-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Whole Exome Re-Sequencing Implicates and Cilia Structure and Function in Resistance to Smoking Related Airflow Obstruction
Very large genome-wide association studies in general population cohorts have successfully identified at least 26 genes or gene regions associated with lung function and a number of these also show association with chronic obstructive pulmonary disease (COPD). However, these findings explain a small proportion of the heritability of lung function. Although the main risk factor for COPD is smoking, some individuals have normal or good lung function despite many years of heavy smoking. We hypothesised that studying these individuals might tell us more about the genetics of lung health. Re-sequencing of exomes, where all of the variation in the protein-coding portion of the genome can be measured, is a recent approach for the study of low frequency and rare variants. We undertook re-sequencing of the exomes of “resistant smokers” and used publicly available exome data for comparisons. Our findings implicate CCDC38, a gene which has previously shown association with lung function in the general population, and genes involved in cilia structure and lung function as having a role in resistance to smoking.
Published in the journal: Whole Exome Re-Sequencing Implicates and Cilia Structure and Function in Resistance to Smoking Related Airflow Obstruction. PLoS Genet 10(5): e32767. doi:10.1371/journal.pgen.1004314
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004314Summary
Very large genome-wide association studies in general population cohorts have successfully identified at least 26 genes or gene regions associated with lung function and a number of these also show association with chronic obstructive pulmonary disease (COPD). However, these findings explain a small proportion of the heritability of lung function. Although the main risk factor for COPD is smoking, some individuals have normal or good lung function despite many years of heavy smoking. We hypothesised that studying these individuals might tell us more about the genetics of lung health. Re-sequencing of exomes, where all of the variation in the protein-coding portion of the genome can be measured, is a recent approach for the study of low frequency and rare variants. We undertook re-sequencing of the exomes of “resistant smokers” and used publicly available exome data for comparisons. Our findings implicate CCDC38, a gene which has previously shown association with lung function in the general population, and genes involved in cilia structure and lung function as having a role in resistance to smoking.
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of global morbidity and mortality [1] and whilst smoking remains the single most important risk factor, it is also clear that COPD risk is heritable [2]. The genetics underlying COPD are still not fully understood although genome-wide association studies have identified 26 genomic regions showing robust association with lung function [3]–[6] and, of these, 11 have also now shown association with airflow obstruction [7]–[9]. However, the proportion of the variance accounted for by the 26 common genetic variants representing these regions remains modest (∼7.5% for the ratio of forced expired volume in 1 second (FEV1) to forced vital capacity (FVC)) [5].
Although over a quarter of the population with a significant smoking history go on to develop COPD [10], some individuals are observed to have preserved lung function as measured by a normal or high FEV1 despite many years of heavy smoking. We hypothesised that these “resistant smokers” may harbour rare variants with large effect sizes which protect against lung function decline caused by smoking. Identification of these variants, and the genes that harbour them, could provide further insight into the mechanisms underlying airflow obstruction.
We undertook whole exome re-sequencing of 100 heavy smokers (>20 pack years of smoking) who had healthy lung function when age, sex, height and amount smoked were taken into account. We employed 3 complementary approaches to investigate the genetic architecture of the resistant smoker genotype (Figure 1). Firstly, we screened these 100 “resistant smokers” for novel rare variants (i.e. not previously identified and deposited in a public database) with a putatively functional effect on protein product and tested for enrichment of these novel variants in functionally related genes and pathways. Secondly, using a comparision with two independent control sets with exome re-sequencing data, we looked for signals of association with the resistant smoker phenotype for individual variants (including variants of all minor allele frequencies). Thirdly, we looked for association of the resistant smoker phenotype with the combined effects of multiple rare and common variants within genes.
Fig. 1. Flowchart describing each of the 3 main analytical pathways used to explore the genetic architecture of smoking resistance. 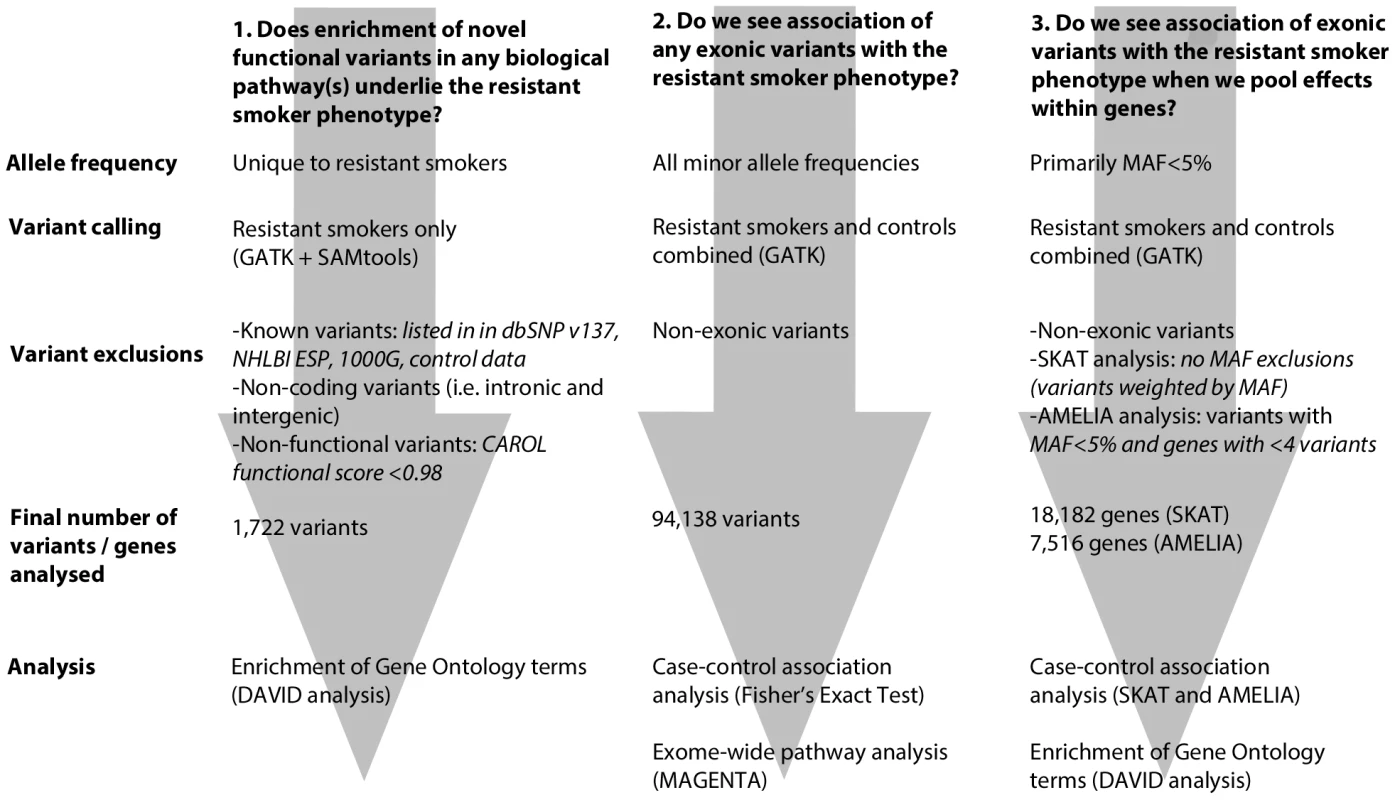
Analyses are described in full in the methods. MAF: minor allele frequency. We found the strongest evidence of association with resistance to smoking for a non-synonymous variant in CCDC38, a gene encoding a coiled-coil domain protein with a role in motor activity, previously identified as showing an association with lung function. We also show evidence of cytoplasmic expression of CCDC38 in bronchial columnar epithelial cells. In addition, we found evidence for an enrichment of novel rare functional variants in resistant smokers in gene pathways related to cilia structure and function. Given that abnormalities of ciliary function are already known to play a role in reduced mucociliary clearance in COPD sufferers and smokers, these data suggest that genetic factors may play a significant role in determining the ciliary response of the airway to inhaled tobacco smoke.
Results
Sample characteristics
100 individuals from the Gedling [11], [12] and Nottingham Smokers cohorts with good lung function (FEV1/FVC>0.7 and % predicted FEV1>80%) when age, sex, height and smoking history (>20 pack years) were taken into account were selected as “resistant smoker” cases. Characteristics of the 100 resistant smoker case samples are shown in Table S1A and Figure S1. Exome re-sequencing and alignment was undertaken as described in the methods.
Two independent control sets were used; the robustness of findings using the primary control set (n = 166) were further assessed using a secondary control set (n = 230).
Identification of novel putatively functional variants in cases
We searched for novel variants among the resistant smokers, i.e. genetic variants which were not observed in either control set and which were not documented in public databases. Bioinformatic tools allow for scoring of likely functional impact, including whether a variant is likely to be “deleterious”; here we use the term “putatively functional” since some variants which have a deleterious effect on the function of a given gene may result in a protective phenotype. A total of 24,098 variants which were not already in databases of known variants or within segmental duplications were identified with high confidence using two independent calling algorithms. A total of 6587 coding SNPs were scored using CAROL (including non-synonymous, loss/gain of stop codon, synonymous and splice site/UTR variants) and 1722 were predicted as being putatively functional (CAROL score>0.98) and were within 1533 genes. 16 of these 1533 genes each contained three novel putatively functional variants (Table S2) (no gene contained more than three such variants). GBF1 contained three novel putatively functional variants of which one, chr10 : 104117872, was identified in two case samples. A further 157 genes each contained 2 novel putatively functional variants and the remaining 1360 genes contained one novel putatively functional variant.
In the resistant smokers, there was no overall enrichment or depletion of novel putatively functional variants among the 26 regions reported to be associated with lung function [5], (16 were observed, the same number would have been predicted based on the sequence length of exons) and no novel putatively functional variants were identified within the CHRNA3/5 region which has been previously associated with smoking [13] and airflow obstruction [9].
Eight of the 1722 novel variants predicted to be putatively functional were identified in >1 case sample. These are listed in Table S3. ATAD3C contained a novel putatively functional variant for which six case samples were heterozygous, SHANK2 contained a novel putatively functional variant for which three cases were heterozygous, and the remaining six genes each contained such a variant for which two cases were heterozygous.
