-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Age-Dependent Enterocyte Invasion and Microcolony Formation by
Non-typhoidal Salmonella are among of the most prevalent causative agents of infectious diarrheal disease worldwide but also very significantly contribute to infant sepsis and meningitis particularly in developing countries. The underlying mechanisms of the elevated susceptibility of the infant host to systemic Salmonella infection have not been investigated. Here we analyzed age-dependent differences in the colonization, mucosal translocation and systemic spread in a murine oral infection model. We observed efficient entry of Salmonella in intestinal epithelial cells of newborn mice. Enterocyte invasion was followed by massive bacterial proliferation and the formation of large intraepithelial bacterial colonies. Intraepithelial, but not non-invasive, extracellular Salmonella induced a potent immune stimulation. Also, enterocyte invasion was required for translocation through the mucosal barrier and spread of Salmonella to systemic organs. This requirement was due to the absence of M cells, specialized epithelial cells that forward luminal antigen to the underlying immune cells, in the neonate host. Our results identify age-dependent factors of host susceptibility and illustrate the initial phase of Salmonella infection. They further present a new small animal model amenable to genetic manipulation to investigate the interaction of this pathogen with epithelial cells and characterize the early steps in Salmonella pathogenesis.
Published in the journal: Age-Dependent Enterocyte Invasion and Microcolony Formation by. PLoS Pathog 10(9): e32767. doi:10.1371/journal.ppat.1004385
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004385Summary
Non-typhoidal Salmonella are among of the most prevalent causative agents of infectious diarrheal disease worldwide but also very significantly contribute to infant sepsis and meningitis particularly in developing countries. The underlying mechanisms of the elevated susceptibility of the infant host to systemic Salmonella infection have not been investigated. Here we analyzed age-dependent differences in the colonization, mucosal translocation and systemic spread in a murine oral infection model. We observed efficient entry of Salmonella in intestinal epithelial cells of newborn mice. Enterocyte invasion was followed by massive bacterial proliferation and the formation of large intraepithelial bacterial colonies. Intraepithelial, but not non-invasive, extracellular Salmonella induced a potent immune stimulation. Also, enterocyte invasion was required for translocation through the mucosal barrier and spread of Salmonella to systemic organs. This requirement was due to the absence of M cells, specialized epithelial cells that forward luminal antigen to the underlying immune cells, in the neonate host. Our results identify age-dependent factors of host susceptibility and illustrate the initial phase of Salmonella infection. They further present a new small animal model amenable to genetic manipulation to investigate the interaction of this pathogen with epithelial cells and characterize the early steps in Salmonella pathogenesis.
Introduction
Non-typhoidal Salmonella are one of the major causal agents of bacterial gastrointestinal infections in all age groups worldwide. In addition, they also significantly contribute to systemic infection particularly in the pediatric population. Non-typhoidal Salmonella together with pneumococci and group B streptococci are among the most frequent causal agents of sepsis and meningitis in neonates and young infants in Africa [1]–[5]. Transmission of this highly endemic bacterium occurs perinatally from infected mothers or after birth from infected family members via the fecal-oral route. The underlying mechanisms of the enhanced susceptibility to systemic disease after infection during the postnatal period have not been systematically investigated.
Salmonella pathogenicity is conferred by horizontally acquired chromosomal regions so called Salmonella pathogenicity islands (SPI) that encode sets of virulence factors required for the various steps in microbial virulence. SPI1 encodes a type III secretion systems (T3SS) and a set of effector molecules that are translocated into the target cell. It mediates pathogen-induced internalization in non-phagocytic cells [6]–[8]. The critical importance of cellular invasion by Salmonella and intracellular proliferation in non-phagocytic cells is supported by epidemiological data and has been extensively studied in vitro [6], [9]–[10]. Although intracellular Salmonella have been observed in epithelial cells and cells of the lamina propria also in vivo, this host cell interaction and the functional importance have remained less defined [7]–[8], [11]–[15]. Instead, translocation through microfold (M) cells has been demonstrated in adult animals. Such cells overlay Peyer's patches and forward luminal antigens and bacteria to the underlying immune cells [16]–[18]. Similarly, uptake via lamina propria resident myeloid cells that generate membrane extrusions extending into the intestinal lumen has been proposed to facilitate Salmonella barrier penetration [19]. More recently, rapid translocation via the colonic epithelium in the absence of intracellular proliferation was observed [15]. The underlying host and bacterial factors facilitating enterocyte invasion, intraepithelial proliferation and mucosal translocation have, however, remained largely undefined.
Here we comparatively analyzed oral Salmonella infection of neonate and adult mice. We demonstrate marked age-dependent differences in intestinal colonization, mucosal translocation and systemic spread. We observed Salmonella enterocyte invasion in neonate mice in vivo and characterize intraepithelial microcolony formation, epithelial translocation and epithelial innate immune stimulation after oral infection. We characterize developmental peculiarities of the newborn's intestinal epithelium that allow enterocyte invasion and characterize the contribution of Salmonella virulence mechanisms. Our results identify age-dependent factors of infection susceptibility and provide a novel in vivo model that allows the use of genetically modified hosts to investigate the intimate host microbial interaction at the intestinal epithelium.
Results
Mucosal translocation in neonates depends on Salmonella-induced internalization by non-phagocytic cells
One-day-old C57BL/6 neonates were orally infected with various inocula (102–105) of mid-log grown Salmonella enterica subsp. enterica sv. Typhimurium (S. Typhimurium). Colony counts and visualization of bioluminescent S. Typhimurium in the intestine using optical imaging technology demonstrated rapid colonization of the small intestine and colon (Fig. 1A–C). Even low dose infection (102 CFU) resulted in systemic spread with maximal bacterial counts in spleen and liver at day 4 post infection (p.i.) (Fig. 1D–E). The absence of bacteria in lung tissue excluded accidental primary pulmonary infection (Fig. S1A). Comparative analysis of 1-day-old, 6-day-old and streptomycin pretreated adult animals at day 4 after oral administration of 102, 5×102, and 5×108 CFU S. Typhimurium, respectively, demonstrated the efficient colonization of the neonate intestine and the marked spread to liver and spleen tissue in 1-day-old animals (Fig. 1F–G). We therefore focused on 1-day-old animals and applied low dose infection and analyzed animals at 4 days p.i. for all subsequent experiments, if not stated otherwise.
Fig. 1. Intestinal colonization and systemic spread of S. Typhimurium. 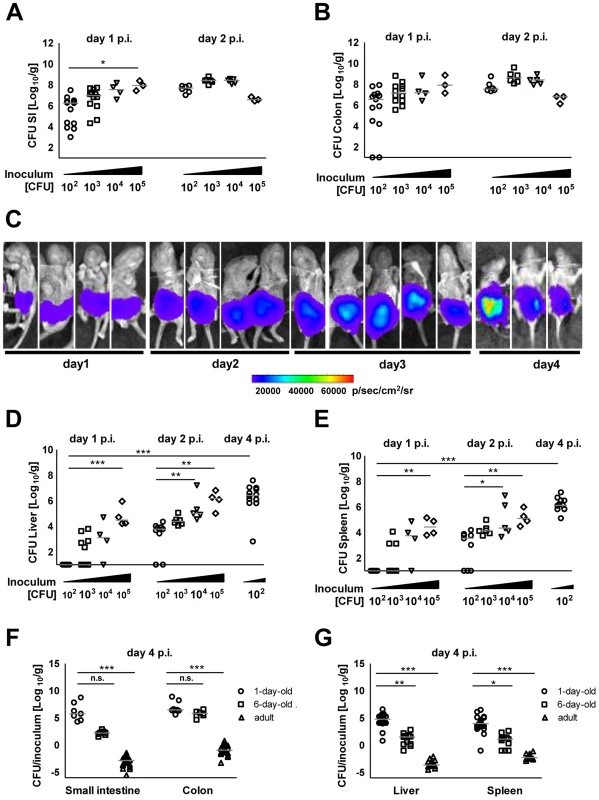
Number of viable bacteria in total (A) small intestine and (B) colon at day 1 and 2 p.i. The results represent the median values from 2–3 independent experiments (n = 3–6 per group). (C) Whole body IVIS imaging of 1-day-old mice orally infected with 5×103 CFU of a lux expressing S. Typhimurium strain. Strength of the detected light emission in p/sec/cm2/sr. 4 mice were used per group in 2 independent experiments. (D and E) Number of viable bacteria in total liver (D) and spleen (E) at day 1, 2, and 4 p.i. with the indicated inoculum. The results represent the median values from 2–3 independent experiments (n = 3–6 per group). (F and G) Colonization of the small intestine and colon (F) as well as organ counts of liver and spleen (G) 4 days after oral infection of 1-day-old (102 CFU), 6-day-old (5×102 CFU) and streptomycin pre-treated adult (6 week-old) mice (5×108 CFU). The values indicate the total organ CFU divided by the inoculum. The results represent the median values from 2–3 independent experiments (n = 3–6 per group). As SPI1 is involved in Salmonella internalization in non-phagocytic cells, we next analyzed the requirement of Salmonella SPI1 effector translocation for oral infection of neonatal mice. Spread to liver (p<0.001), spleen (p<0.001) and mesenteric lymph nodes (MLN, p<0.001) was practically abolished in the absence of a functional SPI1 system (ΔinvC) (Fig. 2A–C). In fact, spleen tissue of all mice and liver and MLN tissue of the majority of neonates (8/14 and 18/23, respectively) remained sterile at day 2 and 4 after infection by SPI1 deficient Salmonella. Similarly, systemic dissemination of ΔinvC Salmonella was also highly significantly reduced after high dose (105 CFU) infection (Fig. S2D). In accordance with these results, immunostaining (Fig. 2D), plating of isolated epithelial cells after gentamicin-treatment to remove extracellular Salmonella (Fig. 2E–F) and flow cytometry (Fig. 2G) detected enterocytes infected by WT but not SPI1-deficient Salmonella. Both, WT and ΔinvC Salmonella after high and low dose infection spontaneously colonized the intestinal tract (Fig. S2A–C). The reduced total small intestinal organ counts at later points after low dose infection may result from the lack of intraepithelial bacteria (Fig. 2E–F).
