-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Dissemination of Cephalosporin Resistance Genes between Strains from Farm Animals and Humans by Specific Plasmid Lineages
The rapid global rise of infections caused by Escherichia coli that are resistant to clinically relevant antimicrobials, including third-generation cephalosporins, is cause for concern. The intestinal tract of livestock, in particular poultry, is an important reservoir for drug resistant E. coli, but it is unknown to what extent these bacteria can spread to humans. Food is thought to be an important source because drug-resistant E. coli have been detected in animals raised for meat consumption and in meat products. Previous studies that used traditional, low-resolution, genetic typing methods found that drug resistant E. coli present in humans and poultry were indistinguishable from each other, suggesting dissemination of these bacteria through the food-chain to humans. However, by applying high-resolution, whole-genome sequencing methods, we did not find evidence for such transmission of bacteria through the food-chain. Instead, by employing a novel approach for the reconstruction of mobile genetic elements from whole-genome sequence data, we discovered that genetically unrelated E. coli isolates from both humans and animal sources carried nearly identical plasmids that encode third-generation cephalosporin resistance determinants. Our data suggest that cephalosporin resistance is mainly disseminated via the transfer of mobile genetic elements between animals and humans.
Published in the journal: Dissemination of Cephalosporin Resistance Genes between Strains from Farm Animals and Humans by Specific Plasmid Lineages. PLoS Genet 10(12): e32767. doi:10.1371/journal.pgen.1004776
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004776Summary
The rapid global rise of infections caused by Escherichia coli that are resistant to clinically relevant antimicrobials, including third-generation cephalosporins, is cause for concern. The intestinal tract of livestock, in particular poultry, is an important reservoir for drug resistant E. coli, but it is unknown to what extent these bacteria can spread to humans. Food is thought to be an important source because drug-resistant E. coli have been detected in animals raised for meat consumption and in meat products. Previous studies that used traditional, low-resolution, genetic typing methods found that drug resistant E. coli present in humans and poultry were indistinguishable from each other, suggesting dissemination of these bacteria through the food-chain to humans. However, by applying high-resolution, whole-genome sequencing methods, we did not find evidence for such transmission of bacteria through the food-chain. Instead, by employing a novel approach for the reconstruction of mobile genetic elements from whole-genome sequence data, we discovered that genetically unrelated E. coli isolates from both humans and animal sources carried nearly identical plasmids that encode third-generation cephalosporin resistance determinants. Our data suggest that cephalosporin resistance is mainly disseminated via the transfer of mobile genetic elements between animals and humans.
Introduction
Antibiotic resistance among opportunistic pathogens is rapidly rising globally, hampering treatment of infections and increasing morbidity, mortality and health care costs [1], [2]. Of particular concern is the increased incidence of infections caused by Escherichia coli isolates producing extended-spectrum β-lactamases (ESBLs), which has rendered the use of third generation cephalosporins increasingly ineffective against this pathogen [3].
During the 1990s, the most commonly encountered ESBL genes were blaTEM and blaSHV, and their spread occurred mainly through cross-transmission in hospitals. However, the epidemiology of ESBL-producing E. coli has changed. Nowadays, the most prevalent ESBL gene type is blaCTX-M [4] and infections with ESBL-producing E. coli also occur in the community [5], [6]. The intestinal tracts of mammals and birds are important reservoirs for ESBL-producing E. coli [7], but it is unclear to what extent these bacteria can spread to humans. Food may be an important source, since ESBL genes have been detected in food-producing animals, especially poultry [8], [9], and on retail meat [10]. The presence of ESBL-producing bacteria in food has been attributed to widespread use of antimicrobials, including third generation cephalosporins, in industrial farming practices [11].
In The Netherlands, antibiotic use and prevalence of antibiotic resistance in humans are among the lowest in Europe [12], whereas antibiotic use in food-producing animals ranks among the highest in Europe [13]. These circumstances render The Netherlands particularly suitable to study the transfer of third-generation cephalosporin-resistant bacteria through the food-chain. Recent studies performed in The Netherlands suggested clonal transfer of ESBL-producing E. coli from poultry to humans [14]–[16]. However, these interpretations were based on typing methods that target a limited number of genes, and which may not have provided sufficient resolution to accurately monitor the epidemiology of pathogens [17]. In this study, we have therefore sequenced 28 ESBL-producing and four ESBL-negative E. coli strains that had previously been collected from humans, poultry, retail chicken meat and pigs and tested whether previous claims on the relationship between strains from different reservoirs could be confirmed at the whole-genome sequence level. Furthermore, we investigated the relatedness of cephalosporin resistance gene-carrying plasmids, which were derived from different backgrounds and reservoirs, at the genomic level.
Results
Sequencing of ESBL-producing E. coli
We assessed the relatedness of ESBL-producing E. coli from humans, animals and food by using Whole-Genome Sequencing (WGS). The genomes of 32, mostly ESBL-producing, E. coli strains isolated in The Netherlands in the period 2006–2011 were sequenced (Table 1). One set of isolates (n = 24) included five pairs of human and poultry-associated strains that had previously been found indistinguishable based on Multi Locus Sequence Typing (MLST), plasmid typing (pMLST) and ESBL gene sequencing [15], [18]. This set also included 11 human and poultry-associated isolates that carried an AmpC-type β-lactamase gene on an IncK plasmid [18]. The second set of isolates contained eight ESBL-producing strains that were isolated from pigs (n = 4) and their farmers (n = 4) (Table 1).
Tab. 1. E. coli strains sequenced in this study. 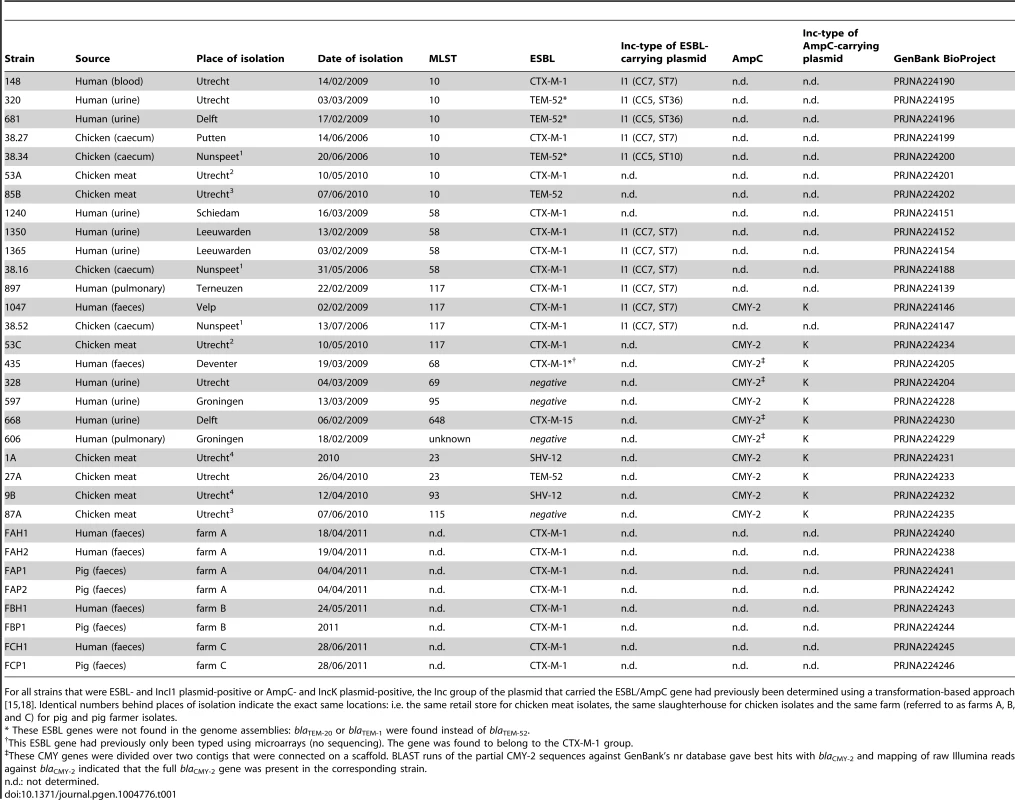
For all strains that were ESBL- and IncI1 plasmid-positive or AmpC- and IncK plasmid-positive, the Inc group of the plasmid that carried the ESBL/AmpC gene had previously been determined using a transformation-based approach [15], [18]. Identical numbers behind places of isolation indicate the exact same locations: i.e. the same retail store for chicken meat isolates, the same slaughterhouse for chicken isolates and the same farm (referred to as farms A, B, and C) for pig and pig farmer isolates. Illumina sequencing yielded draft genomes with an average assembly size of 5.2 Mbp (±0.17 Mbp), consisting of an average number of 133 scaffolds (±41) of size ≥500 bp and a mean N50 of 153 kbp (±47.9 kbp) (S1 Table). WGS-based MLST and ESBL gene analysis provided good agreement with previous typing data. Previously obtained MLST profiles and WGS-based MLST profiles were in complete agreement with each other. Although ESBL genes had previously been detected by both microarray-based methods and Sanger sequencing [15], the previously typed ESBL genes of four (out of 28) strains were absent from their assembled genomes. In three of these cases (strains 681, 320 and 38.34), we detected a blaTEM-1 or blaTEM-20 gene in the assembled genome, whereas a blaTEM-52 gene should have been found according to the typing data. Mapping the Illumina reads of these strains against their own assemblies showed that the assembled blaTEM genes contained several ambiguous positions pointing to the presence of more than one type of blaTEM gene (most likely a combination of blaTEM-1 and blaTEM-52) in these strains (S2 Table). In comparison, no ambiguous positions were found in the assembled blaTEM genes of other strains using the same mapping approach. In addition, the relative coverage of the assembled blaTEM genes of strains 681, 320 and 38.34 was higher than that of the assembled blaTEM genes of other strains (S2 Table). These findings suggested that strains 681, 320 and 38.34 contain multiple nearly identical blaTEM genes (i.e. blaTEM-1 and blaTEM-52) that hampered the correct assembly of these genes. The fourth inconsistency between WGS and typing data was the absence of blaCTX-M-1 from the assembly of strain 435. Mapping the reads of strain 435 against the blaCTX-M-1 gene sequence did suggest the presence of this gene in the WGS data, but with a depth of around 1/10th the average genomic sequencing depth. Possible explanations include a relatively poor isolation efficiency of the blaCTX-M-1-carrying plasmid and/or the loss of this plasmid from the bacterial cells during culturing in the absence of antibiotics. The previous AmpC typing data [18] and our WGS data were in complete agreement.