One hundred and ninety two Gene Ontology (GO) terms reached nominal significance for the set of 1533 genes containing novel putatively functional variants in resistant smoker cases. Of these, 22 high level GO terms were significant after Bonferroni correction for multiple testing and are listed in Table 1 [14]. The most significant GO term was the molecular function term “motor activity” which describes molecules involved in catalysis of movement along a polymeric molecule such as a microfilament or microtubule, coupled to the hydrolysis of a nucleoside triphosphate. Other related GO terms also feature amongst the significant signals from this analysis (including “cytoskeleton”, “microtubule motor activity”, “myosin complex”, “axoneme”, “cilium” and “cilium part”) (Table 1 and Table S4).
Tab. 1. Gene Ontology (GO) terms reaching Bonferroni corrected significance for enrichment amongst the 1533 genes harbouring novel putatively functional variants in the resistant smokers, using DAVID. 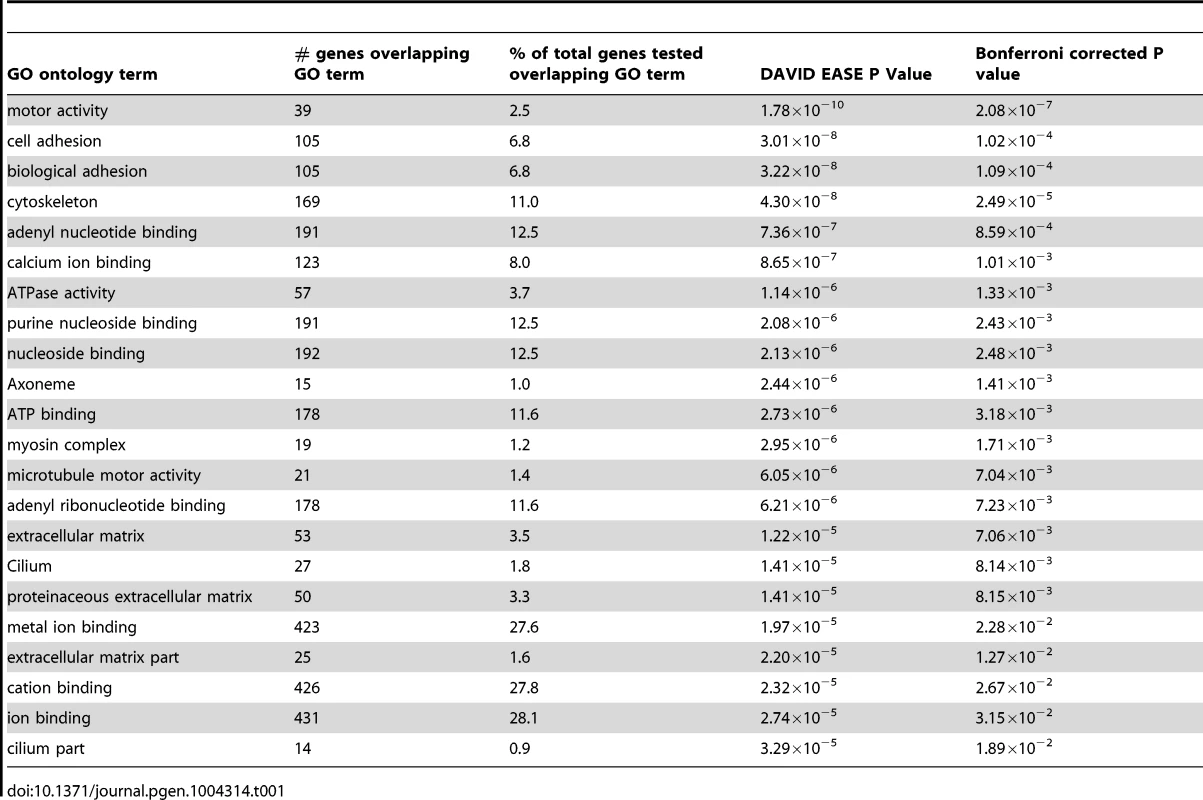
Single-variant association testing
We tested for association of known and novel exonic variants with the resistant smoker phenotype. After exclusion of variants which were missing in >5% of either cases or controls, 269,822 (of which 215,747 were listed in dbSNP137) variants remained. Of the 269,822 variants, 94,138 were exonic and included in further analyses. Similar distributions of variants across the minor allele frequency spectrum were observed for the cases, primary, and secondary controls (results not shown).
After testing for association with resistant smoker status using primary controls, no SNPs reached genome-wide significance (P<5×10−7, based on Bonferroni correction for 94,138 tests). Substantial under-inflation of the test statistics was seen (lambda = 0.6) (Figure 2A), possibly due to the large number of rare variants (lambda = 0.92 if only variants with MAF>5% [n = 25,646] were considered, Figure 2B). Twenty exonic SNPs showed nominal evidence of association with P<10−3 (Table 2).
Fig. 2. QQ plots of single-variant association test statistics for all exonic variants (A) and for exonic variants with MAF>5% (B). 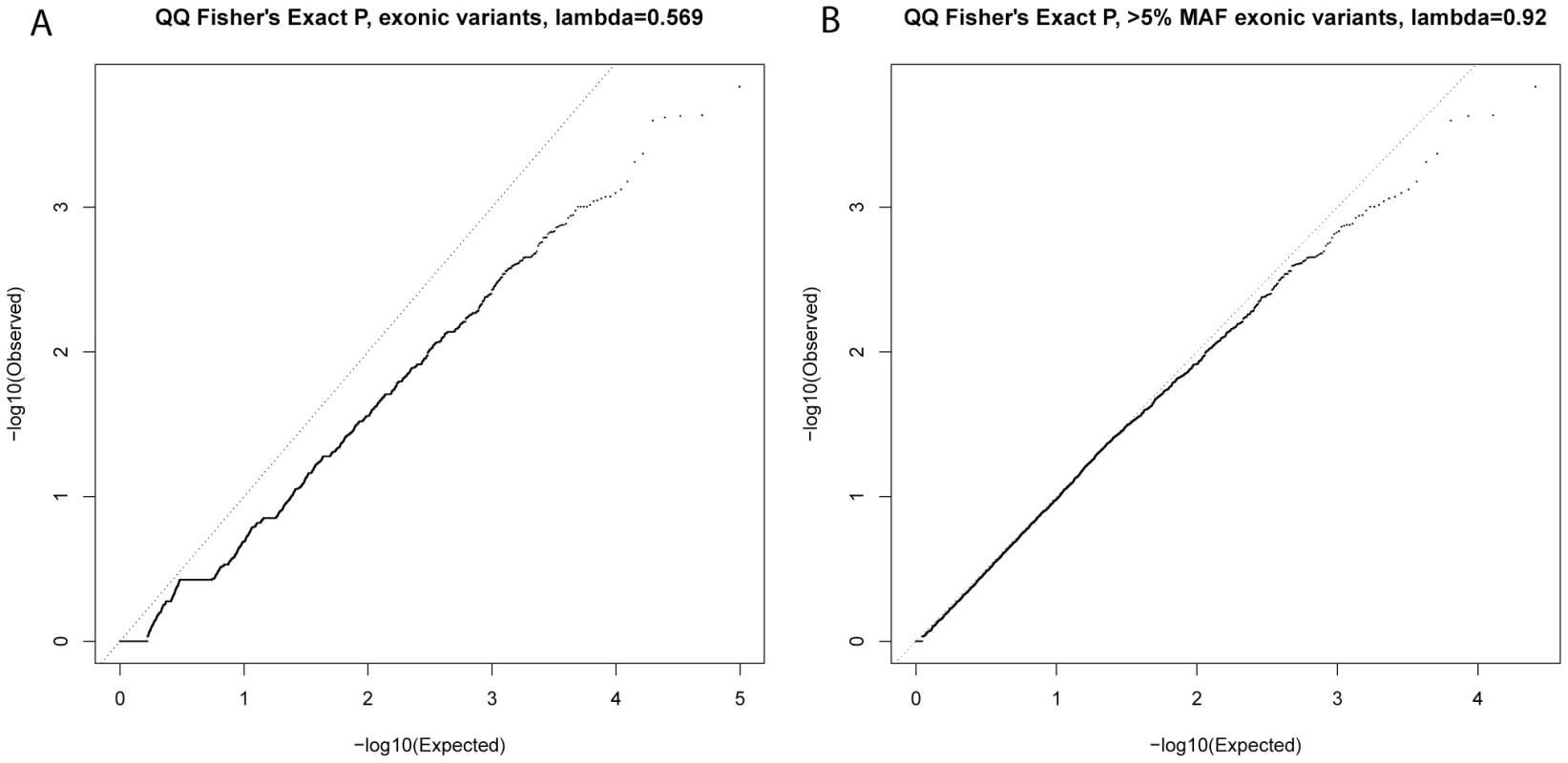
Tab. 2. Single variant association results for 20 top exonic SNPs. 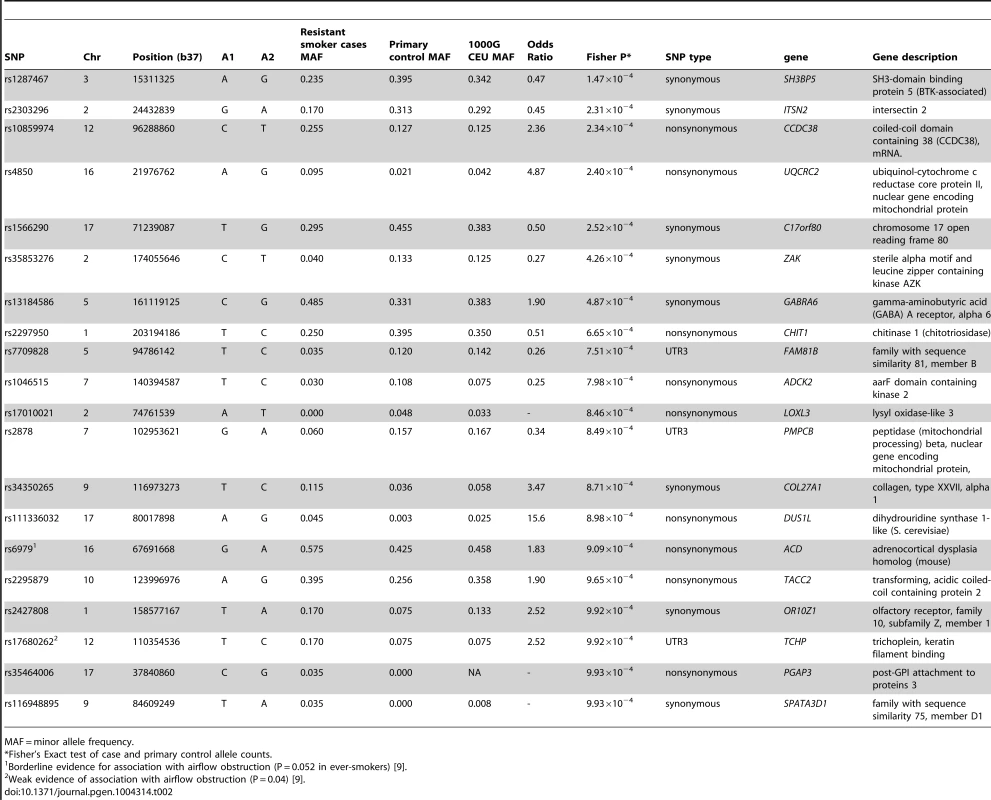
MAF = minor allele frequency. The strongest signal from a non-synonymous SNP was within a region previously identified as being associated with lung function [5]. The non-synonymous SNP in CCDC38 (rs10859974, OR = 2.36, P = 2.34×10−4) is 17.43 kb away from, but statistically independent of, rs1036429 (intronic, r2 = 0.064) which has previously shown genome-wide significant association with FEV1/FVC [5]. SNP rs10859974 itself has shown weak evidence of association with FEV1/FVC (P = 0.032) [5]. This SNP is predicted to cause a methionine to valine substitution at protein position 227; the valine allele is predicted to be protective. Investigations into CCDC38 expression in bronchial tissue via immunohistochemistry identified moderate staining of CCDC38 in the cytoplasm of columnar epithelial cells, with weak staining in the sub-epithelial layer (Figure 3). We found no evidence that rs10859974 or any of its proxy SNPs (r2>0.3) were lung eQTLs for CCDC38 itself, although rs11108320 which is intronic in CCDC38 and in strong LD with rs10859974 (r2 = 1) is an eQTL for nearby gene NTN4 (significant at 10% False Discovery Rate (FDR) threshold). Many additional SNPs located near or within CCDC38 and SNRPF were eQTLs for NTN4 (Table S5). Nearby CCDC38 intronic SNPs in weaker LD (r2 = 0.3) with rs10859974 were eQTLs for SNRPF (Table S5).
