-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
MicroRNAs (miRNAs) are small, non-coding RNAs that regulate important cellular processes by inhibiting the expression of gene targets. In recent years, it has become clear that miRNAs play a critical role in the regulation of the immune response to infection, a highly complex phenotype involving the activation of both generic and infection-specific responses. However, it remains unclear to what extent miRNAs are involved in the regulation of these two types of response. Here, focusing on the miRNA response to mycobacteria, pathogens of major public health importance, we present the first comparative, deep sequencing-based analysis of the miRNA response to a panel of bacterial infections. We define a set of miRNAs that play an essential role in basic cellular responses to stress and identify pathogen-specific miRNA responses that reflect mechanisms by which certain pathogens interfere with the host response to infection. In addition, we show that infection can alter the expression level and proportions of miRNA isoforms, transcripts originating from the same miRNA but with slight differences in their nucleotide sequences. This study highlights a novel aspect of miRNA expression dynamics upon infection and increases our understanding of miRNA-mediated mechanisms involved in host cellular responses to infection.
Published in the journal: Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs. PLoS Genet 11(3): e32767. doi:10.1371/journal.pgen.1005064
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005064Summary
MicroRNAs (miRNAs) are small, non-coding RNAs that regulate important cellular processes by inhibiting the expression of gene targets. In recent years, it has become clear that miRNAs play a critical role in the regulation of the immune response to infection, a highly complex phenotype involving the activation of both generic and infection-specific responses. However, it remains unclear to what extent miRNAs are involved in the regulation of these two types of response. Here, focusing on the miRNA response to mycobacteria, pathogens of major public health importance, we present the first comparative, deep sequencing-based analysis of the miRNA response to a panel of bacterial infections. We define a set of miRNAs that play an essential role in basic cellular responses to stress and identify pathogen-specific miRNA responses that reflect mechanisms by which certain pathogens interfere with the host response to infection. In addition, we show that infection can alter the expression level and proportions of miRNA isoforms, transcripts originating from the same miRNA but with slight differences in their nucleotide sequences. This study highlights a novel aspect of miRNA expression dynamics upon infection and increases our understanding of miRNA-mediated mechanisms involved in host cellular responses to infection.
Introduction
The response of host cells to microbial infection or immune activation is among the most-well studied examples of cellular responses to external stimuli. This response is characterised by marked changes in gene expression [1–6], which require precise coordination to establish appropriate immunological outcomes, ensuring maximal protection against infection while avoiding tissue damage. The crucial role of microRNAs (miRNAs) – small regulatory RNAs that mediate degradation or translational repression of thousands of target mRNAs [7–9] – in regulating mammalian immune systems is increasingly well established. MiRNAs regulate the development and function of immune cells and can have pro-inflammatory or anti-inflammatory effects [10–14]. Furthermore, experimental data indicate that microbial infection alters the miRNA repertoire of host cells [15,16] and that, when aberrantly expressed, miRNAs can contribute to immunity-related pathological conditions, such as infectious or inflammatory diseases, autoimmunity or cancer [12,14,17,18].
The advent of next-generation sequencing, in particular RNA-sequencing, has enabled the exploration of a myriad of novel questions related to miRNA diversity. For example, besides the detection of many novel miRNAs and the description of an increasingly broad array of non-canonical biogenesis pathways producing functional miRNAs [19–21], RNA-seq studies have highlighted the highly dynamic relative abundance of the 5p and 3p arms of the miRNA duplex, a process known as arm-switching [22]. Indeed, following cleavage by Dicer, the miRNA hairpin produces a ∼22-bp RNA duplex, one strand of which is preferentially incorporated into the RNA-induced silencing complex (RISC) as a mature, functional miRNA, whereas the other strand has often been thought to be degraded [23]. Previously believed to be static and dictated by the thermodynamic and structural properties of the duplex [24,25], the choice of the dominant miRNA arm has recently been shown to be flexible across species, tissues and developmental stages [22,26–31].
Deep sequencing has also revealed the presence of sequence variation among mature miRNAs – known as isomiRs – shifting the view of miRNAs from single sequences to heterogeneous repertoires of multiple isoforms [32–34]. For a given miRNA, the distribution of different isomiRs appears to be non-random and can differ between tissues and developmental stages [27,28,30,35–41]. Moreover, that isomiRs appear to act co-operatively with canonical miRNA sequences, targeting common pathways to reduce the signal-to-noise ratio of mRNA targeting [28], suggests that changes in their proportions may have functional implications for gene regulation. Although these studies indicate that arm dominance and isomiR expression are dynamic, the extent to which they can be altered by external stimuli, such as infection, remains unknown.
The characterisation of the host miRNA responses to bacteria has progressed at a slower pace than that of viral and parasitic infections [16], despite the fact that a number of bacteria are responsible for some of the most devastating infectious diseases today. Notable among these is Mycobacterium tuberculosis (MTB), the aetiological agent of tuberculosis (TB), the most deadly disease caused by a single bacterial agent [42]. A large number of miRNAs have been recently described as being involved in the response to MTB and other mycobacteria [43–55]. However, the highly heterogeneous nature of these studies—i.e., the use of different mycobacteria, experimental settings (patients, human cells, mice), cell types or tissues, and times post-infection—has precluded any comparison among them, so a clear understanding of the miRNA transcriptional response to MTB is missing. More generally, the extent to which alterations in miRNA expression upon infection are specific to particular pathogens or strains, or instead reflect general responses of host cells to infection, cell activation or inflammation remains to be explored.
Here, we use deep sequencing to characterise the miRNA transcriptional response over time of a key immune cell type – the dendritic cell (DC) – to various mycobacterial strains differing in their virulence as well as to other intracellular bacteria outside the Mycobacterium genus. This global, unbiased approach provides a truly comparative picture of the miRNA repertoire, including novel miRNAs, involved in immunity to MTB and, more broadly, bacteria. We defined core bacterial miRNA responses, as well as responses shared between smaller groups of pathogens or detected in a single condition that may reflect particular mechanisms of virulence or suppression. Furthermore, we explored, for the first time, the extent to which infection impacts both the relative abundance of the arms of the miRNA hairpin and the expression dynamics of miRNA isoforms.
Results
Expression of annotated and novel miRNAs in dendritic cells
To assess the variability of the genome-wide miRNA response to infection, we exposed human monocyte-derived DCs from healthy donors to a diverse set of bacterial species. This panel included three bacteria of the Mycobacterium tuberculosis complex (MTBC), a group of closely related mycobacteria that cause TB in humans or other species. Specifically, we used two virulent strains of Mycobacterium tuberculosis – the reference strain H37Rv of the Euro-American lineage (MTB-Rv) and a member of the Beijing strain of the East Asian lineage (MTB-Bj) – as well as the attenuated strain Mycobacterium bovis BCG (BCG), widely used as a vaccine against TB. In addition, we included a Gram-positive species, Staphylococcus epidermidis (STP), and two Gram-negative bacteria, Salmonella typhimurium (SLM) and Yersinia pseudotuberculosis (YRS) (S1 Fig.). To study variation in miRNA transcript levels at high resolution, we performed small RNA-sequencing (sRNA-seq) from matched non-infected and infected cells at three time points (4h, 18h and 48h). In total, we generated 1.1 billion reads of 50 bp, corresponding to 116 samples with an average of 9.1 million reads per sample after filtering (GSE64142). Of these, 98% were mappable to the human genome while 85% of reads mapped to miRNAs in miRBase v20 (www.mirbase.org).
Following processing of the data (Methods and S1 Fig.), we detected 387 annotated miRNAs that were present in three or more donors in at least one experimental condition (i.e. cells infected with a given bacterium, or left uninfected, at a given time point) at a depth ≥ 50 reads (S1 Table). To identify putative novel miRNAs, we applied a two-step discovery and quantification approach using miRDeep2 [56] (see Methods). Of the 369 predicted hairpins, we detected 18 putative mature miRNAs that were present at a depth ≥ 50 reads in three or more donors in at least one experimental condition (S2 Fig.). Of these, 5 corresponded to known snoRNAs, 8 were located in introns, 4 were antisense to genes and 1 was intergenic (S2 Table). Together, this dataset presents a comprehensive, unbiased characterisation of the miRome of steady-state and activated DCs.
Highly overlapping miRNA responses to diverse bacterial infections
To identify miRNAs whose expression was altered after bacterial infection, we compared infected samples to the corresponding non-infected time point using the package DESeq [57]. A total of 152 miRNAs (38%), of which 145 were previously annotated and 7 were novel, were significantly differentially expressed (FDR-adjusted p<0.01 and |log2 fold change|>1) upon encounter with at least one of the six bacteria. For all bacteria, the number of differentially expressed miRNAs increased over time (Fig. 1A and S1 Table). The bacteria that showed the greatest impact on miRNA expression were YRS, MTB-Bj and MTB-Rv (“high-responders”), while BCG, SLM and STP elicited more modest responses (“low-responders”) (Fig. 1A). In addition, unsupervised hierarchical clustering clearly separated experimental conditions into three distinct clusters corresponding to the length of infection, with all non-infected conditions clustering with the 4h time point (Fig. 1B), consistent with principle component analysis (S3 Fig.). Overall, these results suggest that the length of infection is a stronger driver of the miRNA response than the identity of the bacterium.
