-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
Neurons of the statoacoustic ganglion (SAG) transmit impulses from the inner ear necessary for hearing and balance. SAG cells exhibit a complex pattern of development, regulation of which remains poorly understood. Here we show that transcription factor Tfap2a coordinates multiple cell signaling pathways needed to regulate the quantity and pace of SAG neuron production. SAG progenitors originate within the developing inner ear and then migrate out of the ear towards the hindbrain before forming mature neurons. We showed previously that Fgf initiates formation of SAG progenitors in the inner ear, but rising levels of Fgf signaling eventually terminate this process. Elevated Fgf also stimulates proliferation of SAG progenitors outside the ear and delays their maturation. Notch signaling is also known to limit SAG development. Tfap2a governs the strength of Fgf and Notch signaling by activating expression of Bmp7a, which inhibits Fgf and Notch. Together these signals stabilize the pool of SAG progenitors outside the ear by equalizing rates of maturation and proliferation. This balance is critical for sustained accumulation of SAG neurons during larval growth as well as regeneration following neural damage. These findings could inform development of stem cell therapies to correct auditory neuropathies in humans.
Published in the journal: Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling. PLoS Genet 11(3): e32767. doi:10.1371/journal.pgen.1005037
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005037Summary
Neurons of the statoacoustic ganglion (SAG) transmit impulses from the inner ear necessary for hearing and balance. SAG cells exhibit a complex pattern of development, regulation of which remains poorly understood. Here we show that transcription factor Tfap2a coordinates multiple cell signaling pathways needed to regulate the quantity and pace of SAG neuron production. SAG progenitors originate within the developing inner ear and then migrate out of the ear towards the hindbrain before forming mature neurons. We showed previously that Fgf initiates formation of SAG progenitors in the inner ear, but rising levels of Fgf signaling eventually terminate this process. Elevated Fgf also stimulates proliferation of SAG progenitors outside the ear and delays their maturation. Notch signaling is also known to limit SAG development. Tfap2a governs the strength of Fgf and Notch signaling by activating expression of Bmp7a, which inhibits Fgf and Notch. Together these signals stabilize the pool of SAG progenitors outside the ear by equalizing rates of maturation and proliferation. This balance is critical for sustained accumulation of SAG neurons during larval growth as well as regeneration following neural damage. These findings could inform development of stem cell therapies to correct auditory neuropathies in humans.
Introduction
Vestibular and auditory information is transmitted from the inner ear to the hindbrain via neurons of VIII.th cranial ganglion, also known as the stato-acoustic ganglion (SAG). SAG neurons are formed by a complex but poorly understood multi-step process that begins in the otic vesicle, the precursor of the inner ear. In the first step, SAG neuroblasts are specified in the floor of the otic vesicle and are marked by the expression of proneural gene neurogenin1 (ngn1) [1,2]. After specification, neuroblasts delaminate from the otic epithelium via epithelial-mesenchymal transition and migrate to a region between the otic vesicle and hindbrain. In zebrafish, markers of later stages of differentiation are usually not expressed within the otic epithelium. Upon delamination, however, neuroblasts quickly lose expression of ngn1 and upregulate the related factor neurod [1,3]. neurod-expressing cells form a population of migrating and proliferating precursors called the transit-amplifying (TA) pool [4]. As cells in the TA pool differentiate into mature neurons they lose expression of neurod and upregulate early neuronal markers islet1 and islet2b [5]. Newly formed neurons extend processes bi-directionally to connect sensory epithelia with central targets in the hindbrain. SAG development in chick and mouse embryos follows a similar course except that transit-amplification and expression of NeuroD and Isl1/2 begin while neuroblasts still reside within the otic epithelium [2,6,7].
We previously showed that Fgf signaling regulates each step in SAG development in zebrafish [5]. Specification of SAG neuroblasts is initiated by a low level of Fgf signaling. As SAG neurons mature they begin to express fgf5 such that rising levels of Fgf eventually become inhibitory to ngn1 expression in the otic vesicle. Consequently, neuroblast specification starts to decline after 24 hpf and ceases entirely by 42 hpf [5,8]. Elevated Fgf also delays terminal differentiation of cells in the TA pool. The TA pool is thereby maintained as a relatively stable population in which the rate of proliferation closely matches the rate of terminal differentiation.
