-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
Methyl farnesoate (MF) is the immediate precursor of juvenile hormone (JH) III in the JH biosynthetic pathway, and lacks the epoxide moiety characteristic of JHs. The potential role of MF as a JH in arthropods has been an issue of a long-standing debate. In this report, comprehensive molecular genetics studies demonstrated that MF plays a dual role in regulating Drosophila metamorphosis. MF is produced by the larval CA and released into the hemolymph, from where it exerted its anti-metamorphic effects indirectly after conversion to JHB3, as well as acting as a hormone itself through a direct interaction with Met and Gce, the two JH receptors.
Published in the journal: Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis. PLoS Genet 11(3): e32767. doi:10.1371/journal.pgen.1005038
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005038Summary
Methyl farnesoate (MF) is the immediate precursor of juvenile hormone (JH) III in the JH biosynthetic pathway, and lacks the epoxide moiety characteristic of JHs. The potential role of MF as a JH in arthropods has been an issue of a long-standing debate. In this report, comprehensive molecular genetics studies demonstrated that MF plays a dual role in regulating Drosophila metamorphosis. MF is produced by the larval CA and released into the hemolymph, from where it exerted its anti-metamorphic effects indirectly after conversion to JHB3, as well as acting as a hormone itself through a direct interaction with Met and Gce, the two JH receptors.
Introduction
Juvenile hormones (JHs) are members of a family of sesquiterpenoid compounds synthesized primarily by the corpus allatum (CA) of insects. Several forms of JH have been identified, including JH 0, JH I, 4-methyl JH I, JH II, JH III, JH bisepoxide (JHB3) and JH skipped bisepoxide. JH III is found in most insect orders, whereas JH 0, JH I, and JH II are exclusive to Lepidoptera [1]. JHB3 is unique to higher Diptera, such as the fruit fly, Drosophila melanogaster [2], and JH skipped bisepoxide has been described in Heteroptera [3]. Methyl farnesoate (MF) is the major sesquiterpenoid identified in the hemolymph of crustaceans, in which it might play the role of a JH [4]. MF lacks the epoxide moiety present in other JHs, and it is usually considered as an immediate precursor of JH III in Insecta [1]. The potential role of MF as a true JH in insects has been an issue of a long-standing debate; it has JH activity in the Drosophila white puparial bioassays and is abundant in the hemolymph of several insects [5–10].
The biosynthetic pathway of JH III in the CA of insects involves 13 discrete enzymatic reactions and is conventionally divided into early and late steps (S1 Fig) [1]. The early steps follow the mevalonic acid pathway to form farnesyl pyrophosphate [11]. 3-hydroxy-3-methylglutaryl CoA reductase (HMGCR), the rate-limiting enzyme for mevalonic acid biosynthesis in mammals, is also an important enzyme in the early steps of JH biosynthesis [11]. In the late steps of JH III biosynthesis, farnesyl pyrophosphate is sequentially transformed to farnesol, farnesal and farnesoic acid (FA) [1]. The order of the last two biosynthetic steps, methyl esterification and epoxidation, catalyzed by a JH acid (JHA) methyltransferase (JHAMT) and a P450 epoxidase, differs among insect species: epoxidation precedes methylation in Lepidoptera, whereas epoxidation follows methylation in Diptera, Orthoptera, Dictyoptera, Coleoptera and probably most other insect orders [12–17]. The Drosophila CA produces and releases three sesquiterpenoids: JHB3, JH III, and MF [2, 9, 10, 18, 19]. However, the entire JH biosynthetic pathway in Drosophila has not been well defined to date.
One major function of JH is to inhibit action of the molting hormone (20-hydroxyecdysone, 20E) for preventing metamorphosis during the larval molts [1]. In JH-deficient animals in which the CA is genetically ablated, JH prevents 20E-triggered apoptosis of the larval fat body [20, 21] and precocious differentiation of the optic lobe in the adult brain [22] in Drosophila. JH serves an equally important function, regulating various aspects of reproductive maturation in most insects [1]. For example, incomplete ablation of the CA results in a partial deficiency of JH with an associated reduction in reproductive capacity in Drosophila [23].
The recent discovery that the JH-resistance gene, Methoprene-tolerant (Met), plays a critical role in insect metamorphosis has been followed by a rapid increase in our understanding of JH signaling [24]. Met and Gce, two paralogous bHLH transcription factors in Drosophila, are involved in JH action [25, 26]. Although both the Met and gce null mutants are viable, the Met gce double mutant dies during the larval-pupal transition [21], similar to that observed in JH-deficient animals [20, 22]. Functionally, Met and Gce mediate JH action to prevent the 20E-triggered metamorphic events [20–22]. Moreover, Met and Gce bind to JH at physiological concentrations in vitro [27, 28], suggesting that they are JH receptors. In parallel, Met is also involved in JH action as a receptor in the red flour beetle, Tribolium castaneum [28, 29]. Downstream of Met, the anti-metamorphic action of JH is transduced by Krüppel-homolog 1 (Kr-h1), a transcription factor involved in JH action. A number of studies in Drosophila [21, 30, 31] and several other insect species [24] have shown that Kr-h1 is a JH primary-response gene.
As shown in previous studies [20, 22], genetic ablation of the CA results in JH deficiency and pupal lethality in Drosophila. To further clarify the roles of JHs in Drosophila, we generated a jhamt mutant. Surprisingly, the jhamt mutant is viable and its MF biosynthesis was not affected. Further, MF was demonstrated to exert crucial roles for completion of Drosophila metamorphosis, by both acting directly as a JH and indirectly after conversion to JHB3.
Results
Mutation of jhamt did not increase JH-dependent lethality
Genetic ablation of the CA results in JH deficiency and pupal lethality in Drosophila [20, 22], while traces of the CA cells are often still present in the ring gland (RG) of the ablated animals during the early larval stages. To further clarify the roles of JHB3, JH III, and MF in Drosophila, we generated a jhamt mutant, which was expected to disrupt the JH biosynthetic pathway and to result in lethality at pupal or earlier stages. The ends-out gene targeting method was utilized to replace the entire jhamt open reading frame with the white gene via homologous recombination [32] (Fig. 1A). Three independent jhamt mutant lines (jhamt1, jhamt2, and jhamt3) were obtained and validated by PCR analysis of genomic DNA (Fig. 1B). The mRNA of jhamt was not detectable in the CA of the jhamt mutants at 3 h after the initiation of wandering (3h AIW), a time when JH titer [10], JH biosynthesis [2] and jhamt mRNA levels [13] are high (Fig. 1C). Immunohistochemical studies revealed the absence of JHAMT protein in the CA of the jhamt mutants at 3h AIW (Fig. 1D and 1D’). Taken together, these studies showed that jhamt1 and jhamt2 are null alleles. For consistence, jhamt2 was used in all the subsequent studies.
Fig. 1. Generation and characterization of the jhamt mutants. 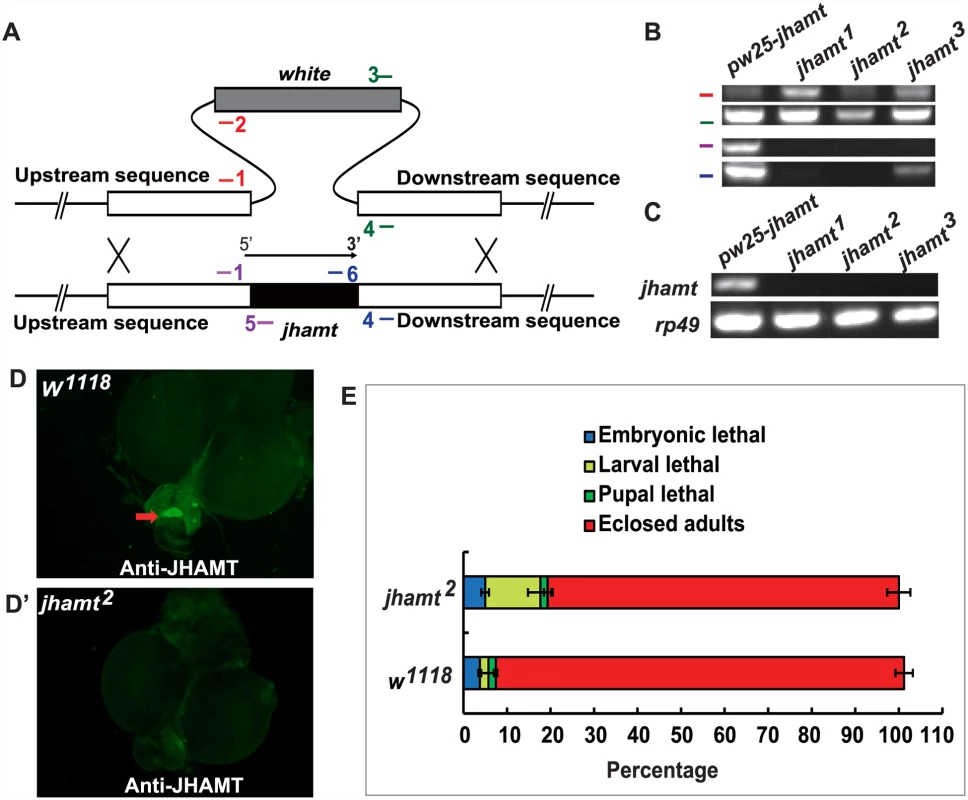
(A) Scheme for jhamt targeting. jhamt (black box) is replaced with white (gray box) by homologous recombination of the flanking sequences (white boxes). Red bars represent the primer pairs jhamt-1/jhamt-2; green bars, jhamt-3/jhamt-4; purple bars, jhamt-1/jhamt-5; blue bars, jhamt-6/jhamt-4. (B) Genomic DNA PCR to detect white and jhamt DNA using the above-mentioned primer pairs and genomic DNA extracted from pw25-jhamt and jhamt1, jhamt2, and jhamt3 lines. (C) Reverse transcription PCR to detect jhamt mRNA (rp49 as the internal control) from pw25-jhamt and jhamt1, jhamt2, and jhamt3. All of the mRNAs were isolated from the brain-RG complexes at 3 hours after initiation of wandering (3hAIW). (D and D’) Immunohistochemistry to detect JHAMT in the CA of w1118 (D) and jhamt2 (D’) at 3hAIW. The red arrow points to the CA showing JHAMT expression. (E) The lethality of w1118 and jhamt2 homozygous mutant during embryonic, larval and pupal stages. JH-dependent phenotypes were evaluated in jhamt2 in comparison with w1118, the wild type fly used to generate jhamt mutants. Approximately 10% of jhamt2 larvae died during the larval stage, with the rest surviving to adulthood (Fig. 1E). In addition, the initiation of wandering was delayed for about 4 hours in jhamt2 larvae (S2A Fig), whereas body weight was not affected (S2B Fig). The fecundity of jhamt2 adult females decreased by about 80%, whereas topical application of methoprene (0.5×10-3 μmol per female) partially restored fecundity (S2C Fig). The ovary size of the 6-day-old jhamt2 virgin females was significantly reduced. However, methoprene partially restored ovary growth (S2C’ Fig). The CA-specific Aug21-GAL4 was used for genetic ablation of the CA in previous studies [20, 22]. We performed a genetic rescue experiment with Aug21-GAL4 driving UAS-jhamt overexpression in a jhamt2 background. Importantly, fecundity and ovary growth of jhamt2/jhamt2; Aug21-GAL4>UAS-jhamt were restored to similar levels to those in w1118 (S2D and S2D’ Fig), showing that the reproductive capacity in jhamt2 was fully rescued by CA-specific jhamt overexpression. Overall, the phenotypic changes in jhamt2 were similar to those described for Aug21-GAL4>UAS-reaper::UAS-hid animals, in which the CA is incompletely ablated and JH is partially deficient [23]. However, jhamt2 showed less robust effects than those observed in JH-deficient Aug21-GAL4>UAS-Grim (Aug21>Grim) animals, in which CA activity is efficiently disrupted [20, 22].
