-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Interferon-γ: The Jekyll and Hyde of Malaria
article has not abstract
Published in the journal: Interferon-γ: The Jekyll and Hyde of Malaria. PLoS Pathog 11(10): e32767. doi:10.1371/journal.ppat.1005118
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1005118Summary
article has not abstract
Introduction
Interferon gamma (IFN-γ) is a key mediator of inflammatory immune responses induced primarily by interleukin-12 (IL-12). IFN-γ secretion by both innate and adaptive immune cells is essential for control of intracellular pathogens and tumors, yet aberrant production of IFN-γ contributes to autoimmunity and inflammation in certain disease settings. These divergent roles are well illustrated in the context of malaria, a disease caused by infection with protozoan parasites of the genus Plasmodium. IFN-γ is a central cytokine in controlling Plasmodium infection in both the liver and blood stages of the parasite life cycle, but it can also exacerbate the severity of malarial disease depending on the temporal and spatial production of IFN-γ. Here, we review the types of immune cells that produce IFN-γ during malaria and discuss the IFN-γ-induced effector mechanisms that can aid in killing Plasmodium parasites but also contribute to the pathogenesis of malaria.
Which Immune Cells Produce IFN-γ during Malaria?
Plasmodium infection induces IFN-γ production from a variety of innate and adaptive immune cell subsets at different stages of the life cycle. Studies in mice have demonstrated that natural killer (NK) cells are one of the earliest sources of IFN-γ during the liver stage [1], as well as blood stage [2], of malaria. For example, C57BL/6J mice depleted of NK cells and infected with a nonlethal Plasmodium yoelli strain showed a 58% abrogation of IFN-γ production at 24 hours postinfection [2]. Human NK cells have also been shown to rapidly produce IFN-γ upon incubation with Plasmodium falciparum-infected red blood cells (iRBCs) in vitro [3]. Bridging innate and adaptive immunity, both natural killer T (NKT) cells and γδ T cells can contribute to IFN-γ production during Plasmodium infection. Studies suggest a significant proportion (50%) of γδ T cells from humans infected with P. falciparum secrete IFN-γ [4], while NKT cells in mice secrete IFN-γ in response to sporozoites and liver stage parasites [5]. While there is likely significant redundancy in IFN-γ production from leukocytes in response to both liver stage and blood stage Plasmodium parasites, studies using IFN-γ eYFP reporter mice infected with P. berghei ANKA suggest that NK cells contribute greater to IFN-γ production than both NKT and γδ T cells at early time points postinfection, and the production of IFN-γ from NKT and γδ T cells remains fairly stable over time [6].
Once an adaptive immune response is initiated, both CD4+ and CD8+ T cells become a major source of IFN-γ in response to both liver stage [7] and blood stage malaria. The finding that both CD4+ [8] and CD8+ [9] T cells isolated from Plasmodium-infected humans produce IFN-γ is also observed in many mouse models of malaria. Secretion of IFN-γ by both CD4+ and CD8+ T cells increases around day seven postinfection with blood stage P. berghei ANKA in both the spleen and brain [6]. While IFN-γ is the canonical cytokine that has been used to define CD4+ T cells as Th1 cells, it has been widely observed that Th1 cells can simultaneously produce other inflammatory cytokines including IL-2, TNF-α, and IL-17 during an adaptive immune response. A subset of IFN-γ/IL-10 double-producing CD4+ T cells have been observed in humans infected with Plasmodium [8,10], and mouse models of malaria suggest that IFN-γ/IL-10 double-producing cells are an important source of IL-10 that limit immunopathogenesis of malaria [11] at the cost of inhibiting control of the infection [12].
What Evidence Suggests That IFN-γ Is Protective during Malaria?