Fig. 2. Comparative analysis of WT and invasion-deficient Salmonella. 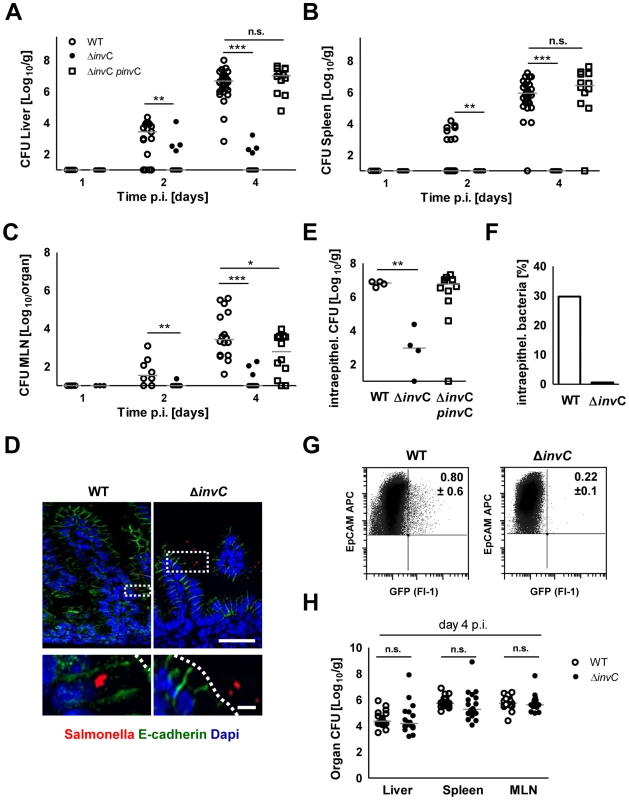
(A–C) Organ counts in liver (A), spleen (B) and MLN (C) 1, 2 and 4 days after oral infection of 1-day-old mice with 102 CFU WT (open circles), ΔinvC SPI1 mutant (filled circles) or complemented ΔinvC pinvC (open squares) S. Typhimurium. The results represent the median values from 3–4 independent experiments (n = 9–15 per group). (D) Immunostaining of S. Typhimurium (red) in small intestinal tissue sections of 1-day-old mice 4 days p.i. with 102 CFU WT or SPI1 mutant (ΔinvC) S. Typhimurium. Counterstaining with E-cadherin (green) and Dapi (blue). Bar, upper panel = 20 µm; lower panel = 5 µm. (E) Viable bacteria cultured from gentamicin treated primary enterocytes isolated at day 4 p.i. with WT or SPI1 mutant (ΔinvC) S. Typhimurium. The results represent the median values from one out of two independent experiments (n = 4–6 per group). (F) Median of the number of intraepithelial gentamicin-protected bacteria as shown in (E) divided by the total number of bacteria cultured from untreated isolated IECs (in %). (G) Flow cytometric analysis of infected enterocytes (Fl-1) isolated at day 4 after infection of 1-day-old mice with 102 CFU WT or SPI1 mutant (ΔinvC) (GFP+) S. Typhimurium. Cells were gated for the epithelial cell marker EpCAM (APC). The number of GFP-positive enterocytes of all EpCAM+ cells is indicated (%). One representative data set of three independent experiments is shown. Note that due to the relatively high autofluorescence of enterocytes, cell infected by low numbers of bacteria may remain undetected; the indicated gating might therefore underestimate the number of Salmonella infected cells. (H) Organ counts in liver, spleen and MLN after oral infection of 6-week-old streptomycin (20 mg) pretreated mice infected with 2×108 CFU WT (open circles, n = 21) or isogenic ΔinvC SPI1 mutant (filled circles, n = 18) S. Typhimurium (n = 6). The results represent the median values from three experiments. Requirement for enterocyte invasion in the absence of M cells in the neonate intestine
In contrast to the critical role of SPI1 in neonate mice, intestinal colonization but also spread to spleen, liver and MLN in adult streptomycin-pretreated animals was largely SPI1-independent (Fig. 2H and Fig. S2E). To identify differences that might account for the requirement of SPI1 in neonate but not adult animals, the gene expression profile of isolated primary neonate and adult epithelial cells was compared. Unexpectedly, genes of differentiated M cells such as Spi-B and Ccl9 were found to be markedly reduced in neonate epithelial cells (Fig. 3A–B). In adult hosts, M cell-mediated bacterial translocation represents the major entry pathway of Salmonella [16], [17]. To confirm the age-dependent differentiation of M cells, mRNA expression of the M cell specific transcription factor Spi-B was examined [18]. As expected, Spi-B expression was found to be strongly diminished in neonates and an increase was only observed starting at day 8 after birth (Fig. 3C). Also, immunostaining of intestinal tissue for the established M cell markers glycoprotein 2 (gp2), Ccl9 and Ulex europaeus agglutinin (UEA)-1 confirmed the appearance of M cells only after the neonatal period (Fig. 3D). Finally, wildtype (WT) and fimD mutant Salmonella disseminated to a similar degree to the spleen and liver tissue after 4 days infection of 1-day-old neonates (Fig. S3A). fimD mutant Salmonella are unable to express type I pili and cannot attach to the M cell surface protein gp2. This significantly reduces their ability to invade the adult host via M cells [20]. In contrast to a recent report on Salmonella-induced Spi-B expression in adult animals, Spi-B expression in neonate mice was reduced rather than enhanced following Salmonella infection (Fig. S3B) [21]. Hence, Salmonella in neonate mice spontaneously colonize the intestine, invade enterocytes, penetrate the mucosal barrier and spread to systemic organs in a SPI1-dependent but M cell-independent fashion.
Fig. 3. Absence of mature M cells in the neonatal small intestine. 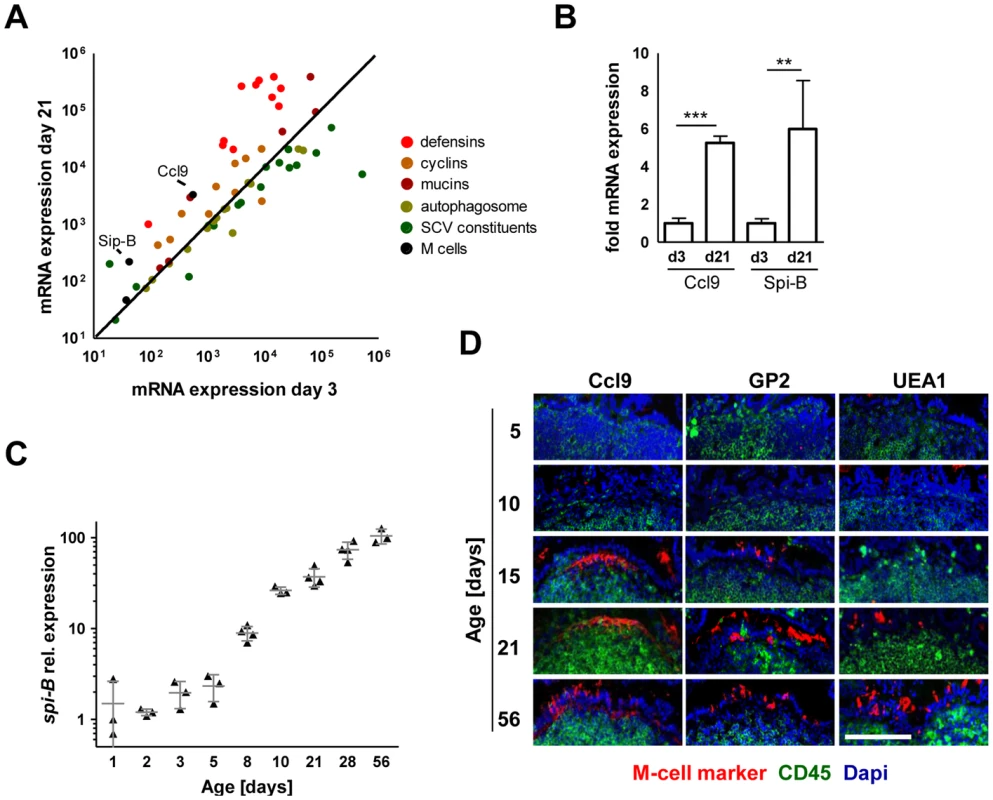
(A) Comparative gene expression analysis of M cell specific genes (black: Spi-B, Ccl9), antimicrobial peptide or protein genes (red: Defcr3, Defcr4, Defcr6, Defcr20, Defcr22, Defcr23, Defcr-rs1, Defcr-rs7, Defcr-rs12, Reg3g, Saa1), cyclin genes (brown: Ccna2, Ccnb1, Ccnb2, Ccnc, Ccnd1, Ccnd2, Ccnd3, Ccne1, Ccne2, Ccnf), mucin genes (dark red: Muc2, Muc3, Muc4, Muc5b, Muc6, Muc13, Muc20), autophagosome constituents (light green: Atg3, Atg4a, Atg4b, Atg4c, Atg4d, Atg5, Atg7, Atg10, Atg12, Atg16l1, Atg16l2, LC3, p62, NDP52), and SCV constituents (dark green: gp91-phox, p22-phox, iNOS, vATPase, Eea1, Lamp1, Lamp2, Rab5a, Rab5b, Rab5c, Rab7, lysosomal acid phosphatase, mannose-6-phosphate receptor, cathepsin A exopeptidase, cathepsin D endopeptidase, cathepsin L endopeptidase, G6pc, Frap1) obtained from a two-color comparative gene expression array of isolated highly pure primary intestinal epithelial obtained from healthy 3-day-old versus 21-days-old C57BL/6 mice. The expression array data are accessible through GEO Series accession numbers GSE35596 and GSE35597. (B) Fold mRNA expression analysis of Ccl9 and Spi-B in primary enterocytes from 21-day-old mice as compared to 3-day-old neonate animals. The results represent the mean ± SD (n = 4 per group). (C) Quantitative RT-PCR analysis for Spi-B mRNA in total enterocytes isolated from healthy C57BL/6 mice at the indicated age (n = 3–4 per group). (D) Immunostaining for the M cell markers Ccl9, gp2 and UEA-1 (red) in small intestinal tissue sections obtained from healthy 5-, 10-, 15-, 21-, and 56-day-old adult C57BL/6 mice. Peyer's patches were identified using an anti-CD45 antibody (green). Counterstaining with Dapi (blue). Bar = 100 µm. Epithelial invasion leads to intraepithelial proliferation and microcolony formation
Salmonella infection of neonatal enterocytes in vivo was subsequently studied in more detail. Immunostaining revealed Salmonella-positive enterocytes (Fig. 4A). Unexpectedly, Salmonella generated multi-bacterial intraepithelial colonies of variable size consistent with the formation of Salmonella-containing vacuoles (SCV) previously observed in epithelial cell lines in vitro (Fig. 4A–B). Quantification of Salmonella-positive cells was achieved by flow cytometric analysis on isolated enterocytes. 0.24±0.12% and 0.80±0.61% of all murine epithelial cell adhesion molecule (EpCAM)+ cells at 2 and 4 days p.i., respectively were found to be infected (uninfected controls: 0.07±0.03%, p<0.05). Co-infection using a 1∶1 mixed inoculum of genetically green - and red-labeled bacteria revealed solely single-colored intracellular colonies (Fig. 5A). This suggests that individual microcolonies originated from a single event of bacterial invasion.
