-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
RA and FGF Signalling Are Required in the Zebrafish Otic Vesicle to Pattern and Maintain Ventral Otic Identities
The vertebrate inner ear is a complex three-dimensional structure with hearing and balance functions. To form a functional ear in the embryo, it is crucial that the right cells develop at the right time and in the right place. These cells include the sensory hair cells that detect sound and movement, neurons that relay sensory information to the brain, and structural cells. We have investigated patterning and maintenance events in the developing ear of the zebrafish embryo. We show that two signalling pathways, FGF and Retinoic Acid (RA), act in an antagonistic manner to regulate the numbers of sensory hair cells that develop, together with the expression of a key gene, otx1b, required for the development of structural cells. However, the two signalling pathways act in concert to regulate the emergence of neuronal cells. Our data also indicate that FGF and RA signalling form a feedback loop, placing them at the heart of the regulatory network that ensures correct patterning is maintained in the ear. Both FGF and RA signalling are employed to generate hair cells and neurons for replacement therapies to treat hearing loss. Understanding the roles of FGF and RA signalling underpins the development of such therapies.
Published in the journal: RA and FGF Signalling Are Required in the Zebrafish Otic Vesicle to Pattern and Maintain Ventral Otic Identities. PLoS Genet 10(12): e32767. doi:10.1371/journal.pgen.1004858
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004858Summary
The vertebrate inner ear is a complex three-dimensional structure with hearing and balance functions. To form a functional ear in the embryo, it is crucial that the right cells develop at the right time and in the right place. These cells include the sensory hair cells that detect sound and movement, neurons that relay sensory information to the brain, and structural cells. We have investigated patterning and maintenance events in the developing ear of the zebrafish embryo. We show that two signalling pathways, FGF and Retinoic Acid (RA), act in an antagonistic manner to regulate the numbers of sensory hair cells that develop, together with the expression of a key gene, otx1b, required for the development of structural cells. However, the two signalling pathways act in concert to regulate the emergence of neuronal cells. Our data also indicate that FGF and RA signalling form a feedback loop, placing them at the heart of the regulatory network that ensures correct patterning is maintained in the ear. Both FGF and RA signalling are employed to generate hair cells and neurons for replacement therapies to treat hearing loss. Understanding the roles of FGF and RA signalling underpins the development of such therapies.
Introduction
Most cell types of the inner ear arise from the otic placode, a region of specialised ectoderm lying adjacent to the developing hindbrain. To form the complex three-dimensional structure of the adult inner ear, cells in the otic region integrate information from both extrinsic and intrinsic factors over time, thereby gradually restricting the competence of the different regions in the emerging inner ear. In the developing zebrafish otic vesicle (OV), one of the first subdivisions to occur is the emergence of sensory, neurogenic and non-neural domains in the ventral otic epithelium. The sensory domain gives rise to the sensory patches or maculae at the anterior and posterior poles of the OV, consisting of sensory hair cells and supporting cells. Neurons of the statoacoustic ganglion (SAG) arise from the neurogenic domain in an anteroventral position. The non-neural domain, located ventrolaterally, gives rise to non-neural epithelium, including the ventral pillar of the lateral semicircular canal.
The emergence of these domains is a dynamic process, and key intrinsic regulators of these early otic cell fate decisions include tbx1 and otx1b, which are both expressed in the otic placode and OV and code for transcription factors. The posteroventral OV expresses tbx1, a gene that has been associated with DiGeorge syndrome in humans. Both mice (Tbx1−/−) and zebrafish (van gogh (vgo)) mutant for tbx1 show an expansion of otic neurogenic anteroventral territories towards more posterior positions, demonstrating that Tbx1 functions to restrict neurogenesis in posterior otic regions [1], [2]. otx1b is expressed in a discrete ventrolateral domain in the zebrafish OV from around the 18 somite (18S) stage [3], [4], [5]. Injection of zebrafish embryos with a morpholino to otx1b results in loss of the non-neural epithelium separating the two maculae, together with the ventral semicircular canal pillar and lateral semicircular canal [6]. Expression of otx1b is lost in the ears of vgo/tbx1 mutant embryos, suggesting that otx1b acts downstream of Tbx1 [7]. These observations, together with the dramatic ventral otic phenotypes observed when tbx1 or otx1b are disrupted, led us to examine the regulatory signalling events that function upstream of otx1b.
In zebrafish, fibroblast growth factor (FGF) signalling emanating from rhombomere 4 at early somitogenesis stages is an early inducer of otic placodal fate [8], [9], [10], [11]. At late placode and early vesicle stages, FGF is required for the specification of anterior otic fates [12]. In addition, evidence from several model systems indicates that FGF signalling is required for the generation of otic sensory neuroblasts [8], [13], [14] (reviewed in [15]), which emerge from an anteroventral position in the zebrafish OV.
Retinoic acid (RA) signalling is another extrinsic factor that has been implicated in otic patterning at early developmental stages. RA plays a role in determining the extent of the region competent to respond to otic inducing signals [16]. Whilst FGF signalling is required for anteroventral otic fates, RA signalling has been implicated in the specification of posteroventral otic territories. The RA-producing enzyme gene aldh1a2 is expressed in head mesenchyme posterior to the otic placode [17], [18], while aldh1a3 is expressed in the developing ear from OV stages [19]. Recently, RA signalling from late gastrula stages has been shown to promote early otic non-neural fates. Here, it acts via tbx1 to regulate the expression of the bHLH transcription factor her9, which is expressed in the posteroventral region of the zebrafish OV and is required for the repression of neurogenic fates [2].
Expression of FGF and RA pathway members persists at later OV stages (Fig. 1), both in surrounding tissues and in the OV epithelium, raising the intriguing possibility that these signalling pathways continue to act at later stages of inner ear development. Thus we investigated the roles of FGF and RA signalling after the initial induction and patterning phase to examine whether these two signalling pathways act during the refinement and maintenance phase of OV patterning (from the 18S stage onward). We have characterised the expression patterns of genes coding for components of both of these signalling pathways during later otic development. In addition, we have employed conditional approaches to manipulate FGF and RA signalling to analyse the late requirement of both FGF and RA signalling during OV stages and provide loss of function data for aldh1a3. We provide evidence that ventral otic otx1b expression, a marker of non-neural fates, requires ongoing high levels of FGF signalling, whereas lower levels of FGF favour sensory (hair cell) development. RA signalling, on the other hand, promotes sensory fates and restricts otx1b expression. In addition, we show that tbx1 and otx1b expression are differentially regulated by RA signalling. This suggests that both FGF and RA signalling continue to function during late phases of ventral OV development. The analysis of aldh1a3 morphant embryos pinpoints the anterior OV as the source of RA at these stages. Expression of aldh1a3 in the OV is itself perturbed in embryos mutant for fgf3 and fgf8a, or in embryos where FGF signalling has been blocked chemically, indicating that FGF signalling is required for aldh1a3 expression at later stages. Moreover, we provide evidence that RA can suppress anterior otic fgf3 and fgf8a expression. As fgf3 and fgf8a start to be expressed in the OV before otx1b or aldh1a3, this suggests a temporal sequence of events that interconnects FGF and RA in a feedback loop.
Fig. 1. Expression of FGF and RA signalling pathway components during zebrafish OV development. 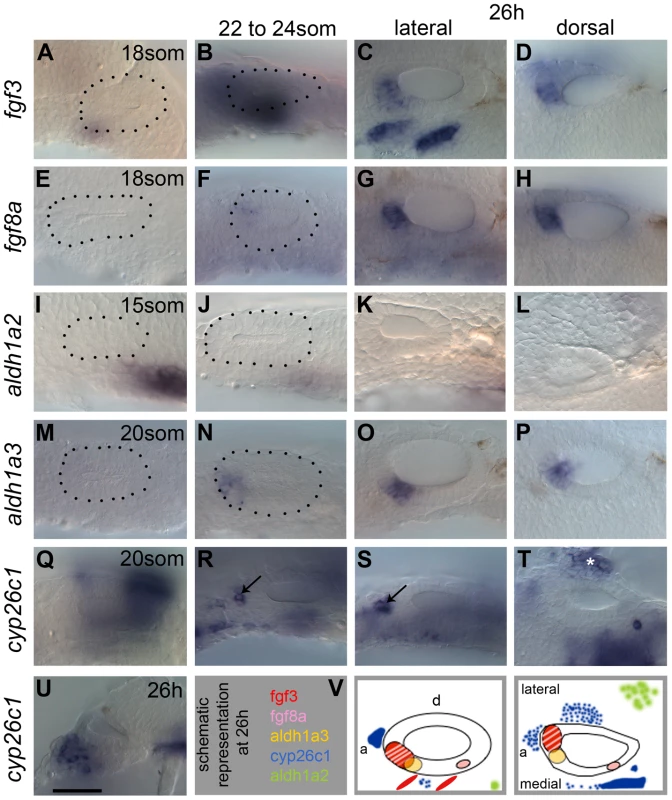
All panels are lateral views with anterior to the left, apart from D,H,L,P,T (dorsal views; anterior left, lateral up), and U (transverse section, lateral left, dorsal up). (A–D) fgf3 is expressed in pharyngeal pouch mesenchyme underlying the anteroventral OV from 18S. At 26 hpf (C,D,V), two stripes of expression underlie the OV in an anteroventral lateral position, and the OV itself expresses fgf3 in the anterior. (E–H) fgf8a expression is very weak at early stages, but is strongly expressed in the anterior OV at 26 hpf (G,H,V). (I–L) At 15S, aldh1a2 is strongly expressed in the mesenchyme posteroventral to the otic placode (I). At later stages, mesenchymal aldh1a2 expression becomes weaker and shifts to a position further away from the OV (J–L,V). (M–P) Expression of aldh1a3 can be detected in the OV from 22S in an anteroventral position. At 26 hpf, the aldh1a3-expressing cells are located in an anteroventral medial position in the OV (O,P,V). (Q–U) Expression of cyp26c1 can be detected in periotic mesenchyme from early stages and at 26 hpf is expressed strongly in the hindbrain (out of focus). Groups of cells expressing cyp26c1 abut the OV anteriorly (R,S, arrows) and ventrolaterally (T, asterisk). (V) Schematic representation of the expression domains of fgf3, fgf8a, aldh1a3, cyp26c1 and aldh1a2 in the OV at 26 hpf in a lateral and dorsal view. Scale bar: 50 µm. Results
Expression of FGF and RA signalling pathway members in the zebrafish otic vesicle
To address the role of FGF and RA signalling in otic patterning at otic vesicle (OV) stages, we first examined the expression of pathway members in and around the OV from 18 hours post fertilisation/18 somites (18 hpf/18S). As previously reported, both fgf3 and fgf8a are expressed in the anterior OV from around 18 hpf (Fig. 1A–H), with weak fgf8a expression in the posterior OV (Fig. 1G,H) [8]. In addition, strong expression of fgf3 is observed in the pharyngeal pouches underneath the ventral OV floor, whereas fgf8a is expressed only weakly in this region (Fig. 1C,G). In addition, fgfr1a, fgfr2 and fgfr4, but not fgfr3, are expressed in the OV in partially overlapping but distinct patterns (S1 Figure), indicating that the ventral OV is competent to respond to FGF signalling. fgfr4 shows the strongest expression, in a ventromedial stripe at 22S. At 26 hpf, expression of fgfr4 is excluded from the neurogenic region, but remains strongly expressed at the OV poles. fgfr1a and fgfr2 are expressed in the posterior part of the OV weakly from 22S and expression persists at 26 hpf. Transverse sections through the OV at 26 hpf reveal that fgfr1a is expressed fairly ubiquitously, while fgfr2 is expressed posteromedially (S1 Figure).
RA levels in the embryo are thought to reflect a balance between expression levels of RA-producing (aldh) and RA-degrading (cyp) enzyme genes. Two aldh genes are expressed in or near the zebrafish ear at the relevant stages. aldh1a2 is expressed in the mesenchyme adjacent to the otic placode posteriorly at the 15S stage (Fig. 1I) [18]. As development proceeds, weak expression persists in the mesenchyme, but the distance of the expression domain from the OV increases (Fig. 1J–L), indicating that aldh1a2 is not likely to influence later stages of OV development. This is further corroborated by the analysis of aldh1a2/neckless (nls) mutant embryos at 26 hpf, which show only subtle defects in OV patterning (S2 Figure). aldh1a3 is expressed in the anteroventral OV from 20/21S onwards (Fig. 1M–P) [19]. At 20S, the RA-degrading enzyme gene cyp26c1 is expressed in the hindbrain rhombomere 5/6 and in the head mesenchyme from the 6S stage (Fig. 1Q–U) [20]. As development proceeds, an additional expression domain is detected in a cluster of cells adjacent to the anterior of the OV (Fig. 1R,S). At 26 hpf, cyp26c1 expression continues in rhombomere 5/6 of the hindbrain and in cells anterior and lateral to the OV, which are likely to form the main RA sink sites surrounding the OV (Fig. 1S–U). A schematic summary of these expression data at 26 hpf is shown in Fig. 1V. The four retinoic acid receptor genes show distinct and spatially regulated expression patterns in the zebrafish OV at 26 hpf (S1 Figure). Both rarab and rargb appear to mark the two developing sensory patches, while rarga shows a strikingly similar pattern to that of tbx1, with expression excluded from an anteroventral domain. raraa is only weakly expressed at the anterior of the OV. Taken together, these data raise the interesting possibility that FGF and RA signalling influence patterning or maintenance events in the OV between 18S and 26 hpf.
Graded FGF signalling is required for otic vesicle patterning at later stages of development
We first tested whether FGF signalling is required during OV stages. To bypass the early requirements of FGF signalling in otic induction and anterior-posterior patterning, we exposed embryos to different concentrations of the chemical pan-FGF inhibitor SU5402 [21], [22], from 18/20S to 26 hpf. In our hands, SU5402 was a very effective inhibitor of the FGF response genes etv4 and dusp6 throughout the embryo, including in the OV (S3 Figure). To assess the effects of FGF inhibition on the OV at 26 hpf we used the following markers for different subregions of the OV: (1) To identify the non-neural domain, we used tbx1, which at 26 hpf is most strongly expressed in the posteroventral OV, and otx1b, which is expressed in the ventrolateral OV floor. (2) To identify the neurogenic domain, we analysed the expression of neurog1 (expressed in the neurogenic region in the OV in specified neuronal cells and in some of the emerging neuroblasts) and neurod1 (expressed in delaminating otic neuroblasts and those forming the statoacoustic ganglion beneath the OV). (3) To assess sensory patch spacing and development we analysed the expression of sox2, atoh1a and tecta, which are all expressed in the emerging utricular and saccular maculae (S4 Figure).