The otic vesicle originates from an ectodermal thickening called the otic placode. The otic placode, along with all other cranial placodes, emerges from a contiguous region of pre-placodal ectoderm (PPE) that forms around the anterior neural plate by the end of gastrulation [9,10]. In zebrafish competence to form PPE is regulated by four transcription factors: Tfap2a, Tfap2c, Gata3 and Foxi1 [9,11]. These transcription factors are also essential for later development of a subset of cranial placodes, including the otic placode. For example, in response to inductive Fgf signaling foxi1 expression upregulates in nascent otic/epibranchial placodes; and disruption of foxi1 leads to severe deficiencies of epibranchial and otic tissue in zebrafish [12–14]. A similar role has been shown recently for Foxi3 in mouse and chick [15,16]. Expression of gata3 also regulates otic development, becoming localized to the nascent otic placode [17] and to discrete regions of the otic vesicle [18–20]. Disruption of Gata3 in mouse causes severe defects in otic vesicle development [18], including deficiencies of sensory epithelia and improper wiring of auditory neurons [21–23]. In contrast, less is known about later roles of tfap2a/c. tfap2a is best known for its role in the early differentiation and survival of neural crest cells [24–32] and together with tfap2c is indispensible for neural crest specification in zebrafish [26,28]. However, whether tfap2a/c genes regulate later development in the otic placode and vesicle has not been investigated.
Here we report that tfap2a is expressed throughout the nascent otic placode and is later restricted to a ventrolateral region in the otic vesicle overlapping with the neurogenic domain. Misexpression of tfap2a leads to excess specification and precocious differentiation of SAG neurons whereas knockdown of tfap2a causes reduced neurogenesis and delayed differentiation of SAG precursors. Further investigation revealed that tfap2a acts non-autonomously through upregulation of bmp7a, which in turn restricts Fgf and Notch signaling to promote specification and differentiation of SAG precursors.
Results
Expression of tfap2a during otic development
To begin to assess potential functions of tfap2a in otic development, we examined expression of tfap2a in the otic placode and early otic vesicle in zebrafish. At 14 hpf (10 somites) when otic cells first form a morphological placode, tfap2a is expressed broadly throughout the placode as shown by co-staining for the early otic marker pax2a (Fig. 1A-B). The level of tfap2a expression varies, with higher levels in dorsal and lateral otic cells. Otic expression in general appears much weaker than in surrounding neural crest cells. By 24 hpf, expression of tfap2a in the otic vesicle is restricted to ventrolateral cells. This domain partially overlaps with the neurogenic domain of the otic vesicle, marked by the proneural gene ngn1 (Fig. 1C-D). Neurogenesis declines sharply by 30 hpf and ceases entirely by 42 hpf [5,8]. Similarly, the level of tfap2a gradually declines in the neurogenic domain after 30 hpf and is no longer detectable by 48 hpf (Fig. 