-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
During embryonic development, stem cell-like primordial germ cells travel across the developing embryo to the genital ridge, which gives rise to the gonad. Around the time of their arrival, the primordial germ cells gain the capacity to undertake sexual specialization and meiosis—a process called germ cell licensing. Based on the observation that meiosis and sexual differentiation can occur when primordial germ cells stray into the area of the adrenal gland, the primordial germ cell has been thought to be responsible for its own licensing. We tested this notion by examining the licensing process in mutant mouse embryos that did not form a genital ridge. We discovered that in the absence of the genital ridge, primordial germ cells migrate across the developing embryo properly, but instead of undergoing licensing, these cells retain their primordial germ cell characteristics. We conclude that licensing of embryonic primordial germ cells for gametogenesis is dependent on signaling from the genital ridge.
Published in the journal: Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling. PLoS Genet 11(3): e32767. doi:10.1371/journal.pgen.1005019
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005019Summary
During embryonic development, stem cell-like primordial germ cells travel across the developing embryo to the genital ridge, which gives rise to the gonad. Around the time of their arrival, the primordial germ cells gain the capacity to undertake sexual specialization and meiosis—a process called germ cell licensing. Based on the observation that meiosis and sexual differentiation can occur when primordial germ cells stray into the area of the adrenal gland, the primordial germ cell has been thought to be responsible for its own licensing. We tested this notion by examining the licensing process in mutant mouse embryos that did not form a genital ridge. We discovered that in the absence of the genital ridge, primordial germ cells migrate across the developing embryo properly, but instead of undergoing licensing, these cells retain their primordial germ cell characteristics. We conclude that licensing of embryonic primordial germ cells for gametogenesis is dependent on signaling from the genital ridge.
Introduction
In mammals, both the testis and ovary derive from a common precursor structure, the bipotential gonad [1]. The development of the bipotential gonad involves two simultaneously occurring processes. The coelomic epithelium on the ventromedial surface of the mesonephros transforms from a monolayer into a thickened, multilayer epithelial structure, the genital ridge. Meanwhile, primordial germ cells (PGCs) that have migrated from the base of the allantois start arriving at the genital ridge, as early as the monolayer stage, and multiply as the genital ridge thickens. The formation of the bipotential gonad in mouse embryos begins at embryonic (E) day 10.0 and continues until E11.5-E12.0, when sexual differentiation takes place [2–4].
Migratory PGCs maintain a genomic program associated with pluripotency [5,6]. They express core pluripotency genes (Oct4, Nanog, and Sox2) and are able to form teratomas following their injection into postnatal mouse testes [7]. Around the time of their arrival at the genital ridge, PGCs undergo a global change in gene expression [8–10]. Specifically, the PGCs turn on a set of genes that enable them to undergo sexual differentiation and gametogenesis, and to switch off their pluripotency program. Following this transition, germ cells are referred to as gametogenesis-competent cells (GCCs), and are poised to initiate meiosis as well as male or female differentiation [11–13]. Upon the development of the genital ridge into either a testis or an ovary (at ~E12.5 in mouse embryos), GCCs respond to cues from the somatic environment and enter either the spermatogenic or oogenic pathway accordingly. The transition from PGC to GCC is referred to as germ cell licensing [11], and it represents a critical transformation of germ cells to a sexually competent state.
One of the genes upregulated in germ cells at the time of licensing is Dazl, which encodes an evolutionarily conserved and germ-cell-specific RNA-binding protein [14]. In mouse embryos of C57BL/6 genetic background, germ cell licensing is dependent on Dazl [11,15]. In Dazl-null embryos, germ cells retain characteristics of PGCs and fail to embark upon the pathways to oogenesis or spermatogenesis in the fetal ovary or testis, respectively. However, what triggers Dazl expression and germ cell licensing remains unknown.
One hypothesis, based on observational studies, states that licensing is triggered in a cell-autonomous and gonad-independent manner. As PGCs migrate to the genital ridge, a fraction of them are left in places along the migratory path, such as in the allantois, tail, midline, spinal cord, and adrenal gland [16,17]. While most of these ectopic PGCs die, those migrating to the adrenal gland survive until ~3 weeks after birth [16,18–20]. Upadhyay and Zamboni [19] observed that these adrenal germ cells, regardless of the sex of the fetus, enter meiosis according to the schedule of normal ovarian germ cell development. Based on these findings, the authors hypothesized that PGCs transition into meiotic germ cells (oocytes) in a gonad-independent, and therefore cell-autonomous, manner. This hypothesis was further supported by several in vitro studies [13,21–23], showing, for instance, that PGCs isolated from E10.5 mouse embryos of both sexes continue to develop in vitro and initiate meiosis at approximately the same time as meiotic entry occurs in vivo [13,22,23].
Previous studies from our lab and others led us to question this hypothesis and suggest an alternative: PGCs undergo germ cell licensing in response to external signals, upon migration to the genital ridge. The authors who proposed the cell-autonomous hypothesis considered E10.5 PGCs to be pre-gonadal germ cells [22,23]. However, we recently showed that the marker of genital ridge formation, GATA4, is expressed as early as E10.0 [2]. It is plausible that the E10.5 PGCs used in the in vitro studies had already been exposed to gonadal factors. In addition, the claim that the PGCs in the adrenal gland transition to meiotic germ cells without exposure to the genital ridge belies the fact that the adrenal anlagen and genital ridge derive from a common precursor, called the adrenogonadal primordium. These two organs are not segregated completely until ~E11.5 [24,25]. Adrenal PGCs would therefore be exposed to the genital ridge, or its equivalent, during a short interval in their development. These findings raise doubts about whether the transition of PGCs to meiosis-competent cells is gonad-independent, or induced by factors shared by the developing gonad and adrenal gland.
Germ cell licensing precedes meiotic entry [11,15]. Since the occurrence of licensing coincides with the arrival of PGCs at the genital ridge, we suspected that the genital ridge provides extrinsic signals required for inducing germ cell licensing. Initiation of genital ridge formation depends on the transcription factor GATA4, which is expressed in the somatic compartment, but not in germ cells [2]. We therefore utilized Gata4 conditional knockout (cKO) embryos, which lack the genital ridge, to test the hypothesis of genital ridge-dependent licensing. If true, we would expect that in the absence of the genital ridge, PGCs would fail to undergo licensing and subsequent meiotic entry. The result of this study would provide fundamental insight into how germ cells switch off their pluripotency program and acquire competence for meiosis and sexual differentiation.