Embryos treated with 5, 10 or 15 µM SU5402 from the 18/20S stage onwards all showed a severe reduction (5, 10 µM) or loss (15 µM) of tbx1 expression (Fig. 2G,M,S), and a loss of otx1b expression, as previously reported [8] (Fig. 2H,N,T), suggesting that FGF signalling is required for normal development of the non-neural domain. Concentrations of 20 µM SU5402 were toxic. Expression of neurod1 decreased with rising SU5402 concentration (Fig. 2I,O,U), in line with results reported previously [14], confirming that FGFs are required for normal levels of otic neurogenesis (neuroblast specification and/or proliferation), even in the absence of tbx1 expression. Interestingly, expression of sensory patch markers varied with the different inhibitor concentrations. Low and intermediate levels of SU5402 (5 and 10 µM) resulted in expansion of the sox2 expression domain, which now extended across the entire ventral OV floor (Fig. 2J,P), whereas expression of sox2 was lost at high SU5402 levels (15 µM; Fig. 2V). Expression of both atoh1a and tecta, more mature sensory patch markers, was decreased or lost at 26 hpf at all concentrations tested (Fig. 2).
Fig. 2. Inhibition of FGF signalling by SU5402 reveals a concentration-dependent effect of FGF signalling during OV development. 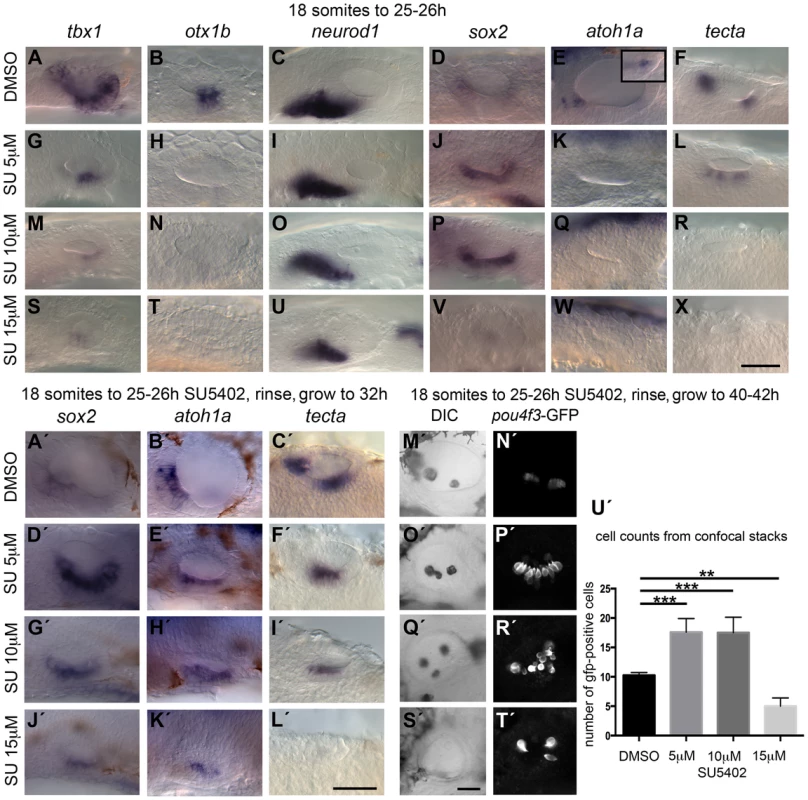
Wild-type (WT) embryos were treated with DMSO or SU5402 from 18S to 26 hpf (A–X) or rinsed and grown on to 32 hpf (A′–L′) or 40–42 hpf (M′–T′). (A–F) WT embryos treated with DMSO show normal expression of non-neural markers tbx1 (n = 51) and otx1b (n = 61; A,B), the neuronal marker neurod1 (n = 64; C), and the sensory markers sox2 (n = 56), atoh1a (n = 26) and tecta (n = 44; D–F). Boxed area in panel (E) depicts the atoh1a expression in posterior sensory patch in a different focal plane. (G–R) Treatment with 5 µM SU5402 (G–L) or 10 µM SU5402 (M–R) reduces the expression of tbx1 (n = 56; G,I) and neurod1 (n = 76; M,O). Expression of otx1b is lost (n = 32; H,N) and expression of sox2 is extended in the ventral OV floor (n = 45; J,P). The expression of atoh1a is lost (n = 18; K,Q) and expression of tecta is weak but extended (n = 27; L, 5 µM SU5402) or lost (n = 40; R, 10 µM SU5402). (S–X) Treatment with 15 µM SU5402 reduces the expression of tbx1 (n = 38; S) and neurod1 (n = 65; U); expression of otx1b (n = 57), sox2 (n = 58), atoh1a (n = 35), and tecta (n = 51) is lost (T,V–X). (A′–C′) WT embryos treated with DMSO show normal expression of the sensory markers sox2 (n = 23), atoh1a (n = 31) and tecta (n = 25) at 32 hpf. (D′–I′) In embryos treated with 5 µM SU5402 (D′–F′) or 10 µM SU5402 (G′–I′), expression of sox2 (n = 23) and atoh1a (n = 25) is extended in the ventral OV floor; tecta expression is misplaced in a single ventromedial patch (n = 24). (J′–L′) Treatment with 15 µM SU5402 reduces or abolishes the expression of sox2 (n = 18), atoh1a (n = 16), and tecta (n = 14). (M′,N′) Tg(pou4f3:GFP) embryos treated with DMSO show normal hair cell patterns marked with GFP (n = 26). (O′,P′) In Tg(pou4f3:GFP) embryos treated with 5 µM SU5402, GFP-positive cells extended between the anterior and posterior sensory patch in the ventral OV floor (n = 18). (Q′,R′) In Tg(pou4f3:GFP) embryos treated with 10 µM SU5402, GFP-positive cells appear less orderly and are extended in the ventral OV floor (n = 12). (S′,T′) Treatment with 15 µM SU5402 reduced the number of GFP-positive cells in the OV (n = 14). (U′) Graphical representation of counts of GFP-positive cells at 40–42 hpf. Error bars represent standard error of the mean. One-way ANOVA with Dunnett's multiple comparison post-test: **p = 0.0065, ***p<0.0005. All panels are lateral views with anterior to the left, apart from the panels depicting tecta (dorsal views; anterior to the left, lateral up). Scale bar: 50 µm. To assess whether sensory development was blocked at the sox2-expressing stage during low and intermediate level SU5402 treatment, we treated wild-type (WT) and Tg(pou4f3:gfp) embryos, in which mature hair cells express GFP, with SU5402, washed at 26 hpf and grew on further to analyse sox2, atoh1a and tecta expression at 32 hpf in WT embryos (Fig. 2A′–L′) or GFP expression at 40–42 hpf in transgenic embryos (Fig. 2M′–U′). At these later stages, embryos exposed to 5 and 10 µM SU5402 now showed an up-regulation of sox2 and atoh1a expression in the ventral OV floor, while tecta expression is misplaced (Fig. 2D′–I′), whereas all three markers were lost or reduced at high SU5402 levels (15 µM, Fig. 2J′–L′). The maculae in the Tg(pou4f3:gfp) transgenic embryos treated with 5 µM SU5402 appeared orderly but were enlarged and merged in the middle (Fig. 2D′–F′,P′), whereas at 10 µM SU5402 the merged maculae were more disarrayed (Fig. 2G′–I,′, R′). A significant increase in the number of GFP-positive cells, indicative of mature hair cells, was observed in these embryos (Fig. 2U′). This indicates that despite a block in hair cell differentiation during SU5402 treatment, the spatial domain of sensory development was expanded in embryos treated with low and intermediate concentrations of the FGF inhibitor, and that hair cell differentiation could proceed once FGF inhibition was relieved. There was a concomitant disruption to otolith formation, with ectopic and misplaced otoliths observed in the ears of treated embryos (Fig. 2O′,Q′,S′). At the highest SU5402 concentration, hair cells were almost entirely lost; the few that appeared might be early hair cells that formed before inhibitor treatment commenced. Interestingly, otoliths failed to form in embryos exposed to 15 µM SU5402, indicating that the otolith precursor-producing cells might be missing in these embryos as well (Fig. 2S′–U′).
Taken together, our results suggest a dose-dependent requirement for FGF signalling during OV development. High levels of FGF are required to maintain ventral, non-sensory and non-neural OV floor character as indicated by the loss or reduction of both otx1b and tbx1 expression with low levels of SU5402. On the other hand, low levels of FGF signalling favour sensory development; a reduction in Fgf signalling is sufficient to lead to an up-regulation of sensory markers, and the differentiation of supernumerary hair cells, in the non-neural region of the OV. Our results also confirm a requirement for Fgf signalling for normal otic neurogenesis, as shown previously [8], [13], [14].
FGF3 and FGF8A have different roles during otic vesicle development
We were intrigued by the strong requirement for FGF signalling in the ventral OV floor revealed by the SU5402 treatments. Given the differences in expression patterns between fgf3 and fgf8a in the pharyngeal pouch region underneath the OV (Fig. 1), and in their receptor specificity [23], we tested whether the two genes had different roles in OV patterning. To distinguish FGF3 from FGF8A signalling we employed lia (fgf3−/−) and ace (fgf8a−/−) mutant embryos. According to the literature, the fgf8ati282a allele ‘strongly or completely’ inactivates the fgf8a gene [24], but the severity of the OV phenotype can vary in these mutants [8]. The observed effects described below could be detected across the range of OV size reduction that occurs in ace (fgf8a−/−) mutants. The fgf3t21142 allele is likely to be null [25]. We examined expression of a subset of markers used in the SU5402 treatments at 26–28 hpf. Interestingly, with regard to sensory markers, lia (fgf3−/−) and ace (fgf8a−/−) mutant embryos showed qualitatively distinct phenotypes: in embryos deficient for fgf3, the two maculae appeared closer together or merged (Fig. 3D–F; see also [12]), resembling the phenotype observed at low levels of SU5402 treatment, whereas in fgf8a-deficient embryos, two sensory patches developed with the correct spacing in the smaller ear, but were reduced in size (Fig. 3G–I) (see also [8]). This was corroborated in lia (fgf3−/−) and ace (fgf8a−/−) mutant embryos stained for actin and analysed at 54 hpf. In lia (fgf3−/−) embryos the anterior and posterior maculae remained juxtaposed, and the posterior macula contained more hair cells than normal (Fig. 3J,K,N), whereas in fgf8a-deficient ace embryos the two sensory patches developed with the correct spacing in the smaller ear, but were reduced in size, with fewer hair cells in the posterior patch (Fig. 3L–N). We never observed a fusion of the sensory patches either at 26–28 hpf or at 54 hpf as has been reported for 1 out of 8 ace (fgf8a−/−) mutant embryos analysed at 5 dpf [8]. Expression of tbx1 and otx1b was also affected differently in each of the FGF mutants at 26 hpf. In lia (fgf3−/−) mutants, tbx1 expression extended ectopically into the anteroventral domain (normally devoid of tbx1 expression; S5 Figure), most likely due to earlier functions of fgf3 during otic development. In addition, otx1b expression was reduced (S5 Figure), although appeared normal at later stages [12]. By contrast, both genes were patterned normally, albeit in smaller domains, in the smaller ace (fgf8a−/−) mutant ear (S5 Figure) [8].
Fig. 3. Expression of sensory markers in lia (fgf3−/−) and ace (fgf8a−/−) mutant embryos. 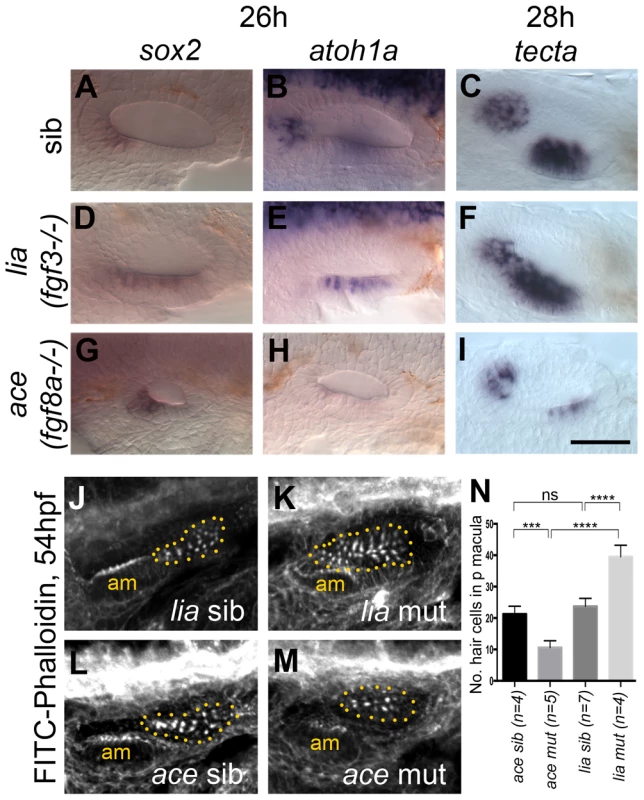
(A–C) Phenotypically wild-type sibling (sib) embryos show normal expression of the sensory markers sox2, atoh1a and tecta. (D–F) In lia (fgf3−/−) embryos, expression of sox2 (n = 12/41 embryos from a heterozygous cross), atoh1a (n = 13/45) and tecta (n = 14/42) is extended in the ventral OV floor. (G–I) In ace (fgf8a−/−) embryos, expression of sox2 (n = 12/42), atoh1a (n = 10/39) and tecta (n = 14/54) shows normal spatial patterning, but while sox2 expression levels are slightly increased, atoh1a and tecta levels are reduced. (J–N) Dotted yellow line demarcates the area of the posterior macula in which FITC-Phalloidin-positive hair bundles were counted. (J,L,N) Phenotypically wild-type sibling (sib) embryos show normal numbers of hair cells (n = 7 ears counted, lia sib; n = 4, ace sib). (K) In lia (fgf3−/−) embryos the posterior macula is enlarged; only FITC-phalloidin-positive cells that appeared posterior-like and were located in the demarcated area were counted (n = 4 mutant ears). (M) In ace (fgf8a−/−) embryos, hair cells show a normal spatial pattern, but the size of the maculae appears reduced (n = 6 mutant ears). (N) Graphical representation of counts of Phalloidin-positive cells at 53–56 hpf in the demarcated area. Error bars represent standard deviation. One-way ANOVA with Šídák's multiple comparison post-test: ***p<0.001, ****p<0.0001, ns = not significant. All panels are lateral views with anterior to the left, apart from the panels depicting tecta, which are dorsal views with anterior to the left, lateral up. Scale bar: 50 µm. The role FGF3 plays in otic patterning is further strengthened by over-expression of FGF3 using the Tg(hsp70:fgf3)-line (Fig. 4). The space between the two sensory patches was increased due to a specific reduction of the posterior sensory patch and instead, non-neurogenic (otx1b) and neuronal (neurog1, neurod1) markers were up-regulated (Fig. 4A–Z). Hair cell numbers in the posterior macula were specifically reduced at 49–53 hpf (Fig. 4A′), but overall ear morphology was only mildly perturbed (Fig. 4B′–E′). These data suggest that FGF3 is mainly responsible for the correct spacing of the sensory patches in the ventral otic floor, and influences hair cell number in the posterior macula. We speculate that the underlying pharyngeal pouches act as a source for FGF3 in this process, but further experiments are needed to test this conclusively. The smaller ear and reduced sensory patches in ace mutants are likely to reflect the earlier functions of FGF8A in otic induction, together with its requirement for sensory and neuronal development, as previously reported [26], [27].
