-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Species
article has not abstract
Published in the journal: On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Species. PLoS Pathog 11(7): e32767. doi:10.1371/journal.ppat.1004982
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004982Summary
article has not abstract
What Are Trichosporon Species?
Trichosporon species are basidiomycetous, yeast-like organisms capable of filamentous growth, i.e., dimorphic (Fig 1), that are distributed throughout nature [1]. They are important from a biotechnological point of view as they are capable of decontaminating polluted environments by accumulating large amounts of oils [2–5]. A limited number of reports also show their presence in the human microbiome, such as Trichosporon asahii, which has been isolated from human fecal samples, the skin of healthy individuals, and patients with atopic dermatitis [1,6–10]. In addition, Trichosporon spp. can cause white piedra, hypersensitivity pneumonitis, superficial infections, and invasive trichosporonosis [11].
Fig. 1. Colony morphology of Trichosporon species. 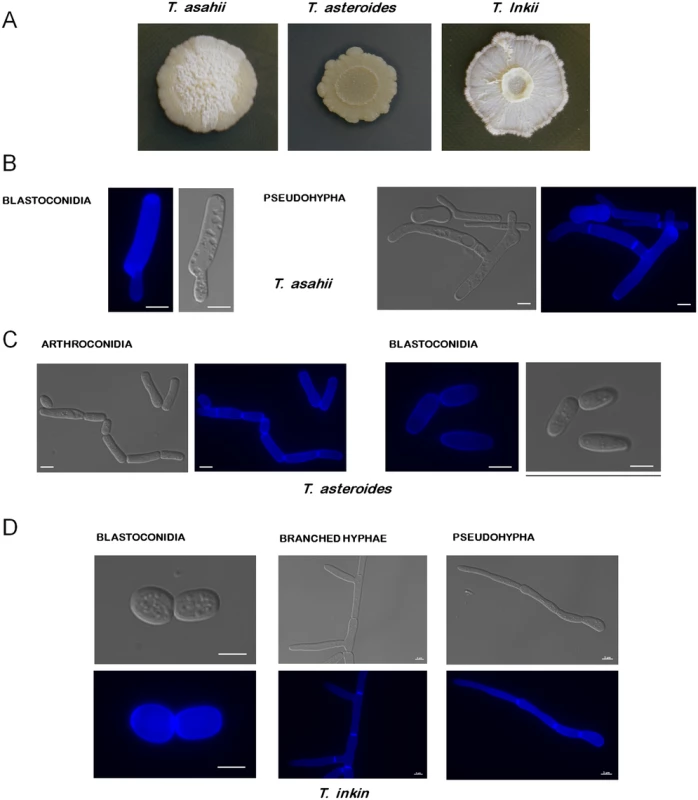
(A) T. asahii, T. asteroides, and T. inkii were grown at 30°C for 15 days on Sabouraud, YNB+tributyrin 1%, and YPD media, respectively. T. asteroides is releasing lipases, producing a halo where tributyrin is degraded. (B) T. asahii blastoconidia and pseudohypha. (C) T. asteroides arthroconidia and blastoconidia. (D) T. inkin pseudohypha, branched hypha, and blastoconidia. The blue fluorescence on the structures is due to their incubation with calcofluor white. Bars, 5 μm. Designated 150 years ago, the genus Trichosporon was for many years a collection of many different yeast-like organisms. Until the end of the 20th century, a wide range of species were included under the name of Trichosporon beigelii or synonyms, which were later shown to be phylogenetically distinct. With the advent of molecular techniques, the genus was rearranged, and many of its species were reassigned to other genera and new ones were described. Currently there are 51 accepted Trichosporon species, 16 of which have clinical relevance [1,12–13] (these species are highlighted in Fig 2).
