrounded by 6 patches of bnl expressing cells, prefiguring the stereotypic directions of the common primary branches (S1A Fig.). This general pattern diversifies in the cephalic region of stage 12 embryos, where the DTa1 migrates toward a more dorsal bnl expression spot (S1B Fig.). Despite this difference, kni induction in DTa1 is still detected in btl mutants (S1C,D Fig.), excluding a major role of bnl in kni induction in DTa1. dEGFR/faint little ball/torpedo (top) [63] encodes an RTK that is suggested to positively act on salm expression [64] upon binding the Spitz/EGF ligand [65]. In embryos mutant for rhomboid (rho), encoding a protease required for generating the active Spitz [66,67] or for dEGFR, the expression of kni in the DTa1/CB still occurs (S1E,F Fig.). This argues against a role of localized dEGFR activation in controling kni expression in DTa1.
dpp and wg are known inducers of kni/knrl in DB [24] and salm in DT [27,28], respectively, in a “typical” metamere. Thus, variations of their expression in the Tr1 proximity might influence the specialized expression patterns of kni or salm in DTa1. However, the expression of both dpp and wg is comparable around Tr1-3 (S1G, H Fig.), arguing against an instructive role of these two factors in kni induction in DTa1. Indeed, neither mutants of tkv, encoding one of the two dpp receptor subunits [68,69] nor arm mutants lacking an essential component of wg signaling [70–72], showed major defects in kni induction and outgrowth of DTa1/CB (S1I, J Fig.).
hh is a signaling molecule that binds its receptor patched (ptc), thereby relieving ptc-mediated inhibition of the 7 transmembrane domain protein smoothened (smo) [73,74]. hh is expressed in stripes in the ectoderm, abutting the anterior edge of the airway primordia at stage 10 and overlying the anterior part of the invaginated airway cells of each metamere at stage 11 (S1K, L Fig.) [34]. Glazer and Shilo showed that hh induces marker gene expression in the anteriorly migrating branches of central metameres [34], arguing that hh signaling patterns the anterior primary branch fates of the “typical”, central metameres. We found that just after invagination of the airway primordial cells, expression of ptc, a transcriptional target of hh [75,76] is upregulated in the DTa1 precursors (Fig. 2A), suggesting that hh signaling is active there. In hh mutants the dorsalward CB extension is hardly detectable and salm expression is expanded in the entire DT1 (Fig. 2B-D). This suggests that hh signaling in DTa1 induces kni and thereby represses salm expression. Among the ectopically salm expressing cells in DTa1, some cells also express kni while others do not. We suggest that in the absence of hh signaling, hh responsive kni induction is lost while dpp signaling may take over to induce kni expression in some salm positive cells. Such an ectopic activation of kni in the absence of hh could induce ectopic kni/salm-double positive cells resembling DB1 cells. This interpretation is consistent with the de-repression of kni-(dpp)-lacZ in Df(kni/knrl) mutants (Fig. 1E). Thus, we conclude that hh signaling is required to induce kni and to repress salm in DTa1. The earlier function of hh is also required for the maintenance of the striped expression of wg [75,77], which induces salm expression in DT. Thus, the variability of salm expression either in DTa1 or in DTa/DTp of any metamere in hh mutants may partly reflect a reduction or loss of epidermal wg expression.
In hh mutants, bnl expression guiding DB migration in central metameres is lost but bnl expression in surrounding cells guiding CB is still detectable at stage 12 (S1M Fig.). Nevertheless, the dorsal extension of a CB-like branch is not detected in hh mutants at later stages (S1N Fig.). To more directly address the effect of hh signaling in DTa1 we attempted to inactivate its components specifically in the airways. hh signaling modifies the transcriptional activity of cubitus interruptus (ci) [74]. In the absence of hh, Ci is proteolytically processed and acts as a repressor [78], while upon hh pathway activation, smo mediated signaling suppresses this proteolysis and turns Ci into an activator [78–80]. The balance of loss of the repressor form and generation of the activator form of Ci determines the hh signaling outputs [79–81]. We generated embryos expressing dominant negative forms of ci, ciDN (cirep [82,83] and/or ci75 [78,84]) exclusively in the airways and assessed the expression levels of kni-(dpp)-lacZ (a DB and LT/GB marker) [25] and of salm-TSE-lacZ (a reporter of salm expression) [30] in metamere 1. Both markers are ectopically induced in the DTa1 of these embryos at stage 13 (Fig. 2E-H) although the cell number in this branch did not significantly change (20 cells, standard deviation SD = 0.707 for 5 wild type embryos at stage 13 and 19.2 cells, SD = 0.447 for 5 btlX2> cirep embryos). We interpret that the incomplete inactivation of hh signaling in the airways by ciDN, partially transformed DTa1 cells to DTp1. These cells are still receiving enough Dpp to express kni-(dpp)-lacZ. The weaker effects of ciDN expressing embryos compared to hh mutants may reflect ineffectiveness of CiDN or the delayed btl-Gal4 mediated expression [58] of CiDN, which starts slightly later than the initiation of hh action on DTa1. Additionally, the airway-specific overexpression of CiDN or general hh inactivation in smo mutants frequently resulted in DTa1/VB1 co-segregation defects and CB misrouting at stage 16 (Fig. 2I, J and S1O Fig.). A similar CB misrouting phenotype has been described in mutants of unplugged (unpg) encoding a TF expressed in CB [54]. Indeed, the expression of an unpg enhancer trap in the CB of wild type embryos is lost upon CiDN overexpression (Fig. 2I, J).
Collectively, these results identify a selective, direct role of hh signaling in inducing the distinct cell identities of DTa1 compared to the cells of the remaining DT branches.
Fig. 2. hh signaling in the airways is required for DTa1 development. 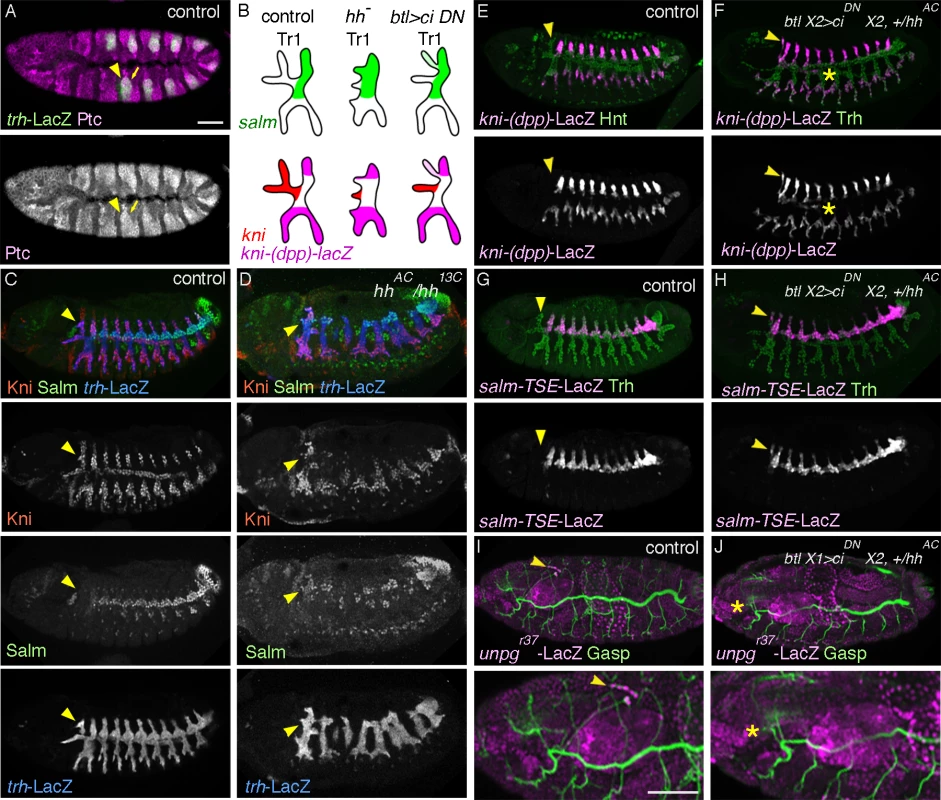
(A) At stage 11, Ptc is detected strongly in all of the invaginated airway primodia (arrow) marked with trh-LacZ, with the strongest expression in the anterior part (arrowhead). hh pathway overactivation transforms DB1 and DTp1 into DTa1-like branches
The transformation of DTa1 to DTp1/DB1 upon inhibition of hh signaling suggests that its overactivation may be sufficient to transform DTp1/DB1 to DTa1. To examine this, we analyzed ptc mutants, where hh signaling is inappropriately activated due to the loss of ptc-mediated inhibition of smo [73,74]. In ptc mutants, the dorsal part of metamere 1 expresses Kni but not Salm already at late stage 11 (Fig. 3A, B). Correspondingly at stage 13, expression of salm-TSE-lacZ is specifically lost from metamere 1, suggesting a defect in both DTp1 and DB1 specification (Fig. 3C and S3A Fig., note that in wild type, salm is expressed in DB1 and DTp1). Consistent with a loss of the DB1 fate in ptc mutants, kni-(dpp)-lacZ expression in the dorsal part of metamere 1 is completely lost (Fig. 3E) while Kni protein is expressed in the whole distal part of Tr1 (S2B Fig.). Although ptc mutants contain fewer airway cells [33], presumably due to an early upregulation of wg [85], a negative regulator of the airway primodia size [86], reduction of cell number in CycA mutants [38] does not significantly abrogate DB1/DT1 fates (S2D, H Fig.). This suggests that the effect of ptc on DB1/DT1 specification is more direct and not due to a general reduction in the number of airway cells. Because btl-Gal4 driven Cirep (Fig. 3D, F) or Ci75 can restore the expression of both kni-(dpp)-lacZ and sal-TSE-lacZ in the Tr1 cells of ptc mutants and because Ptc is expressed in all airway primordia including the entire Tr1 primordium (Fig. 2A), these results suggest that overactivation of hh signaling in Tr1 abolishes the DTp1/DB1 fates.
