-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
MIF Contributes to Associated Immunopathogenicity Development
Uncontrolled inflammation is a major contributor to pathogenicity development during many chronic parasitic infections, including African trypanosome infections. Hence, therapies should aim at re-establishing the balance between pro - and anti-inflammatory responses to reduce tissue damage. Our experiments uncovered that macrophage migration inhibitory factor (MIF) plays a pivotal role in trypanosomiasis-associated pathogenicity development. Hereby, MIF-deficient and neutralizing anti-MIF antibody-treated wild type (WT) T. brucei-infected mice exhibited decreased inflammatory responses, reduced liver damage and anemia (i.e. the most prominent pathogenicity features) compared to WT control mice. The reduced tissue damage coincided with reduced infiltration of pathogenic monocytic cells and neutrophils, whereby neutrophil-derived MIF contributed more significantly than monocyte-derived MIF to tissue damage. MIF also promoted anemia development by suppressing red blood cell production and enhancing their clearance. The clinical significance of these findings follows from human genetic data indicating that low-expression (protective) MIF alleles are enriched in Africans. The current findings therefore offer promise for human translation and open the possibility of assessing MIF levels or MIF genotype as an indication of an individual's risk for severe trypanosomiasis. Furthermore, given the unmet medical need of African trypanosomiasis affecting millions of people, these findings highlight MIF as a potential new therapeutic target for treatment of trypanosomiasis-associated pathogenicity.
Published in the journal: MIF Contributes to Associated Immunopathogenicity Development. PLoS Pathog 10(9): e32767. doi:10.1371/journal.ppat.1004414
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004414Summary
Uncontrolled inflammation is a major contributor to pathogenicity development during many chronic parasitic infections, including African trypanosome infections. Hence, therapies should aim at re-establishing the balance between pro - and anti-inflammatory responses to reduce tissue damage. Our experiments uncovered that macrophage migration inhibitory factor (MIF) plays a pivotal role in trypanosomiasis-associated pathogenicity development. Hereby, MIF-deficient and neutralizing anti-MIF antibody-treated wild type (WT) T. brucei-infected mice exhibited decreased inflammatory responses, reduced liver damage and anemia (i.e. the most prominent pathogenicity features) compared to WT control mice. The reduced tissue damage coincided with reduced infiltration of pathogenic monocytic cells and neutrophils, whereby neutrophil-derived MIF contributed more significantly than monocyte-derived MIF to tissue damage. MIF also promoted anemia development by suppressing red blood cell production and enhancing their clearance. The clinical significance of these findings follows from human genetic data indicating that low-expression (protective) MIF alleles are enriched in Africans. The current findings therefore offer promise for human translation and open the possibility of assessing MIF levels or MIF genotype as an indication of an individual's risk for severe trypanosomiasis. Furthermore, given the unmet medical need of African trypanosomiasis affecting millions of people, these findings highlight MIF as a potential new therapeutic target for treatment of trypanosomiasis-associated pathogenicity.
Introduction
African trypanosomiasis is a parasitic disease of medical and veterinary importance that adversely affects the public health and economic development of sub-Saharan Africa. The causative agents, trypanosomes transmitted by the tsetse fly (Glossina spp), are extracellular hemoflagellated protozoans that cause fatal diseases in mammals, commonly called sleeping sickness in humans (HAT, Human African Trypanosomiasis) or nagana in domestic livestock [1]. In the case of bovine trypanosomiasis, anemia is considered to be the most prominent pathogenicity feature and the major cause of death associated with the disease [2]. In fact, the main difference between trypanosusceptible and trypanotolerant animals relies in their capacity to control anemia development. The underlying mechanisms mediating trypanosome-associated anemia have been scrutinized in murine models [3]. The data collectively suggest that a strong pro-inflammatory/type I immune response, involving classically activated myeloid cells/macrophages (M1), is required for initial parasite growth control. Yet, if maintained, this response contributes to pathogenicity in general and anemia in particular in trypanosusceptible mice, resulting in reduced survival of the host. Hereby, myeloid cell hyperactivation was proposed to be involved in the extravascular destruction of red blood cells (RBCs) due to enhanced erythrophagocytosis by spleen and liver-associated M1 cells of the infected host [3], [4] causing trypanosome-associated anemia. Such type I immune response driven anemia resembles anemia of inflammation, also termed anemia of chronic disease (ACD), that is associated with chronic infections and sterile inflammations [5], [6].
Uncontrolled inflammation associated with persistence of M1 cells is also a major cause of liver injury and cachexia observed in trypanosusceptible animals, whereby the anti-inflammatory cytokine IL-10 was found to be detrimental to prevent these pathogenic features [7]. Hence, therapies should aim at re-establishing the balance between pro - and anti-inflammatory signals during the disease to avoid tissue damage. In this context, the glycosylphosphatidylinositol (GPI)-anchor of the Variant Surface Glycoprotein (VSG) coat was identified as a major parasite-derived molecule with a M1-activating potential [8]. Interestingly, a GPI-based treatment was found to protect against infection-associated cachexia, liver damage, anemia, and to prolong survival by modulating the myeloid cell activation state, i.e. forcing a transition from M1 to M2 (alternatively) activated myeloid cells during the course of infection [9].
The possibility to render trypanosusceptible animals more tolerant by modulating the activation state of myeloid cells offers an attractive model to identify genes and gene-products involved in the pathogenicity of African trypanosomiasis. In this context, a comparative gene expression analysis revealed that the macrophage migration inhibitory factor (MIF) expression was significantly reduced in mice rendered trypanotolerant upon GPI treatment. This “early response” cytokine is expressed by numerous cell types, including myeloid cells, and plays a key role in innate and adaptive immunity [10], [11]. MIF is a prominent inducer of systemic inflammation in many inflammatory diseases [12], [13]. It functions by recruiting myeloid cells to the site of inflammation [14], by inducing their differentiation towards M1 cells secreting TNF [15] and by suppressing p53-dependent apoptosis of inflammatory cells [16]. Since African trypanosomes trigger a persistent type I/M1 immune response in trypanosusceptible (e.g. T. brucei brucei (T. brucei)) infected mice, we evaluated the potential role of MIF in the development of infection-associated pathogenicity. More specifically, the effect of MIF on the infiltration of myeloid cells, liver damage and anemia development was investigated.
Results
1. MIF expression levels show two distinct waves during T. brucei infection
As a first step towards evaluating the potential role of MIF during the course of T. brucei infection, we analysed its gene expression in different organs. As shown in Fig. 1A-C, MIF gene expression level in liver, spleen and bone marrow was characterized by two distinct phases, i.e. an initial increase during the acute phase of infection that returns back to the level of non-infected mice, followed by a second more progressive increase during the chronic phase of infection. Serum MIF protein levels followed the same kinetic as in the tested organs (Fig. 1D).
Fig. 1. MIF expression exhibits biphasic profiles during T. brucei infection. 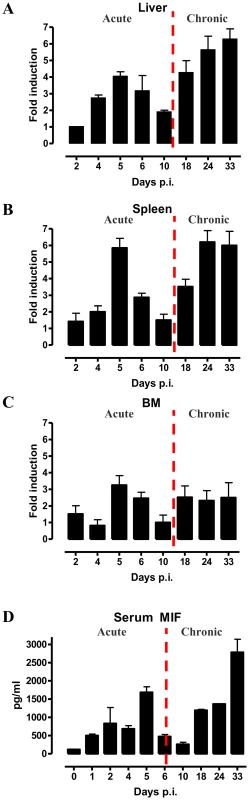
Mif gene expression levels in the liver (A), spleen (B), bone marrow (BM) (C) and serum MIF protein levels (D) during infection in C57Bl/6 mice. The dashed line delineates the transition from the acute to the chronic phase of infection. Gene expression levels were normalized against s12 and expressed relative to expression levels in non-infected mice. Results are representative for 2–3 independent experiments and presented as mean of 2–3 individual mice ± SEM. 2. MIF deficiency correlates with reduced type I inflammation during T. brucei infection
To evaluate the potential role of MIF in inflammation-associated pathogenicity occurring during T. brucei infection, two strategies targetting MIF production/activity were evaluated, (i) a comparison between wild type (WT) and MIF-deficient (Mif−/−) mice and (ii) a comparison between monoclonal anti-MIF IgG or isotype control antibody treated WT mice. As shown in Fig. 2A, first peak parasitemia and the further progression of parasitemia development were similar in WT and Mif−/− mice. However, there was a small, yet significant prolongation in median survival time in Mif−/− mice (Fig. 2B).