There have been several correlations between IFN-γ levels in the periphery and protection against severe malaria in humans. The protective capacity of IFN-γ in malaria appears to be, in part, related to the timing of IFN-γ production with the early appearance of IFN-γ after infection in humans correlated with protection against the development of clinical symptoms of malaria in some studies [13]. However, study conclusions are often complicated by factors that include differing patterns of Plasmodium transmission between study sites or varying levels of pathogen coinfection giving rise to conflicting data. Experiments in mice also suggest that early IFN-γ production is protective against experimental cerebral malaria (ECM), and peripheral levels of IFN-γ can drop just before the onset of ECM [14] with a similar phenomenon potentially occurring in humans [15]. This introduces a time-dependent sampling variable that can pose problems when attempting to establish a correlation between disease severity and peripheral IFN-γ levels. Nevertheless, in a study where human volunteers were infected over time with several low doses of Plasmodium iRBCs and treated to clear the infection, protection from a challenge infection was positively correlated with numbers of circulating IFN-γ-producing CD4+ T cells [16]. The natural resistance of the Fulani tribe in Mali to Plasmodium infection has also been correlated with elevated levels of IFN-γ [17], suggesting a protective role for IFN-γ against malaria.
Similar to human malaria, IFN-γ also appears to play a protective role against blood stage Plasmodium infection in mice. Mice lacking IFN-γ experience higher and more prolonged blood stage parasitemia compared to IFN-γ-sufficient mice when infected with the rodent parasites P. yoelii yoelii or P. chabaudi adami [18]. Additionally, a separate study found that IFN-γ levels were markedly higher 24 hours post blood stage infection in mice infected with nonlethal strains of P. chabaudi or P. yoelli when compared to mice infected with lethal strains of P. yoelli or P. berghei [2] emphasizing the potential benefits of IFN-γ to disease control in mice.
The prevalence of IFN-γ-producing CD4+ and CD8+ T cells has been associated with a greater likelihood of uncomplicated malaria [8], as well as reduced severe malarial anemia [9] in humans. However, experiments in mice have demonstrated that IFN-γ production by NK cells, NKT cells, and γδ T cells can also play a major role in the control of Plasmodium infection. NKT cells have been shown to inhibit parasite growth within hepatocytes in a partially IFN-γ-dependent manner [5]. Also, despite the prominent role of CD4+ and CD8+ T cells in contributing to serum IFN-γ levels during malaria, γδ T cells in mice are able to control liver stage Plasmodium infection in the absence of αβ T cells [19], demonstrating that γδ T cells are an important source of IFN-γ with respect to parasite control.