Phylogeny and epidemiology of ESBL-producing E. coli
To assess the phylogenetic context of the sequenced strains within the genus Escherichia and Shigella, we used publicly available genome sequences of Escherichia (n = 126) and Shigella (n = 12) strains. Based on COG assignments, we identified 215 core proteins in the 170 analysed genomes, from which a concatenated core genome alignment of 170461 bp was built. A phylogenetic tree based on the 18169 variable positions in this alignment confirmed previous clustering based around phylogroups A, B1, B2, D, E and F (Fig. 1) [19]. The sequenced strains clustered together in accordance with their ST. Strains did not cluster based on isolation source, year, plasmid or ESBL gene. The ESBL-producing strains were spread throughout the tree, indicating that acquisition of ESBLs arises in different E. coli genetic backgrounds and has occurred multiple times during evolution (Fig. 1).
Fig. 1. Phylogeny of Escherichia and Shigella species, including ESBL- and AmpC-positive strains sequenced for the purpose of this study. 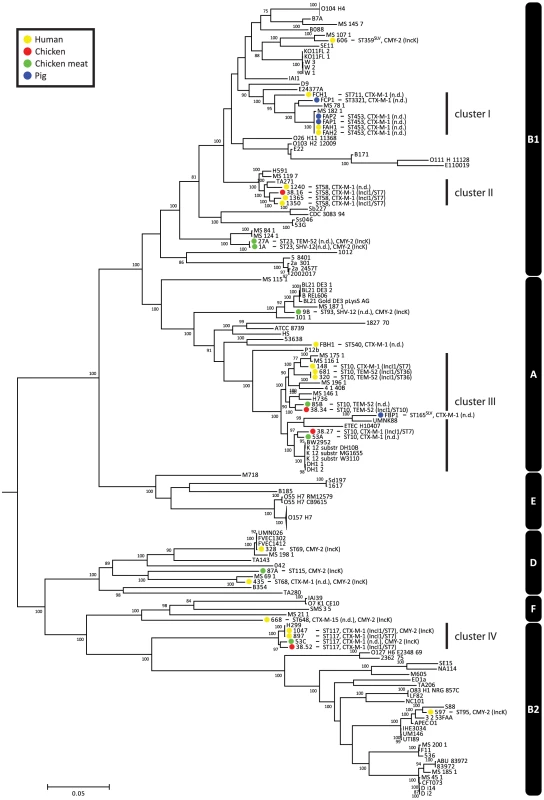
The tree was built using 18169 variable positions present in 215 core genes. The strains sequenced in this study are indicated in coloured bullets according to isolation source. Typing characteristics (Table 1) are given behind strain names. In case the MLST had not been determined before, it was determined using MLST v1.6 [55]. Clusters I–IV (see main text) are indicated behind the tree. Phylogroups are also indicated behind the tree (white text on black bars). The O104:H4 and O157:H7 branches are collapsed and represent 11 and 20 strains, respectively. Bootstrap support was implemented by running 100 bootstrap replicates. Values <75% are not displayed. SLV indicates Single Locus Variants of corresponding MLSTs. There were four clusters of ESBL-producing strains isolated from humans and animals/meat (clusters I–IV, Fig. 1). Cluster I contained human and pig isolates from two pig farms, with strains from farm A being particularly closely related. The other three clusters contained the five pairs of human and chicken isolates that had previously been considered indistinguishable based on traditional typing methods [15].
Among the five pairs of human and chicken isolates, the most closely related pairs were in cluster IV. The COG-based core genome alignment showed 171 SNPs between these strains, corresponding to 1003 SNPs/Mbp. To better elucidate the minimum number of SNPs between human and chicken isolates, we performed a core genome analysis using OrthoMCL [20] on the strains in cluster IV. For comparison, ten clonal O104:H4 strains from the 2011 German EHEC outbreak [21] and the four strains from pig farm A (cluster I) were included in this analysis (Fig. 1). We identified 3574 core proteins in this dataset translating to a concatenated nucleotide alignment of 3.34 Mbp. Within cluster IV there were 4216 SNPs between the most closely related isolates, corresponding to 1263 SNPs/Mbp. In contrast, only 0–6 SNPs (0–1.8 SNPs/Mbp) were found between any two strains in the German EHEC outbreak and only 6 SNPs were found between farmer isolate FAH2 and any of its two related pig isolates, suggesting recent clonal transmission of E. coli between pig and human in farm A (Fig. 2).
Fig. 2. Phylogeny and SNP analysis of closely related ESBL-producing E. coli strains from human and poultry. 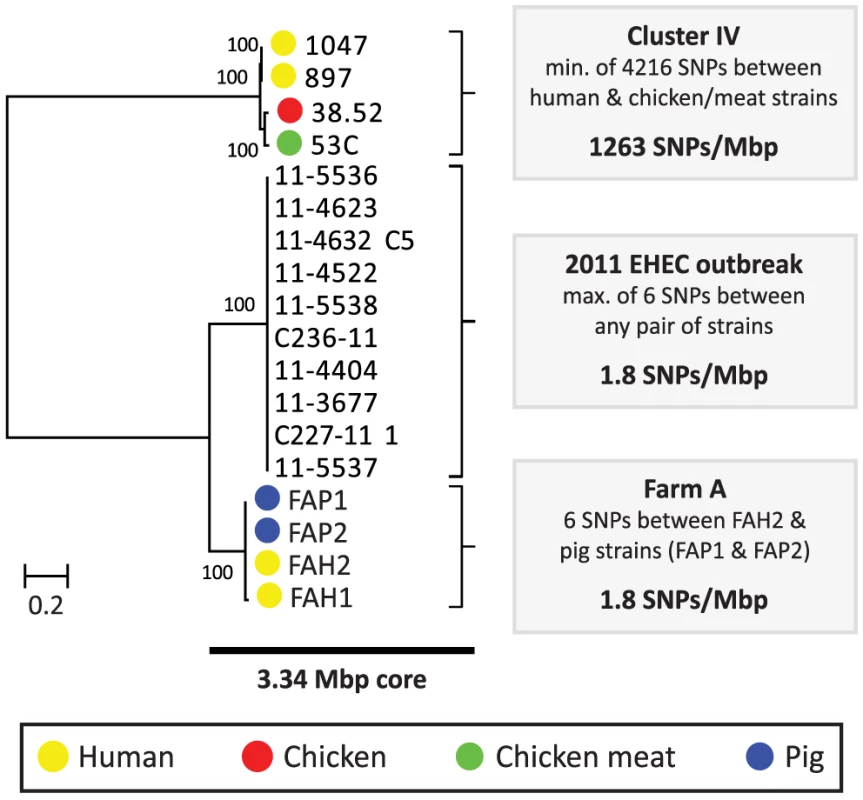
A high resolution core genome analysis was performed for a subset of strains which, based on an initial phylogenetic analysis (Cluster IV, Fig. 1), included the most closely related pairs of human and poultry-associated ESBL-producing strains within our dataset. Strains within Cluster IV had previously been found to be identical with respect to MLST, ESBL gene and ESBL-carrying plasmid (Table 1). For comparative purposes, clonally related E. coli strains from the 2011 German EHEC outbreak [21] and four potentially clonally related strains isolated from a single pig farm (Farm A) were included in this analysis. A phylogenetic tree, built from the 107919 variable positions present in the resulting 3.34 Mbp core genome alignment is shown to the left. Bootstrap support was implemented by running 1000 bootstrap replicates. Coloured bullets refer to the isolation source. The number of SNPs found in each of the three clusters (Cluster IV, EHEC outbreak, Farm A) is shown to the right. Given an estimated E. coli mutation rate of 2.3×10−7 to 3.0×10−6 substitutions per site per year [21], [22] and an average E. coli genome size of 5.2 Mbp, the number of SNPs (1263/Mbp) between the two most closely related human and chicken isolates largely exceeded the number of 3–41 SNPs that is expected to arise in 2.6 years (the difference in isolation dates between both strains, Table 1). Even if 10% of the detected SNPs were due to recombination, which is considerably more than the reported upper limit for recombinant DNA (∼3.5%) in E. coli [19], the number of SNPs due to mutation would exceed the expected maximum number of SNPs in case of recent clonal transmission. As the genetic distance between all other pairs of human and poultry isolates was even larger, our findings do not support a scenario of recent clonal transmission of ESBL-producing E. coli strains between humans and poultry.
Reconstruction of plasmids from WGS data
To investigate the possibility of horizontal spread of ESBLs via plasmids, we employed a Plasmid Constellation Networks (PLACNET) approach to reconstruct plasmids from WGS data [23]. Application of this approach resulted in the reconstruction of 147 plasmids (average of 4.6±2.1 plasmids per strain), with plasmid sizes ranging from 1.1 kbp to 290.4 kbp (Table 2). The plasmid sizes showed a trimodal distribution (Fig. 3) that was similar to the distribution previously reported for plasmids from a wide range of bacterial taxa [24]. The median size of large (conjugative) plasmids was 93.6 kbp (n = 91). Small plasmids could be further subdivided into two groups: one with a median size of 5.9 kbp (n = 41), predominated by mobilizable plasmids (i.e. containing MOB genes) and one with a median size of 1.7 kbp (n = 15), predominated by non-mobilizable plasmids. Based on the classification of their MOB genes [25] and using a hierarchical clustering analysis of gene content (Fig. 4), reconstructed plasmids belonged to a limited number of plasmid families, of which the most abundant ones were IncF-MOBF12 (n = 38; average size of 107.4±57.7 kbp) and IncI1-MOBP12 (n = 26; average size of 95.7±20.0 kbp). Other abundant families included MOBP5 (n = 25), IncK (n = 12) and MOBQ (n = 11). Finally, there were 18, mostly small-sized, plasmids (median size of 1.6 kbp; range of 1.1–106.3 kbp) that were scattered throughout the dendrogram and could not be clearly subdivided into any family. A comparison between previous typing data and the PLACNET reconstructions showed that both data types were in excellent agreement with each other. First of all, the 11 strains that were previously found to contain an IncI1 plasmid were also found to contain such a plasmid using PLACNET. The sizes of these 11 reconstructed plasmids (average size of 92.7 kbp±5.7 kbp) were also in agreement with their previously estimated sizes on the basis of gel electrophoresis (average size of 97.7 kbp±3.8 kbp) [15]. Furthermore, the reconstructed plasmids for ten of these 11 strains had exactly the same ST as was previously found using pMLST. The only inconsistency was found for strain 38.34, which should contain an IncI1/ST10 plasmid according to pMLST, whereas we reconstructed an IncI1/ST36 plasmid. However, IncI1/ST10 and IncI1/ST36 are single locus variants that differ by only one SNP (http://pubmlst.org/plasmid/), indicating that this inconsistency was not a result of PLACNET, but was likely due to typing errors. Of the 11 strains that had previously been found to contain an IncK plasmid, ten were also found to contain such a plasmid using PLACNET, the only exception being strain 1047.
