-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Mutations in the ATP-Binding Groove of the Rad3/XPD Helicase Lead to -Cockayne Syndrome-Like Phenotypes
TFIIH is a protein complex that functions in the repair of bulky adducts distorting the DNA via the pathway of Nucleotide Excision Repair, and in transcription initiation and transactivation, the latter being a specific transcription activation process occurring in response to hormones. We have taken advantage of the powerful genetics and molecular biology of the model organism Saccharomyces cerevisiae to characterize the impact on cell fitness of a particular kind of mutations of one of the two helicases of the TFIIH complex, Rad3, called rem mutations for their increased levels of recombination and mutation. We have realized that these mutations affect a particular site of the protein, its ATP-binding groove, and modify the dynamics of TFIIH, leading to unfinished repair reactions and DNA break accumulation. Finally, we recreated these mutations in the human homolog XPD protein and found that their phenotypes recapitulated those of human mutations leading to a combination of the two hereditary diseases Xeroderma pigmentosum and Cockayne syndrome (XP-D/CS), whose molecular basis remains elusive. As these mutations also affect the ATP-binding groove of XPD, this study permits to propose a model to explain the molecular basis of XP-D/CS.
Published in the journal: The Mutations in the ATP-Binding Groove of the Rad3/XPD Helicase Lead to -Cockayne Syndrome-Like Phenotypes. PLoS Genet 10(12): e32767. doi:10.1371/journal.pgen.1004859
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004859Summary
TFIIH is a protein complex that functions in the repair of bulky adducts distorting the DNA via the pathway of Nucleotide Excision Repair, and in transcription initiation and transactivation, the latter being a specific transcription activation process occurring in response to hormones. We have taken advantage of the powerful genetics and molecular biology of the model organism Saccharomyces cerevisiae to characterize the impact on cell fitness of a particular kind of mutations of one of the two helicases of the TFIIH complex, Rad3, called rem mutations for their increased levels of recombination and mutation. We have realized that these mutations affect a particular site of the protein, its ATP-binding groove, and modify the dynamics of TFIIH, leading to unfinished repair reactions and DNA break accumulation. Finally, we recreated these mutations in the human homolog XPD protein and found that their phenotypes recapitulated those of human mutations leading to a combination of the two hereditary diseases Xeroderma pigmentosum and Cockayne syndrome (XP-D/CS), whose molecular basis remains elusive. As these mutations also affect the ATP-binding groove of XPD, this study permits to propose a model to explain the molecular basis of XP-D/CS.
Introduction
Accuracy of DNA enzymatic processes, such as transcription, replication and repair, is essential to guarantee genome integrity and, at a higher scale, cell and organism fitness. Such processes are functionally connected to checkpoint mechanisms that respond to DNA damage and stresses compromising cell cycle progression [1]. One relevant player in DNA repair and the maintenance of genome integrity is the multifunctional eukaryotic complex TFIIH. It is formed by 10 subunits and functions in Nucleotide Excision Repair (NER), transcription initiation and transactivation. During NER, bulky adducts that distort the DNA helix are recognized as lesions to which TFIIH binds to allow DNA unwinding, damaged DNA strand recognition and recruitment of the specific nucleases that excise the damaged DNA segment. During transcription, TFIIH facilitates DNA strand opening at promoter regions allowing full association of the transcription machinery and transcription initiation. Promoter escape, which allows transition from transcription initiation to elongation, is achieved by the ability of the cAMP-kinase CAK subcomplex of TFIIH to phosphorylate the C-terminal domain of RNA polymerase II (RNAPII) [2], [3]. During transactivation, TFIIH phosphorylates nuclear receptors to allow their entry into the nucleus, which in turn activates expression of downstream genes.
Central to TFIIH performance is Rad3/XPD (as named in yeast/mammals), an essential and conserved eukaryotic protein with 5′>3′ DNA helicase activity. During NER, Rad3 catalyzes DNA-strand opening. This creates the substrate for the action of the DNA-incision endonucleases Rad1-10/XPF-ERCC1 and Rad2/XPG. It is believed that removal of TFIIH is required to allow re-filling of the ssDNA gap generated by the endonucleases [4]. In contrast, the role of Rad3 in transcription initiation is structural. The activity required to open the promoter is provided by a second helicase present in TFIIH, Rad25/XPB [5]. Rad3 serves as a bridge between the core TFIIH and the CAK subcomplex. Since, as mentioned above, CAK phosphorylates RNAPII to clear the promoter and is responsible for the phosphorylation of nuclear receptors during transactivation, Rad3 integrity is fundamental for CAK attachment to TFIIH and its correct performance during transcription and transactivation.
Altogether, this explains why mutations in XPD/RAD3 may lead to NER failures as well as transcriptional and developmental defects. In humans, XPD mutations lead to Xeroderma pigmentosum (XP) and trichothiodystrophy (TTD), as well as combinations of XP with Cockayne Syndrome (XP-CS) and with TTD (XP-TTD). The clinical features of XP patients are explained by a NER deficiency, while TTD is seen as a consequence of transcriptional defects. However, our understanding of the XP-CS clinical features is less clear [6]. CS phenotypes are attributed to the inability to perform transcription-coupled repair (TCR), a NER subpathway in which lesions in the transcribed DNA strand encountered by an elongating RNAPII are efficiently repaired as compared to those of the non-transcribed strand [7], [8]. In a simplified way, XP-D/CS patient phenotypes could thus be explained by a TCR defect and/or a transcription defect, which would also explain their developmental defects and extreme sunlight sensitivity. Nevertheless, additional issues complicate this view: DNA breaks happen in trans upon UV irradiation in XP-D/CS cells [9] and the cancer proneness of XP-D/CS mice exceeds that of the most NER-deficient mice, those lacking XPA [10]. Therefore, there is still a lack of comprehension regarding the molecular basis of XPD-associated XP-CS phenotypes.
A particularly interesting class of rad3 mutations in Saccharomyces cerevisiae is that comprising the semi-dominant rad3-101 (Rad3-A237T) and rad3-102 (Rad3-H661Y) alleles [11]. They were named rem alleles, as they displayed increased levels of recombination and mutation [12], [13]. rem mutants differ from canonical NER-deficient RAD3/XPD mutants in their moderate UV sensitivity, increased levels of allelic recombination in heterozygous diploid cells, and inviability in the absence of the homologous recombination (HR) factor Rad52 [13], [14]. We have previously shown that, in contrast to most NER-deficient mutations, the rad3-102 (Rad3-H661Y) allele blocks NER at a post-incision step causing an extended retention of TFIIH at the damaged DNA that in turn provokes replication fork breakage and channeling of bulky lesions into HR-mediated Double Strand Break (DSB) repair [15]. The longer stay of Rad3-H661Y-containing TFIIH complexes at the site of NER action may be explained by an elevated affinity for ssDNA, given that the rad3-102 mutation lies in the ATP-binding groove of Rad3 and, when ATP hydrolysis by TFIIH is prevented, a gain of affinity for ssDNA manifests [16]. We have proposed that a parallelism may exist between rad3-102 cells and the mutations causing XP-CS in humans [15] because of several reasons. First, re-creation in Sulfolobus acidocaldarius of the equivalent human XPD-G675R mutant protein, associated with XP-CS disease, lies at the ATP-binding groove and displays a ssDNA-binding affinity 164% above that of the WT [17]. Second, this same mutation causes DNA breaks upon UV irradiation [9], reminiscent of rad3-102 cells. Third, as in rad3-102, removal of early NER proteins, such as XPA in mice, suppresses the phenotype of break accumulation conferred by the XP-D/CS mutation XPD-G602D [14], [18].
To gain insight into the molecular nature of the rem mutations and the possibility that they could relate to specific defects such as XP-CS, we analyzed two other yeast rem mutants, rad3-101 [12], [14] and the new and uncharacterized rad3-107 (Rad3-E236G; this paper). Both mutations also lie in the ATP-binding groove of the protein and, relevantly, all above-mentioned mutations are perfectly conserved between S. cerevisiae and humans (S1 Figure). We first determined the levels of NER deficiency and the dependency on HR of these two rem mutants, establishing a gradient of phenotypes. In vivo experiments show that when the Rad3 ATP-binding groove is mutated, TFIIH gains a higher affinity for DNA. Since mutations in the ATP-binding groove of Sulfolobus acidocaldarius XPD may provoke both a loss of helicase activity and a gain of ssDNA affinity [17], our results suggest that the balance between these two effects determines the ability of TFIIH to open DNA during NER and its recruitment to both DNA lesions and promoters. This would impair transcription resumption after DNA damage and NER, consistent with the CS phenotype. Last, we extended our study to human cells and show that the mutation equivalent to yeast rad3-102 in human cell lines recapitulates the XP-D/CS phenotypes, opening a parallelism between S. cerevisiae rem alleles and XP-D/CS mutations.
Results
RAD3 rem mutants display a gradient of responses to UV irradiation
To define the molecular basis of the different phenotypes of rad3-101 and rad3-107, we first studied the UV response of rad3-101 and rad3-107 mutants in comparison with that of the WT strain and the NER-deficient mutant rad3-2. Notably, rad3-101 cells respond to UV as the WT strain and in contrast to rad3-102 cells, which were slightly UV sensitive to increasing doses of UV. Instead rad3-107, as rad3-2, was highly UV-sensitive (Fig. 1A).