Fig. 3. Immunohistochemistry staining for Coiled-Coil Domain-Containing Protein 38 (CCDC38) in a normal human bronchial epithelium at ×40 magnification. 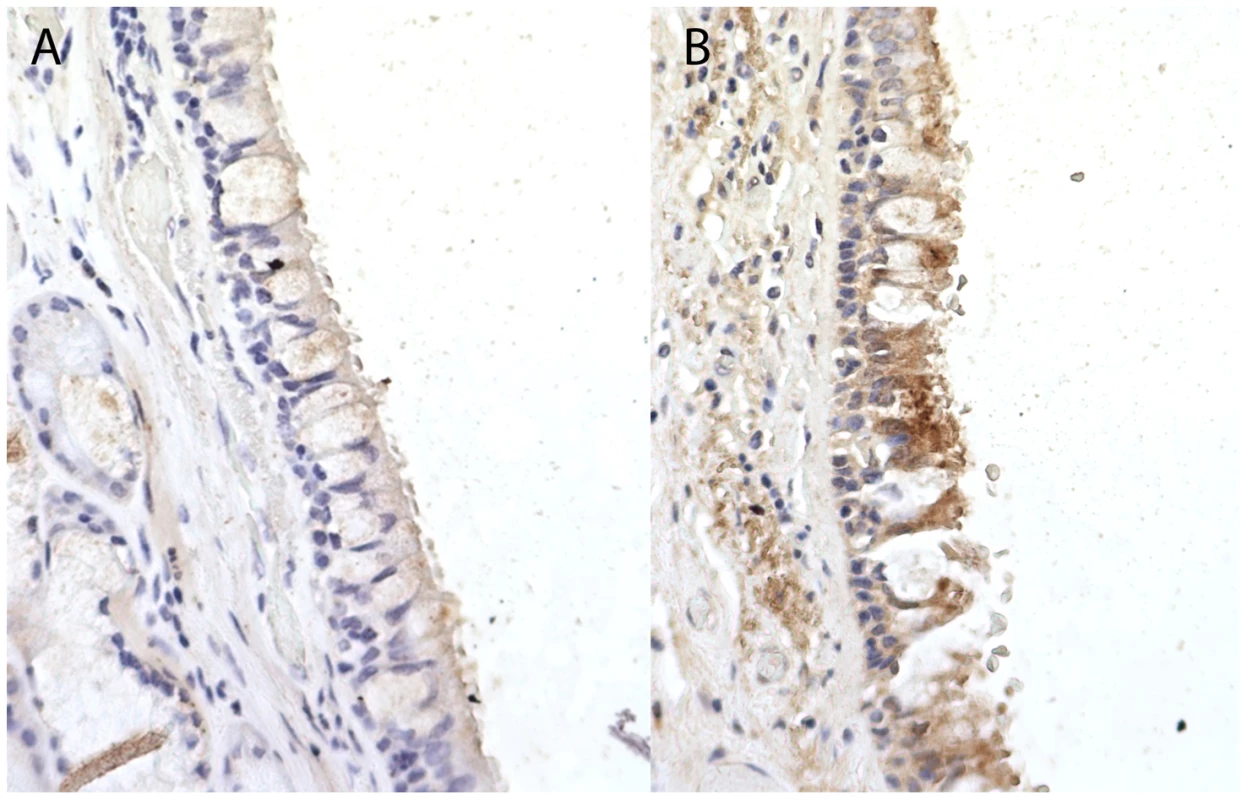
When compared to the unstained isotype control (Rabbit IgG) (A), staining identifies moderate cytoplasmic expression of CCDC38 particularly in the columnar epithelial cells and the bronchial smooth muscle layer (B). Image representative of three independent staining procedures. The strongest signal of association in the single-variant analysis was from a synonymous SNP, rs1287467, in SH3BP5 (OR = 0.47, P = 1.47×10−4) (Table 2). A SNP downstream of SH3BP5 (rs1318937, 1000G CEU MAF = 0.108, 16 kb from rs1287467, r2 = 0.018) has shown evidence of association with alcohol dependence and alcohol and nicotine co-dependence [15].
Synonymous SNP rs2303296 in ITSN2 was the second strongest signal of association (OR = 0.45, P = 2.31×10−4) and had previously shown weak evidence of association with FEV1 (P = 0.02) [5] but was not near to any previously identified genome-wide significant associations with lung function and has not shown evidence of association with COPD [9]. Another SNP in ITSN2, rs6707600 (intronic, 1000 G CEU MAF = 0.017, 89 Kb from rs2303296, r2 = 0.02), has shown some evidence of association with antipsychotic response in schizophrenia patients [16].
The second strongest signal from a non-synonymous SNP was rs4850 in UQCRC2 (OR = 4.87, P = 2.4×10−4). There were no nearby associations reported with any other trait for this gene.
The third strongest signal from a non-synonymous SNP was rs2297950 (OR = 0.51, P = 6.65×10−4) in CHIT1 which encodes chitinase 1 (Chit1). The chitinase pathway has been implicated in asthma and lung function [17] and lung function decline in COPD patients [18]. Chit1 expression in mice has been shown to be correlated with severity of bleomycin-induced pulmonary fibrosis (with overexpression leading to increased severity and Chit1−/− mice exhibiting reduced pulmonary fibrosis) [19].
A non-synonymous SNP in LOXL3, rs17010021, was the only SNP with an association P<10−3 regardless of whether the primary or the secondary controls were used (Table S6). This variant had a minor allele frequency of 0.048 and 0.061 in the primary and secondary control sets respectively, but the minor allele was not observed in any of the resistant smoker cases.
Synonymous SNP rs1051730, in CHRNA3 (15q25.1), has previously shown very strong evidence of association with smoking behaviour (particularly cigarettes per day) [13], [20], [21]. This SNP showed weak evidence of association with the resistant smoker phenotype in our study (P = 0.03 when the secondary control set was used and P = 0.06 when primary control set was used). Association results for SNPs within 500 Kb of rs1051730 are in Table S7.
No nominally significant enrichment of association signals in known pathways was identified in the exome-wide results of the single-variant analysis using MAGENTA [22].
Gene-based association testing
SKAT [23] and AMELIA [24] analyses were undertaken to assess whether multiple variants within a gene collectively showed evidence of association; these tests are agnostic to whether a given variant is previously known. Quantile-Quantile plots for SKAT and AMELIA analyses are shown in Figure 4. Genes with nominally significant association (P<10−3) for SKAT or AMELIA analysis using the primary controls are shown in Table 3 (results of SKAT and AMELIA analyses using the secondary controls are shown in Table S8). No genes showed significant association after Bonferroni correction for multiple testing (P<0.05/18000 = 2.8×10−6) for either analysis (Table 3 and Table S8). Since the genes are likely to be correlated (through LD structure or overlapping reading frames), SKAT provides a resampling function to control Family Wise Error Rate (FWER). No genes were significant after controlling FWER = 0.05. None of the genes in Table 3 and Table S8 were within any of the 26 lung function associated regions [3]–[5], the CHRNA3/5 smoking-associated region [13] or SERPINA1 (mutations in which are known to cause alpha-1-antitrypsin deficiency) [25].
