-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
α-Actinin-3: Why Gene Loss Is an Evolutionary Gain
article has not abstract
Published in the journal: α-Actinin-3: Why Gene Loss Is an Evolutionary Gain. PLoS Genet 11(1): e32767. doi:10.1371/journal.pgen.1004908
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1004908Summary
article has not abstract
Introduction
Large-scale sequencing of human populations has revealed many regions of the genome that have undergone positive selection during recent human evolution [1]. For most such regions, the genes and the nucleotide variants under selection are challenging to identify, and one can only guess about the cellular and physiological mechanisms. In this issue of PLOS Genetics, Head et al. [2] shed light on this question for one of the most fascinating examples of selection, in part because the variant undergoing selection is a loss-of-function, and in part because it was discovered long before the human genome sequence was completed.
Originally identified during a search for muscular dystrophy defects [3], deficiency of α-actinin-3 later turned out to be surprisingly common [4]. Roughly 18% of the world population is homozygous for a nonsense mutation (R577X) in ACTN3 deficiency, and the derivative allele (ACTN3 577xx) frequency correlates with greater latitude and lower temperature [5]. There is an intriguing correlation with athletic performance—the derivative allele is overrepresented among elite marathoners and other endurance athletes, but underrepresented among elite sprinters—indeed, the ancestral allele has been referred to as “the gene for speed” [6]. The evidence for positive selection of the derivative allele in European and East Asian populations is strong, but the phenotype being selected is uncertain and the underlying cell biology is even less clear. The article by Head et al. [2] provides some clarity and, together with earlier work from our group (Bruton et al. [7]), a unifying hypothesis.
Background
To put the work on mechanism into context, it is helpful to review some of the basics of ACTN3 biology. The ACTN3 gene is only expressed in glycolytic, fast-twitch (type II) skeletal muscle fibers, where it binds to actin and is part of the Z-line in the sarcomere structure [8]. Considerable insight into function has come from knockout mice: fast-twitch muscle fibers of Actn3 knockout (KO) mice have increased aerobic capacity with increased citrate synthase (CS) activity and higher expression of mitochondrial proteins, such as cytochrome c oxidase and porin [4]. The Actn3 KO mice can cover more distance on a treadmill, and therefore exhibit adaptations also observed in response to endurance exercise [9].
One interesting aspect of Actn3 KO muscle is an increase in calcineurin (CaN) signaling [10]. CaN, together with calmodulin kinase (CaMK), acts as a Ca2+ decoder that responds to increases in Ca2+ and trigger intracellular signaling [11]. Wright et al. showed that mitochondrial biogenesis is activated in skeletal muscle by artificially increasing cytosolic [Ca2+] with caffeine; e.g., increases in citrate synthase and cytochrome c oxidase mRNA were observed 24 hours after caffeine exposure [12]. They also observed an increase in peroxisome proliferator-activated receptor ɣ coactivator 1-α (PGC-1α) [12], which is regarded as key promoter of mitochondrial biogenesis [13, 14].
Work from our group (Bruton et al.) showed that in cold-exposed mice, there was also a link between sarcoplasmic reticulum (SR) Ca2+ leak and mitochondrial biogenesis. Non-shivering muscles of cold-exposed mice displayed increased expression of PGC-1α with subsequent increases in citrate synthase activity and endurance [7].
Bringing It All Together
In this issue of PLOS Genetics, Head et al. [2] observed marked changes in cellular Ca2+ handling in fast-twitch muscles of Actn3 KO mice. These muscles expressed more of the SR Ca2+ ATPase 1 (SERCA1) and the SR Ca2+ buffering proteins calsequestrin 1 and sarcolumenin. Muscle fibers of Actn3 KO mice showed 3 - to 4-fold increases in SR Ca2+ leak and Ca2+ reuptake. Moreover, cytoplasmic Ca2+ transients were better maintained during repeated tetanic stimulation, which is in accordance with previously published data showing increased fatigue resistance in muscles of Actn3 KO mice (Fig. 1).