Fig. 1. rad3 mutants display a gradient response to UV irradiation. 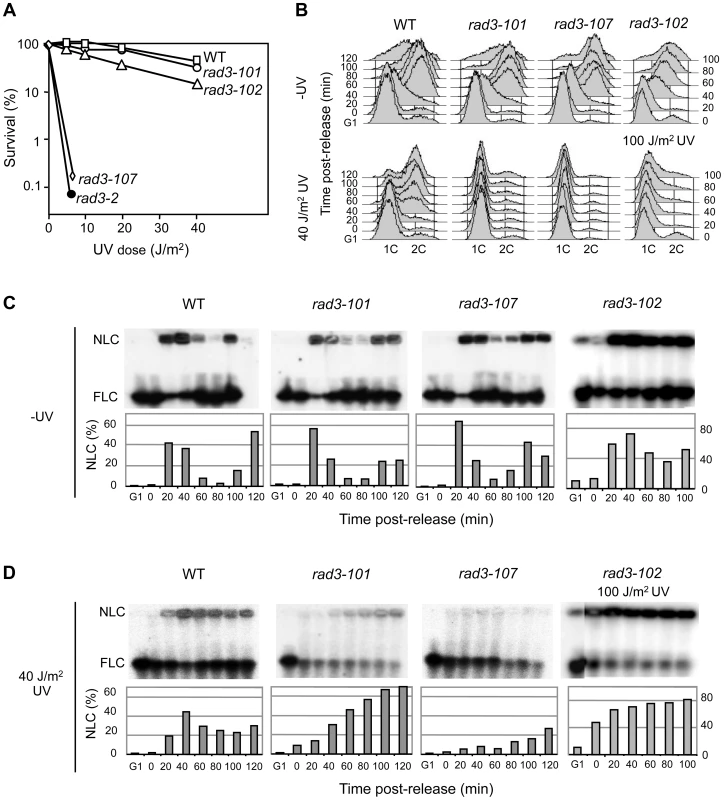
(A) Survival curves of WT and different yeast rad3 mutants after UV-C exposure. (B) FACS profiles from WT, rad3-101, rad3-107 and rad3-102 cells synchronized in G1 with α-factor, untreated or UV-irradiated with 40 or 100 J/m2 and released after 2 h. (C) Pulsed-field gel electrophoresis (PFGE) of DNA from WT, rad3-101, rad3-107 and rad3-102 cells synchronized in G1 with α-factor and further released into S phase. Bands reveal chromosome VII by hybridization with a probe of the ADE5,7 locus. Nonlinear (NLC) and full-length linear (FLC) chromosomes include replication intermediates, in the well, and pre- and post-replicated chromosomes, which enter the gel, respectively. Bars represent the quantification of NLC with respect to the total of signal of each lane. The bottom part of the membrane below the FLC is not shown since no signals, as expected from broken DNA molecules, were revealed by hybridization in any lane. (D) All details as in (C) except that after G1 synchronization, cells were UV-irradiated with 40 J/m2 or 100 J/m2 and released into S phase 2 h later. We have reported that rad3-102 was moderately resistant to UV because DNA gaps generated by an unfinished NER reaction could be resolved during the S phase via recombination [15]. It is thus possible that UV lesions are differently processed in each of the rad3 mutants analyzed, which would explain the different degrees of UV sensitivity. To test this possibility, we analyzed cell cycle progression through the S phase of cells synchronized in G1 with α-factor, irradiated with a 40 J/m2 UV-C dose and released 2 hours later from the G1 arrest. Without UV irradiation none of the mutant strains showed a cell cycle delay. However, while rad3-102 cells were able to progress through S phase almost as readily as the WT after a similar UV dose [15], UV-treated rad3-101 and rad3-107 cells were not able to progress throughout the S phase (Fig. 1B). Since FACS analysis cannot differentiate between a block in G1 or early S phase, next we analyzed replication fork progression by PFGE. This technique allows us to determine the fraction of chromosomes that are under active replication as the fraction of DNA unable to enter the gel, staying stacked in the gel well during electrophoresis [15]. In agreement with the FACS analysis, replication kinetics was mostly unchanged without irradiation in the different assayed strains (Fig. 1C). When UV-irradiating the cells, the analysis reveals that rad3-101 cells are able to initiate replication. Up to 70% of the DNA molecules were stacked in the well 120 min after G1 release (Fig. 1D). Nevertheless, replication in rad3-101 was much slower than in the WT as it took longer to accumulate replicating chromosomes. Instead, the same UV dose seems to fully prevent the rad3-107 UV-sensitive cells from initiating replication. In this case accumulation of replicating chromosomes in the well was poor (Fig. 1D). This rad3-107 phenotype is reminiscent of that of the canonical NER-deficient mutant rad3-2, which is unable to progress into S phase after UV irradiation [15]. Therefore, the different replication efficiencies seem to match the distinct abilities of TFIIH to resolve DNA lesions via NER. According to this hypothesis, we would expect that, at higher UV doses, the UV-resistant rad3-102 mutant should show a similar S-phase delay to that of the rad3-101 and rad3-107. On the contrary, no arrest during the S phase would be expected for rad3-101 cells at lower UV doses. Indeed, PFGE analysis showed a strong DNA retention in the well in rad3-102 cells in early S phase at UV doses of 100 J/m2, reaching up to 80% of DNA molecules (Fig. 1B and D). Instead, after a 20 J/m2 UV dose, rad3-101 and rad3-102 cells entered S phase and progressed normally throughout the cell cycle, while rad3-107 cells were still incapable to do so (S2 Figure). Altogether, these results indicate that NER is differently affected in the three mutants analyzed, the intensity of the defect correlating with a different degree of replication impairment.
Recombinational repair in rem mutants
The spontaneous and UV-induced hyper-recombination of rad3-102 mutants has been explained by the accumulation of ssDNA gaps derived from NER abortive reactions that are converted by replication into DSBs that are repaired by HR [15]. If this is the case for all rem-like mutants, the different amounts of DNA lesions accumulated in S phase as a consequence of abortive NER would be revealed by the levels of recombination. As genetically scored recombination events only inform about the DNA breaks that are successfully repaired by HR, we analyzed the global accumulation of Rad52 recombination foci as a way to evaluate whether a correlation existed between UV sensitivity and the accumulation of HR intermediates. Spontaneous and UV-induced Rad52 foci at 2 hours after irradiation with 10 or 20 J/m2 UV-C were thus analyzed and only cells in S and G2 phase of the cell cycle were considered. Both rad3-101 and rad3-107 cells accumulated more spontaneous Rad52 foci than wild-type cells (Fig. 2A), as previously shown for rad3-102 ([15] and Fig. 2A). However, after 10 J/m2 of UV-C dose, rad3-101 and rad3-107 displayed a 4 - and 2-fold increase with respect to the wild type, respectively (Fig. 2A), both mutants having reached a maximum level of Rad52 foci. After a 20 J/m2 UV-C dose, foci increased only in the wild type (Fig. 2A), consistent with the data showing that rad3-101 and rad3-107 cells did not progress through S phase properly (Fig. 1B). Notably, Rad52 foci accumulated in rad3-107 despite its strong UV sensitivity, which contrasts with the canonical NER-deficient mutant rad3-2 [15]. Altogether, these results suggest that a specific feature of rem mutants is their ability to convert bulky DNA adducts into DNA breaks that are repaired by HR, even though this may not be equally efficient in all mutants.
Fig. 2. Hyper-recombination and genetic interactions of rem mutations with recombination and replication functions. 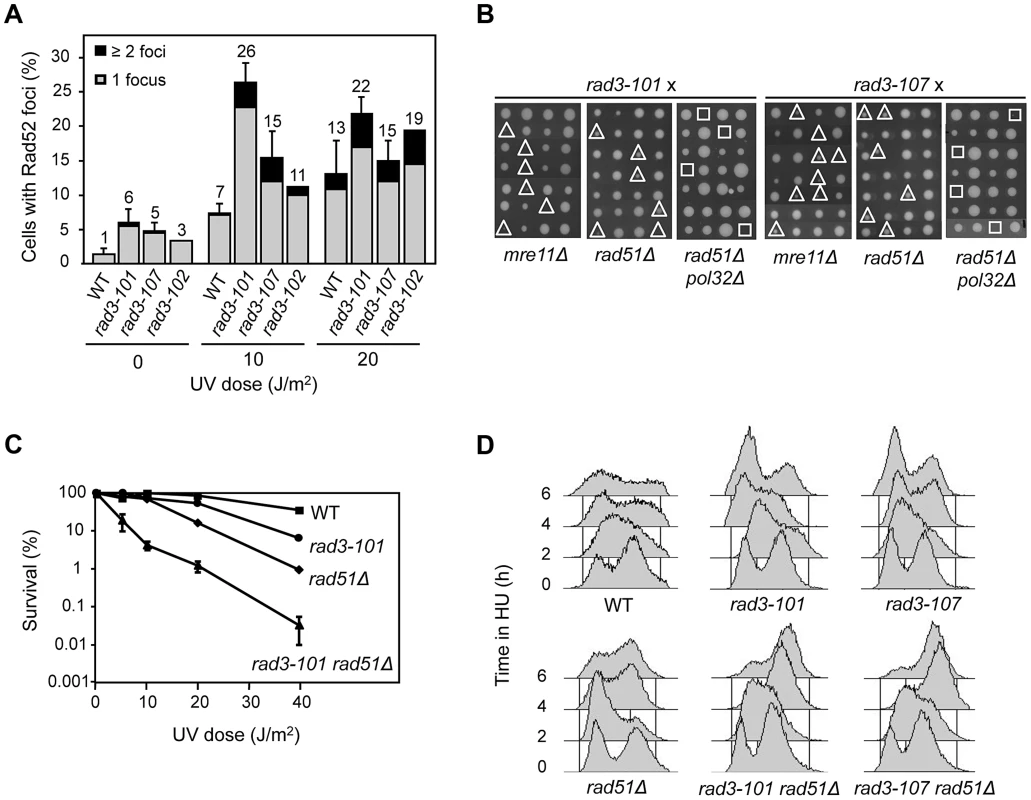
(A) Analysis of Rad52 foci after exposure to UV-C. Only cells in S and G2 were considered. Error bars represent the SD of three independent experiments. One representative experiment is shown for rad3-102 in order to facilitate comparison with previous published data [15]. (B) Analysis of genetic interactions of rem alleles of RAD3 with mre11Δ, rad51Δ and pol32Δ. Tetrads dissected on rich medium are shown. The sites of double and triple mutants are indicated by triangles and squares, respectively. (C) Survival curves of WT, rad3-101, rad51Δ and their corresponding double mutant cells after UV-C exposure. (D) FACS profiles of mid-log cultures from WT, rad3-101, rad51Δ and their corresponding double mutant cells taken every 2 h after addition of 40 mM HU. Consequently, we wondered whether early recombinational DSB repair functions such as Rad52 and the MRX complex become essential in rad3-101 and rad3-107 mutants, as previously shown for rad3-102 and in contrast to rad3-2 [13]-[15]. First we asked whether rad3-101 and rad3-107 relied on HR for their viability. rad3-101 was either lethal or showed a synthetic growth defect phenotype when combined with mre11Δ in non-irradiated cells (Fig. 2B), consistent with the previous lethality reported for rad3-101 rad52-1 [13]. The results suggest that, in rad3-101, spontaneous and UV-induced DNA breaks accumulate at a high frequency. rad3-107 also proved to be dependent on HR functions for viability, as rad3-107 mre11Δ double mutants showed a clear synthetic growth defect (Fig. 2B). Moreover, surviving rad3-101 mre11Δ cells and rad3-107 mre11Δ cells were extremely UV-sensitive (S3 Figure). Data are consistent with the idea that a specific feature of rem mutants is the accumulation of DNA breaks that need to be repaired by HR, which thus becomes essential for viability, even though to different extents in different mutants.