To further test the role of hh signaling in determining branch identities in Tr1 we analyzed the RNA expression of unpg. In control embryos at early stage 12, unpg expression is strongly detected in DTa1 [54] and weakly in the anterior part of TC1. Both of these regions correspond to hh signaling activation (Fig. 3G). At stage 13, unpg RNA is detected in CB and GB0/GB1 in Tr1 and also in the GBs of the more posterior metameres Tr2-Tr9 (Fig. 3H) [54]. Consistent with the loss of unpg-lacZ expression in CB upon CiDN overexpression, unpg RNA expression is lost in DTa1/CB of hh mutants (Fig. 3K, L). Conversely in ptc mutants, it is expanded posteriorly to cover the positions of DB1/DTp1/TC1 (Fig. 3I, J), indicating their transformation to CB-like fates. Consistent with the expanded unpg expression, we often detected a duplication of CB-like branches in ptc mutants (Fig. 3P). We additionally noted that unpg expression in GB is lost in ptc mutants (Fig. 3J) while unpg is derepressed in LTa in hh mutants (Fig. 3L), in accord with the notion that hh confers the anterior branch identity in the central metameres [34].
At stage 16, ptc mutants show variable branching defects including stalled GBs, DBs and DT breaks [33]. Concomitantly with the loss of DTp1 fate marker (salm-TSE-lacZ), DT1 and DT2 never fuse in ptc mutants (Fig. 3N-P). In wild type, one of DTp1 cells takes the fusion cell fate, activates dys expression and attaches to a fusion cell in DTa2 (S2E Fig.), [18]. In ptc mutants, dys is not activated in DTp1 while dys expression is variably expanded in more cells of the DT branches in posterior metameres (S2E-G Fig.). This may reflect an increase of epidermal expression of wg [85], an inducer of the fusion cell fates [27,28]. The failure of dys activation and DT1 fusion in ptc mutants is significantly rescued by btl-Gal4 mediated overexpression of Cirep (S2I Fig.). Moreover, both loss of dys expression in DT1 as well as DT1/2 fusion defects are variably observed when dominant active Ci, Ciact [84,87] is overexpressed in the airway cells (S2J Fig.). Notably however, the Ciact overexpression by btl-Gal4 did not diminish salm-TSE-lacZ or kni-(dpp)-lacZ expression.
In summary, we suggest that hh signaling instructs the DTa1 fate at the expense of DB1/DTp1 fates. In DTp1, hh signaling must be kept low to allow the proper selection of the fusion cell fate and subsequent DT1/DT2 branch fusion.
Fig. 3. Overactivation of hh signaling transforms the primary branch identities in Tr1. 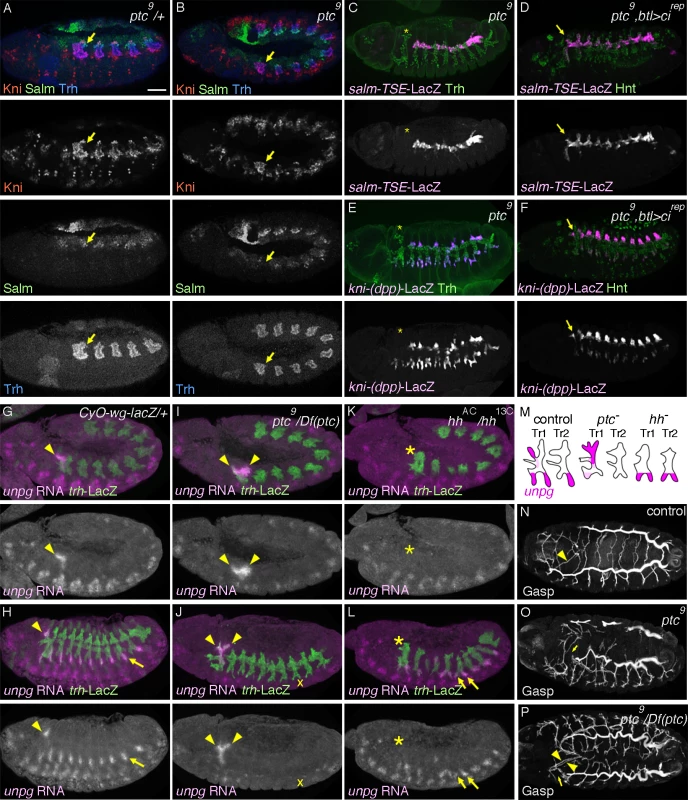
(A, B) Expression of Salm, Kni and Trh at late stage 11. In contrast to the control (A), where Salm is upregulated in DTp1 (arrow), in ptc mutants (B), Salm upregulation in DTp1 (arrow) is not detected and Kni occupies the whole distal part of Tr1. Note that the airway primordia look smaller in ptc mutants [33]. Abdominal Hox genes shunt hh signaling from induction of kni in DTa
The hh induction of kni expression in the DTa of wild type embryos as well as the loss of DT/DB fates in ptc mutants are confined to Tr1. However, hh signaling outcomes are expected to be equally profound in the more posterior metameres of both wild type [34] and ptc mutant embryos [33]. The exclusive restriction of Hh responses within Tr1 implies the presence of an inhibitory mechanism preventing kni activation in the DTa branches of posterior metameres. The BX-C genes represent obvious candidate regulators of posterior metamere identity and modulators of hh signaling outcomes along the A-P axis of the airways. Indeed, unpg expression is de-repressed in progressively more posterior metameres in Ubx mutants and Ubx abdA AbdB triple mutants [54].
BX-C gene expression is graded along the A-P axis of the airway metameres (S3A-D Fig.). Ubx expression starts in Tr2 (PS5) and peaks at Tr3 (PS6) (S3A Fig.). abdA expression starts in Tr4 (PS7) and peaks in Tr6 (PS9) (S3B Fig.) while AbdB expression starts in Tr7 (PS10) and peaks in Tr10 (PS13) (S3C Fig.). These expression patterns are in register with the expression of BX-C genes in the ectoderm [53], which is the origin of the airway primordia.
To explore the function of BX-C genes in DTa fates, we first monitored dys expression in various BX-C mutants. In Ubx mutants, single fusion cells are detected in Tr1, Tr2 and Tr3 (S3E, F Fig.) suggesting that DTa2 and 3 are transformed to become DTa1/CB. abdA single mutants do not show dys expression defects in the DT (S3G Fig.) while a superfluous fusion cell in DTp10 is detected in both AbdB single and abdA AbdB double mutants (S3H, I Fig.). This suggests that DT10, which normally contains only a single fusion cell in its DTa branch, is transformed into a more anterior identity. Compared to Ubx single mutants, Ubx abdA double mutants have single fusion cells in Tr1–8 and often in Tr9 (S3J Fig.). In Ubx abdA AbdB triple mutants, the DT stumps of all metameres contain single fusion cells (S3K Fig.). This implies that DTa branches in progressively more posterior metameres are transformed to become DTa1 upon progressive loss of BX-C genes [39]. Any single gene of the BX-C is sufficient to suppress the DTa1 fate. Consistently, we detected expansion of kni expression and a loss of salm in the transformed DTa in Ubx single, Ubx abdA double and Ubx abdA AbdB triple mutants (Fig. 4A-C). These phenotypes are often accompanied with the appearance of dorsally extending branches that are positive for Kni but negative for kni-(dpp)-LacZ, Salm and salm-TSE-LacZ, resembling the CBs of wild type embryos (S3K Fig.) [54]. The marker expression analysis in BX-C mutants suggests that in the posterior metameres, Ubx, abdA and AbdB interfere with the outcomes of hh signaling.
If the antagonistic role of BX-C on hh-mediated kni induction reflects an essential function of the BX-C in posterior metameres, one might expect some rescue of the branching defects of BX-C mutants upon simultaneous loss of hh or kni/knrl. Indeed, salm expression is de-repressed in DTa1-9 of Ubx abdA hh and of Ubx abdA kni/knrl mutants (Fig. 4D, E). Additionally, DT fusion is weakly restored in both the triple and quadruple mutants (S3L, M Fig.).
Taken together, we suggest that BX-C genes antagonize hh-mediated induction of kni in DTa branches.