Fig. 4. QQ plots for SKAT and AMELIA analyses using primary controls. 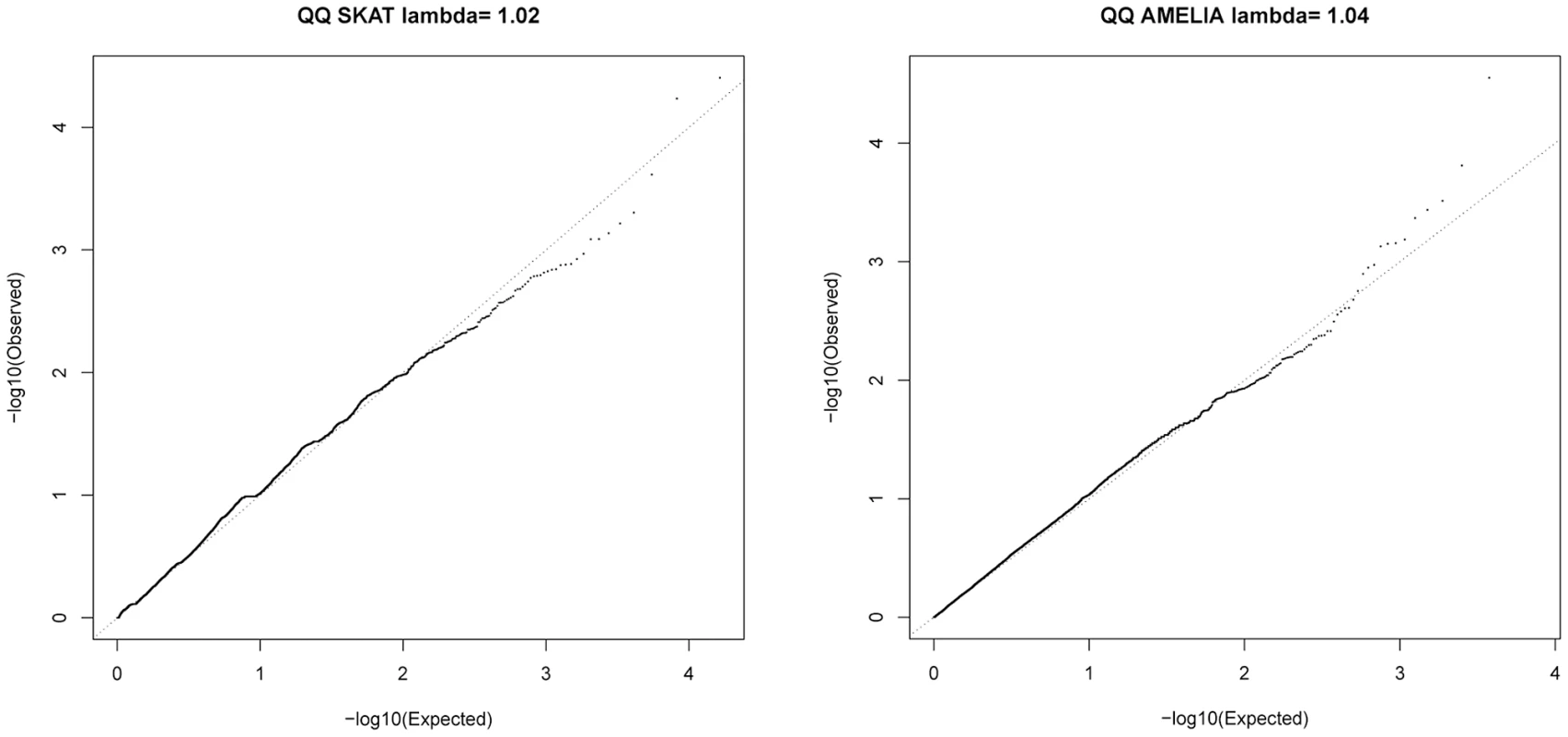
Tab. 3. SKAT and AMELIA analysis results using primary controls, ranked by SKAT P value. 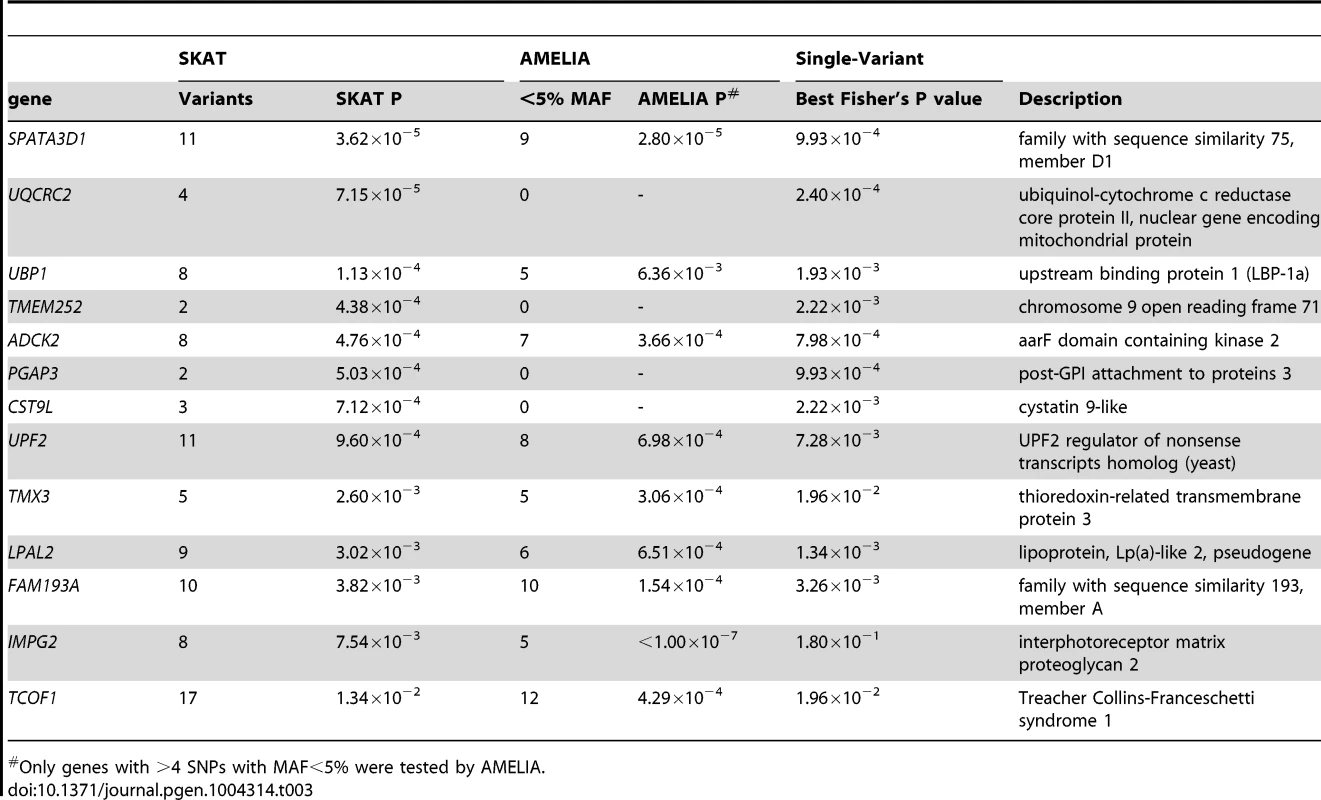
Only genes with >4 SNPs with MAF<5% were tested by AMELIA. We also checked overlap between the gene-based association testing and single-variant tests. A signal in TMEM252 (which showed P<10−3 in the SKAT analysis regardless of which control set was used) was driven by rs117451470, a non-synonymous SNP, which had P = 2.2×10−3 in the single-variant association analysis (the other SNP in TMEM252, a singleton novel synonymous variant, had P = 0.38 in the single-variant analysis). Signals in UQCRC2 (strongest signal using SKAT), SPATA3D1, PGAP3 and ADCK2 were also driven by variants with P<10−3 in the single-variant analysis. Signals from TMX3, IMPG2 and TCOF1 were not driven by single-variant signals (all SNPs within these genes had P>0.01 in the single variant analysis). IMPG2 was the strongest signal from the AMELIA analysis and all 8 SNPs within IMPG2 had no evidence of association in the single-variant analysis (P> = 0.18).
We tested for enrichment of GO terms within the set of genes showing association with P<0.01 in the SKAT analyses. Ten high level GO terms reached nominal significance (P<0.05) for the set of 150 genes identified using SKAT but none were significant after Bonferroni correction for multiple testing [14].
Discussion
Understanding why some heavy smokers seem to show resistance to the detrimental effects of cigarette smoke on lung function should provide further insight into the genetics of lung function and COPD. We undertook pathway enrichment, single-variant association testing and gene-based association testing analyses on whole exome re-sequencing data from a set of resistant smokers. Although no individual SNP achieved genome-wide statistical significance (P<5×10−7), our strongest association signal for a non-synonymous SNP was in CCDC38; a gene which has previously shown strong and robust evidence of association with lung function [5]. The intronic SNP previously shown to be associated with lung function (FEV1/FVC) and the non-synonymous SNP showing nominally significant association with the resistant smoker phenotype in this study are located close together (17.4 kb apart) but are not well correlated (the non-synonymous SNP has previously shown nominally significant evidence [P<0.05] of association with FEV1/FVC). A conditional analysis of these two SNPs was consistent with no statistical correlation between these signals. Although the function of CCDC38 is not yet well understood, members of the coiled-coil domain protein family are known to have a role in cell motor activity (e.g. myosin) [26] and cilia assembly [27], [28]. Expression of CCDC38 has been identified in the human bronchi of two subjects, with strong cytoplasmic staining in the epithelium and moderate staining in the airway smooth muscle (Human Protein Atlas [http://www.proteinatlas.org] [29]: ENSG00000165972). We experimentally confirmed these findings using immunohistochemistry on lung sections. We observed moderate cytoplasmic CCDC38 staining in bronchial columnar epithelial cells and some potential airway smooth muscle staining. There is no evidence that SNP rs10859974 is an eQTL for CCDC38 itself, although proxies for rs10859974 are eQTLs for a nearby downstream gene, NTN4, encoding Netrin-4 which may play a role in embryonic lung development [30]. Gene Ontology terms shown to be significantly enriched among the novel putatively functional variants identified only in the resistant smokers also pointed to pathways relating to motor activity and the cytoskeleton, including cilia. Another locus showing association with lung function (1p36.13, [5]) also contains a gene encoding a component of cilia (CROCC which encodes rootletin, another coiled-coil domain protein) and Crocc-null mice have been shown to have impaired cilia with pathogenic consequences to the airways [31]. The enrichment of genes involved in cilia function amongst the results of our analyses supports the importance of cilia function in lung health. Cilia abnormalities are known to be associated with smoking [32], [33], asthma [34], and play a role in COPD [35] where reduced cilia function leads to reduced mucus clearance of the airways. Improving mucociliary clearance is one of the aims of drug therapy for chronic bronchitis in COPD patients (reviewed in [36]).