Our previous findings that rad3-102 is not viable if both Rad51 and Pol32 are removed suggest that these proteins control two different but non-mutually exclusive Rad52-dependent HR pathways for the repair of replication-mediated DNA breaks [15]. To assay whether this was also the case for the DNA lesions accumulated in rad3-101 and rad3-107 mutants, we characterized the corresponding double and triple mutants with rad51Δ and pol32Δ. As previously shown for rad3-102 [15], double rad3-101 rad51Δ and rad3-107 rad51Δ mutants were viable. In addition, rad3-101 rad51Δ was clearly UV sensitive when compared with each of the single mutants (Fig. 2C). As can be seen in Fig. 2B, both triple mutants rad3-101 rad51Δ pol32Δ and rad3-107 rad51Δ pol32Δ were not viable, as previously reported for rad3-102. To assay whether Rad51 becomes critical for the repair of broken forks and viability under HU-induced replicative stress, we deleted it in rad3-101 and rad3-107 cells. Notably, when 40 mM HU was added to asynchronous cultures of both rad3-101 rad51Δ and rad3-107 rad51Δ double mutants, cells arrested in late S/G2 phase, in contrast to WT and single rad3 mutants (Fig. 2D), as previously shown for rad3-102 [15]. This suggests that cells are unable to progress through the S phase, likely due to the incapacity of the broken forks to re-start in a Rad51-dependent manner. The results are consistent with the idea that, in rem mutants, replication forks break at the damaged sites as a consequence of unfinished NER, channeling repair of the resulting DSBs to HR, which in turn becomes essential.
Higher DNA affinity of TFIIH in vivo in ATP-binding groove mutants
As mentioned above, the three rem mutations so far identified localize at the ATP-binding groove of Rad3 (S4A Figure). Defects in Rad3 ATP binding cause an ATP-hydrolysis defect, which is known to increase the affinity of TFIIH for DNA [16]. This could explain the low efficiency of late NER steps in rad3-102 cells [4], [15]. Therefore we wondered whether the different levels of damage capable of being repaired by HR in the three rem mutants would correlate with a gain of DNA affinity of the Rad3 mutant proteins. We hypothesized that any ATP-binding groove mutant should show a rem-like phenotype. Since ATP hydrolysis failure should also compromise the helicase activity, the exception would be the helicase-null mutants, in which the incision step of the NER reaction cannot occur and would therefore behave as NER-null mutants. This would be the case of rad3-2, also located in the ATP-binding groove (S4A Figure), which is unable to excise the damaged ssDNA [19].
First, we asked whether mutations in the ATP-binding groove of Rad3, such as those of the rem strains studied here, could cause a gain in DNA affinity in vivo, independently of whether or not being masked by a helicase activity defect. For this we analyzed TFIIH retention at promoters, in which the helicase activity needed to open the DNA is provided by Rad25 and not by Rad3 [5]. We performed Tfb4-TAP chromatin immunoprecipitation (ChIP) in asynchronous cultures of the wild-type strain, the three rem mutants and the NER-null mutant rad3-2, plus the rad3-25 mutant carrying a E548K amino acid change that maps at the DNA binding channel (S4A Figure), outside of the ATP-binding groove and that was therefore expected not to show a significant gain of ssDNA affinity. To minimize any possible effect caused by transcription, we determined TFIIH binding at the ALG9 promoter, since ALG9 is constitutively expressed at a constant rate independently of environmental and cellular conditions [20] and we verified that it was transcribed in all mutants to similar levels as the WT (S4B Figure). The ChIP analyses show that TFIIH is more abundant at the ALG9 promoter in the three rem mutants, while in the rad3-25 control recruitment was indistinguishable from the WT (Fig. 3A). The rad3-2 NER-null mutant displayed the strongest promoter retention of all ATP-groove mutants, up to 3-fold the WT levels (Fig. 3A).
Fig. 3. Analysis of TFIIH recruitment to promoters in rad3 mutants. 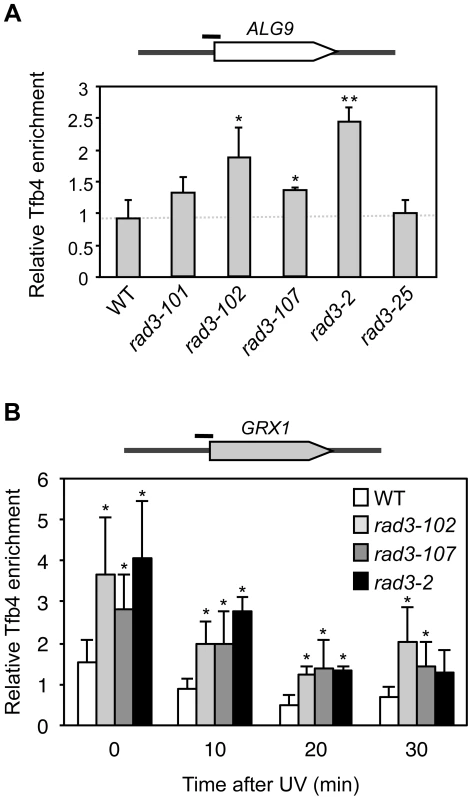
(A) Chromatin Immunoprecipitation (ChIP) analysis of Tfb4-TAP. Cells were grown in synthetic complete (SC) medium until the exponential phase. ChIP analysis was performed at the ALG9 promoter and normalized with respect to the MFA2 promoter in MATα cells, which is constitutively repressed. (B) ChIP analysis of Tfb4-TAP after UV damage. Cells were grown in SC medium until the exponential phase, and then irradiated with 80 J/m2. Analysis of the different time-point samples was performed at the GRX1 promoter and normalized with respect to the MFA2 promoter in MATα cells, which is constitutively repressed. The mean and the SD of triplicate assays of four independent experiments are depicted for each condition. *, p<0.05, **, p<0.01 (Student's t-test). Then we analyzed promoter occupancy upon UV irradiation, as UV is known to drive TFIIH out of the promoters presumably to facilitate its action at NER sites [21]. We took samples at different time-points from asynchronous cultures during 30 minutes after UV irradiation of the WT and the strains showing a significantly enhanced promoter retention in Fig. 3A, namely rad3-102, rad3-107 and rad3-2 mutants, all of them mutated in the ATP-binding groove. We assayed TFIIH recruitment to the GRX1 promoter, where we found the same relative increase as in the ALG9 promoter under conditions of no irradiation. The high basal transcription levels of GRX1 allow a better detection of falling off promoters upon UV treatment. Recruitment values for the control rad3-25 were similar as those of the WT (S4C Figure). After an 80 J/m2 UV dose, the relative fall-off promoters experienced by TFIIH in each mutant background was similar (Fig. 3B), implying that the shut-off of transcription in response to UV to accomplish NER is intact. Nevertheless, a significant amount of TFIIH remained bound to the GRX1 promoter in the rad3-102, rad3-107 and rad3-2 ATP-binding groove mutants up to 30 minutes, whereas almost most TFIIH was released from the promoter in WT cells (Fig. 3B). Altogether, these results indicate that mutants in the ATP-binding groove of Rad3 experience a gain in DNA affinity in vivo and, in particular, for promoters both with and without UV irradiation.
Rad3 ATP-binding groove mutants generate highly diffusible TFIIH
We had established the affinity for DNA in vivo of the mutant Rad3 proteins by studying the residence of TFIIH at promoters. We then assayed globally the capacity of mutant TFIIH to bind to DNA at NER sites in vivo by using Fluorescence Recovery After Photo-bleaching (FRAP) using the tagged Tfb4-yEGFP protein. The rationale behind was that an impairment in the ATPase activity would lead to helicase activity defects and consequently to a defective performance of the protein during the repair reaction. Therefore, after UV, if rad3 mutations prevented these activities of the protein, a bigger diffusing fraction should be observed for the mutants. We first confirmed that Tfb4-yEGFP behaves as a wild-type non-tagged Tfb4 by showing that the EGFP signal was detected all over the nuclei, that cells were as UV-resistant as WT cells and that an expected kinetics of fluorescence recovery was observed after photo-bleaching (Fig. 4A, and S5A and S5B Figure). In untreated WT cells, full recovery of fluorescence was observed in less than 12 seconds after bleaching (Fig. 4A and S5C Figure), consistent with the observation that most of the TFIIH complex within the nucleus is diffusible [22]. After UV irradiation a variable fraction of TFIIH is expected to move to the sites of DNA lesions to be engaged in their repair, leading to a low-diffusible fraction that reduces fluorescence recovery after bleaching. Accordingly, fluorescence recovery reached only up to 80% in cells treated with an 80 J/m2 UV dose (S5C Figure).