Fig. 2. MIF deficiency confers protection and reduces inflammatory immune responses during T. brucei infection. 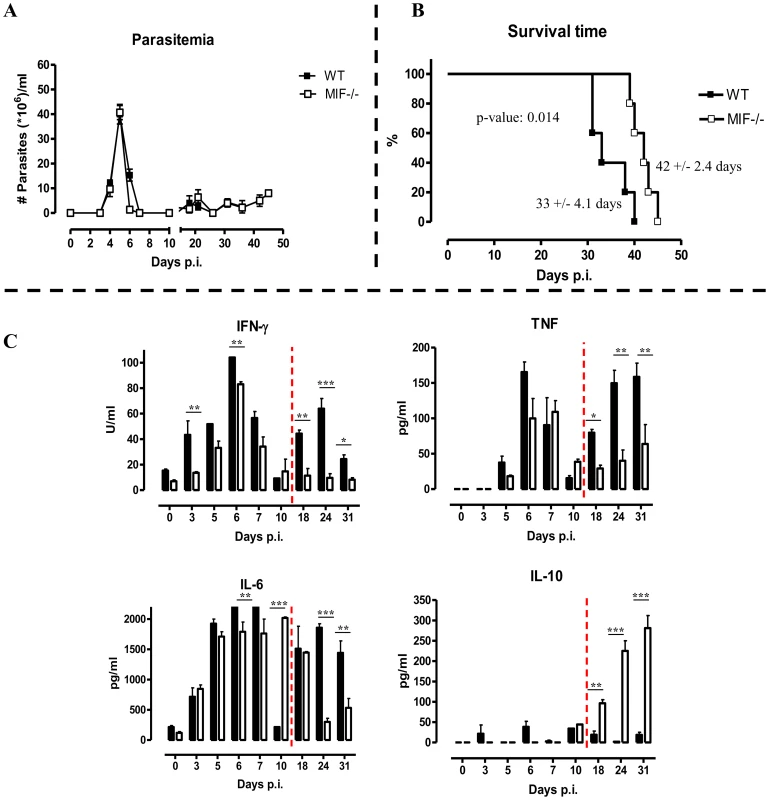
Parasitemia (A) and survival (B) during infection in C57Bl/6 (WT, black box; Mif−/−, white box) Mice. Results are representative of 2–5 independent experiments and expressed as mean of 5 individual mice ± SEM. (C) Serum cytokine levels of IFN-γ (upper left panel) (1 U = 100 pg), TNF (upper right panel), IL-6 (lower left panel) and IL-10 (lower right panel) in Mif−/− (white bars) and WT (black bars) mice. The dashed line delineates the transition from the acute to the chronic phase of infection. Results are representative of 3 independent experiments and presented as mean of 3 individual mice ± SEM (*: p-values ≤0.05, **: p-values ≤0.01, p-value: ***≤0.001). Next, we investigated whether infected WT and Mif−/− mice exhibited different cytokine immune responses. The results shown in Fig. 2C indicate that during the course of infection, both strains of mice mounted a prominent pro-inflammatory immune response as evidenced by the elevated serum levels of IFN-γ, TNF and IL-6. Yet, these cytokine levels were lower in Mif−/− mice than in WT mice, especially during the chronic stage of infection. Conversely, serum IL-10 levels progressively increased during the chronic stage of infection (day 18 p.i. till the end) in Mif−/− mice whereas they were low/marginal in WT mice.
Similar to Mif−/− mice, anti-MIF IgG treatment did not affect parasitemia development but increased the median survival time compared to control antibody treated mice (Fig. S1A-B), suggesting a role for extracellular MIF in disease pathogenesis. As in Mif−/− mice, the pro-inflammatory cytokine production was decreased and IL-10 production was elevated during the chronic stage of infection upon anti-MIF IgG treatment of WT mice (Fig. S1C).
In a tsetse fly-mediated T. brucei infection model that mimics the natural route of infection, Mif deficiency did not affect parasitemia development but resulted in a prolonged survival (Fig. S2A-B) and a reduced pro-inflammatory cytokine profile (mainly IFN-γ) together with an increased IL-10 production during the chronic stage of infection (Fig. S2C).
3. Tissue pathogenicity and infiltration of CD11b+Ly6chighLy6G− and CD11b+Ly6cintLy6G+ myeloid cells are reduced in Mif−/− mice during the chronic phase of T. brucei infection
A persistent pro-inflammatory immune response contributes to liver damage in the chronic stage of T. brucei infection [7], [17]. Interestingly, at this stage (day 25 p.i.), Mif−/− mice exhibited significantly reduced liver pathogenicity than WT mice, as evidenced by lower hepatomegaly and reduced ALT (alanine aminotransferase) levels (Fig. 3A left and middle panel, respectively). The reduced serum AST (aspartate aminotransferase) levels further confirmed lower tissue pathogenicity in infected Mif−/− mice (Fig. 3A right panel).
Fig. 3. MIF deficiency reduces liver damage and alters liver cell composition during T. brucei infection. 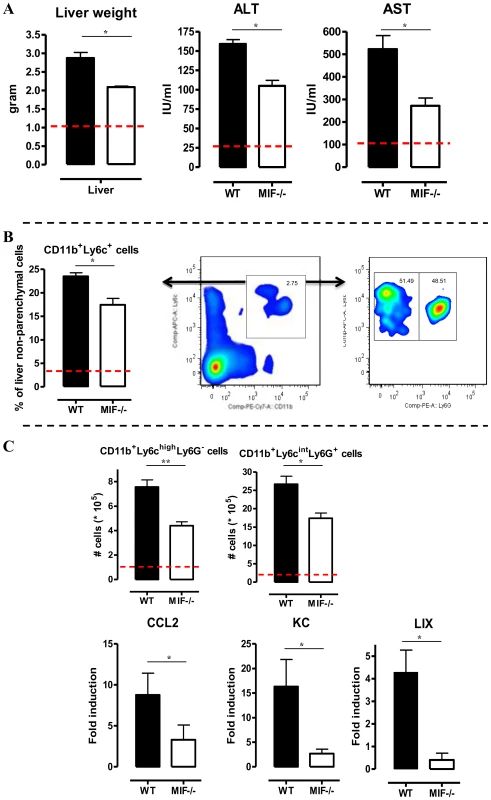
(A) Liver weight (left panel), serum ALT (middle panel) and AST (right panel) levels in Mif−/− (white bars) and WT (black bars) C57Bl/6 mice at day 25 p.i. (B) Representative liver immune cell gating strategy: Ly6c versus CD11b plot following gating on CD45+ and 7AAD- cells allows identifying CD11b+Ly6C+ cells (middle panel). This population was plotted in a Ly6C versus Ly6G plot to distinguish CD11b+Ly6ChighLy6G− inflammatory monocytes and CD11b+Ly6CintLy6G+ neutrophils (right panel). Percentage of CD11b+Ly6C+ cells within the liver CD45+ cells in WT (black bars) and Mif−/− (open bars) mice (left panel). (C) Number of CD11b+Ly6ChighLy6G− and CD11b+Ly6CintLy6G+ cells within the liver CD45+ cells (upper left panels). The dashed line represents values in non-infected mice. Total liver CCL2, KC (CXCL1) and LIX (CXCL5) gene expression levels in Mif−/− (white bars) and WT (black bars) mice at day 25 p.i. (lower panels). Gene expression levels are normalised using s12 and expressed relatively to expression levels of non-infected mice. Results are representative of 2 independent experiments and presented as mean of 3 individual mice ± SEM (*: p-values ≤0.05, **: p-values ≤0.01). We have documented that infiltration of CD11b+Ly6c+ myeloid cells in the chronic stage of T. brucei infection contributes to liver pathogenicity in WT mice [7], [18]. Upon gating on CD45+ liver non-parenchymal cells (see gating strategy Fig. S3A-C), we found that the infiltration of CD11b+Ly6c+ myeloid cells (Fig. 3B, middle panel) was reduced by 25% in infected Mif−/− mice compared to WT mice (Fig. 3B, left panel). The CD11b+Ly6c+ cells (Fig. 3B, middle panel) were further subdivided into CD11b+Ly6chighLy6G− inflammatory monocytes and CD11b+Ly6cintLy6G+ neutrophils (Fig. 3B, right panel). Both cell populations were significantly less represented in the liver of infected Mif−/− mice as compared to WT mice (Fig. 3C, upper panels), correlating with a reduced gene expression level of the inflammatory monocyte chemoattractant CCL2 and of the neutrophil chemoattractants CXCL1 (KC) and CXCL5 (LIX) in total liver (Fig. 3C, lower panels). These observations in Mif−/− mice were corroborated by anti-MIF IgG treatment of WT infected mice (Fig. 4A-B).
