-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Depletion of . GlmU from Infected Murine Lungs Effects the Clearance of the Pathogen
The synthesis of the Mtb cell wall involves a cascade of reactions catalyzed by cytosolic and cell membrane-bound enzymes. The reaction catalyzed by GlmUMtb (an enzyme with acetyltransferase and uridyltransferase activities) generates UDP-GlcNAc, a central nucleotide-sugar building block of the cell wall. Apart from cell wall synthesis UDP-GlcNAc is an essential metabolite participating in other cellular processes including disaccharide linker and mycothiol biosynthesis. GlmUMtb shares very little sequence similarity with eukaryotic acetyltransferase and uridyltransferase enzymes. Many pathogens have alternative pathway(s) for foraging GlcNAc from the host. The present study was undertaken to see the effects of depleting GlmUMtb on pathogen survival in the host animal. We have generated a conditional gene replacement mutant of glmUMtb and find that depletion of GlmUMtb at any stage of bacterial growth or in mice infected with Mtb including a well-established infection, results in irreversible bacterial death due to perturbation of cell wall synthesis. We have developed a novel anti-GlmUMtb inhibitor (Oxa33), identified its binding site on GlmUMtb, and shown its specificity for GlmUMtb. The study demonstrates that GlmUMtb is a promising target for therapeutic intervention and Oxa33 can be pursued as a lead molecule.
Published in the journal: Depletion of . GlmU from Infected Murine Lungs Effects the Clearance of the Pathogen. PLoS Pathog 11(10): e32767. doi:10.1371/journal.ppat.1005235
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005235Summary
The synthesis of the Mtb cell wall involves a cascade of reactions catalyzed by cytosolic and cell membrane-bound enzymes. The reaction catalyzed by GlmUMtb (an enzyme with acetyltransferase and uridyltransferase activities) generates UDP-GlcNAc, a central nucleotide-sugar building block of the cell wall. Apart from cell wall synthesis UDP-GlcNAc is an essential metabolite participating in other cellular processes including disaccharide linker and mycothiol biosynthesis. GlmUMtb shares very little sequence similarity with eukaryotic acetyltransferase and uridyltransferase enzymes. Many pathogens have alternative pathway(s) for foraging GlcNAc from the host. The present study was undertaken to see the effects of depleting GlmUMtb on pathogen survival in the host animal. We have generated a conditional gene replacement mutant of glmUMtb and find that depletion of GlmUMtb at any stage of bacterial growth or in mice infected with Mtb including a well-established infection, results in irreversible bacterial death due to perturbation of cell wall synthesis. We have developed a novel anti-GlmUMtb inhibitor (Oxa33), identified its binding site on GlmUMtb, and shown its specificity for GlmUMtb. The study demonstrates that GlmUMtb is a promising target for therapeutic intervention and Oxa33 can be pursued as a lead molecule.
Introduction
The cell wall, which contains a number of virulence determinants, is the first line of defence for survival of the pathogen in the hostile host environment [1]. The mycobacterial cell envelope includes three layers of cell membrane and a cell wall made up of peptidoglycan, mycolic acid, arabinogalactan and lipoarabinomannan (LAM) [2–4]. Most existing first line and second line drugs used to treat TB such as isoniazid, ethambutol, ethionamide and cycloserine, act on enzymes engaged in the synthesis of different cell wall components [5]. The current high mortality rates of infected individuals as well as increasing incidence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis (TB) among patients underscore the importance of finding new targets for therapeutic intervention.
GlmUMtb is a bi-functional enzyme, with acetyltransferase and uridyltransferase activities catalyzed by the C - and N - terminal domains respectively (Fig 1A) [6,7]. The carboxy-terminal domain of GlmUMtb transfers the acetyl moiety from acetyl CoA onto glucosamine-1-phosphate to generate N-acetylglucosamine-1-phosphate (GlcNAc-1-P). The N-terminal uridyltransferase domain of GlmUMtb then catalyzes the transfer of UMP (from UTP) to GlcNAc-1-P to form UDP-GlcNAc (Fig 1A) [6]. The UDP-GlcNAc thus produced is among the central metabolites that is required for the synthesis of peptidoglycan, lipid A of LAM, arabinogalactan, Rha-GlcNAc linkers, mycothiol (required for maintaining redox homeostasis) [8–14]. The crystal structure of M. tuberculosis GlmU (GlmUMtb) displays two-domain architecture with an N-terminal α/β - like fold and a C-terminal left-handed parallel-β-helix structure [15,16]. Unlike its orthologs, GlmUMtb has a long carboxy-terminal tail which displays little secondary structure [17]. Results from transposon mutagenesis experiments have indicated glmUMtb to be an essential gene, supported by the fact that M. smegmatis is unable to grow in the absence of glmUsmeg [18–20]. However, no studies have addressed the question of whether both the activities of GlmUMtb are independently essential for the growth or survival of the bacterium.
Fig. 1. Generation of glmUMtb conditional deletion mutant. 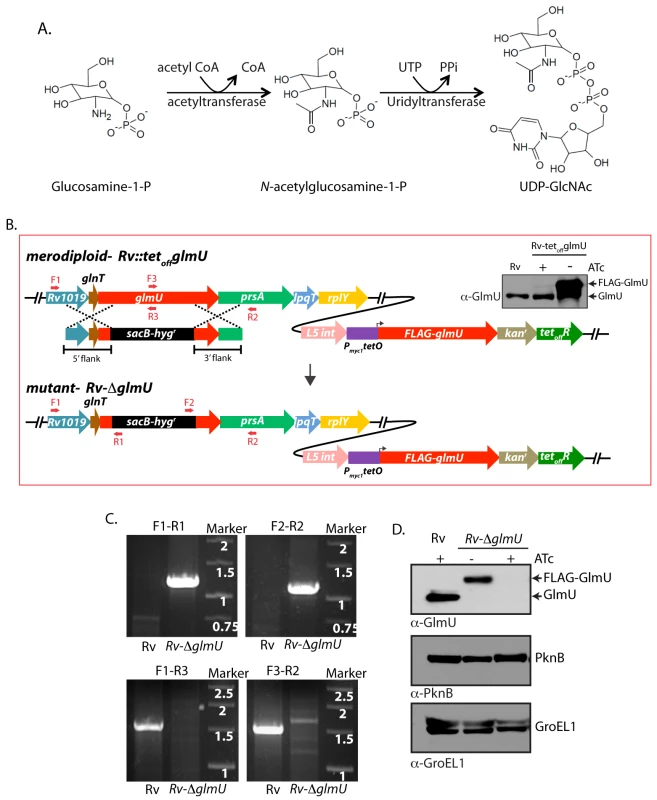
(A) Schematic depicting the biochemical reaction catalyzed by GlmUMtb. (B) Schematic diagram representing the genomic location of glmUMtb (Rv1018c) and homologous recombination between flanking sequence in the phagemid and the genomic locus. Primers used for the PCR amplification are depicted. Upper right panel shows the immunoblot. WCLs from Rv or Rv::glmU grown in the presence or absence of ATc were resolved and probed with anti-GlmU antibodies. Bands corresponding to the endogenous GlmU and ectopic FLAG-GlmU are indicated. (C) Agarose gel showing the PCR amplification of the Rv & putative Rv∆glmU mutant using specific primers. Primers F1 and R2 are beyond the flanks, R1 and F2 belong to resolvase sites in sacB/hygR cassette and F3 and R3 binds to the native glmU. Amplification with F1-R1 or F2-R2 primers results in 1.23 kb or 1.17 kb size products with Rv∆glmU strain but none with the Rv. Whereas PCRs with F1-R3 or F3-R2 primers amplifies 1.5 kb band with the Rv and none with the Rv∆glmU mutant. (D) Whole cell lysates (WCL) were prepared from the large scale cultures of Rv and Rv∆glmU grown in the absence and presence of ATc for five days. 20 μg of WCLs were resolved and probed with anti-GlmUMtb, anti-PknB and anti-GroEL1 antibodies. Band corresponding to endogenous GlmUMtb and FLAG-GlmUMtb are indicated. While the enzymes required for the synthesis of UDP-GlcNAc are well conserved among prokaryotes, they are very different from those found in eukaryotes, making GlmUMtb an attractive putative drug target [21,22]. Researchers have developed compounds that inhibit the activities of the orthologs of GlmUMtb (GlmU from T. brucei, P. aeruginosa, E. coli, H. influenza and X. oryzae) in vitro [23–30]. Bioinformatic analyses and kinetic modelling data advocate GlmUMtb to be a potential target for the development of suitable inhibitors [31]. In concurrence with these predictions, effective inhibitors have been developed against, the acetyltransferase and uridyltransferase domains of GlmUMtb [32,33]. However, the precise sites of inhibitor-protein interactions and the efficacy of the inhibitors ex vivo or in vivo have not been investigated. Subjecting Mtb cultures in vitro to gradual decrease of oxygen (hypoxic stress) results in reprogramming of metabolic pathways and up-regulation of stress response genes, and is considered to be an in vitro model for the dormancy [34,35]. The importance of GlmUMtb for growth under hypoxic conditions and in an in vivo infection model is yet to be investigated. In the present study we have generated a conditional gene replacement mutant of glmUMtb and used this mutant to investigate any role GlmUMtb may play in modulating the growth of the bacterium in vitro, ex vivo and in vivo. The data presented here demonstrate that GlmUMtb is a viable and promising target for therapeutic intervention against tuberculosis.
Results
GlmUMtb depletion perturbs cell wall structure and affects the bacterial survival in normoxia
As the tetracycline-inducible system is an effective means to regulate gene expression [36], we introduced the integration-proficient pST-KirT-glmU construct (wherein glmUMtb gene was cloned under a promoter that shuts down upon ATc addition; S1A Fig) into Mtb H37Rv (Fig 1B). Whereas the expression of GlmUMtb from its native locus remained unaltered, the expression of FLAG-GlmUMtb in Rv::glmU strain was drastically compromised in the presence of ATc (Western blot inset, Fig 1B). This merodiploid strain was transduced with temperature sensitive phage, and the fidelity of homologous recombination at the native locus was confirmed by amplification across the replacement junctions using appropriate primers (Fig 1C). A comparison of GlmUMtb expression in the presence and absence of ATc revealed that the protein was not detectable by western blot analysis after 6 days of growth in the presence of ATc (Fig 1D). While the growth of Rv∆glmU in the absence of ATc was similar to Rv, in the presence of ATc the growth was drastically compromised (Fig 2A). A comparative analysis of growth by spotting of serially diluted cultures of Rv and Rv∆glmU grown in the presence versus absence of ATc showed that GlmUMtb depletion by addition of ATc led to complete inhibition of growth, with no growth detected after 6 days (Fig 2B). Interestingly, analysis of GlmUMtb expression every 24 hours post-ATc addition uncovered significant reduction in GlmUMtb expression by the third day itself (Fig 2C). These results indicate that GlmUMtb is required for the Mtb survival. To determine the impact of GlmUMtb depletion on cellular morphology we carried out SEM and TEM imaging analysis of Rv and Rv∆glmU cells grown for three days in the absence or presence of ATc. SEM analysis revealed severe morphological perturbations in the absence of GlmUMtb, with the bacilli showing wrinkled surface and fused cells (Fig 2D). TEM analysis showed that whereas in Rv and Rv∆glmU cell wall structure and thickness are comparable, there was a marked decrease in cell wall thickness in Rv∆glmU cells where GlmUMtb was not expressed (cells grown in the presence of ATc; Fig 2E and 2F).
