-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Mutations in Four Glycosyl Hydrolases Reveal a Highly Coordinated Pathway for Rhodopsin Biosynthesis and N-Glycan Trimming in
As newly synthesized glycoproteins move through the secretory pathway, the asparagine-linked glycan (N-glycan) undergoes extensive modifications involving the sequential removal and addition of sugar residues. These modifications are critical for the proper assembly, quality control and transport of glycoproteins during biosynthesis. The importance of N-glycosylation is illustrated by a growing list of diseases that result from defects in the biosynthesis and processing of N-linked glycans. The major rhodopsin in the Drosophila (fruit fly) eye, Rh1, is highly unique among glycoproteins, as the N-glycan appears to be completely removed during Rh1 biosynthesis and maturation. However, much of the deglycosylation pathway for Rh1 remains unknown. To elucidate the key steps in Rh1 deglycosylation, we conducted a genetic dissection of glycoprotein processing in vivo. We have demonstrated that four glycosyl hydrolases play essential and unique roles in a highly coordinated pathway for N-glycan trimming during Rh1 biosynthesis. Our results reveal novel insights into the functions of glycosyl hydrolases in the secretory pathway and provide fundamental advances towards understanding N-glycosylation in vivo.
Published in the journal: Mutations in Four Glycosyl Hydrolases Reveal a Highly Coordinated Pathway for Rhodopsin Biosynthesis and N-Glycan Trimming in. PLoS Genet 10(5): e32767. doi:10.1371/journal.pgen.1004349
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004349Summary
As newly synthesized glycoproteins move through the secretory pathway, the asparagine-linked glycan (N-glycan) undergoes extensive modifications involving the sequential removal and addition of sugar residues. These modifications are critical for the proper assembly, quality control and transport of glycoproteins during biosynthesis. The importance of N-glycosylation is illustrated by a growing list of diseases that result from defects in the biosynthesis and processing of N-linked glycans. The major rhodopsin in the Drosophila (fruit fly) eye, Rh1, is highly unique among glycoproteins, as the N-glycan appears to be completely removed during Rh1 biosynthesis and maturation. However, much of the deglycosylation pathway for Rh1 remains unknown. To elucidate the key steps in Rh1 deglycosylation, we conducted a genetic dissection of glycoprotein processing in vivo. We have demonstrated that four glycosyl hydrolases play essential and unique roles in a highly coordinated pathway for N-glycan trimming during Rh1 biosynthesis. Our results reveal novel insights into the functions of glycosyl hydrolases in the secretory pathway and provide fundamental advances towards understanding N-glycosylation in vivo.
Introduction
Many proteins that are synthesized in the endoplasmic reticulum (ER) and transported through the secretory pathway are posttranslationally modified with an asparagine (N)-linked carbohydrate moiety, termed an N-glycan (Figure 1). N-glycosylation is key to the proper assembly, quality control, and transport of glycoproteins during biosynthesis [1], [2]. Not only does the N-glycan directly reduce protein aggregation, but it also serves as a signal for interaction with key lectins and glycosyl hydrolases. N-glycosylation begins in the ER, where preformed Glc3Man9GlcNAc2 (Figure 1) is transferred, en bloc, to asparagine (Asn) residues on nascent polypeptide chains containing the consensus sequence, NXS/T [3], [4]. As the newly synthesized glycoprotein moves through the secretory pathway, the oligosaccharide precursor undergoes extensive modifications involving sequential removal and addition of sugar residues in the Golgi [5]. All of the enzymes that function in carbohydrate trimming during biosynthesis are exoglycosidases and thus require their substrate residue to be exposed at a terminal position on the N-glycan. Accordingly, carbohydrate trimming proceeds sequentially, with the most terminal residues being removed first. These modifications serve critical functions in glycoprotein recognition, folding, targeting, and degradation [1], [6].
Fig. 1. A schematic of the asparagine (Asn, N)-linked oligosaccharide precursor, Glc3Man9GlcNAc2. 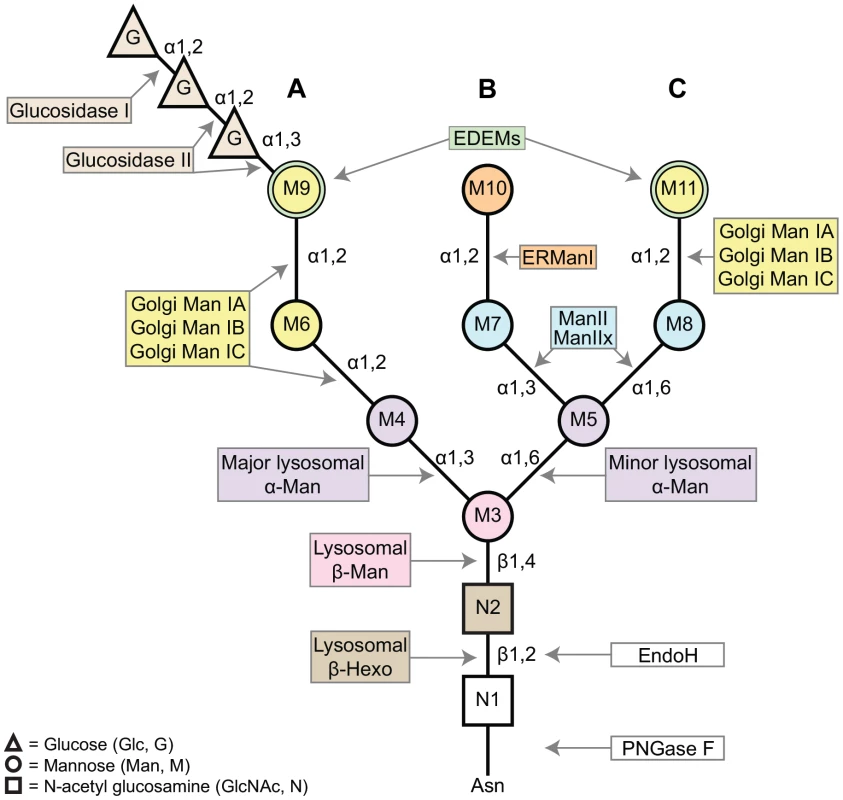
The N-glycan contains three glucose residues (triangles, G), nine mannose residues (circles, M3–11), and two β-N-acetylglucosamine residues (squares, N1–2). A, B, and C refer to the major branches on the N-glycan structure. Colors complement the enzyme list provided in Figure 2, indicating the glycosyl hydrolases (GH) responsible for trimming each residue. The α1,2-linked mannose residues (M6, M9, M10, and M11) are removed by Class I α-mannosidases (GH Family 47). The α1,3- and α1,6-linked mannose residues (M4, M5, M7, and M8) are removed by Class II α-mannosidases (GH Family 38). M3 is attached to the N-glycan by a β1,4-linkage and is removed by a β-mannosidase (GH Family 2). Finally, the terminal GlcNAc residue is attached via a β1,2-linkage and is removed by a β-hexosaminidase (GH Family 20). Man (mannosidase), Hexo (hexosaminidase). N-glycosylation in Drosophila melanogaster proceeds in a similar fashion to the well-characterized glycosylation pathways identified in mammalian systems, beginning with addition of the Glc3Man9GlcNAc2 precursor to newly synthesized proteins in the ER [2], [7]–[9]. Likewise, steps in the subsequent processing and trimming of the N-glycan are highly conserved between humans and Drosophila [2]. Here, we show that there is at least one Drosophila homolog for nearly all of the human glycosyl hydrolase (GH) enzymes involved in N-glycosylation (Figures 2 and S2–S7).
Fig. 2. N-glycan processing enzymes in humans and Drosophila. 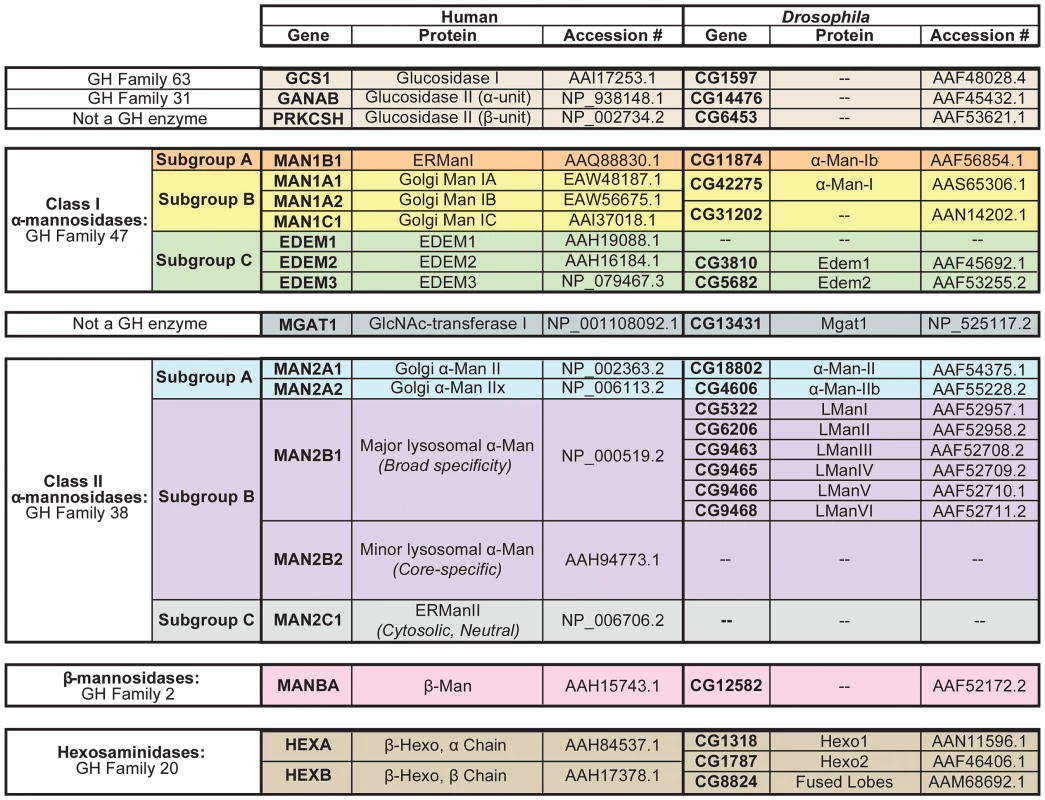
Human and Drosophila enzymes in numerous glycosyl hydrolase (GH) families are involved in the processing and/or catabolism of N-glycans. These proteins are divided into six major groups and are listed in the order in which they are thought to function in the cascade (See Figure 4). (1) Glucosidase I and the α-subunit of glucosidase II are from GH Families 63 and 31, respectively (tan). The β-subunit of glucosidase II is not a GH enzyme (tan). (2) The Class I α-mannosidases from GH Family 47 can be classified into three functionally distinct subgroups: Subgroup A includes the ER α1,2-mannosidases (orange), Subgroup B includes the Golgi α1,2-mannosidases (yellow), and Subgroup C includes the EDEMs (green). (3) GlcNAc-transferase is not a GH enzyme (dark blue). (4) The Class II α-mannosidases from GH Family 38 can also be classified into several functionally distinct subgroups: Subgroup A includes the Golgi α1,3(1,6)-mannosidases (light blue), Subgroup B includes the lysosomal α-mannosidases (purple), and Subgroup C includes an ER/cytosolic α-mannosidase, which is not found in Drosophila (grey). (5) The β-mannosidases from GH Family 2 (pink) and (6) the hexosaminidases from GH Family 20 (brown) are also listed. Accession numbers presented here indicate the protein sequences that were used for all amino acid alignments and sequence analyses performed in this study. Man (mannosidase), Hexo (hexosaminidase), L (lysosomal). One notable difference between vertebrate and invertebrate glycan processing is reflected in the relative abundance of the final N-glycan structures produced on glycoproteins [2]. In vertebrates, glycoproteins typically harbor hybrid - or complex-type glycan structures, in which one or two additional N-acetylglucosamine (GlcNAc) residues serve as the building blocks for the elongation and elaboration of the N-glycan. In contrast, glycoproteins in Drosophila and other invertebrates typically have high - or pauci-mannosidic glycan structures, in which the GlcNAc residues are removed prior to elongation, and thus elaboration of the N-glycan begins directly from the trimannosyl core. These differences are explained by the presence of a β-N-acetylglucosaminidase in Drosophila, called fused lobes (Fdl), which functions to remove the terminal GlcNAc residue. Accordingly, mutations in Drosophila fused lobes lead to the formation of the hybrid - and complex-type glycans that are more typical of vertebrates [10].