Fig. 1. Differential expression of miRNAs in DCs upon infection with a panel of bacteria. 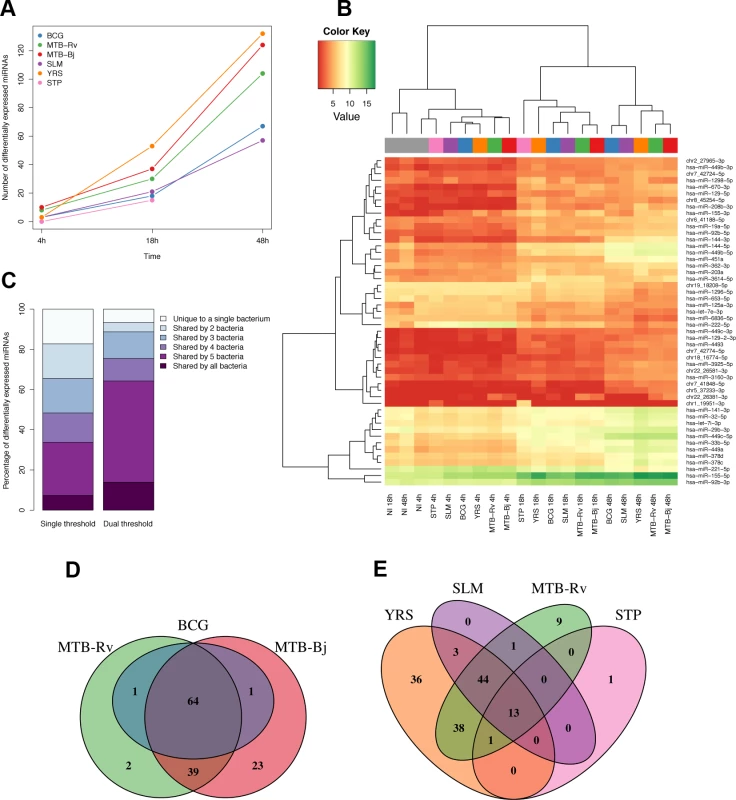
(A) Numbers of significantly differentially expressed miRNAs upon infection at each time point for each bacterium. As we did not have expression profiles for the 48h time point for STP infection, this point is missing from the plot. (B) Heatmap illustrating the hierarchical clustering of experimental conditions based on the mean expression levels of the 50 most variable miRNAs. (C) Overlap of differentially expressed miRNAs between bacteria using two different significance cut-offs. Left-bar shows overlap using a single cut-off of FDR-adjusted p<0.01 and |log2 fold change|>1, while the right bar shows the overlap using a secondary cut-off where a miRNA was called as significant if the absolute log2 fold change was less than 1, if it passed the first more stringent fold-change cut-off upon infection with at least one of the six bacteria. (D and E) Venn diagrams showing the overlap of significantly differentially expressed miRNAs between bacteria of the MTBC (D) and between MTB-Rv and all other non-mycobacterial infections (E). To qualitatively assess the similarity of miRNA responses to the various bacterial infections, we studied the overlap of differentially expressed miRNA sets. We found that over 30% of miRNAs were differentially expressed upon infection with five or more different bacteria, with over 80% shared between at least two independent infections (Fig. 1C). To avoid inflating the dissimilarities between bacteria due to slight differences in fold changes, which were generally highly correlated (S4 Fig.), we also defined miRNAs as significantly differentially expressed when the absolute log2 fold change was less than 1 if in at least one other experimental condition the change upon infection exceeded this cut-off. Using this threshold, 64% of differentially expressed miRNAs were altered upon infection with at least five of the six bacteria (Fig. 1C). Consistent with their close genetic similarity, MTBC bacteria showed highly overlapping miRNA responses (Fig. 1D). At the same time, less than 10% of miRNAs differentially expressed following MTB-Rv infection were unique to this bacterium, taken as a representative of the MTBC, when compared to more distantly related, non-mycobacterial infections (Fig. 1E). Overall, these results suggest a remarkably consistent miRNA response across diverse bacterial pathogens.
Temporal miRNA dynamics identifies a core response to bacterial infection
We next investigated whether miRNAs that were differentially expressed upon infection showed similar temporal responses across bacteria, using the Short Time-series Expression Miner (STEM) [58,59]. This program, specifically designed for short time-series datasets, uses the changes in expression observed at each time point to cluster miRNAs according to a set of pre-determined model temporal response profiles. We identified 14 miRNAs that were assigned to the same model profiles in all bacteria and 35 additional miRNAs that were assigned to highly correlated model profiles in all bacteria (see Methods, Fig. 2A and S3 Table). These 49 miRNAs, which represent the basis of the core miRNA response to bacterial infection in DCs, comprised 27 miRNAs that were upregulated upon infection, 21 that were downregulated and one, miR-222-5p, which was upregulated at 4h but downregulated at later time points. In addition, two novel miRNAs (chr6_41188-5p and chr19_18208-5p) were assigned to highly correlated model profiles in all bacteria (S5 Fig.). To check that these core response miRNAs indeed showed consistent responses across bacteria, we chose to test two of them – miR-155-5p and miR-92b-3p – by qPCR and confirmed our observations (S6A Fig.).
Fig. 2. Shared and specific miRNA responses to bacterial infection. 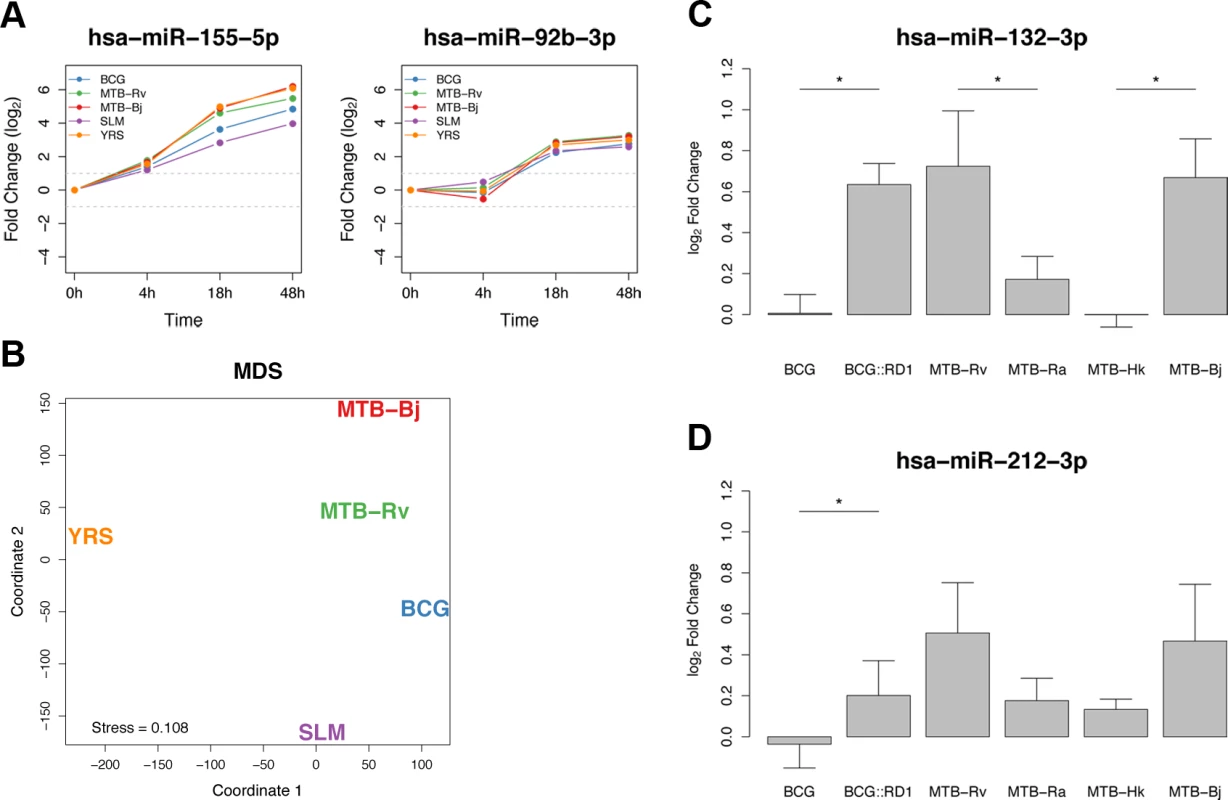
(A) Plots of the temporal dynamics of two core response miRNAs. As we did not have expression profiles for the 48h time point for STP infection, this bacterium was excluded from the analysis. (B) Multidimensional scaling analysis (MDS) representing the distances between the temporal miRNA responses to different bacterial infections. Distances were based on the sum of edit distances, for each miRNA, between bacteria using STEM-assigned model temporal profiles. (C) The expression of miR-132-3p increased following infection with MTB-Rv and MTB-Bj but not BCG. Transformation of BCG with RD1 from MTB resulted in a significant increase in miR-132-3p expression, not significantly different to that induced by MTB-Rv. Both MTB-Ra and MTB-Hk showed significantly lower miR-132-3p expression than virulent mycobacteria. (D) The induction of miR-212-3p was significantly higher following BCG::RD1 infection, compared to infection with control BCG, and was not significantly different to the response to MTB-Rv. Though the difference between the induction of this miRNA upon infection with virulent or avirulent MTB strains was not significant, the tendencies observed were consistent with miR-132-3p. Significance was calculated using a Mann-Whitney test (* = p < 0.05). To provide insight into the impact of this core miRNA response on innate immune cell function, we performed gene ontology category enrichment analysis for gene targets of core miRNAs as predicted by TargetScan [9]. We further restricted our analysis to those targets whose mRNA transcripts have been found to interact with the miRNAs concerned in at least three independent CLIP-seq experiments [60]. We found that a number of relevant biological processes showed some enrichment in high-confidence targets of up-regulated miRNAs, most notably “cellular response to lipopolysaccharide” (S4 Table), while no enrichment was found in high-confidence targets of down-regulated miRNAs. To complement this analysis, we used miRNA and mRNA expression profiles from a previous study in the same cellular system upon MTB-Rv infection [55], to delineate mRNAs whose expression was significantly correlated with that of our core response miRNAs. Interestingly, mRNAs correlated with four core response miRNAs – miR-155–5p, miR-505–3p, miR-7-5p and miR-940 – showed an enrichment in innate immune functions (e.g. innate immune response, immune system process and response to bacterium) [55].
We next used hierarchical clustering to assess the correlation structure among core response miRNAs (Methods). Using the dynamic tree cut algorithm [61], we identified six clusters of highly correlated miRNAs (S3 Table). The two largest clusters contained 20 and 17 miRNAs, respectively. To identify upstream regulators that may explain the coexpression of miRNAs in these two large clusters, we searched for an enrichment of transcription factor binding in the regions surrounding these miRNAs (Methods). Using transcriptional regulatory relationships from ChIP-seq data obtained from ChIPBase [62], we found that hairpins coding for core response miRNAs belonging to the largest of these clusters (i.e., cluster 1 in S3 Table) were most strongly enriched in the binding of the transcription factor MED12 in their promoter regions (p = 4×10–3). Interestingly, MED12 has been found to be significantly upregulated upon MTB infection of DCs [63], suggesting a role of this gene in the regulation of this miRNA cluster in response to bacterial infection.
Distinct miRNA signatures in response to infection by virulent mycobacteria
Despite the generally strong similarity of miRNA responses to all bacteria in our panel (Fig. 1), closer examination revealed a number of more subtle signatures of variability that could be related to differences between bacteria. At the genome-wide level, hierarchical clustering based on miRNA expression levels indicated the presence of sub-clustering at each time point, with some bacteria showing more similar expression profiles following infection (Fig. 1B). This sub-clustering was further supported by a multidimensional scaling analysis (MDS) using the sum of edit distances calculated for each miRNA between bacteria based on their STEM model temporal response profiles (Methods). This showed a strong similarity between the responses to the two virulent MTBC bacteria (MTB-Rv and MTB-Bj), while the response to the attenuated BCG was intermediate between those of MTB-Rv and SLM (Fig. 2B). The separation of YRS in coordinate 1, indicating that the miRNA response to this bacterium was the most distinctive, may be due to the secretion of Yersinia outer proteins (Yops), which are unique to this bacterium and have previously been described to modulate host signalling pathways [64].
