-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
Host-directed therapeutics (HDTs) for infectious diseases target cellular mechanisms used by pathogens to move into, through, or out of cells. The Abl tyrosine kinase (TK) inhibitor and cancer therapeutic imatinib mesylate (Gleevec), for example, has activity against bacterial and viral pathogens via effects on pathogen entry (polyomaviruses), intracellular transit (Mycobacteria) and exit (poxviruses and filoviruses). Other HDTs target the host immune system by suppressing or activating circulating innate and adaptive cells. Here we report that imatinib at doses that are effective in clearing Mycobacterial infections but which are 10-fold lower than those used for cancer, mimics a physiological innate response to infection in the bone marrow, called the “emergency response,” in which hematopoietic stem cells and multipotent progenitors expand and differentiate into mature myeloid cells that migrate to peripheral sites. Imatinib effects occur in part via partial inhibition of c-Kit, suggesting a mechanism by which c-Kit controls the earliest stages of hematopoiesis. Mimicking a physiological antimicrobial response may make imatinib broadly useful. Accordingly, imatinib also has efficacy against infections caused by Franciscella spp., which do not use imatinib-sensitive TKs for pathogenesis. These observations identify myelopoiesis as an important target for HDTs, and provide information on how to dose imatinib for clinical use.
Published in the journal: Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity. PLoS Pathog 11(3): e32767. doi:10.1371/journal.ppat.1004770
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004770Summary
Host-directed therapeutics (HDTs) for infectious diseases target cellular mechanisms used by pathogens to move into, through, or out of cells. The Abl tyrosine kinase (TK) inhibitor and cancer therapeutic imatinib mesylate (Gleevec), for example, has activity against bacterial and viral pathogens via effects on pathogen entry (polyomaviruses), intracellular transit (Mycobacteria) and exit (poxviruses and filoviruses). Other HDTs target the host immune system by suppressing or activating circulating innate and adaptive cells. Here we report that imatinib at doses that are effective in clearing Mycobacterial infections but which are 10-fold lower than those used for cancer, mimics a physiological innate response to infection in the bone marrow, called the “emergency response,” in which hematopoietic stem cells and multipotent progenitors expand and differentiate into mature myeloid cells that migrate to peripheral sites. Imatinib effects occur in part via partial inhibition of c-Kit, suggesting a mechanism by which c-Kit controls the earliest stages of hematopoiesis. Mimicking a physiological antimicrobial response may make imatinib broadly useful. Accordingly, imatinib also has efficacy against infections caused by Franciscella spp., which do not use imatinib-sensitive TKs for pathogenesis. These observations identify myelopoiesis as an important target for HDTs, and provide information on how to dose imatinib for clinical use.
Introduction
Signaling by protein tyrosine kinases (PTKs) mediates a variety of cellular processes including migration, morphogenesis, stress responses, and cytoskeletal reorganization [1,2]. Dysregulation of PTK activity causes a variety of diseases, including cancer. One such cancer, chronic myelogenous leukemia (CML), is associated with a characteristic translocation between chromosomes 9 and 22, called the “Philadelphia chromosome (Ph),” which encodes a fusion protein composed of breakpoint cluster region (BCR) protein and the PTK Abl1, called BCR-ABL[3]. Expression of BCR-ABL in hematopoietic stem cells (HSCs) results in aberrant proliferation of Ph+ stem cells and the accumulation of myeloid cells in the bone marrow and blood.
Over the last decade, development of small molecule inhibitors of Abl1 and BCR-ABL, such as imatinib mesylate (imatinib, Gleevec), have dramatically reduced mortality rates in patients with CML and related cancers [4–8]. Imatinib selectively kills Ph+ myeloid lineage cells in the bone marrow and periphery, whose survival depends on expression of BCR-ABL. However, the drug does not appear to affect survival of Ph+ hematopoietic stem cells (HSCs), nor of Ph- cells [9].
Imatinib inhibits several other structurally related PTKs in a dose-dependent manner [10–13]. Nanomolar concentrations of imatinib inhibit c-Abl1 and c-Abl2, platelet-derived growth factor receptor alpha (PDGFRα) and beta (PDGFRβ), and the stem cell receptor (c-Kit), whereas micromolar concentrations inhibit macrophage colony-stimulating factor receptor (m-CSFR or c-fms)[13]. Accordingly, imatinib also has efficacy against gastrointestinal stromal tumors (GISTs), which are caused by dysregulated c-Kit or PDGFRα [14].
Various pathogens utilize activation of Abl1 and related PTKs to facilitate intracellular survival, intracellular trafficking, and spread from cell to cell [15]. These include diarrheagenic Escherichia coli, Pseudomonas, Salmonella, Shigella, Helicobacter, Anaplasma, Chlamydia, and pathogenic mycobacteria amongst bacteria, and filoviruses, HIV, Coxsackie virus, Kaposi sarcoma virus, Polyomaviruses, and orthopoxviruses amongst viruses, as well as the human parasite Leishmania [16–33]. For pathogenic mycobacteria including Mycobacterium tuberculosis (Mtb) and Mycobacterium marinum (Mm), imatinib enhances trafficking of the bacteria into acidified vesicles [30,33], whereas for orthopoxviruses, the drug prevents Abl-dependent dissemination of the virus [31,32].
In contrast to the wealth of information on the role of PTKs in cancer and microbial pathogenesis, information on how PTK inhibitors function in vivo remains more limited. Historically, the therapeutic effects of imatinib have been attributed to its cell autonomous effects on tumor cells expressing oncogenic kinases, or to its inhibition of cellular kinases and pathogenesis in infected cells. However, recent evidence suggests that imatinib also regulates the immune response. Imatinib inhibits T cell signaling in vitro, and reportedly causes immunosuppression and even neutropenia in some patients, especially at high doses [34]. By contrast, other data suggests that the therapeutic effect of imatinib may actually require the immune system. Thus, imatinib remains effective in vivo even against engrafted GIST cells that are unresponsive to the drug in vitro, an effect attributed to stimulation of cross talk between dendritic cells and natural killer cells, which have attendant anti-tumor activity [35]. Moreover, recent reports indicate that imatinib relieves c-Kit-dependent immunosuppression by regulatory T cells, which in turn potentiate anti-tumor CD8+ T cell responses [36].
Such immunostimulatory effects may be important for infectious diseases as well. We measured the effects of imatinib on innate immune responses, particularly the increased numbers of myeloid cells typically seen following bacterial infection. We demonstrate that low doses of imatinib activate myelopoiesis in the bone marrow and increase the number of myeloid cells in the bone marrow, blood, and spleen, which enhances a physiological antimicrobial response to infection.
Results
Imatinib induces expansion of myeloid cells in spleen and blood
Previous studies have shown that imatinib maximally reduces mycobacterial load in mice when administered at 66mg/kg/d, whereas higher doses (e.g. 200mg/kg/d) proved much less effective [30]. Doses of 66mg/kg/d resulted in steady state serum levels of 57+/-21ng/ml (~100nM) in mice. Such serum levels would be considered sub-therapeutic in humans, where doses of 400mg QD result in serum levels of ~1500 to ~3000 ng/ml (2–5 μM)[37,38]. To characterize the effect of imatinib at a dose of 66mg/kg/d on the composition of immune cells, the drug was delivered to uninfected mice or those infected with Mm beginning one day prior to infection and continuing for the duration of the experiment. Blood and spleens were harvested seven days after infection and lymphoid, myeloid and dendritic cell (DC) populations enumerated by flow cytometry (Figs. 1A,B; S1A-S1C). Imatinib significantly increased the number of myeloid-derived cells in the blood and spleen in both infected and uninfected animals. No difference in cell numbers was evident in animals left untreated or treated with the carrier (water). The number of neutrophils, defined as CD11b+ Ly6Cint Gr-1hi SSCint, increased on average by 14-fold in blood, and 22-fold in spleen in imatinib-treated mice compared to uninfected untreated controls (Fig. 1A; representative plots in S1B Fig.). Infection with Mm alone increased neutrophil numbers by ~3 and 6-fold in the blood and spleen, respectively. Neutrophil numbers in animals treated with imatinib and infected increased by 15 - and 25-fold in blood and spleen, respectively, compared to numbers observed in uninfected controls (Figs. 1A; S1B). Monocytes, eosinophils, natural killer (NK) cells, and CD8− DCs likewise increased in number following imatinib treatment with or without infection, though to a lesser extent than neutrophils (Figs. 1A,B; S1C). Imatinib also increased numbers of CD8+DCs in the spleen compared to uninfected controls (S1C Fig). By contrast, imatinib produced no change in the numbers of T cells or B cells (Fig. 1B). Thus, imatinib caused a large-scale increase in cells of the myeloid lineage, as well as changes in CD8+ DCs and NK cells.
Fig. 1. Imatinib treatment induces expansion of myeloid cells in mouse spleen and blood. 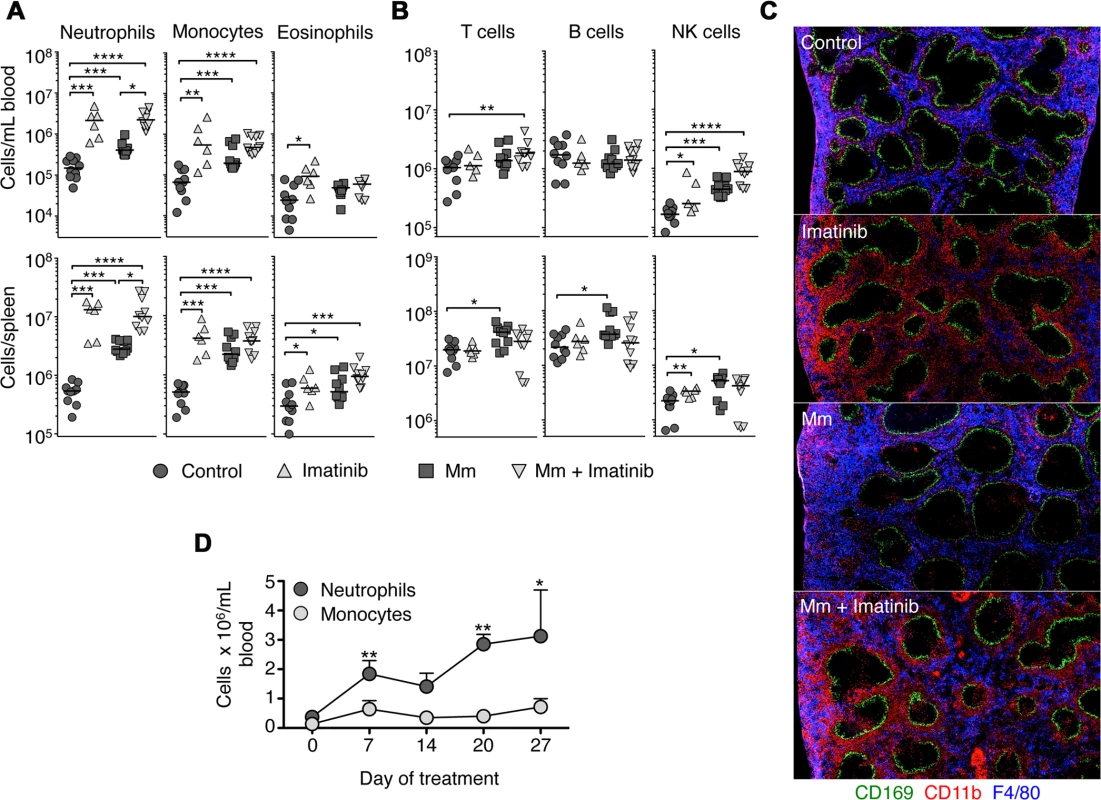
C57Bl/6 mice were administered imatinib at 66mg/kg/day (Imatinib) or left untreated (Control). For some experiments, mice were either injected in the tail vein with 105 CFU Mm 1218R one day after administration of drug (Mm + imatinib) or carrier (Mm). Neutrophils (CD11b+ Ly6Cint Gr-1hi SSCint), monocytes (CD11b+ Ly6Chi Gr-1int SSClow and F4/80+) and eosinophil (CD11b+ Ly6Clo Gr-1int SSChi and F4/80+) (A), or T cells (Thy1.2+), B cells (B220+ CD19+) and NK cells (NK1.1+) (B) were enumerated by flow cytometry in the blood (top panel) or spleen (bottom panel) at d7 post infection or treatment. Each symbol represents one mouse. Data shown are representative of six independent experiments. (C) Sections of spleens taken from mice left untreated or treated with imatinib at 66mg/kg/day plus or minus infection with Mm. Sections were stained with anti-CD169 to recognize marginal zone macrophages (green), anti-F4/80 to recognize red pulp myeloid cells (blue), and anti-CD11b to recognize neutrophils and monocytes (red). (D) C57Bl/6 mice were administered imatinib at 66 mg/kg/day for 28 days. On day 0, 7, 14, 21, 28 of treatment, mice were bled from the tail vein and numbers of neutrophils and monocytes enumerated by flow cytometry (three mice per time point). Data shown are from two independent experiments. For all data sets, the line in each group of data points represents the median. For A, and B, a Mann-Whitney test was used for pairwise comparisons (e.g. +/- drug or +/- infection only), and a Kruskal Wallis test for multiple comparisons across groups. For all panels, p values less than 0.05 were considered significant; p values < 0.05 are denoted by *, values <.001 by **, and values <.0001 by ***, and values <.00005 by ****. To validate the expansion of the myeloid compartment, histological sections of spleens were analyzed seven days after imatinib treatment and/or infection with Mm. As shown in Fig. 1C, the splenic architecture was largely maintained with drug treatment or infection, with CD169+ marginal zone macrophages (green) clearly demarcating lymphocyte areas from F4/80+ CD11b+ red pulp (blue, Fig. 1C). However, the expansion of myeloid cells following imatinib treatment was clearly evident, with accumulation of CD11b+ cells evident (red, Fig. 1C), particularly in the areas of the red pulp adjacent to the marginal zones.