Mutation of jhamt decreased JHB3 but not MF biosynthesis
To verify whether jhamt2 might be only partially JH-deficient, we measured the activity of methyltransferase in the brain-RG complexes isolated from 3h AIW larvae using either FA or JHA as substrates [14, 20, 23, 33]. In w1118 larvae, the methyltransferase activity using FA as substrate was at least 10-fold higher than that using JHA (Fig. 2A). In jhamt2 larvae, the activity of methyltransferase using JHA as the substrate was similar to that of wild-type glands, whereas the activity of methyltransferase using FA as the substrate decreased by 90% when compared to that in wild-type glands (Fig. 2A).
Fig. 2. Mutation of jhamt decreases JHB3 but not JH III and MF biosynthesis. 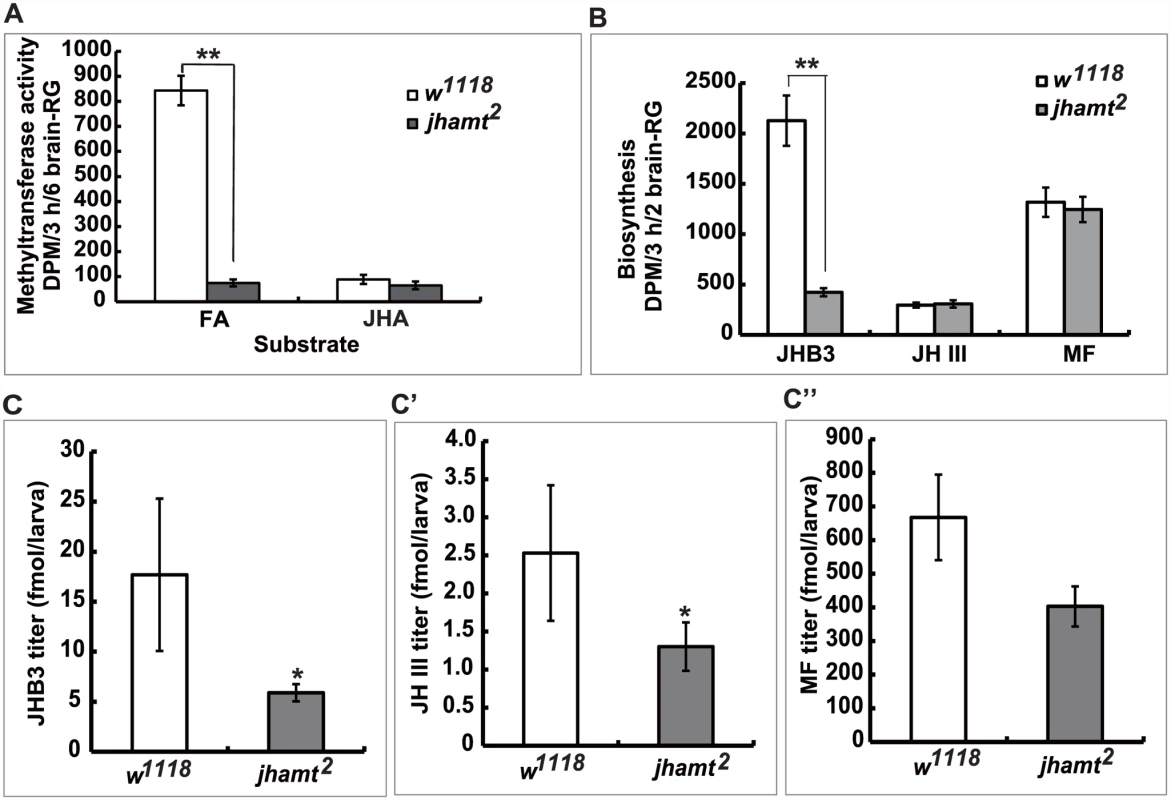
(A) Measurements of methyltransferase activity in the brain-RG complexes in w1118 and jhamt2 at 3hAIW using FA or JHA as the substrate. (B) Measurements of JH biosynthesis in the brain-RG complexes in w1118 and jhamt2 at 3hAIW using the RCA-TLC method. (C-C”) Quantitative measurements of whole body titers of JHB3 (C), JH III (C’), and MF (C”) in w1118 and jhamt2 at 3hAIW using the HPLC-FD protocol. Using the radiochemical assay followed by thin layer chromatography analysis, we studied the biosynthesis of JHB3, JH III, and MF by the brain-RG complexes dissected from 3h AIW larvae. As previously reported [2, 18, 19], JHB3 was the most abundant product released by wild-type glands, the amount of MF released was about half that of JHB3, whereas JH III was produced at the lowest rate. Remarkably, although JHB3 biosynthesis in jhamt2 larval glands decreased by 75% when compared to that in wild-type glands, the rates of JH III and MF biosynthesis were not affected (Fig. 2B).
Finally, using a recently developed HPLC-FD protocol [34], we measured whole body titers of JHB3, JH III, and MF in 3h AIW larvae. In w1118 larvae, MF was the most abundant sesquiterpenoid (~670 fmol/larva), followed by JHB3 (~18 fmol/larva) and JH III (~2.5 fmol/larva) (Fig. 2C–2C”). Although JHB3 showed higher biosynthetic rates, MF showed a higher titer in the larvae, suggesting that MF could be more stable than JHB3 in the body. Whole body titers of JHB3, JH III, and MF in jhamt2 larvae decreased by approximately 70%, 50%, and 30% (no statistical difference) to their respective control levels (Fig. 2C–2C”). Our data thus suggest that 1) jhamt is critical for JHB3 biosynthesis, but not for the biosynthesis of MF and JH III, and 2) the highly abundant MF might play important roles during Drosophila metamorphosis.
Decrease in biosynthesis and titers of the three sesquiterpenoids result in complete lethality
To better understand the relation between the JH-deficient lethal phenotypes and the biosynthesis of the three sesquiterpenoids by the larval CA, we further explored the effect of additional loss-of-function of enzymes in the JH biosynthetic pathway. Drosophila CG10527 is an ortholog of a crustacean FA methyltransferase [35], which has been reported as not involved in JH biosynthesis in Drosophila [33, 36]. We generated a jhamt CG10527 double mutant, jhamt2 CG10527187 (S3 Fig). Mutation of CG10527 in a jhamt2 background did not increase JH-deficient phenotypes (S4 Fig), confirming that CG10527 is not involved in FA or JHA methylation in Drosophila.