Impaired cilia function is known to cause a wide range of diseases (collectively known as ciliopathies) many of which include pulmonary symptoms [37]. Primary Ciliary Dyskinesia (PCD) is a rare genetic disorder where respiratory tract cilia function is impaired leading to reduced (or absent) mucus clearance. Mutations in genes which encode components of the cilia have been found to cause several forms of PCD and include the dynein, axonemal heavy chain encoding genes DNAH11 [38], [39] and DNAH5 [40] within which resistant smoker-specific novel putatively functional variants were identified in this study (2 such variants were discovered in DNAH11). Whilst PCD affects resistance to infection and results in bronchiectasis, abnormal lung function can manifest early in life and progressive airflow obstruction has been observed in later life, although aggressive treatment may prevent the latter [41]. Retinitis pigmentosa is a feature of several ciliopathies, including some with pulmonary involvement (for example, Alstrom Syndrome). Low frequency variants in IMPG2 (interphotoreceptor matrix proteoglycan 2) collectively showed strong evidence of association (using AMELIA). Variants in IMPG2 are associated with a form of retinitis pigmentosa [42]. Another retinitis pigmentosa gene, RP1, was amongst the 16 genes containing 3 novel putatively functional variants in the resistant smokers. RP1 encodes part of the photoreceptor axoneme [43], a central component of cilia.
A recent study identified modulators of ciliogenesis using a high throughput assay of in vitro RNA interference of 7,784 genes in human retinal pigmented epithelial cells (htRPE) and identified 36 positive modulators and 13 negative modulators of ciliogenesis [44]. These modulators included many genes which did not encode structural cilia proteins and thus were not obvious candidates for a role in cilia function. None of the genes highlighted by the single-variant or gene-based analyses were confirmed as modulators of ciliogenesis although ITSN2, which contained one of the top signals in our single-variant analysis, was included in the screen and showed suggestive evidence of a positive role in ciliogenesis but this was not confirmed in a second screen. Two of the genes found to harbour a novel putatively functional variant in the resistant smokers were identified as positive modulators of ciliogenesis: GSN (gelsolin) which is a known cilia gene with a role in actin filament organisation and AGTPBP1 (ATP/GTP binding protein 1) which has a role in tubulin modification.
Collectively, our data show an enrichment of novel putatively functional variants in genes related to cilia structure and function in resistant smokers. Association between smoking and shorter cilia has been reported [32]. The largest genome-wide association with lung function to date supports the notion that the majority of associated variants, including those associated with COPD risk, affect lung function development rather than decline in lung function in adults [5]. If confirmed in other studies, it would be interesting to assess whether genetic influences on the function of cilia primarily affect growth or whether these affect more directly the extent of damage caused by tobacco smoke.
Very large GWAS have identified up to hundreds of common variants each with a modest effect on a variety of phenotypes. However, collectively, these still only explain a very modest proportion of the additive polygenic variance. It has been widely hypothesised that rare variation may account for some of this missing variance [45]. Commercially available SNP arrays have tended to include mostly variants with minor allele frequencies upwards of 5% and rare variants have not been reliably imputed from these. Re-sequencing approaches provide the most accurate platform for the study of exome-wide and genome-wide rare variation. However, there is increasing evidence that rare variants may not account for the missing heritability for all traits [46]. Our study did not find evidence for any individual rare variants with large effects in any of the known lung function associated loci or elsewhere in the exome (albeit in a modest overall sample size), although we did identify significant enrichment of novel rare variants in sets of genes with known functions in pathways which are known to have a role in lung health.
For the single variant analyses, we used Fisher's Exact Test. Whilst this is an appropriate test to use for small cell counts (for example, where minor allele counts are low), alternatives have been recently proposed including the Firth test, and although the optimal approach in the size of study we undertook is not clear from the comparisons shown to date, the Fisher's Exact Test can be more conservative than the Firth test and this may have had some impact on the power of the study [47]. Methods for the analysis of rare variant data are continuing to evolve.
Although this is the first exome re-sequencing study of resistance to airways obstruction among heavy smokers, our study does have potential limitations. Sample size was limited both by availability of individuals with such an extreme phenotype as that we were able to study, and also by current sequencing costs. We were able to utilise re-sequencing data available to the scientific community as control data and therefore maximise the discovery potential of our resources by re-sequencing to a sufficient sequencing read depth for confident rare variant calling. By doing so, and selecting an extreme phenotype group from our sampling frame, we adopted a suitable design to test whether there was enrichment of rare variants of large effect in resistant smokers. The same limitations also impact on the availability of suitable replication studies. In particular, it would have been desirable to undertake replication to support the statistically significant findings of the pathway analysis. However, in the absence of a suitable replication resource, the prior evidence for the role of cilia in lung health does lend support to our findings. As it becomes possible to sample and re-sequence from very large biobanks it should become possible to circumvent these issues in years to come, particularly if the cost of sequencing falls.
As limited information was available on smoking status among the controls, we did not restrict controls to heavy smokers and there is therefore potential for genetic associations to be driven via an effect on smoking behaviour. Nevertheless, our design is also consistent with the detection of association due to primary effect on airways and previous genome-wide association studies of lung function not fully adjusted for smoking have detected loci associated with lung function and COPD which were not associated with smoking behaviour [4], [5]. We saw only a weak association with variants at the CHRNA3/CHRNA5 locus (the locus at which variants have shown the strongest effect with smoking behaviour [13], [20], [21]).
Misclassification impacts on power; we would have underestimated the power to detect SNP and gene-based associations if the prevalence of resistance to airways obstruction among heavy smokers was greater than we assumed. In a cross-sectional study of this kind, survivor bias could occur if genetic variants influencing survival were under-represented or over-represented in the resistant smokers, but as the mean age of the resistant smokers was 56.4, any survivor bias, if present, is unlikely to have had a major impact. Finally, although we would expect the allele frequencies of the control sets we used to be representative of a general population control set across the vast majority of the genome, biases could potentially be introduced for any genetic variants related to the ascertainment strategy of the control sets. For the main findings we report in this paper, we also present allele frequencies from a public database (1000 Genomes Project); any such bias does not explain our main findings.
In the first deep whole exome re-sequencing study of the resistant smoker phenotype, we have identified an association signal in a region that has already shown robust association with lung function (CCDC38) and demonstrate significant enrichment of novel putatively functional variants in genes related to cilia structure. These findings provide insights into the mechanisms underlying preserved lung function in heavy smokers and may reveal mechanisms shared with COPD aetiology.
Materials and Methods
Ethics statement
The Gedling study was approved by the Nottingham City Hospital and Nottingham University Ethics committees (MREC/99/4/01) and written informed consent for genetic study was obtained from participants. The Nottingham Smokers study was approved by Nottingham University Medical School Ethical Committee (GM129901/) and written informed consent for genetic study was obtained from participants. The Edinburgh MR-psychosis sample set was compliant with the UK10K Ethical Governance Framework (http://www.uk10k.org/ethics.html) and no restrictions were placed on the use of the genetic data by the scientific community. For TwinsUK, ethics committee approval was obtained from Guy's and St Thomas' Hospital research ethics committee. Tissue for immunohistochemistry was from Nottingham Health Science Biobank (Nottingham, UK) with the required ethical approval (08/H0407/1).
For lung eQTL datasets: At Laval, lung specimens were collected from patients undergoing lung cancer surgery and stored at the “Institut universitaire de cardiologie et de pneumologie de Québec” (IUCPQ) site of the Respiratory Health Network Tissue Bank of the “Fonds de recherche du Québec – Santé” (www.tissuebank.ca). Written informed consent was obtained from all subjects and the study was approved by the IUCPQ ethics committee. At Groningen, lung specimens were provided by the local tissue bank of the Department of Pathology and the study protocol was consistent with the Research Code of the University Medical Center Groningen and Dutch national ethical and professional guidelines (“Code of conduct; Dutch federation of biomedical scientific societies”; http://www.federa.org). At Vancouver, the lung specimens were provided by the James Hogg Research Center Biobank at St Paul's Hospital and subjects provided written informed consent. The study was approved by the ethics committees at the UBC-Providence Health Care Research Institute Ethics Board.
Sample selection
100 individuals with prolonged exposure to tobacco smoke and unusually good lung function (resistant smokers) were selected from the Gedling and Nottingham Smokers studies, described below.
The Gedling cohort is a general population sample recruited in Nottingham in 1991 (18 to 70 years of age, n = 2,633) [11] and individuals were then followed-up in 2000 (n = 1346) when blood samples were taken for DNA extraction, and FEV1 and FVC were measured using a calibrated dry bellows spirometer (Vitalograph, Buckingham, UK), recording the best of three satisfactory attempts [12].
The Nottingham Smokers cohort is an ongoing collection in Nottingham using the following criteria; European ancestry, >40 years of age and smoking history of >10 pack years (currently n = 538). Lung function measurements (FEV1 and FVC) were recorded at enrolment using a MicroLab ML3500 spirometer (Micro Medical Ltd, UK) recording the best of three satisfactory attempts.