Fig. 2. GlmUMtb depletion affects the bacterial survival by perturbing the cell wall structure. 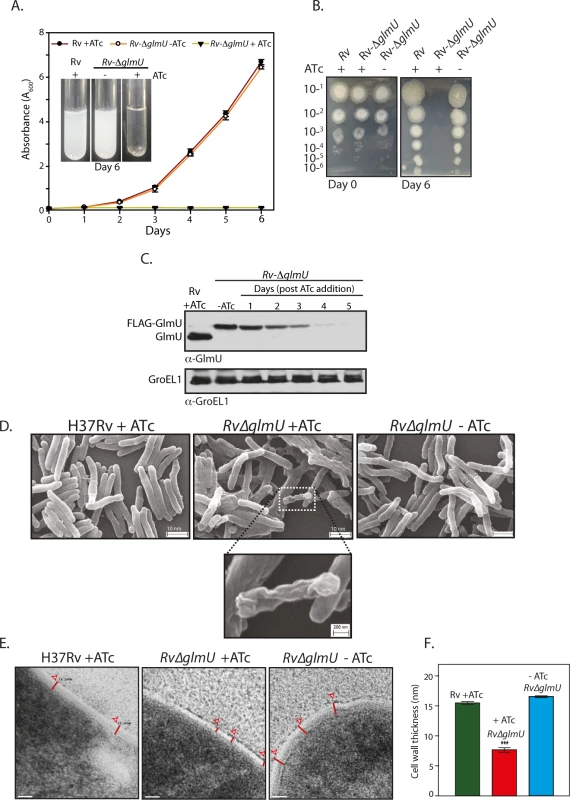
(A) Rv and Rv∆glmU cultures grown to an A600 of 0.8 were used to seed fresh cultures at an initial A600 of 0.1 in the presence or absence of ATc as indicated and the day wise growth was monitored for six days. Inset shows the growth in the culture tubes after six days. The experiment was performed in triplicates (n = 3). Error bars indicate standard error of mean (s.e.m). (B) Day 0 and Day 6 cultures grown in the presence or absence of ATc were serially diluted and spotted on 7H11 agar plates. (C) Large scale cultures were inoculated at A600 of 0.1 in the presence or absence of ATc and the WCLs were prepared on different days post ATc addition (as indicated). WCLs were resolved and probed with anti-GlmUMtb and anti-GroEL1 antibodies. (D) Scanning electron microscopy of Rv and Rv∆glmU grown for 72 h with or without ATc as indicated. Scale bars in upper panel were 10 nm and for the inset was 200 nm. The experiment was repeated thrice. (E) Transmission electron micrographs results at 50,000X of Rv and Rv∆glmU cultures grown with or without ATc. Red lines depict the cell wall thickness. Scale bar: 20 nm. (F) Cell wall thickness was measured in nm for 15 to 22 cells for each sample. ***p<0.0001, two tailed non parametric t-test, mean, s.e.m. Impact of GlmUMtb depletion on dormant bacteria
Next we used the Wayne model to investigate the consequence of GlmUMtb depletion on the dormant bacteria under hypoxic conditions [35]. Accordingly, hypoxia was established and maintained for 42 days with depletion of GlmUMtb or addition of INH for either 22 days, or for 2 days (Fig 3A, line diagram). In agreement with previous reports, we observed that bacteria were tolerant to INH under hypoxic conditions [37] (Fig 3C), with a thicker cell wall being observed under hypoxic conditions compared with the normoxic cultures (Fig 3D and 3E). Depletion of GlmUMtb for 22 days resulted in complete clearance of growth (Fig 3B), which was also reflected in severe morphological perturbations and drastic reduction in cell wall thickness (Fig 3D and 3E). Significantly, GlmUMtb depletion for as less as 2 days decreased cell viability by three orders of magnitude (Fig 3B) and decrease in cell wall thickness (~18%; Fig 3D and 3E). Taken together, the data suggests that the absence of GlmUMtb in hypoxic condition leads to aberrant cell wall thickness and morphology, eventually leading to the death of the cell.
Fig. 3. Effect of GlmUMtb depletion of dormant bacteria. 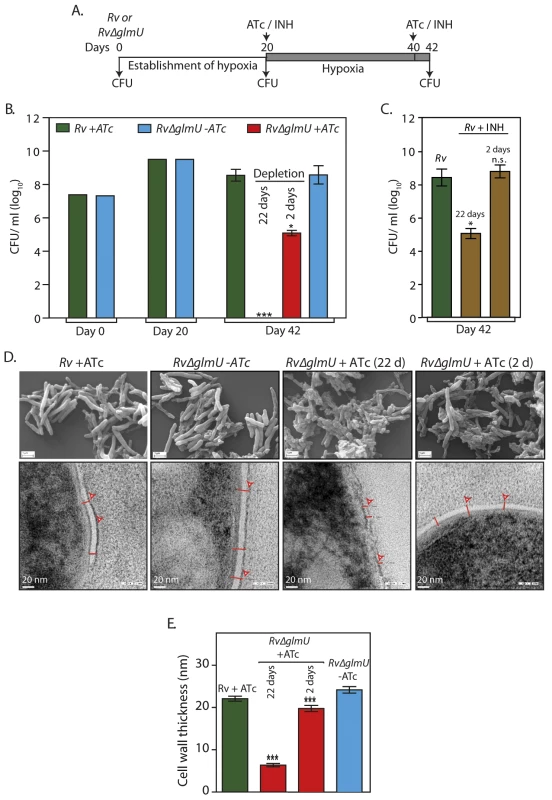
(A) Schematic outline of the experiment. (B) Rv and Rv∆glmU cultures were seeded at an initial A600 of 0.1 in 1.5 HPLC tubes or in 500 ml flasks containing penetrable caps. Establishment of hypoxia was monitored with the help of methylene blue color change (blue to colorless). ATc was added to Rv on 20th day and to Rv∆glmU on 20th and 40th day. CFUs were enumerated on day 0, day 20 and day 42. *p<0.05 or ***p<0.005, two tailed non parametric t-test, Error bars indicate s.e.m. and the experiment was performed in triplicates (n = 3). (C) INH (50 ng/ml) was added to the hypoxic Rv cultures on 20th and 40th day. CFUs were enumerated on day 42. *p<0.05 or NS: non- significant, two tailed non parametric t-test, Error bars indicate s.e.m. and n = 3. (D) Large scale hypoxic cultures were pelleted down on day 42 and processed for scanning electron microscopy (upper panel) or transmission electron microscopy (lower panel) imaging. (SEM: scale bars: 1 μm; TEM scale bar: 20 nm). Cell wall thickness is indicated by red lines. (E) Cell wall thickness was measured in nm for 20 to 54 cells from the TEM images of different samples (representative image shown in Fig 3C). ***p< 0.0001 and 0.0005, two tailed non parametric t-test, Error bars indicate s.e.m. Acetyl and uridyltransferase activities of GlmUMtb are independently essential
Biochemical investigations have shown that the N-terminal fragment (1–352 amino acids) and C-terminal fragment (150–495 amino acids) of GlmUMtb can independently undertake uridyltransferase and acetyltransferase activities respectively (Fig 4A and 4B) [15,17]. The active site residues that are necessary for these activities have also been identified (Fig 4A and 4B) [17]. To investigate if both activities are essential for cell survival, we have generated previously reported truncation mutants GlmU-N and GlmU-C [38]. We also generated GlmUK26A and GlmUH374A, the uridyltransferase and acetyltransferase active site mutants, and GlmUDM wherein both the active site residues were concomitantly mutated. GlmUMtb wild type and mutant proteins were purified (Fig 4C) and their uridyltransferase and acetyltransferase activities were assayed. While GlmU-C and GlmUK26A mutants showed acetyltransferase activity, as expected they did not show any uridyltransferase activity (Fig 4D). On the other hand GlmU-N and GlmUH374A had uridyltransferase activity but not the acetyltransferase activity (Fig 4D). As expected the double mutant did not have either uridyl or acetyltransferase activity (Fig 4D). Next complementation assays using one or other truncations / active site mutants were carried out. The FLAG-GlmUMtb and the complemented untagged wt-GlmUMtb proteins were found to be expressed at similar levels (Fig 4E). The episomally expressed wt-GlmUMtb could rescue the Rv∆glmU phenotype in the presence of ATc (Fig 4F). Contrastingly, while the various GlmUMtb mutant proteins were expressed at levels comparable to that of FLAG-GlmUMtb (Fig 4E); none of them rescued the growth defects of the Rv∆glmU strain in the presence of ATc (Fig 4F). These results indicate that both uridyltransferase and acetyltransferase activities of GlmUMtb are essential for pathogen survival and imply that the only source of the metabolites GlcNAc-1-P and UDP-GlcNAc is through the GlmUMtb mediated synthesis pathway.
Fig. 4. Acetyl and uridyltransferase activities are independently essential. 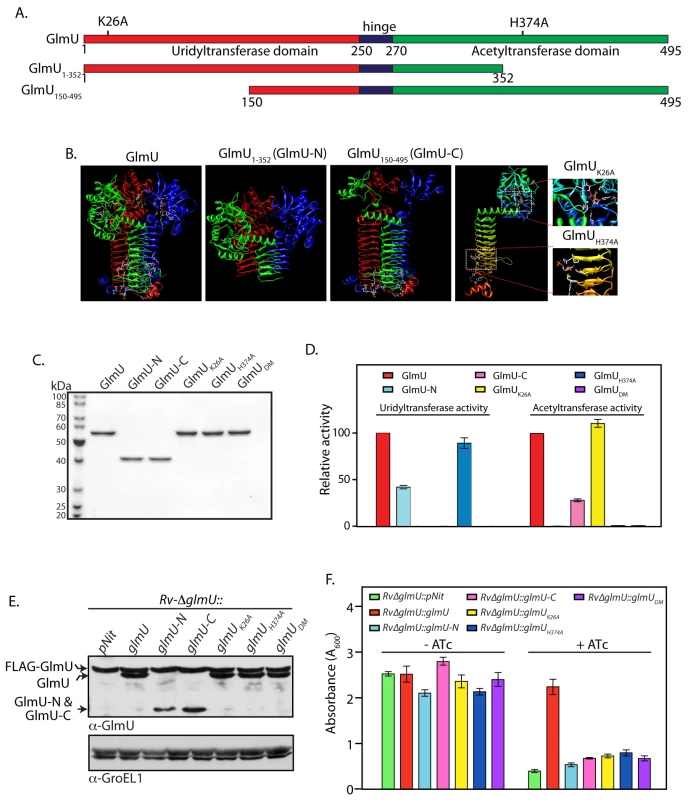
(A) and (B) Schematic and cartoon representation of GlmUMtb depicting different domains, active site residues and the deletion mutants. (C) GlmUMtb and GlmUMtb-mutants were purified as described earlier [38] and the 1 μg of the purified proteins were resolved on 10% SDS-PAGE and stained with coomassie. (D) Uridyltransferase (left panel) and acetyltransferase (right panel) activities were carried out as describe in Methods using 0.5 to 20 pmoles of wild type or mutant GlmUMtb proteins. Activity was defined as μM product formed / min / pmole of enzyme. Relative activities of the mutants were calculated with respect to the activity of GlmUMtb, which was normalized to 100%. The experiment was repeated three times and the error bars indicate s.e.m. (E) Wild type and mutated GlmUMtb genes were cloned into pNit vector without any N- or C- terminal tag. pNit-glmUwt or pNit-glmUmutant constructs were electroporated into Rv∆glmU, and the WCLs prepared from Rv∆glmU and Rv∆glmU::glmUmutant cultures were resolved and probed with anti-GlmU and anti-GroEL1 antibodies. Bands corresponding to FLAG-GlmUMtb, complemented GlmUwt/mutant and the deletion fragments of GlmU are indicated. (F) Rv∆glmU and Rv∆glmU::glmUmutant cultures were seeded at an initial A600 of 0.1 and grown for five days in the absence or presence of ATc. The experiment was performed in triplicates and the error bars represent s.e.m. Presence of GlmUMtb is obligatory for the survival of Mtb in the host
Mtb cells devoid of an intact cell wall have been found to be capable of surviving inside the host [39,40]. Some pathogens have been reported to resort to cell wall “recycling” for the synthesis of UDP-GlcNAc, and others have been known to utilize GlcNAc from the host for this purpose [41–44]. However, such mechanisms have not yet been reported in Mtb. To investigate these possibilities we examined the impact of GlmUMtb depletion on survival of the pathogen in the host. Using an ex vivo THP-1 infection model we observed ~80% phagolysosome fusion in the absence of GlmUMtb (Fig 5A and 5B; compare Rv∆glmU with Rv∆glmU +ATc). This was also reflected in the survival pattern of the pathogen upon depletion of GlmUMtb (Fig 5C), with survival being strongly compromised in absence of GlmUMtb. The impact of GlmUMtb depletion was evident as early as 24 h post-infection, with a dramatic drop in survival by 48 hours post-infection (Fig 5C). The consequences of GlmUMtb depletion on survival of the pathogen in vivo were evaluated using guinea pig infection model. CFUs obtained 24 h after infection suggested efficient and equivalent implantation of both wild type and mutant bacilli in the lungs of guinea pigs (Fig 5D). Discrete bacilli were observed in the lungs of guinea pigs infected with Rv and Rv∆glmU 28 days post-infection (Fig 5E and 5F). In contrast, the lungs of the guinea pigs infected with Rv∆glmU in the presence of doxycycline were clear (Fig 5E and 5F). In addition splenomegaly was significantly reduced upon depletion of GlmUMtb (Rv∆glmU + Dox; S2A and S2B Fig). Whereas the bacillary load in the lungs and spleen of guinea pigs infected with Rv and Rv∆glmU were comparable, we did not detect any bacilli when the Rv∆glmU infected guinea pigs were administered Dox (Fig 5D). In accordance with these observations, while the gross pathology of lungs infected with Rv and Rv∆glmU displayed considerable granulomatous architecture, normal lung parenchyma was observed upon GlmUMtb depletion (Fig 5E). These results suggest that the presence of GlmUMtb is obligatory for mycobacteria to survive in the host.