What Immune Effector Mechanisms Responsible for Controlling Plasmodium Infection Are Activated by IFN-γ?
IFN-γ secreted by CD4+ Th1 cells is critical for optimal activation of CD8+ T cells, B cells, and macrophages, all of which perform vital roles in the control of Plasmodium infection (Fig 1). The primary immune effector mechanisms by which IFN-γ can influence destruction of Plasmodium-infected cells include increasing the cytotoxic potential of CD8+ T cells, inducing production of cytophilic antibodies by B cells and enhancing phagocytic abilities of immune cells such as macrophages. It should be noted that the latter two functions are not mutually exclusive.
Fig. 1. Effector mechanisms induced by IFN-γ during malaria. 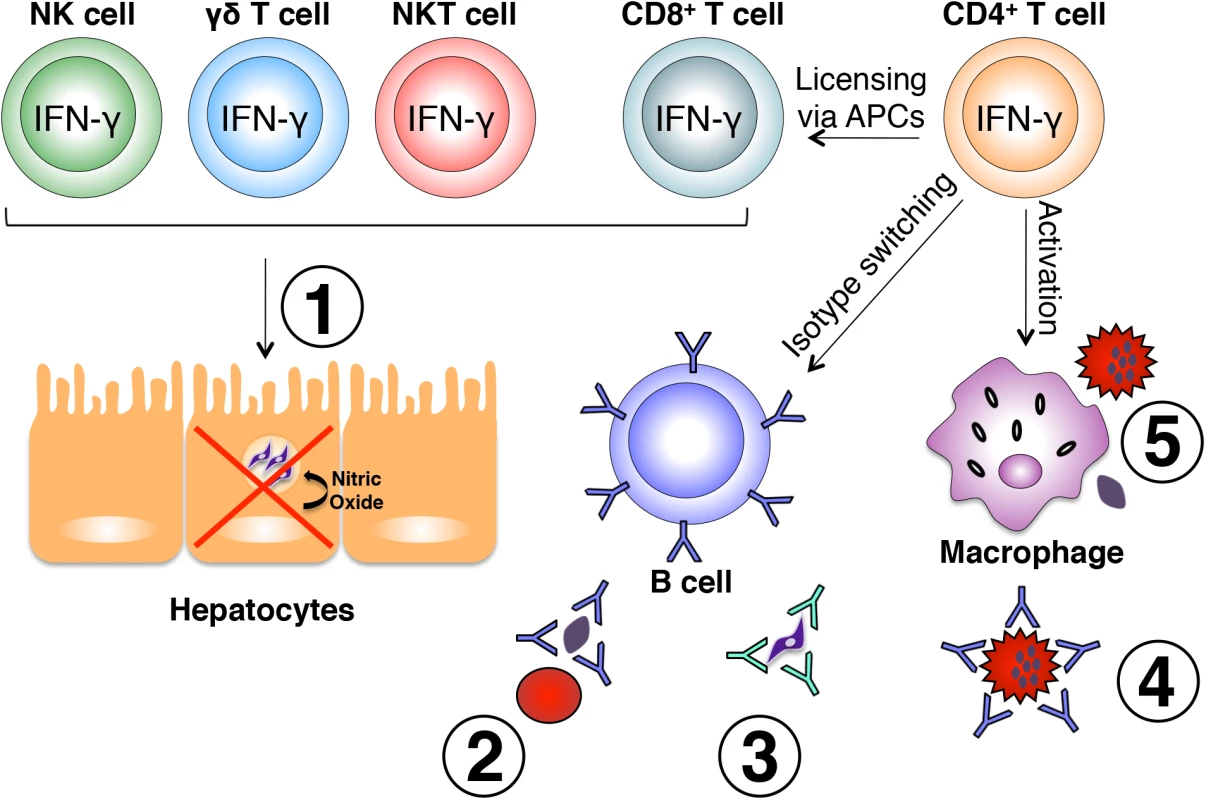
Various immune cell subsets produce IFN-γ in response to Plasmodium infection. NK, γδ, and NKT cells are largely responsible for early production of IFN-γ in response to liver and blood stages of the parasite and play a role in early control of parasite growth. IFN-γ-producing CD8+ T cells have also been shown to limit intrahepatic parasite growth through an IFN-γ-inducible, nitric oxide-dependent mechanism (1). Once an adaptive immune response is initiated, IFN-γ produced by CD4+ T cells optimally activates CD8+ T cells, B cells, and macrophages. IFN-γ influences isotype switching in B cells leading to production of cytophilic antibodies capable of binding free parasites and blocking red blood cell invasion (2), mediating parasite clearance through opsonization (3), and binding the surface of infected red blood cells promoting antibody-dependent phagocytosis (4). Production of IFN-γ from CD4+ T cells also optimally activates macrophages to phagocytose infected red blood cells and free parasites (5). All of these mechanisms are important for optimal control of parasite growth during Plasmodium infection. IFN-γ exerts its effects on immune cells that express the IFNGR1/2 cell surface receptor, and signaling through this receptor results in activation of transcription factors such as IRF1, STAT1, JAK2, IRF9, CIITA [20], and T-bet [21]. This leads to expression of a number of proteins such as nitric oxide synthase and FcγRI (CD64, a high-affinity Fc receptor) [20], as well as induction of B cell class-switching to the IgG2a antibody isotype [21]. As a result, the aforementioned events can lead to enhanced phagocytosis and destruction of intracellular pathogens.
Regarding liver stage parasite development in mice, CD8+ T cells induced by a DNA vaccination encoding the gene for a P. yoelli liver stage antigen were shown to be absolutely essential for protection of mice from a P. yoelli sporozoite challenge infection [22]. This protection was entirely dependent on IFN-γ production from Plasmodium-specific CD8+ T cells as well as IFN-γ-inducible nitric oxide synthase production from Plasmodium-infected hepatocytes [22] leading to direct intracellular parasite killing.