To determine whether the number of myeloid cells with imatinib transiently increased, uninfected mice were treated with drug for up to 27 days, the longest time tested, and the numbers of immune cells in the blood determined at weekly intervals. Increases in numbers of neutrophils and monocytes persisted for as long as the drug was administered, whereas the numbers of eosinophils, DCs, B cells and T cells did not significantly increase over this time period (e.g. Fig. 1D).
Imatinib increases number of myeloid cells in the bone marrow
We next determined whether the increased number of myeloid cells in blood and spleen seen at 66mg/kg/d resulted from increased production in the bone marrow. Giemsa staining of femurs from imatinib-treated mice revealed a pronounced increase in marrow cellularity (Fig. 2A). Moreover, centrifuged pellets of bone marrow from mice treated with imatinib appeared white, a hallmark of neutrophilia, compared to marrow from untreated mice, which was pink. The numbers of neutrophils and monocytes in bone marrow from imatinib-treated mice increased by 4 - and 3-fold, respectively (Fig. 2B; gating schema in S2A Fig). The numbers of mature B cells, T cells, DCs, eosinophils, and NK cells in the bone marrow did not change significantly upon treatment with imatinib (Figs. 2B; S2B). Thus, accumulation of mature myeloid cells in the marrow was positively correlated with increases in mature cells in the blood and spleen at a dose of 66mg/kg/d. By contrast, with infection the number of mature myeloid cells in the bone marrow did not significantly increase with or without imatinib. Because increases in numbers of mature cells were evident in blood and peripheral tissues (Fig. 1), we surmise that infection augmented migration of mature cells from the bone marrow.
Fig. 2. Effects of imatinib on neutrophils and monoctyes in bone marrow. 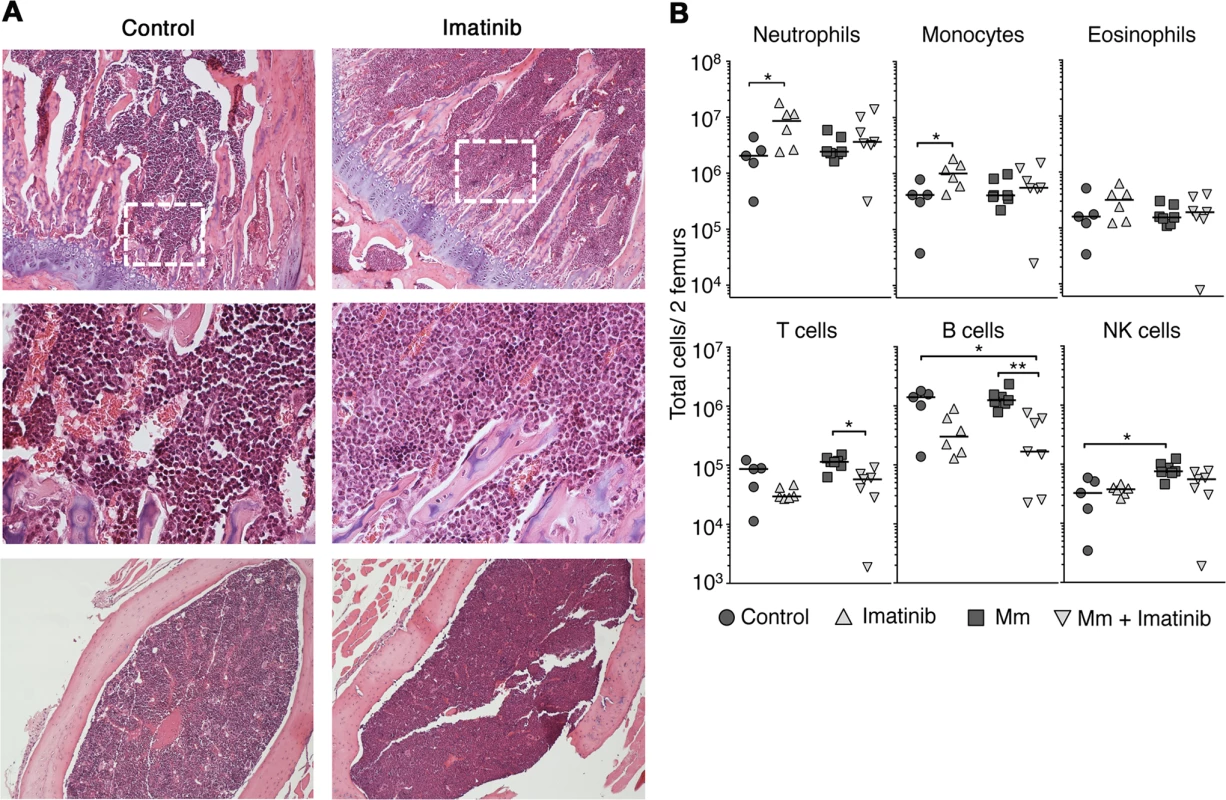
(A) Geimsa staining of femurs from C57Bl/6 mice administered imatinib at 66 mg/kg/d for seven days or left untreated as indicated. Top panels: coronal sections (10X). Middle panels: Insets (40x) derived from the area denoted by a dashed white box. Bottom panels: sagittal sections (10x). (B) C57Bl/6 mice were administered imatinib at 66 mg/kg/d for seven days or left untreated, as indicated. Beginning 24h after onset of drug, mice were either injected in the tail vein with 105 CFU Mm 1218R or left uninfected. At seven days post-treatment bone marrow was collected from femurs. Data shown are representative of three independent experiments. For all data sets, the line in each group of data points represents the median. A Mann-Whitney test was used for pairwise comparisons, and a Kruskal Wallis test for multiple comparisons. Imatinib increases numbers of multipotent progenitors in the bone marrow
The bone marrow is the primary site of post-natal hematopoiesis, where dormant hematopoietic stem cells (HSC) become activated [39,40] and sequentially differentiate into four identifiable multipotent progenitor cell types (MPP1-MPP4)[41], myeloid/lymphoid or erythroid/myeloid progenitors [42,43], and finally, into a variety of both immature cells and mature cells, which migrate out of the bone marrow. HSCs are phenotypically distinguished from more mature cells primarily based on their capacity for efficient immune reconstitution upon transplant into lethally irradiated animals [39,40].
We next assessed the effects of imatinib on numbers of neutrophil precursors, HSCs, and multipotent progenitors in bone marrow by flow cytometry. Despite increases in the numbers of mature neutrophils in the bone marrow, no effects of imatinib were evident on the number of neutrophil precursors, including promyelocytes, myelocytes, and metamyelocytes (S3A and S3B Fig). By contrast, the fraction of LineagenegSca1+c-Kit+ (LSK) cells [44], which include MPPs and HSCs, increased by 2-fold on average with imatinib treatment at 66 mg/kg/day, and to an even greater extent with infection (Figs. 3A; S4A and S4B). Notably accumulation of LSK cells evident with infection markedly decreased with imatinib treatment (Fig. 3A). Using markers defined by Wilson et al. [41] (S4A Fig), we next determined whether the observed increase in numbers of LSK cells was due to an effect on HSCs, MPP1, MPP2, MPP3, or MPP4 cells. As shown in Fig. 3B, the number of HSCs remained unchanged with imatinib alone at 66 mg/kg/day, but increased by up to 5-fold with Mm infection. Notably, reduced accumulation of HSCs in infected animals was evident upon treatment with imatinib. Numbers of MPP2, MPP3 and MPP4 cells increased with imatinib at 66 mg/kg/day, with the greatest accumulation compared to control animals evident with MPP3 and MPP4 cells (Fig. 3C). Infection alone produced an accumulation of MPP1, MPP2, MPP3, and MPP4 cells, and, as with HSCs, MPP1 and MPP2 cells accumulated to a lesser extent with infection plus imatinib compared to infection alone (Fig. 3C). Together, these data indicate that (i) imatinib at 66 mg/kg/day does not induce accumulation of HSCs, whereas infection does; (ii) imatinib nevertheless reduces accumulation of HSCs upon infection, suggesting that the drug may increase flux of HSCs to progenitors; and (iii) imatinib regulates accumulation of subsets of MPPs, an effect also evident with infection.
Fig. 3. Effects of Imatinib on HSCs and multipotent progenitors in vivo. 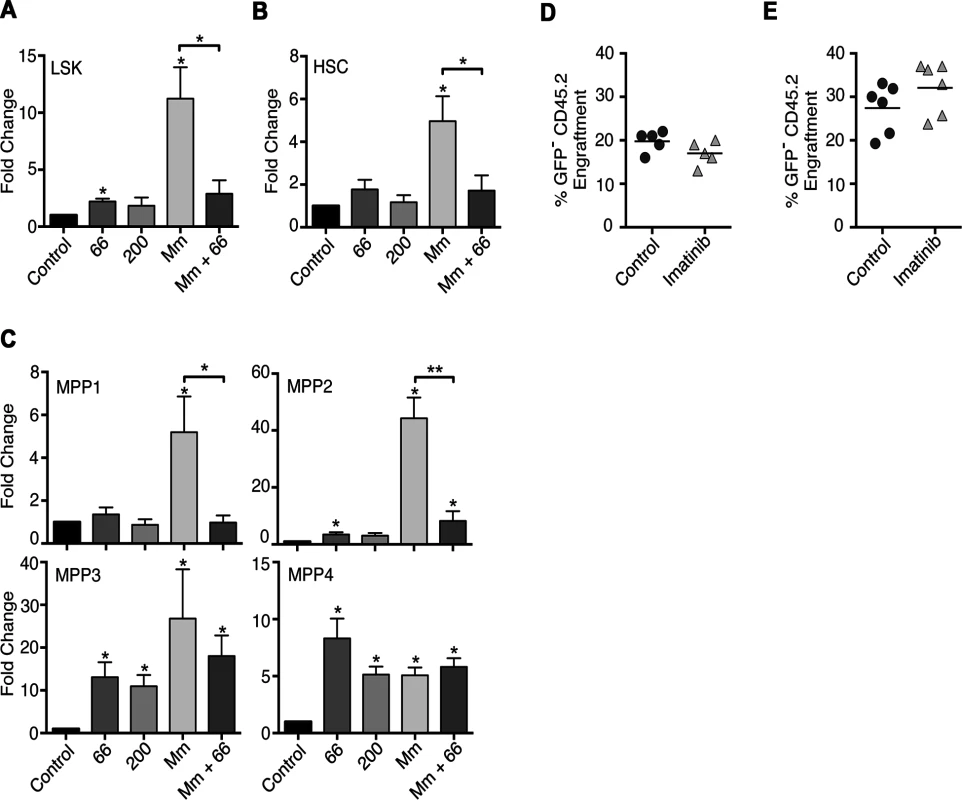
(A-C) C57Bl/6 mice were administered imatinib at 66 or 200mg/kg/d or left untreated. Beginning 24h post-treatment mice were either injected in the tail vein with 105 CFU Mm 1218R or left uninfected. Bone marrow was collected from femurs and HSCs and MPPs enumerated by flow cytometry[41]. (A-C) Effect of imatinib or infection on Linnegsca1+CD117+ (LSK) cells (A), HSCs (CD34−CD48−CD150+CD135−) (B) or MPP1 (CD34+CD48-CD150+CD135−), MPP2 (CD34+CD48+CD150+CD135−), MPP3 (CD34+CD48+CD150−CD135−) and MPP4 (CD34+CD48+CD150−CD135+) cells (C). Each bar represents the average fold change relative to control in 2–6 independent experiments with 3–6 animals per condition per experiment. An asterisk (*) above the error bar indicates statistical significance compared to control as determined by t-test. Statistical significance was also assessed for infected (Mm) versus infected, drug treated animals (Mm + imatinib) and is indicated by a bracket over the bars. (D-E) Competitive bone marrow transplants with whole marrow (D) or LSK cells (E) from control animals or animals treated with 66mg/kg/d imatinib for 7d. (D) Congenic CD45.1+ C57Bl/6 mice were lethally irradiated and transplanted with 2 x 106 whole bone marrow cells from untreated GFP+ C57Bl/6 donors mixed with 2 x 106 whole bone marrow cells from C57Bl/6 (CD45.2) donors that were either treated with imatinib for seven days or left untreated. Numbers of leukocytes derived from the different donor sources or the recipient mice were determined by flow cytometry at 4 months post transplant (n = 5 recipient mice per condition). (E) GFP+ C57Bl/6 marrow (3x105 cells) was mixed with 5x103 sorted LSK cells before transplantation, and donor chimerism was determined using blood drawn 4 months post-transplantation (n = 5 recipient mice per condition). A Mann-Whitney nonparametric test was used to determine significance. Imatinib does not increase numbers of transplantable HSCs
To determine whether imatinib caused an expansion of transplantable HSCs, we assessed the engraftment capacity of these cells in a competitive repopulation assay [45]. Marrow derived from control CD45.2 C57Bl/6 mice or CD45.2 C57Bl/6 mice treated with imatinib for seven days was mixed with marrow derived from untreated CD45.2 GFP+C57Bl/6 mice. Irradiated congenic CD45.1+ C57Bl/6 mice were then injected with either the control/GFP mixture or the imatinib/GFP mixture. At 4 and 12 months post-transplant, mice were bled and the relative numbers of blood cells derived from GFP-, naïve - or imatinib-treated donor mice, or recipient mice determined by flow cytometry. At all time points, the proportion of blood cells derived from naïve or imatinib-treated animals were similar, indicating a lack of competitive advantage of bone marrow from imatinib-treated mice (Fig. 3D), in accordance with the absence of a statistically significant accumulation of HSCs (Fig. 3B). Because HSCs represent less than ~0.003% of the total marrow [41], it remained possible that the competition assay with whole marrow might not resolve small differences in the HSC numbers between imatinib-treated or naive mice. To assess this possibility, competitive transplant experiments using just the LSK fraction from naïve or imatinib-treated animals were performed. As shown in Fig. 3E, at four months post transplant, proportions of mature cells derived from imatinib-treated mice were unchanged compared to their naïve counterparts. Together, these data suggest that imatinib does not cause an increase in the number of transplantable HSCs, in accordance with observations showing that HSCs do not accumulate upon treatment with the drug (Fig. 3B). However, these data do not rule out the possibility that imatinib increases the capacity of a fraction of activated HSCs to asymmetrically divide and rapidly differentiate into MPPs, a result suggested by data with infection plus imatinib (Fig. 3A-C).
