-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
In the genome of eukaryotic cells, domains of active and repressive chromatin alternate along the chromosome arms. Insulator elements are necessary to shield these different environments from each other. In the fission yeast Schizosaccharomyces pombe, the chromatin remodeler Fft3 is required to maintain the repressed subtelomeric chromatin. Here we show that Fft3 maintains nucleosome structure of insulator elements at the subtelomeric borders. We also observe that subtelomeres and insulator elements move away from the nuclear envelope in cells lacking Fft3. The nuclear periphery is known to harbor repressive chromatin in many eukaryotes and has been implied in insulator function. Our results suggest that chromatin remodeling through Fft3 is required to maintain proper chromatin structure and nuclear organization of insulator elements.
Published in the journal: The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast. PLoS Genet 11(3): e32767. doi:10.1371/journal.pgen.1005101
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005101Summary
In the genome of eukaryotic cells, domains of active and repressive chromatin alternate along the chromosome arms. Insulator elements are necessary to shield these different environments from each other. In the fission yeast Schizosaccharomyces pombe, the chromatin remodeler Fft3 is required to maintain the repressed subtelomeric chromatin. Here we show that Fft3 maintains nucleosome structure of insulator elements at the subtelomeric borders. We also observe that subtelomeres and insulator elements move away from the nuclear envelope in cells lacking Fft3. The nuclear periphery is known to harbor repressive chromatin in many eukaryotes and has been implied in insulator function. Our results suggest that chromatin remodeling through Fft3 is required to maintain proper chromatin structure and nuclear organization of insulator elements.
Introduction
Nuclear architecture, i.e. non-random positioning of chromosomal loci and nuclear components in three dimensions, is important in organizing genome processes such as transcription, DNA replication and DNA repair [1–3]. One crucial aspect of this organization is the interaction between chromatin and the nuclear periphery. In many eukaryotic species, chromosomal domains near the nuclear envelope show low expression levels, repressive chromatin marks and low gene density [4–7]. These domains interact with the nuclear lamina [4,5] or inner nuclear membrane (INM) proteins, such as the LEM-domain proteins [6,7]. How chromosomal loci are targeted to the nuclear envelope is still poorly understood, although step-wise methylation of lysine 9 on histone H3 (H3K9me) [8,9] and interaction with different nuclear membrane proteins [10] are known to contribute.
In the fission yeast Schizosaccharomyces pombe, the INM protein Man1 interacts with about a third of the genome, mainly at lowly transcribed genes [7]. Especially striking, we observed Man1 binding to large domains adjacent to the telomeres of chromosomes I and II, which are characterized by a unique type of chromatin [11]. These subtelomeric domains show low levels of histone methylation, in both the repressive mark H3K9me2 and the active mark H3K4me2, as well as lower histone acetylation and H2A.Z levels compared to euchromatin [11,12]. Many genes in these regions are lowly expressed in rapidly growing cells, but are induced during nutritional stress or meiosis [13]. The borders of the subtelomeric domains are bound by the chromatin remodeling factor Fft3 [14]. Maintenance of the special chromatin state inside these domains in rapidly growing cells depends on Fft3, since a deletion of the remodeler results in a strong upregulation of subtelomeric genes and spreading of euchromatin marks into the domains.
Fft3 belongs to a highly conserved subfamily of SNF2 remodeling enzymes. Fft3 homologs are present in all eukaryotes examined, including Fun30 in S. cerevisiae, ETL1 in mouse and SMARCAD1 in humans [15,16]. Fun30 subfamily enzymes act in regulating chromatin, maintaining silent chromatin domains and preserving genome stability [14,16–22].
Here, we explore the interplay between genome organization and transcriptional regulation. We show that Fft3 maintains chromatin structure and peripheral positioning of subtelomeres by binding to and remodeling nucleosomes at their borders. In addition to the subtelomeres, Fft3 interacts with TFIIIC and Pol III at tRNA genes and affects nucleosome positioning and interaction with the nuclear periphery. We propose that Fft3 maintains nucleosome stability and peripheral positioning of these elements and thereby preserves proper genome organization.
Results
Deletion of fft3 results in altered chromatin properties and decreased nuclear envelope interactions at subtelomeres
We previously showed that subtelomeric genes are upregulated in cells lacking the chromatin remodeler Fft3 [14]. To see whether these expression changes coincide with an altered chromatin structure, we performed genome-wide ChIP-chip for three hallmarks of active chromatin: RNA polymerase II (Pol II), the histone variant H2A.Z and the histone modification H4K12Ac. All three marks show a striking increase over the subtelomeric chromatin domains on chromosomes I and II (Fig. 1A) in fft3Δ cells compared to wild type, allowing for a chromatin structure more permissive to transcription. These increases are significant when compared with the rest of the genome (p<0.002, Fig. 1 B-D) and could be verified by ChIP qPCR (S1 Fig). Based on these observations, we conclude that Fft3 affects both expression levels and chromatin properties at the subtelomeres.
Fig. 1. Chromatin changes in fft3Δ cells are pronounced at the subtelomeres of chromosomes I and II. 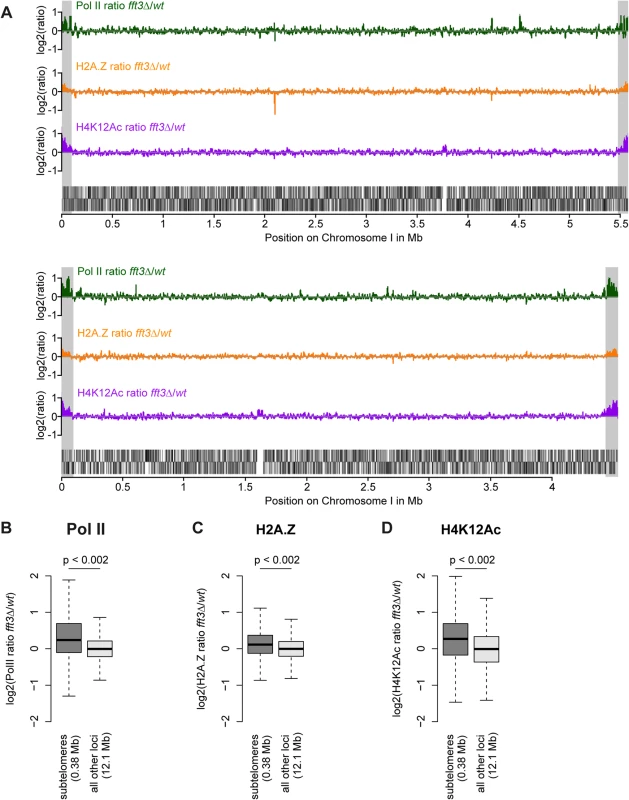
(A) Subtelomeric regions show strong changes in Pol II and H2A.Z occupancy, as well as H4K12Ac levels. H2A.Z and H4K12Ac ChIP-chip data from [14]. Subtelomeric regions are marked in gray. (B-D) Pol II, H2A.Z and H4K12Ac are significantly enriched over subtelomeric regions of chromosomes I and II in fft3Δ cells compared to wild type cells. All data is shown as boxplot, with p-values calculated by circular permutation test (see Materials and Methods).
When we mapped interactions between the INM protein Man1 and chromatin, we observed that subtelomeres are strongly enriched for Man1 [7]. We hypothesized that the expression and chromatin changes in fft3Δ cells might be accompanied by an altered nuclear organization. Indeed, DamID mapping revealed that Man1 interactions with the subtelomeres are strongly reduced in fft3Δ cells (Fig. 2A-C, S2 Fig). Interestingly, Fft3 itself does not bind the subtelomeric domains, only their borders (Fig. 2A,D).
Fig. 2. Chromatin changes at subtelomeres in fft3Δ cells coincide with changes in nuclear organization. 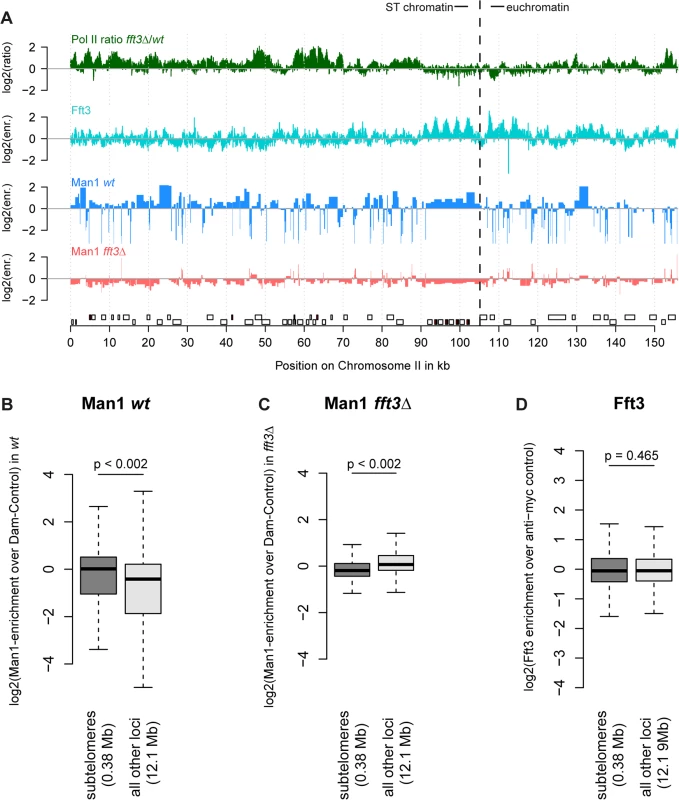
(A) Association with the nuclear envelope in wild type is lost in fft3Δ cells. Shown over the left subtelomere of chromosome II are log2-ratios of Pol II enrichment in fft3Δ vs. wt, log2-enrichment of Fft3-myc ChIP over anti-myc control ChIP, log2-enrichment Man1-Dam over the Dam only control in wild type, log2-enrichment Man1-Dam over Dam only control in fft3Δ cells. (B) Subtelomeric chromatin is associated with the nuclear periphery in wild type cells. Man1-Dam enrichment over Dam-only control is shown as boxplot. (C) Association of the subtelomeres with the nuclear periphery is lost in fft3Δ cells. Man1-Dam enrichment over Dam-only control is shown as boxplot. (D) Fft3 is not enriched at subtelomeres. Fft3-enrichment over anti-myc control is shown as boxplot. Taken together, these results suggest that deleting fft3 leads to drastic changes in chromatin composition and positioning of these large domains while interacting only with the domain borders.
