-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Chromatin Landscape Dictates HSF Binding to Target DNA Elements
Sequence-specific transcription factors (TFs) are critical for specifying patterns and levels of gene expression, but target DNA elements are not sufficient to specify TF binding in vivo. In eukaryotes, the binding of a TF is in competition with a constellation of other proteins, including histones, which package DNA into nucleosomes. We used the ChIP-seq assay to examine the genome-wide distribution of Drosophila Heat Shock Factor (HSF), a TF whose binding activity is mediated by heat shock-induced trimerization. HSF binds to 464 sites after heat shock, the vast majority of which contain HSF Sequence-binding Elements (HSEs). HSF-bound sequence motifs represent only a small fraction of the total HSEs present in the genome. ModENCODE ChIP-chip datasets, generated during non-heat shock conditions, were used to show that inducibly bound HSE motifs are associated with histone acetylation, H3K4 trimethylation, RNA Polymerase II, and coactivators, compared to HSE motifs that remain HSF-free. Furthermore, directly changing the chromatin landscape, from an inactive to an active state, permits inducible HSF binding. There is a strong correlation of bound HSEs to active chromatin marks present prior to induced HSF binding, indicating that an HSE's residence in “active” chromatin is a primary determinant of whether HSF can bind following heat shock.
Published in the journal: Chromatin Landscape Dictates HSF Binding to Target DNA Elements. PLoS Genet 6(9): e32767. doi:10.1371/journal.pgen.1001114
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001114Summary
Sequence-specific transcription factors (TFs) are critical for specifying patterns and levels of gene expression, but target DNA elements are not sufficient to specify TF binding in vivo. In eukaryotes, the binding of a TF is in competition with a constellation of other proteins, including histones, which package DNA into nucleosomes. We used the ChIP-seq assay to examine the genome-wide distribution of Drosophila Heat Shock Factor (HSF), a TF whose binding activity is mediated by heat shock-induced trimerization. HSF binds to 464 sites after heat shock, the vast majority of which contain HSF Sequence-binding Elements (HSEs). HSF-bound sequence motifs represent only a small fraction of the total HSEs present in the genome. ModENCODE ChIP-chip datasets, generated during non-heat shock conditions, were used to show that inducibly bound HSE motifs are associated with histone acetylation, H3K4 trimethylation, RNA Polymerase II, and coactivators, compared to HSE motifs that remain HSF-free. Furthermore, directly changing the chromatin landscape, from an inactive to an active state, permits inducible HSF binding. There is a strong correlation of bound HSEs to active chromatin marks present prior to induced HSF binding, indicating that an HSE's residence in “active” chromatin is a primary determinant of whether HSF can bind following heat shock.
Introduction
Signal-dependent activation of transcription is a highly regulated process that is dictated by transcriptional activators that selectively identify and function at sequence-specific DNA motifs. The most basic function of sequence specific activators is to discriminate between binding sites in the context of the entire genome [1]–[4], but the mechanism by which this occurs is poorly understood. Two main mechanisms have been proposed that explain the observed in vivo binding specificity (reviewed in [5]): TFs are occluded from cognate site by chromatin structure or TF binding is facilitated by cooperative interactions with cofactors. In vivo, TFs are in competition with chromatin factors, which may limit TF access to cognate binding sites [6], [7]. Early sequence-specific ChIP experiments of homeoproteins revealed that binding sites are preferentially accessible if target motifs are located within active genes [1]. More recently, advances in genome-wide characterization of histone modifications and chromatin structure have begun to identify additional requirements for the binding of TFs. In human cells, it has been shown that the H3K4me1 and H3K4me3 modifications are present at inducible STAT1 binding sites prior to interferon-gamma stimulation [8]. In Drosophila, H3K36me3 has been revealed as an important histone mark for male-specific lethal (MSL) complex binding [9], [10]. However, the Hox proteins primarily discriminate between equivalent predicted binding sites by cooperative interactions with DNA-bound cofactors (reviewed in [11]). These findings indicate that the binding of TFs depend upon the chromatin landscape as well as specific sequence elements, and we set out to determine the extent to which chromatin affects TF binding genome-wide. Characterizing the mechanistic parameters by which TFs locate and bind to target DNA sequences will provide insight into a critical early step in a cell's ability to orchestrate patterns of gene expression in response to developmental, nutritional, and environmental signals.
Heat Shock Factor (HSF) has a conserved function as the master regulator of the heat shock (HS) response from organisms as distantly related as yeast and humans [12]. The HS genes of Drosophila melanogaster are an attractive model system to study the general functions of HSF and its induced transcriptional regulation [13]. HSF is present as a nuclear-localized monomer during non-stress conditions [14]; upon stress, HSF homotrimerization [15] mediates binding to HSF Sequence-binding Element (HSE) motifs within seconds [16], [17], which strongly activates a set of HS genes. While transcription factor binding to DNA is necessary for cis regulation of target genes, not all TF binding is necessarily functional [18]. For instance, HSF has been mapped to over 164 cytological sites on the polytene choromosomes of Drosophila salivary gland cells after HS [19], but only 9 cytological loci exhibit HS-induced transcription elongation factor recruitment and activation [20]–[23]. It remains unclear how HSF discriminates between sites and selectively stimulates functional gene activation.
In this study, we set out to determine the comprehensive set of HSF binding sites in the Drosophila genome and the molecular basis for the binding. We used ChIP (chromatin immunoprecipitation) followed by sequencing [24], adapted for high throughput detection (ChIP-seq) [25]–[27], to map the sites of HSF binding in an unbiased manner with high sensitivity and resolution. We made use of the ChIP-chip datasets from the model organism ENCyclopedia Of DNA Elements (modENCODE) consortium [28], [29], which profiles histone modifications, histone variants [30], insulators [31], [32], and Pol II. These datasets describe critical features of the chromatin landscape in unstressed cells. Using this data, we contrasted the chromatin landscape before HS induction at induced HSF-bound HSE motifs and HSE motifs that remain HSF-free. The roles of many of the modENCODE chromatin features are well established [30]–[33], thus the absence or presence of one or many of these features provides insight into the mechanism of HSF binding.
Results
ChIP-seq in HSF depleted cells is a critical control for optimizing sensitivity and specificity
To determine the comprehensive set of HSF binding sites, we performed two highly correlated, independent ChIP-seq experiments in Drosophila S2 cells [34] for both non-heat shock (NHS) and 20′ HS conditions (Figure S1). We used well-characterized ChIP-grade HSF antiserum [23], [35] which specifically recognizes one HSF-RNAi sensitive Western blot band from whole S2 cell extract (Figure 1A) [35] and generates the expected global HSF-binding pattern observed by indirect immunofluorescence (IF) polytene staining [19], [36], [37]. Despite the specificity observed in these assays, we set out to directly assess specificity in genome-wide ChIP by identifying any HSF-non-specific DNA pull-down. We performed two independent HSF antiserum ChIP-seq control experiments, for each condition (NHS and 20′ HS), in cells that were depleted of HSF by RNAi. This approach approximates a control immunoprecipitation (IP) from cells that lack the factor of interest [38], [39].
Fig. 1. HSF depletion filters false positive peaks. 
(A) Densitometry of the loading control (TFIIS) confirmed that the intensity of each band was proportional to the number of cells loaded. The HSF-KD HSF band is 1.6 times the intensity of the most dilute HSF band of the standard curve, indicating a 40-fold depletion of HSF. (B) The UCSC Genome Browser is used to show a locus that contains two legitimate HS inducible/HSF-RNAi-sensitive binding sites (represented by asterisks) and a false-positive peak that is neither inducible nor sensitive to HSF depletion (represented by “×”). The y-axis scale is linear (from 2 to 110) and normalized for each experiment (shifted tags/10 bp/10 million sequences in the library). Mock IP with the pre-inoculated animal serum served as a background dataset (Pre-immune IP). HSF-knock down (KD) depleted endogenous levels of HSF to less than 2.5% of control cells as measured by quantitative Western blot (Figure 1A). Importantly, the level of HSF in RNAi depleted cells was reduced at the promoters of well-characterized HS genes, including the highest affinity Hsp83 promoter (Figure S2). Due to the unique presence of tandem HSEs and cooperative HSF binding, the in vitro dissociation constant for the HSF/Hsp83 promoter interaction is on the order of single-digit femtomolar [40], and the Hsp83 promoter harbors the only strongly bound sites during NHS [19] (Figure S1). Since our KD of HSF was successful at reducing HSF levels at the highest affinity binding site, the signal intensity of all HSF-specific peaks should be susceptible to HSF-RNAi depletion as well. Therefore, we discarded peaks that were resistant to HSF depletion, as these are very likely false positives (Figure 1B, Figure S3, Figure S4 and Materials and Methods).