Imatinib increases myeloid progenitors in mouse and human marrow
To further characterize the effects of imatinib on the expansion and differentiation of myeloid progenitors, bone marrow from imatinib-treated mice was plated in semi-solid media and analyzed by Colony Forming Cell (CFC) assay to detect and quantify colonies of granulocyte-macrophage hematopoietic progenitors (CFU-GM). As shown in Fig. 4A, bone marrow derived from mice treated with imatinib at 66mg/kg/d or 200 mg/kg/d for seven days yielded ~34% more CFU-GM colonies compared to marrow from naïve mice. These data suggest that treatment with imatinib induces an irreversible commitment of HSCs into progenitors that can differentiate ex vivo into myeloid cells.
Fig. 4. Effects of imatinib on progenitor differentiation in culture, and effects of anti-c-Kit neutralizing antibody. 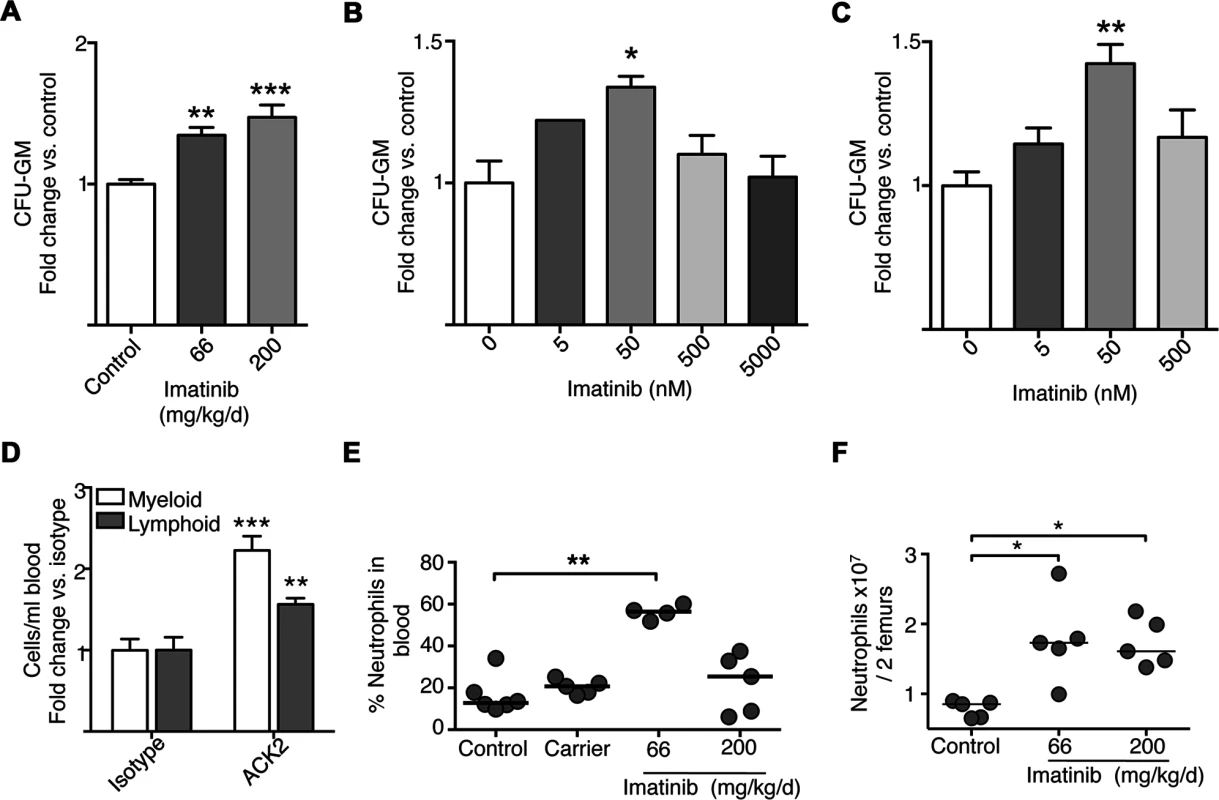
(A) Colony forming cell (CFC) assays with bone marrow derived from mice treated with imatinib at 66 or 200mg/kg/d for seven days or left untreated (Control). Bone marrow was cultured in the absence of imatinib, CFU-GM colonies were counted after 10 days. (B) CFC assays with bone marrow derived from naïve mice and cultured in the presence of increasing concentrations of imatinib. CFU-GM colonies were counted after 10 days. (C) CFC assays with human donor bone marrow cultured in the absence or presence of increasing concentrations of imatinib. CFU-GM colonies were counted after 14 days. All CFU results are presented as a percentage of untreated controls. Results represent combined data from three independent experiments with three replicates per experiment. A Kruskal-Wallis nonparametric test was used to determine significance of each concentration compared to control. (D) Effect of anti-c-Kit neutralizing antibody. 3μg of either anti-c-Kit neutralizing antibody (ACK2) or the IgG2b isotype control was administered for five to seven days, and the number of myeloid cells, including neutrophils, monocytes, and eosinophils, and lymphoid cells, including B cells and T cells and NK cells, in the blood determined by flow cytometry. Average fold changes compared to the isotype control on combined myeloid or lymphoid cell populations are presented. (E) Mice were treated with imatinib at 66 or 200mg/kg/day or with pumps filled with water for 28 days, the vehicle for imatinib. On day 7, mice were bled from the tail vein and numbers of neutrophils determined by flow cytometry with 6 mice per condition. For all data sets, the line in each group of data points represents the median. (F) Effects of dosage on neutrophil and monocyte numbers in bone marrow. Gleevec was administered at 66 or 200 mg/kg/d for 7 days without infection, and neutrophils were enumerated by flow cytometry. A Mann-Whitney nonparametric test was used to determine significance. Data shown are representative of three independent experiments. To determine whether cells from naïve animals could likewise be induced to differentiate into myeloid-type colonies when treated with imatinib in culture, CFC assays were performed on naïve bone marrow cultured with various concentrations of drug. As shown in Fig. 4B, addition of imatinib at 50 nM caused a 33% increase in CFU-GM, whereas concentrations exceeding 500 nM, were without effect. We also assessed the effects of PTK inhibitors in CFC assays using marrow derived from human donors. Addition of low concentrations of imatinib (50 nM) to the media maximally increased the number of CFU-GM by 42% compared to untreated marrow (Fig. 4C). By contrast, and in accordance with previous reports [46] concentrations at or exceeding 500nM were without effect. Together, these data suggest that imatinib induced an irreversible differentiation of HSCs or progenitors into myeloid cells in a dose-dependent fashion, and that imatinib effects on myelopoiesis in vivo are recapitulated in cultures of murine and human cells in vitro.
Partial inhibition of c-Kit affects myeloid and lymphoid cells in the blood. Previous studies suggested that administration of high concentrations (1mg) of the c-Kit neutralizing antibody ACK2 to C57Bl/6 mice ablated hematopoietic progenitors [47]. However, because the effects of imatinib appeared strongly dose-dependent, we reasoned that partial inhibition of c-Kit might recapitulate some or all effects of the drug. To test this hypothesis, we assessed the effects of ACK2 at lower concentrations (0.3ng, 3ng, 3μg and 30μg; Fig. 4D). Increased overall numbers of myeloid cells, including neutrophils, eosinophils and monocytes, were evident in the blood of mice treated with 3 μg of ACK2 (~2 fold on average), relative to an isotype control (Fig. 4D), though the effect was not to the same extent as that seen with imatinib. However, unlike imatinib, ACK2 also increased the numbers of lymphoid lineage cells by ~1.5 fold (Fig. 4D). We were unable to assess effects of ACK2 on numbers of MPPs directly because the antibody interfered with bone marrow staining panels. Nevertheless, low doses of ACK2, which may partially inhibit c-Kit kinase activity or reduce levels of c-Kit protein, appeared sufficient to induce expansion of leukocytes in the blood. Such an effect is consistent with an increase in myeloid and lymphoid progenitors. The observation that ACK2 affects both myeloid and lymphoid cells whereas imatinib predominantly affects myeloid cells suggests that inhibition of other kinases governs myeloid lineage commitment.
We also tested the effect of the ACK2 antibody in the context of infection. Although treatment with the antibody resulted in a ~2-fold decrease in CFU on average (S4C Fig). Although these data did not reach the 0.05 level of statistical significance, they “trended” in the right direction. We surmise that the limited efficacy is due to the fact that the antibody is not as efficient as the drug in inducing myelopoiesis.
Imatinib-induced expansion of myeloid cells depends on dose
We next assessed numbers of myeloid cells in the blood and bone marrow of uninfected mice treated with imatinib at 200mg/kg/d, a dose previously shown to have no anti-mycobacterial effects. As shown in Fig. 4E, whereas imatinib at 66mg/kg/d increased the percentage of neutrophils in the blood compared to untreated controls from 18% to 60% (see also Fig. 1), with 200mg/kg/d no such increase was evident; similar dosage effects were apparent with monocytes in the blood. Interestingly, in the bone marrow imatinib at 66 or 200mg/kg/d induced comparable increases in numbers of mature neutrophils and monocytes (Fig. 4F). Accordingly, bone marrow derived from mice treated with imatinib 200 mg/kg/d for seven days yielded ~40% more CFU-GM colonies compared to marrow from naïve mice, an increase comparable to that observed with marrow from animals treated with 66mg/kg/d (Fig. 4A). Likewise, comparable increases in numbers of LSKs, HSCs, and MPP2, MPP3 and MPP4 cells were evident with imatinib at doses of 66 and 200mg/kg/d (Fig. 3A-C). Thus, accumulation of mature myeloid cells in the marrow was positively correlated with increases in mature cells in the blood and spleen at 66mg/kg/d, but no such correlation was evident at doses of 200mg/kg/d or higher. Notably, infection plus 200mg/kg/d imatinib did not induce substantial increases in the numbers of myeloid cells in the blood and spleen beyond that seen with infection alone. Together, these data suggest that imatinib facilitates exodus of myeloid cells from the bone marrow only at lower doses, but inhibits this process at higher doses. Because doses of 66mg/kg/d have proven most effective in bacterial infection studies [30], our subsequent analysis focused on effects of the drug at this dose and not at higher doses.