Fig. 4. In vivo TFIIH complex dynamics in rad3 mutants. 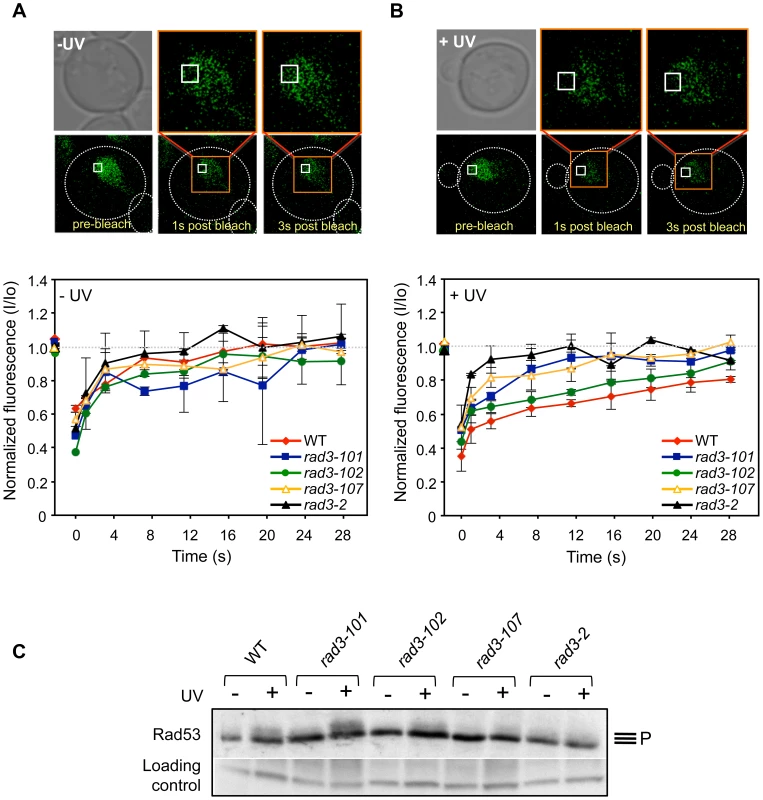
(A) FRAP analysis of asynchronous non-irradiated cultures expressing yEGFP-Tfb4. Pictures show Tfb4-yEGFP fluorescence in the nucleus of WT cells. The white square indicates the bleached area. Curves show the evolution of the normalized fluorescence in the bleached area of the nucleus [fluorescence intensity at each time-point (I) between the initial value of fluorescence intensity (Io)]. Each represented value corresponds to the median value calculated from four consecutive time-points. Error bars indicate the SD of two independent experiments. (B) FRAP analysis of asynchronous 80 J/m2 UV-C-irradiated cultures expressing yEGFP-Tfb4. Details as in (A). (C) Total protein extracts of G1-synchronized cells that were either UV-irradiated (+) or not (-) with 100 J/m2 were probed for Rad53 phosphorylation. Control and Rad3 ATP-binding groove mutant cells are compared. Unspecific hybridization of the antibody is used as an inner loading control. In spontaneous conditions, the TFIIH complex in ATP-groove mutants showed a similar dynamics to that of the WT (Fig. 4A). Nevertheless, after an 80 J/m2 UV dose, two observations could be made. First, fluorescence recovered completely in all mutants, indicating that the diffusing TFIIH fraction is bigger than in the WT (Fig. 4B). Second, the maximal recovery of fluorescence is achieved in shorter times in all mutants with respect to WT (Fig. 4B). Thus, it took only 8 seconds in rad3-2 cells for maximal recovery, in sharp contrast to the 24 seconds needed for the WT. This is better appreciated when each time point is plotted relative to an identical normalized maximum (S5D Figure), in which it can be seen the difference in the slopes of the initial time points among the different strains. This result is consistent with the idea that a defect in the ATP-binding groove of Rad3 may hinder TFIIH ability to bind to repair sites, leading to a larger diffusion fraction.
The rem phenotype recapitulates XP-D/CS-associated features
Accumulation of DNA breaks upon UV irradiation has been previously reported in XPD-deficient human XP8BR (XP-D/CS) cells [18] bearing a mutation in the ATP-binding groove of XPD. Interestingly, the equivalent mutant protein in Sulfolobus acidocaldarius exhibits an increased affinity for ssDNA [17]. Moreover, removal of the early-acting NER protein XPA suppressed the accumulation of DNA breaks [18]. Given that yeast rem mutants seem reminiscent of XP-D/CS cells, we investigated this putative parallelism.
In the first place, we analyzed the ability of rem mutants to activate the checkpoint in response to UV insults, since NER-deficient mutants do not accomplish damage processing, which in turn prevents checkpoint activation [23]. This feature is recapitulated in human cell lines defective for NER, including XPD-defective ones. Interestingly, cells from XP-D/CS patients, contrary to what was just mentioned, manage to activate the checkpoint [24]. Therefore, if XP-D/CS and rem mutations functionally relate, rem mutants should lead to checkpoint activation in response to UV. This prediction should apply to rad3-101 and rad3-102 mutants, who display an acute HR dependency, implying that initial NER steps are accomplished and therefore checkpoint activation can presumably occur. We synchronized cells in G1 and irradiated them with 100 J/m2 as previously described [23]. We monitored checkpoint activation in response to UV by following Rad53 phosphorylation. We could observe the expected phosphorylation of Rad53 in the WT strain (Fig. 4C). In agreement with the prediction, rad3-101 and rad3-102 displayed an even better, or slightly worse, respectively, Rad53 phosphorylation when compared with the WT (Fig. 4C). Very UV-sensitive mutants as rad3-107 and rad3-2, related to poor damage processing, displayed negligible or absent checkpoint activation, respectively (Fig. 4C). As a whole, these data suggest a parallelism between rem and XP-D/CS-causing mutations and a molecular explanation for the reported checkpoint activation after UV described for XP-D/CS cells [24].
In the second place, we wanted to test whether the rem alleles had the same impact on human cells as in S. cerevisiae. For this, we first assayed whether the yeast rad3-102 feature of accumulation of spontaneous DNA breaks was recreated in human cells. To achieve it, we overexpressed an XPD allele, XPD-102 (XPD-H659Y), carrying the equivalent of the yeast semi-dominant rad3-102 (rad3-H661Y) mutation (Fig. 5A). As a control we overexpressed the wild-type version of XPD from the same plasmid. mRNA levels of XPD and XPD-102 increased 300-fold with respect to cells transfected with the empty vector after 24 hours of transfection, and this correlated with increased expression at the protein level (S6A Figure). As an additional control, we verified that XPD-102 overexpression did not alter basic transcriptional patterns of the cell. Analysis by qPCR of levels of two relevant housekeeping mRNAs denoted no change in transcription between control and XPD-102-overexpressing cells (S6B Figure).
Fig. 5. Evidences of DNA damage in XPD-102 human cells. 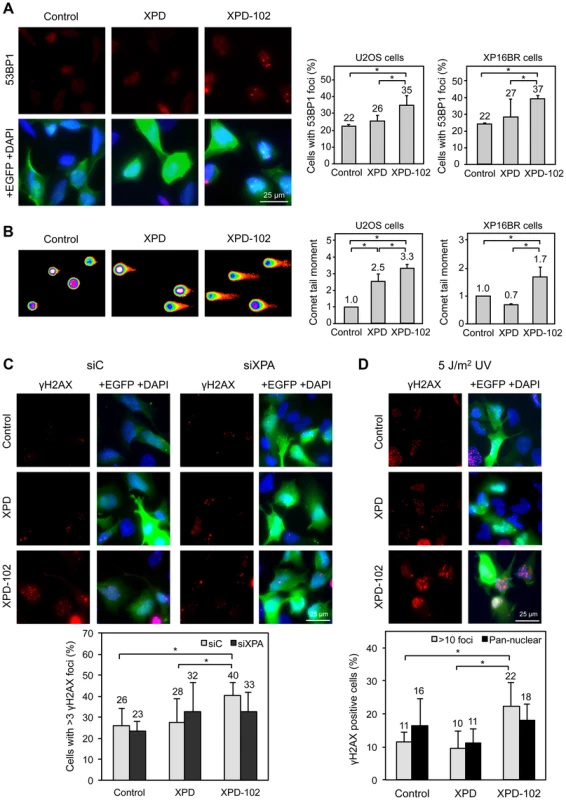
(A) Immunofluorescence of U2OS cells and quantification of the number of U2OS and XP16BR cells containing 53BP1 foci after transfection with pIRES2-EGFP empty vector (Control), pIRES2-EGFP-XPD overexpressing the wild-type XPD allele (XPD) and pIRES2-EGFP-XPD-102 overexpressing the XPD-102 rem allele (XPD-102). Expression of EGFP is used as a marker of transfection. Error bars indicate SD of three independent experiments. (B) Density map of the DNA single-cell electrophoresis from U2OS cells and quantification of the comet tail moments of U2OS and XP16BR cells transfected with the control, XPD and XPD-102 constructions. Details as in (A) (C) Immunofluorescence and quantification data of the number of U2OS Control, XPD and XPD-102 cells containing more than 3 γH2AX foci after transfection with siControl (siC) or siXPA. Error bars indicate the SD of four independent experiments. (D) Immunofluorescence and quantification of the number of U2OS control, XPD and XPD-102 cells containing either pan-nuclear staining or more than 10 discrete γH2AX foci 5 hours after irradiation with 5 J/m2 UV-C. Error bars indicate the SD of four independent experiments. *, p<0.05 (Mann-Whitney U test). As DNA breaks accumulate in yeast rad3-102 cells [15], we analyzed by immunofluorescence 53BP1 foci, known to accumulate early at sites of DSBs [25]. U2OS cells overexpressing wild-type XPD show a similar percentage of 53BP1 foci-containing cells as U2OS cells transfected with the empty plasmid (22% and 26%, respectively). However, the percentage of cells with 53BP1 foci was clearly increased in cells overexpressing XPD-102 (35%) (Fig. 5A). For further evidence of the accumulation of DNA breaks, we directly analyzed the accumulation of broken DNA fragments using the single cell gel electrophoresis assay (comet assay) under alkaline conditions to detect both single-stranded and double-stranded DNA breaks. In these assays, XPD-102 cells showed a larger tail moment than XPD and control cells (Fig. 5B), which demonstrates that physical breaks are spontaneously produced in these cells. Conclusions were confirmed in U2OS cells by additional immunofluorescence analysis. While the percentage of U2OS cells containing γH2AX foci was similar in control and XPD-overexpressing cells, XPD-102 U2OS cells showed a significantly higher accumulation (Fig. 5C). We also validated this notion in HeLa cells, where the γH2AX signal increased 1.5 times after XPD-102 overexpression with respect to XPD-overexpressing cells as determined by FACS (S6C Figure). As thus far experiments were performed overexpressing XPD-102 in XPD+/+ cells, next we asked whether results were the same in primary XP16BR (XPD-R683W/R616P, XP-D) fibroblasts, in which one XPD allele is hypothesized to be null and the other one severely affected in its enzymatic activity [26]. In these primary fibroblasts, 53BP1 foci and comet tail moments were similar to those obtained in XPD+/+ U2OS cells, with a 1.7-fold increase above the control cells transfected either with the empty vector or the one overexpressing the wild-type allele of XPD (Fig. 5A and 5B). Altogether, these results imply that the basal level of damage recruiting TFIIH is sufficient to uncover the effects caused by the action of mutant XPD-102, which results in the production of breaks. We therefore conclude that the XPD-102 mutation causes similar effects both in humans and yeast.