Although CD8+ T cell responses have also been implicated in control of blood stage Plasmodium infection in an IFN-γ-dependent manner [23], the mechanism by which this could occur remains unclear since infected red blood cells do not express the major histocompatibility complex class I (MHC-I) which is required for CD8+ T cell recognition. On the contrary, antibodies are known to be key effector molecules in Plasmodium infection and perform many well-characterized functions important for parasite control and clearance such as blocking parasite reinvasion, parasite opsonization, and targeting of parasites for phagocytosis. IFN-γ impacts the antibody response and isotypes of malaria-specific antibodies produced, which is evident in IFN-γ-/- mice that produce significantly less parasite-specific IgM, IgG3, and cytophilic IgG2a than wild-type mice [24]. Thus, the role of IFN-γ in the isotype switching of B cells to a cytophilic antibody isotype such as IgG2a increases the opsonic potential of antibodies.
IFN-γ is a potent activator of macrophages and can increase canonical macrophage activities such as phagocytosis and the production of both proinflammatory cytokines and reactive oxygen intermediates. In mice, IFN-γ has been shown to enhance phagocytosis of P. chabaudi AS iRBCs and free merozoites by peritoneal macrophages with mice lacking IFN-γ exhibiting lower levels of parasite phagocytosis than wild-type mice [25]. The phagocytosis-enhancing role of IFN-γ has been further demonstrated in vitro as macrophages isolated from C57BL/6J mice treated with IFN-γ exhibit enhanced phagocytosis of iRBCs, an effect inhibited by IL-10 treatment [25]. Since phagocytosis of iRBCs can occur in an antibody-dependent or antibody-independent manner, the role of IFN-γ on B cells and phagocytes is likely synergistic leading to increased phagocytosis of opsonized parasites and parasite products.
How Does IFN-γ Cause Pathology during Malaria?
The pathology associated with malaria is caused by the blood stages of Plasmodium infection and, in particular, immune responses targeting iRBCs sequestered in various organs. In susceptible C57BL/6J mice infected with P. berghei ANKA, which develop experimental cerebral malaria (ECM), the IFN-γ-induced immune response against iRBCs sequestered in the brain and lung is required for pathogenesis of infection [6]. Furthermore, IFN-γ-/- mice that are resistant to ECM show decreased parasite and leukocyte accumulation in the brain [26]. This finding has been attributed to robust IFN-γ-induced expression of canonical adhesion molecules such as ICAM-1 [26], as well as CD4+ T cell IFN-γ-induced expression of the chemokines CXCL9 and CXCL10 that recruit IFN-γ-producing CD8+ T cells to the brain during ECM [6]. These pathogenic T cells induce cerebral pathology [6] most likely through perforin - and granzyme-dependent disruption of the blood-brain barrier upon recognition of malaria-derived peptides presented in the context of MHC-I on brain endothelial cells [27]. Thus, while IFN-γ is necessary for the recruitment of CD8+ T cells to the brain during ECM [28], other effector molecules produced by activated CD8+ T cells such as perforin and granzyme B are the critical mediators of pathology [29].
Perspectives
Studies in both humans and mice suggest a fine line between IFN-γ-associated protection versus immunopathology in the immune response to malaria in which a threshold level is needed for optimal control of parasitemia, yet aberrant expression can lead to pathology and the complications of severe malaria. It is tempting to explore boosting the IFN-γ response during malaria therapeutically, given the associations between IFN-γ and improved disease outcome. For example, phase IIa clinical trials for the RTS,S pre-erythrocytic malaria vaccine candidate showed a correlation between prolonged CD4+ and CD8+ T cell IFN-γ responses against a malaria-specific protein and protection upon challenge infection in human volunteers [30]. In mice, the loss of protective immunity against P. chabaudi is associated with a decrease in memory CD4+ Th1 cells after parasite clearance along with a concomitant decrease in IFN-γ production from splenocytes [31]. Splenic IFN-γ has been shown to be required for optimal priming of effector and effector memory T cells by splenic innate cells [32], demonstrating the importance of this cytokine in maintenance of optimal immunity. However, it is clear that the dynamic roles of IFN-γ during malaria are complex, and more work is needed to understand the delicate balance of IFN-γ necessary for achieving optimal protection while minimizing pathology.