Fig. 4. Visualization of intraepithelial Salmonella. 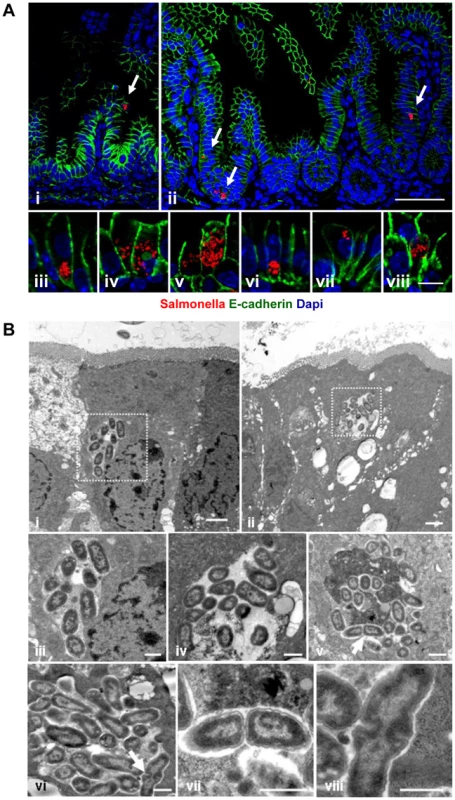
(A) Immunostaining in small intestinal tissue 4 days after 102 CFU S. Typhimurium (red) infection of 1-day-old neonate mice. Counterstaining with E-cadherin (green) and Dapi (blue). i and ii, bar = 20 µm; iii–viii, bar = 5 µm. (B) Transmission electron microscopy of enterocytes from 1-day-old mice obtained at day 4 p.i. with S. Typhimurium. Image iii and iv represent enlarged parts of image i and ii, respectively. Image vii and viii represent enlarged images of the bacteria marked with an arrow in image v and vi, respectively. Bar: i and ii = 2 µm; iii–viii = 1 µm. Fig. 5. Characterization of S. Typhimurium infected enterocytes. 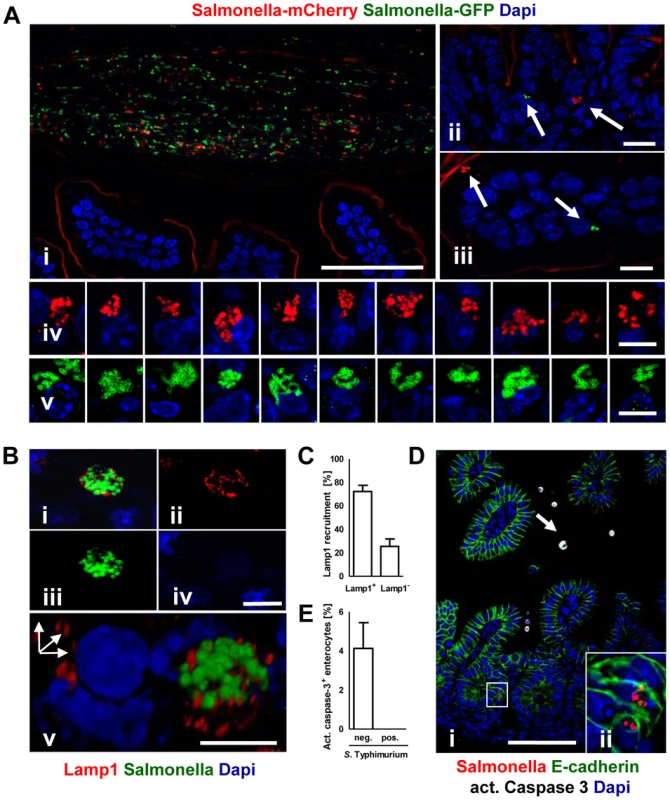
(A) Immunostaining of C57BL/6 mice simultaneously infected with two genetically labelled S. Typhimurium strains orally administrated at a ratio of 1∶1. Staining was performed simultaneously with fluorophore-conjugated anti-GFP (green) and anti-mCherry antibodies (red). Note the presence of equal numbers of both green and red bacteria in the intestinal lumen in (i). Note also that the anti-mCherry antibody cross-reacted to some extend with the epithelial brush border. Counterstaining with Dapi (blue). Bar, i = 50 µm; ii and iii = 10 µm; iv and v = 5 µm. (B) Co-immunostaining of the SCV marker Lamp1 (red) and S. Typhimurium (green). i represents the merged image, ii–iv single color channel images. v shows a three-dimensional reconstruction to illustrate the punctuate Lamp1 staining surrounding the SCV. Counterstaining with Dapi (blue). Bar, i = 5 µm. (C) Quantitative analysis of the percentage of S. Typhimurium microcolonies associated with Lamp1 staining. All detected microcolony positive cells in 3 sections obtained from 3 S. Typhimurium infected animals were analyzed at day 4 p.i. The results represent the mean ± SD. (D) Co-immunostaining for active caspase 3 (white, arrow) and Salmonella (red) in small intestinal tissue sections obtained at day 4 p.i. Image ii represents an enlarged part of image i as indicated. Counterstaining with E-cadherin (green) and Dapi (blue). Bar, i = 50 µm. (E) Quantitative analysis of the percentage of active caspase 3 positive enterocytes among S. Typhimurium-infected and non-infected cells. 20 image areas (Magnification ×20) of intestinal sections from 3 S. Typhimurium infected animals were analyzed at day 4 p.i. The results represent the mean ± SD. Electron microscopy showed that bacteria were enclosed in most instances by a detectable endosomal membrane (Fig. 4B). The intra-endosomal material appeared heterogeneous with hyper - or hypodense areas (Fig. 4Biv–v). Consistently, the well-established SCV transmembrane marker protein lysosomal-associated membrane protein (Lamp) 1 was detected in close proximity to the majority of intraepithelial microcolonies by immunostaining (Fig. 5B–C). In some instances, however, direct contact of individual bacteria with the host cell cytosol could not be excluded. Clear morphological signs of intracellular bacterial proliferation were noted (Fig. 4Bv–viii) in accordance with a rise in the mean fluorescence intensity (MFI) of infected EpCAM+ enterocytes from 59.5±35.1 to 98.4±62.1 at 2 and 4 days p.i., respectively. Despite invasion and intracellular proliferation, Salmonella-positive cells appeared morphologically intact (Fig. 4B). Whereas a low number of uninfected enterocytes stained positive for active caspase 3, no apoptotic Salmonella-positive cells were identified (Fig. 5D–E).
In sharp contrast, only minute numbers of (questionably) intraepithelial Salmonella without any indication of bacterial proliferation were detected in tissue sections of adult mice after high dose (1–2×108 CFU) infection despite numerous attempts both with and without streptomycin pretreatment (data not shown). Consistently, flow cytometric analysis did not detect Salmonella-infected intestinal epithelial cells (Fig. S2F and). To identify molecular differences that might account for the age-dependent susceptibility of the epithelium to Salmonella invasion, the gene expression profile of neonate and adult enterocytes was compared (Fig. 3A). Known SCVs constituents (with the notable exception of iNOS) and autophagosomal markers were equally expressed in neonate and mature adult epithelial cells. However, the neonate intestine exhibited a markedly reduced mucus layer thickness and mucin glycoprotein expression (Fig. S3C–D). Also, reduced cyclin expression was noted in accordance with minimal epithelial cell turn-over and reduced crypt-villus migration in neonates [22], [23]. Finally, a severely reduced spectrum of antimicrobial peptides was observed consistent with previous reports [24]. All of these factors might facilitate epithelial invasion, prolong the lifetime of infected epithelial cells and thereby ultimately allow intraepithelial proliferation and microcolony formation.
Invasion-dependent innate immune stimulation of epithelial cells
We next investigated the neonate's host response to Salmonella infection. Highly enriched primary enterocytes (Fig. S4) were subjected to quantitative RT-PCR. A significant time-dependent increase in Cxcl2 and Cxcl5 mRNA expression was measured after infection by WT or the complemented ΔinvC pinvC but not SPI1-deficient non-invasive Salmonella (Fig. 6A–B). Global gene array analysis confirmed the absence of any detectable increase in epithelial gene expression upon administration of invC deficient, non-invasive Salmonella (Fig. 6C). It further identified a large number of additional genes involved in metabolism, cellular responses and intercellular communication induced after Salmonella WT infection (Fig. 6D). In accordance with the requirement of enterocyte invasion for innate immune stimulation (Fig. 6C) and the observed age-dependent susceptibility to enterocyte invasion (Fig. 2D–G and S2F), Cxcl2 and Cxcl5 mRNA expression in enterocytes isolated at day 4 p.i. from infected 6-day-old or adult mice was severely reduced (Fig. 6E–F).
