-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
Bacteria make use of two-component transduction systems, composed of a sensor-kinase and a response regulator, to perceive environmental signals and orchestrate an appropriate response. The virulence regulon of the whooping cough agent Bordetella pertussis is controlled by the two-component system BvgAS. The sensor-kinase BvgS harbor extra-cytoplasmic Venus flytrap perception domains similar to those found in neuronal receptors, and it is the prototype of a large bacterial protein family. We report the atomic structure of the extra-cytoplasmic moiety of BvgS, which shows a novel dimeric arrangement. We show that the virulent phase of B. pertussis that occurs by default corresponds to a specific conformation of BvgS generated by the periplasmic architecture itself and by the differential dynamics of its Venus flytrap domains. The perception of negative signals by the periplasmic domains causes BvgS to shift to a different conformation that corresponds to the avirulent phase of the bacteria. In addition to contributing to our understanding of virulence regulation by B. pertussis at a time of whooping cough re-emergence, this study also paves the way to the mechanistic exploration of the homologous sensor-kinases found in various bacterial pathogens.
Published in the journal: Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS. PLoS Pathog 11(3): e32767. doi:10.1371/journal.ppat.1004700
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004700Summary
Bacteria make use of two-component transduction systems, composed of a sensor-kinase and a response regulator, to perceive environmental signals and orchestrate an appropriate response. The virulence regulon of the whooping cough agent Bordetella pertussis is controlled by the two-component system BvgAS. The sensor-kinase BvgS harbor extra-cytoplasmic Venus flytrap perception domains similar to those found in neuronal receptors, and it is the prototype of a large bacterial protein family. We report the atomic structure of the extra-cytoplasmic moiety of BvgS, which shows a novel dimeric arrangement. We show that the virulent phase of B. pertussis that occurs by default corresponds to a specific conformation of BvgS generated by the periplasmic architecture itself and by the differential dynamics of its Venus flytrap domains. The perception of negative signals by the periplasmic domains causes BvgS to shift to a different conformation that corresponds to the avirulent phase of the bacteria. In addition to contributing to our understanding of virulence regulation by B. pertussis at a time of whooping cough re-emergence, this study also paves the way to the mechanistic exploration of the homologous sensor-kinases found in various bacterial pathogens.
Introduction
Two-component sensory transduction systems (TCSs) regulate various physiological processes in response to environmental changes [1]. They are abundant throughout the phylogenetic tree except for vertebrates and represent major bacterial signaling pathways [2,3]. TCSs notably regulate the cell cycle, motility, biofilm formation or antibiotic resistance, as well as the virulence of major pathogens [4–8]. TCSs are typically composed of a sensor-kinase activated by environmental stimuli and a response regulator mediating phosphorylation-dependent effects [9,10]. Upon perception of a physical or chemical signal, auto-phosphorylation of a conserved cytoplasmic His residue of the sensor-kinase is followed by transfer of the phosphoryl group to a conserved Asp residue of the response regulator. The phosphorylated response regulator mediates a specific, frequently transcriptional, cellular response [11]. There is considerable diversity among TCSs regarding domain composition and organization [9,10].
Bordetella pertussis, the whooping cough agent, colonizes the upper respiratory tract of humans [12]. Transcription of its virulence regulon is positively regulated by the TCS BvgAS [13]. Over one hundred genes belong to the Bvg regulon, including those coding for the adhesins and toxins and their secretion and assembly machineries [14]. The virulent, Bvg+ phase, in which phosphorylated BvgA trans-activates the expression of the virulence regulon, is essential for the development of the infection cycle of B. pertussis and other pathogenic Bordetella species [13,15]. The kinase and phosphotransfer activities of BvgS are maximal (referred to below as the ‘kinase-on’ state) without specific chemical stimuli and at 37°C, the B. pertussis host body temperature, while low temperatures and specific negative modulators turn these activities off in laboratory conditions (referred to below as the ‘kinase-off’ state). Thus, millimolar concentrations of nicotinate or sulfate ions result in the dephosphorylation of BvgA, switching the bacteria to the avirulent, Bvg- phase [16,17]. Virulence genes are no longer expressed, while a smaller set of virulence-repressed genes (vrgs) are upregulated [18,19]. At low modulator concentrations, an intermediate Bvgi phase occurs in which the reduced concentration of phosphorylated BvgA is sufficient to transactivate ‘early’ virulence genes as well as specific intermediate genes [13,20,21]. Thus, BvgAS operates like a rheostat, determining several states of gene expression that might correspond to distinct temporal or spatial situations in the course of infection. BvgS is composed of periplasmic Venus flytrap (VFT) domains, a transmembrane segment, a PAS domain, and a kinase and additional domains that make up a phosphorelay (Fig. 1A). The cytoplasmic moiety of BvgS dimerizes, similar to the other TCS sensor-kinases [22,23].
Fig. 1. Function of BvgS and selected homologs. 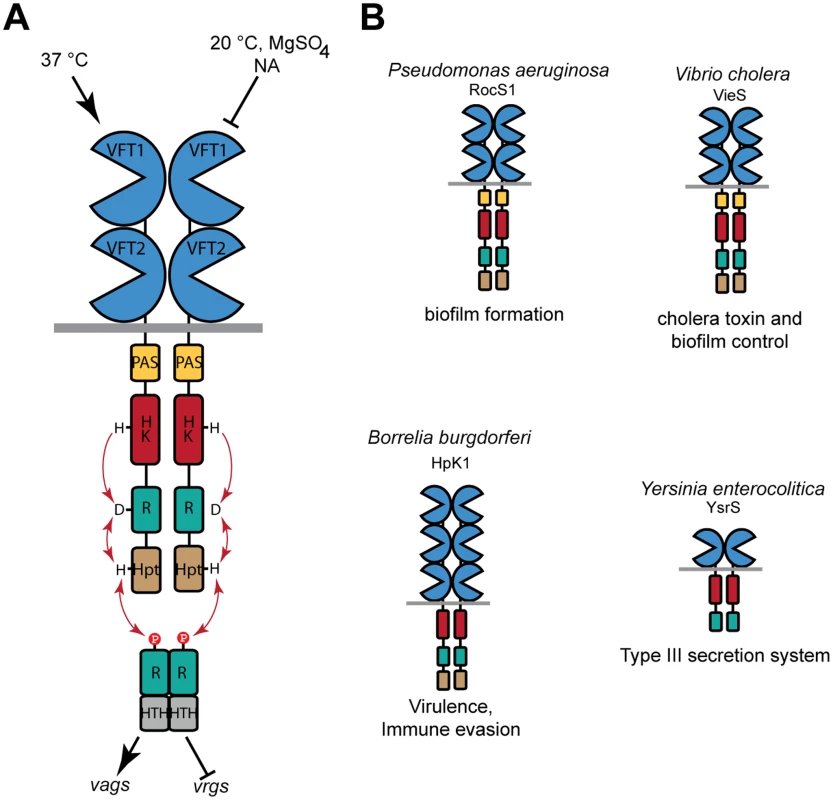
A. Schematic representation of virulence regulation by BvgAS in B. pertussis. Only the virulent (Bvg+) and avirulent (Bvg-) phases of the bacterium are represented for simplicity. Conditions that turn the bacteria to the avirulent phase include low temperatures and negative modulators such as sulfate or nicotinate (NA) ions. The vags (virulence-activated genes) are trans-activated by phosphorylated BvgA, while the vrgs (virulence-repressed genes) are upregulated in the avirulent phase. An intermediate phase occurs at low modulator concentrations (see text). From N to C terminus, 135 kDa-BvgS is composed of two periplasmic VFT domains, a transmembrane segment, a PAS domain, followed by a histidine-kinase (HK), a receiver (R) and a Histidine phosphotransfer (Hpt) domains that make up a phosphorelay (represented by arrows). BvgA is composed of a receiver domain and a helix-turn-helix DNA-binding domain (HTH). B. Structural organization of selected BvgS homologs, with the same color code as for BvgS. Note that the domain composition varies in the family. The cellular functions regulated by these sensor-kinases are also indicated. BvgS is the prototype of a family of bacterial VFT-domain-containing sensor-kinases [24]. VFT domains have a bi-lobed structure with two mobile jaws delimitating a putative ligand-binding cavity [25,26]. They exist in open and closed conformations that interconvert by clamshell motions. Typically, binding of a ligand in the cavity stabilizes the closed conformation, which triggers downstream cellular events such as transport or signaling. The periplasmic moiety of BvgS is composed of two VFT domains, membrane-distal VFT1 and membrane-proximal VFT2. We have previously reported the structure of the isolated VFT2 domain and showed that nicotinate and related negative modulators bind to VFT2 [27]. There are currently more than 2000 predicted BvgS homologs, containing from one to five VFT domains. Some of them are found in major pathogens, including Pseudomonas aeruginosa, Vibrio cholerae, Yersinia enterocolitica and Borrelia burgdorferi, in which they regulate various responses that contribute to pathogenicity [28–32] (Fig. 1B). Unlike those of classical TCSs, the molecular mechanisms of signal perception and transduction by these VFT-containing sensor-kinases are largely unknown.