Zdroje
1. Miller JL, Sack BK, Baldwin M, Vaughan AM, Kappe SH. Interferon-mediated innate immune responses against malaria parasite liver stages. Cell Rep. 2014 Apr 4;7(2):436–47. doi: 10.1016/j.celrep.2014.03.018 24703850
2. De Souza JB, Williamson KH, Otani T, Playfair JH. Early gamma interferon responses in lethal and nonlethal murine blood-stage malaria. Infect Immun. 1997 May 4;65(5):1593–8. 9125535
3. Artavanis-Tsakonas K, Riley EM. Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol. 2002 Sep;169(6):2956–63. 12218109
4. Hviid L, Kurtzhals JA, Adabayeri V, Loizon S, Kemp K, Goka BQ, et al. Perturbation and proinflammatory type activation of V delta 1(+) gamma delta T cells in African children with Plasmodium falciparum malaria. Infect Immun. 2001 May 2;69(5):3190–6. 11292740
5. Pied S, Roland J, Louise A, Voegtle D, Soulard V, Mazier D, et al. Liver CD4-CD8 - NK1.1+ TCR alpha beta intermediate cells increase during experimental malaria infection and are able to exhibit inhibitory activity against the parasite liver stage in vitro. J Immunol. 2000 Feb 2;164(3):1463–9. 10640763
6. Villegas-Mendez A, Greig R, Shaw TN, de Souza JB, Gwyer Findlay E, Stumhofer JS, et al. IFN-γ-producing CD4+ T cells promote experimental cerebral malaria by modulating CD8+ T cell accumulation within the brain. J Immunol. 2012 Jul;189(2):968–79. doi: 10.4049/jimmunol.1200688 22723523
7. Connelly M, King CL, Bucci K, Walters S, Genton B, Alpers MP, et al. T-cell immunity to peptide epitopes of liver-stage antigen 1 in an area of Papua New Guinea in which malaria is holoendemic. Infect Immun. 1997 Dec 1;65(12):5082–7. 9393799
8. Walther M, Jeffries D, Finney OC, Njie M, Ebonyi A, Deininger S, et al. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog. 2009 Apr 3;5(4):e1000364. doi: 10.1371/journal.ppat.1000364 19343213
9. Ong’echa JM, Lal AA, Terlouw DJ, Ter Kuile FO, Kariuki SK, Udhayakumar V, et al. Association of interferon-gamma responses to pre-erythrocytic stage vaccine candidate antigens of Plasmodium falciparum in young Kenyan children with improved hemoglobin levels: XV. Asembo Bay Cohort Project. Am J Trop Med Hyg. 2003 May 4;68(5):590–7. 12812352
10. Jagannathan P, Eccles-James I, Bowen K, Nankya F, Auma A, Wamala S, et al. IFNγ/IL-10 co-producing cells dominate the CD4 response to malaria in highly exposed children. PLoS Pathog. 2014 Jan 3;10(1):e1003864. doi: 10.1371/journal.ppat.1003864 24415936
11. Freitas do Rosário AP, Lamb T, Spence P, Stephens R, Lang A, Roers A, et al. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol. 2012 Feb 3;188(3):1178–90. doi: 10.4049/jimmunol.1102755 22205023
12. Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, et al. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog. 2008 Feb 5;4(2):e1000004. doi: 10.1371/journal.ppat.1000004 18401464
13. D’Ombrain MC, Robinson LJ, Stanisic DI, Taraika J, Bernard N, Michon P, et al. Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin Infect Dis. 2008 Dec 1;47(11):1380–7. doi: 10.1086/592971 18947328
14. Mitchell AJ, Hansen AM, Hee L, Ball HJ, Potter SM, Walker JC, et al. Early cytokine production is associated with protection from murine cerebral malaria. Infect Immun. 2005 Sep 4;73(9):5645–53. 16113282
15. Prakash D, Fesel C, Jain R, Cazenave P-AA, Mishra GC, Pied S. Clusters of cytokines determine malaria severity in Plasmodium falciparum-infected patients from endemic areas of Central India. J Infect Dis. 2006 Jul 6;194(2):198–207. 16779726
16. Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. 2002 Aug 6;360(9333):610–7. 12241933