Fig. 5. Presence of GlmUMtb is obligatory for the survival of Mtb in the host. 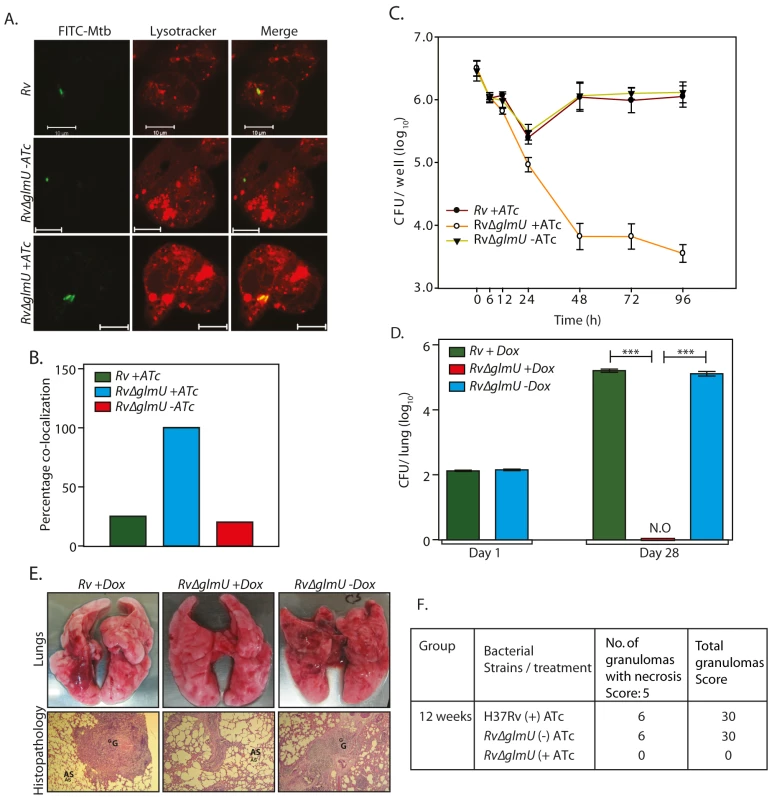
(A) Confocal microscopy images of THP1 cells infected with Rv and Rv∆glmU in the presence or absence of ATc as indicated were taken 48 h post infection. Bacteria were stained with FITC (green) and lysosomes were stained with Lyso Tracker red DND 99 (red). Scale bars, 10 μm. (B) Quantification of percentage co-localization of bacterium and lysosomes in Fig 3A. n = 10 to 15. (C) PMA differentiated THP1 cells were infected with Rv and Rv∆glmU and either ATc (400 ng/ml) or the vehicle were added at 0 h. CFUs were enumerated at different time points post infection. The experiment was performed in triplicates and error bars represent s.e.m. (D) Guinea pigs were infected with 150–200 bacilli CFUs of Rv or Rv∆glmU and Dox was provided in the water as indicated. Guinea pigs (n = 2) were sacrificed on day 1 and homogenates from the lungs were plated in triplicates to determine the implantation. Guinea pigs (6 guinea pigs /group) were sacrificed four weeks post infection and CFUs were determined in the lung homogenates and results were plotted with log10 /lung on the Y-axis and samples on the X-axis. At four weeks post infection mean CFUs for Rv +Dox or Rv∆glmU +Dox and Rv∆glmU–Dox were 5.2, 0 and 5.11 on log10 scale.***p<0.0001, two tailed non parametric t-test, error bars represent s.e.m. (E) Overall pathology (upper panel) and histopathology (lower panel, HE, 40X) of the infected guinea pig lungs four weeks post infection. Both Rv +Dox and Rv∆glmU -Dox were having prominent granuloma and necrotic lesions in the central and epithelioid cells and lymphocytes around it. Guinea pigs infected with Rv∆glmU +Dox were showing normal lung parenchyma with clear alveolar spaces. G = Granuloma, AS = Alveolar Space. (F) Granuloma scores from histopathology results. Depletion of GlmUMtb from infected lungs leads to clearance of pathogen
It was apparent from the data presented above that the addition of ATc or Dox at the time of inoculation or at the time of infection does not allow mycobacterial cell growth or survival in the host. In the ideal candidate for therapeutic intervention, inhibiting the activity of/ depleting the enzyme at any stage of the infection should result in pathogen clearance. We assessed this parameter of GlmUMtb by providing ATc at different stages of bacterial growth (early, log and stationary phases) and investigating its influence on cell survival in liquid cultures. Addition of ATc to Rv∆glmU cultures on the 2nd, 4th or 6th day after inoculation significantly thwarted growth (Fig 6A). A similar analysis of bacterial growth by serial dilution of cultures followed by spotting on solid medium also revealed that viability was compromised by ~2 log fold 48 h after the addition of ATc, indicating that GlmUMtb depletion negatively impacted cell survival regardless of which stage of cell growth it was depleted at (S3A Fig).
Fig. 6. Depletion of GlmUMtb from infected lungs leads to clearance of pathogen. 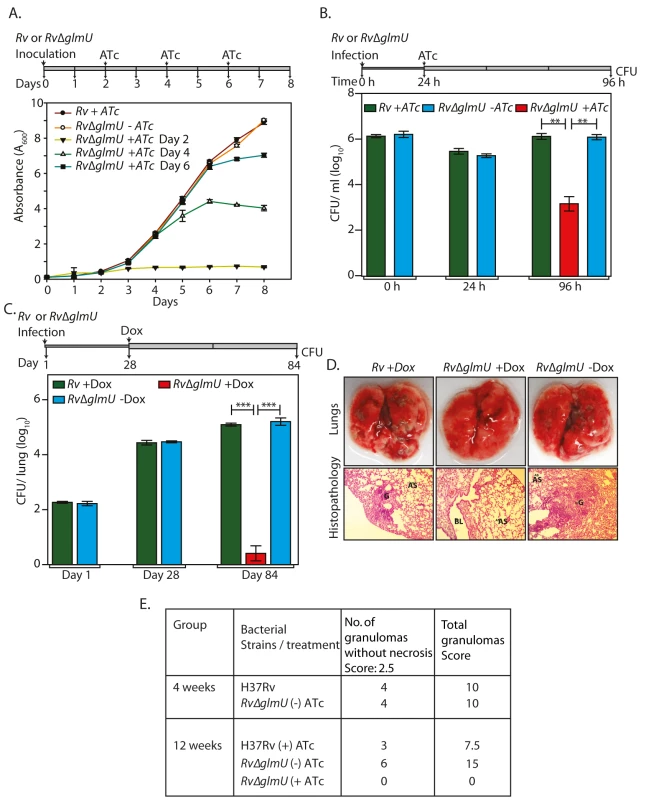
(A) Rv and Rv∆glmU cultures were inoculated at an initial A600 of 0.1 and the growth was monitored every day for eight days. ATc was added to the Rv culture on day 2 and Rv∆glmU cultures were either grown in the absence of ATc or was supplemented with ATc in the growth media on 2nd, 4th or 6th day. Experiment was performed in triplicates and the error bars represent s.e.m. (B) Differentiated THP-1 cells were infected Rv or Rv∆glmU cultures and the infection was allowed to be established for 24 h. 24 h post infection ATc was supplemented in the media for Rv and Rv∆glmU infected cultures. As a control THP1 cells infected with Rv∆glmU were grown without ATc. CFUs were enumerated at 0, 24 and 96 h post infection. **p<0.005, two tailed non parametric t-test, Experiment was performed in triplicates and the error bars represent s.e.m. (C) BALB/c mice (6 to 9 mice / group) were infected with Rv and Rv∆glmU strains and the infection was established for next 28 days. Subsequently, Dox was provided (with 5% dextrose on every alternate day) in the drinking water for Rv infected mice. One group of Rv∆glmU infected mice were administered with Dox and other with vehicle control for the next 56 days. CFUs were enumerated on day 1, day 28 and day 84 post infection. At 28th days post infection mean CFUs for Rv and Rv∆glmU infected mice were 4.43 and 4.47 on log10 scale and 84th days post infection mean CFUs for Rv +Dox or Rv∆glmU +Dox and Rv∆glmU–Dox were 5.1, 0.42 and 5.2 on log10 scale. ***p<0.0005, two tailed non parametric t-test, mean, error bars represent s.e.m.. (D) Overall pathology and histopathology of infected BALB/c mice lungs. Infected lungs dissected on 84th day after infection from Rv (+Dox) and Rv∆glmU (-Dox) infected mice shows clear lesions (upper panel) and granuloma with lymphocytes and foamy histiocytes (lower panel, HE stain, 100x) while Rv∆glmU (+Dox) was showing normal lung parenchyma and no granuloma. G = Granuloma, BL = Bronchial Lumen, AS = Alveolar Space. (E) Granuloma scores from 4 and 12 weeks of histopathology results. The influence of GlmUMtb depletion on an established ex vivo infection was estimated by providing ATc 24 h post-infection in a THP-1 infection model. As expected the bacillary load in THP-1 cells infected with Rv and Rv∆glmU were similar at 0 and 24 h after infection (Fig 6B). In contrast, while at 96 h post-infection the bacillary load for Rv and Rv∆glmU- infected THP-1 cells remained the same, the addition of ATc to Rv∆glmU- infected THP-1 cells 24 h after infection decreased the pathogen load by ~2.5 log fold, indicating that the reduction of GlmUMtb levels impacts pathogen survival even in an established ex vivo infection (Fig 6B). We extended this investigation to analyze the effect of GlmUMtb depletion from a fully-infected lung using murine infection model. As anticipated, the bacillary load in the lungs of mice infected with Rv and Rv∆glmU were comparable both on Day 1 and on Day 28. Administration of Dox to Rv∆glmU infected mice for the next 56 days (Day 28 to Day 84) drastically decreased the CFUs in the lungs (Fig 6C) and the pathogen was completely cleared from the spleen (S3B Fig). Unlike the lungs of mice infected with Rv and Rv∆glmU, mice infected with Rv∆glmU to whom Dox was administered displayed a total absence of lesions and granulomas in the lungs (Fig 6D and 6E). Collectively, these data suggest a fundamental role for UDP-GlcNAc, the end product of the GlmUMtb -mediated enzymatic reaction, in modulating the persistence of Mtb infection.