Fig. 2. Maximum-likelihood phylogenetic tree of Trichosporon species, based on analysis of the ITS1 and ITS2 regions and the D1/D2 region of the LSU. 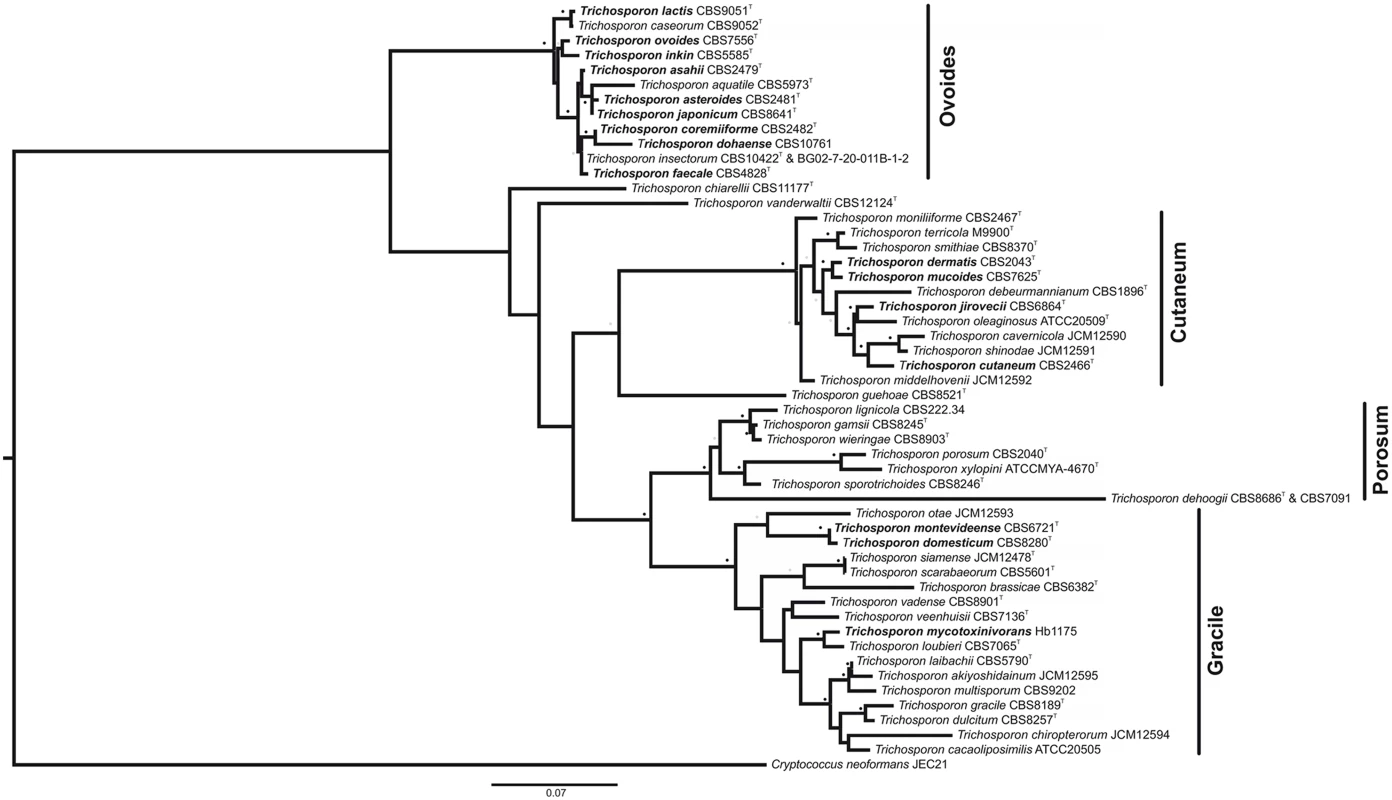
Strain names appear after the species name. Trichosporon species of clinical significance appear in bold. See S1 Table for the GenBank accession numbers. Cryptococcus neoformans was used as an outgroup. Sequences of each individual region were structurally aligned using MXSCARNA [58] and then concatenated into a supermatrix using FASConCAT [59]. Phylogenetic inference was carried out with the software RAxML [60]. For the loop regions, the evolutionary model GTR was used. The stem regions were analyzed under the S16 evolutionary model, thus taking into account secondary structure topology, i.e., compensatory mutations [61–63]. Substitution rate heterogeneity was taken into account using the gamma model of Yang [64]. Bootstrap values were computed over 1,000 replicates. Grey dots represent bootstrap values 50%–75% and black dot bootstrap values 76%–100%. Bar, 0.07 substitutions per nucleotide position. T: Type strain. Formerly associated with uncommon hair and skin infections, research on Trichosporon has fallen behind more life-threatening fungi such as Candida or Cryptococcus species, until the rise of opportunistic, deeply seated, disseminated Trichosporon infections (especially T. asahii) over the last decades. A lack of background knowledge impairs the proper diagnosis and treatment of Trichosporon infections. Fortunately in the recent years, studies of Trichosporon epidemiology [14], virulence factors [15,16], antifungal susceptibility [16,17], and animal models of infection [18,19] have been conducted. There are a few reports of genetic transformation systems for T. cutaneum, which used both dominant and auxotrophic markers [20,21]. However, forward and reverse genetics is still not common practice in the study of Trichosporon.
Molecular techniques have already proved necessary for the detection and correct identification of Trichosporon species [1,17]. However, the use of whole genome sequencing and gene manipulation techniques in Trichosporon is in its infancy, in comparison to other fungi, such as Candida or Aspergillus species. Genome sequences for a T. asahii environmental strain and a strain isolated from a progressive psoriatic lesion are available [22,23]. Both T. asahii genomes are of approximately 25 Mbp and are predicted to encode approximately 9,000 genes. A comparison of these two Trichosporon genomes revealed >99% chromosomal and mitochondrial sequence identity. When compared to the genomes of other skin-associated or pathogenic basidiomycetes, the T. asahii genome is larger and is predicted to encode a greater number of genes than the basidiomycetes Malassezia restricta, M. globosa, and M. sympodialis, which range from 7.6 to 9.0 Mbp and are predicted to encode around 3,500 to 4,300 genes [24,25]. In contrast, the genomes of Cryptococcus neoformans and C. gattii are between 17 to 20 Mbp and are predicted to encode around 6,500 to 8,300 genes [26,27].
Along with the advances in health care, rarer opportunistic pathogens are gaining more attention, and through the anticipated application of modern pathogenomics to the study of trichosporonosis, this may represent a turning point in the understanding of Trichosporon species and their virulence determinants.
What Is the Clinical Importance of Trichosporon?