The breaks described to occur in XP-D/CS human XPD-G675R cells and in mouse XpdG602 cells are UV-induced, and in the latter case have been shown to depend on the NER-initiating factor XPA [18]. To prove the analogy between the rem-like mutation XPD-102 and XP-D/CS cells, we first assessed whether XPA depletion by siRNA suppressed the breaks provoked by XPD-102 without UV irradiation. XPA mRNA levels were similarly reduced in all cells after 96 hours of siRNA depletion, and this correlated with a decrease at the protein level (S6D Figure). XPA depletion did not have any suppressive effect on the number of γH2AX foci in the control or in cells overexpressing XPD (Fig. 5C). In contrast, the increase in the percentage of γH2AX foci-containing U2OS cells upon XPD-102 expression was reduced back to the level of cells overexpressing wild-type XPD and depleted of XPA (Fig. 5C). This argues in favor of the idea that, as in XP-D/CS cells, the accumulation of DNA breaks in XPD-102 cells depends on a functional NER pathway.
If our hypothesis is correct, the XPD-102-induced breaks should be exacerbated by UV treatment, as more substrates would become available for the mutant XPD-102 protein. Following the same approach as above, the percentage of γH2AX foci-containing U2OS cells was studied. UV light greatly increased γH2AX foci in all cells (compare Figs. 5C and 5D). We differentiated pan-nuclear staining, a response to UV irradiation dependent on initial attack of the damage by the NER components, from discrete foci formation, a readout of replication-associated DSBs, among others [27]. While pan-nuclear staining was virtually similar in all assayed cellular contexts, the increase of discrete γH2AX foci was significantly exacerbated for cells overexpressing XPD-102 in comparison with both controls (Fig. 5D).
Altogether, the results suggest a functional relationship between human XP-D/CS and yeast rem mutations, in particular rad3-102, all of them affecting the ATP-binding groove.
Discussion
The TFIIH complex has central roles in cell physiology, as it is involved in both transcription and DNA repair. This explains that defects in any of its subunits lead to inherited diseases in humans of important clinical relevance. Understanding the molecular basis underlying the phenotypes shown by TFIIH patients bearing a combination of both Xeroderma pigmentosum and Cockayne Syndrome has been long pursued. The classical view invokes a defect in TCR that accounts for both the NER deficiency and the transcriptional defects [6]. We have previously discussed several similarities between XP-D/CS-causing mutations and the S. cerevisiae rad3-102 rem allele [28]. Nevertheless, there are also important differences between them. Thus, rad3-102 cells are slightly sensitive to UV, while XP-D/CS cells are strongly UV-sensitive. Also, rad3-102 cells do not show clear transcriptional defects, while XP-D/CS cells do. In this work, by addressing directly the molecular basis for the analogies and differences between several yeast rem mutants (rad3-101, rad3-102 and the newly constructed rad3-107) and by characterizing the effects of XPD-102 in human cells, we show that rem mutants can be used as a model to understand XP-D/CS-associated molecular phenotypes.
We first show that the rad3-102 ability to tolerate UV irradiation is not by itself a rem feature. On the contrary, there exists a gradient of UV sensitivity depending on the rem mutant analyzed (Fig. 1). rad3-102 tolerance to UV is based on the fact that TFIIH is able to remain bound to the damage site longer, thus inhibiting gap filling and generating a DSB during replication. As a result HR becomes essential for survival [13]-[15]. This is also the case of rad3-101 cells, which display UV resistance comparable to that of the WT strain and exhibit full dependence on HR factors Rad52 and the MRX complex for survival (Fig. 2B). Instead, rad3-107 cells, which are extremely UV-sensitive, display only a synthetic growth defect in the absence of HR factors (Fig. 2B). A lower ability of TFIIH to load onto DNA and to remain bound, thus resulting in a lower frequency of NER reactions ending in DSBs, would explain the inverse correlation between the need of HR and UV sensitivity. Nevertheless, even if the amount of damage leading to DSBs is different in each rem mutant, replication forks could still break in most cases. Consistently, removal in the three rem mutants tested of both Pol32 and Rad51, which block HR-mediated fork restart in S. cerevisiae, lead to inviability (Fig. 2B) [15]. Therefore, UV-resistance is not an obligatory feature of rem mutants, their variable degree of UV sensitivity depending on the balance between the amount of NER-repairable damage that remains unprocessed and the amount that is directed into HR repair.
As rem mutations map to the ATP-binding groove of Rad3 (S4A Figure), and are fully conserved (S1 Figure), they provide a useful tool to understand the possible consequences of impairing ATP binding on NER processing. XPD alterations leading to ATP-binding defects may compromise helicase activity and, consequently, could influence helix opening and helicase translocation on the DNA [29]. Indeed, the mutants tested displayed levels of fluorescence recovery after UV superior to the WT, as determined by FRAP analysis (Fig. 4B), a measure indicating a problem to attach to damage sites. Additionally, difficulties in hydrolyzing ATP would also lead to a putative TFIIH gain in ssDNA affinity [16]. Since, during transcription, Rad25 helicase activity is in charge of promoter opening, while Rad3 is only needed for structural reasons [5], it was possible to use promoters to detect putative TFIIH retentions at DNA. Notably, at basal transcription levels, all ATP-binding groove mutants, including the rad3-2 NER-null mutant, show higher levels of TFIIH recruitment to promoters than the WT strain or than a UV-sensitive strain [30] whose mutation did not map to the ATP-binding groove, a behavior maintained after UV irradiation (Fig. 3A and 3B). Nevertheless, the relative fall-off promoters upon UV irradiation experienced by all mutants tested was comparable to that of the WT, indicating a normal response to abrogate transcription and migrate to NER sites (Fig. 3B). These data support the notion that the TFIIH complexes in rem ATP-binding groove mutants display an enhanced affinity for DNA. Remarkably, all XPD mutations causative of XP-CS known to date are located in the ATP-binding groove (S4A Figure). Re-creation of these four mutations in Sulfolobus acidocaldarius XPD demonstrated a dramatic loss of both ATPase and helicase activities. Importantly, half of them exhibited an increased ssDNA affinity, a property not seen in any other category of XPD mutants [17].
Our first molecular observation to functionally link Rad3 rem mutations and XP-CS-causing XPD ones is the ability to activate the checkpoint in response to UV of both XPD-CS cells [24] and HR-dependent rem mutants (Fig. 4C). This is a remarkable parallelism since canonical rad3 NER-deficient yeast and XPD-deficient human cells are unable to activate the checkpoint upon UV insult [23], [24]. Moreover, this provides an explanation for the mechanism leading to such checkpoint activation in XP-D/CS cells: partial processing of the lesion by accomplishment of the initial steps of NER, as it occurs in HR-dependent rem cells.
To search for additional molecular support for the rem/XP-D/CS link, we moved to human cells. Overexpression of an XPD-102 (XPD-H659Y) mutation leads to DNA break accumulation, already in the absence of UV, as detected by single-cell electrophoresis as well as 53BP1 and γH2AX foci accumulation (Fig. 5 and S6C Figure) in three different human cell lines. This detection is possible since there exist substrates for NER under basal conditions that are processed by the defective XPD-102. Therefore, we are able to re-create in human cells this known feature of yeast rad3-102. Yet, if the notion that rem and XP-D/CS mutations are equivalent is correct, several predictions should prove right. First, such an accumulation of DNA breaks should be XPA-dependent. Indeed, depletion of XPA led to a reduction in the accumulation of DNA breaks to levels comparable to those caused by overexpression of the control XPD (Fig. 5C), as seen in XP-D/CS [18] and rad3-102 cells, where early-acting factors such as Rad4 suppress this defect [14]. Second, the formation of breaks should be further increased by providing the cells with more substrates, namely by UV-irradiation. Again, a significant fraction of γH2AX foci accumulated specifically upon overexpression of XPD-102, while overexpression of the wild type allele of XPD did not even increase this value up to the level seen in the control itself (Fig. 5D). Last, even though XP-D/CS breaks were originally described as being transcription-dependent [18], this notion was recently dismissed using XpdG602D mouse cells, which mimic an XP-D/CS mutation [31]. Indeed, it has been demonstrated that early steps of the DNA damage-signaling cascade, as those under study here, still occur even if transcription is inhibited, and that the action of the mutant protein has been exerted [31]. Altogether, the results support the view that rem alleles and human mutations causing XP-D/CS phenotype are functionally equivalent.