Fig. 4. Effects of fgf3 over-expression from the 18 somite stage on otic vesicle patterning. 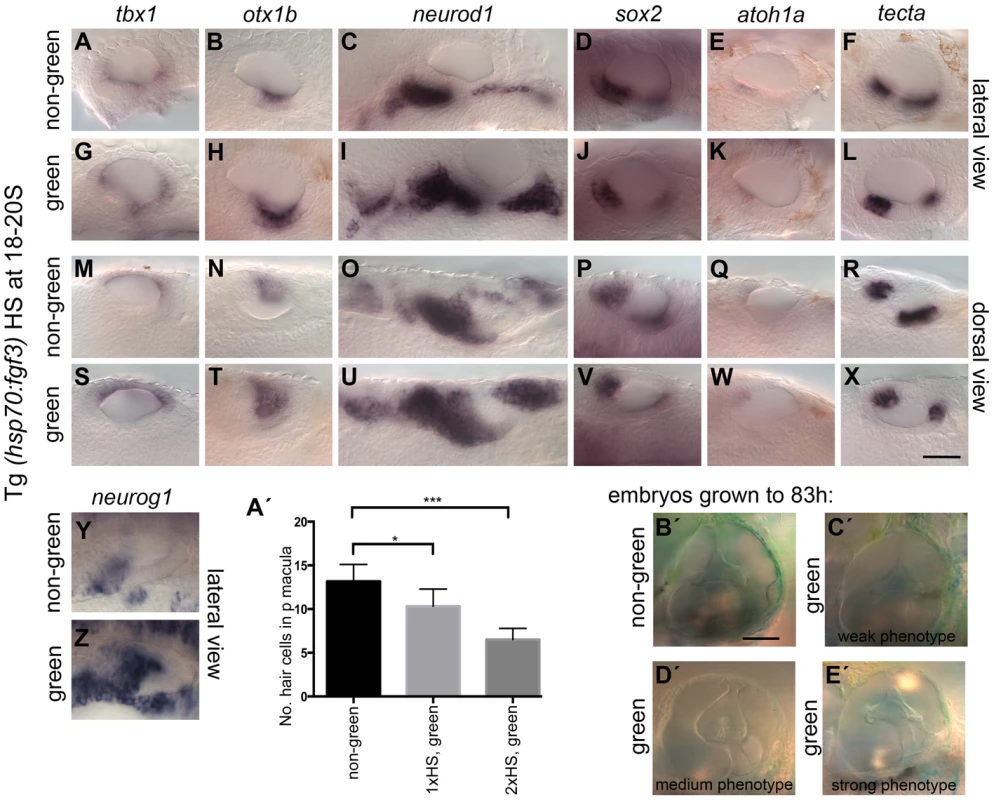
Tg(hsp70:fgf3) embryos were heat shocked (HS) from 18S and sorted as non-green, non-transgenic siblings (A–F, M–R, Y, A′) or green transgenic embryos (G–L, S–X, Z, B′). (A–F, M–R, A′) In non-green embryos, expression of the non-neural markers tbx1 (n = 79; A,M) and otx1b (n = 52; B,N), the neuronal marker neurod1 (n = 18; C,O), and the sensory markers sox2 (n = 11), atoh1a (n = 40), tecta (n = 32) (D–F, P–R) and the neurogenic marker neurog1 (Y, n = 13) is normal. (G–L, S–X, B′) In Tg(hsp70:fgf3) embryos sorted as green, the expression of tbx1 is unaffected or slightly up-regulated (n = 97; G,S), while expression of otx1b (n = 56; H,T), neurog1 (n = 25; Z) and otic and non-otic neurod1 (n = 35; I,U) is strongly up-regulated. The expression of sox2 (n = 24), atoh1a (n = 83) and tecta (n = 100) is slightly reduced and the anterior part of the posterior domain marked by tecta is missing (J–L, V–X). (A′) Graphical representation of counts of GFP-positive sensory hair cells (pou4f3:GFP expression) in the demarcated area from embryos heterozygous for both Tg(hsp70:fgf3) and Tg(pou4f3:GFP). Error bars represent standard deviation. One-way ANOVA with Šídák's multiple comparison post-test: *p<0.05, ***p<0.001. (B′–E′) Live images of Tg(hsp70:fgf3) embryos HS at 18S and grown to 83 hpf. The inner ear morphology of non-green non-transgenic embryos appears normal (n = 10; B′). (C′–E′) Green Tg(hsp70:fgf3) embryos display only minor ventral patterning defects; the ventral pillar for the lateral semicircular canal is always present (n = 10; C′–E′), but is sometimes displaced posteriorly (E′). Scale bar: 50 µm. RA is required for the spatial patterning of the ventral otic vesicle
RA signalling has been shown to regulate otic tbx1 expression positively at early (late gastrula, 10 hpf) stages in zebrafish [2], a result that we were able to replicate with DEAB (RA inhibitor) treatment, albeit at higher concentrations (S6 Figure). To investigate the role of RA signalling during ventral OV development at later stages, we used a number of different approaches. Firstly, we used the Tg(hsp70:dnRAR) line to block RA signalling in a conditional manner, by heat-shocking embryos from heterozygous incrosses from 18S/20S (Fig. 5; S7 Figure). To our surprise, at these later stages of OV development, tbx1 expression was no longer dependent on RA signalling and persisted in the OV, even in embryos expressing high levels of GFP in the OV, indicative of strong repression of RA signalling. There even appeared to be some ectopic expression of tbx1 in the anteroventral domain (Fig. 5G,M). In all cases, otx1b expression was expanded anteriorly (Fig. 5H,N), while neurod1 expression was severely reduced (Fig. 5I,O). The expansion of the otx1b domain occurred despite the overall reduction in size of the otic vesicle as a result of the dnRAR over-expression. Treatment with the chemical RA-inhibitor DEAB revealed that in addition to neurod1, the expression of the two neuronal markers neurog1 (expressed in specified neuroblasts in the OV and some delaminated neuroblasts) and isl1 (initiated after cell cycle exit in delaminated neuroblasts) is reduced in embryos with blocked RA signalling (S8 Figure). The reduction observed in neurod1-positive cells in embryos in which RA signalling had been abolished from 18S was more pronounced than after FGF inhibition, suggesting that the neuroblast lineage requires RA as well as FGF for proper development. In addition, sensory development was perturbed in embryos with reduced or blocked RA signalling (Fig. 5D–F, J–L, P–R).
Fig. 5. Heatshock of Tg(hsp70:dnRAR) embryos leads to an expansion of otic otx1b and a loss of neurod1 expression. 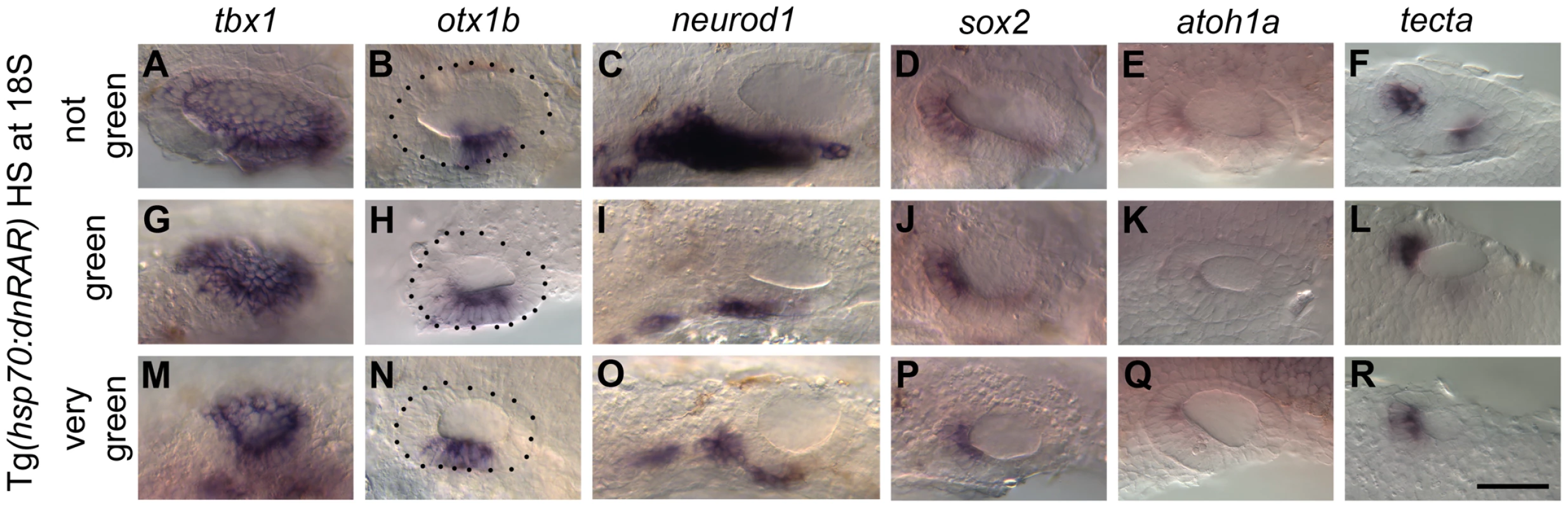
Tg(hsp70:dnRAR) embryos were heat shocked at 18S and sorted into non-green (non-transgenic siblings), green and very green embryos. (A–F) Non-transgenic sibling embryos show normal pattering of the OV assessed by the exp`ression of the non-neural markers tbx1 and otx1b (A,B), the neuronal marker neurod1 (C), and the sensory markers sox2, atoh1a and tecta (D–F) (at least 50 embryos per marker). (G–R) Embryos with low (G–L) or very low (M–R) levels of RA signalling show only a slight change in tbx1 expression (n = 46; G,M), but expression of otx1b is expanded anteroventrally (n = 109; H,N) and expression of neurod1 is reduced (n = 60; I,O). Expression of sox2 (n = 106), atoh1a (n = 150) and tecta (n = 98) is reduced posteriorly (J–L,P–R) and anteriorly (P–R). All panels are lateral views with anterior to the left, apart from the panels depicting tecta (dorsal views; anterior to the left, lateral up). Scale bar: 50 µm. The expression of aldh1a3 in the anterior otic vesicle and the lack of otic phenotype in aldh1a2 (nls) mutants suggested that aldh1a3 is the likely source of RA signalling to pattern the otic epithelium at OV stages. To test this, we injected a translation-blocking morpholino to knock down aldh1a3 function from early cleavage stages. The results corroborated the dnRAR data presented above, but were even more dramatic: in aldh1a3 morphants, the otx1b expression domain expanded to cover the entire otic vesicle floor, while tbx1 expression was slightly expanded into the anteroventral domain. Otic neurog1 and neurod1 expression was severely down-regulated (Fig. 6). Importantly, the morpholino did not result in any significant change in overall otic vesicle size (Fig. 6D–F). Taken together, these data indicate that aldh1a3 is the source for RA signalling in the OV, where it is required to restrict otx1b expression in anterior OV regions and to establish or maintain normal levels of otic neurog1 and neurod1 expression.
Fig. 6. aldh1a3 is required to pattern the zebrafish otic vesicle. 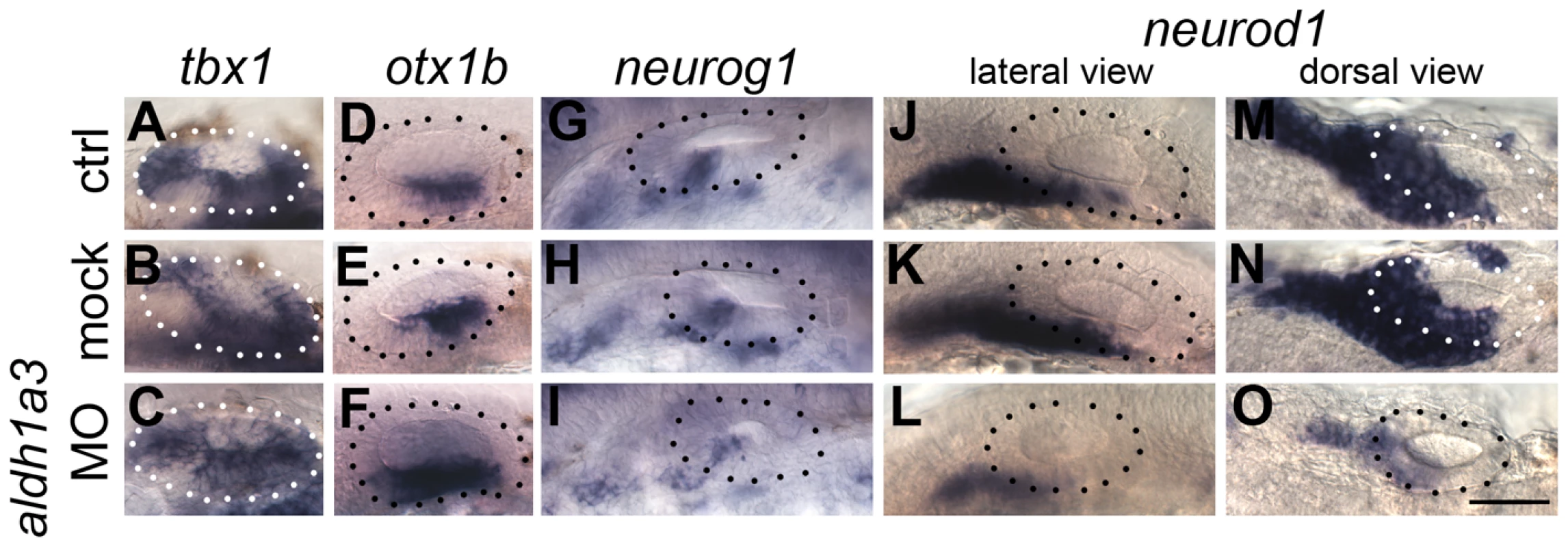
(A–C) Control uninjected (A, n = 36) and mock-injected (B, n = 14) embryos show normal expression of tbx1. Embryos injected with the aldh1a3 morpholino (C) show an expansion of expression of tbx1 in the anteroventral part of the OV (n = 38/46). (D–F) Control uninjected (D, n = 98) and mock-injected (E, n = 25) embryos show normal expression of otx1b. Embryos injected with the aldh1a3 morpholino (F) show a marked expansion of otx1b expression in the anteroventral part of the OV (n = 95/122). (G–I) Control uninjected (G, n = 11) and mock-injected (H, n = 9) embryos show normal expression of neurog1. Embryos injected with the aldh1a3 morpholino (I) show a marked reduction of neurog1 expression in the OV (n = 33). (J–O) Control uninjected (J,M, n = 42) and mock-injected (K,N, n = 18) embryos show normal expression of neurod1. Embryos injected with the aldh1a3 morpholino (L,O) show a marked reduction of neurod1 expression (n = 70/76). (A–L) Lateral views; (M–O) dorsal views; anterior to the left. Scale bar: 50 µm. To test whether RA functions directly in the neuroblast lineage or acts indirectly by suppressing otx1b expression in the neurogenic domain, we employed otx1b−/− and vgo (tbx1−/−) mutant embryos (Fig. 7). vgo mutant embryos lack expression of otx1b in the OV, and in both otx1b−/− and vgo (tbx1−/−) embryos the otic neurogenic domain is increased and neuroblasts emerge in a more posterior position in the OV floor (Fig. 7B,D) [2], [28]. We reasoned that inhibiting RA signalling in the absence of otx1b expression should reveal otx1b-independent regulatory roles of RA signalling on neuroblast development. We cultured WT, otx1b−/−, vgo (tbx1−/−) mutant and sibling embryos together in the RA inhibitor DEAB (400 µM) from 18S to 26 hpf and analysed expression of neurod1. While expression of neurod1 is normal in embryos treated with DMSO (Fig. 7A–E) it occurred in a smaller domain at reduced levels in WT, sibling and mutant embryos treated with DEAB (Fig. 7F–J), suggesting that RA is required directly in the neuroblast lineage, rather than acting through suppression of otx1b function.