To further identify miRNA responses that were specific to virulent MTBC (vMTBC) bacteria, we fitted a generalized linear mixed model to test for the effect of infection with a vMTBC strain, while accounting for variability between infection conditions (Methods). We found that the magnitude of the response of 6, 5 and 14 miRNAs, at 4h, 18h and 48h respectively, was specific to infection with MTB-Rv and MTB-Bj (FDR-adjusted p < 0.01; S5 Table). This suggests that these miRNAs are part of a virulence-dependent response to mycobacterial infections.
Of the 20 miRNAs that showed a vMTBC-specific response, it is worth noting the presence of miR-132-3p, the only miRNA that was significant at all time points. This miRNA has previously been implicated in the regulation of the inflammatory response [65]. Additionally, both arms of miR-212—the other member of the miR-132/212 family due to their sequence homology and co-localisation on chromosome 17p13.3—also showed a vMTBC-specific response at 18h or 48h. To further investigate the role of virulence in the altered expression of the miR-132/212 family, we studied their response to an extended set of mycobacterial strains that differ in their virulence (Methods). Interestingly, we found that the attenuation and/or inactivation of MTB leads to a significantly lower induction of miR-132–3p, and to a lesser extent miR-212–3p, compared to virulent mycobacteria (Fig. 2C,D and S6 Table). Furthermore, infection with BCG::RD1 – a recombinant strain of BCG containing the RD1 locus, the absence of which accounts, to a large extent, for the attenuation of BCG [66] – significantly increased the induction of miR-132-3p and miR-212-3p, with respect to BCG, attaining a level that was not significantly different from cells infected with the virulent strains (Fig. 2C,D and S6 Table). Overall, these results indicate that the altered expression of the miR-132/miR-212 family is dependent on mycobacterial virulence and, more specifically, that the presence of the virulence-associated RD1 locus is sufficient to account for the stronger induction of the miR-132/212 family among virulent mycobacteria.
Infection induces changes in the relative expression of the arms of the miRNA hairpin
We next sought to move beyond considering miRNAs as single units to assess the impact of infection on other aspects of miRNA dynamics, from the broader context of the miRNA hairpin to the finer level of internal sequence variability. We first assessed whether infection induces changes in the relative expression of sequences derived from the 5p and 3p arms of the miRNA hairpin. In general, we found that one of the two arms is usually highly dominant. Of the 341 detected mature miRNAs that had a unique genomic alignment, 63% had only one arm expressed at detectable levels, of which one third had no annotated second sequence (Fig. 3A). Of the remaining 27%, 78% had at least a 10-fold dominance of one of the two arms, while only 15% showed less than a 2-fold difference between the expression levels of the two arms (Fig. 3A). These figures, however, are likely to underestimate the true dominance as we applied the same expression criteria for the detection of both arms of the hairpin.
Fig. 3. Changes in relative miRNA arm expression upon infection. 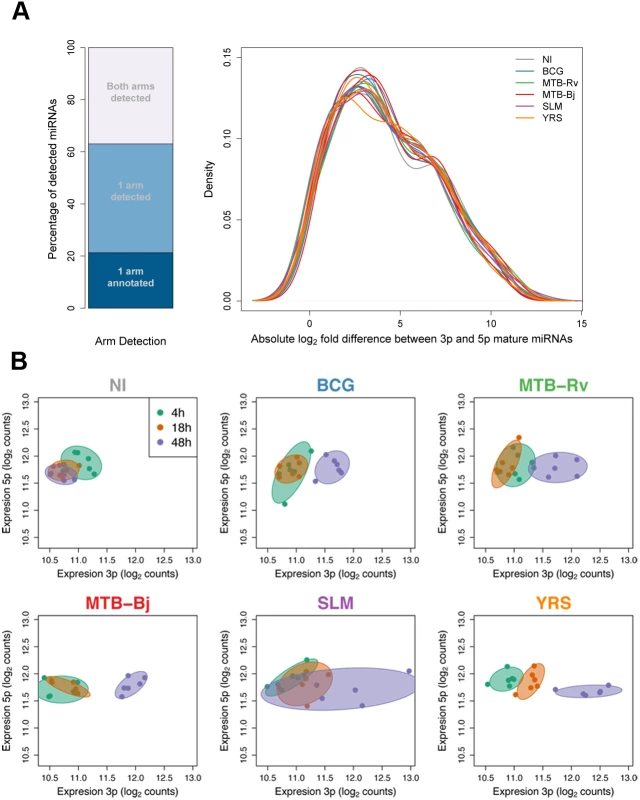
(A) Detection and relative expression of miRNA hairpin arms. Stacked bar plot shows the proportion of detected miRNA hairpins for which either one or both mature miRNA sequences were detected. Density plot shows the distribution of log2 expression ratios for hairpins where both arms were expressed. (B) An example of one miRNA, miR-361, which showed a strong infection-dependent change in relative arm expression. Axes show the expression of the 3p and 5p arms of all sequenced individuals at each time point. In each panel, colours denote the different time points, and infection conditions are plotted in separate panels. The displacement of points towards the right of the plot in infected samples at later time points shows that the change in the expression ratio of the two arms is due to the increased expression of the 3p arm that, in some conditions, becomes dominant. We then examined the extent to which infection alters the relative expression of the arms of the miRNA hairpin for the 92 miRNAs for which both arms were detected (Methods). We detected 40 miRNAs that showed a significant change in relative arm expression in at least one experimental condition (FDR-corrected p < 0.01), with changes being broadly consistent, in tendency if not in statistical significance, across all bacteria (S7 Table). The majority of these changes grew in magnitude over time. Indeed, 13 hairpins showed differences in relative arm expression between time points in non-infected samples (p<0.01), suggesting that time also has a marked, albeit more modest, impact on this feature of miRNA abundance. Of the 27 hairpins (30% of those tested) for which the observed change in the relative expression of the hairpin arms was exclusively associated with infection, three of them (miR-199b, miR-361, miR-582) showed a change in the identity of the dominant miRNA arm upon infection with at least one bacterium (Fig. 3B, S7 Fig. and S7 Table). In most conditions, this change reflected the loss of arm dominance due to a change in the abundance of one of the two arms. However, in two cases (miR-361 and miR-582 expression following YRS infection for 48h) we observed a clear switch in the dominant miRNA sequence. To validate these observations, we measured the expression of one arm-switch miRNA – miR-361 – by qPCR, and obtained a highly concordant tendency of the change in relative expression upon infection (S6B Fig.).
Abundant changes in isomiR diversity in response to infection
We finally explored the internal sequence diversity of expressed miRNAs (i.e., isomiRs) at the steady-state and upon infection. In general, we detected much greater variability in the end site of isomiRs compared to their start positions (S8A Fig.). Moreover, this variability was dependent on the hairpin arm from which a miRNA was derived, with greater start-site variability among 3p miRNAs and greater end site variability in 5p miRNAs (p = 3.39×10−14 and p = 3.15×10−20, respectively; S8B Fig.). We next classified reads aligning to miRNAs into six groups according to their differences from the canonical miRNA sequence (Fig. 4A). We found that the canonical sequence was the dominant isomiR for only ∼50% of miRNAs, with the most common alteration being a change in the end position of the miRNA (3PC), a pattern that was consistently observed across conditions (Fig. 4B and S9 Fig.). Additionally, we observed high isomiR diversity for most miRNAs with the dominant read accounting for only half (median = 49.9%) of all reads aligning to a given miRNA (Fig. 4C).
Fig. 4. Dynamic expression of miRNA isomiRs upon infection. 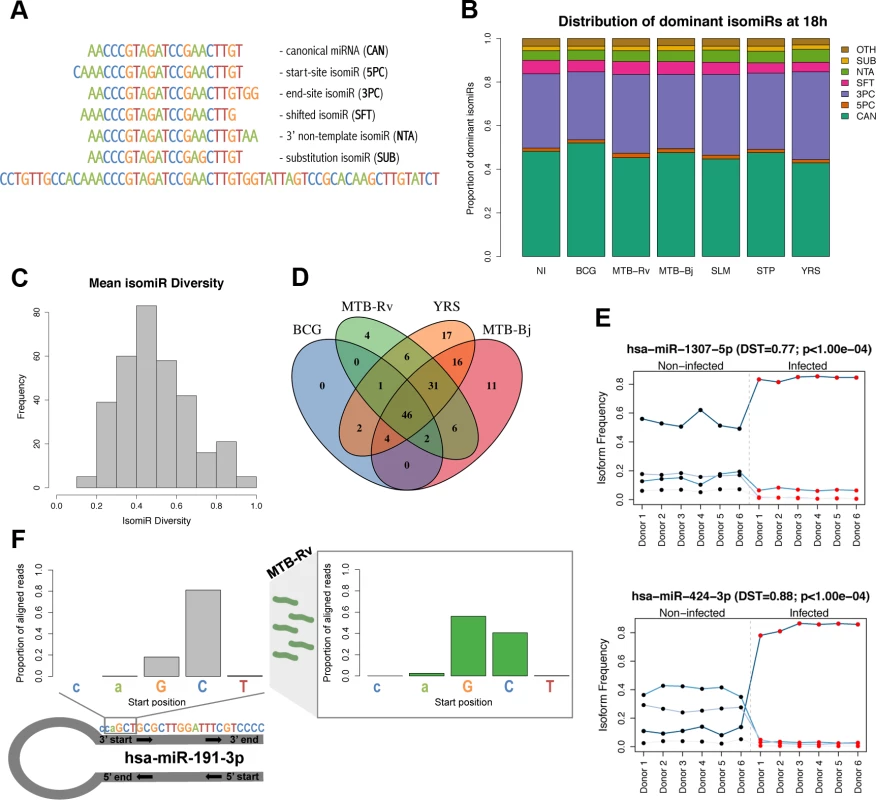
(A) Classification of isomiRs depending on their difference from the annotated mature miRNA sequence (based on [28]). (B) Cumulative barplots showing the proportion of miRNAs for which the dominant sequence was the canonical sequence or, instead, one of six classes of isomiR. Data shown is for 18h post-infection, for other time points see S9 Fig. (C) Histogram showing the mean miRNA diversity (expression of dominant isomiR / total expression of miRNA) across all experimental conditions. (D) Venn diagram showing the overlap of miRNAs showing a significant change in isomiR distribution upon infection with different bacteria. Due to the smaller sample size, we did not perform the analysis upon STP infection. We obtained no significant changes in isomiR distribution following SLM infection, as one individual did not respond to infection (S3 Fig.). (E) Examples of the two characteristic profiles observed for miRNAs showing a change in isomiR distribution upon infection; top: a difference in the proportional expression of each isomiR upon infection, bottom: a change in the dominant isomiR upon infection. Examples are taken from MTB-Rv infection at 48h. Each line represents an individual isomiR and the proportion of the total miRNA expression level accounted for by each isomiR, per donor, is shown before (black dots) and after (red dots) infection. Points are joined together for legibility. (F) The distribution of starting bases of reads aligning to miR-191-3p, which showed a seed shift upon infection with MTB-Rv, MTB-Bj and YRS. Before infection (left), less than 20% of reads start at the first “G” position of the canonical sequence, whereas after infection (right), over 50% of reads start at this position. Proportions of aligned reads shown in the figure are following MTB-Rv infection at 48h. To assess the impact of infection at the level of individual isomiRs, we searched for differentially expressed isomiRs using DESeq (see Methods) and detected 1,595 isomiRs, corresponding to 235 miRNAs, whose expression was altered upon infection (FDR-corrected p-value < 0.01 and |log2 fold change| > 1) (S8 Table). We found a significant overlap between these miRNAs and those that were differentially expressed at the level of total miRNA expression across experimental conditions (p-values = 1.15×10−4–2.03×10−10). However, we detected 132 additional miRNAs that show a response to infection at the isomiR level yet are missed when searching only for expression changes at the total miRNA level. Such changes may reflect (i) modest changes in the expression of one or a small number of isoforms that do not have a sufficiently strong cumulative effect on total miRNA levels to be detected, or (ii) changes in the relative proportions of isomiRs upon infection that do not result in an appreciable net gain or loss of reads aligning to the miRNA. When we tested for such changes in the relative expression of isomiRs in response to infection (see Methods), we identified 146 miRNAs that showed a significant change in isomiR proportions upon infection with at least one bacterium (S9 Table). Interestingly, although miRNAs showing changes in isomiR distribution upon infection showed a significant overlap with miRNAs that had one or more significantly differentially expressed isoforms (p-values = 2.48×10−5–7.27×10−10), we found only limited overlap between miRNAs showing changes in isomiR distribution and miRNAs that were differentially expressed at the whole miRNA level (p-values = 0.045–1), suggesting that this approach captures an additional aspect of miRNA variation in response to infection.