Fig. 4. Anti-MIF treatment reduces serum ALT/AST levels and affects liver cell composition during T. brucei infection. 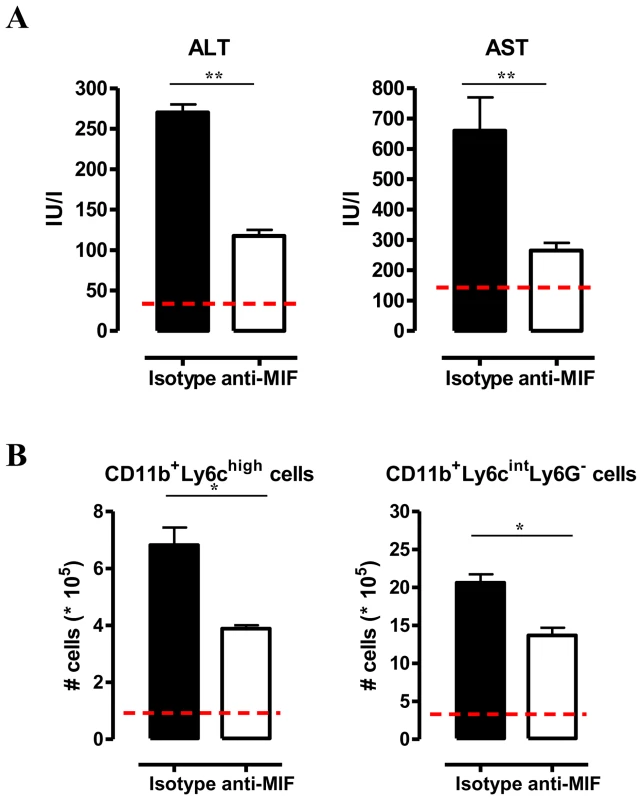
(A) Serum ALT and AST levels of non-infected (dashed line), isotype control antibody treated (black box) and anti-MIF IgG treated (white box) WT C57Bl/6 mice at day 25 p.i. (B) Total numbers of liver CD11b+Ly6ChighLy6G− and CD11b+Ly6CintLy6G+ cells in the chronic (day 25 p.i.) phase of infection calculated using the gating strategy described in Fig. 3B. Results are representative of 2 independent experiments and presented as mean of 3 individual mice ± SEM (*: p-values ≤0.05, **: p-values ≤0.01). Neutrophils can represent an important source of MIF [19] and so far their contribution to African trypanosomiasis remains unknown. We addressed the possible involvement of neutrophils to T. brucei infection outcome in first instance by measuring the myeloperoxidase (MPO) activity as read-out of neutrophil activity. We observed that MPO levels increased more in WT than in Mif−/− mice during the course of infection (Fig. S4). Secondly, we performed adoptive transfer experiments whereby bone marrow-derived neutrophils (i.e. CD11b+Ly6cintLy6G+ cells) isolated from T. brucei infected (day 24 p.i.) WT or Mif−/− mice were transferred into infected Mif−/− mice. Using neutrophils from T. brucei infected ubiquitin-GFP mice we could demonstrate that these cells were still present within the liver of recipient mice 18 hours post-transfer (Fig. S3D-E). Upon transfer of WT but not Mif−/− neutrophils, TNF levels in the liver cell culture supernatants as well as serum ALT/AST levels of Mif−/− recipient mice increased to the levels of infected WT mice (Fig. 5). MIF levels also increased in liver cells culture supernatants of Mif−/− recipient mice treated with WT neutrophils (Fig. 5), reflecting the contribution of neutrophils to MIF production. On the other hand, adoptive transfer of WT CD11b+Ly6chighLy6G− inflammatory monocytes in infected Mif−/− recipient mice resulted in a lower increase in TNF and MIF levels in the liver cell culture supernatants than transfer of WT neutrophils (Fig. S3F-G; Fig. 5). The serum ALT/AST levels increased to a similar level in Mif−/− recipient mice treated with WT monocytes or WT neutrophils (Fig. 5). Together, these data indicate that during T. brucei infection neutrophils can produce more MIF than monocytes and hereby are more important contributors than monocytes to liver pathogenic TNF production. Neutrophils and monocytes contribute to similar extent to liver injury in infected mice. However, while neutrophil-derived MIF is primarily responsible for liver injury, the pathogenic activity of monocytes is relatively less MIF-dependent.
Fig. 5. Neutrophil-derived MIF and monocyte-derived MIF contribute to different extent to TNF production and liver injury in T. brucei infected mice. 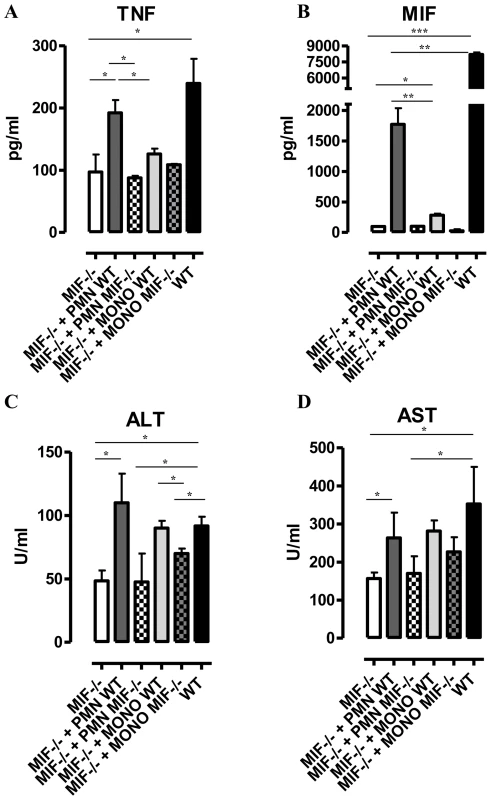
TNF and MIF levels from liver cell cultures (A, B), serum ALT (C) and AST (D) levels of infected (day 24 p.i.) Mif−/− mice (white box), of infected Mif−/− mice treated with neutrophils from WT (dark grey box) or Mif−/−(white hatched box) infected mice, of infected Mif−/− mice treated with monocytes from WT (light grey box) or Mif−/− (darker grey hatched box) infected mice and of infected WT mice (black box). Results are representative of 3–4 independent experiments and presented as mean of 3 individual mice ± SEM (*: p-values ≤0.05, **: p-values ≤0.01, ***: p-value ≤0.001). 4. MIF deficiency correlates with reduced anemia during the chronic phase of T. brucei infection
Besides liver damage, persistent inflammation during T. brucei infection causes anemia [5], which is characterized by two distinct phases; (i) a rapid decline in RBC levels followed by partial recovery during the acute phase of infection and (ii) a more progressive decline in RBC levels during the chronic phase of infection (Fig. 6A). During the acute phase of infection, the initial drop in RBC percentages starts at the same time (day 5–10 p.i.) in both mouse strains but was more severe in WT than Mif−/− mice. Indeed, Mif−/− mice lost about 30% of the total percentage of RBCs while WT mice lost about 50% as compared to non-infected mice (Fig. 6A). Subsequently, a partial RBC recovery phase reaching about 70% of that of non-infected mice occurred (day 10–14 p.i.), whereas in Mif−/− mice this recovery reached about 90% over a longer time period (day 10–18 p.i.). During the chronic phase of infection (following recovery) the RBC levels declined progressively and remained lower in WT than Mif−/− mice. Furthermore, treatment of infected WT mice with anti-MIF IgG resulted in a better recovery of RBCs similar as in infected Mif−/− mice (Fig. S5A). Finally, Mif−/− mice also showed reduced anemia upon tsetse fly-based infection (Fig. S5B).