Our analysis of the ChIP-seq data aimed to increase the sensitivity of HSF detection without compromising confidence. To this end, we relied upon two peak calling programs [41], [42] to determine HSF binding sites (see Materials and Methods and Figure S3). Lower confidence peaks were initially considered and later filtered out if found resistant to HSF-RNAi. We detected 464 HSF-specific peaks after 20′ of HS (Dataset S1). We recovered 118 RNAi-sensitive peaks that would have otherwise been discarded because of high false discovery rates (FDR) (Figure S3). In addition, we filtered out 310 non-specific peaks that had FDRs below 0.1 (Figure S3), because they were completely insensitive (and actually increased in intensity) to HSF-KD and exhibit comparable NHS intensity (Figure 1B). Therefore, performing ChIP-seq in cells that were depleted of HSF by RNAi increased the sensitivity and specificity of peak calling.
HSF inducibly binds to a specific consensus motif
We derived a position-specific weight matrix (PSWM) [43] and generated an in vivo composite HSF binding site using all 464 HSF peaks occupied after 20′ HS (Figure 2A bottom). Greater than 95% (442/464) of the peaks contained at least one HSE (Figure 2A bottom) with a p-value below 0.001 (Figure S4 and Materials and Methods), indicating that we are primarily detecting HSF directly bound to DNA. In contrast, the distribution of HSE motifs surrounding the HSF-RNAi resistant peaks approximates random expectation (Figure S4). This analysis indicates that the majority of RNAi resistant peaks are false positives that likely result from antiserum cross-reaction with another DNA binding protein, as these peaks are not present in the pre-immune IP. Consistent with the high affinity motif derived by in vitro band shift assays [16] (Figure 2A top), the in vivo HSE is a tandem array of three oppositely oriented five base pair units: AGAAN. In vitro HSF can bind to elements containing three five base pair units, regardless of their orientation relative to one another—although the opposite orientation of three 5 base pair units bound more tightly than direct repeats [16]. Our ChIP-seq study reveals that the opposite orientation of the tandem 5 base pair units is absolutely critical for detectable binding in vivo.
Fig. 2. Characterization of HSF binding sites. 
(A) The PSWM derived from in vitro band shift assays [16] (top) and this study (bottom) are compared. Sequence logos were generated using WebLogo [93]. (B) The 67B locus harbors known heat shock protein (hsp) genes. The y-axis scale is linear (from 2 to 180) and directly comparable for each condition (shifted tags/10 bp/10 million sequences in the library). HSF binding sites, detected by our peak calling criteria (asterisks), increased in signal intensity or appeared de novo as cells were shifted from NHS to HS. (C) HSF binding sites are found within the body of RefGenes (72%: 316 sites), in the 500 bases upstream of TSS (22%: 97 sites), and within intergenic regions (18%: 81). A precise genomic sequence can be both within a gene and within the promoter of an upstream gene; 52 binding sites (the 12% slice) fall in this category. At those peaks that contain HSE motifs, we inferred the HSF binding sites at base pair resolution using the consensus-binding motif derived from this study (Figure 2A bottom). If multiple HSEs were within the 442 HSE containing peaks, the motif closest to the peak center was scored as the HSF binding site (Dataset S2). Our analysis recovered all previously well-characterized HSF binding sites within the promoters of HS responsive genes (Figure 2B, Figure S2, and Dataset S3), including the multi-copy Hsp70 gene (Figure S5). We found that only 20 of the high-confidence HS peaks are detected during NHS conditions, and with a much lower density of tag counts (Figure 2B). Despite the fact that a corresponding NHS peak could not be detected at 422 of the 442 HS peaks, sequence tags are associated with these regions and signal may be above background, but below our threshold for detecting peaks. We considered that true signal should still be susceptible to HSF-KD (Figure S6) and concluded that the majority of these 422 sites are either completely devoid of HSF or contain extremely low, thus undetectable, levels of HSF under NHS conditions. Taken together, our analysis reveals that HSF behaves as we expected from previous molecular analyses of particular genes [16], [17] and from comprehensive, but low resolution, cytological analyses [19]: HSF binds strictly to HSEs and these sites are absent or show drastically reduced occupancy during NHS conditions.
Previous independent reports indicate that ChIP signal intensity positively correlates with motif conformity [3], [4], [44]. We find, however, that HSF binding sites conforming more stringently to the PSWM contain a comparable density of sequence tags as degenerate HSF binding sites (Figure S7A and S7B), suggesting that sequence alone is not driving HSF binding affinity.
HSF binds to only a fraction of potential motifs in vivo
Although bona fide HSF binding sites contain highly specific HSE motifs, only a small fraction of potential HSE motifs are occupied by HSF. To search for HSF-free binding sites, we employed a conservative cut-off for conformity to the consensus HSE by using a p-value of 5×10−6 or less [43], while ensuring that the flanking region is mappable [45]. There are 708 HSF-free motifs (Dataset S4 and Figure S8A) that meet these criteria. Less than 15% (107/815) of the mappable HSE motifs with a p-value of 5×10−6 or less are detectably bound by HSF after HS. Upon closer inspection (Figure S6), we find that HSF-free motifs are absolutely HSF-free during NHS, and these same motifs are either unoccupied or infrequently occupied after HS. In contrast, HSF-bound motifs are either very weakly occupied or unoccupied prior to HS, and show strong inducible binding after HS induction. Therefore, these two categories of motifs, HSF-free and HSF-bound, are distinct from one another and are compared below.
We determined the distribution of HSF binding sites relative to annotated genes and promoter regions. Annotated genes account for 60.6% of the Drosophila reference genome (Figure S8B), however, 72% of the HSF-bound motifs are found within gene boundaries (Figure 2C). HSF-bound motifs within promoters (500 bp upstream of a transcription start site (TSS)) were also enriched, accounting for 22% of the total bound motifs (Figure 2C), while such promoter regions only account for 3.4% of the total reference genome (Figure S8B). In contrast, the classification of the 708 HSF-free motifs is much closer to a background distribution; 63% HSF-free motifs are within genes and 5.5% are within promoters (Figure S8A). These results indicate that HSE motifs are not simply enriched within gene and promoter boundaries, but that HSF preferentially interacts with HSEs that are present within genes and promoters.
HSF discriminates between HSEs based on local signatures of active chromatin
We hypothesized that HSF discriminates between equivalent HSE sequences in vivo based on the chromatin landscape in which motifs reside. Previous work shows that HSF preferentially binds acetylated nucleosomes in vitro and more recently that the androgen receptor preferentially binds nucleosomes modified with methylated H3K4 in vivo [46], [47]. To determine the extent to which HSF binding is influenced by chromatin in vivo, we compared the NHS chromatin state between the motifs that become HSF-bound or remain HSF-free following HS, excluding the 20 HSF-bound motifs in which HSF was detected during NHS (Dataset S5). Using modENCODE S2 ChIP-chip data [28]–[32], we examined the composite intensity of microarray signal in the region surrounding each HSE. We found that HSF-bound motifs were generally associated with marks of active chromatin, even though these modENCODE signals were generated under NHS conditions (Figure S9). The HSF-free motifs, as a class, were neither enriched nor depleted for any particular factor, histone modification, or histone variant.
Nucleosome occupancy of potential TF binding sites generally restricts TF binding [6], [7], so we examined the distribution of histones and histone variants around HSF motifs. We expected the HSF-bound motifs to be depleted of nucleosomal H3. The composite profiles show that nucleosomal H3 is clearly not depleted (Figure 3); in fact, we observe a slight increase in H3 levels at bound HSEs compared to free HSEs. This observation is in contrast to the general inhibitory nature of nucleosomes and the previous view of HSF binding, as the small set of well-characterized HSF binding sites are devoid of canonical nucleosomes prior to HS [48], [49]. The histone variant H3.3, which associates with active genes [30], displays a peak centered on the HSE motif (Figure 3). These results indicate that HSF binding specificity is not simply dictated by nucleosome-free DNA sequence.
Fig. 3. Bound HSE motifs contain marks of active chromatin prior to HSF binding. 