Results
Anterior-to-posterior expression of the germ cell licensing marker Dazl
The genital ridge develops in an anterior-to-posterior (A-P) direction starting at E10.0 [1,2], as PGCs are entering the region. Dazl is expressed in germ cells during licensing for gametogenesis [8,9,11,14,15]. If the genital ridge regulates germ cell licensing, we would expect to find a similar A-P induction of licensing, along with Dazl expression. To test this prediction, we quantified Dazl transcript levels in individual germ cells along the A-P axis of the genital ridge using single-molecule fluorescence in situ hybridization (smFISH) [26]. We first confirmed that Dazl expression was below the detectable level in migratory PGCs at E9.5, as expected (S1A Fig). When examining post-migratory germ cells at E11.5, we detected a gradient of Dazl transcript levels along the genital ridge; expression is highest in the anterior portion and decreases in an A-P direction (Figs. 1 and S1B). As a comparison, we also quantified the transcript level of Oct4 (Pou5f1), a pluripotency gene that is expressed in both migratory PGCs and post-migratory germ cells. We did not observe an A-P gradient of Oct4 transcript levels in germ cells. Instead, germ cell Oct4 transcript levels were relatively consistent along the length of the genital ridge. Therefore, the A-P expression pattern of Dazl supports the possibility that the genital ridge induces germ cell licensing.
Fig. 1. Dazl expression in germ cells displays an A-P gradient along the genital ridge. 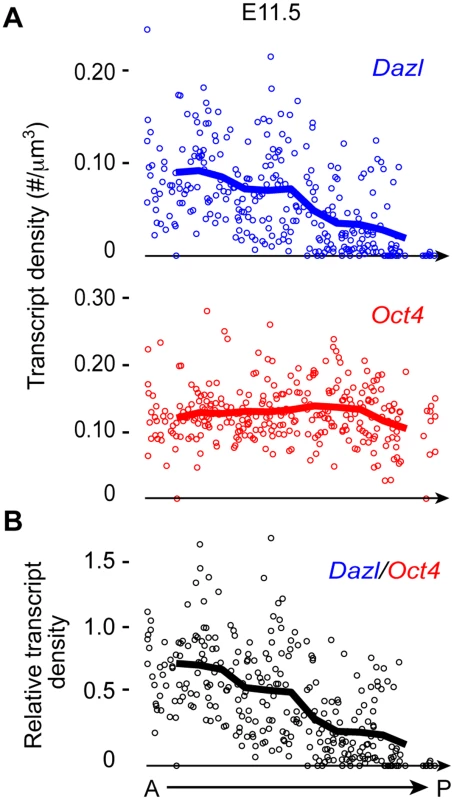
(A) Scatterplots represent transcript densities of Dazl and Oct4 in individual germ cells along A-P axis of genital ridge of E11.5 embryos (n = 3), as measured by smFISH. (B) Dazl transcript density was normalized against Oct4 transcript density for each individual cell to obtain relative Dazl:Oct4 transcript density per cell. Lines in plots represent average transcript density of cells (A) or ratio of densities (B) at a particular A-P position. Genital ridge formation is required for germ cell licensing
We next asked whether germ cell licensing requires the presence of the genital ridge. We examined DAZL expression in transverse sections of E11.5 embryos in which we had prevented genital ridge formation by ubiquitous deletion of Gata4 (Gata4 cKO) through tamoxifen injection at E8.75 (Fig. 2A, Gata4flox/∆; CAG-CreER [2]). Sections were immunostained for SSEA1, DAZL, and GATA4 expression. SSEA1 was used to identify all germ cells at this time point, as it identifies both migratory PGCs and post-migratory germ cells. In sections from littermate controls (Gata4+/flox), we found that the majority of germ cells that had colonized the genital ridge expressed DAZL, consistent with our smFISH data (Fig. 1). In contrast, germ cells in Gata4 cKO embryos migrated to the ventromedial side of the mesonephros (the location of the genital ridge in wild type animals) but failed to initiate DAZL expression (95% vs. 4%, respectively; Fig. 2B and C). These results indicate that genital ridge formation is essential for DAZL expression in PGCs, but not for their migration.
Fig. 2. Germ cells in Gata4 cKO embryos do not express DAZL or MVH. 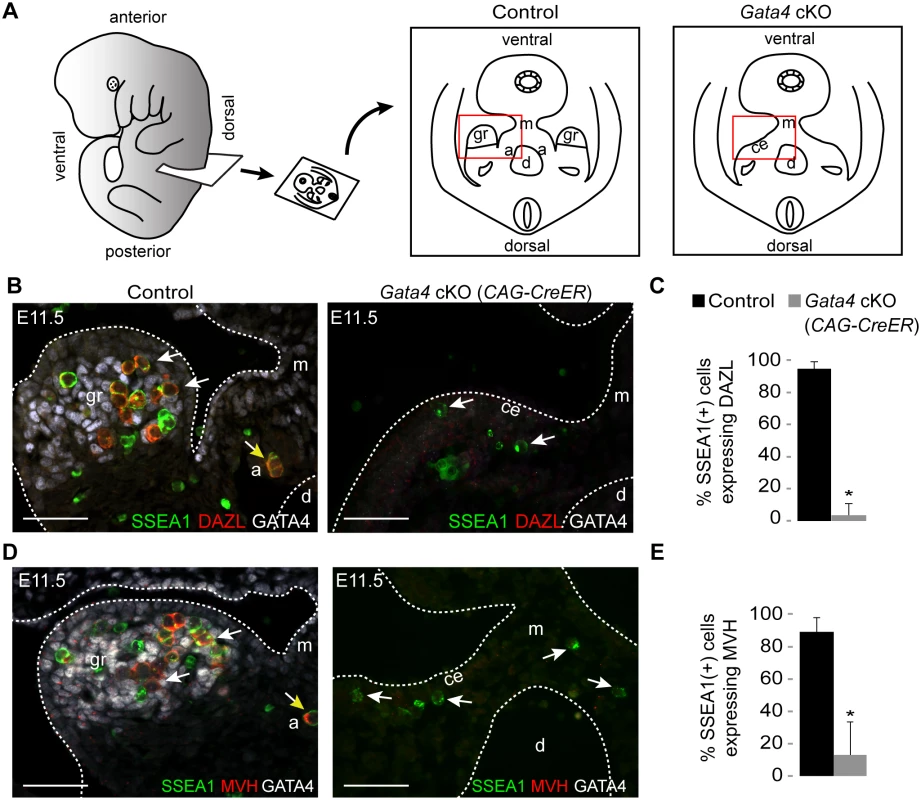
(A) Schematic illustration of transverse section through embryo trunk that contains urogenital ridge. Red boxes indicate areas imaged, shown in B and D. a, adrenal gland; ce, coelomic epithelium; d, dorsal aorta; gr, genital ridge; m, mesentery. (B and D) Immunofluorescent staining for SSEA1, DAZL, MVH, and GATA4 protein in transverse sections of control (Gata4+/flox) and Gata4 ubiquitous cKO (CAG-CreER) embryos on a mixed genetic background. Representative germ cells are indicated by white arrows. Germ cells mis-migrating to the adrenal gland (yellow arrows) also express DAZL and MVH. Scale bars: 50 μm. (C and E) Percentage of germ cells that are positive for DAZL or MVH expression in control and Gata4 cKO (CAG-CreER) embryos. SSEA1 marks all germ cells at this age. Plotted here are means ± standard deviation from biological replicates (n ≥ 3 for each genotype). *, P < 0.05 (two-tailed Student’s t-test). Like Dazl, Mouse vasa homolog (Mvh, also known as Ddx4) is also expressed in germ cells around the time of their arrival at the genital ridge [27]. In Dazl-null embryos of the C57BL/6 genetic background, germ cells do not undergo licensing, but MVH is still expressed in these cells, suggesting that MVH expression is independent of Dazl [11]. We then examined MVH expression in the germ cells of Gata4 cKO embryos. In transverse sections from littermate controls, the majority of germ cells that had colonized the genital ridge expressed MVH, whereas germ cells in Gata4 cKO embryos failed to express MVH (89% vs. 13%; Fig. 2D and E). These results indicate that, like DAZL, MVH expression is also dependent upon genital ridge formation.