In this work, we describe the structure of the periplasmic portion of BvgS, revealing a novel homo-dimeric architecture with two highly intricate polypeptide chains wound around each other. A combination of site-directed mutagenesis, functional analyses in vivo and molecular modeling indicated that the integrity of the periplasmic domain is necessary both to maintain BvgS in a kinase-on state by default and to bring about conformational changes that switch the protein to the kinase-off state in response to negative modulation. This study shows that BvgS represents a new paradigm of bacterial two-component sensor-kinases and contributes to our understanding of virulence regulation in Bordetella.
Results
Structure of the periplasmic domain of BvgS
The periplasmic domain of BvgS (residues Ala29-Leu544, which includes VFT1 and VFT2) was produced in Escherichia coli and crystallized as a recombinant protein with a 60-residue-long GB1 domain at the N terminus and a 6-His tag at the C terminus. The structure was solved to a resolution of 3.1 Å (Fig. 2, S1 Table). BvgS forms intricate butterfly-shaped dimers in which the A and B polypeptide chains (‘protomers’) wind around each other, with an extensive dimeric interface of ≈ 4000 Å2. The two protomers overlap with an RMSD of 1.184 Å. The N-terminal GB1 domain and C-terminal His tag are not visible in the electron density maps.
Fig. 2. General organization of the BvgS periplasmic domain. 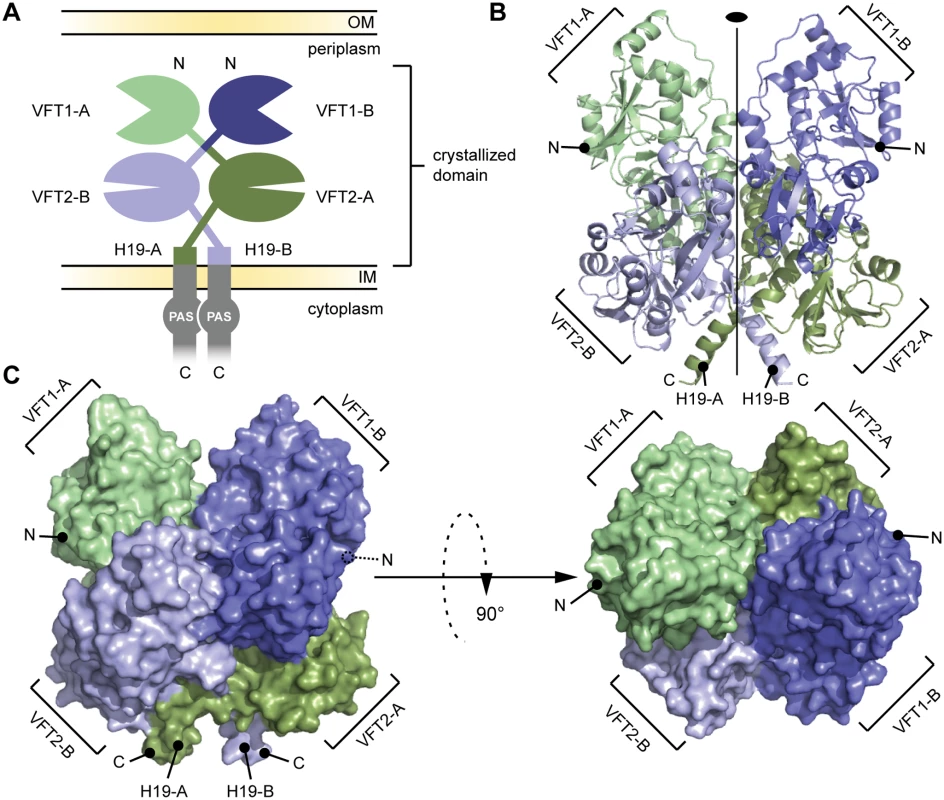
A. Schematic representation of the homodimeric BvgS periplasmic portion. The protomers A and B are shown in shades of green and blue, respectively. One protomer consists of two VFT domains and a C-terminal H19 α helix. B. Ribbon representation of the X-ray structure of the BvgS periplasmic domain, the same color code as in (A) is used to show the different VFTs. The two-fold symmetry axis is indicated. C. Surface representation of the periplasmic domain of BvgS. On the left, the view angle is similar to (B), while on the right, a 90° clockwise rotation along the x-axis was applied. N and C denote the N and C termini of the two protomers. A two-fold symmetry axis runs parallel to the long axis of the BvgS dimer. The N termini of the two protomers are located on the outer surface of the dimer, and their C termini interrupt α helices at the membrane-proximal end of the structure. VFT1 and VFT2 adopt typical Venus flytrap architectures consisting of two α/β subdomains called lobes 1 and 2 (hereafter L1 and L2) separated by a cleft. They have similar topologies with two crossings between the lobes (S1 Fig). The hinge is formed of anti-parallel β strands in VFT2 and flexible loops in VFT1. The VFT2s are followed by the C-terminal (Ct) domains that encompass the Gly527-Pro532 Ct loops and the H19 Ct helices (Figs. 2 and S1). In the absence of membrane constraints, the H19s adopt divergent orientations in the crystal structure. In full-length BvgS they are predicted to continue across the membrane down to the cytoplasmic PAS domain, with a total length of 60 residues.
The two VFT1s are open, while atypically the VFT2s are closed with no ligand in their inter-lobe cavities (Fig. 3), consistent with the structure of VFT2 alone [27]. The VFT1 cavities are each oriented toward the hinge of the VFT2 domain of the other protomer, and the cavities of the VFT2s are each oriented toward the H19 helix of the opposite protomer (Fig. 3).
Fig. 3. Characterization of the VFT domains. 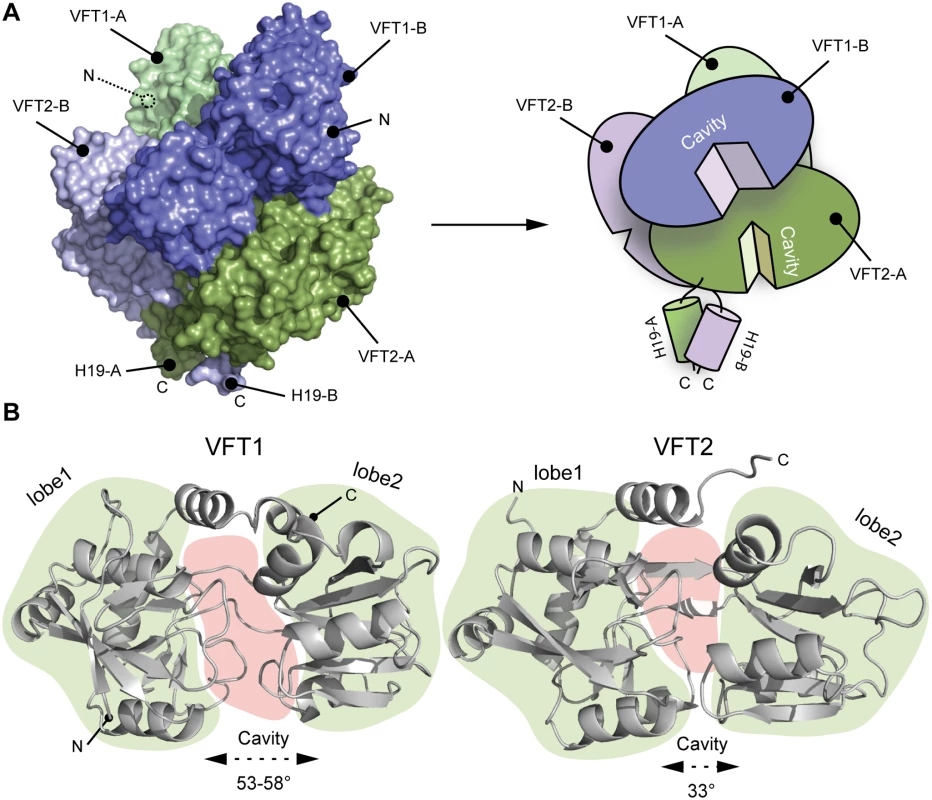
A. Surface and cartoon representation of BvgS showing that VFT1-B is open and VFT2-A is in an apo-closed conformation. B. Ribbon representation of the open VFT1 and closed VFT2 domains. The lobes are delimited in light green and the cavities in light red. The opening angles for the VFTs are given. The linker (H9) joining VFT1 and VFT2 and the Ct loop that follows VFT2 have been included in the representation of the VFT1 and VFT2 domains, respectively. N and C indicate the N and C termini of each protomer (in A) or VFT domain (in B). The VFT1L1s interact with each other through several hydrogen bonds between their H8s, while the VFT2s are not directly interconnected. Both lobes of the VFT1s, VFT1L1 and VFT1L2, contact the hinge and lobes of VFT2 of the opposite protomer (Fig. 4), forming the largest dimeric interfaces. Other large interfaces occur between VFT1L2 and VFT2 of the same protomer, and between VFT2L2 and the Ct domains. In particular, both the Ct loop and the N terminus of H19 strongly interact with VFT2L2 of the opposite protomer through hydrogen bonds and through π-stacking interactions that involve a conserved residue in the BvgS family, Trp535 (Fig. 4).