Fig. 7. RA signalling is required for normal levels of otic neurod1 expression in the absence of tbx1 and otx1b activity. 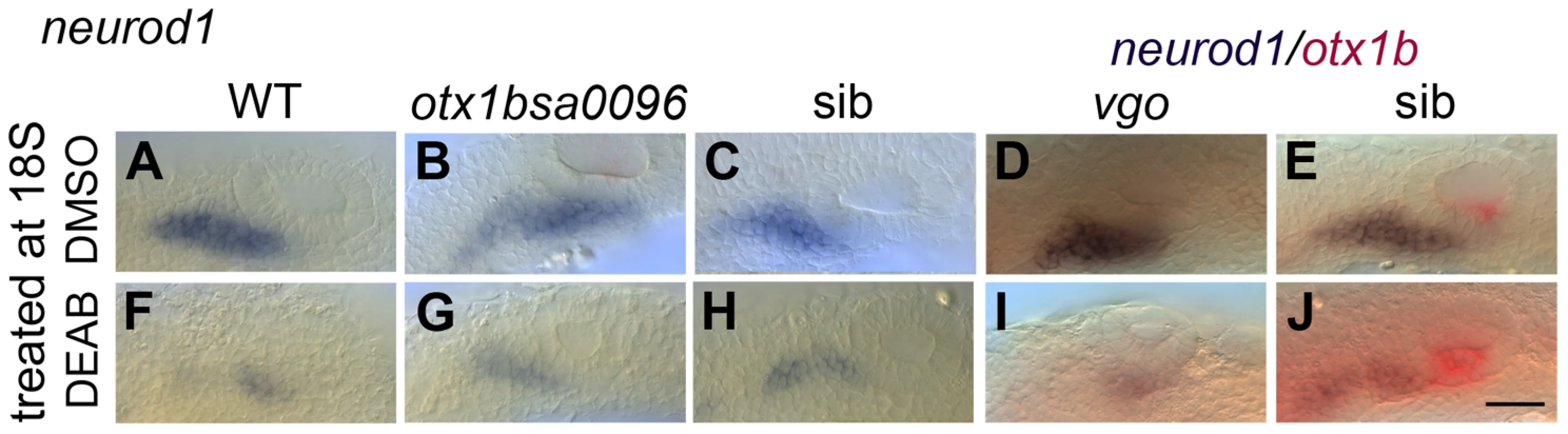
Embryos were treated with DMSO or DEAB from 18S to 26 hpf. (A) WT embryos treated with DMSO show normal expression of neurod1 (n = 10). (F) Treatment of WT embryos with 400 µM DEAB (n = 18) leads to a reduction in the size of the OV and a reduction neurod1 expression. (B) In otx1bsa96−/− embryos, expression of neurod1 is shifted posteriorly (n = 4/16 embryos from a heterozygous cross). (G) In otx1bsa96−/− embryos treated with DEAB, the OV is smaller and expression of neurod1 is reduced (n = 9/37). (C) In otx1bsa96 sibling (sib; wild-type or heterozygote) embryos, expression of neurod1 is normal (n = 12/16 embryos from a heterozygous cross), but levels are reduced after treatment with DEAB (n = 28/37; H). (D) In tbx1−/− (vgo) embryos, expression of otx1b is lost and expression of neurod1 is shifted posteriorly (n = 9/38). (I) tbx1−/− embryos treated with DEAB (n = 16/62) do not express otx1b (lack of red stain); expression of neurod1 is still shifted posteriorly, but is reduced. (E) vgo sibling embryos treated with DMSO show normal expression of neurod1 (purple) and otx1b (red) (n = 29/38 embryos from a heterozygous cross). (J) Treatment of sibling embryos with DEAB (n = 46/62) leads to a reduction in neurod1 expression. All panels are lateral views with anterior to the left. Dotted outline marks position of the OV; vertical lines mark posterior extent of the neurod1-expressing domain relative to the OV. Scale bar: 50 µm. Next we addressed the effects of exogenous RA signalling on OV development. We cultured embryos in 5, 10 and 20 nM RA at the 18S/20S stages, and assessed non-neural, neurogenic and sensory markers at 26 hpf (Fig. 8). Exogenous RA signalling affected the non-neural markers tbx1 and otx1b differently. While an up-regulation of tbx1 expression was observed, with expression extending anteriorly (Fig. 8I,Q,Y), otic otx1b expression was severely down-regulated or lost in embryos treated with 5,10 or 20 nM RA (Fig. 8J,R,Z). In addition, the size of the otic neurod1-expressing domain was expanded in embryos treated with the lower concentration of RA (Fig. 8K,L) but normal or only a little expanded in embryos treated with 10 or 20 nM RA (Fig. 8K,L,S,T,A′,B′). Analysis of the neuronal markers neurog1 and isl1 in concert with neurod1 reveals that while delaminating (neurod1-positive) and postmitotic (isl1-positive) neuroblast domains are increased in embryos treated with RA, the neurog1-positive domain in the OV is unchanged or slightly decreased in embryos treated with RA (S8 Figure). This suggests that RA promotes the transition from the neurog1-expressing to the neurod1-expressing state during neuroblast maturation. Expression of sox2 was expanded, and as with the low level SU5402 treatments, expression of tecta was blocked (Fig. 8). The effect on sensory markers was also seen if treatment continued for longer time points, up to 32 hpf. Supernumerary atoh1a-positive cells emerged in the maculae and in the OV floor (Fig. 8I,K′M′), and the spacing between the two sensory patches was reduced (tecta, Fig. 8J′,L′,N′). An increase in the number of GFP-positive cells at 55 hpf was also observed in Tg(pou4f3:gfp) embryos treated with 10 nM RA from 20S (Fig. 8Q′,R′,U′), whereas inhibition of RA signalling by DEAB arrested hair cell and otolith development (Fig. 8S′,T′,U′).
Fig. 8. Effects of RA treatment on otic vesicle patterning. 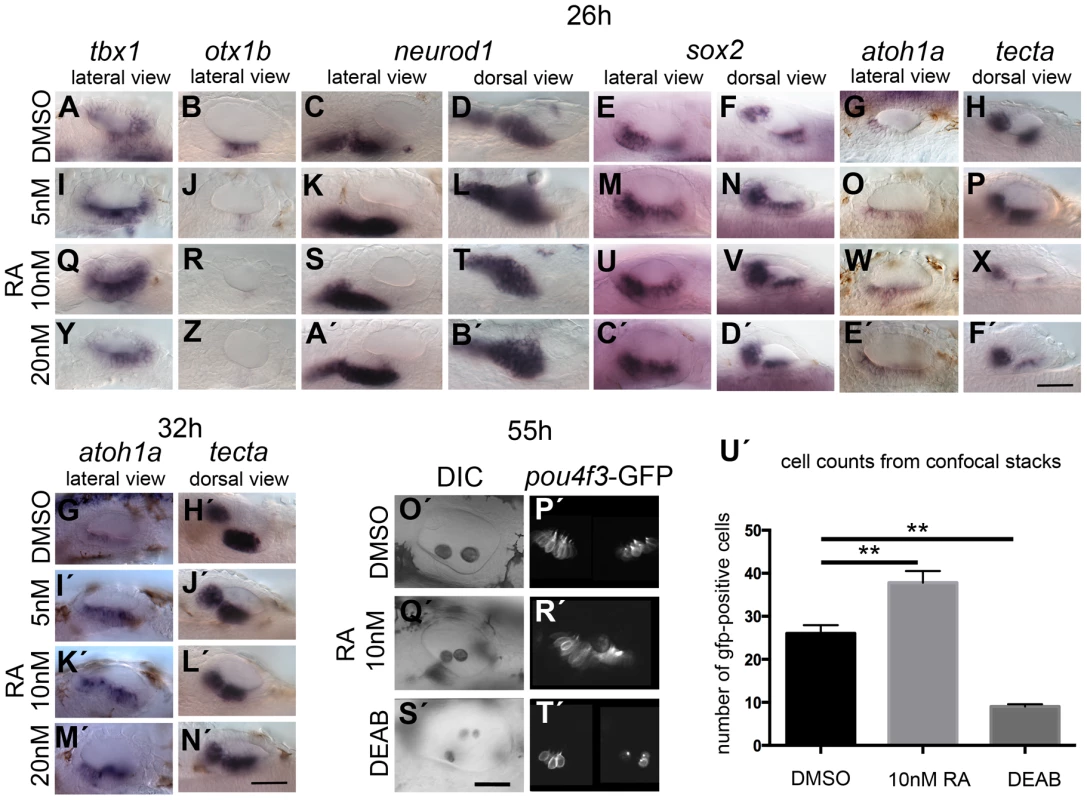
WT embryos were treated with DMSO or RA from 20S to 26 hpf (A–F′), 32 hpf (G′–N′) or 55 hpf (O′–T′). (A–H) WT embryos treated with DMSO show normal expression of non-neural markers tbx1 (n = 71; A) and otx1b (n = 52; B), the neuronal marker neurod1 (n = 50; C,D), and the sensory markers sox2 (n = 54), atoh1a (n = 63) and tecta (n = 40) (E–H). (I–X) Treatment with 5 nM RA (I–P) or 10 nM RA (Q–X) results in expanded tbx1 expression (n = 80/110; I,Q), down-regulation of otx1b expression (n = 104; J,R), and expansion of the neurod1 expression domain (n = 94/100). The expression of sox2 (n = 72/86) and atoh1a (n = 68) is extended in the ventral OV floor (M–O, U–W). Expression of tecta (n = 109) is reduced, but tecta-positive cells emerge in the inter-patch space (P,X). (Y–F′) Treatment with 20 nM RA results in a slight down-regulation, but anteroventral expansion, of tbx1 expression (n = 42/62; Y), down-regulation of otx1b expression (n = 64; Z), and an expansion of the neurod1 expression domain (n = 55; A′,B′). Expression of sox2 extends in the ventral OV floor (n = 51/62; C′,D′), while the expression of atoh1a (n = 38/40) is extended. Expression of tecta (n = 44/54) is down-regulated, but tecta-positive cells emerge in the inter-patch space (E′,F). (G′,H′) WT embryos treated with DMSO show normal expression of the sensory markers atoh1a (n = 34) and tecta (n = 49) when grown to 32 hpf. (I′–L′) In embryos treated with 5 nM (I′,J′), 10 nM (K′,L′) or 20 nM RA (M′–N′), expression of atoh1a extends in the ventral OV floor (n = 65, all concentrations). The expression of tecta (n = 96, all concentrations) indicates that the two sensory patches are closer together or fused (J′,L′,N′). (O′–U′) Tg(pou4f3:GFP) embryos treated with DMSO show normal hair cell development in two clearly separated sensory patches (n = 23; O′,P′). In Tg(pou4f3:GFP) embryos treated with 10 nM RA, GFP-positive cells differentiate between the anterior and posterior sensory patch in the ventral OV floor (n = 18; Q′,R′). In Tg(pou4f3:GFP) embryos treated with DEAB, GFP-positive cells are severely reduced in the OV (n = 13; S′,T′). (U′) Graphical representation of the number of GFP-positive cells per ear for each treatment. Error bars represent standard error of the mean. One-way ANOVA with Dunnett's multiple comparison post-test: RA vs. DMSO: **p = 0.0082; DEAB vs. DMSO: **p = 0.0019. All panels are lateral views with anterior to the left, apart from the panels depicting tecta (dorsal views; anterior to the left). Scale bar: 50 µm. Taken together, our results support a model where RA, most likely emanating from the aldh1a3-expressing cells in the anterior OV, is required and sufficient to repress otx1b expression in the emerging sensory and neurogenic domains of the anterior OV. In addition, development of otic neuroblasts seems to depend critically on RA signalling: inhibition of RA signalling leads to a decrease in neurog1 and neurod1 expression (most likely indicating a smaller neuronal domain with fewer emerging neuroblasts), suggesting that levels of RA have to be tightly regulated for proper neurogenesis to occur. Most intriguingly, elevated levels of RA signalling are sufficient to induce ectopic sensory hair cell development in the ventral OV, a phenotype that mimics the low level inhibition of FGF signalling, suggesting that RA and FGF signalling have opposing roles in otic sensory development.
FGF and RA signalling in the developing ear—A possible feedback loop
The similarities between the effect of FGF inhibition and RA over-exposure with regard to sensory markers prompted us to explore whether FGF and RA signalling influence each other in the OV. Since fgf3 and fgf8a are expressed in the otic epithelium before aldh1a3 expression can be observed, we asked whether FGF signalling is required for aldh1a3 expression and sufficient to induce it ectopically. Otic aldh1a3 expression was lost in ace (fgf8a−/−) and lia (fgf3−/−) mutant embryos, and in embryos treated with 10 µM SU5402 at 18–20S (Fig. 9A–L), suggesting that high levels of FGF signalling are required for otic aldh1a3 expression. In addition, elevating FGF3 levels by heat-shocking Tg(hsp70:fgf3) embryos for 60 min at 18S/20S was sufficient to up-regulate aldh1a3 expression exogenously in the dorsomedial part of the OV, indicating that FGF is upstream of aldh1a3 expression, but that only part of the OV is competent to respond (Fig. 9M–P). The sufficiency of FGF for ectopic otic aldh1a3 expression was even seen with heat shock at 20S, arguing strongly that the requirement for FGF for endogenous aldh1a3 expression is independent of early FGF functions in otic induction or anteroposterior patterning. Although this result predicts an epistatic relationship, indicating that RA inhibition would not exacerbate the otic phenotype caused by loss of FGF function, we found that simultaneous treatment with both FGF and RA inhibitors (10 µM SU5402, 300 µM DEAB) resulted in whole embryo developmental arrest and death by 24 hpf. We were therefore unable to examine the effects of combined inhibition of FGF and RA at OV stages on otic patterning.