Fig. 4. The airway intrinsic BX-C code determines the outcome of hh signaling. 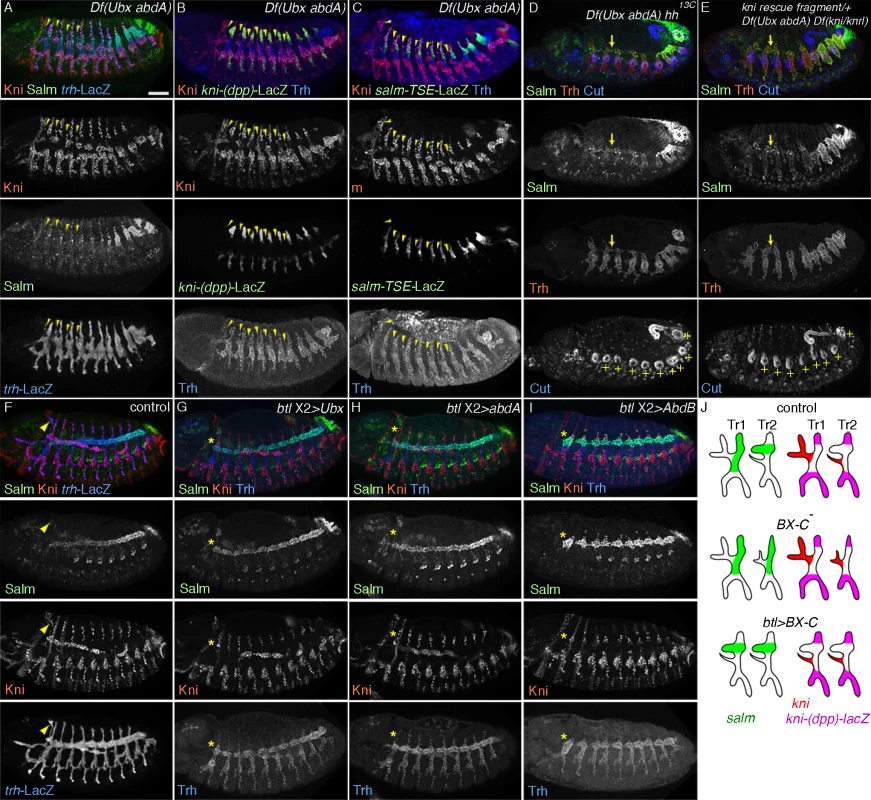
(A-C) Effects of loss of Ubx and abdA on the DTa fate in the central metameres. In Df(Ubx abdA) mutants at stage 13, ectopic dorsalword migration of cells that are positive for Kni and negative for Salm is variably detected in metameres 1–7 (A). These transformed DTa/CB-like cells (arrowheads) are negative for both kni-(dpp)-lacZ (B) and salm-TSE-lacZ (C). Note that expression of Salm/salm-TSE-LacZ is weakly detected in DB, DTp and TC in the transformed metameres as well as in Tr1. Airway-specific expression of abdominal Hox genes diverts hh signaling from kni activation
In addition to the airways, BX-C genes are expressed in many embryonic tissues. Where do they act to divert hh signaling from kni induction in the DT branches? In lack of reagents for the reliable conditional inactivation of the BX-C genes in the airways, we monitored the effects of airway-specific ectopic expression of BX-C genes on DT1 cell specification. The first metamere does not express the BX-C genes (S3A-D Fig.) and thereby may provide a naïve environment for assessing the effects of their overexpression on marker gene activation [41]. btl-Gal4 mediated overexpression of any of the BX-C genes in wild type background, variably decreases kni expression in the DTa1 and concomitantly leads to increased salm levels at stage 13/14 (Fig. 4F-J). At later stages, DTa1 branches are thick, resembling typical DTa branches of posterior metameres (see below) in agreement with the previously reported loss of CBs upon abdA overexpression [88].
Similarly, btl-Gal4 mediated overexpression of either Ubx or abdA restores the fusion defects of DT1 and DT2 in ptc mutants (S3N, O Fig.). We detected that expression of both salm-TSE-lacZ and kni-(dpp)-lacZ is restored in the dorsal part of Tr1 of ptc mutants upon abdA overexpression (S3P, Q Fig.). These results argue that the Hox code in the airway cells autonomously changes hh-signaling outputs in Tr1 both in wild type and in ptc mutants, where the hh pathway is hyper activated. Finally, we asked if transgenic expression of abdA or Ubx in the airways could restore the branch fusion defects along the entire DT of Ubx abdA double mutants. Again, this manipulation rescues the branch fusion phenotypes (S3R-T Fig.) arguing that BX-C genes autonomously shunt hh signaling from inducing kni in the DTa branches of all metameres to promote continuous DT formation.
BX-C genes control tube tapering
A common characteristic of biological tubes is the tapering of tube diameter along their length. The Drosophila larval airways receive air only from the PSP and distribute it anteriorly. Correspondingly, the tubes show a posterior to anterior tapering [38], which presumably gradually increases the flow rates to the anterior and facilitates air diffusion from the PSP to the most distant anterior organs (http://hyperphysics.phy-astr.gsu.edu/hbase/pfric.html).
salm is a master selector gene for DT identity. Intriguingly, its expression levels in the DT tubes at stage 13/14 show a largely proportional decrease from posterior to anterior metameres matching the tapering of the airways (Fig. 4F)[30]. The graded diameter along the airway length also coincides with the graded expression of BX-C proteins along the A-P axis. To explore the potential regulatory roles of BX-C factors and salm in tube shaping, we first analyzed airway shapes in BX-C mutants and detected 2 kinds of effects of BX-C genes on tube diameter, metamere-autonomous and systemic (see below).
Consistent with graded AbdB expression in PS 10–13, in AbdB mutants, tube diameter in DT7-10 lost its tapering and became narrower suggesting that the amount of AbdB proportionally controls the tube diameter. (Fig. 5A, C, I, J and S1 Table). Similarly, in abdA mutants, the gradient of tube diameter in DT4-6 was lost and DT4-9 became narrower (Fig. 5B, I, J and S1 Table) suggesting again that AbdA levels proportionally control DT tube diameter. In abdA AbdB double mutants the shape of the airways is changed further (Fig. 5D, I, J and S1 Table). The airways of Tr4-10 acquire a more cylindrical shape compared to the conically shaped tubes of wild type embryos. We suggest that in wild type embryos the gradient of abdA and AbdB activities would superimpose on a weak but clear, abdA and AbdB-independent gradient of DT tube thickness (Fig. 5I and S1 Table). This may explain why abdA mutants, where PS7-9 (DT4-6) are expected to transform to PS6 (DT3) still show a clear difference in tube diameter between DT3 and DT4 and why AbdB mutants, where PS10-13 (DT7-10) are expected to transform to PS9 (DT6) show a distinct tube caliber in DT6 and DT7 (Fig. 5I and S1 Table). Thus, in accord with their known functions in determining cell fates and morphogenesis in the embryonic ectoderm [39,44,53], the BX-C genes control the tapering of airways along the A-P axis autonomously. We note however that there is also a systemic effect of BX-C mutations along the entire DT. In either abdA, AbdB single or in abdA AbdB double mutants, the diameter of the more anterior metameres, where corresponding BX-C genes are not expressed also show a slight reduction of tube diameter (Fig. 5I and S1 Table). Among different possibilities, these results may suggest that the activities of abdA or AbdB control the hydrostatic pressure in the lumen to non-autonomously assure proportional growth of all the DT tubes [89] (see below). The residual tapering of abdA AbdB double mutants implies a mechanism of A-to-P gradient formation independent of abdA and AbdB. Ubx could exert such a function in the absence of abdA and AbdB. We analyzed bxd113 or bxd100 mutants, where Ubx expression levels in PS5 become similar to that of PS4 but its ectodermal expression is lost from PS7 onwards [90] (S4A Fig.). In these embryos, DT3 tube diameter approaches that of DT2 and there is also an overall reduction of tube diameter in more posterior metameres (Fig. 5I, S4C-E Fig. and S1 Table), suggesting that the endogenous Ubx level controls DT diametric tube expansion. However, in abdA AbdB double mutants, Ubx levels are largely uniform in DT4-10, which correspond to PS7-13 [91] (S5B Fig.). This suggests the presence of an additional, BX-C-independent cue in DT tube shaping. In Ubx abdA AbdB triple mutants, the transformed Salm positive residual DT/TC branches in posterior metameres are slightly thicker than those in anterior metameres, further arguing for the existence of the postulated BX-C-independent mechanism in tube shaping (S3K, S4F Figs.).
Fig. 5. BX-C genes control DT tube tapering. 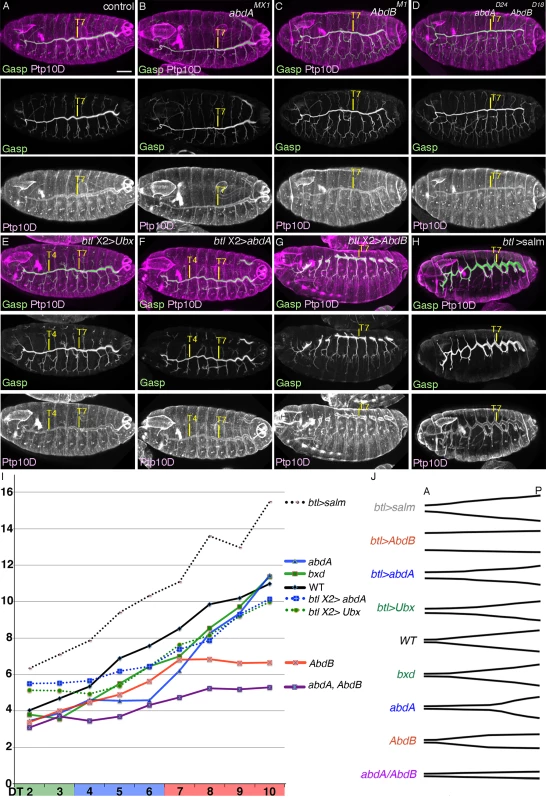
(A-H) BX-C control of DT tube tapering visualized by luminal Gasp and the apical protein PTP10D. Is there any causative link between the BX-C mediated DT expansion control and the A-to-P gradual increase of salm expression levels in the DT (Fig. 4F) [30]? We noticed that Salm levels are reduced in central and posterior metameres of abdA, AbdB or abdA AbdB double mutants (S4G-J Fig.). This reduction is largely consistent with the changes in shape and DT tube diameters in the corresponding mutants. Conversely, upon overexpression of AbdB, higher Salm amounts are detected in the DT of all metameres at stage 13 (Fig. 4I). The DT branches of these embryos often stall and fail to fuse making evaluation of tube diameter difficult. Nevertheless, the DT diameter in all metameres appears comparable to the diameter of the most posterior DT branches (Fig. 5G). Overexpression of Ubx or abdA renders Salm expression levels uniform in the anterior metameres (Fig. 4G, H). Correspondingly, the DT diameter in the anterior metameres becomes thicker (Fig. 5E, F, I, J and S1 Table).