Skin and hair infections due to Trichosporon were considered rare for a long time, but the misdiagnosis of superficial trichosporonosis might have led to an underestimation of its prevalence [28]. Being a part of the natural microbiota of the skin, these Trichosporon species can be misdiagnosed as contaminants, resulting in the infection being attributed to dermatophytes, when in fact Trichosporon might be the etiological agent in 10%–40% of superficial infections depending on the geographic area and population [29,30]. The old designation T. beigelii has been used years after the rearrangement of the genus [29]. The carryover of older names is common in clinical practice and can interfere in the correct diagnosis and treatment. In order to overcome this issue, fast and efficient molecular-based tools are being developed to effectively identify Trichosporon species in clinical settings [31]. In addition, MALDI-TOF mass spectrometry has proven to be a reliable tool for the identification of Trichosporon species and could become a cheaper and complementary alternative to gene sequencing [32,33]. Currently T. inkin, T. cutaneum, T. ovoides, and T. loubieri are considered the most prominent species involved in superficial trichosporonosis, while T. asahii, T. ateroides, and T. mucoides are associated with invasive infections in immunocompromised patients [11]. T. asahii stands out as the leading cause of disseminated infections and is not usually associated with superficial infections [2,34]. However, a rare case was recently reported of a T. asahii cutaneous infection that progressed into subcutaneous tissues and resulted in a fatal outcome in an immunocompetent patient [35].
The vast majority of cases of disseminated trichosporonosis occur in patients that are immunocompromised or those that have received cytotoxic chemotherapy, steroids, or broad-spectrum antibiotics [14,36]. A major risk factor is reported to be the use of venous catheters or drains that may facilitate the penetration of the fungus beyond the colonized skin [37]. In a global surveillance study, Trichosporon species accounted for a 0.5% of total yeast isolates and 10.7% of non-Candida species in invasive and mucosa-associated infections [38]. Invasive Trichosporon infections have been reported in 0.4% of patients with acute leukemia and are associated with a high mortality rate of 64% [14]. This is also a common trend for hematological patients, since high mortality rates, ranging from 42% to 76%, have been widely reported for Trichosporon infections [39]. Apart from cancer and neutropenia, other underlying conditions, such as burns, surgery, and organ failure, were reported to be associated with invasive Trichosporon infections and a mortality rate as high as 87.5% in adult patients in Brazil [34].
How Can Trichosporon spp. Infections Be Treated?
Superficial Trichosporon infections, such as white piedra, often respond well to topical or oral azole treatments combined with improved hygienic habits to avoid relapses [40]. However, invasive infections represent a therapeutic challenge, and no consensus exists for a recommended treatment. Several studies report minimal success with amphotericin B or fluconazole [11,37], which correlates with the commonly reported high minimal inhibitory concentrations for these drugs [34,36]. The echinocandins display minimal activity against basidiomycetes, and several cases of breakthrough infections caused by Trichosporon on patients undergoing echinocandin therapy have been reported [41,42]. Only a single case has been reported of the successful treatment of peritonitis caused by T. inkin with caspofungin alone [43]. Newer azole drugs such as voriconazole and posaconazole have shown excellent in vitro activity against Trichosporon [16,17]. In vivo efficacy and increasing reports of successful treatments with voriconazole highlight this drug as a potential therapeutic option to combat disseminated Trichosporon infections [11,44,45]. Posaconazole, on the other hand, has also shown encouraging results in a murine model of infection [19] but lacks clinical evidence of its efficacy. Combined antifungal therapy is considered a way to broaden the antimycotic spectrum of treatments, especially applicable to cases of rare or refractory fungal infections. However, the use of multiple antifungal agents can lead to antagonistic interactions and cause interference with other therapies the patient might be receiving. Hence, this option is only used as a last resort, unless there is an important patient background that encourages its use [46]. Concomitant or sequential usage of antifungal drugs has been reported for Trichosporon infections and generally yielded undistinguished results [14,37]. However, experimental results and recent reports of successful treatments [18,47] suggest that combined therapies are an option to take into account against trichosporonosis.
How Do Trichosporon spp. Colonize and Infect the Hosts?
With the exception of C. neoformans, basidiomycete disseminated infections are rare and tend to occur in patients with severe underlying conditions. Cryptococcus disseminated infections are generally associated with a primary pulmonary infection that disseminates through the bloodstream and tends to affect the central nervous system [48]. Several filamentous basidiomycetes, such as Hormographiella aspergillata and Tyromyces fissilis [49,50], have also been reported to cause pulmonary infections in severely immunocompromised patients, supposedly by spore inhalation. Conversely, several basidiomycetous yeasts, such as Trichosporon, Rhodotorula, Malassezia, and Sporobolomyces species, are considered to colonize or even infect the skin or mucosa, a location from which they take advantage of a barrier disruption caused by their own means or by trauma, such as a catheter implantation.