Fig. 1. Ca2+, heat, and mitochondrial biogenesis. 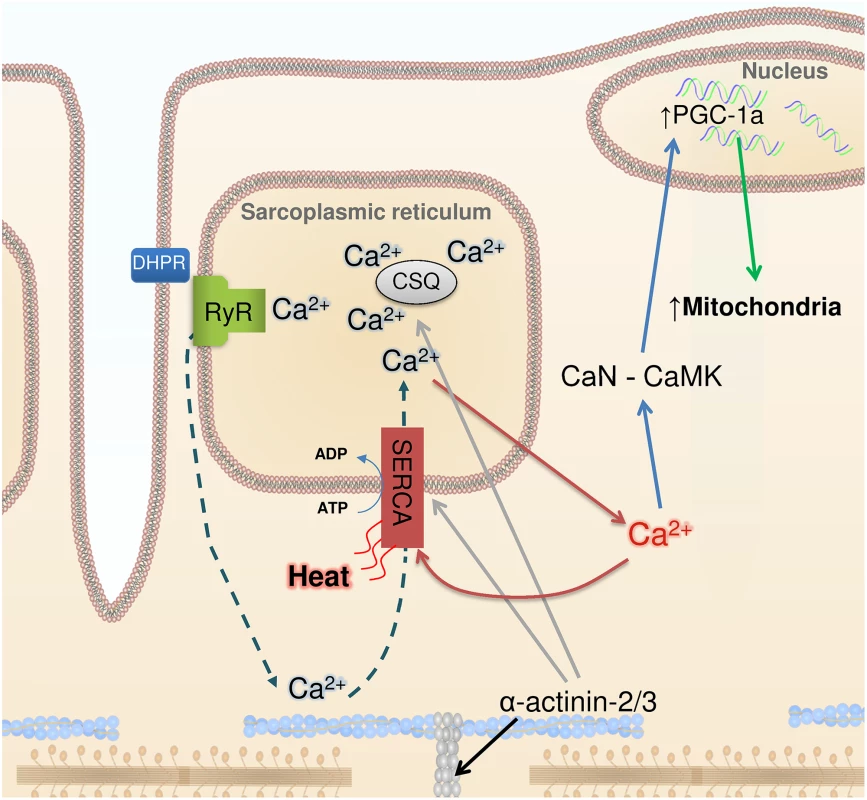
The contraction of skeletal muscle fibers is initiated by sarcoplasmic reticulum (SR) Ca2+ release via the ryanodine receptors (RyR), which is triggered by action potential activation of the transverse tubular voltage sensors, the dihydropyridine receptors (DHPR). Ca2+ activates the contractile machinery and is subsequently pumped back into the SR via SERCA (dashed arrows). α-Actinin 3 deficiency results in increased protein expression of SERCA and the SR Ca2+ buffers calsequestrin (CSQ) (grey arrows) and sarcalumenin (not shown). These changes are accompanied by increased SR Ca2+ leak and, subsequently, increased Ca2+ reuptake (red arrows), which generates heat. Increased [Ca2+] in the cytosol can trigger calcineurin (CaN) and calmodulin kinase (CaMK), resulting in PGC-1α activation (blue arrows) and subsequent mitochondrial biogenesis (green arrow). Head et al. highlight the similar adaptations in Actn3 KO muscles and non-shivering muscles of cold-acclimated mice, which also show increased SR Ca2+ leak and are more fatigue resistant [7]. An increased SR Ca2+ leak would require increased SR Ca2+ re-uptake and increased SERCA1 ATP hydrolysis, which would generate heat. Thus, in addition to heat from activation of brown adipose tissue [15], fatigue-resistant muscle fibers with leaky SR would contribute to non-shivering thermogenesis, providing a tentative explanation for the evolutionary advantage of carrying the ACTN3 577xx gene in a cold climate.
Unanswered Questions and Future Perspectives
From a cell biologic perspective, the source of the SR Ca2+ leak in Actn3 KO muscle is not yet clear. Head et al. [2] suggest that the major source is via SERCA [16]; alternatively, it might be due to destabilized SR Ca2+ release channel (ryanodine receptor, RyR) protein complexes [7, 17, 18]. Regardless, the SR Ca2+ leak seems to enhance the oxidative capacity of muscle in a number of settings: development, as with the Actn3 KO mice; stress, such as cold exposure; and, possibly, endurance exercise.
From an evolutionary perspective, the SR Ca2+ leak may be good for ancestral humans in cold climates and good for endurance athletes, but it is also known to be deleterious in aging-associated muscle weakness [19], in muscular dystrophies [18], and in response to excessive endurance training (“overtraining”) [17]. In this respect, the evolutionary balance between the functional and non-functional ACTN3 alleles may be “playing with fire”, as exemplified by results from cold-exposed mice. In these animals, we noted that minor modifications in the RyR protein complex were accompanied by larger cytosolic [Ca2+] during contractions and increased fatigue resistance [7] in non-shivering muscle. In more stressed, shivering muscle, however, severe RyR modifications led to decreased tetanic [Ca2+] and muscle weakness [20].