Fig. 4. Interfaces between the VFT domains important for the kinase-on state. 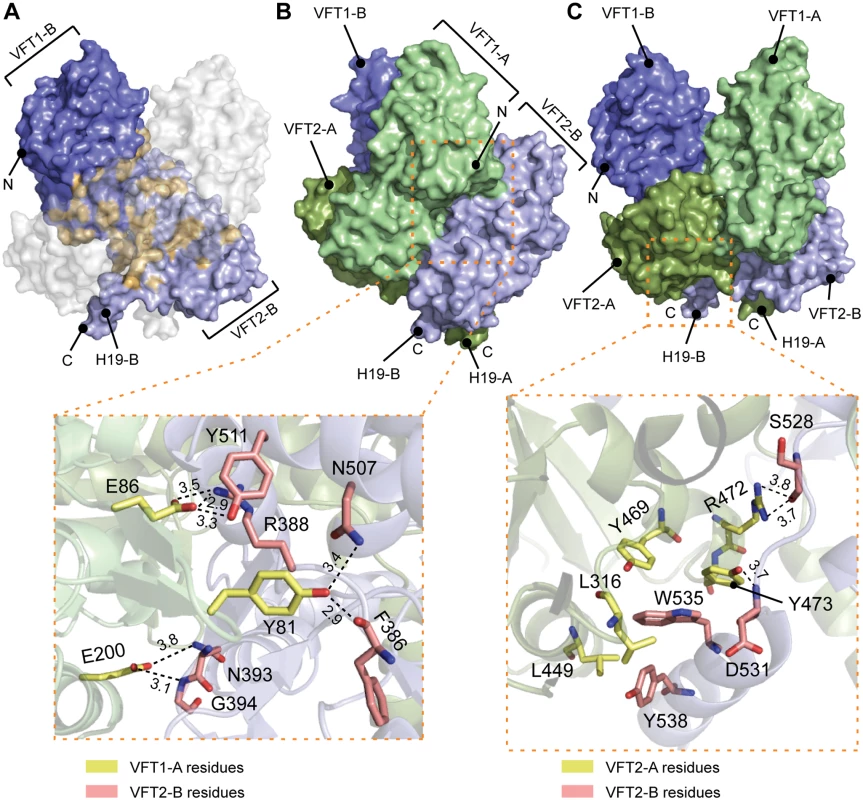
A. Surface representation of protomer B (in blue); the residues interacting with protomer A are shown in orange. To help visualizing these interactions, a “ghost” protomer A is represented in transparent white on top of protomer B. B. Illustration of the VFT1-VFT2 inter-protomer interface. A side view of BvgS is shown in surface representation, with the VFT1 of one protomer in green and the VFT2 of the other protomer in pale blue. A zoom delimited by a dashed orange box shows specific residues that are critical for BvgS function, as shown by mutagenesis. The side chains of Tyr81 and Glu86 of the β hairpin in VFT1L1 form hydrogen bonds with Phe386 and Arg388 at one extremity of the VFT2 hinge, and with residues of the α helix H17. Glu200 belongs to VFT1L2, and its side chain makes hydrogen bonds with Asn393 and Gly394 at the other extremity of the VFT2 hinge. C. Illustration of the VFT2-Ct domain inter-protomer interface. In the upper panel, BvgS is shown in surface representation, with protomer A in green and protomer B in blue. A zoom shows specific residues involved in critical interactions for BvgS kinase activity. Thus, Trp535 from H19 stacks in a hydrophobic and aromatic pocket mainly lined with VFT2L2 residues of the other protomer, and Arg472 and Tyr473 from helix H16 in VFT2L2 interact with Ser528 and Asp531 in the Ct loop of the other protomer. Hydrogen-bond distances are reported in angstroms. Conformation and dynamics of the VFT domains
In the crystal structure, the VFT1 domains are open and unliganded, while conversely the VFT2 domains are closed without ligands. We performed normal mode analyses of BvgS motions based on a Gaussian network model to identify the main global motions that are accessible to the protein based on its tridimensional structure. The first, lowest-frequency normal modes are usually most relevant to function. For BvgS, the first two modes of motion consist of large motions of one VFT1L1 (S2 Fig). In contrast, the VFT2s move together as a rigid body, as shown in mode #3. Mode #4 consists of motions of both VFT1L2s together with the VFT2s. Thus, the first lobes of the VFT1s in particular can make large motions, while the VFT2 motions are more restrained and mainly coupled to each other and to those of the VFT1s. This was confirmed by performing molecular dynamics simulations to measure the evolution of the opening angles of the VFTs over time. In the first parts of the simulations, the VFT1s make clamshell motions, while motions of the VFT2s are limited around their closed conformations (S2 Fig). As the simulations progress the VFT1 mobility is reduced, which suggests that sustained VFT motions may require the feedback from the transmembrane and cytoplasmic portions of BvgS absent from our model. These in silico analyses thus indicate that the X-ray structure reflects bona fide differences between the VFT1 and VFT2 domains in terms of conformation and dynamics.
We then asked whether VFT1 closing—as might happen upon binding of a ligand—would affect BvgS activity. We locked the VFT1 domains in closed conformations by generating a disulfide (S-S) bonds across their cavity [33,34]. Two residues located on the edges of the lobes were replaced by Cys to obtain BvgSE113C+N177C (S3 Fig). The corresponding point mutations were inserted into the chromosomal bvg locus by allelic exchange, and we verified the production of the protein and the formation of the S-S bond by immunoblotting (S4 Fig). The in vivo effect of the substitution on BvgS function was then measured by using a reporter system with the lacZ gene under the control of the Bvg-regulated ptx promoter [35]. In vivo formation of the S-S bond in VFT1 abrogates the kinase activity of BvgS (Fig. 5). This phenotype is reverted by the addition of a reducing agent, TCEP, to the growth medium (S5 Fig), which confirms that the S-S bond forms in vivo and shows that the loss of function is related to its presence and not to the Cys substitutions.
Fig. 5. In vivo effects of the substitutions in BvgS. 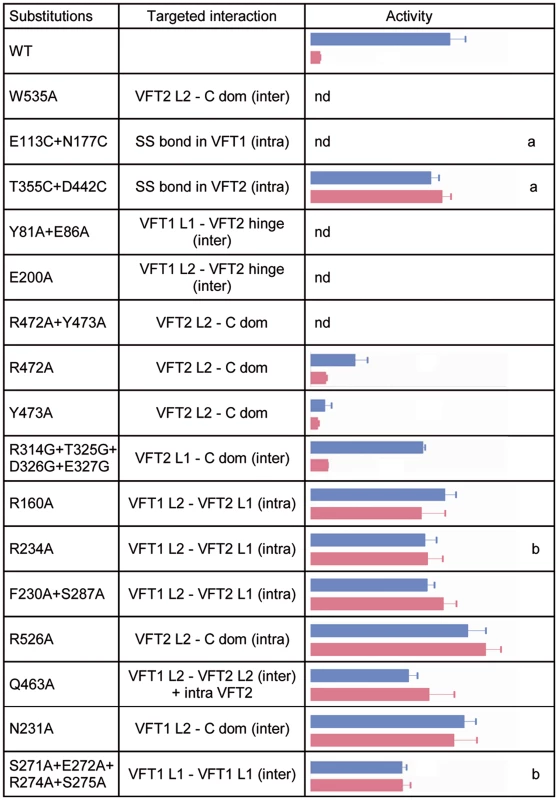
A lacZ reporter gene under the control of the Bvg-regulated ptx promoter was used for determination of BvgS kinase activity in standard or modulated culture conditions. Blue and pink bars indicate kinase activity levels of bacteria producing the indicated BvgS variants and grown without or with 8 mM nicotinate, respectively, with the standard errors of the mean calculated from three distinct experiments. The middle column indicates the interfaces in which the targeted interactions are located, with inter- and intra-protomer interfaces designated ‘inter’ and ‘intra’, respectively. Nd, no β-gal activity detected; a, wild type activity and/or modulation recovered when cells were grown in the presence of TCEP; b, BvgS variants only responsive to high nicotinate concentrations (20 mM). The full set of data is shown in S5 Fig. The VFT2s remain closed even when isolated [27]. Nevertheless, to maintain them closed in vivo we also generated an S-S bond between their lobes using a similar method as above, yielding BvgST355C+D442C. We checked that the S-S bond was formed (S4 Fig; see also below). In contrast to VFT1, closing VFT2 was found to have no effect on the BvgS kinase activity as determined with the ptx-lacZ reporter (Figs. 5 and S5). Altogether thus, closing of the VFT1 domains and/or restraining their mobility abrogate BvgS kinase activity. In contrast, closed VFT2 domains correspond to the kinase-on state of BvgS. The different conformations and dynamics of the two VFT domains thus contribute to BvgS function.
Importance of periplasmic domain integrity for BvgS kinase activity
B. pertussis is in the virulent, Bvg+ phase by default at 37°C. To determine the role of the periplasmic domain of BvgS in maintaining this kinase-on state, we loosened the connections between the periplasmic and cytoplasmic moieties of BvgS by replacing Trp535 with Ala. This residue is located in the C-terminal helix H19 and it contributes to connecting each H19 to the VFT2L2 of the opposite protomer (Fig. 4C). After allelic exchange, the effect of the substitution on BvgS function was measured by using the ptx-lacZ reporter system. The BvgSW535A variant has no kinase activity (Fig. 5). The presence of BvgSW535A in B. pertussis membranes was verified, showing that the substitution does not affect the structure of the protein in such a way as to prevent its integration in the membrane or to cause its proteolytic degradation in vivo (S4 Fig). Thus, the kinase-on state of BvgS depends on tight connections between the periplasmic domains and the transmembrane H19 helices.