Oxa33: A novel allosteric GlmUMtb inhibitor
In addition to the acetyltransferase and uridyltransferase active site pockets, GlmUMtb also contains an allosteric site. Binding of any suitable molecule/inhibitor to the allosteric site would prevent the conformational change essential for GlmUMtb uridyltransferase catalytic activity. To target the allosteric site on GlmUMtb we drew on crystal structure data of H. influenza GlmU (GlmUHI) bound to its allosteric small molecule inhibitor (S4A and S4B Fig) [27]. Alignment of the GlmUMtb and GlmUHI allosteric pocket residues suggested that the interacting residues were conserved between the two proteins (S4C and S4D Fig). The Asinex database was screened against shape as described (S5A Fig) and the resulting 43 hits were biochemically characterized for their ability to inhibit GlmUMtb uridyltransferase activity. One of the promising molecules was used for further structural optimization (S5A Fig). Of the 53 structurally optimized compounds one molecule, namely (4Z)-4-(4-benzyloxybenzylidene)-2-(naphthalen-2-yl)-1,3-oxazol-5(4H)-one (Oxa33; Synthesis scheme provided in Figs 7A and S5B), was found to be an efficient inhibitor of GlmUMtb activity with an IC50 of 9.96±1.1 μM (Fig 7B). Isothermal titration analysis suggested an adequately high affinity binding for the compound (Ka = 2.35×106 M-1), with a binding stoichiometry of 0.7 (S6A Fig). We sought to identify the residues in GlmUMtb that are critical for interacting with Oxa33. Docking and MD simulation studies revealed polar, non-polar and hydrophobic interactions between Oxa33 and the allosteric site residues (Figs 7C, S6B and S6C). Based on the obtained data a panel of GlmUMtb proteins each carrying a single mutation was created, the mutant proteins were purified (S6D Fig), and their uridyltransferase activity assayed. While all the mutants had similar levels of uridyltransferase activity there was a substantial increase in their IC50 values, suggesting a loss of interaction with Oxa33 (Fig 7D and 7E).
Fig. 7. Oxa33, a novel inhibitor against GlmUMtb binds at allosteric site of uridyltransferase domain. 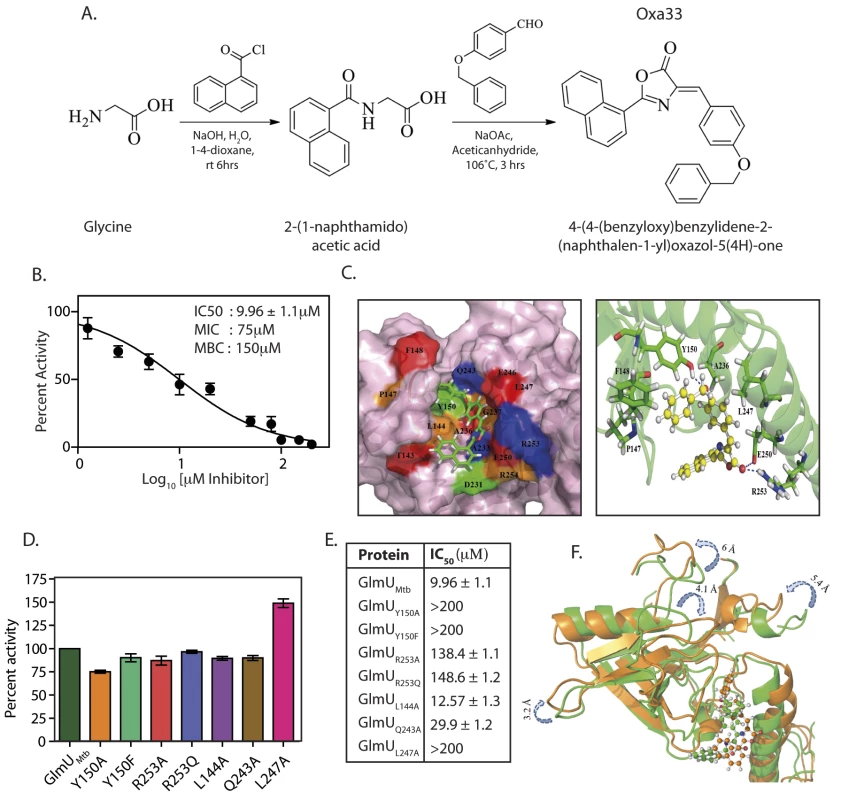
(A) Synthetic scheme for the preparation of 4-(4-(benzyloxy)benzylidene)-2-(naphthalen-1-yl)oxazol-5(4H)-one (Oxa33). (B) IC50 values were determined by varying the concentration of Oxa33 and calculating the percent activity with respect to the uridyltransferase activity of 0.75 pM conc GlmUMtb in the presence of 5% DMSO (vehicle). IC50 value was calculated by plotting percentage activities against different log10 values of inhibitor concentrations (μM). Experiment was performed in triplicates and the error bars represent s.e.m. (C) Left panel depicts docking surface representation of the allosteric site on the of the GlmUMtb allosteric site in complex with Oxa33. Right panel represents ribbon cartoon showing the amino acids residues of allosteric site and hinge region interacting with Oxa33 (in ball and stick model). Tyr150, Glu250 and Arg 253 were found to be in hydrogen bonding with carbonyl oxygen over the oxazole ring. While Leu144, Pro147, Phe148, Tyr150, Ala233, Ala236 and Leu247 participate in strong hydrophobic interactions with Oxa33. (D) Based on the data above GlmUMtb allosteric site mutants were created, proteins were purified and the uridyltransferase activity of GlmUMtb mutants was determined. Wild type and mutant GlmUMtb proteins show almost comparable activity. (E) IC50 values in μM were determined as described above for the wild type and the mutant GlmUMtb proteins. Tables shows the values obtained for wild type and mutant enzymes. Experiments were performed in triplicates and the error bars represent s.e.m. (F) Superimposed view of GlmUMtb-Oxa33 complex before (green) and after (orange) the simulation time period. Showing a partial closure of uridyltransferase site (decreased volume) thereby making it unavailable for its substrates for catalysis. To decipher the mechanism of Oxa33 mediated inhibition of uridyltransferase activity, we superimposed the GlmUMtb-Oxa33 complex with the unbound GlmUMtb structure. Upon Oxa33 binding, the loop regions (in the range of 3–6 Å) at the uridyltransferase active site undergo significant conformational changes, decreasing the active site volume, which results in occlusion of the substrates (Fig 7F). Differential scanning fluorimetry (DSF) analysis of GlmUMtb in the presence of Oxa33 showed a 3°C shift in protein melting temperature (Tm) validating the conformational changes (S5E Fig). Interestingly we also observed much higher relative fluorescence units (~10000 vs 2500) in the presence of Oxa33, which is likely due to the compound induced structural changes facilitating increased binding of the dye (S6E Fig). Together, these data demonstrate that Oxa33 binds to the allosteric site at N-terminal domain of GlmUMtb and inhibits its uridyltransferase activity by causing structural changes.
Specificity and efficacy of Oxa33
Subsequently we investigated the ability of Oxa33 to inhibit the in vitro growth of Mtb H37Rv. Oxa33 inhibited the in vitro growth of Mtb H37Rv with a minimum inhibitory concentration (MIC) of ~75 μM (~30 μg / ml) and a maxium bacteriocidal concentration (MBC) of ~150 μM (~60 μg / ml). To ascertain if this inhibitory effect was due to inhibition of GlmUMtb activity we overexpressed GlmUMtb in the cells prior to drug treatment and determined the effect of this on the MIC value (Figs 8A and S6A). Whereas the inhibition of growth in the presence of INH was similar with or without GlmUMtb overexpression in the cells (Fig 8A, lower panel), Oxa33 failed to inhibit cell growth even at concentrations as high as 150 μM (60 μg/ ml) (Fig 8A, upper panel). Interestingly when sub lethal concentration of Oxa33 was provided, the MIC of INH decreased from 32 to 16 ng/ml (S7B Fig). The impact of Oxa33 on THP1 cells 24 h after infection with either Rv or Rv::glmUtet-on was also investigated. In concurrence with the in vitro growth data, overexpression of GlmUMtb alleviated Oxa33-mediated clearance of Mtb from THP-1 cells (Figs 8B, S6D and S6E). These results suggest that the inhibition of mycobacterial growth by Oxa33 is specifically due to inhibition of endogenous GlmUMtb.
Fig. 8. Specificity and efficacy of Oxa33. 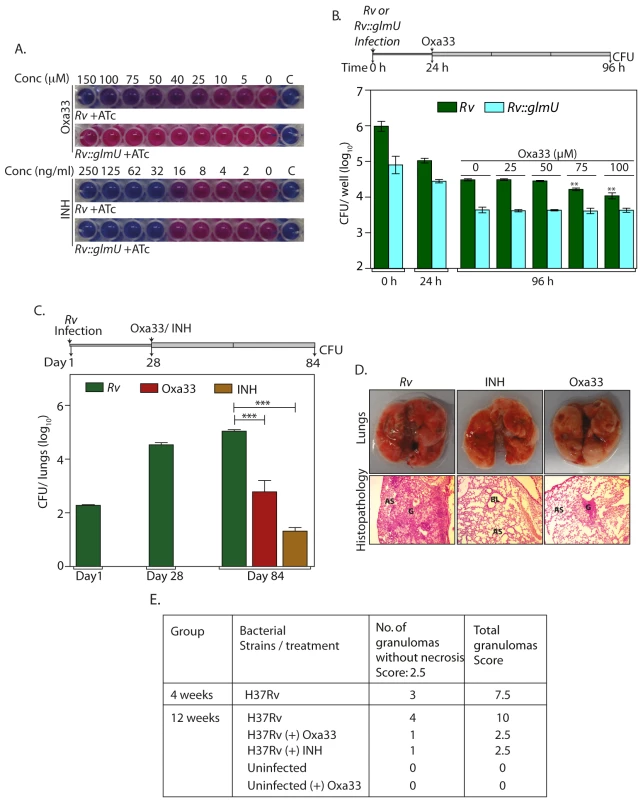
(A) Rv and Rv::glmUtet-on cultures were grown in presence of 1 μg/ml ATc and different concentrations of Oxa33 or INH (as indicated). 0.02% resazurin dye was added on day 7. Pink colour indicates viable bacteria while blue colour indicates dead bacteria. ‘C’ denotes culture media control. The experiment was performed thrice. (B) Differentiated THP1 were infected either with Rv or Rv::glmUtet-on and grown in RPMI media containing 400 ng/ml ATc. 24 h post infection different concentrations (as indicated) of Oxa33 or vehicle control was supplemented to the media for the next 72 h followed by CFU enumeration. Experiment was performed in triplicates and the error bars represent s.e.m. (C) BALB/c mice (7 to 11 mice / group) were infected with Rv and the infection was allowed to be established for 28 day. Oxa33 (50 mg/kg, intraperitoneal, in 2.5% Tween 80) or INH (25 mg/kg, in drinking water with 5% sucrose) were administered to the mice for a subsequent period of 56 days. CFU in the lungs of mice were determined on 1, 28 and 84 days post infection. Day 1st and day 28th CFUs show successful implantation and establishment of infection, respectively. 56 days of Oxa33 or INH treatment partially reduces the mean bacillary load to 2.78 and 1.3 on log10 scale. ***p<0.0005 or 0.0006, two tailed non parametric t-test, error bars represent s.e.m. (D) While the overall pathology of lungs (upper panel) illustrates robust Mtb infection in untreated mice, relatively smaller and fewer lesions and granulomas were observed in Oxa33 or INH treated mice. Lower panel exhibit hematoxylin and eosin stained photomicrograph of lungs from Rv, INH treated and Oxa33 treated mice at 100x magnification. This histopathological analysis shows large or small granuloma with lymphocytes and foamy histiocytes in Rv infected mice or Oxa treated mice respectively G = Granuloma, BL = Bronchial Lumen, AS = Alveolar space. (E) Granuloma scores from lungs of infected and treated mice for 4 weeks and 12 weeks. Finally, we analysed the efficacy of Oxa33 in clearing bacilli from infected lungs using a murine infection model. Oxa33 compound is highly hydrophobic in nature. After trying many solvents, we could successfully resuspend it in 2.5% Tween-80. Prior to performing the experiments we examined the maximum dose tolerance and survival analysis to determine the toxicity (S8A and S8B Fig). Based on the data obtained we chose 50 mg / kg as the appropriate dose. Since it was difficult to predict the fate of Oxa33 during the process of digestion, we avoided using the oral administration route. We chose intra peritoneal route for administering the compound as the intravenous (I/V) injection of Tween 80 (solvent) in the animals was known to cause hypersensitivity and anaphylactic shock [45,46].