Mutations in other Drosophila glycosyl hydrolases that function in the N-glycan processing pathway have also been described in vivo. The first α-mannosidase to be cloned and characterized in Drosophila was α-mannosidase-I (α-Man-I, G42275) [7]. Null mutations in α-man-I (also known as mas1) were shown to cause developmental defects in the embryonic peripheral nervous system (PNS), in the wing, and in the adult eye [7]. It was recently shown that α-mannosidase-II (α-Man-II, CG18802) and α-mannosidase-IIb (α-Man-IIb, CG4606) are required for rhodopsin deglycosylation in Drosophila [11]. The authors further demonstrated that α-Man-II activity is strictly regulated by a phosphatase, Drosophila metallophosphoesterase (dMPPE). In addition to mutant analysis, Drosophila α-Man-II has been the subject of extensive structural analysis over the last decade. Its crystal structure now serves as the standard model for all Class II α-mannosidases [12]–[22]. While some of the GH enzymes involved in N-glycosylation have been isolated and well characterized in Drosophila, many remain unannotated and/or uncharacterized.
The importance of N-glycosylation is illustrated by a growing list of diseases that result from defects in the biosynthesis and processing of N-linked glycans. Congenital disorders of glycosylation (CDG) are a group of autosomal recessive diseases that result from defects in the synthesis, attachment, and/or processing of glycans [23]. Patients with CDG manifest a variety of symptoms and many of these diseases affect the eye. Patients with CDG Type Ia and Ic may develop progressive vision loss, retinitis pigmentosa (RP), impaired night vision, convergent strabismus, abnormal eye movements, as well as other ocular pathologies [24]–[26]. Several additional diseases have been associated with defects in N-glycan processing, such as α-mannosidosis and β-mannosidosis, which are autosomal recessive lysosomal storage diseases caused by genetic disruption of an α-mannosidase (MAN2B1) or β-mannosidase (MANB1) enzyme, respectively [27], [28] (Figure 2). These disorders are characterized by the accumulation of undegraded N-glycan products, which retain either α - or β-linked mannose residues, respectively. Patients often suffer from mental retardation, among a variety of other clinical manifestations [27], [29]. Other symptoms include late-onset retinal dystrophy, peripheral neuropathy, hearing loss, speech impairment, epileptic encephalopathy, angiokeratome, and skeletal/facial dysmorphism [30], [31]. Finally, two loci encoding the hexosaminidase enzymes, HEXA and HEXB (Figure 2), have been directly linked to Tay-Sachs and Sandhoff disease [32].
There is an overwhelming diversity of oligosaccharide structures that can be generated during glycoprotein processing, thus determining the roles of distinct oligosaccharide structures in vivo is a formidable challenge. Limited clues have come from studies of cell lines that are defective in certain processing enzymes [33]. However, these studies present some limitations, as cell culture experiments do not always mimic the complex environments that exist in vivo, in multi-cellular organisms. Other insights have come from studies involving the treatment of cells or animals with inhibitors that block various enzymatic steps in N-glycan processing [34]. The caveat with these studies is that the inhibitors often target multiple enzymes, complicating interpretation of the results [35]. One of the most powerful techniques for elucidating the specific roles of individual processing enzymes in animals involves the generation and analysis of the corresponding mutants in vivo. Such a systematic genetic dissection of N-glycosylation is entirely feasible in Drosophila. Additionally, the eye is not required for viability and therefore null mutations in the eye can be easily investigated to uncover the roles of glycosyl hydrolases in vivo.
In this study, we identify four glycosyl hydrolases in Drosophila that are critical for N-glycan processing during protein biosynthesis, namely α-mannosidase-II, α-mannosidase-IIb, a β-N-acetylglucosaminidase called fused lobes, and hexosaminidase 1. We have used the major visual protein in Drosophila, rhodopsin 1 (Rh1), as a substrate for the identification and characterization of mutants that are defective in specific N-glycan processing enzymes in the eye. Rh1 is highly unique among glycoproteins as we, and others, have shown that the N-glycan is extensively trimmed as Rh1 matures through the secretory pathway during biosynthesis [36]–[39]. Here, we demonstrate that Rh1 deglycosylation requires the sequential actions of a defined set of enzymes from numerous glycosyl hydrolase families, unveiling a highly coordinated pathway for Rh1 maturation. Furthermore, we show that in order to trim the final mannose residues (M3, M4, and M5) and the final N-acetylglucosamine residues (N1 and N2) at the base of the N-glycan (Figure 1), Rh1 requires the actions of several lysosomal and plasma membrane enzymes that, to date, have not been characterized within the context of N-glycan processing in the secretory pathway. Therefore, our analysis of Rh1 deglycosylation has uncovered novel roles for these glycosyl hydrolase enzymes during protein biosynthesis. Our findings on the glycosyl hydrolases in Drosophila provide insights for future studies aimed at unraveling the complex process of N-glycosylation in vivo, as well as for understanding mechanisms that underlie human disease.
Results
Identification of mutant alleles affecting N-glycosylation of Rh1
Using heat pulse-chase experiments, we have shown that Rh1 is initially synthesized in high-molecular weight (MW) glycosylated forms, that are processed down to the mature form (Figure 3A) [40]. Therefore, unlike all other known glycoproteins, the N-glycan on Rh1 is trimmed but not subsequently built upon to form larger carbohydrate structures [36]. Because of this highly unique characteristic, Rh1 offers a powerful model for studying the glycosyl hydrolases without their action being obscured by subsequent carbohydrate addition.
Fig. 3. Rh1 is deglycosylated in a step-wise fashion. 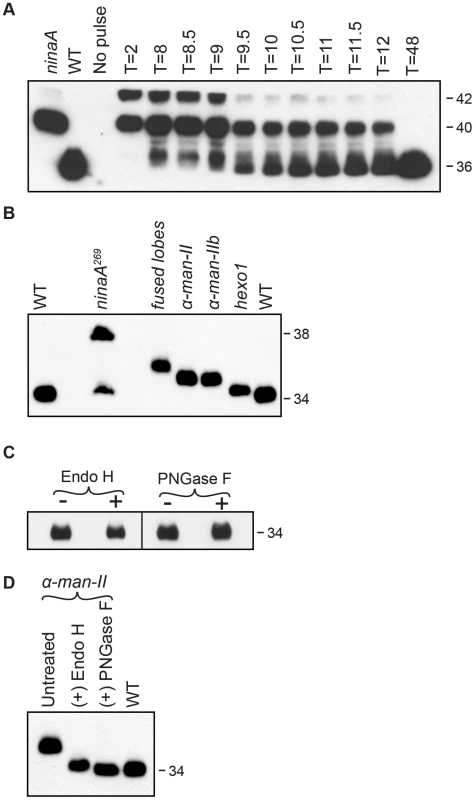
(A) Western blot of Rh1 protein from transgenic flies carrying the P[hs:Rh1-bov] construct, probed with an antibody (1D4) directed to the bov epitope tag on Rh1. Following a 1 hour heat pulse at 37°C, flies were shifted to 22°C and assayed at the indicated times (T, in hours). Lanes 1 and 2 show Rh1-bov under the control of the endogenous Rh1 promoter, expressed in either ninaA269 mutant or wild-type (WT) flies. The epitope tag adds ∼2 kD to Rh1, such that the immature forms are detected at 42 and 40 kD and the mature form is detected at 36 kD. Five fly heads were loaded per lane, with the exception of lane 2 (WT), in which 1/6 head was loaded. (B) Western blot of Rh1 protein from wild-type (WT), ninaA269, fdld03111, α-man-IILL01094, α-man-IIbf02524, and hexo1e00001 mutant flies. One half of a fly head was loaded per lane, with the exception of lane 2 (ninaA), in which 15 heads were loaded per lane. (C) Western blot of Rh1 protein from Canton S. wild-type tissue, treated (+) with either Endo H or PNGase F enzyme. (D) Western blot of Rh1 protein from α-man-IILL01094 mutant tissue (Lane 1), treated (+) with either Endo H (Lane 2) or PNGase F (Lane 3), and compared alongside a 1-head wild-type sample (Lane 4, WT). To identify genetic loci critical for Rh1 deglycosylation during biosynthesis, we analyzed flies from the Zuker collection of EMS-generated alleles [41] for defects in carbohydrate trimming by monitoring the molecular weight (MW) of Rh1. In wild-type adult flies, Rh1 is detected in a mature 34 kD form (Figure 3B). Mutations that result in defective trimming of the oligosaccharide chain during Rh1 biosynthesis lead to the accumulation of Rh1 in abnormal high MW forms. For example, NinaA is a chaperone required for the exit of Rh1 from the ER [39], [42]–[44]. Accordingly, in ninaA269 mutants, Rh1 is detected in an immature, 38 kD hyperglycosylated form (Figure 3B) [39], [45].
In our EMS screen, we observed defects in Rh1 deglycosylation in two mutant alleles and performed deficiency mapping, followed by sequence analysis, to identify each mutant locus. We identified a point mutation that leads to a G572E substitution in CG4606, which encodes a Class II α-mannosidase enzyme from GH Family 38, termed α-mannosidase-IIb (α-Man-IIb). We also identified a substitution leading to a pre-mature stop codon (Q592X) in CG1318, which encodes a β-N-acetylglucosaminidase enzyme from GH Family 20, termed hexosaminidase 1 (Hexo1).
Identification of the α-man-IIbG572E and hexo1Q592X mutants served as the starting point for a larger analysis of the role of glycosyl hydrolases from these GH families in Rh1 deglycosylation. Specifically, we focused on Class II α-mannosidases from GH Family 38 [46] and hexosaminidases from GH Family 20 [47] with potential roles in N-glycosylation. We identified additional mutant alleles of α-man-IIb and hexo1, as well as mutant alleles corresponding to a second Class II α-mannosidase from GH Family 38, α-mannosidase-II (α-Man-II, CG18802), and a second hexosaminidase from GH Family 20, termed fused lobes (Fdl, CG8824). Here, we characterize the roles of α-Man-II, α-Man-IIb, fused lobes, and Hexo1 during Rh1 deglycosylation in vivo (Figure 3B). Again, the ninaA269 mutant is shown for reference, as Rh1 is retained in the ER in this mutant, and thus N-glycan trimming is blocked at a very early stage during biosynthesis. Accordingly, mutations in ninaA lead to the accumulation of a hyperglycosylated, immature form of Rh1 that is significantly larger than the mutant forms observed in the glycosyl hydrolase mutants, which function for N-glycan trimming downstream in the Golgi (Figure 3B).
To determine the extent of Rh1 deglycosylation during its biosynthesis, we performed digestions with endoglycosidase H (Endo H) and peptide N-glycosidase F (PNGase F) and found that the mature form of Rh1 is completely insensitive to treatment with either enzyme (Figure 3C). These results suggest that mature Rh1 protein is completely void of carbohydrate. However, it is possible that mature Rh1 is highly trimmed, but retains a small amount of carbohydrate that is not resolved by SDS-PAGE. To determine whether we are able to detect single oligosaccharide differences via Western blot analysis, we took advantage of Rh1's sensitivity to both Endo H and PNGase F in the α-man-II mutants. Endo H cleaves between the first and second N-acetyl glucosamine (GlcNAc) residues, N1 and N2, leaving N1 attached to the protein (Figure 1). In contrast, PNGase F cleaves between N1 and the asparagine residue on Rh1, thus removing the final GlcNAc residue (Figure 1). Therefore, following PNGase F digestion, no carbohydrate remains. We compared the MW of Rh1 protein in α-man-II mutant tissue that had been treated with each enzyme. Here, we show that when α-man-II mutant fly extracts were treated with Endo H, the Rh1 protein was slightly larger than when treated with PNGase F (Figure 3D). These results demonstrate that we can detect the presence or absence of a single GlcNAc residue. Further, the MW of mature Rh1 protein in wild-type flies is indistinguishable from the Rh1 protein in the PNGase F-treated sample (Figure 3D). These results support the concept that the final GlcNAc residue has been removed from mature Rh1 and are consistent with the hypothesis that Rh1 is fully deglycosylated during biosynthesis.
The results presented here demonstrate that Rh1 deglycosylation is a highly complex process involving defined and sequential trimming steps, carried out by a cascade of glycosyl hydrolase enzymes. In addition to α-Man-II, α-Man-IIb, fused lobes, and Hexo1, we have identified numerous glycosyl hydrolases in Drosophila with potential functions in N-glycosylation. The Drosophila glycosyl hydrolases that we identified displayed a high degree of amino acid identity with corresponding enzymes in humans, allowing us to place them into well-defined families and subfamilies (Figures 2 and S2, S3, S4, S5, S6, S7). Therefore, in addition to our characterization of α-Man-II, α-Man-IIb, fused lobes, and Hexo1, we provide an informatics-based analysis of the full complement of human and Drosophila glycosyl hydrolases involved in N-glycan processing (see Supporting Information and Figure 2).
