-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
The importance of antibody and B-cell responses for control of the erythrocytic-stage of the malaria parasite, Plasmodium, was first described when immune serum, passively transferred into Plasmodium falciparum-infected children, reduced parasitemia. This was later confirmed in experimental models in which mice deficient in B cells were unable to eliminate erythrocytic-stage infections. The signals required to activate these protective long-lasting B cell responses towards Plasmodium have not been investigated. IL-21 has been shown to be important for development of B-cell responses after immunization; however, a direct requirement for IL-21 in the control of infection via B-cell dependent mechanisms has never been demonstrated. In this paper, we have used mouse models of erythrocytic P. chabaudi and P. yoelii 17X(NL) infections in combination with IL-21/IL-21R deficiency to show that IL-21 from CD4+ T cells is required to eliminate Plasmodium infection by activating protective, long-lasting B-cell responses. Disruption of IL-21 signaling in B cells prevents the elimination of the parasite resulting in sustained high parasitemias, with no development of memory B-cells, lack of antigen-specific plasma cells and antibodies, and thus no protective immunity against a second challenge infection. Our data demonstrate the absolute requirement of IL-21 for B-cell control of this systemic infection. This has important implications for the design of vaccines against Plasmodium.
Published in the journal: Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria. PLoS Pathog 11(3): e32767. doi:10.1371/journal.ppat.1004715
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004715Summary
The importance of antibody and B-cell responses for control of the erythrocytic-stage of the malaria parasite, Plasmodium, was first described when immune serum, passively transferred into Plasmodium falciparum-infected children, reduced parasitemia. This was later confirmed in experimental models in which mice deficient in B cells were unable to eliminate erythrocytic-stage infections. The signals required to activate these protective long-lasting B cell responses towards Plasmodium have not been investigated. IL-21 has been shown to be important for development of B-cell responses after immunization; however, a direct requirement for IL-21 in the control of infection via B-cell dependent mechanisms has never been demonstrated. In this paper, we have used mouse models of erythrocytic P. chabaudi and P. yoelii 17X(NL) infections in combination with IL-21/IL-21R deficiency to show that IL-21 from CD4+ T cells is required to eliminate Plasmodium infection by activating protective, long-lasting B-cell responses. Disruption of IL-21 signaling in B cells prevents the elimination of the parasite resulting in sustained high parasitemias, with no development of memory B-cells, lack of antigen-specific plasma cells and antibodies, and thus no protective immunity against a second challenge infection. Our data demonstrate the absolute requirement of IL-21 for B-cell control of this systemic infection. This has important implications for the design of vaccines against Plasmodium.
Introduction
Malaria is the leading parasitic cause of morbidity and mortality worldwide; about half of the world's population is at risk of infection [1]. There is an urgent need for an effective vaccine able to bring about high levels of protection.
Immunity to the erythrocytic stages of malaria is thought to be primarily dependent on an antibody response. In endemic areas of Plasmodium falciparum transmission, there are associations between Plasmodium-specific antibody responses and protection against infection [2–5]. Elimination of the erythrocytic-stages of Plasmodium falciparum in infected children can be achieved by passive transfer of immune serum [2, 6], and studies in experimental models show that B cells and antibodies are important for elimination of chronic infections, and immunity to re-infection [7, 8]. A better understanding of the signals underlying activation of protective, long lasting, B-cell responses would be of great value in malaria vaccine development.
The cytokine IL-21, produced by follicular helper CD4+ T cells (Tfh) and other cells, is important for the generation of B-cell responses in germinal centers (GC), isotype switching, affinity maturation, antibody production, and development of memory B cells (MBC) [9, 10]. However, a requirement of IL-21 for activation and maintenance of Tfh cell is still controversial [11–23]. Most of our knowledge about the role of IL-21 in humoral responses has come from studies using immunization with protein antigens, where IL-21 is critical for the development of a T-cell dependent IgG response in GCs [11, 15, 16, 21, 23, 24]. Contrary to its importance in generating B cell responses after immunization, IL-21 seems not to be necessary for all aspects of T-cell-dependent B cell responses in different infection models [14, 19, 20, 22, 25, 26].
An investigation into Tfh cell development and the role of IL-21 in malaria has not been carried out, but this would be an excellent infection model in which to determine the importance of IL-21 in protective humoral immunity to a systemic pathogen, and would shed light on the induction, control and impairment of humoral responses in malaria. Here we have used a mouse model of malaria, Plasmodium chabaudi chabaudi AS in C57BL/6 mice, and have shown that IL-21 and Tfh cells are prominently induced and maintained in an erythrocytic-stage infection, suggesting that this crucial element of the humoral response is not impaired. Tfh cells producing IL-21 are multifunctional, with a majority also producing IFN-γ. Importantly, IL-21 produced by CD4+ T cells, acting directly on B cells, is crucial for triggering protective long-lasting Plasmodium-specific IgG B cell responses that are required to control and resolve the chronic phase of erythrocytic-stage malaria infection and for immunity to re-infection.
Results
IL-21 signaling is essential to control the chronic phase of blood stage P. chabaudi infection
Injection of 105 red blood cells (rbc) infected with P. chabaudi (irbc) into C57BL/6 mice gives rise to an erythrocytic infection, with an early acute parasitemia occurring at day 8 post-infection. Thereafter, the infection is rapidly controlled, reaching very low parasitemia levels by day 20 post-infection. This is followed by a chronic phase of infection, characterized by a prolonged sub-patent parasitemia with small patent recrudescence for up to 60 days before parasite elimination [27].
To explore a role for IL-21 during erythrocytic-stage P. chabaudi infection, we first determined the pattern of IL-21 mRNA expression in spleens of C57BL/6 mice during a P. chabaudi infection by real-time quantitative RT-PCR. Although IL-21 mRNA was detected over basal naïve levels as early as 2 days post-P. chabaudi infection, there was a striking increase in the spleen by day 7 post-infection, when IL-21 mRNA levels were approximately 130-fold higher than the basal level. IL-21 mRNA decreased thereafter, but remained higher than the basal level for at least 20 days (Fig 1 A).
Fig. 1. IL-21 is produced during P. chabaudi infection and required to control chronic infection. 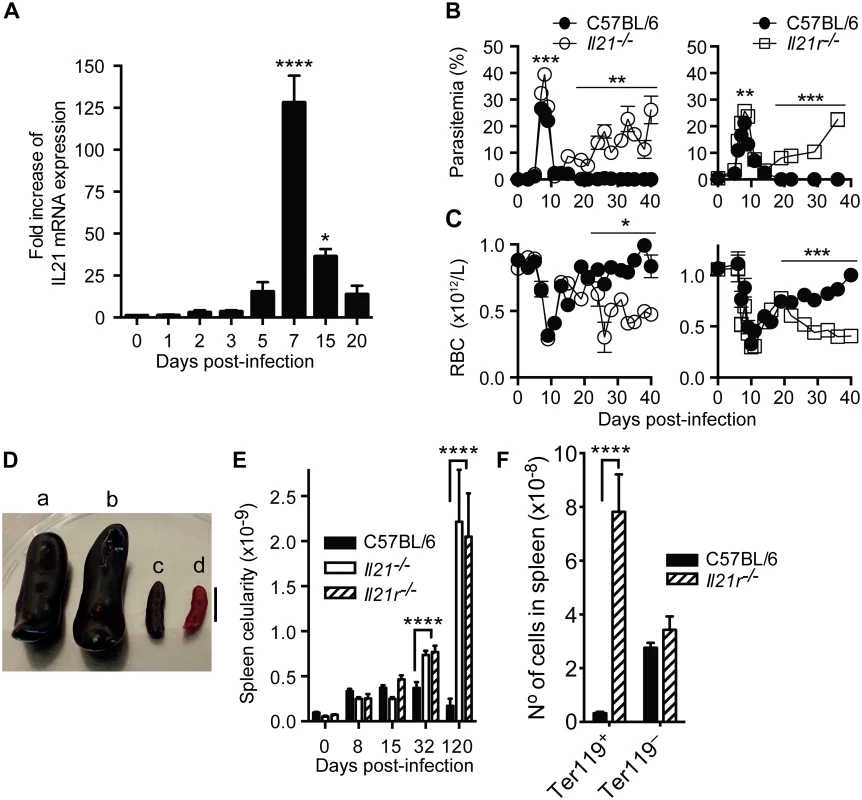
(A) IL-21 mRNA in spleen cells of P. chabaudi-infected mice measured by real-time quantitative RT-PCR. Parasitemia (B) and total rbc counts (C) were determined in WT C57BL/6 (closed circles), Il21-/- (open circles) and Il21r-/- (open squares) mice. (D) Individual examples of spleens from Il21r-/- (a) Il21-/- (b) and WT C57BL/6 (c) mice at day 120 post-infection, and a spleen from an age-matched WT C57BL/6 naïve mouse (d). Bar, 1 cm. (E) Total number of nucleated live splenocytes were determined with a hemocytometer in WT C57BL/6 (black bars), Il21-/- (open bars) and Il21r-/- (stripped bars) mice. (F) Numbers of Ter119+ and Ter119– cells in the spleen of WT C57BL/6 (black bars) and Il21r-/- (striped bars) at day 32 post-infection. Data are representative of two or more independent experiments and are obtained in groups of 5–10 mice per time point. Statistical significance was obtained using Mann Whitney U test or Kruskal-Wallis test. *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001. Error bars correspond to mean ± SEM. We next investigated whether IL-21, or signaling through its receptor, was necessary to control a primary P. chabaudi infection. C57BL/6 mice carrying a targeted deletion of the Il21 gene, or its receptor, Il21r, were infected as described above. They were able to control the acute phase of P. chabaudi infection, despite showing slightly but significantly higher peak parasitemias than infected WT C57BL/6 mice (Fig 1 B). Similar to WT C57BL/6 mice, both Il21-/- and Il21r-/- mice had very low parasitemias by 11–14 days post-infection. However, in stark contrast to WT mice, they developed sustained high parasitemias during the chronic phase of infection (e.g., parasitemias of 17% in Il21-/- and 23% in Il21r-/- mice compared with less than 0.04% in WT C57BL/6 at day 36 post-infection) (Fig 1 B). These high parasitemias were maintained in both Il21-/- and Il21r-/- after 120–150 days of infection (44±4% in Il21-/- and 56±3% in Il21r-/- mice at day 120 post-infection), without mortality. The non-resolving P. chabaudi infection was accompanied by increased anemia in both Il21-/- and Il21r-/- mice from days 19–22 post-infection onwards (Fig 1 C).
P. yoelii 17X(NL) gives rise to a non-lethal erythrocytic infection in WT C57BL/6 mice that is completely cleared after the acute phase without showing a chronic phase (S1 Fig, A and B). Similar to the P. chabaudi infection, both Il21-/- and Il21r-/- mice failed to clear a P. yoelii 17X(NL) erythrocytic infection, and developed sustained high parasitemias (S1 Fig, A and B). These data show that the requirement for IL-21 signaling is necessary to control infection with different Plasmodium species.
P. chabaudi-infected Il21-/- and Il21r-/- mice showed significantly greater spleen cellularity compared with WT C57BL/6 controls as early as 32 days post-infection, and dramatic splenomegaly at later time points (Fig 1, D and E). Despite increased anemia and splenomegaly, neither Il21-/- nor Il21r-/- mice had any other clinical signs. At day 32 post-infection, the numbers of Ter119+ erythrocytic cells in the spleen in the absence of IL-21 signaling were dramatically increased (Fig 1F), thus contributing to the large splenomegaly observed in Il21-/- and Il21r-/- mice from day 32 post-infection onwards.