Fig. 6. MIF deficiency correlates with reduced anemia, restored hemoglobin/serum iron levels and restored iron homeostasis during T. brucei infection. 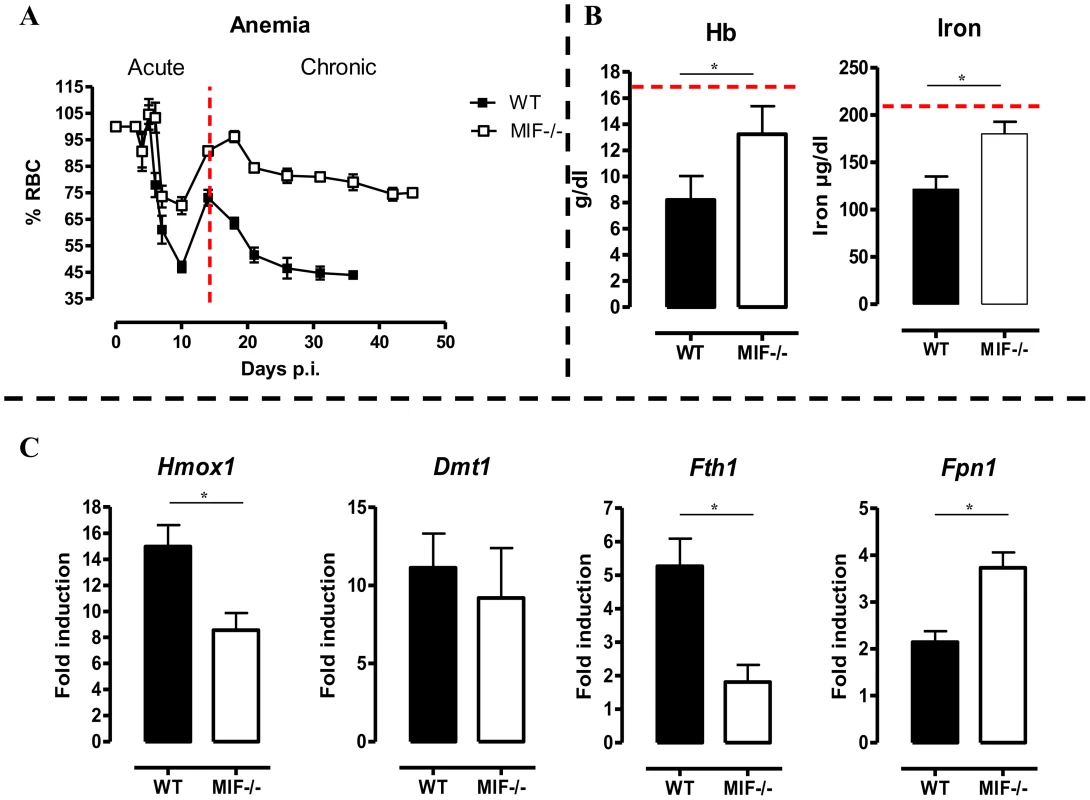
(A) Anemia development during infection in C57Bl/6 (WT, black box; Mif−/−, white box) mice. Results are representative of 2–5 independent experiments and expressed as mean of 3–5 individual mice ± SEM. (B) At day 18 p.i., (left panel) hemoglobin levels and (right panel) serum iron levels in Mif−/− (open bars) and WT (black bars) mice. (C) Expression levels of the iron-homeostasis associated genes Hmox1 (iron import), Dmt1 (iron transport), Fth1 (iron storage) and Fpn1 (iron export) were quantified by RT-QPCR in total livers from Mif−/− (white bars) and WT (black bars) mice at day 18 p.i. Gene-expression levels are normalised using s12 and expressed relatively to expression levels of non-infected mice. Results are representative of 2 independent experiments and presented as mean of 3 individual mice ± SEM (*: p-values ≤0.05). 5. Mif−/− mice exhibit restored iron homeostasis during the chronic phase of T. brucei infection
Anemia development during persistent inflammation can result from iron accumulation within the mononuclear phagocyte system (MPS) leading to iron deprivation from erythropoiesis. Hence, the reduced pro-inflammatory immune response in Mif−/− mice during the chronic stage of T. brucei infection as compared to WT mice might impact on iron-homeostasis and hemoglobin levels. At day 18 p.i., the time-point when the differences in anemia development and cytokine levels between WT and Mif−/− mice become apparent (see Fig. 6A and Fig. 2C), hemoglobin and serum iron levels were less reduced in Mif−/− mice as compared to WT mice (Fig. 6B). These differences were corroborated at the level of expression of genes implicated in iron homeostasis in the liver. In this context, it should be emphasized that under physiological conditions, tissue-associated myeloid cells, in particular liver myeloid cells, recover ferrous iron (Fe2+) via engulfment of senescent RBCs and hemoglobin recycling. The Fe2+ iron from hemoglobin is extracted by myeloid cells via heme oxygenase-1 (HO-1), then transported into the cytosol by the divalent metal transporter 1 (DMT-1/Nramp2), from where it can be either exported from or stored inside the myeloid cells via ferroportin-1 (FPN-1) or ferritin (FHC), respectively, to prevent toxicity, depending on the iron demand of the host [6]. As shown in Fig. 6C, there was a lower liver HO-1 (Hmox1) gene expression level in infected Mif−/− than WT mice (suggesting lower iron extraction), while the DMT-1 (Dmt1) gene expression levels were similar in both mouse groups (suggesting similar iron transport). In addition, the FHC (Fth1) gene expression levels were lower in infected Mif−/− mice (Fig. 6C), indicating reduced iron accumulation in Mif−/− than WT mice. Finally, Fpn1 mRNA levels were higher in infected Mif−/− mice (Fig. 6C), suggesting that iron export in these mice is less impaired as compared to WT mice. Together these data indicate that there is less iron extracted and retained in the MPS from infected Mif−/− than WT mice, which in turn allows increased iron availability for erythropoiesis.
6. Mif−/− mice display enhanced erythropoiesis during the chronic phase of T. brucei infection
The persistent pro-inflammatory immune response in T. brucei infection (see Fig. 2C), leading to iron accumulation within the MPS and iron deprivation (see Fig. 6B-C) could impair erythropoiesis. Therefore, the efficiency of the constitutive (i.e. bone marrow) and inflammation-induced (i.e. spleen) erythropoiesis was determined in WT and Mif−/− mice during the chronic stage of infection (day 18 p.i.) using three different approaches. First, as shown in Fig. 7A (left panel) we measured the relative abundance of immature (Ter-119+CD71+) and mature (Ter-119+CD71−) RBCs as described by [20]. Infected Mif−/− mice had relatively more mature RBCs in the bone marrow and the spleen than WT mice (Fig. 7A, right panels). Concomitantly, the reduction in the percentage of mature RBCs in the blood was less pronounced in infected Mif−/− mice than in WT mice. Secondly, we determined the expression levels of representative genes involved in RBC differentiation (Gata1, Epor, Tal1, Maea and Gas6) in the bone marrow and spleen. As shown in Fig. 7B, the expression of most genes was not induced (with the exception of Tal1) during infection in WT mice in both organs, while they increased in infected Mif−/− mice. Thirdly, we quantified the different stages of erythropoiesis (from nucleated erythroblasts (P1) till enucleated erythrocytes (P5)) using a recently described protocol [21] based on a CD44 versus FSC profile following gating on the Ter-119+ cells (Fig. 8A). It appeared that compared to WT mice, infected Mif−/− mice had a more efficient RBC maturation mainly at the later stage of differentiation (reflected by the increased percentage of P5) in the spleen and to a lesser extent in the bone marrow (Fig. 8B-C, respectively). Together, these data demonstrate a higher level of erythropoiesis in the absence of MIF in T. brucei infected mice.
Fig. 7. MIF deficiency correlates with restored/enhanced erythropoiesis during T. brucei infection. 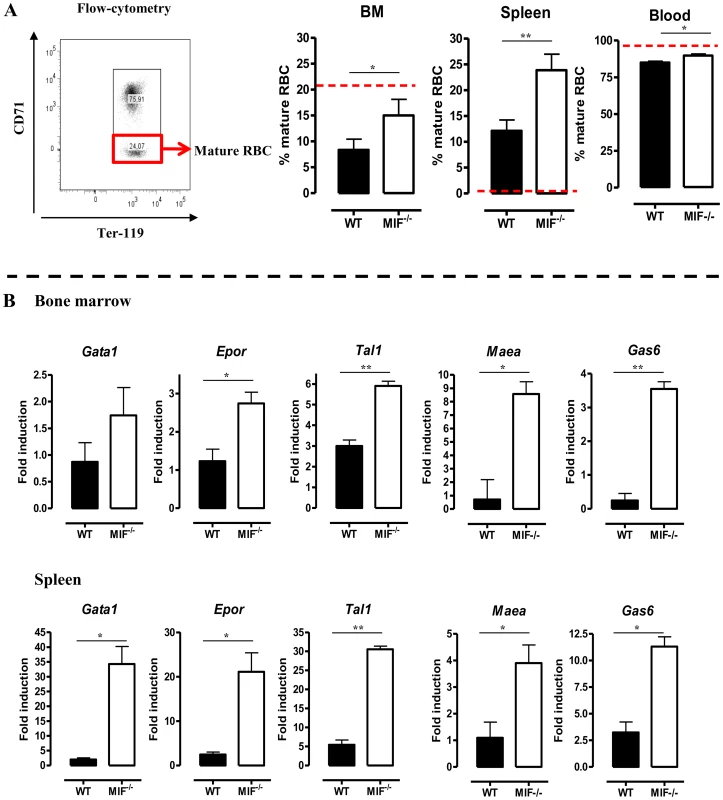
(A) Representative profile for the gating of mature (Ter-119+ CD71−) and immature (Ter-119+ CD71+) RBCs in bone marrow of non-infected C57Bl/6 mice (left panel). The percentage of mature RBCs within the total Ter-119+ population in bone marrow, spleen and blood at day 18 p.i. (right panels). Dashed line represents percentage of mature RBCs in non-infected mice, which was similar in WT and Mif−/− mice. Results are representative of 3 independent experiments and shown as mean of 3 individual mice ± SEM. (B) Expression levels of genes involved in erythropoiesis in total bone marrow (upper panels) and spleen (lower panels) from Mif−/− (white bars) and WT (black bars) mice at day 18 p.i. Gene-expression levels are normalised using s12 and expressed relatively to expression levels of non-infected mice. Results are representative of 2 independent experiments and presented as mean of 3 individual mice ± SEM (*: p-values ≤0.05, **: p-values ≤0.01). Fig. 8. MIF deficiency correlates with enhanced terminal RBC differentiation and reduced RBC clearance during T. brucei infection. 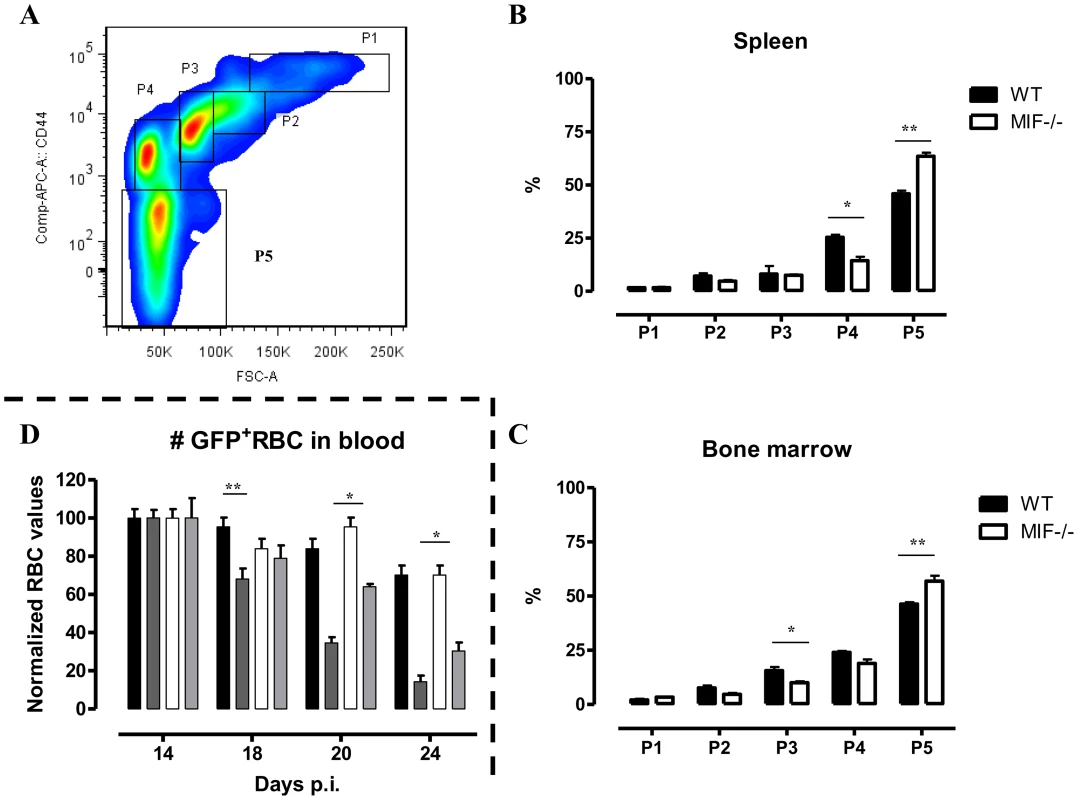