How does the Salm gradient along the DT A-P axis correlate to the graded DT tube expansion? salm overexpression confers DT identity to other primary branches [28,62]. We noted that salm overexpression also generally expands tube diameter not only in the transformed branches [28] but also in the DT, which endogenously expresses salm (Fig. 5H, I, J and S1 Table). The programmed secretion of luminal and apical proteins has been proposed to drive tube dilation of the Drosophila airways [92–95]. Tenectin (Tnc) is a luminal glycoprotein accumulating in the DT and hindgut tubes during diametric expansion [96]. Tnc overexpression in the airways drives DT tube dilation in a dose-dependent manner potentially through increasing hydrostatic pressure [89] and the tnc mRNA levels increase in a characteristic graded fashion along the A-P axis of the DT tubes in wild type embryos [89]. We found that the diametric increase caused by salm overexpression in the airways is accompanied by an increase in the luminal levels of Tnc and conversely, Tnc becomes undetectable in the tracheal tubes of salm mutants (S4K-M Fig.). This suggests that Salm adjusts the graded expression levels of Tnc and presumably other proteins during tube dilation.
Additionally, the diameter increase in the branches of salm over expressing embryos is still most pronounced in the posterior metameres. (Fig. 5H, I, J and S1 Table). The accentuated tube enlargement in posterior metameres upon salm overexpression suggests a salm-independent control mode of A-to-P gradient of DT tube diameter. This is consistent with the observation that tube diameter of the remaining branches in salm mutants are still thickest in Tr10 (S4N Fig.).
In conclusion, our work suggests two interdependent mechanisms by which the tube intrinsic Hox code controls branch identity and tube shape in the Drosophila respiratory network (Fig. 6A, B). Firstly, Hox genes autonomously divert extrinsic hh signaling from kni induction in the DT thereby generating continuous, salm positive DT airways. We suggest that this allows a second tier of DT tube shape regulation, where the Hox activity gradient both locally and systemically organizes graded tube dilation via salm dependent and independent modes. The Ci/BX-C circuit may control tube morphology directly by binding to the regulatory regions of kni and salm, as recent genome-wide TF binding studies suggest that kni is a direct target of ci [37] and that salm is a direct target of Ubx [97]. In our model, tube tapering and thereby luminal fluid flow are calibrated by the balance between extrinsic signals and the intrinsic Hox code. Since Hox selector genes are regionally expressed in other developing tubular organs like the mammalian lung [98] and vasculature [9,10], a similar regulatory logic of tube branching and shaping may apply to other systems.
Fig. 6. Models for the BX-C dependent control of branching patterns and of DT tube diameter. 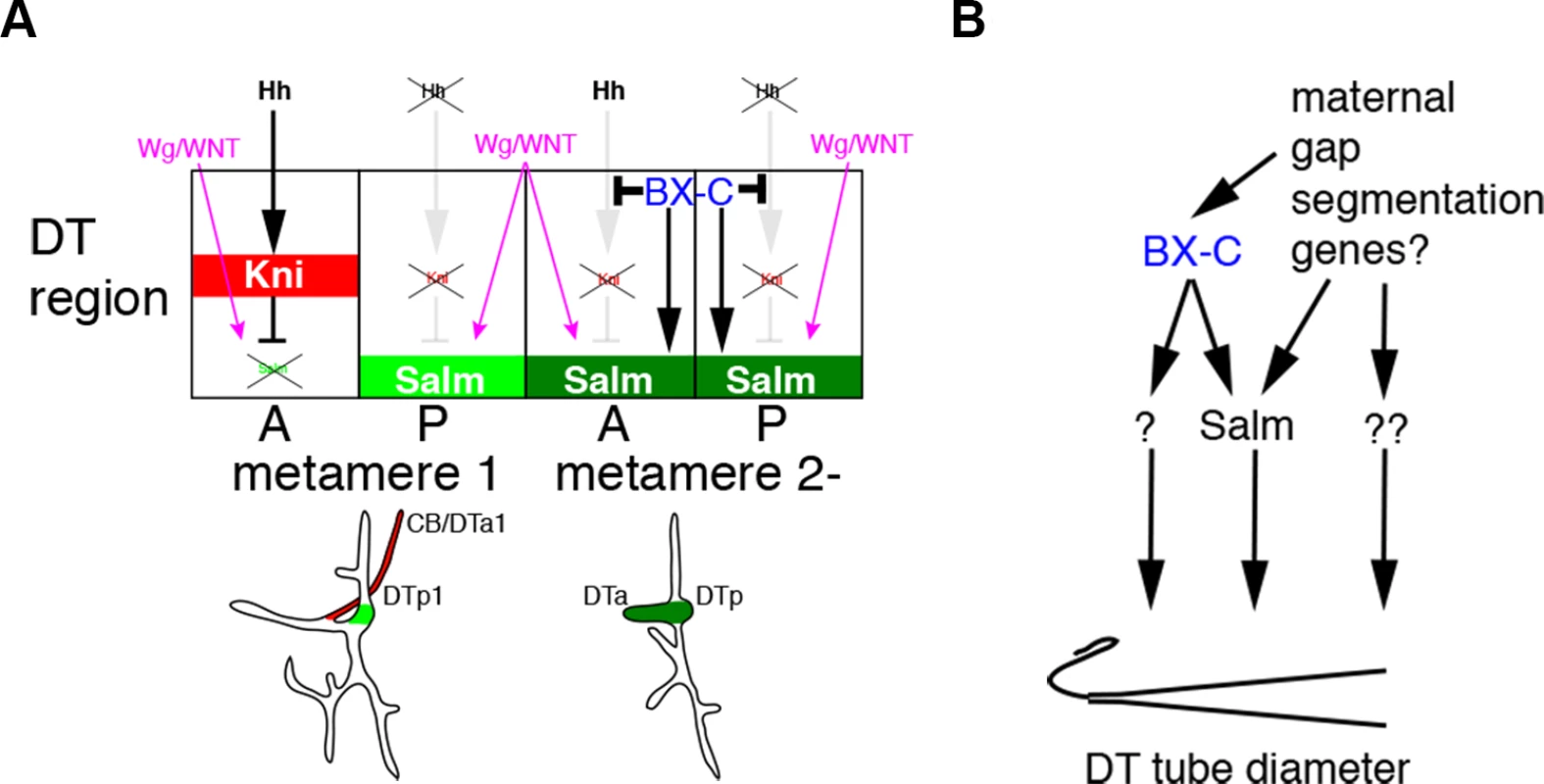
(A) In all metameres, expression of hh and wg in cells surrounding the tracheal primordia are comparable. In metamere 1, where there is no input from BX-C, hh signaling induces kni in DTa1 in wild type, and additionally in DTp1 in ptc mutants. kni represses wg/WNT mediated upregulation of salm, making DTa1 branches thinner. In the posterior metameres, BX-C shunts hh signaling from induction of kni, allowing salm upregulation by wg/WNT. BX-C additionally enhances salm expression in the DT. Materials and Methods
Fly genetics
Flies kept over balancer chromosomes [99] were grown in standard medium. We obtained the appropriate genotypes by standard genetic crosses. For overexpression of genes, we used the Gal4/UAS system [100]. Mutant embryos were identified by the expression of twi-lacZ, ftz-lacZ, Ubx-lacZ or dfd-GFP [101] constructs inserted on balancer chromosomes. We identified mutants harboring Ubx or AbdB mutations by selecting embryos with previously reported phenotypes in the anterior spiracle or PSP. For collection of large amount of virgins, we used a Y chromosome harboring hs-hid construct developed by R. Lehmann and M. Van Doren [102]. See Flybase [103] for details of strains described below.
Mutant strains. abdAM1 (a gift from B. Gebelein) [104], AbdBM1, AbdBM5 and Df(3R)P115 as Df(Ubx abdA AbdB) (gifts from I. Lohmann) [105], btl∆oh10 and btl∆oh24-1[106], Df(5) as Df(salm/salr) (a gift from M. Llimargas) [28,107], hh13C [108], kni early rescue fragment; Df(3L)riXT (a gift from R. Schuh) [25], rhod38 (a gift from D. Andrew and A. Salzberg) [109,110], rho7M (a gift from J. Skeath) [111], topf24 (a gift from K. Moses) [112]. arm4 and CycA3 were obtained from Natinal Institute of Genetics (NIG), Mishima, Japan. Ubx1, abdAMX1, abdAD24 AbdBD18, Ubx1 abdAD24 AbdBD18[113], Df(109) as Df(Ubx,abdA), CycAC8LR1, Df(3L)Exel6115 as Df(CycA), hhAC, Df(3L)BSC448 homozygous or its transheterozygote over Df(3L)riXT as Df(kni/knrl), ptc9, Df(2R)Exel7098 as Df(ptc), smo3, tkv7 and Df(2R)Exel6076 as Df(top) were obtained from Bloomington stock center (BDSC), Indiana, USA.
Enhancer trap strains. 1-eve-1 as trh-lacZ (a gift from N. Perrimon) [114] and unpgr37 (a gift from F. J. Diaz-Benjumea, R. Urbach and G. M. Technau) [115,116]. hhP30 [117] was obtained from BDSC.