In addition, at this time the numbers of NK cells, granulocytes and monocytes were greater in the Il21r-/- spleens compared with WT C57BL/6 controls (S2 Fig). On the other hand, the numbers of B cells in the spleen from Il21r-/- mice were substantially reduced compared to WT C57BL/6 control (S2 Fig).
Taken together, these data demonstrate a central role for IL-21 signaling in the resolution of erythrocytic-stage P. chabaudi and P. yoelii 17X(NL) infections.
IL-21 is produced by CD4+ T cells during P. chabaudi infection, and co-expressed with IFN-γ and IL-10
To identify which cells were responsible for the production of IL-21, intracellular cytokine staining and multiparameter flow cytometry performed on splenic cells from WT C57BL/6 mice revealed that IL-21 production was observed only in CD4+ T cells throughout the acute phase of a P. chabaudi infection (Fig 2, A and B). At no point during the infection was IL-21 detectable in NK cells, CD19+ cells or CD3–NK1.1– cells. In accordance with the kinetics observed for IL-21 mRNA expression (Fig 1 A), the percentage and total number of IL-21-producing CD4+ T cells in the spleen increased early after infection, (Fig 2, C and D). Both percentages and total numbers decreased thereafter, but remained higher than basal naïve levels at least up to day 32 post-infection. By 120 days post-infection, the frequency and total numbers of IL-21-producing CD4+ T cells in the spleen were comparable to those observed in naïve WT C57BL/6 mice.
Fig. 2. IL-21 is produced by CD4+ T cells during P. chabaudi infection. 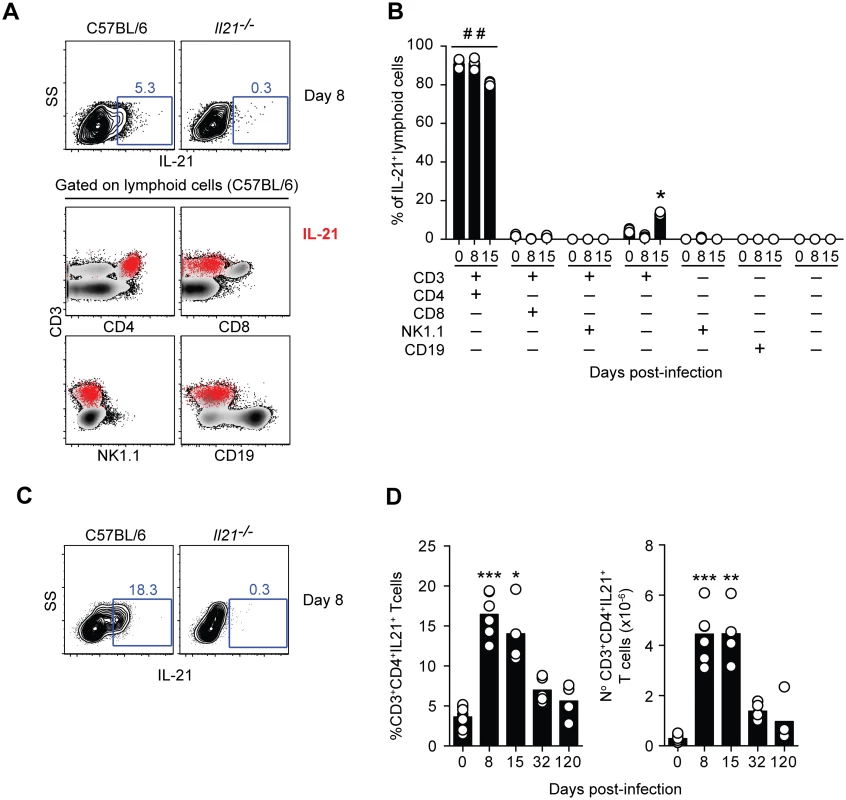
(A) Flow cytometry plots showing individual examples of IL-21 expression on mononuclear cells from WT C57BL/6 and Il21-/- mice at day 8 post-infection (top row). For the gating strategy, singlet cells were first selected, followed by live cells and mononuclear cells. In the bottom row, the IL-21-producing mononuclear cells detected in WT C57BL/6 mice, identified by red dots, were overlaid on the plots corresponding to the different combinations of surface biomarkers. (B) Cumulative data showing the differential combination of expression (+) or absence of expression (–) of each surface marker (indicated in the bottom left) on IL-21-producing mononuclear cells. (C) Flow cytometry plots showing individual examples of IL-21 expression on CD3+CD4+ T cells at day 8 post-infection. (D) Cumulative data showing the percentage (left) and total numbers (right) of IL-21-producing CD4+ T cells in the spleen of WT C57BL/6 mice at different days post-infection. Data are representative of at least two independent experiments and were obtained in groups of 4–5 mice per time point. Statistical significance was obtained using the Kruskal-Wallis test comparing each time point with its respective basal level (day 0 post-infection) (*, P<0.05; **, P<0.01; ***, P<0.001); or comparing each surface marker combination with every other surface marker combination within each time point (# #, P<0.01). Bars represent median values. The majority of IL-21-producing CD4+ T cells in the spleens of WT C57BL/6 mice co-expressed IFN-γ; in particular at the peak of infection, when over 70% of the IL-21-producing CD4+ T cells in the spleen also expressed IFN-γ (Fig 3, A, E and F). The total number of CD4+ T cells co-expressing IL-21 and IFN-γ showed a dramatic increase by day 8 post-infection, remained high at day 15 post-infection, and decreased thereafter (Fig 3 F). Interestingly, some of the IL-21-producing CD4+ T cells expressing IFN-γ also expressed IL-10 (Fig 3, C-F). The frequency of these triple producers increased with the progression of the infection until day 32 post-infection, when a maximum of approximately 30% of IL-21-producing CD4+ T cells co-expressed IFN-γ and IL-10 (Fig 3 D and E). The highest number of IL-21-producing CD4+ T cells co-expressing IFN-γ and IL-10 was detected at day 15 post-infection (Fig 3 F). By day 120–140 post-infection, the pattern of cytokines co-expressed with IL-21 resembled that of naïve WT C57BL/6 mice (Fig 3, E and F). Altogether, these data show that CD4+ T cells are the main source of IL-21 in the spleens of WT C57BL/6 mice during P. chabaudi infection, and demonstrate the occurrence of multifunctional CD4+ T cells co-expressing IFN-γ and IL-10 together with IL-21.
Fig. 3. IL-21 is co-expressed with IFN-γ and IL-10 during P. chabaudi infection. 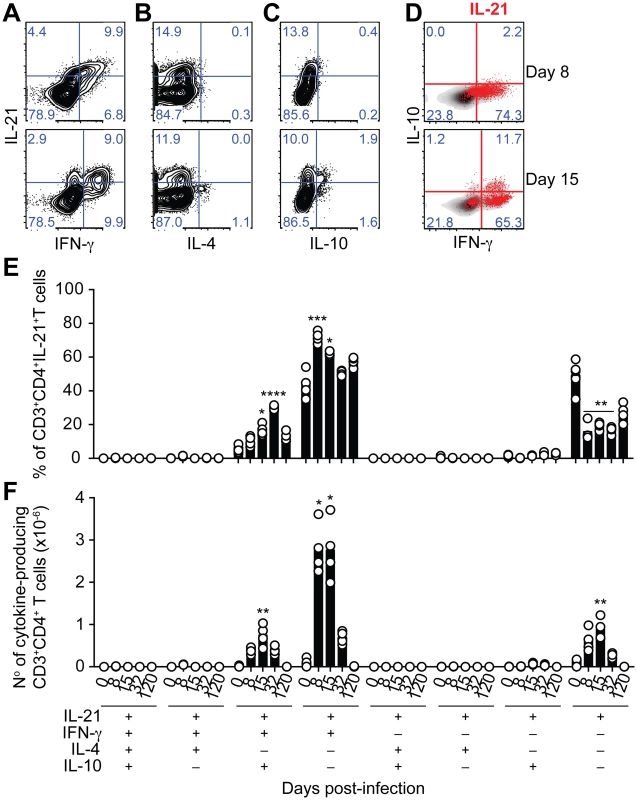
(A-C) Flow cytometry plots showing individual examples for days 8 and 15 post-infection of different cytokine combinations studied in CD3+CD4+ T cells from the spleen of WT C57BL/6 mice. (D) IL-21-producing CD4+ T cells (red) overlaid on the plots corresponding to IFN-γ vs IL-10 on gated CD3+CD4+ T cells. Cumulative data showing the percentage (E) and total numbers (F) of IL-21-producing CD4+ T cells co-expressing IFN-γ, IL-4 and IL-10 in the spleen of WT C57BL/6 mice. The differential combination of expression (+) or absence of expression (–) of each cytokine (indicated in the bottom left) is shown for each subset at different days post-infection. Data are representative of at least two independent experiments and were obtained in groups of 4–6 mice per time point. Statistical significance was obtained using the Kruskal-Wallis test comparing each time point, corresponding to each cytokine combination with its respective basal level (day 0 post-infection). *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001. Bars represent median values. Having identified CD4+ T cells as the producers of IL-21 during P. chabaudi infection, we wanted to confirm that IL-21 production by T cells was necessary to control P. chabaudi infection. To this end, we generated mixed bone marrow (BM) chimeras in which the deletion of the Il21 gene, or its receptor, was restricted to T cells (Fig 4 A). Control groups consisted of mixed BM chimeric mice reconstituted with BM obtained from WT C57BL/6 mice, and from Tcra-/- [28] and Ighm [29] mice mixed in an 80 : 20 ratio. Similar to Il21-/- and Il21r-/- mice, mixed BM chimeric mice bearing Il21-/- T cells failed to control the chronic phase of infection and showed increasingly and sustained high parasitemias during this phase of infection (Fig 4 B). IL-21R signaling on T cells was not required to control P. chabaudi infection, as mixed BM chimeric mice bearing Il21r-/- T cells showed a normal course of P. chabaudi infection when compared with WT C57BL/6 mice or mixed BM chimeric control groups (Fig 4 C). Together, these data demonstrate the requirement for IL-21 production by CD4+ T cells to control chronic P. chabaudi infection.