Different promoters can be used to drive CA-specific expression in Drosophila. We have previously shown that jhamt-GAL4 has a more robust CA-specific expression than Aug21-GAL4 [21]. Therefore, we generated jhamt-GAL4>UAS-GFP flies, which exhibited strong CA-specific expression of GFP (Fig. 3A and 3A’). As expected, similar to jhamt2/jhamt2; Aug21-GAL4>UAS-jhamt, fecundity and ovary growth of jhamt2/jhamt2; jhamt-GAL4>UAS-jhamt were restored to levels similar to those in w1118 (S2D and S2D’ Fig). We then generated Aug21-GAL4>UAS-hmgcr dsRNA and jhamt-GAL4>UAS-hmgcr dsRNA animals, in which hmgcr expression is specifically reduced in the CA by RNAi. As detected by quantitative real-time PCR (qPCR), hmgcr expression in the brain-RG complexes at 3h AIW decreased by ~35% in Aug21-GAL4>UAS-hmgcr dsRNA animals and ~50% in jhamt-GAL4>UAS-hmgcr dsRNA animals (S5A Fig). Lethality of ~55% and ~70% was observed in Aug21-GAL4>UAS-hmgcr dsRNA (S5B Fig) and jhamt-GAL4>UAS-hmgcr dsRNA animals (Fig. 3B), respectively. Moreover, the lethality in jhamt2/jhamt2; Aug21-GAL4>UAS-hmgcr dsRNA was about 93% (S5C Fig), whereas 100% lethality before adult emergence was observed in jhamt2/jhamt2; jhamt-GAL4>UAS-hmgcr dsRNA (Fig. 3B’). Most jhamt2/jhamt2; jhamt-GAL4>UAS-hmgcr dsRNA animals died during the pupal stage (60%), exhibiting a variety of developmental defects (Fig. 3C). These data not only confirmed that jhamt-GAL4 has a more robust CA-specific expression than Aug21-GAL4, but also demonstrated that reduction of hmgcr expression in the CA in a jhamt2 background causes stronger lethal phenotypes than the jhamt mutant alone.
Fig. 3. Reduction of hmgcr expression in the CA of jhamt mutant results in complete lethality. 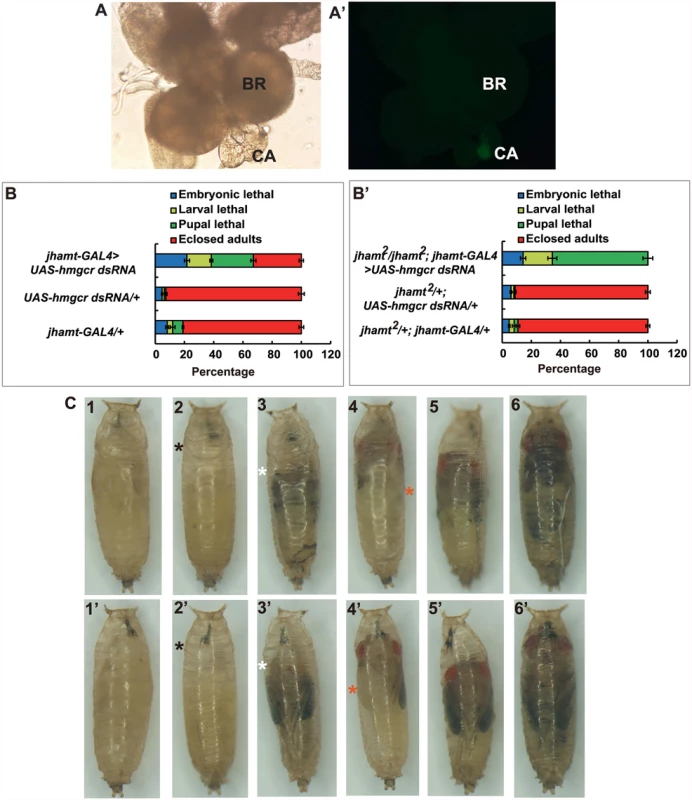
(A and A’) The brain-RG complex in jhamt-GAL4>UAS-GFP. BR, brain; CA, corpus allatum. Observed under bright-field (A) or fluorescence (A’) using the same microscope. The CA cells expressing JHAMT were labeled with GFP. (B and B’) (B) Lethality of jhamt-GAL4>UAS-hmgcr dsRNA during the embryonic, larval, and pupal stages. jhamt-GAL4/+ and UAS-hmgcr dsRNA/+ were used as the controls. (B’) Lethality of jhamt2/jhamt2; jhamt-GAL4>UAS-hmgcr dsRNA during the embryonic, larval, and pupal stages. jhamt2/+; jhamt-GAL4/+ and jhamt2/+; UAS-hmgcr dsRNA/+ were used as the controls. (C) Images of various pupal lethal phenotypes of jhamt2/jhamt2; jhamt-GAL4>UAS-hmgcr dsRNA. (1–6) the abdominal sides; (1’-6’) the dorsal sides. The black asterisks point to empty portions of the pupae; the white asterisks, eye defects showing no pigmentation; the red asterisks, wing defects showing a unilateral wing loss. Overall, these experiments suggest that reduction of hmgcr expression in the CA in a jhamt2 background decreases biosynthesis and titers of the three sesquiterpenoids to very low levels, resulting in complete lethality. In the following experiments, jhamt-GAL4>UAS-hmgcr dsRNA (hmgcrRNAi) and jhamt2/jhamt2; jhamt-GAL4>UAS-hmgcr dsRNA (jhamt2 hmgcrRNAi) were used to further confirm the above hypothesis. We measured JH biosynthesis in larval brain-RG complexes isolated from four different lines at 3h AIW: w1118, jhamt2, hmgcrRNAi, and jhamt2 hmgcrRNAi. In comparison with the w1118 larvae, JHB3 biosynthesis decreased by 75% in jhamt2 and hmgcrRNAi larvae and by more than 90% in jhamt2 hmgcrRNAi larvae. JH III biosynthesis was not altered in jhamt2 larvae, but decreased by 30–40% in hmgcrRNAi and jhamt2 hmgcrRNAi larvae. MF biosynthesis was not altered in jhamt2 larvae, but decreased to about 50% in hmgcrRNAi and jhamt2 hmgcrRNAi larvae (Fig. 4A).
Fig. 4. Reduction of hmgcr expression in the CA of jhamt mutant dramatically decreases biosynthesis and titers of the three sesquiterpenoids. 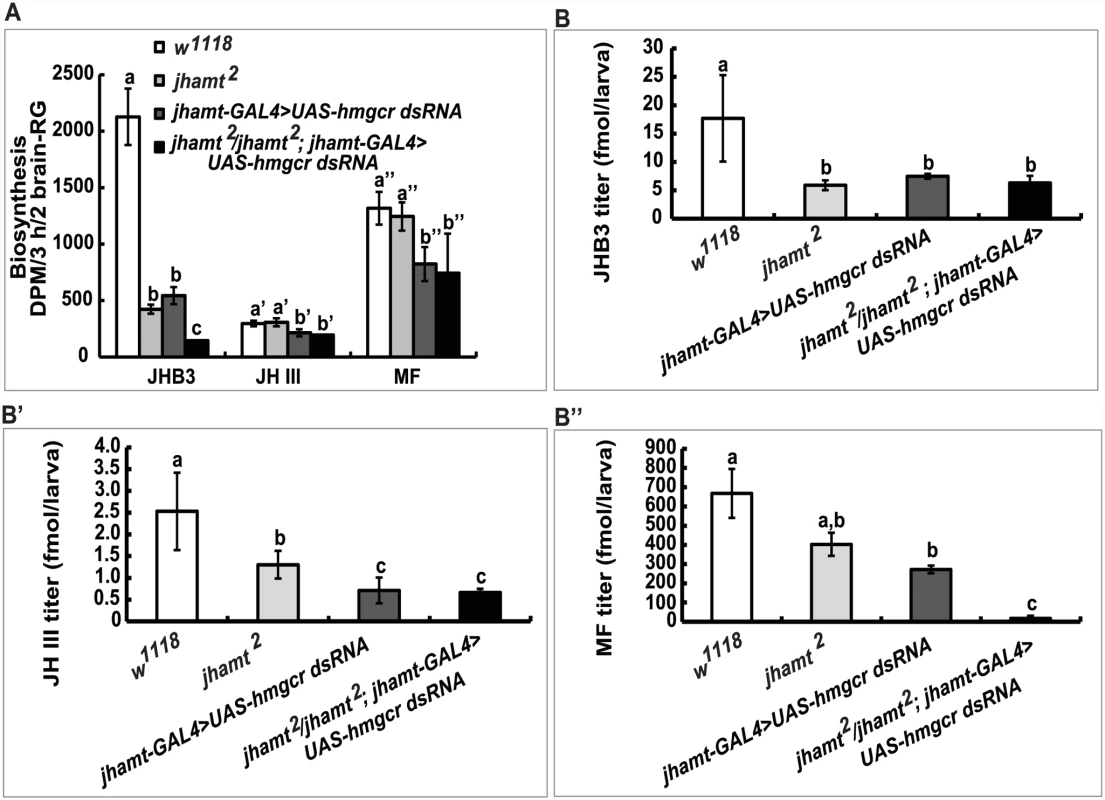
Measurements of biosynthesis of JHB3, JH III, and MF in the brain-RG complexes (A) and whole body titers of JHB3 (B), JH III (B’), and MF (B”) titer in w1118, jhamt2, jhamt-GAL4>UAS-hmgcr dsRNA, and jhamt2/jhamt2; jhamt-GAL4>UAS-hmgcr dsRNA at 3h AIW. We also measured titers of the three sesquiterpenoids in the whole larval bodies of the four above mentioned genotypes at 3h AIW. In comparison with the w1118 larvae, JHB3 titer decreased by 60–70% in jhamt2, hmgcrRNAi, and jhamt2 hmgcrRNAi larvae (Fig. 4B). JH III titer decreased by 50% in jhamt2 larvae, whereas it decreased by 70–75% in hmgcrRNAi and jhamt2 hmgcrRNAi larvae (Fig. 4B’). MF titer decreased by 30% (not statistically significant difference) in jhamt2 larvae, whereas the decrease was approximately 40% in hmgcrRNAi larvae (Fig. 4B”). Interestingly, MF titer decreased by 98% in jhamt2 hmgcrRNAi larvae (Fig. 4B”), implying that most of MF is converted to JHs in jhamt2 hmgcrRNAi larvae. Overall, these experiments suggest that the three sesquiterpenoids synthesized and released by the larval CA are required for Drosophila to survive to adulthood; in particular, that the very abundant MF plays essential anti-metamorphic roles during Drosophila development (Table 1).