Fig. 9. RA signalling and FGF signalling are linked in a regulatory feedback loop in the ear. 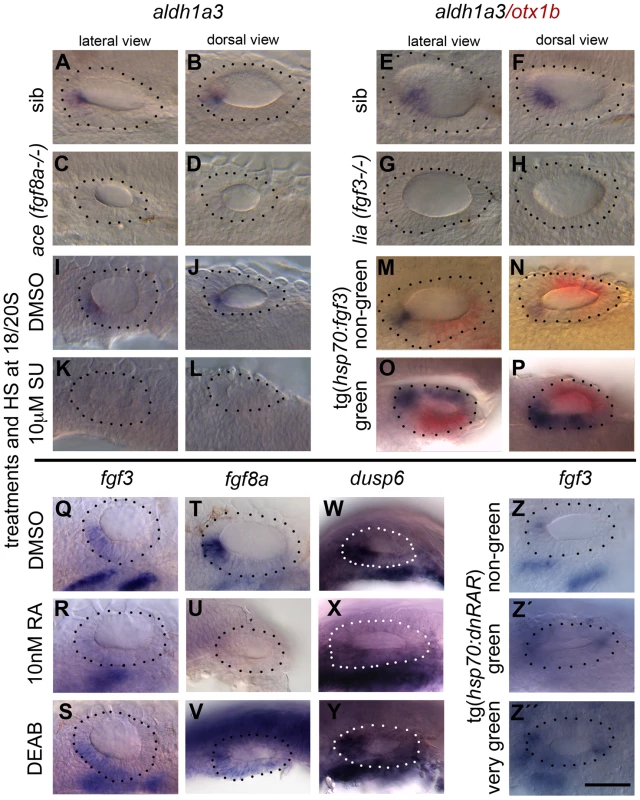
Embryos were treated with DMSO, SU5402 and DEAB from 18S to 26 hpf, with RA from 20S to 26 hpf, and heatshock was performed at the 18/20S stage. (A–H) aldh1a3 expression is normal in sibling embryos (n = 24/31 embryos from a heterozygous cross (ace sib), n = 31/39 (lia sib)) but lost in embryos mutant for fgf8a (ace, n = 7/31; C,D) or fgf3 (lia, n = 8/39; G,H). (I–L) aldh1a3 expression is normal in WT embryos treated with DMSO (n = 15; I,J), but lost in embryos treated with 10 µM SU5402 (n = 35; K,L). (M–P) Tg(hsp70:fgf3) embryos were heat shocked (HS) from 18S and sorted as non-green non-transgenic siblings (M,N), or green transgenic embryos (O,P), and double stained for aldh1a3 (blue) and otx1b (red). In non-green non-transgenic embryos, expression of aldh1a3 is normal (n = 31). In green Tg(hsp70:fgf3) embryos, expression of aldh1a3 and otx1b is up-regulated (n = 48). (Q–S) fgf3 expression is normal in WT embryos treated with DMSO (n = 15; Q) but down-regulated in the otic epithelium in embryos treated with 10 nM RA (n = 28; R); the domain is expanded in embryos treated with DEAB (n = 23; S). (T–V) fgf8a expression is normal in WT embryos treated with DMSO (n = 16; T) but down-regulated in the otic epithelium in embryos treated with 10 nM RA (n = 22; U) and expanded posteroventrally in embryos treated with DEAB (n = 19; V). (W–Y) dusp6 expression is normal in WT embryos treated with DMSO (n = 9; W) but down-regulated in the otic epithelium in embryos treated with 10 nM RA (n = 26; X) and expanded posteroventrally in embryos treated with DEAB (n = 17; Y). (Z–Z″) Tg(hsp70:dnRAR) embryos were heat shocked (HS) from 18S and sorted as non-green non-transgenic siblings (Z) or green transgenic embryos (Z′,Z″). In non-green, non-transgenic embryos, the expression of fgf3 is normal (n = 18; Z). In green and very green Tg(hsp70:dnRAR) embryos the expression fgf3 is up-regulated (n = 28; Z′,Z″). Dotted line demarcates the OV. Scale bar: 50 µm. To test whether FGF expression is dependent on RA signalling, we manipulated RA signalling and analysed expression of fgf3 and fgf8a, together with the FGF target gene dusp6, at 26 hpf. Exposure of embryos to 10 nM RA decreased otic fgf3, fgf8a and dusp6 expression (Fig. 9R,U,X), whereas treatment with the RA antagonist DEAB resulted in a modest expansion of the otic fgf3, fgf8a and dusp6 expression domains in the medial and posterior OV floor (Fig. 9S,V,Y). For both treatments, only a single stripe of fgf3 expression in the pharyngeal pouch mesenchyme remained (Fig. 9S–T). Heat-shock of Tg(hsp70:dnrar) embryos at 18S resulted in the up-regulation of fgf3 expression in a subset of otic cells, which we assume reflects the mosaic expression of the transgene (Fig. 9W–Y). Taken together, our results suggest that while FGF activity regulates RA production positively (via aldh1a3 expression), RA itself acts to down-regulate FGF activity. This raises the interesting possibility that FGF and RA signalling form a feedback loop in the anterior OV that is required for the correct patterning of the ventral OV.
RA signalling has FGF-dependent and independent functions during otic vesicle development
Since exogenous RA can inhibit expression of fgf3 in the OV and influences sensory development in a manner similar to low level FGF inhibition with SU5402 or lia (fgf3−/−) mutant phenotypes, we wanted to test if the results obtained through RA treatment are caused simply by inhibiting fgf3 expression and thus lowering FGF levels. If that were the case, raising levels of FGF3 in RA-treated embryos should rescue the observed phenotype. Tg(hsp70:fgf3) embryos were treated with 10 nM (Table 1) or 20 nM RA (Fig. 10; Table 1) from 18S and immediately heat-shocked once, twice, three or four times for 1 hour to generate embryos with different levels of FGF3 rescue, and stained for sox2, otx1b, neurog1 and neurod1. While in 95–100% of RA-treated control (heat shocked, non-green) embryos, the anterior and posterior sensory patch marked by sox2 was closer together or fused, fewer (1×HS, 2×HS) or no (3×HS, 4×HS) embryos displayed this phenotype in the transgenic heat-shocked siblings that over-express fgf3 (Fig. 10A–L; Table 1). Instead, the two sox2 patches were now more widely spaced, and the posterior patch was reduced in size. This resembles the phenotype generated by 1×HS of Tg(hsp70:fgf3) embryos without RA treatment, which we designated ‘Fgf3-like’ (Table 1; Fig. 4). These data suggest that RA acts on sox2 expression through its ability to influence fgf3 expression. By contrast, in RA-treated, heat-shocked green embryos, compared with RA-treated non-green embryos, elevated levels of FGF signalling were not sufficient to override the suppressive effect RA signalling has on otx1b expression (Fig. 10M–R; compare with Fig. 4). These results indicate that RA can down-regulate otx1b expression directly, independent of its inhibitory effect on FGF signalling.
Fig. 10. Elevated levels of FGF3 can counteract the effect of elevated RA signalling on sensory development. 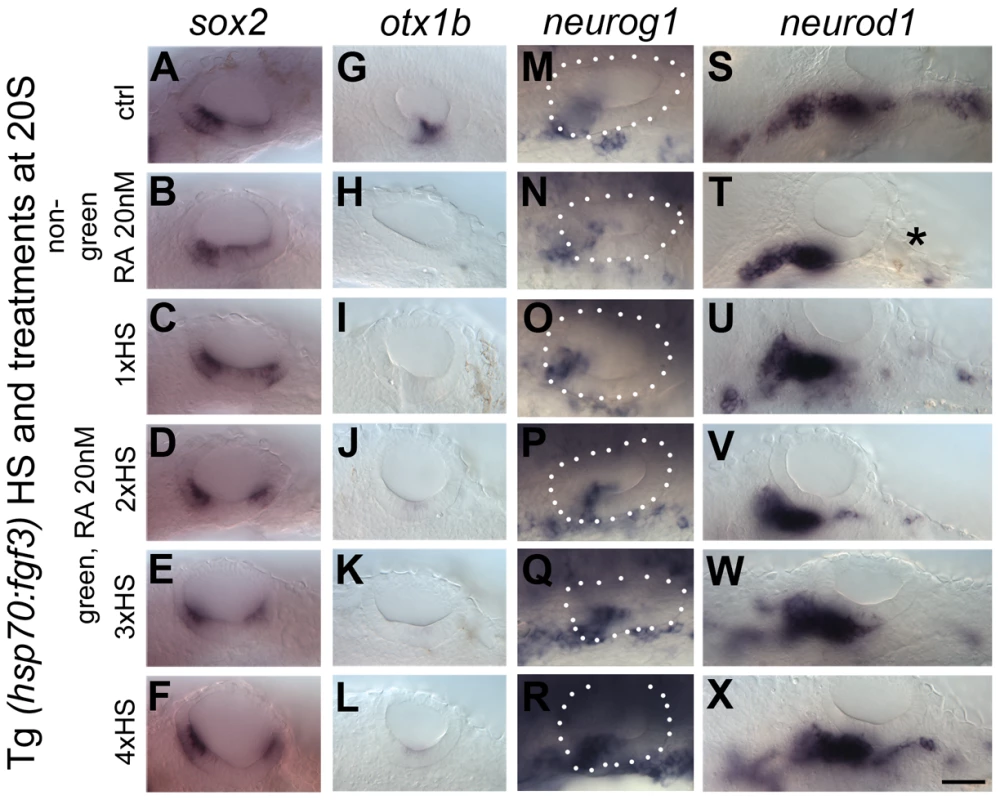
Tg(hsp70:fgf3) embryos were treated with DMSO (A,G,M,S) or 20 nM RA from 20S and the first heat shock applied at the same time. Embryos were heat shocked never (A,G,M,S), once (1×HS; C,I,O,U), twice (2×HS; D,J,P,V), three times (3×HS; E,K,Q,W) or four times (4×HS; F,L,R,X) and sorted as non-green non-transgenic siblings (B,H,N,T) or green transgenic embryos (C–X). Non-green non-transgenic siblings were pooled; different HS treatments did not affect the phenotype (B,H,N,T). (A,G,M,S) Tg(hsp70:fgf3) embryos treated with DMSO show normal expression of the sensory marker sox2 (A) and the non-neurogenic marker otx1b (G) and the otic markers neurog1 (M) and neurod1 (S). (B) In non-green sibling embryos treated with 20 nM RA, the expression of sox2 extends across the ventral OV floor. (C–F) In Tg(hsp70:fgf3) embryos treated with 20 nM RA and given 1×HS (C) or 2×HS (D), the expression of sox2 is present in two extended domains at the anterior and posterior of the OV. (E) In Tg(hsp70:fgf3) embryos treated with 20 nM RA and 3×HS the expression of sox2 is normal or slightly reduced. (F) In Tg(hsp70:fgf3) embryos treated with 20 nM RA and 4×HS the expression of sox2 is slightly reduced or normal; the posterior domain of expression is shifted laterally. (H) In non-green sibling embryos treated with 20 nM RA, the expression of otx1b is reduced. (I–L) In Tg(hsp70:fgf3) embryos treated with 20 nM RA and given 1×HS (I), 2×HS (J), 3×HS (K) or 4×HS (L), the expression of otx1b is greatly reduced. (N) In non-green sibling embryos treated with 20 nM RA, the expression of neurog1 is reduced in the OV. (O,P) In Tg(hsp70:fgf3) embryos treated with 20 nM RA and given 1×HS (O) or 2×HS (P), the expression of neurog1 is reduced in the OV. (Q) In Tg(hsp70:fgf3) embryos treated with 20 nM RA and 3×HS the expression of neurog1 is normal but slightly shifted towards posterior. (R) In Tg(hsp70:fgf3) embryos treated with 20 nM RA and 4×HS the expression of neurog1 is increased but shifted to a more posterior position in the floor of the OV. (S,T) In non-green sibling embryos treated with 20 nM RA, the expression of otic neurod1 is slightly increased, while expression is lost in prospective posterior lateral line and vagal ganglion cells posterior to the OV (asterisk). (U–W) In Tg(hsp70:fgf3) embryos treated with 20 nM RA and given 1×HS (U), 2×HS (V) or 3×HS (W) the expression of neurod1 is increased in the OV. In embryos heatshocked three times neuroblasts emerge in a more posterior position in the OV floor (W). (X) In Tg(hsp70:fgf3) embryos treated with 20 nM RA and 4×HS the expression of neurod1 is increased and otic neuroblasts emerge from a more posterior position in the floor of the OV. In addition, neurod1-positive cell populations re-emerge below the OV. All panels are lateral views with anterior to the left. For numbers see Table 1. Scale bar: 50 µm. Tab. 1. Distribution of sox2 phenotypes after RA and FGF manipulation. 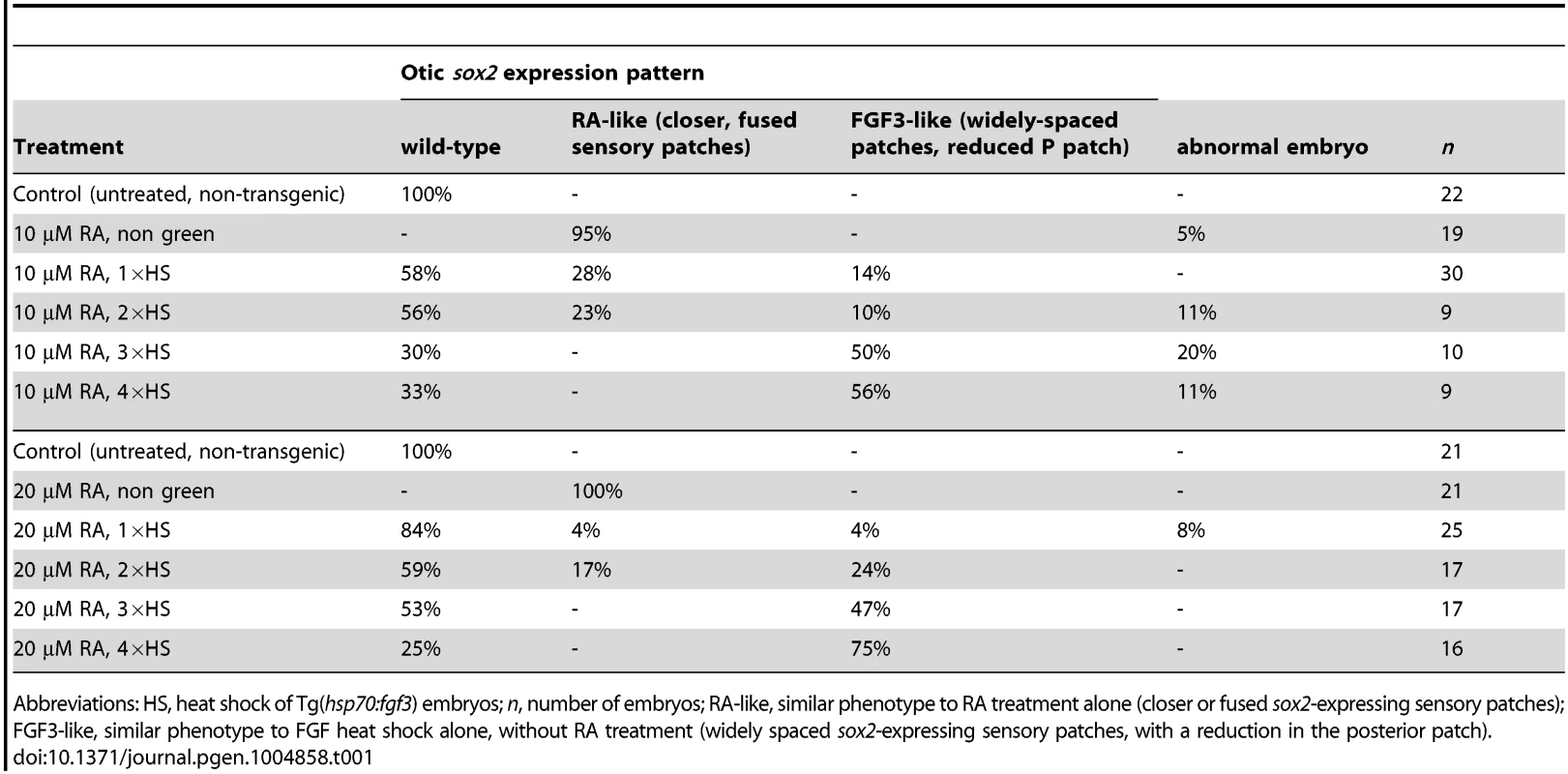
Abbreviations: HS, heat shock of Tg(hsp70:fgf3) embryos; n, number of embryos; RA-like, similar phenotype to RA treatment alone (closer or fused sox2-expressing sensory patches); FGF3-like, similar phenotype to FGF heat shock alone, without RA treatment (widely spaced sox2-expressing sensory patches, with a reduction in the posterior patch). Both FGF and RA signalling have a positive effect on emerging neurod1-positive otic neuroblasts [12], [14] (Figs 4, 8, 10) but while exogenous RA has little effect on otic neurog1 expression (Fig. 10M,N; S8 Figure), neurog1 is up-regulated in embryos with elevated FGF3 levels (Fig. 4Y,Z). Expression of neurog1 is up-regulated in embryos treated with 20 nM RA and higher levels of fgf3 over-expression (3×HS, 4×HS) (Fig. 10Q,R). Expression of otic neurod1 is increased in all combinations of FGF and RA compared with embryos treated with RA alone (Fig. 10S–X). Note that RA signalling in this context leads to a loss of neurod1 expression in a domain posterior to the ear, likely to include posterior lateral line and vagal ganglia (Fig. 10), which is expanded in embryos with elevated fgf3 levels alone (Fig. 4). The increase in neurod1-positive otic neuroblasts in embryos with both RA treatment and elevated fgf3 expression probably reflects an enhancement of the neurog1-positive pool by FGF3 and a drive to neuroblast maturation (expression of neurod1) by RA signalling.
