-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Role for Circadian Clock Genes in Seasonal Timing: Testing the Bünning Hypothesis
The circadian clock consists of an extensive genetic network that drives daily rhythms of physiological, biochemical and behavioural processes. The network is evolutionary conserved and has been extensively studied in a broad range of organisms. Another genetic network constitutes the photoperiodic clock and monitors the seasonal change in day-length. Here, we address a major and long-standing question in chronobiology: whether the circadian clock is involved in photoperiodic timing, also known as the Bünning hypothesis. Drosophila, as with many other insects in temperate regions, exhibits a photoperiodic response that allows the insect to anticipate and survive the winter. Here we show that the cold-tolerance of the fly is regulated by the photoperiod. We use this phenotype to test day-length timing in various circadian clock mutants and observe that in null clock mutants, the photoperiodic response is abolished, whereas in mutants that exhibit short or long daily cycles, the photoperiodic response is modified, further supporting a circadian-clock function. Overall, these results provide the first evidence in Drosophila that support for the Bünning hypothesis, and pave the way for the genetic dissection of seasonal timing in Drosophila melanogaster.
Published in the journal: Role for Circadian Clock Genes in Seasonal Timing: Testing the Bünning Hypothesis. PLoS Genet 10(9): e32767. doi:10.1371/journal.pgen.1004603
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004603Summary
The circadian clock consists of an extensive genetic network that drives daily rhythms of physiological, biochemical and behavioural processes. The network is evolutionary conserved and has been extensively studied in a broad range of organisms. Another genetic network constitutes the photoperiodic clock and monitors the seasonal change in day-length. Here, we address a major and long-standing question in chronobiology: whether the circadian clock is involved in photoperiodic timing, also known as the Bünning hypothesis. Drosophila, as with many other insects in temperate regions, exhibits a photoperiodic response that allows the insect to anticipate and survive the winter. Here we show that the cold-tolerance of the fly is regulated by the photoperiod. We use this phenotype to test day-length timing in various circadian clock mutants and observe that in null clock mutants, the photoperiodic response is abolished, whereas in mutants that exhibit short or long daily cycles, the photoperiodic response is modified, further supporting a circadian-clock function. Overall, these results provide the first evidence in Drosophila that support for the Bünning hypothesis, and pave the way for the genetic dissection of seasonal timing in Drosophila melanogaster.
Introduction
Seasonal changes in day-length provide a reliable environmental cue used by many temperate species to adapt to their fluctuating environments. While the available evidence suggests that changes in day-length are monitored by an internal photoperiodic timer [1], intensive studies of photoperiodicity in animals over the last 80 years have yet to identify an underlying molecular mechanisms [2] (although significant progress has been made in plants and mammals [3]–[5]).This is in marked contrast to the level of understanding of the circadian timer that regulates daily rhythms, where studies in various model organisms, particularly Drosophila, led to the discovery of principles and molecules that are highly conserved in diverse phyla [6].
The Bünning hypothesis [7] invoked a link between the circadian and the photoperiodic mechanisms and suggested that circadian rhythmicity is required for day-length measurement. Bünning's original model assumed that circadian oscillations consist of light (‘photophil’) and dark (‘scotophil’)-requiring phases. In short days, ambient light is present only during the photophil phase, and the dark phase is not exposed to light. As days become longer, light coincides with the scotophil phase. The relative size of the photophil and scotophil phases encodes the critical photoperiod (time of the year) that induces the seasonal response. A modified version of this model was later named the ‘external coincidence model’ [8]. An alternative hypothesis, the ‘internal coincidence model’, was also proposed, where light plays only an indirect role, and the critical photoperiod is encoded by unique phase relationships between two internal oscillators. Several experimental protocols have been devised to test the Bünning hypothesis, one of which is the Nanda-Hamner protocol [9], which employs exotic light-dark cycles of ultra-long periods (T>72 hr). If the seasonal response peaks at 24 hr intervals (‘positive Nanda-Hamner’), a link, not necessarily causal, with the circadian system in photoperiodic timing is indicated [9], [10].
Drosophila melanogaster, which was instrumental in identifying higher eukaryotic circadian clock genes [11], also exhibits a photoperiodic response [12], providing an opportunity to test the link between the two timers. This response is manifested as a developmental arrest of the ovaries (i.e. reproductive diapause) under short (autumnal) days and lower temperatures, presumably enhancing the fly's ability to survive the winter in temperate regions. Although Nanda-Hamner experiments in Drosophila revealed an underlying 24 h oscillation [13], experiments using the period (per) clock mutants [12] suggested that the two systems are independent, as the per null mutants were still capable of discriminating between long and short days (albeit with a shifted critical day-length). Later, natural allelic variation in the timeless (tim) locus was associated with diapause in Drosophila [14], [15], and in Chymomyza costata [16]. Knockdown of per and the positive circadian regulator, cycle, in the bean bug Riptortus pedestris by RNAi caused simultaneous disruption of both circadian output (cuticle deposition rhythm) and photoperiodic diapause [17]. In Drosophila triauraria, genetic variation in tim and cry (but not in per, Clk or cyc) was significantly associated with the photoperiodic response [18]. Yet, because the impact of a given clock gene mutation on the photoperiodic response can be interpreted as a pleiotropic effect on diapause, the application of the Bünning hypothesis to these results has been questioned [19].
Given the shallow photoperiodic diapause of D. melanogaster [14], we have sought an alternative seasonal phenotype in this species that could be used for testing Bünning's hypothesis. Measuring chill comma recovery times (CCRt) is an established approach for studying insect thermal adaptation [20]. Here, we build on the earlier observation that flies raised in different photoperiods show differing CCRt [21], and use this phenotype to test day-length timing in various circadian clock mutants.
Results
The chill-coma recovery times (CCRt) of wild-type flies raised at different photoperiods is sexually dimorphic (Figure 1). Females that developed under short winter-like photoperiod exhibit significantly shorter CCRt than females that were raised under long summer-like photoperiods (log rank test, χ2 = 11.8, df = 1, p<0.001). However, CCRt did not differ significantly between males raised on long vs. short days (Figure 1). Females kept in covered vials (in darkness, DD) within the same chambers also showed a moderate but significant differential response, driven by the low-amplitude (∼2°C) thermoperiod that was generated by the lighting system (log-rank test χ2 = 7.9, p<0.01, see Methods). In another set of experiments where the heat cycles produced by the two photoperiods were offset by a counteracting temperature cycle, there was no significant difference in the CCRt between long and short photoperiods in females kept in covered vials (in darkness, DD). The difference in median CCRt between long and short days in females exposed to light (driven by both photoperiod and thermoperiod) was twice as large as the difference exhibited by the thermoperiod only (DD) females (14 vs. 7 min). This difference in photoperiod was significant after statistically accounting for the temperature effect (via non-parametric ANCOVA, W = 664, p<0.001) confirming the interaction between photoperiod and thermoperiod.