Tab. 1. Comparisons of JH biosynthesis, JH titer, and lethality among three genotypes. 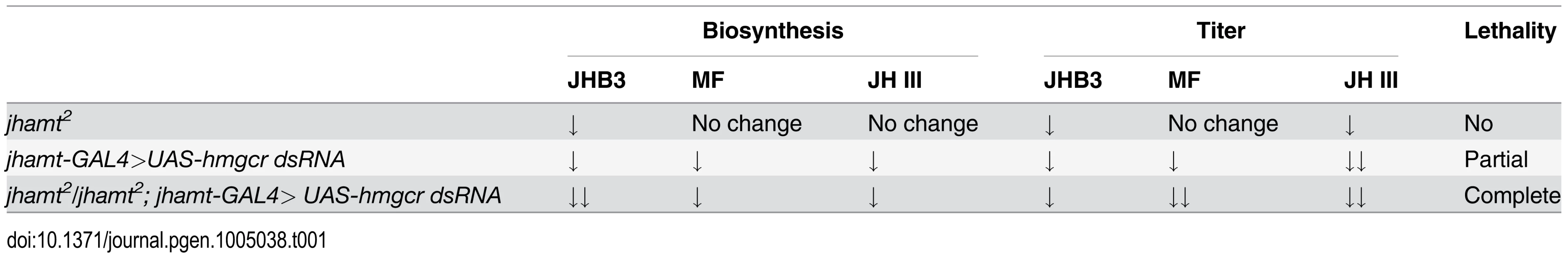
MF acts through Met/Gce to induce Kr-h1 expression and prevents lethality of JH-deficient flies but not Met gce double mutant
To further understand the anti-metamorphic roles of each of the three sesquiterpenoids synthesized by the larval CA, we performed a series of experiments by treating JH-deficient animals with methoprene or sesquiterpenoids to evaluate their ability to prevent lethality, as well as their efficiency in inducing expression of the JH-responsive gene Kr-h1. Topical application of high doses of methoprene, JHB3, JH III, and MF (0.5×10-2 μmol per larva) to third instar larvae when JH titers are low (at 96h AEL: 96 hours after egg laying) [10] was able to decrease mortality significantly (40–75%) in the two JH-deficient animals (Aug21>Grim and jhamt2 hmgcrRNAi). By contrast, neither methoprene nor sesquiterpenoids (0.5×10-2 μmol per larva) prevented the lethality of Met27 gce2.5k (Fig. 5A). Additional experiments were performed on jhamt2 hmgcrRNAi to evaluate the dose-responses for methoprene and the three sesquiterpenoids in preventing lethality. These compounds showed significant effects at 0.5×10-4 μmol/larva, with MF being the most effective, followed by JH III, methoprene, and JHB3. At higher doses (0.5×10-3 and 0.5×10-2 μmol/larva), only the effects of JHB3 and JH III continued to increase (Fig. 5B).
Fig. 5. MF plays a dual role: as a JHB3 precursor and as a hormone. 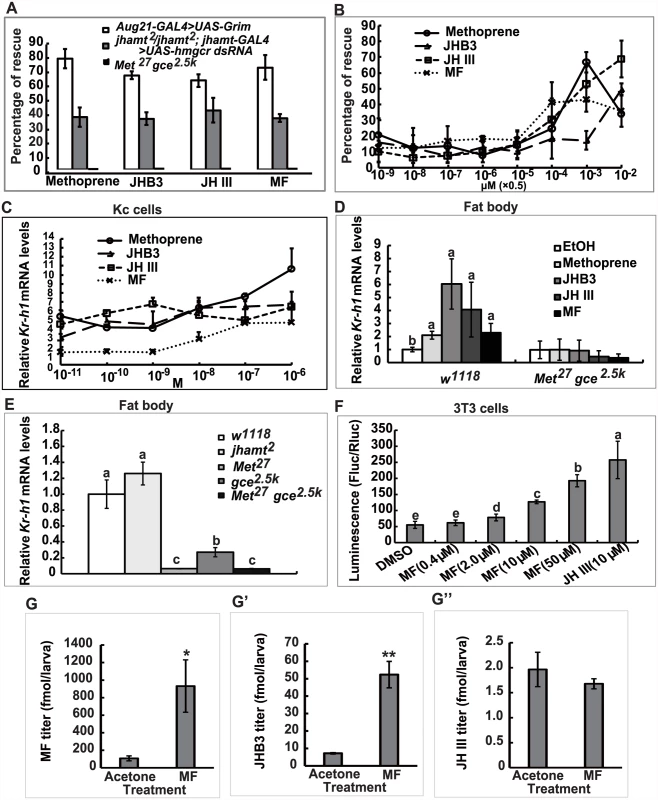
(A) Percentage of rescuing Aug21-GAL4>UAS-Grim, jhamt2/jhamt2; jhamt-GAL4>UAS-hmgcr dsRNA, and Met27 gce2.5k to adults by topical application of methoprene, JHB3, JH III and MF (0.5×10-2 μmol per larva) at 96h AEL. (B) Percentage of rescuing jhamt2/jhamt2; jhamt-GAL4>UAS-hmgcr dsRNA to adults by topical application of a dose gradient of methoprene, JHB3, JH III, and MF (0.5×10-9~-2 μmol per larva) at 96h AEL. (C) qPCR measurements of fold-changes of relative Kr-h1 mRNA levels in Kc cells treated with methoprene, JHB3, JH III, and MF (1×10-10~-6 M) for 30 min. (D) qPCR measurements of relative Kr-h1 mRNA levels in fat body tissues isolated from w1118 and Met27 gce2.5k at 96h AEL after treatments with methoprene, JHB3, JH III, and MF (1×10-6 M) for 30 min. (E) qPCR measurements of the relative Kr-h1 mRNA levels in the fat body tissues isolated from w1118, jhamt2, Met27, gce2.5k, and Met27 gce2.5k at 3h AIW. (F) MF promotes interaction of Met and SRC in mouse embryonic fibroblast 3T3 cells. 3T3 cells were transiently transfected with GAL4:TcMet and TcSRC. And the transfected cells were cultured in the medium containing different concentrations of MF and JH III (DMSO as control). After 24 hours exposure to the ligands, cells were assayed for luciferase reporter activity. The luciferase activity was normalized based on the total protein concentration determined for cells in each well. (G-G”) Measurements whole body titers of JHB3 (G), JH III (G’), and MF (G”) in jhamt2/jhamt2; jhamt-GAL4>UAS-hmgcr dsRNA at 3hAIW after topical application of MF (0.5×10-2 μmol per larva; dissolved in acetone) at 96h AEL (about 24 hours after treatments). qPCR was utilized to examine whether MF acts through Met/Gce to induce Kr-h1 expression [20, 30, 31]. Methoprene and the three sesquiterpenoids induced Kr-h1 expression in both Drosophila Kc cells (1×10-10~-6 M) (Fig. 5C) and cultured fat body tissues isolated from w1118 larvae at 96h AEL (1×10-7 M) (Fig. 5D, left panel); although induction with MF was weaker than JHB3 and JH III. We also determined Kr-h1 mRNA levels in jhamt2 larvae, wherein JHB3 biosynthesis but not MF biosynthesis is reduced (Fig. 2A). Kr-h1 expression was normal in 3h AIW jhamt2 larvae, indicating that the other two sesquiterpenoids (in particular the very abundant MF) were sufficient to induce Kr-h1 expression to control levels. In contrast, as previously reported [21], in Met27 gce2.5k larvae, Kr-h1 mRNA levels were reduced by about 95% when compared to its levels in w1118 larvae (Fig. 5E). As expected, methoprene and the three sesquiterpenoids failed to induce Kr-h1 expression in cultured fat body tissues isolated from Met27 gce2.5k larvae at 96h AEL (Fig. 5D, right panel). These data from in vitro and in vivo experiments revealed that, in addition to JHB3 and JH III, MF also has an anti-metamorphic or “JH-like” role in Drosophila larvae, acting through Met/Gce to induce Kr-h1 expression.
We then extended our study to Tribolium, in which JH III directly induces heterodimerization of the JH receptor (TcMet) and its partner (TcSRC) in mouse embryonic fibroblast 3T3 cells [37]. Here we found that MF also induced heterodimerization of TctMet and TcSRC in 3T3 cells in a dose-dependent manner, although its induction ability was weaker than JH III (Fig. 5F). This experiment provides strong evidence that MF acts as a hormone itself through a direct interaction with the JH receptor Met in Tribolium, supporting the above findings in Drosophila.