The average factor or histone modification occupancy was assigned in 100 base windows (step size of 50) around HSF-free HSE motifs (red) and HSF-bound HSE motifs (green). HSF-bound motifs are categorized by annotation class: motifs within promoters (magenta), RefGene bodies (blue), and intergenic regions (black). Canonical active chromatin marks are enriched at HSF-bound motifs (purple). H3K27me3 is depleted at HSF-bound motifs (orange). In recent years, considerable attention has focused on the plethora of covalent histone modifications that occur on the N-terminal tails of histones, the enzymes responsible for catalyzing histone modifications, and the functional consequence of each modification. Acetylation of histone residues H3K9, H3K18, H3K27, H4K5, H4K8, and H4K16 were found to associate with HSF-bound motifs (Figure 3 and Figure S10). Each one of these acetylation marks has previously been shown to mark active chromatin [33], [50]. We find that the methylation marks H3K4me3 and H3K79me2, which associate with active genes [33], [51], are also enriched around the HSF-bound HSEs (Figure 3 and Figure S10). Mono-ubiquitylation (Ub) of H2B, a modification that is necessary for methylation of H3K4 [52], correlates with HSF-bound motifs as well (Figure S10). Conversely, marks of repressive chromatin, H3K27me3 and H3K9me3, were found to be depleted or at background levels (Figure 3 and Figure S10).
We considered that HSEs and histone marks cooperate to specify HSF binding. Transcription factors can bind acetylation and methylation marks through specific domains such as bromodomains, chromodomains and PHD domains (reviewed in [53]). For example, the MSL complex harbors a chromodomain, accounting for preferential recognition and binding of the H3K36me3 mark in Drosophila [9]. Interestingly, the HSF protein is devoid of all of these domains, and thus cannot be binding to DNA and histone methyl or acetyl marks cooperatively by any of these well-characterized interactions.
Comparison of HSF-bound motifs with TF binding data reveals that HSF co-localizes with factors that are associated with active transcription. The presence of Pol II is the foremost indicator of an active gene or a gene that is primed to be activated. The composite Pol II profile at HSF-bound HSEs exhibits a striking peak, even in instances where bound HSEs are within intergenic regions (Figure 3). Likewise, we observe a strong BEAF (boundary element-associated factor) signal centered on HSF-bound motifs (Figure 3). BEAF is an insulator that localizes to transcriptionally active and paused polymerase-harboring genes [32]. The multifaceted TF, GAGA Associated Factor (GAF), is associated with both paused polymerases and HSF-bound motifs [54] (Figure 3). Taken together, these profiles indicate that HSF binds to sites that contain hallmarks of open and active chromatin.
These composite profiles provide an average view of HSF-binding, which could potentially be influenced by a small population of binding sites. We used the available “Regions of Significant Enrichment” tracks from modENCODE to determine which motifs (HSF-bound or HSF-free) were present within the significantly enriched regions of a given factor or modification. We employed the Fisher exact test to determine whether HSF-bound motifs were associated with each factor compared to HSF-free motifs and vice versa (Table S1). Depicted in Figure 4 and Figure S11 are the fractions of HSEs that are present within a given region of enrichment (enriched is colored yellow, unenriched is blue). Strikingly, only 30 (7%) inducible HSF-bound sites do not contain any tested activation marks prior to HS. This analysis reveals a statistically significant association (p-value<0.05) of HSF-bound motifs with 17 different histone modifications or chromatin-bound factors that have previously been shown to be associated with active chromatin (Table S1, Figure 4, and Figure S11), regardless of whether the motifs are classified as intergenic, promoter proximal or within genes (Table S2, Table S3, and Table S4). Unlike previous genome-wide TF binding data that show the co-occupancy of many TFs and histone marks, we are able to show that these chromatin features are present before any detectable HSF binding (Figure S12).
Fig. 4. Bound HSE motifs are statistically associated with marks of active chromatin, compared to HSF-free motifs. 
For each factor shown, the Fisher exact test was used to determine the statistically significant association of HSF-bound motifs (left bar in each panel) with each modENCODE factor or histone modification, compared to HSF-free motifs (right bar). The yellow fraction of the bar chart represents HSF binding sites that are within regions of significant enrichment, while blue depicts all non-enriched sites. We have shown that the presence of activation marks strongly influences the pattern of HSF binding, so we next determined whether quantitative differences in individual marks play a role in the degree of HSF binding. For each HSE that is enriched for a mark or factor in Figure 4, we compared the ChIP-chip intensity of each mark or factor during NHS to the intensity of induced HSF binding following HS. We found a modest, but significant (p-value<0.05), correlation between the intensity of BEAF, tetra-acetylated H4, and H3K18ac with HSF binding intensity (Figure S13).
Considering that the intensity of any one mark only modestly affects HSF binding, we set out to determine whether distinct patterns of TF profiles and histone modifications affect HSF binding intensity. Sets of histone modifications and TFs occur together in distinct combinations on the genome-wide scale in eukaryotic cells [18], [33], [55], [56], and this chromatin landscape can be used to predict and characterize functional regions of the genome [57], [58]. We used cluster analysis [59] to determine whether TF factors and histone modifications showed clear binding patterns at both classes of HSE motifs (Figure 5). This clustering shows that, generally, any single HSF-bound motif is enriched for many activation marks. HSF-free motifs are primarily found in regions with background levels or depleted levels of activation marks. Consistent with our composite profiles, nucleosomal H3 and H2A were not depleted at the bound HSEs prior to HSF binding and H3.3 is generally enriched. Our findings indicate that HSF-accessible chromatin is not synonymous with nucleosome vacancy, but rather, with marks of loose or active chromatin.
Fig. 5. Active chromatin marks are clustered at HSE motifs. 
K-means clustering analysis, specifying five clusters, reveals that the histone modifications tend to occur together at HSF-bound motifs. Each motif corresponds to an individual row. Columns represent the average microarray intensity of all the probes in a 400 base window centered on the motif for a given factor or histone modification. Cluster and Treeview were used to generate and visualize the clustering data [59], [94]. Clusters are not absolutely delineated by the presence or absence of a given factor or set of factors; however, we note general properties of individual clusters. For instance, ubiquitous acetylation of histone residues and high levels of H3K4me1 characterize HSF-bound cluster three, while cluster four contains modest levels of every factor and modification tested (Figure 5). Considering that motif conformity does not significantly affect HSF-signal (Figure S7A and S7B), we tested whether clustering HSEs cleanly separated strong and weak binding sites. We observe that cluster four generally exhibits less intense HSF binding, while cluster one, which is driven by intense Pol II and GAF signal, contains stronger HSF binding sites (Figure S7C). These patterns, however, are not sufficient to account for differences in HSF binding intensity, as the HSF intensity in any p-value quartile or cluster overlaps with all other classes. Ultimately, it is likely that the rules that govern TF binding and intensity of binding are a complex nonlinear system, which results in motif accessibility.
Chromatin landscape dictates HSF binding to a target motif
The strong correlation between open chromatin and HS-induced HSF suggests that open chromatin dictates HSF accessibility. To test this hypothesis, we directed a change in the chromatin landscape, from the restrictive to the permissive state, at an unbound HSE and then examined HSF binding following HS. HSF has been shown to selectively occupy the ecdysone inducible 75B cytological locus, only when the locus is transcriptionally “puffed”, in salivary gland cells [19]. We found an HSF-free motif that resides within the body of an ecdysone inducible gene isoform, Eip75B, which can be inducibly expressed in S2 cells (Figure 6A) [60]. We confirmed that this motif is minimally bound by HSF after HS, and is below the threshold for peak detection by ChIP-seq (Figure 6B). Ecdysone treatment alone results in RNA Pol II recruitment to the body of the Eip75B gene, but does not affect HSF occupancy of the HSE motif (Figure 6B). H3K9ac and tetra-acetylated H4 increase above the background threshold (top dashed line), while H3 levels are unaffected after a 30′ ecdysone treatment (Figure 6B). Recall that prior to HS, between 70% and 80% of the HSF-bound HSEs are significantly enriched for each RNA Pol II, tetra-acetylated H4 and H3K9ac (Figure 4). A 30-minute ecdysone pre-treatment changes the chromatin landscape and allows HSF to strongly occupy the motif following HS (Figure 6B).
Fig. 6. Changing the chromatin landscape converts an HSF-free motif to an HSF-bound site. 
