-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Requires Host Rab1b for Survival in Macrophages
Yersinia pestis is the bacterial agent that causes the human disease known as plague. While often considered a historic disease, Y. pestis is endemic in rodent populations on several continents and the World Health Organization considers plague to be a reemerging disease. Much of the success of this pathogen comes from its ability to evade clearance by the innate immune system of its host. One weapon in the Y. pestis arsenal is its ability to resist killing when engulfed by macrophages. Upon invasion of macrophages, Y. pestis actively manipulates the cell to generate a protective vacuolar compartment, called the Yersinia containing vacuole (YCV) that allows the bacterium to evade the normal pathogen killing mechanisms of the macrophage. Here we demonstrate that the host protein Rab1b is recruited to the YCV and is required for Y. pestis to inhibit both the acidification and normal maturation of the phagosome to establish a protective niche within the cell. Rab1b is the first protein, either from the host or Y. pestis, shown to contribute to the biogenesis of the YCV. Furthermore, our data suggest a previously unknown impact of Rab1b recruitment in the phagosome maturation pathway.
Published in the journal: Requires Host Rab1b for Survival in Macrophages. PLoS Pathog 11(10): e32767. doi:10.1371/journal.ppat.1005241
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005241Summary
Yersinia pestis is the bacterial agent that causes the human disease known as plague. While often considered a historic disease, Y. pestis is endemic in rodent populations on several continents and the World Health Organization considers plague to be a reemerging disease. Much of the success of this pathogen comes from its ability to evade clearance by the innate immune system of its host. One weapon in the Y. pestis arsenal is its ability to resist killing when engulfed by macrophages. Upon invasion of macrophages, Y. pestis actively manipulates the cell to generate a protective vacuolar compartment, called the Yersinia containing vacuole (YCV) that allows the bacterium to evade the normal pathogen killing mechanisms of the macrophage. Here we demonstrate that the host protein Rab1b is recruited to the YCV and is required for Y. pestis to inhibit both the acidification and normal maturation of the phagosome to establish a protective niche within the cell. Rab1b is the first protein, either from the host or Y. pestis, shown to contribute to the biogenesis of the YCV. Furthermore, our data suggest a previously unknown impact of Rab1b recruitment in the phagosome maturation pathway.
Introduction
Yersinia pestis is a facultative intracellular pathogen and causative agent of the disease known as plague. There have been three human plague pandemics in history; the most notable being the Black Death in the 14th century [1, 2]. Y. pestis can infect humans either through the bite of an infected flea or inhalation of contaminated aerosols. Flea inoculation can lead to the development of bubonic plague, a form of plague highlighted by bacterial dissemination to, and replication within, lymph nodes [1]. Inhalation of Y. pestis contaminated aerosols can result in rapid colonization of the lungs and development of pneumonic plague [1]. Both forms of plague are associated with acute disease progression and high mortality rates in the absence of timely antibiotic treatment. Furthermore, the potential for person-to-person transmission and use as a biological weapon in the absence of a vaccine highlights the risks associated with this pathogen [3].
During its natural life cycle, Y. pestis cycles between two different hosts, the mammal and the flea. The bacterium requires different virulence factors to colonize each host, and coordinates the expression of these factors accordingly [1]. Y. pestis has several well characterized antiphagocytic mammalian virulence factors, such as the Ysc type three secretion system (T3SS), secreted Yop effectors and the Caf1 capsule [1]. However, these virulence factors are down regulated in the flea vector and at the time of initial colonization of the mammalian host [1]. During this transitional period, Y. pestis is highly susceptible to phagocytosis by macrophages and neutrophils [4, 5]. Initial colonization of Y. pestis induces a rapid and early influx of neutrophils to the site of infection [4, 6]. Upon phagocytosis by neutrophils, Y. pestis is readily killed by these professional phagocytes [7–9]. However, Y. pestis has demonstrated an increased ability to survive phagocytosis by monocytes and macrophages [4, 5, 10–12]. Upon entry into the macrophage, Y. pestis actively circumvents the natural maturation of the phagolysosome by remodeling the phagosome into a hospitable replicative niche called the Yersinia containing vacuole (YCV) [11–15]. In vitro studies have highlighted three key characteristics of the biogenesis of the YCV. First, Y. pestis is able to actively inhibit the normal acidification of the phagosome and maintain a pH between 6.5–7.5 within the YCV throughout the course of intracellular infection [12]. Second, a significant portion of YCVs appear to become autophagosomes, which is highlighted by colocalization with LC3-II and the presence of double membranes surrounding the bacteria [12, 16]. While the contribution of autophagy to intracellular survival is unclear, data indicates that autophagy contributes to the metabolism of intracellular bacteria [16, 17]. Finally, approximately eight hours after phagocytosis, the tight fitting vacuolar membrane of the YCV begins to expand in size to form a spacious vacuolar compartment that can be observed by both light and electron microscopy [5, 12, 13, 18]. Bacterial replication within the YCV usually coincides with spacious vacuole formation. Importantly, while the fate of Y. pestis in the macrophage has been characterized, the mechanisms used to generate the YCV and avoid macrophage killing have not been defined.
The ability of Y. pestis to survive within macrophages also appears to impact virulence of the bacterium. In vivo, intracellular Y. pestis are recovered from macrophages isolated from both infected nonhuman primates and rodents, but rarely from neutrophils isolated from the same animals [7, 19, 20]. Ye and colleagues further showed lower bacterial burdens in transgenic MaFIA mice selectively depleted of macrophage/dendritic cell populations, suggesting that macrophages are required to establish acute infection [21]. Y. pestis phoPQ mutants, which are defective for intracellular survival, are also attenuated during subcutaneous infection of BALB/c (75-fold change in LD50) and Swiss Webster mice (no change in LD50 but a significant delay in time to death for mutant infected animals) [22, 23]. Moreover, macrophages isolated from canines, a species that are relatively resistant to plague [24], are significantly more capable in killing Y. pestis than macrophages isolated from laboratory mice, a species highly susceptible to plague, suggesting that the ability of macrophages to kill Y. pestis may contribute to resistance to infection [18]. Together, these data highlight the importance of Y. pestis survival within the macrophage during pathogenesis.
Rab GTPases are the largest member of the Ras Superfamily of small guanine triphosphatases and are central mediators of vesicle trafficking within eukaryotic cells [25, 26]. These GTPases mediate vesicle trafficking by cycling through active GTP-bound and inactive GDP-bound conformations [25, 26]. When bound to GTP, the Rab protein integrates into specific vesicle membranes to mediate the trafficking of that vesicle through interactions with other trafficking proteins. Hydrolysis of the bound GTP to GDP results in extraction of the Rab from the membrane. While approximately 60 different Rab proteins have been identified, the contributions of only a few Rabs to specific vesicle trafficking steps have been experimentally described. For example, Rab5, Rab7, and Rab9 have been well studied as key mediators of important steps in the phagosome maturation process [27–32]. Rab5 is recruited to the early endosome/phagosome and is required for phagocytosis [27–32]. Following phagocytosis, Rab5 disassociates from the early endosome and Rab7 is recruited to the endosome to facilitate recruitment of Rab9 and subsequent fusion with the lysosome [27–32]. A single disruption in the recruitment of a Rab protein to the maturing vesicle can stall and even terminate trafficking of that particular endocytic vesicle to its intended destination.
Due to the central role of Rab proteins for endosome sorting and phagosome maturation, many intracellular pathogens target Rab proteins to subvert these processes (see [25] for review). A classic example of Rab manipulation is seen in Mycobacterium infection of macrophages. M. avium and M. tuberculosis alter the normal distribution of Rab5 and Rab7 on their vacuole—retention of Rab5 and exclusion of Rab7 –to inhibit phagosomal fusion with the lysosome and subsequent killing of the bacteria [33–38]. More recently, Rab1 has emerged as a common target required for the intracellular survival of many pathogens [37, 39–49]. Rab1 has two isoforms, Rab1a and Rab1b, which share 92% amino acid similarity and are thought to be functionally redundant [50, 51]. Both isoforms have been shown to be involved in ER-to-Golgi trafficking [43, 52]. More recently Rab1a has been associated with proper endosome sorting during receptor mediated endocytosis and Rab1b has also been linked to autophagosome formation [44, 53–56]. Several pathogen containing vacuoles (PCVs) have been shown to associate with Rab1, and this recruitment is essential for subsequent survival of the pathogens contained within the PCV [39–47, 57]. Coxiella burnetii requires Rab1 for the Coxiella replicative vacuole (CRV) to expand in both Chinese hamster ovary (CHO) and RAW264.7 macrophage cells [39]. This expansion is significantly hindered in the presence of a GTP restricted form of Rab1 [39]. Similarly, Anaplasma phagocytophilum also recruits Rab1 directly to the Anaplasma containing vacuole (APV) and it has been speculated that recruitment of Rab1 to the APV allows the bacteria to hijack endocytic trafficking [43]. Perhaps the best studied subversion of Rab1 by a pathogen comes from Legionella pneumophila. Rab1 has been shown to accumulate on the L. pneumophila containing vacuole (LCV) as early as 10 min after bacterial uptake and Rab1 knockdown has been shown to inhibit L. pneumophila intracellular replication [40, 42, 46]. Furthermore, several L. pneumophila secreted effectors have been identified that specifically target and modify Rab1 to alter its localization [40, 42, 46, 47, 57–62]. In contrast to the requirement of Rab1 for the survival of these intracellular pathogens that exist within vacuoles, Shigella flexneri, which replicates in the host cytoplasm, is hindered by Rab1 [41]. Inactivation of Rab1 by S. flexneri is critical for bacterial survival and is mediated by the VirA/EspG secreted effector family [41]. Together, these studies suggest a distinct role for host Rab1 GTPases for intracellular survival of pathogens that replicate within vacuolar compartments.
Since Rab1 appears to be targeted by several pathogens that reside within vacuoles in order to survive intracellularly, we investigated the role of Rab1 in the survival of Y. pestis within macrophages. We demonstrate that siRNA knockdown of Rab1b in macrophages infected with Y. pestis significantly increases YCV acidification and association with the lysosomal marker Lamp1, resulting in decreased intracellular survival of Y. pestis. Furthermore, we show Rab1b is recruited to the YCV, suggesting a direct interaction with Rab1b is required for proper YCV maturation. Importantly, Rab1b is the first host protein to be identified that is required by Y. pestis to alter phagosome maturation and YCV acidification and impact the ability of this pathogen to survive within the eukaryotic cell. Finally, we also demonstrate for the first time that Rab1b recruitment to the L. pneumophila containing vacuole also impacts vacuole pH, suggesting a conserved mechanism for the recruitment of Rab1b to pathogen containing vacuoles.
Results
Rab1b is required for Y. pestis survival in macrophages
Since Y. pestis exists within a vacuolar compartment within macrophages [5, 11, 14], and Rab1 has been linked to survival of several other intracellular pathogens that exist within vacuoles [39, 40, 43–46, 48], we sought to determine if Rab1 is required for Y. pestis intracellular survival. Toward this goal, we initially screened whether either isoform of Rab1 is required for Y. pestis to survive in macrophages. RAW264.7 macrophages were transfected with either Rab1a or Rab1b specific siRNAs (pool of 3 siRNAs targeting each gene). 48 h after transfection, macrophages were infected with Y. pestis CO92 pCD1(-) LuxPtolC, which contains a bioluminescent bioreporter to monitor Y. pestis numbers [63]. Extracellular bacteria were killed with gentamicin, and intracellular bacterial survival was monitored via bioluminescent signal (Fig 1A). While no change in Y. pestis bioluminescence was observed in Rab1a siRNA treated cells compared to scrambled siRNA treated controls, we observed a significant decrease in bioluminescence in Rab1b siRNA treated cells, indicating that Rab1b, but not Rab1a, is required for Y. pestis survival within macrophages.