Fft3 and Bqt4 cooperate in anchoring subtelomeres to the nuclear envelope
Our data suggest that Man1 and Fft3 have essential roles in tethering subtelomeres to the NE. To explore this further, we monitored the intranuclear position of the telomeres in live cells using the telomere associated protein Taz1. Based on their relative distance to the nuclear envelope, the Taz1 signals in each cell were assigned to one of three zones of equal volume (Fig. 3A). As expected, about 75% of telomere signals are localized in the outermost zone, close to the nuclear envelope, in wild type cells (Fig. 3B). We observed no change in telomere localization in fft3Δ cells (Fig. 3B), suggesting that the telomeres themselves are unaffected by the changes in the subtelomeres. The anchoring of telomeres to NE has been shown to depend on the Bqt4 protein [23]. Indeed, we observed that deleting bqt4 reduced the number of telomeres in zone 1 to below 60%, with an increased number of cells showing telomere signals in the inner zones (Fig. 3C). The loss of peripheral localization is exacerbated in cells lacking both Bqt4 and Fft3 (Fig. 3D-E), with more than 50% of the cells now showing telomere signals in the two innermost zones. We obtained similar results using fluorescence in situ hybridization (FISH, S3 Fig). Signals from a subtelomeric FISH probe showed a modest shift towards the interior in fft3Δ cells compared to wildtype. In fft3Δ bqt4Δ cells, the majority of FISH signals were found in the two innermost zones, while deletion of bqt4 by itself had no effect on localization. Taken together, these results demonstrate that Bqt4 and Fft3 work together in anchoring the subtelomeres to the nuclear envelope, with Bqt4 attaching the telomere end and Fft3 preserving the interaction with Man1 over the subtelomeres.
Fig. 3. Fft3 and Bqt4 anchor subtelomeres at the nuclear envelope. 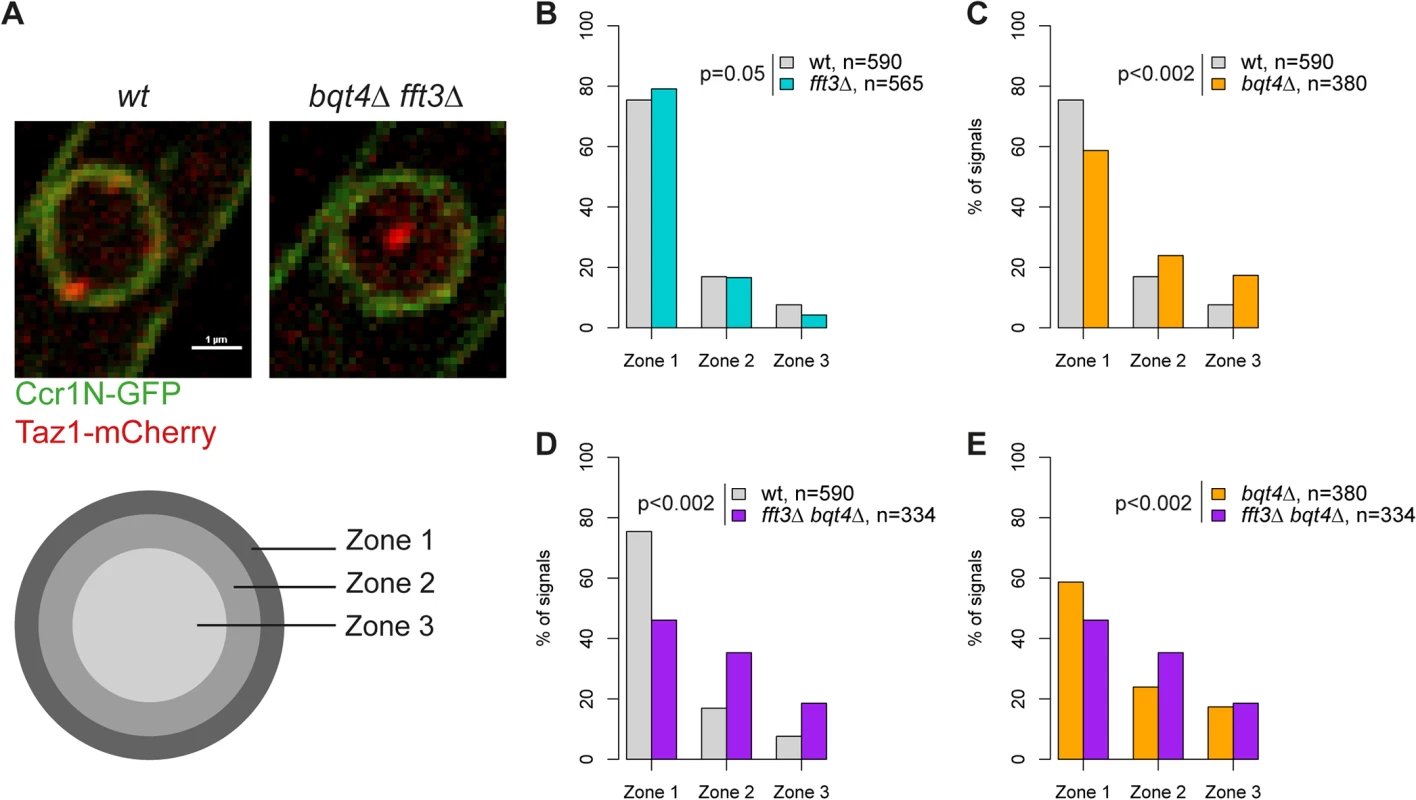
(A) Live cell microscopy examples for wild type and bqt4Δ fft3Δ cells. The nuclear envelope is stained by Ccr1N-GFP (green) and the telomeres by Taz1-mCherry (red). Cells were scored as belonging to either of three equal volume zones depending on the distance between the nuclear envelope and the telomere signal. (B-E) Telomeres are released from the nuclear envelope in bqt4Δ cells, but move even further into the nuclear interior in bqt4Δ fft3Δ cells, where subtelomeres lose association with the nuclear envelope. Percentages of cells in each of the zones in different strains are shown, with number of cells measured in the legend. Significance testing was done using a two-sided Chi-square test. Fft3 interacts with solo LTRs at subtelomeric borders and genome-wide
We next asked what marks the subtelomeric borders aside from Fft3 binding. Interestingly, all four borders feature long terminal repeat elements (LTRs) either at the Fft3 binding sites or in close proximity (Fig. 4A). Assuming that this is a sequence feature that Fft3 recognizes, we looked at all LTRs in the S. pombe genome and found that Fft3 is strongly enriched over these elements genome-wide (Fig. 4B). To study how Fft3 affects nucleosome positioning and occupancy, we performed micrococcal nuclease digestion followed by sequencing (MNase-seq). Interestingly, we observed a significant decrease in nucleosome occupancy over LTR elements in fft3Δ cells compared to wild type (Fig. 4C). This suggests that Fft3 is required for either positioning or maintaining a nucleosome over LTRs.