Groups of mice were infected with Rv and were treated with vehicle, INH, or Oxa33 at 28 days post-infection, for a duration of 56 days (Fig 8C, line diagram). Compared with the vehicle-treated group where we observed a marginal increase in bacillary load, a significant reduction in the bacillary load was observed in the lungs and spleen of both, INH - and Oxa33-treated groups (~4 and 2.5 log fold, respectively for lungs) (Figs 8C and S9A). This was also reflected in the gross pathology and histopathology of lungs (Fig 8D and 8E). Although in vitro MBCvalues of Oxa33 was ~150 μM (60 μg/ml), it seems to be a relatively more efficacious in vivo, which could be due to its accumulation in the lungs of the infected mice. To investigate this possibility uninfected mice were treated with 50 mg/kg Oxa33 for a period of 3 weeks or 8 weeks. In order to estimate the concentration of Oxa33 in the lung, we first determined the absorbance spectra for Oxa33, which gave a clear peak at 401 nm (S10A Fig). We determined the A401 at different concentrations of Oxa33 and the standard curve was plotted (S10B and S10C Fig). Oxa33 was extracted from the lungs and its concentration was determined. The concentrations of Oxa33 in the lungs were in the range of ~200–300 μg /lung at 3 weeks and ~800–1300 μg/lung at 8 weeks (S10D Fig). The accumulation of Oxa33 in the lungs is ~13 to 18 fold higher than the MBC values, which may be the reason for higher potency of Oxa33 in vivo compared with the in vitro experiments. Taken together, the results presented in this study establish GlmUMtb to be an effective target against which new sets of inhibitors may be developed.
Discussion
Cell wall provides the structural rigidity and protects bacteria from various environmental and physiological insults. Biosynthesis of the cell wall of bacteria is a complex process requiring enzymes localized to different cellular compartments [47]. Due to the essentiality of the enzymes involved they are considered attractive targets for anti-microbial therapies. The majority of the first line and second line anti-tuberculosis drugs from the existing regimen target enzymes involved in cell wall synthesis [5]. These include Isoniazid and Ethionamide targeting enoyl-[acyl-carrier-protein] reductase and inhibiting mycolic acid synthesis, Ethambutol targeting arabinosyl transferase and inhibiting arabinogalactan biosynthesis, and Cycloserine targeting D-alanine racemase and ligase, which inhibits peptidoglycan synthesis [5]. However most of these drugs are not very effective against dormant/ latent Mtb [48].
UDP-GlcNAc is a critical metabolite both in prokaryotes and eukaryotes. In eukaryotes it is mainly utilized for O- or N- GlcNAcylation, sialic acid biosynthesis and hylauronic acid biosynthesis [49–51]. In addition to the peptidoglycan synthesis [10], in gram negative bacteria UDP-GlcNAc is required for the synthesis Lipid A subunit of lipopolysaccharide [52] and in gram positive bacteria it is required for Rha-GlcNAc linker [53], arabinogalactan [54], teichioc acid synthesis [55]. In few prokaryotes, UDP-GlcNAc has also been shown to be required for sialic acid [56], N-GlcNAcylation [57] and poly (-GlcNAc-)n [58]. GlmUMtb, an enzyme with dual activity, synthesizes a core metabolite necessary for the synthesis of cell wall peptidoglycan, UDP-GlcNAc [6]. Interestingly, we found that depleting GlmUMtb during both normoxic and hypoxic growth resulted in substantial decrease in cell viability (Fig 3). This may be due to the requirement of UDP-GlcNAc, which in addition to participating in cell wall synthesis is also required for other cellular processes such as mycothiol biosynthesis (to maintain redox homeostasis) [14,59]. However, the TEM data clearly shows decreased cell envelop thickness even in hypoxic conditions (Fig 3). Although the CFUs do not change significantly the cells may be undergoing significant replication, which might be balanced by death [60]. Alternatively, new cell envelop may be required even if the bacteria are not replicating. Thus one can rule out the possibility that decreased viability may well be due to requirement of UDP-GlcNAc for the cell envelop synthesis.
While UDP-GlcNAc is a critical metabolite for both prokaryotes and eukaryotes, the enzymes involved in its de novo synthesis are significantly different [10]. In addition, both prokaryotes and eukaryotes can utilize GlcNAc from different sources to synthesize UDP-GlcNAc through salvage pathways [61–63] (S11 Fig). Capnocytophaga canimorsus, a member bacteria from Bacteroidetes phylum lacks endogenous GlmM and GlmU required for the synthesis of GlcNAc and it instead relies on GlcNAc obtained from forages glycans from the host mucin and N-linked glycoproteins [42]. Depending on the enzymes of the salvage pathway present in the bacterial system, it would require either both the activities or only the uridyltransferase activity of GlmUMtb for UDP-GlcNAc synthesis. Till date the presence of alternate salvage pathway in Mtb has not been demonstrated. However, even with an operating salvage pathway GlmUMtb is essential for the utilization of host GlcNAc to form UDP-GlcNAc (S11 Fig). In line with this, we find that depletion of GlmUMtb during ex vivo or in vivo infection either at the start or after infection has been definitively established leads to clearance of pathogen.
GlmUMtb and the acetyltransferase and uridyltransferase enzymes found in eukaryotes share very little sequence similarity. Although efforts have been made by different groups to target bacterial GlmU proteins, the specificity of these inhibitors for GlmU in vivo have not been established [23–30]. Most GlmU inhibitors characterized till date target either the acetyl - or uridyltransferase active sites. In contrast, inhibitors of GlmUHI target the allosteric site near the uridyltransferase active site [27]. The interaction of the inhibitor with the enzyme via this allosteric site perturbs the active site conformation of the protein, thus inhibiting uridyltransferase activity [27]. In the present study we have used shape based designing and developed a novel oxazolidine molecule, Oxa33, and characterized its ability to bind to the GlmUMtb allosteric site. MD simulation and mutation of critical interacting residues to defined the possible allosteric site residues required for Oxa33 binding (Fig 7). DSF (S6E Fig) and structural superimposition (Fig 7) supports that inhibition of uridyltransferase activity is due to structural changes in the N-terminal domain of GlmUMtb. Further in order to determine the specificity of Oxa33, GlmUMtb over expressing strains of Rv was used to determine the MIC. Both in vitro and ex vivo results (increased MIC or MBC) validate that Oxa33 specifically binds to GlmUMtb inside the bacteria. Administrating the Oxa33 to fully infected (28 days) mice resulted in partial ablation of pathogen load in the lungs. Taken together results presented here demonstrates that GlmUMtb is a viable and promising target for therapeutic intervention and Oxa33 can be pursued as a lead molecule, which needs to be developed further to improve its efficacy.
Materials and Methods
Chemicals and reagents
Restriction enzymes and Phu DNA polymerase were purchased from New England Biolabs. pENTR/directional TOPO cloning kit (Invitrogen), pQE2 (Qiagen), were procured from the respective sources. Analytical grade chemicals and oligonucleotide primers were procured from Sigma. Malachite green phosphate assay kit (POMG-25H) was purchased from BioAssay System (Gentaur). Electron microscopy reagents were purchased from Electron Microscopy Sciences. Media components were purchased from BD Biosciences. Doxycycline hydrochloride was purchased from Biochem pharmaceutical.
Generation of glmU conditional gene mutant in M. tuberculosis
The hexa-His tag in the pST-KiT construct[15] was replaced with an N-terminal FLAG tag, and the tetracycline repressor gene (tetR) was replaced with a reverse tetR (r-tetR) from pTC28S15-OX [64] to create plasmid pST-KirT. To generate the integrating shuttle plasmid pST-KirT-glmUMtb, the glmUMtb gene was excised from pQE2-glmUMtb using NdeI-HindIII digestion and was subcloned into the corresponding sites on pST-KirT. The resulting pST-KirT-glmU construct expresses GlmUMtb in the absence of inducer ATc. Upon addition of ATc, ATc binds to the r-TetR repressor resulting in the conformational change that would allow it to bind to the operator seqeunces in PmyctetO (S1 Fig) [64]. The integration-proficient plasmid containing the inducible glmUMtb gene was electroporated into mycobacterial cells to create the merodiploid strain Rv::glmUMtb. 5’ and 3’ genomic flank sequences of glmUMtb (approximately 1 kb on either side) were amplified, the amplicons digested with PflMI, and ligated with the antibiotic resistance cassette along with the oriE and cosλ fragments generated from pYUB1474 construct, to generate the allelic exchange substrate (AES) [65]. The AES was linearized using the unique PacI site and then cloned into temperature sensitive shuttle phagemid phAE159 at the PacI site. A conditional gene replacement mutant of RvΔglmU was created from the merodiploids with the help of specialized transduction methodology (S1A Fig) [66]. RvΔglmU recombinants obtained were analyzed by PCR amplification to verify the fidelity of the recombination event.
Analysis of growth patterns
H37Rv (Rv) and RvΔglmU cultures were grown in Middlebrook 7H9 medium supplemented with 10% ADC (albumin, dextrose and catalase), or in 7H11 medium supplemented with 10% OADC (oleic acid, ADC). To analyze bacterial growth in vitro, Rv and Rv∆glmU mutant bacterial cultures were inoculated at A600 of 0.1, in the presence or absence of anhydrotetracycline (ATc), and A600 was measured every 24 h for 6 or 8 days. For spotting analysis, cells were harvested by centrifugation, washed twice with PBST (0.05% Tween 80) to remove ATc, resuspended in 7H9 medium, and serially diluted in the same medium, followed by spotting 10 μl aliquots of the various cell dilutions on 7H11 agar plates to assess cell viability. To determine the impact of GlmUMtb depletion during hypoxia in Rv and RvΔglmU strains, we established hypoxia in 1.5 ml HPLC tubes or 500 ml flasks with penetrable caps, following modified Wayne model [35]. ATc (2 μg/ml) or isoniazid (INH) (50 ng/ml) were injected into the cultures at different time points and the number of CFUs were determined after 42 days. Scanning and transmission electron microscopy (SEM & TEM) analysis of Rv and Rv∆glmU mutant grown in the presence or absence of ATc were performed as described earlier [67]. Transmission electron microscopy was performed using standard protocols. Briefly, bacteria was fixed in 2.5% gluteraldehyde and 4% paraformaldehyde, dehydrated in graded series of alcohol and embedded in Epon 812 resin. Ultrathin sections were cut and stained with uranyl acetate and lead citrate [68]. SEM images were procured using Carl Zeiss Evo LS scanning electron microscope, and TEM images were captured using Tecnai G2 20 twin (FEI) transmission electron microscope.
Generation of glmUMtb mutant constructs and western blotting analysis
Site directed mutations of glmUMtb were generated with the help of overlapping PCR and the amplicons were cloned into NdeI-HindIII sites of pQE-2, pNit and pST-KT vectors [15,69]. The tetracylin repressor (TetR) expressed from the plasmids binds to the operator sequence in the promoter PmyctetO in the absence of ATc (S1B Fig) [70]. Addition of ATc to TetR alleviates the repression thus inducing the expression of GlmU. pST-KT-glmU was electroporated into Rv to generate Rv::glmUtet-on strain. pNit-glmU (wild type and mutated) constructs were electroporated into Rv∆glmU to generate Rv∆glmU::glmUwt/mutant strains. Rv and Rv∆glmU::glmUwt/mutant strains were grown in the presence or absence of ATc as described above. GlmUMtb was expressed and purified using plasmid pQE2-GlmUMtb, as described earlier [15]. Whole cell lysates (WCL) isolated from Rv, Rv∆glmU and Rv∆glmU::glmUwt/mutant strains that had been grown for 5 days in presence or absence of Atc, were resolved on 10% SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti-GlmUMtb and anti-GroEL1 antibodies as described earlier [15,67].