Human evolution and athletic performance are fascinating, but the findings of Head et al. provide additional avenues for future studies with important implications for human health, since the benefits of improved mitochondrial function span far beyond increased exercise capacity. Obesity and the metabolic syndrome are associated with impaired mitochondrial function, and of course, constitute a widespread and rapidly increasing health problem. Could strategies that phenocopy the effects of the ACTN3 577xx allele promote increased energy expenditure and improved mitochondrial function without requiring an increase in physical activity? Perhaps treatments to induce a controlled SR Ca2+ leak provide such an opportunity, but then the risk of causing impaired muscle function due to excessive Ca2+ leakage has to be overcome.
Zdroje
1. Danecek P, Auton A, Abecasis G, Albers CA, Banks E, et al. (2011) The variant call format and VCFtools. Bioinformatics 27 : 2156–2158. doi: 10.1093/bioinformatics/btr330 21653522
2. Head SI, Chan S, Houweling PJ, Quinlan KGR, Murphy R, et al. (2015) Altered Ca2+ kinetics associated with α-actinin-3 deficiency may explain positive selection for ACTN3 null allele in human evolution. PLoS Genet 11: e1004862.
3. North KN, Beggs AH (1996) Deficiency of a skeletal muscle isoform of alpha-actinin (alpha-actinin-3) in merosin-positive congenital muscular dystrophy. Neuromuscul Disord 6 : 229–235. doi: 10.1016/0960-8966(96)00361-6 8887951
4. MacArthur DG, Seto JT, Raftery JM, Quinlan KG, Huttley GA, et al. (2007) Loss of ACTN3 gene function alters mouse muscle metabolism and shows evidence of positive selection in humans. Nat Genet 39 : 1261–1265. doi: 10.1038/ng2122 17828264
5. Friedlander SM, Herrmann AL, Lowry DP, Mepham ER, Lek M, et al. (2013) ACTN3 allele frequency in humans covaries with global latitudinal gradient. PLoS ONE 8: e52282. doi: 10.1371/journal.pone.0052282 23359641
6. Vincent B, De Bock K, Ramaekers M, Van den Eede E, Van Leemputte M, et al. (2007) ACTN3 (R577X) genotype is associated with fiber type distribution. Physiol Genomics 32 : 58–63. doi: 10.1152/physiolgenomics.00173.2007 17848603
7. Bruton JD, Aydin J, Yamada T, Shabalina IG, Ivarsson N, et al. (2010) Increased fatigue resistance linked to Ca2+-stimulated mitochondrial biogenesis in muscle fibres of cold-acclimated mice. J Physiol 588 : 4275–4288. doi: 10.1113/jphysiol.2010.198598 20837639
8. Mills M, Yang N, Weinberger R, Vander Woude DL, Beggs AH, et al. (2001) Differential expression of the actin-binding proteins, alpha-actinin-2 and -3, in different species: implications for the evolution of functional redundancy. Hum Mol Genet 10 : 1335–1346. doi: 10.1093/hmg/10.13.1335 11440986
9. Tonkonogi M, Harris B, Sahlin K (1997) Increased activity of citrate synthase in human skeletal muscle after a single bout of prolonged exercise. Acta Physiol Scand 161 : 435–436. doi: 10.1046/j.1365-201X.1997.00233.x 9401597
10. Seto JT, Quinlan KG, Lek M, Zheng XF, Garton F, et al. (2013) ACTN3 genotype influences muscle performance through the regulation of calcineurin signaling. J Clin Invest 123 : 4255–4263. doi: 10.1172/JCI67691 24091322
11. Tavi P, Westerblad H (2011) The role of in vivo Ca2+ signals acting on Ca2+–calmodulin-dependent proteins for skeletal muscle plasticity. J Physiol 589 : 5021–5031. doi: 10.1113/jphysiol.2011.212860 21911615
12. Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO (2007) Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem 282 : 18793–18799. doi: 10.1074/jbc.M611252200 17488713
13. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, et al. (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98 : 115–124. doi: 10.1016/S0092-8674(00)80611-X 10412986
14. Arany Z (2008) PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr Opin Genet Dev 18 : 426–434. doi: 10.1016/j.gde.2008.07.018 18782618
15. Cannon B, Nedergaard J (2011) Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 214 : 242–253. doi: 10.1242/jeb.050989 21177944
16. Murphy RM, Larkins NT, Mollica JP, Beard NA, Lamb GD (2009) Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast - and slow-twitch fibres of rat. J Physiol 587 : 443–460. doi: 10.1113/jphysiol.2008.163162 19029185
17. Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, et al. (2008) Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. PNAS 105 : 2198–2202. doi: 10.1073/pnas.0711074105 18268335
18. Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, et al. (2009) Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 15 : 325–330. doi: 10.1038/nm.1916 19198614
19. Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, et al. (2011) Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 14 : 196–207. doi: 10.1016/j.cmet.2011.05.014 21803290
20. Aydin J, Shabalina IG, Place N, Reiken S, Zhang SJ, et al. (2008) Nonshivering thermogenesis protects against defective calcium handling in muscle. FASEB J 22 : 3919–3924. doi: 10.1096/fj.08-113712 18687806
Štítky
Genetika Reprodukčná medicína
Článek Phosphorylation of Elp1 by Hrr25 Is Required for Elongator-Dependent tRNA Modification in YeastČlánek Naturally Occurring Differences in CENH3 Affect Chromosome Segregation in Zygotic Mitosis of HybridsČlánek Insight in Genome-Wide Association of Metabolite Quantitative Traits by Exome Sequence AnalysesČlánek ALIX and ESCRT-III Coordinately Control Cytokinetic Abscission during Germline Stem Cell DivisionČlánek Deciphering the Genetic Programme Triggering Timely and Spatially-Regulated Chitin Deposition
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 1- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- The Combination of Random Mutagenesis and Sequencing Highlight the Role of Unexpected Genes in an Intractable Organism
- Ataxin-3, DNA Damage Repair, and SCA3 Cerebellar Degeneration: On the Path to Parsimony?
- α-Actinin-3: Why Gene Loss Is an Evolutionary Gain
- Origins of Context-Dependent Gene Repression by Capicua
- Transposable Elements Contribute to Activation of Maize Genes in Response to Abiotic Stress
- No Evidence for Association of Autism with Rare Heterozygous Point Mutations in Contactin-Associated Protein-Like 2 (), or in Other Contactin-Associated Proteins or Contactins
- Nur1 Dephosphorylation Confers Positive Feedback to Mitotic Exit Phosphatase Activation in Budding Yeast
- A Regulatory Hierarchy Controls the Dynamic Transcriptional Response to Extreme Oxidative Stress in Archaea
- Genetic Variants Modulating CRIPTO Serum Levels Identified by Genome-Wide Association Study in Cilento Isolates
- Small RNA Sequences Support a Host Genome Origin of Satellite RNA
- Phosphorylation of Elp1 by Hrr25 Is Required for Elongator-Dependent tRNA Modification in Yeast
- Genetic Mapping of MAPK-Mediated Complex Traits Across
- An AP Endonuclease Functions in Active DNA Demethylation and Gene Imprinting in
- Developmental Regulation of the Origin Recognition Complex
- End of the Beginning: Elongation and Termination Features of Alternative Modes of Chromosomal Replication Initiation in Bacteria
- Naturally Occurring Differences in CENH3 Affect Chromosome Segregation in Zygotic Mitosis of Hybrids
- Imputation of the Rare G84E Mutation and Cancer Risk in a Large Population-Based Cohort
- Polycomb Protein SCML2 Associates with USP7 and Counteracts Histone H2A Ubiquitination in the XY Chromatin during Male Meiosis
- A Genetic Strategy for Probing the Functional Diversity of Magnetosome Formation
- Interactions of Chromatin Context, Binding Site Sequence Content, and Sequence Evolution in Stress-Induced p53 Occupancy and Transactivation
- The Yeast La Related Protein Slf1p Is a Key Activator of Translation during the Oxidative Stress Response
- Integrative Analysis of DNA Methylation and Gene Expression Data Identifies as a Key Regulator of COPD
- Proteasomes, Sir2, and Hxk2 Form an Interconnected Aging Network That Impinges on the AMPK/Snf1-Regulated Transcriptional Repressor Mig1
- Functional Interplay between the 53BP1-Ortholog Rad9 and the Mre11 Complex Regulates Resection, End-Tethering and Repair of a Double-Strand Break
- Estrogenic Exposure Alters the Spermatogonial Stem Cells in the Developing Testis, Permanently Reducing Crossover Levels in the Adult
- Protein Poly(ADP-ribosyl)ation Regulates Immune Gene Expression and Defense Responses