Fig. 1. Photo/thermoperiodic-dependent temperature-tolerance in Drosophila. 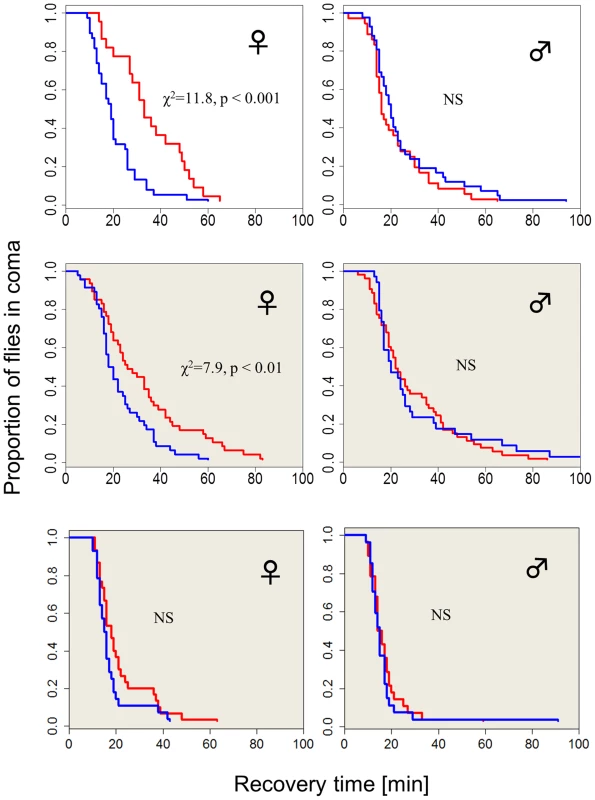
Survival curves showing recovery from coma of wild-type flies (Hu) that were developed in either short winter-like (blue) or long, summer-like (red) daylengths (N1, N2 = 38, 22). Flies exposed to diurnal light and temperature cycles are shown in the top panel, flies exposed only to the thermoperiod are depicted in the middle panel (shaded grey, N1, N2 = 47, 46). Wild-type females (but not males) that develop under short days exhibit significantly faster chill-coma recovery times (CCRt) than flies that were raised under long day photo- or thermoperiods. In a control experiment (bottom panel), where the flies are maintained in the photoperiod but in covered vials (DD), and with the thermoperiod overridden by a reversed temperature cycle (i.e. Δ T = 0°C), there is no detectable difference in CCRt (N1, N2 = 29, 24). We also tested the CCRt of wild-type females over a range of five photoperiods (Figure S1). For short photoperiods 8, 10 and 12 hr, the median CCRt was 15 min (with 95% CI overlapping 13–33). In the 14 hr photoperiod, the median was 13 min (12–19) and at 16 hr was 24 (20–30). Thus, an intermediate photoperiod (comparable to the critical day length in diapause studies) would lie within the 14–16 hr interval. We also examined the association between the CCRt and diapause propensity (Figure S2). Newly emerging females were maintained in diapausing inducing conditions (Methods). After 12 days their CCRt was tested, followed by ovary dissection for determining their reproductive state. The CCRt did not differ significantly between diapausing (n = 82) and non-diapausing (n = 39) females (χ2 = 0.1, df = 1, p = 0.70).
There was however a significant difference in female weight between long vs. short day (Figure S3): Fresh weight in short days was higher (F1,8 = 9.68, p<0.05), due to higher water content (F1,8 = 6.57, p<0.05), since dry weight was similar (F1,8 = 4.13, p = 0.07). Males also showed weight difference between long and short day, which was significant, both for fresh and dry weight (Figure S3). Thus, size or water differences cannot fully explain the photoperiodic CCRt response (which is absent in males). In addition, the CCRt of males resembles that of females in short days (although the size difference between the sexes is substantial).
The finding that female flies were able to discriminate between long and short days provided us with the opportunity to test the role of circadian clock genes in this response. The CCRt of females from congenic strains carrying per01, tim01, and ClkJrk mutations did not show any photo/thermoperiodic effect (Figure 2). In general, the mutant curves resembled the WT short-day response (median CCRt 19 min, range 15–25). For example, in long days, the median recovery time for per01 was 14 min (13–15), for ClkJrk 19 min (18–23) and for tim01 23.5 min (21–35). We did notice however, some differences in cold-tolerance among the mutants, particularly between per and the two other mutants (note the overlapping CIs).
Fig. 2. Photo/thermo-periodic response is disrupted in clock mutants. 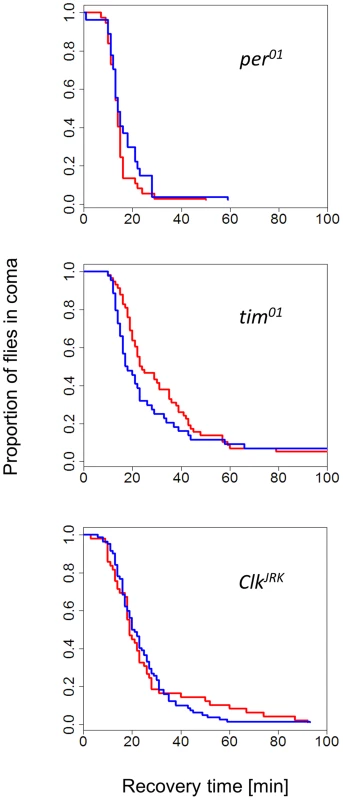
In clock mutant females per01 (N1, N2 = 37, 27), tim01 (N1, N2 = 58, 44), ClkJrk (N1, N2 = 49, 82) the differential CCRt response to day-length (flies exposed to photo/thermo-period cycles) is abolished. All strains have the same genetic background (Hu). Flies were developed in either short winter-like (blue) or long, summer-like (red) day-lengths. To gain insight into the metabolic correlates of the photoperiodic response, we measured glycogen, free fatty acids and protein levels (Figure S4). In wild-type females, glycogen was significantly higher in long days (F1,46 = 4.05, p = 0.05), while four time points taken during the day did not show any significant differences (F1,46 = 2.22, p = 0.14). In contrast, in ClkJrk females glycogen levels did not differ between photoperiods (F1,22 = 0.85, p = 0.37). For free fatty acids, neither the photoperiod nor the time of the day showed significant differences (Figure S4) in WT or ClkJrk. Similarly, total protein also did not differ between photoperiods, but intriguingly there was a significant photoperiod:Zt interaction in the ClkJrk mutants (F2,21 = 7.41, p<0.01).