To establish that the failure of germ cell licensing was due to the loss of Gata4 in somatic tissues, we used an additional Cre line, Osr1-CreER, that is expressed in genital ridge (somatic) precursor cells but not in the germ line. As with the ubiquitous Gata4 cKO described above, Osr1-driven loss of Gata4 in the soma resulted in PGCs migrating to the ventromedial side of the mesonephros, without initiating either DAZL or MVH expression (S2 Fig). These results confirm that licensing of germ cells depends upon Gata4 function in somatic cells.
Consistent with previous observations that mis-migrated adrenal germ cells can initiate meiosis, we noticed that germ cells that had migrated to the adrenal gland expressed DAZL and MVH at E11.5 (Fig. 2B and D). These findings suggest that germ cells in the adrenal gland undergo licensing as they do in the genital ridge, which makes germ cells there capable of entering meiosis [19,20]. Licensing of the adrenal germ cells is likely to be dependent on factors shared between the genital ridge and the adrenal, as both organs derive from the same primordium.
To confirm that genital ridge formation is sufficient to induce the germ cell licensing factor DAZL, we examined embryos lacking either Wt1 or Osr1, both of which are required for development and maintenance of the embryonic gonad [28–30]. We found that, in the absence of either Wt1 or Osr1, GATA4 is expressed in the coelomic epithelium, and the genital ridge is initially formed, although its growth is severely retarded and degeneration ensues (S3 and S4 Figs). In both Wt1 KO and Osr1 KO embryos, we observed DAZL expression in germ cells that migrated to the GATA4-expressing cells of the genital ridge, indicating that the association of PGCs with the nascent genital ridge is sufficient for licensing (S3 and S4 Figs). Taken together, our findings demonstrate that genital ridge formation is both necessary and sufficient to induce expression of DAZL in newly arrived PGCs.
Germ cells in cultured urogenital ridges from Gata4 cKO embryos retain PGC markers and fail to express GCC markers
Having found that germ cells in Gata4 cKO embryos do not express DAZL and MVH, we wondered if these cells fail to transition into GCCs and instead retain characteristics of PGCs, such as continued expression of the pluripotency gene program and inability to sexually differentiate. Because Gata4 cKO embryos die between E11.5-E12.0, we investigated subsequent germ cell development in urogenital ridge (UGR) cultures. The UGR was dissected from E11.5 control and Gata4 ubiquitous cKO embryos, cultured for 3 days, and subjected to paraffin sectioning and immunofluorescent staining. In control UGR cultures (n = 5; 3 XX and 2 XY), all germ cells that we examined expressed markers seen in GCCs and sexually differentiating germ cells, including DAZL, MVH, GCNA, and MILI (Fig. 3). In addition, these germ cells switched off pluripotency markers, including NANOG, OCT4, SOX2, and SSEA1. In contrast, all germ cells in Gata4 cKO UGR cultures (n = 4; 2 XX and 2 XY) retained an expression program similar to that of PGCs, with NANOG, OCT4, SOX2, and SSEA1 being expressed (Fig. 3). Conversely, markers of GCCs and sexually differentiating germ cells, including DAZL, MVH, GCNA and MILI, were not expressed in cultured UGRs from Gata4 cKO embryos.
Fig. 3. Germ cells in Gata4 cKO embryos retain characteristics of PGCs. 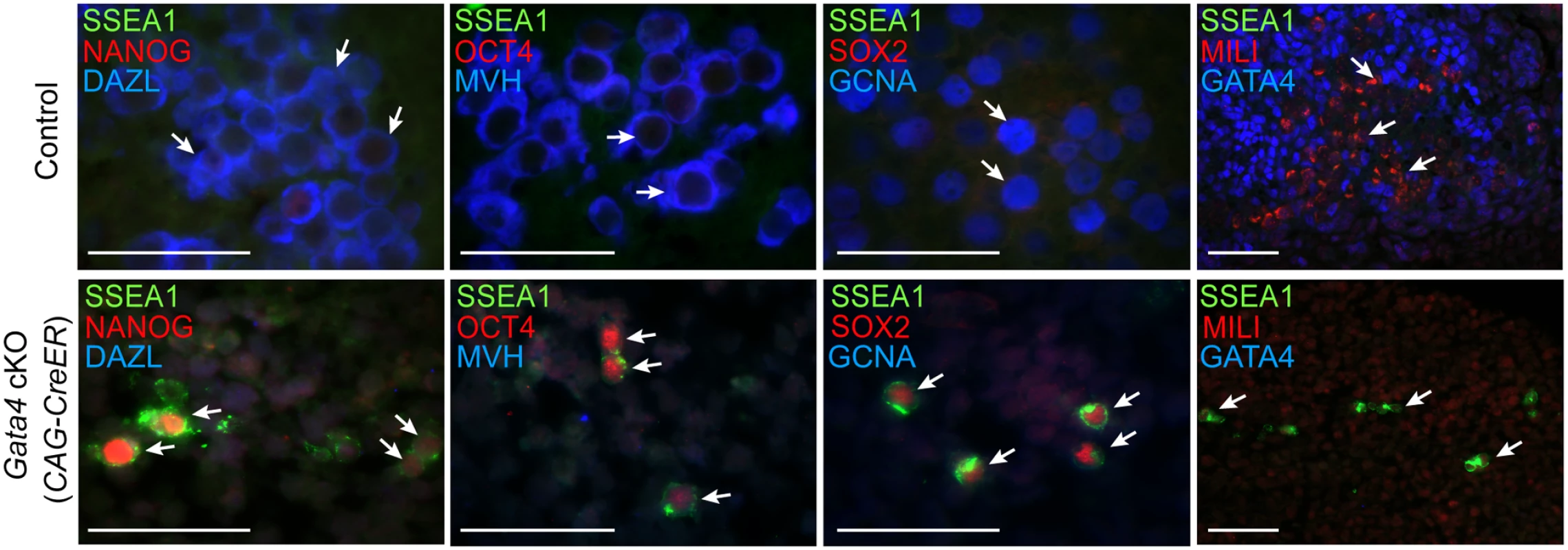
Immunofluorescent staining for PGC and GCC marker proteins in transverse sections of control (Gata4+/flox) and Gata4 cKO (CAG-CreER) urogenital ridge cultures on a mixed genetic background. Arrows indicate representative germ cells. Scale bars: 50 μm. To confirm that the conversion of PGCs to GCCs depends upon Gata4 function in somatic tissues and not the germline, we studied similar UGR cultures generated using the soma-specific Cre line, Wt1-CreER; Osr1-CreER. After 3 days of UGR culture, we observed that germ cells in controls expressed both DAZL and MVH, whereas germ cells in Gata4 soma-specific cKO tissues expressed neither marker, and instead retained expression of the pluripotency marker SSEA1 (S5 Fig). These results indicate that the genital ridge is indispensable for licensing of PGCs to GCCs.