Early steps in N-glycan processing
N-glycan processing begins with the sequential removal of three terminal glucose residues from branch A by α-glucosidase I and α-glucosidase II (Figures 1, 2 and 4) [48]. Following glucose removal, a family of ER and Golgi α1,2-mannosidases collectively work to cleave the four α1,2-linked mannose residues from the N-glycan structure to yield Man5GlcNAc2 (Figures 1 and 4) [49]. These enzymes belong to GH Family 47 and are also referred to as Class I α1,2-mannosidases (Figure 2) [50]. Following trimming to Man5GlcNAc2, the classical pathway for oligosaccharide maturation involves the action of GlcNAc-transferase I, which functions to add a single β1,2-linked N-acetylglucosamine (GlcNAc) to the terminal mannose (M4) on branch A (Figures 1, 2 and 4) [51], [52]. The presence of the terminal GlcNAc residue serves as a signal for additional trimming events mediated by a group of Class II α-mannosidases belonging to GH Family 38 (Figure 2) [35], [51], [53]. Class II α-mannosidases have complementary specificity compared to the Class I enzymes from GH Family 47, primarily cleaving α1,3 - and α1,6-linked mannose residues from the N-glycan structure [54], as opposed to α1,2-linked mannose residues (Figure 1). We have identified Drosophila homologs for each of the components in this pathway (Figures 2 and 4), and provide detailed amino acid (aa) alignments and analyses in Supporting Figures S2–S5.
Fig. 4. Pathway for Rh1 oligosaccharide trimming. 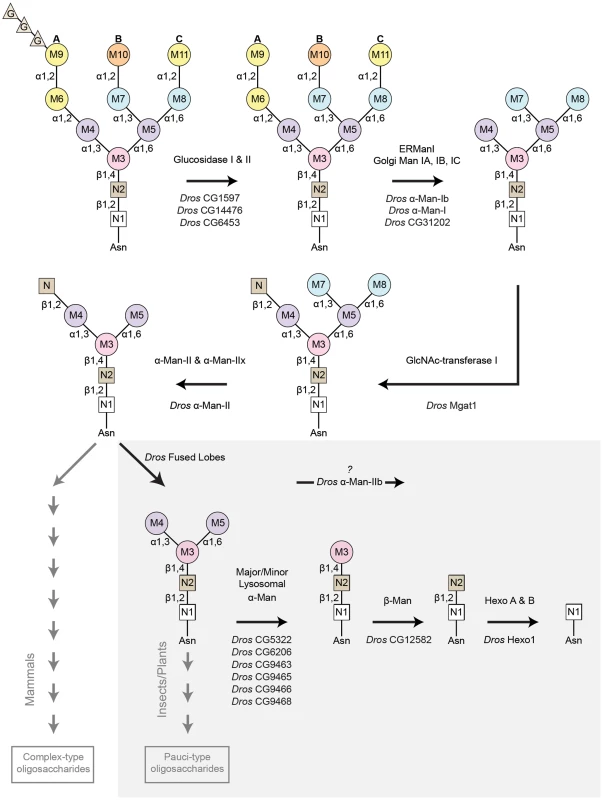
Rh1 deglycosylation is a highly coordinated pathway that requires the sequential actions of a large number of glycosyl hydrolase (GH) enzymes and families. Here, we illustrate the major steps that are thought to occur during Rh1 deglycosylation, beginning with the precursor oligosaccharide, Glc3Man9GlcNAc2. The enzymes responsible for equivalent hydrolysis reactions in mammals are shown above each arrow. The Drosophila enzymes predicted to perform these steps during Rh1 maturation are indicated below each arrow. Of particular note, the step mediated by Drosophila fused lobes is unique to insects and plants. Removal of this terminal GlcNAc residue has not been shown to occur in mammals. This explains why elongation of human glycoproteins leads to the formation of complex-type oligosaccharides, whereas elongation of Drosophila glycoproteins leads to the formation of pauci-mannosidic structures. Importantly, the subsequent steps contained within the grey box represent a novel pathway for Rh1 maturation. That is, the mammalian enzymes responsible for these cleavage events have only been characterized in the context of carbohydrate catabolism in the lysosome, but have no known roles in glycoprotein processing. Here, we propose that the corresponding enzymes in Drosophila play a critical role in Rh1 deglycosylation during biosynthesis, leading to the complete deglycosylation of Rh1. G (glucose), M (mannose), N (β-N-acetylglucosamine, GlcNAc), Man (mannosidase), Hexo (hexosaminidase). Drosophila α-Man-II functions upstream from α-Man-IIb for Rh1 maturation
The presence of the terminal GlcNAc residue, added by GlcNAc-transferase I, serves as a signal for additional trimming events mediated by a group of Class II α-mannosidases belonging to GH Family 38 (Figure 4) [35], [51], [53]. The first trimming events mediated by the Class II enzymes involve the sequential removal of two mannose residues, M7 and M8 (Figure 1), from the GlcNAc1Man5GlcNAc2 structure [35]. Removal of these residues represents the “committed” step in N-glycan processing and is responsible for the conversion from high-mannose, Endo H-sensitive structures, to complex-type mannose structures that are insensitive to Endo H [3], [55]. This step occurs in the Golgi and is mediated by a pair of isozymes from GH Family 38 (Subgroup A) termed α-mannosidase II (α-Man II) and α-mannosidase IIx (α-Man IIx) in mammals [56]. These enzymes are encoded by MAN2A1 [57] and MAN2A2 [58] in humans, respectively (Figure 2). We have identified two GH Family 38 members in Drosophila from Subgroup A, namely α-mannosidase-II (α-Man-II, CG18802) and α-mannosidase-IIb (α-Man-IIb, CG4606) (Figure 2).
The evolutionary history between the human and Drosophila enzymes may yield insight into their potential functions. The mammalian enzymes, α-Man-II and α-Man-IIx, arose from a fairly recent gene duplication event (Figure S5A) and are isozymes, with identical substrate specificities. Therefore, these enzymes have yet to undergo functional specialization. They have, however, developed tissue-specific expression patterns [57], [59]. Based on aa identity, Drosophila α-Man-II appears to represent the ortholog of these isozymes (Figure S5A). Drosophila α-Man-II displays 40% overall aa identity with both human Man II and Man IIx, and 56–57% aa identity within the GH Family 38 N-terminal catalytic domain (Figure S5B). By comparison, Drosophila α-Man-IIb is significantly more divergent (Figure S5A), displaying only 33–34% overall aa identity with the human enzymes and only 32–46% aa identity within the GH Family 38 N-terminal catalytic domain (Figure S5B). It is therefore possible that Drosophila α-Man-IIb has evolved a specialized function, unique from the other three mannosidases. To investigate this possibility and to further understand the role of Drosophila α-Man-II and α-Man-IIb in Rh1 biosynthesis, we assessed defects in Rh1 maturation in mutant alleles corresponding to these loci.
Mutations in Drosophila α-man-II are homozygous lethal. Therefore, we employed a variety of techniques to obtain viable mutant alleles. First, we generated mosaic clones of a homozygous lethal P-element allele, α-man-lILL01094, in the eye, using a FLP/FRT recombination method [60]. Second, we utilized tissue-targeted RNA interference (RNAi) to reduce α-man-lI transcript levels, specifically in the eye, using the α-man-lIv5838 UAS-RNAi construct and an eye-specific GAL4 driver line [61]. Finally, we obtained two hypomorphic P-element alleles, α-man-lIG4901 and α-man-lId06422, whose insertional sites lie in non-coding regions rendering them homozygous viable. All four alleles are depicted in Figure 5A. Mutations in α-man-II lead to defects in the maturation of Rh1 protein, as indicated by the accumulation of a high MW form of Rh1 in all four mutant alleles (Figure 5C, left). The mosaic allele (α-man-lILL01094) and RNAi allele (α-man-lIv5838) are strong alleles as Rh1 is present exclusively in a high MW form, with no mature Rh1 detected (Figure 5C, left). In contrast, some Rh1 protein is present in the mature, wild-type form in the hypomorphic P-element mutants (α-man-lIG4901 and α-man-lId06422) (Figure 5C, left). These results indicate that α-Man-II is required for proper deglycosylation of Rh1.
Fig. 5. Drosophila α-Man-II functions upstream from α-Man-IIb during Rh1 deglycosylation. 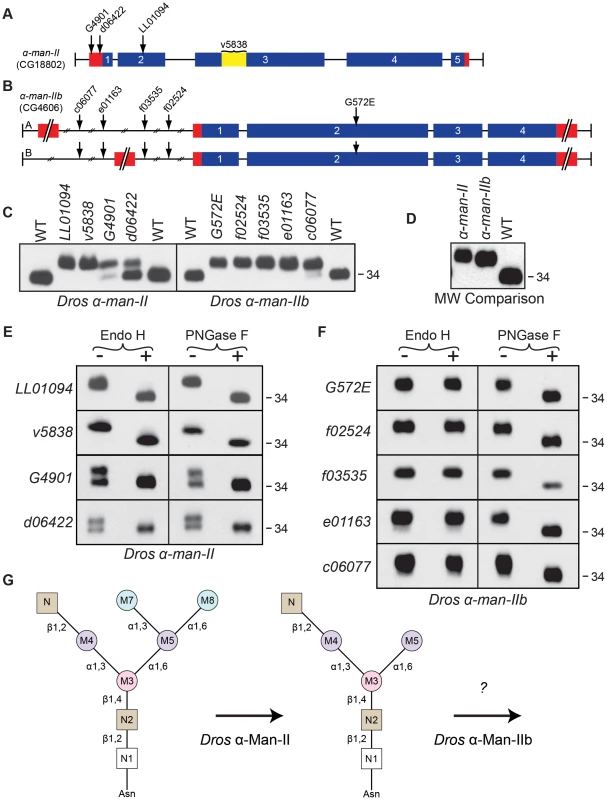
Intron/Exon structures for (A) α-mannosidase-II (α-man-II, CG18802) and (B) α-mannosidase-IIb (α-man-IIb, CG4606) are shown to indicate the alleles used in this study: P[EP]α-man-IIG4901, P[XP]α-man-IId06422, Pbac[SAstopDsRed]α-man-IILL01094, P[GD2875]v5838, Pbac[PB]α-man-IIbc06077, Pbac[RB]α-man-IIbe01163, Pbac[WH]α-man-IIbf03535, Pbac[WH]α-man-IIbf02524, and α-man-IIbG572E. Blue = coding sequence, red = additional mRNA, yellow = RNAi target. (C) Western blots of Rh1 protein from α-man-II (Left) and α-man-IIb (Right) mutant flies. Left Lanes: (1) Wild-type (WT), (2) α-man-IILL01094, (3) α-man-IIv5838, (4) α-man-IIG4901, (5) α-man-IId06422, and (6) WT. Right Lanes: (1) WT, (2) α-man-IIbG572E, (3) α-man-IIbf02524, (4) α-man-IIbf03535, (5) α-man-IIbe01163, (6) α-man-IIbc06077, and (7) WT. One head was loaded per lane, with the exception of lane 3 on the left side (α-man-IIv5838), in which 4 heads were loaded. (D) Western blot, comparing the molecular weight of Rh1 in the α-man-IILL01094 and α-man-IIbf02524 mutants, indicating that Rh1 is slightly larger in α-man-II mutants. One half of a head was loaded per lane. (E and F) Western blot of Rh1 protein from the α-man-II and α-man-IIb alleles described in (C), treated (+) with either Endo H or PNGase F enzyme and labeled for Rh1. (G) Proposed role for Drosophila α-Man-II in trimming the M7 and M8 mannose residues (blue) during Rh1 biosynthesis. Our data indicate that Drosophila α-Man-IIb functions downstream at a step that is distinct from α-Man-II during Rh1 deglycosylation. For the α-man-IIb locus, we obtained a single EMS-generated allele, α-man-IIbG572E, and four P-element alleles, α-man-IIbf02524, α-man-IIbf03535, α-man-IIbe01163, and α-man-IIbc06077, all of which are depicted in Figure 5B. As with α-man-II, mutations in α-man-IIb lead to defects in Rh1 maturation, as indicated by the accumulation of a high MW form of Rh1 in all five mutant alleles (Figure 5C, right). In the strongest alleles, Rh1 is present exclusively in a high MW form, whereas in others a small amount of Rh1 is detected in the wild-type form (Figure 5C, right), indicating that these weaker mutations are hypomorphic. Indeed, certain alleles are temperature-sensitive and these data are shown in Figure S1. These results indicate that α-Man-IIb is also required for proper deglycosylation of Rh1.
The phenotypes observed in the strongest alleles of both α-man-II and α-man-IIb show that Rh1 is present exclusively in a high MW form, with no mature Rh1 detected (Figure 5C). These results suggest that, unlike the human α-Man II and α-Man IIx isozymes, Drosophila α-Man-II and α-Man-IIb do not perform redundant functions, but rather are uniquely required for distinct steps in N-glycan processing during Rh1 maturation. The lethality of α-man-II mutants also supports this hypothesis. Still additional support for their independent roles during Rh1 biosynthesis comes from a close comparison in the MW of Rh1 detected in α-man-II versus α-man-IIb mutants. Specifically, the high MW form of Rh1 in α-man-II mutants is slightly larger than the high MW form observed in α-man-IIb mutants (Figure 5D) [11], indicating that further trimming has occurred in α-man-IIb mutants. These data support the hypothesis that Drosophila α-Man-II functions upstream from α-Man-IIb for carbohydrate trimming during Rh1 biosynthesis.