Fig. 3. Distribution of plasmid sizes in the collection of 32 sequenced E. coli strains. 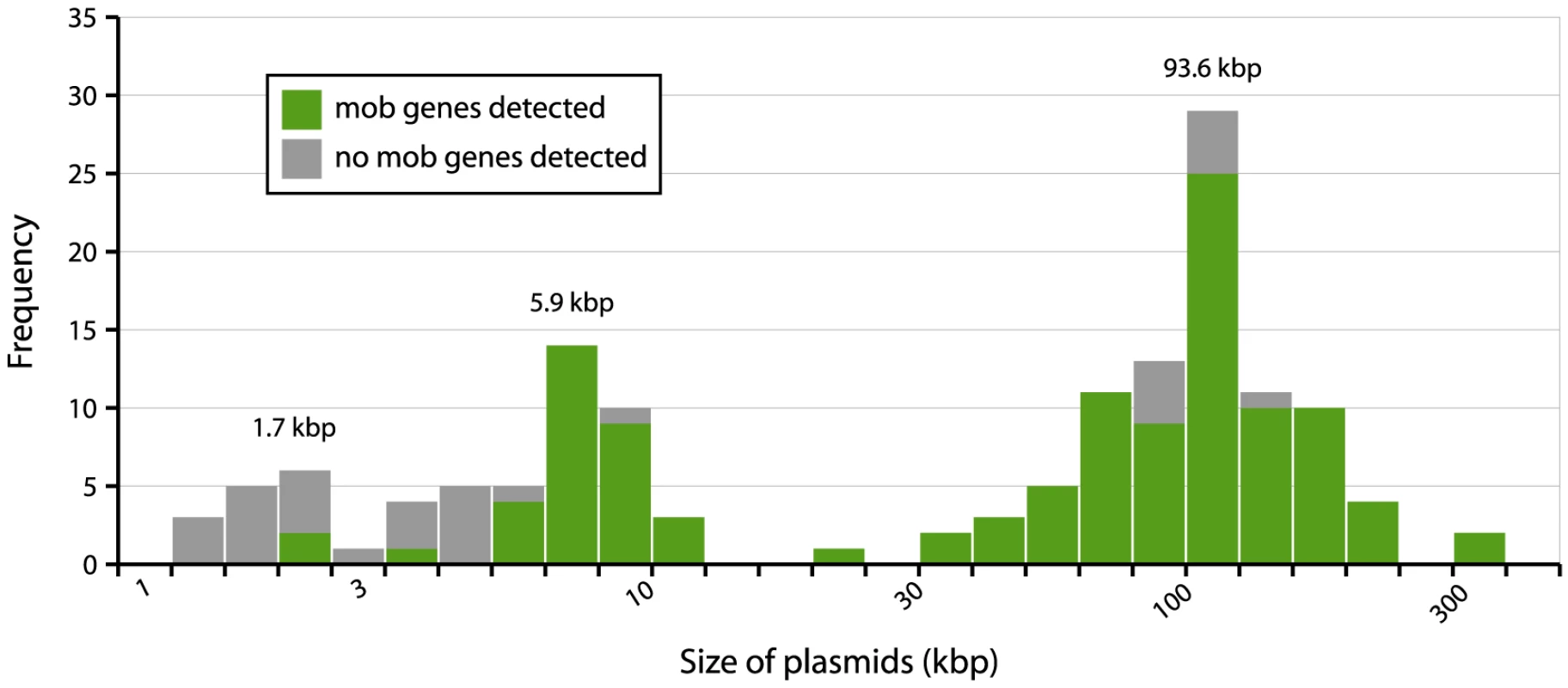
The histogram shows the total number of reconstructed plasmids corresponding to each size class (in a logarithmic scale). The plasmid size distribution shows a trimodal abundance curve. Numbers above the three peaks refer to the median size for each class. Plasmids in which a relaxase gene was detected are shown in green and those in which it was not detected are shown in grey. Fig. 4. Hierarchical clustering dendrogram of reconstructed plasmids contained in the collection of 32 sequenced E. coli strains. 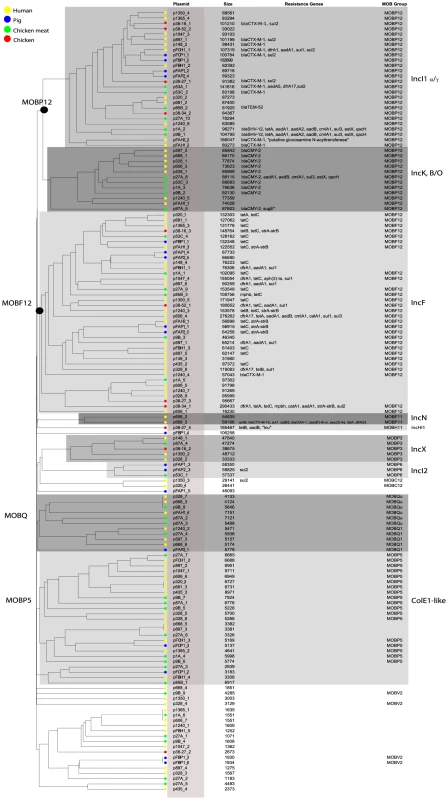
The dendrogram was constructed as explained in Methods. Reconstructed plasmids are indicated with colored bullets according to isolation source. The dendrogram construction automatically grouped plasmids into Inc families, which are shown by background colours. Mob types are also indicated. Additional columns show plasmid sizes, resistance genes and MOB subfamilies. Tab. 2. Plasmids reconstructed using PLACNET. 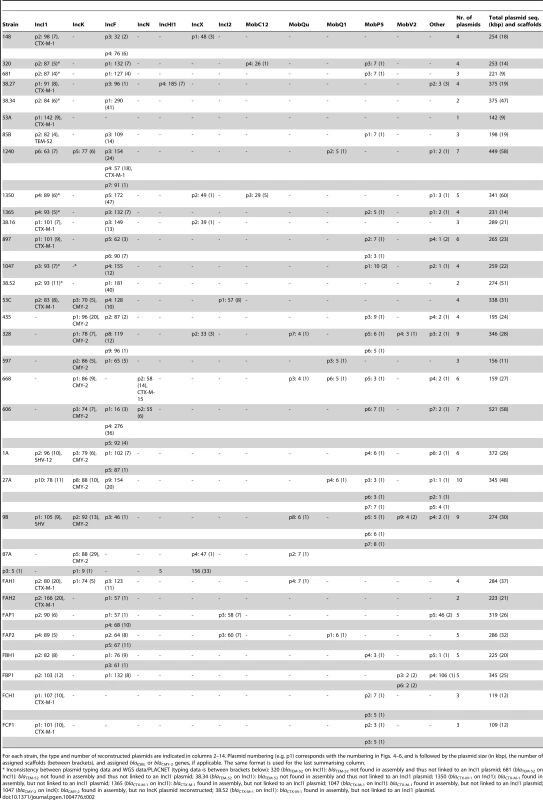
For each strain, the type and number of reconstructed plasmids are indicated in columns 2–14. Plasmid numbering (e.g. p1) corresponds with the numbering in Figs. 4–6, and is followed by the plasmid size (in kbp), the number of assigned scaffolds (between brackets), and assigned blaESBL or blaCMY-2 genes, if applicable. The same format is used for the last summarising column. We also examined to what extent we were able to correctly connect ESBL and AmpC genes to reconstructed plasmids. Of the 28 previously typed ESBL genes, 24 were correctly identified in their genomes (Table 1) and among these, 15 were connected to a reconstructed plasmid. Four of the remaining nine unconnected ESBL genes (blaCTX-M-1 in strains 1350, 1365, 1047 and 38.52) should have been connected to an IncI1 plasmid according to previous typing data (Tables 1–2). The reason that these ESBL genes remained unassigned was because they were located on relatively small scaffolds (average size of 6.6 kbp) that did not contain enough genetic information to unequivocally match them to a single plasmid using our reference database. For the 15 cases where we were able to connect an ESBL gene to a reconstructed plasmid, typing data indicating where the ESBL gene should be located was available for four cases (strains 148, 897, 38.16 and 38.27) and for all these cases we had connected the ESBL gene (blaCTX-M-1) to the correct plasmid (IncI1/ST7) (Tables 1–2). Of the 11 AmpC (blaCMY-2) genes, ten were connected to their correct plasmid (IncK). The only exception was found again for strain 1047 for which we could not reconstruct an IncK plasmid (Table 2). The above findings show that PLACNET worked efficiently to assemble plasmids from WGS data, although the assignment of small scaffolds to plasmids can be problematic, as is illustrated above by the ESBL genes that were not linked to a specific reconstructed plasmid (see also discussion below and in [23]).
Identification of distinct ESBL-associated plasmid lineages
Fifteen ESBL genes were connected to a reconstructed plasmid, of which 13 were connected to an IncI1 plasmid. Frequently (eight out of 13), these IncI1 plasmids were also unequivocally linked to other antibiotic resistance genes, such as sul, dfrA, aadA or tet. We also found IncK plasmids that were commonly (ten out of 12 plasmids) associated with the AmpC β-lactamase-encoding gene blaCMY-2 (Fig. 4).
As IncI1 and IncK were the only plasmid families that included reconstructed ESBL-/AmpC-containing plasmids in strains from both humans and animals/meat, we further investigated their potential role in the transfer of resistance genes through the food-chain. To this aim we built a gene content-based dendrogram that also included closely related and publicly available plasmid sequences. In the resulting dendrogram, all reconstructed ESBL-containing IncI1 plasmids, except the blaSHV-12-carrying plasmids p1A_2 and p9B_1, clustered into one specific branch that did not contain any other previously sequenced plasmid (Fig. 5). This branch also contained 12 of the 13 reconstructed IncI1 plasmids that did not include an ESBL gene. Similarly, all of the reconstructed IncK plasmids, except p87A_5, clustered into one specific branch that did not include any previously sequenced plasmid. These findings suggest the existence of IncI1 and IncK plasmids with a genetic profile distinct from previously characterised plasmids. We did not find any single gene that unequivocally explained the formation of the IncK branch, pointing to a delicate configuration of genes that gives these plasmids their unique genetic profile. However, for the IncI1 branch, we found a characteristic shufflon-related gene (UniProt P10487) that was present in all 26 reconstructed IncI1 plasmids, but which was absent from related IncI1 plasmids (Fig. 5).