To confirm that the periplasmic portion imposes a specific conformation on the cytoplasmic moiety, we introduced other substitutions in the inter-protomer interfaces between the VFT2s and the Ct domains, by targeting residues whose side chains connect the VFT2L2s and the Ct loops that precede the H19s (Fig. 4C). Thus, Arg472 and Tyr473 located in helix H16 of VFT2L2 form hydrogen bonds with residues of the Ct loop of the other protomer. Their simultaneous replacement by Ala abolishes BvgS kinase activity, while the single-substitution variants BvgSR472A and BvgSY473A are partially active (Figs. 5 and S5). This indicates that the inter-protomer interface between H16 in VFT2 and the Ct loop is critical and that it is maintained by partly redundant interactions. In contrast, substitutions at the tip of a β hairpin in VFT2L1 whose residues interact with the other face of the Ct loop do not affect BvgS function, as shown with BvgSR324G/T325G/D326G/E327G. The effect of disrupting of specific interactions between the VFT2L2s and the Ct loops preceding the H19s is consistent with the effect of the W535A substitution, showing that the kinase-on state depends on VFT2-Ct domain inter-protomer connections.
To identify additional architectural features of the periplasmic dimer critical to maintain BvgS in its kinase-on state, we disrupted specific interactions in other intra-dimer interfaces of BvgS by site-directed mutagenesis. We targeted residues in the large interfaces between the VFT1s and the VFT2s of the opposite protomers (Fig. 4B). The side chains of Tyr81 and Glu86 in a β hairpin of VFT1L1 and that of Glu200 in helix H5 of VFT1L2 form hydrogen bonds with residues at the N - and C-terminal sides of the first hinge strand of VFT2, respectively. Two BvgS variants, BvgSY81A+E86A and BvgSE200A were generated and analyzed as above (Figs. 5, S4 and S5). Neither of them is functional, demonstrating that connections between the two lobes of VFT1 and the hinge of VFT2 of the opposite protomer are essential to maintain the kinase-on state of BvgS. In contrast, the replacement of Gln463 by Ala in the same large inter-protomer VFT1-VFT2 interface does not affect activity (Figs. 5 and S5). Gln463 is part of VFT2 but not located in the hinge, unlike the residues of VFT2 in contact with Tyr81, Glu86 and Glu200. The loss of kinase activity of the BvgSY81A+E86A and BvgSE200A variants might result from the loss of constraints applied by the VFT1 lobes on the VFT2 hinge.
In contrast, disruption of specific interactions in other dimeric interfaces (S3 Fig), including the H8-mediated VFT1-VFT1 inter-protomer interface, the VFT1-VFT2 intra-protomer interfaces, the VFT2-Ct domains intra-protomer interfaces or the VFT1-Ct domains inter-protomer interfaces, does not markedly affect Bvg kinase activity (Figs. 5 and S5).
Altogether, thus, we have identified interactions in the inter-protomer interfaces between VFT1 and the VFT2 hinge and between VFT2L2 and the Ct domain that are necessary to maintain BvgS in its kinase-on state. In particular, the substitutions A472A+Y473A and W535A support the idea that the periplasmic domain exerts a strain on the transmembrane domains, causing the cytoplasmic moiety to adopt a specific conformation corresponding to the kinase-on state. The VFT1s contribute to the strain via the close contacts of their two lobes with the hinges of the tight VFT2 domains. Loosening the periplasmic portion or its connections with the transmembrane helices releases the strain, and therefore the cytoplasmic moiety switches to a distinct, kinase-off state.
Modulation by nicotinate requires multiple intra-dimer interactions
Negative modulators turn BvgS to the kinase-off state at millimolar concentrations in laboratory conditions, and they possibly mimic in vivo ligands that might decrease or turn off virulence genes expression at specific stages of the infection. The sites of interaction of these negative modulators are mostly unknown. We have shown that nicotinate binds to isolated VFT2, even though additional sites cannot be ruled out in the dimer [29], and therefore we used nicotinate to determine how the periplasmic moiety contributes to the response of BvgS to negative modulation. The ability of the BvgS variants described above to respond to nicotinate was thus assessed.
The BvgST355C+D442C variant with a S-S bond across the VFT2 cavity variant is unresponsive to nicotinate but reverts to the wild type (wt) modulation phenotype when the growth medium is supplemented with TCEP (Figs. 5 and S5). This confirms the in vivo formation of the S-S bond and also shows that it, rather than the Cys substitutions, hampers the response to nicotinate. The S-S bond might prevent nicotinate from binding or hamper a conformational changes involved in the response to the negative modulator.
A number of other substitutions similarly abrogate the effect of nicotinate (Figs. 5 and S5). Interestingly, both inter-protomer and intra-protomer interactions are required for BvgS response to negative modulation. These interactions map to the VFT1L1-VFT1L1, VFT1L2-VFT2L1, and VFT1L2-VFT2L2 inter-protomer interfaces and to the VFT1L2-VFT2L1 and VFT2L2-Ct domain intra-protomer interfaces (Figs. 5 and S3). Altogether, a large set of both inter - and intra-protomer interactions is required for the response of BvgS to nicotinate. The fact that the response to negative modulation strongly depends on the integrity of the periplasmic moiety indicates that the transition from the kinase-on state to the kinase-off state implies a concerted conformational change.
Function of BvgS heterodimers
The importance of the structural integrity of the periplasmic domain for the kinase-on state and for the transition to the kinase-off state was further probed by generating in vivo BvgS heterodimers that harbor one wt periplasmic domain and another one with a substitution. A merodiploid containing two inactive but complementary bvgS copies, one with a substitution of the phosphorylable Asp of the receiver domain (D1023N) and the other with a substitution of the phosphorylable His of the Hpt domain (H1172Q), will form inactive homodimers and active heterodimers (Fig. 6) [36,37]. Indeed, only heterodimers will be able to restore the phsophorylation cascade of BvgS. We set up this merodiploid expression system in B. pertussis. As shown in Fig. 6, the homodimers formed by BvgSD1023N or by BvgSH1172Q are inactive using the ptx reporter, but the heterodimer BvgSD1023N/H1172Q is functional, displaying kinase activity in the default state and responding to nicotinate like wt BvgS.
Fig. 6. BvgS heterodimers. 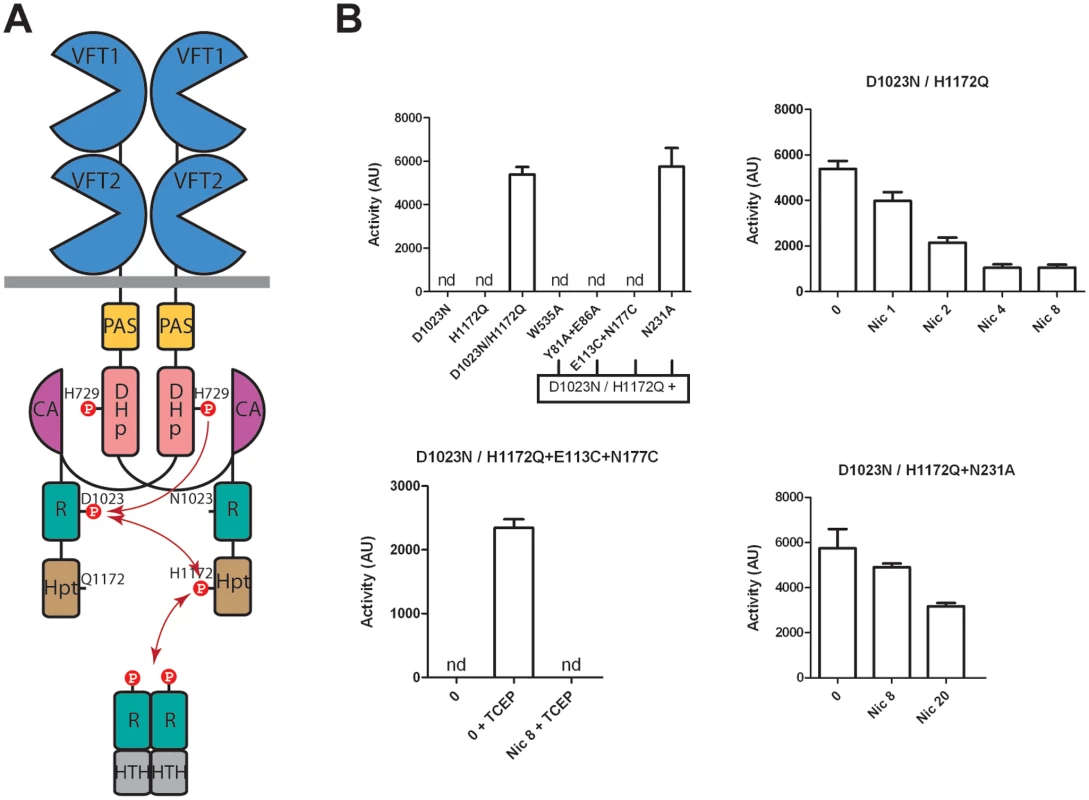
A. Schematic representation of the BvgS heterodimers. The dimerisation/Histidine phosphotransfer domain (DHp) and the catalytic ATP-binding domain (CA) of the kinase are represented separately to show the phosphorylation cascade (arrows). B. Kinase activity levels as determined using the ptx-lacZ reporter for B. pertussis harboring the indicated BvgS variants and grown in standard or modulation conditions. The first panel shows the activities of the various strains. The first two express inactive homodimers, and the last four express heterodimers in which one protomer harbors a wt periplasmic portion combined with the D1023N substitution and the other protomer harbors the indicated periplasmic substitution(s) combined with the H1172Q substitution. The last three panels show the β-gal activities of the strains expressing the indicated heterodimers, with the standard errors of the mean calculated from three distinct experiments. Nicotinate (nic) and TCEP were added at the indicated concentrations (in mM). nd, no activity detected. We disrupted critical contacts in one side of the dimer by combining the W535A variant with the wt periplasmic protomer. The kinase activity of BvgS was measured using the ptx-lacZ system as above. The resulting BvgS homodimer is not functional, similar to the homo-dimeric BvgSW535A variant (Fig. 6). Another variant that harbors the Y81A+E86A substitutions in the inter-protomer VFT1-VFT2 interface was similarly combined with the wt periplasmic moiety. The heterodimer is also not functional, a phenotype similar to that of the BvgSY81A+E86A homodimer (Fig. 6). Both results support the model that the periplasmic architecture and more specifically the crucial inter-protomer interfaces identified above impose a kinase-on conformation onto the cytoplasmic moiety via the H19 helices. Releasing the strain in one half of the dimer is sufficient to lose the kinase-on conformation.