The availability of mutants that exhibit a long or short circadian period provided us with a further opportunity for testing the Bünning hypothesis. We compared long and short mutant alleles of three genes (Figure 3): perL, perS (28.8 vs 19.3 hr circadian period [22]), dbtL, dbtS (26.8 vs 19.3 hr, [23]) and timUL, timS1 (32.7 vs 21.1 hr, [24]). An omnibus ANOVA for analysing the data of all genes simultaneously including experiments at different photo/thermo-periods resulted in a highly significant ‘allele’ factor (short v long period, F1,813 = 28.46, p<0.001) but not ‘photoperiod’ (F1,813 = 0.57, p = 0.45). There was no significant gene:photoperiod interaction (F2,813 = 1.39, p = 0.25). In all three genes, the CCRt of the long alleles was consistently shorter (Figure 3, particularly evident in per mutants), in both photoperiods, suggesting that the long period mutant perceive the day as shorter compared to short period mutants. This result is consistent with Bünning's original model (Figure 3). We also observed that CCRt does not fluctuate significantly throughout the day in WT, (χ2 = 1.4, df = 3, p = 0.7; Figure S5) so that the differences between the mutants is not due to our sampling of CCRt at different subjective phases. We have also compared the average phase angle of the light-entrained activity of the long and the short-period mutants (Figure S6). We estimated the phase values using the pooled locomotor activity profile (16–35 flies in each experiment), averaged over four days. Across genes (n = 3, each tested for two alleles, at two photoperiods, giving 12 data points), there was a significant difference between the long and the short alleles for both morning peak (MP; F1,9 = 140.8, p<0.0001) and evening peak (EP; F1,9 = 18.3, p<0.01). Both the MP and EP were advanced in short allele flies, but for the MP, the advance was enhanced in short day, resulting in significant allele: photoperiod interaction (F1,9 = 105, p<0.001).
Fig. 3. CCRt of long and short period alleles of clock genes. 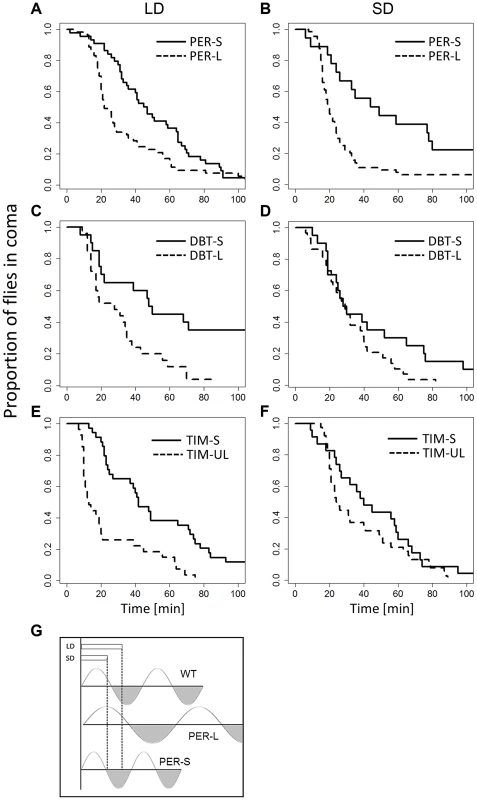
The response of mutant females maintained in long (left column) and short (right column) days (thermoperiods) is depicted. Response of perL and perS is shown in (A) (44, ÷2 = 11.9, p<0.001; N1, N2 = 53) and (B) (χ2 = 11.1, df = 1, p<0.001; N1, N2 = 53,44). The response of dbtL, dbtS is depicted in (C) (χ2 = 9.5,df = 1, p<0.05, N1, N2 = 29, 20) and (D) (χ2 = 2.8, df = 1, p = 0.093; N1, N2 = 29,20). The response of timS1 and timUL is shown in (E) (χ2 = 18.4, df = 1, p<0.001, N1, N2 = 27, 51) and (F) χ2 = 1.7, df = 1, P = 0.19; N1, N2 = 38, 23). G. Schematic diagram showing Bünnings' external coincidence detector in wild-type (WT) and mutant flies. In WT short days coincide with the photophil phase while long day extend to the schotophil phase (note that daylength may be either encoded by photoperiod or the thermoperiod). In long-period mutants, long days still coincide with the photophil phase, and are interpreted as short days, leading to a constitutively short day response. In short period mutants the photophil phase may be brief so even short day are interpreted as a long one. In both type of mutants, long and short daylength may coincide with the same phase of the detector, resulting in loss of the photo/thermo-periodic response. We have also explored the role of alternative splicing in the per locus that was previously associated with seasonal adaptation [25]–[27]. Specifically, under low temperatures, as well as short photoperiods, the splicing level of intron 8 in the 3′UTR of per is increased. To test the role of per splicing in CCRt, we used transgenic lines in which the splicing signal is missing and the intronic sequence cannot be spliced (type A), or a construct that does not contain the intron (type B′) [28]. The perA and perB′ transgenes were expressed in per01 flies. For each transgene two independent insertion lines were tested (see Methods) and their data were pooled. As shown in Figure 4, flies carrying the type B′ transgene showed shorter CCRt both under long or short photo/thermoperiod or thermoperiod alone. ANOVA incorporating all the conditions in which the transgenic flies were tested revealed a significant splice type factor (F1,634 = 8.53, p<0.01). In contrast to the wild-type Hu strains, the splice variants were not photoperiodic (Figure 4), presumably due to the different genetic background of the transformant flies (yw). The control lines perG did show a thermoperiodic response (in DD; χ2 = 10.1, p<0.01; N1,N2 = 128,112), but were not photoperiodic (LD: χ2 = 0.1 p = 0.77; N1,N2 = 110,121). In general the CCRt of perG resembled the response of perA with relatively longer medians (LD:SD = 33,35 min; in DD, LD:SD = 60, 39.5 min).