Fig. 5. Hierarchical clustering dendrogram of reconstructed IncI1 and IncK plasmids contained in the collection of 32 sequenced E. coli strains together with relevant and similar reference plasmids. 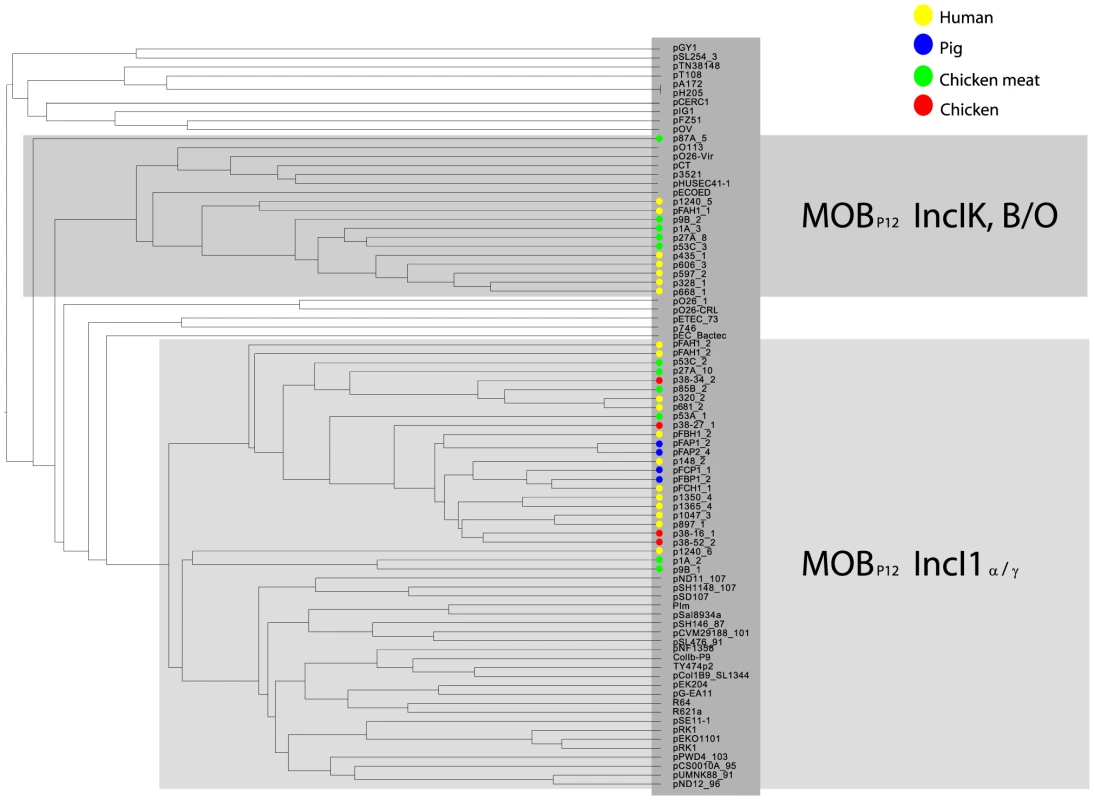
The dendrogram was constructed as explained in Methods. Reconstructed plasmids are indicated with colored bullets according to isolation source. All other (reference) plasmids were taken from public sequence repositories. To further characterise the IncI1 and IncK resistance plasmids, phylogenetic trees were built from the sequences of the reconstructed plasmids and their closest plasmid relatives. For the IncI1 phylogenetic reconstruction, the 23 plasmids belonging to the specific IncI1 branch as well as 27 related plasmids were included. An OrthoMCL analysis of these plasmids resulted in 8 core proteins (S3 Table), corresponding to a concatenated nucleotide alignment of 8.6 kbp, including 763 variable positions. In the phylogenetic tree built from these variable positions the reconstructed IncI1 plasmids were assigned to four distinct branches (Fig. 6A), each of which also contained previously characterised plasmids. However, the reconstructed plasmids within each branch were always more similar to each other than to any of these previously characterised plasmids. Two of the four branches, corresponding to IncI1/ST3 and IncI1/ST7, contained reconstructed ESBL-harbouring plasmids from both humans and animals or meat. Further rounds of OrthoMCL analyses showed that the reconstructed plasmids within each of these two sets were highly similar to each other: a maximum of only four SNPs (all attributable to p53C_2) was found in the 40 kbp plasmid core of the IncI1/ST3 subset, whereas no SNPs were found in the almost 50 kbp plasmid core of the IncI1/ST7 subset (Fig. 6A). Similarly, a subset of the blaCMY-2-carrying IncK plasmids contained a plasmid core of almost 37 kbp with a maximum of 27 SNPs (Fig. 6B), which were mostly attributable to p435_1. Leaving out p435_1 from the comparisons revealed a maximum of only seven SNPs. These data strongly support the existence of ESBL-associated IncI1 and AmpC-associated IncK plasmids that have spread through phylogenetically distinct E. coli populations, possibly contributing to the dissemination of ESBLs and AmpC-type β-lactamases through the food-chain.
Fig. 6. Phylogeny of reconstructed IncI1 and IncK plasmids and their closest relatives. 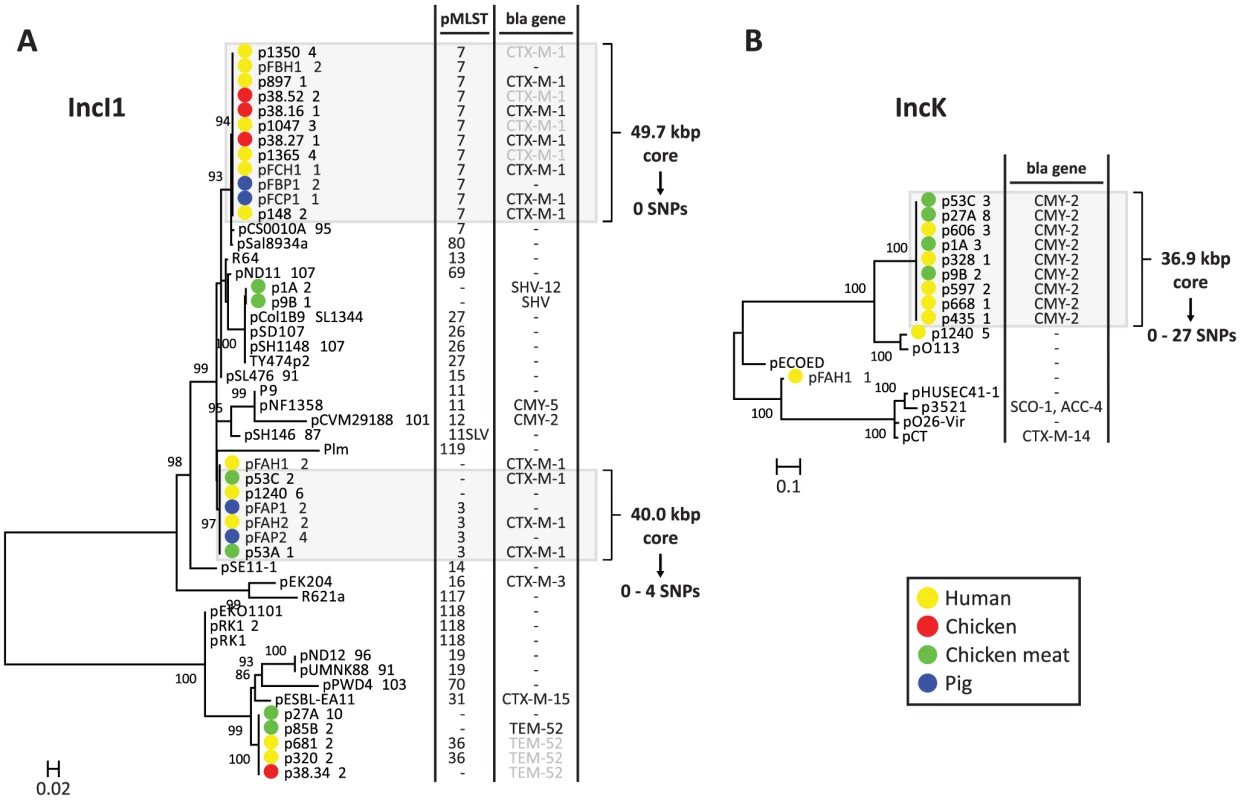
Phylogenetic tree of IncI1 plasmids built from 763 variable positions present in an 8.6 kbp alignment, representing 8 core proteins (S3 Table) (A). Phylogenetic tree of IncK plasmids built from 2724 variable positions present in a 19.9 kbp alignment, representing 27 core proteins (S3 Table) (B). Bootstrap support was implemented by running 1000 bootstrap replicates. Reconstructed plasmids are indicated with coloured bullets according to isolation source (plasmid names include associated strain names, followed by a unique plasmid identifier). All other plasmids were taken from public sequence repositories. Plasmid STs (for IncI1 only) and encoded β-lactamases (with the exception of TEM-1, which does not provide resistance to third generation cephalosporins) are indicated to the right of the trees. 11SLV indicates a single locus variant of ST11. pMLST negative means that the reconstructed plasmid lacks one or more pMLST loci. Bla genes in light grey were not connected to the reconstructed plasmid, but should be located on this plasmid according to typing data. Light grey panels indicate potential epidemic blaCTX-M-1- and blaCMY-2-carrying plasmids. Core genome analysis of these plasmid subsets revealed virtually identical backbones of up to 50 kbp. Validation of plasmid reconstructions by PacBio sequencing of strains 53C and FAP1
To validate the conclusions drawn from the PLACNET reconstructions, we sequenced two strains (53C and FAP1) using long-read DNA sequencing technology (Pacific Biosciences). Strain 53C was selected because it has both an IncI1 and an IncK plasmid, carrying blaCTX-M-1 and blaCMY-2, respectively. Strain FAP1 was selected because it contained an IncI1 plasmid of the same lineage as the one in strain 53C (Fig. 6A). The total amount of reconstructed plasmid sequence for strains 53C and FAP1 was 338 kbp and 319 kbp, respectively (Table 2). Genomes were assembled to an average depth of 66.7 - and 77.0-fold, respectively, resulting in 11 contigs for strain 53C and five contigs for strain FAP1 (S4 Table). Inspection of the contig sequences showed the presence of four large plasmids in both strains. These were assigned to Inc groups F, I1 (carrying blaCTX-M-1), I2, and K (carrying blaCMY-2) in strain 53C and F, I1 (carrying blaCTX-M-1), and I2, in strain FAP1. A single plasmid in strain FAP1 could not be assigned to an Inc group. Except for the IncI1 plasmid of FAP1, all plasmid contigs could be circularized (S4 Table). The plasmid content was in agreement with our reconstructions, except for two inconsistencies in strain FAP1: (i) PLACNET did not assign a blaCTX-M-1 gene to its IncI1 plasmid, and (ii) PLACNET reconstructed two IncF plasmids. Blast analysis of both reconstructed IncF plasmids against the FAP1 long-read assembly suggested that they should indeed have been merged into one single plasmid. The reason for this incorrect prediction by PLACNET is unclear, but in the constellation network the two plasmids were relatively far away from each other, suggesting that the IncF plasmid in FAP1 is a fusion between previously observed IncF plasmids present in the reference database. These data show that caution must be taken in case PLACNET predicts multiple plasmids of the same Inc group in one strain. For the remaining plasmids, blast analysis showed that the precision rate of PLACNET was high, ranging from 97–100% (Table 3). Also in terms of sensitivity, PLACNET performed well being able to recover 72.1–99.7% of the plasmids (Table 3). The plasmid regions that were not reconstructed by PLACNET mostly aligned with small scaffolds (average size of 2.0±1.9 kbp, n = 33) in the assemblies built from Illumina short-read data, which indicates that these regions are difficult to assemble. Notably, these small scaffolds encoded many mobile element-, phage-, transposon - and integrase-associated proteins (29.7% of all predicted proteins in these scaffolds) as compared to the correctly assigned scaffolds, where only 6.7% of the proteins had these predicted roles. These observations are in line with results obtained from the PLACNET validation analyses described in [23] and show that PLACNET efficiently reconstructs plasmids from WGS data. Finally, the PLACNET-based prediction that both IncI1 plasmids from strains 53C and FAP1 are highly similar (Fig. 6A) was confirmed by aligning the two complete IncI1 plasmid sequences assembled from the long-read sequencing data. Filtering out repetitively aligning regions resulted in a pairwise alignment of 94.8 kbp containing only 4 SNPs. These data further substantiate our conclusions regarding highly successful plasmid lineages disseminating cephalosporin resistance.