Imatinib treatment alters CXCR2 expression on neutrophils in the bone marrow
Retention of neutrophils within the bone marrow is correlated with expression of CXCR4 on the cell surface [48], whereas migration of neutrophils into the blood and to peripheral sites is associated with surface expression of CXCR2 [48–50]. Treatment with imatinib at 66mg/kg/d increased the percentage of CXCR2hiCXCR4low neutrophils by 17% in uninfected mice and by 9% in infected mice, compared to control animals (Fig. 5A). Accordingly, imatinib increased the median fluorescence intensity (MFI) of CXCR2 on neutrophils by 1.8-fold and 1.5-fold in uninfected and infected mice, respectively (Fig. 5B). Thus, imatinib at 66mg/kg/d increased the surface expression of CXCR2 and the proportion of CXCR2hiCXCR4low neutrophils in the bone marrow. These data are consistent with an increased capacity of neutrophils to migrate from the bone marrow to the blood at 66mg/kg/d, and with the observed increase of neutrophils in the blood at this dose.
Fig. 5. Effects of Imatinib on CXCR2 expression, activation, and apoptosis in neutrophils. 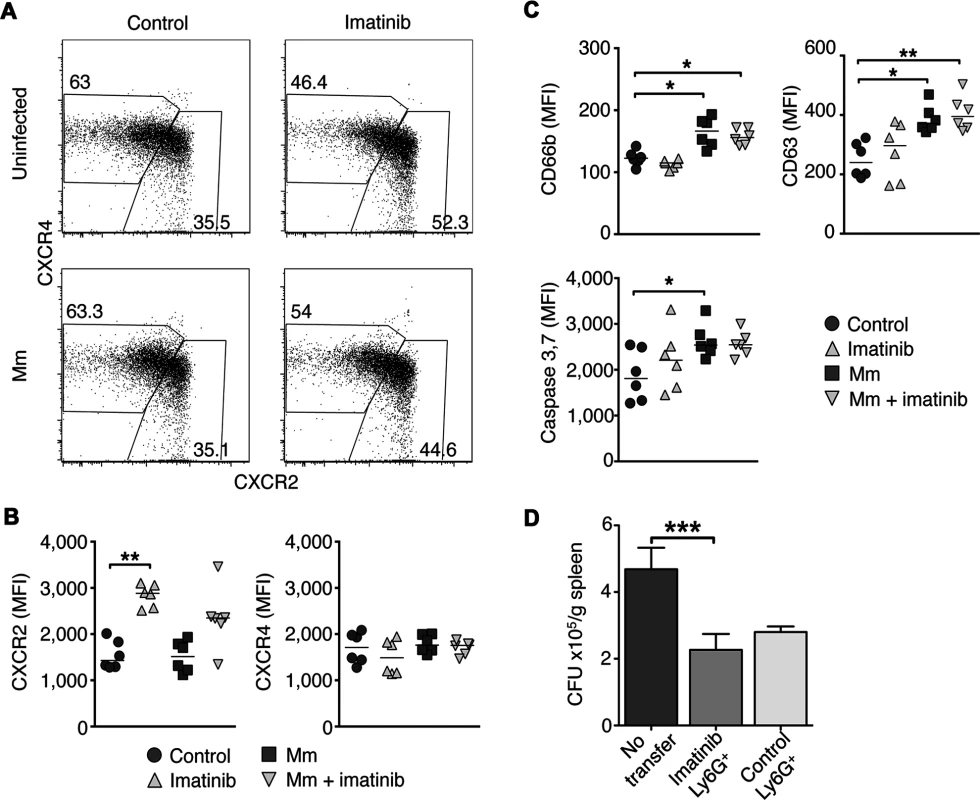
C57Bl/6 mice were administered imatinib at 66 mg/kg/d or left untreated. Beginning 24h post-treatment mice were either injected in the tail vein with 105 CFU Mm 1218R or left uninfected. At 7d post-treatment, bone marrow was collected from both femurs. (A) Representative flow cytometry plots of CXCR4 and CXCR2 expression on neutrophils from bone marrow using flow cytometry. CXCR2hiCXCR4lo and CXCR4hiCXCR2lo subsets were identified, and frequencies of total neutrophils are displayed in boxes. (B) Median fluorescence intensity (MFI) of CXCR2 and CXCR4 on neutrophils from bone marrow. Cumulative data from two independent experiments are presented with six mice per condition. The line in each data set represents the median. (C) C57Bl/6 mice were treated as in A. Activation status was assessed by surface expression of CD66b (secondary granules), CD63 (primary granules) and apoptosis by intracellular staining for caspase 3 and 7 activity of the total neutrophils (Ly6G+Ly6C+) from spleen. Cumulative data from two independent experiments are presented with 6 mice per condition. (D) C57Bl/6 mice were administered imatinib at 66mg/kg/d or left untreated. On d7, splenic neutrophils were isolated using Ly6G+ microbeads and 4 x106 cells were injected via the left tail vein into each naïve recipient mice, and infected via the right tail vein with 105 CFU Mm. Forty eight hours later, spleens were harvested and CFU/gram were determined. CFU/g spleen from mice injected with carrier, or with neutrophils from imatinib-treated mice or mice treated with carrier (water). Cumulative data from three independent experiments are presented with 15–25 animals per condition. The line represents the median. A Mann-Whitney test was used for pairwise comparisons, and a Kruskal Wallis test for multiple comparisons. Neutrophils from imatinib-treated mice are not intrinsically activated, but become activated and undergo apoptosis in response to Mm infection
Proinflammatory stimuli cause neutrophils to become activated and mobilize secondary and primary granules, which fuse with the plasma membrane and release antimicrobial compounds [51]. Neutrophil degranulation can be quantified by the surface expression of CD66b, a marker for secondary granules, or CD63, a marker for primary granules (Fig. 5C). In naïve mice, imatinib at 66 mg/kg/d did not alter the surface expression of CD66b or CD63 on neutrophils in the bone marrow, blood or spleen, indicating that, although the neutrophil numbers increased, the neutrophils were not activated (Figs. 5C and S5A). However, in the context of a Mm infection, neutrophils from control animals or animals treated with imatinib displayed increased surface expression of CD66b and CD63 relative to uninfected controls (Fig. 5C). This effect was most pronounced in the spleen, the site of the greatest concentration of bacteria [30]. Together, these data suggest that neutrophils are not activated by imatinib at low doses, but retain the capacity to become so upon infection.
Following activation or phagocytosis, neutrophils undergo apoptosis [52], which is characterized by cleavage of pro-caspase-3 or -7 (caspase-3/7) into enzymatically active forms [53]. Neutrophils from mice treated with imatinib at 66 mg/kg/d did not display significant differences in levels of active caspase-3/7 compared to untreated mice (Figs. 5C; S5A). However, neutrophils in the spleens of Mm infected mice showed significantly elevated levels of caspase-3 and 7 whether treated with imatinib or not (Figs. 5C; S5A), in accordance with reports that neutrophils become activated in response to mycobacterial infection [52]. These data indicate that imatinib does not alter apoptosis in activated neutrophils.
Increased numbers of neutrophils are sufficient to reduce Mm bacterial load
To determine whether an increase in neutrophil numbers alone was sufficient to reduce bacterial load following infection with Mm, adoptive transfer experiments were performed. Neutrophils were purified from either control or imatinib-treated animals. Recipient mice were then injected with 4x106 neutrophils derived from either control or imatinb-treated animals, and then infected with Mm. This number of transferred neutrophils represented less than half that found in the spleens of animals treated with imatinib; however, levels equivalent to that with drug could not be achieved because purifying larger numbers proved unfeasible. Bacterial load in infected organs was assessed 48h after adoptive transfer and infection. As shown in Fig. 5D, increasing the overall number of neutrophils, whether derived from PBS - or imatinib-treated mice, reduced CFUs in the spleen by ~2-fold, and no significant difference was evident between neutrophils derived from control or imatinib-treated animals. Thus, increasing neutrophil numbers alone is sufficient to reduce mycobacterial load, and imatinib does not augment the specific killing capacity of neutrophils. Notably, other myeloid cell types that increase in number with imatinib may also contribute to the observed reduction in CFU. Moreover, despite administration of sufficient anti-Ly6G antibody (1A8; [54]) to saturate binding sites on neutrophils in imatinib-treated mice, only 30–40% of the neutrophils could be depleted (S5B and S5C Figs). These data suggest that administration of high concentrations of 1A8 under neutrophilic conditions saturates cellular removal mechanisms. Because such mechanisms may also contribute to removal of infected cells, and because only a fraction of the cells can be depleted, using such methods to evaluate the role of neutrophils in imatinib-mediated reduction of CFUs has proven untenable.
Imatinib reduces bacterial load in mice infected with pathogenic Francisella species
Myeloid cells, and particularly neutrophils, are required to contain infections caused by a variety of pathogenic bacteria [55–57]. The observation that imatinib dramatically increased myeloid cell numbers led us to ask whether the drug might be effective against other bacterial infections, which, unlike mycobacteria [30,33], do not utilize Abl or other imatinib-sensitive kinases for pathogenesis. Growth and intracellular survival of the Francisella species F. novicida (Fn) and F. holarctica (LVS, the live vaccine strain), in either broth or in macrophages remained insensitive to imatinib (S6A-S6D Fig). Because these bacterial strains are lethal in mice within a few days of infection, imatinib was provided at 66 mg/kg/d for one week prior to infection with Fn or LVS, and throughout the course of infection (48hrs for Fn and 5 days for LVS). Imatinib reduced Fn and LVS CFU in the spleen and skin of infected animals by up to 10-fold compared to untreated animals (Fig. 6A,B). In addition, pathology at the site of infection with LVS was assessed. Lesions in mice treated with imatinib were either reduced in size or absent compared to controls (Fig. 6C,D). Imatinib was likewise effective against F. tularensis (Ft), reducing CFUs in blood and spleen by on average 8-fold and 15-fold, respectively (Fig. 6E). By contrast, imatinib at a dose of 200mg/kg/d was without effect on CFU (S6E Fig). Unlike Mm, Franciscella infection did not activate a strong emergency response, and appeared to suppress immune cell numbers. Thus, with LVS infection, numbers of neutrophils remained constant, but numbers of monocytes, B, T, and NK cells decreased (Figs. 6F and S6F), perhaps reflecting a partial suppression of immune function or killing of infected cells by the bacteria. With infection plus imatinib (66mg/kg/d), numbers of neutrophils and monocytes increased, although only the neutrophil increase reached statistical significance (p<0.05); imatinib was without effect on T, B, or NK cells (S6F Fig). Thus, imatinib may counter myelosuppressive effects of Franciscella infection by increasing myelopoiesis, or decreasing bacterial CFU, or both. Moreover, these data suggest that imatinib may provide a protective effect against a broad range of pathogens, including those whose intracellular survival does not depend on the activity of Abl1 and other imatinib-sensitive kinases.