Fig. 4. Chill coma recovery in per splicing transgenic flies. 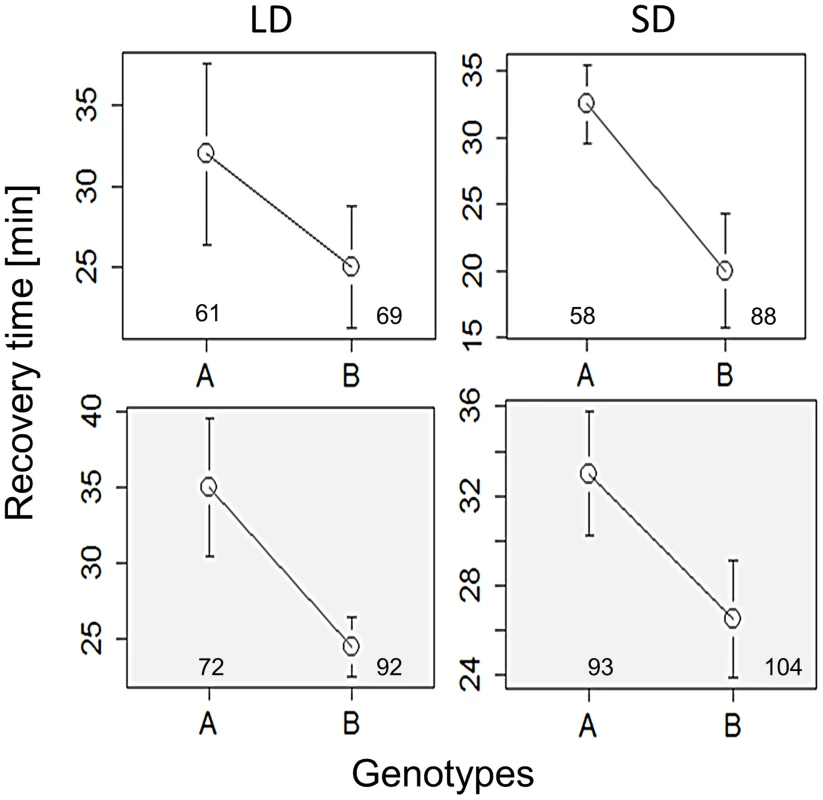
Medians of CCRt under long (left panels) and short day (right panels) driving by photo/thermo-periods, or by the thermoperiod only (shaded grey). Data were pooled for each of the two strains expressing each of the splice variant (A, B). Flies carrying Type B′ transgene, which is locked into the constitutive per 3′ UTR splice mode, show consistently shorter CCRt. Error bars represent SE. Number of females is also shown. Taken together, the results indicate some effect on cold tolerance for per splicing, further supporting the notion that the circadian clock or signalling to the circadian clock is involved in this seasonal adaptation.
Discussion
The chill-coma recovery test has been used in various insect species for studying cold tolerance and adaptation [29]. Recent studies in D. montana have demonstrated that the CCRt in this species is under photoperiodic regulation [30], [31], consistent with the expectation that the autumnal shortening of the day induces various process, including nutrient regulation and reserve accumulation that allows the flies to survive the winter.
Here, we have shown that a similar day-length regulation is present in D. melanogaster, and we exploited this response to study the link between the circadian clock and seasonal timing. While our experiments have not disentangled entirely the thermo - and photo-periodic effects, the difference in CCRt response in LD (day-length encoded by both photoperiod and thermoperiod), and DD (thermal information only) would suggest that both cues contribute to the response (Figure 1).
We show that in clock mutant strains per01, tim01 and ClkJrk the day-length measurement is disrupted. The lack of photoperiodic CCRt in per01 is in apparent contradiction to the previously reported photoperiodic diapause in this mutant [12]. Differences between the CCR and diapause phenotypes may represent two separate photoperiodic circuits that use different genetic networks, a situation which resembles the different circadian locomotor and eclosion circuits [32], [33]. Interestingly, Helfrich-Förster [34] analysed the bimodal locomotor activity profile of per mutants and suggested that the morning peak is derived from a per-independent circadian component (see also [35]), and that this component might be involved in photoperiodic timing. In addition, a recent study [36] using temperature entrainment suggests that per01 (and tim01) are not entirely clockless.
However, it should be noted that the reported photoperiodic response of diapause in per01 (and per deficiency flies) is altered because the critical day length (CDL) for inducing diapause is several hours shorter than in wild-type [12], [37]. In our experiments, the per01 mutants mirror their CDL and exhibit the shortest CCRt. This correlation may reflect the situation in the wild, where populations in colder environments (presumably more cold-adapted flies) would be expected to show a shorter CDL that will trigger diapause later in the season.
The substantial difference in CCRt between long - and short circadian period alleles is particularly informative (Figure 3). The constitutively ‘short-day’ response of the long-period mutants fits well with the ‘external co-incidence’ model underlying day-length measurements (Figure 3G). In wild-type flies, short days coincide with the photophil phase of the pacemaker, while long days extend to the scotophil phase of the oscillations. In long-period mutants (where photophil phase is longer), various daylengths always coincide with the photophil phase and are interpreted as short days, while in short-period mutants both long and short day-length may overlap with the scotophil phase and be interpreted as long days. The main weakness of this model is the requirement for a uniform waveform of the oscillation under long and short days. In Drosophila however, this model is unlikely to be valid, as the oscillation waveform of overt rhythms (locomotor activity) and level of clock proteins change during the seasons [35], [38]. Furthermore, the model (Figure 3) disregards the entrainment of the mutant oscillation during the seasons [35]. Depending of the phases of the mutants, different outcomes may be predicted (Figure S7). Indeed, we have observed a consistent phase difference between long and short period alleles (phase advance in short-period alleles, Figure S6). Interestingly, our data mirrors an early study of the eclosion rhythm of D. pseudoobscura [39], where wild-type flies kept in T cycles longer than the circadian period (resembling the short-period mutants in our study, kept under 24 hr cycle) showed a phase advance, and flies kept in T<τ exhibited phase delays. While the link between the circadian phase and the photoperiodic response is yet not clear, the different photoperiodic phenotypes of the slow and fast clock mutants seems to suggest a causative role for the circadian pacemaker in day-length measurement, but further experiments are required to identify the underlying model (external - vs. internal-coincidence, or any other model). A further analysis of the critical day length of the CCRt (Figure S1) in the long and short clock mutants may provide more insights about the link between the circadian system and the photoperiodic timer, and this will be published elsewhere. Similarly, using the Nanda-Hamner or Bünsow protocols [9] would provide more ways for testing the circadian role in the photoperiodic CCRt.
Our results also show that the regulation of per splicing, a process which was previously implicated in the fly's seasonal response [26], [27], is also involved in the CCRt, as flies carrying the type B′ transgene exhibited shorter recovery times compared with flies carrying type A (Figure 4). This fits well with the enhanced splicing at cold conditions in the type B′ transgenic per but contrasts with the observation that the seasonal locomotor activity profile of the two type of transgenic flies is similar [25]. This may suggests that retention or removal of the per 3′ intron is affecting the CCRt, while the splicing process itself is critical for the cold-induced phase advance in the locomotor activity rhythm. Our results reflect the seasonally adaptive nature of per splicing because during the autumnal shortening of the photoperiods (and decreasing temperatures), per splicing will inevitably increase [26], [35]. This will lead to locomotor changes but also, we suggest, to further physiological changes that may allow the fly to tolerate lower temperatures, as manifested by shorter CCRt (Figure 4).