Tab. 3. PLACNET precision and sensitivity rates for seven reconstructed plasmids. 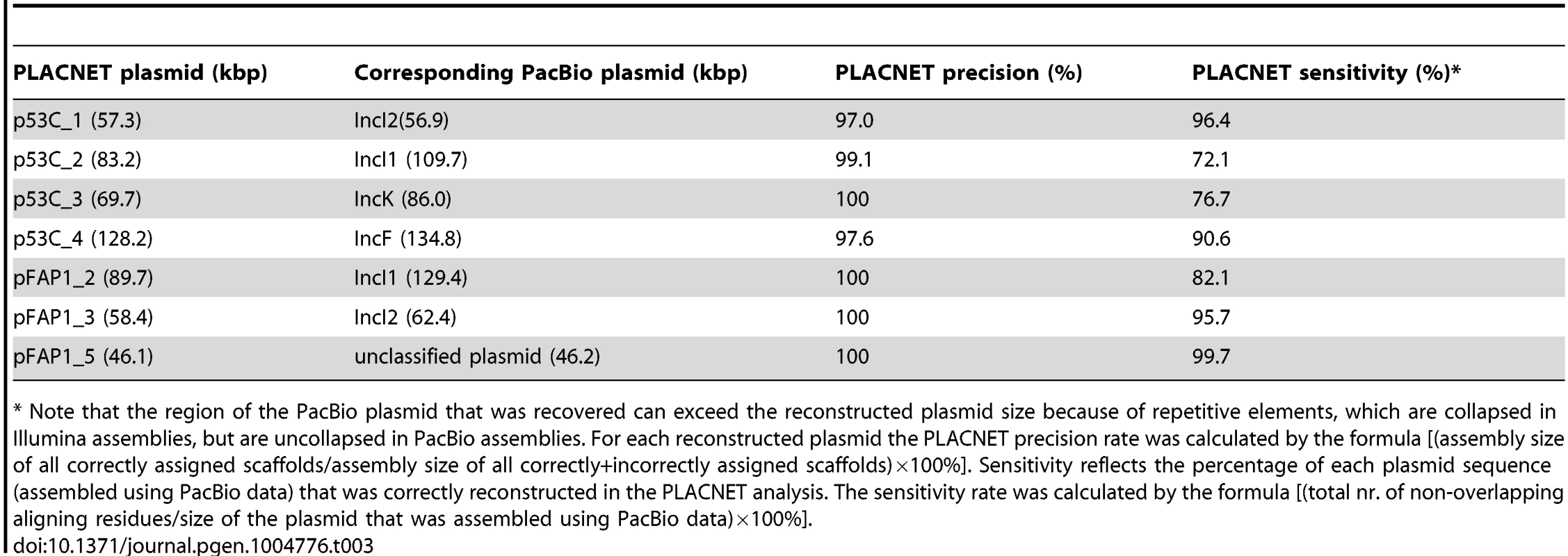
* Note that the region of the PacBio plasmid that was recovered can exceed the reconstructed plasmid size because of repetitive elements, which are collapsed in Illumina assemblies, but are uncollapsed in PacBio assemblies. For each reconstructed plasmid the PLACNET precision rate was calculated by the formula [(assembly size of all correctly assigned scaffolds/assembly size of all correctly+incorrectly assigned scaffolds)×100%]. Sensitivity reflects the percentage of each plasmid sequence (assembled using PacBio data) that was correctly reconstructed in the PLACNET analysis. The sensitivity rate was calculated by the formula [(total nr. of non-overlapping aligning residues/size of the plasmid that was assembled using PacBio data)×100%]. Discussion
We assessed the epidemiology of ESBL-producing E. coli from humans, animals and food using WGS. Our findings strongly suggest the existence of highly successful ESBL-carrying plasmids facilitating transmission of ESBL genes between different reservoirs. This has important implications for our understanding of the dynamics of the spread of ESBL genes and for evaluating control measures.
Several strains that were sequenced in this study and which originated from humans and poultry had previously been considered indistinguishable based on MLST, plasmid and ESBL gene typing, suggesting clonal transfer of these strains through the food-chain, to humans [15]. The claim that ESBL-producing E. coli strains from humans and poultry are frequently identical was also made in other studies that made use of traditional sequence-based typing methods [14], [16]. However, as has been demonstrated for different bacterial pathogens and in varied contexts, especially bacterial outbreak investigations, WGS provides superior resolution over traditional typing methods in terms of ruling in and out epidemiological connections between strains [26]–[28]. Similarly, we demonstrate that conclusions on the clonal spread of ESBL-producing E. coli through the food-chain cannot realistically be drawn on the basis of traditional sequence-based typing methods, due to their insufficient discriminative power. More specifically, we found that none of the five pairs of human and poultry-associated isolates, previously typed as indistinguishable, were particularly closely related. The most similar pair of isolates differed by 1263 SNPs/Mbp compared to a difference of 1.8 SNPs/Mbp for known/expected clonally related isolates. Hence, inferences from classical typing-based studies regarding the extent of transfer of ESBL-producing E. coli strains from animals via food to humans and the burden of disease and mortality due to the use of third-generation cephalosporins in food production must be considered as highly speculative [11].
In fact, our findings strongly suggest that distinct plasmids disproportionately contribute to the spread of antibiotic resistance between different reservoirs. We have demonstrated the existence of highly similar cephalosporin resistance-encoding IncI1/ST3 (40.0 kbp core, 0–4 SNPs), IncI1/ST7 (49.7 kbp core, 0 SNPs), and IncK (36.9 kbp core, 0–27 SNPs) plasmids in different reservoirs. Reconstructed blaCTX-M-1-carrying IncI1/ST3 plasmids were found in one human and two poultry isolates, blaCTX-M-1-carrying IncI1/ST7 plasmids were found in three human, two poultry, and one pig isolate; and blaCMY-2-carrying IncK plasmids were found in five human and four poultry isolates. The isolates carrying these plasmids belonged to evolutionarily distinct backgrounds (IncI1 in phylogroups A, B1 and B2; IncK in phylogroups A, B1, B2, D and F), suggesting that these plasmids efficiently spread through E. coli populations and play an important role in the dissemination of ESBL and AmpC-type β-lactamases between different reservoirs.
Based on their genetic content, the IncI1 and IncK plasmids in our dataset clustered into specific sub-branches that did not contain any previously characterised plasmid. However, phylogenetic analyses revealed that these sub-branches could be split into evolutionarily distinct plasmids, some of them being distantly related to previously sequenced plasmids. These findings suggest that evolutionarily distinct plasmids have been accumulating genes from the same genetic reservoir, resulting in plasmids with a similar genetic inventory. The reconstructed IncI1 plasmids all harboured a characteristic shufflon-related gene that was absent from previously characterised IncI1 plasmids. Shufflons are site-specific recombination systems that produce variable C-terminal extensions of the PilV adhesin, resulting in variations of recipient ability in IncI1 plasmid mating [29]. Whether this shufflon explains the promiscuous nature of ESBL-carrying IncI1 plasmids remains to be determined.
One important question is to what extent the IncI1 and IncK resistance plasmids found in this study have spread beyond The Netherlands. Given the trees in Fig. 6, it is clear that currently available plasmid sequences in public databases do not contain any plasmids that are particularly closely related to our reconstructed IncI1 and IncK plasmids. The pMLST repository (http://pubmlst.org/plasmid/) shows that blaCTX-M-1-carrying IncI1/ST3 plasmids have been isolated from six different European countries, whereas blaCTX-M-1-carrying IncI1/ST7 plasmids have until now been isolated only from The Netherlands and Germany. The location of blaCMY-2 on an IncK plasmid, as found here, has only been occasionally reported before, in The Netherlands [30],[31], but also in Sweden [32] and Canada [33]. Future sequencing projects are needed to determine whether the previously identified plasmids isolated outside The Netherlands are closely related to those described here.
We found that none of the human E. coli strains in our dataset were closely related to strains from poultry. In contrast, nine out of 17 human isolates (53%) contained a blaCTX-M-1 or a blaCMY-2 gene located on plasmids that were highly similar to those found in poultry. These data cannot be interpreted to mean that clonal transfer of antibiotic resistant E. coli strains between poultry and humans does not occur, but rather that such transfer occurs less frequently than the transfer of resistance plasmids between both reservoirs. One drawback of our study is that we have used a relatively small sample size (32 strains). Future studies, using larger sample sizes, are needed in order to make more accurate estimates of the relative (and absolute) contributions of clonal versus plasmid transfer towards the spread of antibiotic resistance and the associated health-care burden. In addition, our study focuses on IncI1 and IncK plasmids. Future studies are needed that also focus on other plasmid families, such as IncF plasmids, which are commonly detected in E. coli from human infections and are associated with the dissemination of many virulence and antibiotic resistance determinants [34],[35].