Fig. 1. Rab1b knockdown inhibits the survival of Y. pestis within macrophages. 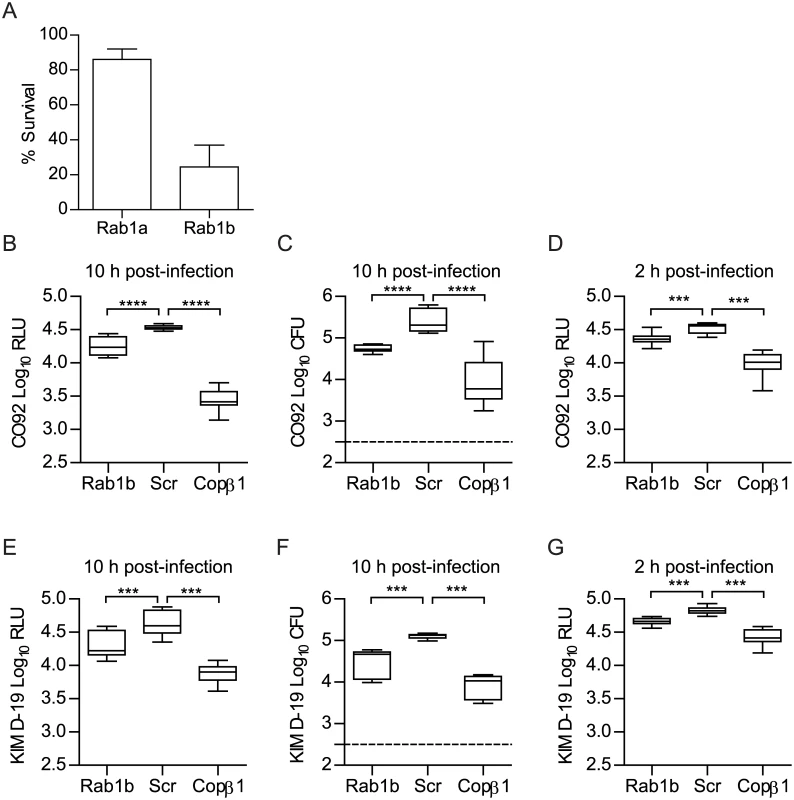
RAW264.7 macrophages were reverse transfected with Rab1a, Rab1b, scrambled (Scr), or Copβ1 siRNA. 48 h after transfection cells were infected with Y. pestis (MOI 10). (A) Percent survival of intracellular CO92 pCD1(-) LuxPtolC in Rab1a or Rab1b siRNA treated macrophages as compared to Scr siRNA treated macrophages. (B) Bioluminescence of intracellular bacteria from macrophages infected for 10 h with Y. pestis CO92 pCD1(-) LuxPtolC. (C) Conventional enumeration of intracellular bacteria from macrophages infected for 10 h with Y. pestis CO92 pCD1(-) LuxPtolC. (D) Bioluminescence of intracellular bacteria from macrophages infected for 2 h with Y. pestis CO92 pCD1(-) LuxPtolC. (E) Bioluminescence of intracellular bacteria from macrophages infected for 10 h with Y. pestis KIM D-19 LuxPtolC. (F) Conventional enumeration of intracellular bacteria from macrophages infected for 10 h with Y. pestis KIM D-19 LuxPtolC. (G) Bioluminescence of intracellular bacteria from macrophages infected for 2 h with Y. pestis KIM D-19 LuxPtolC. The limit of detection for conventional enumeration is denoted by the dotted line. RLU = Relative Light Units; CFU = Colony Forming Units. *** = p<0.001, **** = p<0.0001. To confirm Rab1b is required for Y. pestis intracellular survival, RAW264.7 macrophages were transfected with a single Rab1b siRNA optimized for Rab1b knockdown and cell viability (S1 Fig) and infected with Y. pestis CO92 pCD1(-) LuxPtolC 48 h post-transfection. As a positive control, we also infected macrophages transfected with Copβ1 siRNA. Copβ1 is a component of the cotamer complex and has been shown to alter both invasion and survival of other intracellular pathogens [64, 65]. As expected, Copβ1 knockdown resulted in a significant decrease in intracellular Y. pestis CO92 pCD1(-) LuxPtolC bioluminescence at 10 h post-infection as compared to scramble siRNA treated cells (Fig 1B; p≤0.0001). Rab1b knockdown also resulted in a significant decrease in bioluminescent signal; Y. pestis CO92 pCD1(-) LuxPtolC bioluminescence was ~50% less in Rab1b siRNA treated cells (Fig 1B; p≤0.0001). To confirm that Y. pestis CO92 pCD1(-) LuxPtolC bioluminescence accurately represents viable intracellular bacteria, cells were lysed and bacterial numbers were determined by conventional serial dilution enumeration (Fig 1C). Conventional enumeration supported our bioluminescent data and demonstrated a significant decrease in viable intracellular colony forming units (CFU) in Rab1b siRNA treated cells (p≤0.001). No differences in survival were observed if bacteria were grown at 37°C prior to infection (S2 Fig). Importantly, the direct correlation between bioluminescent signal and bacterial enumeration support the use of bioluminescent data to monitor intracellular Y. pestis numbers.
To confirm that the pCD1 encoded Ysc type three secretion system (T3SS) does not impact Rab1b mediated Y. pestis survival, Rab1b transfected cells were also infected with Y. pestis KIM D-19 LuxPtolC, which contains the pCD1 plasmid and the Ysc T3SS, and bacterial survival was monitored by bioluminescence and conventional bacterial enumeration (Fig 1E and 1F). As observed for Y. pestis CO92 pCD1(-) LuxPtolC, we observed an ~50% decrease in Y. pestis KIM D-19 LuxPtolC survival in Rab1b siRNA treated cells (p≤0.001). We also monitored Y. pestis intracellular bioluminescence temporally over the course of the infection to determine how early during infection Y. pestis intracellular survival was impacted by Rab1b knockdown. This analysis revealed that intracellular bacterial numbers for both strains were significantly decreased in Rab1b treated cells as early as 2 h post-infection, which is the earliest time point we can monitor after gentamicin removal (Fig 1D and 1G; p≤0.001). Finally, to determine if the Rab1b impact on intracellular survival is conserved in the Yersinia genus, transfected macrophages were infected with Y. pseudotuberculosis and Y. enterocolitica. As observed for Y. pestis, both enteric species were attenuated in survival when Rab1b was knocked down (S3 Fig). Together these data demonstrate that Rab1b is required for Yersinia intracellular survival, which is independent of the Ysc T3SS, and bacterial survival is impacted by Rab1b very early during the infection process.
Rab1b is not required for Y. pestis invasion of macrophages
We observed a difference in Y. pestis intracellular numbers in Rab1b siRNA treated cells within 2 h of macrophage infection (Fig 1D and 1G). The difference in recovered bacteria at this early time point could be due to an inability of Y. pestis to avoid phaogolysomal killing in the absence of Rab1b. However, Rab1b may also be required for efficient phagocytosis and the difference in Y. pestis numbers at 2 h post-infection could be a result of less bacteria gaining entry into the macrophages prior to gentamicin treatment. Because phagolysosome fusion and bacterial killing can occur within 120 minutes of phagocytosis [25, 27], we could not rely on the conventional gentamicin protection assay, which requires a 1 h incubation period, to differentiate between invasion and bacterial killing in Rab1b siRNA treated cells. Therefore, we used a differential staining procedure to specifically label extracellular Y. pestis and determine if Rab1b knockdown impacted Y. pestis invasion of macrophages by confocal microscopy. Rab1b siRNA transfected RAW264.7 macrophages were infected with Y. pestis CO92 pCD1(-) pGEN-PEM7::DsRED [66], which constitutively expresses the DsRED fluorescent protein. At 20 and 80 min post-infection, cells and total bacteria were fixed with paraformaldehyde. Extracellular bacteria were then specifically labeled with anti-Y. pestis polyclonal antibody and Alexa Fluor 488 anti-rabbit secondary antibody (Fig 2A). As a positive control, macrophages were treated with Copβ1 siRNA, which has been previously shown to be required for efficient phagocytosis [64]. As expected, cells treated with Copβ1 had significantly less intracellular Y. pestis than scrambled siRNA treated macrophages at both 20 and 80 min post-infection (Fig 2B and 2C; p≤0.001). Conversely, we observed no difference in the proportion of intracellular Y. pestis in Rab1b siRNA treated cells compared to scrambled siRNA treated cells. These data demonstrate that Rab1b is not required for phagocytosis of Y. pestis and suggest that the differences in intracellular bacterial numbers in Rab1b siRNA treated cells is due to a decreased ability of Y. pestis to avoid macrophage killing in the absence of Rab1b.
Fig. 2. Rab1b knockdown does not impact Y. pestis invasion of macrophages. 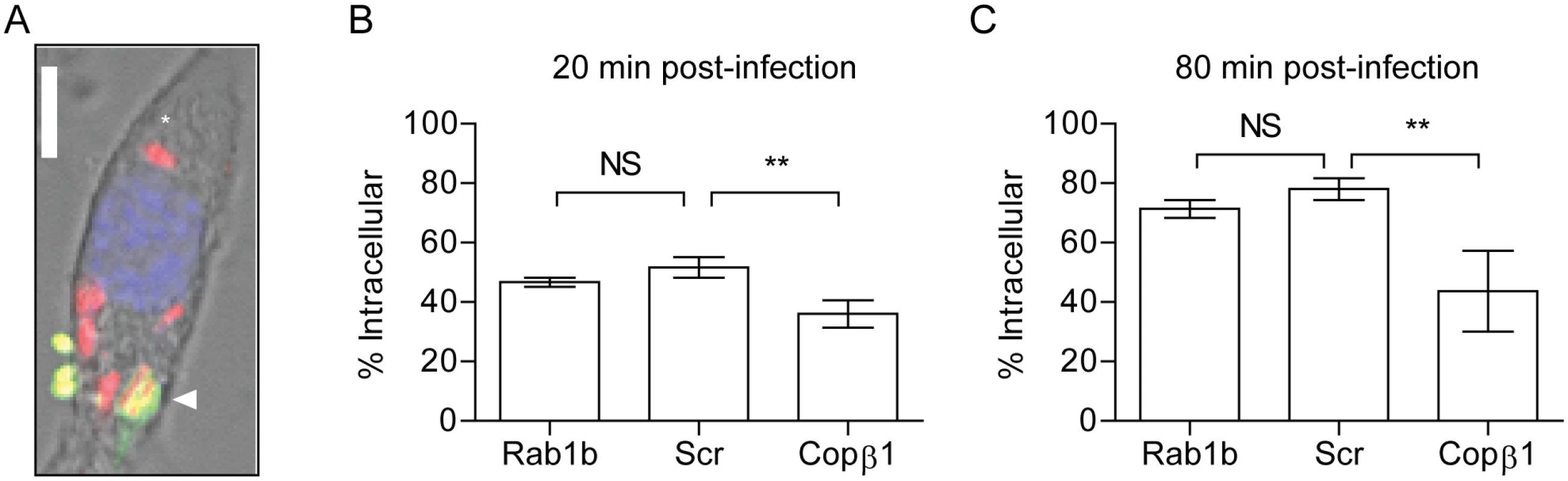
RAW264.7 macrophages were reverse transfected with Rab1b, scrambled (Scr), or Copβ1 siRNA. 48 h after transfection cells were infected with Y. pestis CO92 pCD1(-)pGEN-PEM7::DsRED (MOI 7.5). 20 or 80 min post-infection cells and bacteria were fixed with paraformaldehyde and extracellular bacteria were stained by indirect immunofluorescence with anti-Y. pestis antibody. (A) Representative image showing differential staining of intracellular (red) and extracellular (green or yellow) bacteria. Scale bar is 5μm. Asterisk denotes intracellular Y. pestis. (B and C) Percentage of intracellular bacteria calculated at 20 and 80 min post-infection, respectively. ** = p<0.01, ns = not significant. Rab1b is required for Y. pestis to avoid YCV acidification
A hallmark characteristic of Y. pestis infection of the macrophage is that the bacterium is able to rapidly subvert normal acidification of the YCV [12]. Because acidification is one of the earliest steps in phagosome maturation and is required for both efficient lysosomal fusion and degradation of phagolysosomal contents [27], we next investigated whether Rab1b is required for Y. pestis to avoid YCV acidification. RAW264.7 macrophages were transfected with Rab1b siRNA and then treated with Lysotracker Red DND-99 prior to infection with Y. pestis CO92 pCD1(-) pGEN222, which constitutively expresses EGFP. Lysotracker Red DND-99 fluorescence is pH dependent (fluoresces below pH 5.5), and therefore, allows for identification of acidified vacuoles. As Y. pestis inhibition of YCV acidification is an active process, untransfected cells were infected with paraformaldehyde killed Y. pestis CO92 pCD1(-) pGEN222 to serve as a positive control for YCV acidification. As previously reported for untransfected macrophages [12], Y. pestis CO92 pCD1(-) pGEN222 efficiently avoided YCV acidification in scramble siRNA treated macrophages, with <25% of Y. pestis found within acidified vacuoles by 80 min post-infection (Fig 3). This was significantly lower than paraformaldehyde killed Y. pestis, which were already within acidified vacuoles >80% of the time by 20 min post-infection (p≤0.01). The ability of Y. pestis to inhibit YCV acidification was greatly attenuated in Rab1b knocked down cells, where ~70% of the bacteria were observed within acidified vacuoles within 20 min post-infection (p≤0.01). Furthermore, Y. pestis remained within acidified vacuoles in Rab1b siRNA treated macrophages at 80 min post-infection. These data demonstrate that Y. pestis requires the host Rab1b GTPase to inhibit or avoid YCV acidification.