The circadian clock and the photoperiodic timer appear to act as two modules, each consisting of a group of functionally related genes [40]. Genes interact primarily with genes within the same module, although individual genes may have an effect on the other module. Such pleiotropic effect of individual clock genes on diapause was proposed as an alternative explanation to the “Bünning hypothesis” [10]. In the current work however, the fact that a battery of circadian clock genes are implicated in daily light and temperature measurements strongly indicates that the two modules functionally interact, not simply as isolated pleiotropic effects of one gene on another module. Under pleiotropy, knockdown of different clock genes may results in different outcomes. For example, RNAi targeting either per or cyc in the bean bug Riportus pedestris led to aberrant photoperiodic response, but in opposite directions [17]. In contrast, in our experiments all null mutants exhibit the same trend (loss of long day response; Figure 2), further suggesting that the Bünning hypothesis provides the most parsimonious explanation.
The shortening of CCRt in flies exposed to short photoperiod, which was also recently reported for D. montana [30], reflects an enhanced cold tolerance acquired during development. This cold acclimation is presumably mediated by cold hardening, a process which involves changes in phospholipid fatty acids composition of cell membranes [41], [42], as well as polyols, sugars and other metabolites [43]. The ecological relevance of the improved cold tolerance following cold hardening was previously demonstrated in enhanced D. melanogaster survivorship [44] and reproductive behaviour [45] in flies primed for experiencing low temperatures.
Beyond demonstrating the role of the circadian clock in seasonal timing, our results here juxtapose the CCRt phenotype against female reproductive diapause, the classic readout for insect seasonality [46]. While cold hardiness is often associated with diapause our results suggest that the responses are triggered by different mechanisms (Figure S1). Similarly studies in D. montana shows that the CCRt is not always correlated with diapause and is strain-dependent [30].
For studying seasonal timing, analysing diapause involves extremely laborious dissection of ovaries, and the binary nature of the phenotype (diapause status) requires the processing of large sample sizes for detecting appreciable effects. In contrast, the automated CCRt phenotyping allows for high-throughput screening and the protocol requires that flies are maintained at 20°C, which is more conducive to GAL4 misexpression studies than diapause experiments that are usually performed at 12°C. CCRt thus provides a powerful and efficient method for dissecting the genetic and anatomical basis of seasonal timing in D. melanogaster.
Methods
Fly strains
The strains per01, tim01, ClkJrk were used. All strains were crossed to an isofemale strain originating from a wild Dutch population in Houten [14]. The progeny were screened for individuals carrying the mutation using PCR genotyping as previously described [47] and backcrossed to Hu, a process which was repeated 8 times, resulting in all the mutations inserted in the genomic Hu background (>99%) that also carries the ls-tim natural allelic variant [14]. Mutants were made homozygous by further crossing to balancer strains (also on a Hu background). In addition, the strains perL and perS [13], dbtS, dbtL [23], timUL and timS1 [48] were used (the genetic background of these mutants is not Hu). The mutant's circadian locomotor activity was verified at 19.5°C, which was used for the CCRt experiments (Figure S6).
To investigate the effect of per splicing on CCRt we used congenic transgenic lines that generate either type A (PERA-18, PERA-29) or type B′ (PERB′-11, PERB′-12) per RNA and have been used to rescue per01 flies. We have also tested transgenic flies expressing both type A and type B′ (perG). These lines have been described previously [28]. All strains were maintained at 25°C in LD 12∶12 on a standard cornmeal media.
The CCR protocol
Around 100 flies were kept on egg-collection food for 18 hr, and four replicates of 40 eggs each were transferred to new vials. The vials were placed in either long (16 hr) or short (8 hr) day using fluorescent light boxes. Temperature within the light boxes fluctuated during the LD cycle, due to heat produced by the florescent light, from 21°C during the light phase to 19°C during scotophase. Temperature was monitored by data loggers (Tinytag UK). Each experiment was replicated twice with two different light boxes (total of 8 vials). DD samples (vials covered by aluminum foil, providing constant darkness) were also included, and were used for analysing the effect of the thermoperiod (2°C cycling). The flies were developed under these conditions for 20 days, and emerging adults (age 3–4 days) were tested for their chill coma recovery as follows: At ZT 3.5 (ZT, Zeitgeber time, hr after lights on) the flies were anesthetized by ice, sexed and transferred individually to glass activity tubes (outer diameter = 5 mm, 80 mm) and cotton plugs were used to place the fly at the middle of the tube. The flies were kept on ice at 4°C for 3 hours. At ZT 6.5, the glass tubes were loaded into the Drosophix locomotor activity monitor (Padova, Italy), which was previously described [27] at 25°C. This infra-red based system uses the same glass tubes used by the Trikinetics system, but the space of the tube was reduced to 2 cm by cotton plugs (i.e. the fly was approximately 1 cm from the light beam).
The loading time (t0) for each fly was recorded by the system. A custom written script in “R” [49] was used to calculate the recovery time (CCRt), by subtracting t0 from the time of first movement detected by the system (consequently, our calculated recovery times are slightly longer, by definition, from previous studies, where recovery time was defined as the time in which the fly was first observed standing).
Given the exponential distribution of the CCRt, these data are best analysed by survival curves. We used the Survival R library to fit Kaplan-Meier curves, and log-rank tests to compare the different curves, using χ2 statistics with one degree of freedom [50] We used ANOVA to test the contribution of various factors across different experiments (e.g. day-length, sex, photo - vs. thermoperiod entrainment, etc.). For this purpose we used log transformation for variance stabilisation. To compare the effect of photoperiod after correcting for temperature effect, a non-parametric ANCOVA was carried using the Quade procedure [51]. Briefly, the CCRt irrespective of group membership (photo - or thermo-periodic) were ranked, and regressed over day-length. The residuals were then compared by the Wilcoxon rank sum test.
Diapause
Male and female flies (Hu genetic background) were collected within a six hour post eclosion window and placed under 8∶16LD (light∶Dark) at 12.2±0.2°C. After 12 days, CCRt was measured and immediately followed by dissection of the female ovaries in PBS. Reproductive arrest was determined as previously described [14]. The diapause level in females that were maintained under the same conditions but were not tested for CCRt was not significantly different from females that were exposed to coma inducing temperature (F1,14 = 0.44, p = 0.51), indicating that the cold treatment did not contribute to diapause state.