Conjugal transfer of plasmids carrying antibiotic resistance genes has been shown to frequently occur among Enterobacteriaceae in different environments, including milk, meat, and feces, even in the absence of antibiotic pressure [36],[37]. Moreover, it has been shown that bla-carrying plasmids are readily transferred from invading Enterobacteriaceae to Enterobacteriaceae that are indigenous to the animal and human intestine and that the invading clone itself generally does not persist in the intestine [38], [39]. Nonetheless, it is difficult to infer to what extent the reservoir of bla-type resistance genes in poultry contributes to the carriage of such genes by human E. coli strains. If successful plasmids are largely responsible for the rising prevalence of ESBL - and AmpC-producing E. coli in healthy humans, their emergence in poultry and humans may simply be a reflection of selection of strains carrying these plasmids due to antibiotic usage in human and veterinary medicine.
A better understanding of the dynamics of ESBLs and other resistance genes in different hosts is needed to design effective control measures, both in the community and within health care settings. Our findings strongly suggest the occurrence of clonal transfer of ESBL-producing E. coli between pigs and pig farmers, which may well occur through direct contact or through aerosols. Whether such events represent a public health threat remains to be determined. The occurrence of transmission of ESBL-producing E. coli from poultry through the food-chain is less evident. The occurrence of highly-related plasmids that carry ESBL - and AmpC-type resistance genes among genotypically distinct E. coli strains from different sources is cause for concern because this suggests that plasmids can spread with relative ease between the different reservoirs and the spread of these plasmids may be exceedingly difficult to control. Clearly, there still remains an urgent need to minimize the use of third-generation cephalosporins in animal husbandry as this is an important selective pressure for the occurrence of ESBL - and AmpC-producing E. coli in animals raised for food production.
Materials and Methods
Isolates and molecular analyses
The genomes of 32, mostly ESBL-producing, E. coli strains isolated from different reservoirs in The Netherlands in the period 2006–2011, were sequenced. One set of isolates (n = 24) has been studied previously using classical typing methods [15], [18]. This set contained strains from human clinical infections (n = 13) which had been obtained from geographically dispersed laboratories in The Netherlands, servicing secondary and tertiary care hospitals, general practitioners and long-term care facilities. Additional isolates were from chickens raised on production farms (n = 4) and chicken retail meat (n = 7) (Table 1). All 24 isolates were previously genotyped by MLST [40] (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli) and plasmid characterization was previously performed using PCR-based replicon typing [31], [41] and additional pMLST for IncI1 plasmids [42], [43] (http://pubmlst.org/plasmid/). Detection of ESBL genes had been performed for all 24 strains using microarray analysis and gene sequencing [44]. In addition, detection of AmpC-type β-lactamase-encoding genes had been performed for 11 strains, using gene sequencing [18]. The association between ESBL/AmpC genes and plasmids was previously determined by both Southern blot hybridization and transformation [31]. Four non-ESBL-producing isolates were included as controls and were analysed for the carriage of plasmids that can incorporate ESBL genes via horizontal gene transfer. The second set of isolates contained eight ESBL-producing strains that had been isolated from three different pig farms in The Netherlands in 2011 (Table 1). These farm strains were part of a larger cohort that will be described in detail elsewhere (Dohmen et al., unpublished data). For one farm (farm A), four strains were collected, two from different fecal pools of six unique pigs and two from the feces of different farmers. For each of the other two farms (farms B and C), one strain was collected from a fecal pool of six pigs and one from the feces of a farmer. Detection of the ESBL (blaCTX-M-1) gene was performed using a CTX-M-1 group-specific PCR and additional gene sequencing (Dohmen et al., unpublished data).
Genome sequencing and assembly
Genomic DNA was isolated from cell pellets using the Ultraclean Microbial DNA isolation kit (Mo Bio Laboratories, Inc., Carlsbad, CA, USA) according to the manufacturer's instructions. Strains were sequenced using Illumina HiSeq 2000 sequencing technology (Illumina, Inc., San Diego, CA, USA) generating 90 bp paired-end reads from a library with an average insert size of 500 bp and a total amount of quality-filtered raw sequence of over 600 Mbp per strain. Quality filtering included the removal of duplicate reads and reads that contained ≥15 bp overlap with the adapter sequences. The corresponding paired-end reads were also removed in these cases. Reads were assembled de novo using SOAPdenovo v1.05 [45]. For each Illumina dataset, a range of different k-mer lengths (21–63 bp) was empirically tested to obtain the assembly with the lowest number of scaffolds of size ≥500 bp. In cases where more than one assembly contained the lowest number of scaffolds, the parameters of choice to pick the best assembly were: the lowest number of contigs of size ≥200 bp, the highest N50 for the scaffolds, and the highest N50 for the contigs, in order of priority. Assembly statistics are reported in S1 Table. Two strains (53C and FAP1, Table 1) were also sequenced on a Pacific Biosciences RS II instrument (Pacific Biosciences, Inc., Menlo Park, CA, USA). Libraries were prepared using the PacBio 20 kbp library preparation protocol. Size selection (5 kbp cut-off) of the final libraries was performed using a BluePippin instrument (Sage Science, Inc., Beverly, MA, USA). Sequencing was performed using P4-C2 chemistry. Three and five SMRT cells were used for sequencing strains FAP1 and 53C, respectively, generating 159191 and 95263 reads and a total of 997.1 and 471.5 Mbp, respectively. Reads were assembled using HGAP v3 (Pacific Biosciences, SMRT Analysis Software v2.2.0). Minimus2, part of the AMOS package [46], was used to circularize contigs. The SMRT Analysis Software was used to map reads back to the contigs and correct sequences after circularization. Assembly statistics are reported in S4 Table.
Sequence data analysis
Publicly available sequence data were retrieved from GenBank (ftp://ftp.ncbi.nih.gov/genomes/Bacteria and ftp://ftp.ncbi.nih.gov/genomes/Bacteria_DRAFT). Whole genome sequence data for 126 Escherichia and 12 Shigella species were downloaded in June 2012, whereas sequence data for 4188 completely sequenced plasmids, 797 of them from Enterobacteriaceae, were downloaded in June 2013. The strains that were sequenced in this study were annotated with RAST v4.0 [47] using default settings. Predicted proteins were assigned to Clusters of Orthologous Groups (COG) [48] as described previously [49]. On the basis of COG assignments, a core proteome was defined by (i) extracting, per analysed genome, all proteins with one or more COGs assignments and which represented the only protein in that given COG or combination of COGs and by (ii) selecting from those proteins the ones that occurred in all genomes analysed. Alternatively, in the cases where smaller genomic datasets were analysed (see Results), core proteomes were determined by first subjecting all associated protein sequences to an all-vs-all blastp similarity search (defaults settings, except for: -F ‘m S’; -e 1×10−5; -z [the total number of proteins used in the analysis]). Groups of orthologous proteins were determined from the blastp output using OrthoMCL v2.0.2 [20]. Orthologous groups with exactly one representative protein from each input genome were considered to be part of the core proteome. Core genome alignments were built as follows: for each group of orthologous proteins, the corresponding nucleotide sequences were extracted and aligned using Muscle v3.7 [50], after which gaps were stripped from each alignment using trimAl v1.2 [51]. The resulting alignments were concatenated to yield a core genome alignment. Phylogenies were reconstructed by building maximum likelihood phylogenetic trees from the variable positions in core genome alignments using RAxML v7.2.8 [52] under the GTRCAT model. Confidence was inferred by running 100 or 1000 bootstrap replicates under the same model. Trees were mid-point rooted and visualised in MEGA v5.05 [53]. Bowtie2 [54] was used for mapping Illumina reads against scaffolded assemblies and gene sequences. MLST profiling of sequenced bacteria was performed using MLST v1.6 [55]. Pairwise large-scale nucleotide alignments were built using NUCmer v3.23 (with –mum option), which is part of the MUMmer package [56].
Plasmid reconstructions from WGS data
Plasmid reconstructions were based on the Plasmid Constellation Networks (PLACNET) method of genome representation [23]. In short, for all genomes, a PLACNET representation that clusters all plasmid-associated contigs was built using (i) contig similarities with reference genomes, (ii) all possible contig linkages, and (iii) plasmid-specific relaxase and replication initiator genes. This information was implemented in a network, where genomic contigs, together with reference plasmid and genome sequences are shown as nodes. The nodes are linked by edges of homology and scaffolding information. As a result, contigs fall into clusters, the largest one being the chromosome and additional ones being plasmids. Manual curation of the resulting networks helped solving most of the remaining ambiguities. Reference data from GenBank contained 4188 plasmids and 2728 chromosomes. Contig similarity analysis was performed using megablast against these reference data. Contig homology edges were defined by the five best blast hits (e-value <1×10−20). Scaffolds were determined by mapping all reads against contigs using Bowtie2 [54], and allocating as scaffold links all discordant paired-end reads that matched two different contigs. To provide additional evidence for the plasmid origin of a cluster, a blastp search against in-house databases containing plasmid-specific relaxases and replication initiator proteins was performed. Contigs encoding these proteins were tagged in the PLACNET. Plasmid Neighbour-Joining dendrograms were built based on previously described methodologies [57] using CD-HIT [58] to construct protein profiles and the Jaccard formula to calculate distance metrics between profiles. PLACNET results were validated as follows: for the two strains 53C and FAP1, the scaffolds assigned to the reconstructed plasmids were queried using megablast against the assemblies resulting from Pacific Biosciences (PacBio) sequencing. The best blast hit (e-value ≤1×10−10) was inspected to assess whether the scaffolds had been assigned to the correct plasmid. For each reconstructed plasmid the PLACNET precision rate was calculated by the formula [(assembly size of all correctly assigned scaffolds/assembly size of all correctly+incorrectly assigned scaffolds)×100%]. To assess PLACNET sensitivity (the percentage of each plasmid sequence size that was recovered) blast hits against the corresponding PacBio plasmids were collected (e-value ≤1×10−10, minimum of 250 identical residues). The sensitivity rate was calculated by the formula [(total nr. of non-overlapping aligning residues found by blast/size of the PacBio plasmid)×100%].
Accession numbers
All sequence data have been deposited at DDBJ/EMBL/GenBank. Accession numbers for the Illumina sequence data are listed in Table 1. Pacific Biosciences sequence data have been deposited with accession numbers PRJNA260957 for strain 53C and PRJNA260958 for strain FAP1.