Fig. 4. Fft3 binds LTR elements at subtelomeric borders and genome-wide, affecting nucleosome occupancy and peripheral positioning. 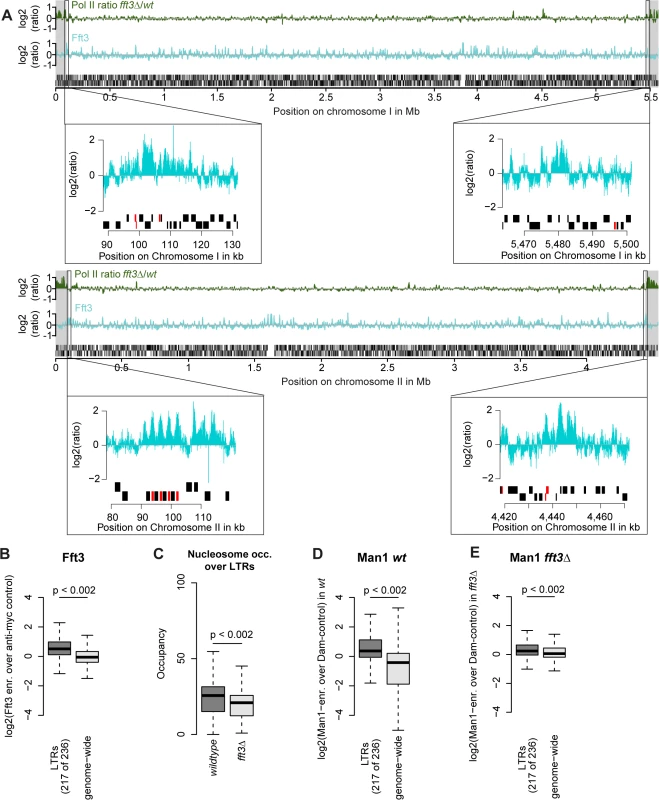
(A) Fft3 binding at subtelomeric borders occurs over or in close proximity to LTR elements. Graphs show Pol II enrichment in fft3Δ compared to wild type (green) and Fft3 enrichment compared to anti-myc control (blue) over chromosomes I and II. Subtelomeric regions are marked in gray and small panels show Fft3 binding at subtelomeric borders, with LTR elements marked in red. (B) Fft3 interacts with LTRs genome-wide. Fft3-enrichment over anti-myc control is shown as boxplot. (C) Nucleosome occupancy at LTRs is reduced in fft3Δ cells. The average number of reads mapping to each LTR is shown for wild type and fft3Δ. P-value was obtained using paired, two-sided Mann-Whitney U test. (D) LTRs interact with Man1 at the nuclear periphery. Man1-Dam enrichment over Dam-only control is shown as boxplot. (E) Interaction with Man1 is reduced in fft3Δ cells. Man1-Dam enrichment over Dam-only control is shown as boxplot. Since Fft3 alters interactions with the nuclear envelope at subtelomeres, we wondered whether LTRs are affected similarly. In wild type cells, LTR elements are enriched for binding of both Man1 and the nucleoporin Nup85 [24] (Fig. 4D, S4A Fig). Binding for both is slightly reduced when Fft3 is deleted (Fig. 4E, S4B Fig), but still higher than at other loci in the genome in the case of Man1. When we looked at the LTRs in the border of subtelomere IIL specifically, we observed a marked decrease in Man1 association (S4C Fig), suggesting that LTRs in the subtelomeric borders lose their interaction with the nuclear envelope. We wondered whether inserting an LTR into a locus is sufficient to recruit Fft3, but found no increase in Fft3-myc occupancy by ChIP adjacent to the inserted LTR (S4D Fig). This argues against a sequence-specific recognition of LTRs by Fft3 and rather suggests that other factors such as local chromatin structure are necessary for recruitment. In summary, we conclude that Fft3 binds to LTR elements, affecting their nucleosome occupancy and—in part—their peripheral positioning.
Catalytic activity of Fft3 is required for subtelomeric silencing
This raises the question how Fft3 affects chromatin structure at subtelomeric borders. MNase-seq data shows several changes in nucleosome occupancy close to the Fft3 binding sites at the borders (Fig. 5A, S5 Fig). In most cases, nucleosome occupancy was reduced in fft3Δ cells compared to wild type, suggesting that loss of Fft3 leads to reduced nucleosome stability in these regions. Based on these observations, we hypothesized that the catalytic activity of Fft3 is essential for its functions in subtelomeric chromatin regulation. We therefore constructed a variant of Fft3 with a point mutation in the ATPase domain (fft3-K418R, Fig. 5B), which is known to lead to loss of enzymatic activity [25,26].
Fig. 5. Subtelomeric silencing requires remodeling activity of Fft3. 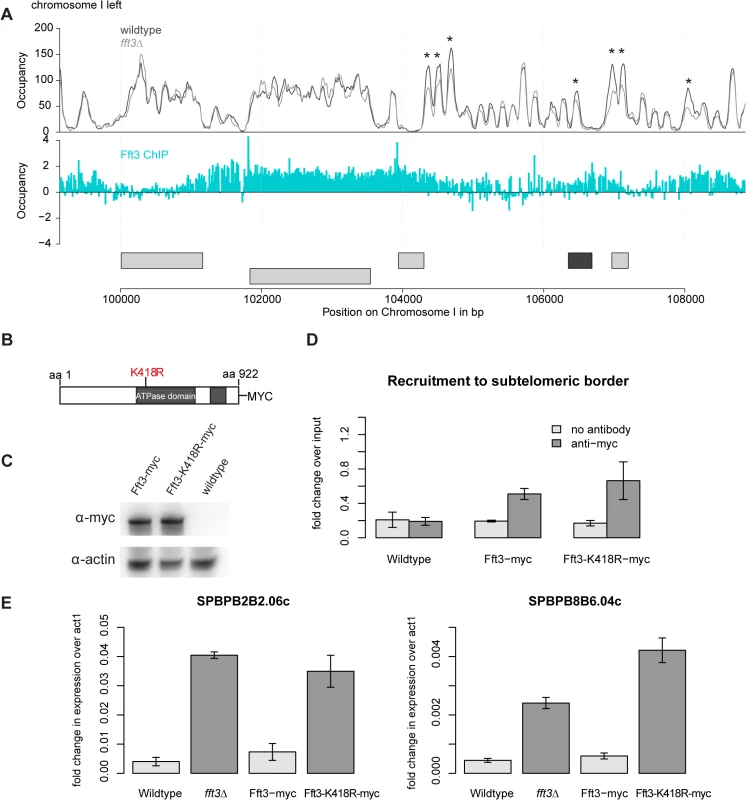
(A) Nucleosome occupancy at subtelomeric borders is affected by Fft3. Map shows nucleosome occupancy profiles over the border of the left subtelomere on chromosome I (top panel) and Fft3 enrichment (blue). Genes are shown in light grey, LTRs in dark grey. Asterisks mark positions were nucleosome occupancy is reduced. (B) Schematic representation of the Fft3 ATPase mutant containing a point mutation in the ATPase domain that replaces lysine at position 418 (AAA) by arginine (AGA) and a C-terminal Myc-tag. The split ATPase domain is highlighted in grey [16]. (C) The Fft3 ATPase mutant (fft3-K418R) is expressed at similar levels as the wild type protein (Fft3-myc). Whole-cell lysate was immunoblotted with anti-myc antibodies. A non-tagged wild type strain was included as a control for the specificity of the antibody and actin blot confirms equal loading. (D) The Fft3 ATPase mutant is recruited to the LTR elements of the subtelomeric border. Data from duplicate ChIP-qPCR of non-tagged wild type, Fft3-myc and Fft3-K418R-myc is shown as fold difference in enrichment at LTR over dg, normalized to input. Error bars represent the standard deviation. (E) The Fft3 ATPase mutant shows an upregulation of subtelomeric genes. Total RNA was extracted in duplicate and reversely transcribed into cDNA. The expression levels of two subtelomeric genes were measured with qPCR. Error bars represent the standard deviation. The resulting catalytically inactive variant of Fft3 is expressed at similar levels (Fig. 5C) and recruited to the same targets as unmodified Fft3 (Fig. 5D, S6A–C Fig). However, fft3-K418R cells mimic the phenotype observed in fft3Δcells: they grow slightly slower than wild type cells at 30°C and show severe growth defects at 25°C and 37°C (S6D Fig). Importantly, subtelomeric genes are upregulated in cells carrying the point mutation in Fft3 (Fig. 5E), as observed in cells lacking Fft3. Furthermore, we observed similar increases in PolII, H3K9Ac and H2A.Z occupancy in fft3-K418R and fft3Δcells (S7A–C Fig). As in fft3Δ cells, Man1-interaction is reduced in fft3-K418R cells compared to wild-type (S7D Fig). Together, these results strongly suggest that ATP-dependent remodeling by Fft3 is directly required to maintain silencing of subtelomeric genes and chromatin structure at the boundaries to the subtelomeres.
Fft3 colocalizes with the Pol III machinery and TFIIIC
After observing the function of Fft3 at subtelomeres and LTR, we set out to explore whether Fft3 also plays a role in regulating chromatin structure elsewhere in the genome. Among other features, we found a preference of Fft3 for snRNA genes, snoRNA genes and replication origins (S8A Fig). Most prominently, we observed a significant enrichment of Fft3 at tRNA genes (Fig. 6A) compared to the rest of the genome. tRNA genes are transcribed by the RNA polymerase III (Pol III) machinery and require the transcription factor TFIIIC for recruitment of Pol III (reviewed in [27]). We also observed Fft3 enrichment at 5S rRNA genes, which are transcribed by Pol III (S8B Fig). When we compared the Fft3 binding profile to Pol III and TFIIIC maps [28], we found a strong overlap in binding sites (Fig. 6B). Several reports have identified loci called ETC (Extra-TFIIIC) or COC (chromosome-organizing clamps) sites across the genome that are occupied by TFIIIC but not by any other components of the Pol III machinery [28–30]. While Fft3 binding coincides with Pol III binding, we did not observe enrichment of Fft3 at ETC/COC sites (Fig. 6C). This suggests that Fft3 binds to actively transcribed Pol III sites.