Our inclusion criteria was; aged over 40 with more than 20 pack years of smoking and no known history of asthma. A total of 184 samples were eligible for this project after further exclusion of individuals with either FEV1, FVC or FEV1/FVC less than the Lower Limit Normal (LLN) (based on age, sex and height). We calculated residuals after adjusting % predicted FEV1 for pack years of smoking and selected the 100 samples with the highest residuals for exome re-sequencing (Figure S1).
Primary controls were from the Edinburgh MR-psychosis set (n = 166) of the UK10K project (http://www.uk10k.org/) and consisted of subjects with schizophrenia, autism or other psychoses, and with mental retardation. No additional phenotype information was available for the primary controls. The TwinsUK secondary control samples (n = 230) were all unrelated females selected from the high and low ends of the pain sensitivity distribution of 2500 volunteers from TwinsUK [48], [49]. Characteristics of the secondary controls are given in Table S1B (note that phenotype information was only available for a subset of the samples). These secondary controls were not included in the main analyses due to the difference in exome coverage. Further phenotype information was not available for either control sample set.
Exome sequencing
For the 100 resistant smoker case samples, DNA was extracted from whole blood and the Agilent SureSelect All Exon 50 Mb kit was used for enrichment. The 100 resistant smoker samples were individually indexed and grouped into 20 pools of 5 samples. Each pool was sequenced in one lane (20 sequencing lanes in total) using an Illumina HiSeq2000. Sequences were generated as 100 bp paired-end reads. Exome-wide coverage of 97 out of 100 samples was >20 (Figure S2). Three samples had mean sequence depth coverage <20, of these, one appeared to have had poor enrichment (high number of off-target reads), one had a low overall sequence yield and high number of duplicate reads and one had a high number of duplicate reads (but good sequence yield). To preserve power, and because there was no evidence that the sequence data quality for these samples was lower than for the other samples, these 3 samples were not excluded from further analyses.
A total of 166 exomes from the Edinburgh MR-psychosis study: a subset of the neurodevelopmental disease group of the UK10K project (http://www.uk10k.org/), were used as primary controls. These were enriched using the Agilent SureSelect All Exon 50 Mb kit and sequenced using an Illumina HiSeq2000 to a mean coverage depth of ∼70x (75 bp paired-end reads). The sequencing of the secondary controls from the TwinsUK pain study has been described elsewhere [49]. In brief, raw sequence data was available for 230 exomes which had been enriched using the NimbleGen EZ v2 (44 Mb) array and sequenced on an Illumina HiSeq2000 to a mean depth of coverage of 71x (90 bp paired-end reads).
Sequence alignment
The sequence alignment of the primary control exomes has been described elsewhere (http://www.uk10k.org/). The 100 resistant smoker case exomes and 230 TwinsUK controls were aligned using BWA v0.6.1 [50] with -q15 for read-trimming. Samtools v0.1.18 [51] was used to convert sort, remove duplicates and index the alignment .bam files. GATK v1.4-30 [52] was used to undertake local realignment around indels and to recalibrate quality scores for all 3 datasets.
Identification of novel putatively functional case-only variants
In order to identify novel variant calls in the 100 resistant smoker exomes, GATK and SAMtools mpileup were run on a per sample basis for all 100 exomes. Only bases with a base quality score >20 were included. The variants called were then compared with dbSNP137, 1000 Genomes Project (1000G) and NHLBI Exome Sequencing Project calls and all known variants were excluded in order to identify novel rare variants which were unique to the 100 resistant smoker exomes. The novel GATK variant calls were then excluded if they had a Phred scaled probability (QUAL) score <30, quality by unfiltered depth (non-REF) (QD) <5, largest contiguous homopolymer run of the variant allele in either direction >5, strand bias >−0.1 or Phred-scaled P value using Fisher's Exact Test to detect strand bias >60. The novel SAMtools mpileup variants were excluded if they had a QUAL<30, mapping quality <25 or genotype quality <25. Variants called at sites with a depth of coverage less than 4 or greater than 2000 were also excluded. The intersect of variants which were identified and passed filtering using both GATK and SAMtools mpileup was taken forward for further analysis.
CAROL (http://www.sanger.ac.uk/resources/software/carol/) was used to predict the consequence of all coding variants. This method combines the results of the functional scoring tools SIFT and Polyphen2. SNPs were predicted as being putatively functional if they had CAROL score>0.98. Amino acid changes were predicted using ENSEMBL.
Variant calling and QC across cases and controls
For each comparison (resistant smoker cases vs. primary controls and resistant smoker cases vs. secondary controls), variant calling was undertaken across cases and controls together using the GATK v1.5-20 Unified Genotyper. Only bases with a base quality score >20 were included. Coverage was down-sampled to 30 (reads are drawn at random where coverage is greater than 30). This was done to improve comparability between cases and controls and to speed up computation. A minimum QUAL of 30 was used as the threshold for calls. The GATK VQSR approach was used to filter variants across all samples. Variants with QUAL<30 and VQSLOD score equivalent to truth< = 99.9 were excluded (VQSLOD score<2.2989). Only single nucleotide polymorphism variants (SNPs) were called. There was >99% genotype concordance with genotype array data (Illumina 660k) for ∼5000 exonic SNPs with MAF>5% in the 100 resistant smokers.
Association analyses
Single-variant association testing was undertaken using the Fisher's exact test for a comparison of resistant smoker cases and primary controls. A secondary comparison of the resistant smoker cases and the secondary controls was also undertaken although results were interpreted with caution due to disparity of the exome coverage at the pre-sequencing enrichment stage between the cases and secondary controls. Two approaches to analyse the effect of multiple variants within genes were used: SKAT (v0.92) [23] and AMELIA [24]. Variants were assigned to RefSeq genes using Annovar [53]: a total of 16439 genes were identified as containing variants in the resistant smoker cases vs. primary controls analysis. Analysis with SKAT was undertaken using default weighting to account for the assumption that rare variants are likely to have bigger effect sizes.
An alternative method, AMELIA [24], was run using a subset of the variants with MAF<5%. A total of 18182 genes were identified as containing variants (with MAF<5%), of which 7516 contained more than 4 variants and so could be reliably tested by AMELIA.
For both SKAT and AMELIA, only variants which were annotated as exonic, 5′UTR or 3′UTR were included.
Power calculations
Power estimates for the identification of novel putatively functional variants in cases only, single-variant association tests and SKAT analysis were undertaken.
For a given variant unique to, and with a minor allele frequency of 0.005 in, resistant smokers, the probability of identifying at least one copy of the minor allele in 100 such individuals is 0.63 (0.86 for a minor allele frequency of 0.01).
Estimates of power for the single-variant association tests were undertaken for a sample size of 100 cases (resistant smokers) and 166 controls assuming a prevalence of the resistant smoker phenotype of 2% in the controls. Power calculations for detecting single variants were undertaken using Quanto and are shown in Figure S3. As an example, power to detect a variant with an allele frequency of 0.01 and an OR of 10 would be 10% for an alpha level of 5×10−8, and 81% for an alpha level of 0.001.
SKAT power calculations were run using the R package SKAT. The simulated dataset that the R package provides based on the coalescent populations genetic model was used to assess LD and MAF. The “Log” option was used to specify that the logOR distribution varies with allele frequency (logOR increases as minor allele frequency decreases), the effect size of each variant is equal to c|log10(MAF)|, where c is estimated assuming that the maximum OR corresponds to a MAF of 10−4. It was assumed that no logOR for causal variants was negative (results were broadly consistent if 20% of the causal variants were assumed to have negative logOR, results not shown). One thousand simulations were run for a region length of 17.7 kb (median of all gene lengths analysed in the real data), maximum OR of all variants analysed ranged from 5 to 10, significance (alpha) thresholds of 2.8×10−6 (Bonferroni correction for testing of 18,000 genes) and 0.01 (nominal significance threshold used to define genes as input to DAVID Gene Ontology analysis) were used and the percentage of causal variants with MAF<1% (only variants with MAF<1% were considered causal) given were 25% and 50%.
Power to detect a region of length 17.7 kb with a maximum OR of 10, assuming that 50% of variants with MAF<1% are causal is 53% for a Bonferroni-corrected significance threshold of 2.8×10−6, and 89% for a nominal significance threshold of 0.01 (Figure S4).
Functional annotation and pathway enrichment analyses
We tested for enrichment of Gene Ontology terms and enrichment of signals in known biological pathways within the results of the single-variant, gene-based and case-only analyses.
A total of 150 genes had P<0.01 in the SKAT analyses (of these, 28 also had P<0.01 in the AMELIA analysis but many genes were not analysed using both SKAT and AMELIA and so only SKAT results, which included all SNPs with no MAF cut-off, were included in this analysis). A total of 1533 genes contained novel putatively functional variants in the resistant smoker cases. We tested for enrichment of Gene Ontology categories within each of these gene lists using DAVID [14] with an EASE (modified Fisher's Exact) P<0.05.
We tested for pathway enrichment within the single-variant association results using MAGENTA v2 [22]. Briefly, MAGENTA tests for deviation from a random distribution of strengths of association signals (P values) for each pathway and includes all available exome-wide single-variant association results (n = 94,138). Six databases of biological pathways were tested: including Ingenuity Pathway (June 2008, number of pathways n = 92), KEGG (2010, n = 186), PANTHER Molecular Function (January 2010, n = 276), PANTHER Biological Processes (January 2010, n = 254), PANTHER Pathways (January 2010, n = 141) and Gene Ontology (April 2010, n = 9542). Significance thresholds were Bonferroni corrected for each database.
Immunohistochemistry
Fixed lung tissue was sectioned and mounted. Slides were treated in Histo-Clear and then re-hydrated using 100% ethanol and 95% ethanol washes. Antigen retrieval was carried out by steaming the tissue samples for 30 minutes in sodium citrate buffer (2.1 g Citric Acid [Fisons - C-6200-53]+13 ml 2M NaOH [Fisher - S-4880/53] in 87 ml H2O). Tissue was then treated with peroxidise blocking solution (Dako - S2023), followed by treatments with a 1 in 50 dilution of rabbit anti-CCDC38 antibody (Sigma HPA039305; 0.2 mg/ml) or a 1 in 50 dilution of the Rabbit IgG Isotype control (Invitrogen 10500C, diluted to 0.2 mg/ml). Secondary antibody staining and DAB treatment was carried out using the EnVision Detection Systems Peroxidase/DAB, Rabbit/Mouse kit (Dako – K5007). Tissue was then counterstained with Mayers Hematoxylin solution (Sigma – 51275) before being dehydrated using 95% ethanol and 100% ethanol washes. Slides were mounted using Vectamount (Vector Laboratories - H-5000).