Fig. 3. Rab1b knockdown alters YCV acidification. 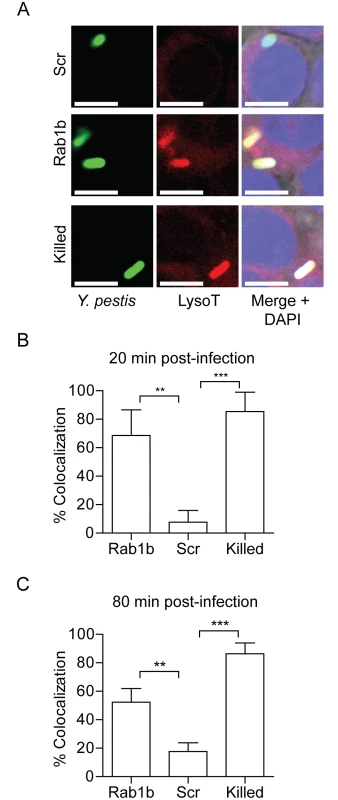
RAW264.7 macrophage cells were reverse transfected with scrambled (Scr), Rab1b or Copβ1 siRNA. 48 h after transfection cells were incubated with Lysotracker Red DND-99 for 1 h and then infected with live or paraformaldehyde-killed Y. pestis CO92 pCD1(-) pGEN222 expressing EGFP (MOI 7.5). Colocalization of Lysotracker Red DND-99 and Y. pestis CO92 pCD1(-) pGEN222 was determined by confocal microscopy. (A) Representative images showing colocalization of Lysotracker Red DND-99 and Y. pestis. Scale bar is 5μm. (B) Percent of YCVs that colocalized with Lysotracker Red DND-99 at 20 min post-infection. (C) Percent of YCVs that colocalized with Lysotracker Red DND-99 at 80 min post-infection. ** = p<0.01, *** = p<0.001. Rab1b is necessary for Y. pestis to avoid fusion with the lysosome
Acidification of the phagosome precedes or coincides with fusion to lysosomes and degradation of foreign particles such as bacteria [27]. As Rab1b knockdown resulted in increased acidification of the YCV, we next determined if Rab1b is required for Y. pestis to avoid fusion with lysosomes. RAW264.7 macrophages were transfected with Rab1b siRNA and infected with live or paraformaldehyde killed Y. pestis CO92 pCD1(-) pGEN-PEM7::DsRED. At 20 and 80 min post-infection, cells were washed, fixed with paraformaldehyde, and stained with anti-Lamp1 antibody, a marker for lysosomal fusion (Fig 4A). In scrambled siRNA treated cells, we observed minimal association of live Y. pestis with Lamp1 (<25%) at 20 and 80 min post-infection, indicating limited association between the YCV and lysosomes at these time points (Fig 4B and 4C). As observed for YCV acidification, there was a significant increase in the association between Lamp1 and paraformaldehyde killed Y. pestis (>60%), supporting an active avoidance of lysosomal fusion by Y. pestis during macrophage infection (Fig 4B and 4C; p≤0.001). Rab1b knockdown also significantly altered Lamp1 association with the YCV compared to scramble siRNA (Fig 4B and 4C; p≤0.001 and p≤0.01, respectively). At 20 min post-infection, Lamp1 associated with ~55% of YCVs in Rab1b siRNA treated cells, and was maintained at this elevated level at 80 min post-infection. These data indicate that Rab1b is required not only for Y. pestis to inhibit YCV acidification but also to avoid lysosomal fusion. Importantly, the ~2-fold increase in association with Lamp1 directly correlates to a similar 2-fold decrease in Y. pestis survival in Rab1b siRNA treated macrophages (Fig 1).
Fig. 4. Rab1b knockdown increases YCV association with Lamp1. 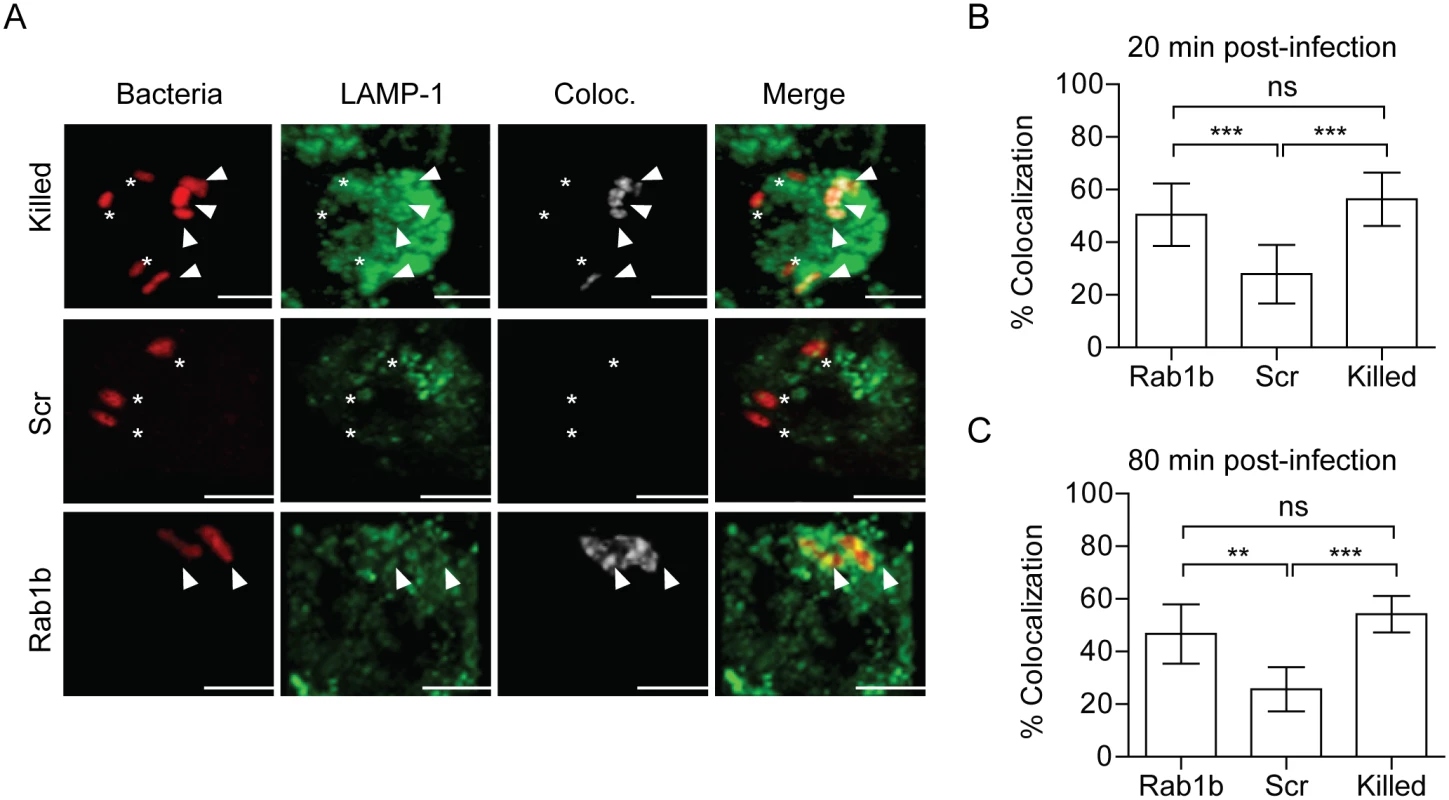
RAW264.7 macrophage cells were reverse transfected with either scrambled (Scr) or Rab1b siRNA. 48 h after transfection cells were infected with live or paraformaldehyde-killed Y. pestis CO92 pCD1(-) pGEN-EM7::DsRED (MOI 3). Cells were stained for Lamp1 and colocalization was determined by confocal microscopy. (A) Representative images showing bacterial colocalization with Lamp1 at 20 min post-infection. Colocalization channel was defined using Imaris software. Asterisks denote bacteria not colocalized with Lamp1; arrowheads denote bacteria colocalized with Lamp1. Scale bar is 5μm. (B) Percent of YCVs that colocalized with Lamp1 at 20 min post-infection. (C) Percent of YCVs that colocalized with Lamp1 at 80 min post-infection. ** = p<0.01, *** = p<0.001. Rab1b is not required for early Y. pestis association with LC3
Autophagy has been linked to both Y. pestis and Y. pseudotuberculosis intracellular infection and may be required for sustained bacterial metabolism within cells [12, 16]. Furthermore, studies have shown a recruitment of LC3, a marker for autophagosomes, to the YCV during Y. pseudotuberculosis infection of HeLa cells and BMDMs [16, 17]. Recently, Huang and colleagues demonstrated a potential role for Rab1b in autophagy and intracellular survival of Salmonella enterica Typhimurium [44]. Given the link of Rab1b to autophagy and autophagy to Yersinia intracellular infection, we next investigated if knockdown of Rab1b impacted early association of LC3 to the YCV during macrophage infection. RAW264.7 macrophages were transfected with Rab1b siRNA and infected with live or paraformaldehyde killed Y. pestis CO92 pCD1(-) pGEN-PEM7::DsRED. 20 and 80 min post-infection cells were washed, fixed with paraformaldehyde, and stained with anti-LC3 antibody (Fig 5A). In contrast to reported infection of epithelial cells with Y. pseudotuberculosis [17], we observed a very low incidence in the association between live or killed Y. pestis with LC3 during early stages of macrophage infection (Fig 5B and 5C) and this association was not significantly altered in Rab1b siRNA treated cells (~20% association in all samples). These data support previous data that LC3 association with the YCV is lower in macrophages than epithelial cells [16, 17] and demonstrate that Rab1b knockdown does not alter YCV-LC3 association during the early stages of Y. pestis infection when we observe changes in YCV maturation and intracellular survival of the bacteria.