Fig. 6. Invasion-associated epithelial innate immune stimulation. 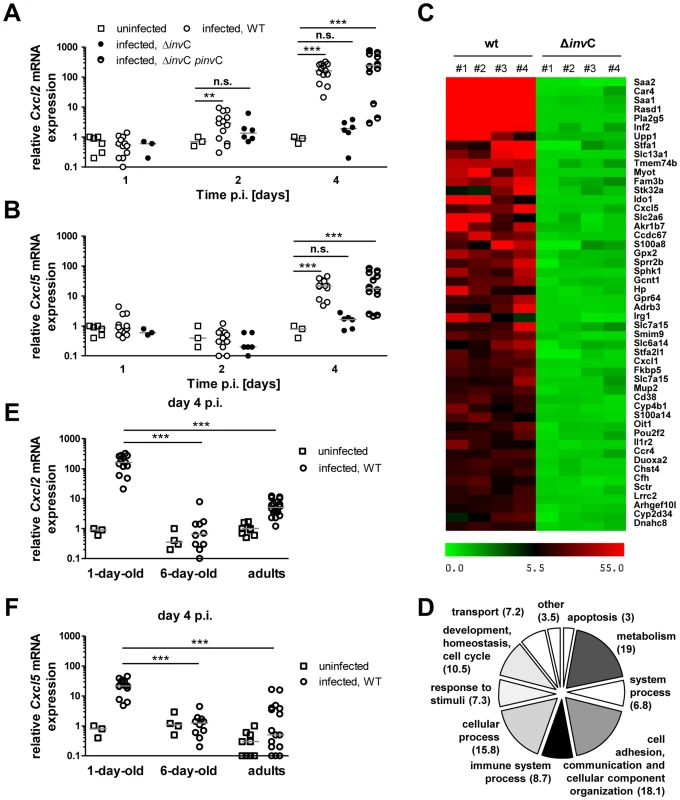
(A and B) Quantitative RT PCR for (A) Cxcl2 and (B) Cxcl5 mRNA in enterocytes isolated at 1, 2 and 4 days p.i. with 102 CFU WT (open circles, n = 13), ΔinvC SPI1 mutant (filled circles, n = 6) or complemented ΔinvC pinvC (semi-filled circles, n = 12) S. Typhimurium. The results represent the median values from 2–5 independent experiments (n = 6–13 per group). (C) Heat map of the relative gene expression in enterocytes of each 4 individual WT or SPI1 mutant (ΔinvC) S. Typhimurium infected animals (#1–#4) as compared to 4 non-infected control animals (fold increase). The 50 most highly expressed genes following WT S. Typhimurium infection are shown. (D) COG analysis of >2.5-fold induced genes in primary isolated intestinal epithelial cells following low dose (102 CFU) wildtype S. Typhimurium infection. (E and F) Quantitative RT PCR for (A) Cxcl2 and (B) Cxcl5 mRNA in enterocytes isolated at 4 days p.i. after infection of 1-day-old neonate (1–2×102 CFU), 6-day-old neonate (5×102 CFU), 6 week-old adult (2×108 CFU) mice with WT S. Typhimurium (open circles) or uninfected age-matched control animals (open squares). The results represent the median values from three independent experiments (n = 3–6 per group). We next examined the innate immune receptors and signaling adaptors involved in Salmonella-induced enterocyte stimulation. Expression of Cxcl2 and Reg3γ mRNA by epithelial cells was severely reduced in the absence of toll-like receptor (Tlr)4, MyD88 as well as Unc93B1 and Tlr9. Although some variability in the expression levels was noted, significantly decreased mRNA expression was also noted in epithelial cells devoid of Tlr2, Tlr5, or Nod2 (Fig. 7A–B). Of note, intestinal colonization (Fig. 7C), enterocyte invasion (Fig. 7E–G) and dissemination to systemic organs (Fig. 7C–D) was observed also in the absence of the most potently stimulated innate immune receptor, Tlr4. Although the production of proinflammatory mediators significantly enhanced the recruitment of polymorphonuclear cells and blood macrophages to the site of infection, no gross loss of epithelial barrier integrity or histopathological signs of tissue destruction were observed at day 4 p.i. in contrast to the situation in the adult host (Fig. 8) [25]. Thus, Salmonella in the neonate efficiently invades small intestinal enterocytes, activates innate immune responses mainly via Tlr stimulation and induces the formation of intraepithelial microcolonies, a hallmark of the Salmonella-enterocyte interaction.
Fig. 7. Epithelial innate immune stimulation during neonatal Salmonella infection. 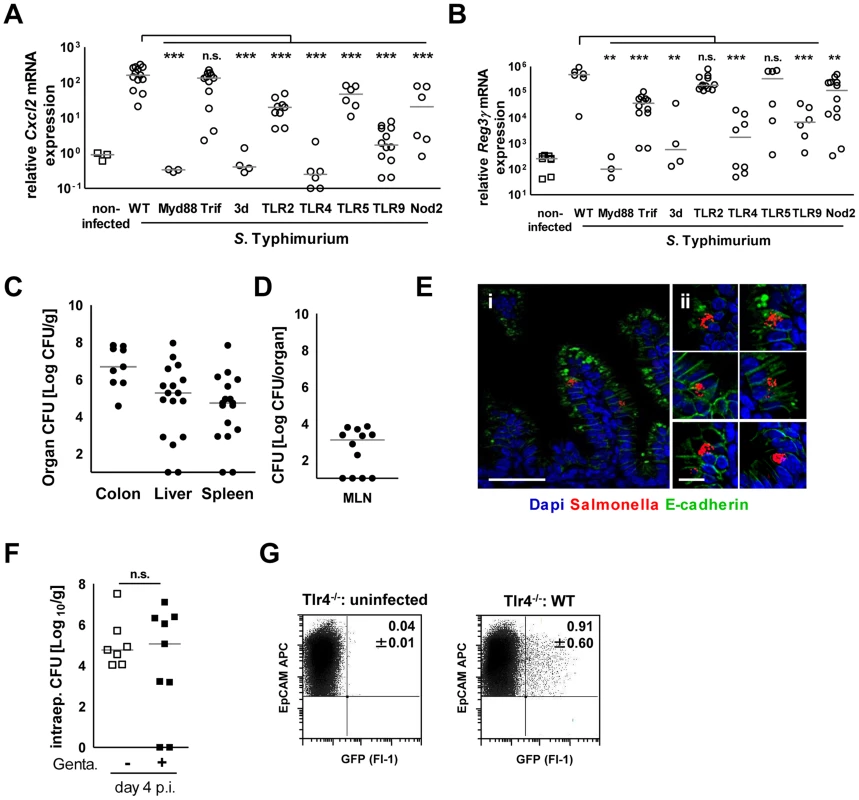
(A and B) Quantitative RT PCR for (A) Cxcl2 and (B) Reg3γ mRNA in enterocytes isolated from the indicated gene-deficient mice at 4 days p.i. with 102 CFU WT S. Typhimurium. The results represent the median values from 2–3 independent experiments (n = 3–14 per group: wt, n = 14; Tlr4−/−, n = 9; Tlr5−/−, n = 6; Myd88−/−, n = 3; Trif−/−, n = 12; TLR2−/−, n = 12; TLR9−/−, n = 12; 3d (Unc93B1 H412R), n = 3; NOD2−/−, n = 12). (C and D) Organ counts in (C) colon, spleen, liver and (D) mesenteric lymph nodes (MLN) of 1-day-old Tlr4−/− neonate mice after oral infection with 102 CFU WT S. Typhimurium. The results represent the median values from 3 independent experiments (n = 3–6 per group). (E) Immunostaining of small intestinal tissue 4 days after infection of 1-day-old Tlr4−/− neonate mice with 102 CFU S. Typhimurium (red). Counterstaining with E-cadherin (green) and Dapi (blue). i, bar = 20 µm; ii, bar = 10 µm. (F) Viable bacteria cultured from primary enterocytes isolated from small intestinal tissue at day 4 p.i. with WT S. Typhimurium and left untreated or incubated in gentamicin. The results represent the median values from one out of two independent experiments (n = 7–9 per group). (G) Flow cytometric analysis of enterocytes isolated at day 4 p.i. from 1-day-old Tlr4−/− mice infected with 102 CFU WT (GFP+) S. Typhimurium or from age-matched control animals. Cells were gated for the epithelial cell marker EpCAM (APC). The number of GFP-positive enterocytes of all EpCAM+ cells is indicated (%). One representative data set of three independent experiments is shown. Fig. 8. Mucosal tissue response following S. Typhimurium infection in neonate mice. 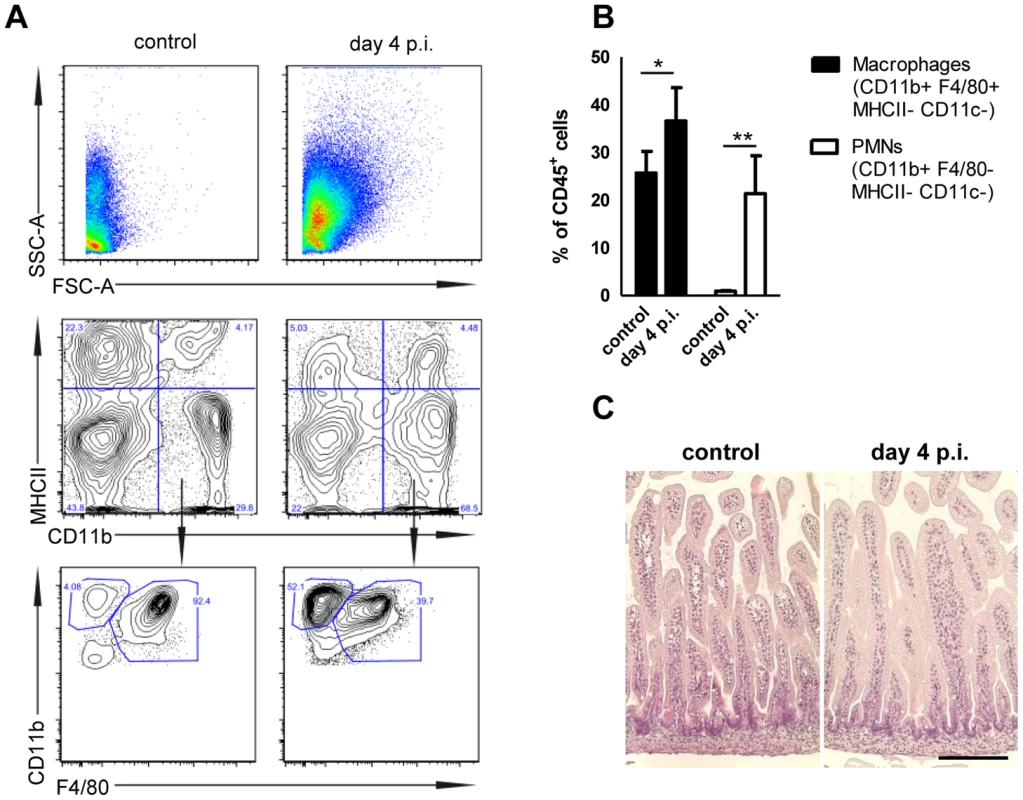
(A) Flow cytometric analysis of lamina propria myeloid immune cells prepared from 1-day-old neonates infected for 4 days with 102 CFU S. Typhimurium or non-infected age-matched control animals. Representative images are shown. (B) Quantitative analysis of CD11b+ F4/80+ MHCII− CD11c− tissue invading macrophages and CD11b+ F4/80− MHCII− CD11c− polymorphonuclear cells relative to total CD45+ cells in the lamina propria of 1-day-old neonates infected for 4 days with 102 CFU S. Typhimurium or non-infected age-matched control animals. The results represent the mean ± SD from 2 independent experiments (n = 4). (C) Hematoxilin and eosin staining of intestinal tissue sections of neonatal mice (small intestine) left untreated or 4 day p.i. with S. Typhimurium (102 CFU). Bar = 50 µm. Discussion
Previous work had demonstrated enterocyte invasion in oral and intestinal loop infection models and ex vivo tissue explants of calfs, rabbits, swine and guinea pigs [26]–[32]. However, these host animals are not amenable to genetic manipulation and studies are restricted to the early course of infection. Enterocyte invasion, rapid egress at the basolateral side of the epithelium and the presence of Salmonella in lamina propria cells has also been described in the mouse large intestine [7], [11]–[15]. The present study now provides a new small animal model that allows the use of genetically modified hosts to analyse intracellular proliferation in small intestinal epithelial cells and the formation of microcolonies by Salmonella in vivo. It might thereby facilitate a better understanding of a hallmark of Salmonella pathogenesis in vitro, the internalization by non-phagocytic epithelial cells and the formation of intraepithelial microcolonies, so called Salmonella containing vacuoles (SCVs).