Fig. 4. Mice bearing T cells deficient in IL-21 fail to control chronic P. chabaudi infection. 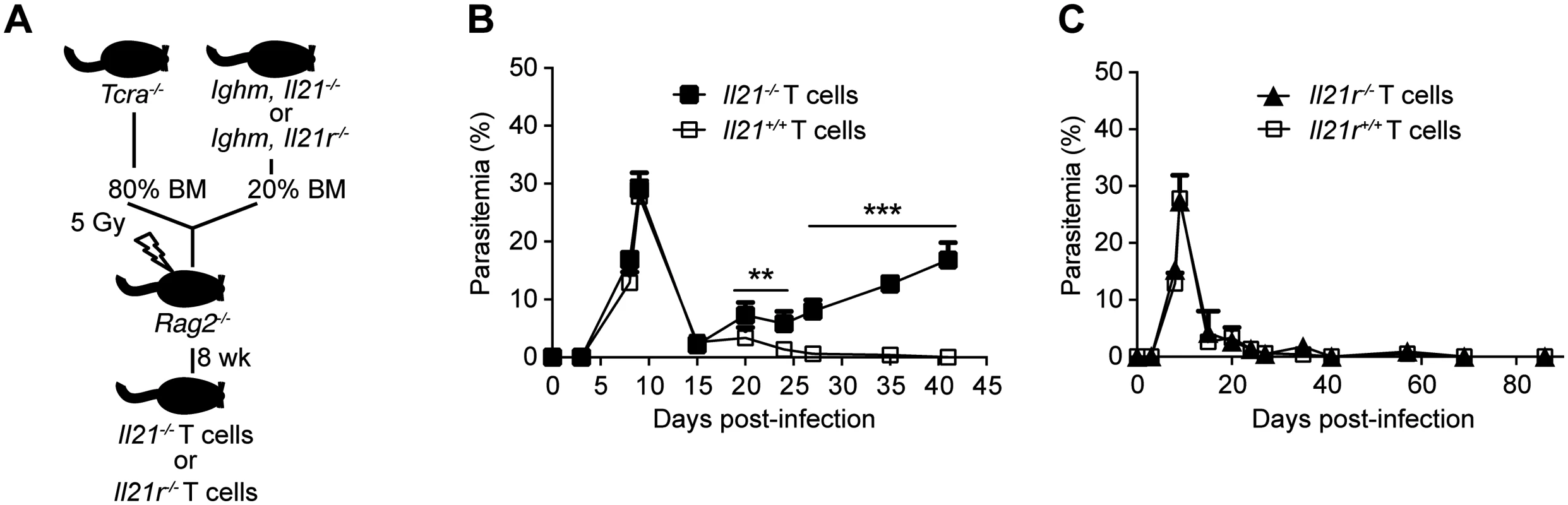
Course of a P. chabaudi infection in mixed BM chimeric mice generated as described with the scheme in (A) and detailed in Materials and Methods and S1 Table, (B) with fully functional B cells and T cells deficient in the Il21 gene (Il21-/- T cells, closed squares), and (C) with fully functional B cells and T cells deficient in the Il21r gene (Il21r-/- T cells, closed triangles) infected with P. chabaudi. As controls, mixed BM chimeric mice with BM from Tcra-/- and Ighm mice were generated (Il21+/+ and Il21r+/+ T cells, open squares, details in S1 Table). Statistical significance was obtained using Mann Whitney U test. **, P<0.01; ***, P<0.001. The graphs show the mean ± SEM of the parasitemia at different time points in 7–10 mice per group. Data are representative of two independent experiments. P. chabaudi infection promotes a robust Tfh cell response, even in the absence of IL-21 signaling
Tfh cells collaborate with B cells and are considered to be critical for the development of antigen-specific B cell responses during GC reactions [9, 10]. Tfh cells are a major source of IL-21, and this cytokine has been shown to be important for Tfh cell functionality. Therefore, we assessed the numbers of Tfh cells generated during a P. chabaudi infection, whether these cells were generated, whether they were the source of IL-21, and whether IL-21 was required for their generation and maintenance.
For multiparameter flow cytometry analysis, we defined Tfh cells as CD3+CD4+CD44highCXCR5+PD-1+ (S3A Fig), and confirmed the identity of Tfh cells by intranuclear staining of the master regulator of Tfh cell differentiation, the transcription factor Bcl-6 (S3B Fig). Infection of WT C57BL/6 mice with P. chabaudi led to an increase in Tfh cells, as evinced by a greater than 14-fold and 60-fold increase in their frequency and total numbers, respectively, in the spleen, 8 days post-infection, when compared to basal levels (Fig 5, A-C). At the peak of P. chabaudi infection, approximately 60% of the IL-21-producing CD4+ T cells in the spleen of WT C57BL/6 mice showed a Tfh cell phenotype (Fig 5, D and E). The majority of the IL-21-producing Tfh cells at the peak of P. chabaudi infection co-expressed IFN-γ (74.3±1.3%, Fig 5 F). Both the frequency and total numbers of Tfh cells in the spleen decreased at day 15 post-infection, reached basal levels by day 32 post-infection, and remained low at later time points of the study (Fig 5, A-C).
Fig. 5. IL-21-producing Tfh cells are activated during acute P. chabaudi infection. 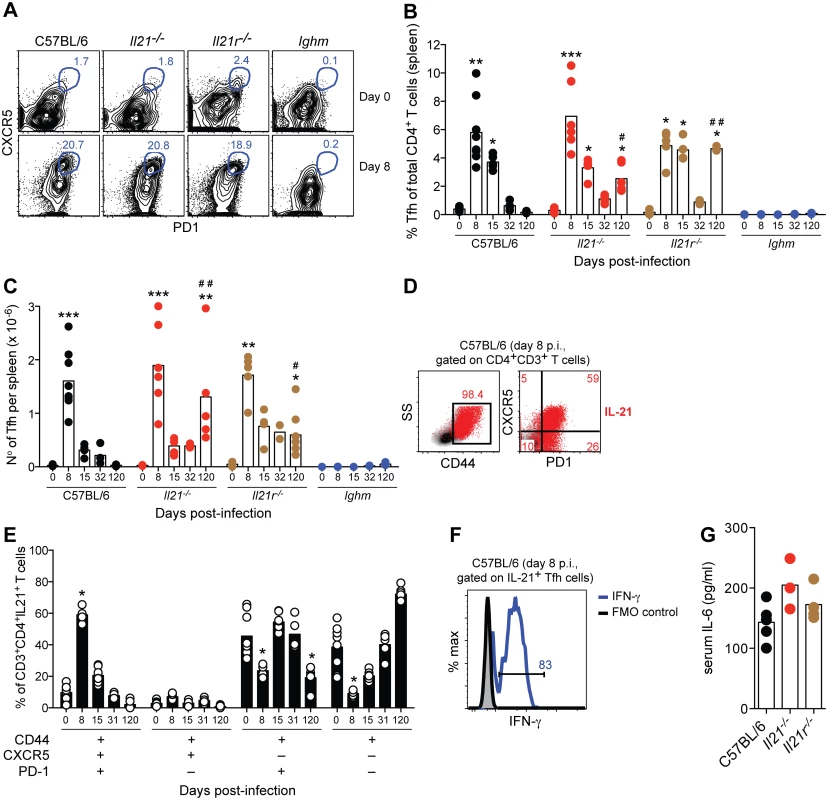
(A) Flow cytometric analysis of representative naïve (top row) and infected mice (8 days post-infection, bottom row). Gates show frequency of CD3+CD4+CD44high cells expressing CXCR5 and PD-1. (B) Frequency and (C) total numbers of Tfh cells, defined as CD3+CD4+CD44highCXCR5+PD-1+, in WT C57BL/6, Il21-/-, Il21r-/- and Ighm mice. (D) Flow cytometric analysis representative of infected WT C57BL/6 mice (8 days post-infection) corresponding to IL-21 intracellular staining on CD4+ T cells (red), overlaid on side scatter light vs CD44 (left) and CXCR5 vs PD-1 (right) from CD3+CD4+ T cells. Numbers show frequency of IL-21-producing CD4+ T cells with high expression of CD44 (left), and their differential expression of CXCR5 and PD-1 (right). (E) Differential combination of expression (+) or absence of expression (–) of CD44, CXCR5 and PD-1 (bottom left) on IL-21-producing CD3+CD4+ T cells at different days post-infection in the spleen of WT C57BL/6 mice. (F) Flow cytometric analysis of IFN-γ (green line) on CD3+CD4+CD44highCXCR5+PD-1+IL-21+ T cells from the spleen of WT C57BL/6 mice, 8 days post-P. chabaudi infection (representative of 4 mice). (G) Serum IL-6 at day 6 post-P. chabaudi infection. Statistical significance was obtained using the Kruskal-Wallis test comparing each time point with its respective basal level (day 0 post-infection) (*, P<0.05; **, P<0.01; ***, P<0.001), or comparing with the data obtained from the WT C57BL/6 group (#, P<0.05; # #, P<0.01). Bars represent median values. Data are representative of at least two independent experiments and were obtained in groups of 4–7 mice per time point. Neither IL-21, nor IL-21R, was required to generate a Tfh cell response during P. chabaudi infection, as the kinetics and magnitude of Tfh cell responses in Il21-/- and Il21r-/- was essentially similar to those observed in WT C57BL/6 mice (Fig 5, A-C). As IL-6 has also been implicated in Tfh differentiation [9], we determined whether infected Il21-/- and Il21r-/- mice could produce IL-6, which might explain their ability to generate Tfh cells. In both knockout strains, IL-6 was detected in the plasma at day 6 of infection at levels not significantly different from those of infected WT C57BL/6 controls (Fig 5 G). The slightly higher numbers of Tfh cells in Il21-/- and Il21r-/- at day 120 post-infection compared with those in WT C57BL/6 mice might have been a consequence of the on-going infection promoting continued T cell activation. In accordance with this idea, both Il21-/- and Il21r-/- mice showed higher frequencies of CD44high and PD-1+ CD4+ T cells in the spleen at day 120 post-infection when compared to WT C57BL/6 mice (frequencies CD4+CD44high of 82±5% and 62±14% for Il21-/- and Il21r-/- vs 14±2% for WT C57BL/6; frequencies CD4+PD-1+ of 74±5 and 61±7 for Il21-/- and Il21r-/- vs 8±2 for WT C57BL/6; P<0.01, Kruskal-Wallis test). P. chabaudi infection failed to activate Tfh cells in B-cell-deficient Ighm mice (Fig 5, A-C), which is in agreement with previous findings showing a requirement for the presence of B cells [30] and direct interactions with B cells [31] for the activation of Tfh cells.
These data show a strong activation of Tfh cell responses during acute P. chabaudi infection, which is not affected by the lack of signaling through the IL-21R. This T cell subset represents an important source of IL-21 at the peak of P. chabaudi infection, and co-expresses IFN-γ.
IL-21 is required to generate P. chabaudi-specific B cell responses, and is necessary for protective immunity against a secondary challenge infection
As IL-21 signaling is required to control the chronic phase of P. chabaudi infection and Tfh cells are an important source of IL-21, we reasoned that the lack of IL-21 signaling would impair the development of protective B cell responses and consequently prevent the resolution of the infection.
Flow cytometric analysis of the different B cell compartments in spleen and BM showed no significant differences in naïve Il21-/- and Il21r-/- mice compared with those of naïve WT C57BL/6 controls (Fig 6). At day 32 post-infection, the numbers of B cells in the spleen of Il21-/- and Il21r-/- mice were reduced compared to WT C57BL/6 controls (S2 Fig), and this was reflected in the numbers of mature (M), transitional 1 (T1) and transitional 2 (T2) B cells (Fig 6, A-D). A robust CD19+IgD–GL-7highCD38low GC B cell response in the spleen of WT C57BL/6 controls was observed at day 15 post-infection and sustained at day 32 post-infection (Fig 6, E-G). In stark contrast, both Il21-/- and Il21r-/- mice failed to generate this GC response during P. chabaudi infection (Fig 6, E-G). The numbers of B220+CD138+ plasmablasts in the spleen of WT C57BL/6 controls were increased at day 32 post-infection (Fig 6, H and I). Interestingly, the numbers of B220+CD138+ plasmablasts in the spleen of Il21-/- and Il21r-/- mice were similar to those of WT C57BL/6 mice (Fig 6, H and I). However, the numbers of B220+CD138high plasmablasts in BM from Il21-/- and Il21r-/- mice, and the numbers of B220–CD138high plasma cells in BM from Il21-/- mice were reduced at day 32 post-infection compared to those of WT C57BL/6 controls (Fig 6, J-L).