Fig. 6. Imatinib decreases bacterial load of pathogenic Francisella spp. in vivo. 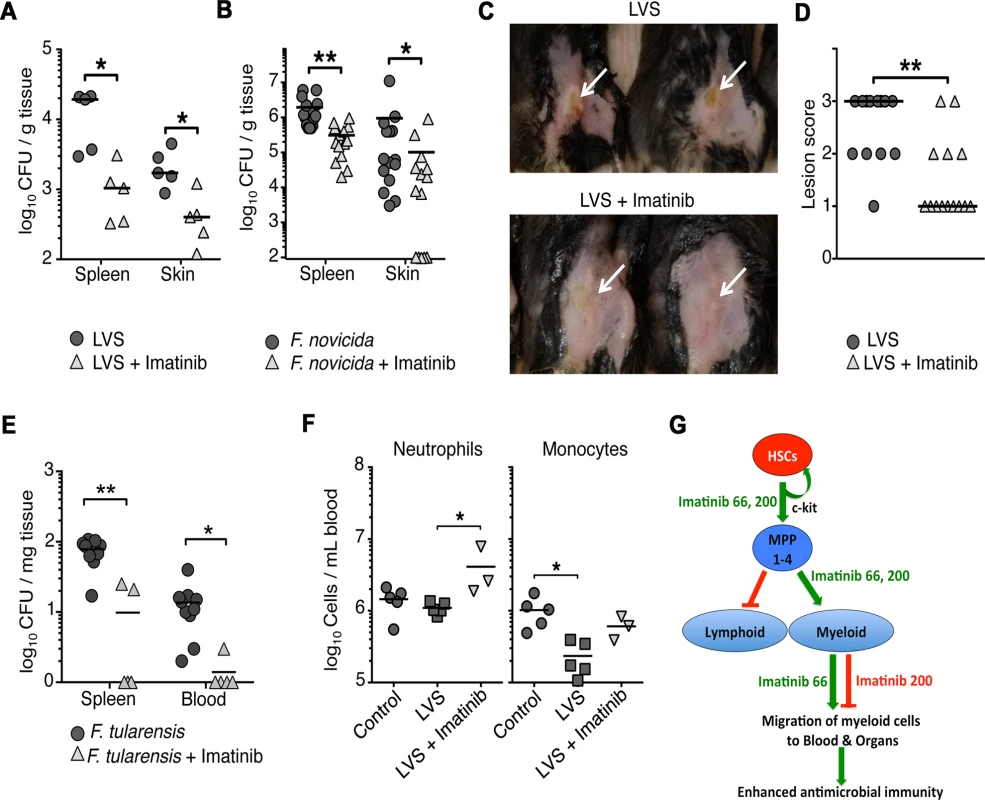
(A-B) C57Bl/6 mice were treated with imatinib at 66 mg/kg/d or water for 7d prior to infection and for the duration of the experiment, and then injected subcutaneously with either ~6x106 Fn (A) or ~2x105 LVS (B). After 48h for Fn or 5d for LVS, skin and spleens were collected and CFU/gram of tissue was determined. The limit of detection of the assay was 100 CFUs. (C) Images of lesions on control or imatinib-treated mice infected with LVS. Hair was removed from injection site. The lesions are indicated by white arrows. (D) Quantitation of lesions from control or imatinib-treated mice. Lesions were scored on a scale of one to three, with a score of one denoting no visible lesion, two a visible lesion but without a break in the skin, and three an open wound. 15 animals were scored per condition. (E) Four days following infection with strain Ft Schu S4, CFU/gram of tissue was determined. The limit of detection of the assay was 100 CFU/gm for Ft. The line in each data set represents the median. A Mann-Whitney nonparametric test was used to determine significance. Combined data from three independent experiments are shown. (F) Effects of imatinib at 66mg/kg/d on neutrophils and monocytes upon infection with LVS. Data from a representative experiment are shown. (G) Summary of effects of Imatinib on myelopoiesis and migration of mature myeloid cells to the blood and organs. Imatinib at doses of 66 or 200 mg/kg/day induces activation, expansion, and maturation of hematopoietic stem cells (HSCs) and multipotent hematopoietic progenitors 1–4 (MPP1-4) in the bone marrow. Imatinib at these doses also stimulates a lineage determination step that induces MPPs to differentiatiate into myeloid precursors and then mature cells (green arrow), but not into cells of the lymphoid lineage (red arrow). Data with low doses of neutralizing antibodies against the c-Kit tyrosine kinase support the hypothesis that effects on HSCs and MPPs are mediated by partial inhibition of c-Kit, whereas effects of the drug on lineage determination appears to be independent of c-Kit. Ultimately, imatinib increases the numbers of mature myeloid cells in the bone marrow, an effect seen with drug alone; the accumulation of mature cells is not evident with the drug in context of infection, which itself appears to mobilize migration of mature cells from the bone marrow to the periphery. Notably, increased numbers of mature cells are only evident in the blood and tissues at lower doses (66mg/kg/d), suggesting that at low doses, Imatinib induces migration of mature cells out of the bone marrow; alternatively, the drug may inhibit migration at higher doses (200mg/kg/d). Importantly, Imatinib mimics the “emergency response” to infection, which likewise induces HSC activation, MPP expansion, myelopoiesis, and migration of mature myeloid cells from the bone marrow to the periphery (green arrows). Discussion
Several lines of evidence suggest that imatinib, at the low doses used in this study, activates dormant HSCs, which rapidly differentiate into MPPs and mature myeloid cells. Low levels of imatinib did not cause accumulation of HSCs nor augment their transplantability. Nevertheless, the drug reduced accumulation of HSCs and MPP1 and MPP2 in infected mice (Fig. 3B,C), suggesting that the drug facilitates flux of early stem cells and progenitors into more differentiated cell types.
Data presented here suggest that partial inhibition of c-Kit may result in expansion of HSCs and/or MPPs but not their differentiation. Thus, low doses of anti-c-Kit mAb ACK2 increased numbers of cells of both myeloid and lymphoid origin (Fig. 4D), whereas imatinib appeared to both expand HSCs and MPPs, and direct MPPs predominantly towards myeloid lineages (e.g. Figs. 1–3). HSCs express c-Kit, and c-Kit ligand both promotes self-renewal of HSCs and maintains quiescence [58–61]. In accordance with our observations, G-CSF, which mobilizes HSCs and augments granulopoiesis [41], also induces the production of proteases that cleave c-Kit and its ligand, thereby reducing c-Kit activity [62]. Finally, mice with mutations in kit regulators (C57BL/6J-KitW-sh) display both increased numbers of myeloid cells in the bone marrow and peripheral neutrophilia [63]. However, because these mice contain more than thirty other mutations, it has not been possible to ascribe these effects to c-Kit directly.
Recent transplantation experiments have highlighted the importance of c-Kit surface expression and signaling levels in regulating self-renewal of HSCs (c-Kitlo) versus their differentiation (c-Kithi), with the c-Kitlo cells giving rise to c-Kithi cells, but not vice versa [61]. c-Kithi HSCs in turn support long-term lympho-myeloid grafts, although they exhibit a bias towards the megakaryocytic lineage [61]. Our data with the low doses of ACK2 antibody suggest that partial inhibition of c-Kit may govern transition of activated HSCs into MPPs and expansion of MPPs. However, the observation that low doses of the ACK2 antibody induce increases in both myeloid and lymphoid cells suggests that lineage determination by imatinib is regulated by kinases other than c-Kit. In this regard, inhibition of PDGFR, also an imatinib target, has been associated with differentiation of megakaryocytes [64]. Imatinib may cause differentiation of myeloid lineage cells by either inhibiting lymphoid differentiation, or alternatively, augmenting myeloid differentiation, perhaps via effects on lymphoid-myeloid progenitors distal to MPPs [42,43].
Finally, although accumulation of MPPs and mature myeloid cells in the bone marrow was evident at doses of 66 and 200mg/kg/d, accumulation of myeloid cells in the blood occurred only at the lower dose (Figs. 3 and 4). Surface expression of CXCR2 increased on mature neutrophils in the marrow in animals treated with 66mg/kg/d, consistent with an increased capacity to migrate out of the marrow. Notably, even with infection, fewer myeloid cells were evident in the periphery at high doses compared to low doses. Thus, whereas low doses of imatinib facilitate exodus of myeloid cells out of the bone marrow, higher doses appear to inhibit this process.
As summarized in Fig. 6G, imatinib doses of 66mg/kg/d appear to regulate hematopoiesis at three distinct steps: (i) flux of HSCs and MPPs, (ii) maturation of myeloid but not lymphoid progenitors and (iii) migration of mature myeloid cells to the blood and peripheral organs. At higher doses (200mg/kg/d) migration appears inhibited. In all these ways, imatinib at doses of 66mg/kg/d mimics “emergency hematopoiesis” [65], a natural response to infection that results in increased numbers of circulating myeloid cells, particularly monocytes and neutrophils. Notably, at optimal doses, the effect of imatinib on myelopoiesis appears more pronounced than that seen with infection (Figs. 1A, 3, 4).
Differences in half-life of imatinib (~1.5 hrs in mice versus ~15 hours in humans) preclude direct comparisons of applied doses between the two species, However, comparisons can be made by considering steady state levels of the drug. In mice, imatinib at doses of 66mg/kg/d yields steady state levels in the serum of ~57ng/ml (~100nM), which corresponds to less than 5% of that observed in CML patients treated with the minimal clinical dose of imatinib at 400mg QD (~1500ng/ml trough to 3000 peak, or 2.5–5μM)[37,38]. Thus, increased myelopoiesis seen in vivo in mice and in vitro using cultured murine and human bone marrow cells (Figs. 1,2, and 4), would likely not be evident at doses currently prescribed for CML patients. Indeed, myelo-suppression has been most commonly associated with doses higher than 400mg QD or with long-term administration of the drug in people [66]. However, it is noteworthy that in rare instances, GIST patients exhibit a dermal rash called Sweet’s syndrome [67], which is characterized by a localized neutrophilia. The rash abates when the drug is discontinued. It remains possible that individuals who contract Sweet’s syndrome are either non-compliant with the treatment regimen, or rapidly metabolize imatinib, either of which could result in lower levels of drug in the blood. In addition, Druker and colleagues noted in their initial clinical study with imatinib that half of those assigned to receive 25, 50, or 85 mg imatinib QD were removed from the study within two months because of elevated white-cell or platelet counts, which required therapy prohibited by the protocol [68]. Although not definitive because of the underlying leukemia, these doses are at the upper range we would expect might cause induction of myelopoiesis, and taken together with our data showing increases in CFC numbers with human marrow at concentrations of 5–50 nM (Fig. 4), suggest that this effect will be evident in humans, a prospect we are currently testing.
Our results raise the possibility that imatinib may be useful in treating several conditions associated with dysregulation of neutrophil homeostasis, which result in increased risk of severe infection. These include hereditary disorders such as cyclical neutropenia, cancer, autoimmune diseases, microbial infections and myelosuppressive chemotherapeutics. Administration of G-CSF mitigates neutropenia in a variety of conditions [69–71] by stimulating the production of neutrophils in the marrow and their migration into the blood [72]. However, G-CSF has been associated with deleterious outcomes against infections. For example, G-CSF blunts helper T cell responses and increases regulatory T cell responses and recapitulates a “super-shedder” phenotype characterized by excretion of high levels of Salmonella and hyperinflammation [73]. Imatinib, by contrast, has the opposite effect, causing reductions in bacterial load [30], decreased regulatory T cell responses, and augmented antigen presentation [36,74,75]. The difference may be that imatinib induces myelopoiesis and an overall increase in all myeloid cells, whereas G-CSF only induces granulopoiesis and neutrophilia, or, alternatively, that G-CSF has additional effects on other cell types [71]. Thus, imatinib may be useful for patients suffering neutropenia and have fewer deleterious side effects than G-CSF, a prospect we are currently testing.
We have proposed that imatinib may be useful in treating a broad range of infections caused by bacterial and viral pathogens that use Abl1, Abl2 or other imatinib-sensitive PTKs for pathogenesis [76]. These include, for example, poxviruses, filoviruses (Ebola), and Mtb [23,28,30–32,76,77]. Different dosing strategies may apply to different pathogens depending on the mechanism of action. Thus, for poxviruses and filoviruses, imatinib effects likely depend on inhibition of viral dissemination, which requires Abl-family kinases [23,31,32]. Notably, the optimal dose for inhibiting Abl-family kinases and treating poxvirus infections with imatinib is 200mg/kg/d, a dose that stimulates myelopoiesis but without attendant increases in myeloid cell numbers in blood and spleen.
By contrast, other pathogens, particularly those that trigger antimicrobial responses mediated by neutrophils and macrophages, may be more susceptible at lower doses of imatinib. Mycobacteria infections are optimally responsive to imatinib at 66mg/kg/d [30], a dose that triggers both myelopoiesis in the marrow, and increases in myeloid cells in blood and spleen. In acute Mtb infections, macrophages, followed by neutrophils, transiently increase in numbers in the lungs, reaching the highest levels just prior to arrival of DCs [54]. Both neutrophils and macrophages become infected, and depleting neutrophils increases the frequency of Mtb-infected DCs in the lungs, but decreases trafficking of DCs to the mesenteric lymph nodes, which precludes DC-initiated adaptive responses [54]. Thus, neutrophils may promote adaptive responses to Mtb by delivering bacterial antigens to DCs in a way that enables DC migration, and allows more effective antigen presentation and activation of naive CD4 T cells. Other evidence suggests that the anti-infective state induced by imatinib in the host and mediated by myeloid cells, resembles that seen in human patients who are protected from TB. First, studies of initially IGRA-negative TB household contacts indicate that low baseline neutrophil count is a predictor of subsequent IGRA conversion [78]. Thus, protected individuals may have, by virtue of continuous or repeated exposure, a heightened basal myeloid response that provides protection, and which resembles that induced by imatinib. Moreover, Kaushal and colleagues have shown in primates that mutants of Mtb, which are cleared by the immune response, induce a strong hematopoietic response, whereas Mtb does not [79]. Thus, Mtb may suppress the emergency response, which may be overcome by imatinib. Finally, Fletcher and colleagues have shown that BCG vaccinees who remain unprotected from TB have transcriptional signatures that may be indicative of either low myeloid responses or hyperactive ones, whereas protected individuals have an intermediate response (H. Fletcher, personal communication). Current efforts are aimed at determining whether these protective responses resemble those seen with imatinib.
Like Mtb, resolution of Franciscella infections depends on neutrophils [57], and Franciscella appears to suppress both the emergency response (Fig. 6F), and adaptive responses (S6F Fig). Our observations suggest that Francisella spp. do not require imatinib-sensitive kinases for pathogenesis in vitro, yet are still susceptible to imatinib in vivo (Figs. 6 and S6). Together, our data raise the possibility that imatinib may have utility against a wide range of pathogens that do not necessarily utilize Abl-family kinases for pathogenesis, by overcoming pathogen strategies to limit or subvert the emergency response. Moreover, agents such as imatinib may even be efficacious against strains resistant to conventional antibiotics, and may even act synergistically with co-administered antibiotics, a result suggested by our previous studies [30].