The ability of Trichosporon to invade the skin and other tissues requires several pathogenic traits, such as biofilm formation; enzymatic activities, including phospholipases and proteases; and the production of hyphae or pseudohyphae. Both Trichosporon and Malassezia are good examples of host-adapted pathogens that possess, to some extent, all the aforementioned traits. Biofilm formation has been observed in several Trichosporon species [16], a trait that is closely related to the ability to grow on exogenous surfaces such as a catheter. Central venous catheter removal is strongly recommended in cases of blood-positive cultures of Trichosporon, Malasezzia, and Rhodotorula in the same manner as it is recommended in bloodstream infections by Candida [11,51,52]. C. neoformans has a wide array of pathogenic traits that permit it to avoid the host immune system and to cross natural barriers within the body; however, not much is known about its ability to form biofilms, which is proposed to be lower than that of the other pathogenic yeasts mentioned above [8]. Accordingly, clinical reports about C. neoformans association to a central venous catheter are scarce [53]. One of the most well-known virulence traits of Cryptococcus species is the production of the polysaccharide glucuronoxylomannan that protects the cells from phagocytosis, collaborates in evasion of the immune system, and promotes intracellular survival [49]. Trichosporon species also produce glucuronoxylomannan, which if demonstrated to have a role in virulence, would represent a possible therapeutic target [54].
How Do Trichosporon spp. Adapt to the Host?
The production of enzymes capable of degrading various components of host tissues is also a major virulence trait present in many pathogenic fungi [11]. A recent study demonstrated that several species of Trichosporon are capable of producing a variety of proteases, lipases phospholipases, and DNAases [15]. This hydrolytic activity is a well-known trait that allows other basidiomycetous yeasts, such as Malassezia and Rhodotorula, to obtain fatty acids from host lipids and breakdown phospholipids present in cell membranes. The proteolytic activity of Trichosporon is a trait shared with dermatophytes that produce a plethora of proteases, which in accordance with their ecological niche, play an important role in overcoming host immunological barriers [25].
In contrast to Trichosporon species, dermatophytes and Malassezia have undergone a severe loss of genes encoding for carbohydrate-degrading enzymes, which indicates a high degree of adaptation to animal hosts [25]. The animal skin is the common target of dermathophytes and Malassezia; however, they adopt different approaches to pathogenesis. Malassezia predominately obtain nourishment from host fatty acids, to the point that they have lost the metabolic routes to synthesize them [25]. Dermatophytes, on the other hand, are specialized in the degradation of keratin and other proteins [55]. These ascomycetes also present a wide arsenal of immunomodulators, antimicrobial and toxic compounds, as well as many of secondary metabolites of unknown function that reveal a high degree of specialization to the host environment [25]. Differential expression of proteases in various species of dermatophytes has been related to their adaptation to a diverse range of hosts and course of infections [55,56]. Higher expression could induce higher inflammatory reactions and immune system responses, while lower expression might lead to a more subtle and chronic infection [55,56].
Trichosporon species seem to maintain their fitness in a wider range of environments than other skin pathogens and may possibly be less specialized. In this sense, it was suggested that the high level of protease expression in Trichosporon might indicate that these species are still in the process of adaptation to the animal hosts [55,56]. Accordingly, there are prominent differences in genome size and predicted encoding genes between Trichosporon spp. and Malassezia. However, further research on Trichosporon–host interactions is required to prove such hypotheses.
What Can Molecular Biology Do to Improve Our Knowledge about Trichosporon Infections?
Exciting times are coming in the study of Trichosporon, with the development of biological tools that will facilitate the enhancement of our understanding of Trichosporon pathogenicity determinants. Genome and RNA sequencing projects will contribute considerably to the understanding of which genes are shared between Trichosporon species and/or isolates with differing virulence profiles and which genes are expressed under different pathogenic conditions. Diagnostics, epidemiology, and drug-resistance studies can take dramatic advantage of the genome sequencing of multiple clinical isolates. Protocols for genetic transformation and genetic markers have already been developed for T. cutaneum, and it should become relatively straightforward to transfer this technology to other Trichosporon species. The construction of deletion strains will allow us to interrogate about the participation of several genes as virulence determinants and the role played by the different morphological phases of this fungus in the infection process. In combination with the already established murine and Galleria mellonella model systems [19,57], this will permit the use of forward and reverse genetics to interrogate the determinants of pathogenicity, in turn facilitating the development of novel drugs to combat this important fungal pathogen.
Supporting Information
Zdroje
1. Sugita T Trichosporon behrend (1890). in: Kurtzman C.P., Fell J.W., Boekhout T. (Eds.) The yeasts: a taxonomic study. 5th ed. Elsevier Science, Amsterdam. 2004; 2011 : 2015–2061.
2. Ageitos JM, Vallejo JA, Veiga-Crespo P, Villa TG Oily yeasts as oleaginous cell factories. Appl Microbiol Biotechnol 2011; 90 : 1219–1227. doi: 10.1007/s00253-011-3200-z 21465305
3. Xiong L, Huang C, Li XM, Chen XF, Wang B, Wang C,et al. Acetone-Butanol-Ethanol (ABE) Fermentation Wastewater Treatment by oleaginous yeast Trichosporon cutaneum. Appl Biochem Biotechnol 2015. In press.