Ex vivo and in vivo infections
THP1 infection experiments were carried out with either unlabelled or FITC-labelled Rv and Rv∆glmU strains at 1 : 10 MOI, as described earlier [71]. For examination of cells under a fluorescence microscope, infected cells (48 h post-infection) were labelled with Lyso Tracker red DND 99 dye (50 nM) and mounted with Antifade (Invitrogen) mounting agent. To determine CFUs per infected cell, the infected cells were lysed in PBS containing 0.1% TritonX-100 for 15 min and different dilutions were plated on OADC-containing 7H11 agar plates. For animal infection experiments, Rv and Rv∆glmU strains grown till A600 of 0.6 were used to infect 3 to 4 week old guinea pigs or ~ 2 month old mice as described previously [72,73]. We initially used guinea pig model system as it has robust immune response. However, for the remaining experiments we chose to use Balb/C mice model of infection as the cost associated with performing the experiments and the amount of Oxa33 required for guinea pig experiments was prohibitive. To determine the implantation dosage, the bacillary load in the lungs of guinea pigs or mice was determined 24 h post-infection. To investigate the impact of glmUMtb depletion on survival of the pathogen, doxycycline hydrochloride (Dox, 1 mg/ kg with 5% dextrose in drinking water) was provided to Rv and Rv∆glmU-infected animals as indicated, either from the time of the infection (guinea pig experiment), or 4 weeks post-infection (mice infection experiments). To assess the impact of INH or Oxa33 treatment on pathogen survival, Rv-infected mice (4 weeks post-infection) were supplied with INH (25 mg/ kg body weight, with 5% dextrose in drinking water) or Oxa33 (50 mg/ kg body weight, with 2.5% Tween 80, injected intra peritoneally) every third day for 8 weeks. Bacillary loads in the lungs and spleens of infected guinea pigs and mice were determined 4 weeks and 12 weeks post-infection. Histopathological evaluation of the harvested organs was performed as described earlier [67,72,73].
Shape based screening and molecular docking studies
ROCS (Rapid Overlay of Chemical Structures), a shape based technique for rapid similarity analysis was used to assess the compounds. Gaussians and shape tanimoto were used to assess the volume and shape overlaps of the compounds, respectively. As the chemical functionality is critical, the chemical feature based similarity was also considered using ROCS colour score whose force field was composed of SMARTS patterns of the chemical functions [74,75]. The shape tanimoto score and scaled color score were considered during the selection of the compounds for further virtual screening. The compounds selected were subjected to molecular docking studies using Glide v5.8 of Schrödinger molecular modelling suite 2012 (Glide v5.8, Schrödinger). The compounds were subjected to a series of docking protocols–high throughput virtual screening (HTVS), standard precision (SP) and extra precision (XP) docking. As the docking progresses from HTVS to XP, the algorithm differs, which starts from a simple docking of compounds and ends with docking protocol with high precision and parameterization while cutting off the number of compounds.
Synthesis of 4-(4-(benzyloxy)benzylidene)-2-(naphthalen-1-yl)oxazol-5(4H)-one
To the glycine solution (3.0 g, 39.89 mmol) in water under constant stirring at 0°C, NaOH (3.19 g, 79.78 mmol) was added. This was followed few minutes later by the addition of 1-naphthoyl chloride (7.20 mL, 47.86 mmol) in 1, 4-Dioxane (20 ml) and the contents were stirred at room temperature for 6 h. The reaction mixture was concentrated to half the volume and 60 ml EtOAc was added. The EtOAc layer was washed with sat NaHCO3 (2 × 30 mL) followed by H2O (2 × 20 mL). The separated organic layer was dried and concentrated over anhydro Na2SO4 to obtain solid compound, which was washed with hexanes to get 2-(1-naphthamido) acetic acid (8.30 g, 90%) as a white solid (M.P. 152°C1). 2-(1-naphthamido)acetic acid (2.0 g, 8.73 mmol), NaOAc (0.21 g, 2.62 mmol) and 4-benzyloxybenzaldehyde (1.85 g, 8.73 mmol) were taken in acetic anhydride and heated at 106°C for 3 h. The solid formed were filtered and washed with water to remove traces of acetic anhydride, and ethanol to remove unreacted aldehyde and other organic impurities. Final compound 4-(4-(benzyloxy)benzylidene)-2-(naphthalen-1-yl)oxazol-5(4H)-one (Oxa33; 3.14 g, 88%), purified as a yellow solid, was confirmed with nuclear magnetic resonance (NMR) [76].
Isothermal titration calorimetry
To investigate the binding of Oxa33 to GlmUMtb, we performed Isothermal Titration Calorimetry (MicroCal 2000 VP-ITC, GE Healthcare) [28]. Oxa33 was re-suspended in dialysis buffer (25 mM Tris pH 7.4, NaCl 140 mM and 15% glycerol), 100 μM of MgCl2 containing 2% DMSO. 625 μM of Oxa33 was injected for titrations from syringe (rotating at 307 rpm) into ITC cell containing 25 μM of GlmU or blank buffer at 25°C. Each injection lasted for 20 sec with 300 sec interval between every step. The quantity of heat associated by every injection was calculated by combining the area beneath every heat burst curve (microcalories/second vs. seconds). Data was corrected for the buffer signal and fitting was done by one-site binding model. Origin software (version 7.0) was used to obtain different thermodynamic binding parameters.
In vitro cytotoxicity
Oxa33 was evaluated for its cytotoxic activity in THP1 cells with the help of alamar blue assay. Serially diluted inhibitors (in 2.5% DMSO) incubated with 5 x 103 differentiated THP-1 cells in 96 well plates for 3 days. After 3 days cells were incubated for 5 h with 10 μl of alamar blue and color development was measured using micro-plate reader at 570 nm.
Docking and molecular dynamics simulations studies of GlmUMtb with Oxa33
Molecular dynamics (MD) simulation for the protein-ligand complex was carried out for a time scale of 20 ns so as to analyze the stability of molecular interactions between ligand and protein employing Newton’s Laws of Motions. Desmond molecular dynamics system v3.1 was used for carrying out the simulations employing OPLS-AA force field [77]. The protein-ligand complex was solvated using TIP3P water model which was setup as an orthorhombic solvent box, keeping a cut-off of 10 Å from any solute atom in all directions [78]. Na+ counter ions were added in order to neutralize the system. A cut-off of 14 Å was maintained for calculating the solvent-solvent and solute-solvent non-bonded interactions. Initially, the system was minimized keeping the convergence threshold criteria of 1.0 kcal.mol-1.Å-1 so as to allow the adjustment of atoms to the system environment. A simulation for each system was performed using isothermal-isobaric ensemble (NPT) including a relaxation process. Under NPT, the system was simulated for 12 ps using a Berendsen thermostat and a Berendsen barostat with temperature of 10K and a pressure of 1 atm. The later step of relaxation protocol included the simulation of the system for 24 ps with a temperature of 300 K and 1 atm pressure with and without restraints on solute heavy atoms. M-SHAKE algorithm was used with an integration time step of 2 fs for rearranging the hydrogen bonds in the simulation [79]. The temperature and pressure of the system were maintained at 300 K and 1.013 atm respectively. The molecular dynamics simulation was run for 20 ns recording the trajectory frames at an interval of every 4.8 ps and the trajectory analysis was carried out using the Simulation Event Analysis of Desmond.
Determination of percentage inhibition, IC50 and MIC
Uridyltranferase assays were performed using malachite green phosphate detection kit as described previously [17]. Acetyltransferase activity of GlmUMtb was carried out in the presence of 500 μM each of GlcN-1-P and acetyl-CoA in a 30 μl reaction volume for 30 min at 30°C as described earlier [80]. To determine the percent inhibition by different compounds the enzyme was preincubated with either 5% DMSO or 100 μM compounds for 30 min prior to performing uridyltransferase activity assays. In order to determine the IC50 values, GlmUwt/mutant proteins were preincubated with different concentrations of Oxa33 compound for 30 min followed by the uridyltransferase assay. To determine minimum inhibitory concentration (MIC), 5x105 bacteria of Rv or Rv::glmUtet-on (overexpressing GlmUMtb) cultures (grown in the presence or absence of 2 μg/ml ATc) were mixed with 100 μl of 2.5% DMSO or different concentrations of Oxa33/ INH in 96-well plates, and incubated at 37°C for 6 days. After 6 days, 40 μl of resazurin dye (0.02% in 5% Tween-80) was added to each well and the colour change was observed after 12 h.
Ethical statement
Experimental protocol for the animal experiments was approved by the Institutional Animal Ethics Committee of National Institute of Immunology, New Delhi, India (the approval number is IAEC# 315/13). The approval is as per the guidelines issued by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Govt. of India.
Statistical analysis
Student’s t-test (two tailed non parametric) was used to analyze the significance of cell wall thickness, THP1 and animal infection experimental results. SigmaPlot version 10.0 and GraphPad Prism version 5.0 was used for the statistical analysis and for plotting the results.