(A) Gating strategy used to discriminate different stages of erythroid development, starting from nucleated erythroblasts (P1 (pro), P2 (Basophilic + polychromatic) and P3 (orthochromatic)) till enucleated erythrocytes (P4 (reticulocyte) and P5 (erythrocyte)). 7AAD−CD45−Ter-119+ cells were selected and plotted in a CD44 versus FSC plot (upper panel). The percentage of the different erythroid populations at day 18 p.i. in WT (black box) and Mif−/− (white box) mice is shown for the spleen (B) and bone marrow (C). Results are representative of 2 independent experiments and presented as mean of 3 individual mice ± SEM. (D) RBC clearance in non-infected (WT, black bars; Mif−/−, white bars) and day 12 p.i. T. brucei infected (WT, dark grey bars; Mif−/−, light grey bars) C57Bl/6 mice following i.v injection with 200 µl GFP+ RBCs. At different time points after injection the presence of GFP+ RBCs following gating on Ter-119+ cells in the blood was evaluated. GFP+ RBC numbers were normalized, whereby GFP+ RBCs present after 1 day post infection are being referred as 100%. Results are representative of 2 independent experiments and presented as mean of 5 individual mice ± SEM (*: p-values ≤0.05, **: p-values ≤0.01). 7. Mif−/− mice display a reduced RBC clearance during the chronic phase of T. brucei infection
An increased RBC elimination may also contribute to anemia in T. brucei infected mice. To address this question, we injected green fluorescent protein positive (GFP+) RBCs i.v. at the start of the chronic phase of T. brucei infection (day 12 p.i.) in WT and Mif−/− mice and analyzed their clearance as the infection progressed. Non-infected WT and Mif−/− mice were treated similarly with GFP+ RBCs as controls. As shown in Fig. 8D, there was no difference in GFP+ RBC clearance between non-infected WT and Mif−/− mice. However, the GFP+ RBC clearance during infection was faster compared to non-infected mice. In addition, relatively more GFP+ RBCs remained in infected Mif−/− compared to WT mice (Fig. 8D). These data suggest that a reduction in RBC elimination observed in Mif−/− mice could contribute to less severe anemia during T. brucei infection.
Discussion
We investigated herein the role of MIF, an upstream regulator of the inflammatory response, in the immunopathogenicity of experimental African trypanosomiasis. We found that during T. brucei infection in trypanosusceptible wild type (WT) mice, the MIF gene expression profile in lymphoid tissues and the systemic serum protein level consists of two distinct waves; an upregulation during the acute phase of infection that declines after the first peak of parasitemia, followed by a second increase as the infection progresses to the chronic phase. Our results are in line with observations documenting that the Mif mRNA levels were increased in spleen cells during T. brucei infection in rats [22]. Using Mif−/− mice and neutralizing anti-MIF IgG treatment, we revealed that absence/blockade of MIF did not affect parasite growth. Yet, absence of MIF slightly increased survival time and reduced serum IFN-γ, TNF, and IL-6 concentrations while inducing IL-10 production in the chronic stage of infection. This observation most likely reflects a switch from a pro-inflammatory/pathogenic type I to an anti-inflammatory/anti-pathogenic type 2 response in the host. The higher TNF and IFN-γ response observed in WT mice compared to Mif−/− mice during the chronic stage of infection could result from the reported positive feedback loop between MIF, TNF and IFN-γ [23].
The switch from a pro-inflammatory to an anti-inflammatory immune response in Mif−/− or anti-MIF IgG treated WT mice during the chronic stage of infection was reflected by a reduction in pathogenicity, as evidenced by less liver damage and anemia, which are the main pathogenic manifestations of T. brucei infection [3], [7]. CD11b+Ly6chighLy6G− inflammatory monocytes are reported to contribute to T. brucei-induced pathogenicity development through their production of TNF, whereby in the acute phase of infection their emigration from the bone marrow is CCL2/CCR2-dependent [18], [24]. Although MIF can induce CCL2 [25] and trigger monocyte recruitment and subsequent arrest in tissue [14], the bone marrow emigration and recruitment of inflammatory monocytes in tissues during the acute stage of infection was found independent of MIF [24]. We now report that MIF contributed to the recruitment of inflammatory monocytes into the liver in the chronic stage of T. brucei infection. The lower accumulation of inflammatory monocytes in Mif−/− mice at this stage of infection could result from the reduced expression of Ccl2 and/or increased production of IL-10. Indeed, during T. brucei infection, IL-10 has been reported to limit the CCR2-mediated egress of monocytes from the bone marrow and to suppress their differentiation/maturation into TNF producing cells, thereby preventing tissue damage [18], [24]. The more sustained MIF production during the chronic stage of infection compared to the acute stage could also account for the observed accumulation of inflammatory monocytes in WT infected mice.
Our data further revealed that CD11b+Ly6cintLy6G+ neutrophils accumulated in the liver of WT mice during the chronic stage of T. brucei infection, coinciding with increased gene expression of the neutrophil-specific chemokines KC and LIX in total liver [26]. Yet, this increase was higher in WT than Mif−/− mice. Of note, besides indirect effects of MIF on monocyte and neutrophil recruitment mediated via induction of CCL2 and KC/LIX, respectively, we can't exclude that direct MIF effects account for the observed difference in cell recruitment/retention between WT and Mif−/− infected mice. Indeed, MIF features structural motives shared by canonical CXC+ ligands and is therefore considered a non-cognate ligand of the chemokine receptors CXCR2 and CXCR4 that, through interaction with these receptors, can be instrumental in inflammatory pathogenic leukocyte recruitment [14]. Moreover, MIF was also shown to bind CD74 (Ia-associated invariant chain), a single-pass membrane-receptor, which in turn can form functional complexes with either CXCR2 or CXCR4 and subsequently mediates MIF-specific signaling [14].