4. Tamis J, Sorokin DY, Jiang Y, van Loosdrecht MC, Kleerebezem R Lipid recovery from a vegetable oil emulsion using microbial enrichment cultures. Biotechnol Biofuels 2015; 8 : 39. doi: 10.1186/s13068-015-0228-9 25798194
5. Nhi Cong le T, Ngoc Mai CT, Thanh VT, Nga le P, Minh NN Application of a biofilm formed by a mixture of yeasts isolated in Vietnam to degrade aromatic hydrocarbon polluted wastewater collected from petroleum storage. Water Sci Technol 2014; 70 : 329–336. doi: 10.2166/wst.2014.233 25051481
6. Middelhoven WJ, Scorzetti G, Fell JW Systematics of the anamorphic basidiomycetous yeast genus Trichosporon Behrend with the description of five novel species: Trichosporon vadense, T. smithiae, T. dehoogii, T. scarabaeorum and T. gamsii. Int J Syst Evol Microbiol 2004; 54 : 975–986. 15143052
7. Zhang E, Sugita T, Tsuboi R, Yamazaki T, Makimura K The opportunistic yeast pathogen Trichosporon asahii colonizes the skin of healthy individuals: analysis of 380 healthy individuals by age and gender using a nested polymerase chain reaction assay. Microbiol Immunol 2011; 55 : 483–4888. doi: 10.1111/j.1348-0421.2011.00341.x 21707737
8. Zhang E, Tanaka T, Tajima M, Tsuboi R, Nishikawa A, Sugita T Characterization of the skin fungal microbiota in patients with atopic dermatitis and in healthy subjects. Microbiol Immunol 2011; 55 : 625–632. doi: 10.1111/j.1348-0421.2011.00364.x 21699559
9. Hamad I, Sokhna C, Raoult D, Bittar F Molecular detection of eukaryotes in a single human stool sample from Senegal. PLoS One 2012; 7: e40888. doi: 10.1371/journal.pone.0040888 22808282
10. Gouba N, Raoult D, Drancourt M Eukaryote culturomics of the gut reveals new species. PLoS One 2014; 9: e106994. doi: 10.1371/journal.pone.0106994 25210972
11. Silvestre AM Jr, Miranda MAR, Camargo ZP Trichosporon species isolated from the perigenital region, urine and catheters of a Brazilian population. Braz J Microbiol 2010; 41 : 628–634. doi: 10.1590/S1517-83822010000300013 24031538
12. Colombo AL, Padovan ACB, Chaves GM Current knowledge of Trichosporon spp. and trichosporonosis. Clin Microbiol Rev 2011; 24 : 682–700. doi: 10.1128/CMR.00003-11 21976604
13. Gueho E, de Hoog GS, Smith MT Neotypification of the genus Trichosporon. Antonie Van Leeuwenhoek 1992; 61 : 285–288 1497333
14. Motaung TE, Albertyn J, Kock JL, Lee CF, Suh SO, et al. Trichosporon vanderwaltii sp. nov., an asexual basidiomycetous yeast isolated from soil and beetles. Antonie Van Leeuwenhoek 2013; 103 : 313–319. doi: 10.1007/s10482-012-9811-2 22996387
15. Girmenia C, Pagano L, Martino B, D’Antonio D, Fanci R, Blackweel M, et al. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: a retrospective multicenter study from Italy and review of the literature. J Clin Microbiol 2005; 43 : 1818–1828. 15815003
16. Donnarumma H, Bentubo L, Fischman-Gompertz O Effects of temperature and incubation time on the in vitro expression of proteases, phospholipases, lipases and DNases by different species of Trichosporon. SpringerPlus 2014; 3 : 377. doi: 10.1186/2193-1801-3-377 25161862
17. Taj-Aldeen SJ, Al-Ansari N, El Shafei S, Meis JF, Curfs-Breuker I, Theelen B, et al. Molecular identification and susceptibility of Trichosporon species isolated from clinical specimens in Qatar: isolation of Trichosporon dohaense Taj-Aldeen, Meis & Boekhout sp. nov. J Clin Microbiol 2009; 47 : 1791–1799. doi: 10.1128/JCM.02222-08 19321719
18. Iturrieta-González IA, Padovan AC, Bizerra FC, Hahn RC, Colombo AL Multiple species of Trichosporon produce biofilms highly resistant to triazoles and amphotericin B. PLoS One 2014; 9: e109553. doi: 10.1371/journal.pone.0109553 25360765
19. Serena C, Pastor FJ, Gilgado F, Mayayo E, Guarro J Efficacy of micafungin in combination with other drugs in a murine model of disseminated trichosporonosis. Antimicrob Agents Chemother 2005; 49 : 497–502. 15673724
20. Treviño-Rangel RD, López LJ, Palma-Nicolás JP, Hernández-Bello R, González JG, González GM Therapeutic efficacy of posaconazole in a murine model of disseminated trichosporonosis. J Antimicrob Chemother 2014; 69 : 1075–1078. doi: 10.1093/jac/dkt466 24252752
21. Ochsner UA, Glumoff V, Kälin M, Fiechter A, Reiser J Genetic transformation of auxotrophic mutants of the filamentous yeast Trichosporon cutaneum using homologous and heterologous marker genes. Yeast 1991; 7 : 513–524. 1897316
22. Reiser J, Glumoff V, Ochsner UA, Fiechter A Molecular analysis of the Trichosporon cutaneum DSM 70698 argA gene and its use for DNA-mediated transformations. J Bacteriol 1994; 176 : 3021–3032. 8188603
23. Yang RY, Li HT, Zhu H, Zhou GP, Wang M, Wang L. Draft genome sequence of CBS 2479, the standard type strain of Trichosporon asahii. Eukaryot Cell 2012; 11 : 1415–1416. doi: 10.1128/EC.00237-12 23104369