The explanation underlying the molecular basis of XP-CS has remained elusive. The XP defect is explained by deficient NER, but the explanation for the CS phenotype, generally invoking a defect in TCR, is unclear. In our view, an integrated mechanism for DNA damage removal inability and transcription retardation can be proposed. In WT cells, TFIIH will promote NER and then resume transcription (Fig. 6, middle). Rad3/XPD null mutants will be unable to open DNA at a damaged site therefore not affecting transcription resumption (Fig. 6, left). However, in mutants of the ATP-binding groove (rem-like mutants, Fig. 6, right), there would co-exist an enhanced DNA affinity and a reduced DNA opening capacity. The increased DNA affinity would easily manifest at locations where TFIIH binding does not depend on Rad3/XPD, as it is the case of promoters. The enhanced affinity for other DNA sites, such as damaged DNA, nevertheless, would depend on the ability of TFIIH to open the DNA first. If the opening occurs, TFIIH could be engaged in NER but will stay bound to the damaged DNA for longer due to a gain in affinity for DNA. This may delay transcription resumption and also provoke replication fork collapse and breakage that would demand the intervention of the HR machinery. If this process were efficient, UV sensitivity would be mild. However, mutations in the ATP-binding groove may also have a strong impact, so that TFIIH may stay associated with promoters (Fig. 6, right, dashed lines). This model would be in agreement with the proposal that genomic DNA is cut in trans upon transfection of damaged plasmids into XP-CS cells [9]. In our view, therefore, the transcription impairment after DNA damage would occur in a TCR-independent manner. In further agreement with this view, recent data reveal that RNA recovery defects following UV in XP-CS cells are restricted to genes whose expression was shut-off specifically in response to UV, leaving damage-inducible genes unaffected, thus arguing against a general defect in TCR, which impairs all types of transcription [32]. Moreover, authors demonstrate a specific heterochromatinization happening at promoters that do not resume transcription [32], temptingly as a consequence of a too lasting period without TFIIH components coming back.
Fig. 6. Model to explain the molecular defects occurring in XP-D/CS cells. 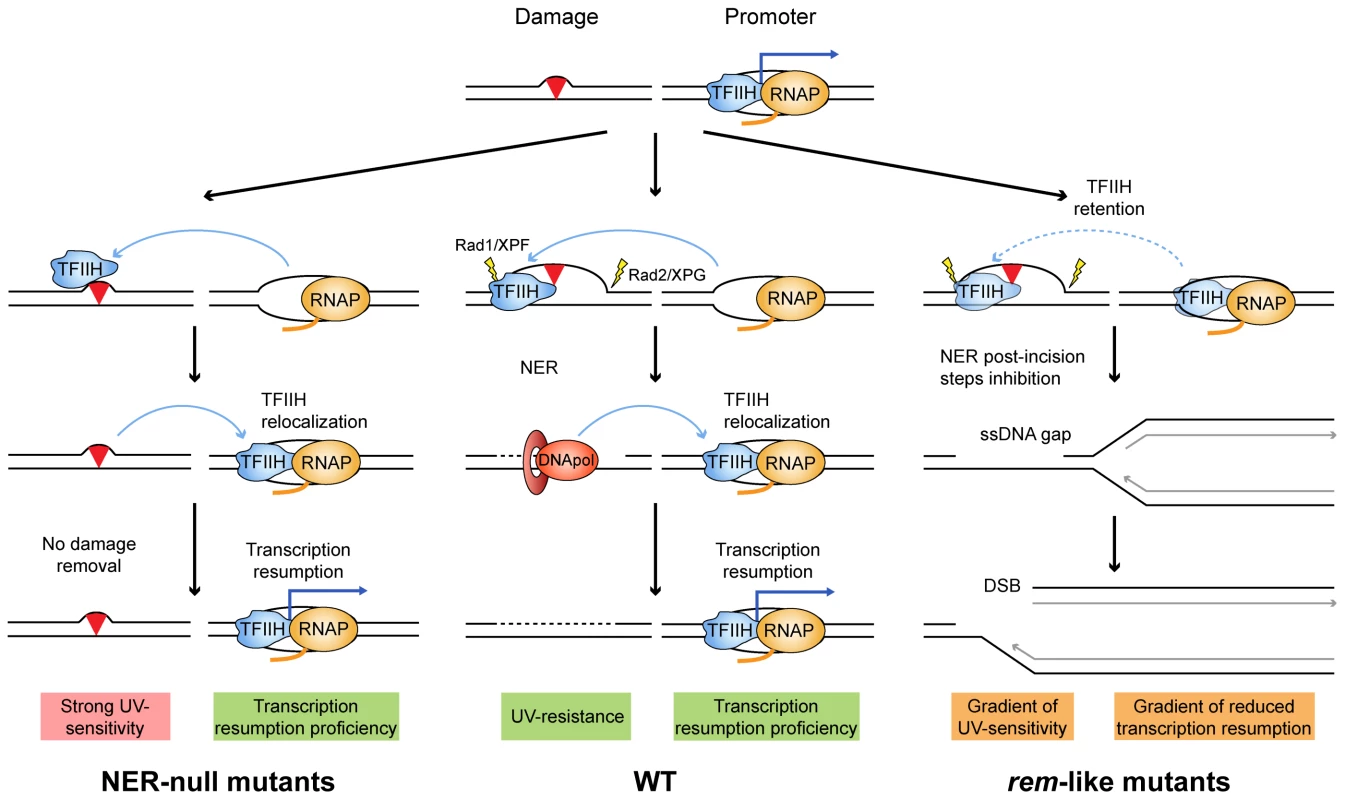
Mutations in the ATP-binding groove of Rad3/XPD would compromise both its ATPase and its helicase activities. Depending on the mutation, both capacities would be compromised to different extents. A simplified scheme of each situation is illustrated by a paradigmatic yeast rad3 mutation. The different envisioned stages of the NER engagement in repair and its impact on on-going transcription in each situation are depicted. The main molecular outcomes are highlighted in pink, green and orange. In conclusion, we propose that, as in the rem alleles, the XP features seen in XP-D/CS patients arise from the inability of their cells to initiate and/or accomplish a proficient NER reaction, whereas the CS features would be the consequence of increased levels of the repair conformation of TFIIH that may compromise resumption of transcription. Consistent with our model, it has recently been observed in XpdG602D mice cells mimicking an XP-D/CS mutation that defective NER leads to the accumulation of long stretches of ssDNA upon UV, suggesting that the long-lasting aberrant NER intermediate extends the time of repair and therefore may inhibit transcription for long periods [31]. Subsequent accumulation of replication-mediated DNA breaks that demand an intact HR for its repair and replication restart would explain the high levels of genetic instability that characterizes both rem yeast and XP-CS human cells.
Materials and methods
Strains and plasmids
Strains used are described in S1 Table. The rad3-107 (rad3-E236G) mutation was constructed by oligonucleotide directed mutagenesis using the primer 5'TTTTGATG(CGT)AGCGCACA3' and a plasmid pPF1 containing the BamHI-SalI fragment of RAD3 cloned into pGEM7Zf (Promega Biotech, Madison, WI). The E236G mutation was confirmed by sequencing and was used to substitute the wild type BamHI-SalI fragment of pBM3 [14] to make pBM3-107. Plasmids pBM3-101 and pBM3-107, containing the rad3-101 and rad3-107 mutant alleles, were used to substitute the endogenous RAD3 gene by the respective mutant alleles followed by the hygromycin resistance cassette to generate strains YREC57-41 and YREC57-45. Plasmid pKT127 [33] was used to construct Tfb4-yEGFP-based strains. All other single, double and triple mutants were obtained by backcrosses. Plasmid pWJ1344 carrying the RAD52-YFP construct has been previously described [34]. The XPD cDNA sequence was obtained by PCR from the RZPD/ImaGenes clone IRATp970F12108D (Berlin, Germany). The XPD-102 allele containing the C1980T substitution was obtained by directed mutagenesis using two overlapping PCR primers (GGACCTGTAAGGCTCGAGATGAAGCTCAACGTGGA and GGGCCGCGTAGCGCATGGCATCGAAGGTAAGAAA for the 5′ site and ACTTTCTTACCTTCGATGCCATGCGCTACGCGGCCCAGT and GCGCTCGATACGGAATTCTCAGAGCTGCTGAGCAA for the 3′ site) sharing the mutation. Both cDNAs were cloned into the XhoI-EcoRI sites of the pIRES2-EGFP vector (Clontech, Palo Alto, CA, USA).
Cell culture and transfection
U2OS and HeLa cells were cultured in DMEM (Gibco, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS). Primary human XP16BR fibroblasts (provided by Dr. A.R. Lehmann) were cultured in Eagle's minimum essential medium (Biowest) with 15% heat-inactivated FBS. All cells were maintained at 37°C and 5% CO2.
Short interfering RNAs (siRNA) used were on-target plus non-targeting pool (siC) and on-target plus smartpool human XPA (siXPA) (Dharmacon). Cells were transfected with plasmid (2 µg/ml) or siRNA (100 nM) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) or DharmaFECT 1 (Dharmacon) respectively, according to the manufacturer's instructions. Immunostaining and single-cell electrophoresis assays were performed 24 or 96 hours after the plasmid or siRNA transfection, respectively.
Chromatin immunoprecipitation and quantitative PCR
Strains were cultured until 0.7O.D.600nm in SC. A 50 mL sample was taken for the non-irradiated control experiments. The culture was centrifuged, resuspended in distilled, sterile water, and irradiated in plates using a 80 J/m2 UV dose. Cells were immediately resuspended in fresh medium, and 50 mL samples were taken every 10 min. Samples processing was performed as described [35]. IgG Sepharose (GE Healthcare) was incubated with samples over night to precipitate TAP-tagged Tfb4. The WizardR SV DNA clean-up system (Promega) was used for the last DNA purification step. Quantitative PCR (qPCR) was performed against the ALG9 or GRX1 loci promoters in Matα cells. Normalization was done with values of amplification at the MFA2 promoter, as described for Matα cells in order to study TFIIH recruitment in the absence of transcription [36]. Damage of templates by UV irradiation did not impede proper qPCR amplification, since controls at sites of active transcription displayed high amplification signals, and in vitro repair of templates prior to qPCR as described [37] did not alter results. Primers used were: ALG9-fw: TGGCTCTTTTTTCACCCTGAA; ALG9-rv: TGGTTACCGCCTTGCAATTC; MFA2-fw: TGCATGTCAGAGGAAAAAGAACAAAG; MFA2-rv: CGGTGAACGACAGAAGAAGTGG; GRX1-fw: TCACGTGAATCAGGAGGCG; GRX1-rv: GGCGTTTCCAGATTGCGAT.