MF plays a dual role: As a JHB3 precursor and as a hormone
Finally, we examined whether once released by the CA, MF could be converted to JHB3 or JH III in the fly hemolymph or peripheral tissues. The jhamt2 hmgcrRNAi larvae were topically treated with acetone or MF (0.5×10-2 μmol per larva) at 108h AEL, and the three sesquiterpenoid titers were measured at 3h AIW (about 24 hours after treatment). While JH III titer did not change, MF and JHB3 titers in the MF-treated animals increased approximately 9 - and 7-fold respectively when compared to control animals treated with acetone (Fig. 5G–5G”). The topical application experiments showed that a portion of the exogenous MF was converted to JHB3 in the hemolymph or peripheral tissues, consistent with the results obtained from jhamt2 (Fig. 2C–2C”) and jhamt2 hmgcrRNAi larvae (Fig. 4B–4B”). We conclude that MF is required for completion of Drosophila metamorphosis, playing a dual role: as a JHB3 precursor and as a hormone (Fig. 6).
Fig. 6. A possible model showing the last two steps of biosynthesis and the molecular actions of the three sesquiterpenoids in Drosophila. 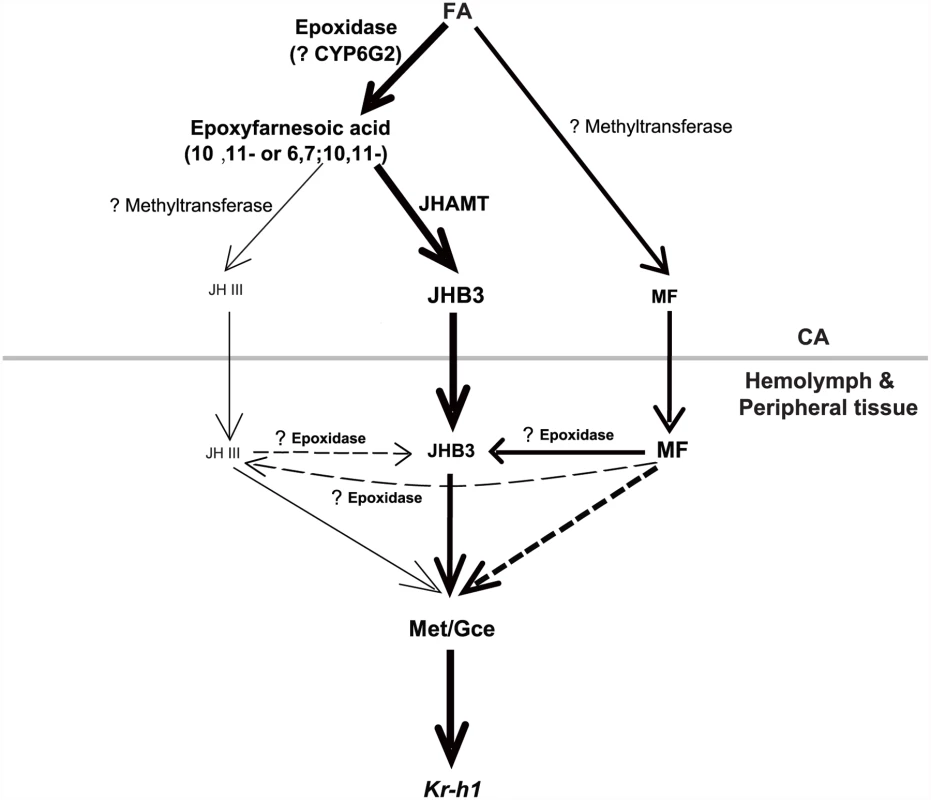
In the CA, FA is the common precursors for JHB3, JH III, and MF biosynthesis. In the hemolymph and peripheral tissues, MF either directly acts through Met/Gce or is converted to JHB3. JHAMT only accounts for JHB3 biosynthesis; and other methyltransferases and P450 epoxidase with question marks have not been identified. Please see Discussion for details on the model. Text and arrow sizes convey magnitude of treatment and response. The gray line separates CA from hemolymph and peripheral tissues. Discussion
Requirement of the three sesquiterpenoids for completion of Drosophila metamorphosis
This study (Table 1; Figs 1–4) confirmed and expanded previous studies, showing that genetic ablation of the CA caused JH deficiency and pupal lethality in Drosophila [20, 22]. Knockdown and/or knockout of enzymes in the early and late steps of the JH biosynthetic pathway generated different phenotypes depending on the background of the animals: 1) null mutation of jhamt resulted in significant decrease in JHB3 biosynthesis, as well as JHB3 and JH III titers, without compromising development and survival, 2) RNAi-mediated reduction of hmgcr expression in the CA decreased biosynthesis and titers of the three sesquiterpenoids produced by the larval CA, resulting in partial lethality, and 3) RNAi-mediated reduction of hmgcr expression in the CA of the jhamt mutant further decreased JHB3 biosynthesis and MF titer, leading to complete lethality. These results lead us to conclude that only dramatic decreases in biosynthesis of the three sesquiterpenoids resulted in very low circulating titers and caused complete lethality in the two JH-deficient animals (Aug21>grim and jhamt2 hmgcrRNAi). Moreover, the requirement of the three sesquiterpenoids for Drosophila metamorphosis was further strengthened by the rescue experiments in the two JH-deficient animals (Fig. 5A and 5B), showing that JHB3, JH III, and MF are able to functionally replace one another.
MF plays a dual role: As a JHB3 precursor and as a hormone during Drosophila metamorphosis
Although accepted as the anti-metamorphic hormone in Crustacea, the potential role of MF as a true JH in Insecta has been an issue of a long-standing debate [1, 4, 24, 37]. Our experiments provide additional evidence that supports the anti-metamorphic or “JH-like” role of MF in Drosophila, including: 1) the fact that MF is released by the CA and is the most abundant sesquiterpenoid present in extracts of larval body, 2) the ability to phenocopy anti-metamorphic roles following topical application to JH-deficient animals (“rescue” experiments), 3) the capability to act through the JH receptors (Met and Gce) and induce a dose-dependent expression of Kr-h1, a JH primary-response anti-metamorphic gene, and 4) the conversion to JHB3 in the hemolymph or peripheral tissues.
The presence of high circulating MF levels has been previously described in Drosophila larvae [9, 10], as well as the production of MF by the larval brain-RG complexes [3]. MF might also play an anti-metamorphic role during early larval development in Bombyx; high levels of MF might exist in Bombyx dimolting, a P450 epoxidase mutant, that contains no detectable JH I, JH II, and JH III in the hemolymph [16].
The ability of MF to phenocopy anti-metamorphic roles has been previously established in the white puparia JH bioassay [6, 7]. The importance of MF during Drosophila metamorphosis was validated by the RNAi-mediated reduction of hmgcr expression in the CA of the jhamt mutant, in which only MF was further decreased leading to complete lethality (Table 1); as well as by the observation that JHB3, JH III and MF efficiently precluded lethality in two JH-deficient lines.
It has been suggested that MF could play anti-metamorphic roles acting through ultraspiracle (USP, an ortholog of the retinoid X receptor and a molecular partner of the 20E receptor, EcR) [9]. On the other hand, MF efficiently competes with JH III for binding to Met and Gce in Drosophila [28], MF directly induces heterodimerization of Met and SRC of Crustacea in mammalian cells [38], and MF induces Kr-h1 promoter activity in mammalian cells in the presence of Bombyx Met and SRC [39]. We validated and expanded those results, showing that MF induces a dose-dependent Kr-h1 expression in Drosophila cell lines and fat body tissues isolated from JH-deficient animals (Fig. 5C–5E). Moreover, MF induces heterodimerization of Met and SRC of Tribolium in mammalian 3T3 cells in a dose-dependent manner (Fig. 5F). Data included in this paper show that MF acts through Met/Gce (Fig. 5C–5F), but not USP (S6 Fig), at least in the induction of Kr-h1 expression and Met-SRC heterodimerization.
Finally we showed that MF can be converted in the hemolymph or peripheral tissues to other active JHs in Drosophila. In jhamt2 larvae, JHB3 biosynthesis is dramatically reduced and MF and JH III biosynthesis are unaffected (Fig. 2B), whereas whole body titers of JHB3, JH III, and MF decreased by approximately 70%, 50%, and 30% (no statistical difference) relative to their respective control levels (Fig. 2C–2C”). The decrease in whole body levels of MF could be the consequence of a portion of the MF pool undergoing conversion to JHB3 in jhamt2 larvae. In comparison with hmgcrRNAi larvae, JHB3 biosynthesis is further reduced in jhamt2 hmgcrRNAi larvae, whereas the biosynthesis of MF and JH III is unaffected (Fig. 4A). Similarly, although MF titer decreased to almost zero in jhamt2 hmgcrRNAi larvae, JHB3 and JH III titers remained at the same levels (Fig. 4B–4B”), suggesting again that most of MF is converted to JHB3 in jhamt2 hmgcrRNAi larvae. The possibility that MF can be converted to other JHs was further confirmed by topical application of MF to jhamt2 hmgcrRNAi larvae (Fig. 5G–5G”).
We conclude that MF plays a dual role in regulating Drosophila metamorphosis: through its conversion to JHB3, as well as through its role as a bona fide juvenoid (Fig. 6).