The hypothesis that α-Man-II functions upstream from α-Man-IIb is further supported by the results of Endo H and PNGase F digestions on the mutant tissues. In all four alleles of α-man-II and all five alleles of α-man-IIb, the high MW form of Rh1 was sensitive to digestion with PNGase F, demonstrating that the abnormal, high MW Rh1 protein was indeed hyperglycosylated (Figures 5E and 5F). The α-man-II mutants were also sensitive to digestion with Endo H (Figure 5E), indicating that the M7 and M8 mannose residues had not yet been removed from the N-glycan. These findings are consistent with the predicted role for α-Man-II in the removal of M7 and M8. This is also the function of the mammalian isozymes, α-Man II and α-Man IIx [56]. In contrast, the α-man-IIb mutants were insensitive to treatment with Endo H (Figure 5F), indicating that the M7 and M8 residues had already been removed and that Rh1 had progressed in the Golgi past this step. Taken together, these data strongly support the notion that α-Man-IIb functions downstream from α-Man-II, and downstream from M7 and M8 removal, for Rh1 deglycosylation during biosynthesis. Again, the finding that Rh1 is detected exclusively in a high MW form in strong alleles of α-man-II and α-man-IIb indicates that, not only do they function at unique steps during Rh1 deglycosylation, but also that there is no functional redundancy between the two Drosophila enzymes. These results are consistent with the highly divergent amino acid sequence of Drosophila α-Man-IIb, compared with the other members of GH Family 38, Subgroup A (Figure S5). Although our results clearly indicate a role for α-Man-IIb in Rh1 deglycosylation, downstream from α-Man-II and removal of M7 and M8, the specific role of this enzyme in trimming is not yet known (Figure 5G). These data are consistent with a possible role for α-Man-IIb in trimming M5.
Drosophila fused lobes is essential for Rh1 deglycosylation
Following removal of the M7 and M8 residues, the N-glycan trimannosyl core is built upon to form a variety of N-glycan structures. In mammals, the N-glycan typically retains the terminal GlcNAc residue (attached to M4) and is additionally modified with fucosyl, galatosyl, and/or sialyl residues to form complex-type N-glycan structures in the Golgi (Figure 4) [62], [63]. In contrast, insect cells hydrolyze this terminal GlcNAc by the action of a β-N-acetylhexosaminidase, leading to the generation of paucimannosidic N-glycan structures (Figure 4) [2], [64]. The hexosaminidase responsible for this step in Drosophila, termed fused lobes (CG8824), is a member of GH Family 20 [10]. This gene was originally named due to the mushroom body fused lobes phenotype in the corresponding mutant [65]. Interestingly, mammalian β-hexosamindases from GH Family 20 are generally present as soluble proteins in the lysosome and have well-characterized roles in the catabolism and recycling of sugar residues. Unique among the β-hexosaminidases, Drosophila fused lobes is a membrane-associated protein that has been implicated as a processing enzyme responsible for removing the terminal GlcNAc residue attached to the M4 residue on nascent glycoproteins (Figure 4). This event was proposed to be a routine step during glycoprotein maturation in the secretory pathway [10].
To investigate the role of fused lobes in Rh1 deglycosylation, we obtained a single P-element allele, fdld03111, as well as two RNAi alleles, fdlv4637 and fdlv4638, all of which are shown in Figure 6A. In all three fused lobes mutants, Rh1 was detected in an abnormal high MW form (Figure 6B). These results indicate that fused lobes is required for the proper maturation of Rh1 during biosynthesis. The high MW form of Rh1 detected in fused lobes mutants was sensitive to digestion with PNGase F, indicating that the protein was hyperglycosylated (Figure 6C). In contrast, Rh1 was insensitive to treatment with Endo H in the fused lobes mutants, indicating that M7 and M8 had been removed from the N-glycan in the Golgi (Figure 6C). These results are consistent with the predicted role of fused lobes, downstream from α-Man-II (and removal of M7 and M8), in the Golgi during N-glycan trimming.
Fig. 6. Drosophila Fdl is essential for Rh1 deglycosylation. 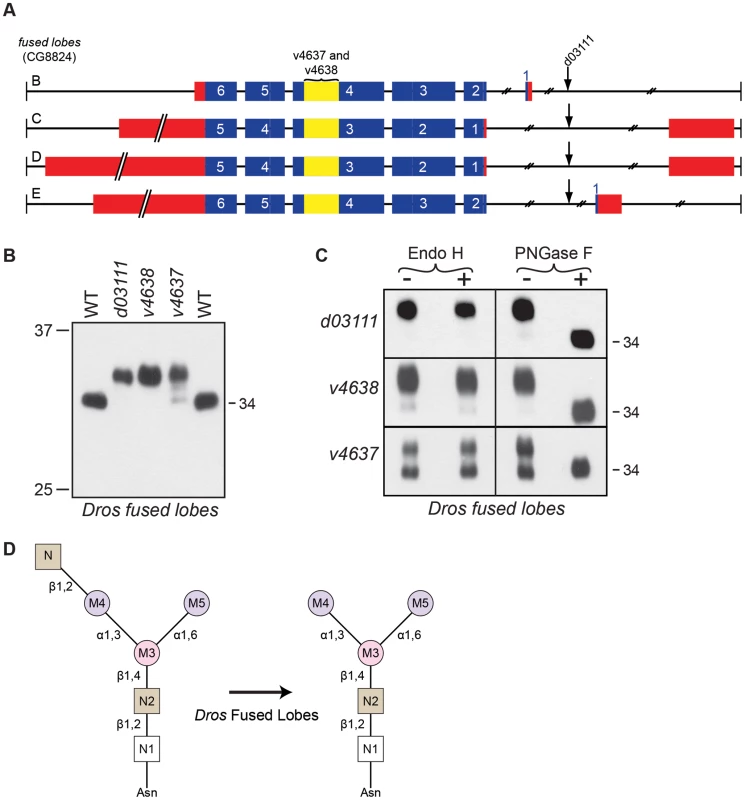
(A) Intron/Exon structure for fused lobes (fdl, CG8824), indicating the alleles used in this study: P[GD1956]v4637, P[GD1956]v4638, and P[XP]fdld03111. Blue = coding sequence, red = additional mRNA, yellow = RNAi target. (B) Western blot of Rh1 protein from fused lobes mutants. Lanes: (1) Wild-type (WT), (2) fdld03111, (3) fdlv4638, (4) fdlv4637, and (5) WT. One half of a head was loaded in Lanes 1, 2, and 5, whereas 4 heads were loaded in Lanes 3 and 4. (C) Western blot of Rh1 protein from the fused lobes alleles described in (B), treated (+) with either Endo H or PNGase F enzyme. (D) Proposed role for Drosophila fused lobes in trimming the terminal GlcNAc residues attached to M4 during Rh1 biosynthesis. Surprisingly, the MW of Rh1 in fused lobes mutants was larger than the MW observed in α-man-II mutants (Figure 3B). Given that fused lobes functions downstream from α-Man-II, these data suggest that the N-glycan not only retains its terminal GlcNAc residue in the absence of fused lobes, but must also undergo some form of elongation. Elongation or elaboration of the N-glycan, triggered by the abnormal retention of GlcNAc, would account for the larger size of Rh1 in fused lobes mutants. Our data are consistent with previous findings that mutations in Drosophila fused lobes lead to the abnormal generation of hybrid - and complex-type glycans that are more typically seen in vertebrates [10]. Therefore, mutations in fused lobes may promote the formation of a complex N-glycan structure on Rh1.
Trimming the trimannosyl core during Rh1 deglycosylation
Whether the terminal GlcNAc residue is removed (as it is in plants and insects) or retained (as it is in mammals), the subsequent stages in N-glycosylation almost always involve elongation and elaboration of the N-glycan structure by the addition of diverse sugar moieties (Figure 4). In stark contrast, the N-glycan on Drosophila Rh1 is further trimmed during biosynthesis (Figure 3A) [36]–[39]. Indeed, our data are consistent with the hypothesis that the mature form of Rh1 is completely void of carbohydrate (Figures 3C and 3D). Step-wise reduction of the remaining trimannosyl core would theoretically involve removal of the residual α-linked mannose residues, M4 and M5, the β-linked mannose, M3, and the two GlcNAc residues at the base of the N-glycan structure, N1 and N2 (Figure 1). Glycosyl hydrolase enzymes capable of performing these steps have been identified, but they are generally present as soluble proteins in the lysosome.
Specifically, there are two lysosomal α-mannosidases from GH Family 38 Subgroup B: the major lysosomal α-mannosidase (MAN2B1) [66]–[68] and the minor lysosomal α-mannosidase (MAN2B2) (Figure 2) [69]–[71]. These enzymes are required for removal of M4 and M5, respectively, during catabolism of the N-glycan (Figure 1). There is also a lysosomal β-mannosidase from GH Family 2 (Figure 2) that is involved in the removal of the β-linked M3 residue from N-glycan structures during catabolism (Figure 1) [31], [72], [73]. Finally, at the very base of the N-glycan structure lie two GlcNAc residues, N1 and N2 (Figure 1). In mammals, the bond between these residues is cleaved by a pair of β-hexosaminidase enzymes from GH Family 20, Hex A and Hex B [32], [74]–[76]. Again, this particular reduction occurs in the context of N-glycan catabolism and the recycling of sugar residues in the lysosome. We have identified Drosophila homologs for each of the components in this catabolic pathway (Figures 2 and 4), and provide detailed amino acid (aa) alignments and analyses in Supporting Figures S5–S7.
The finding that none of these lysosomal enzymes had predicted functions in the biosynthetic processing of glycoproteins presented a conundrum for the case of Rh1 deglycosylation, given that the trimannosyl core structure is removed during Rh1 maturation. One possible explanation is that the residual trimannosyl core is not removed in a step-wise fashion by exo-acting enzymes, but rather an endo-acting hydrolase removes the remaining structure from Rh1 in bulk. If this were the case, the lysosomal enzymes would not be required for Rh1 deglycosylation. Another possible explanation is that some of the enzymes previously characterized as ‘lysosomal’, have in fact evolved functions in glycoprotein processing and are involved in the step-wise deglycosylation of Rh1. Indeed, non-traditional roles have been assigned to a number of enzymes formerly thought to function solely in the lysosome. For example, a number of ‘lysosomal’ enzymes have been shown to localize to the sperm plasma membrane and facilitate sperm-egg interaction during fertilization [77]. Here, we present evidence that one such enzyme in Drosophila, Hexo1, plays a role in Rh1 biosynthesis in the secretory pathway. Our results lend support to the hypothesis that, indeed, Rh1 is deglycosylated, residue by residue, until the protein is completely void of carbohydrate.
Drosophila Hexo1 is uniquely required for Rh1 deglycosylation
In humans, there are two loci encoding β-N-acetylhexosaminidase enzymes from GH Family 20, termed HEXA and HEXB (Figure 2). The Hex A enzyme is a heterodimer (αβ) containing one α-subunit encoded by the HEXA locus [75] and one β-subunit encoded by the HEXB locus [76]. In contrast, the Hex B enzyme is a homodimer of β-subunits (ββ), and thus only requires the HEXB gene [32]. These enzymes have been extensively investigated, due to their known roles in Sandhoff and Tay-Sachs disease, and function to remove terminal GlcNAc or GalNAc residues from a variety of carbohydrate and glycolipid structures. We have identified three hexosaminidases belonging to GH Family 20 in Drosophila, namely hexosaminidase 1 (Hexo1, CG1318), hexosaminidase 2 (Hexo2, CG1787), and a third hexosaminidase characterized above, fused lobes (Fdl, CG8824) (Figure 2). Although β-hexosamindases from GH Family 20 are generally thought of as lysosomal enzymes, involved in the recycling of sugar residues, numerous non-traditional roles have been identified for these ‘catabolic’ enzymes. Both the human and Drosophila hexosaminidases have been identified on the plasma membrane of various cell types [47], [78] and, of particular interest, human Hex B has been shown to localize to the sperm acrosome, which is derived from the Golgi apparatus [79]. Given these uncharacteristic qualities, and the known role of fused lobes in glycoprotein processing, we were interested in the potential role of Drosophila Hexo1 and Hexo2 in cleaving the final GlcNAc residues on Rh1 in the secretory pathway.