Fig. 6. Fft3 interacts with TFIIIC at Pol III transcribed loci and affects peripheral localization of tRNA genes. 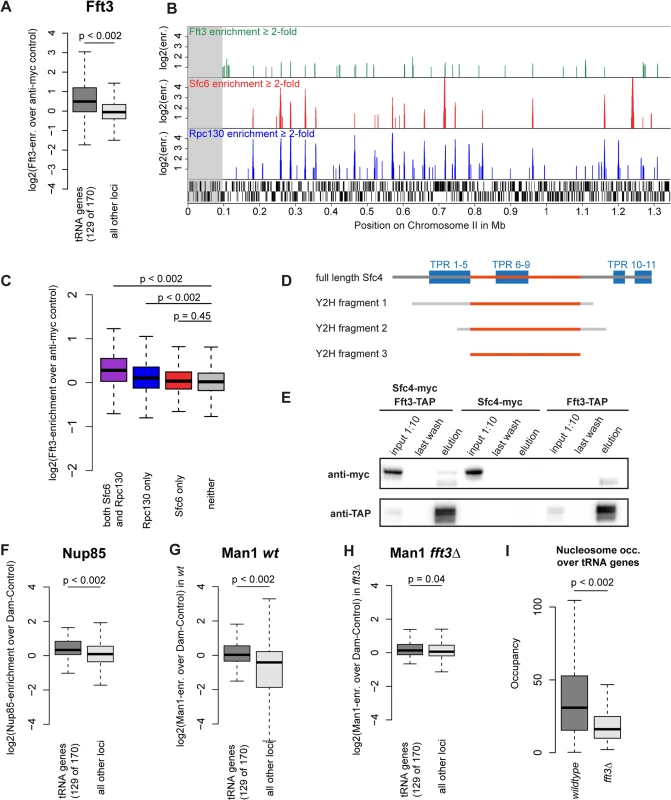
(A) Fft3 is significantly enriched at tRNA genes. Fft3-enrichment over anti-myc control is shown as boxplot. Fft3-myc ChIP-chip data from [14]. (B) Fft3-myc binds to loci also bound by the TFIIIC-subunit Sfc6 and the Pol III-component Rpc130. Shown are ChIP-enrichments over 2-fold over input on 1.5 Mb on the left arm of chromosome 2. The subtelomeric chromatin is marked in gray. Fft3-myc ChIP-chip data from [14], Sfc6 and Rpc130 ChIP-chip data from [28]. (C) Fft3 is significantly enriched at loci bound by TFIIIC and Pol III together and Pol III alone, but not at loci bound by TFIIIC alone. Fft3-enrichment over input is shown as boxplot. Targets were defined as probes with more than two-fold enrichment (both Sfc6 and Rpc130: 896 probes (53.8 kb), Rpc130 only: 1656 probes (99.3 kb), Sfc6 only: 750 probes (45 kb), neither: 37917 (2,275 kb)). (D) Fft3 and Sfc4 interact in a Yeast-2-hybrid screen. Bait fragments of Sfc4 are shown aligned to full-length Sfc4, with overlap between them marked in red. (E) Sfc4 is co-purified in a Fft3-TAP purification. Shown are input (diluted to 10%), final wash and eluate for the double-tagged strain (Sfc4-Myc Fft3-TAP) and the two single tagged strains (Sfc4-Myc and Fft3-TAP). (F) Nup85 is significantly enriched at tRNA genes. Nup85-Dam enrichment over Dam-only control is shown as boxplot. Nup85-Dam data from [24]. (G) The INM protein Man1 is enriched at tRNA genes in wild type S. pombe. Man1-Dam enrichment over Dam-only control is shown as boxplot. (H) Man1 is no longer enriched at tRNA genes in fft3Δ cells. Man1-Dam enrichment over Dam-only control in fft3Δ cells is shown as boxplot. (I) Nucleosome occupancy at tRNA genes is reduced in fft3Δ cells. The average number of reads mapping to each tRNA is shown for wild type and fft3Δ. P-value was obtained using paired, two-sided Mann-Whitney U test. To search for interaction partners of Fft3, we carried out a yeast two-hybrid screen using the full-length Fft3 protein as the bait against an S. pombe cDNA library. This screen identified several cDNAs encoding Sfc4 (Fig. 6D), a subunit of TFIIIC and homologous to S. cerevisiae Tfc4p and human TFIIIC102 [31]. The clones cover a set of tetratricopeptide repeats (TPR) which function as sites for protein-protein interactions [32], suggesting that Fft3 could interact with Sfc4 through this domain. To examine whether Fft3 and Sfc4 also interact in vivo, we constructed a double-tagged strain (Fft3-TAP / Sfc4-Myc) and performed a co-immunoprecipitation assay. We observed an enrichment of Sfc4-Myc in the anti-TAP purified material from the double-tagged strain compared to the single-tagged strain (Fig. 6E), confirming that Fft3 and Sfc4 physically interact in vivo. Taken together, our data indicate that the Fft3 chromatin remodeler directly interacts with the TFIIIC transcription complex at Pol III transcribed loci.
Fft3 affects peripheral positioning and nucleosome occupancy of tRNA genes
Although tRNA genes are dispersed throughout the fission yeast genome, most of them cluster into a few foci in close proximity to centromeres, facilitated by condensin [33,34]. We found condensin binding strongly to tRNA genes both in wild type cells and fft3Δ cells (S9 Fig), suggesting that clustering is unaffected the absence of Fft3. Since the tRNA genes cluster close to the centromeres and therefore the nuclear periphery [34], we asked whether their nuclear positioning changes in fft3Δ cells. While tRNA genes are enriched for Nup85 and Man1 interactions in wild type (Fig. 6F-G), we observed a reduction of Man1 association in fft3Δ cells (Fig. 6H). This suggests that tRNAs move away from the nuclear envelope in the absence of Fft3. Interestingly, this effect seems to be specific to tRNA genes, since the 5S rRNA genes stay enriched for Man1 association when Fft3 is deleted (S8B–D Fig).
We then wondered if there are further effects at tRNA genes in fft3Δ cells. Therefore, we examined expression levels of two tRNA classes, proline and alanine, by northern blot and RT-qPCR (S10A–C Fig). We observed an increase in transcription for proline, but not for alanine, suggesting there is no clear-cut effect of Fft3 on tRNA transcription. A clearer trend emerged when we looked at MNase sequencing data: like LTRs, tRNA genes show a pronounced decrease in nucleosome occupancy (Fig. 6I). Similarly, 5S rRNA genes also showed a decrease in nucleosome occupancy (S6E Fig). Together, these data indicate that Fft3 is involved in maintaining chromatin structure and intra-nuclear positioning of Pol III transcribed genes.
Discussion
It has been established in different eukaryotic systems that the periphery of the cell nucleus harbors chromosomal domains with transcriptionally repressed chromatin. Insulators contribute to this three-dimensional nuclear organization by forming clusters to separate different chromatin domains [35].
Here we show that cells lacking the Fft3 remodeling enzyme or carrying a catalytically inactive version display drastic changes in chromatin marks and gene expression in the subtelomeric regions (Fig. 7A). Fft3 is required to ensure the association of these domains with the nuclear envelope through the LEM domain protein Man1. The budding yeast homolog of Man1, Heh1p, is involved in regulating silencing of rDNA repeats [36]. Also in budding yeast, subtelomeres are associated with the INM protein Src1p [37] and subtelomeric genes change in expression when the Fft3 homolog Fun30 is missing [38]. Our data highlights that Fft3—even though itself not enriched at the subtelomeres per se—affects gene expression in these domains by acting on their borders. We show that local chromatin structure at the borders is altered in fft3Δ cells. This agrees with observations on the action of other chromatin remodelers at insulators, such as incorporation of H3.3 by PBAP at border elements in fruit flies [39] and binding of ISWI to ArsI insulators in sea urchins [40].