Fig. 6. Flow cytometry analysis of B cell responses during P. chabaudi infection in WT C57BL/6, Il21-/- and Il21r-/- mice. 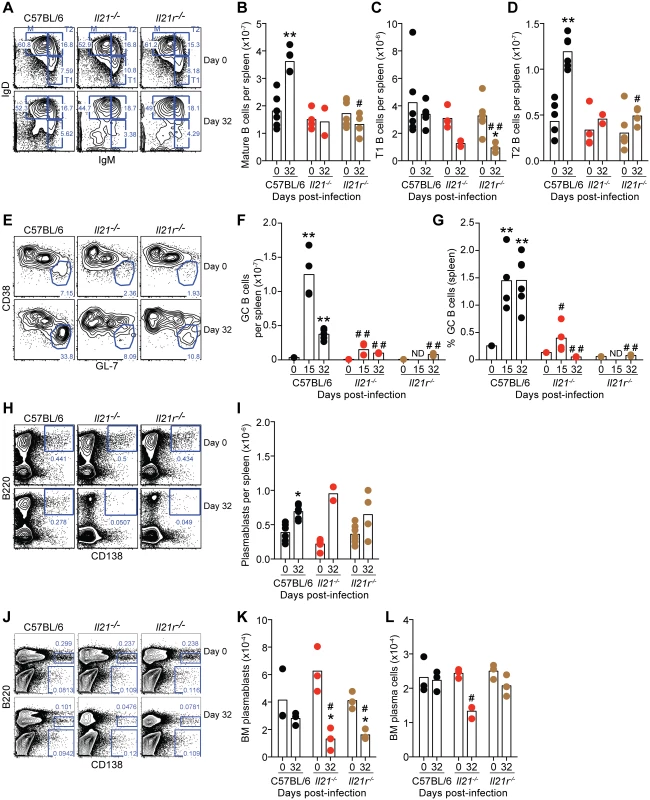
(A-D) Analysis of Mature (M) Transitional 1 (T1) and Transitional 2 (T2) on CD19+B220+ gated B cells in the spleen based on the pattern of IgD and IgM expression. (E-G) Analysis of GL-7highCD38low GC cells on CD19+IgD– gated B cells in the spleen. (H-I) Analysis of B220+CD138+ plasmablasts in the spleen. (J-L) Analysis of B220+CD138high plasmablasts and B220–CD138high plasma cells in the BM (1 femur plus 1 tibia per mouse). Statistical significance was obtained using the Kruskal-Wallis test comparing each time point with its respective basal level (day 0 post-infection) (*, P<0.05; **, P<0.01), or comparing with the data obtained from the WT C57BL/6 group (#, P<0.05; # #, P<0.01). The Mann Whitney U test was used to compare with its respective basal level when sets of data of only 2 time points were available (*, P<0.05). Bars represent median values. Data are representative of at least two independent experiments and were pooled from groups of 3–4 mice per time point. Although there were no differences in the initial numbers of B cells, P. chabaudi-specific IgG antibodies of all isotypes were absent in both Il21-/- and Il21r-/- mice at all evaluated time points (Fig 7, A and B). IgM antibodies, on the other hand, were not significantly affected by the lack of IL-21 signaling (Fig 7 C). In line with the absence of IgG antibodies, there was also a loss of both P. chabaudi-specific IgG antibody-secreting-cells (ASC) in the BM (Fig 7 D), and of P. chabaudi-specific IgG MBC in the spleen (Fig 7 E) at day 32 post-infection in both Il21-/- and Il21r-/- mice when compared with WT C57BL/6 controls.
Fig. 7. P. chabaudi-specific IgG B-cell responses are abrogated in the absence of IL-21 signaling. 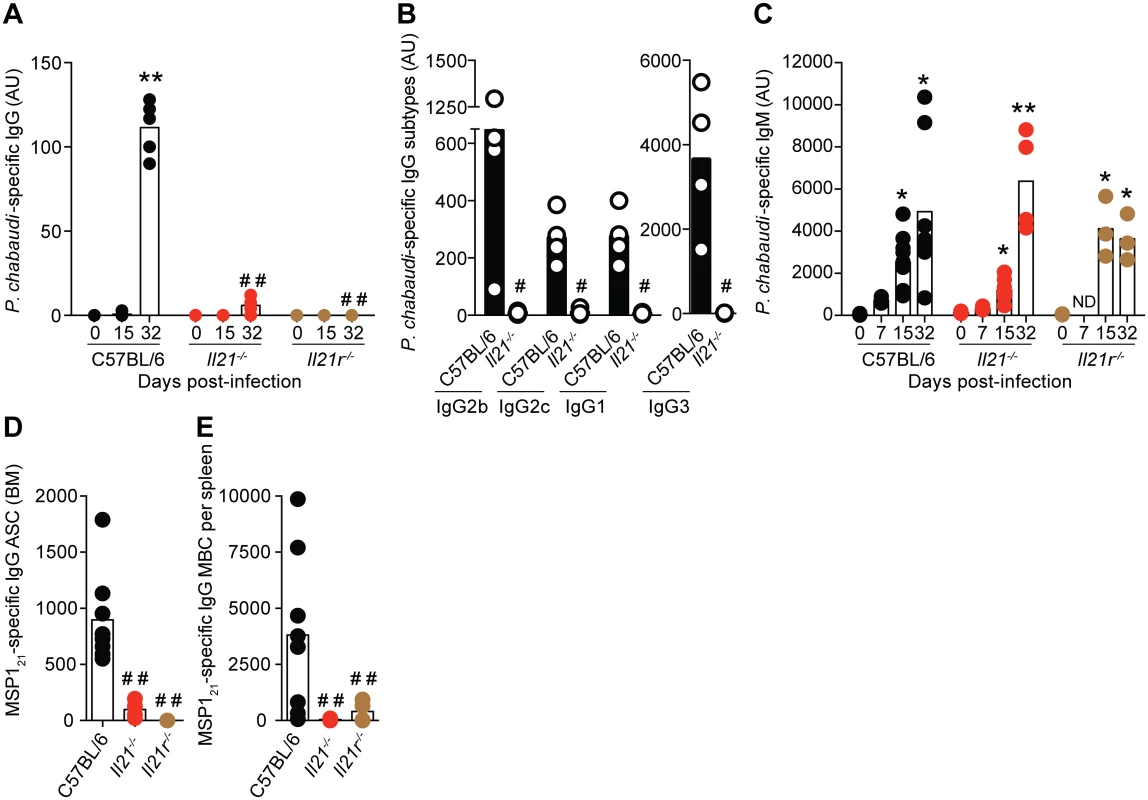
(A) IgG, (B) IgG subtypes (day 32 post-infection) and (C) IgM antibodies specific for a lysate of P. chabaudi-infected rbc determined by ELISA. Antibody units (AU) were calculated based on the P. chabaudi-specific antibody levels of a hyper-immune standard plasma defined as 1000 U. In the cases where levels of antibodies were below background, arbitrary values of 2 log lower than the mean value observed in WT C57BL/6 mice were set to be able to perform the statistical test. (D) MSP121-specific IgG-producing ASC in BM obtained from one femur and one tibia, and (E) MBC per spleen, determined by ELISPOT 32 days post-infection. Statistical significance was obtained using the Kruskal-Wallis test comparing each time point with its respective basal level (day 0 post-infection) (*, P<0.05; **, P<0.01), or comparing with the data obtained from the WT C57BL/6 group (# #, P<0.01). The Mann Whitney U test was used in the case of IgG subtypes, comparing Il21-/- vs WT C57BL/6 mice (#, P<0.05). Bars represent median values. Data are representative of at least two independent experiments and were obtained in groups of 3–8 mice per time point. To evaluate whether IL-21 directly signaled B cells to mount an antibody response, and thus to resolve the chronic phase of P. chabaudi infection, we generated mixed BM chimeric mice in which a targeted deletion of the Il21r gene was restricted to B cells (Fig 8 A). Control groups consisted of mixed BM chimeric mice reconstituted with BM obtained from WT C57BL/6 mice, and from Tcra-/- [28] and Ighm [29] mice mixed in a 20 : 80 ratio. Similar to the Il21r-/- mice, the mixed BM chimeric mice bearing Il21r-/- B cells eventually developed a non-resolving chronic-stage parasitemia (Fig 8 B). Furthermore, the mixed BM chimeric mice bearing Il21r-/- B cells, as well as the mixed BM chimeric mice bearing Il21-/- T cells, but not those bearing Il21r-/- T cells or Il21-/- B cell, showed a dramatic decrease in the levels of P. chabaudi-specific IgG antibodies and in the numbers of P. chabaudi-specific IgG MBC in the spleen (Fig 8, C and D).
Fig. 8. Mice bearing IL-21R-deficient B cells fail controlling chronic P. chabaudi infection and activating P. chabaudi-specific-IgG-responses. 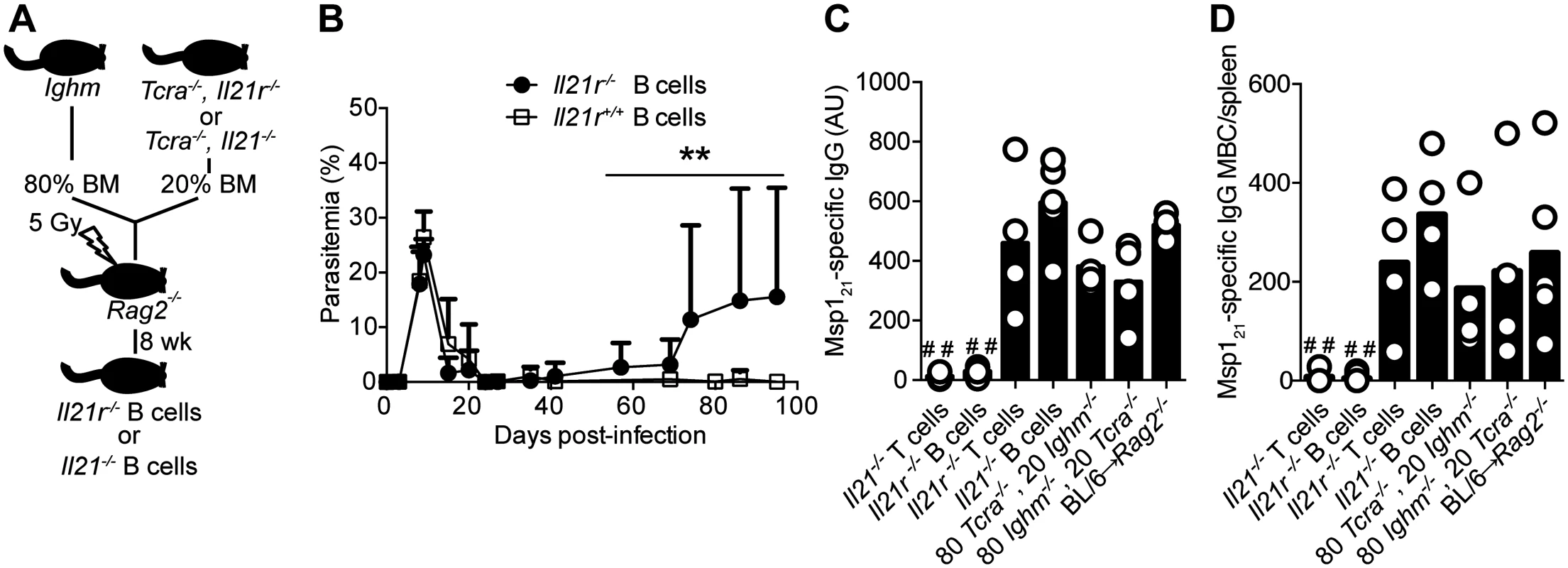
(A) Scheme describing the approach applied to generate mice with fully functional T cells and B cells deficient in the Il21 or the Il21r gene (details in Materials and Methods and S1 Table). (B) Course of a P. chabaudi infection in mixed BM chimeric mice with B cells deficient in the Il21r gene (Il21r-/- B cells, closed circles); as controls, mixed BM chimeric mice with BM from Tcra-/- and Ighm mice were generated (Il21r+/+ B cells, open squares, details in S1 Table). Statistical significance was obtained using the Mann Whitney U test (**, P<0.01). The graph shows the mean ± SEM of the parasitemia at different time points in 7–10 mice per group. (C) MSP121-specific IgG antibodies determined by ELISA 32 days post-infection in different mixed BM chimeric groups (4–5 mice per group, details in S1 Table). (D) Total number of MSP121-specific IgG MBC in spleens from different mixed BM chimeric groups, determined by ELISPOT on days 120–150 post-infection (4–5 mice per group, details in S1 Table). Statistical significance was obtained using the Kruskal-Wallis test comparing the data obtained from the group of Rag2-/- mice reconstituted with BM from WT C57BL/6 mice (BL/6→ Rag2-/-. # #, P<0.01). Bars represent median values. Data are representative of two independent experiments. C57BL/6 mice develop significant immunity to a second infection with the same strain of P. chabaudi and are able control the challenge infection at very low parasitemias [27]. Because infected Il21-/- and Il21r-/- mice cannot make P. chabaudi-specific IgG responses, and in particular MBC responses, we reasoned that lack of IL-21 signaling would render these mice unable to control a re-infection. We therefore infected Il21-/-, Il21r-/- and WT C57BL/6 mice with 105 P. chabaudi-irbc, treated them with chloroquine 30–45 days post-infection to eliminate the chronic primary infection, and re-challenged them with 105 P. chabaudi-irbc of the same strain 3 weeks after drug treatment (Fig 9 A). In accordance with our previous data, WT C57BL/6 similarly treated with chloroquine during the primary chronic infection were immune to a second challenge with very low parasitemias. In stark contrast, both Il21-/- and Il21r-/- mice failed to resolve the second infection and showed sustained high parasitemias (Fig 9, B and C). Interestingly, neither Il21-/- nor Il21r-/- mice showed the peak of parasitemia characteristic of an acute primary infection after receiving the second P. chabaudi challenge, suggesting that some initial immune control could take place in the absence of an IgG B-cell response (Fig 9, B and C). Similarly, Il21r-/- mice also failed to resolve a second infection with P. yoelii 17X(NL), in contrast to WT C57BL/6 controls, which controlled parasitemias at very low levels (S1B Fig).