1E-I). Despite initial expression of tfap2a in at least some neuroblasts in the otic vesicle, expression is lost in most neural precursors as they delaminate from the otic vesicle. Mature neurons of the statoacoustic ganglion (SAG), marked by expression of Isl1, show no detectable expression of tfap2a (Fig. 1J). These data are consistent with the possibility that tfap2a is involved in at least some aspects of neurogenesis in the otic vesicle. Expression patterns of tfap2a, ngn1, and other key genes involved in SAG development are summarized in Fig. 1K.
Fig. 1. Conserved expression of tfap2a during otic neurogenesis. 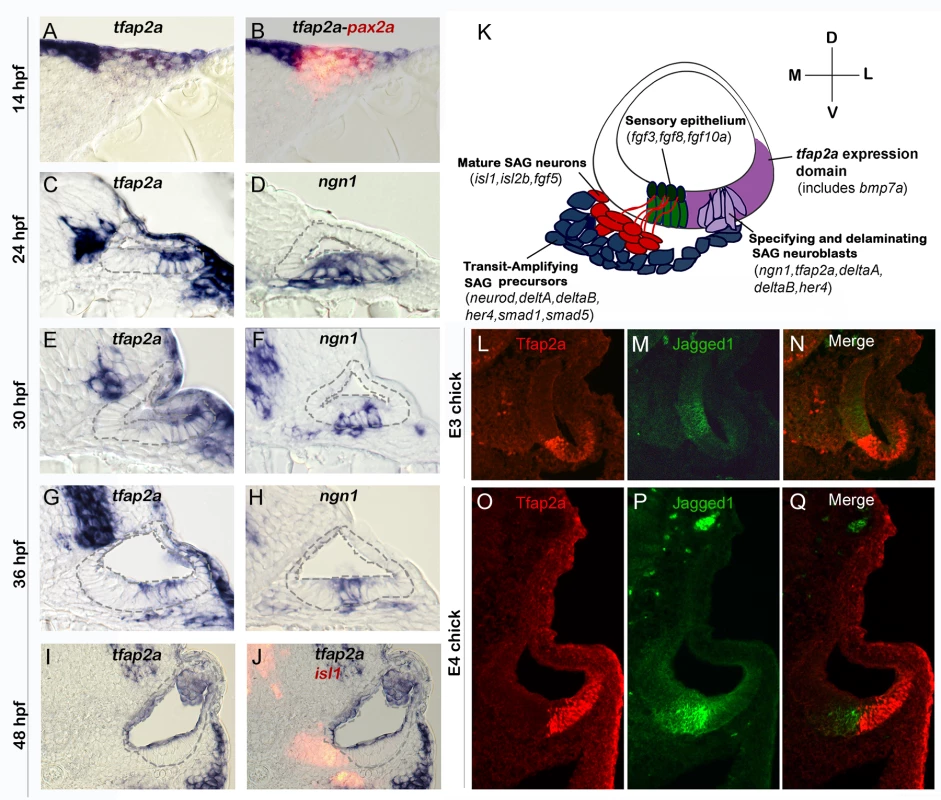
All images show cross-sections of the otic placode or vesicle in wild type zebrafish embryos (A-J) or chick embryos (L-Q) with a dorsal up and medial to the left. (A, B) At 14 hpf (10 somites) pax2a (red) marks the precursor cells in the emerging otic placode that are co-labeled with tfap2a (blue). (C-H) Cross-sections through the widest part of the neurogenic domain of the otic vesicle, just posterior to the utricular macula. The outer and inner edges of the otic vesicle are outlined. Patterns of ngn1 or tfap2a are shown at the indicated times. tfap2a is expressed in the ventrolateral part of the otic vesicle, which partially overlaps the domain of ngn1 expression. (I-J) Cross-sections passing through the utricular macula of specimens co-stained for Isl1 (red) and tfap2a (blue) at 48 hpf. Expression of tfap2a is not detected in the floor of the otic vesicle or in the mature SAG neurons at this time. (K) Schematic summary of SAG development in zebrafish, including regional markers. Neuroblasts are specified and delaminate from the otic vesicle (light purple) adjacent to nascent sensory epithelia (green). Recently delaminated neuroblasts migrate towards hindbrain and continue to proliferate, forming the transit-amplifying pool (blue). Neuroblasts then stop dividing and differentiate into mature neurons (red). Relevant genes expressed in each domain are indicated. Expression of tfap2a (dark purple) overlaps the neurogenic domain, as well as the domain of bmp7a expression. Note that all of the tissues indicated express Fgf-target genes (etv5b and spry4) and transducers of Bmp (smad1 and smad5), but transit amplifying SAG precursors show specific upregulation of smad1 and smad5 [37]. (L-Q) Cross-sections through the otic vesicle of chick embryos at days 3 and 4 (E3 and E4). The sensory region is labeled with Jagged-1 (green). Tfap2a (red) is expressed in the ventrolateral otic domain in chick embryos similar to the pattern observed in zebrafish. To examine the possibility that expression is conserved in amniote vertebrates, we examined expression of Tfap2a in chick embryos. In agreement with the patterns observed in zebrafish, chick embryos also show expression in ventrolateral regions of the otic vesicle (Fig. 1L-Q). Moreover, in chick as in zebrafish, the domain of Tfap2a expression abuts the sensory domain with little or no overlap. The similar expression patterns seen in zebrafish and chick potentially reflect a broadly conserved role in early otic development.