Invasion of non-phagocytic epithelial cells is facilitated by virulence factors encoded by the pathogenicity island SPI1 [6]. The critical role of SPI1 for enteric disease is illustrated by its presence in all subspecies of Salmonella enterica [33] and its high prevalence in clinical Salmonella isolates [9]. In accordance, SPI1 effector proteins are required to cause diarrhea and mucosal inflammation in several in vivo models although also SPI2 effector molecules contribute to intraepithelial survival and proliferation [25], [34]–[36]. The analysis of the interaction of S. Typhimurium with polarized epithelial cells within their anatomical environment represents a prerequisite to understand the functional role of individual bacterial virulence factors.
Whereas previous reports using streptomycin pretreated adult animals failed to demonstrate infection of the small intestinal epithelium, we observe efficient Salmonella invasion of enterocytes in neonate mice. Known host constituents of mature SCVs and autophagosome factors are similarly expressed by neonatal and adult epithelial cells [37]–[39]. However, a number of fundamental differences exist between the neonate and adult gut epithelium in mice. For example, we demonstrate that the synthesis of various mucin glycoproteins and thus the thickness of the mucus layer are significantly enhanced in adult animals. The mucus layer was shown to significantly impair mucosal translocation of Salmonella in adult mice [40]. Also, we and others have shown that the antimicrobial peptide repertoire is severely reduced in the neonate intestine in the absence of mature Paneth cells. In adult mice, Paneth cell-derived antimicrobial peptides cells significantly influence the course of enteropathogen infection [41], [42] and cooperate with the mucus layer to generate an antibacterial and antiinflammatory shield [43]. Finally, the well-established constant renewal of the epithelium associated with enterocyte migration and exfoliation at the villus tip is not yet established in newborn mice [22], [23]. The reduced epithelial cell turn-over is the consequence of the lack of crypts and the reduced pool of rapidly proliferating cells during this early developmental stage. Thus, enterocytes during the neonatal period remain longer at the same anatomical position. This might allow Salmonella to proliferate intracellularly and form microcolonies. Shedding of Salmonella-infected enterocytes at the villus tip was previously observed in bovine, pig and rabbit intestinal loop models and might represent an important mechanism to remove infected enterocytes [28], [30], [44]. Finally, the prolonged presence of the more diverse enteric microbiota in the adult host may contribute to the enhanced resistance to Salmonella enterocyte invasion and this issue requires future investigations.
In the neonate host both Salmonella enterocyte invasion and mucosal translocation were dependent on SPI1. This suggests that penetration of the neonate's intestinal barrier occurs secondary to the observed enterocyte invasion. This is in contrast to the situation in adult mice but also other species. Here, M cells were shown to represent the major port of entry [16]–[18], [29]. M cells as part of the follicle-associated epithelium overlaying Peyer's patches facilitate uptake of particulate antigen [17], [18], [45]. Thus, translocation takes place independent of bacteria-induced internalization [16]. In contrast, the absence of M cells in the neonate mouse intestine shifts the major entry pathway to enterocyte invasion. It thereby pronounces the requirement for SPI1 for mucosal translocation. This may also explain the recent finding that Yersinia enterocolitica translocation is highly reduced in neonate mice [46]. Mucosal translocation of enteropathogenic Yersinia is also mostly mediated through M cell transport [47]. However, we cannot formally exclude that additional host factors also contribute to the enhanced requirement for a functional SPI1 system in the neonate host.
Strong innate immune receptor-mediated stimulation of the epithelium was noted upon infection in accordance with previous analyses of infected total mucosal tissue [48], [49]. The present study provides a global gene expression analysis of isolated primary enterocytes after Salmonella infection in vivo. The restriction of innate immune stimulation to invasion-competent Salmonella is consistent with reports on the requirement of SPI1 for mucosal inflammation and clinical disease [25], [34]–[35], [49]. Epithelial stimulation was mediated by recognition of Salmonella lipopolysaccharide (LPS) through Tlr4 and signaling via MyD88 consistent with previous reports on the involvement of Tlr4 in the adult host defense against Salmonella [50], [51]. Similarly, deficiency in Tlr9 and the processing molecule Unc93B1 significantly reduced the epithelial cell response to Salmonella infection. The strong effect observed in 3d UNC93B1 mutant mice indicates the possible involvement of additional UNC93B1 dependent innate immune receptors such as Tlr3, 7 or 13 [52]. Our results indicate the synergistic action of Tlr4 and Tlr9 in the epithelial response to Salmonella in accordance with previous reports [53]. A minor but significant role was also found for Tlr2, Tlr5 and Nod2 previously implicated in the antimicrobial response of the adult host to Salmonella [54]. The synergistic stimulation of Tlr2, 4 and 9 was shown to be required for endosomal acidification and SPI2 effector protein translocation in bone marrow-derived macrophages [53]. A similar scenario may apply to intestinal epithelial cells. In accordance, lack of Tlr4 alone did not significantly influence enterocyte invasion or intraepithelial proliferation in vivo. Further studies are required to investigate the role of cooperative immune signaling by different receptors for the process of enterocyte infection and microcolony formation.
Colonization of the neonate intestine occurred largely independent of the invasion-dependent innate immune stimulation consistent with a low degree of colonization resistance in the neonate host. Reduced organ counts in the total small intestine after low dose infection of SPI1 mutant Salmonella might result from the lack of proliferating intraepithelial Salmonella. Alternatively, invasion-induced immune stimulation might promote metabolic changes that favor pathogen colonization of the neonate intestine as recently described in adult animals [55]. In accordance, a number of metabolic genes were significantly influenced during bacterial challenge supporting the idea of a heavily altered intestinal host metabolism during neonatal infection [56]. In addition, several innate immune response genes such as the chemokines, serum amyloid proteins, iNOS, calprotectin, mucins and antimicrobial lectins were strongly upregulated in infected epithelial cells in accordance with previous reports [57]. As a consequence, large numbers of polymorphonuclear cells (PMNs) but also macrophages were recruited to the site of infection. The cellular immune response at this time point, however, did not lead to significant tissue alteration. The anti-apoptotic effect of NF-κB stimulation might have prevented the induction of epithelial apoptosis previously reported in both Salmonella-infected and non-infected enterocytes [44].
In conclusion, we analyzed age-dependent differences in the interaction of Salmonella with the host epithelium. We identify host and bacterial factors responsible for the infection process in the neonate host and characterize the epithelial innate immune response. Our results demonstrate enterocyte invasion and intracellular proliferation of Salmonella in differentiated and polarized epithelial cells and established a new animal model amenable to genetic manipulation to study the enterocyte-pathogen interaction in vivo. However, neonate mice exhibit an immature mucosal immune system, which might contribute to the observed phenotype and requires further characterization. Also, limitations such as the small animal size and lack of a suitable anesthesia required for intravital microscopy as well as the possible exchange of bacteria between newborn mice and the dam exist. Nevertheless, we believe that the described model opens new avenues of research to unravel the functional role of individual effector proteins in the Salmonella–enterocyte interaction and to better understand the cellular and immunological events of the Salmonella pathogenesis.