Fig. 9. Mice deficient in IL-21 signaling fail to develop immunity to a secondary P. chabaudi infection. 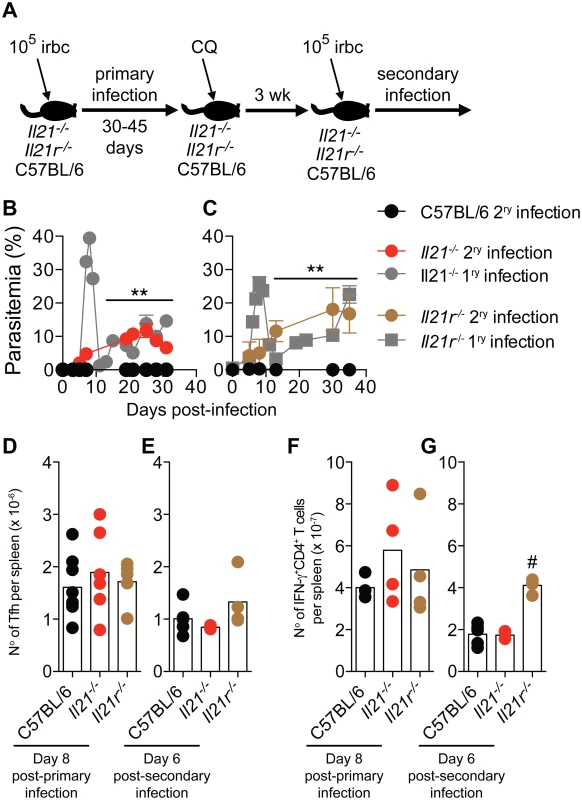
(A) Scheme describing the experimental approach. CQ = chloroquine. (B and C) P. chabaudi-infected mice were treated with chloroquine to eliminate parasitemia as described in the materials and methods, and re-infected with 105 P. chabaudi-infected rbc (day 0 post-secondary infection). The graphs show the course of secondary P. chabaudi infection in WT C57BL/6 (black circles), Il21-/- (red circles) and Il21r-/- (brown circles) mice; course of primary infection in Il21-/- (gray circles) and Il21r-/- (gray squares) are overlaid. (D and E) Number of Tfh cells per spleen post-primary and secondary infection, respectively. (F and G) Number of IFN-γ+CD4+ T cells per spleen post-primary and secondary infection, respectively. Data are representative of two independent experiments and are obtained in groups of 3–10 mice per time point. Statistical significance was obtained using Mann Whitney U test (**, P<0.01) or Kruskal-Wallis test (#, P<0.05). Error bars correspond to mean ± SEM. Similar to Tfh responses during primary infection (Fig 5 A-C and Fig 9 D), Tfh responses during second P. chabaudi infection (Fig 9 E) and IFN-γ responses of CD4+ T cells during primary and second P. chabaudi infection (Fig 9 F and G) were not altered by the absence of IL-21 signaling.
These data show that IL-21 is necessary to activate P. chabaudi-specific IgG B-cell responses, by direct signaling through the IL-21R on B cells, thus resolving the chronic infection. Moreover, IL-21 signaling is required to activate P. chabaudi-specific IgG MBC responses, and to develop immunity to homologous secondary P. chabaudi and P. yoelii 17X(NL) infections.
Discussion
Here we demonstrate, for the first time, a direct requirement of IL-21 signaling on B cells for the elimination of a systemic infection, and its importance in the control of chronic malaria. In the model of P. chabaudi infection in C57BL/6 mice, IL-21 produced by CD4+ T cells, predominantly Tfh cells, during the acute phase of an erythrocytic-stage P. chabaudi infection, is necessary for development of ASC, specific IgG antibodies and MBC, and to bring about the resolution of a chronic P. chabaudi infection. In a similar way, IL-21 signaling was required to control and eliminate a P. yoelii 17X(NL) infection. Our data highlight, for the first time, the importance of IL-21-dependent B-cell responses in the acquisition of immunity to re-infection and suggest that long-lived B-cell responses are required to achieve immunity to malaria re-infection.
Both Il21-/- and Il21r-/- mice failed to produce P. chabaudi-specific IgG antibodies, and did not generate P. chabaudi-specific IgG-producing MBC in the spleen or ASC in the bone marrow showing that IL-21 is required for class switching, and demonstrating that class switching to IgG responses is required for effective control of a P. chabaudi infection. P. chabaudi-specific IgM responses were not altered in the absence of IL-21 signaling. IgM can be produced by short-lived plasmablasts in the spleen, which have not undergone development in GC [32], perhaps explaining why total plasmablast numbers in the spleen were unaffected by the lack of IL-21 signaling. As we show that IL-21 signaling is required to activate GC B-cell responses upon P. chabaudi infection, we believe that IL-21 signaling is required to activate early stages of T-dependent B-cell responses to the parasites. However, we cannot rule out a direct requirement of IL-21 in the signal pathways leading to class switch recombination and MBC generation. As mixed BM chimeric mice with B cells deficient in IL-21R showed impairment of the humoral response, and did not resolve the chronic phase of P. chabaudi infection, it appears that there is a direct requirement for IL-21 from T cells to signal through the IL-21R on B cells to induce the differentiation of B cells into plasma cells, and thereby for antibody production. Our data is in accordance with those studies showing the critical importance of IL-21 signaling in IgG responses following immunization [11, 15, 23], but contrasts with studies of other pathogens, where there are variable requirements for IL-21 signaling for B-cell responses. In LCMV - and Influenza-infected mice, the lack of IL-21 signaling results only in slight, or no alterations in induction of B-cell responses, but the IgG antibody response is poorly sustained [19, 22, 25, 26]. Despite decreased levels of IgG1, isotype-switching and GC formation are not altered in Il21r-/- mice infected with H. polygyrus [14]. Primary B-cell responses, but not MBC responses, against live rabies virus-based vaccines, require IL-21 signaling [12]. The reasons for these differential requirements for IL-21 in the different infections/immunizations are not known. It is possible that other signals such as co-stimulation via TLR ligation, cytokines like IL-4, or strength of the BCR signal through extensive BCR cross-linking, could partially compensate for the lack of IL-21 signaling. Intrinsic differences in the antigens and co-stimulations delivered by different infections or immunizations could then differentially engage signals able to compensate different aspects of the B-cell response that would otherwise require signaling through IL-21. In this regard, signaling through TLR7 is known to be able to restore defective B-cell activation in Il21r-/- mice [11].
The impaired control of parasitemia and loss of the humoral response to P. chabaudi in Il21-/- and Il21r-/- mice were not due to lack of development of phenotypically-defined Tfh cells. Mice lacking IL-21 or the IL-21R in all cells were able to generate WT levels of Tfh cells; and mice lacking IL-21R only in T cells also had specific B-cell responses similar to those of WT mice. The requirement of IL-21 for the generation or maintenance of Tfh cell responses is controversial. Some studies have shown that IL-21 signaling is required for development, maintenance, or functional competence [15, 16, 19–21]. However, similar to our studies, others have shown that Tfh cell responses are normal or even elevated in the complete absence of IL-21 signaling [11–14, 17, 18, 22, 23]. Thus, the requirement for IL-21 to activate Tfh responses seems to be highly dependent on the model of immunization or infection studied. In this P. chabaudi infection, direct IL-21 signaling on T cells is not required to activate a functional Tfh program. It is possible that IL-6, which we show here is produced at levels similar to those of infected WT mice, compensates for the lack of IL-21 in the generation of Tfh cells, as has been described [13, 16, 33, 34].
CD8+ T cells have been shown to require IL-21 for protective responses in chronic viral infections in humans and mice [22, 25, 26, 35–37], and are known to play some role in controlling erythrocytic-stage Plasmodium infections in mice [38–42]. Our data rule out a mechanism by which IL-21 acts on or via CD8+ T cells to resolve the chronic P. chabaudi infection, since, in mixed BM chimeric mice in which there is a deficiency of IL-21R only in T cells, there was no exacerbation of the chronic infection.
Despite the failure of Il21-/- and Il21r-/- mice to resolve their chronic infection, they did not succumb to a fulminating parasitemia within the 100–150 days of the study. One possible factor that may limit the chronic parasitemia is the preference of P. chabaudi for mature rbc [43]. The presence of large numbers of Ter119+ rbc in the spleens of Il21-/- and Il21r-/- mice indicates increased hematopoiesis in response to anemia, presumably leading to production of many new rbc, which then controls the level of parasitemia. In addition, there are likely to be numerous B-cell-independent innate effector mechanisms activated, which could partially control parasitemia, but unable to completely clear the infection by themselves. Different from infections in Il21-/- and Il21r-/- mice, mixed BM chimeric mice with B cells deficient in IL-21R did not show a significant difference in relapsing parasitemia compared to WT C57BL/6 mice until days 70–80 post-infection. The higher variability in the parasitemias in these mixed BM chimeric mice compared to Il21r-/- late in infection may reflect B-cell-independent alternate mechanisms, which require IL-21. In general agreement with this idea, IL-21 has been shown to be involved in activation of macrophages and NK cells [44], both cell types implicated in the control of Plasmodium infection [45].