Enhancer reporter strains. kni-(dpp)-lacZ [25] and salm-TSE-lacZ [30] (gifts from R. Schuh).
Gal4 and UAS strains. btl-gal4 on 2nd and 3rd chromosomes (gifts from S. Hayashi) [58], UAS-abdA (a gift from F. J. Diaz-Benjumea) [115], UAS-ci75 and UAS-ciH4P (gifts from S. Ishii) [84], UAS-cirep (a gift from A. Moore) [83], UAS-kni and UAS-knrl (gifts from R. Schuh) [25]. UAS-AbdB, UAS-Axn-GFP, UAS-salm and UAS-Ubx were obtained from BDSC.
In situ hybridization and immunostaining
Egg collection was done with apple/grape juice plate at 25°C. Embryos were bleached and fixed as previously described [118] for 15–30 minutes with a 1 : 1 mixture of heptane and a fix solution (3.7% formaldehyde, 0.1M Hepes pH6.9, 2mM MgSO4). Embryos were dechorionated with methanol and incubated in 0.1% PBT supplemented with 0.5% BSA. Staging of embryos was done as previously described [119].
For immunostaining the following primary antibodies were used:
Guinea-pig anti-AbdA (1 : 500, a gift from B. Gebelein) [104], rabbit anti-Dys (1 : 500, a gift from L. Jiang) [18], Guinea-pig anti-Gasp (1 : 1000) [95], mouse anti-Ubx (1 : 10, a gift from R. White) [120], Guinea-pig anti-Kni (1 : 300), (developed by J. Reinitz and distributed by Y. Hiromi, East Asian Segmentation Antibody Center, Mishima, Japan) [121], rabbit anti-Salm (1 : 200, gifts from R. Barrio and T. Cook) [107,122], rabbit anti-Tnc C-terminal (1 : 1000, a gift from Z. A. Syed and A. Uv) [89], rat anti-Trh (1 : 200, a gift from D. Andrew) [123] and rabbit anti-Trh (1 : 50). Mouse anti-Abd-B (1 : 10, donated by S. Celniker) [124], mouse anti-Cut (1 : 10, donated by G. M. Rubin) [125], mouse anti-Hnt (1 : 10, donated by H. D. Lipshitz) [126], mouse mab2A12 (anti-Gasp) (1 : 5, donated by M. Krasnow, N. Patel and C. Goodman) [17,95], mouse anti-Ptc (1 : 10, donated by I. Guerrero) [127] and mouse anti-PTP10D (1 : 10, donated by K. Zinn) [128] were obtained from Developmental Studies Hybridoma Bank (DSHB), Iowa, USA. Commercially available antibodies were anti-LacZ (E.coli. β-Galactosidase) antibodies made in goat (1 : 500, Biogenesis) or rabbit (1 : 1000, Capel) and anti-GFP antibodies made in rabbit (1 : 500, JL-8 Clontech), mouse (1 : 1000, GFP20 Sigma) or goat (1 : 500, ab6673 Abcam).
Donkey or goat biotin - or fluorescently labeled secondary antibodies made against the host species of primary antibodies were purchased from Jackson Laboratories. Streptavidin coupled with AMCA, FITC or Cy5 were used when necessary. For mab2A12 detection TSA amplification (PerkinElmer) was used.
Double fluorescent labeling with RNA probe and antibody was carried out as described [129]. The following cDNA clones were used to make hybridization probes; bnl (a gift from M. Krasnow) [20], dpp [108] and unpg (a gift from P. A. Beachy) [54]. wg, salm and kni clones were obtained from Drosophila Genomics Resource Center (DGRC), Indiana, USA.
Confocal images were taken by Biorad MRC1024, Oympus Fluoview 1000 or Zeiss LSM780. Images of controls and mutants taken by the same confocal microscopes were used for comparison. Images were processed by ImageJ and figures were prepared with Photoshop and Illustrator.
Quantification of Trh positive cell number of CB was done for stage 13 embryos stained with antibodies against Trh and DE-cad, scanned at 40× magnification with 0.6 um intervals.
For quantification of DT tube thickness, mid-late stage 16 embryos stained with antibodies against PTP10D, Gasp and DE-cad were scanned at 40× magnification with 0.6 um intervals. Using ImageJ, 3 points next to the bases of DB were selected from each Z-stacked image for each metamere to measure the maximal distance of PTP10D positive apical membranes perpendicular to the longitudinal tube axis. 3 embryos for each genotype were measured. Average, SD and p-value of student t-test were calculated by Excel.
Supporting Information
Zdroje
1. Lubarsky B, Krasnow MA (2003) Tube morphogenesis: making and shaping biological tubes. Cell 112 : 19–28. 12526790
2. Andrew DJ, Ewald AJ (2010) Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Dev Biol 341 : 34–55. doi: 10.1016/j.ydbio.2009.09.024 19778532
3. Ochoa-Espinosa A, Affolter M (2012) Branching morphogenesis: from cells to organs and back. Cold Spring Harb Perspect Biol 4.
4. Kusumbe AP, Ramasamy SK, Adams RH (2014) Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507 : 323–328. doi: 10.1038/nature13145 24646994
5. Eilken HM, Adams RH (2010) Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol 22 : 617–625. doi: 10.1016/j.ceb.2010.08.010 20817428
6. Ghabrial A, Luschnig S, Metzstein MM, Krasnow MA (2003) Branching morphogenesis of the Drosophila tracheal system. Annu Rev Cell Dev Biol 19 : 623–647. 14570584
7. Cantile M, Schiavo G, Terracciano L, Cillo C (2008) Homeobox genes in normal and abnormal vasculogenesis. Nutr Metab Cardiovasc Dis 18 : 651–658. doi: 10.1016/j.numecd.2008.08.001 19013779
8. Kachgal S, Mace KA, Boudreau NJ (2012) The dual roles of homeobox genes in vascularization and wound healing. Cell Adh Migr 6 : 457–470. doi: 10.4161/cam.22164 23076135
9. Pruett ND, Hajdu Z, Zhang J, Visconti RP, Kern MJ, et al. (2012) Changing topographic Hox expression in blood vessels results in regionally distinct vessel wall remodeling. Biol Open 1 : 430–435. doi: 10.1242/bio.2012039 23213434
10. Pruett ND, Visconti RP, Jacobs DF, Scholz D, McQuinn T, et al. (2008) Evidence for Hox-specified positional identities in adult vasculature. BMC Dev Biol 8 : 93. doi: 10.1186/1471-213X-8-93 18826643
11. Schottenfeld J, Song Y, Ghabrial AS (2010) Tube continued: morphogenesis of the Drosophila tracheal system. Curr Opin Cell Biol 22 : 633–639. doi: 10.1016/j.ceb.2010.07.016 20739171
12. Affolter M, Caussinus E (2008) Tracheal branching morphogenesis in Drosophila: new insights into cell behaviour and organ architecture. Development 135 : 2055–2064. doi: 10.1242/dev.014498 18480161
13. Manning G, Krasnow MA (1993) The Development of Drosophila melanogaster. Plainview, N.Y.: Cold Spring Harbor Laboratory Press.
14. Samakovlis C, Hacohen N, Manning G, Sutherland DC, Guillemin K, et al. (1996) Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development 122 : 1395–1407. 8625828
15. Hartenstein V, Jan YN (1992) Studying Drosophila Embryogenesis with P-Lacz Enhancer Trap Lines. Rouxs Archives of Developmental Biology 201 : 194–220.