Germ cells in Gata4 cKO embryos fail to enter meiosis
A key functional characteristic that distinguishes GCCs from PGCs is their ability to enter meiosis. To investigate whether germ cells from Gata4 cKO embryos are able to enter meiosis, we performed immunostaining on sections of cultured UGRs for SYCP3 and SSEA1 expression. We found that in control UGR cultures (n = 5 cultures), germ cells that expressed GCC markers (Fig. 3) showed SYCP3 assembly onto chromosomes—a characteristic of prophase of meiosis I—while the pluripotency marker SSEA1 was not detectable (Fig. 4). In contrast, germ cells in Gata4 cKO UGR cultures (n = 4 cultures) expressed neither GCC markers (Fig. 3) nor SYCP3 (Fig. 4). Instead, they continued to express SSEA1, a marker of PGC identity. We conclude that germ cells in Gata4 cKO UGR cultures were not competent to enter meiosis, functionally validating the earlier evidence that these cells had not become GCCs.
Fig. 4. Germ cells in Gata4 cKO embryos do not enter meiosis. 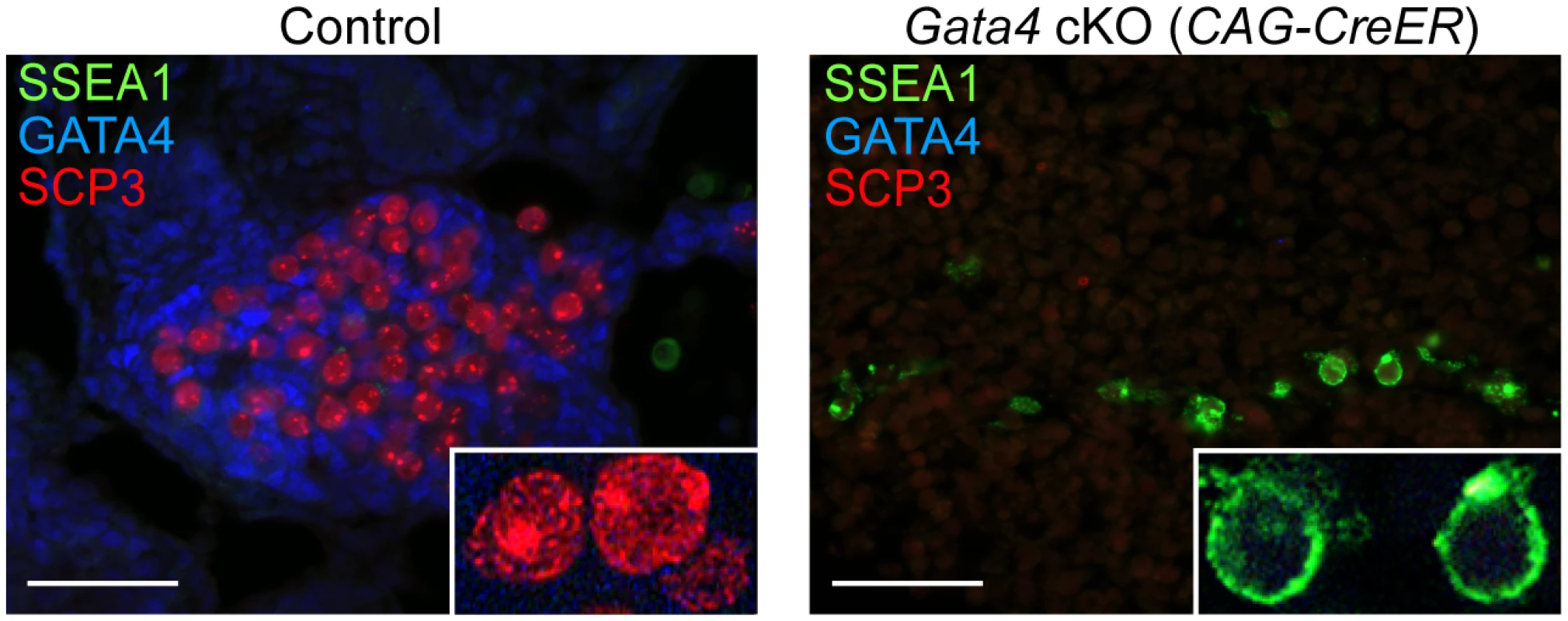
Immunofluorescent staining for SSEA1, SYCP3, and GATA4 proteins in transverse sections of control (Gata4+/flox) and Gata4 cKO (CAG-CreER) urogenital ridge cultures on a mixed genetic background. Inset shows higher magnification of cells. Scale bars: 50 μm. Discussion
We have identified a previously unrecognized role of the genital ridge in germ cell development, prior to sex determination. We show that germ cell licensing—the transition of PGCs to GCCs—is induced by cues from the genital ridge (Fig. 5). If the genital ridge is not formed due to loss of the somatic transcription factor Gata4, germline cells remain at the PGC stage, failing to become GCCs and lacking the competence to undergo sexual differentiation and initiate meiosis. Thus, we provide genetic evidence that the transition of PGCs into meiotic germ cells is not a purely cell-autonomous process and is instead dependent on the somatic gonad.