Previous studies have shown that Th1, Th2 and Th17 CD4+ T cell subsets can also produce IL-21 [9], and that Tfh cells can express cytokines characteristic of Th1/Th2/Th17 subsets [46]. This suggests either that other subsets of CD4+ T cells can produce IL-21, or that Th1/Th2/Th17 subsets activated in infections can acquire an additional Tfh phenotype. The generation of different CD4+ T cell subsets during infections is a very dynamic process, and activated CD4+ T cells, including Tfh cells, show substantial plasticity with overlapping phenotypes [47]. In the case of P. chabaudi infection, IL-21 production by CD4+ T cells was strongly linked to that of IFN-γ, the hallmark cytokine of Th1 CD4+ T cells, reflecting the strong bias to a Th1 response during acute P. chabaudi infection. We found no IL-4/IL-21 double-producing cells, and there is little induction of IL-17 in the spleens of P. chabaudi-infected mice [48]. Later in the course of P. chabaudi infection (13–15 days), we observed the appearance of splenic CD4+ T cells co-expressing IL-21, IFN-γ and IL-10. IL-10 may have been induced in these cells in order to regulate the inflammatory response and thus immune-mediated pathology, as we have shown previously [49, 50].
IL-21-producing CD4+ T cells are present in peripheral blood mononuclear cells from malaria-exposed immune adults [51, 52] and correlate with P. falciparum-specific IgG antibodies in children with acute falciparum malaria [53]. These observations encourage us to believe that use of the mouse model of P. chabaudi is a valid approach to dissect the regulation and role of IL-21, Tfh and B-cell responses, which is also relevant to other infections dependent on humoral immunity for their elimination.
In summary, we show that the absence of IL-21 signaling on B cells results in a loss of capacity to activate Plasmodium-specific IgG antibodies and memory B cells, resulting in a failure to resolve a chronic erythrocytic-stage infection with P. chabaudi and an inability to control a secondary challenge infection. This important immune mechanism should receive particular attention when exploring novel vaccine strategies.
Materials and Methods
Ethics statement
All animal experiments were approved by the MRC NIMR institutional Ethical Review Panel and carried out according to UK National guidelines (Scientific Procedures) Act 1986 under license PPL80/2385 approved by the British Home Office.
Mice
C57BL/6, Ighm [29], Tcra-/- [28] and Rag2-/- [54] mouse strains were bred in the specific pathogen-free facilities of the MRC NIMR and were backcrossed for at least 10 generations onto NIMR C57BL/6 mice. Mice with a targeted deletion of the Il21 gene, originally from NIH MMRRC (F2 129/ SvEvBrd x C57BL6/J), were obtained from Manfred Kopf, Institute for Molecular Health Sciences, Zürich, Switzerland. They were backcrossed 3 generations to C57BL/6J at the Garvan Institute, Sydney, and then backcrossed for 5 generations by Manfred Kopf and a further 3 generations onto NIMR C57BL/6 mice. Il21r-/- mice backcrossed to C57BL/6 (N7) were a kind gift from Manfred Kopf [55], and were further backcrossed 3 times onto NIMR C57BL/6. In all experiments, NIMR C57BL/6 bred in the same animal house were used as controls. From a panel of 768 SNPs, NIMR C57BL/6 and C57BL/6/J mice differ only by 2 SNPs; one each on chromosomes 2 and 12.
Mixed BM chimeras
The Il21-/- and Il21r-/- mice were crossed with Ighm and Tcra-/- to obtain double knockout strains Ighm Il21-/-, Ighm Il21r-/-, Tcra-/- Il21-/-, and Tcra-/- Il21r-/-. To generate mixed BM chimeric mice in which either B or T cells were deficient in the expression of IL-21 or IL-21R, femurs and tibias from donor mice were excised and cleaned of flesh using forceps and scalpel, and BM was obtained by flushing out with IMDM supplemented with 2 mM L-glutamine, 0.5 mM sodium pyruvate, 100 U penicillin, 100 mg streptomycin, 6 mM Hepes buffer, and 50 mM 2-ME (all from Gibco, Invitrogen), using a syringe with a needle. Thereafter, single BM cell suspensions were obtained by mashing through a 70 μm filter mesh, further sieved through 40 μm filter mesh and washed once. Live cells were resuspended in sodium chloride solution 0.9% (Sigma) at 4x106cells/200 μl. Rag2-/- mice were sub-lethally irradiated (5 Gy) using a [137Cs] source and reconstituted less than 24hr after irradiation by i.v. injections of different combinations of donor BM cells (S1 Table and S4 Fig). Recipient mice were maintained on acidified drinking water and analyzed for reconstitution after 8 weeks. Only mixed BM chimeric mice showing frequencies of T and B cells similar to those of WT C57BL/6 control mice were included in experiments.
Infection of mice with P. chabaudi and P. yoelii
Mice were infected with 105 P. chabaudi chabaudi AS–infected or 103 P. yoelii 17X(NL)-infected rbc by i.p. injections, and parasitemias monitored by Giemsa-stained blood films, as previously described [56]. The total number of rbc and hemoglobin concentrations were determined using a Vetscan (Abaxis), and body ventral temperature was measured using a MiniTemp MT6 infrared thermometer (Raytek).
Drug treatment
Drug-mediated elimination of P. chabaudi infection in Il21-/-, Il21r-/- and WT C57BL/6 mice was accomplished with 10 i.p. injections of 40 mg chloroquine (Sigma)/kg body weight in 0.9% sodium chloride solution given in 10 consecutive days, starting on day 30–45 post-primary infection. Drug-mediated elimination of P. yoelii in Il21r-/- and WT C57BL/6 was accomplished with chloroquine given in drinking water (600mg/L) during 6 consecutive days, followed by 3 i.p. injections of 0.25 mg pyrimethamine (Sigma) given in 3 consecutive days, followed by 3 i.p. injections of 1.25 mg of artesunat (Pharbaco) given in 3 consecutive days. Elimination of parasitemia was monitored by Giemsa-stained blood films and, in the case of P. chabaudi infection, to verify complete elimination of parasitemia after chloroquine treatment, 50 μl of blood were obtained from each mouse, diluted in 350 μl Kreb’s saline containing glucose (11 mM) and heparin (50 mU) and subinoculated into Rag2-/- mice. Giemsa-stained thin blood films from the recipient Rag2-/- mice were monitored for 3 weeks [27]. A second infection of 105 P. yoelii-infected rbc was given to Il21r-/-and WT C57BL/6 control mice 3 days after the last artesunat injection.
Quantitative real-time PCR
Expression of IL-21 mRNA was measured as previously described [48]. Briefly, total RNA was extracted from splenocytes from uninfected and P. chabaudi-infected C57BL/6 mice with TRIzol reagent (Life Technologies), reverse transcribed, and IL-21 mRNA was measured using real-time quantitative PCR with the primers, forward TCATCATTGACCTCGTGGCCC and reverse ATCGTACTTCTCCACTTGCAATCCC.
Flow cytometry
Spleens were dissected and single cell suspension was obtained by mashing the organs through a 70 μm filter mesh in HBSS (Gibco, Invitrogen). After removal of rbc by treatment with lysing buffer (Sigma), the remaining cells were resuspended in complete IMDM [IMDM supplemented with 10% FBS Serum Gold (PAA Laboratories, GE Healthcare), 2 mM L-glutamine, 0.5 mM sodium pyruvate, 100 U penicillin, 100 mg streptomycin, 6 mM Hepes buffer, and 50 mM 2-ME (all from Gibco, Invitrogen)] and viable cells were counted using trypan blue exclusion (Sigma) and a hemocytometer. Cells were then resuspended in PBS containing 2% FBS, 0.1% NaN3 (staining buffer), with the monoclonal anti-mouse CD16/32 [24G2, [57]] to block Fc-mediated binding of antibodies. To identify Tfh cells, 2x106 cells were first incubated with biotin anti-CXCR5 in complete IMDM (BD Pharmingen), washed twice with staining buffer, resuspended in PBS and incubated with appropriate dilutions of PE or APC streptavidin, PE/Cy7 anti-PD-1, APC/Cy7 anti-CD4, FITC or PerCP/Cy5.5 anti-CD44 and APC or Pacific Blue anti-CD3e (Biolegend). Cells were fixed with 2% paraformaldehyde and stored in staining buffer at 4°C until acquisition. For B cell analysis, spleens and BM (1 femur plus 1 tibia) were prepared as described above, and 2x106 cells were first incubated with anti-mouse CD16/32, followed by surface staining with different combinations of BV605 or BV785 anti-CD19, APC or PE/Cy7 anti-B220, BV421 anti-IgD, PerCP/Cy5.5 anti-IgM (all from Biolegend), FITC anti-GL-7, PE anti-CD138 (BD Pharmingen) and APC anti-CD38 (eBioscience), and acquired after two washes with PBS. To identify the different cell populations in the spleen, 2x106 cells were first incubated with anti-mouse CD16/32, followed by surface staining with different combinations of BV785 anti-CD19, BV711 anti-NK1.1, BV650 anti-CD8, BV605 anti-CD4, BV421 anti-γδTCR, PerCP/Cy5.5 anti-Ly6G, FITC anti-MHCII, PE anti-Ly6C, APC/Cy7 anti-Ter119, alexa fluor 700 anti-CD3 and alexa fluor 647 anti-CD11c (all from Biolegend), and acquired after two washes with PBS.
For intracellular cytokine staining, cells were stimulated for 5h in complete IMDM with PMA (50 ng/mL; Sigma), Ionomycin (500 ng/mL; Sigma), and Brefeldin A (10 mg/mL; Sigma), and surface stained as described above. Intracellular staining for IL-21-producing Tfh cells was performed as described [58–60]. Briefly, cells were fixed in 2% paraformaldehyde and stored in staining buffer overnight at 4°C, permeabilized in Perm/Wash buffer (BD Pharmingen), and incubated in Perm/Wash with recombinant mouse IL-21 receptor/ human Fc chimera (5mg/mL; R&D Systems). Cells were then stained with R-Phycoerythrin-conjugated AffiniPure F(ab’)2 Fragment Goat Anti-Human IgG (Jackson ImmunoResearch). For the study of cytokines co-expressed with IL-21, cells were further stained with PE/Cy7 anti-IFN-γ, FITC anti-IL-10, alexa fluor 647 anti-IL-4 (all from Biolegend) or FITC anti-IFN-γ (eBioscience). Intranuclear staining of Bcl-6 was performed using PE anti-Bcl-6 (eBioscience) and the Foxp3/Transcription Factor Staining Buffer Set (eBioscience), following manufacturer’s manual. Cells were acquired on CyAn ADP (Beckman Coulter), BD FACSVerse, BD LSRII or BD LSRFortessa X-20 (BD Biosciences) flow cytometers. Dead cells were excluded by staining with LIVE/DEAD Fixable Aqua or Blue stain (Invitrogen). Singlets were selected based on FCS-A vs FCS-W and further based on SSC-A vs SSC-W. “Fluorescence minus one” (FMO) controls, in combination with isotype controls, were used to set the thresholds for positive/negative events. Analysis was performed with FlowJo software version 9.6 or higher (Tree Star).