Fig. 7. Proposed mechanism of how Fft3 regulates subtelomeric chromatin and tRNA positioning. 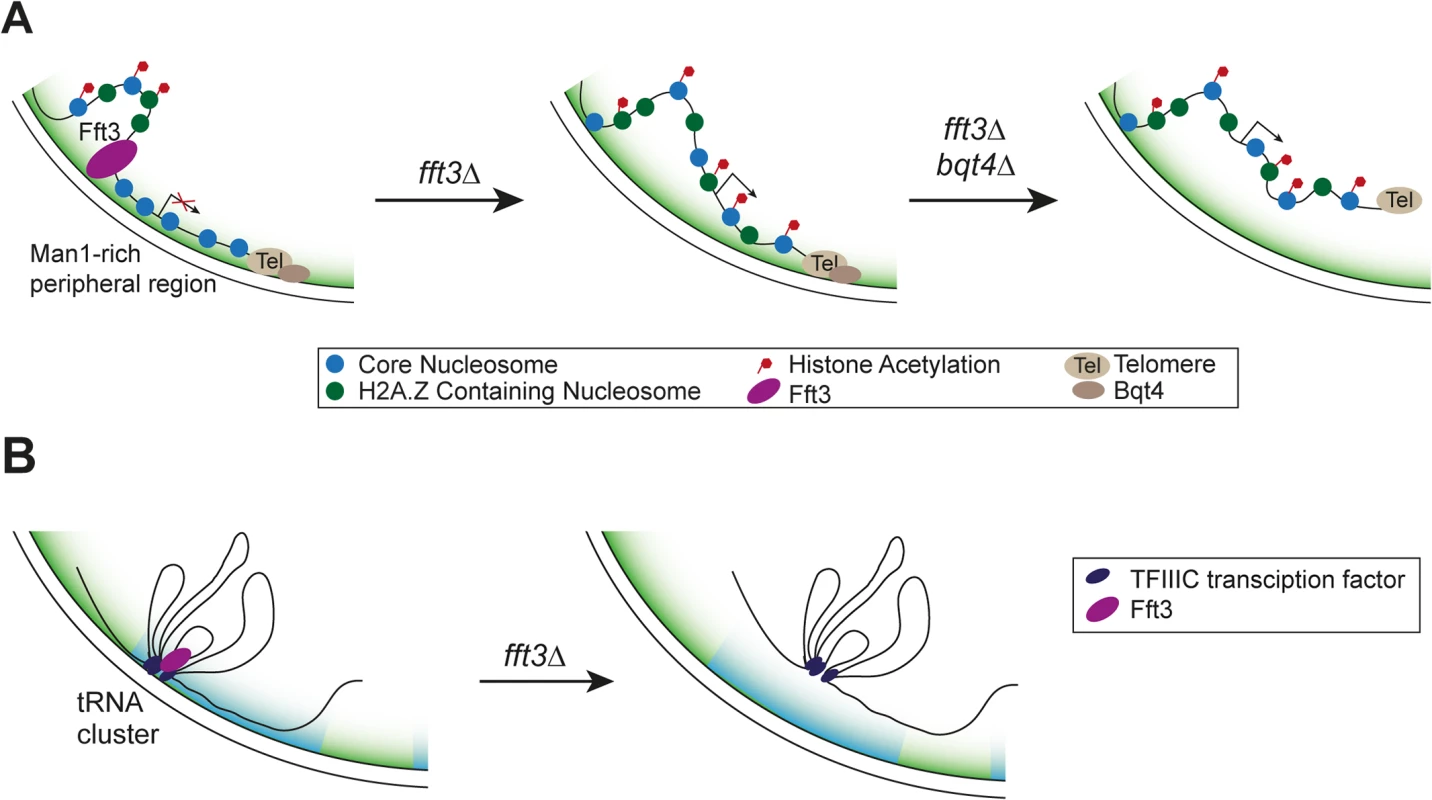
(A) Fft3 maintains subtelomeric chromatin through binding and remodeling activity at boundary elements. In the absence of Fft3, active chromatin marks and transcription in the subtelomeric domain increase and the domain is released from the nuclear periphery. When Bqt4 is deleted in addition to Fft3, the entire telomere can move away from the nuclear envelope towards the interior. (B) tRNA clusters at the nuclear envelope dissociate from the periphery in fft3Δ cells and lose their transcriptional regulation. The borders of subtelomeres in S. pombe coincide with retrotransposon-derived LTR elements. In mouse cells, LTR elements can function as insulators [41]. Furthermore, the well-characterized gypsy insulator in Drosophila is derived from the gypsy retrotransposon (reviewed in [42]). The Tf2 retrotransposons in fission yeast cluster within the nucleus to form Tf-bodies, and de-cluster in response to oxidative stress [43]. Our results show that Fft3 binds at or near LTR elements and affects their nucleosome occupancy and position in the nucleus. Taken together, these findings point to a conserved role for transposable elements in genome organization and insulation.
It has been suggested that insulator elements function by changing higher-order chromatin structure (reviewed in [44]). Insulators can interact with each other and tether the chromatin fiber to structural elements within the nucleus, e.g. nuclear pores. In this way, chromatin loops can form which separate euchromatin and heterochromatin domains. In S. pombe, Tf2 transposons cluster into Tf bodies that play a role in genome organization [43]. Furthermore, the Drosophila gypsy insulators form specialized insulator bodies at the nuclear periphery, and transposon-derived MAR/SAR sequences can create chromatin loops [42,45]. In agreement with this, we found that S. pombe LTR elements localize close to the nuclear periphery and nuclear pores. It is possible that this anchoring of LTR elements helps to divide loops of chromatin with different properties, such as the subtelomeric chromatin and neighboring euchromatin.
In addition to LTR elements, Fft3 binds to tRNA genes and physically interacts with the Pol III transcription factor TFIIIC. These features may be conserved in the Fun30 remodeling family, since the S. cerevisiae homolog, Fun30, is enriched over tRNA genes and the human homolog, SMARCAD1, purifies together with the TFIIIC complex [16,17,21]. Based on our findings in S. pombe it is plausible that budding yeast and human Fun30 homologues also directly interact with TFIIIC. Like in S. pombe, tRNA genes function as insulators in other eukaryotes and play a role in genome organization by clustering at specific sites in the nucleus (reviewed in [46]). It is noteworthy that both the insulating function and clustering of tRNA genes in yeast depend on TFIIIC [46]. It is therefore likely that Fft3 is recruited to tRNA genes through its interaction with TFIIIC and maintains the chromatin state at these insulators. Fft3 does not appear to be involved in condensin recruitment to tRNA genes, suggesting that condensin-dependent clustering of tRNA genes may not be affected. However, our results indicate that Fft3 is required for their anchoring at the nuclear envelope (Fig. 7B).
At the nucleosome level, we observed a reduction in nucleosome occupancy at Fft3 targets when the remodeler was missing. A similar decrease has also been observed at genes regulated by Fun30 in budding yeast [38]. This suggests that Fft3 is required to incorporate these nucleosomes or to stabilize them after incorporation. The nucleosomes could then serve either as a platform for binding or as a barrier for other proteins. Alternatively, Fft3 remodeling could affect chromatin mobility. INO80, another Snf2 remodeling factor, facilitates chromatin movement inside the budding yeast nucleus by altering the stiffness of the chromatin fiber [47]. It is conceivable that Fft3 acts in a similar manner to allow for correct positioning of its targets within the nucleus.
Beyond yeasts, subtelomeres are important in several other eukaryotic systems. In parasites such as Trypanosoma brucei and Plasmodium falciparum, the subtelomeres habor genes encoding surface markers that can be varied through recombination [48] and contribute to antigen variation. In T. brucei, these loci move from the nuclear periphery towards the nuclear interior when activated during differentiation [49]. In human cells, the D4Z4 insulator is located in the subtelomere of chromosome 4q and maintains subtelomeric heterochromatin [50]. Deletion of D4Z4 causes a type of muscular dystrophy, Facio-Scapulo-Humeral Dystrophy (FSHD). Interestingly, insulator function of D4Z4 depends on CTCF and lamin A, and is involved in peripheral positioning of the telomere [51,52]. Taken together, these studies highlight the importance of links between subtelomeric chromatin states and nuclear positioning.
In this study, we provide an example how a chromatin remodeler affects both local chromatin structure and genome-wide nuclear organization. Further studies will be necessary to shed light on this interplay between nuclear architecture and transcriptional regulation that seems to have a major role genome function in eukaryotes.
Materials and Methods
Strain construction and cell culture
S. pombe cells were grown at 30°C and in YES medium unless stated otherwise. The S. pombe strains used in this study are listed in S1 Table. The point mutation in the ATPase domain of Fft3 was created using PCR with a primer containing the mutation. Genomic DNA was isolated from the Fft3-myc::hph strain (Hu1911) and part of the gene, the myc-tag, the hygromycin resistance gene and part of the 3’UTR were amplified by PCR using primers ATPase-F and ATPase-R (see S2 Table). The ATPase-F primer contained a mismatch introducing the K418R substitution (AAA to AGA). To improve the recombination efficiency, the fragment was elongated by a second PCR using ATPaseL-F and ATPase-R primers. The PCR product was then purified and electroporated into a wild type strain (Hu0029). Strains in which the construct had replaced the wild type fft3+ gene through homologous recombination were selected by hygromycin resistance and confirmed by DNA sequencing.
Chromatin immunoprecipitation
DNA was immunoprecipitated as described earlier [53] using 2μl of anti-H4K12Ac (ab1761, abcam), 2μl of anti-myc (9E10, Sigma), 1 μl of anti-GFP (ab290, abcam), or 3μl anti-RNA polymerase II CTD repeat (ab5408, abcam) antibodies per 100μl chromatin extracts.
For microarray hybridization, immunoprecipitated DNA was amplified to 5 μg DNA as described in [53], with the exception that in the second PCR, 5 mM dUTP was added to the reaction. Fragmentation, labeling and hybridization to the Affymetrix GeneChip S. pombe Tiling 1.0FR was performed by the Affymetrix core facility at Novum (http://apt.bea.ki.se) according to Affymetrix standard protocols.
All experiments were done as biological duplicates.
For real-time quantitative PCR, immunoprecipitated DNA was amplified in the presence of SYBR Green using Applied Biosystems 7500 real-time PCR machine. The primers used are listed in S2 Table.
DamID
Genomic DNA was extracted as described previously [7], with an additional clean-up of genomic DNA using the DNeasy Blood and Tissue Kit (Qiagen). DamID experiments were done as described by [54]. The amplified material was cleaned using the Qiagen PCR purification kit and fragmented with DNAse. Fragmented DNA was labeled and hybridized to the Affymetrix GeneChip S. pombe Tiling 1.0FR using standard protocols by the Affymetrix core facility at Novum (http://apt.bea.ki.se).
All experiments were done as biological duplicates.
RNA extraction
RNA was extracted using the hot phenol method as described in [55] and reverse transcribed using random hexamers and the SuperScript II Reverse Transcriptase kit (Invitrogen). Real-time quantitative PCR was performed in the presence of SYBR Green using an Applied Biosystems 7500 real-time PCR machine. Primers used are listed in S2 Table.