Fig. 5. A proposed model for somatic induction of germ cell differentiation, in three steps. 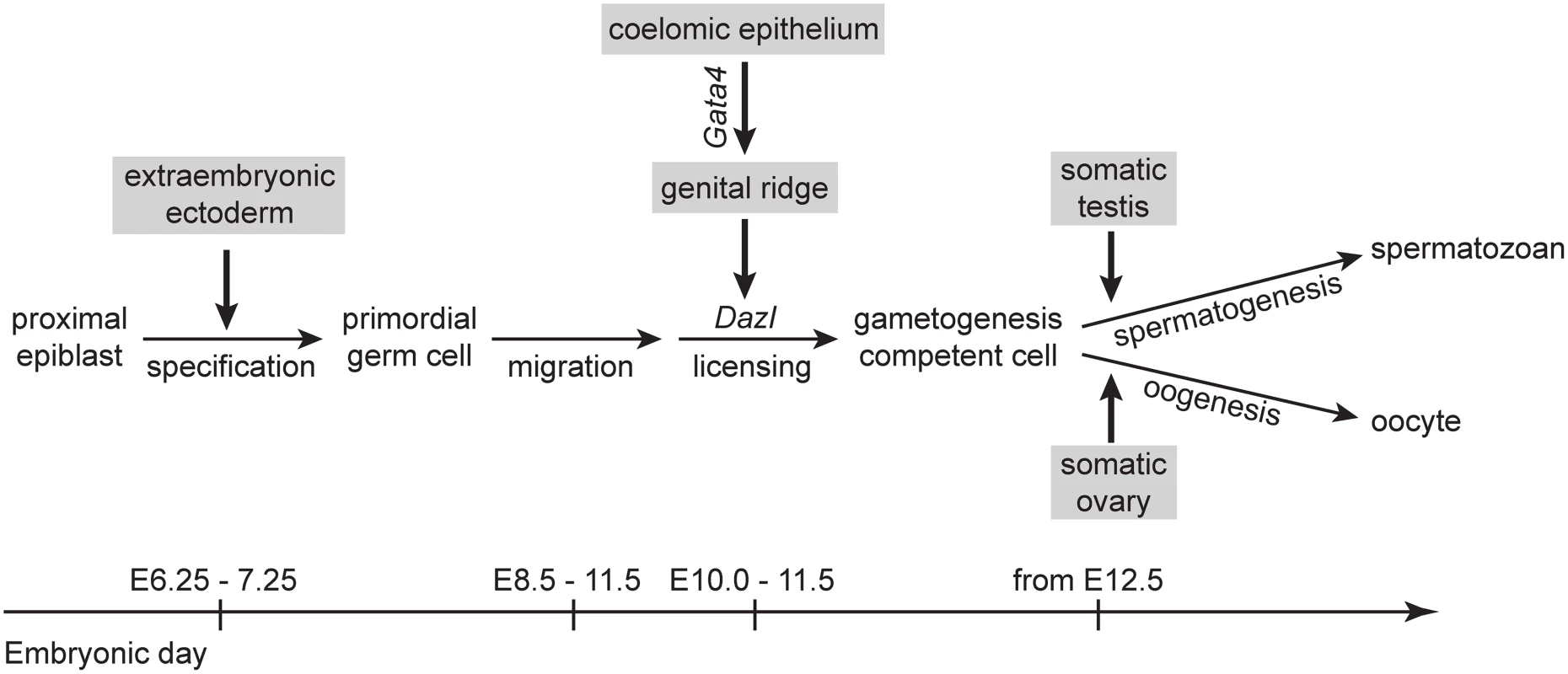
1) Germ cell specification induced by signals, such as BMP4, from extraembryonic ectoderm [53,54]; 2) germ cell licensing induced by the genital ridge, which arises from the coelomic epithelium following Gata4 expression; and 3) GCCs embark on either spermatogenesis or oogenesis in response to cues from somatic testis or ovary, respectively [12,13]. The genital ridge-dependent induction of germ cell licensing is compatible with the observation that ectopic germ cells in the adrenal gland also enter meiosis [19]. Given that the genital ridge and adrenal gland share a common precursor, the adrenogonadal primordium [24,25], it is plausible that these two organs produce the same factors required to induce licensing and meiosis. Our finding also helps explain previous studies in which PGCs isolated from E10.5 embryos were shown to initiate meiosis in culture [13,22,23]. Because genital ridge formation is initiated as early as E10.0 [2], it is likely that a portion of E10.5 germ cells had already been exposed to the genital ridge environment. These licensed germ cells, therefore, had acquired meiotic competence by E10.5, before being cultured.
We have previously shown that Dazl is required for licensing of PGCs to GCCs in mouse embryos from an inbred C57BL/6 genetic background [11]. The present study extends our understanding of germ cell licensing for gametogenesis. We now appreciate that germ cell licensing is induced by the soma; indeed, the soma induces expression in germ cells of at least two key factors, DAZL and MVH, independently. Our findings also indicate that germ cell licensing depends upon the genital ridge in embryos of either mixed (Figs 2–4) or C57BL/6 genetic background (S2 and S5 Figs). The molecular mechanism by which the genital ridge regulates germ cell licensing requires further exploration.
We propose that the genital ridge—a somatic structure—induces germ cell licensing. However, we cannot formally exclude the possibility that the failure of licensing in germ cells of Gata4 cKO embryos is a secondary effect of other activities at the genital ridge. The genital ridge has previously been shown to regulate germ cell motility, as PGCs become nonmotile after arriving at the genital ridge [18,31]. The genital ridge also produces factors that stimulate PGC proliferation [28,32,33]. Although germ cells colonize the coelomic epithelium in similar numbers in control and Gata4 cKO embryos at E10.3 [2], Gata4 cKO embryos display a reduced number of germ cells at E11.5, likely due to the absence of genital ridge-derived factors that stimulate proliferation. It is unlikely that this reduction in germ cell numbers is responsible for the licensing defects observed in the Gata4 cKO, given that other mutants with reduced germ cell number display qualitatively normal germ cell development in males [34,35]. Similarly, we cannot yet exclude the possibility that systemic defects (e.g., in the gut, liver, or heart) contribute to the failure of germ cell licensing observed in the Gata4 cKO. The generation of a genital ridge-specific Cre mouse will be required to rule this out.
We can now reconstruct the series of events in soma and germline (Fig. 5) that result in post-migratory PGCs initiating meiosis in the female fetal gonad, in an anterior-to-posterior (A-P) wave. In the soma, Gata4 expression initiates the transformation of the coelomic epithelium (on the ventromedial surface of the mesonephros) into the genital ridge, in an anterior-to-posterior (A-P) wave [2]. Concurrently, PGCs migrate to the developing genital ridge as early as the monolayer stage. Based on our findings—that Dazl is expressed in germ cells in an A-P progression (Fig. 1), and that this expression is dependent upon the genital ridge (Fig. 2)—we propose that the progressive A-P development of the genital ridge induces Dazl expression and licensing in a similar A-P wave. Upon expression of DAZL, GCCs acquire the competence to interpret retinoic acid as a meiosis-inducing signal [36,37], and then express Stra8, the gene required for meiotic initiation [15,38], along with Rec8 [39] and Dmc1—all in an A-P manner [40,41].
Licensing for gametogenesis constitutes a major transition during early germ cell development, allowing PGCs to acquire competence for sexual differentiation and gametogenesis. Our results indicate that the genital ridge triggers germ cell licensing. Thus, PGCs undergo licensing upon their arrival at the genital ridge, ensuring that gametogenesis occurs at the correct time and place.