Effects of Tfap2a on neurogenesis in the otic vesicle
To explore the role of tfap2a, we characterized the effects of tfap2a loss of function or misexpression on neurogenesis in the otic vesicle in zebrafish. Disruption of tfap2a causes no overt defects in morphogenesis of the otic vesicle [27]. However, tfap2a-/- (lockjaw) mutants produced only half the normal number of ngn1-positive neuroblasts in the otic epithelium at 24 hpf, the stage when neurogenesis normally peaks in wild-type embryos (Fig. 2A, C). At later stages, too, tfap2a-/- mutants continued to show significant deficiencies in neuroblast specification (Fig. 2D, F, M). Similar results were seen in tfap2a morphants (tfap2a-MO, Fig. 2M). We next used a heat-shock inducible transgenic line, hs:tfap2a, to misexpress tfap2a at various developmental stages. Activation of hs:tfap2a at 20 hpf increased the peak number of neuroblasts in the otic vesicle at 24 hpf by 30% (58.33±3.21 ngn1+ cells in hs:tfap2a embryos vs. 44.5±2.65 cells in controls, Fig. 2B). Activation of hs:tfap2a at 24 hpf prolonged the phase of peak neurogenesis, resulting in twice the normal number of neuroblasts at 28 hpf (Fig. 2D, E, M). Despite the initial surge, however, the number of ngn1+ neuroblasts subsequently declined sharply in transgenic embryos, dropping below the level seen in control embryos at 30 hpf and thereafter (Fig. 2G, H, M). Because transgene activity decays 5 hours after heat shock (S1 Fig.), we tested the effects of serial heat shocks at 24 hpf and 29 hpf. This resulted in elevated neurogenesis through at least 32 hpf, when transgenic embryos had twice as many ngn1+ cells in the otic epithelium as in control embryos (Fig. 2G, I, N). Neurogenesis in the ear normally ceases by 42 hpf [5,8], prompting us to investigate whether termination of neurogenesis could be altered by later misexpression of tfap2a. Indeed, activation of hs:tfap2a at 38 hpf prolonged specification of neural precursors, as ngn1+ neuroblasts were still present in the otic vesicle through at least 43 hpf (Fig. 2J, K). In contrast, activation of hs:tfap2a at 40 hpf was not sufficient to prevent or delay the cessation of neurogenesis in the otic vesicle (Fig. 2L). Overall these results indicate that tfap2a enhances neurogenesis in the ear but cannot induce ectopic neurogenesis beyond the floor of the otic vesicle nor reactivate neurogenesis after it has stopped.
Fig. 2. Tfap2a enhances otic neurogenesis. 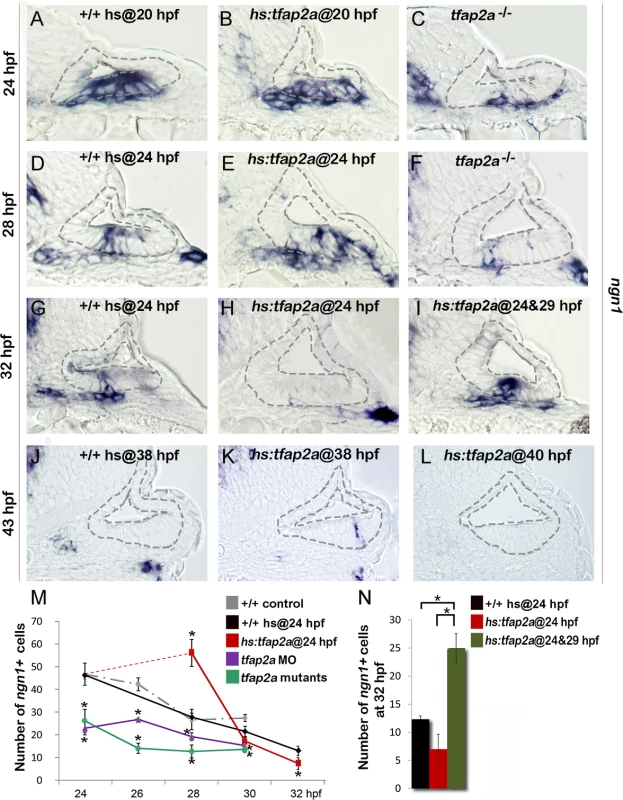
(A-L) Cross-sections (medial left, dorsal up) through the otic region just posterior to the utricular macula showing ngn1 expression in +/+ control embryos, hs:tfap2a embryos and tfap2a-/- mutants at the indicated times. Wild-type and hs:tfap2a embryos were heat shocked as indicated in each panel. Overexpression of tfap2a increases the number of neuroblasts in the otic vesicle whereas loss of tfap2a slows down and decreases otic neurogenesis. The outer and inner edges of the otic vesicle are outlined in each image. (M, N) Mean and standard deviation of the total number of ngn1 positive cells in the otic epithelium from 24 to 32 hpf for the genotypes indicated in the color key (counted from serial sections, n = 3–7 ears per time point). Asterisks (*) indicate statistically significant differences between groups indicated by brackets (N) or compared to control embryos (M). Effects of Tfap2a on later stages of SAG development