Discussion
We have analysed signalling requirements in patterning the ventral floor of the zebrafish otic vesicle (OV), which gives rise to sensory, neuronal and non-neural fates. We provide evidence that FGF signalling is required for normal OV development at a time point after its function in otic placode induction and anterior patterning. In the OV, FGF3 is required to restrict sensory fates and is also both required and sufficient to promote expression of the non-neural marker otx1b in the ventral floor of the OV. RA signalling has the opposite role, promoting sensory fates and restricting otx1b expression and the development of non-neural territories. Both RA and FGF, however, are required for the development of the neurogenic domain and formation of the correct number of neuroblasts. We have identified separate roles for FGF3 and FGF8A in ventral OV patterning. Moreover, our results indicate that FGF and RA signalling form a feedback loop in the OV.
Specification of the non-neural (otx1b-expressing) region
We have identified FGF signalling as a key regulator of otx1b expression and thus non-neural otic ventral floor fates, including the ventral pillar of the lateral semicircular canal duct. otx1b is regulated by Tbx1: expression of otx1b is lost in the tbx1 (vgo) mutant [7] and both tbx1 mutant (vgo) and otx1b mutant or morphant embryos display an expansion of the neurogenic domain and a loss of non-neural ventral otic floor identities [2], [6], [28] (and this work). Embryos treated with the FGF inhibitor SU5402 from 18/20S onwards until 26 hpf lose the expression of tbx1 and otx1b, indicating the loss of non-neural ventral fates. In comparison, treatment with 10 µM SU5402 from earlier stages (10S for 3 h (to 16S) or 5 h (to 20S)) does not affect otic otx1b expression; likewise, the ventral pillar is still present under these conditions [12]. This indicates that FGF signalling after the 18S stage is required for correct development of the non-neural domain of the ventral OV floor.
During otic placodal stages (10.5 hpf in the zebrafish, 9 hours before the treatments in our study at 20S) mesodermal RA emanating from aldh1a2/Raldh2-expressing cells is required to specify tbx1/Tbx1-positive cells in zebrafish, chick and mice [2], [29], promoting posteroventral otic identities. Since protein synthesis is not required for this process in chick, this seems to be a direct regulatory effect exerted by RA on Tbx1 [30]. Interestingly, an expansion of Tbx1 expression in chick otocysts was only observed when a RA bead was implanted at E1.5 (∼HH10, 10S) but not at E2 (HH13, 19S) [29], suggesting a narrow temporal window in which RA can influence Tbx1 expression directly. This change in RA-responsiveness precedes the onset of Raldh3 expression in the chick otocyst itself at HH18 by 24 h [31], suggesting later non-described functions of RA signalling during inner ear development.
Our results indicate that RA signalling emanating from the aldh1a3 expression domain in the anterior OV acts at later stages of inner ear development in the zebrafish. Exogenous RA application at these later stages inhibits the expression of otx1b, but not tbx1, suggesting a Tbx1-independent restriction of otx1b by RA. Moreover, abrogation of RA signalling at these later stages (by chemical inhibition, over-expression of dnRAR or morpholino-mediated aldh1a3 knockdown) only slightly alters tbx1 expression in the zebrafish ear, while otx1b expression is expanded anteriorly. aldh1a3 expression in the OV starts at 22S, slightly after the onset of otx1b expression; both aldh1a3 and otx1b depend on FGF signalling for their expression. This suggests that RA signalling through aldh1a3 at the OV stage is required in the anterior OV to restrict the anterior expansion of otx1b expression induced by FGF, thus regulating the position of the neural/non-neural boundary in the ventral OV floor. In medaka, however, aldh1a3 has been lost from the genome, and aldh1a2 takes over the ventral expression and subfunction of aldh1a3 in the developing eye [32]. It will be interesting to see whether there is a similar replacement by aldh1a2 in medaka of the specific role for aldh1a3 in ear patterning that we have uncovered here for the zebrafish.
Specification and spacing of the sensory maculae
An early sign of sensory differentiation in the zebrafish inner ear is the expression of atoh1b from otic placodal stages, marking the differentiation of pairs of tether cells at the anterior and posterior poles of the OV [26]. Perturbing FGF or RA signalling at the OV stages results in abnormal sensory patch development and spacing, suggesting that even though differentiation has been initiated, not all cells in and outside the sensory region are fully committed. If FGF signalling is abolished completely, only a few hair cells develop and sensory markers are down-regulated or lost, indicating that FGF signalling is required for sensory development, in line with results from the Riley group [26].
Interestingly, in our study, treatment with low and intermediate levels of SU5402 resulted in the up-regulation of sox2 expression and the development of supernumerary hair cells at later stages after relief from FGF inhibition, mimicking the result observed when embryos are treated with RA. Thus transient low level FGF inhibition or exogenous activation of the RA signalling pathway both lead to an expansion of the sensory domain. In the zebrafish, expansion of the sensory region occurs in a spatially restricted manner, along the ventromedial OV. This is likely to correspond to the region of sensory competence thought to be marked by dlx3b/4b expression in the placodal ectoderm [33], which could explain the spatial preference in sensory expansion that we observe with either partial FGF inhibition or increased RA signalling.
Analysis of sensory markers in embryos mutant for either fgf8a (ace) or fgf3 (lia) reveals a differential requirement for these FGFs in zebrafish OV development. While sensory development is reduced in fgf8a (ace) mutant embryos, the two sensory patches appear fused in fgf3 (lia) mutants and the posterior patch is enlarged, suggesting a re-specification of the inter-sensory patch epithelium. In embryos with elevated levels of FGF3 from the 18/20S stage, the anterior part of the posterior patch is missing, strengthening the notion that FGF3 is sufficient to suppress sensory fate specification in ventromedial otic regions. FGF3 and FGF8A belong to two different FGF subfamilies and preferentially signal through specific FGFR isoforms [34]. In zebrafish, our data indicate that fgfr4 is mainly expressed in the sensory region of the OV at 26 hpf; FGF8A but not FGF3 signals preferentially through FGFR4 [23], further supporting the non-redundant functions of different FGF ligands in OV development. FGF3 signalling has been implicated in non-sensory development in mouse. In mice mutant for the FGF3 - and FGF10 - specific FGFR2(IIIb) isoform [23], which is expressed in the non-sensory epithelia of the otocyst, inner ear development initially occurs normally, but morphogenesis is disturbed [35].
In mice, several studies have implicated FGF signalling in cell fate decisions in the cochlea [36], [37], [38], [39], [40], [41]. In the auditory sensory epithelium, FGF8 is expressed in the earliest differentiating cells, the inner hair cells, where it controls differentiation of the adjacent pillar cells [37], [38], [39]. In zebrafish, a model has been proposed in which FGF8A can confer sensory competence to cells in the OV when over-expressed ectopically together with atoh1a [27]. Our results suggest that the role FGF signalling plays in the context of otic sensory development is more diverse. High levels of FGF signalling suppressed the acquisition of sensory fates, while lowering endogenous levels of FGF signalling from 18/20S onwards was sufficient to induce sensory fates, without additional over-expression of atoh1a.
RA signalling can inhibit otic fgf3 and fgf8a expression, with application of exogenous RA resulting in a similar expansion of sensory fates in the zebrafish ear as that seen with lowered FGF signalling. Our results show that elevated levels of FGF can rescue the sensory expansion induced by RA, indicating that RA acts through lowering FGF levels in this case. Nevertheless, inhibition of RA signalling reveals that RA is required for sensory patch development and hair cell differentiation. The posterior patch responds more strongly to lowered levels of endogenous RA, and we speculate that this might be because endogenous RA levels at around 1 dpf are lower at the posterior pole of the OV, and thus inhibition here might be more complete. Again, loss of RA signalling shows similarities to a gain in FGF signalling. We have shown that down-regulation of RA signalling can lead to an expansion of fgf3 and fgf8a expression in the OV. This suggests that RA might be either directly required in the sensory lineage or might act indirectly through its FGF regulatory function. Taken together, our data suggest that endogenous levels of RA and FGF signalling have to be tightly regulated for correct sensory development to occur, and only slight perturbations can have significant effects for overall ear morphology and function.
Specification and restriction of the neurogenic region
Inhibition of RA signalling at OV stages decreases the otic neurog1 - and neurod1-positive domain dramatically. The decrease is even more pronounced with RA signalling inhibition compared with FGF signalling inhibition in our hands. The role of FGF in otic neurogenesis is well established [8], [13], [14], but our results indicate that RA signalling is also required in the otic neurogenic lineage at later stages of OV development. Because otx1b expression is expanded in embryos in which RA signalling is inhibited, the action of RA on neurogenesis might be direct or indirect. Embryos mutant for otx1b have an expanded neurod1-positive domain, suggesting that Otx1b has a role in limiting neurogenesis. Support for a direct role of RA signalling in otic neuroblast development comes from our analysis of neurod1 expression in tbx1−/− (vgo) mutant embryos. Here, otic otx1b expression is absent, but RA inhibition still leads to a decrease in neurod1 expression, suggesting that RA might act directly in the neurogenic domain. Embryos with elevated levels of RA signalling have a slightly decreased neurog1-positive domain in the OV, while both the neurod1 - and the isl1-positive domains are increased. This suggests that RA acts in the neurogenic lineage by driving neurod1 expression prematurely, depleting the neurog1-positive pool of specified progenitor cells. In all our treatments, we cannot distinguish unequivocally between an effect on size of the neurogenic domain within the epithelium, and an effect on neuroblast proliferation after cells have left the otic epithelium. Impaired neuronal development has also been reported in mice embryos double mutant for RARα/RARγ, in which the VIIIth ganglion is hypoplastic [42].
Feedback and integration of the FGF and RA signalling pathways in the zebrafish ear
Both FGF and RA signalling are implicated in the earliest phases of otic development, including otic induction, which in zebrafish occurs at around 11–14 hpf. We have identified a time window for their requirement beginning five hours later, at the 20S stage, coinciding approximately with the onset of otx1b expression in the non-neural regions of the ventral OV. Soon afterwards, at around 22S, aldh1a3 starts to be expressed in the anterior ventral OV. Both otx1b and aldh1a3 depend on ongoing high levels of FGF signalling, but RA signalling is sufficient to restrict both FGF signalling from reaching too far posteriorly and otx1b expression from spreading too far anteriorly, providing a beneficial signalling niche for the emerging otic neuroblasts.
Taken together, our results support the following model of OV patterning and refinement (Fig. 11): fgf3 expression is required for the normal otic expression of otx1b from around 20S. High FGF signalling induces aldh1a3 expression in an anterior, ventromedial position in the OV from around 22S. Elevated levels of FGF3 are sufficient to up-regulate aldh1a3 expression in the dorsal and intermediate OV, suggesting that these regions are competent to respond to FGF. As development proceeds, the cells expressing aldh1a3 in the OV itself provide a local source of RA. RA signalling could then act in a negative feedback loop to restrict fgf3- and fgf8a-expressing cells anteriorly. In mice, the negative regulatory effect exogenous RA exerts on Fgf3 is well established [9], [43], [44], and in mouse embryos mutant for the RA-degrading enzyme CYP26, elevated RA levels repress Fgf8 expression [45]. In addition, RA emanating from the somites has been shown to inhibit FGF signalling in emerging spinal cord [46] and forebrain [47]. While FGF8 emerging from the pre-neural tube inhibits Raldh2 expression in the adjacent presomitic mesoderm, RA in turn down-regulates Fgf8 expression [46].