Materials and Methods
Mice
All experiments involving mice were approved by the Committee on Animal Care at the Massachusetts Institute of Technology. The following mice were obtained from Jackson Laboratory (Stock Numbers 008194, 004682, 009061, 009387, 010912 and 002332): Gata4flox/+ [42]; CAG-CreER (ubiquitously expressed) [43]; Osr1eGFP-CreERt2/+ (somatically expressed) [44]; Osr1tm1Jian/+ [30]; Wt1CreERt2/+ (somatically expressed) [45]; and Wt1tm1Jae/+[29], respectively. In some cases (as described in the text), these mice were backcrossed to the C57BL/6 strain (Taconic Farms) for at least 10 generations. Gata4 cKO embryos were generated by mating Gata4flox/flox females with Gata4+/∆ males carrying the indicated CreER. Where applicable, Gata4+/flox littermate embryos were used as controls. Tamoxifen (Sigma) was dissolved in corn oil (Sigma) at a concentration of 30 mg/ml. Dams were injected intraperitoneally at 8.75 days postcoitum with a single shot of tamoxifen (4 mg/40 g body weight) to induce excision of the floxed Gata4 allele. The injection scheme was optimized for maximum embryo survival and Gata4 excision efficiency [2]. Embryos were collected at the indicated time and immediately genotyped by PCR according to protocols from the Jackson Laboratory website.
Single-molecule fluorescence in situ hybridization
Whole E9.5 embryos or urogenital ridges dissected from E11.5 embryos on C57BL/6 genetic background were fixed 2 hours at 4°C in 4% paraformaldehyde, equilibrated in 30% sucrose/4% paraformaldehyde in PBS overnight, and frozen and stored in OCT (Tissue-Tek) at -80°C before cryosectioning (8 μm thick). Probes were synthesized and hybridization performed as previously described [26]. Probes to Dazl and Oct4 transcripts were conjugated to Cy5, A594, or TMR. AlexaFluor 488-conjugated anti-SSEA1 (560271, BD Biosciences) was added to the hybridization to label germ cells. Images were taken with a Nikon Ti-E inverted fluorescence microscope equipped with a 100x oil-immersion objective and a Photometrics Pixis 1024B CCD camera. We recorded stacks of images (z spacing 0.3 μm) at adjacent x-y positions covering the entire A-P length of the genital ridge. Images were stitched based on stage coordinates, and stitching coordinates were optimized locally by cross-correlation. Data analysis was performed in MATLAB (MathWorks) using custom-written code. Individual transcript molecules were identified and counted semi-automatically as previously described [26]. Individual germ cells were identified and outlined manually using a combination of SSEA1 expression and DAPI morphology. Dazl probe sequences used in this study are listed in S1 Table. Oct4 probe sequences were published elsewhere [46].
Urogenital ridge (UGR) cultures
Urogenital ridges, comprised of genital ridges, mesonephroi, primitive kidneys, and dorsal aorta, were dissected from E11.5 control and Gata4 cKO embryos. The dissected UGRs were cultured on agar blocks, as previously described [47], for 3 days in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, non-essential amino acids, glutamine and penicillin/streptomycin. In this culture condition, as reported previously [13,23,48,49], E11.5 XY germ cells (which are sexually bipotential) develop toward the oogenic pathway, in part due to impaired testis cord formation and the presence of meiosis-inducing factors in the culture medium.
Immunofluorescence
Immunofluorescent staining of embryonic sections was carried out as described previously [50]. Briefly, whole embryos or cultured urogenital organs were fixed at 4°C overnight in 4% paraformaldehyde, paraffin embedded, and sectioned. Slides were then dewaxed, rehydrated, and antigen-retrieved by microwaving in citrate buffer (10mM sodium citrate, 0.05% Tween 20, pH6.0). After blocking, slides were incubated with primary antibodies at 4°C overnight. Slides were then incubated with donkey secondary antibodies conjugated to FITC, Rhodamine Red X or DyLight 649 (Jackson ImmunoResearch) and mounted with ProLong Gold Antifade reagent with DAPI (Life Technologies).
Primary antibodies against GATA4 (sc-25310, Santa Cruz Biotechnology), DAZL (ab34139, Abcam), SSEA1 (MAB4301, Millpore), MVH (AF2030, R&D Systems), GCNA (a gift from George Enders, University of Kansas Medical Center, Kansas City, KS) [51], SOX2 (ab97959, Abcam), NANOG (IHC-00205, Bethyl Laboratories), OCT4 (560186, BD), MILI (a gift from Gregory J. Hannon, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) [52], and SYCP3 (sc-33195, Santa Cruz Biotechnology) were used in the study.
Supporting Information
Zdroje
1. Brambell FWR (1927) The development and morphology of the gonads of the mouse—Part I The morphogenesis of the indifferent gonad and of the ovary. Proc R Soc Lond B Biol Sci 101 : 391–409.
2. Hu YC, Okumura LM, Page DC (2013) Gata4 is required for formation of the genital ridge in mice. PLoS Genet 9: e1003629. doi: 10.1371/journal.pgen.1003629 23874227
3. Hacker A, Capel B, Goodfellow P, Lovell-Badge R (1995) Expression of Sry, the mouse sex determining gene. Development 121 : 1603–1614. 7600978
4. Schmahl J, Eicher EM, Washburn LL, Capel B (2000) Sry induces cell proliferation in the mouse gonad. Development 127 : 65–73. 10654601
5. Hayashi K, de Sousa Lopes SM, Surani MA (2007) Germ cell specification in mice. Science 316 : 394–396. 17446386
6. Saitou M, Yamaji M (2012) Primordial germ cells in mice. Cold Spring Harb Perspect Biol 4: a008375. doi: 10.1101/cshperspect.a008375 23125014
7. Chuma S, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, et al. (2005) Spermatogenesis from epiblast and primordial germ cells following transplantation into postnatal mouse testis. Development 132 : 117–122. 15576408
8. Molyneaux KA, Wang Y, Schaible K, Wylie C (2004) Transcriptional profiling identifies genes differentially expressed during and after migration in murine primordial germ cells. Gene Expr Patterns 4 : 167–181. 15161097
9. Rolland AD, Lehmann KP, Johnson KJ, Gaido KW, Koopman P (2011) Uncovering gene regulatory networks during mouse fetal germ cell development. Biol Reprod 84 : 790–800. doi: 10.1095/biolreprod.110.088443 21148109
10. Seisenberger S, Andrews S, Krueger F, Arand J, Walter J, et al. (2012) The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol Cell 48 : 849–862. doi: 10.1016/j.molcel.2012.11.001 23219530
11. Gill ME, Hu YC, Lin Y, Page DC (2011) Licensing of gametogenesis, dependent on RNA binding protein DAZL, as a gateway to sexual differentiation of fetal germ cells. Proc Natl Acad Sci U S A 108 : 7443–7448. doi: 10.1073/pnas.1104501108 21504946
12. Adams IR, McLaren A (2002) Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development 129 : 1155–1164. 11874911
13. McLaren A, Southee D (1997) Entry of mouse embryonic germ cells into meiosis. Dev Biol 187 : 107–113. 9224678
14. Seligman J, Page DC (1998) The Dazh gene is expressed in male and female embryonic gonads before germ cell sex differentiation. Biochem Biophys Res Commun 245 : 878–882. 9588208
15. Lin Y, Gill ME, Koubova J, Page DC (2008) Germ cell-intrinsic and-extrinsic factors govern meiotic initiation in mouse embryos. Science 322 : 1685–1687. doi: 10.1126/science.1166340 19074348
16. Stallock J, Molyneaux K, Schaible K, Knudson CM, Wylie C (2003) The pro-apoptotic gene Bax is required for the death of ectopic primordial germ cells during their migration in the mouse embryo. Development 130 : 6589–6597. 14660547