16. Tanaka-Matakatsu M, Uemura T, Oda H, Takeichi M, Hayashi S (1996) Cadherin-mediated cell adhesion and cell motility in Drosophila trachea regulated by the transcription factor Escargot. Development 122 : 3697–3705. 9012491
17. Samakovlis C, Manning G, Steneberg P, Hacohen N, Cantera R, et al. (1996) Genetic control of epithelial tube fusion during Drosophila tracheal development. Development 122 : 3531–3536. 8951068
18. Jiang L, Crews ST (2003) The Drosophila dysfusion basic helix-loop-helix (bHLH)-PAS gene controls tracheal fusion and levels of the trachealess bHLH-PAS protein. Mol Cell Biol 23 : 5625–5637. doi: 10.1128/MCB.23.16.5625-5637.2003 12897136
19. Uv A, Cantera R, Samakovlis C (2003) Drosophila tracheal morphogenesis: intricate cellular solutions to basic plumbing problems. Trends Cell Biol 13 : 301–309. 12791296
20. Sutherland D, Samakovlis C, Krasnow MA (1996) branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell 87 : 1091–1101. 8978613
21. Klambt C, Glazer L, Shilo BZ (1992) breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev 6 : 1668–1678. 1325393
22. Reichman-Fried M, Dickson B, Hafen E, Shilo BZ (1994) Elucidation of the role of breathless, a Drosophila FGF receptor homolog, in tracheal cell migration. Genes Dev 8 : 428–439. 8125257
23. Lee T, Hacohen N, Krasnow M, Montell DJ (1996) Regulated Breathless receptor tyrosine kinase activity required to pattern cell migration and branching in the Drosophila tracheal system. Genes Dev 10 : 2912–2921. 8918892
24. Vincent S, Ruberte E, Grieder NC, Chen CK, Haerry T, et al. (1997) DPP controls tracheal cell migration along the dorsoventral body axis of the Drosophila embryo. Development 124 : 2741–2750. 9226445
25. Chen CK, Kuhnlein RP, Eulenberg KG, Vincent S, Affolter M, et al. (1998) The transcription factors KNIRPS and KNIRPS RELATED control cell migration and branch morphogenesis during Drosophila tracheal development. Development 125 : 4959–4968. 9811580
26. Ribeiro C, Ebner A, Affolter M (2002) In vivo imaging reveals different cellular functions for FGF and Dpp signaling in tracheal branching morphogenesis. Dev Cell 2 : 677–683. 12015974
27. Chihara T, Hayashi S (2000) Control of tracheal tubulogenesis by Wingless signaling. Development 127 : 4433–4442. 11003842
28. Llimargas M (2000) Wingless and its signalling pathway have common and separable functions during tracheal development. Development 127 : 4407–4417. 11003840
29. Llimargas M, Lawrence PA (2001) Seven Wnt homologues in Drosophila: a case study of the developing tracheae. Proc Natl Acad Sci U S A 98 : 14487–14492. doi: 10.1073/pnas.251304398 11717401
30. Kuhnlein RP, Schuh R (1996) Dual function of the region-specific homeotic gene spalt during Drosophila tracheal system development. Development 122 : 2215–2223. 8681802
31. Ribeiro C, Neumann M, Affolter M (2004) Genetic control of cell intercalation during tracheal morphogenesis in Drosophila. Curr Biol 14 : 2197–2207. 15620646
32. Shaye DD, Casanova J, Llimargas M (2008) Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat Cell Biol 10 : 964–970. doi: 10.1038/ncb1756 18641639
33. Buti E, Mesquita D, Araujo SJ (2014) Hedgehog is a positive regulator of FGF signalling during embryonic tracheal cell migration. PLoS One 9: e92682. doi: 10.1371/journal.pone.0092682 24651658
34. Glazer L, Shilo BZ (2001) Hedgehog signaling patterns the tracheal branches. Development 128 : 1599–1606. 11290298
35. Kato K, Chihara T, Hayashi S (2004) Hedgehog and Decapentaplegic instruct polarized growth of cell extensions in the Drosophila trachea. Development 131 : 5253–5261. 15456724
36. Llimargas M, Casanova J (1997) ventral veinless, a POU domain transcription factor, regulates different transduction pathways required for tracheal branching in Drosophila. Development 124 : 3273–3281. 9310322
37. Biehs B, Kechris K, Liu S, Kornberg TB (2010) Hedgehog targets in the Drosophila embryo and the mechanisms that generate tissue-specific outputs of Hedgehog signaling. Development 137 : 3887–3898. doi: 10.1242/dev.055871 20978080
38. Beitel GJ, Krasnow MA (2000) Genetic control of epithelial tube size in the Drosophila tracheal system. Development 127 : 3271–3282. 10887083
39. Lewis EB (1978) A gene complex controlling segmentation in Drosophila. Nature 276 : 565–570. 103000
40. Carroll SB (1995) Homeotic genes and the evolution of arthropods and chordates. Nature 376 : 479–485. 7637779
41. Mann RS, Morata G (2000) The developmental and molecular biology of genes that subdivide the body of Drosophila. Annu Rev Cell Dev Biol 16 : 243–271. 11031237
42. Shubin N, Tabin C, Carroll S (2009) Deep homology and the origins of evolutionary novelty. Nature 457 : 818–823. doi: 10.1038/nature07891 19212399
43. Mann RS, Carroll SB (2002) Molecular mechanisms of selector gene function and evolution. Curr Opin Genet Dev 12 : 592–600. 12200165
44. Pearson JC, Lemons D, McGinnis W (2005) Modulating Hox gene functions during animal body patterning. Nat Rev Genet 6 : 893–904. 16341070
45. Mallo M, Wellik DM, Deschamps J (2010) Hox genes and regional patterning of the vertebrate body plan. Dev Biol 344 : 7–15. doi: 10.1016/j.ydbio.2010.04.024 20435029
46. Castelli-Gair J, Greig S, Micklem G, Akam M (1994) Dissecting the temporal requirements for homeotic gene function. Development 120 : 1983–1995. 7925003
47. Averof M, Akam M (1995) Hox genes and the diversification of insect and crustacean body plans. Nature 376 : 420–423. 7630416
48. Averof M, Patel NH (1997) Crustacean appendage evolution associated with changes in Hox gene expression. Nature 388 : 682–686. 9262403
49. Merabet S, Hombria JC, Hu N, Pradel J, Graba Y (2005) Hox-controlled reorganisation of intrasegmental patterning cues underlies Drosophila posterior spiracle organogenesis. Development 132 : 3093–3102. 15930099
50. Sanchez-Higueras C, Sotillos S, Castelli-Gair Hombria J (2014) Common origin of insect trachea and endocrine organs from a segmentally repeated precursor. Curr Biol 24 : 76–81. doi: 10.1016/j.cub.2013.11.010 24332544
51. Kaufman TC, Lewis R, Wakimoto B (1980) Cytogenetic Analysis of Chromosome 3 in DROSOPHILA MELANOGASTER: The Homoeotic Gene Complex in Polytene Chromosome Interval 84a-B. Genetics 94 : 115–133. 17248988
52. Sanchez-Herrero E, Vernos I, Marco R, Morata G (1985) Genetic organization of Drosophila bithorax complex. Nature 313 : 108–113. 3917555
53. Maeda RK, Karch F (2006) The ABC of the BX-C: the bithorax complex explained. Development 133 : 1413–1422. 16556913
54. Chiang C, Young KE, Beachy PA (1995) Control of Drosophila tracheal branching by the novel homeodomain gene unplugged, a regulatory target for genes of the bithorax complex. Development 121 : 3901–3912. 8582298
55. Hu N, Castelli-Gair J (1999) Study of the posterior spiracles of Drosophila as a model to understand the genetic and cellular mechanisms controlling morphogenesis. Dev Biol 214 : 197–210. 10491268
56. Nusslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287 : 795–801. 6776413
57. Chung S, Chavez C, Andrew DJ (2011) Trachealess (Trh) regulates all tracheal genes during Drosophila embryogenesis. Dev Biol 360 : 160–172. doi: 10.1016/j.ydbio.2011.09.014 21963537
58. Shiga Y, TanakaMatakatsu M, Hayashi S (1996) A nuclear GFP beta-galactosidase fusion protein as a marker for morphogenesis in living Drosophila. Development Growth & Differentiation 38 : 99–106.
59. Hamada F, Tomoyasu Y, Takatsu Y, Nakamura M, Nagai S, et al. (1999) Negative regulation of Wingless signaling by D-axin, a Drosophila homolog of axin. Science 283 : 1739–1742. 10073940
60. Willert K, Logan CY, Arora A, Fish M, Nusse R (1999) A Drosophila Axin homolog, Daxin, inhibits Wnt signaling. Development 126 : 4165–4173. 10457025
61. Cliffe A, Hamada F, Bienz M (2003) A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol 13 : 960–966. 12781135
62. Caviglia S, Luschnig S (2013) The ETS domain transcriptional repressor Anterior open inhibits MAP kinase and Wingless signaling to couple tracheal cell fate with branch identity. Development 140 : 1240–1249. doi: 10.1242/dev.087874 23444354
63. Price JV, Clifford RJ, Schupbach T (1989) The maternal ventralizing locus torpedo is allelic to faint little ball, an embryonic lethal, and encodes the Drosophila EGF receptor homolog. Cell 56 : 1085–1092. 2493993
64. Wappner P, Gabay L, Shilo BZ (1997) Interactions between the EGF receptor and DPP pathways establish distinct cell fates in the tracheal placodes. Development 124 : 4707–4716. 9409686
65. Rutledge BJ, Zhang K, Bier E, Jan YN, Perrimon N (1992) The Drosophila spitz gene encodes a putative EGF-like growth factor involved in dorsal-ventral axis formation and neurogenesis. Genes Dev 6 : 1503–1517. 1644292
66. Bier E, Jan LY, Jan YN (1990) rhomboid, a gene required for dorsoventral axis establishment and peripheral nervous system development in Drosophila melanogaster. Genes Dev 4 : 190–203. 2110920
67. Urban S, Lee JR, Freeman M (2001) Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell 107 : 173–182. 11672525
68. Affolter M, Nellen D, Nussbaumer U, Basler K (1994) Multiple requirements for the receptor serine/threonine kinase thick veins reveal novel functions of TGF beta homologs during Drosophila embryogenesis. Development 120 : 3105–3117. 7720555
69. Nellen D, Affolter M, Basler K (1994) Receptor serine/threonine kinases implicated in the control of Drosophila body pattern by decapentaplegic. Cell 78 : 225–237. 8044837
70. Wieschaus E, Nussleinvolhard C, Jurgens G (1984) Mutations Affecting the Pattern of the Larval Cuticle in Drosophila-Melanogaster .3. Zygotic Loci on the X-Chromosome and 4th Chromosome. Wilhelm Rouxs Archives of Developmental Biology 193 : 296–307.