Although neutrophilia has been documented before [27], the role of neutrophils in the outcome and pathogenesis of African trypanosomiasis is poorly investigated. In this regard, we report that the serum myeloperoxidase activity, as indicator of neutrophil activity which can play a pro-inflammatory pathogenic role during chronic infections [28], increased significantly during T. brucei infection in WT mice and to a lower extent in Mif−/− mice. An accumulation of neutrophils in the liver of T. brucei infected mice can be relevant. Indeed, neutrophils represent an important source of MIF that can be released upon activation or TNF-induced apoptosis [19]. Moreover, neutrophils can enhance hepatocyte death that release necrotic products into the circulation triggering a systemic inflammatory immune response [29], which is the major cause of tissue pathogenicity in T. brucei infected mice. Accordingly, liver MIF protein levels increased drastically during the chronic phase of infection. Moreover, we observed a pathogenic role for WT neutrophils upon their adoptive transfer into infected Mif−/− recipient mice, which resulted in increased TNF production and increased ALT/AST levels. Since the transfer of neutrophils from Mif−/− mice into WT recipient mice did not affect TNF and liver injury, it seems that MIF production by neutrophils evidenced in this report plays a prominent, previously unappreciated role in trypanosomiasis-associated pathogenicity. On the other hand, the transfer of WT monocytes, that contribute to MIF production in acute T. brucei infection stage [24], into Mif−/− mice recipient mice only resulted in a moderate increase in MIF and TNF production but induced similar level of tissue injury than the transfer of WT neutrophils. Hence, neutrophil-derived MIF is a more important contributor than monocyte-derived MIF to pathogenicity in infected mice. To our knowledge, this is the first time that the neutrophil is identified as the more prominent source of MIF in a disease situation. Although the pathogenic activity of monocytes is relatively less dependent from their MIF production and TNF induction than the one of neutrophils, monocytes could contribute to liver injury through production of other pathogenic type I immune response associated molecules such as Cxcl10, Cxcl9 or Ccl3 [24].
Besides liver pathogenicity, a clinically significant anemia develops during African trypanosomiasis [3], [30]. We observed reduced anemia and preserved serum iron levels during the course of T. brucei infection in Mif−/− mice. These data are in concordance with our previous results showing that reducing the inflammatory immune responses using a GPI-based treatment coincides with the alleviation of anemia and improved iron-homeostasis resulting in higher iron-bioavailability and increased erythropoiesis [31]. Similarly, key genes involved in iron homeostasis were differentially modulated in T. brucei infected WT and Mif−/− mice. Indeed, Hmox1 which is involved in RBC catabolism and iron extraction from hemoglobin, together with the iron storage molecule Fth1, showed reduced expression in infected Mif−/− mice. Conversely, the iron cell exporter Fpn1 expression increased upon T. brucei infection. Given that TNF and IFN-γ can induce Hmox1, that TNF and IL-6 are important inducers of Fth1 and that IFN-γ can suppress Fpn1 expression [6], the reduced levels of TNF and IL-6, as well as of IFN-γ may explain for the lower Hmox1, Fth1 and higher Fpn1 expression levels in Mif−/− mice.
The reduced inflammatory immune response coupled with restored iron-homeostasis in infected Mif−/− mice could alleviate the suppression of erythropoiesis that occurs during the chronic phase of T. brucei infection [32]. Our data indeed show that the absence of MIF correlated with improved erythropoiesis as evidenced by the higher percentage of mature RBCs and increased expression of genes involved in erythropoiesis (Gata1, Epor, Tal1, Maea and Gas6) in both the bone marrow and spleen during the chronic phase of infection. In this context, elevated MIF levels in the spleen and bone marrow of P. chabaudi-infected mice were found to inhibit the early stages of erythropoiesis as well as hemoglobin production [33]. In the T. brucei African trypanosomiasis model, the suppressive effect of MIF was observed at the later stage of erythroid differentiation. The increased gene expression levels in T. brucei infected Mif−/− mice of Maea, which is crucial for the enucleation of reticulocytes [34], and of Gas6, which reduces TNF-mediated apoptosis of erythroid progenitor cells [35], may contribute to the more efficient RBC maturation. The reduced anemia observed in T. brucei infected Mif−/− mice could also be attributed to the better RBC recovery/reduced RBC clearance in the chronic phase of infection, which in turn could result from reduced myeloid cell activation and/or lower IFN-γ as compared to WT infected mice.
Collectively, our results suggest that in the chronic phase of T. brucei infection, MIF contributed to inflammation-associated pathogenicity by (i) sustaining a persistent pro-inflammatory type I immune response and (ii) maintaining/enhancing the recruitment of pathogenic monocytic cells and neutrophils in the liver. Hereby, neutrophil-derived MIF contributed significantly to enhanced TNF production and liver damage. In addition, MIF contributed to (iii) iron-accumulation in liver myeloid cells, suppressing erythropoiesis at later stages of erythroblast differentiation and (iv) enhanced RBC clearance. Despite reduced pathogenicity, Mif−/− mice exhibit only a moderate increase in survival compared to WT mice, inferring that MIF-independent mechanisms determine survival of infected mice. Yet, so far the exact cause of death in murine African trypanosomiasis is unknown and is suggested to be due to Systemic Inflammatory Response Syndrome (SIRS), thus likely multifactorial [36] and going beyond the role of MIF.
Interestingly, polymorphisms in the Mif gene have been shown to contribute to susceptibility in several inflammatory diseases (reviewed in [37]). Moreover, low-expression MIF alleles, which occur more commonly in Africans, may offer protection from disease manifestations in trypanosomiasis endemic settings [38]. Therefore, interfering with MIF signaling could be a novel approach to limit inflammation-associated complications during T. brucei trypanosomiasis.
Materials and Methods
Ethics statement
All experiments complied with the ECPVA guidelines (CETS n° 123) and were approved by the VUB Ethical Committee (Permit Number: 08-220-8). T. brucei tsetse fly infections were approved by the Environmental administration of the Flemish government.
Parasites, mice and infections
Clonal pleomorphic T. brucei AnTat 1.1E parasites were a kind gift from N. Van Meirvenne (Institute for Tropical Medicine, Belgium) and stored at −80°C. Tsetse flies infected with non-clonal T. brucei AnTAR1 parasites were maintained at the Institute of Tropical Medicine. Wild type (WT) C57Bl/6 mice were obtained from Janvier. MIF deficient (Mif−/−) [39] and ubiquitin-GFP (Jackson Laboratories) C57Bl/6 mice were bred in our animal facility.
Female mice (7–8 weeks old) were infected with 5×103 AnTat1.1E trypanosomes (intraperitonealy (i.p.)) or using one individual tsetse fly with a mature salivary gland infection which was allowed to feed per mouse.
Parasite and red blood cell (RBC) numbers in blood were determined via haemocytometer by tail-cut (2.5 µl blood in 500 µl RPMI). Anemia was expressed as percentage of RBCs remaining in infected mice compared to that of non-infected mice. MIF-neutralisation experiments were performed by i.p. injection of mice with 500 µg neutralizing anti-MIF IgG1 [40] or matching isotype control IgG (University of Louvain, Experimental Immunology Unit) every second day starting from day 1 post infection (p.i.).
Measurement of serum iron and myeloperoxidase (MPO) content as well as of blood hemoglobin levels
Total serum iron was measured using the IRON FZ kit (Chema Diagnostics) as recommended by the suppliers. Serum MPO concentrations were measured as described by the suppliers (R&D systems). Blood Hemoglobin levels were quantified colorimetrically. Briefly, 2 µl blood was diluted in 200 µl distilled water in a 96 well round bottom plate (Falcon) and incubated for 30 min. at 37°C. After centrifugation (600 g, 10 min.) the supernatant was collected and the OD540nm measured in an Ultra Microplate reader (ELx808, Bio-Tek instruments.inc). The hemoglobin concentration was calculated using a hemoglobin standard (Sigma).
Liver cell isolation protocol
Livers from CO2 euthanized mice were perfused with 30 ml heparinized saline (10 units/ml; Leo Pharma) containing 0.05% collagenase type II (Clostridium histolyticum; Sigma-Aldrich), excised and rinsed in saline. Following liver mincing in 10 ml digestive media (0.05% collagenase type II in Hanks' Balanced Salt Solution (HBSS) without calcium or magnesium; Invitrogen) and incubation at 37°C for 30 min, the digested tissue was homogenized and filtered (40 µm pore filter). The cell suspension was centrifuged (7 min, 300×g, 4°C) and the pellet treated with erythrocyte-lysis buffer. Following centrifugation (7 min, 300×g, 4°C) the pellet was resuspended in 2–5 ml RPMI/5%FCS and cells counted to bring them at 107 cells/ml for flow-cytometric analysis, RT-QPCR and cell culturing. For RT-QPCR analysis 107 cells were resuspended in 1 ml TRIzol (Gibco-Invitrogen Life Technologies) and stored at −80°C.
Spleen and bone marrow cell isolation
Spleen and bone marrow (tibia and femur) cells were obtained by homogenizing the organs in 10 ml RPMI medium, passing the suspension through a 40 µm pore filter and centrifugation (7 min, 300×g, 4°C). After resuspending the pellet, cells were counted and brought at 107 cells/ml for flow cytometry (RBC) analysis. Remaining cells were pelleted (7 min, 300×g, 4°C) and processed as described for the liver (see above).
Cytokine analysis
Concentrations of MIF and TNF (R&D Systems) as well as IFN-γ, IL-6 and IL-10 (Pharmingen) in serum and/or culture medium were determined by ELISA as recommended by the suppliers.