Fig. 11. A model of ventral otic patterning in the zebrafish. 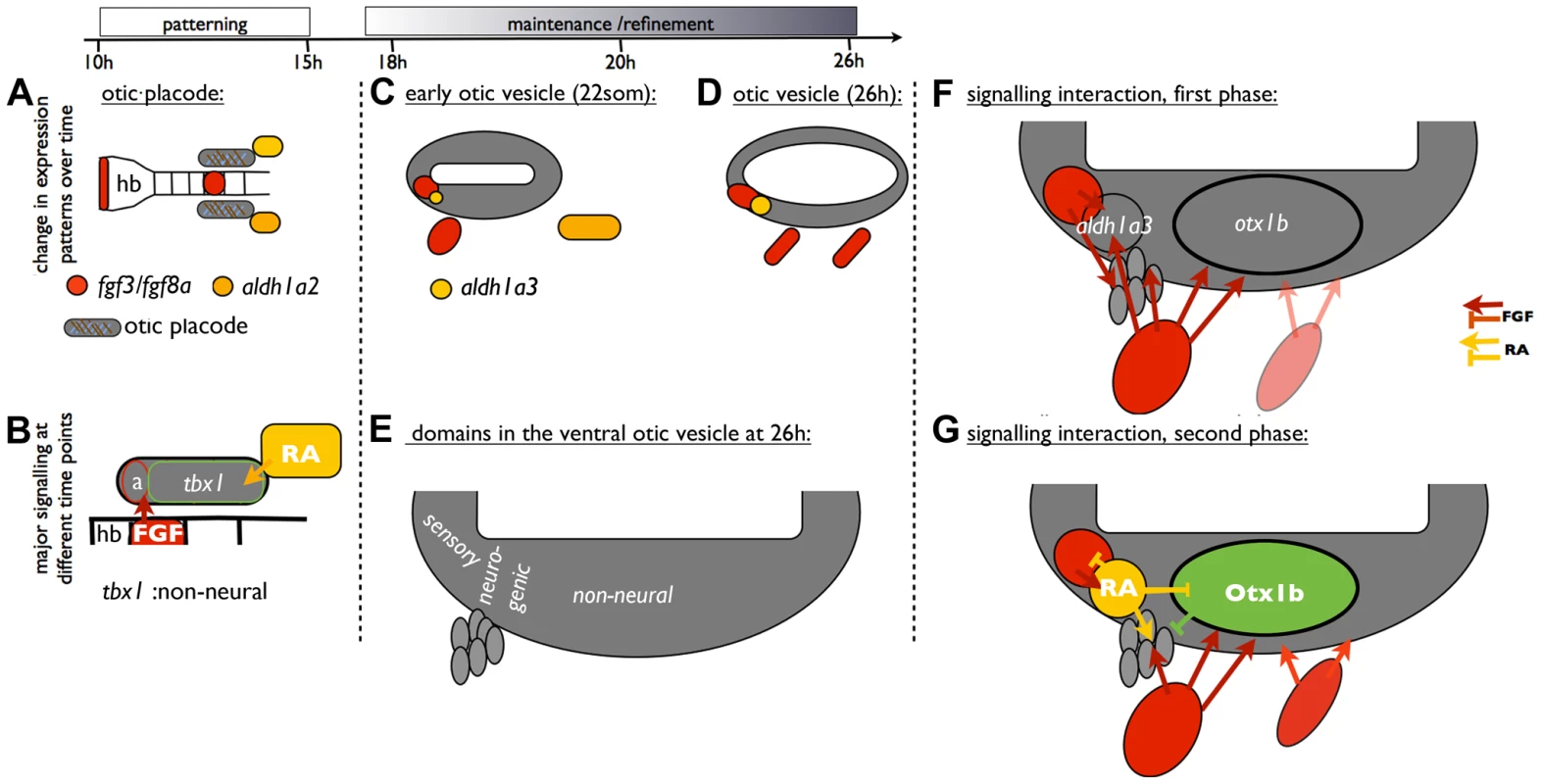
(A,B) At otic placode stages, FGF3 from the hindbrain patterns anterior otic regions, while RA signalling is required for the expression of tbx1 and subsequent non-neural development (based on [2], [12]. (C–E) At OV stages, FGF and RA signalling pathway members contribute to maintenance of otic patterning. It is likely that both otic and pharyngeal fgf3 expression sources influence otic patterning, although our experiments have not distinguished these. In addition, expression of otx1b in the OV starts from around 18S. (F) FGF signalling is required for the expression of aldh1a3 and otx1b in the OV. (G) Cells expressing aldh1a3 in the OV itself provide a local source of RA. RA signalling acts in a negative feedback loop to restrict fgf-expressing cells anteriorly. In addition, RA signalling prevents otx1b expression from spreading into anteroventral neurogenic regions of the OV and promotes the maturation of otic neuroblasts. Abbreviations: a, anterior otic placode; hb, hindbrain. Both FGF and RA signalling pathways play a positive role in neuroblast formation in the OV. Inhibition of either FGF or RA signalling decreases neurod1 expression. One idea is that, independent from its function to repress the anterior spread of otx1b expression, RA is also required for neuroblast maturation in the statoacoustic ganglion. It has been suggested that opposing actions of FGF and RA signalling control the timing of the epithelial-mesenchymal transition in trunk neural crest cells and consequently emigration of NCC from the neural tube [48]. It will be interesting to see whether FGF and RA signalling have a similar function during neuroblast emigration from the OV.
Materials and Methods
Ethics statement
All animal experiments were performed under licence from the UK Home Office and passed University of Sheffield local ethical review.
Animals
Zebrafish lines used were AB, London wild-type (LWT), aceti282a (fgf8a−/−) [24], [49], liat21142 (fgf3−/−) [25], nacw2/w2 (mitfa−/−) [50], nls (aldh1a2−/−) [18], otx1bsa96−/− (Sanger Institute Zebrafish Mutation Resource), vgotm208 (tbx1−/−) [7], [51], Tg(hsp70l:dnraraa-EGFP) (pd18Tg), hereafter referred to as Tg(hsp70:dnRAR) [52], Tg(hsp70:fgf3) [53] and Tg(pou4f3:gfp) [54].
Chemical treatment, heat shock and embryo culture conditions
Embryos were raised in E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, 0.0001% methylene blue). Embryonic stages are given as hours post fertilisation (hpf) at 28.5°C or as somite stages (S) [55], [56]. Embryos were dechorionated prior to chemical treatment. Embryos were treated with 5, 10,15 or 20 µM SU5402 (Calbiochem), 5, 10 or 20 nM retinoic acid (RA, Sigma) or DEAB (Alfa Aesar, 2 year old batch) in DMSO or with DMSO alone. To titrate our batch of DEAB we repeated a published experiment with 20 µM, 50 µM, 100 µM, 200 µM or 400 µM DEAB: we found 400 µM to block RA signalling efficiently and used this concentration (S6 Figure). The age of our DEAB batch may explain why our treatments required higher concentrations than those previously published. DMSO volume was always matched to that used for the highest experimental treatment.
Tg(hsp70:dnRAR) transgenic and sibling embryos from a Tg(hsp70:dnRAR/+)×Tg(hsp70:dnRAR/+) or a Tg(hsp70:dnRAR/+)×nac/nac cross were incubated at 39°C for 45 min. Transgenic and sibling embryos were distinguished by the expression of GFP. Because the Tg(hsp70:dnRAR) line drives transgenic expression in a mosaic manner, transgenic embryos were sorted as ‘weak green’, ‘green’ and ‘very green’ depending on GFP expression. Only ‘green’ and ‘very green’ embryos were used for further analysis (see S7 Figure). Tg(hsp70:fgf3) transgenic and sibling embryos from a Tg(hsp70:fgf3/+)×Tg(hsp70:fgf3/+) cross were incubated at 38°C for 1 hour (1×HS). To increase the strength of the heat shock, embryos were allowed to cool down for 30 min before repeating the procedure two, three or four times (2×HS, 3×HS and 4×HS). Transgenic Tg(hsp70:fgf3) embryos were identified by their green hearts at 24 hpf.
Morpholino injection
To knock down aldh1a3, we used a previously published translation-blocking morpholino: 5′-TATAGTCCCGTTCTGTGCCATAGCA-3′ [57]. The morpholino was dissolved in water (with fluorescent dextran added for visibility) and injected at 1 mM into one - or two-cell stage embryos (1–1.5 nl per injection). Controls were uninjected stage-matched sibling embryos and sibling embryos injected with a matched volume of water and fluorescent dextran alone (‘mock-injected’) to exclude injection artifacts.
In situ hybridisation
Single or double whole-mount in situ hybridisation was carried out as described [58], [59]. Probes used were aldh1a2 [18], aldh1a3 [19], atoh1a [60], cyp26c1 [20], dusp6 [61], etv4 [62], fgf3 [63], fgf8a [24], fgfr1a, fgfr3, fgfr4 [64], fgfr2 [65], isl1 [66], neurod1 [67], neurog1 [68], otx1b [3], raraa, rarab, rarga, rargb [69], sox2 [70], tbx1 [51], and tecta (G. Stooke-Vaughan and TTW, unpublished data).
FITC-Phalloidin staining
Staining was carried out as described [71].
Live imaging and cell counting
Embryos were anaesthetised in 0.5 mM MS222 (3-aminobenzoic acid ethyl ester) and mounted in 3% methylcellulose. Tg(pou4f3:gfp) embryos or FITC-Phalloidin stained embryos were imaged with a 20× objective on an Olympus FV-1000 confocal microscope. Fiji was used to adjust brightness and contrast and to count cells. Cell count data were analysed using GraphPad Prism.
n numbers
For chemical treatments and morpholino experiments, we have indicated the number of embryos treated or injected (e.g. n = 10). If the phenotype was not consistent, the number of embryos showing the phenotype out of the total is given (e.g. n = 9/10). For analysis of batches of embryos from a cross between heterozygous carriers of a mutation, we have indicated the numbers of siblings (wild-type or heterozygous, e.g. n = 9/12) and the presumed homozygous mutants (e.g. n = 3/12) that show an expression pattern or phenotype. If all embryos showed the same phenotype, the number indicates the number of embryos used (e.g. n = 12).
Supporting Information
Zdroje
1. RaftS, NowotschinS, LiaoJ, MorrowBE (2004) Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development 131 : 1801–1812.
2. RadosevicM, Robert-MorenoA, CoolenM, Bally-CuifL, AlsinaB (2011) Her9 represses neurogenic fate downstream of Tbx1 and retinoic acid signaling in the inner ear. Development 138 : 397–408.
3. LiY, AllendeML, FinkelsteinR, WeinbergES (1994) Expression of two zebrafish orthodenticle-related genes in the embryonic brain. Mech Dev 48 : 229–244.
4. MercierP, SimeoneA, CotelliF, BoncinelliE (1995) Expression pattern of two otx genes suggests a role in specifying anterior body structures in zebrafish. Int J Dev Biol 39 : 559–573.
5. ThisseB, PflumioS, FürthauerM, LoppinB, HeyerV, et al. (2001) Expression of the zebrafish genome during embryogenesis. ZFIN Direct Data Submission
6. HammondKL, WhitfieldTT (2006) The developing lamprey ear closely resembles the zebrafish otic vesicle: otx1 expression can account for all major patterning differences. Development 133 : 1347–1357.
7. WhitfieldTT, GranatoM, van EedenFJM, SchachU, BrandM, et al. (1996) Mutations affecting development of the zebrafish inner ear and lateral line. Development 123 : 241–254.
8. LégerS, BrandM (2002) Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech Dev 119 : 91–108.
9. LiuD, ChuH, MavesL, YanY-L, MorcosPA, et al. (2003) Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development 130 : 2213–2224.
10. MaroonH, WalsheJ, MahmoodR, KieferP, DicksonC, et al. (2002) Fgf3 and Fgf8 are required together for formation of the otic placode and vesicle. Development 129 : 2099–2108.
11. PhillipsBT, BoldingK, RileyBB (2001) Zebrafish fgf3 and fgf8 encode redundant functions required for otic placode induction. Dev Biol 235 : 351–365.
12. HammondKL, WhitfieldTT (2011) Fgf and Hh signalling act on a symmetrical pre-pattern to specify anterior and posterior identity in the zebrafish otic placode and vesicle. Development 138 : 3977–3987.
13. AlsinaB, AbellóG, UlloaE, HenriqueD, PujadesC, et al. (2004) FGF signaling is required for determination of otic neuroblasts in the chick embryo. Dev Biol 267 : 119–134.
14. VemarajuS, KantarciH, PadanadMS, RileyBB (2012) A spatial and temporal gradient of Fgf differentially regulates distinct stages of neural development in the zebrafish inner ear. PLoS Genet 8: e1003068.
15. MaierE, SaxenaA, AlsinaB, BronnerM, WhitfieldT (2014) Sensational placodes: Neurogenesis in the otic and olfactory systems. Dev Biol 389 : 50–67.
16. HansS, WesterfieldM (2007) Changes in retinoic acid signaling alter otic patterning. Development 134 : 2449–2458.
17. GrandelH, LunK, RauchGJ, RhinnM, PiotrowskiT, et al. (2002) Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development 129 : 2851–2865.
18. BegemannG, SchillingTF, RauchG-J, GeislerR, InghamPW (2001) The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development 128 : 3081–3094.
19. PittlikS, DominguesS, MeyerA, BegemannG (2008) Expression of zebrafish aldh1a3 (raldh3) and absence of aldh1a1 in teleosts. Gene Exp Patt 8 : 141–147.
20. GuX, XuF, WangX, GaoX, ZhaoQ (2005) Molecular cloning and expression of a novel CYP26 gene (cyp26d1) during zebrafish early development. Gene Exp Patt 5 : 733–739.
21. MohammadiM, McMahonG, SunL, TangC, HirthP, et al. (1997) Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276 : 955–960.
22. RaibleF, BrandM (2001) Tight transcriptional control of the ETS domain factors Erm and Pea3 by Fgf signaling during early zebrafish development. Mech Dev 107 : 105–117.
23. OrnitzD, XuJ, ColvinJ, McEwenD, MacArthurC, et al. (1996) Receptor specificity of the fibroblast growth factor family. J Biol Chem 271 : 15292–15297.
24. ReifersF, BöhliH, WalshEC, CrossleyPH, StainierDYR, et al. (1998) Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development 125 : 2381–2396.
25. HerzogW, SonntagC, von der HardtS, RoehlHH, VargaZM, et al. (2004) Fgf3 signaling from the ventral diencephalon is required for early specification and subsequent survival of the zebrafish adenohypophysis. Development 131 : 3681–3692.
26. MillimakiBB, SweetEM, DhasonMS, RileyBB (2007) Zebrafish atoh1 genes: classic proneural activity in the inner ear and regulation by Fgf and Notch. Development 134 : 295–305.
27. SweetEM, VemarajuS, RileyBB (2011) Sox2 and Fgf interact with Atoh1 to promote sensory competence throughout the zebrafish inner ear. Dev Biol 358 : 113–121.