To realize the potential of imatinib as an immunomodulatory therapeutic for Mtb infections will require a balanced inflammatory response, without favoring hyper - or hypo-inflammation, which have been shown to be deleterious (e.g. [80–83]). Notably, the hematopoietic response generated by imatinib is titratable with dose. This response comprises an increase in numbers of all myeloid cells, thereby providing a limit on inflammation [84], rather than an increase in a single cell type, such as neutrophils, which can by themselves induce significant damage [85]. Nevertheless, heterogeneity in immune response (e.g. [82]) or disease stage could affect how an individual responds to up-regulation of the emergency response. Thus, careful dosing regimens and treatment at appropriate disease stages, in conjunction with assessments of diagnostic biomarkers and clinical signs will be required to ensure optimal activity of the drugs with minimal toxicity. A more complete discussion of the promise and caveats associated with host directed therapeutics for TB, including imatinib, as well as trial design and measures or efficacy, is reviewed elsewhere by one of us (D.K.;[77]).
In summary, we demonstrate a surprising immune-stimulatory effect of imatinib on myelopoiesis, which depends in part on c-Kit and occurs at subclinical doses. These observations have important implications for the use of imatinib as an immunostimulatory therapeutic against neutropenia and against infectious pathogens, including those that do not utilize host imatinib-sensitive kinases for pathogenesis.
Materials and Methods
Flow cytometry
For analysis of immune cells in whole blood, bone marrow and collagenase-digested splenocytes [86] were incubated with blocking mAb 2.4G2 anti-FcγRIII/I and live/dead probe (Alexa Fluor 430; Invitrogen, Grand Island, NY). Cells were labeled with CD11b (M1/70), B220 (RA3-6B2), and Ly6C (AL-21) antibodies from BD Biosciences (San Jose CA), CD19 (MB19-1), Thy1.2 (53–2.1), F4/80 (BM8), CD11c (N418), CD8α (53–6.7), Gr-1 (RB6-8C5) from eBioscience (San Diego, CA) and NK1.1 (PK136) from BioLegend. Cells were then stained with Streptavidin (QDot655; Invitrogen) before fixation.
To isolate neutrophils for activation and degranulation assays, blood, bone marrow and spleen cell samples were processed on ice and in PBS-EDTA buffer to prevent activation of the cells. Bone marrow and spleen cells were homogenized and then filtered. Then, cells were incubated with blocking mAb 2.4G2 anti-FcγRIII/I (BD Biosciences) and live/dead probe (Yellow; Invitrogen) along with labeled CD11b (M1/70), B220 (RA3-6B2), Ly6C (AL-21), and CD66b (G10F5) antibodies from BD Biosciences, Ly6G (1A8), CXCR2 (TG11), CD63 (MEM-259) from BioLegend (San Diego, CA), CXCR4 (TG12) from eBiosciences and FLICA probe for caspases 3/7 from Novus Biologicals (Littleton, CO). Notably, there is good cross-species reactivity with mouse neutrophils with the anti-human CD66b antibody (clone G10F5; [87]). All samples were acquired on a BD Biosciences LSR II (BD Biosciences and analyzed using FlowJo (TreeStar, Inc; Ashland, OR).
To deplete neutrophils in naïve or imatinib treated mice, 300ug of anti-Ly6G antibody clone 1A8 or 2A3 isotype control were administered one day prior to imatinib treatment and 2 days post treatment as described previously [54]. Blood was collected on the third day after imatinib treatment and neutrophil numbers were determined by flow cytometry. Both fluorescently labeled anti-Ly6G (clone 1A8) and anti-Gr-1 (specific for Ly6G and Ly6C) antibodies were unable to bind some neutrophils from 1A8-treated naïve or imatinib-treated mice owing to continued presence of 1A8 depleting antibody occluding the epitope shared by both antibodies. Thus, full enumeration of blood neutrophils was achieved with an anti-DEC-205 fluorescently labeled antibody in conjunction with an anti-Ly6C antibody. Neutrophil depletion of mice with 1A8 reduced median numbers of blood neutrophils to ~6% of the number seen in naïve animals, in line with previous reports [54]. However, depletion of imatinib-treated mice reduced numbers to only 62% of that seen in naïve animals or to 20% of that seen in imatinib-treated animals. Thus, our data suggest that in the presence of imatinib, the maximum number of neutrophils possible were depleted with 1A8, and increasing the concentration of 1A8 would not deplete more, and therefore that the mechanism by which neutrophils were removed from circulation appeared to be saturated in the presence of imatinib plus 1A8. Together these data suggest that we could not efficiently deplete neutrophils under these conditions. Moreover, because of this inefficiency and because cellular depletion mechanisms likewise are required to remove infected cells, we could not evaluate whether neutrophils were necessary for imatinib-mediated reduction of CFU.
To identify LSK, HSC, and multipotent progenitor populations (MPP1-4), bone marrow was flushed from femurs with DMEM plus 10% fetal bovine serum and labeled with the following mAbs: CD34 (HM34) and erythroid cells (TER-119) from Biolegend, CD135 (A2F10) from eBiosciences, sca-1 (D7), c-Kit/CD117 (2B8) and the following biotinylated mAbs (CD19 lineage (ID3), NK1.1 (PK136), B220 (RA3-6B2), GR1 (RB6-8C5), CD11b (M1/70), CD19 (1D3), CD4 (GK1.5), CD8 (53–6.7) CD150 (Q38-480) CD48 (HM48-1), followed by a secondary stain with streptavidin, from BD Biosciences. Cell numbers are expressed as per two femurs based on cell counts or, alternatively fluorescent beads to measure the concentration of cells during flow cytometry. Both methodologies yielded similar numbers of cell subsets.
FACS sorting of LSK cells
Bone marrow was flushed from donor femurs with sterile PBS containing 1% heat-inactivated fetal calf serum (PBS/FCS). Bone marrow cells were stained with a cocktail of antibodies from BD Biosciences: biotinylated lineage 1 antibodies (CD3, CD11b, CD19, CD49b, IgM, and Ter119), PE-conjugated lineage 2 antibodies (CD4, CD8, GR-1, and I-Ab), as well as, B220 PE-Cy5, c-kit APC, and Sca-1 PE-Cy7, followed by streptavidin APC-Cy7. For some experiments, cells were sorted using a FACS-Aria cell sorter and data analyzed using Diva Version 5.1 software (both from BD Biosciences). After initial scatter-based gating to exclude doublets, the B220− population was further gated to identify and sort the lineage- Sca-1+ c-Kit+ (LSK) cell population that contains hematopoietic stem cells [44].
Bone marrow colony-forming cell assays
Murine bone marrow (BM) cells were flushed from femurs and tibias using Iscove’s MDM (Stem Cell Technologies; Vancouver, BC, Canada) containing 2%FBS. Cells were triturated and filtered through a nylon screen to obtain a single-cell suspension. Human BM was obtained by aspiration from the posterior iliac crest in an IRB-approved protocol that enrolled normal volunteers (see below) and was depleted of RBCs by treatment with 0.8% Ammonium Chloride Solution (Stem Cell Technologies) according to manufacturer’s instructions. BM was plated in duplicate (2 × 104 nucleated cells/35 mm dish for murine BM, 5 x 104 nucleated cells/35 mm dish for human BM) in semisolid methylcellulose medium containing stem cell factor (SCF), interleukin-3 (IL-3), erythropoietin (Epo), and either interleukin-6 (IL-6) for murine BM (MethoCult M3434, Stem Cell Technologies), or granulocyte/macrophage colony stimulating factor (GM-CSF) for human BM (MethoCult H4434, Stem Cell Technologies), plus or minus the indicated concentrations of imatinib. Plates were incubated at 37°C, 5% CO2 and >95% humidity and Granulocyte-Macrophage colonies (CFU-GM) were identified by morphology and counted after 10–12 (murine) or 14–16 (human) days of incubation.
Competitive BM transplant experiments
In the first experiment, 2x106 bone marrow cells from naïve CD45.2 C57Bl/6 mice or CD45.2 C57Bl/6 mice treated with imatinib for 7 days were mixed with 2x106 bone marrow cells from untreated CD45.2 GFP-C57Bl/6 mice, and either the naïve-GFP BM mixture or the imatinib-GFP BM mixture injected I.V. into the tail vein of a congenic CD45.1+ C57Bl/6 recipient mice. At four and twelve months post-transplantation, mice were bled and the relative numbers of WBC derived from GFP animals, or from either naïve - or imatinib-treated donors, or recipient mice were analyzed by flow cytometry, taking advantage of the GFP label and CD45.1 congenic marker. In the second experiment, 5000 FACS-sorted LSK cells from naïve - or imatinib-treated mice were co-transplanted with 300,000 untreated BM cells from GFP+ mice. The relative numbers of WBC derived from GFP, or either naïve - or imatinib-treated donors, or recipient mice were determined 4 to 12 months post-transplantation.
Delivery of drugs in vivo
For experiments with imatinib, the mesylate salt was dissolved in water and loaded into Alzet pumps (Braintree Scientific, 1007D or 2002; Cupertino, CA) capable of dispensing a continuous flow of drug at doses ranging from 1, to 300mg/kg/day. Pumps were inserted subcutaneously into anesthetized 6-week old male C57Bl/6 mice (Jackson Laboratories; Bar Harbor, ME). At all doses tested, no weight loss or other adverse events were evident in uninfected animals. Alzet pumps were inserted 24 h to 7 days prior to manipulation or infection, and drug delivery was maintained for the duration of the experiment (7 to 28 days).
Some variation in CFU in Mm infection, or with plaque forming units (PFU) with vaccinia virus was evident when using different sources or lots of drug. The drug lot used in this study achieved a steady state serum concentration in the blood of 57+/-21ng/ml at 66mg/kg/d, and 165ng/ml +/ - 66ng/ml at 200mg/kg/d. Discrepancies between lots or sources were accounted for by the fact that different lots of drug applied at the same dose sometimes yielded different steady state concentrations of drug in the blood; however, no phenotypic differences were evident when animals were dosed such that serum levels were equivalent. All the data shown in this paper used a single lot of drug, but the phenotypes have been reproduced with different lots from different manufacturers. Together, these data highlight the utility of normalizing phenotypes to the steady state concentration of drug in the blood rather than to the administered dose.
c-Kit neutralization in vivo
Mice were injected intravenously with 3μg of c-Kit neutralizing antibody ACK2 (eBiosciences), or 3μg of IgG2b isotype control (eBiosciences), every 48h for seven days at the indicated concentrations. Blood was collected from mice at day seven and the number of myeloid cells and lymphocytes was determined by flow cytometry.
Bacterial strains and ex vivo assays
M. marinum (Mm) strain 1218R (ATCC 927), a fish outbreak isolate, was grown in Middlebrook 7H9 broth (7H9) (BBL Microbiology Systems, Cockeysville, MD) supplemented with ADC (Difco Laboratories, Detroit, MI,) and 0.05% Tween 80 (Mtb) (Sigma-Aldrich, St. Louis, MO) or 0.025% Tween 80 (Mm). For CFU assays 7H10 agar supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC) was used (Difco Laboratories, Sparks, MD). For in vivo Mm infections, bacterial stocks were grown at 30°C for 2 days to an OD600 of 0.4 (Eppendorf, BioPhotometer; Hamburg, Germany), the cells were diluted with PBS to 105 CFU/100ul. F. novicida (Fn) strain U112 overnight cultures were grown at 37°C with aeration in tryptic soy broth (TSB; Difco) supplemented with 0.02% L-cysteine (Sigma-Aldrich) while LVS cultures were grown in modified Mueller-Hinton broth (mMHB) supplemented with 1 mM CaCl2, 1 mM MgCl2, 0.1% glucose (Sigma-Aldrich), 2% Isovitalex (Difco), and 0.025% ferric pyrophosphate. For macrophage CFU assays, Fn was plated for enumeration on tryptic soy agar (TSA; Difco) and supplemented with 0.01% L-cysteine. Mouse macrophage cell line J774A.1 (ATCC TIB-67) was maintained in Dulbecco’s modified Eagle Medium (DMEM). For in vivo CFU assays, Fn experiments were plated on modified Mueller Hinton (mMH) (Difco/BD) plates supplemented with 0.025% ferric pyrophosphate (Sigma-Aldrich), 0.1% glucose, and 0.01% L-cysteine. For both macrophage and in vivo assays, LVS was plated on mMH agar supplemented with 2% Isovitalex.