We also combined the wt periplasmic moiety with that harboring a S-S bond across the VFT1 cavity. The resulting BvgS heterodimer has no kinase activity (Fig. 6). Thus, both protomers must have the proper conformation and dynamics for BvgS function.
We finally used the heterodimer strategy to test the effect of a substitution that makes BvgS unresponsive to nicotinate. We thus combined a protomer harboring a wt periplasmic domain with that harboring the N231A substitution. Asn231 from VFT1L2 makes interactions with the Ct loop of the other protomer (S3 Fig), and the BvgSN231A homodimer does not respond to nicotinate (Figs. 5 and S5). The recombinant strain expressing the heterodimer has β-galactosidase activity and interestingly, its sensitivity to nicotinate is partially restored. Thus, the heterodimer responds to 20 mM nicotinate, although it is not fully modulated (Fig. 6). This intermediary phenotype indicates that the transition to the kinase-off state requires higher modulator concentrations when the integrity of the periplasmic domain is slightly compromised.
Discussion
Although the BvgAS system was identified more than 25 years ago [38], the mode of regulation of Bordetella virulence has remained a puzzle. With its kinase-on state by default and its extracytoplasmic domain different from those of classical ‘PDC’ (for PhoB/ DcuS/CitA) TCS sensor-kinases, BvgS was initially considered an oddity. However, the realization that many bacterial sensor-kinases harbor similar sensor domains and the first clues about its structure and mode of action have made BvgS a model for the family [23,24,27]. Importantly, some of the BvgS homologs are found in major pathogens, including other Bordetella species as well as P. aeruginosa, E. coli, V. cholerae, Y. enterocolitica and B. burgdorferi, in which they control programs such as biofilm formation, efflux pump expression, type III secretion, or nutritional adaptation [28–32]. The BvgS structure establishes the foundations to decipher the molecular mode of action of this poorly characterized family of VFT-containing sensor-kinases, and it may pave the way to develop new, highly specific, anti-infective therapeutic strategies [39].
Our functional analyses based on the BvgS structure support the following model. Specific inter-protomer interactions are necessary to maintain the kinase-on state. The tight architecture of the periplasmic moiety together with the differential dynamics of the VFTs imposes a strain onto the transmembrane H19 helices. In response, the cytoplasmic moiety, beginning with the PAS domain, adopts specific conformation and dynamics that support the kinase and phosphotransfer activities of BvgS. The bacteria are thus in the virulent, Bvg+ phase, and they can establish an infection. Switching BvgS to the kinase-off state involves a conformational change of the periplasmic moiety, which modifies the conformation, and possibly the dynamics, of the downstream cytoplasmic PAS and kinase domains. The roles of the avirulent or intermediate phases of B. pertussis are unclear, and in vivo stimuli that may trigger the shift to phosphatase or lower kinase states of activity remain to be identified. However, this work shows that the shift to the kinase-off state can easily be hampered by point mutations at various periplasmic sites. That the ability to reversibly perform the shift that regulates BvgS activity has been conserved through evolution supports the importance of the avirulent or intermediate phases in the lifestyle of B. pertussis. It also strongly argues that the VFT domains perceive negative in vivo signals, which explains the good conservation of their cavities in Bordetella [35].
As shown in this work, one can artificially turn BvgS to the kinase-off state by disrupting specific inter-protomer interactions between the VFT2 domains and the H19 helices. The release of constraints on these helices causes the cytoplasmic portion to adopt an alternative conformation in which BvgS functions as a phosphatase. We have shown that other events putatively relevant to BvgS function, i.e. the closing of VFT1 domains, which might mimic the binding of a ligand, or the binding of nicotinate to VFT2 [27], also turn BvgS to the kinase-off state. Both most likely cause conformational—and/or dynamic - changes to the periplasmic domain, with repercussions below the membrane. A number of BvgS variants with looser connections between the VFT domains are blocked in the kinase-on state and cannot respond to nicotinate, which shows that the shift to the kinase-off state implies a concerted conformational change. Modulation therefore facilitates the transition by shifting the equilibrium from the kinase-on to the kinase-off conformations. It is likely that these two stable states will also differ in their dynamics, and we have indeed obtained preliminary indications that VFT1 dynamics is modified in the modulated state. Similarly, VFT1 dynamics probably contributes to the transition, in line with the emerging paradigm that the dynamics of signaling proteins relates to their function [40].
In the default situation—i.e., at 37°C and without modulators-, the equilibrium is strongly shifted towards the kinase-on state of BvgS, which is therefore fully populated, while conversely the equilibrium is strongly shifted towards the kinase-off state in the presence of high modulator concentrations. This two-state model is compatible with intermediate levels of activity of the BvgAS system, such as those obtained at intermediate modulator concentrations [15], in which kinase-on and-off BvgS proteins may co-exist in equilibrium. It is also most likely the case for the BvgSwt/N231A heterodimer, in which the lack of a critical interaction on one side of the dimer hampers the transition, and therefore only a proportion of the BvgS molecules shift to the kinase-off state at high modulator concentrations. The transition between the two conformations will likely imply relative rotation, translation or shearing movements of the helices that join the periplasmic and cytoplasmic domains, similar to what has been proposed in other signaling proteins [41–44].
With its clamshell motions, VFT1 behaves like a typical VFT domain. As stated above, the conservation of the VFT1 cavity residues in Bordetella [35] suggests that it binds specific ligand(s) in vivo, and if so our results show that ligand binding to VFT1 will likely cause BvgS to shift to the kinase-off state. In contrast, the VFT2s remain closed in the kinase-on state with no bona fide ligand in their cavity. Whether nicotinate binding to VFT2 opens the cavity or causes another type of deformation remains unknown, but the thermal stabilization of VFT2 upon nicotinate binding argues against the former possibility [27]. The crystal structure of the single VFT domain of a BvgS homolog, the HK29 histidine-kinase of Geobacter sulfurreducens interestingly shows that this VFT is also closed unliganded [45]. Its hinge is composed of two β strands, like that of VFT2 in BvgS, leading those authors to propose that it might not be able to open. Sequence analyses of BvgS homologs indicate that the regions forming the hinge of the membrane-proximal VFT domain contain fewer Gly and more Pro residues than those of classical VFT domains. Therefore, we speculate that in the BvgS family the membrane-proximal VFT domain should be closed and tight for the regulation of sensor-kinase activity. BvgS also responds to various organic and inorganic ions [46]. The binding of these modulating molecules might not necessarily involve the cavity but possibly also interfaces, as in some other VFT-containing receptors [47,48].
The periplasmic moiety of BvgS adopts a highly compact dimeric structure. The helical and strongly intertwined architecture of BvgS may explain how some of its homologs could be functional with three, four or even five predicted VFT domains in tandem [24]. The multiple VFT domains of these sensor-kinases potentially enable the perception of several chemical signals that must be integrated to determine the appropriate response. A compact structure like that of BvgS appears to be better suited for inter-domain communication than more linear arrangements such as those found in the VFT-based iGlu receptors of higher eukaryotes, which might dissipate information coming from the most distal VFT domains [49,50] (S6 Fig). This study of BvgS will undoubtedly serve as a basis to elucidate the function of the other family members. Not all BvgS homologs are in a kinase-on state by default [51], but our mechanistic model can perfectly accommodate sensor-kinases that are regulated in the opposite manner.