24. Yang RY, Li HT, Zhu H, Zhou GP, Wang M, Wang L Genome sequence of the Trichosporon asahii environmental strain CBS 8904. Eukaryot Cell 2012; 11 : 1586–1587. doi: 10.1128/EC.00264-12 23193141
25. Xu J, Saunders CW, Hu P, Grant RA, Boekhout T, Kuramae EE, et al. Dandruff-associatedMalassezia genomes reveal convergent and divergent virulence traits shared with plant and human fungal pathogens. Proc Natl Acad Sci 2007; 104 : 18730–18735. 18000048
26. White TC, Findley K, Dawson TL Jr, Scheynius A, Boekhout T, Cuomo CA et al. Fungi on the skin: dermatophytes and Malassezia. Cold Spring Harb Perspect Med 2014; 4:a019802 doi: 10.1101/cshperspect.a019802 25085959
27. Loftus BJ, Fung E, Roncaglia P, Rowley D, Amedeo P, Bruno D, et al. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 2005; 307 : 1321–1324. 15653466
28. D'Souza CA, Kronstad JW, Taylor G, Warren R, Yuen M, Hu G, et al. Genome variation in Cryptococcus gattii, an emerging pathogen of immunocompetent hosts. MBio 2011; 2: e00342–10. doi: 10.1128/mBio.00342-10 21304167
29. Gold I, Sommer B, Urson S, Schewach-Millet M White piedra. A frequently misdiagnosed infection of hair. Int J Dermatol 1984; 23 : 621–623. 6542905
30. Han MH, Choi JH, Sung KJ, Moon KC, Koh JK Onychomycosis and Trichosporon beigelii in Korea. Int J Dermatol 2000; 39 : 266–269. 10809974
31. Archer-Dubon C, Orozco-Topete R, Leyva-Santiago J, Arenas R, Carbajosa J, Ysunza A Superficial mycotic infections of the foot in a native pediatric population: a pathogenic role for Trichosporon cutaneum? Pediatr Dermatol 2003; 20 : 299–302. 12869147
32. Kolecka A, Khayhan K, Groenewald M, Theelen B, Arabatzis M, Velegraki A, et al. Identification of medically relevant species of arthroconidial yeasts by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 2013; 51 : 2491–2500. doi: 10.1128/JCM.00470-13 23678074
33. de Almeida Júnior JN, Figueiredo DS, Toubas D, Del Negro GM, Motta AL, Rossi F, et al. Usefulness of matrix-assisted laser desorption ionisation-time-of-flight mass spectrometry for identifying clinical Trichosporon isolates. Clin Microbiol Infect 2014; 20 : 784–790. doi: 10.1111/1469-0691.12502 24355037
34. Diaz MR, Fell JW High-throughput detection of pathogenic yeasts of the genus Trichosporon. J Clin Microbiol 2004; 42 : 3696–3706 15297519
35. Chagas-Neto TC, Chaves GM, Melo AS, Colombo AL Bloodstream infections due to Trichosporon spp.: species distribution, Trichosporon asahii genotypes determined on the basis of ribosomal DNA intergenic spacer 1 sequencing, and antifungal susceptibility testing. J Clin Microbiol 2009; 47 : 1074–1081. doi: 10.1128/JCM.01614-08 19225102
36. Baka S, Tsouma I, Kouskouni E Fatal lower limb infection by Trichosporon asahii in an immunocompetent patient. Acta Dermatovenerol Croat 2013; 21 : 241–244 24476611
37. Rodriguez-Tudela JL, Diaz-Guerra TM, Mellado E, Cano V, Tapia C, Perkins A, et al. Susceptibility patterns and molecular identification of Trichosporon species. Antimicrob Agents Chemother 2005; 49 : 4026–4034. 16189076
38. Rodrigues Gda S, de Faria RR, Guazzelli LS, Oliveira Fde M, Severo LC Nosocomial infection due to Trichosporon asahii: clinical revision of 22 cases. Rev Iberoam Micol. 2006 : 23 : 85–89. 16854183
39. Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Bijie H, Dzierzanowska D, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007 : 10.5-year analysis of susceptibilities of noncandidal yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol 2009; 47 : 117–123. doi: 10.1128/JCM.01747-08 19005141
40. Caira M, Trecarichi EM, Tumbarello M, Leone G, Pagano L Uncommon yeast infections in hematological patients: from diagnosis to treatment. Expert Rev Anti Infect Ther 2011; 9 : 1067–1075. doi: 10.1586/eri.11.124 22029524
41. Fischman O, Bezerra FC, Francisco EC, da Silva FC, Nishikaku AS, Cavalcanti SD Trichosporon inkin: an uncommon agent of scalp white piedra. Report of four cases in brazilian children. Mycopathologia 2014; 178 : 85–89. doi: 10.1007/s11046-014-9750-8 24952012
42. Matsue K, Uryu H, Koseki M, Asada N, Takeuchi M Breakthrough trichosporonosis in patients with hematologic malignancies receiving micafungin. Clin Infect Dis 2006; 42 : 753–757. 16477548
43. Madariaga MG, Tenorio A, Proia L Trichosporon inkin peritonitis treated with caspofungin. J Clin Microbiol 2003; 41 : 5827–5829. 14662994
44. Serena C, Gilgado F, Mariné M, Pastor FJ, Guarro J Efficacy of voriconazole in a guinea pig model of invasive trichosporonosis. Antimicrob Agents Chemother 2006; 50 : 2240–2243. 16723595
45. Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; European Confederation of Medical Mycology. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect Suppl 2014; 3 : 76–98.