(A) The ecdysone inducible gene Eip75B harbors an HSF motif that conforms to the consensus with a p-value of 1.2×10−7. (B) The blue bars represent the changes in factor and histone modification occupancy after ecdysone is added to the cells. The pink bars indicate the changes in occupancy after HS treatment in cells that were pre-treated with ecdysone. Precipitation with Rabbit IgG controls for non-specific pull-down at this site for each condition (first sub-panel) and dashed and solid lines indicate the range of background intensities for non-specific background pull-down by each antibody (see Materials and Methods) and provides an estimated threshold for assessing enrichment over background. Pre-treatment with ecdysone, followed by HS, not only allows HSF binding at this HSE, but also causes a concomitant increase in local H4 and H3K9 acetylation and decrease in RNA Pol II intensity (Figure 6B and Figure S12). Increased acetylation of histones is consistent with HSF's ability to recruit the acetyltransferase CREB Binding Protein (CBP) to HSF bound sites [61], [62]. At first glance, it is unintuitive that RNA Pol II intensity is compromised following heat shock (Figure 6B and Figure S12). However, this molecular analysis confirms a long-standing observation that following HS, HSF has the ability to repress ecdysone inducible puffs and general protein synthesis [19], [63]. While the mechanism of HSF-mediated repression is unknown in Drosophila, it is tempting to speculate that HSF can act as a roadblock to RNA Pol II within the bodies of active genes (Figure S14).
Promoter-bound HSF does not necessarily lead to gene activation
It has long been known that HSF inducibly binds to many sites and only a subset of sites are transcriptionally activated by HS [19], [64]. These studies, however, did not have the resolution to determine if HSF binding sites did not lead to mRNA production simply because HSF was not promoter-bound. In all well-characterized cases of Drosophila HSF-induced transcription, HSF binds to the promoter. To determine whether promoter-bound HSF is sufficient to upregulate the local gene, we measured mRNA abundance at candidate genes during NHS and after a 20′ HS (Figure 7). Note that HSF is inducibly bound at each gene after 2′ minutes of HS (Figure S15), allowing sufficient time for mRNA accumulation (reviewed in [65]). We observe a continuum of induced mRNA accumulation, from the highly induced Hsp26 gene, to genes that are unaffected by HSF binding (Figure 7).
Fig. 7. Induced mRNA accumulation after a 20′ HS shows that promoter-bound HSF has varying induction effects. 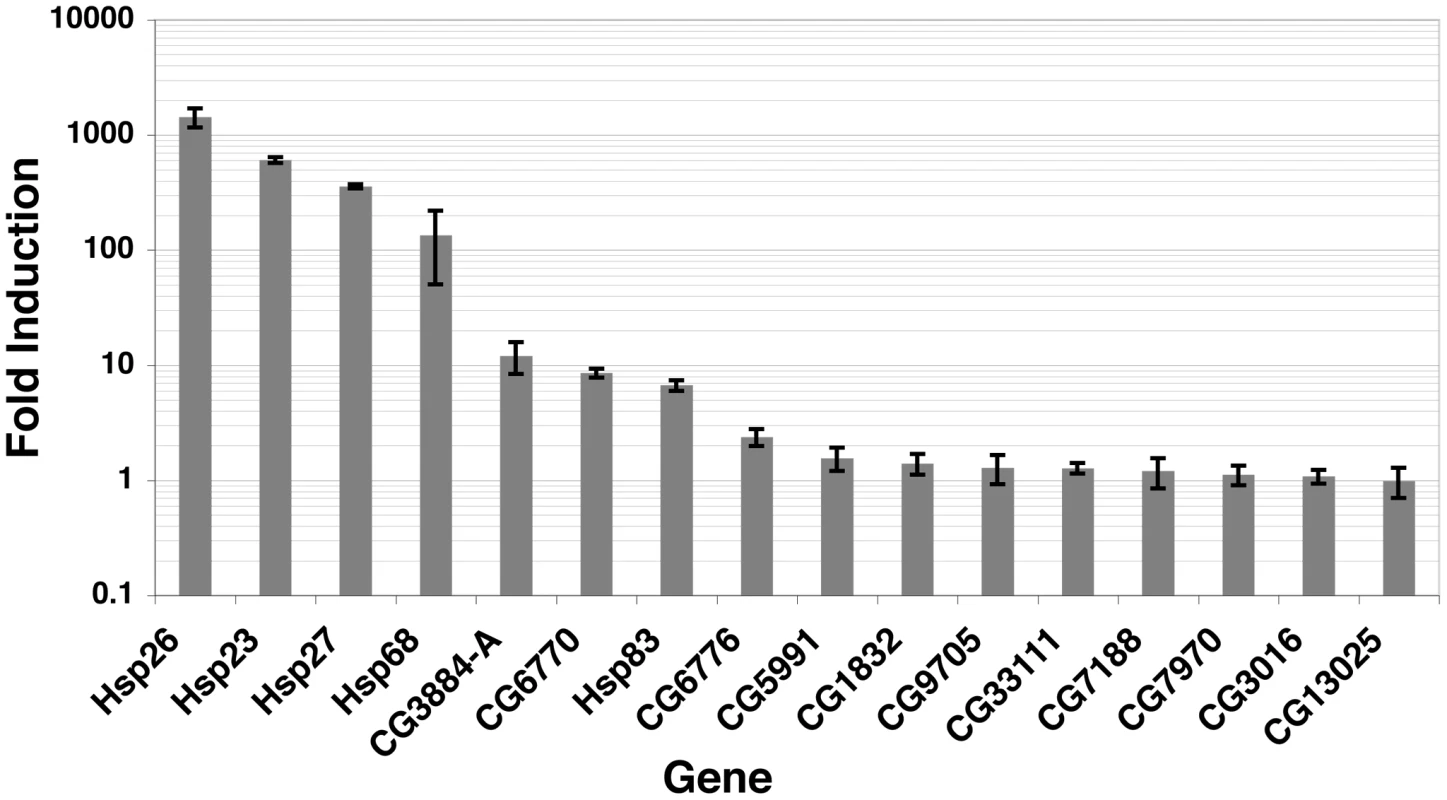
Oligo dT-reverse transcribed RNA was subjected to real-time qPCR with the primers illustrated in Figure S15 (sequences available within Dataset S6), during NHS and 20′ HS conditions. All mRNA levels were normalized to RpL32 [95] and are represented as HS mRNA levels divided by NHS levels. Three independent biological replicates and two technical replicates for each biological sample were performed. Previous genome-wide ChIP experiments report that TF binding intensity generally correlates with functional binding [18], [66]. The ChIP-seq signals that we observe are directly comparable to qPCR quantified ChIP material, indicating that the quantitative properties of ChIP were retained in our sample preparation (Figure S16). Because HSF acts as a potent acidic activator [67], we hypothesized that all genes that exhibit inducible and strong promoter binding of HSF would be activated. HSF can activate when bound moderately to the promoters of genes, as is the case for the CG3884 and CG6770 genes (Figure 7 and Figure S15). Surprisingly, HSF binds inducibly and intensely to the CG3016 and CG13025 promoters (Figure S15), but these mRNA levels remain unchanged (Figure 7). Selective activation is not unique to HSF, as both ER and p53 bind the promoters of genes in a signal-dependent manner, but transcription of some local genes remains unaffected [2], [68].
To investigate how HSF may be selectively activating local genes, we used the ChIP-chip data to look for patterns of histone modification and TF binding that separates functional promoter-bound HSF sites, which can activate gene expression, from promoter-bound HSF that does not result in gene activation. We noticed that GAF was present at many up-regulated genes, and in contrast, BEAF was present at unregulated genes (Figure S17). Previous work has shown that GAF is important for the activation of HS genes [69], but our results indicate that GAF is not necessary for HSF activation (Figure S17). BEAF has been shown to function as an insulator [31], [32]; therefore, we speculate that BEAF is blocking the activation function of HSF at unregulated genes. Previous work has implicated paused polymerase as an important criterion for activation from an Hsp70 promoter [70]. Using promoter-proximal enriched Pol II and pausing factor (NELF) data [54], however, we did not see a significant correlation between these pausing hallmarks and activation potential using these 16 genes. In the same way that chromatin signatures affect the binding of HSF to a motif in vivo, we expect that chromatin landscape and individual gene properties act together to dictate the activation potential of activator-bound genes.
Discussion
We present an experimental approach that increases the sensitivity and power of determining TF-bound sites by ChIP-seq, and we use this approach to characterize the binding profile for HSF under both NHS and HS conditions. Our analysis revealed that HSF binding is dependent upon an underlying HSE motif, although the primary HSE sequence is not sufficient to confer HSF binding. HSF-bound HSEs were found to be associated with a chromatin landscape that harbors active marks prior to HSF binding. Lastly, we demonstrated that promoter-bound HSF is not sufficient to activate local genes.