Supporting Information
Zdroje
1. Smith I (2003) Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev 16 : 463–496. 12857778
2. McNeil MR, Brennan PJ (1991) Structure, function and biogenesis of the cell envelope of mycobacteria in relation to bacterial physiology, pathogenesis and drug resistance; some thoughts and possibilities arising from recent structural information. Res Microbiol 142 : 451–463. 1871433
3. Brennan PJ, Nikaido H (1995) The envelope of mycobacteria. Annu Rev Biochem 64 : 29–63. 7574484
4. Lee A, Wu SW, Scherman MS, Torrelles JB, Chatterjee D, et al. (2006) Sequencing of oligoarabinosyl units released from mycobacterial arabinogalactan by endogenous arabinanase: identification of distinctive and novel structural motifs. Biochemistry 45 : 15817–15828. 17176104
5. Zumla A, Nahid P, Cole ST (2013) Advances in the development of new tuberculosis drugs and treatment regimens. Nat Rev Drug Discov 12 : 388–404. doi: 10.1038/nrd4001 23629506
6. Mengin-Lecreulx D, van Heijenoort J (1994) Copurification of glucosamine-1-phosphate acetyltransferase and N-acetylglucosamine-1-phosphate uridyltransferase activities of Escherichia coli: characterization of the glmU gene product as a bifunctional enzyme catalyzing two subsequent steps in the pathway for UDP-N-acetylglucosamine synthesis. J Bacteriol 176 : 5788–5795. 8083170
7. Gehring AM, Lees WJ, Mindiola DJ, Walsh CT, Brown ED (1996) Acetyltransfer precedes uridylyltransfer in the formation of UDP-N-acetylglucosamine in separable active sites of the bifunctional GlmU protein of Escherichia coli. Biochemistry 35 : 579–585. 8555230
8. Crick DC, Mahapatra S, Brennan PJ (2001) Biosynthesis of the arabinogalactan-peptidoglycan complex of Mycobacterium tuberculosis. Glycobiology 11 : 107R–118R. 11555614
9. Alderwick LJ, Birch HL, Mishra AK, Eggeling L, Besra GS (2007) Structure, function and biosynthesis of the Mycobacterium tuberculosis cell wall: arabinogalactan and lipoarabinomannan assembly with a view to discovering new drug targets. Biochem Soc Trans 35 : 1325–1328. 17956343
10. Barreteau H, Kovac A, Boniface A, Sova M, Gobec S, et al. (2008) Cytoplasmic steps of peptidoglycan biosynthesis. Fems Microbiology Reviews 32 : 168–207. doi: 10.1111/j.1574-6976.2008.00104.x 18266853
11. Birch HL, Alderwick LJ, Bhatt A, Rittmann D, Krumbach K, et al. (2008) Biosynthesis of mycobacterial arabinogalactan: identification of a novel alpha(1—>3) arabinofuranosyltransferase. Mol Microbiol 69 : 1191–1206. doi: 10.1111/j.1365-2958.2008.06354.x 18627460
12. Mills JA, Motichka K, Jucker M, Wu HP, Uhlik BC, et al. (2004) Inactivation of the mycobacterial rhamnosyltransferase, which is needed for the formation of the arabinogalactan-peptidoglycan linker, leads to irreversible loss of viability. J Biol Chem 279 : 43540–43546. 15294902
13. Dover LG, Cerdeno-Tarraga AM, Pallen MJ, Parkhill J, Besra GS (2004) Comparative cell wall core biosynthesis in the mycolated pathogens, Mycobacterium tuberculosis and Corynebacterium diphtheriae. FEMS Microbiol Rev 28 : 225–250. 15109786
14. Vilcheze C, Av-Gay Y, Attarian R, Liu Z, Hazbon MH, et al. (2008) Mycothiol biosynthesis is essential for ethionamide susceptibility in Mycobacterium tuberculosis. Mol Microbiol 69 : 1316–1329. doi: 10.1111/j.1365-2958.2008.06365.x 18651841
15. Parikh A, Kumar D, Chawla Y, Kurthkoti K, Khan S, et al. (2013) Development of a new generation of vectors for gene expression, gene replacement, and protein-protein interaction studies in mycobacteria. Appl Environ Microbiol 79 : 1718–1729. doi: 10.1128/AEM.03695-12 23315736
16. Verma SK, Jaiswal M, Kumar N, Parikh A, Nandicoori VK, et al. (2009) Structure of N-acetylglucosamine-1-phosphate uridyltransferase (GlmU) from Mycobacterium tuberculosis in a cubic space group. Acta Crystallogr Sect F Struct Biol Cryst Commun 65 : 435–439. doi: 10.1107/S1744309109010252 19407371
17. Jagtap PK, Soni V, Vithani N, Jhingan GD, Bais VS, et al. (2012) Substrate-bound crystal structures reveal features unique to Mycobacterium tuberculosis N-acetyl-glucosamine 1-phosphate uridyltransferase and a catalytic mechanism for acetyl transfer. J Biol Chem 287 : 39524–39537. doi: 10.1074/jbc.M112.390765 22969087
18. Sassetti CM, Boyd DH, Rubin EJ (2003) Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol 48 : 77–84. 12657046
19. Zhang YJ, Ioerger TR, Huttenhower C, Long JE, Sassetti CM, et al. (2012) Global Assessment of Genomic Regions Required for Growth in Mycobacterium tuberculosis. PLoS Pathog 8: e1002946. doi: 10.1371/journal.ppat.1002946 23028335
20. Zhang W, Jones VC, Scherman MS, Mahapatra S, Crick D, et al. (2008) Expression, essentiality, and a microtiter plate assay for mycobacterial GlmU, the bifunctional glucosamine-1-phosphate acetyltransferase and N-acetylglucosamine-1-phosphate uridyltransferase. Int J Biochem Cell Biol 40 : 2560–2571. doi: 10.1016/j.biocel.2008.05.003 18573680
21. Jackson M, McNeil MR, Brennan PJ (2013) Progress in targeting cell envelope biogenesis in Mycobacterium tuberculosis. Future Microbiol 8 : 855–875. doi: 10.2217/fmb.13.52 23841633
22. Moraes GL, Gomes GC, Monteiro de Sousa PR, Alves CN, Govender T, et al. (2015) Structural and functional features of enzymes of Mycobacterium tuberculosis peptidoglycan biosynthesis as targets for drug development. Tuberculosis (Edinb) 95 : 95–111.
23. Urbaniak MD, Collie IT, Fang W, Aristotelous T, Eskilsson S, et al. (2013) A novel allosteric inhibitor of the uridine diphosphate N-acetylglucosamine pyrophosphorylase from Trypanosoma brucei. ACS Chem Biol 8 : 1981–1987. doi: 10.1021/cb400411x 23834437
24. Moir DT, Di M, Moore RA, Schweizer HP, Woods DE (2008) Cellular reporter screens for inhibitors of Burkholderia pseudomallei targets in Pseudomonas aeruginosa. Trans R Soc Trop Med Hyg 102 Suppl 1: S152–162. doi: 10.1016/S0035-9203(08)70033-6 19121678
25. Pereira MP, Blanchard JE, Murphy C, Roderick SL, Brown ED (2009) High-throughput screening identifies novel inhibitors of the acetyltransferase activity of Escherichia coli GlmU. Antimicrob Agents Chemother 53 : 2306–2311. doi: 10.1128/AAC.01572-08 19349513
26. Doig P, Boriack-Sjodin PA, Dumas J, Hu J, Itoh K, et al. (2014) Rational design of inhibitors of the bacterial cell wall synthetic enzyme GlmU using virtual screening and lead-hopping. Bioorg Med Chem 22 : 6256–6269. doi: 10.1016/j.bmc.2014.08.017 25262942
27. Mochalkin I, Lightle S, Narasimhan L, Bornemeier D, Melnick M, et al. (2008) Structure of a small-molecule inhibitor complexed with GlmU from Haemophilus influenzae reveals an allosteric binding site. Protein Sci 17 : 577–582. doi: 10.1110/ps.073271408 18218712
28. Buurman ET, Andrews B, Gao N, Hu J, Keating TA, et al. (2011) In vitro validation of acetyltransferase activity of GlmU as an antibacterial target in Haemophilus influenzae. J Biol Chem 286 : 40734–40742. doi: 10.1074/jbc.M111.274068 21984832
29. Larsen NA, Nash TJ, Morningstar M, Shapiro AB, Joubran C, et al. (2012) An aminoquinazoline inhibitor of the essential bacterial cell wall synthetic enzyme GlmU has a unique non-protein-kinase-like binding mode. Biochem J 446 : 405–413. doi: 10.1042/BJ20120596 22721802
30. Min J, Lin D, Zhang Q, Zhang J, Yu Z (2012) Structure-based virtual screening of novel inhibitors of the uridyltransferase activity of Xanthomonas oryzae pv. oryzae GlmU. Eur J Med Chem 53 : 150–158. doi: 10.1016/j.ejmech.2012.03.051 22521370
31. Singh VK, Das K, Seshadri K (2012) Kinetic modelling of GlmU reactions—prioritization of reaction for therapeutic application. PLoS One 7: e43969. doi: 10.1371/journal.pone.0043969 22952829
32. Li Y, Zhou Y, Ma Y, Li X (2011) Design and synthesis of novel cell wall inhibitors of Mycobacterium tuberculosis GlmM and GlmU. Carbohydr Res 346 : 1714–1720. doi: 10.1016/j.carres.2011.05.024 21704310
33. Tran AT, Wen D, West NP, Baker EN, Britton WJ, et al. (2013) Inhibition studies on Mycobacterium tuberculosis N-acetylglucosamine-1-phosphate uridyltransferase (GlmU). Org Biomol Chem 11 : 8113–8126. doi: 10.1039/c3ob41896k 24158720
34. Chao MC, Rubin EJ (2010) Letting sleeping dos lie: does dormancy play a role in tuberculosis? Annu Rev Microbiol 64 : 293–311. doi: 10.1146/annurev.micro.112408.134043 20825351
35. Wayne LG, Hayes LG (1996) An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun 64 : 2062–2069. 8675308
36. Blokpoel MC, Murphy HN, O'Toole R, Wiles S, Runn ES, et al. (2005) Tetracycline-inducible gene regulation in mycobacteria. Nucleic Acids Res 33: e22. 15687380
37. Karakousis PC, Williams EP, Bishai WR (2008) Altered expression of isoniazid-regulated genes in drug-treated dormant Mycobacterium tuberculosis. J Antimicrob Chemother 61 : 323–331. 18156607
38. Parikh A, Verma SK, Khan S, Prakash B, Nandicoori VK (2009) PknB-mediated phosphorylation of a novel substrate, N-acetylglucosamine-1-phosphate uridyltransferase, modulates its acetyltransferase activity. J Mol Biol 386 : 451–464. doi: 10.1016/j.jmb.2008.12.031 19121323
39. Alavi HA, Moscovic EA (1996) Immunolocalization of cell-wall-deficient forms of Mycobacterium tuberculosis complex in sarcoidosis and in sinus histiocytosis of lymph nodes draining carcinoma. Histol Histopathol 11 : 683–694. 8839759
40. BERAN MH V., KAUSTOVA J., DVORSKA L., PAVLIK I. (2006) Cell wall deficient forms of mycobacteria: a review. Veterinarni Medicina 51 : 365–389.
41. Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, et al. (2005) Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307 : 1955–1959. 15790854
42. Renzi F, Manfredi P, Dol M, Fu J, Vincent S, et al. (2015) Glycan-foraging systems reveal the adaptation of Capnocytophaga canimorsus to the dog mouth. MBio 6: e02507. doi: 10.1128/mBio.02507-14 25736888
43. Renzi F, Manfredi P, Mally M, Moes S, Jeno P, et al. (2011) The N-glycan glycoprotein deglycosylation complex (Gpd) from Capnocytophaga canimorsus deglycosylates human IgG. PLoS Pathog 7: e1002118. doi: 10.1371/journal.ppat.1002118 21738475
44. Gisin J, Schneider A, Nagele B, Borisova M, Mayer C (2013) A cell wall recycling shortcut that bypasses peptidoglycan de novo biosynthesis. Nat Chem Biol 9 : 491–493. doi: 10.1038/nchembio.1289 23831760
45. Engels FK, Mathot RA, Verweij J (2007) Alternative drug formulations of docetaxel: a review. Anticancer Drugs 18 : 95–103. 17159596
46. Coors EA, Seybold H, Merk HF, Mahler V (2005) Polysorbate 80 in medical products and nonimmunologic anaphylactoid reactions. Ann Allergy Asthma Immunol 95 : 593–599. 16400901
47. Lovering AL, Safadi SS, Strynadka NC (2012) Structural perspective of peptidoglycan biosynthesis and assembly. Annu Rev Biochem 81 : 451–478. doi: 10.1146/annurev-biochem-061809-112742 22663080
48. Denholm JT, McBryde ES (2010) The use of anti-tuberculosis therapy for latent TB infection. Infect Drug Resist 3 : 63–72. 21694895
49. Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O (2011) Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem 80 : 825–858. doi: 10.1146/annurev-biochem-060608-102511 21391816
50. Tanner ME (2005) The enzymes of sialic acid biosynthesis. Bioorg Chem 33 : 216–228. 15888312
51. Itano N, Kimata K (2002) Mammalian hyaluronan synthases. IUBMB Life 54 : 195–199. 12512858
52. Swoboda JG, Campbell J, Meredith TC, Walker S (2010) Wall teichoic acid function, biosynthesis, and inhibition. Chembiochem 11 : 35–45. doi: 10.1002/cbic.200900557 19899094
53. Mikusova K, Mikus M, Besra GS, Hancock I, Brennan PJ (1996) Biosynthesis of the linkage region of the mycobacterial cell wall. J Biol Chem 271 : 7820–7828. 8631826
54. Skovierova H, Larrouy-Maumus G, Pham H, Belanova M, Barilone N, et al. (2010) Biosynthetic origin of the galactosamine substituent of Arabinogalactan in Mycobacterium tuberculosis. J Biol Chem 285 : 41348–41355. doi: 10.1074/jbc.M110.188110 21030587
55. Wang X, Quinn PJ (2010) Lipopolysaccharide: Biosynthetic pathway and structure modification. Prog Lipid Res 49 : 97–107. doi: 10.1016/j.plipres.2009.06.002 19815028
56. Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM (2004) Diversity of microbial sialic acid metabolism. Microbiol Mol Biol Rev 68 : 132–153. 15007099
57. Dell A, Galadari A, Sastre F, Hitchen P (2010) Similarities and differences in the glycosylation mechanisms in prokaryotes and eukaryotes. Int J Microbiol 2010 : 148178. doi: 10.1155/2010/148178 21490701
58. Ujita M, Misra AK, McAuliffe J, Hindsgaul O, Fukuda M (2000) Poly-N-acetyllactosamine extension in N-glycans and core 2 - and core 4-branched O-glycans is differentially controlled by i-extension enzyme and different members of the beta 1,4-galactosyltransferase gene family. J Biol Chem 275 : 15868–15875. 10747980
59. Alderwick LJ, Molle V, Kremer L, Cozzone AJ, Dafforn TR, et al. (2006) Molecular structure of EmbR, a response element of Ser/Thr kinase signaling in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 103 : 2558–2563. 16477027
60. Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, et al. (2009) A replication clock for Mycobacterium tuberculosis. Nat Med 15 : 211–214. doi: 10.1038/nm.1915 19182798
61. Naseem S, Parrino SM, Buenten DM, Konopka JB (2012) Novel roles for GlcNAc in cell signaling. Commun Integr Biol 5 : 156–159. doi: 10.4161/cib.19034 22808320
62. Handford M, Rodriguez-Furlan C, Orellana A (2006) Nucleotide-sugar transporters: structure, function and roles in vivo. Braz J Med Biol Res 39 : 1149–1158. 16981043