Lung eQTL analysis
The description of the lung eQTL dataset and subject demographics have been published previously [54]–[56]. Briefly, non-tumor lung tissues were collected from patients who underwent lung resection surgery at three participating sites: Laval University (Quebec City, Canada), University of Groningen (Groningen, The Netherlands), and University of British Columbia (Vancouver, Canada). Whole-genome gene expression and genotyping data were obtained from these specimens. Gene expression profiling was performed using an Affymetrix custom array (GPL10379) testing 51,627 non-control probe sets and normalized using RMA [57]. Genotyping was performed using the Illumina Human1M-Duo BeadChip array. Genotype imputation was undertaken using the 1000G reference panel. Following standard microarray and genotyping quality controls, 1111 patients were available including 409 from Laval, 363 from Groningen, and 339 from UBC. Lung eQTLs were identified to associate with mRNA expression in either cis (within 1 Mb of transcript start site) or in trans (all other eQTLs) and meeting the 10% false discovery rate (FDR) genome-wide significant threshold.
Supporting Information
Zdroje
1. LopezAD, ShibuyaK, RaoC, MathersCD, HansellAL, et al. (2006) Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 27 : 397–412.
2. SilvermanEK, ChapmanHA, DrazenJM, WeissST, RosnerB, et al. (1998) Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis. Am J Respir Crit Care Med 157 : 1770–1778.
3. HancockDB, EijgelsheimM, WilkJB, GharibSA, LoehrLR, et al. (2010) Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet 42 : 45–52.
4. RepapiE, SayersI, WainLV, BurtonPR, JohnsonT, et al. (2010) Genome-wide association study identifies five loci associated with lung function. Nat Genet 42 : 36–44.
5. Soler ArtigasM, LothDW, WainLV, GharibSA, ObeidatM, et al. (2011) Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet 43 : 1082–1090.
6. WilkJB, ChenTH, GottliebDJ, WalterRE, NagleMW, et al. (2009) A genome-wide association study of pulmonary function measures in the Framingham Heart Study. PLoS Genet 5: e1000429.
7. CastaldiPJ, ChoMH, LitonjuaAA, BakkeP, GulsvikA, et al. (2011) The association of genome-wide significant spirometric loci with chronic obstructive pulmonary disease susceptibility. Am J Respir Cell Mol Biol 45 : 1147–1153.
8. Soler ArtigasM, WainLV, RepapiE, ObeidatM, SayersI, et al. (2011) Effect of five genetic variants associated with lung function on the risk of chronic obstructive lung disease, and their joint effects on lung function. Am J Respir Crit Care Med 184 : 786–795.
9. WilkJB, ShrineNR, LoehrLR, ZhaoJH, ManichaikulA, et al. (2012) Genome-wide association studies identify CHRNA5/3 and HTR4 in the development of airflow obstruction. Am J Respir Crit Care Med 186 : 622–632.
10. LokkeA, LangeP, ScharlingH, FabriciusP, VestboJ (2006) Developing COPD: a 25 year follow up study of the general population. Thorax 61 : 935–939.
11. BrittonJR, PavordID, RichardsKA, KnoxAJ, WisniewskiAF, et al. (1995) Dietary antioxidant vitamin intake and lung function in the general population. Am J Respir Crit Care Med 151 : 1383–1387.
12. McKeeverTM, ScrivenerS, BroadfieldE, JonesZ, BrittonJ, et al. (2002) Prospective study of diet and decline in lung function in a general population. Am J Respir Crit Care Med 165 : 1299–1303.
13. LiuJZ, TozziF, WaterworthDM, PillaiSG, MugliaP, et al. (2010) Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet 42 : 436–440.
14. Huang daW, ShermanBT, LempickiRA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4 : 44–57.
15. ZuoL, ZhangF, ZhangH, ZhangXY, WangF, et al. (2012) Genome-wide search for replicable risk gene regions in alcohol and nicotine co-dependence. Am J Med Genet B Neuropsychiatr Genet 159B: 437–444.
16. McClayJL, AdkinsDE, AbergK, BukszarJ, KhachaneAN, et al. (2011) Genome-wide pharmacogenomic study of neurocognition as an indicator of antipsychotic treatment response in schizophrenia. Neuropsychopharmacology 36 : 616–626.
17. GuerraS, HalonenM, SherrillDL, VenkerC, SpangenbergA, et al. (2013) The relation of circulating YKL-40 to levels and decline of lung function in adult life. Respir Med 107 : 1923–30.
18. AminuddinF, AkhabirL, StefanowiczD, ParePD, ConnettJE, et al. (2012) Genetic association between human chitinases and lung function in COPD. Hum Genet 131 : 1105–1114.
19. LeeCG, HerzogEL, AhangariF, ZhouY, GulatiM, et al. (2012) Chitinase 1 is a biomarker for and therapeutic target in scleroderma-associated interstitial lung disease that augments TGF-beta1 signaling. J Immunol 189 : 2635–2644.
20. ThorgeirssonTE, GudbjartssonDF, SurakkaI, VinkJM, AminN, et al. (2010) Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet 42 : 448–453.
21. Tobacco, GeneticsC (2010) Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet 42 : 441–447.
22. SegreAV, ConsortiumD, investigatorsM, GroopL, MoothaVK, et al. (2010) Common inherited variation in mitochondrial genes is not enriched for associations with type 2 diabetes or related glycemic traits. PLoS Genet 6: e1001058.
23. WuMC, LeeS, CaiT, LiY, BoehnkeM, et al. (2011) Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet 89 : 82–93.
24. AsimitJL, Day-WilliamsAG, MorrisAP, ZegginiE (2012) ARIEL and AMELIA: testing for an accumulation of rare variants using next-generation sequencing data. Hum Hered 73 : 84–94.
25. LarssonC (1978) Natural history and life expectancy in severe alpha1-antitrypsin deficiency, Pi Z. Acta Med Scand 204 : 345–351.
26. BurkhardP, StetefeldJ, StrelkovSV (2001) Coiled coils: a highly versatile protein folding motif. Trends Cell Biol 11 : 82–88.
27. Becker-HeckA, ZohnIE, OkabeN, PollockA, LenhartKB, et al. (2011) The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat Genet 43 : 79–84.
28. MerveilleAC, DavisEE, Becker-HeckA, LegendreM, AmiravI, et al. (2011) CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat Genet 43 : 72–78.
29. UhlenM, OksvoldP, FagerbergL, LundbergE, JonassonK, et al. (2010) Towards a knowledge-based Human Protein Atlas. Nat Biotechnol 28 : 1248–1250.
30. LiuY, SteinE, OliverT, LiY, BrunkenWJ, et al. (2004) Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr Biol 14 : 897–905.
31. YangJ, GaoJ, AdamianM, WenXH, PawlykB, et al. (2005) The ciliary rootlet maintains long-term stability of sensory cilia. Mol Cell Biol 25 : 4129–4137.
32. LeopoldPL, O'MahonyMJ, LianXJ, TilleyAE, HarveyBG, et al. (2009) Smoking is associated with shortened airway cilia. PLoS One 4: e8157.
33. VerraF, EscudierE, LebargyF, BernaudinJF, De CremouxH, et al. (1995) Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. Am J Respir Crit Care Med 151 : 630–634.
34. ThomasB, RutmanA, HirstRA, HaldarP, WardlawAJ, et al. (2010) Ciliary dysfunction and ultrastructural abnormalities are features of severe asthma. J Allergy Clin Immunol 126 : 722–e722, 722-729, e722.
35. HoggJC, ChuF, UtokaparchS, WoodsR, ElliottWM, et al. (2004) The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350 : 2645–2653.
36. KimV, CrinerGJ (2013) Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 187 : 228–237.
37. WareSM, AygunMG, HildebrandtF (2011) Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc 8 : 444–450.
38. LucasJS, AdamEC, GogginPM, JacksonCL, Powles-GloverN, et al. (2012) Static respiratory cilia associated with mutations in Dnahc11/DNAH11: a mouse model of PCD. Hum Mutat 33 : 495–503.
39. BartoloniL, BlouinJL, PanY, GehrigC, MaitiAK, et al. (2002) Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proc Natl Acad Sci U S A 99 : 10282–10286.
40. OlbrichH, HaffnerK, KispertA, VolkelA, VolzA, et al. (2002) Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat Genet 30 : 143–144.
41. KnowlesMR, DanielsLA, DavisSD, ZariwalaMA, LeighMW (2013) Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med 188 : 913–922.
42. Bandah-RozenfeldD, CollinRW, BaninE, van den BornLI, CoeneKL, et al. (2010) Mutations in IMPG2, encoding interphotoreceptor matrix proteoglycan 2, cause autosomal-recessive retinitis pigmentosa. Am J Hum Genet 87 : 199–208.
43. LiuQ, ZuoJ, PierceEA (2004) The retinitis pigmentosa 1 protein is a photoreceptor microtubule-associated protein. J Neurosci 24 : 6427–6436.
44. KimJ, LeeJE, Heynen-GenelS, SuyamaE, OnoK, et al. (2010) Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464 : 1048–1051.
45. ManolioTA, CollinsFS, CoxNJ, GoldsteinDB, HindorffLA, et al. (2009) Finding the missing heritability of complex diseases. Nature 461 : 747–753.
46. HuntKA, MistryV, BockettNA, AhmadT, BanM, et al. (2013) Negligible impact of rare autoimmune-locus coding-region variants on missing heritability. Nature 498 : 232–235.
47. MaC, BlackwellT, BoehnkeM, ScottLJ, GoTDi (2013) Recommended joint and meta-analysis strategies for case-control association testing of single low-count variants. Genet Epidemiol 37 : 539–550.