Fluorescence recovery after photobleaching
Overnight Tfb4-yEGFP mid-log cultures, grown in YPAD, were either non-treated or 80 J/m2-UV-C-irradiated. FRAP was performed on a Leica TCS SP5 confocal microscope at room temperature. A 0.6 µm2 area into the nucleus was bleached at 50% laser intensity. Recovery of fluorescence into this area was monitored with 1 second intervals at 5% laser intensity. Images were acquired with a 488 nm laser. Fluorescence intensities were measured in bleached and unbleached areas with the MetaMorph v7.5.1.0. software. Normalized fluorescence in each point was calculated as follows: Irel = ((N0 - B0) × (It – Bt))/((Nt – Bt) × (I0 – B0)) where I0, N0 and B0 are the average intensity of the bleached region, an area in the same nucleus, or a randomly selected region outside of the cell during prebleach, respectively. It, Nt and Bt are the average intensity of the bleached region, an area in the same nucleus, or a randomly selected region outside of the cell at each time point, respectively. At least 10 cells for each condition were analyzed.
Immunofluorescence analysis
Cells were cultured on glass coverslips and transfected at 70-80% confluence. For UV irradiation, cells were washed with PBS, irradiated with a 5 J/m2 UV-C dose after PBS removal, further incubated and collected after 5 hours. Cells were fixed in 2% formaldehyde in phosphate-buffered saline buffer (PBS) for 20 min and permeabilized with 70% ethanol for 5 min at −20°C, 5 min at 4°C, and washed twice in PBS. After blocking with 3% bovine serum albumin (BSA) in PBS, the coverslips were incubated with rabbit polyclonal anti-53BP1 (NB100-304 Abyntec Biopharma) or mouse monoclonal anti-γH2AX (JBW301, 05-636 Millipore) primary antibodies (1∶500) diluted in 3% BSA in PBS for 1h at room temperature. Secondary goat anti-rabbit antibody conjugated with Alexa Fluor 568 or goat anti-mouse antibody conjugated with Alexa Fluor 546 (Invitrogen) in 3% BSA in PBS were used. Transfection efficiency was monitored by transfection into cells of pIRES2-EGFP, pIRES2-EGFP-XPD or pIRES2-EGFP-XPD-102 plasmids. DNA was stained with DAPI. Images were captured with a Leica DM6000 microscope equipped with a DFC390 camera (Leica). Data acquisition was performed with LAS AF (Leica) and analyzed with the MetaMorph v7.5.1.0. software. More than 100 cells from each experiment were analyzed.
Single-cell gel electrophoresis
Comet assay was performed using a commercial kit (Trevigen, Gaithersburg, MD, USA) following the manufacturer's protocol. Images were acquired as described above and analyzed with the Comet-score software (version 1.5). More than 100 cells from each experiment were scored.
Reverse transcription qPCR analysis
cDNA was synthesized from total RNA extracted using RNeasy Mini Kit (Qiagen) (1 µg) by reverse transcription using Super-Script TM First strand synthesis for RT-PCR (Invitrogen, Carlsbad, CA) and random primers. RT-qPCR was performed with SYBR qPCR Mix and analyzed on an ABI Prism 7000 (Applied Biosystems, Carlsbad, CA). mRNA expression of the indicated genes were normalized with mRNA expression of the HPRT housekeeping gene. For the analysis of DHFR and GAPDH mRNA expression, data were normalized to the 18S RNA levels. Primers used were AAAGTGTCCGAGGGAATCGA and GGGACGCCAAACATGATGA for XPD, GAACCACTTTGATTTGCCAACTT and TTGCCTCTGTTTTGGTTATAAGCTT for XPA, GGACTAATTATGGACAGGACTG and TCCAGCAGGTCAGCAAAGAA for HPRT, TGCCACCAACTATCCAGACCA and CCTGGTTCTCCATTCCTGAGA for DHFR, CGACCACTTTGTCAAGCTCA and TACTCCTTGGAGGCCATGTG for GAPDH, and ATTCGAACGTCTGCCCTATCA and GTCACCCGTGGTCACCATG for 18S.
Miscellanea
γH2AX signal in HeLa cells by FACS was measured using a commercial kit (FlowCellect Multi-Color DNA Damage Response Kit, Millipore, Germany) following the manufacturer's protocol. The methods for the analyses of yeast UV survival curves, cell cycle profiles, Pulsed-Field Gel Electrophoresis and spontaneous and UV-induced Rad52 foci have been previously described in detail [15]. Cell treatment, protein extraction and Western Blotting for Rad53 activation has been performed strictly as previously described [23]. Rad53 antibody used was previously described [38]. For XPD and XPA Western blots, 25 µg of total amount of protein, extracted from U2OS cells, were used. Antibodies XPD (abcam ab54676, 1∶5000), XPA (abcam ab2352, 1∶1000) and β-Actin (abcam ab8226, 1∶5000) diluted in TBS-Tween 0.1% with 5% milk were incubated overnight at 4°C.
Supporting Information
Zdroje
1. AguileraA, Garcia-MuseT (2013) Causes of genome instability. Annu Rev Genet 47 : 1–32.
2. FeaverWJ, SvejstrupJQ, HenryNL, KornbergRD (1994) Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell 79 : 1103–1109.
3. SchroederSC, SchwerB, ShumanS, BentleyD (2000) Dynamic association of capping enzymes with transcribing RNA polymerase II. Genes Dev 14 : 2435–2440.
4. MocquetV, LaineJP, RiedlT, YajinZ, LeeMY, et al. (2008) Sequential recruitment of the repair factors during NER: the role of XPG in initiating the resynthesis step. EMBO J 27 : 155–167.
5. TirodeF, BussoD, CoinF, EglyJM (1999) Reconstitution of the transcription factor TFIIH: assignment of functions for the three enzymatic subunits, XPB, XPD, and cdk7. Mol Cell 3 : 87–95.
6. NouspikelT (2009) DNA repair in mammalian cells : Nucleotide excision repair: variations on versatility. Cell Mol Life Sci 66 : 994–1009.
7. VenemaJ, MullendersLH, NatarajanAT, van ZeelandAA, MayneLV (1990) The genetic defect in Cockayne syndrome is associated with a defect in repair of UV-induced DNA damage in transcriptionally active DNA. Proc Natl Acad Sci U S A 87 : 4707–4711.
8. FousteriM, VermeulenW, van ZeelandAA, MullendersLH (2006) Cockayne syndrome A and B proteins differentially regulate recruitment of chromatin remodeling and repair factors to stalled RNA polymerase II in vivo. Mol Cell 23 : 471–482.
9. BerneburgM, LoweJE, NardoT, AraujoS, FousteriMI, et al. (2000) UV damage causes uncontrolled DNA breakage in cells from patients with combined features of XP-D and Cockayne syndrome. EMBO J 19 : 1157–1166.
10. AndressooJO, MitchellJR, de WitJ, HoogstratenD, VolkerM, et al. (2006) An Xpd mouse model for the combined xeroderma pigmentosum/Cockayne syndrome exhibiting both cancer predisposition and segmental progeria. Cancer Cell 10 : 121–132.
11. MonteloneBA, MaloneRE (1994) Analysis of the rad3-101 and rad3-102 mutations of Saccharomyces cerevisiae: implications for structure/function of Rad3 protein. Yeast 10 : 13–27.
12. GolinJE, EspositoMS (1977) Evidence for joint genic control of spontaneous mutation and genetic recombination during mitosis in Saccharomyces. Mol Gen Genet 150 : 127–135.
13. MaloneRE, HoekstraMF (1984) Relationships between a hyper-rec mutation (REM1) and other recombination and repair genes in yeast. Genetics 107 : 33–48.
14. MonteloneBA, HoekstraMF, MaloneRE (1988) Spontaneous mitotic recombination in yeast: the hyper-recombinational rem1 mutations are alleles of the RAD3 gene. Genetics 119 : 289–301.
15. Moriel-CarreteroM, AguileraA (2010) A postincision-deficient TFIIH causes replication fork breakage and uncovers alternative Rad51 - or Pol32-mediated restart mechanisms. Mol Cell 37 : 690–701.
16. WinklerGS, SugasawaK, EkerAP, de LaatWL, HoeijmakersJH (2001) Novel functional interactions between nucleotide excision DNA repair proteins influencing the enzymatic activities of TFIIH, XPG, and ERCC1-XPF. Biochemistry 40 : 160–165.
17. FanL, FussJO, ChengQJ, ArvaiAS, HammelM, et al. (2008) XPD helicase structures and activities: insights into the cancer and aging phenotypes from XPD mutations. Cell 133 : 789–800.
18. TheronT, FousteriMI, VolkerM, HarriesLW, BottaE, et al. (2005) Transcription-associated breaks in xeroderma pigmentosum group D cells from patients with combined features of xeroderma pigmentosum and Cockayne syndrome. Mol Cell Biol 25 : 8368–8378.
19. NaumovskiL, ChuG, BergP, FriedbergEC (1985) RAD3 gene of Saccharomyces cerevisiae: nucleotide sequence of wild-type and mutant alleles, transcript mapping, and aspects of gene regulation. Mol Cell Biol 5 : 17–26.
20. TesteMA, DuquenneM, FrancoisJM, ParrouJL (2009) Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Mol Biol 10 : 99.
21. MoneMJ, VolkerM, NikaidoO, MullendersLH, van ZeelandAA, et al. (2001) Local UV-induced DNA damage in cell nuclei results in local transcription inhibition. EMBO Rep 2 : 1013–1017.
22. HoogstratenD, NiggAL, HeathH, MullendersLH, van DrielR, et al. (2002) Rapid switching of TFIIH between RNA polymerase I and II transcription and DNA repair in vivo. Mol Cell 10 : 1163–1174.
23. GiannattasioM, LazzaroF, LongheseMP, PlevaniP, Muzi-FalconiM (2004) Physical and functional interactions between nucleotide excision repair and DNA damage checkpoint. EMBO J 23 : 429–438.
24. MariniF, NardoT, GiannattasioM, MinuzzoM, StefaniniM, et al. (2006) DNA nucleotide excision repair-dependent signaling to checkpoint activation. Proc Natl Acad Sci U S A 103 : 17325–17330.