Was MF the ancestral ‘JH’ of Arthropods? Ongoing studies of the metabolic pathways for JH biosynthesis and degradation in other Arthropods, including Myriapods and Chelicerates, indicate that these groups all possess the requisite enzymes to produce at least MF. In particular, these groups all appear to possess a JHAMT ortholog, indicating that MF may have been synthesized and functional in these groups. These groups also possess enzymes known to be involved in the degradation of the sesquiterpenoids, as well as binding proteins [40, 41]. At present, it is unknown if these groups possess a functional member of the CYP family of cytochrome P450 enzymes that would be responsible for the epoxidation of MF. The apparent absence of this enzyme in crustaceans and possibly in Drosophila argues for the importance of MF in the regulation of metamorphosis. These studies suggest that the ‘JH’ signaling pathway has deep evolutionary roots [40, 41] and our present results on Drosophila support such a view. These authors also suggest that the pathway “might have evolved together with the emergence of the exoskeleton”. This suggestion highlights the importance of MF, particularly in metamorphosis. During evolution in arthropods, MF maintains its anti-metamorphic role from crustaceans to insects and probably across the phylum. Subsequently, different JHs emerged in different orders of insects. Diversification of the JH(s) might contribute to variation and novelty during arthropod evolution. The co-existence of three JHs and two JH receptors in a single organism makes Drosophila a complicated but fascinating system for studying the JH signal transduction pathway, from both molecular and evolutionary perspectives.
The last two steps of JH biosynthesis in Drosophila
Compared with other insects producing only JH III, the last two steps of the JH biosynthetic pathway in Drosophila are much more ambiguous. We propose a JH biosynthetic pathway in which FA is the common precursor for JHB3, JH III, and MF in Drosophila (Fig. 6). Our previous studies [19] and the data included in this paper (Fig. 2) show that overexpression and mutation of jhamt increased and decreased JHB3 biosynthesis, respectively, but did not affect the production of JH III and MF, suggesting that JHAMT is responsible only for JHB3 biosynthesis in the CA. Moreover, mutation of jhamt significantly decreased the activity of methyltransferase using FA but not JHA as substrate, implying the existence of one or more additional methyltransferases converting FA into MF and JHA into JH III in the CA of Drosophila larvae.
It has been suggested that the lack of a clear ortholog of a P450 epoxidase in Drosophila might be explained on the basis of the different chemistry of the fly JHs [15]. The CYP15 of higher flies could have evolved to allow the epoxidation at both the 6, 7 and 10, 11 double bonds, and this evolution resulted in such significant changes so that the sequence is no longer recognizable as a CYP15. A global analysis of CYP enzymes in Drosophila revealed specific expression of CYP6G2 in the CA [42], but whether it functions as a P450 epoxidase is currently unknown. One possibility is that CYP6G2 preferably epoxidizes FA to 6, 7; 10, 11-epoxyfarnesoic acid (JHB3 acid) rather than 6, 7-epoxyfarnesoic acid (JHA), resulting in a much higher JHB3 biosynthesis ratio compared to the JH III biosynthesis ratio. Moreover, we found that a portion of MF was converted to JHB3 in the hemolymph or peripheral tissues (Fig. 2, 4, 6), presumably by an uncharacterized P450 epoxidase. The identification of the methyltransferases and P450 epoxidases that are involved in the last two steps of JH biosynthesis in Drosophila remains as a future challenge.
Materials and Methods
Flies and genetics
To generate the jhamt mutant, we used the homologous recombination—mediated ends-out gene targeting technique [32]. Two genomic DNA fragments flanking the jhamt (CG17330) coding region were amplified by PCR. The upstream flanking region (4245-bp length: -4212 bp to +33 bp from the translational start site of jhamt) was cloned into the pw25 plasmid using the Not I (jhamt-5’end-Not I) and Acc65 I (jhamt-5’end-Acc65 I) restriction sites introduced by PCR primers. Subsequently, the downstream flanking region (3977-bp length: +1050 bp to +5027 bp from the start site of the jhamt gene) was cloned into the above generated vector using the Asc I (jhamt-3’end-Asc I) and BsiW I (jhamt-3’end-BsiW I) restriction sites. The resulting construct of pw25-jhamt (Fig. 1A) was used to generate transgenic flies using P-element-mediated germline transformation. Then, the pw25-jhamt transgenic flies were crossed with yw; p{70FLP}23 p{70I-SceI}4A/TM6 to generate the jhamt knock-out strains (jhamt1, jhamt2, and jhamt3) (Fig. 1B and 1C). Primers used here and elsewhere are listed in S1 Table.
The putative promoter sequence (2540-bp length: -2544 bp to-4 bp, from the translational start site of jhamt) of jhamt was amplified as a Sac II-BamH I fragment, and cloned into the pChsGAL4 plasmid to generate the jhamt-GAL4 construct. The jhamt-GAL4 transgenic flies were then produced.
w1118, Aug21-GAL4, Act-GAL4, UAS-GFP, UAS-grim, CG10527187, Met27, gce2.5k, and Met27 gce2.5k were reported previously [14, 20, 21, 31, 33]. Multiple UAS-hmgcr dsRNA lines (stock number 11635 is reported) were obtained from the Vienna Drosophila RNAi Center. RNAi lines were also obtained from the Bloomington Drosophila Stock Center, and similar results were obtained. Other flies used in this paper were generated by recombination. All fly strains in this paper were grown at 25°C on standard cornmeal/molasses/agar medium.
PCR and western blot analysis
For genomic DNA PCR, genomic DNA was extracted from flies using phenol-chloroform-isoamyl alcohol. To confirm the jhamt mutants and the jhamt2 CG10527187 double mutants, genomic DNA PCR was performed with 4 primer pairs, including jhamt-1 and jhamt-2 (689-bp length), jhamt-3 and jhamt-4 (812-bp length), jhamt-1 and jhamt-5 (671-bp length), and jhamt-6 and jhamt-4 (1259-bp length) (Fig. 1A and 1B). To identify and confirm the CG10527187 mutation in the jhamt2 CG10527187 double mutant, genomic DNA PCR were performed with primer pairs CG10527-F and CG10527-R (1968-bp for wild type and ~600-bp for the CG10527187 mutant) (S3 Fig). For reverse transcription PCR, a primer pair jhamt-7 and jhamt-8 (405-bp) were used to detect jhamt mRNA expression from the brain-RG complexes isolated from larvae at 3hAIW (Fig. 1C). qPCR was performed as previously described [14, 20, 21, 31, 33].
DmCG10527 rat polyclonal antibody [33] was used to conduct the Western blot analysis of the brain-RG complexes isolated from larvae at 3hAIW. The tubulin mouse monoclonal antibody (#AT819, Beyotime, China) was used as an internal control.
Immunohistochemistry
For detecting JHAMT in the CA by immunohistochemistry, the brain-RG complexes were dissected from larvae at the EW stage. The Drosophila JHAMT rabbit polyclonal antibody (1 : 100) [13] and the FITC-conjugated Affinipure Goat Anti-Rabbit IgG secondary antibody (Jackson ImmunoResearch Inc.) were used, and the fluorescence signals were captured with an Olympus IX71 invert fluorescence microscope (Japan) [14, 20, 31].
JH treatments and cell culture
Methoprene (Service Chemical Inc., Germany), JH III (Sigma-Aldrich), and MF (Echelon) were purchased. JHB3 was synthesized from MF using m-chloroperbenzoic acid in dichloromethane (Sigma-Aldrich) [19]. For rescue of fertility of jhamt2, newly eclosed females were placed in vials with standard medium; after 24 hours, virgin females were topically treated with acetone-dissolved methoprene (0.5 μl × 10-3 M per female) [21, 23]. For rescue of pupal lethality of Aug21>grim and jhamt2 hmgcrRNAi, methoprene, JHB3, JH III, and MF (0.5 μl × 10-9~-2 M per larva) were dissolved in acetone and topically applied to the larvae at 96h AIW [14, 20, 21, 31, 33]. For inducing Kr-h1 expression in w1118 and Met27 gce2.5k, fat body tissues were isolated at 96h AIW and treated with methoprene, JHB3, JH III, and MF (1×10-6 M; DMSO as a control) for 30 min. For testing the conversion of MF to other JHs, the jhamt2 hmgcrRNAi larvae were topically treated with acetone or MF (0.5×10-2 μmol per larva) at 108h AEL, and the three sesquiterpenoids titers were measured at 3hAIW (about 24 hours after treatment).
For inducing Kr-h1 expression in Drosophila Kc cells cultured in Schneider’s medium, the cells were treated with methoprene, JHB3, JH III, and MF (1×10-11~-6 M; DMSO as a control) for 30 min [31]. Using the T7 RiboMAX Express RNAi System (Promega), dsRNAs of USP and EGFP (as a control) were synthesized. Reduction of gene expression by RNAi in Kc cells was performed by transfecting dsRNAs using Effectene at a final concentration of 1 μg/ml dsRNA. The transfected cells were cultured for 48 h and treated with MF (1×10-6 M; DMSO as a control) for 30 min [31].
Luciferase assay in 3T3 cells
3T3 cells were grown at 37°C with 5% CO2 in a DMEM (life technology) containing 10% fetal bovine serum. For transfection experiments, 50,000 cells/well were seeded in a 48-well plate. On the following day, the cells were transiently transfected with 67 ng each of receptor/partner and 200 ng each of pFRLUC reporter construct, using a “Polyfect” transfection reagent (Qiagen). After 4 hours, different final concentration of MF (0.4, 2, 10 and 50 μM) were added to the wells along with DMEM medium with 20% FBS as well. DMSO and 10 μM JH III were used as a negative and positive control, respectively. After 24 hours exposure to the ligands, cells were washed with PBS, 60 μl of reporter lysis buffer was added to each well and luciferase reporter activity was measured using the luciferase reporter assay system from Promega (Madison, WI). To standardize the luciferase activity, protein concentration in cells from well was determined using the Bradford reagent. Details on the constructs GAL4:TcMet in the pBIND vector and TcSRC in the pACT vector, as well as JH III treatment experiments were published previously [37].