Materials and Methods
Bacterial strains
The isogenic strain MvP818 harboring a deletion of invC has been described before [58]. For complementation of the invC mutation, plasmids p3545 was generated as follows: The promoter of invF was amplified from S. Typhimurium genomic DNA using PinvF-For-EcoRI (CCGGAATTCTCCATCCAG ATGACAATATC) and PinvF-Rev-SmaI (ATATCTAGATCCATCCAGATG ACAATATCTG). The resulting product was digested by EcoRI and SmaI and subcloned in pWSK29 to obtain p3537. invC was amplified using invC-For–SmaI (atacccgggtttagtcg gtcgctaatgag) and invC-Rev-XbaI (GTATCTAGATTAAT TCTGGTCAGCGA ATGC), the resulting fragment was subcloned as SmaI/XbaI fragment in p3537 to generate p3545. Correct clones were confirmed by DNA sequencing and functional complementation of the invasion defect of MvP818 was verified in non-phagocytic intestinal epithelial m-ICcl2 cells (Fig. S5A–C). S. Typhimurium ATCC 14028 and SPI-1 mutant S. Typhimurium MvP813 ΔinvC (KanaR) carrying a green fluorescent protein (GFP) expression plasmid (pGFP, AmpR, kindly provided by Brendan Cormack, Stanford, USA) and S. Typhimurium ATCC 14028 carrying a mCherry expression plasmid (pFPV-mCherry, AmpR, kindly provided by Leigh Knodler, NIH, Hamilton, USA) were used for in vivo infection experiments. Maintenance of the plasmid pGFP in S. Typhimurium under in vivo conditions was confirmed by comparative plating of spleen and liver tissue simultaneously on selective (100 µg/mL ampicillin) and non-selective LB agar plates (Fig. S6C–D). For oral infection with two different Salmonella strains, a 1∶1 mixture of 102 pGFP and 102 pFPV-mCherry carrying bacteria were administered orally in 2 µl PBS. Life imaging (IVIS) was performed employing an isogenic S. Typhimurium strain with a chromosomal insertion of the lux-operon from Photorhabdus luminescens under the control of the constitutive ß-lactamase promoter.
Infection experiments
Adult C57BL/6 wildtype and B6.B10ScN-Tlr4lps-del/JthJ Tlr4 (stock no. 007227), B6.129S1-Tlr5tm1Flv/J Tlr5 (stock no. 008377), B6.129-Tlr2tm1Kir/J (stock no. 004650), and B6.129S1-Nod2tm1Flv/J Nod2 (stock 005763) deficient, as well as C57BL/6J-Ticam1Lps2/J TRIF mutant (005037) mice were obtained from the Jackson Laboratory (Bar Harbour, USA). 3d [59] and B6.129P2-Tlr9(tm1Aki) Tlr9 deficient mice [60] were obtained from M. Brinkmann, Helmholtz Center for Infection Biology, Braunschweig, Germany. Mice were housed under specific pathogen-free conditions and handled in accordance with regulations defined by FELASA and the national animal welfare body GV-SOLAS (http://www.gv-solas.de).
Salmonella enterica subsp. enterica serovar Typhimurium ATCC14028 (NCTC12023) WT and isogenic mutant strains were cultured in Luria Bertani (LB) broth overnight at 37°C, diluted 1∶10 and incubated at 37°C until reaching the logarithmic phase (OD600 approximately 0.5). Bacteria were washed and adjusted to OD600 0.55–0.60 containing approximately 1.5–2.0×108 CFU/mL and diluted to obtain the appropriate infection dose. All experiments with neonatal mice were performed using 1-day-old C57BL/6 animals. Neonates were infected orally with the indicated number of S. Typhimurium in a volume of 1 µl PBS. The administered inoculum was confirmed by serial dilution and plating. In vivo imaging was performed using the IVIS200 imaging system (PerkinElmer). For image analysis and visualization the LivingImage 4.3.1 software was used. Oral infection of 6 week-old adult female mice was performed as previously described [25]. Bacterial counts were obtained after homogenization of spleen, liver and mesenteric lymph nodes by serial dilution and plating.
Ethics statement
All animal experiments were performed in compliance with the German animal protection law (TierSchG) and approved by the local animal welfare committee (approval 12/0697, 12/0693, 13/1097 and 14/1385 of the Niedersachsische Landesamt für Verbraucherschutz und Lebensmittelsicherheit Oldenburg, Germany).
Primary cell isolation and flow cytometry
Primary intestinal epithelial cells were isolated from small intestinal tissue after incubation in 30 mM EDTA PBS at 37°C for 10 min as described previously [61]. Cells were passed through a 100 µm nylon cell strainer (BD Falcon), washed with 10% FCS/PBS, and harvested by centrifugation. The purity was verified by flow cytometry (Fig. S4). In order to determine the number of cell-associated versus intracellular bacteria, one fraction of epithelial cells was plated directly (total number of epithelium-associated bacteria); another fraction was incubated in 100 µg/mL gentamicin for 1 h prior to plating (number of gentamicin-protected, intracellular bacteria). For flow cytometry analysis of intracellular Salmonella, cells were fixed with 4% PFA for 15 min. on ice. The cells were washed, stained with APC-conjugated anti-EpCAM (anti-CD326 clone 48.8, diluted 1∶500, from eBioscience) and finally resuspended in 5% FCS/PBS. Prior to analysis, cells were filtered through a 35 µm pore size BD Falcon Polystyrene tube (BD, cat. no. 352235). 300,000 events were acquired and GFP expression was detected using a FACS Calibur apparatus (BD). For purity confirmation of isolated primary enterocytes, cells were fixed and incubated on ice with APC-conjugated anti-EpCAM (clone 48.8) and PE-conjugated anti-CD45 antibodies (clone 30-F11, dilution: 1∶200) purchased from eBioscience, for 30 minutes. Cells were washed and filtered. 25,000 events were acquired. Postacquisition analysis of FACS data was performed using the Summit 5.0 software. For flow cytometric analysis of tissue invading myeloid cells, small intestines from neonatal mice were removed and opened longitudinally. Tissue was digested in Liberase (Roche)/DNAse I (Roche)/10%FCS/RPMI at 37°C and separated on a 40%/70% Percoll gradient. Cell were stained with PerCP Cy5.5 conjugated anti-CD45 (Clone 104), Brilliant Violet-conjugated anti-I-A/I-E (Clone M5/114.15.2), APC/Cy7-conjugated anti-CD11c (Clone N418, all purchased from Biolegend), PE-conjugated anti-CD11b (Invitrogen) and APC-conjugated anti-F4/80 (Clone BM8, from eBioscience). The samples were acquired on a LSRII (BD) and analyzed with FlowJo (Treestar).
Immunostaining
Immunostaining using a chicken anti-GFP (dilution 1∶500), a mouse anti-mCherry (diluted 1∶500, both from Abcam), a mouse anti Salmonella O4-antigen (diluted 1∶500, from Abcam), a rat anti-Lamp1 (diluted 1∶500, from the Developmental Studies Hybridoma Bank (DSHB), University of Iowa, US), a rabbit anti-active Caspase 3 (diluted 1∶200, from Cell Signaling), a rabbit anti-Muc2 (diluted 1∶100, generous gift from Gunnar Hansson, Göteborg, Sweden) and a mouse anti-E-cadherin (diluted 1∶100, from BD Transduction Laboratories), was performed on 3 µm paraformaldehyde-fixed paraffin-embedded tissue sections in combination with the appropriate fluorophore conjugated secondary antibody (Jackson ImmunoResearch). Sections were deparaffinized in xylene and rehydrated in ethanol followed by antigen retrieval in 10 mM sodium citrate and blocking with PBS 10% serum 5% BSA. Staining of constitutively GFP expressing bacteria was performed to enhance the sensitivity of detection. In addition, this approach ensured bacterial detection also under low oxygen conditions, which might be present within the intestinal lumen and have been reported to alter GFP emission. The accuracy of the anti-GFP staining to detect plasmid-bearing S. Typhimurium was verified by staining of two consecutive sections cut from the same tissue block with anti-GFP and a biotinylated 1∶100 diluted rat monoclonal anti-O4/O5 antibody generously provided by M. Kim (Kim Laboratories, Champaign, IL). Both staining methods revealed a very similar staining pattern suggesting that the anti-GFP staining method used was both sensitive and specific (Fig. S6A–B). Staining with the rhodamine-conjugated lectin UEA-1 (Vector), a rat anti-GP2 (diluted 1∶100, from MBL) or a goat anti-Ccl9 (diluted 1∶100, from R&D) was performed on 8 µm sections obtained from freshly frozen OCT (Tissue-Tek) embedded tissue. Sections were dried and fixed using methanol at −20°C for 10 min. followed by rehydration in PBS for 15 min. Blocking in 0.2% BSA, 0.2% saponin, 10% serum in PBS was performed prior to immunostaining. Peyer's patches were visualized using a FITC-conjugated anti-CD45 antibody (clone 30-F11, BioLegend). Hematoxylin and eosin staining of paraformaldehyde-fixed tissue sections was performed according to Mayer's protocol using reagents from Roth. Slides were mounted in Vectashield (Vector) supplemented with DAPI and pictures were taken with an Apo-Tome microscope connected to a digital camera (Zeiss).
Electron microscopy
For electron microscopical analysis of infected intestinal tissue, samples were fixed in 150 mM HEPES, pH 7.35, containing 4% formaldehyde and 0.1% glutaraldehyde at room temperature for 1 hour and then stored over night in fixative at 4°C. Samples were dehydrated in acetone and embedded in EPON. 60 nm sections were mounted onto formvar-coated copper grids, stained with 4% uranyl acetate and lead citrate as previously described by Reynolds and visualized in a Morgagni TEM (FEI), operated at 80 kV [61].
Gene expression analysis
Total RNA was extracted from isolated enterocytes using TRIzol (Ambion) and the RNA concentration was determined on a NanoDrop 1000 spectrophotometer (Thermo Scientific). First-strand complementary DNA (cDNA) for quantitative RT-PCR was synthesized from 5 µg of RNA with Oligo-dT primers and RevertAid reverse transcriptase (Fermentas). Taqman technology based RT PCR was performed using absolute QPCR ROX mix (Thermo Scientific), sample cDNA and the Taqman probes hprt (Mm00446368_m1), Spi-B (Mm03048233-m1) Cxcl2 (Mm00436450_m1), Cxcl5 (Mm00436451_g1) and Reg3γ (Mm01181783_g1) from Life Technologies. SYBR green–based real-time PCRs were performed as described in the Extended Experimental Procedures. Microarray analysis was performed using Whole Mouse Genome Oligo Microarray v2 (4×44k) (Agilent Technologies) following the SC_AgilentV5.7 protocol provided by the manufacturer. Heat map analysis was performed using the MeV 4_5_1 software. Clusters of orthologous groups (COG) analysis was performed using the online bioinformatic tool PANTHER (http://www.pantherdb.org/). The expression array data are accessible through GEO Series accession numbers GSE51160. Reviewer can access the raw data files using the following link: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=ehufucgejpwtjwf&acc=GSE51160.
In vitro cell culture
Intestinal epithelial m-ICcl2 cells were cultured for 6 days with medium change every other day to obtain a confluent cell monolayer as previously described [62]. m-ICcl2 cells were incubated with wildtype S. Typhimurium, the SPI1 mutant strain (ΔinvC) or the complemented SPI1 mutant strain (ΔinvC pinvC) at a multiplicity of infection of 1∶10 for 1 h at 37°C, washed and gentamicin (Sigma Aldrich) was added at a concentration of 100 µg/mL for 1 h at 37°C. Cells were washed 3 times in PBS and lysed directly or maintained in culture in the presence of 20 µg/mL gentamicin. At the indicated incubation period cells were washed with PBS and lysed in 500 µl 0.1% Triton X-100 for 2 min. at room temperature. Viable counts were determined by serial dilution and plating on LB agar plates. Flow cytometric analysis was performed on trypsinized m-ICcl2 cells following fixation using a FACS Calibur apparatus (BD).
Statistical analysis
Results for bacterial growth in organ tissues or quantitative RT-PCR show counts for individual animals plus the median. The One-way ANOVA Kruskal-Wallis test (with Dunn's posttest) and the Mann-Whitney test were employed for statistical analysis of bacterial growth in organ tissue at different time points, comparative analysis of quantitative RT PCR results or bacterial counts after infection with WT versus SPI1 mutant Salmonella respectively. The GraphPad Prism Software 5.00 was used for statistical evaluation. p values are indicated as follows: ***p<0.001; **p<0.01, and *p<0.05.
Supporting Information
Zdroje
1. MolyneuxE, WalshA, PhiriA, MolyneuxM (1998) Acute bacterial meningitis in children admitted to the Queen Elizabeth Central Hospital, Blantyre, Malawi in 1996–97. Trop Med Int Health 3 : 610–618.
2. MilledgeJ, CalisJC, GrahamSM, PhiriA, WilsonLK, et al. (2005) Aetiology of neonatal sepsis in Blantyre, Malawi: 1996–2001. Ann Trop Paediatr 25 : 101–110.
3. SigaúqueB, RocaA, MandomandoI, MoraisL, QuintóL, et al. (2009) Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J 28 : 108–113.
4. MakokaMH, MillerWC, HoffmanIF, CholeraR, GilliganPH, et al. (2012) Bacterial infections in Lilongwe, Malawi: aetiology and antibiotic resistance. BMC Infect Dis 12 : 67 doi: 10.1186/1471-2334-12-67
5. McCormickDW, WilsonML, MankhamboL, PhiriA, ChimalizeniY, et al. (2013) Risk factors for death and severe sequelae in Malawian children with bacterial meningitis, 1997–2010. Pediatr Infect Dis J 32: e54–61.
6. QueF, WuS, HuangR (2013) Salmonella pathogenicity island 1(SPI-1) at work. Curr Microbiol 66 : 582–587.
7. HapfelmeierS, StecherB, BarthelM, KremerM, MüllerAJ, et al. (2005) The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J Immunol 174 : 1675–1685.
8. García-Del PortilloF, PucciarelliMG, CasadesúsJ (1999) DNA adenine methylase mutants of Salmonella typhimurium show defects in protein secretion, cell invasion, and M cell cytotoxicity. Proc Natl Acad Sci U S A 96 : 11578–11583.
9. AnjumMF, MarooneyC, FookesM, BakerS, DouganG, et al. (2005) Identification of core and variable components of the Salmonella enterica subspecies I genome by microarray. Infect Immun 73 : 7894–7905.
10. FigueiraR, WatsonKG, HoldenDW, HelaineS (2013) Identification of salmonella pathogenicity island-2 type III secretion system effectors involved in intramacrophage replication of S. enterica serovar typhimurium: implications for rational vaccine design. MBio 4: e00065 doi: 10.1128/mBio.00065-13
11. HapfelmeierS, MüllerAJ, StecherB, KaiserP, BarthelM, et al. (2008) Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J Exp Med 205 : 437–450.
12. FelmyB, SonghetP, SlackEM, MüllerAJ, KremerM, et al. (2013) NADPH oxidase deficient mice develop colitis and bacteremia upon infection with normally avirulent, TTSS-1 - and TTSS-2-deficient Salmonella Typhimurium. PLoS One 8: e77204.
13. SonghetP, BarthelM, StecherB, MüllerAJ, KremerM, et al. (2011) Stromal IFN-γR-signaling modulates goblet cell function during Salmonella Typhimurium infection. PLoS One 6: e22459.
14. MüllerAJ, HoffmannC, GalleM, Van Den BroekeA, HeikenwalderM, et al. (2009) The S. Typhimurium effector SopE induces caspase-1 activation in stromal cells to initiate gut inflammation. Cell Host Microbe 6 : 125–136.
15. MüllerAJ, KaiserP, DittmarKE, WeberTC, HaueterS, et al. (2012) Salmonella gut invasion involves TTSS-2-dependent epithelial traversal, basolateral exit, and uptake by epithelium-sampling lamina propria phagocytes. Cell Host Microbe 11 : 19–32.
16. JonesBD, GhoriN, FalkowS (1994) Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med 180 : 15–23.
17. JangMH, KweonMN, IwataniK, YamamotoM, TeraharaK, et al. (2004) Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl. Acad. Sci. USA 101 : 6110–6115.
18. KanayaT, HaseK, TakahashiD, FukudaS, HoshinoK, et al. (2012) The Ets transcription factor Spi-B is essential for the differentiation of intestinal microfold cells. Nat Immunol 13 : 729–736.
19. FaracheJ, KorenI, MiloI, GurevichI, KimKW, et al. (2013) Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 38 : 581–595.
20. HaseK, KawanoK, NochiT, PontesGS, FukudaS, et al. (2009) Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature 462 : 226–230.
21. TahounA, MahajanS, PaxtonE, MaltererG, DonaldsonDS, et al. (2012) Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion. Cell Host Microbe 12 : 645–656.
22. de Santa BarbaraP, van den BrinkGR, RobertsDJ (2003) Development and differentiation of the intestinal epithelium. Cell Mol Life Sci 60 : 1322–1332.
23. MuncanV, HeijmansJ, KrasinskiSD, BüllerNV, WildenbergME, et al. (2011) Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat Commun 2 : 452 doi: 10.1038/ncomms1463
24. MénardS, FörsterV, LotzM, GütleD, DuerrCU, et al. (2008) Developmental switch of intestinal antimicrobial peptide expression. J Exp Med 205 : 183–193.
25. BarthelM, HapfelmeierS, Quintanilla-MartínezL, KremerM, RohdeM, et al. (2003) Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun 71 : 2839–2858.
26. TakeuchiA (1967) Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol 50 : 109–136.
27. NietfeldJC, TylerDE, HarrisonLR, ColeJR, LatimerKS, et al. (1992) Invasion of enterocytes in cultured porcine small intestinal mucosal explants by Salmonella choleraesuis. Am J Vet Res 53 : 1493–1499.
28. GiannellaRA, FormalSB, DamminGJ, CollinsH (1972) Pathogenesis of salmonellosis. Studies of fluid secretion, mucosal invasion, and morphologic reaction in the rabbit ileum. J Clin Invest 52 : 441–453.
29. FrostAJ, BlandAP, WallisTS (1997) The early dynamic response of the calf ileal epithelium to Salmonella typhimurium. Vet Pathol 34 : 369–386.
30. LaughlinRC, KnodlerLA, BarhoumiR, PayneHR, WuJ, et al. (2014) Spatial segregation of virulence gene expression during acute enteric infection with Salmonella enterica serovar Typhimurium. MBio 5: e00946-13 doi: 10.1128/mBio.00946-13
31. BoltonAJ, OsborneMP, WallisTS, StephenJ (1999) Interaction of Salmonella choleraesuis, Salmonella dublin and Salmonella typhimurium with porcine and bovine terminal ileum in vivo. Microbiology 145 : 2431–2441.
32. SantosRL, ZhangS, TsolisRM, BäumlerAJ, AdamsLG (2002) Morphologic and molecular characterization of Salmonella typhimurium infection in neonatal calves. Vet Pathol 39 : 200–215.
33. OchmanH, GroismanEA (1996) Distribution of pathogenicity islands in Salmonella spp. Infect Immun 64 : 5410–5412.
34. ZhangS, SantosRL, TsolisRM, StenderS, HardtWD, et al. (2002) The Salmonella enterica serotype typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect Immun 70 : 3843–3855.
35. TsolisRM, AdamsLG, FichtTA, BäumlerAJ (1999) Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect Immun 67 : 4879–4885.
36. HapfelmeierS, EhrbarK, StecherB, BarthelM, KremerM, et al. (2004) Role of the Salmonella pathogenicity island 1 effector proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica subspecies 1 serovar Typhimurium colitis in streptomycin-pretreated mice. Infect Immun 72 : 795–809.
37. ConwayKL, KuballaP, SongJH, PatelKK, CastorenoAB, et al. (2013) Atg16l1 is required for autophagy in intestinal epithelial cells and protection of mice from Salmonella infection. Gastroenterology 145 : 1347–1357.