All experiments were done as biological duplicates.
Fluorescence in-situ hybridization
Cells were grown to early log phase at 30°C in YES medium, fixed with adding paraformaldehyde to 1,75% for 40 min and processed for IF with mouse monoclonal anti-myc antibodies (9E10, Sigma, 4μg/ml). For FISH, cells were refixed with 3% paraformaldehyde for 30min at room temperature. Chromosomal DNA was denatured by successive 15 min incubation time in 2SSC, 2SSC 10% formamide, 2SSC 20% formamide and 2SCC 40% formamide at room temperature. Subtelomeric FISH probes were generated using the sequencing cosmid c186 (provided by the Wellcome Trust Sanger Institute, Hinxton, UK) as template. c186 spans 30176bp on chr1R (Range 5524764 to 5554939), from SPNCRNA.283 to SPAC186.09.
Images were taken using a Nikon A1+ laser scanning microscope with a 60x Lambda S oil-immersion objective (NA 1.4). Imaging was set up fulfilling the Nyquist criterion in xy and z, with a minimum zoom of 2. For each cell, z-stacks were acquired with 0.2μm spacing and subjected to a blind 3D deconvolution algorithm using the NIS Elements Advanced Research software version 4.12. After decapping (removing the top three and bottom three z-stacks) of each nucleus, distances from the center of each FISH spot to the nuclear periphery were measured. Measurements were then assigned to three concentric equal volume zones [56]. Significance tests were carried out using the Chi-square test.
Live cell microscopy
Cells were grown over night at 30°C in PMG liquid medium. For mounting, 35 mm glass bottomed culture dishes (MatTek Corp) were coated with soy lectin (Sigma). A log phase cell suspension was added on top of the coated surface and cells were left to sediment for five minutes before pre-warmed PMG medium was added on top. During imaging, the culture dish was kept at 30°C inside the microscope incubator and cells were imaged for no longer than three hours.
Images were taken using an inverted Nikon A1R laser scanning microscope with a 60x Lambda S oil-immersion objective (NA 1.4). Imaging was set up fulfilling the Nyquist criterion in xy and z, with a minimum zoom of 2. For each nucleus, z-stacks were acquired with 0.125μm spacing in a 5 μm range around the center of the nucleus. Deconvolution of each stack was carried out using a 3D non-blind algorithm in NIS Elements (Nikon, High Content Analysis software version 4.20). After decapping (removing the top three and bottom three z-stacks of the nuclear volume) of each nucleus, distances from the center of each mCherry spot to the nuclear periphery were measured in the z-plane with the brightest signal. The radius of each nucleus was measured and used to normalize the distance measurement. Normalized distances were then assigned to one of three concentric equal volume zone [56]. Significance tests were carried out using a two-sided Chi-square test.
Micrococcal nuclease digestion and sequencing
Mononucleosomal DNA fragments were digested and purified as described in [57]. Fragments were then amplified and labeled with the NEBNext ChIP-Seq Library Prep Master Mix Set for Illumina (NEB #E6240) followed by sequencing on an Illumina Miseq v.3. Paired-end reads were mapped to the S. pombe genome using Bowtie2[58] with standard parameters. DANPOS [59] was then used to remove clonal reads, normalize by quantile normalization and calculate nucleosome occupancy. All calculations and visualization were carried out using R. All experiments were done as biological duplicates. MNAse-seq data can be accessed at NCBI GEO under the accession number GSE58013.
Protein extraction and western blot
Proteins were extracted using a FastPrep-24 machine (MP Biomedicals) and separated by SDS PAGE. Immunoblot analysis was carried out using anti-myc (9E10, Sigma) and anti-actin (ab8224, abcam) antibodies.
Yeast-2-hybrid assay
The yeast-2-hybrid screen and data analysis were performed by Hybrigenics, Paris, France (www.hybrigenics.com). The full-length open-reading frame of the fft3 gene (SPAC25A8.01c) was cloned into the pB27 vector as a C-terminal fusion to LexA. The construct was use as bait to screen a S. pombe cDNA library. 62.4 million interactions were tested.
Co-immunoprecipitation
50 ml of log phase growing cells were pelleted and resuspended in 500μl lysis buffer (20 mM Tris pH7.5, 150mM NaCl, 3% glycerol, 0.05% Igepal, 0.5mM EDTA, 0.5 mM DTT and protease inhibitors) and lysed in a FastPrep machine (5x30sec at 6.5) with 1 volume of glass beads. The extract was centrifuged for 20 min, 14,000 rpm at 4°C, and the supernatant was incubated with 60μl 50% IgG-bead slurry for 40 min at 4°C. The beads were washed five times with lysis buffer. Bound proteins were eluted with 30μl NuPAGE LDS Sample Buffer (Invitrogen) at 70°C for 10 min and subjected to immunoblot analysis using anti-myc antibody (9E10, Sigma). The membrane was then stripped and incubated with anti-TAP antibody (CAB1001, Thermo Scientific)
Northern blots
2.5ug of total RNA was electrophoresed in 10% polyacrylamide, 8M urea, 0.5x TBE gel and blotted onto Hybond Nx membrane (GE Healthcare) in 0.3x TBE using a semi dry blotter, followed by UV crosslinking. Oligonucleotide probes were 32P labelled using T4 PNK (USB Affymetrix) and hybridized in 5x Denhardt’s solution, 6xSSC, 10mM EDTA, 0.5% SDS, 0.1mg/ml salmon sperm DNA (Invitrogen) at respective temperatures for 16 hours. The blots were washed 3 times in 2xSSC 0.1% SDS and exposed to Phosphorimager screens (Fuji). Screens were scanned using Molecular Imager FX (BioRad). Band intensities were quantified using the Quantity One software (BioRad). Probe sequences are listed in S2 Table.
Data analysis
All analysis was performed in R (http://www.r-project.org) using the Bioconductor (http://www.bioconductor.org) packages “affy”, “affxparser” and “preprocessCore” with standard parameters. CEL-files were imported and normalized as described in [7]. For visualization, all probes were used, while only probes with one match in the genome were used for calculations and significance tests.
To determine probe distributions for the subtelomeres, probes mapping to the following regions were used: 1–98950 bp and 5496300–5579133 bp on chromosome 1, 1–96400 bp and 4437300–4539804 bp on chromosome 2. For probe distributions across tRNA genes, probes were used which overlapped with sequence features marked “tRNA” in the GFF-annotation file downloaded from the Pombase website (ftp://ftp.sanger.ac.uk/pub/yeast/pombe/GFF/pombe_09052011.gff). For averaging of microarray scores across different genomic features, the “feature” column was used for all items with the following exceptions: features matching “LTRTF2”, “Tf2” (together with the feature category “protein_conding_gene”), “origin_of_replication” and “external_name = intron” were annotated as LTRs, Tf2s, replication origins and introns, respectively.
Microarray data published elsewhere was used as processed data when available or otherwise processed as described above. Boxplot were created in R using the “boxplot” function with standard parameters. Significance tests between data subsets (subtelomeric and tRNA probes vs. all other probes) were performed using a circular permutation as described in [7].
Microarray data can be accessed at NCBI GEO under the accession number GSE58013.
Supporting Information
Zdroje
1. Bermejo R, Kumar A, Foiani M (2012) Preserving the genome by regulating chromatin association with the nuclear envelope. Trends in Cell Biology 22 : 465–473. doi: 10.1016/j.tcb.2012.05.007 22771046
2. Smith OK, Aladjem MI (2014) Chromatin Structure and Replication Origins: Determinants of Chromosome Replication and Nuclear Organization. Journal of Molecular Biology. E-pub ahead of print.
3. Wendt KS, Grosveld FG (2014) Transcription in the context of the 3D nucleus. Current Opinion in Genetics & Development 25C: 62–67.