Measurements of methyltransferase activity, JH biosynthesis, and JH titer
S-Adenosyl-L-methionine (SAM) was purchased from Sigma-Aldrich and S-Adenosyl-L-[methyl-3H] methionine (370GBq mmol, 10 Ci/mmol) from Perkin-Elmer Life Sciences (Waltham). Methyltransferase activity in the brain-RG complexes isolated from larvae at 3hAIW was measured with JHA and FA as substrates, as described previously [14, 20, 23, 33]. L-[Metyl-3H] methionine (2.92–3.70 TBq/mmol) was purchased from Perkin-Elmer Life Sciences and TLC plates (20×20 cm2 plastic plate coated with silica gel F254) from Merck KgaA (Germany). JH biosynthesis in the brain-RG complexes was detected using the radiochemical assay followed by thin layer chromatography analysis as reported previously [18, 19, 37]. JH titers from the whole bodies of each genotype were determined using the recently developed HPLC-FD protocol [34].
Statistics
Experimental data were analyzed with the Student’s t-test and ANOVA. t-test: *, p<0.05; **, p<0.01. ANOVA: the bars labeled with different lowercase letters are significantly different (p<0.05). Throughout the paper, values are represented as the mean ± standard deviation of at least five independent experiments.
Supporting Information
Zdroje
1. Goodman WG, Cusson M (2012) The juvenile hormones. In: Gilbert LI, editor. Insect endocrinology. Amsterdam: Elsevier; 2012 pp. 310–365.
2. Richard DS, Applebaum SW, Sliter TJ, Baker FC, Schooley DA, et al. (1989) Juvenile hormone bisepoxide biosynthesis in vitro by the ring gland of Drosophila melanogaster: a putative juvenile hormone in the higher Diptera. Proc Natl Acad Sci USA 86 : 1421–1425. 2493154
3. Kotaki T, Shinada T, Kaihara K, Ohfune Y, Numata H (2009) Structure determination of a new juvenile hormone from a heteropteran insect. Org Lett. 11 : 5234–5237. doi: 10.1021/ol902161x 19863071
4. Nagaraju GPC (2011) Reproductive regulators in decapod crustaceans: an overview. J Exp Biol 214 : 3–16. doi: 10.1242/jeb.047183 21147963
5. Teal PE, Jones D, Jones G, Torto B, Nyasembe V, et al. (2014) Identification of methyl farnesoate from the hemolymph of insects. J Nat Prod 77 : 402–405. doi: 10.1021/np400807v 24467367
6. Harshman LG, Song KD, Casas J, Schuurmans A, Kuwano E, et al. (2010) Bioassays of compounds with potential juvenoid activity on Drosophila melanogaster: Juvenile hormone III, bisepoxide juvenile hormone III and methyl farnesoates. J Insect Physiol 56 : 1465–1470. doi: 10.1016/j.jinsphys.2010.06.003 20599543
7. Jones G, Jones D, Li X, Tang L, Ye L, et al. (2010) Activities of natural methyl farnesoids on pupariation and metamorphosis of Drosophila melanogaster. J Insect Physiol 56 : 1456–1464. doi: 10.1016/j.jinsphys.2010.06.001 20541556
8. Jones D, Jones G, Teal P, Hammac C, Messmer L, et al. (2010) Suppressed production of methyl farnesoid hormones yields developmental defects and lethality in Drosophila larvae. Gen. Comp. Endocrinol. 165 : 244–254. doi: 10.1016/j.ygcen.2009.07.006 19595690
9. Jones G., Teal P., Henrich V., Krzywonos A., Sapa A., et al. (2013a) Ligand binding pocket function of Drosophila USP is necessary for metamorphosis. Gen Comp Endocrinol 182 : 73–82. doi: 10.1016/j.ygcen.2012.11.009 23211750
10. Jones D, Jones G, Teal P (2013b) Sesquiterpene action, and morphogenetic signaling through the ortholog of retinoid X receptor, in higher Diptera. Gen Comp Endocrinol 194 : 326–335. doi: 10.1016/j.ygcen.2013.09.021 24120505
11. Belles X, Martin D, Piulachs MD (2005) The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu Rev Entomol 50 : 181–199. 15355237
12. Shinoda T, Itoyama K (2003) Juvenile hormone acid methyltransferase: a key regulatory enzyme for insect metamorphosis. Proc Natl Acad Sci USA 100 : 11986–11991. 14530389
13. Niwa R., Niimi T., Honda N., Yoshiyama M., Itoyama K., et al. (2008) Juvenile hormone acid O-methyltransferase in Drosophila melanogaster. Insect Biochem Mol Biol 38 : 714–720. doi: 10.1016/j.ibmb.2008.04.003 18549957
14. Huang J., Tian L., Abdou M., Wen D., Wang Y., et al. (2011) DPP-mediated TGF-β signaling regulates juvenile hormone biosynthesis by upregulating expression of JH acid methyltransferase. Development 138 : 2283–2291. doi: 10.1242/dev.057687 21558376
15. Helvig C, Koener JF, Unnithan GC, Feyereisen R (2004) CYP15A1, the cytochrome P450 that catalyzes epoxidation of methyl farnesoate to juvenile hormone III in cockroach corpora allata. Proc Natl Acad Sci USA 101 : 4024–4029. 15024118
16. Daimon T, Kozaki T, Niwa R, Kobayashi I, Furuta K, et al. (2012) Precocious metamorphosis in the juvenile hormone-deficient mutant of the silkworm, Bombyx mori. PLoS Genet 8: e1002486. doi: 10.1371/journal.pgen.1002486 22412378
17. Defelipe LA, Dolghih E, Roitberg AE, Nouzova M, Mayoral JG, et al. (2011) Juvenile hormone synthesis: “esterify then epoxidize” or “epoxidize then esterify”? Insights from the structural characterization of juvenile hormone acid methyltransferase. Insect Biochem Mol Biol 41 : 228–235. doi: 10.1016/j.ibmb.2010.12.008 21195763
18. Richard DS, Applebaum SW, Gilbert LI (1989) Developmental regulation of juvenile hormone biosynthesis by the ring gland of Drosophila. J Comp Physiol [B] 159 : 383–387. 2509524
19. Bendena WG, Zhang JR, Burtenshaw SM, Tobe SS (2011) Evidence for differential biosynthesis of juvenile hormone (and related) sesquiterpenoids in Drosophila melanogaster. Gen Comp Endocrinol 172 : 56–61. doi: 10.1016/j.ygcen.2011.02.014 21354154
20. Liu Y, Sheng Z, Liu H, Wen D, He Q, et al. (2009) Juvenile hormone counteracts the bHLH-PAS transcriptional factor MET and GCE to prevent caspase-dependent programmed cell death in Drosophila. Development 136 : 2015–2025. doi: 10.1242/dev.033712 19465595
21. Abdou M, He Q, Wen D, Zyaan O, Wang J, et al. (2011) Drosophila Met and Gce are partially redundant in transducing juvenile hormone action. Insect Biochem Mol Biol 41 : 938–945. doi: 10.1016/j.ibmb.2011.09.003 21968404
22. Riddiford LM, Truman JW, Mirth CK, Shen YC (2010) A role for juvenile hormone in the prepupal development of Drosophila melanogaster. Development 137 : 1117–1126. doi: 10.1242/dev.037218 20181742
23. Gruntenko NE, Wen D, Karpova EK, Adonyeva NV, Liu Y, et al. (2010) Altered juvenile hormone metabolism, reproduction and stress resistance in Drosophila adults with genetic ablation of the corpus allatum cells. Insect Biochem Mol Biol 40 : 891–897. doi: 10.1016/j.ibmb.2010.09.001 20849954
24. Jindra M, Palli SR, Riddiford LM (2013) The juvenile hormone signaling pathway in insect development. Annu Rev Entomol 58 : 181–204. doi: 10.1146/annurev-ento-120811-153700 22994547
25. Ashok M, Turner C, Wilson TG (1988) Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc Natl Acad Sci USA 95 : 2761–2766.
26. Godlewski J, Wang SL, Wilson TG (2006) Interaction of bHLH-PAS proteins involved in juvenile hormone reception in Drosophila. Biochem Biophys Res Commun 342 : 1305–1311. 16516852
27. Miura K, Oda M, Makita S, Chinzei Y (2005) Characterization of the Drosophila Methoprene-tolerant gene product. Juvenile hormone binding and ligand-dependent gene regulation. FEBS J 272 : 1169–1178. 15720391
28. Charles J P, Iwema T, Epa V C, Takaki K, Rynes J et al. (2011) Ligand-binding properties of a juvenile hormone receptor, methoprene-tolerant. Proc Natl Acad Sci USA 108 : 21128–21133. doi: 10.1073/pnas.1116123109 22167806
29. Konopova B, Jindra M (2007) Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc Natl Acad Sci USA 104 : 10488–10493. 17537916
30. Minakuchi C, Zhou X, Riddiford LM (2008) Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech Dev 125 : 91–105. 18036785
31. He Q, Wen D, Jia Q, Cui C, Wang J, et al. (2014) Heat shock protein 83 (Hsp83) facilitates methoprene-tolerant (Met) nuclear import to modulate juvenile hormone signaling. J Bio Chem doi: 10.1074/jbc.M114.582825
32. Gong WJ, Golic KG (2003) Ends-out, or replacement, gene targeting in Drosophila. Proc Natl Acad Sci USA 100 : 2556–2561. 12589026
33. Zhang H, Tian L, Tobe S, Xiong Y, Wang S, et al. (2010) Drosophila CG10527 mutants are resistant to juvenile hormone and its analog methoprene. Biochem Bioph Res Co 401 : 182–187.