Real-time quantitative polymerase chain reaction (RT-QPCR) analysis
1 µg total RNA prepared from 107 cells was reverse-transcribed using oligo(dT) and Superscript II Reverse Transcription (Roche Molecular Systems) following the manufacturer's recommendations. RT-QPCR conditions were as described in [41]. Primer sequences are reported in Table S1 and Ct values of the S12 household gene for naive and infected mice samples (within the BM, spleen and liver) is shown in Table S2.
Flow cytometry
Blood, spleen and bone marrow cells were analysed before RBC lysis. Briefly, total blood (2.5 µl diluted in 500 µl RPMI) and spleen and bone marrow (100 µl from a stock solution of 107 cells/ml) were incubated (20 min, 4°C) with Fc-gamma blocking antibody (2.4G2, BD Biosciences) and further stained with phycoerythrin (PE)-conjugated anti-Ter-119, fluorescent isothiocyanate (FITC)-conjugated anti-CD71, Allophycocyanin (APC)-conjugated CD44 (eBioscience, ImmunoSource), PE-Cy7 conjugated rat anti-CD11b (BD Pharmingen) and matching control antibodies. The cells were washed with PBS, measured on FACSCanto II (BD Biosciences) and the results analysed using FlowJo software by gating on Ter-119+ cells.
The bone marrow, spleen and liver cells after RBC lysis were analyzed using APC-Cy7 conjugated CD45 (BD Pharmingen), PE-Cy7 conjugated rat anti-CD11b (BD Pharmingen), Alexa647-conjugated rat anti-Ly6c (Serotec), PE-conjugated rat anti-Ly6G (BD Pharmingen) and matching control antibodies. 7AAD (BD Pharmingen) was used to exclude death cells.
Adoptive transfer experiments
Neutrophils and monocytes were isolated from the bone marrow of infected mice (day 24 p.i). Following isolation and surface staining as described above using PE-Cy7 conjugated rat anti-CD11b (BD Pharmingen) and Alexa647-conjugated rat anti-Ly6c (Serotec), neutrophils were sorted based on their CD11b+Ly6cint surface expression and FSC/SSC profile using FACSAria II (BD) and subsequently stained with PE-conjugated rat anti-Ly6G (BD Pharmingen) to confirm their purity (95–99%). Monocytes were sorted based on their CD11b+Ly6chigh surface expression and FSC/SSC profile using FACSAria II (BD). Next, 3 individual infected (24 days p.i.) WT or Mif−/− mice were injected intravenously (i.v.) with 2×106 neutrophils, 2×106 monocytes or 200 µl RPMI (control group). 18 hours after transfer cells were isolated from the liver of recipient mice and analyzed.
Cell culturing
Liver cells (stock: 107/ml) were diluted till 2×106 cells/ml in RPMI-1640 medium/10% FCS/non-essential amino acids/glutamate/penicillin/streptomycin. Next, 500 µl cell suspension/well were cultured (36 hours, 37°C, 5% CO2) in 48 well plates (Nunc) and the supernatant tested for TNF levels.
Aspartate transaminase (AST) and alanine transaminase (ALT) measurement
Serum AST and ALT levels were determined as described by the suppliers (Boehringer Mannheim Diagnostics).
RBC clearance assay
200 µl heparinized blood from 7–8 weeks old ubiquitin-GFP C57Bl/6 mice was injected i.v. into 3–5 non-infected or infected (12 days p.i.) mice. Every second day, RBC numbers were enumerated via haemocytometer (see above) and the remaining cells analysed via FACS (see above) using PE-conjugated Ter-119 antibody. Following gating on Ter-119+ cells, two distinct RBC populations could be identified (GFP+ or -). To calculate the RBC clearance, the percentage of GFP+ RBCs present at day 1 post injection was referred to as 100% signal. All other time-points were compared with this day.
Statistical analysis
The GraphPad Prism 4.0 software was used for statistical analyses (Two-way ANOVA or student t-test). Values are expressed as mean ± SEM. Values of p≤0.05 are considered statistically significant.
Supporting Information
Zdroje
1. BarrettMP, BurchmoreRJ, StichA, LazzariJO, FraschAC, et al. (2003) The trypanosomiases. Lancet 362 : 1469–1480.
2. d'IeterenGD, AuthieE, WissocqN, MurrayM (1998) Trypanotolerance, an option for sustainable livestock production in areas at risk from trypanosomosis. Rev Sci Tech 17 : 154–175.
3. StijlemansB, VankrunkelsvenA, CaljonG, BockstalV, GuilliamsM, et al. (2010) The central role of macrophages in trypanosomiasis-associated anemia: rationale for therapeutical approaches. Endocr Metab Immune Disord Drug Targets 10 : 71–82.
4. NaessensJ (2006) Bovine trypanotolerance: A natural ability to prevent severe anaemia and haemophagocytic syndrome? Int J Parasitol 36 : 521–528.
5. StijlemansB, VankrunkelsvenA, BrysL, MagezS, De BaetselierP (2008) Role of iron homeostasis in trypanosomiasis-associated anemia. Immunobiology 213 : 823–835.
6. WeissG, GoodnoughLT (2005) Anemia of chronic disease. N Engl J Med 352 : 1011–1023.
7. BosschaertsT, GuilliamsM, StijlemansB, De BaetselierP, BeschinA (2009) Understanding the role of monocytic cells in liver inflammation using parasite infection as a model. Immunobiology 214 : 737–747.
8. MagezS, StijlemansB, BaralT, De BaetselierP (2002) VSG-GPI anchors of African trypanosomes: their role in macrophage activation and induction of infection-associated immunopathology. Microbes Infect 4 : 999–1006.
9. StijlemansB, BaralTN, GuilliamsM, BrysL, KorfJ, et al. (2007) A glycosylphosphatidylinositol-based treatment alleviates trypanosomiasis-associated immunopathology. J Immunol 179 : 4003–4014.
10. CalandraT, RogerT (2003) Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol 3 : 791–800.
11. LarsonDF, HorakK (2006) Macrophage migration inhibitory factor: controller of systemic inflammation. Crit Care 10 : 138.
12. AyoubS, HickeyMJ, MorandEF (2008) Mechanisms of disease: macrophage migration inhibitory factor in SLE, RA and atherosclerosis. Nat Clin Pract Rheumatol 4 : 98–105.
13. Rosado JdeD, Rodriguez-SosaM (2011) Macrophage migration inhibitory factor (MIF): a key player in protozoan infections. Int J Biol Sci 7 : 1239–1256.
14. BernhagenJ, KrohnR, LueH, GregoryJL, ZerneckeA, et al. (2007) MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 13 : 587–596.
15. CalandraT, BernhagenJ, MetzCN, SpiegelLA, BacherM, et al. (1995) MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377 : 68–71.
16. MitchellRA, LiaoH, ChesneyJ, Fingerle-RowsonG, BaughJ, et al. (2002) Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci U S A 99 : 345–350.
17. MagezS, TruyensC, MerimiM, RadwanskaM, StijlemansB, et al. (2004) P75 tumor necrosis factor-receptor shedding occurs as a protective host response during African trypanosomiasis. J Infect Dis 189 : 527–539.
18. GuilliamsM, MovahediK, BosschaertsT, VandenDriesscheT, ChuahMK, et al. (2009) IL-10 dampens TNF/inducible nitric oxide synthase-producing dendritic cell-mediated pathogenicity during parasitic infection. J Immunol 182 : 1107–1118.
19. DaryadelA, GrifoneRF, SimonHU, YousefiS (2006) Apoptotic neutrophils release macrophage migration inhibitory factor upon stimulation with tumor necrosis factor-alpha. J Biol Chem 281 : 27653–27661.
20. SocolovskyM, NamH, FlemingMD, HaaseVH, BrugnaraC, et al. (2001) Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood 98 : 3261–3273.
21. LiuJ, ZhangJ, GinzburgY, LiH, XueF, et al. (2013) Quantitative analysis of murine terminal erythroid differentiation in vivo: novel method to study normal and disordered erythropoiesis. Blood 121: e43–49.
22. NishimuraK, NakayaH, NakagawaH, MatsuoS, OhnishiY, et al. (2011) Differential effects of Trypanosoma brucei gambiense and Trypanosoma brucei brucei on rat macrophages. J Parasitol 97 : 48–54.
23. CalandraT, BernhagenJ, MitchellRA, BucalaR (1994) The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med 179 : 1895–1902.
24. BosschaertsT, GuilliamsM, StijlemansB, MoriasY, EngelD, et al. (2010) Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS Pathog 6: e1001045.
25. GregoryJL, MorandEF, McKeownSJ, RalphJA, HallP, et al. (2006) Macrophage migration inhibitory factor induces macrophage recruitment via CC chemokine ligand 2. J Immunol 177 : 8072–8079.
26. LinM, CarlsonE, DiaconuE, PearlmanE (2007) CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J Leukoc Biol 81 : 786–792.
27. OkaM, NagasawaH, ItoY, HimenoK (1989) Granulocyte-macrophage colony-stimulating activity in the serum of mice stimulated with homogenates of Trypanosoma gambiense. Clin Exp Immunol 78 : 285–291.