71. Noordermeer J, Klingensmith J, Perrimon N, Nusse R (1994) dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature 367 : 80–83. 7906389
72. Siegfried E, Wilder EL, Perrimon N (1994) Components of wingless signalling in Drosophila. Nature 367 : 76–80. 8107779
73. Jiang J, Hui CC (2008) Hedgehog signaling in development and cancer. Dev Cell 15 : 801–812. doi: 10.1016/j.devcel.2008.11.010 19081070
74. Chen Y, Jiang J (2013) Decoding the phosphorylation code in Hedgehog signal transduction. Cell Res 23 : 186–200. doi: 10.1038/cr.2013.10 23337587
75. Hidalgo A, Ingham P (1990) Cell patterning in the Drosophila segment: spatial regulation of the segment polarity gene patched. Development 110 : 291–301. 2081466
76. Ingham PW (1993) Localized hedgehog activity controls spatial limits of wingless transcription in the Drosophila embryo. Nature 366 : 560–562. 8255293
77. DiNardo S, Heemskerk J, Dougan S, O’Farrell PH (1994) The making of a maggot: patterning the Drosophila embryonic epidermis. Curr Opin Genet Dev 4 : 529–534. 7950320
78. Aza-Blanc P, Ramirez-Weber FA, Laget MP, Schwartz C, Kornberg TB (1997) Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell 89 : 1043–1053. 9215627
79. Methot N, Basler K (1999) Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell 96 : 819–831. 10102270
80. Methot N, Basler K (2001) An absolute requirement for Cubitus interruptus in Hedgehog signaling. Development 128 : 733–742. 11171398
81. Parker DS, White MA, Ramos AI, Cohen BA, Barolo S (2011) The cis-regulatory logic of Hedgehog gradient responses: key roles for gli binding affinity, competition, and cooperativity. Sci Signal 4: ra38. doi: 10.1126/scisignal.2002077 21653228
82. Hepker J, Wang QT, Motzny CK, Holmgren R, Orenic TV (1997) Drosophila cubitus interruptus forms a negative feedback loop with patched and regulates expression of Hedgehog target genes. Development 124 : 549–558. 9053330
83. Karim MR, Moore AW (2011) Convergent local identity and topographic projection of sensory neurons. J Neurosci 31 : 17017–17027. doi: 10.1523/JNEUROSCI.2815-11.2011 22114271
84. Dai P, Akimaru H, Ishii S (2003) A hedgehog-responsive region in the Drosophila wing disc is defined by debra-mediated ubiquitination and lysosomal degradation of Ci. Dev Cell 4 : 917–928. 12791275
85. Martizez Arias A, Baker NE, Ingham PW (1988) Role of segment polarity genes in the definition and maintenance of cell states in the Drosophila embryo. Development 103 : 157–170. 3197626
86. Wilk R, Weizman I, Shilo BZ (1996) trachealess encodes a bHLH-PAS protein that is an inducer of tracheal cell fates in Drosophila. Genes Dev 10 : 93–102. 8557198
87. Chen Y, Gallaher N, Goodman RH, Smolik SM (1998) Protein kinase A directly regulates the activity and proteolysis of cubitus interruptus. Proc Natl Acad Sci U S A 95 : 2349–2354. 9482888
88. Merabet S, Litim-Mecheri I, Karlsson D, Dixit R, Saadaoui M, et al. (2011) Insights into Hox protein function from a large scale combinatorial analysis of protein domains. PLoS Genet 7: e1002302. doi: 10.1371/journal.pgen.1002302 22046139
89. Syed ZA, Bouge AL, Byri S, Chavoshi TM, Tang E, et al. (2012) A luminal glycoprotein drives dose-dependent diameter expansion of the Drosophila melanogaster hindgut tube. PLoS Genet 8: e1002850. doi: 10.1371/journal.pgen.1002850 22876194
90. Camprodon FJ, Castelligair JE (1994) Ultrabithorax Protein Expression in Breakpoint Mutants—Localization of Single, Cooperative and Redundant Cis Regulatory Elements. Rouxs Archives of Developmental Biology 203 : 411–421.
91. Struhl G, White RA (1985) Regulation of the Ultrabithorax gene of Drosophila by other bithorax complex genes. Cell 43 : 507–519. 3935322
92. Tsarouhas V, Senti KA, Jayaram SA, Tiklova K, Hemphala J, et al. (2007) Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev Cell 13 : 214–225. 17681133
93. Forster D, Luschnig S (2012) Src42A-dependent polarized cell shape changes mediate epithelial tube elongation in Drosophila. Nat Cell Biol 14 : 526–534. doi: 10.1038/ncb2456 22446736
94. Forster D, Armbruster K, Luschnig S (2010) Sec24-dependent secretion drives cell-autonomous expansion of tracheal tubes in Drosophila. Curr Biol 20 : 62–68. doi: 10.1016/j.cub.2009.11.062 20045324
95. Tiklova K, Tsarouhas V, Samakovlis C (2013) Control of airway tube diameter and integrity by secreted chitin-binding proteins in Drosophila. PLoS One 8: e67415. doi: 10.1371/journal.pone.0067415 23826295
96. Fraichard S, Bouge AL, Chauvel I, Bouhin H (2006) Tenectin, a novel extracellular matrix protein expressed during Drosophila melanogaster embryonic development. Gene Expr Patterns 6 : 772–776. 16510317
97. Choo SW, White R, Russell S (2011) Genome-wide analysis of the binding of the Hox protein Ultrabithorax and the Hox cofactor Homothorax in Drosophila. PLoS One 6: e14778. doi: 10.1371/journal.pone.0014778 21483667
98. Herriges JC, Yi L, Hines EA, Harvey JF, Xu G, et al. (2012) Genome-scale study of transcription factor expression in the branching mouse lung. Dev Dyn 241 : 1432–1453. doi: 10.1002/dvdy.23823 22711520
99. Lindsley DL, Zimm GG, Lindsley DL (1992) The genome of Drosophila melanogaster. San Diego: Academic Press. viii, 1133 p., 1138 leaves of plates p.
100. Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 : 401–415. 8223268
101. Le T, Liang Z, Patel H, Yu MH, Sivasubramaniam G, et al. (2006) A new family of Drosophila balancer chromosomes with a w—dfd-GMR yellow fluorescent protein marker. Genetics 174 : 2255–2257. doi: 10.1534/genetics.106.063461 17057238
102. Starz-Gaiano M, Cho NK, Forbes A, Lehmann R (2001) Spatially restricted activity of a Drosophila lipid phosphatase guides migrating germ cells. Development 128 : 983–991. 11222152
103. St Pierre SE, Ponting L, Stefancsik R, McQuilton P, FlyBase C (2014) FlyBase 102--advanced approaches to interrogating FlyBase. Nucleic Acids Res 42: D780–788. doi: 10.1093/nar/gkt1092 24234449
104. Li-Kroeger D, Witt LM, Grimes HL, Cook TA, Gebelein B (2008) Hox and senseless antagonism functions as a molecular switch to regulate EGF secretion in the Drosophila PNS. Dev Cell 15 : 298–308. doi: 10.1016/j.devcel.2008.06.001 18694568
105. Lohmann I, McGinnis N, Bodmer M, McGinnis W (2002) The Drosophila Hox gene deformed sculpts head morphology via direct regulation of the apoptosis activator reaper. Cell 110 : 457–466. 12202035
106. Ohshiro T, Saigo K (1997) Transcriptional regulation of breathless FGF receptor gene by binding of TRACHEALESS/dARNT heterodimers to three central midline elements in Drosophila developing trachea. Development 124 : 3975–3986. 9374395
107. Barrio R, de Celis JF, Bolshakov S, Kafatos FC (1999) Identification of regulatory regions driving the expression of the Drosophila spalt complex at different developmental stages. Dev Biol 215 : 33–47. 10525348
108. Hosono C, Takaira K, Matsuda R, Saigo K (2003) Functional subdivision of trunk visceral mesoderm parasegments in Drosophila is required for gut and trachea development. Development 130 : 439–449. 12490551
109. Bradley PL, Andrew DJ (2001) ribbon encodes a novel BTB/POZ protein required for directed cell migration in Drosophila melanogaster. Development 128 : 3001–3015. 11532922
110. Inbal A, Volk T, Salzberg A (2004) Recruitment of ectodermal attachment cells via an EGFR-dependent mechanism during the organogenesis of Drosophila proprioceptors. Dev Cell 7 : 241–250. 15296720
111. Skeath JB (1998) The Drosophila EGF receptor controls the formation and specification of neuroblasts along the dorsal-ventral axis of the Drosophila embryo. Development 125 : 3301–3312. 9693134
112. Kumar JP, Tio M, Hsiung F, Akopyan S, Gabay L, et al. (1998) Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development 125 : 3875–3885. 9729495
113. Hopmann R, Duncan D, Duncan I (1995) Transvection in the iab-5,6,7 region of the bithorax complex of Drosophila: homology independent interactions in trans. Genetics 139 : 815–833. 7713434
114. Perrimon N, Noll E, McCall K, Brand A (1991) Generating lineage-specific markers to study Drosophila development. Dev Genet 12 : 238–252. 1651183
115. Estacio-Gomez A, Moris-Sanz M, Schafer AK, Perea D, Herrero P, et al. (2013) Bithorax-complex genes sculpt the pattern of leucokinergic neurons in the Drosophila central nervous system. Development 140 : 2139–2148. doi: 10.1242/dev.090423 23633511
116. Urbach R, Technau GM (2003) Molecular markers for identified neuroblasts in the developing brain of Drosophila. Development 130 : 3621–3637. 12835380
117. Lee JJ, von Kessler DP, Parks S, Beachy PA (1992) Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell 71 : 33–50. 1394430
118. Patel NH (1994) Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. Methods Cell Biol 44 : 445–487. 7707967
119. Campos-Ortega JA, Hartenstein V (1997) The embryonic development of Drosophila melanogaster. Berlin; New York: Springer. xvii, 405 p. p.