For the hexo1 locus, we identified an EMS-generated allele, hexo1Q592X, as well as a P-element allele, hexo1e00001, both of which are depicted in Figure 7A. Mutations in hexo1 lead to defects in Rh1 deglycosylation, as indicated by the accumulation of Rh1 in a slightly higher MW form in both hexo1 mutants (Figure 7B). The very subtle difference in size between the high MW form of Rh1 observed in hexo1 mutants and the wild-type form of Rh1 is consistent with a defect in removing a GlcNAc residue at the very end of the deglycosylation cascade. This high MW form of Rh1 is sensitive to digestion with PNGase F, but insensitive to digestion with Endo H (Figure 7C). These results indicate that the hyperglycosylated Rh1 protein detected in hexo1 mutants has already been processed by the Class II α-mannosidases in the Golgi. Taken together, these results are consistent with a role for Hexo1 in the removal of the N2 GlcNAc residue from the base of the N-glycan during Rh1 biosynthesis (Figure 7D).
Fig. 7. Drosophila Hexo1 is essential for Rh1 deglycosylation. 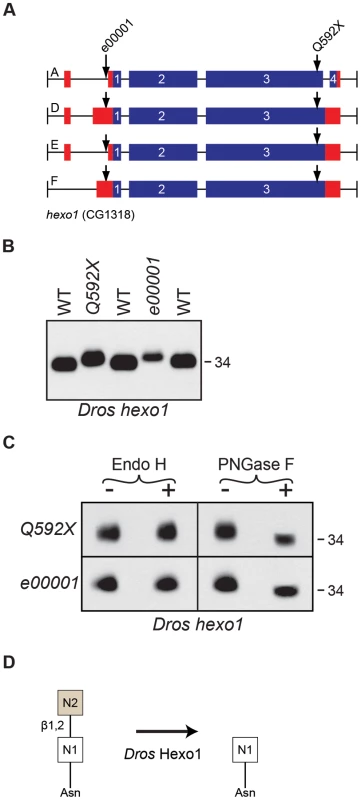
(A) Intron/Exon structures for hexosaminidase1 (hexo1, CG1318), indicating the alleles used in this study: Pbac[RB]hexo1e00001 and hexo1Q592X. Blue = coding sequence, red = additional mRNA. (B) Western blot of Rh1 protein from hexo1 mutant flies. Lanes: (1) WT, (2), hexo1Q592X/Df(3L)ED4341, (3) hexo1e00001, and (4) WT. Deficiency Df(3L)ED4341 deletes cytological region 63F6-64B9 and thus fails to complement the hexo1 locus, which lies at 64A12. 2 heads were loaded per lane. (C) Western blot of Rh1 protein from the hexo1 alleles described in (B), treated (+) with either Endo H or PNGase F enzyme. (D) Proposed role for Drosophila Hexo1 in trimming the N2 GlcNAc residue from the N-glycan during Rh1 biosynthesis. Analysis of two hexo2 P-element alleles, hexo2f04129 and hexo2d06829, did not reveal defects in the MW of Rh1 protein (data not shown). This does not rule out a potential role for Hexo2 in Rh1 deglycosylation, as there could be other enzymes with redundant functions. However, the finding that Rh1 is present exclusively in a high MW form in both the fused lobes (Figure 6B) and hexo1 (Figure 7B) mutants, and that these two MWs are clearly distinct from one another (Figure 3B), indicates that both fused lobes and Hexo1 are independently required for unique steps in Rh1 deglycosylation.
Summary: Rh1 is deglycosylated in a step-wise fashion
Here, we demonstrate that α-Man-II, α-Man-IIb, fused lobes, and Hexo1, all play essential and unique roles during Rh1 maturation. Although three of these enzymes have known roles in N-glycoprotein processing, Hexo1 has not been previously shown to be involved in protein biosynthesis. Our results demonstrate that Hexo1 plays a critical role during Rh1 deglycosylation in the secretory pathway and provide direct evidence that the N-linked oligosaccharide on Rh1 is removed in a step-wise fashion, down to the final GlcNAc residue (Figure 4). Our findings, demonstrating step-wise trimming of the final trimannosyl core residues during Rh1 biosynthesis reveal exciting new possibilities for the complex functions of glycosyl hydrolases in the secretory pathway.
Discussion
It has been known for over 25 years that Rh1 is deglycosylated during biosynthesis [36], however much of the deglycosylation pathway for Rh1 has remained unknown. In this same timeframe, an enormous body of research has led to the identification and characterization of a wide variety of glycosyl hydrolase enzymes involved in N-glycan processing. These studies have focused predominantly on the biochemical characterization of mammalian glycosyl hydrolases in cell culture. Drosophila remains a highly effective genetic model for the dissection of biological pathways and has the potential to yield enormous insight into the functions of N-glycans in vivo. To date, a comprehensive informatics comparison between human and Drosophila glycosyl hydrolases involved in N-glycan processing has not been presented. Here, we present a framework for such a comparison between the human and Drosophila glycosyl hydrolase families (Figures 2 and S2–S7) and describe a role for Drosophila glycosyl hydrolases in the sequential trimming of the N-glycan during Rh1 biosynthesis. We characterize mutations in four critical glycosyl hydrolase enzymes, α-Man-II, α-Man-IIb, fused lobes, and Hexo1, and unveil a highly coordinated pathway for Rh1 deglycosylation in Drosophila photoreceptor cells (Figure 4).
Whereas α-Man-II, α-Man-IIb, and fused lobes have previously been implicated in N-glycoprotein processing, Hexo1 has not, and therefore our findings on the role of Hexo1 in the secretory pathway are particularly intriguing. Although hexosaminidases are typically thought of as lysosomal enzymes involved in N-glycan catabolism, both human and Drosophila hexosaminidases have been previously identified outside of the lysosome. For example, Hex A has been identified on the plasma membrane of human fibroblasts [80] and Hex B has been shown to localize to the sperm acrosome, which is derived from the Golgi apparatus [79]. All three Drosophila hexosaminidases have been identified on the sperm plasma membrane and are thought to mediate egg-sperm binding by forming complexes with oligosaccharide substrates present on the egg zona pellucida (ZP) [47], [78]. Interestingly, it has been suggested that the family of β-hexosaminidases may be posttranslationally and differentially processed in different tissue types, resulting in either a membrane-bound form of the enzyme (typically on the plasma membrane) or a soluble form that is either localized to the cytosol of the lysosome or secreted from the cell [47]. Given this apparent flexibility in cellular localization, and the non-traditional roles that these ‘lysosomal’ enzymes have already been shown to play, a role for Hexo1 during Rh1 maturation in the secretory pathway is not unprecedented.
The predicted evolutionary relationship between the human and Drosophila genes from GH Family 20 provides further insight into the potentially broad functions of hexosaminidases. Based on amino acid analysis, the phylogenetic tree shown in Figure S6A predicts a single hexosaminidase in the last common ancestor between humans and Drosophila. Therefore, the human gene pair and the Drosophila gene triplet have evolved separately, through independent duplication events. As is always the case, gene duplication provides the evolutionary potential for functional specialization and it is very likely that the Drosophila loci have evolved unique and/or additional functions compared to the human loci. This has already been shown for the fused lobes hexosaminidase from this gene family. Finally, as with the HEXA and HEXB loci in humans, the three Drosophila loci from GH Family 20 (fdl, hexo1, and hexo2) are thought to encode subunits that can pair in unique combinations to form dimeric hexosaminidase enzymes [78]. For example, two dimeric hexosaminidase enzymes have been identified on the sperm plasma membrane in Drosophila, including HEXA (which consists of one fused lobes subunit and one Hexo2 subunit) and HEXB (which consists of one Hexo1 subunit and one Hexo2 subunit) [47]. Factoring in homo-dimeric combinations, the human gene pair can yield only three hexosaminidase enzymes. In contrast, the three Drosophila loci can encode a total of six unique enzymes. Again, this allows significantly greater freedom for functional specialization of the various enzymes and makes a role for Hexo1 and Fused Lobes in glycoprotein biosynthesis entirely feasible.
Our results, demonstrating that Hexo1 plays a critical role for carbohydrate trimming during Rh1 biosynthesis in the secretory pathway, reveal intriguing new possibilities for the complex roles of glycosyl hydrolases traditionally thought to function as catabolic enzymes in the lysosome. For example, it is possible that the two α-linked mannose residues, M4 and M5, are removed by one (or more) of the six Drosophila lysosomal α-mannosidases from GH Family 38 Subgroup B (Figures 2 and S4). The pathway for Rh1 deglycosylation would then require the removal of a β-linked mannose, M3, which points to a role for the Drosophila lysosomal β-mannosidase from GH Family 20 (Figures 2 and S6). Given the requirement by Rh1 for these trimming events, which should occur upstream of Hexo1, we hypothesize that, like Hexo1, these ‘catabolic’ enzymes may play roles as processing enzymes during glycoprotein biosynthesis.
Interestingly, the six lysosomal α-mannosidases in Drosophila display significant amino acid identity with the major lysosomal α-mannosidase in humans, MAN2B1 (Figures S5A and S5C), having relatively no similarity to the minor lysosomal α-mannosidase in humans, MAN2B2. Importantly, the major lysosomal α-mannosidase has high specificity for cleavage of M4, whereas the minor lysosomal α-mannosidase preferentially cleaves M5 (Figure 1). The complementary actions of these two enzymes, along with their ubiquitous expression, suggest that they function in tandem for N-glycan degradation in mammals. These findings indicate that, if they play a role in Rh1 deglycosylation, the Drosophila lysosomal α-mannosidases likely function to cleave M4. Given the absence of a direct Drosophila homolog for the mammalian minor lysosomal α-mannosidase (Figure 2), it is difficult to predict which glycosyl hydrolase functions to cleave M5.
Just as there is uncertainty regarding the identity of the enzyme responsible for trimming M5 from the trimannosyl core, there is also uncertainty regarding the role of Drosophila α-Man-IIb in the pathway for Rh1 deglycosylation. In this study, we demonstrate that α-Man-II and α-Man-IIb are both required at distinct steps during Rh1 maturation and that α-Man-IIb functions downstream from α-Man-II (Figure 5). It has previously been shown that α-Man-II functions for the sequential removal of two mannose residues, M7 and M8, during N-glycan processing (Figure 5) [11]. In contrast, the role of α-Man-IIb is currently unknown. Interestingly, Drosophila α-Man-II, α-Man-IIb, and the six lysosomal α-mannosidases are all Class II α-mannosidases from GH Family 38 (Figure 2). Accordingly, these enzymes all have the capacity to remove α1,3 - and α1,6-linked mannose residues from the N-glycan structure, including M4, M5, M7, and M8 (Figure 1). Given the predicted role of the lysosomal α-mannosidases in cleaving M4 and the known role of α-Man-II in cleaving M7 and M8, one possibility is that α-Man-IIb functions to remove M5. While this theoretical pairing addresses two unresolved issues in the pathway, there is currently no direct evidence to support a role for α-Man-IIb in trimming M5.
In recent decades, there has been considerable interest in the fundamental nature of insect glycoprotein processing pathways due to the widespread use of the baculovirus-insect cell expression system [81]. This system is used to produce recombinant mammalian glycoproteins for a variety of different biomedical research applications. The insect system is advantageous, as it produces recombinant glycoproteins more readily than mammalian cell expression systems [82]. A major limitation is that insect-cell-produced glycoproteins have significantly different N-glycan structures than those produced by mammalian cells and are rarely of the complex-type [81], [82]. In part, this is due to the presence of the fused lobes β-N-acetylglucosaminidase, which removes a critical GlcNAc residue that serves as a building block for complex-type N-glycans (Figure 4). This event was proposed to be a routine step during glycoprotein maturation in the secretory pathway and, accordingly, some fused lobes expression was detected in the Golgi when expressed in yeast [10].
A Golgi hexosaminidase with a role in N-glycoprotein processing has also been identified in the lepidopteran insect, Spodoptera frugiperda (Sf21 and Sf9 cells) [64], [83]. This fused lobes ortholog, Sf-Fdl, has a slightly higher pH optimum of 6.0 [64], [83] compared to Drosophila fused lobes, which has a pH optimum of 5.5 [10]. However, Drosophila fused lobes has a range of activity through pH 6–7, and therefore fused lobes is active in the pH range typical of secretory pathway compartments, such as the Golgi [84]. When expressed in yeast, fused lobes was also detected on the plasma membrane and in multivesicular bodies [10]. Likewise, fused lobes was detected on the sperm plasma membrane [47]. Interestingly, there is currently no report of fused lobes expression in lysosomes.