Free fatty acid, glycogen and proteins measurements
Flies developing under LD and SD (see CCR protocol) were collected (4 days old) at four time points (Zt1, 7, 13 and 19) and immediately frozen in liquid nitrogen and stored at −80C. The fresh weight of 10 individuals was recorded after a 5 min thaw in ice with a precision balance (Precisa180A). Glycogen, proteins and free fatty acid content were measured in these samples and expressed as µg or nmol per fresh weight. Glycogen concentration was obtained from samples that were homogenized in 100 µl of water in ice for 30 sec. After centrifugation at top speed for 5 min (4°C), the homogenates (10 µl were saved for protein assay) were boiled for 5 min and Hydrolysis buffer was added to final volume of 120 µl (Sigma-Aldrich, MAK016). Glycogen content was assayed by colorimetric reaction (570 nm) after treatment with Hydrolysis Enzyme and Development Enzyme (Sigma-Aldrich, MAK016). Glucose background was removed from each sample. 10 µl of homogenates were diluted 10 times in water and proteins quantified spectrophotometrically (595 nm) using Bradford reagent (10 µl diluted samples +290 µl reagent; Sigma-Aldrich, B6916). Total proteins were quantified using a BSA (10 mg/ml) standard curve. The free fatty acids were isolated from samples homogenized for 30 sec in ice in 200 ul chloroform-1% Triton X-100. After 10 min centrifugation the organic phase was isolated and vacuum dried for 30 min to remove the chloroform. The lipids were dissolved in 200 ul of fatty acid buffer (ABCAM ab65341). Free fatty acid content was assayed by colorimetric reaction (570 nm) after Acyl-CoA synthesis (ABCAM ab65341). FLUOStar omega plate reader was used for both colorimetric reactions and the Bradford assay.
Fresh, dry weight and water content
Fresh weight (FW, g) was measure from samples collected at Zt 3.5 (LD and SD) using a precision balance (Precisa180A). Dry weight (DW, g) was measured after desiccating the sample at 60°C for 3 days. The difference between FW and DW indicate the water content (WC, g).
Locomotor activity
The locomotor activity of 3–4 days old virgins was measure at 19.5±0.5°C using the Trikinetics system. The activity of flies was recorded during 4 days entrainment (either LD or SD) follow by 5 days of constant darkness (DD). The DD activity was also used to calculate the flies' circadian period of activity using “Cosinor analysis” [52], which employs the least squares method to fit a sine wave to a time series. Monte Carlo simulations (n = 100) were used to estimate 99% significant level. For phase analysis, the morning and the evening peak were recorded and converted into degrees. Because the data are circular, large angles (>270°) were converted to negative values (subtracting 360°).
Supporting Information
Zdroje
1. BradshawWE, HolzapfelCM (2010) Light, time, and the physiology of biotic response to rapid climate change in animals. Annu Rev Physiol 72 : 147–166.
2. SaundersDS, LewisRD, WarmanGR (2004) Photoperiodic induction of diapause: Opening the black box. Physiol Entomol 29(1): 1–15.
3. SawaM, NusinowDA, KaySA, ImaizumiT (2007) FKF1 and GIGANTEA complex is required for day-length measurement in Arabidopsis. Science 318 : 261–265.
4. SawaM, KaySA, ImaizumiT (2008) Photoperiodic flowering occurs under internal and external coincidence. Plant Signal Behav 3 : 269–271.
5. DardenteH, WyseC, BirnieM, DupréS, LoudonA, et al. (2010) A molecular switch for photoperiod responsiveness in mammals. Curr Biol 20 : 2193–2198.
6. HogeneschJB, UedaHR (2011) Understanding systems-level properties: Timely stories from the study of clocks. Nat Rev Genet 12 (6) 407–416.
7. BünningE (1936) Die endonome tagesrhythmik als grundlage der photoperiodischen reaktion. Ber Dtsch Bot Ges 54 : 590–607.
8. PittendrighCS, MinisDH (1964) The entrainment of circadian oscillations by light and their role as photoperiodic clocks. Am Naturalist 98 : 261–294.
9. TauberE, KyriacouBP (2001) Insect photoperiodism and circadian clocks: Models and mechanisms. J Biol Rhythms 16 (4) 381–90.
10. BradshawWE, HolzapfelCM (2010) What season is it anyway? circadian tracking vs. photoperiodic anticipation in insects. J Biol Rhythms 25 (3) 155–165.
11. HardinPE (2005) The circadian timekeeping system of drosophila. Curr Biol 15 (17) R714–22.
12. SaundersDS, HenrichVC, GilbertLI (1989) Induction of diapause in Drosophila melanogaster - photoperiodic regulation and the impact of arrhythmic clock mutations on time measurement. Proc Natl Acad Sci U S A 86 (10) 3748–3752.
13. SaundersDS (1990) The circadian basis of ovarian diapause regulation in Drosophila melanogaster - is the period gene causally involved in photoperiodic time measurement. J Biol Rhythms 5 (4) 315–331.
14. TauberE, ZordanM, SandrelliF, PegoraroM, OsterwalderN, et al. (2007) Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science 316 (5833) 1895–8.
15. SandrelliF, TauberE, PegoraroM, MazzottaG, CisottoP, et al. (2007) A molecular basis for natural selection at the timeless locus in Drosophila melanogaster. Science 316 (5833) 1898–1900.
16. PavelkaJ, ShimadaK, KostalV (2003) TIMELESS: A link between fly's circadian and photoperiodic clocks? Eur J Entomol 100 (2) 255–265.
17. IkenoT, TanakaSI, NumataH, GotoSG (2010) Photoperiodic diapause under the control of circadian clock genes in an insect. BMC Biol 8 : 116.
18. YamadaH, YamamotoMT (2011) Association between circadian clock genes and diapause incidence in Drosophila triauraria. PLoS One 6 (12) e27493.
19. BradshawWE, HolzapfelCM (2010) Circadian clock genes, ovarian development and diapause. BMC Biol 8 : 115.
20. Jean DavidR, GibertP, PlaE, PetavyG, KaranD, et al. (1998) Cold stress tolerance in Drosophila: Analysis of chill coma recovery in D. melanogaster. J Therm Biol 23 (5) 291–299.
21. LancianiCA, LippKE, GieselJT (1992) The effect of photoperiod on cold tolerance in Drosophila melanogaster. J Therm Biol 17 (3) 147–148.
22. RutilaJE, SuriV, LeM, SoWV, RosbashM, et al. (1998) CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell 93 (5) 805–814.