34. Rivera-Perez C, Nouzova M, Noriega FG (2012) A quantitative assay for the juvenile hormones and their precursors using fluorescent tags. PLoS One 7: e43784. doi: 10.1371/journal.pone.0043784 22928033
35. Gunawardene YI, Chow BK, He JG, Chan SM (2001) The shrimp FAMeT cDNA is encoded for a putative enzyme involved in the methylfarnesoate (MF) biosynthetic pathway and is temporally expressed in the eyestalk of different sexes. Insect Biochem Mol Biol 31 : 1115–1124. 11520690
36. Burtenshaw SM, Su PP, Zhang JR, Tobe SS, Dayton L, et al. (2008) A putative farnesoic acid O-methyltransferase (FAMeT) orthologue in Drosophila melanogaster (CG10527): Relationship to juvenile hormone biosynthesis? Peptides 29 : 242–251. doi: 10.1016/j.peptides.2007.10.030 18242777
37. Zhang Z, Xu J, Sheng Z, Sui Y, Palli SR (2011) Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, methoprene tolerant. J Biol Chem 286 : 8437–8447. doi: 10.1074/jbc.M110.191684 21190938
38. Miyakawa H, Toyota K, Hirakawa I, Ogino Y, Miyagawa S, et al. (2013) A mutation in the receptor Methoprene-tolerant alters juvenile hormone response in insects and crustaceans. Nat Comm 4 : 1856.
39. Kayukawa T, Minakuchi C, Namiki T, Togawa T, Yoshiyama M, et al. (2012) Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc Natl Acad Sci USA 109 : 11729–11734. doi: 10.1073/pnas.1204951109 22753472
40. Grbic M, Van Leeuwen T, Clark RM, Rombauts S, Rouze P, et al. (2011) The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479 : 487–492. doi: 10.1038/nature10640 22113690
41. Chipman AD, Ferrier DEK, Brena C, Qu J, Hughes DST, et al. (2014) The First Myriapod Genome Sequence Reveals Conservative Arthropod Gene Content and Genome Organisation in the Centipede Strigamia maritima. PLoS Biol 12:e1002005. doi: 10.1371/journal.pbio.1002005 25423365
42. Chung H, Sztal T, Pasricha S, Sridhar M, Batterham P, et al. (2009) Characterization of Drosophila melanogaster cytochrome P450 genes. Proc Natl Acad Sci USA 106 : 5731–5736. doi: 10.1073/pnas.0812141106 19289821
Štítky
Genetika Reprodukčná medicína
Článek NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY ModuleČlánek Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell LongevityČlánek HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and MiceČlánek LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male FertilityČlánek The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAsČlánek Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick VectorČlánek Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53Článek The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in NematodesČlánek The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse SpermatocytesČlánek The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 3- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
- Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
- A Genomic Duplication is Associated with Ectopic Eomesodermin Expression in the Embryonic Chicken Comb and Two Duplex-comb Phenotypes
- Genome-wide Association Study and Meta-Analysis Identify as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy
- Mutations of Human , Encoding the Mitochondrial Asparaginyl-tRNA Synthetase, Cause Nonsyndromic Deafness and Leigh Syndrome
- Exome Sequencing in an Admixed Isolated Population Indicates Variants Confer a Risk for Specific Language Impairment
- Genome-Wide Association Studies in Dogs and Humans Identify as a Risk Variant for Cleft Lip and Palate
- Rapid Evolution of Recombinant for Xylose Fermentation through Formation of Extra-chromosomal Circular DNA
- The Ribosome Biogenesis Factor Nol11 Is Required for Optimal rDNA Transcription and Craniofacial Development in
- Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
- Maternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity
- Clonality and Evolutionary History of Rhabdomyosarcoma
- HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and Mice
- Methylation-Sensitive Expression of a DNA Demethylase Gene Serves As an Epigenetic Rheostat
- BREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
- Seizures Are Regulated by Ubiquitin-specific Peptidase 9 X-linked (USP9X), a De-Ubiquitinase
- The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
- A Cascade of Iron-Containing Proteins Governs the Genetic Iron Starvation Response to Promote Iron Uptake and Inhibit Iron Storage in Fission Yeast
- Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
- LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male Fertility
- Cis-Regulatory Mechanisms for Robust Olfactory Sensory Neuron Class-restricted Odorant Receptor Gene Expression in
- Effects on Murine Behavior and Lifespan of Selectively Decreasing Expression of Mutant Huntingtin Allele by Supt4h Knockdown
- HDAC4-Myogenin Axis As an Important Marker of HD-Related Skeletal Muscle Atrophy
- A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex
- Selective and Genetic Constraints on Pneumococcal Serotype Switching
- Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
- The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAs
- Accumulation of Glucosylceramide in the Absence of the Beta-Glucosidase GBA2 Alters Cytoskeletal Dynamics
- Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities
- The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair
- Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick Vector
- Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
- The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors
- PHABULOSA Controls the Quiescent Center-Independent Root Meristem Activities in
- DNA Polymerase ζ-Dependent Lesion Bypass in Is Accompanied by Error-Prone Copying of Long Stretches of Adjacent DNA
- Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus
- Zinc Finger Independent Genome-Wide Binding of Sp2 Potentiates Recruitment of Histone-Fold Protein Nf-y Distinguishing It from Sp1 and Sp3
- GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters
- Neurospora Importin α Is Required for Normal Heterochromatic Formation and DNA Methylation
- Ccr4-Not Regulates RNA Polymerase I Transcription and Couples Nutrient Signaling to the Control of Ribosomal RNA Biogenesis
- Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53
- A Systems-Level Interrogation Identifies Regulators of Blood Cell Number and Survival
- Morphological Mutations: Lessons from the Cockscomb
- Genetic Interaction Mapping Reveals a Role for the SWI/SNF Nucleosome Remodeler in Spliceosome Activation in Fission Yeast
- The Role of China in the Global Spread of the Current Cholera Pandemic
- The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
- A Zinc Finger Motif-Containing Protein Is Essential for Chloroplast RNA Editing
- Resistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
- Small Regulatory RNA-Induced Growth Rate Heterogeneity of
- Mitochondrial Dysfunction Reveals the Role of mRNA Poly(A) Tail Regulation in Oculopharyngeal Muscular Dystrophy Pathogenesis
- Complex Genomic Rearrangements at the Locus Include Triplication and Quadruplication
- Male-Biased Aganglionic Megacolon in the TashT Mouse Line Due to Perturbation of Silencer Elements in a Large Gene Desert of Chromosome 10
- Sex Ratio Meiotic Drive as a Plausible Evolutionary Mechanism for Hybrid Male Sterility
- Tertiary siRNAs Mediate Paramutation in .
- RECG Maintains Plastid and Mitochondrial Genome Stability by Suppressing Extensive Recombination between Short Dispersed Repeats
- Escape from X Inactivation Varies in Mouse Tissues
- Opposite Phenotypes of Muscle Strength and Locomotor Function in Mouse Models of Partial Trisomy and Monosomy 21 for the Proximal Region
- Glycosyl Phosphatidylinositol Anchor Biosynthesis Is Essential for Maintaining Epithelial Integrity during Embryogenesis
- Hyperdiverse Gene Cluster in Snail Host Conveys Resistance to Human Schistosome Parasites
- The Class Homeodomain Factors and Cooperate in . Embryonic Progenitor Cells to Regulate Robust Development
- Recombination between Homologous Chromosomes Induced by Unrepaired UV-Generated DNA Damage Requires Mus81p and Is Suppressed by Mms2p
- Synergistic Interactions between Orthologues of Genes Spanned by Human CNVs Support Multiple-Hit Models of Autism
- Gene Networks Underlying Convergent and Pleiotropic Phenotypes in a Large and Systematically-Phenotyped Cohort with Heterogeneous Developmental Disorders
- The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
- Combinatorial Control of Light Induced Chromatin Remodeling and Gene Activation in
- Linking Aβ42-Induced Hyperexcitability to Neurodegeneration, Learning and Motor Deficits, and a Shorter Lifespan in an Alzheimer’s Model
- The Complex Contributions of Genetics and Nutrition to Immunity in
- NatB Domain-Containing CRA-1 Antagonizes Hydrolase ACER-1 Linking Acetyl-CoA Metabolism to the Initiation of Recombination during . Meiosis
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
- Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption
- A Genomic Portrait of Haplotype Diversity and Signatures of Selection in Indigenous Southern African Populations
- Sequence Features and Transcriptional Stalling within Centromere DNA Promote Establishment of CENP-A Chromatin
- Inhibits Neuromuscular Junction Growth by Downregulating the BMP Receptor Thickveins
- Replicative DNA Polymerase δ but Not ε Proofreads Errors in and in
- Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in
- The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
- A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension
- Pervasive Variation of Transcription Factor Orthologs Contributes to Regulatory Network Evolution
- Network Analyses Reveal Novel Aspects of ALS Pathogenesis
- A Role for the Budding Yeast Separase, Esp1, in Ty1 Element Retrotransposition
- Nab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
- A RecA Protein Surface Required for Activation of DNA Polymerase V
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Clonality and Evolutionary History of Rhabdomyosarcoma
- Morphological Mutations: Lessons from the Cockscomb
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