17. Runyan C, Gu Y, Shoemaker A, Looijenga L, Wylie C (2008) The distribution and behavior of extragonadal primordial germ cells in Bax mutant mice suggest a novel origin for sacrococcygeal germ cell tumors. Int J Dev Biol 52 : 333–344. doi: 10.1387/ijdb.072486cr 18415933
18. Molyneaux KA, Stallock J, Schaible K, Wylie C (2001) Time-lapse analysis of living mouse germ cell migration. Dev Biol 240 : 488–498. 11784078
19. Upadhyay S, Zamboni L (1982) Ectopic germ cells: natural model for the study of germ cell sexual differentiation. Proc Natl Acad Sci U S A 79 : 6584–6588. 6959138
20. Zamboni L, Upadhyay S (1983) Germ cell differentiation in mouse adrenal glands. J Exp Zool 228 : 173–193. 6663256
21. Richards AJ, Enders GC, Resnick JL (1999) Differentiation of murine premigratory primordial germ cells in culture. Biol Reprod 61 : 1146–1151. 10491656
22. Chuma S, Nakatsuji N (2001) Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Dev Biol 229 : 468–479. 11203703
23. Tedesco M, Desimio MG, Klinger FG, De Felici M, Farini D (2013) Minimal concentrations of retinoic acid induce stimulation by retinoic acid 8 and promote entry into meiosis in isolated pregonadal and gonadal mouse primordial germ cells. Biol Reprod 88 : 145. doi: 10.1095/biolreprod.112.106526 23636811
24. Hatano O, Takakusu A, Nomura M, Morohashi K (1996) Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1 : 663–671. 9078392
25. Laufer E, Kesper D, Vortkamp A, King P (2012) Sonic hedgehog signaling during adrenal development. Mol Cell Endocrinol 351 : 19–27. doi: 10.1016/j.mce.2011.10.002 22020162
26. Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S (2008) Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods 5 : 877–879. doi: 10.1038/nmeth.1253 18806792
27. Toyooka Y, Tsunekawa N, Takahashi Y, Matsui Y, Satoh M, et al. (2000) Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech Dev 93 : 139–149. 10781947
28. Chen SR, Zheng QS, Zhang Y, Gao F, Liu YX (2013) Disruption of genital ridge development causes aberrant primordial germ cell proliferation but does not affect their directional migration. BMC Biol 11 : 22. doi: 10.1186/1741-7007-11-22 23497137
29. Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, et al. (1993) WT-1 is required for early kidney development. Cell 74 : 679–691. 8395349
30. Wang Q, Lan Y, Cho ES, Maltby KM, Jiang R (2005) Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev Biol 288 : 582–594. 16223478
31. Donovan PJ, Stott D, Cairns LA, Heasman J, Wylie CC (1986) Migratory and postmigratory mouse primordial germ cells behave differently in culture. Cell 44 : 831–838. 3955652
32. Dudley B, Palumbo C, Nalepka J, Molyneaux K (2010) BMP signaling controls formation of a primordial germ cell niche within the early genital ridges. Dev Biol 343 : 84–93. doi: 10.1016/j.ydbio.2010.04.011 20417197
33. Godin I, Wylie C, Heasman J (1990) Genital ridges exert long-range effects on mouse primordial germ cell numbers and direction of migration in culture. Development 108 : 357–363. 2351075
34. Medeiros LA, Dennis LM, Gill ME, Houbaviy H, Markoulaki S, et al. (2011) Mir-290–295 deficiency in mice results in partially penetrant embryonic lethality and germ cell defects. Proc Natl Acad Sci U S A 108 : 14163–14168. doi: 10.1073/pnas.1111241108 21844366
35. Luoh SW, Bain PA, Polakiewicz RD, Goodheart ML, Gardner H, et al. (1997) Zfx mutation results in small animal size and reduced germ cell number in male and female mice. Development 124 : 2275–2284. 9187153
36. Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, et al. (2006) Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A 103 : 2474–2479. 16461896
37. Bowles J, Knight D, Smith C, Wilhelm D, Richman J, et al. (2006) Retinoid signaling determines germ cell fate in mice. Science 312 : 596–600. 16574820
38. Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, et al. (2006) In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 38 : 1430–1434. 17115059
39. Koubova J, Hu YC, Bhattacharyya T, Soh YQ, Gill ME, et al. (2014) Retinoic acid activates two pathways required for meiosis in mice. PLoS Genet 10: e1004541. doi: 10.1371/journal.pgen.1004541 25102060
40. Menke DB, Koubova J, Page DC (2003) Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol 262 : 303–312. 14550793
41. Yao HH, DiNapoli L, Capel B (2003) Meiotic germ cells antagonize mesonephric cell migration and testis cord formation in mouse gonads. Development 130 : 5895–5902. 14561636
42. Watt AJ, Battle MA, Li J, Duncan SA (2004) GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci U S A 101 : 12573–12578. 15310850
43. Hayashi S, McMahon AP (2002) Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244 : 305–318. 11944939
44. Mugford JW, Sipila P, McMahon JA, McMahon AP (2008) Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol 324 : 88–98. doi: 10.1016/j.ydbio.2008.09.010 18835385
45. Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, et al. (2008) Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454 : 109–113. doi: 10.1038/nature07060 18568026
46. Grun D, Kester L, van Oudenaarden A (2014) Validation of noise models for single-cell transcriptomics. Nat Methods 11 : 637–640. doi: 10.1038/nmeth.2930 24747814
47. Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B (1997) Male-specific cell migration into the developing gonad. Curr Biol 7 : 958–968. 9382843
48. Ozdzenski W (1972) Differentiation of the genital ridges of mouse embryos in the kidney of adult mice. Arch Anat Microsc Morphol Exp 61 : 267–278. 4667754
49. Byskov AG, Fenger M, Westergaard L, Andersen CY (1993) Forskolin and the meiosis inducing substance synergistically initiate meiosis in fetal male germ cells. Mol Reprod Dev 34 : 47–52. 8418816
50. Hu YC, de Rooij DG, Page DC (2013) Tumor suppressor gene Rb is required for self-renewal of spermatogonial stem cells in mice. Proc Natl Acad Sci U S A 110 : 12685–12690. doi: 10.1073/pnas.1311548110 23858447
51. Enders GC, May JJ 2nd (1994) Developmentally regulated expression of a mouse germ cell nuclear antigen examined from embryonic day 11 to adult in male and female mice. Dev Biol 163 : 331–340. 8200475
52. Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ (2007) Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 316 : 744–747. 17446352
53. Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, et al. (1999) Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 13 : 424–436. 10049358
54. Tam PP, Zhou SX (1996) The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev Biol 178 : 124–132. 8812114
Štítky
Genetika Reprodukčná medicína
Článek NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY ModuleČlánek Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell LongevityČlánek HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and MiceČlánek LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male FertilityČlánek The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAsČlánek Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick VectorČlánek Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53Článek The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in NematodesČlánek The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse SpermatocytesČlánek The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 3- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
- Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
- A Genomic Duplication is Associated with Ectopic Eomesodermin Expression in the Embryonic Chicken Comb and Two Duplex-comb Phenotypes
- Genome-wide Association Study and Meta-Analysis Identify as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy
- Mutations of Human , Encoding the Mitochondrial Asparaginyl-tRNA Synthetase, Cause Nonsyndromic Deafness and Leigh Syndrome
- Exome Sequencing in an Admixed Isolated Population Indicates Variants Confer a Risk for Specific Language Impairment
- Genome-Wide Association Studies in Dogs and Humans Identify as a Risk Variant for Cleft Lip and Palate
- Rapid Evolution of Recombinant for Xylose Fermentation through Formation of Extra-chromosomal Circular DNA
- The Ribosome Biogenesis Factor Nol11 Is Required for Optimal rDNA Transcription and Craniofacial Development in
- Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
- Maternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity
- Clonality and Evolutionary History of Rhabdomyosarcoma
- HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and Mice
- Methylation-Sensitive Expression of a DNA Demethylase Gene Serves As an Epigenetic Rheostat
- BREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
- Seizures Are Regulated by Ubiquitin-specific Peptidase 9 X-linked (USP9X), a De-Ubiquitinase
- The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
- A Cascade of Iron-Containing Proteins Governs the Genetic Iron Starvation Response to Promote Iron Uptake and Inhibit Iron Storage in Fission Yeast
- Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
- LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male Fertility
- Cis-Regulatory Mechanisms for Robust Olfactory Sensory Neuron Class-restricted Odorant Receptor Gene Expression in
- Effects on Murine Behavior and Lifespan of Selectively Decreasing Expression of Mutant Huntingtin Allele by Supt4h Knockdown
- HDAC4-Myogenin Axis As an Important Marker of HD-Related Skeletal Muscle Atrophy
- A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex
- Selective and Genetic Constraints on Pneumococcal Serotype Switching
- Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
- The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAs
- Accumulation of Glucosylceramide in the Absence of the Beta-Glucosidase GBA2 Alters Cytoskeletal Dynamics
- Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities
- The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair
- Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick Vector
- Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
- The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors
- PHABULOSA Controls the Quiescent Center-Independent Root Meristem Activities in
- DNA Polymerase ζ-Dependent Lesion Bypass in Is Accompanied by Error-Prone Copying of Long Stretches of Adjacent DNA
- Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus
- Zinc Finger Independent Genome-Wide Binding of Sp2 Potentiates Recruitment of Histone-Fold Protein Nf-y Distinguishing It from Sp1 and Sp3
- GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters
- Neurospora Importin α Is Required for Normal Heterochromatic Formation and DNA Methylation
- Ccr4-Not Regulates RNA Polymerase I Transcription and Couples Nutrient Signaling to the Control of Ribosomal RNA Biogenesis
- Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53
- A Systems-Level Interrogation Identifies Regulators of Blood Cell Number and Survival
- Morphological Mutations: Lessons from the Cockscomb
- Genetic Interaction Mapping Reveals a Role for the SWI/SNF Nucleosome Remodeler in Spliceosome Activation in Fission Yeast
- The Role of China in the Global Spread of the Current Cholera Pandemic
- The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
- A Zinc Finger Motif-Containing Protein Is Essential for Chloroplast RNA Editing
- Resistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
- Small Regulatory RNA-Induced Growth Rate Heterogeneity of
- Mitochondrial Dysfunction Reveals the Role of mRNA Poly(A) Tail Regulation in Oculopharyngeal Muscular Dystrophy Pathogenesis
- Complex Genomic Rearrangements at the Locus Include Triplication and Quadruplication
- Male-Biased Aganglionic Megacolon in the TashT Mouse Line Due to Perturbation of Silencer Elements in a Large Gene Desert of Chromosome 10
- Sex Ratio Meiotic Drive as a Plausible Evolutionary Mechanism for Hybrid Male Sterility
- Tertiary siRNAs Mediate Paramutation in .
- RECG Maintains Plastid and Mitochondrial Genome Stability by Suppressing Extensive Recombination between Short Dispersed Repeats
- Escape from X Inactivation Varies in Mouse Tissues
- Opposite Phenotypes of Muscle Strength and Locomotor Function in Mouse Models of Partial Trisomy and Monosomy 21 for the Proximal Region
- Glycosyl Phosphatidylinositol Anchor Biosynthesis Is Essential for Maintaining Epithelial Integrity during Embryogenesis
- Hyperdiverse Gene Cluster in Snail Host Conveys Resistance to Human Schistosome Parasites
- The Class Homeodomain Factors and Cooperate in . Embryonic Progenitor Cells to Regulate Robust Development
- Recombination between Homologous Chromosomes Induced by Unrepaired UV-Generated DNA Damage Requires Mus81p and Is Suppressed by Mms2p
- Synergistic Interactions between Orthologues of Genes Spanned by Human CNVs Support Multiple-Hit Models of Autism
- Gene Networks Underlying Convergent and Pleiotropic Phenotypes in a Large and Systematically-Phenotyped Cohort with Heterogeneous Developmental Disorders
- The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
- Combinatorial Control of Light Induced Chromatin Remodeling and Gene Activation in
- Linking Aβ42-Induced Hyperexcitability to Neurodegeneration, Learning and Motor Deficits, and a Shorter Lifespan in an Alzheimer’s Model
- The Complex Contributions of Genetics and Nutrition to Immunity in
- NatB Domain-Containing CRA-1 Antagonizes Hydrolase ACER-1 Linking Acetyl-CoA Metabolism to the Initiation of Recombination during . Meiosis
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
- Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption
- A Genomic Portrait of Haplotype Diversity and Signatures of Selection in Indigenous Southern African Populations
- Sequence Features and Transcriptional Stalling within Centromere DNA Promote Establishment of CENP-A Chromatin
- Inhibits Neuromuscular Junction Growth by Downregulating the BMP Receptor Thickveins
- Replicative DNA Polymerase δ but Not ε Proofreads Errors in and in
- Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in
- The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
- A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension
- Pervasive Variation of Transcription Factor Orthologs Contributes to Regulatory Network Evolution
- Network Analyses Reveal Novel Aspects of ALS Pathogenesis
- A Role for the Budding Yeast Separase, Esp1, in Ty1 Element Retrotransposition
- Nab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
- A RecA Protein Surface Required for Activation of DNA Polymerase V
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Clonality and Evolutionary History of Rhabdomyosarcoma
- Morphological Mutations: Lessons from the Cockscomb
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