4. Pickersgill H, Kalverda B, de Wit E, Talhout W, Fornerod M, et al. (2006) Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nature Genetics 38 : 1005–1014. 16878134
5. Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, et al. (2008) Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453 : 948–951. doi: 10.1038/nature06947 18463634
6. Ikegami K, Egelhofer TA, Strome S, Lieb JD (2010) Caenorhabditis elegans chromosome arms are anchored to the nuclear membrane via discontinuous association with LEM-2. Genome Biology 11: R120. doi: 10.1186/gb-2010-11-12-r120 21176223
7. Steglich B, Filion GJ, van Steensel B, Ekwall K (2012) The inner nuclear membrane proteins Man1 and Ima1 link to two different types of chromatin at the nuclear periphery in S. pombe. Nucleus 3 : 77–87. 22156748
8. Pinheiro I, Margueron R, Shukeir N, Eisold M, Fritzsch C, et al. (2012) Prdm3 and Prdm16 are H3K9me1 methyltransferases required for mammalian heterochromatin integrity. Cell 150 : 948–960. doi: 10.1016/j.cell.2012.06.048 22939622
9. Towbin BD, González-Aguilera C, Sack R, Gaidatzis D, Kalck V, et al. (2012) Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell 150 : 934–947. doi: 10.1016/j.cell.2012.06.051 22939621
10. Zuleger N, Boyle S, Kelly DA, las Heras de JI, Lazou V, et al. (2013) Specific nuclear envelope transmembrane proteins can promote the location of chromosomes to and from the nuclear periphery. Genome Biology 14: R14. doi: 10.1186/gb-2013-14-2-r14 23414781
11. Buchanan L, Durand-Dubief M, Roguev A, Sakalar C, Wilhelm B, et al. (2009) The Schizosaccharomyces pombe JmjC-protein, Msc1, prevents H2A.Z localization in centromeric and subtelomeric chromatin domains. PLoS Genet 5: e1000726. doi: 10.1371/journal.pgen.1000726 19911051
12. Zofall M, Fischer T, Zhang K, Zhou M, Cui B, et al. (2009) Histone H2A.Z cooperates with RNAi and heterochromatin factors to suppress antisense RNAs. Nature 461 : 419–422. doi: 10.1038/nature08321 19693008
13. Mata J, Lyne R, Burns G, Bähler J (2002) The transcriptional program of meiosis and sporulation in fission yeast. Nature Genetics 32 : 143–147. 12161753
14. Strålfors A, Walfridsson J, Bhuiyan H, Ekwall K (2011) The FUN30 chromatin remodeler, Fft3, protects centromeric and subtelomeric domains from euchromatin formation. PLoS Genet 7: e1001334. doi: 10.1371/journal.pgen.1001334 21437270
15. Flaus A, Martin DMA, Barton GJ, Owen-Hughes T (2006) Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Research 34 : 2887–2905. 16738128
16. Neves-Costa A, Will WR, Vetter AT, Miller JR, Varga-Weisz P (2009) The SNF2-family member Fun30 promotes gene silencing in heterochromatic loci. PLoS ONE 4: e8111. doi: 10.1371/journal.pone.0008111 19956593
17. Rowbotham SP, Barki L, Neves-Costa A, Santos F, Dean W, et al. (2011) Maintenance of silent chromatin through replication requires SWI/SNF-like chromatin remodeler SMARCAD1. Molecular Cell 42 : 285–296. doi: 10.1016/j.molcel.2011.02.036 21549307
18. Yu Q, Zhang X, Bi X (2011) Roles of chromatin remodeling factors in the formation and maintenance of heterochromatin structure. Journal of Biological Chemistry 286 : 14659–14669. doi: 10.1074/jbc.M110.183269 21388962
19. Chen X, Cui D, Papusha A, Zhang X, Chu C-D, et al. (2012) The Fun30 nucleosome remodeller promotes resection of DNA double-strand break ends. Nature 489 : 576–580. doi: 10.1038/nature11355 22960743
20. Costelloe T, Louge R, Tomimatsu N, Mukherjee B, Martini E, et al. (2012) The yeast Fun30 and human SMARCAD1 chromatin remodellers promote DNA end resection. Nature 489 : 581–584. doi: 10.1038/nature11353 22960744
21. Durand-Dubief M, Will WR, Petrini E, Theodorou D, Harris RR, et al. (2012) SWI/SNF-like chromatin remodeling factor Fun30 supports point centromere function in S. cerevisiae. PLoS Genet 8: e1002974. doi: 10.1371/journal.pgen.1002974 23028372
22. Eapen VV, Sugawara N, Tsabar M, Wu W-H, Haber JE (2012) The Saccharomyces cerevisiae chromatin remodeler Fun30 regulates DNA end resection and checkpoint deactivation. Molecular and Cellular Biology 32 : 4727–4740. doi: 10.1128/MCB.00566-12 23007155
23. Chikashige Y, Yamane M, Okamasa K, Tsutsumi C, Kojidani T, et al. (2009) Membrane proteins Bqt3 and -4 anchor telomeres to the nuclear envelope to ensure chromosomal bouquet formation. The Journal of Cell Biology 187 : 413–427. doi: 10.1083/jcb.200902122 19948484
24. Woolcock KJ, Stunnenberg R, Gaidatzis D, Hotz H-R, Emmerth S, et al. (2012) RNAi keeps Atf1-bound stress response genes in check at nuclear pores. Genes & Development 26 : 683–692.
25. Laurent BC, Treich I, Carlson M (1993) The yeast SNF2/SWI2 protein has DNA-stimulated ATPase activity required for transcriptional activation. Genes & Development 7 : 583–591.
26. Corona DF, Längst G, Clapier CR, Bonte EJ, Ferrari S, et al. (1999) ISWI is an ATP-dependent nucleosome remodeling factor. Molecular Cell 3 : 239–245. 10078206
27. Huang Y, Maraia RJ (2001) Comparison of the RNA polymerase III transcription machinery in Schizosaccharomyces pombe, Saccharomyces cerevisiae and human. Nucleic Acids Research 29 : 2675–2690. 11433012
28. Noma K-II, Cam HP, Maraia RJ, Grewal SIS (2006) A role for TFIIIC transcription factor complex in genome organization. Cell 125 : 859–872. 16751097
29. Moqtaderi Z, Struhl K (2004) Genome-wide occupancy profile of the RNA polymerase III machinery in Saccharomyces cerevisiae reveals loci with incomplete transcription complexes. Molecular and Cellular Biology 24 : 4118–4127. 15121834
30. Moqtaderi Z, Wang J, Raha D, White RJ, Snyder M, et al. (2010) Genomic binding profiles of functionally distinct RNA polymerase III transcription complexes in human cells. Nat Struct Mol Biol 17 : 635–640. doi: 10.1038/nsmb.1794 20418883
31. Huang Y, Hamada M, Maraia RJ (2000) Isolation and cloning of four subunits of a fission yeast TFIIIC complex that includes an ortholog of the human regulatory protein TFIIICbeta. J Biol Chem 275 : 31480–31487. 10906331
32. Dumay-Odelot H, Acker J, Arrebola R, Sentenac A, Marck C (2002) Multiple roles of the tau131 subunit of yeast transcription factor IIIC (TFIIIC) in TFIIIB assembly. Molecular and Cellular Biology 22 : 298–308. 11739742
33. Haeusler RA, Pratt-Hyatt M, Good PD, Gipson TA, Engelke DR (2008) Clustering of yeast tRNA genes is mediated by specific association of condensin with tRNA gene transcription complexes. Genes & Development 22 : 2204–2214.
34. Iwasaki O, Tanaka A, Tanizawa H, Grewal SIS, Noma K-II (2010) Centromeric localization of dispersed Pol III genes in fission yeast. Molecular Biology of the Cell 21 : 254–265. doi: 10.1091/mbc.E09-09-0790 19910488
35. Van Bortle K, Corces VG (2013) The role of chromatin insulators in nuclear architecture and genome function. Current Opinion in Genetics & Development.
36. Mekhail K, Seebacher J, Gygi SP, Moazed D (2008) Role for perinuclear chromosome tethering in maintenance of genome stability. Nature 456 : 667–670. doi: 10.1038/nature07460 18997772
37. Grund SE, Fischer T, Cabal GG, Antúnez O, Pérez-Ortín JE, et al. (2008) The inner nuclear membrane protein Src1 associates with subtelomeric genes and alters their regulated gene expression. The Journal of Cell Biology 182 : 897–910. doi: 10.1083/jcb.200803098 18762579
38. Byeon B, Wang W, Barski A, Ranallo RT, Bao K, et al. (2013) The ATP-dependent chromatin remodeling enzyme Fun30 represses transcription by sliding promoter-proximal nucleosomes. Journal of Biological Chemistry 288 : 23182–23193. doi: 10.1074/jbc.M113.471979 23779104
39. Nakayama T, Shimojima T, Hirose S (2012) The PBAP remodeling complex is required for histone H3.3 replacement at chromatin boundaries and for boundary functions. Development 139 : 4582–4590. doi: 10.1242/dev.083246 23136390
40. Yajima M, Fairbrother WG, Wessel GM (2012) ISWI contributes to ArsI insulator function in development of the sea urchin. Development 139 : 3613–3622. doi: 10.1242/dev.081828 22949616
41. Carabana J, Watanabe A, Hao B, Krangel MS (2011) A barrier-type insulator forms a boundary between active and inactive chromatin at the murine TCRβ locus. J Immunol 186 : 3556–3562. doi: 10.4049/jimmunol.1003164 21317385
42. Valenzuela L, Kamakaka RT (2006) Chromatin insulators. Annu Rev Genet 40 : 107–138. 16953792
43. Cam HP, Noma K-II, Ebina H, Levin HL, Grewal SIS (2008) Host genome surveillance for retrotransposons by transposon-derived proteins. Nature 451 : 431–436. 18094683
44. Strålfors A, Ekwall K (2012) Heterochromatin and Euchromatin—Organization, Boundaries, and Gene Regulation. In: Meyers RA, editor. Epigenetic Regulation and epigenomics: Advances in Moleular Biology and Medicine. Wilwy-VCH Verlag GmbH & Co. KGaA. pp. 171–189.
45. Jordan IK, Rogozin IB, Glazko GV, Koonin EV (2003) Origin of a substantial fraction of human regulatory sequences from transposable elements. Trends Genet 19 : 68–72. 12547512
46. Kirkland JG, Raab JR, Kamakaka RT (2012) TFIIIC bound DNA elements in nuclear organization and insulation. Biochim Biophys Acta.
47. Neumann FR, Dion V, Gehlen LR, Tsai-Pflugfelder M, Schmid R, et al. (2012) Targeted INO80 enhances subnuclear chromatin movement and ectopic homologous recombination. Genes & Development 26 : 369–383.