- Sumoylation Influences DNA Break Repair Partly by Increasing the Solubility of a Conserved End Resection Protein
- A Discrete Transition Zone Organizes the Topological and Regulatory Autonomy of the Adjacent and Genes
- Elevated Mutation Rate during Meiosis in
- The Intersection of the Extrinsic Hedgehog and WNT/Wingless Signals with the Intrinsic Hox Code Underpins Branching Pattern and Tube Shape Diversity in the Airways
- MiR-24 Is Required for Hematopoietic Differentiation of Mouse Embryonic Stem Cells
- Tissue-Specific Effects of Genetic and Epigenetic Variation on Gene Regulation and Splicing
- Heterologous Aggregates Promote Prion Appearance via More than One Mechanism
- The Tumor Suppressor BCL7B Functions in the Wnt Signaling Pathway
- , A -Acting Locus that Controls Chromosome-Wide Replication Timing and Stability of Human Chromosome 15
- Regulating Maf1 Expression and Its Expanding Biological Functions
- A Polyubiquitin Chain Reaction: Parkin Recruitment to Damaged Mitochondria
- RecFOR Is Not Required for Pneumococcal Transformation but Together with XerS for Resolution of Chromosome Dimers Frequently Formed in the Process
- An Intracellular Transcriptomic Atlas of the Giant Coenocyte
- Insight in Genome-Wide Association of Metabolite Quantitative Traits by Exome Sequence Analyses
- The Role of the Mammalian DNA End-processing Enzyme Polynucleotide Kinase 3’-Phosphatase in Spinocerebellar Ataxia Type 3 Pathogenesis
- The Global Regulatory Architecture of Transcription during the Cell Cycle
- Identification and Functional Characterization of Coding Variants Influencing Glycemic Traits Define an Effector Transcript at the Locus
- Altered Ca Kinetics Associated with α-Actinin-3 Deficiency May Explain Positive Selection for Null Allele in Human Evolution
- Genetic Variation in the Nuclear and Organellar Genomes Modulates Stochastic Variation in the Metabolome, Growth, and Defense
- PRDM9 Drives Evolutionary Erosion of Hotspots in through Haplotype-Specific Initiation of Meiotic Recombination
- Transcriptional Control of an Essential Ribozyme in Reveals an Ancient Evolutionary Divide in Animals
- ALIX and ESCRT-III Coordinately Control Cytokinetic Abscission during Germline Stem Cell Division
- Century-scale Methylome Stability in a Recently Diverged Lineage
- A Re-examination of the Selection of the Sensory Organ Precursor of the Bristle Sensilla of
- Antagonistic Cross-Regulation between Sox9 and Sox10 Controls an Anti-tumorigenic Program in Melanoma
- A Dependent Pool of Phosphatidylinositol 4,5 Bisphosphate (PIP) Is Required for G-Protein Coupled Signal Transduction in Photoreceptors
- Deciphering the Genetic Programme Triggering Timely and Spatially-Regulated Chitin Deposition
- Aberrant Gene Expression in Humans
- Fascin1-Dependent Filopodia are Required for Directional Migration of a Subset of Neural Crest Cells
- The SWI2/SNF2 Chromatin Remodeler BRAHMA Regulates Polycomb Function during Vegetative Development and Directly Activates the Flowering Repressor Gene
- Evolutionary Constraint and Disease Associations of Post-Translational Modification Sites in Human Genomes
- A Truncated NLR Protein, TIR-NBS2, Is Required for Activated Defense Responses in the Mutant
- The Genetic and Mechanistic Basis for Variation in Gene Regulation
- Inactivation of PNKP by Mutant ATXN3 Triggers Apoptosis by Activating the DNA Damage-Response Pathway in SCA3
- DNA Damage Response Factors from Diverse Pathways, Including DNA Crosslink Repair, Mediate Alternative End Joining
- hnRNP K Coordinates Transcriptional Silencing by SETDB1 in Embryonic Stem Cells
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Global Regulatory Architecture of Transcription during the Cell Cycle
- A Truncated NLR Protein, TIR-NBS2, Is Required for Activated Defense Responses in the Mutant
- Proteasomes, Sir2, and Hxk2 Form an Interconnected Aging Network That Impinges on the AMPK/Snf1-Regulated Transcriptional Repressor Mig1
- The SWI2/SNF2 Chromatin Remodeler BRAHMA Regulates Polycomb Function during Vegetative Development and Directly Activates the Flowering Repressor Gene
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