28. Giuliani G (2011) The role of tbx1 and otx1 in the development of the zebrafish inner ear. PhD thesis, University of Sheffield.
29. BokJ, RaftS, KongKA, KooSK, DrägerUC, et al. (2011) Transient retinoic acid signaling confers anterior-posterior polarity to the inner ear. Proc Natl Acad Sci USA 108 : 161–166.
30. BokJ, Bronner-FraserM, WuDK (2005) Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development 132 : 2115–2124.
31. BlenticA, GaleE, MadenM (2003) Retinoic acid signalling centres in the avian embryo identified by sites of expression of synthesising and catabolising enzymes. Dev Dyn 227 : 114–127.
32. CañestroC, CatchenJ, Rodríguez-MaríA, YokoiH, PostlethwaitJ (2009) Consequences of lineage-specific gene loss on functional evolution of surviving paralogs: ALDH1A and retinoic acid signaling in vertebrate genomes. PLoS Genet 5: e1000496.
33. HansS, IrmscherA, BrandM (2013) Zebrafish Foxi1 provides a neuronal ground state during inner ear induction preceding the Dlx3b/4b-regulated sensory lineage. Development 140 : 1936–1945.
34. ItohN, OrnitzD (2004) Evolution of the Fgf and Fgfr gene families. Trends Genet 20 : 563–569.
35. PirvolaU, Spencer-DeneB, Xing-QunL, KettunenP, ThesleffI, et al. (2000) FGF/FGFR-2(IIIb) signaling is essential for inner ear morphogenesis. J Neurosci 20 : 6125–6134.
36. ColvinJ, BohneB, HardingG, McEwenD, OrnitzD (1996) Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet 12 : 390–397.
37. PirvolaU, YlikoskiJ, TrokovicR, HebertJM, McConnellSK, et al. (2002) FGFR1 is required for the development of the auditory sensory epithelium. Neuron 35 : 671–680.
38. MuellerK, JacquesB, KelleyM (2002) Fibroblast growth factor signaling regulates pillar cell development in the organ of Corti. J Neurosci 22 : 9368–9377.
39. ShimK, MinowadaG, ColingD, MartinG (2005) Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell 8 : 553–564.
40. HayashiT, CunninghamD, Bermingham-McDonoghO (2007) Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev Dyn 236 : 525–533.
41. ZelarayanLC, VendrellV, AlvarezY, Domínguez-FrutosE, TheilT, et al. (2007) Differential requirements for FGF3, FGF8 and FGF10 during inner ear development. Dev Biol 308 : 379–391.
42. RomandR, HashinoE, DolléP, VoneschJ, ChambonP, et al. (2002) The retinoic acid receptors RARalpha and RARgamma are required for inner ear development. Mech Dev 119 : 213–223.
43. FrenzD, LiuW, CveklA, XieQ, WassefL, et al. (2010) Retinoid signaling in inner ear development: A “Goldilocks” phenomenon. Am J Med Genet A 152A: 2947–2961.
44. CadotS, FrenzD, MaconochieM (2012) A novel method for retinoic acid administration reveals differential and dose-dependent downregulation of Fgf3 in the developing inner ear and anterior CNS. Dev Dyn 241 : 741–758.
45. Abu-AbedS, DolléP, MetzgerD, WoodC, MacLeanG, et al. (2003) Developing with lethal RA levels: genetic ablation of Rarg can restore the viability of mice lacking Cyp26a1. Development 130 : 1449–1459.
46. Diez del CorralR, Olivera-MartinezI, GorielyA, GaleE, MadenM, et al. (2003) Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 40 : 65–79.
47. MarklundM, SjödalM, BeehlerB, JessellT, EdlundT, et al. (2004) Retinoic acid signalling specifies intermediate character in the developing telencephalon. Development 131 : 4323–4332.
48. Martínez-MoralesP, Diez del CorralR, Olivera-MartínezI, QuirogaA, DasR, et al. (2011) FGF and retinoic acid activity gradients control the timing of neural crest cell emigration in the trunk. J Cell Biol 194 : 489–503.
49. BrandM, HeisenbergC-P, JiangY-J, BeuchleD, LunK, et al. (1996) Mutations in zebrafish genes affecting the formation of the boundary between midbrain and hindbrain. Development 123 : 179–190.
50. ListerJA, RobertsonCP, LepageT, JohnsonSL, RaibleDW (1999) nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 126 : 3757–3767.
51. PiotrowskiT, AhnD-G, SchillingTF, NairS, RuvinskyI, et al. (2003) The zebrafish van gogh mutation disrupts tbx1, which is involved in the DiGeorge deletion syndrome in humans. Development 130 : 5043–5052.
52. KikuchiK, HoldwayJE, MajorRJ, BlumN, DahnRD, et al. (2011) Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev Cell 20 : 397–404.
53. LecaudeyV, Cakan-AkdoganG, NortonWH, GilmourD (2008) Dynamic Fgf signaling couples morphogenesis and migration in the zebrafish lateral line primordium. Development 135 : 2695–2705.
54. XiaoT, RoeserT, StaubW, BaierH (2005) A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development 132 : 2955–2967.
55. KimmelCB, BallardWW, KimmelSR, UllmannB, SchillingTF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203 : 253–310.
56. Westerfield M (2000) The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). Eugene: University of Oregon Press.
57. MaA, ChungM, LiangR, LeungA (2010) A DEAB-sensitive aldehyde dehydrogenase regulates hematopoietic stem and progenitor cells development during primitive hematopoiesis in zebrafish embryos. Leukemia 24 : 2090–2099.
58. Nüsslein-Volhard C, Dahm R, editors(2002) Zebrafish: A Practical Approach: Oxford University Press.
59. OxtobyE, JowettT (1993) Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nuc Acids Res 21 : 1087–1095.
60. KimCH, BaeYK, YamanakaY, YamashitaS, ShimizuT, et al. (1997) Overexpression of neurogenin induces ectopic expression of HuC in zebrafish. Neurosci Lett 239 : 113–116.
61. TsangM, MaegawaS, KiangA, HabasR, WeinbergE, et al. (2004) A role for MKP3 in axial patterning of the zebrafish embryo. Development 131 : 2769–2779.
62. MünchbergSR, OberEA, SteinbeisserH (1999) Expression of the Ets transcription factors erm and pea3 in early zebrafish development. Mech Dev 88 : 233–236.
63. KwakS-J, PhillipsBT, HeckR, RileyBB (2002) An expanded domain of fgf3 expression in the hindbrain of zebrafish valentino mutants results in mis-patterning of the otic vesicle. Development 129 : 5279–5287.
64. GrovesJ, HammondC, HughesS (2005) Fgf8 drives myogenic progression of a novel lateral fast muscle fibre population in zebrafish. Development 132 : 4211–4222.
65. NechiporukA, LinboT, RaibleDW (2005) Endoderm-derived Fgf3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish. Development 132 : 3717–3730.
66. InoueA, TakahashiM, HattaK, HottaY, OkamotoH (1994) Developmental regulation of islet-1 mRNA expression during neuronal differentiation in embryonic zebrafish. Dev Dyn 199 : 1–11.
67. BladerP, FischerN, GradwohlG, GuillemotF, SträhleU (1997) The activity of Neurogenin1 is controlled by local cues in the zebrafish embryo. Development 124 : 4557–4569.
68. AndermannP, UngosJ, RaibleDW (2002) Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev Biol 251 : 45–58.
69. LinvilleA, RadtkeK, WaxmanJ, YelonD, SchillingT (2009) Combinatorial roles for zebrafish retinoic acid receptors in the hindbrain, limbs and pharyngeal arches. Dev Biol 325 : 60–70.
70. CunliffeVT, Casaccia-BonnefilP (2006) Histone deacetylase 1 is essential for oligodendrocyte specification in the zebrafish CNS. Mech Dev 123 : 24–30.
71. HaddonC, LewisJ (1996) Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol 365 : 113–123.
Štítky
Genetika Reprodukčná medicína
Článek Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart DiseaseČlánek Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the NicheČlánek Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial TetheringČlánek Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature SeasonalityČlánek Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell DivisionsČlánek Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity inČlánek Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration inČlánek ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression inČlánek Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex EnvironmentsČlánek The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 12- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Stratification by Smoking Status Reveals an Association of Genotype with Body Mass Index in Never Smokers
- Genome Wide Meta-analysis Highlights the Role of Genetic Variation in in the Regulation of Circulating Serum Chemerin
- Occupancy of Mitochondrial Single-Stranded DNA Binding Protein Supports the Strand Displacement Mode of DNA Replication
- Distinct Genealogies for Plasmids and Chromosome
- Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart Disease
- Non-coding RNAs Prevent the Binding of the MSL-complex to Heterochromatic Regions
- Plasmid Flux in ST131 Sublineages, Analyzed by Plasmid Constellation Network (PLACNET), a New Method for Plasmid Reconstruction from Whole Genome Sequences
- Epigenome-Guided Analysis of the Transcriptome of Plaque Macrophages during Atherosclerosis Regression Reveals Activation of the Wnt Signaling Pathway
- The Inventiveness of Nature: An Interview with Werner Arber
- Mediation Analysis Demonstrates That -eQTLs Are Often Explained by -Mediation: A Genome-Wide Analysis among 1,800 South Asians
- Generation of Antigenic Diversity in by Structured Rearrangement of Genes During Mitosis
- A Massively Parallel Pipeline to Clone DNA Variants and Examine Molecular Phenotypes of Human Disease Mutations
- Genetic Analysis of the Cardiac Methylome at Single Nucleotide Resolution in a Model of Human Cardiovascular Disease
- Genetic Analysis of Circadian Responses to Low Frequency Electromagnetic Fields in
- The Dissection of Meiotic Chromosome Movement in Mice Using an Electroporation Technique
- Altered Chromatin Occupancy of Master Regulators Underlies Evolutionary Divergence in the Transcriptional Landscape of Erythroid Differentiation
- Syd/JIP3 and JNK Signaling Are Required for Myonuclear Positioning and Muscle Function
- Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the Niche
- Mutation of Leads to Blurred Tonotopic Organization of Central Auditory Circuits in Mice
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- RAN-Binding Protein 9 is Involved in Alternative Splicing and is Critical for Male Germ Cell Development and Male Fertility
- Enhanced Longevity by Ibuprofen, Conserved in Multiple Species, Occurs in Yeast through Inhibition of Tryptophan Import
- Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial Tethering
- Recurrent Loss of Specific Introns during Angiosperm Evolution
- Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature Seasonality
- SEEDSTICK is a Master Regulator of Development and Metabolism in the Arabidopsis Seed Coat
- Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell Divisions
- Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity in
- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression in
- Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex Environments
- Global Analysis of Photosynthesis Transcriptional Regulatory Networks
- Mucolipin Co-deficiency Causes Accelerated Endolysosomal Vacuolation of Enterocytes and Failure-to-Thrive from Birth to Weaning
- Controlling Pre-leukemic Thymocyte Self-Renewal
- How Malaria Parasites Avoid Running Out of Ammo
- Echoes of the Past: Hereditarianism and
- Deep Reads: Strands in the History of Molecular Genetics
- Keep on Laying Eggs Mama, RNAi My Reproductive Aging Blues Away
- Analysis of a Plant Complex Resistance Gene Locus Underlying Immune-Related Hybrid Incompatibility and Its Occurrence in Nature
- Epistatic Adaptive Evolution of Human Color Vision
- Increased and Imbalanced dNTP Pools Symmetrically Promote Both Leading and Lagging Strand Replication Infidelity
- Genetic Basis of Haloperidol Resistance in Is Complex and Dose Dependent
- Genome-Wide Analysis of DNA Methylation Dynamics during Early Human Development
- Interaction between Conjugative and Retrotransposable Elements in Horizontal Gene Transfer
- The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
- is Required for Adult Maintenance of Dopaminergic Neurons in the Ventral Substantia Nigra
- PRL1, an RNA-Binding Protein, Positively Regulates the Accumulation of miRNAs and siRNAs in Arabidopsis
- Genetic Control of Contagious Asexuality in the Pea Aphid
- Early Mesozoic Coexistence of Amniotes and Hepadnaviridae
- Local and Systemic Regulation of Plant Root System Architecture and Symbiotic Nodulation by a Receptor-Like Kinase
- Gene Pathways That Delay Reproductive Senescence
- The Evolution of Fungal Metabolic Pathways
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- Formation of Linear Amplicons with Inverted Duplications in Requires the MRE11 Nuclease
- Identification of Rare Causal Variants in Sequence-Based Studies: Methods and Applications to , a Gene Involved in Cohen Syndrome and Autism
- Rrp12 and the Exportin Crm1 Participate in Late Assembly Events in the Nucleolus during 40S Ribosomal Subunit Biogenesis
- The Mutations in the ATP-Binding Groove of the Rad3/XPD Helicase Lead to -Cockayne Syndrome-Like Phenotypes
- Topoisomerase I Plays a Critical Role in Suppressing Genome Instability at a Highly Transcribed G-Quadruplex-Forming Sequence
- A Cbx8-Containing Polycomb Complex Facilitates the Transition to Gene Activation during ES Cell Differentiation
- Transcriptional Frameshifting Rescues Type VI Secretion by the Production of Two Length Variants from the Prematurely Interrupted Gene
- Association Mapping across Numerous Traits Reveals Patterns of Functional Variation in Maize
- Genome-Wide Analysis of -Regulated and Phased Small RNAs Underscores the Importance of the ta-siRNA Pathway to Maize Development
- Dissemination of Cephalosporin Resistance Genes between Strains from Farm Animals and Humans by Specific Plasmid Lineages
- The Tau Tubulin Kinases TTBK1/2 Promote Accumulation of Pathological TDP-43
- Germline Signals Deploy NHR-49 to Modulate Fatty-Acid β-Oxidation and Desaturation in Somatic Tissues of
- Microevolution of in Macrophages Restores Filamentation in a Nonfilamentous Mutant
- Vangl2-Regulated Polarisation of Second Heart Field-Derived Cells Is Required for Outflow Tract Lengthening during Cardiac Development
- Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice
- A ABC Transporter Regulates Lifespan
- RA and FGF Signalling Are Required in the Zebrafish Otic Vesicle to Pattern and Maintain Ventral Otic Identities
- , and Reprogram Thymocytes into Self-Renewing Cells
- The miR9863 Family Regulates Distinct Alleles in Barley to Attenuate NLR Receptor-Triggered Disease Resistance and Cell-Death Signaling
- Detection of Pleiotropy through a Phenome-Wide Association Study (PheWAS) of Epidemiologic Data as Part of the Environmental Architecture for Genes Linked to Environment (EAGLE) Study
- Extensive Copy-Number Variation of Young Genes across Stickleback Populations
- The and Genetic Modules Interact to Regulate Ciliogenesis and Ciliary Microtubule Patterning in
- Analysis of the Genome, Transcriptome and Secretome Provides Insight into Its Pioneer Colonization Strategies of Wood
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- Echoes of the Past: Hereditarianism and
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