Materials and Methods
Crystallization of BvgS, data collection and processing
The bvgS sequence was amplified by PCR and introduced into pGEV2 [52]. The resulting plasmid encodes the periplasmic portion of BvgS (A29-L544) with N-terminal GB1 and C-terminal His tags. The recombinant protein was purified on a Ni2+-Sepharose affinity column (GE Life Sciences) and eluted in 10 mM Tris—HCl (pH 8.8), 500 mM NaCl, 200 mM at 4°C. BvgS was concentrated by ultrafiltration to 20 mg/mL. The initial crystallization screening was carried out using the sitting-drop, vapor-diffusion technique in 96-wells microplates with a Cybi-Workstation (Cybio) and commercial crystallization kits (Nextal-Qiagen and JBSscreen). Extremely fragile crystals were obtained at 19°C by manual refinement in 100 mM sodium acetate (pH 4.6), 1.6 M NaCl, in 5 to 7 days. All manual crystallization attempts were carried out using the hanging-drop, vapor-diffusion technique in 24-well plates. The crystals were soaked in a stepwise fashion to a final concentration of 20% glycerol in the crystallization buffer.
A preliminary diffraction screening was performed on 80 crystals. On the best crystal, diffracting at 3.10 Å, a single diffraction dataset (160 images with an oscillating range of 1°) was collected at an X-ray wavelength of 1.5418 Å and a temperature of 100 K using an in-house Mardtb goniostat and a Mar345 image plate detector. Diffraction images were indexed and scaled using the XDS program package [53]. The crystal belongs to the space group P212121, with cell parameters a = 72 Å, b = 286 Å and c = 128 Å. According to the calculated Matthews coefficient of 2.52 Å3 Da-1, a solvent content of 51.3% was estimated.
Structure determination and refinement
The crystal structure containing four monomers in the asymmetric unit was determined by molecular replacement using MOLREP [54] and the crystallographic structure of the isolated VFT2 domain (PDB code: 3MPK) as a search model. Eight copies of the model were located, four occupying the actual positions of VFT2 domains and the other four those of VFT1 domains, whose sequence identity to VFT2 is 24%. The former four copies were positioned using the conventional Patterson search. The latter four copies were found using an iterative procedure alternating refinement of a partial structure with REFMAC [55] and molecular-replacement search in the electron density maps [56]. Subsequent model rebuilding and refinement of the 3.10 Å structure were conducted iteratively using Coot [57] and phenix.refine [58], with the use of local non-crystallographic symmetry restraints. Torsion angles of the structure were optimized by using the Godzilla web server (http://godzilla.uchicago.edu/) [59]. The structure was refined to final Rwork of 18.1% and Rfree of 24.4%. The two BvgS homo-dimers (AB and CD) found in the asymmetric unit can be superimposed with a Cα rmsd of 1.234 Å. A Ramachandran analysis performed with the program Phenix indicated that 94.4% of residues are in preferred conformations and 1.4% in disallowed conformations. The GB1 domains are not seen in the electron density. Analysis of crystal packing revealed an empty space close to the N-terminal segment of each polypeptide chain, indicating that they might be unseen because of crystallographic disorder.
Structure analyses
The 1026-residue AB dimer was used for all analyses. The opening angles for VFT1 and VFT2 were measured using three residues structurally equivalent between the two VFTs, one on the lip of each lobe and one in the hinge. They are Tyr70, Gly244 and Ser199 for VFT1 and Leu314, Glu490 and Pro444 for VFT2. The inter-domain interfaces were defined using the PISA server (http://www.ebi.ac.uk/pdbe/prot_int/pistart.html) [60], and http://capture.caltech.edu/ was used to identify cation-π interactions. A model for the closed VFT1 domain was made with Modeller [61] based on its closest homologous structure (PDB code: 1WDN), and residues to be replaced by cysteines were chosen by using http://cptweb.cpt.wayne.edu/DbD/ [62].
Molecular modeling
The methods used for the normal mode analysis and the molecular dynamics simulations with their associated references are described in S1 Protocol. For the analyses of the MD simulations, the opening angles of the VFT domains were calculated based on the geometric centers of the C-α atoms of each lobe and the hinge using a slightly extended definition of the hinge region, encompassing residues 146–151 and 241–246 for that of VFT1, and 390–395 and 486–491 for that of VFT2, to make them less susceptible to noise.
Measurement of BvgS activity
Point mutations were introduced into the chromosome of B. pertussis BPSM by allelic exchange [35]. The BvgS sequence corresponds to that of TohamaI except for a Glu residue at position 705, as in most B. pertussis strains [35]. A ptx-lacZ transcriptional fusion was generated in each recombinant strain [63]. The strains were grown in modified Stainer-Scholte medium [64] non-supplemented or containing 1 to 8 mM of nicotinate. Tris(2-carboxyethyl)phosphine (TCEP, SIGMA) was added to 3–10 mM where indicated. TCEP did not affect the activity or the response to nicotinate of wild type BvgS. The bacteria were grown to mid-exponential phase, harvested by centrifugation, resuspended to an OD600 of 5 and broken by using a Hybaid Ribolyser apparatus for 30 s at speed 6 in tubes containing 0.1 mm silica spheres. β-galactosidase activities were measured and calculated as described [63]. Each experiment was performed with 3 different clones at different times. The bars represent the standard errors of the mean.
Detection of inactive BvgS variants
The inactive proteins were detected by immunoblotting of B. pertussis membrane extracts using anti-BvgS polyclonal antibodies [23] to verify that the substitution(s) generated no major structural defect that might cause BvgS to misfold and to be degraded intracellularly. BPSMΔbvgA and BPMSΔbvgS were described previously [23,35].
Construction of heterodimers
To construct a bvgAS locus deletion strain from BPSM, a Tohama I streptomycin - resistant derivative, sequences on either side of the locus (i.e., the 5’ end of the fhaB gene and the 3’ end of the bvgR gene) were amplified by PCR using the pairs of oligonucleotides iEco-up and Xma-lo, and Xho-up and HindIII-lo (S2 Table). All the amplicons were first introduced into pCRII-TOPO (Invitrogen) and sequenced. The amplicons were introduced as EcoRI-XhoI and XhoI-HindIII fragments into pUC19 by performing a triple ligation, yielding pUC19newΔbvgAS. The EcoRI-HindIII insert was then introduced as in [35] into pSORTP1, a mobilizable plasmid for allelic replacement, resulting in BPSMnewΔ.
The bvgAS locus was then constructed as a mosaic gene for allelic replacement in BPSMnewΔ. We replaced the EcoRI-SpeI part of pUC19mos [35] using a triple ligation with the EcoRI-XmaI fragment obtained as above and a XmaI-SpeI fragment generated using the primers XmaI-up and SpeI-lo. In the latter amplicon, a natural EcoRI site was eliminated by site-directed mutagenesis with a synonymous mutation. The XbaI-HindIII part of pUC19mos was replaced by 3 fragments: a XbaI-NcoI PCR fragment generated using the primers XbaI-up and NcoI-lo, a NcoI-XhoI PCR fragment generated using the primers NcoI-up and XhoI-lo, and the XhoI-HindIII fragment described above. The latter fragment contains a natural NcoI site, which was eliminated as above. The final plasmid was called pUC19mint. The 5.5-kb EcoRI-HindIII insert of pUC19mint was transferred into pSORTP1 for allelic exchange.
A plasmidic construction of the bvgAS locus was also created starting from pUC19mint and replacing the EcoRI-SpeI fragment by that generated using the primers pEcoRI-up and SpeI-lo. The natural EcoRI site of this latter fragment was eliminated as above. Finally, the NcoI-HindIII fragment of pUC19mint was replaced by another fragment generated using the primers NcoI-up and pHindIII-lo, yielding pUC19mpla. The 4.7-kb EcoRI-HindIII insert was transferred into pBBR1-MCS4 [65], a low-copy, mobilizable and replicative plasmid.
The residues Asp1023 and His1172 were replaced by Asn and Gln, respectively, using site-directed mutagenesis (QuikchangeXL, Agilent). The first mutation was inserted in pUC19mint and then in pSORTP1 for allelic replacement in BPSMnewΔ. The second mutation was inserted in pUC19mpla and then in pBBR1-MCS4, yielding pBBRmpla to be introduced in Bordetella as an episome.
Successive conjugations were then performed to generate the merodiploids. The first one introduced pSORTP1 containing the bvgAS locus with the D1023N substitution into BPSMnewΔ, yielding an avirulent strain. Then, pFUS-S1 was integrated to generate the ptx-lacZ transcriptional fusion [63], and the resulting strain was finally transformed with pBBRmpla containing bvgAS with the H1172Q substitution. The mutations of the periplasmic domain were introduced via restriction fragment exchange in pUC19mpla and then in pBBRmpla.
Accession numbers
Atomic coordinates and structure factors for the BvgS periplasmic moiety have been deposited in the Protein Data Bank under the accession number 4Q0C.
Supporting Information
Zdroje
1. Stock AM, Robinson VL, Goudreau PN (2000) Two-component signal transduction. Annu Rev Biochem 69 : 183–215. 10966457
2. Bekker M, Teixeira de Mattos MJ, Hellingwerf KJ (2006) The role of two-component regulation systems in the physiology of the bacterial cell. Sci Prog 89 : 213–242. 17338439
3. Wuichet K, Cantwell BJ, Zhulin IB (2010) Evolution and phyletic distribution of two-component signal transduction systems. Curr Opin Microbiol 13 : 219–225. doi: 10.1016/j.mib.2009.12.011 20133179
4. Szurmant H (2012) Essential two-component systems of Gram-positive bacteria. In: Two-component systems in bacteria. Gross R & Beier D, editors. Norfolk, UK: Caister Academic Press. pp. 127–147.