ELISPOT assays
ELISPOTs to measure ex-vivo frequencies of P. chabaudi-specific ASC in BM and MBC in spleen were performed as previously described [61]. Cells were incubated for 5h on 96-wells Multiscreen-HA filter plates (Millipore) coated with the C-terminal 21kDa part of P. chabaudi merozoite surface protein 1 (MSP121), prepared as described [62], to measure the frequency of MSP121-specific ASC, or coated with affinity-purified goat anti-mouse IgG (Sigma), to measure the frequency of total IgG ASC. Spots were enumerated with an Immunospot analyzer (CTL, Germany).
For MBC ELISPOTs, spleen cells were obtained by mashing the spleens through a 70 μm filter strainer, and rbc were eliminated by treatment with lysing buffer (Sigma). Cells were polyclonally activated by incubation for 6 days in flat-bottomed 96-well plates in the presence of irradiated feeder splenocytes, LPS, and supernatant from concanavalin A-stimulated C57BL/6 spleen cells. Four-fold dilutions of splenocytes were tested in replicates of 22 wells each. Cells were then transferred to antigen-coated 96-well Multiscreen-HA filter plates (Millipore) for ASC ELISPOT performed as described above. Precursor frequencies were calculated with ELDA software [63].
ELISA
Serum samples were obtained periodically after P. chabaudi infection by bleeding the mice from the tail vein. MSP121 and whole parasite lysate were generated and used in ELISAs to measure specific IgM, IgG and IgG subclasses, as previously described [64].
Statistical analysis
Statistical analysis was performed using Mann Whitney U test or Kruskal-Wallis test on Prism software version 6 (GraphPad). P<0.05 was accepted as a statistically significant difference.
Accession numbers
Ighm Ig mu chain C region [Mus musculus]; Gene ID: 102641210; Uniprot ID: P01872
Tcra T-cell receptor alpha chain C region [Mus musculus]; Gene ID: 21473; Uniprot ID: P01849
Rag2 V(D)J recombination-activating protein 2 [Mus musculus]; Gene ID: 19374; UniProt ID: P21784
Il21 Interleukin-21 [Mus musculus]; Gene ID: 60505; Uniprot ID: Q9ES17
Il21r Interleukin-21 receptor [Mus musculus]; Gene ID: 60505; Uniprot ID: Q9JHX3
Bcl6 B cell leukemia/lymphoma 6 [Mus musculus]; Gene ID: 12053; Uniprot ID: P41183
CD16 Low affinity immunoglobulin gamma Fc region receptor III [Mus musculus]; Gene ID: 14131; Uniprot ID: P08508
CD32 Low affinity immunoglobulin gamma Fc region receptor II [Mus musculus]; Gene ID: 14130; Uniprot ID: P08101
CXCR5 chemokine (C-X-C motif) receptor 5 [Mus musculus]; Gene ID: 12145; UniProt ID: Q04683
IFNg Interferon gamma [Mus musculus]; Gene ID: 15978; Uniprot ID: P01580
Il10 Interleukin-10 [Mus musculus]; Gene ID: 16153; Uniprot ID: P18893
TLR7 Toll like receptor 7 [Mus musculus]; Gene ID: 170743; Uniprot ID:Q548J0
Il4 Interleukin-4 [Mus musculus]; Gene ID: 16189; Uniprot ID: P07750
PD1 Programmed cell death 1 [Mus musculus]; Gene ID: 18566; Uniprot ID: Q02242
CD38 antigen [Mus musculus]; Gene ID: 12494; Uniprot ID: P56528
CD19 antigen [Mus musculus]; Gene ID: 12478; Uniprot ID: P25918
B220 protein tyrosine phosphatase, receptor type, C [Mus musculus]; Gene ID: 19264; Uniprot ID: P06800
Ter119 lymphocyte antigen 76 [Mus musculus]; Gene ID: 104231
Supporting Information
Zdroje
1. World Health Organization. (2013) World Malaria Report. 2013 : 1–286.
2. Cohen S, McGregor IA, and Carrington S. (1961) Gamma-globulin and acquired immunity to human malaria. Nature. 192 : 733–7. 13880318
3. Conway DJ, Cavanagh DR, Tanabe K, Roper C, Mikes ZS, et al. (2000) A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat Med. 6(6):689–92. 10835687
4. Fowkes FJI, Richards JS, Simpson JA, and Beeson JG. (2010) The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Med. 7(1):e1000218. doi: 10.1371/journal.pmed.1000218 20098724
5. Osier FHA, Fegan G, Polley SD, Murungi L, Verra F, et al. (2008) Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun. 76(5):2240–8. doi: 10.1128/IAI.01585-07 18316390
6. Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, et al. (1991) Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 45(3):297–308. 1928564
7. Burns JM Jr., Dunn PD, and Russo DM. (1997) Protective immunity against Plasmodium yoelii malaria induced by immunization with particulate blood-stage antigens. Infect Immun. 65(8):3138–45. 9234766
8. von der Weid T, Honarvar N, and Langhorne J. (1996) Gene-targeted mice lacking B cells are unable to eliminate a blood stage malaria infection. J Immunol. 156(7):2510–6. 8786312
9. Crotty S. (2011) Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 29 : 621–63. doi: 10.1146/annurev-immunol-031210-101400 21314428
10. Linterman MA, and Vinuesa CG. (2010) T follicular helper cells during immunity and tolerance. Prog Mol Biol Transl Sci. 92 : 207–48. doi: 10.1016/S1877-1173(10)92009-7 20800823
11. Bessa J, Kopf M, and Bachmann MF. (2010) Cutting edge: IL-21 and TLR signaling regulate germinal center responses in a B cell-intrinsic manner. J Immunol. 184(9):4615–9. doi: 10.4049/jimmunol.0903949 20368279
12. Dorfmeier CL, Tzvetkov EP, Gatt A, and McGettigan JP. (2013) Investigating the Role for IL-21 in Rabies Virus Vaccine-induced Immunity. PLoS Neg Trop Dis. 7(3):e2129. doi: 10.1371/journal.pntd.0002129 23516660
13. Eto D, Lao C, DiToro D, Barnett B, Escobar TC, et al. (2011) IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PloS One. 6(3):e17739. doi: 10.1371/journal.pone.0017739 21423809
14. King IL, Mohrs K, and Mohrs M. (2010) A nonredundant role for IL-21 receptor signaling in plasma cell differentiation and protective type 2 immunity against gastrointestinal helminth infection. J Immunol. 185(10):6138–45. doi: 10.4049/jimmunol.1001703 20926797
15. Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, et al. (2010) IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 207(2):353–63. doi: 10.1084/jem.20091738 20142429
16. Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, et al. (2008) Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 29(1):138–49. doi: 10.1016/j.immuni.2008.05.009 18599325
17. Phares TW, DiSano KD, Hinton DR, Hwang M, Zajac AJ, et al. (2013) IL-21 optimizes T cell and humoral responses in the central nervous system during viral encephalitis. J Neuroimmunol. 263(1–2):43–54. doi: 10.1016/j.jneuroim.2013.08.007 24035008
18. Rankin AL, MacLeod H, Keegan S, Andreyeva T, Lowe L, et al. (2011) IL-21 receptor is critical for the development of memory B cell responses. J Immunol. 186(2):667–74. doi: 10.4049/jimmunol.0903207 21169545
19. Rasheed MAU, Latner DR, Aubert RD, Gourley T, Spolski R, et al. (2013) Interleukin-21 is a critical cytokine for the generation of virus-specific long-lived plasma cells. J Virol. 87(13):7737–46. doi: 10.1128/JVI.00063-13 23637417
20. Stumhofer JS, Silver JS, and Hunter CA. (2013) IL-21 Is Required for Optimal Antibody Production and T Cell Responses during Chronic Toxoplasma gondii Infection. PloS One. 8(5):e62889. doi: 10.1371/journal.pone.0062889 23667536
21. Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, et al. (2008) A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 29(1):127–37. doi: 10.1016/j.immuni.2008.06.001 18602282
22. Yi JS, Du M, and Zajac AJ. (2009) A Vital Role for Interleukin-21 in the Control of a Chronic Viral Infection. Science. 324(5934):1572–6. doi: 10.1126/science.1175194 19443735
23. Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, et al. (2010) IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 207(2):365–78. doi: 10.1084/jem.20091777 20142430
24. Ozaki K, Spolski R, Feng C, Qi C, Cheng J, Sher A, et al. (2002) A Critical Role for IL-21 in Regulating Immunoglobulin Production. Science. 298(5598):1630–4. 12446913
25. Elsaesser H, Sauer K, and Brooks DG. (2009) IL-21 is required to control chronic viral infection. Science. 324(5934):1569–72. doi: 10.1126/science.1174182 19423777
26. Fröhlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, et al. (2009) IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 324(5934):1576–80. doi: 10.1126/science.1172815 19478140
27. Achtman AH, Stephens R, Cadman ET, Harrison V, and Langhorne J. (2007) Malaria-specific antibody responses and parasite persistence after infection of mice with Plasmodium chabaudi chabaudi. Parasite Immunol. 29(9):435–44. 17727567
28. Philpott KL, Viney JL, Kay G, Rastan S, Gardiner EM, et al. (1992) Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science. 256(5062):1448–52. 1604321
29. Kitamura D, Roes J, Kühn R, and Rajewsky K. (1991) A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 350(6317):423–6. 1901381
30. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, et al. (2011) Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 17(8):975–82. doi: 10.1038/nm.2425 21785433
31. Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, et al. (2010) Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 32(2):253–65. doi: 10.1016/j.immuni.2010.01.010 20153220
32. Smith KG, Hewitson TD, Nossal GJ, Tarlinton DM. (1996) The phenotype and fate of the antibody-forming cells of the splenic foci. Eur. J. Immunol. 26 : 444–48. 8617316
33. Karnowski A, Chevrier S, Belz GT, Mount A, Emslie D, et al. (2012) B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J Exp Med. 209(11):2049–64. doi: 10.1084/jem.20111504 23045607
34. Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, et al. (2009) Bcl6 mediates the development of T follicular helper cells. Science. 325(5943):1001–5. doi: 10.1126/science.1176676 19628815
35. Barker BR, Gladstone MN, Gillard GO, Panas MW, and Letvin NL. (2010) Critical role for IL-21 in both primary and memory anti-viral CD8+ T-cell responses. Eur J Immunol. 40(11):3085–96. doi: 10.1002/eji.200939939 21061439
36. Li L, Liu M, Cheng L-W, Gao X-Y, Fu J-J, et al. (2013) HBcAg-specific IL-21-producing CD4+ T cells are associated with relative viral control in patients with chronic hepatitis B. Scand J Immunol. 78(5):439–46. doi: 10.1111/sji.12099 23957859
37. Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, et al. (2011) Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J Virol. 85(5):2316–24. doi: 10.1128/JVI.01476-10 21159862
38. Horne-Debets JM, Faleiro R, Karunarathne DS, Liu XQ, Lineburg KE, et al. (2013) PD-1 Dependent Exhaustion of CD8+ T Cells Drives Chronic Malaria. Cell Rep. 5(5):1204–13. doi: 10.1016/j.celrep.2013.11.002 24316071
39. Imai T, Shen J, Chou B, Duan X, Tu L, et al. (2010) Involvement of CD8+ T cells in protective immunity against murine blood-stage infection with Plasmodium yoelii 17XL strain. Eur J Immunol. 40(4):1053–61. doi: 10.1002/eji.200939525 20101613
40. Mogil RJ, Patton CL, and Green DR. (1987) Cellular subsets involved in cell-mediated immunity to murine Plasmodium yoelii 17X malaria. J Immunol. 138(6):1933–9. 3102605
41. Podoba JE, and Stevenson MM. (1991) CD4+ and CD8+ T lymphocytes both contribute to acquired immunity to blood-stage Plasmodium chabaudi AS. Infect Immun. 59(1):51–8. 1898902
42. Süss G, Eichmann K, Kury E, Linke A, and Langhorne J. (1988) Roles of CD4 - and CD8-bearing T lymphocytes in the immune response to the erythrocytic stages of Plasmodium chabaudi. Infect Immun. 56(12):3081–8. 2903123
43. Carter R, and Walliker D. (1975) New observations on the malaria parasites of rodents of the Central African Republic—Plasmodium vinckei petteri subsp. nov. and Plasmodium chabaudi Landau, 1965. Ann Trop Med Parasitol. 69(2):187–96. 1155987
44. Yi JS, Cox MA, Zajac AJ. (2010) Interleukin-21: a multifunctional regulator of immunity to infections. Microbes Infect. 12(14–15):1111–9. doi: 10.1016/j.micinf.2010.07.015 20691803
45. Liehl P, Mota MM. (2012) Innate recognition of malarial parasites by mammalian hosts. Int J Parasitol. 42 (6):557–6. doi: 10.1016/j.ijpara.2012.04.006 22543040
46. Cannons JL, Lu KT, and Schwartzberg PL. (2013) T follicular helper cell diversity and plasticity. Trends Immunol. 34(5):200–7. doi: 10.1016/j.it.2013.01.001 23395212
47. Nakayamada S, Takahashi H, Kanno Y, and O'Shea JJ. (2012) Helper T cell diversity and plasticity. Curr Opin Immunol. 24(3):297–302. doi: 10.1016/j.coi.2012.01.014 22341735
48. Mastelic B, do Rosario APF, Veldhoen M, Renauld JC, Jarra W, et al. (2012) IL-22 Protects Against Liver Pathology and Lethality of an Experimental Blood-Stage Malaria Infection. Front Immunol. 25;3 : 85.