17. McCall MBB, Hopman J, Daou M, Maiga B, Dara V, Ploemen I, et al. Early interferon-gamma response against Plasmodium falciparum correlates with interethnic differences in susceptibility to parasitemia between sympatric Fulani and Dogon in Mali. J Infect Dis. 2010 Jan 5;201(1):142–52. doi: 10.1086/648596 19929378
18. Van der Heyde HC, Pepper B, Batchelder J, Cigel F, Weidanz WP. The time course of selected malarial infections in cytokine-deficient mice. Exp Parasitol. 1997 Feb 6;85(2):206–13. 9030670
19. Tsuji M, Mombaerts P, Lefrancois L, Nussenzweig RS, Zavala F, Tonegawa S. Gamma delta T cells contribute to immunity against the liver stages of malaria in alpha beta T-cell-deficient mice. Proc Natl Acad Sci USA. 1994 Jan 2;91(1):345–9. 8278391
20. Waddell SJ, Popper SJ, Rubins KH, Griffiths MJ, Brown PO, Levin M, et al. Dissecting interferon-induced transcriptional programs in human peripheral blood cells. PLoS ONE. 2010 Jan 5;5(3):e9753. doi: 10.1371/journal.pone.0009753 20339534
21. Xu W, Zhang JJ. Stat1-dependent synergistic activation of T-bet for IgG2a production during early stage of B cell activation. J Immunol. 2005 Dec 4;175(11):7419–24. 16301649
22. Doolan DL, Sedegah M, Hedstrom RC, Hobart P, Charoenvit Y, Hoffman SL. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell-, interferon gamma-, and nitric oxide-dependent immunity. J Exp Med. 1996 Apr 1;183(4):1739–46. 8666931
23. Imai T, Shen J, Chou B, Duan X, Tu L, Tetsutani K, et al. Involvement of CD8+ T cells in protective immunity against murine blood-stage infection with Plasmodium yoelii 17XL strain. Eur J Immunol. 2010 Apr 4;40(4):1053–61. doi: 10.1002/eji.200939525 20101613
24. Su Z, Stevenson MM. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect Immun. 2000 Aug 2;68(8):4399–406. 10899836
25. Su Z, Fortin A, Gros P, Stevenson MM. Opsonin-independent phagocytosis: an effector mechanism against acute blood-stage Plasmodium chabaudi AS infection. J Infect Dis. 2002 Nov 5;186(9):1321–9. 12402202
26. Amani V, Vigário AM, Belnoue E, Marussig M, Fonseca L, Mazier D, et al. Involvement of IFN-gamma receptor-medicated signaling in pathology and anti-malarial immunity induced by Plasmodium berghei infection. Eur J Immunol. 2000 Jun 4;30(6):1646–55. 10898501
27. Howland SW, Poh CM, Gun SY, Claser C, Malleret B, Shastri N, et al. Brain microvessel cross-presentation is a hallmark of experimental cerebral malaria. EMBO Mol Med. 2013 Jul 1;5(7):916–31. doi: 10.1002/emmm.201202273 23681698
28. Belnoue E, Potter SM, Rosa DS, Mauduit M, Grüner AC, Kayibanda M, et al. Control of pathogenic CD8+ T cell migration to the brain by IFN-gamma during experimental cerebral malaria. Parasite Immunol. 2008 Oct 3;30(10):544–53. doi: 10.1111/j.1365-3024.2008.01053.x 18665903
29. Haque A, Best SE, Unosson K, Amante FH, de Labastida F, Anstey NM, et al. Granzyme B expression by CD8+ T cells is required for the development of experimental cerebral malaria. J Immunol [Internet]. 2011 Jun 3;186(11):6148–56. http://www.jimmunol.org/cgi/pmidlookup?view=long&pmid=21525386
30. McCall MBB, Sauerwein RW. Interferon-γ—central mediator of protective immune responses against the pre-erythrocytic and blood stage of malaria. J Leukoc Biol. 2010 Dec 3;88(6):1131–43. doi: 10.1189/jlb.0310137 20610802
31. Freitas do Rosário AP, Muxel SM, Rodríguez-Málaga SMM, Sardinha LR, Zago CAA, Castillo-Méndez SI, et al. Gradual decline in malaria-specific memory T cell responses leads to failure to maintain long-term protective immunity to Plasmodium chabaudi AS despite persistence of B cell memory and circulating antibody. J Immunol. 2008 Dec 1;181(12):8344–55. 19050251