Due to widespread interest in improving the baculovirus-insect cell expression system, identification of the fused lobes locus in Drosophila melanogaster [10] and the lepidopteran insect, Spodoptera frugiperda [83] was of great interest to a broad audience. Here, using Rh1 protein as a substrate, we confirm that Drosophila fused lobes is indeed required for glycoprotein biosynthesis in vivo. Consistent with a range of activity between pH 6–7 [10], our results demonstrate that fused lobes is capable of functioning in the secretory pathway during Rh1 maturation. These, and future, studies on the function of fused lobes will likely assist in the development of baculovirus-insect cell expression systems capable of producing more mammalian-like recombinant glycoproteins. For example, genetic elimination or inactivation of fused lobes in these cell expression systems might promote the formation of the desired complex carbohydrates on mammalian glycoproteins.
Here, we demonstrate that Drosophila Rh1 serves as an excellent reporter protein for studying N-glycosylation. In the present study, we have used the molecular weight of Rh1 protein, and its sensitivity to Endo H and PNGase F, in order to gain insights into the roles of the glycosyl hydrolases in the N-glycan trimming cascade. While our data are consistent with the hypothesis that the biochemical activities of these enzymes are the same as they are in vertebrates, precise identification of the glyco-intermediates will require mass spectrometry or nuclear magnetic resonance (NMR) analysis. When paired with the genetic and biochemical data, structural analyses of the oligosaccharides present on the various forms of immature Rh1 presented here will be highly useful for characterizing N-glycan structure-function relationships.
Studies involving mutations in N-glycan processing enzymes in intact model organisms provide a rich source of information on the physiological functions of these enzymes in vivo. Analysis of our collection of Drosophila mutants has uncovered unexpected roles for glycosyl hydrolases in Rh1 biosynthesis. Furthermore, given the many severe diseases that result from defects in N-glycosylation, including congenital disorders of glycosylation (CDG), α-mannosidosis, β-mannosidosis, Tay-Sachs disease, and Sandhoff disease, animal models will likely yield important insights into disease mechanisms. In the eye, glycosylation of rhodopsin has long been known to be key for photoreceptor health and function. Mutations that prevent the N-glycosylation of Drosophila rhodopsin cause photoreceptor defects and retinal degeneration [38], [85]–[87]. In humans, mutations at the two sites for N-linked glycosylation have been identified in patients with the hereditary retinal degeneration disorder, autosomal dominant retinitis pigmentosa (T4K, N15S and T17M) [88]–[90]. More recently, failures in oligosaccharide trimming during rhodopsin biosynthesis have also been shown to cause photoreceptor defects in Drosophila [11]. Consistent with these findings, the original mutants identified in this study originated from a deep pseudopupil (DPP) screen, indicating that they display photoreceptor defects and/or undergo retinal degeneration. Therefore, future studies of these glycosyl hydrolases will likely provide insights into mechanisms of hereditary retinal degeneration disorders, as well as other human diseases. While Rh1 deglycosylation appears to represent a highly unique phenomenon, it remains to be seen whether other glycoproteins are similarly processed and how broadly the ‘catabolic’ glycosyl hydrolases influence glycoprotein processing. Our study represents a critical step towards a genetic dissection of N-glycan processing in Drosophila, and yields important insights into the process of N-glycan trimming during Rh1 biosynthesis in the eye.
Materials and Methods
Drosophila strains
Drosophila melanogaster stocks were reared on standard media at 22°C, on a 12∶12 light∶dark cycle. The wild-type stocks used in this study were Canton S. and the parental strain from the EMS-mutagenesis, brown;scarlet (bw;st). To isolate the α-man-IIbG572E and hexo1Q592X mutants, we screened approximately 12,000 EMS (ethyl methyl sulfonate) mutagenized lines from the Zuker collection [41] for the presence or absence of the deep pseudopupil (DPP), as previously described [91]–[93]. Nine hundred DPP-defective stocks were identified and then further screened for defects in Rh1 deglycosylation by Western blot analysis. Deficiency stocks used for mapping the EMS-generated mutations were obtained from the Bloomington Drosophila Stock Center (Indiana University), as were the P[EP]α-Man-IIG4901 (Stock #30106), PBac[RB]Hexo1e00001 (Stock #17805), and Pbac[WH]Hexo2f04129 (Stock #18732) P-element alleles. The remaining P-element alleles used in this study were obtained from the Exelixis Collection (Harvard Medical School): P[XP]α-Man-IId06422, Pbac[WH]α-Man-IIbf02524, Pbac[WH]α-Man-IIbf03535, Pbac[RB]α-Man-IIbe01163, Pbac[PB]α-Man-IIbc06077, P[XP]fdld03111, and P[XP]Hexo2d06829. The following transgenic Drosophila strains, each containing an inducible UAS-RNAi construct directed against either the α-man-II or fused lobes transcripts, were obtained from the Vienna Drosophila RNAi Center (VDRC): P[GD2875]v5838, P[GD1956]v4637, and P[GD1956]v4638. Eye-specific knockdown of the target mRNA transcripts was achieved by performing standard genetic crosses between the UAS-RNAi strains and a Gal4 driver line provided by Claude Desplan: P[UAS-Dcr2];P[ey-Gal4],P[IGMR-Gal4];+. The Gal4 driver line contained both eyeless-Gal4 and lGMR-Gal4 drivers recombined on the second chromosome. Together, these two drivers induced RNAi expression in the whole eye from the time the eye is differentiated through adulthood [61]. The RNAi driver line also harbored a third construct on the X chromosome, UAS-Dicer2, to enhance the efficiency of generating small interfering RNA. Finally, mosaic mutants for α-man-II were generated with the P-element insertion line pBac[SAstopDsRed]α-man-IILL01094. Specifically, we used the FLP-FRT, P[GMR-hid] method using an eyeless-FLP driver to induce genetic mosaics and generate mitotic clones of a single genotype in the eye [60]. Both the α-man-II allele (DGRC Stock #140255) and the ey-FLP driver line (Bloomington Stock #5253) were generously provided by Junhai Han.
Deficiency mapping and DNA sequencing of EMS alleles
We performed deficiency mapping to narrow the cytogenetic locations of the two EMS-generated mutations to 89A5 (α-man-IIbG572E) and 64A12 (hexo1Q592X) on the third chromosome. To identify the mutant loci, we sequenced prioritized candidate genes within the corresponding deficiency regions. Genomic DNA was isolated from the EMS-mutagenized lines (α-man-IIbG572E and hexo1Q592X) and the wild-type parental line (bw;st) using the DNeasy Blood and Tissue Kit, according to the manufacturer's instructions (QIAGEN Inc., Valencia, CA). Primer pairs were designed for numerous loci in the deficiency regions, based on their GenBank sequence accession numbers. PCR-amplified DNA sequences were determined by the DNA Sequencing Facility at the University of Wisconsin Biotechnology Center and were aligned using the GAP v4.7 application (The X Window Systems, X11 1.1.3 – XFree86 4.4.0). In the first mutant, we identified a G to A substitution at nucleotide position 1715 in the coding region of CG4606. This mutation corresponds to a substitution at amino acid (aa) 572, from glycine (G) to glutamate (E) (G572E). CG4606 encodes a Class II α-mannosidase enzyme from GH Family 38, termed α-mannosidase-IIb (α-Man-IIb). In the second mutant, we identified a C to T substitution at nucleotide position 1774 in the coding region of CG1318. This mutation generates a premature stop codon (Och) at aa 592, in place of a glutamine (Q) residue (Q592X). CG1318 encodes a β-N-acetylglucosaminidase enzyme from GH Family 20, termed hexosaminidase 1 (Hexo1). Identification of the α-man-IIbG572E and hexo1Q592X mutants served as the springboard for a larger analysis of the enzymes involved in N-glycosylation.
Western blotting
Flies were reared at 22°C and then aged for 1 week at 29°C before head tissue was collected for Western blot analysis. We loaded enough tissue in each case to determine the MW of Rh1. Proteins were separated by electrophoresis in SDS polyacrylamide gels and electroblotted onto nitrocellulose membranes as previously described [39]. Rh1 protein was detected with a monoclonal antibody (4C5) obtained from the Developmental Studies Hybridoma Bank (University of Iowa) [36]. Rh1-bov protein (Figure 3A) was detected with a monoclonal antibody (1D4) directed to the bov epitope, obtained from Phyllis Robinson. The immunoreactive proteins were visualized using horseradish peroxidase (HRP)-conjugated goat anti-mouse (Invitrogen Corporation, Carlsbad, CA) followed by ECL detection (Amersham Pharmecia Biotech, Piscataway, NJ).
Biochemical procedures
All reagents for the Endo H and PNGase F digestions were obtained from New England BioLabs (Ipswich, MA). Between 20–40 fly heads were homogenized into denaturing buffer, sonicated, and processed according to a modification of the manufacturer's instructions. Specifically, we increased the denaturing buffer to a final concentration of 1% SDS and incubated for 4 hours at 22°C. All samples were mixed with appropriate volumes of sample buffer [94] and assessed via Western blot analysis.
Heat pulse-chase experiments
For heat pulse-chase experiments, we used transgenic flies expressing wild-type Drosophila Rh1 (ninaE) tagged with a 12 amino acid epitope from the C-terminus of bovine rhodopsin (Rh1-bov) [40]. The epitope tag adds ∼2 kD to Rh1, such that the immature forms are detected at 42 and 40 kD, and the mature form is detected at 36 kD. The tag does not affect Rh1 maturation or function [40]. We used transgenic flies expressing Rh1-bov under the control of either the Drosophila hsp70 heat-shock promoter, P[hs:Rh1-bov] [40] or under the control of the endogenous Rh1 promoter, P[ninaE:Rh1-bov] [40]. Wild-type flies expressing the P[hs:Rh1-bov] construct were exposed to a 1 hour heat shock at 37°C and then shifted back to room temperature and assayed at the indicated times, in hours. Wild-type and ninaA269 mutant flies expressing the P[ninaE:Rh1-bov] construct were shown to indicate the mature and immature forms of Rh1 under non-heat shock conditions.
Supporting Information
Zdroje
1. RothJ, ZuberC, ParkS, JangI, LeeY, et al. (2010) Protein N-glycosylation, protein folding, and protein quality control. Mol Cells 30 : 497–506.
2. KatohT, TiemeyerM (2013) The N's and O's of Drosophila glycoprotein glycobiology. Glycoconj J 30 : 57–66.
3. KornfeldR, KornfeldS (1985) Assembly of asparagine linked oligosaccharides. Annu Rev Biochem 54 : 631–664.
4. SilbersteinS, GilmoreR (1996) Biochemistry, molecular biology, and genetics of the oligosaccharyltransferase. FASEB J 10 : 849–858.
5. Stanley P (2011) Golgi glycosylation. Cold Spring Harb Perspect Biol 3: : pii: a005199. doi: 10.1101/cshperspect.a005199.
6. CarameloJJ, ParodiAJ (2007) How sugars convey information on protein conformation in the endoplasmic reticulum. Semin Cell Dev Biol 18 : 732–742.
7. KerscherS, AlbertS, WucherpfennigD, HeisenbergM, SchneuwlyS (1995) Molecular and genetic analysis of the Drosophila mas-1 (mannosidase-1) gene which encodes a glycoprotein processing alpha 1,2-mannosidase. Dev Biol 168 : 613–626.
8. ParkerGF, WilliamsPJ, ButtersTD, RobertsDB (1991) Detection of the lipid-linked precursor oligosaccharide of N-linked protein glycosylation in Drosophila melanogaster. FEBS Lett 290 : 58–60.
9. WilliamsPJ, WormaldMR, DwekRA, RademacherTW, ParkerGF, et al. (1991) Characterisation of oligosaccharides from Drosophila melanogaster glycoproteins. Biochim Biophys Acta 1075 : 146–153.
10. LeonardR, RendicD, RabouilleC, WilsonIB, PreatT, et al. (2006) The Drosophila fused lobes gene encodes an N-acetylglucosaminidase involved in N-glycan processing. J Biol Chem 281 : 4867–4875.
11. CaoJ, LiY, XiaW, ReddigK, HuW, et al. (2011) A Drosophila metallophosphoesterase mediates deglycosylation of rhodopsin. EMBO J 30 : 3701–3713.
12. EnglebienneP, FiauxH, KuntzDA, CorbeilCR, Gerber-LemaireS, et al. (2007) Evaluation of docking programs for predicting binding of Golgi alpha-mannosidase II inhibitors: a comparison with crystallography. Proteins 69 : 160–176.
13. FiauxH, KuntzDA, HoffmanD, JanzerRC, Gerber-LemaireS, et al. (2008) Functionalized pyrrolidine inhibitors of human type II alpha-mannosidases as anti-cancer agents: optimizing the fit to the active site. Bioorg Med Chem 16 : 7337–7346.
14. KawatkarSP, KuntzDA, WoodsRJ, RoseDR, BoonsGJ (2006) Structural basis of the inhibition of Golgi alpha-mannosidase II by mannostatin A and the role of the thiomethyl moiety in ligand-protein interactions. J Am Chem Soc 128 : 8310–8319.