28. KlebanoffSJ (2005) Myeloperoxidase: friend and foe. J Leukoc Biol 77 : 598–625.
29. MarquesPE, AmaralSS, PiresDA, NogueiraLL, SorianiFM, et al. (2012) Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 56 : 1971–1982.
30. NaessensJ, KitaniH, NakamuraY, YagiY, SekikawaK, et al. (2005) TNF-alpha mediates the development of anaemia in a murine Trypanosoma brucei rhodesiense infection, but not the anaemia associated with a murine Trypanosoma congolense infection. Clin Exp Immunol 139 : 405–410.
31. StijlemansB, VankrunkelsvenA, BrysL, RaesG, MagezS, et al. (2010) Scrutinizing the mechanisms underlying the induction of anemia of inflammation through GPI-mediated modulation of macrophage activation in a model of African trypanosomiasis. Microbes Infect 12 : 389–399.
32. NishimuraK, NakayaH, NakagawaH, MatsuoS, OhnishiY, et al. (2011) Effect of Trypanosoma brucei brucei on erythropoiesis in infected rats. J Parasitol 97 : 88–93.
33. McDevittMA, XieJ, ShanmugasundaramG, GriffithJ, LiuA, et al. (2006) A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia. J Exp Med 203 : 1185–1196.
34. SoniS, BalaS, GwynnB, SahrKE, PetersLL, et al. (2006) Absence of erythroblast macrophage protein (Emp) leads to failure of erythroblast nuclear extrusion. J Biol Chem 281 : 20181–20189.
35. Angelillo-ScherrerA, BurnierL, LambrechtsD, FishRJ, TjwaM, et al. (2008) Role of Gas6 in erythropoiesis and anemia in mice. J Clin Invest 118 : 583–596.
36. De MuylderG, DaulouedeS, LecordierL, UzureauP, MoriasY, et al. (2013) A Trypanosoma brucei kinesin heavy chain promotes parasite growth by triggering host arginase activity. PLoS Pathog 9: e1003731.
37. RennerP, RogerT, CalandraT (2005) Macrophage migration inhibitory factor: gene polymorphisms and susceptibility to inflammatory diseases. Clin Infect Dis 41 Suppl 7S513–519.
38. ZhongXB, LengL, BeitinA, ChenR, McDonaldC, et al. (2005) Simultaneous detection of microsatellite repeats and SNPs in the macrophage migration inhibitory factor (MIF) gene by thin-film biosensor chips and application to rural field studies. Nucleic Acids Res 33: e121.
39. Fingerle-RowsonG, PetrenkoO, MetzCN, ForsthuberTG, MitchellR, et al. (2003) The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc Natl Acad Sci U S A 100 : 9354–9359.
40. CalandraT, EchtenacherB, RoyDL, PuginJ, MetzCN, et al. (2000) Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med 6 : 164–170.
41. RaesG, BrysL, DahalBK, BrandtJ, GrootenJ, et al. (2005) Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol 77 : 321–327.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell ResponsesČlánek RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct MechanismsČlánek Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 9- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Virus Control Goes Epigenetic
- The Role of Iron in Prion Disease and Other Neurodegenerative Diseases
- The Ins and Outs of Rust Haustoria
- Prion Strains and Amyloid Polymorphism Influence Phenotypic Variation
- Teaching Fido New ModiFICation Tricks
- Can Enhance Infection in Mosquitoes: Implications for Malaria Control?
- MIF Contributes to Associated Immunopathogenicity Development
- Persistence of Virus Reservoirs in ART-Treated SHIV-Infected Rhesus Macaques after Autologous Hematopoietic Stem Cell Transplant
- Bacillus Calmette-Guerin Infection in NADPH Oxidase Deficiency: Defective Mycobacterial Sequestration and Granuloma Formation
- EhCoactosin Stabilizes Actin Filaments in the Protist Parasite
- Molecular Insights Into the Evolutionary Pathway of O1 Atypical El Tor Variants
- LprG-Mediated Surface Expression of Lipoarabinomannan Is Essential for Virulence of
- Structural Correlates of Rotavirus Cell Entry
- Multivalent Adhesion Molecule 7 Clusters Act as Signaling Platform for Host Cellular GTPase Activation and Facilitate Epithelial Barrier Dysfunction
- The Effects of Vaccination and Immunity on Bacterial Infection Dynamics
- Myeloid Derived Hypoxia Inducible Factor 1-alpha Is Required for Protection against Pulmonary Infection
- Functional Characterisation of Germinant Receptors in and Presents Novel Insights into Spore Germination Systems
- Global Analysis of Neutrophil Responses to Reveals a Self-Propagating Inflammatory Program
- Host Cell Invasion by Apicomplexan Parasites: The Junction Conundrum
- Comparative Phenotypic Analysis of the Major Fungal Pathogens and
- Unravelling the Multiple Functions of the Architecturally Intricate β-galactosidase, BgaA
- Sialylation of Prion Protein Controls the Rate of Prion Amplification, the Cross-Species Barrier, the Ratio of PrP Glycoform and Prion Infectivity
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- Ontogeny of Recognition Specificity and Functionality for the Broadly Neutralizing Anti-HIV Antibody 4E10
- Identification and Characterisation of a Hyper-Variable Apoplastic Effector Gene Family of the Potato Cyst Nematodes
- Crimean-Congo Hemorrhagic Fever Virus Entry into Host Cells Occurs through the Multivesicular Body and Requires ESCRT Regulators
- Age-Dependent Enterocyte Invasion and Microcolony Formation by
- CD160-Associated CD8 T-Cell Functional Impairment Is Independent of PD-1 Expression
- Functional Fluorescent Protein Insertions in Herpes Simplex Virus gB Report on gB Conformation before and after Execution of Membrane Fusion
- The Tudor Domain Protein Spindlin1 Is Involved in Intrinsic Antiviral Defense against Incoming Hepatitis B Virus and Herpes Simplex Virus Type 1
- Transgenic Analysis of the MAP Kinase MPK10 Reveals an Auto-inhibitory Mechanism Crucial for Stage-Regulated Activity and Parasite Viability
- Evidence for a Transketolase-Mediated Metabolic Checkpoint Governing Biotrophic Growth in Rice Cells by the Blast Fungus
- Incomplete Deletion of IL-4Rα by LysM Reveals Distinct Subsets of M2 Macrophages Controlling Inflammation and Fibrosis in Chronic Schistosomiasis
- Identification and Functional Expression of a Glutamate- and Avermectin-Gated Chloride Channel from , a Southern Hemisphere Sea Louse Affecting Farmed Fish
- Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell Responses
- Strong Epistatic Selection on the RNA Secondary Structure of HIV
- Hematopoietic but Not Endothelial Cell MyD88 Contributes to Host Defense during Gram-negative Pneumonia Derived Sepsis
- Delineation of Interfaces on Human Alpha-Defensins Critical for Human Adenovirus and Human Papillomavirus Inhibition
- Exploitation of Reporter Strains to Probe the Impact of Vaccination at Sites of Infection
- RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct Mechanisms
- Helminth Infections Coincident with Active Pulmonary Tuberculosis Inhibit Mono- and Multifunctional CD4 and CD8 T Cell Responses in a Process Dependent on IL-10
- MHC Class II Restricted Innate-Like Double Negative T Cells Contribute to Optimal Primary and Secondary Immunity to
- Reactive Oxygen Species Regulate Caspase-11 Expression and Activation of the Non-canonical NLRP3 Inflammasome during Enteric Pathogen Infection
- Evolution of Plastic Transmission Strategies in Avian Malaria
- A New Human 3D-Liver Model Unravels the Role of Galectins in Liver Infection by the Parasite
- Translocates into the Myocardium and Forms Unique Microlesions That Disrupt Cardiac Function
- Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
- The Cofilin Phosphatase Slingshot Homolog 1 (SSH1) Links NOD1 Signaling to Actin Remodeling
- Kaposi's Sarcoma Herpesvirus MicroRNAs Induce Metabolic Transformation of Infected Cells
- Reorganization of the Endosomal System in -Infected Cells: The Ultrastructure of -Induced Tubular Compartments
- Distinct Dictation of Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality via Triggering TLR3 and TLR4 Signal Pathways
- Exploitation of the Complement System by Oncogenic Kaposi's Sarcoma-Associated Herpesvirus for Cell Survival and Persistent Infection
- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Structural Insight into Host Recognition by Aggregative Adherence Fimbriae of Enteroaggregative
- The CD14CD16 Inflammatory Monocyte Subset Displays Increased Mitochondrial Activity and Effector Function During Acute Malaria
- Infection Induces Expression of a Mosquito Salivary Protein (Agaphelin) That Targets Neutrophil Function and Inhibits Thrombosis without Impairing Hemostasis
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- MIF Contributes to Associated Immunopathogenicity Development
- The Ins and Outs of Rust Haustoria
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