Supporting Information
Zdroje
1. De KrakerMEA, DaveyPG, GrundmannH on behalf of the BURDEN study group (2011) Mortality and Hospital Stay Associated with Resistant Staphylococcus aureus and Escherichia coli Bacteremia: Estimating the Burden of Antibiotic Resistance in Europe. PLoS Med 8: e1001104 doi:10.1371/journal.pmed.1001104
2. MauldinPD, SalgadoCD, HansenIS, DurupDT, BossoJA (2010) Attributable Hospital Cost and Length of Stay Associated with Health Care-Associated Infections Caused by Antibiotic-Resistant Gram-Negative Bacteria. Antimicrob Agents Chemother 54 : 109–115 doi:10.1128/AAC.01041-09
3. CoqueTM, BaqueroF, CantónR (2008) Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill 13: pii: 19044.
4. HawkeyPM, JonesAM (2009) The changing epidemiology of resistance. J Antimicrob Chemother 64: i3–i10 doi:10.1093/jac/dkp256
5. DuboisV, BarbeyracBD, RoguesA-M, ArpinC, CoulangeL, et al. (2010) CTX-M-producing Escherichia coli in a maternity ward: a likely community importation and evidence of mother-to-neonate transmission. J Antimicrob Chemother 65 : 1368–1371 doi:10.1093/jac/dkq153
6. ValverdeA, CoqueTM, Sánchez-MorenoMP, RollánA, BaqueroF, et al. (2004) Dramatic Increase in Prevalence of Fecal Carriage of Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae during Nonoutbreak Situations in Spain. J Clin Microbiol 42 : 4769–4775 doi:10.1128/JCM.42.10.4769-4775.2004
7. KaperJB, NataroJP, MobleyHLT (2004) Pathogenic Escherichia coli. Nat Rev Microbiol 2 : 123–140 doi:10.1038/nrmicro818
8. SmetA, MartelA, PersoonsD, DewulfJ, HeyndrickxM, et al. (2008) Diversity of Extended-Spectrum β-Lactamases and Class C β-Lactamases among Cloacal Escherichia coli Isolates in Belgian Broiler Farms. Antimicrob Agents Chemother 52 : 1238–1243 doi:10.1128/AAC.01285-07
9. MachadoE, CoqueTM, CantónR, SousaJC, PeixeL (2008) Antibiotic resistance integrons and extended-spectrum β-lactamases among Enterobacteriaceae isolates recovered from chickens and swine in Portugal. J Antimicrob Chemother 62 : 296–302 doi:10.1093/jac/dkn179
10. DoiY, PatersonDL, EgeaP, PascualA, López-CereroL, et al. (2010) Extended-spectrum and CMY-type β-lactamase-producing Escherichia coli in clinical samples and retail meat from Pittsburgh, USA and Seville, Spain. Clin Microbiol Infect 16 : 33–38 doi:10.1111/j.1469-0691.2009.03001.x
11. CollignonP, AarestrupFM, IrwinR, McEwenS (2013) Human deaths and third-generation cephalosporin use in poultry, Europe. Emerg Infect Dis 19 : 1339–1340 doi:10.3201/eid.1908.120681
12. Van de Sande-BruinsmaN, GrundmannH, VerlooD, TiemersmaE, MonenJ, et al. (2008) Antimicrobial drug use and resistance in Europe. Emerg Infect Dis 14 : 1722–1730 doi:10.3201/eid1411.070467
13. GraveK, GrekoC, KvaaleMK, Torren-EdoJ, MackayD, et al. (2012) Sales of veterinary antibacterial agents in nine European countries during 2005–09: trends and patterns. J Antimicrob Chemother 67 : 3001–3008 doi:10.1093/jac/dks298
14. OverdevestI, WillemsenI, RijnsburgerM, EustaceA, XuL, et al. (2011) Extended-spectrum β-lactamase genes of Escherichia coli in chicken meat and humans, The Netherlands. Emerg Infect Dis 17 : 1216–1222 doi:10.3201/eid1707.110209
15. Leverstein-van HallMA, DierikxCM, Cohen StuartJ, VoetsGM, van den MunckhofMP, et al. (2011) Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 17 : 873–880 doi:10.1111/j.1469-0691.2011.03497.x
16. KluytmansJAJW, OverdevestITMA, WillemsenI, Kluytmans-van den BerghMFQ, van der ZwaluwK, et al. (2013) Extended-spectrum β-lactamase-producing Escherichia coli from retail chicken meat and humans: comparison of strains, plasmids, resistance genes, and virulence factors. Clin Infect Dis Off Publ Infect Dis Soc Am 56 : 478–487 doi:10.1093/cid/cis929
17. SabatAJ, BudimirA, NashevD, Sá-LeãoR, van Dijl Jm, et al. (2013) Overview of molecular typing methods for outbreak detection and epidemiological surveillance. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 18 : 20380.
18. VoetsGM, FluitAC, ScharringaJ, SchapendonkC, van den MunckhofT, et al. (2013) Identical plasmid AmpC beta-lactamase genes and plasmid types in E. coli isolates from patients and poultry meat in the Netherlands. Int J Food Microbiol 167 : 359–362 doi:10.1016/j.ijfoodmicro.2013.10.001
19. McNallyA, ChengL, HarrisSR, CoranderJ (2013) The evolutionary path to extraintestinal pathogenic, drug-resistant Escherichia coli is marked by drastic reduction in detectable recombination within the core genome. Genome Biol Evol 5 : 699–710 doi:10.1093/gbe/evt038
20. LiL, StoeckertCJJr, RoosDS (2003) OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res 13 : 2178–2189 doi:10.1101/gr.1224503
21. GradYH, LipsitchM, FeldgardenM, ArachchiHM, CerqueiraGC, et al. (2012) Genomic epidemiology of the Escherichia coli O104:H4 outbreaks in Europe, 2011. Proc Natl Acad Sci U S A 109 : 3065–3070 doi:10.1073/pnas.1121491109
22. ReevesPR, LiuB, ZhouZ, LiD, GuoD, et al. (2011) Rates of Mutation and Host Transmission for an Escherichia coli Clone over 3 Years. PLoS ONE 6: e26907 doi:10.1371/journal.pone.0026907
23. LanzaVF, de ToroM, Garcillán-BarciaMP, MoraA, BlancoJ, CoqueTM, de la CruzF Turbulent plasmid flux in Escherichia coli ST131 sublineages, analyzed by plasmid constellation network (PLACNET), a new method for plasmid reconstruction from whole genome sequences. PLoS Gen 10: e1004766.
24. SmillieC, Garcillán-BarciaMP, FranciaMV, RochaEPC, de la CruzF (2010) Mobility of plasmids. Microbiol Mol Biol Rev MMBR 74 : 434–452 doi:10.1128/MMBR.00020-10
25. Garcillán-BarciaMP, FranciaMV, de la CruzF (2009) The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol Rev 33 : 657–687.
26. Den BakkerHC, AllardMW, BoppD, BrownEW, FontanaJ, et al. (2014) Rapid Whole-Genome Sequencing for Surveillance of Salmonella enterica Serovar Enteritidis. Emerg Infect Dis 20 : 1306–1314 doi:10.3201/eid2008.131399
27. KöserCU, HoldenMTG, EllingtonMJ, CartwrightEJP, BrownNM, et al. (2012) Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N Engl J Med 366 : 2267–2275 doi:10.1056/NEJMoa1109910
28. RoetzerA, DielR, KohlTA, RückertC, NübelU, et al. (2013) Whole genome sequencing versus traditional genotyping for investigation of a Mycobacterium tuberculosis outbreak: a longitudinal molecular epidemiological study. PLoS Med 10: e1001387 doi:10.1371/journal.pmed.1001387
29. IshiwaA, KomanoT (2004) PilV Adhesins of Plasmid R64 Thin Pili Specifically Bind to the Lipopolysaccharides of Recipient Cells. J Mol Biol 343 : 615–625 doi:10.1016/j.jmb.2004.08.059
30. DierikxC, van der GootJ, FabriT, van Essen-ZandbergenA, SmithH, et al. (2013) Extended-spectrum-β-lactamase - and AmpC-β-lactamase-producing Escherichia coli in Dutch broilers and broiler farmers. J Antimicrob Chemother 68 : 60–67 doi:10.1093/jac/dks349
31. DierikxC, van Essen-ZandbergenA, VeldmanK, SmithH, MeviusD (2010) Increased detection of extended spectrum beta-lactamase producing Salmonella enterica and Escherichia coli isolates from poultry. Vet Microbiol 145 : 273–278 doi:10.1016/j.vetmic.2010.03.019
32. BörjessonS, JernbergC, BrolundA, EdquistP, FinnM, et al. (2013) Characterization of plasmid-mediated AmpC-producing E. coli from Swedish broilers and association with human clinical isolates. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 19: E309–311 doi:10.1111/1469-0691.12192
33. BaudryPJ, MatasejeL, ZhanelGG, HobanDJ, MulveyMR (2009) Characterization of plasmids encoding CMY-2 AmpC beta-lactamases from Escherichia coli in Canadian intensive care units. Diagn Microbiol Infect Dis 65 : 379–383 doi:\10.1016/j.diagmicrobio.2009.08.011.\
34. VillaL, García-FernándezA, FortiniD, CarattoliA (2010) Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J Antimicrob Chemother 65 2518–2529 : 34.
35. WoodfordN, CarattoliA, KarisikE, UnderwoodA, EllingtonMJ, et al. (2009) Complete nucleotide sequences of plasmids pEK204, pEK499, and pEK516, encoding CTX-M enzymes in three major Escherichia coli lineages from the United Kingdom, all belonging to the international O25:H4-ST131 clone. Antimicrob Agents Chemother 53 : 4472–4482 doi:10.1128/AAC.00688-09
36. KruseH, SørumH (1994) Transfer of multiple drug resistance plasmids between bacteria of diverse origins in natural microenvironments. Appl Environ Microbiol 60 : 4015–4021.