63. Konopka JB (2012) N-acetylglucosamine (GlcNAc) functions in cell signaling. Scientifica (Cairo) 2012.
64. Klotzsche M, Ehrt S, Schnappinger D (2009) Improved tetracycline repressors for gene silencing in mycobacteria. Nucleic Acids Res 37 : 1778–1788. doi: 10.1093/nar/gkp015 19174563
65. Jain P, Hsu T, Arai M, Biermann K, Thaler DS, et al. (2014) Specialized transduction designed for precise high-throughput unmarked deletions in Mycobacterium tuberculosis. MBio 5: e01245–01214. doi: 10.1128/mBio.01245-14 24895308
66. Bardarov S, Bardarov S Jr, Jr., Pavelka MS Jr, Jr., Sambandamurthy V, Larsen M, et al. (2002) Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148 : 3007–3017. 12368434
67. Chawla Y, Upadhyay S, Khan S, Nagarajan SN, Forti F, et al. (2014) Protein kinase B (PknB) of Mycobacterium tuberculosis is essential for growth of the pathogen in vitro as well as for survival within the host. J Biol Chem 289 : 13858–13875. doi: 10.1074/jbc.M114.563536 24706757
68. Gupta A, Sharma Y, Dash KN, Verma S, Natarajan VT, et al. (2014) Ultrastructural Investigations in an Autosomal Recessively Inherited Case of Dyschromatosis Universalis Hereditaria. Acta Derm Venereol.
69. Pandey AK, Raman S, Proff R, Joshi S, Kang CM, et al. (2009) Nitrile-inducible gene expression in mycobacteria. Tuberculosis (Edinb) 89 : 12–16.
70. Ehrt S, Guo XV, Hickey CM, Ryou M, Monteleone M, et al. (2005) Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res 33: e21. 15687379
71. Puri RV, Reddy PV, Tyagi AK (2013) Secreted acid phosphatase (SapM) of Mycobacterium tuberculosis is indispensable for arresting phagosomal maturation and growth of the pathogen in guinea pig tissues. PLoS One 8: e70514. doi: 10.1371/journal.pone.0070514 23923000
72. Hu Y, Coates AR (2009) Acute and persistent Mycobacterium tuberculosis infections depend on the thiol peroxidase TpX. PLoS One 4: e5150. doi: 10.1371/journal.pone.0005150 19340292
73. Reddy PV, Puri RV, Chauhan P, Kar R, Rohilla A, et al. (2013) Disruption of mycobactin biosynthesis leads to attenuation of Mycobacterium tuberculosis for growth and virulence. J Infect Dis 208 : 1255–1265. doi: 10.1093/infdis/jit250 23788726
74. Kirchmair J, Distinto S, Markt P, Schuster D, Spitzer GM, et al. (2009) How to optimize shape-based virtual screening: choosing the right query and including chemical information. J Chem Inf Model 49 : 678–692. doi: 10.1021/ci8004226 19434901
75. Hawkins PC, Skillman AG, Nicholls A (2007) Comparison of shape-matching and docking as virtual screening tools. J Med Chem 50 : 74–82. 17201411
76. György Kóczána GCk, Antal Csámpaic, Balogb Erika, Szilvia Bőszea Pál Sohárc, Hudecza Ferenc (2001) Synthesis and characterization of 4-ethoxymethylene-2-[1]-naphthyl-5(4H)-oxazolone and its fluorescent amino acid derivatives Tetrahedron 57 : 4589–4598.
77. William L. Jorgensen DSM, and Julian Tirado-Rives (1996) Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. Journal of the American Chemical Society 118 : 11225–11236.
78. William L. Jorgensen JC, Jeffry D. Madura, Impey Roger W. and Klein Michael L. (1983) Comparison of simple potential functions for simulating liquid water. The Journal of Chemical Physics 79 : 926–935.
79. Vincent Kräutler WFvGaHH(2001) A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. Journal of Computational Chemistry 22 : 501–508.
80. Zhou Y, Xin Y, Sha S, Ma Y (2011) Kinetic properties of Mycobacterium tuberculosis bifunctional GlmU. Arch Microbiol 193 : 751–757. doi: 10.1007/s00203-011-0715-8 21594607
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized VirusČlánek Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell ProliferationČlánek Fundamental Roles of the Golgi-Associated Aspartyl Protease, ASP5, at the Host-Parasite InterfaceČlánek Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 10- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Expression of Concern: Misregulation of Underlies the Developmental Abnormalities Caused by Three Distinct Viral Silencing Suppressors in Arabidopsis
- Preparing for the Next Epidemic with Basic Virology
- Effectively Communicating the Uncertainties Surrounding Ebola Virus Transmission
- Translating Basic Research into Clinical Applications: Malaria Research at an NIH Lab
- A Gut Odyssey: The Impact of the Microbiota on Spore Formation and Germination
- Papillomavirus E6 Oncoproteins Take Common Structural Approaches to Solve Different Biological Problems
- Chronobiomics: The Biological Clock as a New Principle in Host–Microbial Interactions
- Dimensions of Horizontal Gene Transfer in Eukaryotic Microbial Pathogens
- Addressing the Complications of Ebola and Other Viral Hemorrhagic Fever Infections: Using Insights from Bacterial and Fungal Sepsis
- Time for Chocolate: Current Understanding and New Perspectives on Cacao Witches’ Broom Disease Research
- Ganglioside and Non-ganglioside Mediated Host Responses to the Mouse Polyomavirus
- Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
- Structure Elucidation of Coxsackievirus A16 in Complex with GPP3 Informs a Systematic Review of Highly Potent Capsid Binders to Enteroviruses
- CD39 Expression Identifies Terminally Exhausted CD8 T Cells
- Abiotic Stresses Antagonize the Rice Defence Pathway through the Tyrosine-Dephosphorylation of OsMPK6
- Dissociation of Tissue Destruction and Bacterial Expansion during Bubonic Plague
- Interferon-γ: The Jekyll and Hyde of Malaria
- CCR2 Inflammatory Dendritic Cells and Translocation of Antigen by Type III Secretion Are Required for the Exceptionally Large CD8 T Cell Response to the Protective YopE Epitope during Infection
- A New Glycan-Dependent CD4-Binding Site Neutralizing Antibody Exerts Pressure on HIV-1
- The Suramin Derivative NF449 Interacts with the 5-fold Vertex of the Enterovirus A71 Capsid to Prevent Virus Attachment to PSGL-1 and Heparan Sulfate
- Trans-generational Immune Priming Protects the Eggs Only against Gram-Positive Bacteria in the Mealworm Beetle
- Peripheral Vγ9Vδ2 T Cells Are a Novel Reservoir of Latent HIV Infection
- Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33
- TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized Virus
- Modeling the Effects of Vorinostat Reveals both Transient and Delayed HIV Transcriptional Activation and Minimal Killing of Latently Infected Cells
- Identification of a Novel Lipoprotein Regulator of Spore Germination
- Calcium Regulation of Hemorrhagic Fever Virus Budding: Mechanistic Implications for Host-Oriented Therapeutic Intervention
- Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis
- Comparative Life Cycle Transcriptomics Revises Genome Annotation and Links a Chromosome Duplication with Parasitism of Vertebrates
- The Autophagy Receptor TAX1BP1 and the Molecular Motor Myosin VI Are Required for Clearance of Salmonella Typhimurium by Autophagy
- Carcinogenic Parasite Secretes Growth Factor That Accelerates Wound Healing and Potentially Promotes Neoplasia
- Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell Proliferation
- Dengue Virus Infection of Requires a Putative Cysteine Rich Venom Protein
- Distinct Viral and Mutational Spectrum of Endemic Burkitt Lymphoma
- Fundamental Roles of the Golgi-Associated Aspartyl Protease, ASP5, at the Host-Parasite Interface
- Phenotypic and Functional Alterations in Circulating Memory CD8 T Cells with Time after Primary Infection
- Systematic Identification of Cyclic-di-GMP Binding Proteins in Reveals a Novel Class of Cyclic-di-GMP-Binding ATPases Associated with Type II Secretion Systems
- Influenza Transmission in the Mother-Infant Dyad Leads to Severe Disease, Mammary Gland Infection, and Pathogenesis by Regulating Host Responses
- Myeloid Cell Arg1 Inhibits Control of Arthritogenic Alphavirus Infection by Suppressing Antiviral T Cells
- The White-Nose Syndrome Transcriptome: Activation of Anti-fungal Host Responses in Wing Tissue of Hibernating Little Brown Myotis
- Influenza Virus Reassortment Is Enhanced by Semi-infectious Particles but Can Be Suppressed by Defective Interfering Particles
- Identification of the Mechanisms Causing Reversion to Virulence in an Attenuated SARS-CoV for the Design of a Genetically Stable Vaccine
- Differentiation-Dependent KLF4 Expression Promotes Lytic Epstein-Barr Virus Infection in Epithelial Cells
- The Histone Acetyltransferase Hat1 Regulates Stress Resistance and Virulence via Distinct Chromatin Assembly Pathways
- C-di-GMP Regulates Motile to Sessile Transition by Modulating MshA Pili Biogenesis and Near-Surface Motility Behavior in
- Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes
- Crystal Structure of the Human Cytomegalovirus Glycoprotein B
- Depletion of . GlmU from Infected Murine Lungs Effects the Clearance of the Pathogen
- Immunologic Control of Papillomavirus Type 1
- Requires Host Rab1b for Survival in Macrophages
- Structure Analysis Uncovers a Highly Diverse but Structurally Conserved Effector Family in Phytopathogenic Fungi
- PD-L1 Expression on Retrovirus-Infected Cells Mediates Immune Escape from CD8 T Cell Killing
- Phospho-dependent Regulation of SAMHD1 Oligomerisation Couples Catalysis and Restriction
- IL-4 Induced Innate CD8 T Cells Control Persistent Viral Infection
- Crystal Structures of a Piscine Betanodavirus: Mechanisms of Capsid Assembly and Viral Infection
- BCG Skin Infection Triggers IL-1R-MyD88-Dependent Migration of EpCAM CD11b Skin Dendritic cells to Draining Lymph Node During CD4+ T-Cell Priming
- Antigenic Characterization of the HCMV gH/gL/gO and Pentamer Cell Entry Complexes Reveals Binding Sites for Potently Neutralizing Human Antibodies
- Rescue of a Plant Negative-Strand RNA Virus from Cloned cDNA: Insights into Enveloped Plant Virus Movement and Morphogenesis
- Geminivirus Activates to Accelerate Cytoplasmic DCP2-Mediated mRNA Turnover and Weakens RNA Silencing in
- Disruption of Sphingolipid Biosynthesis Blocks Phagocytosis of
- The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps
- The Timing of Stimulation and IL-2 Signaling Regulate Secondary CD8 T Cell Responses
- Structural and Functional Analysis of Murine Polyomavirus Capsid Proteins Establish the Determinants of Ligand Recognition and Pathogenicity
- The Dual Role of an ESCRT-0 Component HGS in HBV Transcription and Naked Capsid Secretion
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Chronobiomics: The Biological Clock as a New Principle in Host–Microbial Interactions
- Interferon-γ: The Jekyll and Hyde of Malaria
- Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
- Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