Changes in isomiR distribution increased over time and were strongly overlapping across bacteria, with only 22% unique to a single bacterial infection (Fig. 4D). For a subset of these miRNAs, this change in isomiR distribution involved a change in the dominant isomiR sequence between non-infected and infected samples (Fig. 4E and S10 Table). For those miRNAs where the canonical miRNA was the dominant sequence in one of the two conditions (∼70% of cases), a switch from the canonical sequence before infection to a non-canonical isoform upon infection was 3× more common than the reverse trend. Consistent with the expression levels of different isomiR classes, the majority of dominant isomiR switches (77–100% per condition) involved a change in the 3’ terminus of the most abundant sequence. Interestingly, we identified seven miRNAs – miR-98-3p, miR-140–3p, miR-191-3p, miR-342-5p, miR-548e-5p and miR-2116–3p – that showed a change in the 5’ start site of the dominant sequence upon infection with one or more bacteria (Fig. 4F).
To assess the impact of a change in the 5’ start site, and hence the functional seed sequence, on potential miRNA targets, we used TargetScan’s custom target prediction [67]. We found that 45–87% (mean 71%) of the predicted targets of the annotated miRNA sequence are not predicted targets of the alternative 5’ shift isomiR, suggesting that these isomiR changes could profoundly impact upon miRNA-mediated functions. In addition, that miRNAs showing changes in isomiR distribution in response to infection were highly expressed compared to genome-wide miRNA expression levels (Mann-Whitney p-values 8.13×10−4–2.88×10−9) suggests that these changes are sufficiently highly abundant to be of functional relevance. These results, together with our observation that infection can also lead to changes in the relative abundance of the hairpin arms, indicate an even greater dynamism in the regulation of miRNA expression than previously appreciated.
Discussion
In this study, we have shown that the miRNA repertoire involved in the host cellular response to infection is highly similar across a set of different bacteria, both qualitatively – in the identities of differentially expressed miRNAs – and quantitatively – in the high concordance of their expression dynamics upon infection and over time (Fig. 1 and Fig. 2). Specifically, we found that less than 10% of differentially expressed miRNAs are unique to a single bacterium, and defined a set of 49 miRNAs – one third of all differentially expressed miRNAs – that characterises a core response to bacterial infection. Such temporally conserved miRNA responses across bacteria, despite their genetic diversity and differing strategies to manipulate host cell functions [68–70], most likely reflect the activation of common or convergent signalling pathways in response to infection, as has been shown for mRNAs [3,71,72]. For example, the expression of two core response miRNAs—miR-155-5p and let-7i-3p – is known to be induced by the activation of TLRs, key innate immunity receptors that recognise a diverse array of microbial products and the signalling of which is regulated, in part, by miRNAs [73,74].
The consistent changes observed among core miRNAs upon infection raises the question of whether such changes are essential for establishing and maintaining an effective immune response to infection. Though the importance of a small number of miRNAs for the immune response has been described [12,74–76], our understanding of the roles played by miRNAs in the regulation of this and other biological processes remains limited. Some insight can be gained from the study of computationally predicted miRNA targets, however the limited complementarity of animal miRNAs and target sites makes this challenging [7,8]. Additionally, such computational tools are restricted by our current knowledge of the rules of miRNA targeting and do not account for cell-specific interactions, limitations that are reflected in the high false positive and false negative rates of such algorithms [77]. Targets have also been identified through a range of experimental approaches, each of which carry their own limitations [78], and, critically, few targets have been functionally validated. Despite these limitations, the enrichment analyses of both predicted targets and correlated mRNAs, as well as existing knowledge of the function of some miRNAs, point to the involvement of our set of core response miRNAs in the innate immune response. This suggests that this miRNA response plays a genuine role in the regulation of basic cellular responses to stress, at least in DCs, rather than being a side effect of the immunological changes following infection.
Our results also revealed some important elements of variability in miRNA transcriptional responses between bacteria that may provide insight into bacterial pathogenesis. In this context, we demonstrated that the induction of the miR-132/212 family characterises the response to virulent mycobacteria and is dependent on the presence of the virulence-associated RD1 locus (Fig. 2C,D). Moreover, the reduced induction of miR-132/212 after infection with an attenuated strain that secretes lower levels of the RD1-encoded virulence effector protein ESAT-6 [79] indicates that such an induction is associated with the secretion of this virulence factor. These results raise questions regarding the underlying mechanisms and potential functional consequences of this response, and of the other virulence-dependent miRNA signatures we identified. For example, in light of the role of miR-132 in the negative regulation of the inflammatory response [65,74,80], it is tempting to speculate that the stronger miR-132 induction we observe contributes to the dampening of the early inflammatory response to infection by virulent mycobacteria. Interestingly, reduced early inflammatory responses have been observed in response to modern MTBC lineages, which include MTB-Rv and MTB-Bj, and have been associated with faster progression and increased virulence in macrophages [81]. Further experimental work is now needed to substantiate this hypothesis and, more generally, to identify the specific mycobacterial virulence factors associated with host miRNA expression dynamics.
One of the most interesting findings of our study, made possible by the use of sRNA-seq, is that infection induces changes in both the relative expression of the arms of the miRNA duplex and the distribution of isomiRs (Fig. 3 and Fig. 4). In particular, the induction of strong changes in isomiR distributions, which were highly concordant across individuals and bacteria, highlights the dynamism of miRNA biogenesis and raises important questions regarding the regulation of this process. Several features of our results strongly support that these sequence variants represent true isomiRs showing genuine, infection-dependent changes in their distribution and expression. The isomiRs that we report are expressed at appreciable frequencies and involve more frequent changes at the 3’ end of the sequence (S8A Fig.), consistent with the conservation of the seed region [82], which are concordant with known post-transcriptional modifications of miRNAs, such as the non-template addition of, exclusively, “A” and “T” nucleotides [83,84]. In addition, the inverse differences in start and end site variability between miRNAs derived from 3p and 5p arms of the miRNA hairpin (S8B Fig.), supports a greater specificity of Drosha, compared to Dicer, cleavage, as recently suggested [37,41]. More importantly, although specific sequence variants could, theoretically, be the result of errors in the trimming of the sequencing adaptors, these would be expected to occur systematically across conditions and cannot therefore account for the reproducible differences in isomiR expression and/or proportions observed between non-infected and infected cells.
Our results suggest that infection, or the cellular response it elicits, alters one or more of the cellular processes that regulate miRNA expression and isomiR production. The control of miRNA homeostasis is a highly complex and dynamic process involving the transcriptional and post-transcriptional regulation of miRNA expression, biogenesis, loading and decay [19,23]. Even a slight disruption of any of these highly integrated stages could have profound yet variable consequences for miRNA abundance and isomiR diversity as well as, potentially, miRNA functions. For example, isomiR-generating post-transcriptional modifications such as nucleolytic trimming and 3’ uridylation and adenylation have been associated with changes in miRNA stability, loading into the miRISC complex and target gene expression [32,35,83–86], and some viruses have been shown to interfere with these processes [87].
We also found that some miRNAs, including miR-191-3p and miR-342-5p, show a seed shift upon infection. Seed shift isomiRs can have distinct, though overlapping, sets of target genes [28,84,88], as highlighted by our results that show a modest 30% overlap between shifted and canonical sequences, suggesting that the targeting properties of these miRNAs are altered upon infection. More broadly, that the majority of changes at the isomiR level were not detected at the whole miRNA level highlights that focusing only on total miRNA expression misses potentially important changes in miRNA regulation. However, it should be kept in mind that ∼85% of all miRNAs had at least one differentially expressed isomiR, emphasising the need to understand further how much of this variability is tolerated by the cell without any biological impact on gene regulation.
In conclusion, our study has reported extensive changes in miRNA expression upon infection that are highly concordant across diverse bacteria and over time. Our results represent the most comprehensive, unbiased view of the similarities in miRNA responses between bacteria to date, and highlight common miRNA-mediated mechanisms that are likely to be essential in the cellular response to stress. Conversely, the detected differences between bacteria may reflect more subtle variations in magnitude and tempo that could, nonetheless, impact on bacterial pathogenesis, such as the case of the induction miR-132-3p. Overall, our findings highlight a novel aspect of miRNA expression dynamics upon infection and increase our understanding of miRNA-mediated mechanisms involved in host cellular responses to infection. In doing so, they provide new perspectives concerning the ways in which infection leads to changes in cellular processes that regulate miRNA expression and isomiR production.