In vivo bacteria infection assays
For M. marinum infections, six-week old male C57Bl/6 mice were injected in the tail vein with active growing cultures at ~105 CFU/mouse. The number of bacteria injected for each experiment was determined by retrospective plating and was ~2.5x105 CFU/mouse. Seven days after infection, blood, spleen and bone marrow were harvested. For CFU, spleens were weighed and homogenized in a Tissuemise (Fisher Scientific, Hampton, NH) in 1 ml PBS. Each homogenate was diluted and spread on 7H10 agar. Colonies were scored after seven days of incubation at 30°C. Total weight of the organ and colonies per ml of the homogenized organ were used to determine CFU/gram. For Francisella infections, C57Bl/6 mice were infected with ~6x106 F. novicida) or ~2 x 105 F. holartica (LVS strain), or F. tularensis (Ft; strain Schu S4). After 48 hours with Fn, or 5 days with LVS, of 4 days with Ft mice from both types of infections were sacrificed and the spleen, liver, and skin at the site of infection were harvested, homogenized, plated for CFU on MH plates, and incubated overnight at 37°C.
Neutrophil purification and adoptive transfers
Neutrophils were purified from the collagenase-digested spleens derived from control or imatinib-treated mice. Splenocytes were first depleted of B cells with anti-CD19 coated microbeads (Miltenyi; San Diego, CA) then neutrophils were positively selected by anti-Ly6G+ microbeads. Purity was assessed on the Ly6G+ enriched fraction using the parameters listed above. Neutrophils, defined as CD11b+Gr-1hiLy6Clow. Ly6G+ fractions were 100% pure from imatinib-treated mice and 80% pure from naïve mice. We routinely purified ~1x106 neutrophils from the spleen of a naïve mouse and ~8x106 from the spleen of an imatinib treated mouse. For adoptive transfers, 4x106 neutrophils were injected into the left lateral tail vein of naïve recipients. Immediately following the adoptive transfer, 105 Mm were injected into the right lateral tail vein of the mouse. Spleens were harvested 48 hours after the neutrophil transfer and infection, and bacterial CFU determined as described above.
Histology and immunofluorescence microscopy
Imatinib-treated (66.7mg/kg/day) or water control pumps were implanted one day prior to infection. Day 7 post-treatment, spleens were harvested, frozen in optimum cutting temperature (OCT) compound, cut into 6μm sections, mounted on slides, and then fixed with %100 acetone for 10 min at −20°C. Following rehydration in PBS, slides were permeabilized with PBS +0.5% Triton X-100 +1% BSA, blocked with normal rat serum and anti-FcRIII/I antibodies and then incubated with anti-CD169-FITC (Serotec; Oxford, UK), CD11b-PE (eBioscience), and F4/80-biotin (eBioscience). Tissues were incubated with Streptavidin-APC and mounted with Prolong-GOLD (with DAPI) (Invitrogen). Images were captured using the x10 objective on a Zeiss Axioscope (Carl Zeiss, Germany) and analyzed using ImageJ (National Institute of Mental Health) and DoubleTake (Echo One, Denmark) software. Bone slices from naïve mice or mice treated with imatinib at 66 mg/kg/d and stained with Giemsa and imaged on a Zeiss 200M microscope at x100 or x400 magnification.
Statistical analysis
Statistical analysis was done using either of two non-parametric tests including the Mann-Whitney test to compare two samples, or the Kruskal Wallis test to compare multiple subsets within a group (e.g. with or without infection and with or without drug). In both tests, the data were pooled and the values ranked. The statistic calculates the probability that the observed ranks of a subset of observations represent a random sampling from the population as a whole, or a significantly different population compared to the group as a whole. Values less than or equal to 0.05 were considered statistically significant. For comparing amongst averaged data from several experiments, a t-test was used to determine significance.
Ethics statement
For some experiments, bone marrow was obtained from normal human volunteers enrolled in an institutional observational study conducted at the Emoryt transplant center to evaluate the central immune response in healthy volunteers. Samples used in this paper were from normal bone marrow donors who did not have signs of disease including malignancy nor gastrointestinal infection (viral, bacterial, fungal, protozoal) within two weeks of the day of collection. Samples used in this paper were obtained from bone marrow unused for other aspects of the study. The protocol was reviewed by the Emory University Institutional Review Board (IRB-00060350). Since samples for this study involved the use of samples obtained as part of an ongoing study where written informed consent was obtained for storage and use of samples in other studies, it qualifies for a waiver of informed consent for this study. Samples from 5 patients were used in this study. All mouse studies were reviewed and approved by the Emory Institutional Animal Care and Use Committee (DAR-2001392-020615BN), which reviews the animal care and use projects. The committee adheres to specific national and international regulations regarding the ethical treatment of animals as specified by the National Institutes of Health.
Supporting Information
Zdroje
1. Bradley WD, Koleske AJ (2009) Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci 122 : 3441–3454. doi: 10.1242/jcs.039859 19759284
2. Hantschel O, Superti-Furga G (2004) Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol 5 : 33–44. 14708008
3. Koretzky GA (2007) The legacy of the Philadelphia chromosome. J Clin Invest 117 : 2030–2032. 17671635
4. Evans DB, Traxler P, Garcia-Echeverria C (2000) Molecular approaches to receptors as targets for drug discovery. Exs 89 : 123–139. 10997286
5. Garcia-Echeverria C, Traxler P, Evans DB (2000) ATP site-directed competitive and irreversible inhibitors of protein kinases. Med Res Rev 20 : 28–57. 10608920
6. Kantarjian H, O'Brien S, Jabbour E, Garcia-Manero G, Quintas-Cardama A, et al. (2012) Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood 119 : 1981–1987. doi: 10.1182/blood-2011-08-358135 22228624
7. Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, et al. (2012) Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood 119 : 1123–1129. doi: 10.1182/blood-2011-08-376087 22160483
8. Goldman JM, Druker BJ (2001) Chronic myeloid leukemia: current treatment options. Blood 98 : 2039–2042. 11567987
9. Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, et al. (2011) Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest 121 : 396–409. doi: 10.1172/JCI35721 21157039
10. Buchdunger E, Zimmermann J, Mett H, Meyer T, Muller M, et al. (1996) Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res 56 : 100–104. 8548747
11. Carroll M, Ohno-Jones S, Tamura S, Buchdunger E, Zimmermann J, et al. (1997) CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL, and TEL-PDGFR fusion proteins. Blood 90 : 4947–4952. 9389713
12. Dewar AL, Cambareri AC, Zannettino AC, Miller BL, Doherty KV, et al. (2005) Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood 105 : 3127–3132. 15637141
13. Melnick JS, Janes J, Kim S, Chang JY, Sipes DG, et al. (2006) An efficient rapid system for profiling the cellular activities of molecular libraries. Proc Natl Acad Sci U S A 103 : 3153–3158. 16492761
14. Krauss GL, Bar M, Biton V, Klapper JA, Rektor I, et al. (2012) Tolerability and safety of perampanel: two randomized dose-escalation studies. Acta Neurol Scand 125 : 8–15. doi: 10.1111/j.1600-0404.2011.01588.x 21883097
15. Lebeis SL, Kalman D (2009) Aligning antimicrobial drug discovery with complex and redundant host-pathogen interactions. Cell Host Microbe 5 : 114–122. doi: 10.1016/j.chom.2009.01.008 19218083
16. Swimm A, Bommarius B, Li Y, Cheng D, Reeves P, et al. (2004) Enteropathogenic Escherichia coli use redundant tyrosine kinases to form actin pedestals. Mol Biol Cell 15 : 3520–3529. 15155808
17. Pielage JF, Powell KR, Kalman D, Engel JN (2008) RNAi screen reveals an Abl kinase-dependent host cell pathway involved in Pseudomonas aeruginosa internalization. PLoS Pathog 4: e1000031. doi: 10.1371/journal.ppat.1000031 18369477
18. Ly KT, Casanova JE (2008) Abelson Tyrosine Kinase Facilitates Salmonella enterica serovar Typhimurium Entry into Epithelial Cells. Infect Immun.
19. Burton EA, Plattner R, Pendergast AM (2003) Abl tyrosine kinases are required for infection by Shigella flexneri. Embo J 22 : 5471–5479. 14532119
20. Tammer I, Brandt S, Hartig R, Konig W, Backert S (2007) Activation of Abl by Helicobacter pylori: a novel kinase for CagA and crucial mediator of host cell scattering. Gastroenterology 132 : 1309–1319. 17408661
21. Lin M, den Dulk-Ras A, Hooykaas PJ, Rikihisa Y (2007) Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection. Cell Microbiol 9 : 2644–2657. 17587335
22. Elwell CA, Ceesay A, Kim JH, Kalman D, Engel JN (2008) RNA interference screen identifies Abl kinase and PDGFR signaling in Chlamydia trachomatis entry. PLoS Pathog 4: e1000021. doi: 10.1371/journal.ppat.1000021 18369471
23. Garcia M, Cooper A, Shi W, Bornmann W, Carrion R, et al. (2012) Productive replication of Ebola virus is regulated by the c-Abl1 tyrosine kinase. Sci Transl Med 4 : 123ra124.
24. Harmon B, Campbell N, Ratner L (2010) Role of Abl kinase and the Wave2 signaling complex in HIV-1 entry at a post-hemifusion step. PLoS Pathog 6: e1000956. doi: 10.1371/journal.ppat.1000956 20585556
25. Coyne CB, Bergelson JM (2006) Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 124 : 119–131. 16413486
26. Hirsch AJ, Medigeshi GR, Meyers HL, DeFilippis V, Fruh K, et al. (2005) The Src family kinase c-Yes is required for maturation of West Nile virus particles. J Virol 79 : 11943–11951. 16140770
27. Prakash O, Swamy OR, Peng X, Tang ZY, Li L, et al. (2005) Activation of Src kinase Lyn by the Kaposi sarcoma-associated herpesvirus K1 protein: implications for lymphomagenesis. Blood 105 : 3987–3994. 15665117
28. Swimm AI, Bornmann W, Jiang M, Imperiale MJ, Lukacher AE, et al. (2010) Abl family tyrosine kinases regulate sialylated ganglioside receptors for polyomavirus. J Virol 84 : 4243–4251. doi: 10.1128/JVI.00129-10 20181697
29. Wetzel DM, McMahon-Pratt D, Koleske AJ (2012) The Abl and Arg kinases mediate distinct modes of phagocytosis and are required for maximal Leishmania infection. Mol Cell Biol 32 : 3176–3186. doi: 10.1128/MCB.00086-12 22665498
30. Napier RJ, Rafi W, Cheruvu M, Powell KR, Zaunbrecher MA, et al. (2011) Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe 10 : 475–485. doi: 10.1016/j.chom.2011.09.010 22100163
31. Reeves PM, Bommarius B, Lebeis S, McNulty S, Christensen J, et al. (2005) Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases. Nat Med 11 : 731–739. 15980865
32. Reeves PM, Smith SK, Olson VA, Thorne SH, Bornmann W, et al. (2011) Variola and monkeypox viruses utilize conserved mechanisms of virion motility and release that depend on abl and SRC family tyrosine kinases. J Virol 85 : 21–31. doi: 10.1128/JVI.01814-10 20962097
33. Bruns H, Stegelmann F, Fabri M, Dohner K, van Zandbergen G, et al. (2012) Abelson tyrosine kinase controls phagosomal acidification required for killing of Mycobacterium tuberculosis in human macrophages. J Immunol 189 : 4069–4078. doi: 10.4049/jimmunol.1201538 22988030
34. Sinai P, Berg RE, Haynie JM, Egorin MJ, Ilaria RL Jr., et al. (2007) Imatinib mesylate inhibits antigen-specific memory CD8 T cell responses in vivo. J Immunol 178 : 2028–2037. 17277106
35. Borg C, Terme M, Taieb J, Menard C, Flament C, et al. (2004) Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest 114 : 379–388. 15286804
36. Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, et al. (2011) Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med 17 : 1094–1100. doi: 10.1038/nm.2438 21873989
37. Menon-Andersen D, Mondick JT, Jayaraman B, Thompson PA, Blaney SM, et al. (2009) Population pharmacokinetics of imatinib mesylate and its metabolite in children and young adults. Cancer Chemother Pharmacol 63 : 229–238. doi: 10.1007/s00280-008-0730-x 18398615
38. Larson RA, Druker BJ, Guilhot F, O'Brien SG, Riviere GJ, et al. (2008) Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood 111 : 4022–4028. doi: 10.1182/blood-2007-10-116475 18256322
39. Wilson A, Trumpp A (2006) Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 6 : 93–106. 16491134
40. Wilson A, Oser GM, Jaworski M, Blanco-Bose WE, Laurenti E, et al. (2007) Dormant and self-renewing hematopoietic stem cells and their niches. Ann N Y Acad Sci 1106 : 64–75. 17442778
41. Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, et al. (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135 : 1118–1129. doi: 10.1016/j.cell.2008.10.048 19062086
42. Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, et al. (2005) Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 121 : 295–306. 15851035
43. Gorgens A, Radtke S, Mollmann M, Cross M, Durig J, et al. (2013) Revision of the human hematopoietic tree: granulocyte subtypes derive from distinct hematopoietic lineages. Cell Rep 3 : 1539–1552. doi: 10.1016/j.celrep.2013.04.025 23707063
44. Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, et al. (1992) In vivo and in vitro stem cell function of c-kit - and Sca-1-positive murine hematopoietic cells. Blood 80 : 3044–3050. 1281687
45. Harrison DE, Jordan CT, Zhong RK, Astle CM (1993) Primitive hemopoietic stem cells: direct assay of most productive populations by competitive repopulation with simple binomial, correlation and covariance calculations. Exp Hematol 21 : 206–219. 8425559
46. Bartolovic K, Balabanov S, Hartmann U, Komor M, Boehmler AM, et al. (2004) Inhibitory effect of imatinib on normal progenitor cells in vitro. Blood 103 : 523–529. 12969987
47. Ogawa M, Nishikawa S, Yoshinaga K, Hayashi S, Kunisada T, et al. (1993) Expression and function of c-Kit in fetal hemopoietic progenitor cells: transition from the early c-Kit-independent to the late c-Kit-dependent wave of hemopoiesis in the murine embryo. Development 117 : 1089–1098. 7686845