48. Li B (2012) Telomere components as potential therapeutic targets for treating microbial pathogen infections. Front Oncol 2 : 156. doi: 10.3389/fonc.2012.00156 23125966
49. Landeira D, Navarro M (2007) Nuclear repositioning of the VSG promoter during developmental silencing in Trypanosoma brucei. The Journal of Cell Biology 176 : 133–139. 17210949
50. Riethman H, Ambrosini A, Paul S (2005) Human subtelomere structure and variation. Chromosome Res 13 : 505–515. 16132815
51. Ottaviani A, Schluth-Bolard C, Rival-Gervier S, Boussouar A, Rondier D, et al. (2009) Identification of a perinuclear positioning element in human subtelomeres that requires A-type lamins and CTCF. The EMBO Journal 28 : 2428–2436. doi: 10.1038/emboj.2009.201 19644448
52. Ottaviani A, Schluth-Bolard C, Gilson E, Magdinier F (2010) D4Z4 as a prototype of CTCF and lamins-dependent insulator in human cells. Nucleus 1 : 30–36. doi: 10.4161/nucl.1.1.10799 21327102
53. Durand-Dubief M, Ekwall K (2009) Chromatin immunoprecipitation using microarrays. Methods Mol Biol 529 : 279–295. doi: 10.1007/978-1-59745-538-1_18 19381973
54. Vogel MJ, Peric-Hupkes D, van Steensel B (2007) Detection of in vivo protein-DNA interactions using DamID in mammalian cells. Nat Protoc 2 : 1467–1478. 17545983
55. Xue Y, Haas SA, Brino L, Gusnanto A, Reimers M, et al. (2004) A DNA microarray for fission yeast: minimal changes in global gene expression after temperature shift. Yeast 21 : 25–39. 14745780
56. Meister P, Gehlen LR, Varela E, Kalck V, Gasser SM (2010) Visualizing yeast chromosomes and nuclear architecture. Meth Enzymol 470 : 535–567. doi: 10.1016/S0076-6879(10)70021-5 20946824
57. Lantermann A, Strålfors A, Fagerström-Billai F, Korber P, Ekwall K (2009) Genome-wide mapping of nucleosome positions in Schizosaccharomyces pombe. Methods 48 : 218–225. doi: 10.1016/j.ymeth.2009.02.004 19233281
58. Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Meth 9 : 357–359.
59. Chen K, Xi Y, Pan X, Li Z, Kaestner K, et al. (2013) DANPOS: dynamic analysis of nucleosome position and occupancy by sequencing. Genome Research 23 : 341–351. doi: 10.1101/gr.142067.112 23193179
Štítky
Genetika Reprodukčná medicína
Článek NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY ModuleČlánek Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell LongevityČlánek HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and MiceČlánek LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male FertilityČlánek The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAsČlánek Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick VectorČlánek Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53Článek The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in NematodesČlánek The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse SpermatocytesČlánek The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 3- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
- Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
- A Genomic Duplication is Associated with Ectopic Eomesodermin Expression in the Embryonic Chicken Comb and Two Duplex-comb Phenotypes
- Genome-wide Association Study and Meta-Analysis Identify as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy
- Mutations of Human , Encoding the Mitochondrial Asparaginyl-tRNA Synthetase, Cause Nonsyndromic Deafness and Leigh Syndrome
- Exome Sequencing in an Admixed Isolated Population Indicates Variants Confer a Risk for Specific Language Impairment
- Genome-Wide Association Studies in Dogs and Humans Identify as a Risk Variant for Cleft Lip and Palate
- Rapid Evolution of Recombinant for Xylose Fermentation through Formation of Extra-chromosomal Circular DNA
- The Ribosome Biogenesis Factor Nol11 Is Required for Optimal rDNA Transcription and Craniofacial Development in
- Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
- Maternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity
- Clonality and Evolutionary History of Rhabdomyosarcoma
- HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and Mice
- Methylation-Sensitive Expression of a DNA Demethylase Gene Serves As an Epigenetic Rheostat
- BREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
- Seizures Are Regulated by Ubiquitin-specific Peptidase 9 X-linked (USP9X), a De-Ubiquitinase
- The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
- A Cascade of Iron-Containing Proteins Governs the Genetic Iron Starvation Response to Promote Iron Uptake and Inhibit Iron Storage in Fission Yeast
- Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
- LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male Fertility
- Cis-Regulatory Mechanisms for Robust Olfactory Sensory Neuron Class-restricted Odorant Receptor Gene Expression in
- Effects on Murine Behavior and Lifespan of Selectively Decreasing Expression of Mutant Huntingtin Allele by Supt4h Knockdown
- HDAC4-Myogenin Axis As an Important Marker of HD-Related Skeletal Muscle Atrophy
- A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex
- Selective and Genetic Constraints on Pneumococcal Serotype Switching
- Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
- The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAs
- Accumulation of Glucosylceramide in the Absence of the Beta-Glucosidase GBA2 Alters Cytoskeletal Dynamics
- Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities
- The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair
- Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick Vector
- Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
- The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors
- PHABULOSA Controls the Quiescent Center-Independent Root Meristem Activities in
- DNA Polymerase ζ-Dependent Lesion Bypass in Is Accompanied by Error-Prone Copying of Long Stretches of Adjacent DNA
- Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus
- Zinc Finger Independent Genome-Wide Binding of Sp2 Potentiates Recruitment of Histone-Fold Protein Nf-y Distinguishing It from Sp1 and Sp3
- GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters
- Neurospora Importin α Is Required for Normal Heterochromatic Formation and DNA Methylation
- Ccr4-Not Regulates RNA Polymerase I Transcription and Couples Nutrient Signaling to the Control of Ribosomal RNA Biogenesis
- Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53
- A Systems-Level Interrogation Identifies Regulators of Blood Cell Number and Survival
- Morphological Mutations: Lessons from the Cockscomb
- Genetic Interaction Mapping Reveals a Role for the SWI/SNF Nucleosome Remodeler in Spliceosome Activation in Fission Yeast
- The Role of China in the Global Spread of the Current Cholera Pandemic
- The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
- A Zinc Finger Motif-Containing Protein Is Essential for Chloroplast RNA Editing
- Resistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
- Small Regulatory RNA-Induced Growth Rate Heterogeneity of
- Mitochondrial Dysfunction Reveals the Role of mRNA Poly(A) Tail Regulation in Oculopharyngeal Muscular Dystrophy Pathogenesis
- Complex Genomic Rearrangements at the Locus Include Triplication and Quadruplication
- Male-Biased Aganglionic Megacolon in the TashT Mouse Line Due to Perturbation of Silencer Elements in a Large Gene Desert of Chromosome 10
- Sex Ratio Meiotic Drive as a Plausible Evolutionary Mechanism for Hybrid Male Sterility
- Tertiary siRNAs Mediate Paramutation in .
- RECG Maintains Plastid and Mitochondrial Genome Stability by Suppressing Extensive Recombination between Short Dispersed Repeats
- Escape from X Inactivation Varies in Mouse Tissues
- Opposite Phenotypes of Muscle Strength and Locomotor Function in Mouse Models of Partial Trisomy and Monosomy 21 for the Proximal Region
- Glycosyl Phosphatidylinositol Anchor Biosynthesis Is Essential for Maintaining Epithelial Integrity during Embryogenesis
- Hyperdiverse Gene Cluster in Snail Host Conveys Resistance to Human Schistosome Parasites
- The Class Homeodomain Factors and Cooperate in . Embryonic Progenitor Cells to Regulate Robust Development
- Recombination between Homologous Chromosomes Induced by Unrepaired UV-Generated DNA Damage Requires Mus81p and Is Suppressed by Mms2p
- Synergistic Interactions between Orthologues of Genes Spanned by Human CNVs Support Multiple-Hit Models of Autism
- Gene Networks Underlying Convergent and Pleiotropic Phenotypes in a Large and Systematically-Phenotyped Cohort with Heterogeneous Developmental Disorders
- The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
- Combinatorial Control of Light Induced Chromatin Remodeling and Gene Activation in
- Linking Aβ42-Induced Hyperexcitability to Neurodegeneration, Learning and Motor Deficits, and a Shorter Lifespan in an Alzheimer’s Model
- The Complex Contributions of Genetics and Nutrition to Immunity in
- NatB Domain-Containing CRA-1 Antagonizes Hydrolase ACER-1 Linking Acetyl-CoA Metabolism to the Initiation of Recombination during . Meiosis
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
- Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption
- A Genomic Portrait of Haplotype Diversity and Signatures of Selection in Indigenous Southern African Populations
- Sequence Features and Transcriptional Stalling within Centromere DNA Promote Establishment of CENP-A Chromatin
- Inhibits Neuromuscular Junction Growth by Downregulating the BMP Receptor Thickveins
- Replicative DNA Polymerase δ but Not ε Proofreads Errors in and in
- Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in
- The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
- A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension
- Pervasive Variation of Transcription Factor Orthologs Contributes to Regulatory Network Evolution
- Network Analyses Reveal Novel Aspects of ALS Pathogenesis
- A Role for the Budding Yeast Separase, Esp1, in Ty1 Element Retrotransposition
- Nab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
- A RecA Protein Surface Required for Activation of DNA Polymerase V
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Clonality and Evolutionary History of Rhabdomyosarcoma
- Morphological Mutations: Lessons from the Cockscomb
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