Fig. 5. Rab1b knockdown does not affect YCV association with LC3. 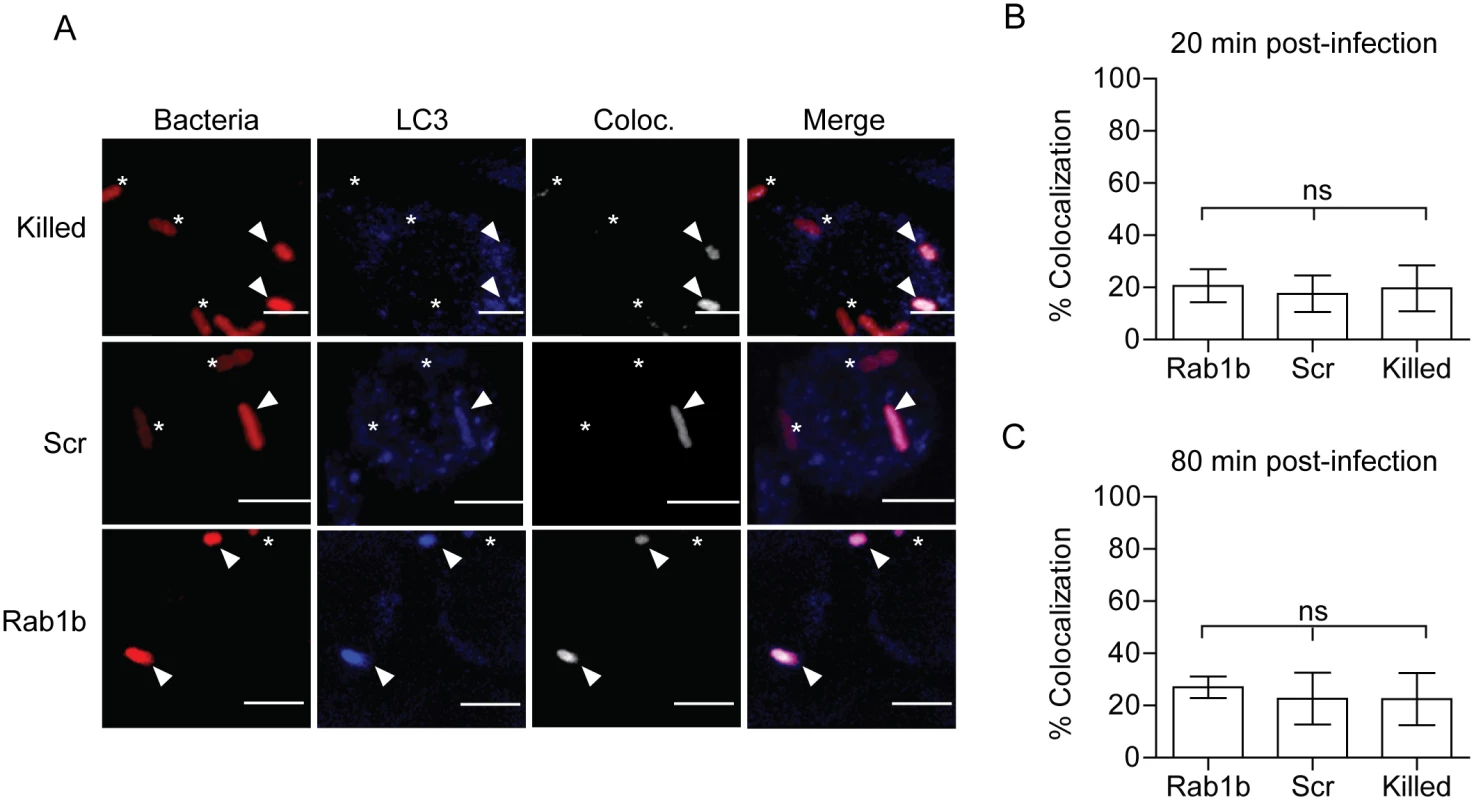
RAW264.7 macrophage cells were reverse transfected with either scrambled (Scr) or Rab1b siRNA. 48 h after transfection cells were infected with live or paraformaldehyde killed Y. pestis CO92 pCD1(-) pGEN-PEM7::DsRED (MOI 7.5). Cells were stained for LC3 and colocalization was determined by confocal microscopy. (A) Representative images showing bacterial colocalization with LC3 at 20 min post infection. The colocalization channel was defined using Imaris software. Asterisks denote bacteria not colocalized with LC3; arrowheads denote bacteria colocalized with LC3. Scale bar is 5μm. (B) Percent of YCVs that colocalized with LC3 at 20 min post-infection. (C) Percent of YCVs that colocalized with LC3 at 80 min post-infection. ns = not significant. Rab1b is recruited to the YCV during macrophage infection
Rab GTPases mediate vesicular trafficking through direct interactions with vesicle membranes (see [25, 26] for review). Therefore, we next sought to determine whether Rab1b is recruited to the YCV during Y. pestis infection. Because Rab interactions with membranes are transient, we transfected RAW264.7 macrophages with a GFP-labelled, constitutively active form of Rab1b [eGFP-Rab1b(CA)]. eGFP-Rab1b(CA) contains a mutation in the GTP binding domain that inhibits the hydrolysis of GTP, resulting in retention of the protein in the membrane in which the Rab GTPase is recruited [43, 46, 67, 68]. Twenty-four hours after transfection, macrophages were infected with either live or PFA killed Y. pestis CO92 pCD1(-) pGEN::mCherry or E. coli K12 pGEN::mCherry, which constitutively express the mCherry fluorescent protein. Cells were washed and fixed with paraformaldehyde at 20 and 80 min post-infection and analyzed by confocal microscopy to determine localization of eGFP-Rab1b(CA) (Fig 6). Less than 25% of E. coli or PFA killed Y. pestis, which traffic to acidified vacuoles, colocalized with eGFP-Rab1b(CA) at 20 min post-infection (Fig 6B). Furthermore, we observed no significant change in colocalization at 80 min post-infection. However, in cells infected with live Y. pestis, we observed a significant increase in eGFP-Rab1b(CA) localization to the YCV at both time points (Fig 6B and 6C; ~57%; p≤0.05). These data demonstrate that while Rab1b is minimally associated with phagosomes containing E. coli or dead Y. pestis, the GTPase is associated with the YCV containing live Y. pestis at a significantly higher frequency, suggesting that Rab1b recruitment or retention to the YCV specifically contributes to Y. pestis survival.
Fig. 6. Rab1b is recruited to the YCV. 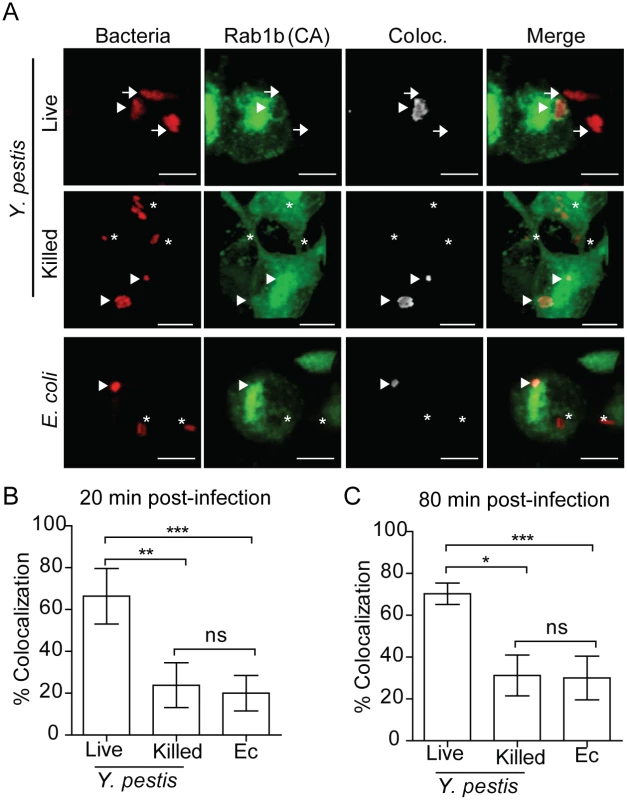
RAW264.7 macrophages were transiently transfected with pEGFP-Rab1B(CA). 24 h after transfection cells were infected with either live or paraformaldehyde killed Y. pestis pMCherry (MOI 7.5) or E. coli pMCherry (MOI 20). Colocalization of EGFP-Rab1b(CA) and bacteria was determined by confocal microscopy. (A) Representative images showing bacterial colocalization with EGFP-Rab1b(CA). Colocalization channel was defined using Imaris software. Asterisks denote bacteria not colocalized with EGFP-Rab1b(CA); arrowheads denote bacteria colocalized with EGFP-Rab1b(CA); arrows denote bacteria in untransfected cells. Scale bar is 5μm. (B and C) Percent of bacteria colocalized with EGFP-Rab1B(CA) at 20 and 80 min post-infection. * = p<0.05; ** = p<0.01; *** = p<0.001. Disruption of the secretory pathway does not alter Y. pestis survival or inhibition of YCV acidification
Rab1b has an important role in mediating ER-to-Golgi trafficking [69, 70]. While Rab1b appears to be directly recruited to the YCV, it is also possible that the effect of Rab1b knockdown on Y. pestis survival is due to changes in Golgi trafficking. To determine if Golgi trafficking, specifically secretory trafficking, is required for Y. pestis to inhibit YCV acidification, we treated RAW264.7 macrophages with Brefeldin A (BFA), which blocks Golgi trafficking independent of Rab1b by targeting Arf1. BFA-treated macrophages were infected with Y. pestis CO92 pCD1(-) LuxPtolC for 20 min, extracellular bacteria were killed with gentamicin, and intracellular bacteria bioluminescence was monitored at 2 and 10 h post infection (Fig 7A and 7B, respectively). At both time points there was no significant difference in the survival of Y. pestis between untreated macrophages or cells treated with increasing concentrations of BFA. Macrophages treated with 10 μM BFA were also incubated with Lysotracker Red DND-99 and subsequently infected with Y. pestis CO92 pCD1(-) pGEN222 to determine if inhibition of the secretory pathway altered YCV acidification. As a control, a separate group of cells were infected with paraformaldehyde killed Y. pestis CO92 pCD1(-) pGEN222. In agreement with the intracellular bacterial survival, there was no significant difference between YCV acidification in BFA-treated macrophages at 20 or 80 min post-infection compared to untreated cells (Fig 7C and 7D). Furthermore, BFA treatment did not alter the acidification of phagosomes containing paraformaldehyde killed Y. pestis. Together these data demonstrate that Y. pestis avoidance of the phagolysosome is independent of retrograde endocytic trafficking and suggests that Rab1b impacts YCV maturation independent of its function in Golgi trafficking.
Fig. 7. Inhibition of the secretory pathway does not inhibit Y. pestis intracellular survival. 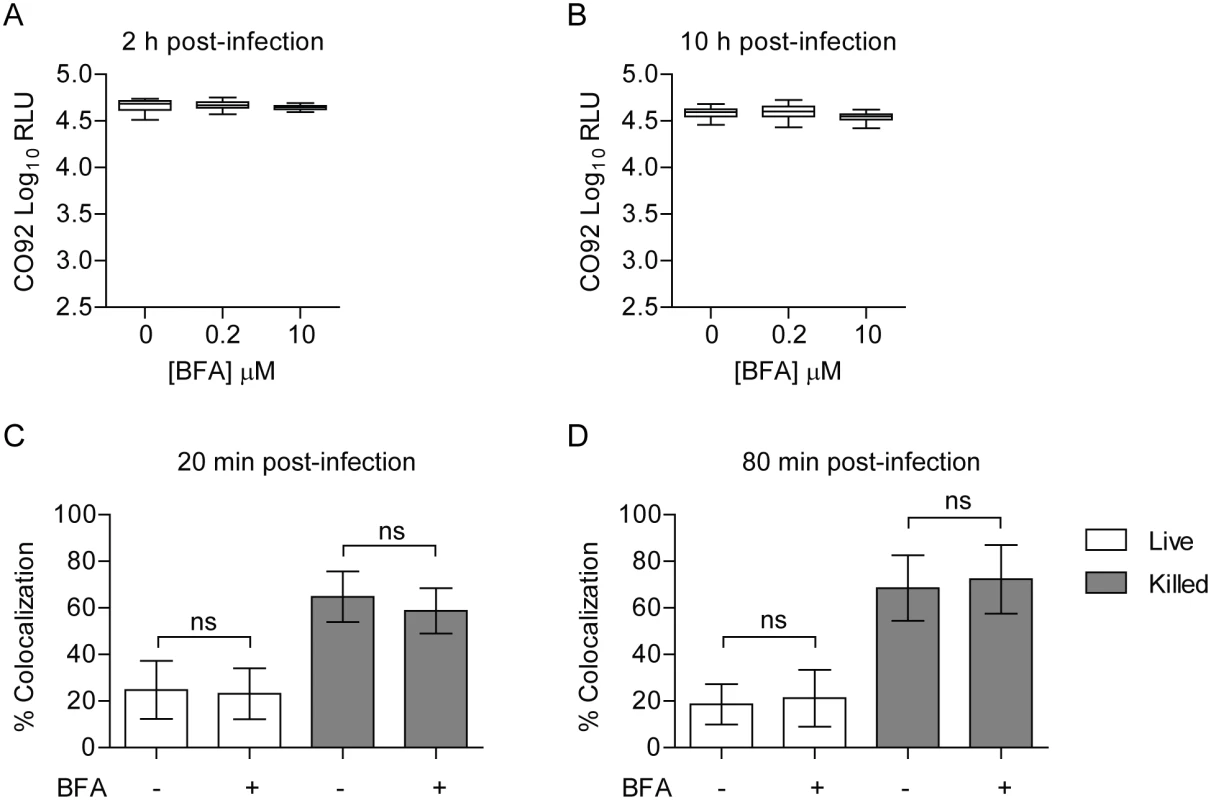
RAW264.7 macrophages were treated with 0, 0.2 or 10 μM BFA prior to infection with Y. pestis CO92 pCD1(-) LuxPtolC (MOI 10). Extracellular bacteria were killed with gentamicin and intracellular bacterial numbers were monitored at (A) 2 h and (B) 10 h post-infection by bioluminescence. To determine if BFA treatment impacted the ability of Y. pestis to inhibit YCV acidification, macrophages treated with 10 μM BFA were incubated with Lysotracker Red DND-99 prior to infection with live or paraformaldehyde killed Y. pestis CO92 pCD1(-) pGEN222 (MOI 3). Bacterial Colocalization with Lysotracker Red DND-99 was determined by confocal microscopy at (C) 20 and (D) 80 min post-infection. ns = not significant. Rab1b inhibition results in increased acidification of the Legionella containing vacuole
Previous studies with L. pneumophila demonstrate the cyclic recruitment and release of Rab1b on the LCV within 2 hours post-infection [45]. The release of Rab1b from the nascent LCV coincides with the transition of the LCV from a neutral to acidic pH [71, 72]. Given that Y. pestis recruits Rab1b to the YCV to prevent vacuole acidification, we hypothesized that L. pneumophila recruitment of Rab1b may also result in arrest of LCV acidification. To test this hypothesis, we transfected RAW264.7 macrophage cells with siRNA targeting Rab1b and treated transfected cells with Lysotracker Red DND-99 prior to infection with L. pneumophila to monitor LCV acidification. As previously reported, we observed that the majority of LCVs did not colocalize with Lysotracker in scramble siRNA treated macrophage (only 30% of L. pneumophila was found in acidified compartments by 80 min post-infection; Fig 8). In contrast, we observed a significant increase in Lysotracker colocalization in macrophages treated with siRNA targeting Rab1b at both 20 and 80 min post-infection (Fig 8B and 8C; p≤0.01 and P≤0.001, respectively). These data demonstrate that like Y. pestis, L. pneumophila requires Rab1b to inhibit LCV acidification during early stages of macrophage infection.