46. Hatipoglu N, Hatipoglu H Combination antifungal therapy for invasive fungal infections in children and adults. Expert Rev Anti Infect Ther 2013; 11 : 523–535. doi: 10.1586/eri.13.29 23627858
47. Hosokawa K, Yamazaki H, Mochizuki K, Ohata K, Ishiyama K, Hayashi T, et al. Successful treatment of Trichosporon fungemia in a patient with refractory acute myeloid leukemia using voriconazole combined with liposomal amphotericin B. Transpl Infect Dis 2012; 14 : 184–187. doi: 10.1111/j.1399-3062.2011.00670.x 22093149
48. Kozubowski L, Heitman J Profiling a killer, the development of Cryptococcus neoformans. FEMS Microbiol Rev 2012; 36 : 78–94. doi: 10.1111/j.1574-6976.2011.00286.x 21658085
49. Suarez F, Olivier G, Garcia-Hermoso D, Randriamalala E, Ghez D, Bruneau J, et al. Breakthrough Hormographiella aspergillata infections arising in neutropenic patients treated empirically with caspofungin. J Clin Microbiol 2011; 49 : 461–465. doi: 10.1128/JCM.01213-10 21068290
50. Chrenkova V, Kolarik M, Hubacek P, Kolarik J, Simonek J, et al. Possible Tyromyces fissilis (Basidiomycota, Polyporales) co-infection in a lung transplant recipient. Folia Microbiol (Praha) 2015; 60 : 33–35.
51. García-Suárez J, Gómez-Herruz P, Cuadros JA, Burgaleta C Epidemiology and outcome of Rhodotorula infection in haematological patients. Mycoses 2011; 54 : 318–324. doi: 10.1111/j.1439-0507.2010.01868.x 20337934
52. Iatta R, Cafarchia C, Cuna T, Montagna O, Laforgia N, Gentile O, et al. Bloodstream infections by Malassezia and Candida species in critical care patients. Med Mycol 2014; 52 : 264–269. doi: 10.1093/mmy/myt004 24576998
53. Tuon FF, Morales HM, Penteado-Filho SR, da-Silva MM, Quadros Id, Hamoui El A Central venous catheter-related bloodstream infection and Cryptococcus neoformans. Braz J Infect Dis 2009; 13 : 317–318. 20231999
54. Fonseca FL, Frases S, Casadevall A, Fischman-Gompertz O, Nimrichter L, Rodrigues ML Structural and functional properties of the Trichosporon asahii glucuronoxylomannan. Fungal Genet Biol 2009; 46 : 496–505. doi: 10.1016/j.fgb.2009.03.003 19285564
55. Achterman RR, White TC A foot in the door for dermatophyte research. PLoS Pathog 2012; 8:e1002564. doi: 10.1371/journal.ppat.1002564 22479177
56. Giddey K, Favre B, Quadroni M, Monod M Closely related dermatophyte species produce different patterns of secreted proteins. FEMS Microbiol Lett 2007; 267 : 95–101. 17156126
57. Mariné M, Pedro Bom VL, de Castro PA, Winkelstroter LK, Ramalho LN, Brown NA, et al. The development of animal infection models and antifungal efficacy assays against clinical isolates of Trichosporon asahii, T. asteroides and T. inkin. Virulence 2015. In press.
58. Tabei Y, Kiryu H, Kin T, Asai K A fast structural multiple alignment method for long RNA sequences. BMC Bioinformatics 2008; 9 : 33. doi: 10.1186/1471-2105-9-33 18215258
59. Kück P, Meusemann K FASconCAT: Convenient handling of data matrices. Mol Phylogenet Evol 2010; 56 : 1115–1118. doi: 10.1016/j.ympev.2010.04.024 20416383
60. Stamatakis A RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006; 22 : 2688–2690. 16928733
61. Higgs PG RNA secondary structure: physical and computational aspects. Q Rev Biophys 2000; 33 : 199–253. 11191843
62. Jow H, Hudelot C, Rattray M, Higgs PG Bayesian phylogenetics using an RNA substitution model applied to early mammalian evolution. Mol Biol Evol 2002; 19 : 1591–1601. 12200486
63. Hudelot C, Gowri-Shankar V, Jow H, Rattray M, Higgs PG RNA-based phylogenetic methods: application to mammalian mitochondrial RNA sequences. Mol Phylogenet Evol 2003; 28 : 241–252. 12878461
64. Yang Z Among-site rate variation and its impact on phylogenetic analyses. Trends Ecol Evol 1996; 11 : 367–372. 21237881
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSSČlánek Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to MalariaČlánek IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 CellsČlánek Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 7- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Basic Prion Science “Spreads” Insight
- Research Driven by Curiosity: The Journey from Basic Molecular Biology and Virology to Studies of Human Pathogenic Coronaviruses
- Cross Kingdom Activators of Five Classes of Bacterial Effectors
- Vaccination Drives Changes in Metabolic and Virulence Profiles of
- Expression of the Blood-Group-Related Gene Alters Susceptibility to Infection
- Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSS
- Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival
- The DSF Family of Cell–Cell Signals: An Expanding Class of Bacterial Virulence Regulators
- Intraperitoneal Infection of Wild-Type Mice with Synthetically Generated Mammalian Prion
- Vpr Promotes Macrophage-Dependent HIV-1 Infection of CD4 T Lymphocytes
- An In-Depth Comparison of Latency-Reversing Agent Combinations in Various and HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression
- α-Macroglobulin Can Crosslink Multiple Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes
- Should Symbionts Be Nice or Selfish? Antiviral Effects of Wolbachia Are Costly but Reproductive Parasitism Is Not