32. Da Silva HB, de Salles EM, Panatieri RH, Boscardin SB, Rodríguez-Málaga SMM, Alvarez JMM, et al. IFN-γ-induced priming maintains long-term strain-transcending immunity against blood-stage Plasmodium chabaudi malaria. J Immunol. 2013 Nov 5;191(10):5160–9. doi: 10.4049/jimmunol.1300462 24133169
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized VirusČlánek Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell ProliferationČlánek Fundamental Roles of the Golgi-Associated Aspartyl Protease, ASP5, at the Host-Parasite InterfaceČlánek Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 10- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Expression of Concern: Misregulation of Underlies the Developmental Abnormalities Caused by Three Distinct Viral Silencing Suppressors in Arabidopsis
- Preparing for the Next Epidemic with Basic Virology
- Effectively Communicating the Uncertainties Surrounding Ebola Virus Transmission
- Translating Basic Research into Clinical Applications: Malaria Research at an NIH Lab
- A Gut Odyssey: The Impact of the Microbiota on Spore Formation and Germination
- Papillomavirus E6 Oncoproteins Take Common Structural Approaches to Solve Different Biological Problems
- Chronobiomics: The Biological Clock as a New Principle in Host–Microbial Interactions
- Dimensions of Horizontal Gene Transfer in Eukaryotic Microbial Pathogens
- Addressing the Complications of Ebola and Other Viral Hemorrhagic Fever Infections: Using Insights from Bacterial and Fungal Sepsis
- Time for Chocolate: Current Understanding and New Perspectives on Cacao Witches’ Broom Disease Research
- Ganglioside and Non-ganglioside Mediated Host Responses to the Mouse Polyomavirus
- Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
- Structure Elucidation of Coxsackievirus A16 in Complex with GPP3 Informs a Systematic Review of Highly Potent Capsid Binders to Enteroviruses
- CD39 Expression Identifies Terminally Exhausted CD8 T Cells
- Abiotic Stresses Antagonize the Rice Defence Pathway through the Tyrosine-Dephosphorylation of OsMPK6
- Dissociation of Tissue Destruction and Bacterial Expansion during Bubonic Plague
- Interferon-γ: The Jekyll and Hyde of Malaria
- CCR2 Inflammatory Dendritic Cells and Translocation of Antigen by Type III Secretion Are Required for the Exceptionally Large CD8 T Cell Response to the Protective YopE Epitope during Infection
- A New Glycan-Dependent CD4-Binding Site Neutralizing Antibody Exerts Pressure on HIV-1
- The Suramin Derivative NF449 Interacts with the 5-fold Vertex of the Enterovirus A71 Capsid to Prevent Virus Attachment to PSGL-1 and Heparan Sulfate
- Trans-generational Immune Priming Protects the Eggs Only against Gram-Positive Bacteria in the Mealworm Beetle
- Peripheral Vγ9Vδ2 T Cells Are a Novel Reservoir of Latent HIV Infection
- Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33
- TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized Virus
- Modeling the Effects of Vorinostat Reveals both Transient and Delayed HIV Transcriptional Activation and Minimal Killing of Latently Infected Cells
- Identification of a Novel Lipoprotein Regulator of Spore Germination
- Calcium Regulation of Hemorrhagic Fever Virus Budding: Mechanistic Implications for Host-Oriented Therapeutic Intervention
- Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis
- Comparative Life Cycle Transcriptomics Revises Genome Annotation and Links a Chromosome Duplication with Parasitism of Vertebrates
- The Autophagy Receptor TAX1BP1 and the Molecular Motor Myosin VI Are Required for Clearance of Salmonella Typhimurium by Autophagy
- Carcinogenic Parasite Secretes Growth Factor That Accelerates Wound Healing and Potentially Promotes Neoplasia
- Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell Proliferation
- Dengue Virus Infection of Requires a Putative Cysteine Rich Venom Protein
- Distinct Viral and Mutational Spectrum of Endemic Burkitt Lymphoma
- Fundamental Roles of the Golgi-Associated Aspartyl Protease, ASP5, at the Host-Parasite Interface
- Phenotypic and Functional Alterations in Circulating Memory CD8 T Cells with Time after Primary Infection
- Systematic Identification of Cyclic-di-GMP Binding Proteins in Reveals a Novel Class of Cyclic-di-GMP-Binding ATPases Associated with Type II Secretion Systems
- Influenza Transmission in the Mother-Infant Dyad Leads to Severe Disease, Mammary Gland Infection, and Pathogenesis by Regulating Host Responses
- Myeloid Cell Arg1 Inhibits Control of Arthritogenic Alphavirus Infection by Suppressing Antiviral T Cells
- The White-Nose Syndrome Transcriptome: Activation of Anti-fungal Host Responses in Wing Tissue of Hibernating Little Brown Myotis
- Influenza Virus Reassortment Is Enhanced by Semi-infectious Particles but Can Be Suppressed by Defective Interfering Particles
- Identification of the Mechanisms Causing Reversion to Virulence in an Attenuated SARS-CoV for the Design of a Genetically Stable Vaccine
- Differentiation-Dependent KLF4 Expression Promotes Lytic Epstein-Barr Virus Infection in Epithelial Cells
- The Histone Acetyltransferase Hat1 Regulates Stress Resistance and Virulence via Distinct Chromatin Assembly Pathways
- C-di-GMP Regulates Motile to Sessile Transition by Modulating MshA Pili Biogenesis and Near-Surface Motility Behavior in
- Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes
- Crystal Structure of the Human Cytomegalovirus Glycoprotein B
- Depletion of . GlmU from Infected Murine Lungs Effects the Clearance of the Pathogen
- Immunologic Control of Papillomavirus Type 1
- Requires Host Rab1b for Survival in Macrophages
- Structure Analysis Uncovers a Highly Diverse but Structurally Conserved Effector Family in Phytopathogenic Fungi
- PD-L1 Expression on Retrovirus-Infected Cells Mediates Immune Escape from CD8 T Cell Killing
- Phospho-dependent Regulation of SAMHD1 Oligomerisation Couples Catalysis and Restriction
- IL-4 Induced Innate CD8 T Cells Control Persistent Viral Infection
- Crystal Structures of a Piscine Betanodavirus: Mechanisms of Capsid Assembly and Viral Infection
- BCG Skin Infection Triggers IL-1R-MyD88-Dependent Migration of EpCAM CD11b Skin Dendritic cells to Draining Lymph Node During CD4+ T-Cell Priming
- Antigenic Characterization of the HCMV gH/gL/gO and Pentamer Cell Entry Complexes Reveals Binding Sites for Potently Neutralizing Human Antibodies
- Rescue of a Plant Negative-Strand RNA Virus from Cloned cDNA: Insights into Enveloped Plant Virus Movement and Morphogenesis
- Geminivirus Activates to Accelerate Cytoplasmic DCP2-Mediated mRNA Turnover and Weakens RNA Silencing in
- Disruption of Sphingolipid Biosynthesis Blocks Phagocytosis of
- The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps
- The Timing of Stimulation and IL-2 Signaling Regulate Secondary CD8 T Cell Responses
- Structural and Functional Analysis of Murine Polyomavirus Capsid Proteins Establish the Determinants of Ligand Recognition and Pathogenicity
- The Dual Role of an ESCRT-0 Component HGS in HBV Transcription and Naked Capsid Secretion
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Chronobiomics: The Biological Clock as a New Principle in Host–Microbial Interactions
- Interferon-γ: The Jekyll and Hyde of Malaria
- Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
- Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