The ChIP-seq method is used routinely to determine genome-wide factor binding profiles; however, important controls and variations in the ChIP protocol more fully exploit this approach. Our implementation of the control RNAi knockdown of HSF allowed us to eliminate the genome-wide set of false positive signals that were resistant to this knockdown, and prevented the elimination of many true positive binding sites. Another rigorous and complementary control for specificity includes performing independent ChIP experiments with multiple antiserum preparations, each of which is affinity purified with nonoverlapping antigens [18], [38]. The details of ChIP-seq chromatin preparation can also enhance peak detection [71]–[73]. Additional crosslinking agents [74] and crosslinkers that target particular types of protein/DNA interactions, such as exclusively probing direct protein/DNA interactions with UV light [1], [75], can also augment the type and quality of information obtained by the basic ChIP-seq strategy.
The non-sequence dependent specificity observed by TFs can be explained by non-mutually exclusive mechanisms: DNA binding is specifically inhibited by repressive chromatin, aided by active chromatin, or mediated by cooperative interactions with chromatin factors. Here, we report that repressive marks contribute minimally to restrict HSF binding, as only a small fraction of HSF-free motifs are associated with repressive chromatin (Figure S11). Additionally, we observe that chromatin containing background levels of active and repressive marks is unfavorable to inducible HSF binding—the default state of an in vivo HSE can be considered inaccessible. In contrast, HSF inducibly binds to sites that contain TFs and marks of active chromatin prior to HS induction. We have shown that the chromatin landscape can be modified to the permissive state and result in recognition and binding of a previously unbound HSE. This result suggests that HSF does not primarily function to bind DNA cooperatively with other factors, but simply co-occupies the same regions as other TFs, due to the accessible nature of the DNA. These results provide a framework for understanding the binding selectivity of HSF, and we look forward to mechanistic studies that solidify the rules of in vivo binding specificity.
Activators are generally thought to bind to promoters and recruit either Pol II or coactivators to produce productively elongating Pol II. HSF recruits the acetyltransferase CREB Binding Protein (CBP) and a methyltransferase, Trithorax, directly to HS genes [61], [62]. Paradoxically, this study shows that the chromatin landscape at HSF binding sites contains considerable histone acetylation and methylation prior to detectable HSF binding. HSF recruits these enzymes after HS to broaden the domain or increase the level of histone modifications (Figure 6 and Figure S12). Another, non-mutually exclusive, possibility is that cofactors other than histones are the functional targets of recruited transferases. Although we describe the landscape at HSF binding sites prior to HS, it still remains unclear which factors are responsible for setting up or maintaining the accessibility of these motifs. Furthermore, many HSF-binding sites are probably passively occupied because they happen to be accessible and HSF binding is non-deleterious [76], but these sites likely have no function in the HS response. The global chromatin landscape is dynamic throughout development and environmental changes; therefore, we expect that the HSF binding profile at non-functional sites is dynamic as well. Nonetheless, the HS response is a ubiquitous cellular response, so functional sites are likely to be evolutionarily constrained at the sequence level [77], [78], and actively maintained in the accessible state at the level of chromatin organization.
The maintenance of functional HSF binding sites may be occurring as a result of a specific class of activators. Non-traditional activators, such as GAF, are known to recruit cofactors that establish an accessible chromatin state, as opposed to directly activating transcription of the local gene (reviewed in [79]). This general mechanism has been characterized at the phaseolin gene in Arabidopsis [80] and at the PHO5 gene in yeast (reviewed in [81]). Taken together, this suggests a step-wise process whereby a repressed site can be potentiated for activator binding and subsequently activated. Additionally, it has been shown that active marks are not simply a product of transcription, as the active marks that are associated with intergenic DNaseI hypersensitive sites and putative enhancers are not correlated with respective gene expression [33]. Our results suggest that the landscape may be marked with active histone modifications to allow binding of activators that can stimulate transcription; therefore, the presence of a modification would not be expected to correlate with gene expression if the activator has yet to bind. Further investigation of activator binding sites during non-induced conditions will determine the generality of this observation.
Our candidate gene analysis shows that HSF is not sufficient to activate local genes. Although inducibly activated genes are occupied by their cognate transcriptional activator near the TSS [4], [82]–[84], it remains unclear how the majority of activators discriminate between locally bound genes to selectively activate. Strikingly, Caudal exhibits promoter element-specific activation, specifically activating genes that contain the Downstream Promoter Element (DPE) [85]. Previously, we presented evidence that the presence of a paused polymerase facilitates activation from an Hsp70 promoter [70], but it is unclear whether or not this is true for the majority HSF-inducible genes. Combinations of promoter features and gene properties are likely necessary for activation. One certainty, however, is that the recent emergence of genome-wide expression and binding data makes the characterization of complex regulatory mechanisms more exciting and promising than ever.
Materials and Methods
ChIP
The ChIP protocol has been previously described [86]. In short, S2-DRSC (lot 181A1) cells were grown in Schneider's media with 10% FBS (lot ASD29137), consistent with modENCODE experiments. Heat shocked cells were instantaneously shifted to 36.5°C by the addition of an equal volume of 48°C media to the 25°C culture. Heat shocked cells were instantaneously cooled to room-temperature and crosslinked with a final concentration of 2% paraformaldhyde for one minute; this shorter duration of crosslinking with higher concentration of paraformaldehyde was found to increase the signal-to-noise ratio. Instant cooling to room temperature and immediate crosslinking allows the heat shock and NHS samples to be crosslinked at the same efficiency and directly compared. We cannot strictly rule out the possibility that instantaneous cooling cells to room-temperature for one minute contributes to the recovery and dissociation of HSF at lower affinity sites, including the 708 HSF-free sites. However, paraformaldehyde penetrates cells quickly to effectively block further cellular changes, and HSF's DNA binding activity is only modestly affected even after a 30 minute recovery from HS [87]. Crosslinking was quenched by the addition of glycine to a final concentration of 250 mM and the extract was sonicated as previously described [86], but for three-times the duration to increase enrichment [88]. The Protein-A beads were blocked with BSA (1 mg/ml) and Polyvinylpyrrolidone (1 mg/ml) prior to the IP and freshly thawed antiserum was used for each IP, which also increased signal compared to noise.
Illumina sample preparation
The sample preparation was previously described [89], with some modifications. Only one size selection, after adapter ligation, was performed. Thirteen cycles of PCR were performed. Quant-iT Pico Green (Invitrogen) staining was used to quantify the DNA sample. Samples were submitted to the Cornell DNA Sequencing and Genotyping Lab and run on the Illumina Genome Analyzer II.
RNAi
RNAi-mediated HSF knockdown was performed as previously described [69]. Primer sequences are available within Dataset S6.
Peak Calling
Sequence tags were aligned to the Drosophila melanogaster April 2006 release of the reference genome using MAQ [90]. We considered those tags that aligned uniquely with less than 4 mismatches. A summary of the sequencing tag counts and unique alignment counts for each condition are supplied in Table S5. The text files containing raw sequence tags and uniquely aligned tags were deposited into NCBI's Gene Expression Omnibus (GEO) [91], accession number GSE19025. Two programs [41], [42] (referred to as MACS and SPP, respectively) were independently used to call peaks with the MAQ mapped sequences for each experimental condition. The parameters we used for each program are indicated in Figure S3. The Subpeaks package was further used to dissect the few areas of broad MACS enrichment. Using SPP, we determined that we achieved saturation at this depth of sequencing. Either of two criteria was used to consider a peak RNAi-sensitive: 1) a peak coordinate was called in both the experimental and RNAi dataset and the peak is depleted in the RNAi data more than the Hsp83 promoter depletion; 2) a peak was only called in the experimental dataset and the corresponding region of the RNAi dataset was depleted by at least 3-fold. The intensity used to calculate depletion was defined by the normalized tag count of mapped 5′ ends in the 240 base window centered on the experimental peak center coordinate. SPP and MACS were considered to have called the same peak if the SPP peak center was within the Subpeak enrichment boundary or the broader MACS enrichment boundary. The window that corresponds to the 60 bases flanking each peak center was used as input for MEME [43]. MAST and Tallymer were used in conjunction to determine the 100% mappable (for 40mer tags in the 400 bp window centered on the motif) HSF-free motifs [43], [45].
Chromatin Landscape Data
The individual labs that generated the chromatin landscape data also validated their results. Table S6 provides the respective modENCODE ID or GEO accession number for each dataset used in this study.