- A Unique Human Norovirus Lineage with a Distinct HBGA Binding Interface
- The Broad Neutralizing Antibody Responses after HIV-1 Superinfection Are Not Dominated by Antibodies Directed to Epitopes Common in Single Infection
- Rapidly Evolving Genes Are Key Players in Host Specialization and Virulence of the Fungal Wheat Pathogen ()
- MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease
- Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection
- Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria
- Dimerization-Induced Allosteric Changes of the Oxyanion-Hole Loop Activate the Pseudorabies Virus Assemblin pUL26N, a Herpesvirus Serine Protease
- Macrophages Subvert Adaptive Immunity to Urinary Tract Infection
- Mycolactone-Dependent Depletion of Endothelial Cell Thrombomodulin Is Strongly Associated with Fibrin Deposition in Buruli Ulcer Lesions
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- K-bZIP Mediated SUMO-2/3 Specific Modification on the KSHV Genome Negatively Regulates Lytic Gene Expression and Viral Reactivation
- Phosphoproteomic Analysis of KSHV-Infected Cells Reveals Roles of ORF45-Activated RSK during Lytic Replication
- CR3 and Dectin-1 Collaborate in Macrophage Cytokine Response through Association on Lipid Rafts and Activation of Syk-JNK-AP-1 Pathway
- IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 Cells
- THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection
- Human Enterovirus Nonstructural Protein 2C Functions as Both an RNA Helicase and ATP-Independent RNA Chaperone
- IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4 T Cells and IFN-γ
- Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation
- Vpu Exploits the Cross-Talk between BST2 and the ILT7 Receptor to Suppress Anti-HIV-1 Responses by Plasmacytoid Dendritic Cells
- Herpesvirus Genome Recognition Induced Acetylation of Nuclear IFI16 Is Essential for Its Cytoplasmic Translocation, Inflammasome and IFN-β Responses
- A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence
- Analysis of the SUMO2 Proteome during HSV-1 Infection
- Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent
- A Novel Antiviral Target Structure Involved in the RNA Binding, Dimerization, and Nuclear Export Functions of the Influenza A Virus Nucleoprotein
- Deploying FLAREs to Visualize Functional Outcomes of Host—Pathogen Encounters
- Mosquitoes Reset Malaria Parasites
- The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease
- Extracellular Virions: The Advance Guard of Poxvirus Infections
- Risks of Antibiotic Exposures Early in Life on the Developing Microbiome
- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Periodontal Diseases: Bug Induced, Host Promoted
- Mechanisms of Host Behavioral Change in Rodent Association
- The Endosymbiotic Bacterium Selectively Kills Male Hosts by Targeting the Masculinizing Gene
- HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days—Implications for HIV Remission
- Ubiquilin 1 Promotes IFN-γ-Induced Xenophagy of
- Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin
- Suppression of Long-Lived Humoral Immunity Following Infection
- The Role of VP1 Amino Acid Residue 145 of Enterovirus 71 in Viral Fitness and Pathogenesis in a Cynomolgus Monkey Model
- Utilizing Chemical Genomics to Identify Cytochrome as a Novel Drug Target for Chagas Disease
- The Emerging Role for RNA Polymerase II in Regulating Virulence Gene Expression in Malaria Parasites
- Turning Up the Heat: Inflammasome Activation by Fungal Pathogens
- On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Species
- EhVps32 Is a Vacuole-Associated Protein Involved in Pinocytosis and Phagocytosis of
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
- The Serine Protease EspC from Enteropathogenic Regulates Pore Formation and Cytotoxicity Mediated by the Type III Secretion System
- Existing Infection Facilitates Establishment and Density of Malaria Parasites in Their Mosquito Vector
- Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice
- Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis
- Epstein-Barr Virus Proteins EBNA3A and EBNA3C Together Induce Expression of the Oncogenic MicroRNA Cluster miR-221/miR-222 and Ablate Expression of Its Target p57
- Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins
- Virulence of Group A Streptococci Is Enhanced by Human Complement Inhibitors
- Identification of Caspase Cleavage Sites in KSHV Latency-Associated Nuclear Antigen and Their Effects on Caspase-Related Host Defense Responses
- Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Infections
- Type VI Secretion System Transports Zn to Combat Multiple Stresses and Host Immunity
- Lv4 Is a Capsid-Specific Antiviral Activity in Human Blood Cells That Restricts Viruses of the SIV/SIV/HIV-2 Lineage Prior to Integration
- Phenylbutyrate Is Bacteriostatic against and Regulates the Macrophage Response to Infection, Synergistically with 25-Hydroxy-Vitamin D₃
- An Internally Translated MAVS Variant Exposes Its Amino-terminal TRAF-Binding Motifs to Deregulate Interferon Induction
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