Materials and Methods
Ethics statement
Blood samples from nine healthy donors were obtained from the Etablissement Français du Sang. Signed, written consent was obtained from all individuals. The biobank has been declared to and recorded by both the French Ministry of Research and the French Ethics Committee under the reference DC-2008-68 collection 2.
Bacterial preparation
We infected DCs from six individuals with a panel of six bacteria comprised of: two strains of Mycobacterium tuberculosis (H37Rv and GC1237), Mycobacterium bovis-BCG Pasteur, Salmonella typhimurium strain Keller, Yersinia pseudotuberculosis and Staphylococcus epidermidis (MTB-Rv, MTB-Bj, BCG, SLM, YRS and STP, respectively). Mycobacteria were grown from a frozen stock to mid-log phase in 7H9 medium supplemented with albumin-dextrose-catalase (Difco). Liquid cultures were grown for up to 12 days and stored at −80°C in 1–2ml aliquots with 10% glycerol. Aliquots were thawed 1 week before infection and bacteria were grown to mid-log phase. Before infection, bacteria were washed 2 times with and re-suspended in 1ml of PBS. Mycobacterial clumps were disassociated by passages through a needle, followed by 5 minutes of sedimentation. Clinical isolates of SLM, STP and YRS were grown on Luria-Bertani agar and stored at −80°C. One day before infection, aliquots were thawed and bacteria grown overnight. 1ml of bacterial culture was grown to mid-log phase shortly prior to infection. Bacterial density in the supernatants was checked at OD600 and confirmed by counting colony-forming units. We infected DCs from three additional individuals with a second panel of six MTBC bacteria comprised of: BCG transformed with the empty cosmid pYUB (BCG) or the same cosmid containing RD1 (BCG::RD1), Mycobacterium tuberculosis H37Ra (MTB-Ra), a non-virulent strain of MTB, heat-killed Mycobacterium tuberculosis H37Rv (MTB-Hk), MTB-Rv and MTB-Bj. All strains were prepared as described above for mycobacteria.
Isolation and infection of DCs
Blood mononuclear cells were isolated by Ficoll-Paque centrifugation. Monocytes were purified from peripheral blood mononuclear cells by positive selection with magnetic CD14 MicroBeads (Miltenyi Biotech). Monocytes were then cultured for 5 days in RPMI-1640 (Invitrogen) supplemented with 10% heat-inactivated FCS (Dutscher), L-glutamine (Invitrogen), GM-CSF (20 ng/mL; Immunotools), and IL-4 (20 ng/mL; Immunotools). Cell cultures were fed every 2 days with complete medium supplemented with the cytokines previously mentioned. The resulting monocyte-derived DCs were infected (∼2×106 cells per condition) at an MOI of 1 : 1 with one of the bacterial panel, or left uninfected, for 1h at 37°C. The cells infected with bacteria of the first panel, or left uninfected, were washed and cultured for a further hour with 50μg/ml gentamycin. The cells were then washed a second time and cultured in complete medium with 5μg/ml gentamycin for an additional 4h, 18h and 48h. In total, we assessed 21 conditions per individual: seven infection conditions (six bacterial infections plus non-infected cells) at three different time points. Due to material limitations and the proliferation rate of the bacteria, we were only able to perform infections and/or recover cells for four of the six individuals and only at 4h and 18h after infection with STP, giving a final total of 116 samples. The cells infected with bacteria of the second panel, or left uninfected, were cultured in complete medium, without gentamycin, for an additional 18h.
Library preparation and sequencing
Total RNA was extracted using the miRNeasy kit (Qiagen). RNA quantity was assessed using the Qubit (Life Technologies) and RNA quality was assessed using the Agilent 2100 Bioanalyzer with the Nano chip (Agilent Technologies). All samples were of very high quality and showed no signs of degradation (mean RNA integrity number = 9). Sequencing libraries were prepared for each of the 116 samples using the Illumina TruSeq protocol following isolation of low molecular weight (small) RNA fragments. Once prepared, indexed cDNA libraries were pooled (8 or 12 libraries per pool) in equimolar amounts and sequenced with single-end 50bp reads on the Illumina HiSeq2000.
Pre-processing of raw sequencing reads
Raw reads were first assigned to individual samples based on their multiplexing index, allowing for 1 mismatch. We obtained an average of 11.9 million raw reads per sample with a minimum yield of 6.3 million reads. Next, sequences matching the 3’ adaptor sequence were identified and trimmed. A minimum matching of the 6 first bases of the adaptor sequence was required giving reads with final real lengths of 0 to 44 bases. Sequence quality was assessed and subsequent processing performed in R using the Bioconductor package ShortRead [89]. Specifically, we confirmed that base quality (Q) values and per-base GC distributions were within expected ranges and that read length distributions showed an enrichment of reads of the same length as mammalian miRNAs (∼22 bases) (S10 Fig.). We further removed repetitive and low complexity reads. Specifically, we discarded all reads that contained a mononucleotide repeat longer than 10 bases, and those that were >75% mono-, di - or tri-nucleotide repeat or >20% “N” bases. Lastly, we discarded all reads shorter than 16 or longer than 26 bases, corresponding to the length distribution of mammalian miRNAs. After these filtering steps, we obtained an average of 9.1 million (minimum 3.8 million) clean, short reads per sample that were used for small RNA quantification.
Sequence alignment
Sequences were aligned to the human reference genome (build GRCh37/hg19) using bowtie (version 0.12.7) [90]. We mapped reads allowing for 2 mismatches (-v 2) and reported all best alignments for reads that mapped equally well to more than one genomic location (-a —best —strata). We suppressed reads with more than 50 possible alignments (-m 50). On average, 98% of reads aligned to the human genome, of which 65% aligned uniquely. As miRNAs are short and tend to occur in families that share highly similar sequences, they are particularly susceptible to spurious multiple alignments, a phenomenon called cross-mapping. Around 35% of reads in the present dataset had more than one best alignment. To avoid cross-mapping artefacts, we used a correction strategy that assigns weights to each of the candidate mapping loci of multiply aligning reads [91]. Weights were calculated based on local expression levels and mismatches in the alignment. Python scripts were obtained from the authors and implemented, without modification, as described in the original manuscript [91].
Prediction of novel miRNAs
We used the program miRDeep2 [56] to detect putative novel miRNAs in our data using a two-step approach. First, we used the mapper module to map all reads to the human reference genome (build GRCh37/hg19) using bowtie (version 0.12.7) with default miRDeep2 alignment parameters [56]. We ran the module on fastq files from all 116 samples by specifying a config file containing a unique identifier for each sample. We removed all sequences containing a base other than A, C, T, G, U or N, collapsed identical reads and output the pooled dataset in fasta format (mapper.pl –d –e –j –h –m –p). We then used the miRDeep2 module to identify novel and known miRNAs in the pooled set of aligned reads from all 116 samples. All reference files containing either mature or precursor sequences of known miRNAs were from miRBase v20, thus we used the –P flag to specify that miRBase identifiers are in post-v18 “5p” and “3p” format. We considered Pan troglodytes, Pan paniscus, Gorilla gorilla and Pongo pygmaeus as related species, as described in the miRDeep2 paper [56]. We validated the miRDeep2 mapping and quantification algorithms by comparing read counts of known miRNAs with our own and confirmed that these were highly correlated. We defined a set of high-confidence putative novel miRNAs using a miRDeep score cut-off of 4 (S2 Fig.). We further removed those predictions that overlapped protein-coding exons, based on Ensembl v75 annotations (www.ensembl.org), as well as those that had a predicted hairpin length less than 45 bases or for which no complementary (star) miRNA was detected.
Expression analysis of annotated and novel miRNA transcripts
We extracted reads aligning to annotated mature miRNA sequences (miRBase v20) or our high confidence set of putative novel miRNAs with at least 75% overlap using BEDTools [92]. As we had libraries that were sequenced to different depths, we normalised the data to give comparable numbers of reads for each sample. Specifically, we used DESeq (version 1.10.1) to calculate a size factor for each library and divided read counts by this factor [57]. To remove lowly or sporadically expressed miRNAs, we kept only those miRNAs with scaled counts of greater than 50 reads in at least three samples from at least one experimental condition.
We used DESeq (version 1.10.1) to identify differentially expressed miRNAs upon infection by fitting a generalized linear model using a negative binomial distribution [57]. Specifically, for each experimental condition we compared miRNA expression levels between non-infected and infected samples at the same time point. As non-infected and infected samples came from the same six donors (four in the case of STP), we controlled for the paired nature of our data by specifying donor identity in our model. We corrected for multiple testing using a stratified false discovery approach, taking a per-condition Benjamini and Hochberg FDR-corrected p-value < 0.01. We also required an absolute log2 fold change greater than 1. We performed unsupervised hierarchical clustering on the 50 most variable miRNAs (i.e. those with the highest variance in expression across all samples) using mean miRNA expression levels, after variance stabilisation, with the heatmap.2 function of the R package gplots.
To test for cases where the miRNA response was specific to virulent MTBC bacteria (MTB-Rv and MTB-Bj), while accounting for variability between conditions, we used the R package glmmADMB [93]. We fit a generalized linear mixed model assuming a negative binomial distribution of miRNA expression:
where μik is the mean read count for individual i in experimental condition k (bacterial strain or non-infected), 1Infectedk is a categorical variable indicating the presence of infection in condition k (irrespective of the bacterial strain), 1MTBk is a categorical variable indicating the presence of infection with a virulent MTBC bacterium in condition k, dk ∼ N(0,σk2) is a random effect of condition k, and where a, b and c are all fixed effects to be estimated from the model. We corrected for multiple testing using a stratified false discovery approach, taking a per-condition Benjamini and Hochberg FDR-corrected p-value < 0.01.We also compared, for annotated miRNAs only, the relative expression level of the 5p and 3p arms of the miRNA duplex between experimental conditions. To do so, we selected only miRNAs where both arms of the hairpin were expressed, had a unique genomic location and did not contain a known polymorphism in their mature sequence (MAF>1% in the European CEU population from the 1,000 Genomes Project [94]). We next calculated, for each sample and miRNA hairpin, the ratio between the expression levels of 5p and 3p mature miRNAs (log2(5p read count / 3p read count)). We then used a paired, two-sided t-test to test for a significant change in this ratio between non-infected and infected samples at the same time point. We used a cut-off of a per-condition Benjamini and Hochberg FDR-corrected p-value < 0.01 to determine significance. To assess the effect of time on the relative expression of 5p and 3p-derived miRNAs, we performed the same analysis comparing, pairwise, non-infected samples at the three time points.