48. SpectorTD, WilliamsFM (2006) The UK Adult Twin Registry (TwinsUK). Twin Res Hum Genet 9 : 899–906.
49. WilliamsFM, ScollenS, CaoD, MemariY, HydeCL, et al. (2012) Genes contributing to pain sensitivity in the normal population: an exome sequencing study. PLoS Genet 8: e1003095.
50. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
51. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079.
52. McKennaA, HannaM, BanksE, SivachenkoA, CibulskisK, et al. (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20 : 1297–1303.
53. WangK, LiM, HakonarsonH (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38: e164.
54. HaoK, BosseY, NickleDC, ParePD, PostmaDS, et al. (2012) Lung eQTLs to help reveal the molecular underpinnings of asthma. PLoS Genet 8: e1003029.
55. LamontagneM, CoutureC, PostmaDS, TimensW, SinDD, et al. (2013) Refining susceptibility loci of chronic obstructive pulmonary disease with lung eqtls. PLoS One 8: e70220.
56. ObeidatM, MillerS, ProbertK, BillingtonCK, HenryAP, et al. (2013) GSTCD and INTS12 regulation and expression in the human lung. PLoS One 8: e74630.
57. IrizarryRA, HobbsB, CollinF, Beazer-BarclayYD, AntonellisKJ, et al. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4 : 249–264.
Štítky
Genetika Reprodukčná medicína
Článek Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin PathwayČlánek Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human DiseasesČlánek G×G×E for Lifespan in : Mitochondrial, Nuclear, and Dietary Interactions that Modify LongevityČlánek PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial MatrixČlánek Rapid Evolution of PARP Genes Suggests a Broad Role for ADP-Ribosylation in Host-Virus ConflictsČlánek The Impact of Population Demography and Selection on the Genetic Architecture of Complex TraitsČlánek Lifespan Extension by Methionine Restriction Requires Autophagy-Dependent Vacuolar AcidificationČlánek The Case for Junk DNA
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 5- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Genetic Interactions Involving Five or More Genes Contribute to a Complex Trait in Yeast
- A Mutation in the Gene in Dogs with Hereditary Footpad Hyperkeratosis (HFH)
- Loss of Function Mutation in the Palmitoyl-Transferase HHAT Leads to Syndromic 46,XY Disorder of Sex Development by Impeding Hedgehog Protein Palmitoylation and Signaling
- Heterogeneity in the Frequency and Characteristics of Homologous Recombination in Pneumococcal Evolution
- Genome-Wide Nucleosome Positioning Is Orchestrated by Genomic Regions Associated with DNase I Hypersensitivity in Rice
- Null Mutation in PGAP1 Impairing Gpi-Anchor Maturation in Patients with Intellectual Disability and Encephalopathy
- Single Nucleotide Variants in Transcription Factors Associate More Tightly with Phenotype than with Gene Expression
- Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin Pathway
- Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human Diseases
- Epistatically Interacting Substitutions Are Enriched during Adaptive Protein Evolution
- Meiotic Drive Impacts Expression and Evolution of X-Linked Genes in Stalk-Eyed Flies
- G×G×E for Lifespan in : Mitochondrial, Nuclear, and Dietary Interactions that Modify Longevity
- Population Genomic Analysis of Ancient and Modern Genomes Yields New Insights into the Genetic Ancestry of the Tyrolean Iceman and the Genetic Structure of Europe
- p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
- Whole Exome Re-Sequencing Implicates and Cilia Structure and Function in Resistance to Smoking Related Airflow Obstruction
- Allelic Expression of Deleterious Protein-Coding Variants across Human Tissues
- R-loops Associated with Triplet Repeat Expansions Promote Gene Silencing in Friedreich Ataxia and Fragile X Syndrome
- PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial Matrix
- The Impairment of MAGMAS Function in Human Is Responsible for a Severe Skeletal Dysplasia
- Octopamine Neuromodulation Regulates Gr32a-Linked Aggression and Courtship Pathways in Males
- Mlh2 Is an Accessory Factor for DNA Mismatch Repair in
- Activating Transcription Factor 6 Is Necessary and Sufficient for Alcoholic Fatty Liver Disease in Zebrafish
- The Spatiotemporal Program of DNA Replication Is Associated with Specific Combinations of Chromatin Marks in Human Cells
- Rapid Evolution of PARP Genes Suggests a Broad Role for ADP-Ribosylation in Host-Virus Conflicts
- Genome-Wide Inference of Ancestral Recombination Graphs
- Mutations in Four Glycosyl Hydrolases Reveal a Highly Coordinated Pathway for Rhodopsin Biosynthesis and N-Glycan Trimming in
- SHP2 Regulates Chondrocyte Terminal Differentiation, Growth Plate Architecture and Skeletal Cell Fates
- The Impact of Population Demography and Selection on the Genetic Architecture of Complex Traits
- Retinoid-X-Receptors (α/β) in Melanocytes Modulate Innate Immune Responses and Differentially Regulate Cell Survival following UV Irradiation
- Genetic Dissection of the Female Head Transcriptome Reveals Widespread Allelic Heterogeneity
- Genome Sequencing and Comparative Genomics of the Broad Host-Range Pathogen AG8
- Copy Number Variation Is a Fundamental Aspect of the Placental Genome
- GOLPH3 Is Essential for Contractile Ring Formation and Rab11 Localization to the Cleavage Site during Cytokinesis in
- Hox Transcription Factors Access the RNA Polymerase II Machinery through Direct Homeodomain Binding to a Conserved Motif of Mediator Subunit Med19
- Drosha Promotes Splicing of a Pre-microRNA-like Alternative Exon
- Predicting the Minimal Translation Apparatus: Lessons from the Reductive Evolution of
- PAX6 Regulates Melanogenesis in the Retinal Pigmented Epithelium through Feed-Forward Regulatory Interactions with MITF
- Enhanced Interaction between Pseudokinase and Kinase Domains in Gcn2 stimulates eIF2α Phosphorylation in Starved Cells
- A HECT Ubiquitin-Protein Ligase as a Novel Candidate Gene for Altered Quinine and Quinidine Responses in
- dGTP Starvation in Provides New Insights into the Thymineless-Death Phenomenon
- Phosphorylation Modulates Clearance of Alpha-Synuclein Inclusions in a Yeast Model of Parkinson's Disease
- RPM-1 Uses Both Ubiquitin Ligase and Phosphatase-Based Mechanisms to Regulate DLK-1 during Neuronal Development
- More of a Good Thing or Less of a Bad Thing: Gene Copy Number Variation in Polyploid Cells of the Placenta
- More of a Good Thing or Less of a Bad Thing: Gene Copy Number Variation in Polyploid Cells of the Placenta
- Heritable Transmission of Stress Resistance by High Dietary Glucose in
- Revertant Mutation Releases Confined Lethal Mutation, Opening Pandora's Box: A Novel Genetic Pathogenesis
- Lifespan Extension by Methionine Restriction Requires Autophagy-Dependent Vacuolar Acidification
- A Genome-Wide Assessment of the Role of Untagged Copy Number Variants in Type 1 Diabetes
- Selectivity in Genetic Association with Sub-classified Migraine in Women
- A Lack of Parasitic Reduction in the Obligate Parasitic Green Alga
- The Proper Splicing of RNAi Factors Is Critical for Pericentric Heterochromatin Assembly in Fission Yeast
- Discovery and Functional Annotation of SIX6 Variants in Primary Open-Angle Glaucoma
- Six Homeoproteins and a linc-RNA at the Fast MYH Locus Lock Fast Myofiber Terminal Phenotype
- EDR1 Physically Interacts with MKK4/MKK5 and Negatively Regulates a MAP Kinase Cascade to Modulate Plant Innate Immunity
- Genes That Bias Mendelian Segregation
- The Case for Junk DNA
- An In Vivo EGF Receptor Localization Screen in Identifies the Ezrin Homolog ERM-1 as a Temporal Regulator of Signaling
- Mosaic Epigenetic Dysregulation of Ectodermal Cells in Autism Spectrum Disorder
- Hyperactivated Wnt Signaling Induces Synthetic Lethal Interaction with Rb Inactivation by Elevating TORC1 Activities
- Mutations in the Cholesterol Transporter Gene Are Associated with Excessive Hair Overgrowth
- Scribble Modulates the MAPK/Fra1 Pathway to Disrupt Luminal and Ductal Integrity and Suppress Tumour Formation in the Mammary Gland
- A Novel CH Transcription Factor that Regulates Expression Interdependently with GliZ in
- Phosphorylation of a WRKY Transcription Factor by MAPKs Is Required for Pollen Development and Function in
- Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics
- Spermatid Cyst Polarization in Depends upon and the CPEB Family Translational Regulator
- Insights into the Genetic Structure and Diversity of 38 South Asian Indians from Deep Whole-Genome Sequencing
- Intron Retention in the 5′UTR of the Novel ZIF2 Transporter Enhances Translation to Promote Zinc Tolerance in
- A Dominant-Negative Mutation of Mouse Causes Glaucoma and Is Semi-lethal via LBD1-Mediated Dimerisation
- Biased, Non-equivalent Gene-Proximal and -Distal Binding Motifs of Orphan Nuclear Receptor TR4 in Primary Human Erythroid Cells
- Ras-Mediated Deregulation of the Circadian Clock in Cancer
- Retinoic Acid-Related Orphan Receptor γ (RORγ): A Novel Participant in the Diurnal Regulation of Hepatic Gluconeogenesis and Insulin Sensitivity
- Extensive Diversity of Prion Strains Is Defined by Differential Chaperone Interactions and Distinct Amyloidogenic Regions
- Fine Tuning of the UPR by the Ubiquitin Ligases Siah1/2
- Paternal Poly (ADP-ribose) Metabolism Modulates Retention of Inheritable Sperm Histones and Early Embryonic Gene Expression
- Allele-Specific Genome-wide Profiling in Human Primary Erythroblasts Reveal Replication Program Organization
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial Matrix
- Null Mutation in PGAP1 Impairing Gpi-Anchor Maturation in Patients with Intellectual Disability and Encephalopathy
- Phosphorylation of a WRKY Transcription Factor by MAPKs Is Required for Pollen Development and Function in
- p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