5. Smith CS, Vicente JJ, Ryan KR (2012) Cell cycle and developmental regulation by two-component signalling proteins in Caulobacter crescentus. In: Two-component systems in bacteria. Gross R & Beier D, editors. Norfolk, UK: Caister Acad Press. pp. 269–291.
6. Keilberg D, Huntley S, Sogaard-Andersen L (2012) Two-component systems involoved in motility and development in Myxococcus xanthus. In: Two-component systems in bacteria. Gross R & Beier D, editors. Norfolk, UK: Caister Acad. Press. pp. 293–314.
7. Cotter PA, DiRita VJ (2000) Bacterial virulence gene regulation: an evolutionary perspective. Annu Rev Microbiol 54 : 519–565. 11018137
8. Clarke DJ (2012) The Rcs phospohorelay: biofilm formation and virulence in the Enterobacteriaceae. In: Two-component systems in bacteria. Gross R & Beier D, editors. Norfolk, UK: Caister Acad Press. pp. 333–353.
9. Ulrich LE, Zhulin IB (2007) MiST: a microbial signal transduction database. Nucleic Acids Res 35: D386–390. 17135192
10. Barakat M, Ortet P, Whitworth DE (2011) P2CS: a database of prokaryotic two-component systems. Nucleic Acids Res 39: D771–776. doi: 10.1093/nar/gkq1023 21051349
11. Casino P, Rubio V, Marina A (2010) The mechanism of signal transduction by two-component systems. Curr Opin Struct Biol 20 : 763–771. doi: 10.1016/j.sbi.2010.09.010 20951027
12. Melvin JA, Scheller EV, Miller JF, Cotter PA (2014) Bordetella pertussis pathogenesis: current and future challenges. Nat Rev Microbiol 12 : 274–288. doi: 10.1038/nrmicro3235 24608338
13. Cotter PA, Jones AM (2003) Phosphorelay control of virulence gene expression in Bordetella. Trends Microbiol 11 : 367–373. 12915094
14. Cummings CA, Bootsma HJ, Relman DA, Miller JF (2006) Species - and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. J Bacteriol 188 : 1775–1785. 16484188
15. Mattoo S, Foreman-Wykert AK, Cotter PA, Miller JF (2001) Mechanisms of Bordetella pathogenesis. Front Biosci 6: E168–186. 11689354
16. Melton AR, Weiss AA (1993) Characterization of environmental regulators of Bordetella pertussis. Infect Immun 61 : 807–815. 8432601
17. Boulanger A, Chen Q, Hinton DM, Stibitz S (2013) In vivo phosphorylation dynamics of the Bordetella pertussis virulence-controlling response regulator BvgA. Mol Microbiol 88 : 156–172. doi: 10.1111/mmi.12177 23489959
18. Knapp S, Mekalanos JJ (1988) Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J Bacteriol 170 : 5059–5066. 2903140
19. Stenson TH, Peppler MS (1995) Identification of two bvg-repressed surface proteins of Bordetella pertussis. Infect Immun 63 : 3780–3789. 7558280
20. Cotter PA, Miller JF (1997) A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol Microbiol 24 : 671–685. 9194696
21. Stockbauer KE, Fuchslocher B, Miller JF, Cotter PA (2001) Identification and characterization of BipA, a Bordetella Bvg-intermediate phase protein. Mol Microbiol 39 : 65–78. 11123689
22. Perraud AL, Rippe K, Bantscheff M, Glocker M, Lucassen M, et al. (2000) Dimerization of signalling modules of the EvgAS and BvgAS phosphorelay systems. Biochim Biophys Acta 1478 : 341–354. 10825546
23. Dupre E, Wohlkonig A, Herrou J, Locht C, Jacob-Dubuisson F, et al. (2013) Characterization of the PAS domain in the sensor-kinase BvgS: mechanical role in signal transmission. BMC Microbiol 13 : 172. doi: 10.1186/1471-2180-13-172 23883404
24. Jacob-Dubuisson F, Wintjens R, Herrou J, Dupré E, Antoine R (2012) BvgS of pathogenic Bordetellae: a paradigm for sensor kinase with Venus Flytrap perception domains. In: Two-component system in bacteria. Gross R & Beier D, editors. Norfolk, UK: Caister Academic Press. pp. 57–83.
25. Quiocho FA, Ledvina PS (1996) Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: variation of common themes. Mol Microbiol 20 : 17–25. 8861200
26. Trakhanov S, Vyas NK, Luecke H, Kristensen DM, Ma J, et al. (2005) Ligand-free and-bound structures of the binding protein (LivJ) of the Escherichia coli ABC leucine/isoleucine/valine transport system: trajectory and dynamics of the interdomain rotation and ligand specificity. Biochemistry 44 : 6597–6608. 15850393
27. Herrou J, Bompard C, Wintjens R, Dupre E, Willery E, et al. (2010) Periplasmic domain of the sensor-kinase BvgS reveals a new paradigm for the Venus flytrap mechanism. Proc Natl Acad Sci USA 107 : 17351–17355. doi: 10.1073/pnas.1006267107 20855615
28. Masuda N, Church GM (2002) Escherichia coli gene expression responsive to levels of the response regulator EvgA. J Bacteriol 184 : 6225–6234. 12399493
29. Sivaneson M, Mikkelsen H, Ventre I, Bordi C, Filloux A (2011) Two-component regulatory systems in Pseudomonas aeruginosa: an intricate network mediating fimbrial and efflux pump gene expression. Mol Microbiol 79 : 1353–1366. doi: 10.1111/j.1365-2958.2010.07527.x 21205015
30. Martinez-Wilson HF, Tamayo R, Tischler AD, Lazinski DW, Camilli A (2008) The Vibrio cholerae hybrid sensor kinase VieS contributes to motility and biofilm regulation by altering the cyclic diguanylate level. J Bacteriol 190 : 6439–6447. doi: 10.1128/JB.00541-08 18676667
31. Walker KA, Miller VL (2004) Regulation of the Ysa of the type III secretion system of Yersinia enterocolitica by YsaE/SycB and YsrS/YsrR. J Bacteriol 186 : 4056–4066. 15205407
32. Caimano MJ, Kenedy MR, Kairu T, Desrosiers DC, Harman M, et al. (2011) The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infect Immun 79 : 3117–3130. doi: 10.1128/IAI.05136-11 21606185
33. Blanke ML, VanDongen AM (2008) Constitutive activation of the N-methyl-D-aspartate receptor via cleft-spanning disulfide bonds. J Biol Chem 283 : 21519–21529. doi: 10.1074/jbc.M709190200 18450751
34. Zhu S, Stroebel D, Yao CA, Taly A, Paoletti P (2013) Allosteric signaling and dynamics of the clamshell-like NMDA receptor GluN1 N-terminal domain. Nat Struct Mol Biol 20 : 477–485. doi: 10.1038/nsmb.2522 23454977
35. Herrou J, Debrie AS, Willery E, Renaud-Mongenie G, Locht C, et al. (2009) Molecular evolution of the two-component system BvgAS involved in virulence regulation in Bordetella. PLoS One 4: e6996. doi: 10.1371/journal.pone.0006996 19750014
36. Beier D, Deppisch H, Gross R (1996) Conserved sequence motifs in the unorthodox BvgS two-component sensor protein of Bordetella pertussis. Mol Gen Genet 252 : 169–176. 8804390
37. Uhl MA, Miller JF (1996) Integration of multiple domains in a two-component sensor protein: the Bordetella pertussis BvgAS phosphorelay. Embo J 15 : 1028–1036. 8605872
38. Arico B, Miller JF, Roy C, Stibitz S, Monack D, et al. (1989) Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci USA 86 : 6671–6675. 2549542
39. Gotoh Y, Eguchi Y, Watanabe T, Okamoto S, Doi A, et al. (2010) Two-component signal transduction as potential drug targets in pathogenic bacteria. Curr Opin Microbiol 13 : 232–239. doi: 10.1016/j.mib.2010.01.008 20138000
40. Smock RG, Gierasch LM (2009) Sending signals dynamically. Science 324 : 198–203. doi: 10.1126/science.1169377 19359576
41. Gao R, Lynn DG (2007) Integration of rotation and piston motions in coiled-coil signal transduction. J Bacteriol 189 : 6048–6056. 17573470
42. Lowe EC, Basle A, Czjzek M, Firbank SJ, Bolam DN (2012) A scissor blade-like closing mechanism implicated in transmembrane signaling in a Bacteroides hybrid two-component system. Proc Natl Acad Sci U S A 109 : 7298–7303. doi: 10.1073/pnas.1200479109 22532667
43. Airola MV, Sukomon N, Samanta D, Borbat PP, Freed JH, et al. (2013) HAMP Domain Conformers That Propagate Opposite Signals in Bacterial Chemoreceptors. PLoS Biol 11: e1001479. doi: 10.1371/journal.pbio.1001479 23424282
44. Brooks AJ, Dai W, O’Mara ML, Abankwa D, Chhabra Y, et al. (2014) Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science 344 : 1249783. doi: 10.1126/science.1249783 24833397
45. Cheung J, Le-Khac M, Hendrickson WA (2009) Crystal structure of a histidine kinase sensor domain with similarity to periplasmic binding proteins. Proteins 77 : 235–241. doi: 10.1002/prot.22485 19544572