15. KuntzDA, ZhongW, GuoJ, RoseDR, BoonsGJ (2009) The molecular basis of inhibition of Golgi alpha-mannosidase II by mannostatin A. Chembiochem 10 : 268–277.
16. KuntzDA, NakayamaS, SheaK, HoriH, UtoY, et al. (2010) Structural investigation of the binding of 5-substituted swainsonine analogues to Golgi alpha-mannosidase II. Chembiochem 11 : 673–680.
17. NumaoS, KuntzDA, WithersSG, RoseDR (2003) Insights into the mechanism of Drosophila melanogaster Golgi alpha-mannosidase II through the structural analysis of covalent reaction intermediates. J Biol Chem 278 : 48074–48083.
18. PolakovaM, SestakS, LattovaE, PetrusL, MuchaJ, et al. (2011) alpha-D-mannose derivatives as models designed for selective inhibition of Golgi alpha-mannosidase II. Eur J Med Chem 46 : 944–952.
19. ShahN, KuntzDA, RoseDR (2003) Comparison of kifunensine and 1-deoxymannojirimycin binding to class I and II alpha-mannosidases demonstrates different saccharide distortions in inverting and retaining catalytic mechanisms. Biochemistry 42 : 13812–13816.
20. ShahN, KuntzDA, RoseDR (2008) Golgi alpha-mannosidase II cleaves two sugars sequentially in the same catalytic site. Proc Natl Acad Sci U S A 105 : 9570–9575.
21. van den ElsenJM, KuntzDA, RoseDR (2001) Structure of Golgi alpha-mannosidase II: a target for inhibition of growth and metastasis of cancer cells. EMBO J 20 : 3008–3017.
22. ZhongW, KuntzDA, EmberB, SinghH, MoremenKW, et al. (2008) Probing the substrate specificity of Golgi alpha-mannosidase II by use of synthetic oligosaccharides and a catalytic nucleophile mutant. J Am Chem Soc 130 : 8975–8983.
23. JaekenJ, MatthijsG (2007) Congenital disorders of glycosylation: a rapidly expanding disease family. Annu Rev Genomics Hum Genet 8 : 261–278.
24. GrunewaldS, MatthijsG (2000) Congenital disorders of glycosylation (CDG): a rapidly expanding group of neurometabolic disorders. Neuropediatrics 31 : 57–59.
25. KahookMY, MandavaN, BatemanJB, ThomasJA (2006) Glycosylation type Ic disorder: idiopathic intracranial hypertension and retinal degeneration. Br J Ophthalmol 90 : 115–116.
26. MoravaE, WosikHN, Sykut-CegielskaJ, AdamowiczM, GuillardM, et al. (2009) Ophthalmological abnormalities in children with congenital disorders of glycosylation type I. Br J Ophthalmol 93 : 350–354.
27. BediluR, NummyKA, CooperA, WeversR, SmeitinkJ, et al. (2002) Variable clinical presentation of lysosomal beta-mannosidosis in patients with null mutations. Mol Genet Metab 77 : 282–290.
28. MalmD, NilssenO (2008) Alpha-mannosidosis. Orphanet J Rare Dis 3 : 21.
29. KleijerWJ, HuP, ThoomesR, BoerM, HuijmansJG, et al. (1990) Beta-mannosidase deficiency: heterogeneous manifestation in the first female patient and her brother. J Inherit Metab Dis 13 : 867–872.
30. HuynhT, KhanJM, RanganathanS (2011) A comparative structural bioinformatics analysis of inherited mutations in beta-D-Mannosidase across multiple species reveals a genotype-phenotype correlation. BMC Genomics 12(Suppl 3): S22.
31. SamraZQ, AtharMA (2008) Cloning, sequence, expression and characterization of human beta-mannosidase. Acta Biochim Pol 55 : 479–490.
32. MahuranDJ (1999) Biochemical consequences of mutations causing the GM2 gangliosidoses. Biochim Biophys Acta 1455 : 105–138.
33. PatnaikSK, StanleyP (2006) Lectin-resistant CHO glycosylation mutants. Methods Enzymol 416 : 159–182.
34. ElbeinAD (1991) Glycosidase inhibitors as antiviral and/or antitumor agents. Semin Cell Biol 2 : 309–317.
35. MoremenKW (2002) Golgi alpha-mannosidase II deficiency in vertebrate systems: implications for asparagine-linked oligosaccharide processing in mammals. Biochim Biophys Acta 1573 : 225–235.
36. de CouetHG, TanimuraT (1987) Monoclonal antibodies provide evidence that rhodopsin in the outer rhabdomeres of Drosophila melanogaster is not glycosylated. Eur J Cell Biol 44 : 50–56.
37. HuberA, SmithDP, ZukerCS, PaulsenR (1990) Opsin of Calliphora peripheral photoreceptors R1-6. Homology with Drosophila Rh1 and posttranslational processing. J Biol Chem 265 : 17906–17910.
38. WebelR, MenonI, O'TousaJ, ColleyNJ (2000) Role of asparagine-linked glycosylation sites in Rhodopsin maturation and association with its molecular chaperone, NinaA. J Biol Chem 275 : 24752–24759.
39. ColleyNJ, BakerEK, StamnesMA, ZukerCS (1991) The cyclophilin homolog ninaA is required in the secretory pathway. Cell 67 : 255–263.
40. ColleyNJ, CassillJA, BakerEK, ZukerCS (1995) Defective intracellular transport is the molecular basis of rhodopsin-dependent dominant retinal degeneration. Proc Natl Acad Sci U S A 92 : 3070–3074.
41. KoundakjianEJ, CowanDM, HardyRW, BeckerAH (2004) The Zuker collection: a resource for the analysis of autosomal gene function in Drosophila melanogaster. Genetics 167 : 203–206.
42. SchneuwlyS, ShortridgeRD, LarriveeDC, OnoT, OzakiM, et al. (1989) Drosophila ninaA gene encodes an eye-specific cyclophilin (cyclosporin A binding protein). Proc Natl Acad Sci U S A 86 : 5390–5394.
43. ShiehBH, StamnesMA, SeavelloS, HarrisGL, ZukerCS (1989) The ninaA gene required for visual transduction in Drosophila encodes a homologue of cyclosporin A-binding protein. Nature 338 : 67–70.
44. StamnesMA, ShiehBH, ChumanL, HarrisGL, ZukerCS (1991) The cyclophilin homolog NinaA is a tissue-specific integral membrane protein required for the proper synthesis of a subset of Drosophila rhodopsins. Cell 65 : 219–227.
45. BakerEK, ColleyNJ, ZukerCS (1994) The cyclophilin homolog NinaA functions as a chaperone, forming a stable complex in vivo with its protein target rhodopsin. EMBO J 13 : 4886–4895.
46. NemcovicovaI, SestakS, RendicD, PlskovaM, MuchaJ, et al. (2013) Characterisation of class I and II alpha-mannosidases from Drosophila melanogaster. Glycoconj J 30 : 899–909.
47. CattaneoF, PasiniME, IntraJ, MatsumotoM, BrianiF, et al. (2006) Identification and expression analysis of Drosophila melanogaster genes encoding beta-hexosaminidases of the sperm plasma membrane. Glycobiology 16 : 786–800.
48. HerscovicsA (1999) Importance of glycosidases in mammalian glycoprotein biosynthesis. Biochim Biophys Acta 1473 : 96–107.
49. HerscovicsA (2001) Structure and function of Class I alpha 1,2-mannosidases involved in glycoprotein synthesis and endoplasmic reticulum quality control. Biochimie 83 : 757–762.
50. MastSW, MoremenKW (2006) Family 47 alpha-mannosidases in N-glycan processing. Methods Enzymol 415 : 31–46.
51. HarpazN, SchachterH (1980) Control of glycoprotein synthesis. Processing of asparagine-linked oligosaccharides by one or more rat liver Golgi alpha-D-mannosidases dependent on the prior action of UDP-N-acetylglucosamine: alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase I. J Biol Chem 255 : 4894–4902.
52. SchachterH, ChenSH, ZhouS, TanJ, YipB, et al. (1997) Structure and function of the genes encoding N-acetylglucosaminyltransferases which initiate N-glycan antennae. Biochem Soc Trans 25 : 875–880.
53. SchachterH (1991) The ‘yellow brick road’ to branched complex N-glycans. Glycobiology 1 : 453–461.
54. HowardS, BraunC, McCarterJ, MoremenKW, LiaoYF, et al. (1997) Human lysosomal and jack bean alpha-mannosidases are retaining glycosidases. Biochem Biophys Res Commun 238 : 896–898.
55. MaleyF, TrimbleRB, TarentinoAL, PlummerTHJr (1989) Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal Biochem 180 : 195–204.
56. AkamaTO, NakagawaH, WongNK, Sutton-SmithM, DellA, et al. (2006) Essential and mutually compensatory roles of alpha-mannosidase II and alpha-mannosidase IIx in N-glycan processing in vivo in mice. Proc Natl Acad Sci U S A 103 : 8983–8988.
57. MoremenKW, RobbinsPW (1991) Isolation, characterization, and expression of cDNAs encoding murine alpha-mannosidase II, a Golgi enzyme that controls conversion of high mannose to complex N-glycans. J Cell Biol 115 : 1521–1534.
58. MisagoM, LiaoYF, KudoS, EtoS, MatteiMG, et al. (1995) Molecular cloning and expression of cDNAs encoding human alpha-mannosidase II and a previously unrecognized alpha-mannosidase IIx isozyme. Proc Natl Acad Sci U S A 92 : 11766–11770.
59. FukudaMN, MasriKA, DellA, LuzzattoL, MoremenKW (1990) Incomplete synthesis of N-glycans in congenital dyserythropoietic anemia type II caused by a defect in the gene encoding alpha-mannosidase II. Proc Natl Acad Sci U S A 87 : 7443–7447.
60. StowersRS, SchwarzTL (1999) A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics 152 : 1631–1639.
61. HsiaoHY, JukamD, JohnstonR, DesplanC (2013) The neuronal transcription factor erect wing regulates specification and maintenance of Drosophila R8 photoreceptor subtypes. Dev Biol 381 : 482–490.
62. MaccioniHJ (2007) Glycosylation of glycolipids in the Golgi complex. J Neurochem 103(Suppl 1): 81–90.
63. VarkiA (1998) Factors controlling the glycosylation potential of the Golgi apparatus. Trends Cell Biol 8 : 34–40.
64. AltmannF, SchwihlaH, StaudacherE, GlosslJ, MarzL (1995) Insect cells contain an unusual, membrane-bound beta-N-acetylglucosaminidase probably involved in the processing of protein N-glycans. J Biol Chem 270 : 17344–17349.
65. BoquetI, HitierR, DumasM, ChaminadeM, PreatT (2000) Central brain postembryonic development in Drosophila: implication of genes expressed at the interhemispheric junction. J Neurobiol 42 : 33–48.
66. LiaoYF, LalA, MoremenKW (1996) Cloning, expression, purification, and characterization of the human broad specificity lysosomal acid alpha-mannosidase. J Biol Chem 271 : 28348–28358.
67. MerkleRK, ZhangY, RuestPJ, LalA, LiaoYF, et al. (1997) Cloning, expression, purification, and characterization of the murine lysosomal acid alpha-mannosidase. Biochim Biophys Acta 1336 : 132–146.
68. WakamatsuN, GotodaY, SaitoS, KawaiH (1997) Characterization of the human MANB gene encoding lysosomal alpha-D-mannosidase. Gene 198 : 351–357.
69. DanielPF, EvansJE, De GasperiR, WinchesterB, WarrenCD (1992) A human lysosomal alpha(1→6)-mannosidase active on the branched trimannosyl core of complex glycans. Glycobiology 2 : 327–336.
70. De GasperiR, DanielPF, WarrenCD (1992) A human lysosomal alpha-mannosidase specific for the core of complex glycans. J Biol Chem 267 : 9706–9712.
71. ParkC, MengL, StantonLH, CollinsRE, MastSW, et al. (2005) Characterization of a human core-specific lysosomal alpha 1,6-mannosidase involved in N-glycan catabolism. J Biol Chem 280 : 37204–37216.
72. AlkhayatAH, KraemerSA, LeipprandtJR, MacekM, KleijerWJ, et al. (1998) Human beta-mannosidase cDNA characterization and first identification of a mutation associated with human beta-mannosidosis. Hum Mol Genet 7 : 75–83.
73. WinchesterB (2005) Lysosomal metabolism of glycoproteins. Glycobiology 15 : 1R–15R.
74. RobinsonD, StirlingJL (1968) N-Acetyl-beta-glucosaminidases in human spleen. Biochem J 107 : 321–327.
75. MyerowitzR, ProiaRL (1984) cDNA clone for the alpha-chain of human beta-hexosaminidase: deficiency of alpha-chain mRNA in Ashkenazi Tay-Sachs fibroblasts. Proc Natl Acad Sci U S A 81 : 5394–5398.