Ecdysone Treatment
Drosophila S2 cells were treated with 1000×20-hydroxyecdysone (20E) in 2% ethanol, at a final concentration of 1 µM for 30 minutes. ChIP was performed immediately after 30 minutes of 20E, for the NHS treated cells, or after a 20 minute HS. Two independent experimental replicates were performed for “20E/NHS” and “20E/HS”. Control cells were treated with 2% ethanol as the vehicle. Two independent control samples were performed, and the values were compared to a no treatment control. Vehicle treatment was comparable to no treatment, so we combined the measurements for a total of three independent biological replicates for both NHS and HS conditions. The error bars indicate the standard error of the mean. Importantly, we calculated two important background measurements. First, we performed ChIP with Rabbit IgG for each condition, to control for non-specific pull-down by IgG or beads. Secondly, we performed ChIP-qPCR at eight regions where each factor or modification is not enriched in untreated conditions [29], which controls for non-specific background pull-down by each antibody. Generally, the background IP by histone modification antibodies is high as measured by raw percent input, presumably do to cross reaction with unmodified histones, so this measurement is necessary in order to assign a threshold for enrichment in ChIP-qPCR assays (the top dashed line).
mRNA Reverse Transcription
RNA levels were measured as previously described [92].
Real-Time qPCR
Dataset S6 contains the primer sets that were used for measuring mRNA abundance. Table S7 contains the primer sequences that were used for ChIP-qPCR.
Supporting Information
Zdroje
1. CarrA
BigginMD
2000 Accessibility of transcriptionally inactive genes is specifically reduced at homeoprotein-DNA binding sites in Drosophila. Nucleic Acids Res 28 2839 2846
2. WeiCL
WuQ
VegaVB
ChiuKP
NgP
2006 A global map of p53 transcription-factor binding sites in the human genome. Cell 124 207 219
3. YangA
ZhuZ
KapranovP
McKeonF
ChurchGM
2006 Relationships between p63 binding, DNA sequence, transcription activity, and biological function in human cells. Mol Cell 24 593 602
4. WelborenWJ
van DrielMA
Janssen-MegensEM
van HeeringenSJ
SweepFC
2009 ChIP-seq of ERalpha and RNA polymerase II defines genes differentially responding to ligands. EMBO J 28 1418 1428
5. BigginMD
McGinnisW
1997 Regulation of segmentation and segmental identity by Drosophila homeoproteins: The role of DNA binding in functional activity and specificity. Development 124 4425 4433
6. SekingerEA
MoqtaderiZ
StruhlK
2005 Intrinsic histone-DNA interactions and low nucleosome density are important for preferential accessibility of promoter regions in yeast. Mol Cell 18 735 748
7. WorkmanJL
KingstonRE
1998 Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem 67 545 579
8. RobertsonAG
BilenkyM
TamA
ZhaoY
ZengT
2008 Genome-wide relationship between histone H3 lysine 4 mono - and tri-methylation and transcription factor binding. Genome Res 18 1906 1917
9. LarschanE
AlekseyenkoAA
GortchakovAA
PengS
LiB
2007 MSL complex is attracted to genes marked by H3K36 trimethylation using a sequence-independent mechanism. Mol Cell 28 121 133
10. GorchakovAA
AlekseyenkoAA
KharchenkoP
ParkPJ
KurodaMI
2009 Long-range spreading of dosage compensation in Drosophila captures transcribed autosomal genes inserted on X. Genes Dev 23 2266 2271
11. MannRS
LelliKM
JoshiR
2009 Hox specificity unique roles for cofactors and collaborators. Curr Top Dev Biol 88 63 101
12. MorimotoRI
2008 Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev 22 1427 1438
13. WuC
1995 Heat shock transcription factors: Structure and regulation. Annu Rev Cell Dev Biol 11 441 469
14. OroszA
WisniewskiJ
WuC
1996 Regulation of Drosophila heat shock factor trimerization: Global sequence requirements and independence of nuclear localization. Mol Cell Biol 16 7018 7030
15. PerisicO
XiaoH
LisJT
1989 Stable binding of Drosophila heat shock factor to head-to-head and tail-to-tail repeats of a conserved 5 bp recognition unit. Cell 59 797 806
16. FernandesM
XiaoH
LisJT
1994 Fine structure analyses of the Drosophila and Saccharomyces heat shock factor—heat shock element interactions. Nucleic Acids Res 22 167 173
17. BoehmAK
SaundersA
WernerJ
LisJT
2003 Transcription factor and polymerase recruitment, modification, and movement on dhsp70 in vivo in the minutes following heat shock. Mol Cell Biol 23 7628 7637
18. MacArthurS
LiXY
LiJ
BrownJB
ChuHC
2009 Developmental roles of 21 Drosophila transcription factors are determined by quantitative differences in binding to an overlapping set of thousands of genomic regions. Genome Biol 10 R80
19. WestwoodJT
ClosJ
WuC
1991 Stress-induced oligomerization and chromosomal relocalization of heat-shock factor. Nature 353 822 827
20. AshburnerM
1970 Patterns of puffing activity in the salivary gland chromosomes of Drosophila. V. responses to environmental treatments. Chromosoma 31 356 376
21. JamrichM
GreenleafAL
BautzEK
1977 Localization of RNA polymerase in polytene chromosomes of Drosophila melanogaster. Proc Natl Acad Sci U S A 74 2079 2083
22. LisJT
MasonP
PengJ
PriceDH
WernerJ
2000 P-TEFb kinase recruitment and function at heat shock loci. Genes Dev 14 792 803
23. SaundersA
WernerJ
AndrulisED
NakayamaT
HiroseS
2003 Tracking FACT and the RNA polymerase II elongation complex through chromatin in vivo. Science 301 1094 1096
24. LawA
HirayoshiK
O'BrienT
LisJT
1998 Direct cloning of DNA that interacts in vivo with a specific protein: Application to RNA polymerase II and sites of pausing in Drosophila. Nucleic Acids Res 26 919 924
25. JohnsonDS
MortazaviA
MyersRM
WoldB
2007 Genome-wide mapping of in vivo protein-DNA interactions. Science 316 1497 1502
26. RobertsonG
HirstM
BainbridgeM
BilenkyM
ZhaoY
2007 Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat Methods 4 651 657
27. BarskiA
CuddapahS
CuiK
RohTY
SchonesDE
2007 High-resolution profiling of histone methylations in the human genome. Cell 129 823 837
28. CelnikerSE
DillonLA
GersteinMB
GunsalusKC
HenikoffS
2009 Unlocking the secrets of the genome. Nature 459 927 930
29. KharchenkoP
AlekseyenkoA
MinodaA
RiddleNC
SchwartzYB
The modENCODE Drosophila chromatin group
30. HenikoffS
HenikoffJG
SakaiA
LoebGB
AhmadK
2009 Genome-wide profiling of salt fractions maps physical properties of chromatin. Genome Res 19 460 469
31. BusheyAM
RamosE
CorcesVG
2009 Three subclasses of a Drosophila insulator show distinct and cell type-specific genomic distributions. Genes Dev 23 1338 1350
32. JiangN
EmberlyE
CuvierO
HartCM
2009 Genome-wide mapping of boundary element-associated factor (BEAF) binding sites in Drosophila melanogaster links BEAF to transcription. Mol Cell Biol 29 3556 3568
33. WangZ
ZangC
RosenfeldJA
SchonesDE
BarskiA
2008 Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet 40 897 903
34. SchneiderI
1972 Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol 27 353 365
35. AndrulisED
GuzmanE
DoringP
WernerJ
LisJT
2000 High-resolution localization of Drosophila Spt5 and Spt6 at heat shock genes in vivo: Roles in promoter proximal pausing and transcription elongation. Genes Dev 14 2635 2649
36. ParkJM
WernerJ
KimJM
LisJT
KimYJ
2001 Mediator, not holoenzyme, is directly recruited to the heat shock promoter by HSF upon heat shock. Mol Cell 8 9 19
37. ShoplandLS
LisJT
1996 HSF recruitment and loss at most Drosophila heat shock loci is coordinated and depends on proximal promoter sequences. Chromosoma 105 158 171
38. WalterJ
DeverCA
BigginMD
1994 Two homeo domain proteins bind with similar specificity to a wide range of DNA sites in Drosophila embryos. Genes Dev 8 1678 1692
39. OhSW
MukhopadhyayA
DixitBL
RahaT
GreenMR
2006 Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat Genet 38 251 257
40. XiaoH
PerisicO
LisJT
1991 Cooperative binding of Drosophila heat shock factor to arrays of a conserved 5 bp unit. Cell 64 585 593
41. ZhangY
LiuT
MeyerCA
EeckhouteJ
JohnsonDS
2008 Model-based analysis of ChIP-seq (MACS). Genome Biol 9 R137
42. KharchenkoPV
TolstorukovMY
ParkPJ
2008 Design and analysis of ChIP-seq experiments for DNA-binding proteins. Nat Biotechnol 26 1351 1359
43. BaileyTL
WilliamsN
MislehC
LiWW
2006 MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res 34 W369 73
44. JothiR
CuddapahS
BarskiA
CuiK
ZhaoK
2008 Genome-wide identification of in vivo protein-DNA binding sites from ChIP-seq data. Nucleic Acids Res 36 5221 5231
45. KurtzS
NarechaniaA
SteinJC
WareD