38. BenjaminJL, SumpterRJr, LevineB, HooperLV (2013) Intestinal epithelial autophagy is essential for host defense against invasive bacteria. Cell Host Microbe 13 : 723–734.
39. ThurstonTL, WandelMP, von MuhlinenN, FoegleinA, RandowF (2012) Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482 : 414–418.
40. ZarepourM, BhullarK, MonteroM, MaC, HuangT, et al. (2013) The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis. Infect Immun 81 : 3672–3683.
41. WilsonCL, OuelletteAJ, SatchellDP, AyabeT, López-BoadoYS, et al. (1999) Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286 : 113–117.
42. SalzmanNH, GhoshD, HuttnerKM, PatersonY, BevinsCL (2003) Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422 : 522–526.
43. DupontA, KaconisY, YangI, AlbersT, WoltemateS, et al. (2014) Intestinal mucus affinity and biological activity of an orally administered antibacterial and anti-inflammatory peptide. Gut [epub ahead of print] doi: 10.1136/gutjnl-2014-307150
44. SchauserK, OlsenJE, LarssonLI (2005) Salmonella typhimurium infection in the porcine intestine: evidence for caspase-3-dependent and -independent programmed cell death. Histochem Cell Biol 123 : 43–50.
45. KunisawaJ, KiyonoH (2012) Alcaligenes is commensal bacteria habituating in the gut-associated lymphoid tissue for the regulation of intestinal IgA responses. Front Immunol 3 : 65 doi: 10.3389/fimmu.2012.0006.
46. EcheverryA, SchesserK, AdkinsB (2007) Murine neonates are highly resistant to Yersinia enterocolitica following orogastric exposure. Infect Immun 75 : 2234–2243.
47. ClarkMA, HirstBH, JepsonMA (1998) M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells. Infect Immun 66 : 1237–1243.
48. GodinezI, HanedaT, RaffatelluM, GeorgeMD, PaixãoTA, et al. (2008) T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect Immun 76 : 2008–2017.
49. LawhonSD, KhareS, RossettiCA, EvertsRE, GalindoCL, et al. (2011) Role of SPI-1 secreted effectors in acute bovine response to Salmonella enterica Serovar Typhimurium: a systems biology analysis approach. PLoS One 6: e26869.
50. WeissDS, RaupachB, TakedaK, AkiraS, ZychlinskyA (2004) Toll-like receptors are temporally involved in host defense. J Immunol 172 : 4463–4439.
51. RoyMF, LarivièreL, WilkinsonR, TamM, StevensonMM, et al. (2006) Incremental expression of Tlr4 correlates with mouse resistance to Salmonella infection and fine regulation of relevant immune genes. Genes Immun 7 : 372–383.
52. BrinkmannMM, SpoonerE, HoebeK, BeutlerB, PloeghHL, et al. (2007) The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol 177 : 265–275.
53. ArpaiaN, GodecJ, LauL, SivickKE, McLaughlinLM, et al. (2011) TLR signaling is required for Salmonella typhimurium virulence. Cell 144 : 675–688.
54. ZengH, CarlsonAQ, GuoY, YuY, Collier-HyamsLS, et al. (2003) Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J Immunol 171 : 3668–3674.
55. WinterSE, WinterMG, XavierMN, ThiennimitrP, PoonV, et al. (2013) Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339 : 708–711.
56. AntunesLC, FinlayBB (2011) A comparative analysis of the effect of antibiotic treatment and enteric infection on intestinal homeostasis. Gut Microbes 2 : 105–108.
57. RaffatelluM, SantosRL, VerhoevenDE, GeorgeMD, WilsonRP, et al. (2008) Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med 14 : 421–428.
58. GerlachRG, CláudioN, RohdeM, JäckelD, WagnerC, et al. (2008) Cooperation of Salmonella pathogenicity islands 1 and 4 is required to breach epithelial barriers. Cell Microbiol 10 : 2364–2376.
59. TabetaK, HoebeK, JanssenEM, DuX, GeorgelP, et al. (2006) The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol 7 : 156–164.
60. HemmiH, TakeuchiO, KawaiT, KaishoT, SatoS, et al. (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408 : 740–745.
61. ReynoldsES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17 : 208–213.
62. LotzM, GütleD, WaltherS, MénardS, BogdanC, et al. (2006) Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med 203 : 973–984.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell ResponsesČlánek RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct MechanismsČlánek Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 9- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Virus Control Goes Epigenetic
- The Role of Iron in Prion Disease and Other Neurodegenerative Diseases
- The Ins and Outs of Rust Haustoria
- Prion Strains and Amyloid Polymorphism Influence Phenotypic Variation
- Teaching Fido New ModiFICation Tricks
- Can Enhance Infection in Mosquitoes: Implications for Malaria Control?
- MIF Contributes to Associated Immunopathogenicity Development
- Persistence of Virus Reservoirs in ART-Treated SHIV-Infected Rhesus Macaques after Autologous Hematopoietic Stem Cell Transplant
- Bacillus Calmette-Guerin Infection in NADPH Oxidase Deficiency: Defective Mycobacterial Sequestration and Granuloma Formation
- EhCoactosin Stabilizes Actin Filaments in the Protist Parasite
- Molecular Insights Into the Evolutionary Pathway of O1 Atypical El Tor Variants
- LprG-Mediated Surface Expression of Lipoarabinomannan Is Essential for Virulence of
- Structural Correlates of Rotavirus Cell Entry
- Multivalent Adhesion Molecule 7 Clusters Act as Signaling Platform for Host Cellular GTPase Activation and Facilitate Epithelial Barrier Dysfunction
- The Effects of Vaccination and Immunity on Bacterial Infection Dynamics
- Myeloid Derived Hypoxia Inducible Factor 1-alpha Is Required for Protection against Pulmonary Infection
- Functional Characterisation of Germinant Receptors in and Presents Novel Insights into Spore Germination Systems
- Global Analysis of Neutrophil Responses to Reveals a Self-Propagating Inflammatory Program
- Host Cell Invasion by Apicomplexan Parasites: The Junction Conundrum
- Comparative Phenotypic Analysis of the Major Fungal Pathogens and
- Unravelling the Multiple Functions of the Architecturally Intricate β-galactosidase, BgaA
- Sialylation of Prion Protein Controls the Rate of Prion Amplification, the Cross-Species Barrier, the Ratio of PrP Glycoform and Prion Infectivity
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- Ontogeny of Recognition Specificity and Functionality for the Broadly Neutralizing Anti-HIV Antibody 4E10
- Identification and Characterisation of a Hyper-Variable Apoplastic Effector Gene Family of the Potato Cyst Nematodes
- Crimean-Congo Hemorrhagic Fever Virus Entry into Host Cells Occurs through the Multivesicular Body and Requires ESCRT Regulators
- Age-Dependent Enterocyte Invasion and Microcolony Formation by
- CD160-Associated CD8 T-Cell Functional Impairment Is Independent of PD-1 Expression
- Functional Fluorescent Protein Insertions in Herpes Simplex Virus gB Report on gB Conformation before and after Execution of Membrane Fusion
- The Tudor Domain Protein Spindlin1 Is Involved in Intrinsic Antiviral Defense against Incoming Hepatitis B Virus and Herpes Simplex Virus Type 1
- Transgenic Analysis of the MAP Kinase MPK10 Reveals an Auto-inhibitory Mechanism Crucial for Stage-Regulated Activity and Parasite Viability
- Evidence for a Transketolase-Mediated Metabolic Checkpoint Governing Biotrophic Growth in Rice Cells by the Blast Fungus
- Incomplete Deletion of IL-4Rα by LysM Reveals Distinct Subsets of M2 Macrophages Controlling Inflammation and Fibrosis in Chronic Schistosomiasis
- Identification and Functional Expression of a Glutamate- and Avermectin-Gated Chloride Channel from , a Southern Hemisphere Sea Louse Affecting Farmed Fish
- Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell Responses
- Strong Epistatic Selection on the RNA Secondary Structure of HIV
- Hematopoietic but Not Endothelial Cell MyD88 Contributes to Host Defense during Gram-negative Pneumonia Derived Sepsis
- Delineation of Interfaces on Human Alpha-Defensins Critical for Human Adenovirus and Human Papillomavirus Inhibition
- Exploitation of Reporter Strains to Probe the Impact of Vaccination at Sites of Infection
- RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct Mechanisms
- Helminth Infections Coincident with Active Pulmonary Tuberculosis Inhibit Mono- and Multifunctional CD4 and CD8 T Cell Responses in a Process Dependent on IL-10
- MHC Class II Restricted Innate-Like Double Negative T Cells Contribute to Optimal Primary and Secondary Immunity to
- Reactive Oxygen Species Regulate Caspase-11 Expression and Activation of the Non-canonical NLRP3 Inflammasome during Enteric Pathogen Infection
- Evolution of Plastic Transmission Strategies in Avian Malaria
- A New Human 3D-Liver Model Unravels the Role of Galectins in Liver Infection by the Parasite
- Translocates into the Myocardium and Forms Unique Microlesions That Disrupt Cardiac Function
- Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
- The Cofilin Phosphatase Slingshot Homolog 1 (SSH1) Links NOD1 Signaling to Actin Remodeling
- Kaposi's Sarcoma Herpesvirus MicroRNAs Induce Metabolic Transformation of Infected Cells
- Reorganization of the Endosomal System in -Infected Cells: The Ultrastructure of -Induced Tubular Compartments
- Distinct Dictation of Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality via Triggering TLR3 and TLR4 Signal Pathways
- Exploitation of the Complement System by Oncogenic Kaposi's Sarcoma-Associated Herpesvirus for Cell Survival and Persistent Infection
- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Structural Insight into Host Recognition by Aggregative Adherence Fimbriae of Enteroaggregative
- The CD14CD16 Inflammatory Monocyte Subset Displays Increased Mitochondrial Activity and Effector Function During Acute Malaria
- Infection Induces Expression of a Mosquito Salivary Protein (Agaphelin) That Targets Neutrophil Function and Inhibits Thrombosis without Impairing Hemostasis
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- MIF Contributes to Associated Immunopathogenicity Development
- The Ins and Outs of Rust Haustoria
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