120. White RA, Wilcox M (1984) Protein products of the bithorax complex in Drosophila. Cell 39 : 163–171. 6091908
121. Kosman D, Small S, Reinitz J (1998) Rapid preparation of a panel of polyclonal antibodies to Drosophila segmentation proteins. Dev Genes Evol 208 : 290–294. 9683745
122. Xie B, Charlton-Perkins M, McDonald E, Gebelein B, Cook T (2007) Senseless functions as a molecular switch for color photoreceptor differentiation in Drosophila. Development 134 : 4243–4253. 17978002
123. Henderson KD, Isaac DD, Andrew DJ (1999) Cell fate specification in the Drosophila salivary gland: the integration of homeotic gene function with the DPP signaling cascade. Dev Biol 205 : 10–21. 9882494
124. Celniker SE, Keelan DJ, Lewis EB (1989) The molecular genetics of the bithorax complex of Drosophila: characterization of the products of the Abdominal-B domain. Genes Dev 3 : 1424–1436. 2575066
125. Blochlinger K, Bodmer R, Jan LY, Jan YN (1990) Patterns of expression of cut, a protein required for external sensory organ development in wild-type and cut mutant Drosophila embryos. Genes Dev 4 : 1322–1331. 1977661
126. Yip ML, Lamka ML, Lipshitz HD (1997) Control of germ-band retraction in Drosophila by the zinc-finger protein HINDSIGHT. Development 124 : 2129–2141. 9187140
127. Sampedro J, Guerrero I (1991) Unrestricted expression of the Drosophila gene patched allows a normal segment polarity. Nature 353 : 187–190. 1653907
128. Jeon M, Zinn K (2009) Receptor tyrosine phosphatases control tracheal tube geometries through negative regulation of Egfr signaling. Development 136 : 3121–3129. doi: 10.1242/dev.033597 19675131
129. Goto S, Hayashi S (1997) Specification of the embryonic limb primordium by graded activity of Decapentaplegic. Development 124 : 125–132. 9006073
130. Merabet S, Ebner A, Affolter M (2005) The Drosophila Extradenticle and Homothorax selector proteins control branchless/FGF expression in mesodermal bridge-cells. EMBO Rep 6 : 762–768. doi: 10.1038/sj.embor.7400462 16007069
Štítky
Genetika Reprodukčná medicína
Článek Phosphorylation of Elp1 by Hrr25 Is Required for Elongator-Dependent tRNA Modification in YeastČlánek Naturally Occurring Differences in CENH3 Affect Chromosome Segregation in Zygotic Mitosis of HybridsČlánek Insight in Genome-Wide Association of Metabolite Quantitative Traits by Exome Sequence AnalysesČlánek ALIX and ESCRT-III Coordinately Control Cytokinetic Abscission during Germline Stem Cell DivisionČlánek Deciphering the Genetic Programme Triggering Timely and Spatially-Regulated Chitin Deposition
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 1- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- The Combination of Random Mutagenesis and Sequencing Highlight the Role of Unexpected Genes in an Intractable Organism
- Ataxin-3, DNA Damage Repair, and SCA3 Cerebellar Degeneration: On the Path to Parsimony?
- α-Actinin-3: Why Gene Loss Is an Evolutionary Gain
- Origins of Context-Dependent Gene Repression by Capicua
- Transposable Elements Contribute to Activation of Maize Genes in Response to Abiotic Stress
- No Evidence for Association of Autism with Rare Heterozygous Point Mutations in Contactin-Associated Protein-Like 2 (), or in Other Contactin-Associated Proteins or Contactins
- Nur1 Dephosphorylation Confers Positive Feedback to Mitotic Exit Phosphatase Activation in Budding Yeast
- A Regulatory Hierarchy Controls the Dynamic Transcriptional Response to Extreme Oxidative Stress in Archaea
- Genetic Variants Modulating CRIPTO Serum Levels Identified by Genome-Wide Association Study in Cilento Isolates
- Small RNA Sequences Support a Host Genome Origin of Satellite RNA
- Phosphorylation of Elp1 by Hrr25 Is Required for Elongator-Dependent tRNA Modification in Yeast
- Genetic Mapping of MAPK-Mediated Complex Traits Across
- An AP Endonuclease Functions in Active DNA Demethylation and Gene Imprinting in
- Developmental Regulation of the Origin Recognition Complex
- End of the Beginning: Elongation and Termination Features of Alternative Modes of Chromosomal Replication Initiation in Bacteria
- Naturally Occurring Differences in CENH3 Affect Chromosome Segregation in Zygotic Mitosis of Hybrids
- Imputation of the Rare G84E Mutation and Cancer Risk in a Large Population-Based Cohort
- Polycomb Protein SCML2 Associates with USP7 and Counteracts Histone H2A Ubiquitination in the XY Chromatin during Male Meiosis
- A Genetic Strategy for Probing the Functional Diversity of Magnetosome Formation
- Interactions of Chromatin Context, Binding Site Sequence Content, and Sequence Evolution in Stress-Induced p53 Occupancy and Transactivation
- The Yeast La Related Protein Slf1p Is a Key Activator of Translation during the Oxidative Stress Response
- Integrative Analysis of DNA Methylation and Gene Expression Data Identifies as a Key Regulator of COPD
- Proteasomes, Sir2, and Hxk2 Form an Interconnected Aging Network That Impinges on the AMPK/Snf1-Regulated Transcriptional Repressor Mig1
- Functional Interplay between the 53BP1-Ortholog Rad9 and the Mre11 Complex Regulates Resection, End-Tethering and Repair of a Double-Strand Break
- Estrogenic Exposure Alters the Spermatogonial Stem Cells in the Developing Testis, Permanently Reducing Crossover Levels in the Adult
- Protein Poly(ADP-ribosyl)ation Regulates Immune Gene Expression and Defense Responses
- Sumoylation Influences DNA Break Repair Partly by Increasing the Solubility of a Conserved End Resection Protein
- A Discrete Transition Zone Organizes the Topological and Regulatory Autonomy of the Adjacent and Genes
- Elevated Mutation Rate during Meiosis in
- The Intersection of the Extrinsic Hedgehog and WNT/Wingless Signals with the Intrinsic Hox Code Underpins Branching Pattern and Tube Shape Diversity in the Airways
- MiR-24 Is Required for Hematopoietic Differentiation of Mouse Embryonic Stem Cells
- Tissue-Specific Effects of Genetic and Epigenetic Variation on Gene Regulation and Splicing
- Heterologous Aggregates Promote Prion Appearance via More than One Mechanism
- The Tumor Suppressor BCL7B Functions in the Wnt Signaling Pathway
- , A -Acting Locus that Controls Chromosome-Wide Replication Timing and Stability of Human Chromosome 15
- Regulating Maf1 Expression and Its Expanding Biological Functions
- A Polyubiquitin Chain Reaction: Parkin Recruitment to Damaged Mitochondria
- RecFOR Is Not Required for Pneumococcal Transformation but Together with XerS for Resolution of Chromosome Dimers Frequently Formed in the Process
- An Intracellular Transcriptomic Atlas of the Giant Coenocyte
- Insight in Genome-Wide Association of Metabolite Quantitative Traits by Exome Sequence Analyses
- The Role of the Mammalian DNA End-processing Enzyme Polynucleotide Kinase 3’-Phosphatase in Spinocerebellar Ataxia Type 3 Pathogenesis
- The Global Regulatory Architecture of Transcription during the Cell Cycle
- Identification and Functional Characterization of Coding Variants Influencing Glycemic Traits Define an Effector Transcript at the Locus
- Altered Ca Kinetics Associated with α-Actinin-3 Deficiency May Explain Positive Selection for Null Allele in Human Evolution
- Genetic Variation in the Nuclear and Organellar Genomes Modulates Stochastic Variation in the Metabolome, Growth, and Defense
- PRDM9 Drives Evolutionary Erosion of Hotspots in through Haplotype-Specific Initiation of Meiotic Recombination
- Transcriptional Control of an Essential Ribozyme in Reveals an Ancient Evolutionary Divide in Animals
- ALIX and ESCRT-III Coordinately Control Cytokinetic Abscission during Germline Stem Cell Division
- Century-scale Methylome Stability in a Recently Diverged Lineage
- A Re-examination of the Selection of the Sensory Organ Precursor of the Bristle Sensilla of
- Antagonistic Cross-Regulation between Sox9 and Sox10 Controls an Anti-tumorigenic Program in Melanoma
- A Dependent Pool of Phosphatidylinositol 4,5 Bisphosphate (PIP) Is Required for G-Protein Coupled Signal Transduction in Photoreceptors
- Deciphering the Genetic Programme Triggering Timely and Spatially-Regulated Chitin Deposition
- Aberrant Gene Expression in Humans
- Fascin1-Dependent Filopodia are Required for Directional Migration of a Subset of Neural Crest Cells
- The SWI2/SNF2 Chromatin Remodeler BRAHMA Regulates Polycomb Function during Vegetative Development and Directly Activates the Flowering Repressor Gene
- Evolutionary Constraint and Disease Associations of Post-Translational Modification Sites in Human Genomes
- A Truncated NLR Protein, TIR-NBS2, Is Required for Activated Defense Responses in the Mutant
- The Genetic and Mechanistic Basis for Variation in Gene Regulation
- Inactivation of PNKP by Mutant ATXN3 Triggers Apoptosis by Activating the DNA Damage-Response Pathway in SCA3
- DNA Damage Response Factors from Diverse Pathways, Including DNA Crosslink Repair, Mediate Alternative End Joining
- hnRNP K Coordinates Transcriptional Silencing by SETDB1 in Embryonic Stem Cells
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Global Regulatory Architecture of Transcription during the Cell Cycle
- A Truncated NLR Protein, TIR-NBS2, Is Required for Activated Defense Responses in the Mutant
- Proteasomes, Sir2, and Hxk2 Form an Interconnected Aging Network That Impinges on the AMPK/Snf1-Regulated Transcriptional Repressor Mig1
- The SWI2/SNF2 Chromatin Remodeler BRAHMA Regulates Polycomb Function during Vegetative Development and Directly Activates the Flowering Repressor Gene
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