49. Freitas do Rosário AP, Lamb T, Spence P, Stephens R, Lang A, et al. (2012) IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol. 188(3):1178–90. doi: 10.4049/jimmunol.1102755 22205023
50. Li C, Corraliza I, and Langhorne J. (1999) Defect in Interleukin-10 Leads to Enhanced Malarial Disease in Plasmodium chabaudi chabaudi Infection in Mice. Infect Immun. 4435–42. 10456884
51. Mewono L, Agnandji ST, Matondo Maya DW, Mouima A-MN, Iroungou BA, et al. (2009) Malaria antigen-mediated enhancement of interleukin-21 responses of peripheral blood mononuclear cells in African adults. Exp Parasitol. 122(1):37–40. doi: 10.1016/j.exppara.2009.01.007 19545527
52. Roetynck S, Olotu A, Simam J, Marsh K, Stockinger B, et al. (2013) Phenotypic and functional profiling of CD4 T cell compartment in distinct populations of healthy adults with different antigenic exposure. PloS One. 8(1):e55195. doi: 10.1371/journal.pone.0055195 23383106
53. Mewono L, Matondo Maya DW, Matsiegui P-B, Agnandji ST, Kendjo E, et al. (2008) Interleukin-21 is associated with IgG1 and IgG3 antibodies to erythrocyte-binding antigen-175 peptide 4 of Plasmodium falciparum in Gabonese children with acute falciparum malaria. Eur Cytokine Netw. 19(1):30–6. doi: 10.1684/ecn.2008.0114 18299268
54. Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, et al. (1992) RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 68(5):855–67. 1547487
55. Fröhlich A, Marsland BJ, Sonderegger I, Kurrer M, Hodge MR, et al. (2007) IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. 109(5):2023–31. 17077330
56. Cadman ET, Abdallah AY, Voisine C, Sponaas A-M, Corran P, et al. (2008) Alterations of splenic architecture in malaria are induced independently of Toll-like receptors 2, 4, and 9 or MyD88 and may affect antibody affinity. Infect Immun. 76(9):3924–31. doi: 10.1128/IAI.00372-08 18559428
57. Unkeless JC. (1979) Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 150(3):580–96. 90108
58. Hiramatsu Y, Suto A, Kashiwakuma D, Kanari H, Kagami S-i, et al. (2010) c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-beta inhibits c-Maf-induced IL-21 production in CD4+ T cells. J Leukoc Biol. 87(4):703–12. doi: 10.1189/jlb.0909639 20042469
59. Kashiwakuma D, Suto A, Hiramatsu Y, Ikeda K, Takatori H, et al. (2010) B and T lymphocyte attenuator suppresses IL-21 production from follicular Th cells and subsequent humoral immune responses. J Immunol. 185(5):2730–6. doi: 10.4049/jimmunol.0903839 20660710
60. Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, et al. (2011) Early Th1 Cell Differentiation Is Marked by a Tfh Cell-like Transition. Immunity. 35(6):919–31. doi: 10.1016/j.immuni.2011.11.012 22195747
61. Ndungu FM, Cadman ET, Coulcher J, Nduati E, Couper E, et al. (2009) Functional Memory B Cells and Long-Lived Plasma Cells Are Generated after a Single Plasmodium chabaudi Infection in Mice. PLoS Pathog. 5(12):e1000690. doi: 10.1371/journal.ppat.1000690 20011127
62. Hensmann M, Li C, Moss C, Lindo V, Greer F, et al. (2004) Disulfide bonds in merozoite surface protein 1 of the malaria parasite impede efficient antigen processing and affect the in vivo antibody response. Eur J Immunol. 34(3):639–48. 14991593
63. Hu Y, and Smyth G. (2009) ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 347(1–2):70–8. doi: 10.1016/j.jim.2009.05.013 19520083
64. Quin SJ, and Langhorne J. (2001) Different regions of the malaria merozoite surface protein 1 of Plasmodium chabaudi elicit distinct T-cell and antibody isotype responses. Infect Immun. 69(4):2245–51. 11254580
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole MembraneČlánek Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and FunctionČlánek Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis
- Is Antigenic Sin Always “Original?” Re-examining the Evidence Regarding Circulation of a Human H1 Influenza Virus Immediately Prior to the 1918 Spanish Flu
- The Great Escape: Pathogen Versus Host
- Coping with Stress and the Emergence of Multidrug Resistance in Fungi
- Catch Me If You Can: The Link between Autophagy and Viruses
- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- Evidence for Ubiquitin-Regulated Nuclear and Subnuclear Trafficking among Matrix Proteins
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
- Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
- α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia
- Induction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
- Intracellular Growth Is Dependent on Tyrosine Catabolism in the Dimorphic Fungal Pathogen
- HCV Induces the Expression of Rubicon and UVRAG to Temporally Regulate the Maturation of Autophagosomes and Viral Replication
- Spatiotemporal Analysis of Hepatitis C Virus Infection
- Subgingival Microbial Communities in Leukocyte Adhesion Deficiency and Their Relationship with Local Immunopathology
- Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
- Establishment of HSV1 Latency in Immunodeficient Mice Facilitates Efficient Reactivation
- XRN1 Stalling in the 5’ UTR of Hepatitis C Virus and Bovine Viral Diarrhea Virus Is Associated with Dysregulated Host mRNA Stability
- γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
- Rhadinovirus Host Entry by Co-operative Infection
- A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane
- Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Transmitted by Fleas
- Elucidation of Sigma Factor-Associated Networks in Reveals a Modular Architecture with Limited and Function-Specific Crosstalk
- A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
- Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and Function
- Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
- Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection
- Diminished Reovirus Capsid Stability Alters Disease Pathogenesis and Littermate Transmission
- Characterization of CD8 T Cell Differentiation following SIVΔnef Vaccination by Transcription Factor Expression Profiling
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission
- Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection
- PKCη/Rdx-driven Phosphorylation of PDK1: A Novel Mechanism Promoting Cancer Cell Survival and Permissiveness for Parvovirus-induced Lysis
- Metalloprotease NleC Suppresses Host NF-κB/Inflammatory Responses by Cleaving p65 and Interfering with the p65/RPS3 Interaction
- Immune Antibodies and Helminth Products Drive CXCR2-Dependent Macrophage-Myofibroblast Crosstalk to Promote Intestinal Repair
- Adenovirus Entry From the Apical Surface of Polarized Epithelia Is Facilitated by the Host Innate Immune Response
- The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family
- Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
- CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies
- Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation
- Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Infection
- TRIM26 Negatively Regulates Interferon-β Production and Antiviral Response through Polyubiquitination and Degradation of Nuclear IRF3
- Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees ()
- A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication
- Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
- Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
- The Role of Misshapen NCK-related kinase (MINK), a Novel Ste20 Family Kinase, in the IRES-Mediated Protein Translation of Human Enterovirus 71
- Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Activates Both IL-1β and IL-1 Receptor Antagonist to Modulate Lung Inflammation during Pneumonic Plague
- Persistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
- An 18 kDa Scaffold Protein Is Critical for Biofilm Formation
- Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children
- Human CD8 T-cells Recognizing Peptides from () Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Inhibitory Phenotype and Represent a Novel Human T-cell Subset
- Decreased HIV-Specific T-Regulatory Responses Are Associated with Effective DC-Vaccine Induced Immunity
- RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets
- Concerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
- Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein
- PPM1A Regulates Antiviral Signaling by Antagonizing TBK1-Mediated STING Phosphorylation and Aggregation
- Lipidomic Analysis Links Mycobactin Synthase K to Iron Uptake and Virulence in .
- Roles and Programming of Arabidopsis ARGONAUTE Proteins during Infection
- Impact of Infection on Host Macrophage Nuclear Physiology and Nucleopore Complex Integrity
- The Impact of Host Diet on Titer in
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Herpesviral G Protein-Coupled Receptors Activate NFAT to Induce Tumor Formation via Inhibiting the SERCA Calcium ATPase
- The Causes and Consequences of Changes in Virulence following Pathogen Host Shifts
- Small GTPase Rab21 Mediates Fibronectin Induced Actin Reorganization in : Implications in Pathogen Invasion
- Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus
- NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target
- β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation
- An O Antigen Capsule Modulates Bacterial Pathogenesis in
- Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape
- Probing the Metabolic Network in Bloodstream-Form Using Untargeted Metabolomics with Stable Isotope Labelled Glucose
- Adhesive Fiber Stratification in Uropathogenic Biofilms Unveils Oxygen-Mediated Control of Type 1 Pili
- Vaccinia Virus Protein Complex F12/E2 Interacts with Kinesin Light Chain Isoform 2 to Engage the Kinesin-1 Motor Complex
- Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis
- Human T-Cell Leukemia Virus Type 1 (HTLV-1) Tax Requires CADM1/TSLC1 for Inactivation of the NF-κB Inhibitor A20 and Constitutive NF-κB Signaling
- Suppression of RNAi by dsRNA-Degrading RNaseIII Enzymes of Viruses in Animals and Plants
- Spatiotemporal Regulation of a T4SS Substrate by the Metaeffector SidJ
- Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells
- Dependence of Intracellular and Exosomal microRNAs on Viral Oncogene Expression in HPV-positive Tumor Cells
- Identification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Dihydrobiopterin in Adult Falciparum Malaria: Association with Disease Severity, Impaired Microvascular Function and Increased Endothelial Activation
- Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses
- Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer
- Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