Expression analysis of isomiRs
We extracted reads aligning to annotated mature miRNA sequences as described above and directly normalised the read counts using the same approach as applied for total miRNA expression levels. We removed all reads aligning to miRNAs with multiple genomic alignments and miRNAs that contained a SNP (MAF>1% in the European CEU population from the 1,000 Genomes Project [94]) in their mature sequence. We considered each unique read as a potential miRNA isoform (isomiR). We classified isoforms into six categories: (i) canonical sequences (according to miRBase v20); (ii) changes in start site; (iii) changes in end site; (iv) shifted sequences; (v) containing a substitution; and (vi) non-templated 3’ additions; as well as a seventh mixed group of reads containing multiple types of change. We identified isomiRs that were differentially expressed upon infection using the same filtering criteria, approach and significance thresholds as described for total miRNA expression levels. To assess the impact of infection on the distribution of isoforms for a given miRNA, we defined a statistic (DST) that estimates differentiation in isoform proportions between populations of samples. DST is analogous to FST [95,96] and VST [97], which are also used for detecting population differentiation. DST varies between 0 and 1 and is calculated by considering,
where Da is the mean Euclidean distance between isomiR proportions of samples from condition a, Db is the mean distance between samples from condition b, and Dall is the mean distance between samples across conditions. Distances were calculated using the R function dist on the proportions of all detected isomiRs for a given miRNA. We calculated p-values based on 10,000 permutations of isomiR proportions, with replacement, per miRNA. We used a cut-off of an empirical p-value < 0.001 to determine significance. We found that 97% of the significant changes identified by DST were also detected using AMOVA [95], in the same experimental condition and using the same statistical cut-off, confirming that our metric captures relevant changes in isomiR distribution.Temporal expression profiles of miRNAs in response to infection
We used the Short Time-series Expression Miner (STEM) to characterise the miRNA responses to each bacterium over time [58,59]. This software assigns observations to a pre-determined set of model temporal response profiles based on the correlation coefficient between observed data (i.e., fold-change at each time point) and model profiles. We used default settings except for the maximum unit change between sequential conditions, which we restricted to 1. To account for inter-individual variability, we simultaneously analysed miRNA expression data for all profiled individuals in a given condition using the “repeat data” option. As we did not measure miRNA expression at 0h, we used the “no normalization / add 0” option to set this value to 0. Fold changes were thus calculated by comparing expression levels before and after infection at the same time-point. To account for the fact that STEM allocates a miRNA to a single model profile, even though it may show a strong correlation with one or more additional profiles, we merged clusters where the model profiles were strongly correlated with each other (r>0.8). We defined the core miRNA response on the basis of these merged model profiles.
We further used these, STEM-assigned, model profiles to calculate edit distances between pairs of bacteria for a given miRNA based on the number of steps required to change between their respective model profiles. For example, the edit distance between the profile 0,0,1 and 0,1,2 would be 2 while the distance between 0,-1,-1 and 0,-1,-2 would be 1. We used the sum of these pairwise edit distances to represent the difference between a given pair of bacteria in terms of their miRNA response and visualised this by nonmetric multidimensional scaling using the R function isoMDS from the MASS package.
Enrichment of predicted miRNA targets in Gene Ontology categories
We identified high-confidence predicted miRNA targets by combinbing TargetScan target predictions [9,67,98] and miRNA-protein interaction data based on CLIP-seq using the StarBase database of high-confidence interactions [60]. Gene Ontology (GO) biological processes were downloaded from the website of the Gene Ontology Consortium (http://geneontology.org/). We first checked that core response miRNAs were not significantly different from all other miRNAs with respect to their conservation, GC content, and number of predicted targets. We then calculated the proportion of high-confidence predicted targets of core miRNAs in each GO category. Next, we calculated the same measure for 10,000 randomly resampled size-matched miRNA sets and used this to calculate an enrichment p-value. This p-value reflects the fraction of random miRNA sets having a greater proportion of predicted targets in a given GO category compared to the test set.
Analysis of miRNA coexpression
We performed hierarchical clustering on miRNA expression levels using the package wgcna with default settings [99,100]. Specifically, we used the dissimilarity of the Topographical Overlap Matrix and average linkage to cluster the 49 core response miRNAs based on normalised miRNA expression levels across all 116 samples. We then used the dynamic tree cut method to cut the branches of the dendrogram to give clusters of highly correlated miRNAs [61]. We used the ChIP-Base database to identify transcription factors (TFs) that bind to miRNA promoter regions, defined as the region from 5kb upstream to 1kb downstream of the transcription start site of the miRNA [62]. As this database contains results from many different tissues and cell lines, we only considered whether binding had been detected, or not, in the promoter region. To identify TFs that were significantly more frequently bound close to coexpressed miRNAs than expected, we compared the average number of bound factors per miRNA to 10,000 randomly sampled size-matched miRNA sets.
Real-time quantification of miRNAs
Total RNA was extracted using the miRNeasy kit (Qiagen). To quantify miRNA expression levels, cDNA was synthesized and quantitative real-time PCR (qPCR) performed using the Qiagen miScript PCR system and primers (miScript II RT Kit: 218161; miScript SYBR Green PCR kit: 218073; miR-92b-3p MS00032144; miR-132-3p MS00003458; miR-155–5p MS00031486; miR-212-3p MS00003815; miR-361-5p MS00004032; miR-361-3p MS00009555; U6 MS00033740) in a 7900 Real-time PCR system (Applied Biosystem). Relative miRNA expression levels, normalized to the endogenous control U6, were calculated using the ΔΔCt method [101].
Supporting Information
Zdroje
1. Chaussabel D, Semnani RT, McDowell MA, Sacks D, Sher A, et al. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 2003;102 : 672–681. 12663451
2. Huang Q, Liu D, Majewski P, Schulte LC, Korn JM, et al. The plasticity of dendritic cell responses to pathogens and their components. Science 2001;294 : 870–875. 11679675
3. Chevrier N, Mertins P, Artyomov MN, Shalek AK, Iannacone M, et al. Systematic discovery of TLR signaling components delineates viral-sensing circuits. Cell 2011;147 : 853–867. doi: 10.1016/j.cell.2011.10.022 22078882
4. Fairfax BP, Humburg P, Makino S, Naranbhai V, Wong D, et al. Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science 2014;343 : 1246949. doi: 10.1126/science.1246949 24604202
5. Gat-Viks I, Chevrier N, Wilentzik R, Eisenhaure T, Raychowdhury R, et al. Deciphering molecular circuits from genetic variation underlying transcriptional responsiveness to stimuli. Nat Biotechnol 2013;31 : 342–349. doi: 10.1038/nbt.2519 23503680
6. Lee MN, Ye C, Villani AC, Raj T, Li W, et al. Common genetic variants modulate pathogen-sensing responses in human dendritic cells. Science 2014;343 : 1246980. doi: 10.1126/science.1246980 24604203
7. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116 : 281–297. 14744438
8. Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 2011;12 : 99–110. doi: 10.1038/nrg2936 21245828
9. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 2009;19 : 92–105. doi: 10.1101/gr.082701.108 18955434
10. Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science 2004;303 : 83–86. 14657504
11. Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, et al. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 2008;451 : 1125–1129. doi: 10.1038/nature06607 18278031
12. O'Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol 2012;30 : 295–312. doi: 10.1146/annurev-immunol-020711-075013 22224773
13. Lodish HF, Zhou B, Liu G, Chen CZ. Micromanagement of the immune system by microRNAs. Nat Rev Immunol 2008;8 : 120–130. doi: 10.1038/nri2252 18204468
14. O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol 2010;10 : 111–122. doi: 10.1038/nri2708 20098459
15. Cullen BR. Viruses and microRNAs: RISCy interactions with serious consequences. Genes Dev 2011;25 : 1881–1894. doi: 10.1101/gad.17352611 21896651
16. Eulalio A, Schulte L, Vogel J. The mammalian microRNA response to bacterial infections. RNA Biol 2012;9 : 742–750. doi: 10.4161/rna.20018 22664920
17. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006;6 : 857–866. 17060945
18. Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 2009;10 : 704–714. doi: 10.1038/nrg2634 19763153
19. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 2009;11 : 228–234. doi: 10.1038/ncb0309-228 19255566
20. Yang JS, Lai EC. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol Cell 2011;43 : 892–903. doi: 10.1016/j.molcel.2011.07.024 21925378
21. Creighton CJ, Reid JG, Gunaratne PH. Expression profiling of microRNAs by deep sequencing. Brief Bioinform 2009;10 : 490–497. doi: 10.1093/bib/bbp019 19332473
22. Griffiths-Jones S, Hui JH, Marco A, Ronshaugen M. MicroRNA evolution by arm switching. EMBO Rep 2011;12 : 172–177. doi: 10.1038/embor.2010.191 21212805
23. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 2010;11 : 597–610. doi: 10.1038/nrg2843 20661255
24. Khvorova A, Reynolds A, Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell 2003;115 : 209–216. 14567918
25. Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, et al. Asymmetry in the assembly of the RNAi enzyme complex. Cell 2003;115 : 199–208. 14567917
26. Chang HT, Li SC, Ho MR, Pan HW, Ger LP, et al. Comprehensive analysis of microRNAs in breast cancer. BMC Genomics 2012;13 Suppl 7: S18. doi: 10.1186/1471-2164-13-S7-S18 23281739
27. Chiang HR, Schoenfeld LW, Ruby JG, Auyeung VC, Spies N, et al. Mammalian microRNAs: experimental evaluation of novel and previously annotated genes. Genes Dev 2010;24 : 992–1009. doi: 10.1101/gad.1884710 20413612
28. Cloonan N, Wani S, Xu Q, Gu J, Lea K, et al. MicroRNAs and their isomiRs function cooperatively to target common biological pathways. Genome Biol 2011;12: R126. doi: 10.1186/gb-2011-12-12-r126 22208850
29. Jagadeeswaran G, Zheng Y, Sumathipala N, Jiang H, Arrese EL, et al. Deep sequencing of small RNA libraries reveals dynamic regulation of conserved and novel microRNAs and microRNA-stars during silkworm development. BMC Genomics 2010;11 : 52. doi: 10.1186/1471-2164-11-52 20089182
30. Li SC, Liao YL, Ho MR, Tsai KW, Lai CH, et al. miRNA arm selection and isomiR distribution in gastric cancer. BMC Genomics 2012;13 Suppl 1: S13. doi: 10.1186/1471-2164-13-S1-S13 22369582
31. Marco A, Hui JH, Ronshaugen M, Griffiths-Jones S. Functional shifts in insect microRNA evolution. Genome Biol Evol 2010;2 : 686–696. doi: 10.1093/gbe/evq053 20817720
32. Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol 2013;14 : 475–488. doi: 10.1038/nrm3611 23800994
33. Glazov EA, Cottee PA, Barris WC, Moore RJ, Dalrymple BP, et al. A microRNA catalog of the developing chicken embryo identified by a deep sequencing approach. Genome Res 2008;18 : 957–964. doi: 10.1101/gr.074740.107 18469162
34. Morin RD, O'Connor MD, Griffith M, Kuchenbauer F, Delaney A, et al. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res 2008;18 : 610–621. doi: 10.1101/gr.7179508 18285502
35. Fernandez-Valverde SL, Taft RJ, Mattick JS. Dynamic isomiR regulation in Drosophila development. RNA 2010;16 : 1881–1888. doi: 10.1261/rna.2379610 20805289
36. Lee LW, Zhang S, Etheridge A, Ma L, Martin D, et al. Complexity of the microRNA repertoire revealed by next-generation sequencing. RNA 2010;16 : 2170–2180. doi: 10.1261/rna.2225110 20876832
37. Humphreys DT, Hynes CJ, Patel HR, Wei GH, Cannon L, et al. Complexity of murine cardiomyocyte miRNA biogenesis, sequence variant expression and function. PLoS One 2012;7: e30933. doi: 10.1371/journal.pone.0030933 22319597
38. Llorens F, Hummel M, Pantano L, Pastor X, Vivancos A, et al. Microarray and deep sequencing cross-platform analysis of the mirRNome and isomiR variation in response to epidermal growth factor. BMC Genomics 2013;14 : 371. doi: 10.1186/1471-2164-14-371 23724959
39. Marti E, Pantano L, Banez-Coronel M, Llorens F, Minones-Moyano E, et al. A myriad of miRNA variants in control and Huntington's disease brain regions detected by massively parallel sequencing. Nucleic Acids Res 2010;38 : 7219–7235. doi: 10.1093/nar/gkq575 20591823
40. Vaz C, Ahmad HM, Bharti R, Pandey P, Kumar L, et al. Analysis of the microRNA transcriptome and expression of different isomiRs in human peripheral blood mononuclear cells. BMC Res Notes 2013;6 : 390. doi: 10.1186/1756-0500-6-390 24073671
41. Zhou H, Arcila ML, Li Z, Lee EJ, Henzler C, et al. Deep annotation of mouse iso-miR and iso-moR variation. Nucleic Acids Res 2012;40 : 5864–5875. doi: 10.1093/nar/gks247 22434881