Fig. 8. Knockdown of Rab1b increases L. pneumophila LCV acidification. 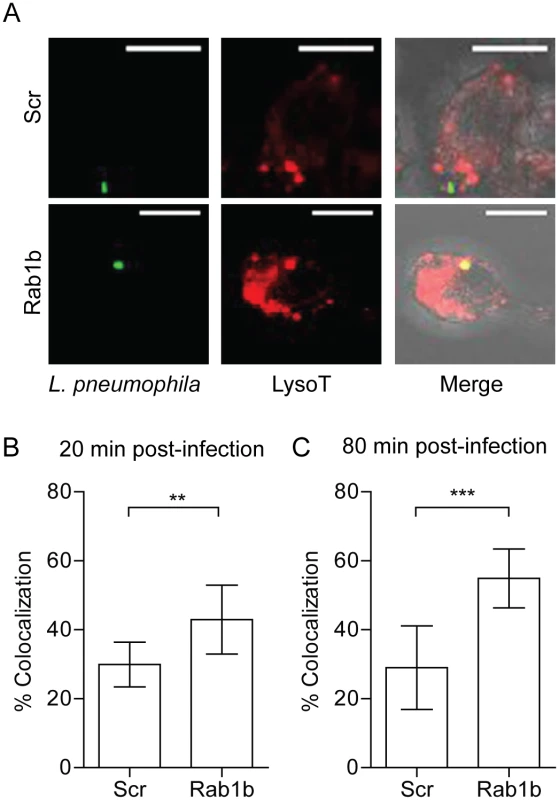
RAW264.7 macrophage cells were reverse transfected with either scrambled (Scr) or Rab1b siRNA. 48 h after transfection cells were incubated with Lysotracker Red DND-99 for 1 h, and infected with L. pneumophila pMIP-GFP (MOI 10). Coverslips were fixed and colocalization of Lysotracker was determined by confocal microscopy. (A) Representative images showing colocalization of Lysotracker with L. pneumophila. Scale bar is 5μm. (B) Percent of LCVs that colocalized with Lysotracker at 20 min post-infection. (C) Percent of LCVs that colocalized with Lysotracker Red DND-99 at 80 min post-infection. ** = p<0.05, *** = p<0.001. Discussion
Rab proteins are central mediators in vesicular trafficking within the cell. As such, intracellular pathogens often target these GTPases to subvert the normal phagosome maturation pathway and survive within host cells (see [25, 73] for reviews). Rab1 was one of the first identified members of this family and has been extensively studied for its role in Golgi trafficking in yeast, Drosophila, and mammalian cells (see [70, 74, 75] for reviews). More recently, both isoforms of Rab1 have been linked to intracellular infection by several pathogens. Chlamydial species [48], L. pneumophila [40, 46], A. phagocytophilum [43], Coxiella burnetii [39], and S. enterica Typhimurium [44] have been shown to recruit Rab1 to the PCV. Furthermore, inhibition of Rab1 by either RNAi or expression of dominant negative Rab1 constructs indicate that Rab1 function is required for the survival/growth of L. pneumophila [40], C. burnetii [39], S. enterica Typhimurium [44, 76], and Brucella melitensis [77]. Our data demonstrate for the first time that Y. pestis also belongs to this group. Specifically, we have demonstrated that Y. pestis recruits Rab1b to the YCV during infection of macrophages and that this GTPase is required for intracellular survival. Interestingly, Rab1 has only been shown to be required for the survival of pathogens that exist within vacuolar compartments, suggesting a role(s) for Rab1 in subverting normal phagosome maturation and generation of a protective PCV. In fact, functional Rab1 has been shown to be detrimental to the survival of the cytoplasmic pathogen Shigella flexnerii through its interaction with the autophagy system within the host cell [41]. However, S. flexnerii has also evolved to target Rab1, through the VirG secreted effector protein, and inactivate the GTPase to inhibit macroautophagy during infection [41].
While Rab1 has been linked to the survival of several intracellular pathogens, the role Rab1 plays in the maturation of individual PCVs is less well understood. In C. burnetii, Rab1 has been shown to be required for the massive expansion of the Coxiella replicative vacuole (CRV) [39]. This requires the acquisition of new membrane in order for the CRV to grow, and Rab1 recruitment to the vacuole may mediate the interception of vesicles (and their membranes) from the secretory pathway. This hypothesis is supported by studies showing that treatment with BFA, which independently inhibits the secretory pathway, also inhibits the expansion of the CRV [39]. Studies from A. phagocytophilum and Chlamydial species, which also form a large replicative vacuole, also suggest that Rab1 recruitment is important for formation of a spacious vacuolar compartment [43, 48]. Therefore, a common goal of bacteria that recruit Rab1 to their PCV may be to subvert the secretory pathway in order to remodel the PCV. Furthermore, Rab1b has also been linked to autophagy [44], which is also associated with the replication of both C. burnetii and A. phagocytophilum [78, 79]. It is possible that in addition to the secretory pathway, Rab1 recruitment may also contribute to the recruitment of autophagsomal membranes to these PCV, though this has yet to be demonstrated. Since the YCV also expands late during infection (though not to the degree of these former pathogens) to form a spacious vacuole [5, 12, 18], it is possible that Rab1b may contribute to YCV expansion. However, we have not observed changes in spacious YCV formation in Rab1b siRNA treated macrophages. Furthermore, our data also suggest that early association with the autophagosome marker LC3 does not appear to protect YCV from acidification, as we observed no difference in YCV-LC3 association in Rab1b siRNA treated cells. More importantly, our data with Y. pestis reveal a potential new benefit of Rab1 recruitment to the PCV, which is to avoid phagosomal acidification and subsequent fusion to the lysosome. While it is currently unclear how Rab1b inhibits YCV acidification, it appears to be independent from its contributions to the secretory pathway, as BFA treatment did not result in similar changes to YCV acidification. Importantly, while knockdown of Rab1B does not alter the expression of Rab 5, 7 or 9, which are required for phagosome maturation (S1A Fig), it is possible that recruitment and retention of Rab1b to the early phagosome inhibits interactions with these Rabs (and/or Rab effector proteins) to inhibit normal phagosome maturation. Rab1 has also been linked to endosomal sorting through direct interactions with the kinesin Kifc1, which in turn affects directional vesicular motility within the cell [55, 56]. Thus, Rab1b recruitment may alter early sorting of the YCV to avoid acidification and lysosomal fusion. Studies to better characterize the early YCV, including differences in Rab composition and vATPase recruitment as compared to the normal phagosome are ongoing and will provide further insight into these mechanisms. Rab1 recruitment to the YCV also occurs significantly earlier than reported for C. burnetii (≤20 min vs. >12 h, respectively) [39], suggesting that timing of recruitment may indicate which function, inhibition of phagosome maturation or membrane acquisition, is contributing to pathogenesis of various pathogens. It should be noted that C. burnetii requires passage through an acidified vacuole to induce the expression of important virulence factors and subsequent intracellular survival [80]. Therefore, our observations that early acquisition of Rab1 inhibits PCV acidification may explain why Rab1 recruitment is delayed during C. burnetii infection. In contrast to C. burnetii, L. pneumophila, which inhibits LCV acidification early during infection [71, 72, 81], recruits Rab1 in a similar time frame as seen during Y. pestis infection (within 10 min) [46]. In support of our hypothesis that early recruitment of Rab1b is a mechanism for pathogens to inhibit phagosome acidification, we demonstrated that knockdown of Rab1b decreased the ability of L. pneumophila to inhibit LCV acidification (Fig 8). Interestingly, L. pneumophila appears to control both recruitment and later release of Rab1 from the LCV (discussed below). The timing of Rab1 modification by L. pneumophila coincides with a transition from a neutral to an acidic LCV [71, 72], suggesting that Rab1 inhibition of acidification may be an active process that is reversible upon removal of Rab1 from the vacuolar membrane.
Phagosome acidification has been shown to be a key step in phagosome maturation. Acidification of the phagosome is believed to work in concert with Rab5, Rab7 and Rab9 to mediate phagosome maturation and ultimately fusion with lysosomes [27, 28]. Initially, the early phagosome, highlighted by association with Rab5, is slightly acidic (~pH 6.0). As the phagosome matures, the pH decreases and Rab7 replaces Rab5 on the phagosome. Rab7 subsequently recruits more vATPase complexes, resulting in further acidification of the phagosome and recruitment of Rab9. By the time Rab9 mediates lysosomal fusion, the pH of the phagosome is approaching 4.0, which is the optimal pH to activate hydrolases and proteases delivered to the phagosome by the lysosome. Several lines of evidence indicate that acidification of the phagosome is required in order for efficient lysosomal fusion and function to occur [31, 32, 82–84], which suggest that inhibition of acidification could influence proper lysosomal fusion to the PCV. In line with these hypotheses, we observed a direct correlation between increased YCV acidification with increased Lamp1 association, and subsequent decreased Y. pestis survival, in Rab1b siRNA treated cells. This direct correlation makes it difficult to separate the impact of acidification directly on Y. pestis survival (acidic killing) from lysosomal fusion, but further supports the importance of inhibiting YCV acidification as mechanism for Y. pestis intracellular survival [12].
While Rab1 is important for the intracellular survival of several pathogens, bacterial virulence factors that target Rab1 have only been identified for Chlamydia [48] and L. pneumophila [45, 47, 85–91]. In the case of L. pneumophila, multiple Dot/Icm secreted factors have been shown to target Rab1 and modify the protein to manipulate localization to the LCV; cycling the host Rab1 between active (anchored to the LCV) and inactive states. The effectors DrrA/SidM, SidD and LepB work in concert to first recruit Rab1 to LCV, and then later remove it [42, 47, 59, 62, 92]. L. pneumophila also manipulates Rab1 independent of recruitment to the LCV through the action of SidC/SdcA, LidA and AnkX [85, 89, 91, 93]. The redundancy in Rab1 targeting proteins indicates that Rab1 manipulation by L. pneumophila is extremely important for the intracellular survival of this pathogen. For Y. pestis, we have yet to define the virulence factors that mediate Rab1b recruitment to the YCV. However, we have shown that Y. pestis does not require the pCD1 plasmid (including the Ysc T3SS) or the high pathogenicity island (pgm locus) to recruit Rab1b and inhibit YCV acidification. These findings are in agreement with previous work that has shown both of these genetic elements are dispensable for intracellular survival [5, 8, 14, 20]. Therefore, virulence factors encoded elsewhere in the genome are mediating both Rab1b interactions and intracellular survival. While the PhoPQ two component regulator has been shown to contribute to intracellular survival, likely through the regulation of other genes [13, 15, 22, 23], we speculate that these genes do not regulate survival through Rab1b because phoPQ mutants still inhibit YCV acidification during infection [13]. However, defining Rab1b recruitment to the phoPQ mutant YCV is needed to confirm this hypothesis. Studies to specifically identify Y. pestis factors involved in Rab1b recruitment to the YCV are ongoing.
In summary, we have shown here for the first time that recruitment of Rab1b to the PCV directly correlates to the ability of a pathogen to inhibit acidification of the vacuole. These findings indicate a novel function for Rab1b in inhibiting phagosome maturation and suggest that other pathogens may use a similar strategy to modify the maturation of the PCV. Furthermore, in the context of Y. pestis infection, Rab1b is the first factor, either host or bacterial, identified that directly impacts acidification of the YCV. Future studies to define how Rab1b impacts phagosome acidification and to identify additional host factors that contribute YCV biogenesis will be important for us to understand how this pathogen evades killing by macrophages.
Materials and Methods
Bacterial strains, plasmids, and macrophages
All bacterial strains used in this study are listed in S1 Table in the Supporting Information. Y. pestis CO92 [94] pCD1(-) and KIM D-19 (pgm(-)) (BEI Resources) were cultivated at 26°C in Brain Heart Infusion (BHI) broth (Difco). When needed, carbenicillin was used at 50μg/mL. Bioluminescent derivatives were generated using the LuxPtolC bioreporter as described previously [63]. To generate fluorescent bacterial strains, Y. pestis and E. coli K12 DH5α were transformed with pGEN222, pGEN-PEM7::DsRED, or pGEN222::mCherry [66]. E. coli was cultivated at 37°C in Luria-Bertani (LB) broth (Difco) supplemented with 50μg/mL carbenicillin. L. pneumophila AA100, a clinical isolate containing pMIP-GFP, was grown on BCYE agar plates for 3 days at 37°C prior to macrophage infection [95–97]. The pGEN222::mCherry plasmid was generated by replacing the EGFP gene from pGEN222 with the mCherry gene using Gibson Cloning [98]. Constitutive active EGFP-Rab1b was generated by site directed mutagenesis of pEGFP-Rab1b [99] using primers 5’ - TGG AAC GGT TCC GGA C -3’ and 5’ - GGC CCG CTG TGT CC -3’ to mutate the Glutamine at residue 67 to a Leucine as previously described [99]. RAW264.7 macrophages were obtained from ATCC and cultured in DMEM, 100 mM glucose + 10% FBS (Hyclone).
Transfection of macrophages
For siRNA transfection, 20 μl of 0.165 μM Silencer siRNA (Life Technologies) diluted in Opti-MEM (Life Technologies) was mixed with 10 μl of 0.03% (v/v) Lipofectamine RNAiMax/Opti-MEM (Life Technologies) as described by the manufacturer. 30 μl of the siRNA-Lipofectamine complex was added to each well of a white flat-bottom 96-well plate (Greiner), incubated at room temperature for 10 min, and then 1x104 RAW264.7 macrophages suspended in 80 μl of DMEM+10% FBS were added. Cells were incubated for 48 h at 37°C with 5% CO2. For 24-well plates used for microscopy, all reagents were increased by 4-fold. For plasmid transfection, 4 μg of plasmid was transfected into 4.4 x 105 RAW264.7 macrophages using Lipofectamine 2000 (Life Technologies) or 0.5 μg of plasmid with JetPrime (Polyplus) as described by the manufacturers. Luminescence was monitored with a Synergy 4 plate reader (BioTek) (1 sec read with sensitivity set at 150).
Bacterial infection of macrophages
Macrophages were infected with Y. pestis strains as previously described [11, 63]. Briefly, bacteria were grown at 26°C in BHI, washed in PBS, and diluted appropriately in prewarmed DMEM+10%FBS. Bacteria were added to macrophages and the infection was synchronized by centrifugation. After 20 min, extracellular bacteria were killed with gentamicin (16μg/mL). One hour after gentamicin treatment, the medium was replaced with DMEM + 10% FBS containing 2μg/mL gentamicin. Intracellular Y. pestis numbers were determined by bioluminescence using a Synergy HT plate reader (Biotek) or conventional bacterial enumeration as described previously [63]. For L. pneumophila, bacteria were swabbed directly from plates and diluted appropriately in prewarmed DMEM+10%FBS. Bacteria were added to macrophages and the infection was synchronized by centrifugation. At 20 minutes and 80 minutes post-infection cell monolayers were washed three times with PBS and fixed as described below [96, 97]. All MOIs were confirmed by conventional enumeration of the inoculum at the time of infection. For vacuole acidification experiments, 75 nM Lysotracker Red DND-99 (Life Technologies) was added to the cells 1 h prior to fixation. Brefeldin A (Sigma) was added to cells 2 h prior to Y. pestis infection and maintained throughout the infection.
Immunofluorescent staining and confocal microscopy
For confocal microscopy, cells were fixed to coverslips with 4% paraformaldehyde for 30 min. For indirect immunofluorescent staining, fixed cells were blocked with 3% BSA overnight and incubated with rabbit anti-Y. pestis serum (1 : 1,000), anti-Lamp1 (0.8ug/ul; Abcam ab24170), or anti-MAP-LC3α/β (1 : 200; Santa Cruz sc-16756) antibodies for 1 h. Unbound primary antibodies were removed by washing and anti-rabbit Alexa Fluor 488 secondary antibody (1 : 4000; Life Technologies) was added for 1 h. All coverslips were mounted with Prolong Gold with DAPI (Life Technologies) and imaged on an Olympus FV100 laser or Zeiss LSM 710 laser confocal microscope. Colocalization of Lysotracker Red DND-99 or proteins to the YCV was determined using the Coloc function in the Imaris image analysis software (BitPlane).
Statistics
All data are shown as mean and standard deviation of three to six biological replicates and each experiment was repeated three times to confirm the phenotypes. For microscopy, at least 50 vacuoles per biological replicate were analyzed. p-values were calculated by one-way ANOVA (or t-test for L. pneumophila experiments) using GraphPad Prism software.
Supporting Information
Zdroje
1. Perry RD, Fetherston JD. Yersinia pestis—etiologic agent of plague. Clinical microbiology reviews. 1997;10(1):35–66. 8993858
2. Butler T. Plague gives surprises in the first decade of the 21st century in the United States and worldwide. The American journal of tropical medicine and hygiene. 2013;89(4):788–93. Epub 2013/09/18. doi: 10.4269/ajtmh.13-0191 24043686
3. Inglesby TV, Dennis DT, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. Jama. 2000;283(17):2281–90. 10807389
4. Spinner JL, Winfree S, Starr T, Shannon JG, Nair V, Steele-Mortimer O, et al. Yersinia pestis survival and replication within human neutrophil phagosomes and uptake of infected neutrophils by macrophages. Journal of leukocyte biology. 2013. Epub 2013/11/15.
5. Straley SC, Harmon PA. Growth in mouse peritoneal macrophages of Yersinia pestis lacking established virulence determinants. Infection and immunity. 1984;45(3):649–54. Epub 1984/09/01. 6469351
6. Gonzalez RJ, Lane MC, Wagner NJ, Weening EH, Miller VL. Dissemination of a highly virulent pathogen: tracking the early events that define infection. PLoS pathogens. 2015;11(1):e1004587. Epub 2015/01/23. doi: 10.1371/journal.ppat.1004587 25611317
7. Lukaszewski RA, Kenny DJ, Taylor R, Rees DG, Hartley MG, Oyston PC. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infection and immunity. 2005;73(11):7142–50. Epub 2005/10/22. 16239508
8. Janssen WA, Surgalla MJ. Plague bacillus: survival within host phagocytes. Science. 1969;163(3870):950–2. Epub 1969/02/28. 5763880
9. Vagima Y, Zauberman A, Levy Y, Gur D, Tidhar A, Aftalion M, et al. Circumventing Y. pestis Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague. PLoS pathogens. 2015;11(5):e1004893. Epub 2015/05/15. doi: 10.1371/journal.ppat.1004893 25974210
10. Burrows TW, Bacon GA. The basis of virulence in Pasteurella pestis: the development of resistance to phagocytosis in vitro. British journal of experimental pathology. 1956;37(3):286–99. Epub 1956/06/01. 13342354
11. Pujol C, Bliska JB. The ability to replicate in macrophages is conserved between Yersinia pestis and Yersinia pseudotuberculosis. Infection and immunity. 2003;71(10):5892–9. 14500510
12. Pujol C, Klein KA, Romanov GA, Palmer LE, Cirota C, Zhao Z, et al. Yersinia pestis can reside in autophagosomes and avoid xenophagy in murine macrophages by preventing vacuole acidification. Infection and immunity. 2009;77(6):2251–61. Epub 2009/03/18. doi: 10.1128/IAI.00068-09 19289509
13. Grabenstein JP, Fukuto HS, Palmer LE, Bliska JB. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infection and immunity. 2006;74(7):3727–41. Epub 2006/06/23. 16790745
14. Straley SC, Harmon PA. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infection and immunity. 1984;45(3):655–9. Epub 1984/09/01. 6469352
15. Grabenstein JP, Marceau M, Pujol C, Simonet M, Bliska JB. The response regulator PhoP of Yersinia pseudotuberculosis is important for replication in macrophages and for virulence. Infection and immunity. 2004;72(9):4973–84. Epub 2004/08/24. 15321989
16. Moreau K, Lacas-Gervais S, Fujita N, Sebbane F, Yoshimori T, Simonet M, et al. Autophagosomes can support Yersinia pseudotuberculosis replication in macrophages. Cellular microbiology. 2010;12(8):1108–23. Epub 2010/02/26. doi: 10.1111/j.1462-5822.2010.01456.x 20180800
17. Ligeon LA, Moreau K, Barois N, Bongiovanni A, Lacorre DA, Werkmeister E, et al. Role of VAMP3 and VAMP7 in the commitment of Yersinia pseudotuberculosis to LC3-associated pathways involving single - or double-membrane vacuoles. Autophagy. 2014;10(9). Epub 2014/07/22.
18. Ponnusamy D, Clinkenbeard KD. Yersinia pestis intracellular parasitism of macrophages from hosts exhibiting high and low severity of plague. PloS one. 2012;7(7):27.
19. Finegold MJ. Pneumonic plague in monkeys. An electron microscopic study. The American journal of pathology. 1969;54(2):167–85. Epub 1969/02/01. 4974722
20. St John AL, Ang WX, Huang MN, Kunder CA, Chan EW, Gunn MD, et al. S1P-Dependent Trafficking of Intracellular Yersinia pestis through Lymph Nodes Establishes Buboes and Systemic Infection. Immunity. 2014;41(3):440–50. Epub 2014/09/23. doi: 10.1016/j.immuni.2014.07.013 25238098
21. Ye Z, Kerschen EJ, Cohen DA, Kaplan AM, van Rooijen N, Straley SC. Gr1+ cells control growth of YopM-negative yersinia pestis during systemic plague. Infection and immunity. 2009;77(9):3791–806. Epub 2009/07/08. doi: 10.1128/IAI.00284-09 19581396
22. Oyston PC, Dorrell N, Williams K, Li SR, Green M, Titball RW, et al. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infection and immunity. 2000;68(6):3419–25. Epub 2000/05/19. 10816493
23. Bozue J, Mou S, Moody KL, Cote CK, Trevino S, Fritz D, et al. The role of the phoPQ operon in the pathogenesis of the fully virulent CO92 strain of Yersinia pestis and the IP32953 strain of Yersinia pseudotuberculosis. Microbial pathogenesis. 2011;50(6):314–21. doi: 10.1016/j.micpath.2011.02.005 21320584
24. Rust JH Jr., Cavanaugh DC, O'Shita R, Marshall JD Jr. The role of domestic animals in the epidemiology of plague. I. Experimental infection of dogs and cats. The Journal of infectious diseases. 1971;124(5):522–6. Epub 1971/11/01. 5115673
25. Stein MP, Muller MP, Wandinger-Ness A. Bacterial pathogens commandeer Rab GTPases to establish intracellular niches. Traffic. 2012;13(12):1565–88. doi: 10.1111/tra.12000 22901006
26. Bhuin T, Roy JK. Rab proteins: the key regulators of intracellular vesicle transport. Experimental cell research. 2014;328(1):1–19. Epub 2014/08/05. doi: 10.1016/j.yexcr.2014.07.027 25088255
27. Kinchen JM, Ravichandran KS. Phagosome maturation: going through the acid test. Nature reviews Molecular cell biology. 2008;9(10):781–95. Epub 2008/09/25. doi: 10.1038/nrm2515 18813294
28. Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: aging gracefully. The Biochemical journal. 2002;366(Pt 3):689–704. Epub 2002/06/14. 12061891
29. Sarantis H, Balkin DM, De Camilli P, Isberg RR, Brumell JH, Grinstein S. Yersinia entry into host cells requires Rab5-dependent dephosphorylation of PI(4,5)P(2) and membrane scission. Cell host & microbe. 2012;11(2):117–28.
30. Luzio JP, Gray SR, Bright NA. Endosome-lysosome fusion. Biochemical Society transactions. 2010;38(6):1413–6. Epub 2010/12/02. doi: 10.1042/BST0381413 21118098
31. Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nature reviews Molecular cell biology. 2007;8(8):622–32. Epub 2007/07/20. 17637737
32. Luzio JP, Pryor PR, Gray SR, Gratian MJ, Piper RC, Bright NA. Membrane traffic to and from lysosomes. Biochemical Society symposium. 2005; (72):77–86. Epub 2005/01/15. 15649132
33. Sturgill-Koszycki S, Schaible UE, Russell DG. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. The EMBO journal. 1996;15(24):6960–8. 9003772
34. Via LE, Deretic D, Ulmer RJ, Hibler NS, Huber LA, Deretic V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. The Journal of biological chemistry. 1997;272(20):13326–31. Epub 1997/05/16. 9148954
35. Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. The Journal of cell biology. 2001;154(3):631–44. Epub 2001/08/08. 11489920
36. Fratti RA, Chua J, Vergne I, Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(9):5437–42. Epub 2003/04/19. 12702770
37. Seto S, Matsumoto S, Ohta I, Tsujimura K, Koide Y. Dissection of Rab7 localization on Mycobacterium tuberculosis phagosome. Biochemical and biophysical research communications. 2009;387(2):272–7. doi: 10.1016/j.bbrc.2009.06.152 19580780
38. Roberts EA, Chua J, Kyei GB, Deretic V. Higher order Rab programming in phagolysosome biogenesis. The Journal of cell biology. 2006;174(7):923–9. Epub 2006/09/20. 16982798
39. Campoy EM, Zoppino FC, Colombo MI. The early secretory pathway contributes to the growth of the Coxiella-replicative niche. Infection and immunity. 2011;79(1):402–13. doi: 10.1128/IAI.00688-10 20937765
40. Derre I, Isberg RR. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infection and immunity. 2004;72(5):3048–53. Epub 2004/04/23. 15102819
41. Dong N, Zhu Y, Lu Q, Hu L, Zheng Y, Shao F. Structurally distinct bacterial TBC-like GAPs link Arf GTPase to Rab1 inactivation to counteract host defenses. Cell. 2012;150(5):1029–41. Epub 2012/09/04. doi: 10.1016/j.cell.2012.06.050 22939626
42. Hardiman CA, Roy CR. AMPylation is critical for Rab1 localization to vacuoles containing Legionella pneumophila. MBio. 2014;5(1):e01035–13. Epub 2014/02/13. doi: 10.1128/mBio.01035-13 24520063
43. Huang B, Hubber A, McDonough JA, Roy CR, Scidmore MA, Carlyon JA. The Anaplasma phagocytophilum-occupied vacuole selectively recruits Rab-GTPases that are predominantly associated with recycling endosomes. Cellular microbiology. 2010;12(9):1292–307. doi: 10.1111/j.1462-5822.2010.01468.x 20345488
44. Huang J, Birmingham CL, Shahnazari S, Shiu J, Zheng YT, Smith AC, et al. Antibacterial autophagy occurs at PI(3)P-enriched domains of the endoplasmic reticulum and requires Rab1 GTPase. Autophagy. 2011;7(1):17–26. Epub 2010/10/29. 20980813
45. Ingmundson A, Delprato A, Lambright DG, Roy CR. Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature. 2007;450(7168):365–9. 17952054
46. Kagan JC, Stein MP, Pypaert M, Roy CR. Legionella subvert the functions of Rab1 and Sec22b to create a replicative organelle. The Journal of experimental medicine. 2004;199(9):1201–11. Epub 2004/05/01. 15117975
47. Neunuebel MR, Chen Y, Gaspar AH, Backlund PS Jr., Yergey A, Machner MP. De-AMPylation of the small GTPase Rab1 by the pathogen Legionella pneumophila. Science. 2011;333(6041):453–6. Epub 2011/06/18. doi: 10.1126/science.1207193 21680813
48. Rzomp KA, Scholtes LD, Briggs BJ, Whittaker GR, Scidmore MA. Rab GTPases are recruited to chlamydial inclusions in both a species-dependent and species-independent manner. Infection and immunity. 2003;71(10):5855–70. Epub 2003/09/23. 14500507
49. Hardiman CA, McDonough JA, Newton HJ, Roy CR. The role of Rab GTPases in the transport of vacuoles containing Legionella pneumophila and Coxiella burnetii. Biochemical Society transactions. 2012;40(6):1353–9. doi: 10.1042/BST20120167 23176480
50. Tisdale EJ, Bourne JR, Khosravi-Far R, Der CJ, Balch WE. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. The Journal of cell biology. 1992;119(4):749–61. Epub 1992/11/01. 1429835
51. Touchot N, Zahraoui A, Vielh E, Tavitian A. Biochemical properties of the YPT-related rab1B protein. Comparison with rab1A. FEBS letters. 1989;256(1–2):79–84. Epub 1989/10/09. 2509243
52. Plutner H, Cox AD, Pind S, Khosravi-Far R, Bourne JR, Schwaninger R, et al. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. The Journal of cell biology. 1991;115(1):31–43. Epub 1991/10/01. 1918138
53. Ao X, Zou L, Wu Y. Regulation of autophagy by the Rab GTPase network. Cell Death Differ. 2014;21(3):348–58. Epub 2014/01/21. doi: 10.1038/cdd.2013.187 24440914
54. Huang J, Brumell JH. Bacteria-autophagy interplay: a battle for survival. Nature reviews Microbiology. 2014. Epub 2014/01/05.
55. Mukhopadhyay A, Quiroz JA, Wolkoff AW. Rab1a regulates sorting of early endocytic vesicles. American journal of physiology Gastrointestinal and liver physiology. 2014;306(5):G412–24. Epub 2014/01/11. doi: 10.1152/ajpgi.00118.2013 24407591
56. Mukhopadhyay A, Nieves E, Che FY, Wang J, Jin L, Murray JW, et al. Proteomic analysis of endocytic vesicles: Rab1a regulates motility of early endocytic vesicles. Journal of cell science. 2011;124(Pt 5):765–75. Epub 2011/02/10. doi: 10.1242/jcs.079020 21303926
57. Arasaki K, Toomre DK, Roy CR. The Legionella pneumophila effector DrrA is sufficient to stimulate SNARE-dependent membrane fusion. Cell host & microbe. 2012;11(1):46–57. Epub 2012/01/24.
58. Mihai Gazdag E, Streller A, Haneburger I, Hilbi H, Vetter IR, Goody RS, et al. Mechanism of Rab1b deactivation by the Legionella pneumophila GAP LepB. EMBO reports. 2013;14(2):199–205. Epub 2013/01/05. doi: 10.1038/embor.2012.211 23288104
59. Muller MP, Peters H, Blumer J, Blankenfeldt W, Goody RS, Itzen A. The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science. 2010;329(5994):946–9. Epub 2010/07/24. doi: 10.1126/science.1192276 20651120
60. Schoebel S, Cichy AL, Goody RS, Itzen A. Protein LidA from Legionella is a Rab GTPase supereffector. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(44):17945–50. doi: 10.1073/pnas.1113133108 22011575
61. Arasaki K, Roy CR. Legionella pneumophila promotes functional interactions between plasma membrane syntaxins and Sec22b. Traffic. 2010;11(5):587–600. Epub 2010/02/19. doi: 10.1111/j.1600-0854.2010.01050.x 20163564
62. Mishra AK, Del Campo CM, Collins RE, Roy CR, Lambright DG. The Legionella pneumophila GTPase activating protein LepB accelerates Rab1 deactivation by a non-canonical hydrolytic mechanism. The Journal of biological chemistry. 2013;288(33):24000–11. Epub 2013/07/04. doi: 10.1074/jbc.M113.470625 23821544
63. Sun Y, Connor MG, Pennington JM, Lawrenz MB. Development of bioluminescent bioreporters for in vitro and in vivo tracking of Yersinia pestis. PloS one. 2012;7(10):e47123. doi: 10.1371/journal.pone.0047123 23071730
64. Agaisse H, Burrack LS, Philips JA, Rubin EJ, Perrimon N, Higgins DE. Genome-wide RNAi screen for host factors required for intracellular bacterial infection. Science. 2005;309(5738):1248–51. Epub 2005/07/16. 16020693
65. Misselwitz B, Dilling S, Vonaesch P, Sacher R, Snijder B, Schlumberger M, et al. RNAi screen of Salmonella invasion shows role of COPI in membrane targeting of cholesterol and Cdc42. Molecular systems biology. 2011;7 : 474. Epub 2011/03/17. doi: 10.1038/msb.2011.7 21407211
66. Galen JE, Nair J, Wang JY, Wasserman SS, Tanner MK, Sztein MB, et al. Optimization of plasmid maintenance in the attenuated live vector vaccine strain Salmonella typhi CVD 908-htrA. Infection and immunity. 1999;67(12):6424–33. Epub 1999/11/24. 10569759
67. Kagan JC, Roy CR. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nature cell biology. 2002;4(12):945–54. 12447391
68. Itoh T, Satoh M, Kanno E, Fukuda M. Screening for target Rabs of TBC (Tre-2/Bub2/Cdc16) domain-containing proteins based on their Rab-binding activity. Genes to cells: devoted to molecular & cellular mechanisms. 2006;11(9):1023–37. Epub 2006/08/23.
69. Satoh A, Wang Y, Malsam J, Beard MB, Warren G. Golgin-84 is a rab1 binding partner involved in Golgi structure. Traffic. 2003;4(3):153–61. 12656988
70. Martinez O, Goud B. Rab proteins. Biochimica et biophysica acta. 1998;1404(1–2):101–12. Epub 1998/08/26. 9714762
71. Horwitz MA, Maxfield FR. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. The Journal of cell biology. 1984;99(6):1936–43. Epub 1984/12/01. 6501409
72. Sturgill-Koszycki S, Swanson MS. Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. The Journal of experimental medicine. 2000;192(9):1261–72. Epub 2000/11/09. 11067875
73. Brumell JH, Scidmore MA. Manipulation of rab GTPase function by intracellular bacterial pathogens. Microbiology and molecular biology reviews: MMBR. 2007;71(4):636–52. Epub 2007/12/08. 18063721
74. Ortiz Sandoval C, Simmen T. Rab proteins of the endoplasmic reticulum: functions and interactors. Biochemical Society transactions. 2012;40(6):1426–32. Epub 2012/11/28. doi: 10.1042/BST20120158 23176493
75. Liu S, Storrie B. Are Rab proteins the link between Golgi organization and membrane trafficking? Cellular and molecular life sciences: CMLS. 2012;69(24):4093–106. Epub 2012/05/15. doi: 10.1007/s00018-012-1021-6 22581368
76. Thornbrough JM, Hundley T, Valdivia R, Worley MJ. Human genome-wide RNAi screen for host factors that modulate intracellular Salmonella growth. PloS one. 2012;7(6):11.