2008 A new method to compute K-mer frequencies and its application to annotate large repetitive plant genomes. BMC Genomics 9 517
46. NightingaleKP
WellingerRE
SogoJM
BeckerPB
1998 Histone acetylation facilitates RNA polymerase II transcription of the Drosophila hsp26 gene in chromatin. EMBO J 17 2865 2876
47. HeHH
MeyerCA
ShinH
BaileyST
WeiG
2010 Nucleosome dynamics define transcriptional enhancers. Nat Genet 42 343 347
48. PeteschSJ
LisJT
2008 Rapid, transcription-independent loss of nucleosomes over a large chromatin domain at Hsp70 loci. Cell 134 74 84
49. WuC
1980 The 5′ ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature 286 854 860
50. MargueronR
TrojerP
ReinbergD
2005 The key to development: Interpreting the histone code? Curr Opin Genet Dev 15 163 176
51. GuentherMG
LawtonLN
RozovskaiaT
FramptonGM
LevineSS
2008 Aberrant chromatin at genes encoding stem cell regulators in human mixed-lineage leukemia. Genes Dev 22 3403 3408
52. ZhuB
MandalSS
PhamAD
ZhengY
Erdjument-BromageH
2005 The human PAF complex coordinates transcription with events downstream of RNA synthesis. Genes Dev 19 1668 1673
53. TavernaSD
LiH
RuthenburgAJ
AllisCD
PatelDJ
2007 How chromatin-binding modules interpret histone modifications: Lessons from professional pocket pickers. Nat Struct Mol Biol 14 1025 1040
54. LeeC
LiX
HechmerA
EisenM
BigginMD
2008 NELF and GAGA factor are linked to promoter-proximal pausing at many genes in Drosophila. Mol Cell Biol 28 3290 3300
55. ZangC
SchonesDE
ZengC
CuiK
ZhaoK
2009 A clustering approach for identification of enriched domains from histone modification ChIP-seq data. Bioinformatics 25 1952 1958
56. HeintzmanND
StuartRK
HonG
FuY
ChingCW
2007 Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39 311 318
57. BermanBP
NibuY
PfeifferBD
TomancakP
CelnikerSE
2002 Exploiting transcription factor binding site clustering to identify cis-regulatory modules involved in pattern formation in the Drosophila genome. Proc Natl Acad Sci U S A 99 757 762
58. HonG
WangW
RenB
2009 Discovery and annotation of functional chromatin signatures in the human genome. PLoS Comput Biol 5 e1000566
59. EisenMB
SpellmanPT
BrownPO
BotsteinD
1998 Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A 95 14863 14868
60. BernardoTJ
DubrovskayaVA
JannatH
MaughanB
DubrovskyEB
2009 Hormonal regulation of the E75 gene in Drosophila: Identifying functional regulatory elements through computational and biological analysis. J Mol Biol 387 794 808
61. HongS
KimSH
HeoMA
ChoiYH
ParkMJ
2004 Coactivator ASC-2 mediates heat shock factor 1-mediated transactivation dependent on heat shock. FEBS Lett 559 165 170
62. SmithST
PetrukS
SedkovY
ChoE
TillibS
2004 Modulation of heat shock gene expression by the TAC1 chromatin-modifying complex. Nat Cell Biol 6 162 167
63. LindquistS
1980 Varying patterns of protein synthesis in Drosophila during heat shock: Implications for regulation. Dev Biol 77 463 479
64. SpradlingA
PenmanS
PardueML
1975 Analysis of Drosophila mRNA by in situ hybridization: Sequences transcribed in normal and heat shocked cultured cells. Cell 4 395 404
65. ArdehaliMB
LisJT
2009 Tracking rates of transcription and splicing in vivo. Nat Struct Mol Biol 16 1123 1124
66. LiangZ
BigginMD
1998 Eve and ftz regulate a wide array of genes in blastoderm embryos: The selector homeoproteins directly or indirectly regulate most genes in Drosophila. Development 125 4471 4482
67. WisniewskiJ
OroszA
AlladaR
WuC
1996 The C-terminal region of Drosophila heat shock factor (HSF) contains a constitutively functional transactivation domain. Nucleic Acids Res 24 367 374
68. KininisM
ChenBS
DiehlAG
IsaacsGD
ZhangT
2007 Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol 27 5090 5104
69. ArdehaliMB
YaoJ
AdelmanK
FudaNJ
PeteschSJ
2009 Spt6 enhances the elongation rate of RNA polymerase II in vivo. EMBO J 28 1067 1077
70. LeeH
KrausKW
WolfnerMF
LisJT
1992 DNA sequence requirements for generating paused polymerase at the start of hsp70. Genes Dev 6 284 295
71. StegerDJ
LefterovaMI
YingL
StonestromAJ
SchuppM
2008 DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells. Mol Cell Biol 28 2825 2839
72. JinC
ZangC
WeiG
CuiK
PengW
2009 H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat Genet 41 941 945
73. AuerbachRK
EuskirchenG
RozowskyJ
Lamarre-VincentN
MoqtaderiZ
2009 Mapping accessible chromatin regions using sono-seq. Proc Natl Acad Sci U S A 106 14926 14931
74. KurdistaniSK
GrunsteinM
2003 In vivo protein-protein and protein-DNA crosslinking for genomewide binding microarray. Methods 31 90 95
75. GilmourDS
LisJT
1985 In vivo interactions of RNA polymerase II with genes of Drosophila melanogaster. Mol Cell Biol 5 2009 2018
76. HahnMW
StajichJE
WrayGA
2003 The effects of selection against spurious transcription factor binding sites. Mol Biol Evol 20 901 906
77. MosesAM
ChiangDY
KellisM
LanderES
EisenMB
2003 Position specific variation in the rate of evolution in transcription factor binding sites. BMC Evol Biol 3 19
78. DermitzakisET
ClarkAG
2002 Evolution of transcription factor binding sites in mammalian gene regulatory regions: Conservation and turnover. Mol Biol Evol 19 1114 1121
79. AdkinsNL
HagermanTA
GeorgelP
2006 GAGA protein: A multi-faceted transcription factor. Biochem Cell Biol 84 559 567
80. NgDW
ChandrasekharanMB
HallTC
2006 Ordered histone modifications are associated with transcriptional poising and activation of the phaseolin promoter. Plant Cell 18 119 132
81. FudaNJ
ArdehaliMB
LisJT
2009 Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature 461 186 192
82. ChengY
WuW
Ashok KumarS
YuD
DengW
2009 Erythroid GATA1 function revealed by genome-wide analysis of transcription factor occupancy, histone modifications, and mRNA expression. Genome Res 19 2172 2184
83. YuM
RivaL
XieH
SchindlerY
MoranTB
2009 Insights into GATA-1-mediated gene activation versus repression via genome-wide chromatin occupancy analysis. Mol Cell 36 682 695
84. ReddyTE
PauliF
SprouseRO
NeffNF
NewberryKM
2009 Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res 19 2163 2171
85. Juven-GershonT
HsuJY
KadonagaJT
2008 Caudal, a key developmental regulator, is a DPE-specific transcriptional factor. Genes Dev 22 2823 2830
86. NiZ
SaundersA
FudaNJ
YaoJ
SuarezJR
2008 P-TEFb is critical for the maturation of RNA polymerase II into productive elongation in vivo. Mol Cell Biol 28 1161 1170
87. FritschM
WuC
1999 Phosphorylation of Drosophila heat shock transcription factor. Cell Stress Chaperones 4 102 117
88. FanX
Lamarre-VincentN
WangQ
StruhlK
2008 Extensive chromatin fragmentation improves enrichment of protein binding sites in chromatin immunoprecipitation experiments. Nucleic Acids Res 36 e125
89. QuailMA
KozarewaI
SmithF
ScallyA
StephensPJ
2008 A large genome center's improvements to the Illumina sequencing system. Nat Methods 5 1005 1010
90. LiH
RuanJ
DurbinR
2008 Mapping short DNA sequencing reads and calling variants using mapping quality scores. Genome Res 18 1851 1858
91. BarrettT
TroupDB
WilhiteSE
LedouxP
RudnevD
2009 NCBI GEO: Archive for high-throughput functional genomic data. Nucleic Acids Res 37 D885 90
92. AdelmanK
MarrMT
WernerJ
SaundersA
NiZ
2005 Efficient release from promoter-proximal stall sites requires transcript cleavage factor TFIIS. Mol Cell 17 103 112
93. CrooksGE
HonG
ChandoniaJM
BrennerSE
2004 WebLogo: A sequence logo generator. Genome Res 14 1188 1190
94. PageRD
1996 TreeView: An application to display phylogenetic trees on personal computers. Comput Appl Biosci 12 357 358
95. NiZ
SchwartzBE
WernerJ
SuarezJR
LisJT
2004 Coordination of transcription, RNA processing, and surveillance by P-TEFb kinase on heat shock genes. Mol Cell 13 55 65
Štítky
Genetika Reprodukčná medicína
Článek Allelic Variation at the 8q23.3 Colorectal Cancer Risk Locus Functions as a Cis-Acting Regulator ofČlánek Allelic Selection of Amplicons in Glioblastoma Revealed by Combining Somatic and Germline AnalysisČlánek Lactic Acidosis Triggers Starvation Response with Paradoxical Induction of TXNIP through MondoAČlánek Rice a Cinnamoyl-CoA Reductase-Like Gene Family Member, Is Required for NH1-Mediated Immunity to pv.Článek Differentiation of Zebrafish Melanophores Depends on Transcription Factors AP2 Alpha and AP2 Epsilon
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2010 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Optimal Strategy for Competence Differentiation in Bacteria
- Mutational Patterns Cannot Explain Genome Composition: Are There Any Neutral Sites in the Genomes of Bacteria?