46. Lacey BW (1960) Antigenic modulation of Bordetella pertussis. J Hyg 31 : 423–434.
47. He XL, Dukkipati A, Wang X, Garcia KC (2005) A new paradigm for hormone recognition and allosteric receptor activation revealed from structural studies of NPR-C. Peptides 26 : 1035–1043. 15911071
48. Mony L, Zhu S, Carvalho S, Paoletti P (2011) Molecular basis of positive allosteric modulation of GluN2B NMDA receptors by polyamines. EMBO J 30 : 3134–3146. doi: 10.1038/emboj.2011.203 21685875
49. Karakas E, Furukawa H (2014) Crystal structure of a heterotetrameric NMDA receptor ion channel. Science 344 : 992–997. doi: 10.1126/science.1251915 24876489
50. Sobolevsky AI, Rosconi MP, Gouaux E (2009) X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462 : 745–756. doi: 10.1038/nature08624 19946266
51. Johnson MD, Bell J, Clarke K, Chandler R, Pathak P, et al. (2014) Characterization of mutations in the PAS domain of the EvgS sensor kinase selected by laboratory evolution for acid resistance in Escherichia coli. Mol Microbiol 93 : 911–927. doi: 10.1111/mmi.12704 24995530
52. Huth JR, Bewley CA, Jackson BM, Hinnebusch AG, Clore GM, et al. (1997) Design of an expression system for detecting folded protein domains and mapping macromolecular interactions by NMR. Protein Sci 6 : 2359–2364. 9385638
53. Kabsch W (2010) XDS. Acta Crystallogr D Biol Crystallogr 66 : 125–132. doi: 10.1107/S0907444909047337 20124692
54. Vagin A, Teplyakov A (2010) Molecular replacement with MOLREP. Acta Crystallogr D Biol Crystallogr 66 : 22–25. doi: 10.1107/S0907444909042589 20057045
55. Murshudov GN, Skubak P, Lebedev AA, Pannu NS, Steiner RA, et al. (2011) REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr 67 : 355–367. doi: 10.1107/S0907444911001314 21460454
56. Vagin AA, Isupov MN (2001) Spherically averaged phased translation function and its application to the search for molecules and fragments in electron-density maps. Acta Crystallogr D Biol Crystallogr 57 : 1451–1456. 11567159
57. Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66 : 486–501. doi: 10.1107/S0907444910007493 20383002
58. Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, et al. (2012) Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr D Biol Crystallogr 68 : 352–367. doi: 10.1107/S0907444912001308 22505256
59. Haddadian EJ, Gong H, Jha AK, Yang X, Debartolo J, et al. (2011) Automated real-space refinement of protein structures using a realistic backbone move set. Biophys J 101 : 899–909. doi: 10.1016/j.bpj.2011.06.063 21843481
60. Krissinel E, Henrick K (2007) Inference of macromolecular assemblies from crystalline state. J Mol Biol 372 : 774–797. 17681537
61. Eswar N, Marti-Renom MA, Webb B, Madhusudhan MS, Eramwan D, et al. (2006) Comparative protein structure modelling with Modeler. In: John Wiley and sons I, editor. Current protocols in Bioinformatics. pp. 5.6.1–5.6.30.
62. Dombkowski AA (2003) Disulfide by Design: a computational method for the rational design of disulfide bonds in proteins. Bioinformatics 19 : 1852–1853. 14512360
63. Antoine R, Alonso S, Raze D, Coutte L, Lesjean S, et al. (2000) New virulence-activated and virulence-repressed genes identified by systematic gene inactivation and generation of transcriptional fusions in Bordetella pertussis. J Bacteriol 182 : 5902–5905. 11004193
64. Imaizumi A, Suzuki Y, Ono S, Sato Y, Sato H (1983) Heptakis (2,6-O-dimethyl)beta-cyclodextrin: a novel growth stimulant for Bordetella pertussis phase I. J Clin Microbiol 17 : 781–786. 6306047
65. Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, et al. (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166 : 175–176. 8529885
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole MembraneČlánek Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and FunctionČlánek Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis
- Is Antigenic Sin Always “Original?” Re-examining the Evidence Regarding Circulation of a Human H1 Influenza Virus Immediately Prior to the 1918 Spanish Flu
- The Great Escape: Pathogen Versus Host
- Coping with Stress and the Emergence of Multidrug Resistance in Fungi
- Catch Me If You Can: The Link between Autophagy and Viruses
- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- Evidence for Ubiquitin-Regulated Nuclear and Subnuclear Trafficking among Matrix Proteins
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
- Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
- α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia
- Induction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
- Intracellular Growth Is Dependent on Tyrosine Catabolism in the Dimorphic Fungal Pathogen
- HCV Induces the Expression of Rubicon and UVRAG to Temporally Regulate the Maturation of Autophagosomes and Viral Replication
- Spatiotemporal Analysis of Hepatitis C Virus Infection
- Subgingival Microbial Communities in Leukocyte Adhesion Deficiency and Their Relationship with Local Immunopathology
- Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
- Establishment of HSV1 Latency in Immunodeficient Mice Facilitates Efficient Reactivation
- XRN1 Stalling in the 5’ UTR of Hepatitis C Virus and Bovine Viral Diarrhea Virus Is Associated with Dysregulated Host mRNA Stability
- γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
- Rhadinovirus Host Entry by Co-operative Infection
- A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane
- Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Transmitted by Fleas
- Elucidation of Sigma Factor-Associated Networks in Reveals a Modular Architecture with Limited and Function-Specific Crosstalk
- A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
- Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and Function
- Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
- Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection
- Diminished Reovirus Capsid Stability Alters Disease Pathogenesis and Littermate Transmission
- Characterization of CD8 T Cell Differentiation following SIVΔnef Vaccination by Transcription Factor Expression Profiling
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission
- Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection
- PKCη/Rdx-driven Phosphorylation of PDK1: A Novel Mechanism Promoting Cancer Cell Survival and Permissiveness for Parvovirus-induced Lysis
- Metalloprotease NleC Suppresses Host NF-κB/Inflammatory Responses by Cleaving p65 and Interfering with the p65/RPS3 Interaction
- Immune Antibodies and Helminth Products Drive CXCR2-Dependent Macrophage-Myofibroblast Crosstalk to Promote Intestinal Repair
- Adenovirus Entry From the Apical Surface of Polarized Epithelia Is Facilitated by the Host Innate Immune Response
- The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family
- Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
- CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies
- Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation
- Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Infection
- TRIM26 Negatively Regulates Interferon-β Production and Antiviral Response through Polyubiquitination and Degradation of Nuclear IRF3
- Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees ()
- A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication
- Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
- Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
- The Role of Misshapen NCK-related kinase (MINK), a Novel Ste20 Family Kinase, in the IRES-Mediated Protein Translation of Human Enterovirus 71
- Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Activates Both IL-1β and IL-1 Receptor Antagonist to Modulate Lung Inflammation during Pneumonic Plague
- Persistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
- An 18 kDa Scaffold Protein Is Critical for Biofilm Formation
- Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children
- Human CD8 T-cells Recognizing Peptides from () Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Inhibitory Phenotype and Represent a Novel Human T-cell Subset
- Decreased HIV-Specific T-Regulatory Responses Are Associated with Effective DC-Vaccine Induced Immunity
- RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets
- Concerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
- Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein
- PPM1A Regulates Antiviral Signaling by Antagonizing TBK1-Mediated STING Phosphorylation and Aggregation
- Lipidomic Analysis Links Mycobactin Synthase K to Iron Uptake and Virulence in .
- Roles and Programming of Arabidopsis ARGONAUTE Proteins during Infection
- Impact of Infection on Host Macrophage Nuclear Physiology and Nucleopore Complex Integrity
- The Impact of Host Diet on Titer in
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Herpesviral G Protein-Coupled Receptors Activate NFAT to Induce Tumor Formation via Inhibiting the SERCA Calcium ATPase
- The Causes and Consequences of Changes in Virulence following Pathogen Host Shifts
- Small GTPase Rab21 Mediates Fibronectin Induced Actin Reorganization in : Implications in Pathogen Invasion
- Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus
- NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target
- β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation
- An O Antigen Capsule Modulates Bacterial Pathogenesis in
- Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape
- Probing the Metabolic Network in Bloodstream-Form Using Untargeted Metabolomics with Stable Isotope Labelled Glucose
- Adhesive Fiber Stratification in Uropathogenic Biofilms Unveils Oxygen-Mediated Control of Type 1 Pili
- Vaccinia Virus Protein Complex F12/E2 Interacts with Kinesin Light Chain Isoform 2 to Engage the Kinesin-1 Motor Complex
- Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis
- Human T-Cell Leukemia Virus Type 1 (HTLV-1) Tax Requires CADM1/TSLC1 for Inactivation of the NF-κB Inhibitor A20 and Constitutive NF-κB Signaling
- Suppression of RNAi by dsRNA-Degrading RNaseIII Enzymes of Viruses in Animals and Plants
- Spatiotemporal Regulation of a T4SS Substrate by the Metaeffector SidJ
- Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells
- Dependence of Intracellular and Exosomal microRNAs on Viral Oncogene Expression in HPV-positive Tumor Cells
- Identification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Dihydrobiopterin in Adult Falciparum Malaria: Association with Disease Severity, Impaired Microvascular Function and Increased Endothelial Activation
- Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses
- Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer
- Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