42. WHO (2013) Global Tuberculosis Report 2013. World Health Organization, Geneva.
43. Fu Y, Yi Z, Wu X, Li J, Xu F. Circulating microRNAs in patients with active pulmonary tuberculosis. J Clin Microbiol 2011;49 : 4246–4251. doi: 10.1128/JCM.05459-11 21998423
44. Kumar R, Halder P, Sahu SK, Kumar M, Kumari M, et al. Identification of a novel role of ESAT-6-dependent miR-155 induction during infection of macrophages with Mycobacterium tuberculosis. Cell Microbiol 2012;14 : 1620–1631. doi: 10.1111/j.1462-5822.2012.01827.x 22712528
45. Liu Y, Wang X, Jiang J, Cao Z, Yang B, et al. Modulation of T cell cytokine production by miR-144* with elevated expression in patients with pulmonary tuberculosis. Mol Immunol 2011;48 : 1084–1090. doi: 10.1016/j.molimm.2011.02.001 21367459
46. Ma F, Xu S, Liu X, Zhang Q, Xu X, et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat Immunol 2011;12 : 861–869. doi: 10.1038/ni.2073 21785411
47. Maertzdorf J, Weiner J 3rd, Mollenkopf HJ, Bauer T, Prasse A, et al. Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc Natl Acad Sci U S A 2012;109 : 7853–7858. doi: 10.1073/pnas.1121072109 22547807
48. Rajaram MV, Ni B, Morris JD, Brooks MN, Carlson TK, et al. Mycobacterium tuberculosis lipomannan blocks TNF biosynthesis by regulating macrophage MAPK-activated protein kinase 2 (MK2) and microRNA miR-125b. Proc Natl Acad Sci U S A 2011;108 : 17408–17413. doi: 10.1073/pnas.1112660108 21969554
49. Sharbati J, Lewin A, Kutz-Lohroff B, Kamal E, Einspanier R, et al. Integrated microRNA-mRNA-analysis of human monocyte derived macrophages upon Mycobacterium avium subsp. hominissuis infection. PLoS One 2011;6: e20258. doi: 10.1371/journal.pone.0020258 21629653
50. Singh Y, Kaul V, Mehra A, Chatterjee S, Tousif S, et al. Mycobacterium tuberculosis controls microRNA-99b (miR-99b) expression in infected murine dendritic cells to modulate host immunity. J Biol Chem 2013;288 : 5056–5061. doi: 10.1074/jbc.C112.439778 23233675
51. Spinelli SV, Diaz A, D'Attilio L, Marchesini MM, Bogue C, et al. Altered microRNA expression levels in mononuclear cells of patients with pulmonary and pleural tuberculosis and their relation with components of the immune response. Mol Immunol 2013;53 : 265–269. doi: 10.1016/j.molimm.2012.08.008 22964481
52. Wang C, Yang S, Sun G, Tang X, Lu S, et al. Comparative miRNA expression profiles in individuals with latent and active tuberculosis. PLoS One 2011;6: e25832. doi: 10.1371/journal.pone.0025832 22003408
53. Wu J, Lu C, Diao N, Zhang S, Wang S, et al. Analysis of microRNA expression profiling identifies miR-155 and miR-155* as potential diagnostic markers for active tuberculosis: a preliminary study. Hum Immunol 2012;73 : 31–37. doi: 10.1016/j.humimm.2011.10.003 22037148
54. Yi Z, Fu Y, Ji R, Li R, Guan Z. Altered microRNA signatures in sputum of patients with active pulmonary tuberculosis. PLoS One 2012;7: e43184. doi: 10.1371/journal.pone.0043184 22900099
55. Siddle KJ, Deschamps M, Tailleux L, Nedelec Y, Pothlichet J, et al. A genomic portrait of the genetic architecture and regulatory impact of microRNA expression in response to infection. Genome Res 2014;24 : 850–859. doi: 10.1101/gr.161471.113 24482540
56. Friedlander MR, Mackowiak SD, Li N, Chen W, Rajewsky N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res 2012;40 : 37–52. doi: 10.1093/nar/gkr688 21911355
57. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol 2010;11: R106. doi: 10.1186/gb-2010-11-10-r106 20979621
58. Ernst J, Bar-Joseph Z. STEM: a tool for the analysis of short time series gene expression data. BMC Bioinformatics 2006;7 : 191. 16597342
59. Ernst J, Nau GJ, Bar-Joseph Z. Clustering short time series gene expression data. Bioinformatics 2005;21 Suppl 1: i159–168. 15961453
60. Yang JH, Li JH, Shao P, Zhou H, Chen YQ, et al. starBase: a database for exploring microRNA-mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Res 2011;39: D202–209. doi: 10.1093/nar/gkq1056 21037263
61. Langfelder P, Zhang B, Horvath S. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics 2008;24 : 719–720. 18024473
62. Yang JH, Li JH, Jiang S, Zhou H, Qu LH. ChIPBase: a database for decoding the transcriptional regulation of long non-coding RNA and microRNA genes from ChIP-Seq data. Nucleic Acids Res 2013;41: D177–187. doi: 10.1093/nar/gks1060 23161675
63. Barreiro LB, Tailleux L, Pai AA, Gicquel B, Marioni JC, et al. Deciphering the genetic architecture of variation in the immune response to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A 2012;109 : 1204–1209. doi: 10.1073/pnas.1115761109 22233810
64. Viboud GI, Bliska JB. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol 2005;59 : 69–89. 15847602
65. Nahid MA, Yao B, Dominguez-Gutierrez PR, Kesavalu L, Satoh M, et al. Regulation of TLR2-mediated tolerance and cross-tolerance through IRAK4 modulation by miR-132 and miR-212. J Immunol 2013;190 : 1250–1263. doi: 10.4049/jimmunol.1103060 23264652
66. Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol 2002;46 : 709–717. 12410828
67. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005;120 : 15–20. 15652477
68. Bueno MJ, Perez de Castro I, Malumbres M. Control of cell proliferation pathways by microRNAs. Cell Cycle 2008;7 : 3143–3148. 18843198
69. Galindo CL, Rosenzweig JA, Kirtley ML, Chopra AK. Pathogenesis of Y. enterocolitica and Y. pseudotuberculosis in Human Yersiniosis. J Pathog 2011;2011 : 182051. doi: 10.4061/2011/182051 22567322
70. van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol 2003;3 : 697–709. 12949494
71. Jenner RG, Young RA. Insights into host responses against pathogens from transcriptional profiling. Nat Rev Microbiol 2005;3 : 281–294. 15806094
72. Amit I, Garber M, Chevrier N, Leite AP, Donner Y, et al. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science 2009;326 : 257–263. doi: 10.1126/science.1179050 19729616
73. He X, Jing Z, Cheng G. MicroRNAs: new regulators of Toll-like receptor signalling pathways. Biomed Res Int 2014;2014 : 945169. doi: 10.1155/2014/945169 24772440
74. O'Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol 2011;11 : 163–175. doi: 10.1038/nri2957 21331081
75. Chen CZ, Schaffert S, Fragoso R, Loh C. Regulation of immune responses and tolerance: the microRNA perspective. Immunol Rev 2013;253 : 112–128. doi: 10.1111/imr.12060 23550642
76. Li Y, Shi X. MicroRNAs in the regulation of TLR and RIG-I pathways. Cell Mol Immunol 2013;10 : 65–71. doi: 10.1038/cmi.2012.55 23262976
77. Alexiou P, Maragkakis M, Papadopoulos GL, Reczko M, Hatzigeorgiou AG. Lost in translation: an assessment and perspective for computational microRNA target identification. Bioinformatics 2009;25 : 3049–3055. doi: 10.1093/bioinformatics/btp565 19789267
78. Martinez-Sanchez A, Murphy CL. MicroRNA Target Identification-Experimental Approaches. Biology (Basel) 2013;2 : 189–205. doi: 10.3390/biology2010189 24832658
79. Frigui W, Bottai D, Majlessi L, Monot M, Josselin E, et al. Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog 2008;4: e33. doi: 10.1371/journal.ppat.0040033 18282096
80. Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 2006;103 : 12481–12486. 16885212
81. Portevin D, Gagneux S, Comas I, Young D. Human macrophage responses to clinical isolates from the Mycobacterium tuberculosis complex discriminate between ancient and modern lineages. PLoS Pathog 2011;7: e1001307. doi: 10.1371/journal.ppat.1001307 21408618
82. Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell 2003;115 : 787–798. 14697198
83. Burroughs AM, Ando Y, de Hoon MJ, Tomaru Y, Nishibu T, et al. A comprehensive survey of 3' animal miRNA modification events and a possible role for 3' adenylation in modulating miRNA targeting effectiveness. Genome Res 2010;20 : 1398–1410. doi: 10.1101/gr.106054.110 20719920
84. Neilsen CT, Goodall GJ, Bracken CP. IsomiRs—the overlooked repertoire in the dynamic microRNAome. Trends Genet 2012;28 : 544–549. doi: 10.1016/j.tig.2012.07.005 22883467
85. Jones MR, Quinton LJ, Blahna MT, Neilson JR, Fu S, et al. Zcchc11-dependent uridylation of microRNA directs cytokine expression. Nat Cell Biol 2009;11 : 1157–1163. doi: 10.1038/ncb1931 19701194
86. Katoh T, Sakaguchi Y, Miyauchi K, Suzuki T, Kashiwabara S, et al. Selective stabilization of mammalian microRNAs by 3' adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes Dev 2009;23 : 433–438. doi: 10.1101/gad.1761509 19240131
87. Backes S, Shapiro JS, Sabin LR, Pham AM, Reyes I, et al. Degradation of host microRNAs by poxvirus poly(A) polymerase reveals terminal RNA methylation as a protective antiviral mechanism. Cell Host Microbe 2012;12 : 200–210. doi: 10.1016/j.chom.2012.05.019 22901540
88. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136 : 215–233. doi: 10.1016/j.cell.2009.01.002 19167326
89. Morgan M, Anders S, Lawrence M, Aboyoun P, Pages H, et al. ShortRead: a bioconductor package for input, quality assessment and exploration of high-throughput sequence data. Bioinformatics 2009;25 : 2607–2608. doi: 10.1093/bioinformatics/btp450 19654119
90. Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 2009;10: R25. doi: 10.1186/gb-2009-10-3-r25 19261174
91. de Hoon MJ, Taft RJ, Hashimoto T, Kanamori-Katayama M, Kawaji H, et al. Cross-mapping and the identification of editing sites in mature microRNAs in high-throughput sequencing libraries. Genome Res 2010;20 : 257–264. doi: 10.1101/gr.095273.109 20051556
92. Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 2010;26 : 841–842. doi: 10.1093/bioinformatics/btq033 20110278
93. Fournier D, Skaug H, Ancheta J, Ianelli J, Magnusson A, et al. AD Model Builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optimization Methods and Software 2012;27 : 233–249.
94. Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, et al. An integrated map of genetic variation from 1,092 human genomes. Nature 2012;491 : 56–65. doi: 10.1038/nature11632 23128226
95. Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 1992;131 : 479–491. 1644282
96. Weir CL, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution 1984;38 : 1358–1370.
97. Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, et al. Global variation in copy number in the human genome. Nature 2006;444 : 444–454. 17122850
98. Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 2007;27 : 91–105. 17612493
99. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008;9 : 559. doi: 10.1186/1471-2105-9-559 19114008
100. Langfelder P, Horvath S. Fast R Functions for Robust Correlations and Hierarchical Clustering. J Stat Softw 2012;46. 23050260
101. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25 : 402–408. 11846609
Štítky
Genetika Reprodukčná medicína
Článek NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY ModuleČlánek Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell LongevityČlánek HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and MiceČlánek LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male FertilityČlánek The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAsČlánek Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick VectorČlánek Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53Článek The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in NematodesČlánek The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse SpermatocytesČlánek The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 3- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
- Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
- A Genomic Duplication is Associated with Ectopic Eomesodermin Expression in the Embryonic Chicken Comb and Two Duplex-comb Phenotypes
- Genome-wide Association Study and Meta-Analysis Identify as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy
- Mutations of Human , Encoding the Mitochondrial Asparaginyl-tRNA Synthetase, Cause Nonsyndromic Deafness and Leigh Syndrome
- Exome Sequencing in an Admixed Isolated Population Indicates Variants Confer a Risk for Specific Language Impairment
- Genome-Wide Association Studies in Dogs and Humans Identify as a Risk Variant for Cleft Lip and Palate
- Rapid Evolution of Recombinant for Xylose Fermentation through Formation of Extra-chromosomal Circular DNA
- The Ribosome Biogenesis Factor Nol11 Is Required for Optimal rDNA Transcription and Craniofacial Development in
- Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
- Maternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity
- Clonality and Evolutionary History of Rhabdomyosarcoma
- HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and Mice
- Methylation-Sensitive Expression of a DNA Demethylase Gene Serves As an Epigenetic Rheostat
- BREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
- Seizures Are Regulated by Ubiquitin-specific Peptidase 9 X-linked (USP9X), a De-Ubiquitinase
- The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
- A Cascade of Iron-Containing Proteins Governs the Genetic Iron Starvation Response to Promote Iron Uptake and Inhibit Iron Storage in Fission Yeast
- Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
- LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male Fertility
- Cis-Regulatory Mechanisms for Robust Olfactory Sensory Neuron Class-restricted Odorant Receptor Gene Expression in
- Effects on Murine Behavior and Lifespan of Selectively Decreasing Expression of Mutant Huntingtin Allele by Supt4h Knockdown
- HDAC4-Myogenin Axis As an Important Marker of HD-Related Skeletal Muscle Atrophy
- A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex
- Selective and Genetic Constraints on Pneumococcal Serotype Switching
- Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
- The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAs
- Accumulation of Glucosylceramide in the Absence of the Beta-Glucosidase GBA2 Alters Cytoskeletal Dynamics
- Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities
- The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair
- Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick Vector
- Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
- The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors
- PHABULOSA Controls the Quiescent Center-Independent Root Meristem Activities in
- DNA Polymerase ζ-Dependent Lesion Bypass in Is Accompanied by Error-Prone Copying of Long Stretches of Adjacent DNA
- Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus
- Zinc Finger Independent Genome-Wide Binding of Sp2 Potentiates Recruitment of Histone-Fold Protein Nf-y Distinguishing It from Sp1 and Sp3
- GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters
- Neurospora Importin α Is Required for Normal Heterochromatic Formation and DNA Methylation
- Ccr4-Not Regulates RNA Polymerase I Transcription and Couples Nutrient Signaling to the Control of Ribosomal RNA Biogenesis
- Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53
- A Systems-Level Interrogation Identifies Regulators of Blood Cell Number and Survival
- Morphological Mutations: Lessons from the Cockscomb
- Genetic Interaction Mapping Reveals a Role for the SWI/SNF Nucleosome Remodeler in Spliceosome Activation in Fission Yeast
- The Role of China in the Global Spread of the Current Cholera Pandemic
- The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
- A Zinc Finger Motif-Containing Protein Is Essential for Chloroplast RNA Editing
- Resistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
- Small Regulatory RNA-Induced Growth Rate Heterogeneity of
- Mitochondrial Dysfunction Reveals the Role of mRNA Poly(A) Tail Regulation in Oculopharyngeal Muscular Dystrophy Pathogenesis
- Complex Genomic Rearrangements at the Locus Include Triplication and Quadruplication
- Male-Biased Aganglionic Megacolon in the TashT Mouse Line Due to Perturbation of Silencer Elements in a Large Gene Desert of Chromosome 10
- Sex Ratio Meiotic Drive as a Plausible Evolutionary Mechanism for Hybrid Male Sterility
- Tertiary siRNAs Mediate Paramutation in .
- RECG Maintains Plastid and Mitochondrial Genome Stability by Suppressing Extensive Recombination between Short Dispersed Repeats
- Escape from X Inactivation Varies in Mouse Tissues
- Opposite Phenotypes of Muscle Strength and Locomotor Function in Mouse Models of Partial Trisomy and Monosomy 21 for the Proximal Region
- Glycosyl Phosphatidylinositol Anchor Biosynthesis Is Essential for Maintaining Epithelial Integrity during Embryogenesis
- Hyperdiverse Gene Cluster in Snail Host Conveys Resistance to Human Schistosome Parasites
- The Class Homeodomain Factors and Cooperate in . Embryonic Progenitor Cells to Regulate Robust Development
- Recombination between Homologous Chromosomes Induced by Unrepaired UV-Generated DNA Damage Requires Mus81p and Is Suppressed by Mms2p
- Synergistic Interactions between Orthologues of Genes Spanned by Human CNVs Support Multiple-Hit Models of Autism
- Gene Networks Underlying Convergent and Pleiotropic Phenotypes in a Large and Systematically-Phenotyped Cohort with Heterogeneous Developmental Disorders
- The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
- Combinatorial Control of Light Induced Chromatin Remodeling and Gene Activation in
- Linking Aβ42-Induced Hyperexcitability to Neurodegeneration, Learning and Motor Deficits, and a Shorter Lifespan in an Alzheimer’s Model
- The Complex Contributions of Genetics and Nutrition to Immunity in
- NatB Domain-Containing CRA-1 Antagonizes Hydrolase ACER-1 Linking Acetyl-CoA Metabolism to the Initiation of Recombination during . Meiosis
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
- Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption
- A Genomic Portrait of Haplotype Diversity and Signatures of Selection in Indigenous Southern African Populations
- Sequence Features and Transcriptional Stalling within Centromere DNA Promote Establishment of CENP-A Chromatin
- Inhibits Neuromuscular Junction Growth by Downregulating the BMP Receptor Thickveins
- Replicative DNA Polymerase δ but Not ε Proofreads Errors in and in
- Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in
- The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
- A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension
- Pervasive Variation of Transcription Factor Orthologs Contributes to Regulatory Network Evolution
- Network Analyses Reveal Novel Aspects of ALS Pathogenesis
- A Role for the Budding Yeast Separase, Esp1, in Ty1 Element Retrotransposition
- Nab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
- A RecA Protein Surface Required for Activation of DNA Polymerase V
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Clonality and Evolutionary History of Rhabdomyosarcoma
- Morphological Mutations: Lessons from the Cockscomb
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy





