-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
Major histocompatibility complex class I (MHCI) molecules are central to immunity and immunological disorders, and constitute a major obstacle in organ transplantation. It is therefore vital to gain insight into the regulation of their expression. NLRC5 was recently found to regulate MHCI gene transcription. However, we lack a thorough understanding of its target gene specificity and mechanism of action. Our work addresses these questions, delineating the unique consensus sequence required for NLRC5 recruitment and pinpointing conserved features conferring its specificity. Furthermore, through genome-wide analyses, we confirm that NLRC5 regulates classical MHCI genes and identify novel target genes, all encoding non-classical MHCI molecules exerting an array of functions in immunity and tolerance. We thereby demonstrate that NLRC5 exclusively transactivates genes of the MHCI pathway, rendering it an attractive target for future therapeutic intervention. The most striking feature of NLRC5 is its restricted and highly focused transcriptional activity, which has been described so far only for one related factor, CIITA. NLRC5 and CIITA therefore emerge as prototypes for a novel kind of extremely specific transcriptional regulator.
Published in the journal: NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module. PLoS Genet 11(3): e32767. doi:10.1371/journal.pgen.1005088
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005088Summary
Major histocompatibility complex class I (MHCI) molecules are central to immunity and immunological disorders, and constitute a major obstacle in organ transplantation. It is therefore vital to gain insight into the regulation of their expression. NLRC5 was recently found to regulate MHCI gene transcription. However, we lack a thorough understanding of its target gene specificity and mechanism of action. Our work addresses these questions, delineating the unique consensus sequence required for NLRC5 recruitment and pinpointing conserved features conferring its specificity. Furthermore, through genome-wide analyses, we confirm that NLRC5 regulates classical MHCI genes and identify novel target genes, all encoding non-classical MHCI molecules exerting an array of functions in immunity and tolerance. We thereby demonstrate that NLRC5 exclusively transactivates genes of the MHCI pathway, rendering it an attractive target for future therapeutic intervention. The most striking feature of NLRC5 is its restricted and highly focused transcriptional activity, which has been described so far only for one related factor, CIITA. NLRC5 and CIITA therefore emerge as prototypes for a novel kind of extremely specific transcriptional regulator.
Introduction
Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) constitute a family of innate immune receptors involved mainly in inflammatory responses and cell death. The NLR family member CIITA instead functions as the master transcriptional regulator of major histocompatibility complex (MHC) class II (MHCII) genes, and mutations in the CIITA gene lead to severe immunodeficiency [1]. Recently, NLR caspase recruitment domain containing protein 5 (NLRC5) was shown to regulate transcription of MHC class I (MHCI) genes, primarily in lymphocytes, where it is highly expressed [2,3,4,5,6]. Overexpression of NLRC5 was initially found to increase mRNA levels for genes encoding human MHCI molecules and proteins functioning in the MHCI-mediated antigen presentation pathway, including beta-2-microglobulin (B2M), transporter associated with antigen processing 1 (TAP1) and the proteasome subunit beta type-9 (PSMB9) [3]. Four independently generated Nlrc5-knockout mice subsequently established that NLRC5 regulates the expression of B2m, Tap1, Psmb9, classical MHCI genes (H2-K, H2-D), and the non-classical MHCI gene H2-M3 [2,4,5,7]. Finally, in vivo promoter occupancy by NLRC5 was demonstrated only for human HLA-A and HLA-B, and mouse H2-K, H2-D, and B2m [2,3].
CIITA-dependent transactivation of MHCII genes requires the SXY motif, a conserved enhancer found in all MHCII promoters. DNA-binding factors recognizing this element form an “enhanceosome” complex that serves as a platform for the recruitment of CIITA [1]. The X-binding regulatory factor X (RFX) complex is essential for enhanceosome assembly and CIITA recruitment. A similar SXY motif is found in MHCI gene promoters, together with more distal regulatory elements, and has been implicated in NLRC5-mediated transactivation [8,9]. Enforced expression of the RFX5, RFXAP, and RFXANK subunits of RFX potentiated NLRC5-driven MHCI transcription, and interaction between NLRC5 and overexpressed RFXANK was observed [8].
The shared use of enhanceosome factors by CIITA and NLRC5 suggests that these NLRs might fulfill partially redundant functions, a hypothesis that has not been tested in vivo. The relevance of endogenous enhanceosome factors for NLRC5-mediated MHCI-transactivation has also not been assessed. Furthermore, a comprehensive set of genes regulated directly by NLRC5 has not been defined. Finally, most NLRC5 target genes are encoded within the MHCI locus, raising the question of whether NLRC5 specifically regulates each one individually or if it instead establishes an open chromatin conformation at the entire locus. To address these questions we compared CIITA and NLRC5-regulated gene expression in various cell types from Rfx5−/−, Nlrc5−/−, CIIta−/− and CIIta−/−Nlrc5−/− mice, as well as in CIITA and RFX-deficient B cell lines, and screened for NLRC5 target genes by means of chromatin immunoprecipitation sequencing (ChIP-seq) experiments performed with T cells from control, Nlrc5−/−, and Rfx5−/− mice.
We found that NLRC5 is remarkably dedicated for a small set of related genes: it selectively occupies the promoters of genes coding for MHCI or related proteins, and identified the non-classical MHCI genes H2-Q4, H2-Q6/7, and H2-T10/22 as novel NLRC5-regulated genes. Analysis of NLRC5-binding in Rfx5-deficient cells demonstrated that Rfx5 is essential for promoter occupancy by NLRC5. Data generated in B cell lines carrying mutations in RFX5, RFXAP, and RFXANK also indicated a key requirement for the enhanceosome in MHCI transactivation. However, despite their recruitment by common factors, analysis of single (CIIta−/−, Nlrc5−/−) and double deficient (CIIta−/−Nlrc5−/−) mice revealed that CIITA and NLRC5 are highly specific for distinct sets of genes. Identification of the consensus sequence occupied in vivo by NLRC5 highlighted unique features that were shown to be responsible for NLRC5 specificity.
Results
Nlrc5−/− and Rfx5−/− mice exhibit similar defects in MHCI expression
Rfx5-deficient mice were exploited to assess the role of the enhanceosome factor Rfx5 in MHCI expression. Analysis of H2-K cell-surface expression by flow cytometry in various immune cell subsets derived from Rfx5+/- and Rfx5−/− littermates demonstrated that Rfx5-deficiency led to a strong decrease in MHCI expression on T cells, NK cells, and NKT cells, a marked reduction on B cells, and a more modest decrease on dendritic cells (DCs) (Fig. 1A). A similar trend, albeit less strong, was observed for H2-D (Fig. 1B). This phenotype was strikingly similar to that of Nlrc5-deficient cells (Fig. 1A and B). However, the defect in MHCI expression observed in the absence of Rfx5 was always slightly less profound as compared to that in Nlrc5-deficient cells, suggesting the existence of mechanisms capable of compensating partially for the deficiency in Rfx5.
Fig. 1. MHCI expression is strongly reduced in Nlrc5−/− and Rfx5−/− lymphocytes. 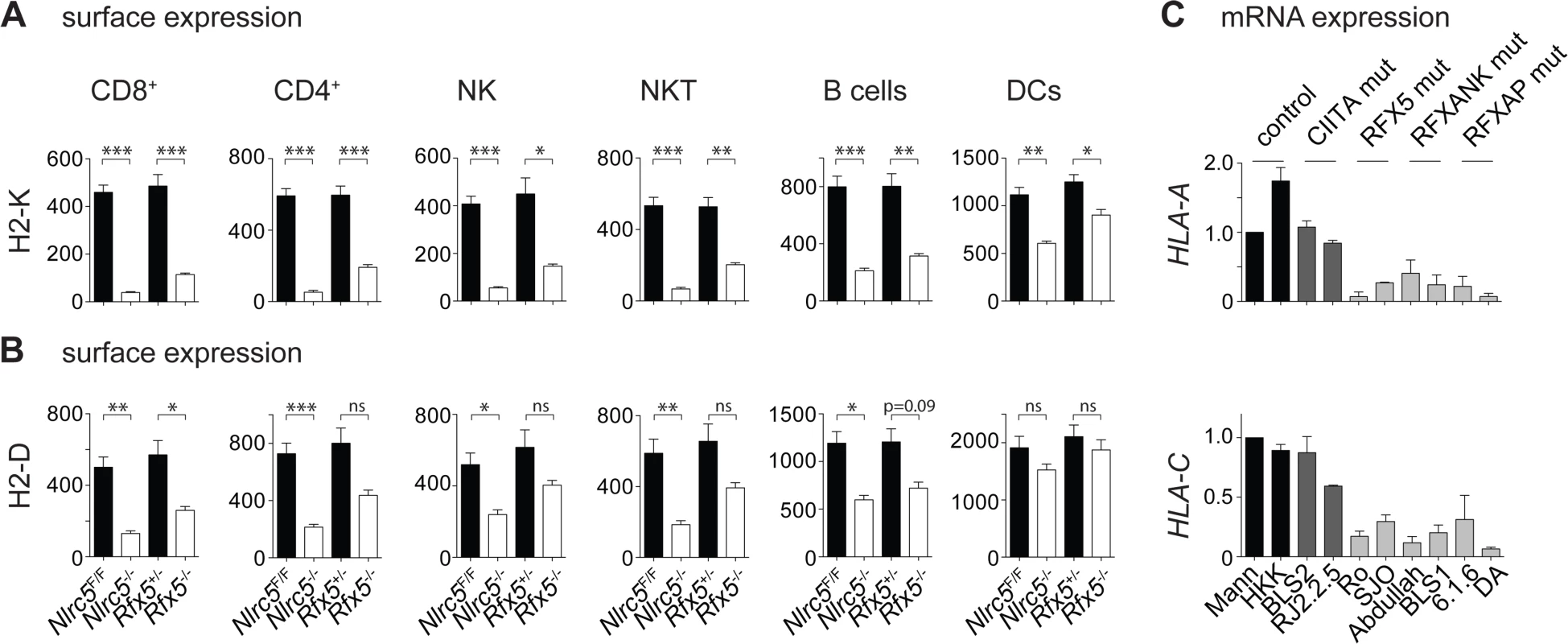
(A and B) MHCI expression on the indicated splenic cells was analyzed in Nlrc5F/F (n = 9), Nlrc5−/− (n = 9), Rfx5+/- (n = 10), and Rfx5−/− (n = 8) mice. Graphs illustrate geometric mean fluorescence intensities (MFIs) of H2-K (A) and H2-D (B) for CD8+ T cells (gated as CD3+CD8+), CD4+ T cells (gated as CD3+CD4+), NK cells (gated as NK1.1+CD3-), NKT cells (gated as NK1.1+CD3+), B cells (gated as CD19+), and DCs (gated as CD11chiCD11bint-hi). Results represent the mean ± SEM from two pooled experiments. Statistical significance was calculated using an unpaired Student’s t-test, two-tailed; adjustment was made using a Bonferroni correction over 12 samples (A and B). (C) HLA-A and HLA-C mRNAs were quantified by qRT-PCR in WT (Mann, HHK), CIITA-deficient (BLS2, RJ2.2.5), RFX5-deficient (Ro, SJO), RFXANK-deficient (BLS1, Abdulla), and RFXAP-deficient (6.1.6, DA) B cell lines. Results were normalized using ACTB, expressed relative to Mann and represent the mean ± SD derived from three independent experiments. We also measured MHCI mRNA expression by quantitative real-time RT-PCR (qRT-PCR) in in vitro-generated B cell mutants and B cell lines derived from bare lymphocyte syndrome (BLS) patients carrying inactivating mutations in CIITA, RFX5, RFXAP, and RFXANK. These experiments underlined the importance of RFX factors for MHCI expression (Fig. 1C) [10,11]. Collectively, these results support a role for the enhanceosome in the recruitment and transcriptional activity of NLRC5, in both human and mouse cells.
NLRC5 and CIITA have non-redundant functions
The fact that both NLRC5 and CIITA dock to similar SXY modules via shared enhanceosome factors raised the question of whether or not these two NLRs are overlapping in their transactivation role. That the two factors might exhibit partial redundancy in MHCI-transactivation was suggested by the findings that decreased MHCI expression caused by NLRC5-deficiency is more pronounced in T, NK, and NKT lymphocytes, which do not express CIITA, than in antigen-presenting cells (APCs) and thymic epithelial cells (TECs) [2], which express high levels of CIITA. Previous studies had also suggested that CIITA can stimulate MHCI transcription [10,11] and that MHCI promoters are occupied by CIITA in APCs [12,13]. We therefore generated double-deficient Nlrc5−/−CIIta−/− mice, and studied MHC expression in different immune cell subsets by flow cytometry. Concomitant ablation of Nlrc5 and CIIta did not substantially reduce H2-K and H2-D levels compared to single Nlrc5-deficiency, neither in any hematopoietic cell type analyzed nor in medullary TECs (Figs. 2A and B, S1A and S1B), although a minor but significant decrease was observed for H2-K in DCs. Accordingly, frequencies of peripheral CD8+ T cells, which require MHCI for their development and maintenance, were not decreased more strongly in Nlrc5−/−CIIta−/− mice than in Nlrc5−/− mice (S1C Fig.). MHCII expression was not reduced further in APCs from double-knockout animals, being already at negligible levels in single CIIta-deficient cells (Fig. 2B). These data indicate that NLRC5 and CIITA are highly specific for transactivating different sets of genes, even though they rely on common DNA binding factors for their recruitment.
Fig. 2. NLRC5 and CIITA exhibit non-redundant functions. 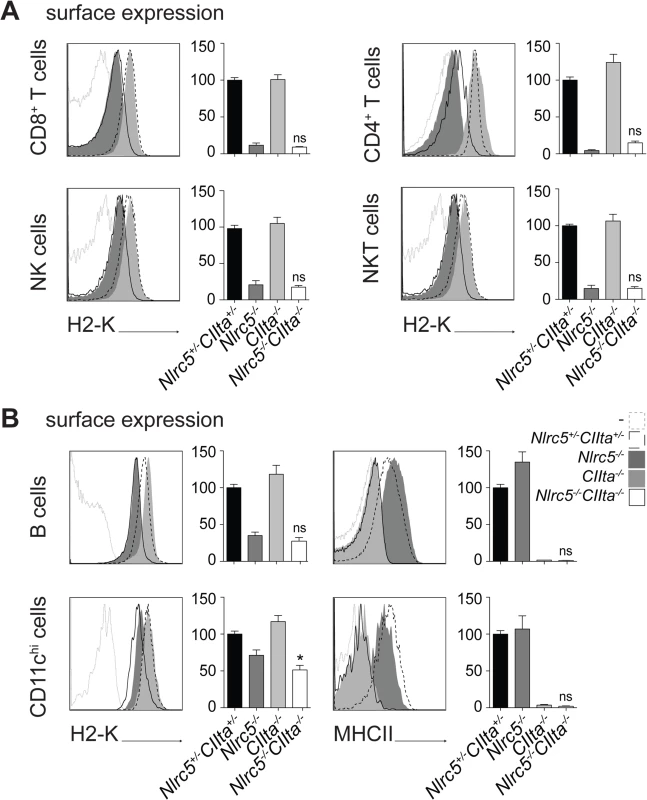
(A and B) MHCI and MHCII expression were assessed in the indicated splenic cells from Nlrc5+/−CIIta+/−, Nlrc5−/−, CIIta−/−, and Nlrc5−/−CIIta−/− mice. Histogram overlays show H2-K or MHCII expression for control (dashed line), Nlrc5−/− (filled dark grey), CIIta−/− (filled light grey), and double-deficient (solid line) mice. Graphs depict the MFIs of H2-K for CD8+ T cells (gated as CD3+CD8+), CD4+ T cells (gated as CD3+CD4+), NK cells (gated as NK1.1+CD3-), and NKT cells (gated as NK1.1+CD3+) (A), or B cells (gated as CD19+) and DCs (gated as CD11chiCD11bint-hi) (B) in control (n = 9), Nlrc5−/− (n = 10), CIIta−/− (n = 9), and double-deficient (n = 8) mice (A and B). For B cells and DCs, graphs also show the MFI of MHCII. MFIs of control mice were set at 100%. (A and B) Results depict the mean ± SEM from three pooled experiments. Statistical significance of the differences between multiple groups was analyzed by 2-way ANOVA adjusted by Bonferroni correction over 6 (H2-K) or 2 (MHCII) samples, and is indicated only for differences between single and double-deficient groups (i.e., the interaction between the two groups). NLRC5 binds to promoters of known and newly identified MHCI genes in an Rfx5-dependent manner
To gain a comprehensive view of genes regulated transcriptionally by NLRC5, we performed ChIP-seq experiments in T cells, which express NLRC5 abundantly and exhibit a dramatic defect in MHCI levels upon its ablation. Chromatin was extracted from T cells derived from control (WT and Nlrc5F/F), Nlrc5−/−, and Rfx5−/− mice. NLRC5-bound chromatin was enriched by ChIP and submitted to deep sequencing.
As previously observed for CIITA [12], and in sharp contrast to most other transcription factors, which typically occupy large numbers of sites in the genome [14], only a restricted number of NLRC5-occupied sites were detected. A total of only 11 NLRC5-binding sites were present in control (WT and/or Nlrc5F/F) cells but absent in Nlrc5−/− cells (Table 1, Figs. 3A, 4A, S2A). Of the NLRC5-occupied sites, 9 resided in the vicinity (-500 to +50) of the transcription start sites (TSSs) of 12 genes. The number of genes exceeds the number of peaks because 3 peaks lie between two closely spaced genes present in divergent orientations. Peaks at these promoter sites were all absent in Rfx5−/− cells (Table 1, Figs. 3A, 4A, S2A). The remaining two NLRC5-occupied sites were situated far from known promoters on chromosomes 1 and 15 and were not Rfx5-dependent (Table 1).
Fig. 3. Identification of NLRC5 targets. 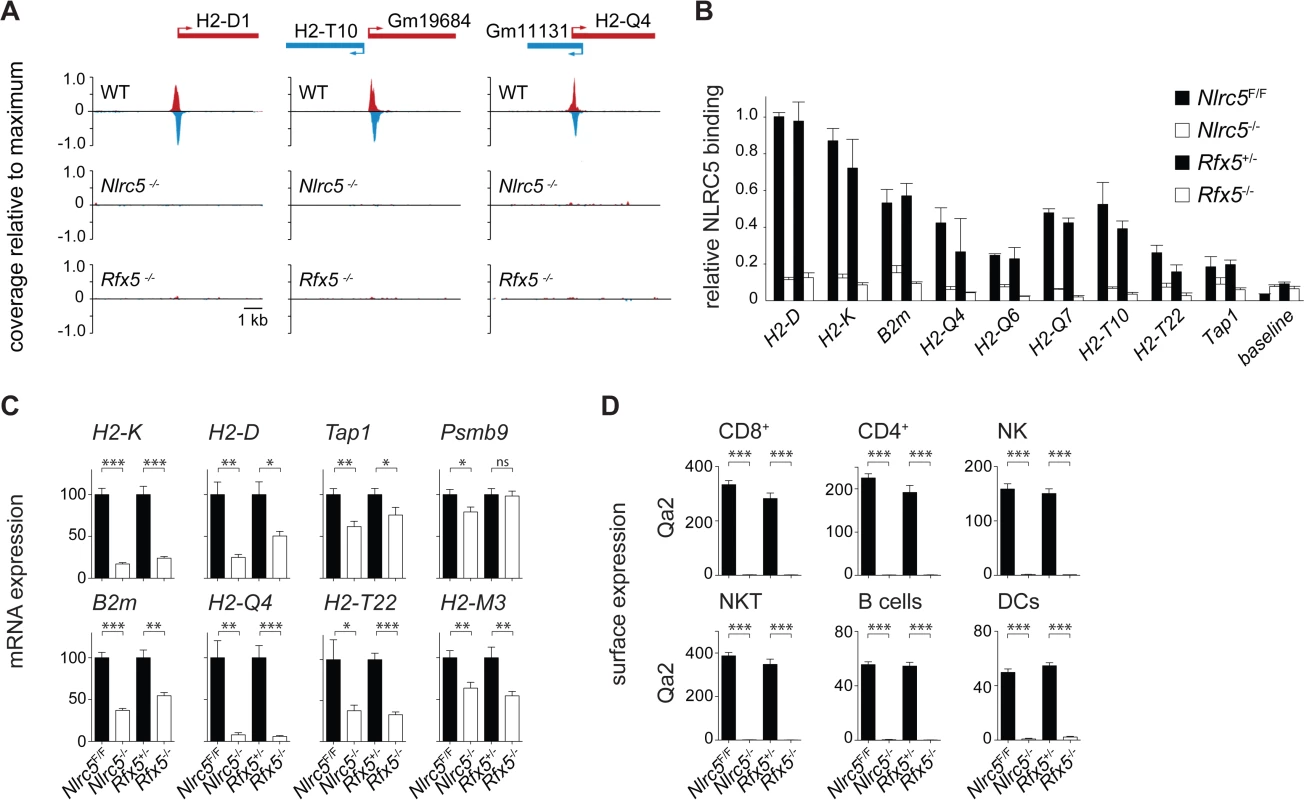
(A) NLRC5-ChIPseq tracks are shown for H2-D1, H2-T10/Gm19684 and Gm11131/H2-Q4. The tracks depict reads mapping to regions spanning between 5kb upstream and 5kb downstream of the peaks. After normalization as rpm (reads per millions), read coverage was expressed relative to the maximal value observed in the region. TSSs are positioned as annotated in ENSEMBL (H2-T10, H2-Q4) or Refseq (H2-D1). (B) Binding of NLRC5 was assessed by quantitative ChIP experiments performed with CD8+ T cells from Nlrc5F/F, Nlrc5−/−, Rfx5+/-, and Rfx5−/− mice. Results are expressed relative to binding of NLRC5 to the H2-D promoter in Nlrc5F/F cells, and depict the mean ± SD derived from three technical replicates and are representative of two independent experiments. (C) mRNAs for the indicated genes were quantified relative to Hprt mRNA in CD8+ T cells purified from a pool of 4–5 mice of each indicated genotype. Values for Nlrc5−/− and Rfx5−/− cells are expressed as percentage of Nlrc5F/F and Rfx5+/-, respectively. Data represent mean ± SD of technical triplicates, and are representative of at least two independent experiments. Statistical significance was calculated with an unpaired Student’s t-test, two-tailed; adjustment was made using a Bonferroni correction over 2 samples. (D) MFI for Qa2 was analyzed in the indicated cell subsets from Nlrc5F/F (n = 9), Nlrc5−/− (n = 9), Rfx5+/- (n = 10), and Rfx5−/− (n = 8) mice. Specific cell populations were gated on CD3+CD8+ for CD8+ T cells, CD3+CD4+ for CD4+ T cells, NK1.1+CD3- for NK cells, NK1.1+CD3+ for NKT cells, CD19+ for B cells, and CD11chiCD11bint-hi for DCs. Results represent the mean ± SEM derived from two pooled experiments. Statistical significance was calculated with an unpaired Student’s t-test, two-tailed; adjustment is made using a Bonferroni correction over 12 samples. Fig. 4. NLRC5 selectively occupies MHCI gene promoters. 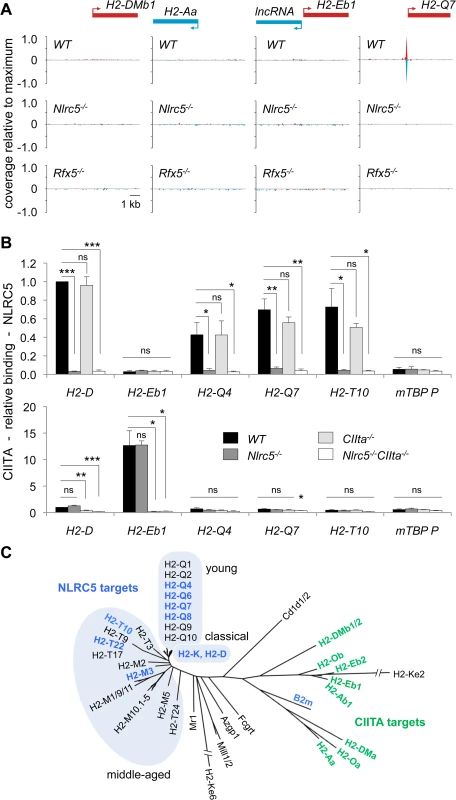
(A) NLRC5-ChIPseq tracks are shown for the CIITA targets H2-DMb1, H2-Aa and IncRNA/H2-Eb1. The tracks depict reads mapping to regions spanning between 5kb upstream and 5kb downstream of the TSS. After normalization as rpm (reads per million), read coverage was expressed relative to the average value observed for all NLRC5-binding peaks. TSSs are positioned as annotated in ENSEMBL. (B) Antibodies specific for NLRC5 and CIITA were used to immunoprecipitate cross-linked chromatin fragments derived from Nlrc5+/-CIIta+/-, Nlrc5−/−, CIIta−/−, and Nlrc5−/−CIIta−/− B cells. Immunoprecipitates were analyzed by quantitative PCR for the abundance of promoter sequences from the indicated genes. Relative promoter binding is shown. The average ± SEM of three experiments are depicted. Statistical significance was calculated using an unpaired Student’s t-test. (C) A phylogenetic tree is shown for classical and non-classical MHCI and MHCII genes. Genes regulated by NLRC5 and those regulated by CIITA are indicated in blue and green font, respectively. Clusters of classical, young and middle-aged MHCI genes are highlighted. Tab. 1. NLRC5-occupied sites and target genes. 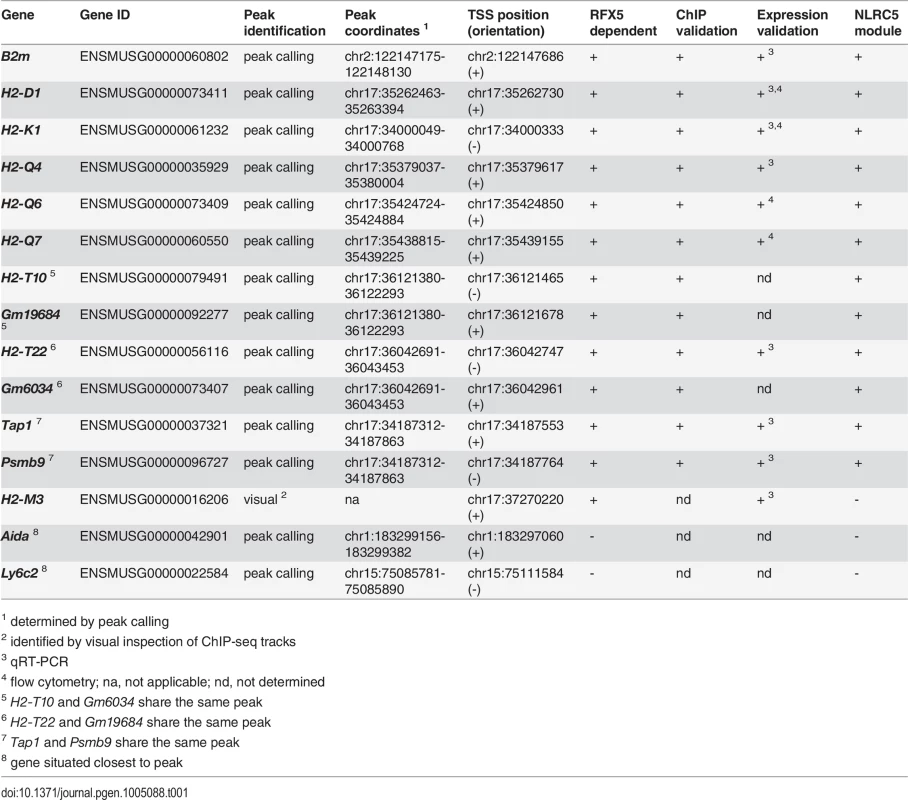
1 determined by peak calling Genes containing NLRC5-occupied sites in their promoter regions included genes previously suggested to be regulated by NLRC5 (H2-K, H2-D, B2m, Psmb9, and Tap1), validating the quality of the ChIP-seq analysis (Table 1, Figs. 3A, S2A). In addition, novel target genes were identified (Table 1, Figs. 3A, 4A). Five of these are non-classical MHCI genes (H2-Q4, H2-Q6, H2-Q7, H2-T10, and H2-T22). Two are predicted genes of unknown function (Gm19684, Gm6034) situated in the reverse orientation immediately upstream of H2-T10 and H2-T22 (Fig. 3A and Table 1).
To ensure that the peak calling procedure had not missed binding sites in other MHC genes, the entire MHC locus was scanned visually for potential binding sites. This identified only one additional non-classical MHCI promoter (H2-M3) (Table 1). The latter was missed by the peak-calling algorithm because of its low intensity. In contrast to CIITA [15], no NLRC5-occupied intergenic enhancers were identified in the MHC locus.
Most NLRC5 targets were validated by classical quantitative ChIP experiments (Fig. 3B). To investigate the relevance of NLRC5 and Rfx5 for transactivation of the target genes identified by ChIP-seq, mRNA expression was quantified by qRT-PCR in control, Nlrc5−/−, and Rfx5−/− CD8+ T cells (Fig. 3C). Transcript abundance of tested NLRC5 targets was reduced in the absence of either Nlrc5 or Rfx5, with the exception of Psmb9, whose expression was not altered in the absence of Rfx5. High homology among non-classical MHCI genes did not allow quantification of H2-T10, H2-Q6, and H2-Q7 by qRT-PCR. However, we measured expression of the Qa2 antigen (encompassing H2-Q6/7/8/9) by flow cytometry and observed a virtually complete loss in all tested cell types in the absence of Nlrc5 or Rfx5 (Fig. 3D). Collectively, these results provide evidence for the critical importance of Rfx5 in recruiting NLRC5 and for the contribution of the Rfx5-NLRC5 axis in activating most of the identified target genes.
NLRC5 and CIITA occupy distinct promoters
Although NLRC5 and CIITA are recruited by common enhanceosome factors, NLRC5-binding was not observed at the promoters of any MHCII genes (Fig. 4A). As the ChIP-sequencing was performed in T lymphocytes, which do not express MHCII genes, we reasoned that an inaccessible chromatin conformation might prevent NLRC5-binding to MHCII promoters in these cells. We therefore immunoprecipitated NLRC5 and CIITA bound chromatin from control, Nlrc5−/−, CIIta−/−, and Nlrc5−/−CIIta−/− B cells, which express high levels of CIITA and MHCII. Quantitative ChIP analysis confirmed that NLRC5 binding was observed at classical and non-classical MHCI promoters but not at the prototypical H2-E MHCII promoter (Fig. 4B). As in T cells, NLRC5 recruitment was dependent on Rfx5 in B cells (S2B Fig.). CIITA binding was evident at the H2-E promoter but not at any of the NLRC5 targets tested (Fig. 4B). These results are consistent with our MHC expression data showing non-redundant functions of NLRC5 and CIITA (Fig. 2).
These results emphasize the striking specificity of NLRC5 and CIITA for phylogenetically related but distinct sets of genes (Fig. 4C). Interestingly, NLRC5-controlled genes encode classical and evolutionarily “middle-aged” and “young” non-classical MHCI molecules [16], with the exception of B2m, which clusters together with MHCII molecules. This suggests that divergent evolution underlies the differentiation of NLRC5 function and specificity.
Identification of a unique consensus motif for NLRC5 binding
A consensus sequence motif with similarity to the X box (Fig. 5A) was derived from promoter-associated NLRC5-occupied sequences using an unbiased motif discovery approach. As organization of the S, X, and Y elements in human MHCII promoters is tightly constrained with respect to their spacing [15,17], we searched for S and Y motifs located at the expected distance ranges from the X box (Fig. 5A). For most NLRC5 targets, we identified S and Y elements situated at distances within 16 and 20–22 base pairs, respectively, from the X box (Figs. 5A, S3, and S4A). Y motifs were not found at 20–22 base-pair distances from the X box in the H2-T10 and H2-T22 promoters. We therefore performed a less stringent search for S and Y motifs situated at more variable distances upstream and downstream of the X box (S5 Fig.). This search revealed the presence of Y motifs situated 48 base pairs downstream of the X box in the H2-T promoters (Figs. 5A, S3, S4B, and S5). It also identified sequences exhibiting similarity to the S box situated 45 base pairs upstream of the X box in these genes (S5 Fig.). Intriguingly, this motif contains a Y sequence, which might influence expression of H2-T10 and H2-T22 genes. At all NLRC5 targets, the identified SXY modules were situated upstream of the TSS, near the center of the NLRC5-binding peak (Fig. 5B).
Fig. 5. Identification of a sequence module for selective NLRC5 recruitment. 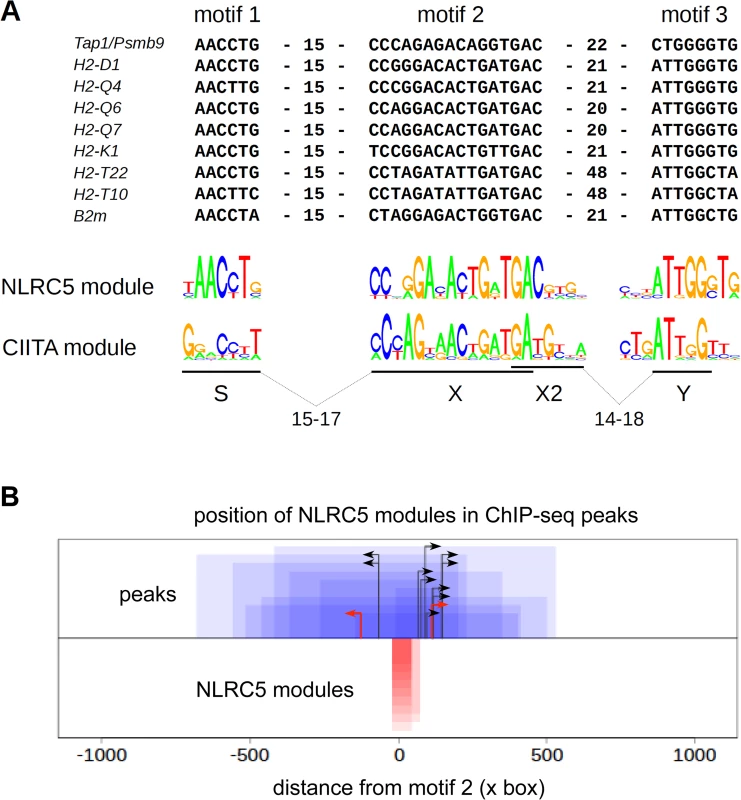
(A) Alignment of sequence motifs situated within NLRC5-occupied peaks found in promoter regions of the indicated genes. S-X and X-Y distance constraints used for identifying S and Y boxes were chosen such that these motifs should be situated within 60 bp windows upstream or downstream of the center of the X box. Distances (bp) between motifs are indicated. The sequence logo for the consensus NLRC5-module is shown below the alignment and is compared with that previously defined for human CIITA. (B) NLRC5-binding peaks (blue boxes) were oriented according to the direction of their SXY modules (red boxes). The 0 position on the x-axis corresponds to the start of the X box. Peaks and modules are paired by their heights on the y-axis. Black arrows represent TSS positions annotated in ENSEMBL. For two genes (H2-D and Psmb9) the predicted TSSs from Refseq (red arrows) are shown because the TSSs annotated in ENSEMBL would be situated upstream of the SXY module (see S2 Table). Irrespectively of the two approaches used for their identification, the SXY module defined for NLRC5-binding diverges substantially from that observed for CIITA, particularly at the level of the S box and at selected positions within the X box (Figs. 5A and S5).
A scan of the entire genome with the consensus motifs defined in Figs. 5A and S5 identified 15 and 173 putative matches, respectively. This indicates that spacing is a critical determinant for NLRC5 binding (S4 Fig.), since relaxing the spacing constraint leads to a larger number of predicted consensus sequences that are not actually occupied by NLRC5 (S4B Fig.). Among the hits obtained with the more stringent screen, 11 were in found in the vicinity of TSSs (S1 Table). These matches corresponded to the promoter-associated NLRC5-occupied sites and no MHCII genes or other CIITA-regulated genes were identified, underscoring the specificity of the consensus motif for NLRC5-recruitment.
The S box sequence determines NLRC5-specific transactivation
To investigate whether differences between the SXY modules bound by NLRC5 and CIITA were sufficient to confer transactivating specificity, we cloned the SXY regions of H2-K and H2-Eb into reporter plasmids. These two SXY modules were chosen based on their high similarity to the consensus motifs defined for NLRC5 and CIITA, respectively (Fig. 6A). NLRC5 exclusively transactivated the H2-K construct, whereas CIITA preferentially activated the H2-Eb construct (Fig. 6B). These results provided direct evidence that the SXY region dictates the differential promoter specificities of NLRC5 and CIITA.
Fig. 6. The S box sequence is required for NLRC5-mediated transactivation. 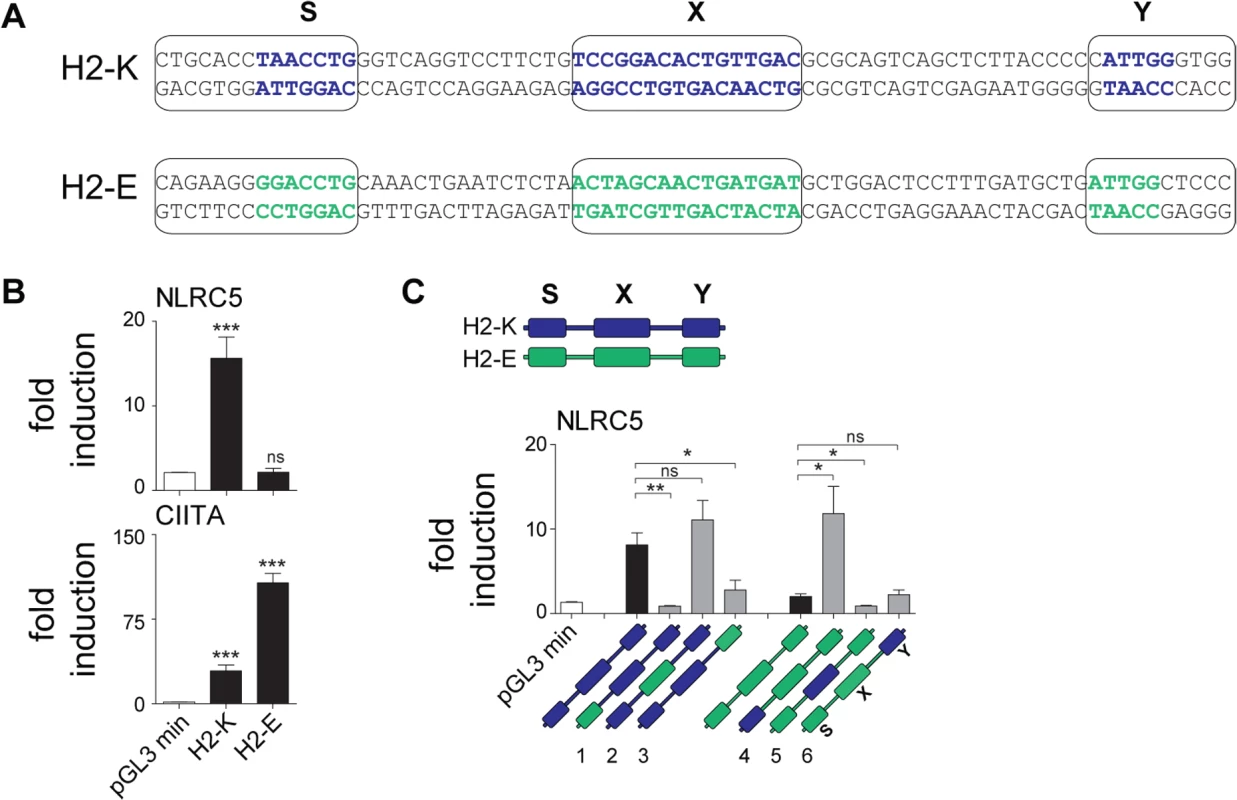
(A) Alignment of SXY regions of H2-K and H2-Eb (H2-E). Bold letters indicate the most conserved sequences in the S, X, and Y motifs; boxes define the regions that were used to generate hybrid promoters 1–6 (see Material and Methods) shown in C. (B) Luciferase reporter gene analyses were performed in HEK293T cells co-transfected with the H2-K or H2-Eb reporter constructs and either empty vector (e.v.) or expression vectors encoding NLRC5 or CIITA. Data represent mean ± SD of technical triplicates expressed as fold induction over e.v. and are representative of at least three experiments. Statistical significance was calculated using an unpaired Student’s t-test, two-tailed. (C) Luciferase reporter gene analyses were performed in HEK293T cells co-transfected with empty or NLRC5-encoding expression vectors and the WT (H2-K, H2-E) or hybrid (H2-K/E 1–6) promoter constructs depicted schematically below: H2-K and H2-Eb derived sequences are represented by blue and green, respectively. Data are expressed as fold induction over e.v. and represent mean ± SEM of four independent experiments. Statistical significance was calculated using an unpaired Student’s t-test, two-tailed. To pinpoint the elements conferring NLRC5 specificity, we generated a series of hybrid promoters in which individual S, X, and Y boxes of H2-K were replaced with the corresponding ones from H2-Eb and vice versa (Fig. 6C). Despite differences in the X box consensus sequences defined for NLRC5 and CIITA (Fig. 5A), reporter assays performed with the hybrid promoters indicated that the X boxes from H2-K and H2-Eb were equally efficient at supporting NLRC5-mediated transactivation (Fig. 6C). The Y box of H2-K partially contributed to NLRC5 activity but was not sufficient per se (Fig. 6C). In contrast, the H2-K S motif proved to be critical for driving NLRC5-mediated activity, as its replacement with the S box of H2-Eb was sufficient to abolish transactivation (Fig. 6C). Furthermore, this element was sufficient for promoting NLRC5-induced transcription when placed into the H2-Eb reporter backbone (Fig. 6C). These results show that the unique S box motif found in the promoters of NLRC5-regulated genes is the major determinant for guiding selective gene activation by NLRC5.
Discussion
Our understanding of NLRC5’s function as a transcriptional regulator of MHCI genes has progressed rapidly during recent years; yet several fundamental aspects remained unexplored. Here, we provide a comprehensive analysis of NLRC5-regulated genes in T cells, leading to the identification of novel target genes and gaining new insights into the molecular mechanisms of NLRC5 recruitment to specific promoters.
Interestingly, NLRC5-transactivated MHCI genes encode classical and evolutionarily “middle-aged” and “young” non-classical MHCI molecules, which generally support T cell receptor engagement and NK cell inhibition [16]. The expression of non-classical MHCI molecules, such as the novel target Qa2, have been shown to be important for the selection of non-conventional T cell subsets and in the development of the preimplantation embryo [18,19,20]. H2-T10 and H2-T22 have been implicated in the selection of gamma-delta T cells with immunoregulatory functions [21,22]. Since the selection of unconventional T cell subsets is mainly driven by hematopoietic cells, and could occur through T cell-T cell interactions, our data generated in T lymphocytes might be particularly relevant for this process [16,23,24]. Taken together, it appears that NLRC5 function has specifically co-evolved with the needs for MHCI-restricted antigen-presentation to conventional or non-conventional T cell subsets, and with NK cell education, suggesting the need to take a closer look at the role of NLRC5 in the development of these subsets.
We provide evidence that Rfx5 serves as a key mediator of NLRC5 binding to the promoter of its target genes, as its absence abolished NLRC5 recruitment to all target genes. Together with evidence that RFX5, RFXAP and RFXANK contribute to HLA class I transcription in human B cells, our findings unambiguously clarify the molecular nature of BLS type III disorders, which are characterized by defects in both MHCI and MHCII expression [8,9,10,11].
Analysis of double-deficient mice demonstrated that CIITA and NLRC5 regulate distinct sets of genes despite the fact that they use common enhanceosome factors and similar promoter sequences. This surprising situation raises the question as to how specificity is achieved. ChIP-seq analysis allowed us to detail the preferential promoter module occupied by NLRC5. Most prominently, selected positions within the X box and the remarkably conserved S box emerged as key features associated with NLRC5 recruitment, thereby distinguishing the SXY region recognized by NLRC5 from that occupied by CIITA. This is consistent with the results of reporter gene assays suggesting that the S box is required for NLRC5-mediated transactivation [8]. We demonstrate here that the distinctive S motif found in the promoters of NLRC5-occupied genes is essential for conferring the transactivation specificity of NLRC5, and that its replacement by the analogous S motif of CIITA-occupied promoters abrogates NLRC5 transactivation. This critical role of the S box suggests that the SXY module occupied by NLRC5 promotes the assembly of an enhanceosome complex differing from that required for the recruitment of CIITA, although the two complexes do share certain DNA-binding proteins. In this respect it should be mentioned that the S box-binding factors remain to be identified and could differ between NLRC5 and CIITA regulated genes.
Polymorphisms within the MHCI locus have been associated with infectious and autoimmune diseases. In many cases, the determining parameter is the MHCI haplotype, as different alleles can present different peptide repertoires. However, it has recently been suggested that various alleles can also be expressed at dissimilar levels, and that their abundance shows significant associations with disease outcomes, as in the case of human immunodeficiency virus infection and Crohn’s disease [25]. Given the fact that the SXY module is conserved between mouse and humans [26], it will be important to establish whether promoter variants of alleles associated with immunological disorders are differentially transactivated by NLRC5. Such correlations could be of high medical relevance as predictive or prognostic markers in selected immunological diseases.
The newly identified NLRC5 target genes encode non-classical MHCI molecules, emphasizing the remarkable selectivity of this NLR for regulating the MHCI system. This renders NLRC5 an attractive candidate for therapeutic intervention aimed at modulating MHCI expression. The high specificity of NLRC5 for a small number of phylogenetically related MHCI genes is strikingly similar to that of CIITA, for which ChIP-on-microarray experiments have revealed high selectivity for genes involved in MHCII-mediated antigen presentation [12]. Although recent ChIP-seq experiments have suggested that there are other CIITA-occupied sites in the genome [27], their functional relevance remains to be demonstrated. The extremely focused activity of NLRC5 and CIITA sets them apart from other transcription factors and transcriptional coactivators, which typically regulate hundreds or thousands of genes and exhibit much more diverse and pleiotropic functions; these two NLRs are instead specialized for the expression of only few phylogenetically and/or functionally related genes, representing a novel type of highly dedicated transcriptional regulator.
Materials and Methods
Mice
Mice were treated in accordance with the Swiss Federal Veterinary Office guidelines. Nlrc5F/F, Nlrc5−/− [2], CIIta−/− [28], and C57BL/6 control mice, all on a C57BL/6 (H2b) background, were bred at the animal facility of the University of Lausanne. Nlrc5−/− and CIIta−/− were intercrossed to generate double-deficient animals. Rfx5−/− [29] and Rfx5+/- littermate controls on a mixed Sv129/C57BL/6 (H2b) background were bred at the animal facility of the University of Geneva Medical School. Sex and age-matched 6 to 12 week-old mice were used.
Cells and flow cytometry
Human BLS cell lines and in vitro generated B cell mutants have been described and are established human cell lines [1,30]. T cells were enriched using anti-CD4 and/or anti-CD8 magnetic beads (Miltenyi Biotec). For all flow cytometric analyses, gating on living cells and exclusion of doublets was performed. Enriched TEC suspensions [2] were washed in PBS, 2% FCS, 5mM EDTA and stained for flow cytometry using death exclusion markers (either DAPI or 7AAD), UEA1 (Sigma) and the following mAb-conjugated mix: α-CD45 (30F11, BioLegend) and α-BP1 (6C3; BioLegend), α-MHCII (M5/114.15.2; eBioscience) and α-EpCAM (G8.8, Developmental Studies Hybridoma Bank, Iowa), α-H2-Db (B22/24g) and α-H2-Kb (B8.24.3). Splenocytes were preincubated with anti-CD16/32 (2.4G2) to block FcRs and stained using Abs against CD8a (Ly-2), CD3e (145-2C11), CD4 (L3T4), CD11b (M1/70), CD11c (N418), CD19 (1D3), H2-Db (28-14-8), H2-Kb (AF6–88.5.5.3), MHCII (M5/114.15.2), NK1.1 (PK136), B220 (RA3-6B2), Qa-2 (69H1-9-9) (all from eBioscience). Streptavidin conjugated to different fluorophores was from eBioscience. Stainings were performed with appropriate combinations of fluorophores. Data was acquired with a FACSCanto flow cytometer (Becton Dickinson) and analyzed using FlowJo software (Tree Star).
ChIP-sequencing
Chromatin was purified from MACS-sorted WT (C57BL/6), Nlrc5F/F, Nlrc5−/−, and Rfx5−/− T cells as described [31]. Five mice were pooled for each genotype. Chromatin immunoprecipitation was performed using anti-NLRC5 antibody as described [2].
Immunoprecipitated DNA was sequenced using the Illumina HiSeq 2000 platform. >300 million reads were obtained for WT samples. >20 million reads were obtained for all other samples. ChIP samples from WT and Nlrc5F/F mice were used as biological repeats. Five pseudo-replicates of 30 million reads each were used for the WT data set, as proposed by the ENCODE consortium [32]. Reads were mapped to the mouse genome (release GRCm38.70) using Bowtie 0.12.7 [33]. Only reads mapping to unique genomic positions were considered for further analysis.
Fragment length was estimated using cross-correlation [32]. The Phantompeakqualtools R package (https://www.encodeproject.org/search/?type=software&used_by=ENCODE&software_type=quality%20metric) [32] was used to measure the quality of the ChIP-seq data, as assessed by the normalized ratio between the fragment-length cross-correlation and the background cross-correlation (normalized strand coefficient, NSC), the ratio between the fragment-length peak and the read-length peak (relative strand correlation, RSC) and the Qtag code. The low NSC scores obtained (< 1.05) (S6A Fig.) are a consequence of the low number of peaks [32]. The RSC (> 1.51–1.85) and Qtag (2, high quality) scores obtained attest to the quality of the ChIP-seq peaks (S6A Fig.) [32].
Peak calling for WT and Nlrc5F/F data sets was first done with MACS2 using the default settings (q-value threshold of 0.05 and without the “–to-large” parameter). This led to the identification of a surprisingly low number of reproducible peaks. The numbers of peaks were 6 and 11, respectively for the WT and Nlrc5F/F datasets. The low number of peaks called using the initial strategy prompted us to use second strategy based on using a lower peak calling stringency followed by Irreproducible Discovery Rate (IDR) analysis. This was done to ascertain that that the low number of peaks identified by our initial procedure was not in fact an artifact resulting from overly-stringent peak selection. Peaks were called using MACS2 2.0.10.20130520 [34] with no-model setting and shift-size parameter set to half of the estimated fragment length. Peak calling stringency was decreased by using p = 0.001 as threshold and applying the “-to-large” setting. Reads obtained from Nlrc5−/− samples were used as negative control for peak calling. Reproducible peaks were obtained by assessing the IDR for all pairs of pseudo-replicates using a threshold of 0.01 (S6B Fig.). Only 11 reproducible peaks were obtained, all of which were confirmed in the biological repeat (Nlrc5F/F) but found to be absent in the Rfx5−/− and Nlrc5−/− samples. These 11 peaks were the same as those identified in the Nlrc5F/F dataset with the first peak identification strategy.
The Fraction of Reads in Peaks (FRiP) [32] was also calculated (S6C Fig.). The low FRiP values obtained (<1%) are consistent with the low number of peaks identified [32].
Sequence analysis
For each gene, all annotated exons (release GRCm38.69) from all isoforms were used to create a unique gene model in which all exons were merged into a single mRNA. The TSS of this unique gene model was defined as the TSS for the corresponding gene. The promoter region was defined as the region spanning −500bp to +50bp of the TSS. Peaks overlapping with promoter regions were used for de novo motif discovery using the package cosmo [35] available for the R project [36]. An initial search identified a motif corresponding to the previously published X box [15]. Peaks were oriented relative to this X motif, and searches for S and Y motifs were then performed within 60 (Figs. 5, S4A, S1 Table) or 100 (S5, S4B Figs.) base-pair windows situated upstream and downstream of the center of the X box. This identified upstream and downstream motifs corresponding, respectively, to the previously described S and Y boxes [15]. Genome wide search for modules containing the 3 motifs was performed using both the Position Weight Matrix (PWM) for each motif and the minimal and maximal distances between the motifs. The consensus for each motif was represented by a PWM obtained by aligning the sequences of the corresponding motif observed in peaks. The score of each sequence versus its PWM was calculated for each peak, and 95% of this minimal score was used as threshold for the genome wide search. Authorized spacing between the motifs in the genome wide search was considered as that observed between the motifs found in peaks plus or minus 5nt. Only sequence modules containing the 3 motifs separated by the authorized distances were accepted.
ChIP
Chromatin was purified as described [31] from Nlrc5F/F, Nlrc5−/−, Rfx5−/− and Rfx5+/-MACS-sorted T cells (Fig. 3B, four to five mice were pooled per genotype), Nlrc5+/−CIIta+/−, Nlrc5−/−, CIIta−/− and Nlrc5−/−CIIta−/− B cells (Fig. 4B, two mice were pooled per genotype), or WT (C57BL/6), Nlrc5−/− and Rfx5−/− B cells (S2B Fig., four to five mice were pooled per genotype). Chromatin immunoprecipitation was performed using anti-NLRC5 and anti-CIITA antibodies as described [2,31]. Analysis of specific DNA regions was performed by qRT-PCR with the primers shown in Table 2.
Tab. 2. Primers used for qRT-PCR analysis after ChIP 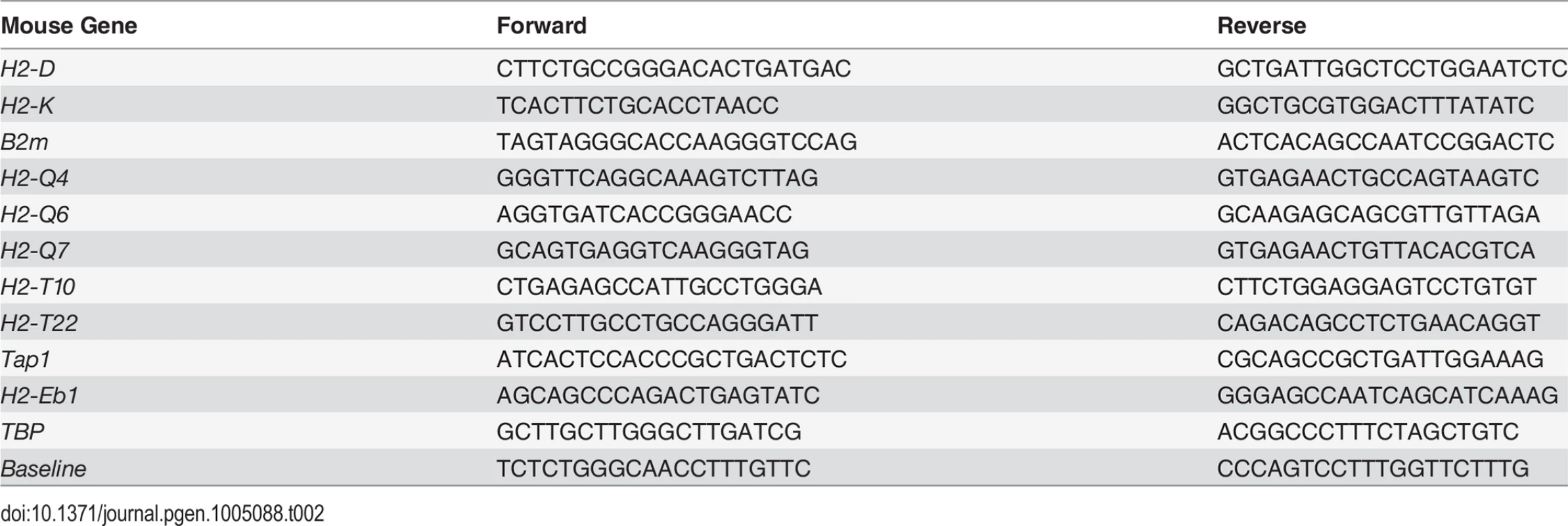
Quantitative RT-PCR
Total RNA was extracted using the TriFastTM reagent according to manufacturer's instructions (PEQLAB Biotechnologie). Retrotranscription to cDNA, quantification, and data analysis have been described [37]. Expression was determined relative the indicated housekeeping gene. Primer sequences used are listed in Table 3.
Tab. 3. Primers used for qRT-PCR analysis 
Phylogenetic tree of mouse MHC molecules
The following amino-acid sequences were downloaded from MGI (http://www.informatics.jax.org/): H2-Ke2 (MGI:95908), H2-K1 (MGI:95904), H2-Ke6 (MGI:95911), H2-Oa (MGI:95924), H2-DMa (MGI:95921), H2-DMb2 (MGI:95923), H2-DMb1 (MGI:95922), H2-Ob (MGI:95925), H2-Ab1 (MGI:103070), H2-Aa (MGI:95895), H2-Eb1 (MGI:95901), H2-Eb2 (MGI:95902), H2-D1 (MGI:95896), H2-Q1 (MGI:95928), H2-Q2 (MGI:95931), H2-Q4 (MGI:95933), H2-Q6 (MGI:95935), H2-Q7 (MGI:95936), H2-Q10 (MGI:95929), H2-T24 (MGI:95958), H2-T23 (MGI:95957), H2-T22 (MGI:95956), H2-T17 (MGI:95949), H2-M10.1 (MGI:1276522), H2-T10 (MGI:95942), H2-T3 (MGI:95959), H2-M10.2 (MGI:1276525), H2-M10.4 (MGI:1276527), H2-M1 (MGI:95913), H2-M9 (MGI:1276570), H2-M10.3 (MGI:1276524), H2-M11 (MGI:2676637), H2-M10.5 (MGI:1276526), H2-M5 (MGI:95917), H2-M3 (MGI:95915), H2-M2 (MGI:95914), Mill1 (MGI:2179988), Cd1d1 (MGI:107674), B2m (MGI:88127), Mr1 (MGI:1195463), Azgp1 (MGI:103163), Mill2 (MGI:2179989), Fcgrt (MGI:103017), Cd1d2 (MGI:107675), H2-Q8 (MGI:95937), H2-Q9 (MGI:95938) and H2-T9 (MGI:95965). Alignment was performed using the Muscle tool [38], the best model to construct the phylogenetic tree was assessed using Prottest [39], and the phylogenetic tree was constructed in PhyML [40] using the JTT substitution model.
Luciferase reporter gene assays
Luciferase reporter plasmids were created by replacing the MluI—BglII fragment spanning the HLA-DRA SXY region in the pDRAprox plasmid [15] with the corresponding H2-K, H2-Eb1 and hybrid SXY regions. The pGL3-min plasmid containing only the HLA-DRA core promoter (from −60 to +10) in the same reporter plasmid was used as negative control. DNA fragments corresponding to the SXY regions were generated using partially complementary primers that were annealed and amplified by PCR using GoTaq polymerase (Promega). Primer sequences used are listed below. Extensions containing the MluI and BglII restriction sites (underlined) used for cloning are indicated in smaller font.
H2-K construct:
5’ATGCACGCGTCCACAGTTTCACTTCTGCACCTAACCTGGGTCAGGTCCTTCTGTCCGGACACTGTTG 3’ (forward primer)
5’TGGTAGATCTCGCCACCCAATGGGGGTAAGAGCTGACTGCGCGTCAACAGTGTC 3’ (reverse primer)
H2-E construct:
5’ATGCACGCGTAACTGCAAGTTTCAGAAGGGGACCTGCAAACTGAATCTCTAACTAGCAACTGATGA 3’ (forward primer)
5’TGGTAGATCTTGGGAGCCAATCAGCATCAAAGGAGTCCAGCATCATCAGTTG 3’ (reverse primer)
Hybrid H2-K/E construct 1:
5’ATGCACGCGTAACTGCAAGTTTCAGAAGGGGACCTGGGTCAGGTCCTTCTGTCCGG ACACTGTTG 3’ (forward primer)
5’TGGTAGATCTCGCCACCCAATGGGGGTAAGAGCTGACTGCGCGTCAACAGTGTC 3’ (reverse primer)
Hybrid H2-K/E construct 2:
5’ATGCACGCGTCCACAGTTTCACTTCTGCACCTAACCTGGGTCAGGTCCTTCTGACTAGCAACTGATGA 3’ (forward primer)
5’TGGTAGATCTCGCCACCCAATGGGGGTAAGAGCTGACTGCGCATCATCAGTTG 3’ (reverse primer)
Hybrid H2-K/E construct 3:
5’ATGCACGCGTCCACAGTTTCACTTCTGCACCTAACCTGGGTCAGGTCCTTCTGTCCGGACACTGTTG 3’ (forward primer)
5’TGGTAGATCTTGGGAGCCAATGGGGTAAGAGCTGACTGCGCGTCAACAGTGTC3’ (reverse primer)
Hybrid H2-K/E construct 4:
5’ATGCACGCGTCCACAGTTTCACTTCTGCACCTAACCTGCAAACTGAATCTCTAACTAGCAACTGATGA 3’ (forward primer)
5’TGGTAGATCTTGGGAGCCAATCAGCATCAAAGGAGTCCAGCATCATCAGTTG 3’ (reverse primer)
Hybrid H2-K/E construct 5:
5’ATGCACGCGTAACTGCAAGTTTCAGAAGGGGACCTGCAAACTGAATCTCTATCCGGACACTGTTG3’ (forward primer)
5’TGGTAGATCTTGGGAGCCAATCAGCATCAAAGGAGTCCAGCGTCAACAGTGTC 3’ (reverse primer)
Hybrid H2-K/E construct 6:
5’ATGCACGCGTAACTGCAAGTTTCAGAAGGGGACCTGCAAACTGAATCTCTAACTAGCAACTGATGA 3’ (forward primer)
5’TGGTAGATCTCGCCACCCAATGCAGCATCAAAGGAGTCCAGCATCATCAGTTG 3’ (reverse primer)
HEK293T cells were subconfluently seeded into a 96-well plate and co-transfected with Polyfect reagent (Qiagen) following the manufacturer’s instructions with 25 ng of empty, human NLRC5 or human CIITA (pIII) expression vectors and 25 ng of the indicated luciferase reporter constructs [2]. 5 ng of Renilla luciferase vector were included for normalization. Cells were harvested 22h post-transfection and cell lysates were analyzed using the Dual-Luciferase Reporter Assay System (Promega) following the manufacturer’s instructions.
Statistical analysis
Statistical differences were calculated as described in the Figure legends. Differences were considered significant when p≤0.05 (*), very significant when p≤0.01 (**) and extremely significant when p≤0.001 (***).
Ethics statement
Mice were treated in accordance with the Swiss Federal Veterinary Office guidelines. Human cell lines are established cell lines.
Supporting Information
Zdroje
1. Reith W, Mach B (2001) The bare lymphocyte syndrome and the regulation of MHC expression. Annu Rev Immunol 19 : 331–373. 11244040
2. Staehli F, Ludigs K, Heinz LX, Seguin-Estevez Q, Ferrero I, et al. (2012) NLRC5 deficiency selectively impairs MHC class I - dependent lymphocyte killing by cytotoxic T cells. J Immunol 188 : 3820–3828. doi: 10.4049/jimmunol.1102671 22412192
3. Meissner TB, Li A, Biswas A, Lee KH, Liu YJ, et al. (2010) NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc Natl Acad Sci U S A 107 : 13794–13799. doi: 10.1073/pnas.1008684107 20639463
4. Robbins GR, Truax AD, Davis BK, Zhang L, Brickey WJ, et al. (2012) Regulation of class I major histocompatibility complex (MHC) by nucleotide-binding domain, leucine-rich repeat-containing (NLR) proteins. J Biol Chem 287 : 24294–24303. doi: 10.1074/jbc.M112.364604 22645137
5. Biswas A, Meissner TB, Kawai T, Kobayashi KS (2012) Cutting edge: impaired MHC class I expression in mice deficient for Nlrc5/class I transactivator. J Immunol 189 : 516–520. doi: 10.4049/jimmunol.1200064 22711889
6. Neerincx A, Castro W, Guarda G, Kufer TA (2013) NLRC5, at the Heart of Antigen Presentation. Front Immunol 4 : 397. doi: 10.3389/fimmu.2013.00397 24319445
7. Yao Y, Wang Y, Chen F, Huang Y, Zhu S, et al. (2012) NLRC5 regulates MHC class I antigen presentation in host defense against intracellular pathogens. Cell Res 22 : 836–847. doi: 10.1038/cr.2012.56 22491475
8. Meissner TB, Liu YJ, Lee KH, Li A, Biswas A, et al. (2012) NLRC5 cooperates with the RFX transcription factor complex to induce MHC class I gene expression. J Immunol 188 : 4951–4958. doi: 10.4049/jimmunol.1103160 22490869
9. Neerincx A, Rodriguez GM, Steimle V, Kufer TA (2012) NLRC5 controls basal MHC class I gene expression in an MHC enhanceosome-dependent manner. J Immunol 188 : 4940–4950. doi: 10.4049/jimmunol.1103136 22490867
10. Gobin SJ, Peijnenburg A, van Eggermond M, van Zutphen M, van den Berg R, et al. (1998) The RFX complex is crucial for the constitutive and CIITA-mediated transactivation of MHC class I and beta2-microglobulin genes. Immunity 9 : 531–541. 9806639
11. Gobin SJ, van Zutphen M, Westerheide SD, Boss JM, van den Elsen PJ (2001) The MHC-specific enhanceosome and its role in MHC class I and beta(2)-microglobulin gene transactivation. J Immunol 167 : 5175–5184. 11673530
12. Krawczyk M, Seguin-Estevez Q, Leimgruber E, Sperisen P, Schmid C, et al. (2008) Identification of CIITA regulated genetic module dedicated for antigen presentation. PLoS Genet 4: e1000058. doi: 10.1371/journal.pgen.1000058 18437201
13. Masternak K, Muhlethaler-Mottet A, Villard J, Zufferey M, Steimle V, et al. (2000) CIITA is a transcriptional coactivator that is recruited to MHC class II promoters by multiple synergistic interactions with an enhanceosome complex. Genes Dev 14 : 1156–1166. 10809673
14. Garber M, Yosef N, Goren A, Raychowdhury R, Thielke A, et al. (2012) A high-throughput chromatin immunoprecipitation approach reveals principles of dynamic gene regulation in mammals. Mol Cell 47 : 810–822. doi: 10.1016/j.molcel.2012.07.030 22940246
15. Krawczyk M, Peyraud N, Rybtsova N, Masternak K, Bucher P, et al. (2004) Long distance control of MHC class II expression by multiple distal enhancers regulated by regulatory factor X complex and CIITA. J Immunol 173 : 6200–6210. 15528357
16. Rodgers JR, Cook RG (2005) MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol 5 : 459–471. 15928678
17. van den Elsen PJ, Peijnenburg A, van Eggermond MC, Gobin SJ (1998) Shared regulatory elements in the promoters of MHC class I and class II genes. Immunol Today 19 : 308–312. 9666603
18. Das G, Gould DS, Augustine MM, Fragoso G, Sciutto E, et al. (2000) Qa-2-dependent selection of CD8alpha/alpha T cell receptor alpha/beta(+) cells in murine intestinal intraepithelial lymphocytes. J Exp Med 192 : 1521–1528. 11085754
19. Fragoso G, Lamoyi E, Mellor A, Lomeli C, Hernandez M, et al. (1998) Increased resistance to Taenia crassiceps murine cysticercosis in Qa-2 transgenic mice. Infect Immun 66 : 760–764. 9453638
20. Wu L, Feng H, Warner CM (1999) Identification of two major histocompatibility complex class Ib genes, Q7 and Q9, as the Ped gene in the mouse. Biol Reprod 60 : 1114–1119. 10208972
21. Crowley MP, Fahrer AM, Baumgarth N, Hampl J, Gutgemann I, et al. (2000) A population of murine gammadelta T cells that recognize an inducible MHC class Ib molecule. Science 287 : 314–316. 10634788
22. Schild H, Mavaddat N, Litzenberger C, Ehrich EW, Davis MM, et al. (1994) The nature of major histocompatibility complex recognition by gamma delta T cells. Cell 76 : 29–37. 8287478
23. Seach N, Guerri L, Le Bourhis L, Mburu Y, Cui Y, et al. (2013) Double-positive thymocytes select mucosal-associated invariant T cells. J Immunol 191 : 6002–6009. doi: 10.4049/jimmunol.1301212 24244014
24. Bendelac A (1995) Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med 182 : 2091–2096. 7500054
25. Apps R, Qi Y, Carlson JM, Chen H, Gao X, et al. (2013) Influence of HLA-C expression level on HIV control. Science 340 : 87–91. doi: 10.1126/science.1232685 23559252
26. van den Elsen PJ, Holling TM, Kuipers HF, van der Stoep N (2004) Transcriptional regulation of antigen presentation. Curr Opin Immunol 16 : 67–75. 14734112
27. Wong D, Lee W, Humburg P, Makino S, Lau E, et al. (2014) Genomic mapping of the MHC transactivator CIITA using an integrated ChIP-seq and genetical genomics approach. Genome Biol 15 : 494. 25366989
28. Chang CH, Guerder S, Hong SC, van Ewijk W, Flavell RA (1996) Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity 4 : 167–178. 8624807
29. Clausen BE, Waldburger JM, Schwenk F, Barras E, Mach B, et al. (1998) Residual MHC class II expression on mature dendritic cells and activated B cells in RFX5-deficient mice. Immunity 8 : 143–155. 9491996
30. Durand B, Sperisen P, Emery P, Barras E, Zufferey M, et al. (1997) RFXAP, a novel subunit of the RFX DNA binding complex is mutated in MHC class II deficiency. EMBO J 16 : 1045–1055. 9118943
31. Masternak K, Peyraud N, Krawczyk M, Barras E, Reith W (2003) Chromatin remodeling and extragenic transcription at the MHC class II locus control region. Nat Immunol 4 : 132–137. 12524537
32. Landt SG, Marinov GK, Kundaje A, Kheradpour P, Pauli F, et al. (2012) ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res 22 : 1813–1831. doi: 10.1101/gr.136184.111 22955991
33. Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. doi: 10.1186/gb-2009-10-3-r25 19261174
34. Liu T (2014) Use Model-Based Analysis of ChIP-Seq (MACS) to Analyze Short Reads Generated by Sequencing Protein-DNA Interactions in Embryonic Stem Cells. Methods Mol Biol 1150 : 81–95. doi: 10.1007/978-1-4939-0512-6_4 24743991
35. Bembom O, Keles S, van der Laan MJ (2007) Supervised detection of conserved motifs in DNA sequences with cosmo. Stat Appl Genet Mol Biol 6: Article8.
36. Team RDC (2011) R: A Language and Environment for Statistical Computing. Vienna, Austria: the R Foundation for Statistical Computing: Available online at http://www.R-project.org/.
37. Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, et al. (2011) Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34 : 213–223. doi: 10.1016/j.immuni.2011.02.006 21349431
38. Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5 : 113. 15318951
39. Abascal F, Zardoya R, Posada D (2005) ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21 : 2104–2105. 15647292
40. Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, et al. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59 : 307–321. doi: 10.1093/sysbio/syq010 20525638
Štítky
Genetika Reprodukčná medicína
Článek Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell LongevityČlánek HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and MiceČlánek LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male FertilityČlánek The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAsČlánek Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick VectorČlánek Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53Článek The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in NematodesČlánek The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse SpermatocytesČlánek The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 3- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
- Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
- A Genomic Duplication is Associated with Ectopic Eomesodermin Expression in the Embryonic Chicken Comb and Two Duplex-comb Phenotypes
- Genome-wide Association Study and Meta-Analysis Identify as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy
- Mutations of Human , Encoding the Mitochondrial Asparaginyl-tRNA Synthetase, Cause Nonsyndromic Deafness and Leigh Syndrome
- Exome Sequencing in an Admixed Isolated Population Indicates Variants Confer a Risk for Specific Language Impairment
- Genome-Wide Association Studies in Dogs and Humans Identify as a Risk Variant for Cleft Lip and Palate
- Rapid Evolution of Recombinant for Xylose Fermentation through Formation of Extra-chromosomal Circular DNA
- The Ribosome Biogenesis Factor Nol11 Is Required for Optimal rDNA Transcription and Craniofacial Development in
- Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
- Maternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity
- Clonality and Evolutionary History of Rhabdomyosarcoma
- HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and Mice
- Methylation-Sensitive Expression of a DNA Demethylase Gene Serves As an Epigenetic Rheostat
- BREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
- Seizures Are Regulated by Ubiquitin-specific Peptidase 9 X-linked (USP9X), a De-Ubiquitinase
- The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
- A Cascade of Iron-Containing Proteins Governs the Genetic Iron Starvation Response to Promote Iron Uptake and Inhibit Iron Storage in Fission Yeast
- Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
- LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male Fertility
- Cis-Regulatory Mechanisms for Robust Olfactory Sensory Neuron Class-restricted Odorant Receptor Gene Expression in
- Effects on Murine Behavior and Lifespan of Selectively Decreasing Expression of Mutant Huntingtin Allele by Supt4h Knockdown
- HDAC4-Myogenin Axis As an Important Marker of HD-Related Skeletal Muscle Atrophy
- A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex
- Selective and Genetic Constraints on Pneumococcal Serotype Switching
- Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
- The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAs
- Accumulation of Glucosylceramide in the Absence of the Beta-Glucosidase GBA2 Alters Cytoskeletal Dynamics
- Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities
- The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair
- Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick Vector
- Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
- The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors
- PHABULOSA Controls the Quiescent Center-Independent Root Meristem Activities in
- DNA Polymerase ζ-Dependent Lesion Bypass in Is Accompanied by Error-Prone Copying of Long Stretches of Adjacent DNA
- Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus
- Zinc Finger Independent Genome-Wide Binding of Sp2 Potentiates Recruitment of Histone-Fold Protein Nf-y Distinguishing It from Sp1 and Sp3
- GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters
- Neurospora Importin α Is Required for Normal Heterochromatic Formation and DNA Methylation
- Ccr4-Not Regulates RNA Polymerase I Transcription and Couples Nutrient Signaling to the Control of Ribosomal RNA Biogenesis
- Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53
- A Systems-Level Interrogation Identifies Regulators of Blood Cell Number and Survival
- Morphological Mutations: Lessons from the Cockscomb
- Genetic Interaction Mapping Reveals a Role for the SWI/SNF Nucleosome Remodeler in Spliceosome Activation in Fission Yeast
- The Role of China in the Global Spread of the Current Cholera Pandemic
- The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
- A Zinc Finger Motif-Containing Protein Is Essential for Chloroplast RNA Editing
- Resistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
- Small Regulatory RNA-Induced Growth Rate Heterogeneity of
- Mitochondrial Dysfunction Reveals the Role of mRNA Poly(A) Tail Regulation in Oculopharyngeal Muscular Dystrophy Pathogenesis
- Complex Genomic Rearrangements at the Locus Include Triplication and Quadruplication
- Male-Biased Aganglionic Megacolon in the TashT Mouse Line Due to Perturbation of Silencer Elements in a Large Gene Desert of Chromosome 10
- Sex Ratio Meiotic Drive as a Plausible Evolutionary Mechanism for Hybrid Male Sterility
- Tertiary siRNAs Mediate Paramutation in .
- RECG Maintains Plastid and Mitochondrial Genome Stability by Suppressing Extensive Recombination between Short Dispersed Repeats
- Escape from X Inactivation Varies in Mouse Tissues
- Opposite Phenotypes of Muscle Strength and Locomotor Function in Mouse Models of Partial Trisomy and Monosomy 21 for the Proximal Region
- Glycosyl Phosphatidylinositol Anchor Biosynthesis Is Essential for Maintaining Epithelial Integrity during Embryogenesis
- Hyperdiverse Gene Cluster in Snail Host Conveys Resistance to Human Schistosome Parasites
- The Class Homeodomain Factors and Cooperate in . Embryonic Progenitor Cells to Regulate Robust Development
- Recombination between Homologous Chromosomes Induced by Unrepaired UV-Generated DNA Damage Requires Mus81p and Is Suppressed by Mms2p
- Synergistic Interactions between Orthologues of Genes Spanned by Human CNVs Support Multiple-Hit Models of Autism
- Gene Networks Underlying Convergent and Pleiotropic Phenotypes in a Large and Systematically-Phenotyped Cohort with Heterogeneous Developmental Disorders
- The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
- Combinatorial Control of Light Induced Chromatin Remodeling and Gene Activation in
- Linking Aβ42-Induced Hyperexcitability to Neurodegeneration, Learning and Motor Deficits, and a Shorter Lifespan in an Alzheimer’s Model
- The Complex Contributions of Genetics and Nutrition to Immunity in
- NatB Domain-Containing CRA-1 Antagonizes Hydrolase ACER-1 Linking Acetyl-CoA Metabolism to the Initiation of Recombination during . Meiosis
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
- Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption
- A Genomic Portrait of Haplotype Diversity and Signatures of Selection in Indigenous Southern African Populations
- Sequence Features and Transcriptional Stalling within Centromere DNA Promote Establishment of CENP-A Chromatin
- Inhibits Neuromuscular Junction Growth by Downregulating the BMP Receptor Thickveins
- Replicative DNA Polymerase δ but Not ε Proofreads Errors in and in
- Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in
- The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
- A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension
- Pervasive Variation of Transcription Factor Orthologs Contributes to Regulatory Network Evolution
- Network Analyses Reveal Novel Aspects of ALS Pathogenesis
- A Role for the Budding Yeast Separase, Esp1, in Ty1 Element Retrotransposition
- Nab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
- A RecA Protein Surface Required for Activation of DNA Polymerase V
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Clonality and Evolutionary History of Rhabdomyosarcoma
- Morphological Mutations: Lessons from the Cockscomb
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