37. WarnesSL, HighmoreCJ, KeevilCW (2012) Horizontal transfer of antibiotic resistance genes on abiotic touch surfaces: implications for public health. mBio 3 doi:10.1128/mBio.00489-12
38. CavacoLM, AbatihE, AarestrupFM, GuardabassiL (2008) Selection and persistence of CTX-M-producing Escherichia coli in the intestinal flora of pigs treated with amoxicillin, ceftiofur, or cefquinome. Antimicrob Agents Chemother 52 : 3612–3616 doi:10.1128/AAC.00354-08
39. GorenMG, CarmeliY, SchwaberMJ, ChmelnitskyI, SchechnerV, et al. (2010) Transfer of carbapenem-resistant plasmid from Klebsiella pneumoniae ST258 to Escherichia coli in patient. Emerg Infect Dis 16 : 1014–1017 doi:10.3201/eid1606.091671
40. WirthT, FalushD, LanR, CollesF, MensaP, et al. (2006) Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 60 : 1136–1151 doi:10.1111/j.1365-2958.2006.05172.x
41. CarattoliA, BertiniA, VillaL, FalboV, HopkinsKL, et al. (2005) Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63 : 219–228 doi:10.1016/j.mimet.2005.03.018
42. García-FernándezA, ChiarettoG, BertiniA, VillaL, FortiniD, et al. (2008) Multilocus sequence typing of IncI1 plasmids carrying extended-spectrum beta-lactamases in Escherichia coli and Salmonella of human and animal origin. J Antimicrob Chemother 61 : 1229–1233 doi:10.1093/jac/dkn131
43. JolleyKA, MaidenMCJ (2010) BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11 : 595 doi:10.1186/1471-2105-11-595
44. Cohen StuartJ, DierikxC, Al NaiemiN, KarczmarekA, Van HoekAHAM, et al. (2010) Rapid detection of TEM, SHV and CTX-M extended-spectrum beta-lactamases in Enterobacteriaceae using ligation-mediated amplification with microarray analysis. J Antimicrob Chemother 65 : 1377–1381 doi:10.1093/jac/dkq146
45. LiY, HuY, BolundL, WangJ (2010) State of the art de novo assembly of human genomes from massively parallel sequencing data. Hum Genomics 4 : 271–277.
46. SchatzMC, PhillippyAM, SommerDD, DelcherAL, PuiuD, et al. (2013) Hawkeye and AMOS: visualizing and assessing the quality of genome assemblies. Brief Bioinform 14 : 213–224 doi:10.1093/bib/bbr074
47. AzizRK, BartelsD, BestAA, DeJonghM, DiszT, et al. (2008) The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9 : 75 doi:10.1186/1471-2164-9-75
48. TatusovRL, NataleDA, GarkavtsevIV, TatusovaTA, ShankavaramUT, et al. (2001) The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res 29 : 22–28.
49. de BeenM, van SchaikW, ChengL, CoranderJ, WillemsRJ (2013) Recent recombination events in the core genome are associated with adaptive evolution in Enterococcus faecium. Genome Biol Evol 5 : 1524–1535 doi:10.1093/gbe/evt111
50. EdgarRC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32 : 1792–1797 doi:10.1093/nar/gkh340
51. Capella-GutiérrezS, Silla-MartínezJM, GabaldónT (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinforma Oxf Engl 25 : 1972–1973 doi:10.1093/bioinformatics/btp348
52. StamatakisA (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinforma Oxf Engl 22 : 2688–2690 doi:10.1093/bioinformatics/btl446
53. TamuraK, PetersonD, PetersonN, StecherG, NeiM, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28 : 2731–2739 doi:10.1093/molbev/msr121
54. LangmeadB, SalzbergSL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9 : 357–359 doi:10.1038/nmeth.1923
55. LarsenMV, CosentinoS, RasmussenS, FriisC, HasmanH, et al. (2012) Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50 : 1355–1361 doi:10.1128/JCM.06094-11
56. KurtzS, PhillippyA, DelcherAL, SmootM, ShumwayM, et al. (2004) Versatile and open software for comparing large genomes. Genome Biol 5: R12 doi:10.1186/gb-2004-5-2-r12
57. TekaiaF, YeramianE (2005) Genome trees from conservation profiles. PLoS Comput Biol 1: e75 doi:10.1371/journal.pcbi.0010075
58. LiW, GodzikA (2006) Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinforma Oxf Engl 22 : 1658–1659 doi:10.1093/bioinformatics/btl158
Štítky
Genetika Reprodukčná medicína
Článek Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart DiseaseČlánek Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the NicheČlánek Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial TetheringČlánek Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature SeasonalityČlánek Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell DivisionsČlánek Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity inČlánek Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration inČlánek ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression inČlánek Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex EnvironmentsČlánek The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 12- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Stratification by Smoking Status Reveals an Association of Genotype with Body Mass Index in Never Smokers
- Genome Wide Meta-analysis Highlights the Role of Genetic Variation in in the Regulation of Circulating Serum Chemerin
- Occupancy of Mitochondrial Single-Stranded DNA Binding Protein Supports the Strand Displacement Mode of DNA Replication
- Distinct Genealogies for Plasmids and Chromosome
- Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart Disease
- Non-coding RNAs Prevent the Binding of the MSL-complex to Heterochromatic Regions
- Plasmid Flux in ST131 Sublineages, Analyzed by Plasmid Constellation Network (PLACNET), a New Method for Plasmid Reconstruction from Whole Genome Sequences
- Epigenome-Guided Analysis of the Transcriptome of Plaque Macrophages during Atherosclerosis Regression Reveals Activation of the Wnt Signaling Pathway
- The Inventiveness of Nature: An Interview with Werner Arber
- Mediation Analysis Demonstrates That -eQTLs Are Often Explained by -Mediation: A Genome-Wide Analysis among 1,800 South Asians
- Generation of Antigenic Diversity in by Structured Rearrangement of Genes During Mitosis
- A Massively Parallel Pipeline to Clone DNA Variants and Examine Molecular Phenotypes of Human Disease Mutations
- Genetic Analysis of the Cardiac Methylome at Single Nucleotide Resolution in a Model of Human Cardiovascular Disease
- Genetic Analysis of Circadian Responses to Low Frequency Electromagnetic Fields in
- The Dissection of Meiotic Chromosome Movement in Mice Using an Electroporation Technique
- Altered Chromatin Occupancy of Master Regulators Underlies Evolutionary Divergence in the Transcriptional Landscape of Erythroid Differentiation
- Syd/JIP3 and JNK Signaling Are Required for Myonuclear Positioning and Muscle Function
- Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the Niche
- Mutation of Leads to Blurred Tonotopic Organization of Central Auditory Circuits in Mice
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- RAN-Binding Protein 9 is Involved in Alternative Splicing and is Critical for Male Germ Cell Development and Male Fertility
- Enhanced Longevity by Ibuprofen, Conserved in Multiple Species, Occurs in Yeast through Inhibition of Tryptophan Import
- Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial Tethering
- Recurrent Loss of Specific Introns during Angiosperm Evolution
- Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature Seasonality
- SEEDSTICK is a Master Regulator of Development and Metabolism in the Arabidopsis Seed Coat
- Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell Divisions
- Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity in
- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression in
- Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex Environments
- Global Analysis of Photosynthesis Transcriptional Regulatory Networks
- Mucolipin Co-deficiency Causes Accelerated Endolysosomal Vacuolation of Enterocytes and Failure-to-Thrive from Birth to Weaning
- Controlling Pre-leukemic Thymocyte Self-Renewal
- How Malaria Parasites Avoid Running Out of Ammo
- Echoes of the Past: Hereditarianism and
- Deep Reads: Strands in the History of Molecular Genetics
- Keep on Laying Eggs Mama, RNAi My Reproductive Aging Blues Away
- Analysis of a Plant Complex Resistance Gene Locus Underlying Immune-Related Hybrid Incompatibility and Its Occurrence in Nature
- Epistatic Adaptive Evolution of Human Color Vision
- Increased and Imbalanced dNTP Pools Symmetrically Promote Both Leading and Lagging Strand Replication Infidelity
- Genetic Basis of Haloperidol Resistance in Is Complex and Dose Dependent
- Genome-Wide Analysis of DNA Methylation Dynamics during Early Human Development
- Interaction between Conjugative and Retrotransposable Elements in Horizontal Gene Transfer
- The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
- is Required for Adult Maintenance of Dopaminergic Neurons in the Ventral Substantia Nigra
- PRL1, an RNA-Binding Protein, Positively Regulates the Accumulation of miRNAs and siRNAs in Arabidopsis
- Genetic Control of Contagious Asexuality in the Pea Aphid
- Early Mesozoic Coexistence of Amniotes and Hepadnaviridae
- Local and Systemic Regulation of Plant Root System Architecture and Symbiotic Nodulation by a Receptor-Like Kinase
- Gene Pathways That Delay Reproductive Senescence
- The Evolution of Fungal Metabolic Pathways
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- Formation of Linear Amplicons with Inverted Duplications in Requires the MRE11 Nuclease
- Identification of Rare Causal Variants in Sequence-Based Studies: Methods and Applications to , a Gene Involved in Cohen Syndrome and Autism
- Rrp12 and the Exportin Crm1 Participate in Late Assembly Events in the Nucleolus during 40S Ribosomal Subunit Biogenesis
- The Mutations in the ATP-Binding Groove of the Rad3/XPD Helicase Lead to -Cockayne Syndrome-Like Phenotypes
- Topoisomerase I Plays a Critical Role in Suppressing Genome Instability at a Highly Transcribed G-Quadruplex-Forming Sequence
- A Cbx8-Containing Polycomb Complex Facilitates the Transition to Gene Activation during ES Cell Differentiation
- Transcriptional Frameshifting Rescues Type VI Secretion by the Production of Two Length Variants from the Prematurely Interrupted Gene
- Association Mapping across Numerous Traits Reveals Patterns of Functional Variation in Maize
- Genome-Wide Analysis of -Regulated and Phased Small RNAs Underscores the Importance of the ta-siRNA Pathway to Maize Development
- Dissemination of Cephalosporin Resistance Genes between Strains from Farm Animals and Humans by Specific Plasmid Lineages
- The Tau Tubulin Kinases TTBK1/2 Promote Accumulation of Pathological TDP-43
- Germline Signals Deploy NHR-49 to Modulate Fatty-Acid β-Oxidation and Desaturation in Somatic Tissues of
- Microevolution of in Macrophages Restores Filamentation in a Nonfilamentous Mutant
- Vangl2-Regulated Polarisation of Second Heart Field-Derived Cells Is Required for Outflow Tract Lengthening during Cardiac Development
- Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice
- A ABC Transporter Regulates Lifespan
- RA and FGF Signalling Are Required in the Zebrafish Otic Vesicle to Pattern and Maintain Ventral Otic Identities
- , and Reprogram Thymocytes into Self-Renewing Cells
- The miR9863 Family Regulates Distinct Alleles in Barley to Attenuate NLR Receptor-Triggered Disease Resistance and Cell-Death Signaling
- Detection of Pleiotropy through a Phenome-Wide Association Study (PheWAS) of Epidemiologic Data as Part of the Environmental Architecture for Genes Linked to Environment (EAGLE) Study
- Extensive Copy-Number Variation of Young Genes across Stickleback Populations
- The and Genetic Modules Interact to Regulate Ciliogenesis and Ciliary Microtubule Patterning in
- Analysis of the Genome, Transcriptome and Secretome Provides Insight into Its Pioneer Colonization Strategies of Wood
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- Echoes of the Past: Hereditarianism and
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