77. Qin QM, Pei J, Ancona V, Shaw BD, Ficht TA, de Figueiredo P. RNAi screen of endoplasmic reticulum-associated host factors reveals a role for IRE1alpha in supporting Brucella replication. PLoS pathogens. 2008;4(7):e1000110. Epub 2008/07/26. doi: 10.1371/journal.ppat.1000110 18654626
78. Gutierrez MG, Vazquez CL, Munafo DB, Zoppino FC, Beron W, Rabinovitch M, et al. Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cellular microbiology. 2005;7(7):981–93. Epub 2005/06/15. 15953030
79. Niu H, Yamaguchi M, Rikihisa Y. Subversion of cellular autophagy by Anaplasma phagocytophilum. Cellular microbiology. 2008;10(3):593–605. Epub 2007/11/06. 17979984
80. Newton HJ, McDonough JA, Roy CR. Effector protein translocation by the Coxiella burnetii Dot/Icm type IV secretion system requires endocytic maturation of the pathogen-occupied vacuole. PloS one. 2013;8(1):e54566. Epub 2013/01/26. doi: 10.1371/journal.pone.0054566 23349930
81. Wieland H, Goetz F, Neumeister B. Phagosomal acidification is not a prerequisite for intracellular multiplication of Legionella pneumophila in human monocytes. The Journal of infectious diseases. 2004;189(9):1610–4. Epub 2004/04/30. 15116296
82. Benes P, Vetvicka V, Fusek M. Cathepsin D—many functions of one aspartic protease. Crit Rev Oncol Hematol. 2008;68(1):12–28. doi: 10.1016/j.critrevonc.2008.02.008 18396408
83. Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nature reviews Molecular cell biology. 2009;10(9):623–35. Epub 2009/08/13. doi: 10.1038/nrm2745 19672277
84. Xu H, Ren D. Lysosomal Physiology. Annual review of physiology. 2015;77 : 57–80. Epub 2015/02/11. doi: 10.1146/annurev-physiol-021014-071649 25668017
85. Horenkamp FA, Mukherjee S, Alix E, Schauder CM, Hubber AM, Roy CR, et al. Legionella pneumophila subversion of host vesicular transport by SidC effector proteins. Traffic. 2014;15(5):488–99. Epub 2014/02/04. doi: 10.1111/tra.12158 24483784
86. Machner MP, Isberg RR. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Developmental cell. 2006;11(1):47–56. Epub 2006/07/11. 16824952
87. Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nature cell biology. 2006;8(9):971–7. Epub 2006/08/15. 16906144
88. Machner MP, Isberg RR. A bifunctional bacterial protein links GDI displacement to Rab1 activation. Science. 2007;318(5852):974–7. Epub 2007/10/20. 17947549
89. Tan Y, Arnold RJ, Luo ZQ. Legionella pneumophila regulates the small GTPase Rab1 activity by reversible phosphorylcholination. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(52):21212–7. Epub 2011/12/14. doi: 10.1073/pnas.1114023109 22158903
90. Tan Y, Luo ZQ. Legionella pneumophila SidD is a deAMPylase that modifies Rab1. Nature. 2011;475(7357):506–9. Epub 2011/07/08. doi: 10.1038/nature10307 21734656
91. Mukherjee S, Liu X, Arasaki K, McDonough J, Galan JE, Roy CR. Modulation of Rab GTPase function by a protein phosphocholine transferase. Nature. 2011;477(7362):103–6. Epub 2011/08/09. doi: 10.1038/nature10335 21822290
92. Chen Y, Tascon I, Neunuebel MR, Pallara C, Brady J, Kinch LN, et al. Structural basis for Rab1 de-AMPylation by the Legionella pneumophila effector SidD. PLoS pathogens. 2013;9(5):e1003382. Epub 2013/05/23. doi: 10.1371/journal.ppat.1003382 23696742
93. Campanacci V, Mukherjee S, Roy CR, Cherfils J. Structure of the Legionella effector AnkX reveals the mechanism of phosphocholine transfer by the FIC domain. The EMBO journal. 2013;32(10):1469–77. Epub 2013/04/11. doi: 10.1038/emboj.2013.82 23572077
94. Doll JM, Zeitz PS, Ettestad P, Bucholtz AL, Davis T, Gage K. Cat-transmitted fatal pneumonic plague in a person who traveled from Colorado to Arizona. The American journal of tropical medicine and hygiene. 1994;51(1):109–14. Epub 1994/07/01. 8059908
95. Abu Kwaik Y, Eisenstein BI, Engleberg NC. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infection and immunity. 1993;61(4):1320–9. Epub 1993/04/01. 8454334
96. Al-Khodor S, Price CT, Habyarimana F, Kalia A, Abu Kwaik Y. A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Molecular microbiology. 2008;70(4):908–23. Epub 2008/09/25. doi: 10.1111/j.1365-2958.2008.06453.x 18811729
97. Pedersen LL, Radulic M, Doric M, Abu Kwaik Y. HtrA homologue of Legionella pneumophila: an indispensable element for intracellular infection of mammalian but not protozoan cells. Infection and immunity. 2001;69(4):2569–79. Epub 2001/03/20. 11254621
98. Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature methods. 2009;6(5):343–5. doi: 10.1038/nmeth.1318 19363495
99. Seto S, Tsujimura K, Koide Y. Rab GTPases regulating phagosome maturation are differentially recruited to mycobacterial phagosomes. Traffic. 2011;12(4):407–20. doi: 10.1111/j.1600-0854.2011.01165.x 21255211
100. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif). 2001;25(4):402–8. Epub 2002/02/16.
101. Kinder SA, Badger JL, Bryant GO, Pepe JC, Miller VL. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O8 and construction of a transformable R-M+ mutant. Gene. 1993;136(1–2):271–5. Epub 1993/12/22. 8294016
102. Chain PS, Carniel E, Larimer FW, Lamerdin J, Stoutland PO, Regala WM, et al. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(38):13826–31. Epub 2004/09/11. 15358858
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized VirusČlánek Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell ProliferationČlánek Fundamental Roles of the Golgi-Associated Aspartyl Protease, ASP5, at the Host-Parasite InterfaceČlánek Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 10- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Expression of Concern: Misregulation of Underlies the Developmental Abnormalities Caused by Three Distinct Viral Silencing Suppressors in Arabidopsis
- Preparing for the Next Epidemic with Basic Virology
- Effectively Communicating the Uncertainties Surrounding Ebola Virus Transmission
- Translating Basic Research into Clinical Applications: Malaria Research at an NIH Lab
- A Gut Odyssey: The Impact of the Microbiota on Spore Formation and Germination
- Papillomavirus E6 Oncoproteins Take Common Structural Approaches to Solve Different Biological Problems
- Chronobiomics: The Biological Clock as a New Principle in Host–Microbial Interactions
- Dimensions of Horizontal Gene Transfer in Eukaryotic Microbial Pathogens
- Addressing the Complications of Ebola and Other Viral Hemorrhagic Fever Infections: Using Insights from Bacterial and Fungal Sepsis
- Time for Chocolate: Current Understanding and New Perspectives on Cacao Witches’ Broom Disease Research
- Ganglioside and Non-ganglioside Mediated Host Responses to the Mouse Polyomavirus
- Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
- Structure Elucidation of Coxsackievirus A16 in Complex with GPP3 Informs a Systematic Review of Highly Potent Capsid Binders to Enteroviruses
- CD39 Expression Identifies Terminally Exhausted CD8 T Cells
- Abiotic Stresses Antagonize the Rice Defence Pathway through the Tyrosine-Dephosphorylation of OsMPK6
- Dissociation of Tissue Destruction and Bacterial Expansion during Bubonic Plague
- Interferon-γ: The Jekyll and Hyde of Malaria
- CCR2 Inflammatory Dendritic Cells and Translocation of Antigen by Type III Secretion Are Required for the Exceptionally Large CD8 T Cell Response to the Protective YopE Epitope during Infection
- A New Glycan-Dependent CD4-Binding Site Neutralizing Antibody Exerts Pressure on HIV-1
- The Suramin Derivative NF449 Interacts with the 5-fold Vertex of the Enterovirus A71 Capsid to Prevent Virus Attachment to PSGL-1 and Heparan Sulfate
- Trans-generational Immune Priming Protects the Eggs Only against Gram-Positive Bacteria in the Mealworm Beetle
- Peripheral Vγ9Vδ2 T Cells Are a Novel Reservoir of Latent HIV Infection
- Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33
- TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized Virus
- Modeling the Effects of Vorinostat Reveals both Transient and Delayed HIV Transcriptional Activation and Minimal Killing of Latently Infected Cells
- Identification of a Novel Lipoprotein Regulator of Spore Germination
- Calcium Regulation of Hemorrhagic Fever Virus Budding: Mechanistic Implications for Host-Oriented Therapeutic Intervention
- Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis
- Comparative Life Cycle Transcriptomics Revises Genome Annotation and Links a Chromosome Duplication with Parasitism of Vertebrates
- The Autophagy Receptor TAX1BP1 and the Molecular Motor Myosin VI Are Required for Clearance of Salmonella Typhimurium by Autophagy
- Carcinogenic Parasite Secretes Growth Factor That Accelerates Wound Healing and Potentially Promotes Neoplasia
- Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell Proliferation
- Dengue Virus Infection of Requires a Putative Cysteine Rich Venom Protein
- Distinct Viral and Mutational Spectrum of Endemic Burkitt Lymphoma
- Fundamental Roles of the Golgi-Associated Aspartyl Protease, ASP5, at the Host-Parasite Interface
- Phenotypic and Functional Alterations in Circulating Memory CD8 T Cells with Time after Primary Infection
- Systematic Identification of Cyclic-di-GMP Binding Proteins in Reveals a Novel Class of Cyclic-di-GMP-Binding ATPases Associated with Type II Secretion Systems
- Influenza Transmission in the Mother-Infant Dyad Leads to Severe Disease, Mammary Gland Infection, and Pathogenesis by Regulating Host Responses
- Myeloid Cell Arg1 Inhibits Control of Arthritogenic Alphavirus Infection by Suppressing Antiviral T Cells
- The White-Nose Syndrome Transcriptome: Activation of Anti-fungal Host Responses in Wing Tissue of Hibernating Little Brown Myotis
- Influenza Virus Reassortment Is Enhanced by Semi-infectious Particles but Can Be Suppressed by Defective Interfering Particles
- Identification of the Mechanisms Causing Reversion to Virulence in an Attenuated SARS-CoV for the Design of a Genetically Stable Vaccine
- Differentiation-Dependent KLF4 Expression Promotes Lytic Epstein-Barr Virus Infection in Epithelial Cells
- The Histone Acetyltransferase Hat1 Regulates Stress Resistance and Virulence via Distinct Chromatin Assembly Pathways
- C-di-GMP Regulates Motile to Sessile Transition by Modulating MshA Pili Biogenesis and Near-Surface Motility Behavior in
- Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes
- Crystal Structure of the Human Cytomegalovirus Glycoprotein B
- Depletion of . GlmU from Infected Murine Lungs Effects the Clearance of the Pathogen
- Immunologic Control of Papillomavirus Type 1
- Requires Host Rab1b for Survival in Macrophages
- Structure Analysis Uncovers a Highly Diverse but Structurally Conserved Effector Family in Phytopathogenic Fungi
- PD-L1 Expression on Retrovirus-Infected Cells Mediates Immune Escape from CD8 T Cell Killing
- Phospho-dependent Regulation of SAMHD1 Oligomerisation Couples Catalysis and Restriction
- IL-4 Induced Innate CD8 T Cells Control Persistent Viral Infection
- Crystal Structures of a Piscine Betanodavirus: Mechanisms of Capsid Assembly and Viral Infection
- BCG Skin Infection Triggers IL-1R-MyD88-Dependent Migration of EpCAM CD11b Skin Dendritic cells to Draining Lymph Node During CD4+ T-Cell Priming
- Antigenic Characterization of the HCMV gH/gL/gO and Pentamer Cell Entry Complexes Reveals Binding Sites for Potently Neutralizing Human Antibodies
- Rescue of a Plant Negative-Strand RNA Virus from Cloned cDNA: Insights into Enveloped Plant Virus Movement and Morphogenesis
- Geminivirus Activates to Accelerate Cytoplasmic DCP2-Mediated mRNA Turnover and Weakens RNA Silencing in
- Disruption of Sphingolipid Biosynthesis Blocks Phagocytosis of
- The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps
- The Timing of Stimulation and IL-2 Signaling Regulate Secondary CD8 T Cell Responses
- Structural and Functional Analysis of Murine Polyomavirus Capsid Proteins Establish the Determinants of Ligand Recognition and Pathogenicity
- The Dual Role of an ESCRT-0 Component HGS in HBV Transcription and Naked Capsid Secretion
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Chronobiomics: The Biological Clock as a New Principle in Host–Microbial Interactions
- Interferon-γ: The Jekyll and Hyde of Malaria
- Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
- Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