- Frail Hypotheses in Evolutionary Biology
- Genetic Architecture of Complex Traits and Accuracy of Genomic Prediction: Coat Colour, Milk-Fat Percentage, and Type in Holstein Cattle as Contrasting Model Traits
- Allelic Variation at the 8q23.3 Colorectal Cancer Risk Locus Functions as a Cis-Acting Regulator of
- Allelic Selection of Amplicons in Glioblastoma Revealed by Combining Somatic and Germline Analysis
- Germline Variation Controls the Architecture of Somatic Alterations in Tumors
- Mice Doubly-Deficient in Lysosomal Hexosaminidase A and Neuraminidase 4 Show Epileptic Crises and Rapid Neuronal Loss
- Analysis of Population Structure: A Unifying Framework and Novel Methods Based on Sparse Factor Analysis
- FliO Regulation of FliP in the Formation of the Flagellum
- Cdc20 Is Critical for Meiosis I and Fertility of Female Mice
- dMyc Functions Downstream of Yorkie to Promote the Supercompetitive Behavior of Hippo Pathway Mutant Cells
- DCAF26, an Adaptor Protein of Cul4-Based E3, Is Essential for DNA Methylation in
- Genome-Wide Double-Stranded RNA Sequencing Reveals the Functional Significance of Base-Paired RNAs in
- An Immune Response Network Associated with Blood Lipid Levels
- Genetic Variants and Their Interactions in the Prediction of Increased Pre-Clinical Carotid Atherosclerosis: The Cardiovascular Risk in Young Finns Study
- The Histone H3K36 Methyltransferase MES-4 Acts Epigenetically to Transmit the Memory of Germline Gene Expression to Progeny
- Long- and Short-Term Selective Forces on Malaria Parasite Genomes
- Lactic Acidosis Triggers Starvation Response with Paradoxical Induction of TXNIP through MondoA
- Identification of Early Requirements for Preplacodal Ectoderm and Sensory Organ Development
- Orphan CpG Islands Identify Numerous Conserved Promoters in the Mammalian Genome
- Analysis of the Basidiomycete Reveals Conservation of the Core Meiotic Expression Program over Half a Billion Years of Evolution
- ETS-4 Is a Transcriptional Regulator of Life Span in
- The SR Protein B52/SRp55 Is Required for DNA Topoisomerase I Recruitment to Chromatin, mRNA Release and Transcription Shutdown
- The Baker's Yeast Diploid Genome Is Remarkably Stable in Vegetative Growth and Meiosis
- Chromatin Landscape Dictates HSF Binding to Target DNA Elements
- The APETALA-2-Like Transcription Factor OsAP2-39 Controls Key Interactions between Abscisic Acid and Gibberellin in Rice
- Accurately Assessing the Risk of Schizophrenia Conferred by Rare Copy-Number Variation Affecting Genes with Brain Function
- Widespread Over-Expression of the X Chromosome in Sterile F Hybrid Mice
- The Characterization of Twenty Sequenced Human Genomes
- The Genome of a Pathogenic : Cooptive Virulence Underpinned by Key Gene Acquisitions
- A Single Element Maintains Repression of the Key Developmental Regulator
- Identification of New Genetic Risk Variants for Type 2 Diabetes
- Effect of Correlated tRNA Abundances on Translation Errors and Evolution of Codon Usage Bias
- Evidence of Selection upon Genomic GC-Content in Bacteria
- Proteomic Changes Resulting from Gene Copy Number Variations in Cancer Cells
- Rice a Cinnamoyl-CoA Reductase-Like Gene Family Member, Is Required for NH1-Mediated Immunity to pv.
- Longitudinal Genome-Wide Association of Cardiovascular Disease Risk Factors in the Bogalusa Heart Study
- Response to Mechanical Stress Is Mediated by the TRPA Channel Painless in the Heart
- DNMT3L Modulates Significant and Distinct Flanking Sequence Preference for DNA Methylation by DNMT3A and DNMT3B
- Identifying Signatures of Natural Selection in Tibetan and Andean Populations Using Dense Genome Scan Data
- Incremental Genetic Perturbations to MCM2-7 Expression and Subcellular Distribution Reveal Exquisite Sensitivity of Mice to DNA Replication Stress
- Loss of Maternal ATRX Results in Centromere Instability and Aneuploidy in the Mammalian Oocyte and Pre-Implantation Embryo
- Comparative Genomic Hybridization (CGH) Reveals a Neo-X Chromosome and Biased Gene Movement in Stalk-Eyed Flies (Genus )
- Differentiation of Zebrafish Melanophores Depends on Transcription Factors AP2 Alpha and AP2 Epsilon
- Gene–Environment Interactions at Nucleotide Resolution
- Dementia Revealed: Novel Chromosome 6 Locus for Late-Onset Alzheimer Disease Provides Genetic Evidence for Folate-Pathway Abnormalities
- Critical Functions of Rpa3/Ssb3 in S-Phase DNA Damage Responses in Fission Yeast
- Preferential Re-Replication of Heterochromatin in the Absence of Geminin
- The Potential for Enhancing the Power of Genetic Association Studies in African Americans through the Reuse of Existing Genotype Data
- Evidence That Mutation Is Universally Biased towards AT in Bacteria
- Perturbation Analysis of Heterochromatin-Mediated Gene Silencing and Somatic Inheritance
- Diversity of Eukaryotic DNA Replication Origins Revealed by Genome-Wide Analysis of Chromatin Structure
- Genetic Deletion of the Desmosomal Component Promotes Tumor Microinvasion in a Mouse Model of Pancreatic Neuroendocrine Carcinogenesis
- The Metabolic Enzyme ManA Reveals a Link between Cell Wall Integrity and Chromosome Morphology
- SNPs Associated with Cerebrospinal Fluid Phospho-Tau Levels Influence Rate of Decline in Alzheimer's Disease
- Synthesizing and Salvaging NAD: Lessons Learned from
- A Central Regulatory System Largely Controls Transcriptional Activation and Repression Responses to Phosphate Starvation in Arabidopsis
- An Insect Herbivore Microbiome with High Plant Biomass-Degrading Capacity
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Synthesizing and Salvaging NAD: Lessons Learned from
- Optimal Strategy for Competence Differentiation in Bacteria
- Long- and Short-Term Selective Forces on Malaria Parasite Genomes
- Identifying Signatures of Natural Selection in Tibetan and Andean Populations Using Dense Genome Scan Data
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