23. PriceJL, BlauJ, RothenfluhA, AbodeelyM, KlossB, et al. (1998) Double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell 94 (1) 83–95.
24. RothenfluhA, YoungMW, SaezL (2000) A TIMELESS-independent function for PERIOD proteins in the Drosophila clock. Neuron 26 (2) 505–514.
25. MajercakJ, SidoteD, HardinPE, EderyI (1999) How a circadian clock adapts to seasonal decreases in temperature and day length. Neuron 24 (1) 219–30.
26. MajercakJ, ChenWF, EderyI (2004) Splicing of the period gene 3′-terminal intron is regulated by light, circadian clock factors, and phospholipase C. Mol Cell Biol 24 (8) 3359–3372.
27. CollinsBH, RosatoE, KyriacouCP (2004) Seasonal behavior in Drosophila melanogaster requires the photoreceptors, the circadian clock, and phospholipase C. Proc Natl Acad Sci U S A 101 (7) 1945–50.
28. ChengY, GvakhariaB, HardinPE (1998) Two alternatively spliced transcripts from the Drosophila period gene rescue rhythms having different molecular and behavioral characteristics. Mol Cell Biol 18 (11) 6505–6514.
29. MacmillanHA, SinclairBJ (2011) Mechanisms underlying insect chill-coma. J Insect Physiol 57 (1) 12–20.
30. VesalaL, HoikkalaA (2011) Effects of photoperiodically induced reproductive diapause and cold hardening on the cold tolerance of Drosophila montana. J Insect Physiol 57 (1) 46–51.
31. VesalaL, SalminenTS, KankareM, HoikkalaA (2012) Photoperiodic regulation of cold tolerance and expression levels of regucalcin gene in Drosophila montana. J Insect Physiol 58 : 704–709.
32. EngelmannW, MackJ (1978) Different oscillators control the circadian rhythm of eclosion and activity in Drosophila. Journal of Comparative Physiology 127 (3) 229–237.
33. SheebaV, ChandrashekaranMK, JoshiA, Kumar SharmaV (2001) A case for multiple oscillators controlling different circadian rhythms in Drosophila melanogaster. J Insect Physiol 47 (10) 1217–1225.
34. Helfrich-ForsterC (2001) The locomotor activity rhythm of Drosophila melanogaster is controlled by a dual oscillator system. J Insect Physiol 47 (8) 877–887.
35. VaninS, BhutaniS, MontelliS, MenegazziP, GreenEW, et al. (2012) Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature 484 (7394) 371–375.
36. BywalezW, MenegazziP, RiegerD, SchmidB, Helfrich-ForsterC, et al. (2012) The dual-oscillator system of Drosophila melanogaster under natural-like temperature cycles. Chronobiol Int 29 (4) 395–407.
37. WulbeckC, SzaboG, ShaferOT, Helfrich-ForsterC, StanewskyR (2005) The novel Drosophila tim(blind) mutation affects behavioral rhythms but not periodic eclosion. Genetics 169 (2) 751–766.
38. MenegazziP, VaninS, YoshiiT, RiegerD, HermannC, et al. (2013) Drosophila clock neurons under natural conditions. J Biol Rhythms 28 (1) 3–14.
39. PittendrighCS, MinisDH (1964) The entrainment of circadian oscillations by light and their role as photoperiodic clocks. Amer Nat 98 : 261–294.
40. EmersonKJ, BradshawWE, HolzapfelCM (2009) Complications of complexity: Integrating environmental, genetic and hormonal control of insect diapause. Trends Genet 25 (5) 217–225.
41. OvergaardJ, SorensenJG, PetersenSO, LoeschckeV, HolmstrupM (2005) Changes in membrane lipid composition following rapid cold hardening in Drosophila melanogaster. J Insect Physiol 51 (11) 1173–1182.
42. GotoSG, UdakaH, UedaC, KatagiriC (2010) Fatty acids of membrane phospholipids in Drosophila melanogaster lines showing rapid and slow recovery from chill coma. Biochem Biophys Res Commun 391 (2) 1251–1254.
43. MichaudMR, DenlingerDL (2007) Shifts in the carbohydrate, polyol, and amino acid pools during rapid cold-hardening and diapause-associated cold-hardening in flesh flies (Sarcophaga crassipalpis): A metabolomic comparison. J Comp Physiol B 177 (7) 753–763.
44. CzajkaMC, LeeREJr (1990) A rapid cold-hardening response protecting against cold shock injury in Drosophila melanogaster. J Exp Biol 148 : 245–254.
45. ShreveSM, KeltyJD, LeeRE (2004) Jr (2004) Preservation of reproductive behaviors during modest cooling: Rapid cold-hardening fine-tunes organismal response. J Exp Biol 207 (Pt 11) 1797–1802.
46. SchiesariL, KyriacouCP, CostaR (2011) The hormonal and circadian basis for insect photoperiodic timing. FEBS Lett 585 (10) 1450–1460.
47. Gesto J. (2010) Circadian clock genes and seasonal behaviour. PhD thesis (University of Leicester, Leicester, UK).
48. RothenfluhA, AbodeelyM, PriceJL, YoungMW (2000) Isolation and analysis of six timeless alleles that cause short - or long-period circadian rhythms in Drosophila. Genetics 156 (2) 665–75.
49. R Development Core Team. (2010) R: A language and environment for statistical computing, 2.10.1.
50. HarringtonDP, FlemingTR (1982) A class of rank test procedures for censored survival data. Biometrika 69 (3) 553–566.