25. SchultzLB, ChehabNH, MalikzayA, HalazonetisTD (2000) p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J Cell Biol 151 : 1381–1390.
26. TaylorEM, BroughtonBC, BottaE, StefaniniM, SarasinA, et al. (1997) Xeroderma pigmentosum and trichothiodystrophy are associated with different mutations in the XPD (ERCC2) repair/transcription gene. Proc Natl Acad Sci U S A 94 : 8658–8663.
27. de FeraudyS, RevetI, BezrookoveV, FeeneyL, CleaverJE (2010) A minority of foci or pan-nuclear apoptotic staining of gammaH2AX in the S phase after UV damage contain DNA double-strand breaks. Proc Natl Acad Sci U S A 107 : 6870–6875.
28. Moriel-CarreteroM, AguileraA (2010) Replication fork breakage and re-start: New insights into Rad3/XPD-associated deficiencies. Cell Cycle 9 : 2958–2962.
29. WolskiSC, KuperJ, KiskerC (2010) The XPD helicase: XPanDing archaeal XPD structures to get a grip on human DNA repair. Biol Chem 391 : 761–765.
30. SongJM, MonteloneBA, SiedeW, FriedbergEC (1990) Effects of multiple yeast rad3 mutant alleles on UV sensitivity, mutability, and mitotic recombination. J Bacteriol 172 : 6620–6630.
31. GodonC, MourguesS, NonnekensJ, MourcetA, CoinF, et al. (2012) Generation of DNA single-strand displacement by compromised nucleotide excision repair. EMBO J 31 : 3550–3563.
32. Velez-CruzR, ZadorinAS, CoinF, EglyJM (2013) Sirt1 suppresses RNA synthesis after UV irradiation in combined xeroderma pigmentosum group D/Cockayne syndrome (XP-D/CS) cells. Proc Natl Acad Sci U S A 110: E212–220.
33. SheffMA, ThornKS (2004) Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast 21 : 661–670.
34. LisbyM, MortensenUH, RothsteinR (2003) Colocalization of multiple DNA double-strand breaks at a single Rad52 repair centre. Nat Cell Biol 5 : 572–577.
35. HechtA, GrunsteinM (1999) Mapping DNA interaction sites of chromosomal proteins using immunoprecipitation and polymerase chain reaction. Methods Enzymol 304 : 399–414.
36. YuY, TengY, LiuH, ReedSH, WatersR (2005) UV irradiation stimulates histone acetylation and chromatin remodeling at a repressed yeast locus. Proc Natl Acad Sci U S A 102 : 8650–8655.
37. GaillardH, WellingerRE, AguileraA (2009) Methods to study transcription-coupled repair in chromatin. Methods Mol Biol 523 : 141–159.
38. SantocanaleC, DiffleyJF (1998) A Mec1 - and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 395 : 615–618.
Štítky
Genetika Reprodukčná medicína
Článek Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart DiseaseČlánek Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the NicheČlánek Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial TetheringČlánek Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature SeasonalityČlánek Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell DivisionsČlánek Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity inČlánek Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration inČlánek ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression inČlánek Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex EnvironmentsČlánek The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 12- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Stratification by Smoking Status Reveals an Association of Genotype with Body Mass Index in Never Smokers
- Genome Wide Meta-analysis Highlights the Role of Genetic Variation in in the Regulation of Circulating Serum Chemerin
- Occupancy of Mitochondrial Single-Stranded DNA Binding Protein Supports the Strand Displacement Mode of DNA Replication
- Distinct Genealogies for Plasmids and Chromosome
- Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart Disease
- Non-coding RNAs Prevent the Binding of the MSL-complex to Heterochromatic Regions
- Plasmid Flux in ST131 Sublineages, Analyzed by Plasmid Constellation Network (PLACNET), a New Method for Plasmid Reconstruction from Whole Genome Sequences
- Epigenome-Guided Analysis of the Transcriptome of Plaque Macrophages during Atherosclerosis Regression Reveals Activation of the Wnt Signaling Pathway
- The Inventiveness of Nature: An Interview with Werner Arber
- Mediation Analysis Demonstrates That -eQTLs Are Often Explained by -Mediation: A Genome-Wide Analysis among 1,800 South Asians
- Generation of Antigenic Diversity in by Structured Rearrangement of Genes During Mitosis
- A Massively Parallel Pipeline to Clone DNA Variants and Examine Molecular Phenotypes of Human Disease Mutations
- Genetic Analysis of the Cardiac Methylome at Single Nucleotide Resolution in a Model of Human Cardiovascular Disease
- Genetic Analysis of Circadian Responses to Low Frequency Electromagnetic Fields in
- The Dissection of Meiotic Chromosome Movement in Mice Using an Electroporation Technique
- Altered Chromatin Occupancy of Master Regulators Underlies Evolutionary Divergence in the Transcriptional Landscape of Erythroid Differentiation
- Syd/JIP3 and JNK Signaling Are Required for Myonuclear Positioning and Muscle Function
- Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the Niche
- Mutation of Leads to Blurred Tonotopic Organization of Central Auditory Circuits in Mice
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- RAN-Binding Protein 9 is Involved in Alternative Splicing and is Critical for Male Germ Cell Development and Male Fertility
- Enhanced Longevity by Ibuprofen, Conserved in Multiple Species, Occurs in Yeast through Inhibition of Tryptophan Import
- Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial Tethering
- Recurrent Loss of Specific Introns during Angiosperm Evolution
- Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature Seasonality
- SEEDSTICK is a Master Regulator of Development and Metabolism in the Arabidopsis Seed Coat
- Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell Divisions
- Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity in
- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression in
- Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex Environments
- Global Analysis of Photosynthesis Transcriptional Regulatory Networks
- Mucolipin Co-deficiency Causes Accelerated Endolysosomal Vacuolation of Enterocytes and Failure-to-Thrive from Birth to Weaning
- Controlling Pre-leukemic Thymocyte Self-Renewal
- How Malaria Parasites Avoid Running Out of Ammo
- Echoes of the Past: Hereditarianism and
- Deep Reads: Strands in the History of Molecular Genetics
- Keep on Laying Eggs Mama, RNAi My Reproductive Aging Blues Away
- Analysis of a Plant Complex Resistance Gene Locus Underlying Immune-Related Hybrid Incompatibility and Its Occurrence in Nature
- Epistatic Adaptive Evolution of Human Color Vision
- Increased and Imbalanced dNTP Pools Symmetrically Promote Both Leading and Lagging Strand Replication Infidelity
- Genetic Basis of Haloperidol Resistance in Is Complex and Dose Dependent
- Genome-Wide Analysis of DNA Methylation Dynamics during Early Human Development
- Interaction between Conjugative and Retrotransposable Elements in Horizontal Gene Transfer
- The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
- is Required for Adult Maintenance of Dopaminergic Neurons in the Ventral Substantia Nigra
- PRL1, an RNA-Binding Protein, Positively Regulates the Accumulation of miRNAs and siRNAs in Arabidopsis
- Genetic Control of Contagious Asexuality in the Pea Aphid
- Early Mesozoic Coexistence of Amniotes and Hepadnaviridae
- Local and Systemic Regulation of Plant Root System Architecture and Symbiotic Nodulation by a Receptor-Like Kinase
- Gene Pathways That Delay Reproductive Senescence
- The Evolution of Fungal Metabolic Pathways
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- Formation of Linear Amplicons with Inverted Duplications in Requires the MRE11 Nuclease
- Identification of Rare Causal Variants in Sequence-Based Studies: Methods and Applications to , a Gene Involved in Cohen Syndrome and Autism
- Rrp12 and the Exportin Crm1 Participate in Late Assembly Events in the Nucleolus during 40S Ribosomal Subunit Biogenesis
- The Mutations in the ATP-Binding Groove of the Rad3/XPD Helicase Lead to -Cockayne Syndrome-Like Phenotypes
- Topoisomerase I Plays a Critical Role in Suppressing Genome Instability at a Highly Transcribed G-Quadruplex-Forming Sequence
- A Cbx8-Containing Polycomb Complex Facilitates the Transition to Gene Activation during ES Cell Differentiation
- Transcriptional Frameshifting Rescues Type VI Secretion by the Production of Two Length Variants from the Prematurely Interrupted Gene
- Association Mapping across Numerous Traits Reveals Patterns of Functional Variation in Maize
- Genome-Wide Analysis of -Regulated and Phased Small RNAs Underscores the Importance of the ta-siRNA Pathway to Maize Development
- Dissemination of Cephalosporin Resistance Genes between Strains from Farm Animals and Humans by Specific Plasmid Lineages
- The Tau Tubulin Kinases TTBK1/2 Promote Accumulation of Pathological TDP-43
- Germline Signals Deploy NHR-49 to Modulate Fatty-Acid β-Oxidation and Desaturation in Somatic Tissues of
- Microevolution of in Macrophages Restores Filamentation in a Nonfilamentous Mutant
- Vangl2-Regulated Polarisation of Second Heart Field-Derived Cells Is Required for Outflow Tract Lengthening during Cardiac Development
- Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice
- A ABC Transporter Regulates Lifespan
- RA and FGF Signalling Are Required in the Zebrafish Otic Vesicle to Pattern and Maintain Ventral Otic Identities
- , and Reprogram Thymocytes into Self-Renewing Cells
- The miR9863 Family Regulates Distinct Alleles in Barley to Attenuate NLR Receptor-Triggered Disease Resistance and Cell-Death Signaling
- Detection of Pleiotropy through a Phenome-Wide Association Study (PheWAS) of Epidemiologic Data as Part of the Environmental Architecture for Genes Linked to Environment (EAGLE) Study
- Extensive Copy-Number Variation of Young Genes across Stickleback Populations
- The and Genetic Modules Interact to Regulate Ciliogenesis and Ciliary Microtubule Patterning in
- Analysis of the Genome, Transcriptome and Secretome Provides Insight into Its Pioneer Colonization Strategies of Wood
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- Echoes of the Past: Hereditarianism and
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