76. O'DowdBF, QuanF, WillardHF, LamhonwahAM, KornelukRG, et al. (1985) Isolation of cDNA clones coding for the beta subunit of human beta-hexosaminidase. Proc Natl Acad Sci U S A 82 : 1184–1188.
77. TulsianiDR, Abou-HailaA (2001) Mammalian sperm molecules that are potentially important in interaction with female genital tract and egg vestments. Zygote 9 : 51–69.
78. CattaneoF, OgisoM, HoshiM, PerottiME, PasiniME (2002) Purification and characterization of the plasma membrane glycosidases of Drosophila melanogaster spermatozoa. Insect Biochem Mol Biol 32 : 929–941.
79. MillerDJ, GongX, ShurBD (1993) Sperm require beta-N-acetylglucosaminidase to penetrate through the egg zona pellucida. Development 118 : 1279–1289.
80. MencarelliS, CavalieriC, MaginiA, TanciniB, BassoL, et al. (2005) Identification of plasma membrane associated mature beta-hexosaminidase A, active towards GM2 ganglioside, in human fibroblasts. FEBS Lett 579 : 5501–5506.
81. HarrisonRL, JarvisDL (2006) Protein N-glycosylation in the baculovirus-insect cell expression system and engineering of insect cells to produce “mammalianized” recombinant glycoproteins. Adv Virus Res 68 : 159–191.
82. TomiyaN, BetenbaughMJ, LeeYC (2003) Humanization of lepidopteran insect-cell-produced glycoproteins. Acc Chem Res 36 : 613–620.
83. GeislerC, AumillerJJ, JarvisDL (2008) A fused lobes gene encodes the processing beta-N-acetylglucosaminidase in Sf9 cells. J Biol Chem 283 : 11330–11339.
84. KimJH, LingwoodCA, WilliamsDB, FuruyaW, ManolsonMF, et al. (1996) Dynamic measurement of the pH of the Golgi complex in living cells using retrograde transport of the verotoxin receptor. J Cell Biol 134 : 1387–1399.
85. O'TousaJE (1992) Requirement of N-linked glycosylation site in Drosophila rhodopsin. Visual Neuroscience 8 : 385–390.
86. BrownG, ChenDM, ChristiansonJS, LeeR, StarkWS (1994) Receptor demise from alteration of glycosylation site in Drosophila opsin: electrophysiology, microspectrophotometry, and electron microscopy. Visual Neuroscience 11 : 619–628.
87. KatanosakaK, TokunagaF, KawamuraS, OzakiK (1998) N-Linked glycosylation of Drosophila rhodopsin occurs exclusively in the amino-terminal domain and functions in rhodopsin maturation. FEBS 424 : 149–154.
88. LiZY, JacobsonSG, MilamAH (1994) Autosomal dominant retinitis pigmentosa caused by the threonine-17-methionine rhodopsin mutation: retinal histopathology and immunocytochemistry. Experimental Eye Research 58 : 397–408.
89. DaigerSP, SullivanLS, RodriguezJA (1995) Correlation of phenotype with genotype in inherited retinal degeneration. Behavioral and Brain Sciences 18 : 452–467.
90. PapermasterDS, WindleJ (1995) Death at an early age. Apoptosis in inherited retinal degenerations. Investigative Ophthalmology & Visual Science 36 : 977–983.
91. RosenbaumEE, BrehmKS, VasiljevicE, GajeskiA, ColleyNJ (2012) Drosophila GPI-mannosyltransferase 2 is required for GPI anchor attachment and surface expression of chaoptin. Vis Neurosci 29 : 143–156.
92. RosenbaumEE, BrehmKS, VasiljevicE, LiuCH, HardieRC, et al. (2011) XPORT-dependent transport of TRP and rhodopsin. Neuron 72 : 602–615.
93. RosenbaumEE, HardieRC, ColleyNJ (2006) Calnexin is essential for rhodopsin maturation, Ca2+ regulation, and photoreceptor cell survival. Neuron 49 : 229–241.
94. LaemmliUK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227 : 680–685.
Štítky
Genetika Reprodukčná medicína
Článek Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin PathwayČlánek Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human DiseasesČlánek G×G×E for Lifespan in : Mitochondrial, Nuclear, and Dietary Interactions that Modify LongevityČlánek PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial MatrixČlánek Rapid Evolution of PARP Genes Suggests a Broad Role for ADP-Ribosylation in Host-Virus ConflictsČlánek The Impact of Population Demography and Selection on the Genetic Architecture of Complex TraitsČlánek Lifespan Extension by Methionine Restriction Requires Autophagy-Dependent Vacuolar AcidificationČlánek The Case for Junk DNA
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 5- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Genetic Interactions Involving Five or More Genes Contribute to a Complex Trait in Yeast
- A Mutation in the Gene in Dogs with Hereditary Footpad Hyperkeratosis (HFH)
- Loss of Function Mutation in the Palmitoyl-Transferase HHAT Leads to Syndromic 46,XY Disorder of Sex Development by Impeding Hedgehog Protein Palmitoylation and Signaling
- Heterogeneity in the Frequency and Characteristics of Homologous Recombination in Pneumococcal Evolution
- Genome-Wide Nucleosome Positioning Is Orchestrated by Genomic Regions Associated with DNase I Hypersensitivity in Rice
- Null Mutation in PGAP1 Impairing Gpi-Anchor Maturation in Patients with Intellectual Disability and Encephalopathy
- Single Nucleotide Variants in Transcription Factors Associate More Tightly with Phenotype than with Gene Expression
- Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin Pathway
- Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human Diseases
- Epistatically Interacting Substitutions Are Enriched during Adaptive Protein Evolution
- Meiotic Drive Impacts Expression and Evolution of X-Linked Genes in Stalk-Eyed Flies
- G×G×E for Lifespan in : Mitochondrial, Nuclear, and Dietary Interactions that Modify Longevity
- Population Genomic Analysis of Ancient and Modern Genomes Yields New Insights into the Genetic Ancestry of the Tyrolean Iceman and the Genetic Structure of Europe
- p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
- Whole Exome Re-Sequencing Implicates and Cilia Structure and Function in Resistance to Smoking Related Airflow Obstruction
- Allelic Expression of Deleterious Protein-Coding Variants across Human Tissues
- R-loops Associated with Triplet Repeat Expansions Promote Gene Silencing in Friedreich Ataxia and Fragile X Syndrome
- PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial Matrix
- The Impairment of MAGMAS Function in Human Is Responsible for a Severe Skeletal Dysplasia
- Octopamine Neuromodulation Regulates Gr32a-Linked Aggression and Courtship Pathways in Males
- Mlh2 Is an Accessory Factor for DNA Mismatch Repair in
- Activating Transcription Factor 6 Is Necessary and Sufficient for Alcoholic Fatty Liver Disease in Zebrafish
- The Spatiotemporal Program of DNA Replication Is Associated with Specific Combinations of Chromatin Marks in Human Cells
- Rapid Evolution of PARP Genes Suggests a Broad Role for ADP-Ribosylation in Host-Virus Conflicts
- Genome-Wide Inference of Ancestral Recombination Graphs
- Mutations in Four Glycosyl Hydrolases Reveal a Highly Coordinated Pathway for Rhodopsin Biosynthesis and N-Glycan Trimming in
- SHP2 Regulates Chondrocyte Terminal Differentiation, Growth Plate Architecture and Skeletal Cell Fates
- The Impact of Population Demography and Selection on the Genetic Architecture of Complex Traits
- Retinoid-X-Receptors (α/β) in Melanocytes Modulate Innate Immune Responses and Differentially Regulate Cell Survival following UV Irradiation
- Genetic Dissection of the Female Head Transcriptome Reveals Widespread Allelic Heterogeneity
- Genome Sequencing and Comparative Genomics of the Broad Host-Range Pathogen AG8
- Copy Number Variation Is a Fundamental Aspect of the Placental Genome
- GOLPH3 Is Essential for Contractile Ring Formation and Rab11 Localization to the Cleavage Site during Cytokinesis in
- Hox Transcription Factors Access the RNA Polymerase II Machinery through Direct Homeodomain Binding to a Conserved Motif of Mediator Subunit Med19
- Drosha Promotes Splicing of a Pre-microRNA-like Alternative Exon
- Predicting the Minimal Translation Apparatus: Lessons from the Reductive Evolution of
- PAX6 Regulates Melanogenesis in the Retinal Pigmented Epithelium through Feed-Forward Regulatory Interactions with MITF
- Enhanced Interaction between Pseudokinase and Kinase Domains in Gcn2 stimulates eIF2α Phosphorylation in Starved Cells
- A HECT Ubiquitin-Protein Ligase as a Novel Candidate Gene for Altered Quinine and Quinidine Responses in
- dGTP Starvation in Provides New Insights into the Thymineless-Death Phenomenon
- Phosphorylation Modulates Clearance of Alpha-Synuclein Inclusions in a Yeast Model of Parkinson's Disease
- RPM-1 Uses Both Ubiquitin Ligase and Phosphatase-Based Mechanisms to Regulate DLK-1 during Neuronal Development
- More of a Good Thing or Less of a Bad Thing: Gene Copy Number Variation in Polyploid Cells of the Placenta
- More of a Good Thing or Less of a Bad Thing: Gene Copy Number Variation in Polyploid Cells of the Placenta
- Heritable Transmission of Stress Resistance by High Dietary Glucose in
- Revertant Mutation Releases Confined Lethal Mutation, Opening Pandora's Box: A Novel Genetic Pathogenesis
- Lifespan Extension by Methionine Restriction Requires Autophagy-Dependent Vacuolar Acidification
- A Genome-Wide Assessment of the Role of Untagged Copy Number Variants in Type 1 Diabetes
- Selectivity in Genetic Association with Sub-classified Migraine in Women
- A Lack of Parasitic Reduction in the Obligate Parasitic Green Alga
- The Proper Splicing of RNAi Factors Is Critical for Pericentric Heterochromatin Assembly in Fission Yeast
- Discovery and Functional Annotation of SIX6 Variants in Primary Open-Angle Glaucoma
- Six Homeoproteins and a linc-RNA at the Fast MYH Locus Lock Fast Myofiber Terminal Phenotype
- EDR1 Physically Interacts with MKK4/MKK5 and Negatively Regulates a MAP Kinase Cascade to Modulate Plant Innate Immunity
- Genes That Bias Mendelian Segregation
- The Case for Junk DNA
- An In Vivo EGF Receptor Localization Screen in Identifies the Ezrin Homolog ERM-1 as a Temporal Regulator of Signaling
- Mosaic Epigenetic Dysregulation of Ectodermal Cells in Autism Spectrum Disorder
- Hyperactivated Wnt Signaling Induces Synthetic Lethal Interaction with Rb Inactivation by Elevating TORC1 Activities
- Mutations in the Cholesterol Transporter Gene Are Associated with Excessive Hair Overgrowth
- Scribble Modulates the MAPK/Fra1 Pathway to Disrupt Luminal and Ductal Integrity and Suppress Tumour Formation in the Mammary Gland
- A Novel CH Transcription Factor that Regulates Expression Interdependently with GliZ in
- Phosphorylation of a WRKY Transcription Factor by MAPKs Is Required for Pollen Development and Function in
- Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics
- Spermatid Cyst Polarization in Depends upon and the CPEB Family Translational Regulator
- Insights into the Genetic Structure and Diversity of 38 South Asian Indians from Deep Whole-Genome Sequencing
- Intron Retention in the 5′UTR of the Novel ZIF2 Transporter Enhances Translation to Promote Zinc Tolerance in
- A Dominant-Negative Mutation of Mouse Causes Glaucoma and Is Semi-lethal via LBD1-Mediated Dimerisation
- Biased, Non-equivalent Gene-Proximal and -Distal Binding Motifs of Orphan Nuclear Receptor TR4 in Primary Human Erythroid Cells
- Ras-Mediated Deregulation of the Circadian Clock in Cancer
- Retinoic Acid-Related Orphan Receptor γ (RORγ): A Novel Participant in the Diurnal Regulation of Hepatic Gluconeogenesis and Insulin Sensitivity
- Extensive Diversity of Prion Strains Is Defined by Differential Chaperone Interactions and Distinct Amyloidogenic Regions
- Fine Tuning of the UPR by the Ubiquitin Ligases Siah1/2
- Paternal Poly (ADP-ribose) Metabolism Modulates Retention of Inheritable Sperm Histones and Early Embryonic Gene Expression
- Allele-Specific Genome-wide Profiling in Human Primary Erythroblasts Reveal Replication Program Organization
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial Matrix
- Null Mutation in PGAP1 Impairing Gpi-Anchor Maturation in Patients with Intellectual Disability and Encephalopathy
- Phosphorylation of a WRKY Transcription Factor by MAPKs Is Required for Pollen Development and Function in
- p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