51. QuadeD (1967) Rank analysis of covariance. Journal of the American Statistical Association 62 : 1187–1200.
52. NelsonW, TongYL, LeeJK, HalbergF (1979) Methods for cosinor-rhythmometry. Chronobiologia 6 (4) 305–323.
Štítky
Genetika Reprodukčná medicína
Článek An Evolutionarily Conserved Role for the Aryl Hydrocarbon Receptor in the Regulation of MovementČlánek Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced ActivityČlánek Co-regulated Transcripts Associated to Cooperating eSNPs Define Bi-fan Motifs in Human Gene NetworksČlánek Identification of a Regulatory Variant That Binds FOXA1 and FOXA2 at the Type 2 Diabetes GWAS LocusČlánek tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa CellsČlánek Derlin-1 Regulates Mutant VCP-Linked Pathogenesis and Endoplasmic Reticulum Stress-Induced ApoptosisČlánek A Genetic Assay for Transcription Errors Reveals Multilayer Control of RNA Polymerase II FidelityČlánek The Proprotein Convertase KPC-1/Furin Controls Branching and Self-avoidance of Sensory Dendrites inČlánek Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell SenescenceČlánek BMPs Regulate Gene Expression in the Dorsal Neuroectoderm of and Vertebrates by Distinct MechanismsČlánek Unkempt Is Negatively Regulated by mTOR and Uncouples Neuronal Differentiation from Growth Control
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Translational Regulation of the Post-Translational Circadian Mechanism
- An Evolutionarily Conserved Role for the Aryl Hydrocarbon Receptor in the Regulation of Movement
- Eliminating Both Canonical and Short-Patch Mismatch Repair in Suggests a New Meiotic Recombination Model
- Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced Activity
- Co-regulated Transcripts Associated to Cooperating eSNPs Define Bi-fan Motifs in Human Gene Networks
- Targeted H3R26 Deimination Specifically Facilitates Estrogen Receptor Binding by Modifying Nucleosome Structure
- Role for Circadian Clock Genes in Seasonal Timing: Testing the Bünning Hypothesis
- The Tandem Repeats Enabling Reversible Switching between the Two Phases of β-Lactamase Substrate Spectrum
- The Association of the Vanin-1 N131S Variant with Blood Pressure Is Mediated by Endoplasmic Reticulum-Associated Degradation and Loss of Function
- Identification of a Regulatory Variant That Binds FOXA1 and FOXA2 at the Type 2 Diabetes GWAS Locus
- Regulation of Flowering by the Histone Mark Readers MRG1/2 via Interaction with CONSTANS to Modulate Expression
- The Actomyosin Machinery Is Required for Retinal Lumen Formation
- Plays a Conserved Role in Assembly of the Ciliary Motile Apparatus
- Hidden Diversity in Honey Bee Gut Symbionts Detected by Single-Cell Genomics
- Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria
- tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa Cells
- Causal Variation in Yeast Sporulation Tends to Reside in a Pathway Bottleneck
- Tissue-Specific RNA Expression Marks Distant-Acting Developmental Enhancers
- WC-1 Recruits SWI/SNF to Remodel and Initiate a Circadian Cycle
- Clonal Expansion of Early to Mid-Life Mitochondrial DNA Point Mutations Drives Mitochondrial Dysfunction during Human Ageing
- Methylation QTLs Are Associated with Coordinated Changes in Transcription Factor Binding, Histone Modifications, and Gene Expression Levels
- Differential Management of the Replication Terminus Regions of the Two Chromosomes during Cell Division
- Obesity-Linked Homologues and Establish Meal Frequency in
- Derlin-1 Regulates Mutant VCP-Linked Pathogenesis and Endoplasmic Reticulum Stress-Induced Apoptosis
- Stress-Induced Nuclear RNA Degradation Pathways Regulate Yeast Bromodomain Factor 2 to Promote Cell Survival
- The MAPK p38c Regulates Oxidative Stress and Lipid Homeostasis in the Intestine
- Widespread Genome Reorganization of an Obligate Virus Mutualist
- Trans-kingdom Cross-Talk: Small RNAs on the Move
- The Vip1 Inositol Polyphosphate Kinase Family Regulates Polarized Growth and Modulates the Microtubule Cytoskeleton in Fungi
- Myosin Vb Mediated Plasma Membrane Homeostasis Regulates Peridermal Cell Size and Maintains Tissue Homeostasis in the Zebrafish Epidermis
- GLD-4-Mediated Translational Activation Regulates the Size of the Proliferative Germ Cell Pool in the Adult Germ Line
- Genome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
- Translational Regulation of the DOUBLETIME/CKIδ/ε Kinase by LARK Contributes to Circadian Period Modulation
- Positive Selection and Multiple Losses of the LINE-1-Derived Gene in Mammals Suggest a Dual Role in Genome Defense and Pluripotency
- Out of Balance: R-loops in Human Disease
- A Genetic Assay for Transcription Errors Reveals Multilayer Control of RNA Polymerase II Fidelity
- Altered Behavioral Performance and Live Imaging of Circuit-Specific Neural Deficiencies in a Zebrafish Model for Psychomotor Retardation
- Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development
- Meta-analysis of Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments
- The Proprotein Convertase KPC-1/Furin Controls Branching and Self-avoidance of Sensory Dendrites in
- Hydroxymethylated Cytosines Are Associated with Elevated C to G Transversion Rates
- Memory and Fitness Optimization of Bacteria under Fluctuating Environments
- Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell Senescence
- Interspecific Tests of Allelism Reveal the Evolutionary Timing and Pattern of Accumulation of Reproductive Isolation Mutations
- PRO40 Is a Scaffold Protein of the Cell Wall Integrity Pathway, Linking the MAP Kinase Module to the Upstream Activator Protein Kinase C
- Low Levels of p53 Protein and Chromatin Silencing of p53 Target Genes Repress Apoptosis in Endocycling Cells
- SPDEF Inhibits Prostate Carcinogenesis by Disrupting a Positive Feedback Loop in Regulation of the Foxm1 Oncogene
- RRP6L1 and RRP6L2 Function in Silencing Regulation of Antisense RNA Synthesis
- BMPs Regulate Gene Expression in the Dorsal Neuroectoderm of and Vertebrates by Distinct Mechanisms
- Unkempt Is Negatively Regulated by mTOR and Uncouples Neuronal Differentiation from Growth Control
- Atkinesin-13A Modulates Cell-Wall Synthesis and Cell Expansion in via the THESEUS1 Pathway
- Dopamine Signaling Leads to Loss of Polycomb Repression and Aberrant Gene Activation in Experimental Parkinsonism
- Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation
- Bipartite Recognition of DNA by TCF/Pangolin Is Remarkably Flexible and Contributes to Transcriptional Responsiveness and Tissue Specificity of Wingless Signaling
- The Olfactory Transcriptomes of Mice
- Muscular Dystrophy-Associated and Variants Disrupt Nuclear-Cytoskeletal Connections and Myonuclear Organization
- Interplay of dFOXO and Two ETS-Family Transcription Factors Determines Lifespan in
- Evidence for Widespread Positive and Negative Selection in Coding and Conserved Noncoding Regions of
- Genome-Wide Association Meta-analysis of Neuropathologic Features of Alzheimer's Disease and Related Dementias
- Rejuvenation of Meiotic Cohesion in Oocytes during Prophase I Is Required for Chiasma Maintenance and Accurate Chromosome Segregation
- Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals
- Local Effect of Enhancer of Zeste-Like Reveals Cooperation of Epigenetic and -Acting Determinants for Zygotic Genome Rearrangements
- Differential Responses to Wnt and PCP Disruption Predict Expression and Developmental Function of Conserved and Novel Genes in a Cnidarian
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals
- Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development
- Genome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
- Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