48. Eash KJ, Greenbaum AM, Gopalan PK, Link DC (2010) CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest 120 : 2423–2431. doi: 10.1172/JCI41649 20516641
49. Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, et al. (2004) Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood 104 : 565–571. 15054039
50. Eash KJ, Means JM, White DW, Link DC (2009) CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood 113 : 4711–4719. doi: 10.1182/blood-2008-09-177287 19264920
51. Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13 : 159–175. doi: 10.1038/nri3399 23435331
52. Fox S, Leitch AE, Duffin R, Haslett C, Rossi AG (2010) Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease. J Innate Immun 2 : 216–227. doi: 10.1159/000284367 20375550
53. Taylor RC, Cullen SP, Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9 : 231–241. 18073771
54. Blomgran RaE J. (2011) Lung Neutrophils Facilitate Activation of Naive Antigen-Specific CD4 + T Cells during Mycobacterium tuberculosis Infection. Journal of Immunology doi: 10.4049/jimmunol.1100001 23264931
55. Elkins KL, Rhinehart-Jones TR, Culkin SJ, Yee D, Winegar RK (1996) Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect Immun 64 : 3288–3293. 8757866
56. Conlan JW (1997) Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun 65 : 630–635. 9009323
57. Sjostedt A, Conlan JW, North RJ (1994) Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect Immun 62 : 2779–2783. 8005668
58. Orlic D, Fischer R, Nishikawa S, Nienhuis AW, Bodine DM (1993) Purification and characterization of heterogeneous pluripotent hematopoietic stem cell populations expressing high levels of c-kit receptor. Blood 82 : 762–770. 7687891
59. Bodine DM, Seidel NE, Zsebo KM, Orlic D (1993) In vivo administration of stem cell factor to mice increases the absolute number of pluripotent hematopoietic stem cells. Blood 82 : 445–455. 7687160
60. Thoren LA, Liuba K, Bryder D, Nygren JM, Jensen CT, et al. (2008) Kit regulates maintenance of quiescent hematopoietic stem cells. J Immunol 180 : 2045–2053. 18250409
61. Shin JY, Hu W, Naramura M, Park CY (2014) High c-Kit expression identifies hematopoietic stem cells with impaired self-renewal and megakaryocytic bias. J Exp Med 211 : 217–231. doi: 10.1084/jem.20131128 24446491
62. Levesque JP, Hendy J, Winkler IG, Takamatsu Y, Simmons PJ (2003) Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol 31 : 109–117. 12591275
63. Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, et al. (2008) Genetic inversion in mast cell-deficient (Wsh) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol 173 : 1693–1701. doi: 10.2353/ajpath.2008.080407 18988802
64. Boitano AE, de Lichtervelde L, Snead JL, Cooke MP, Schultz PG (2012) An image-based screen identifies a small molecule regulator of megakaryopoiesis. Proc Natl Acad Sci U S A 109 : 14019–14023. doi: 10.1073/pnas.1212545109 22891346
65. Panopoulos AD, Watowich SS (2008) Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and 'emergency' hematopoiesis. Cytokine 42 : 277–288. doi: 10.1016/j.cyto.2008.03.002 18400509
66. Druker BJ (2003) Imatinib alone and in combination for chronic myeloid leukemia. Semin Hematol 40 : 50–58. 12783376
67. Cohen PR (2007) Sweet's syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis 2 : 34. 17655751
68. Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, et al. (2001) Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344 : 1031–1037. 11287972
69. Carulli G (1997) Effects of recombinant human granulocyte colony-stimulating factor administration on neutrophil phenotype and functions. Haematologica 82 : 606–616. 9407734
70. Gabrilove JL, Jakubowski A, Scher H, Sternberg C, Wong G, et al. (1988) Effect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional-cell carcinoma of the urothelium. N Engl J Med 318 : 1414–1422. 2452983
71. Greenbaum AM, Link DC (2011) Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia 25 : 211–217. doi: 10.1038/leu.2010.248 21079612
72. Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, et al. (1991) Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 325 : 164–170. 1711156
73. Gopinath S, Hotson A, Johns J, Nolan G, Monack D (2013) The systemic immune state of super-shedder mice is characterized by a unique neutrophil-dependent blunting of TH1 responses. PLoS Pathog 9: e1003408. doi: 10.1371/journal.ppat.1003408 23754944
74. Held SA, Duchardt KM, Tenzer S, Ruckrich T, von Schwarzenberg K, et al. (2012) Imatinib mesylate and nilotinib affect MHC-class I presentation by modulating the proteasomal processing of antigenic peptides. Cancer Immunol Immunother.
75. Sato N, Narita M, Takahashi M, Yagisawa K, Liu A, et al. (2003) The effects of STI571 on antigen presentation of dendritic cells generated from patients with chronic myelogenous leukemia. Hematol Oncol 21 : 67–75. 12802811
76. Napier RJ, Shinnick TM, Kalman D (2012) Back to the future: host-targeted chemotherapeutics for drug-resistant TB. Future Microbiol 7 : 431–435. doi: 10.2217/fmb.12.19 22439718
77. Hawn TR, Shah JA, Kalman D (2015) New tricks for old dogs: countering antibiotic resistance in tuberculosis with host-directed therapeutics. Immunol Rev 264 : 344–362. doi: 10.1111/imr.12255 25703571
78. Tan BH, Meinken C, Bastian M, Bruns H, Legaspi A, et al. (2006) Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol 177 : 1864–1871. 16849498
79. Mehra S, Foreman T, Didier P, Ahsan M, Hudock T, et al. (2015) The DosR regulon modulates adaptive immunity and is essential for Mycobacterium tuberculosis persistence. Am J Resp Crit Care Med (in press).
80. Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, et al. (2014) Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 511 : 99–103. doi: 10.1038/nature13489 24990750
81. Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, et al. (2005) TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med 202 : 1715–1724. 16365150
82. Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, et al. (2012) Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 148 : 434–446. doi: 10.1016/j.cell.2011.12.023 22304914
83. Tobin DM, Ramakrishnan L (2013) TB: the Yin and Yang of lipid mediators. Curr Opin Pharmacol 13 : 641–645. doi: 10.1016/j.coph.2013.06.007 23849093
84. Serhan CN (2007) Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol 25 : 101–137. 17090225
85. Nandi B, Behar SM (2011) Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J Exp Med 208 : 2251–2262. doi: 10.1084/jem.20110919 21967766
86. Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, et al. (2006) Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest 116 : 916–928. 16543948
87. Thom SR, Yang M, Bhopale VM, Huang S, Milovanova TN (2011) Microparticles initiate decompression-induced neutrophil activation and subsequent vascular injuries. J Appl Physiol (1985) 110 : 340–351. doi: 10.1152/japplphysiol.00811.2010 20966192
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole MembraneČlánek Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and FunctionČlánek Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis
- Is Antigenic Sin Always “Original?” Re-examining the Evidence Regarding Circulation of a Human H1 Influenza Virus Immediately Prior to the 1918 Spanish Flu
- The Great Escape: Pathogen Versus Host
- Coping with Stress and the Emergence of Multidrug Resistance in Fungi
- Catch Me If You Can: The Link between Autophagy and Viruses
- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- Evidence for Ubiquitin-Regulated Nuclear and Subnuclear Trafficking among Matrix Proteins
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
- Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
- α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia
- Induction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
- Intracellular Growth Is Dependent on Tyrosine Catabolism in the Dimorphic Fungal Pathogen
- HCV Induces the Expression of Rubicon and UVRAG to Temporally Regulate the Maturation of Autophagosomes and Viral Replication
- Spatiotemporal Analysis of Hepatitis C Virus Infection
- Subgingival Microbial Communities in Leukocyte Adhesion Deficiency and Their Relationship with Local Immunopathology
- Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
- Establishment of HSV1 Latency in Immunodeficient Mice Facilitates Efficient Reactivation
- XRN1 Stalling in the 5’ UTR of Hepatitis C Virus and Bovine Viral Diarrhea Virus Is Associated with Dysregulated Host mRNA Stability
- γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
- Rhadinovirus Host Entry by Co-operative Infection
- A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane
- Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Transmitted by Fleas
- Elucidation of Sigma Factor-Associated Networks in Reveals a Modular Architecture with Limited and Function-Specific Crosstalk
- A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
- Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and Function
- Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
- Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection
- Diminished Reovirus Capsid Stability Alters Disease Pathogenesis and Littermate Transmission
- Characterization of CD8 T Cell Differentiation following SIVΔnef Vaccination by Transcription Factor Expression Profiling
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission
- Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection
- PKCη/Rdx-driven Phosphorylation of PDK1: A Novel Mechanism Promoting Cancer Cell Survival and Permissiveness for Parvovirus-induced Lysis
- Metalloprotease NleC Suppresses Host NF-κB/Inflammatory Responses by Cleaving p65 and Interfering with the p65/RPS3 Interaction
- Immune Antibodies and Helminth Products Drive CXCR2-Dependent Macrophage-Myofibroblast Crosstalk to Promote Intestinal Repair
- Adenovirus Entry From the Apical Surface of Polarized Epithelia Is Facilitated by the Host Innate Immune Response
- The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family
- Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
- CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies
- Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation
- Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Infection
- TRIM26 Negatively Regulates Interferon-β Production and Antiviral Response through Polyubiquitination and Degradation of Nuclear IRF3
- Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees ()
- A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication
- Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
- Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
- The Role of Misshapen NCK-related kinase (MINK), a Novel Ste20 Family Kinase, in the IRES-Mediated Protein Translation of Human Enterovirus 71
- Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Activates Both IL-1β and IL-1 Receptor Antagonist to Modulate Lung Inflammation during Pneumonic Plague
- Persistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
- An 18 kDa Scaffold Protein Is Critical for Biofilm Formation
- Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children
- Human CD8 T-cells Recognizing Peptides from () Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Inhibitory Phenotype and Represent a Novel Human T-cell Subset
- Decreased HIV-Specific T-Regulatory Responses Are Associated with Effective DC-Vaccine Induced Immunity
- RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets
- Concerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
- Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein
- PPM1A Regulates Antiviral Signaling by Antagonizing TBK1-Mediated STING Phosphorylation and Aggregation
- Lipidomic Analysis Links Mycobactin Synthase K to Iron Uptake and Virulence in .
- Roles and Programming of Arabidopsis ARGONAUTE Proteins during Infection
- Impact of Infection on Host Macrophage Nuclear Physiology and Nucleopore Complex Integrity
- The Impact of Host Diet on Titer in
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Herpesviral G Protein-Coupled Receptors Activate NFAT to Induce Tumor Formation via Inhibiting the SERCA Calcium ATPase
- The Causes and Consequences of Changes in Virulence following Pathogen Host Shifts
- Small GTPase Rab21 Mediates Fibronectin Induced Actin Reorganization in : Implications in Pathogen Invasion
- Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus
- NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target
- β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation
- An O Antigen Capsule Modulates Bacterial Pathogenesis in
- Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape
- Probing the Metabolic Network in Bloodstream-Form Using Untargeted Metabolomics with Stable Isotope Labelled Glucose
- Adhesive Fiber Stratification in Uropathogenic Biofilms Unveils Oxygen-Mediated Control of Type 1 Pili
- Vaccinia Virus Protein Complex F12/E2 Interacts with Kinesin Light Chain Isoform 2 to Engage the Kinesin-1 Motor Complex
- Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis
- Human T-Cell Leukemia Virus Type 1 (HTLV-1) Tax Requires CADM1/TSLC1 for Inactivation of the NF-κB Inhibitor A20 and Constitutive NF-κB Signaling
- Suppression of RNAi by dsRNA-Degrading RNaseIII Enzymes of Viruses in Animals and Plants
- Spatiotemporal Regulation of a T4SS Substrate by the Metaeffector SidJ
- Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells
- Dependence of Intracellular and Exosomal microRNAs on Viral Oncogene Expression in HPV-positive Tumor Cells
- Identification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Dihydrobiopterin in Adult Falciparum Malaria: Association with Disease Severity, Impaired Microvascular Function and Increased Endothelial Activation
- Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses
- Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer
- Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