We next examined whether altered levels of neuroblast specification caused by manipulating tfap2a function were followed by changes in later stages of neuronal differentiation. Normally, newly specified neuroblasts delaminate from the otic vesicle, lose expression of ngn1 and initiate expression of neurod, a marker of the “transit-amplifying” (TA) stage of development [1,5] (Fig. 1K). Surprisingly, despite reduced neurogenesis in tfap2a-/- mutants and tfap2a morphants, the number of neurod+ TA cells was greater than normal at every time point examined (Fig. 3B, E, H, J). Conversely, despite the large increase in neurogenic specification caused by overexpression of tfap2a at 24 hpf, the number of neurod-expressing TA cells was reduced at all time points through 48 hpf (Fig. 3C, F, I, J). We hypothesized that changes in the size of the TA pool reflect changes in the overall pace of neuronal differentiation. To test this we examined expression of Isl1, a marker of mature SAG neurons. We observed that tfap2a-/- mutants and tfap2a morphants produced fewer than normal neurons despite the increased number of neurod+ cells (Fig. 4E-H, T). The deficiency in neuronal maturation persisted in tfap2a-/- mutants through at least 72 hpf (S2 Fig.). In contrast, activation of hs:tfap2a had the opposite effect. Activation of hs:tfap2a during placodal stages elevated accumulation of Isl1+ neurons at 30 hpf, and the fold-stimulation was progressively increased with successively later stages of activation (Fig. 4M). Activation of hs:tfap2a at 24 hpf led to maximal accumulation of neurons, with nearly twice the normal number of Isl1+ neurons observed in transgenic embryos at 30 hpf and 37 hpf (Fig. 4I-L, M, T). Interestingly, transgene activation at these early stages enhanced accumulation of anterior (vestibular) SAG neurons but not posterior (auditory) neurons (Fig. 4D, L). However, activating hs:tfap2a expression at 29 hpf increased accumulation of both anterior and posterior neurons (Fig. 4N-P), consistent with our previous findings that auditory neurons are specified at later stages than vestibular neurons [5]. Together these data indicate that disruption of tfap2a inhibits neurogenesis and slows neural maturation, whereas misexpression of tfap2a stimulates neuroblast specification and accelerates subsequent differentiation.
Fig. 3. Tfap2a regulates the number of transit-amplifying SAG precursors. 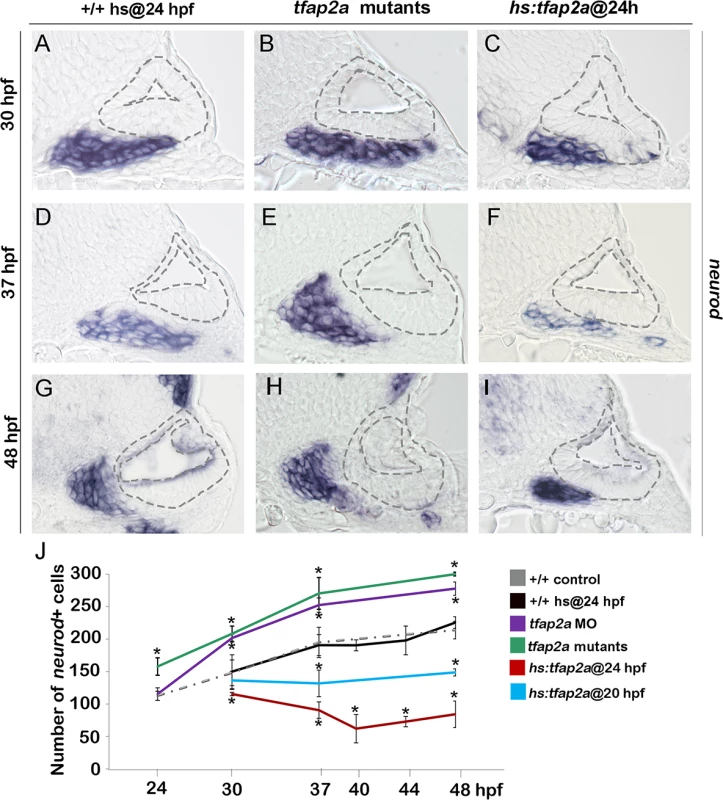
(A-I) Cross-sections (medial left, dorsal up) at the level of the utricular macula showing neurod expression in +/+ control, tfap2a mutants and hs:tfap2a embryos. Wild-type and hs:tfap2a embryos were heat shocked at 24 hpf. Disruption of tfap2a leads to accumulation of excess TA cells whereas tfap2a overexpression decreases the number of the TA cells. The outer and inner edges of the otic vesicle are outlined in each image. (J) Mean and standard deviation of the total number of neurod positive SAG precursors for the genotypes and conditions indicated in the color key (counted from serial sections, n = 3–6 ears per time point). Asterisks (*) indicate significant differences from control specimens. Fig. 4. Tfap2a regulates maturation of SAG neurons. 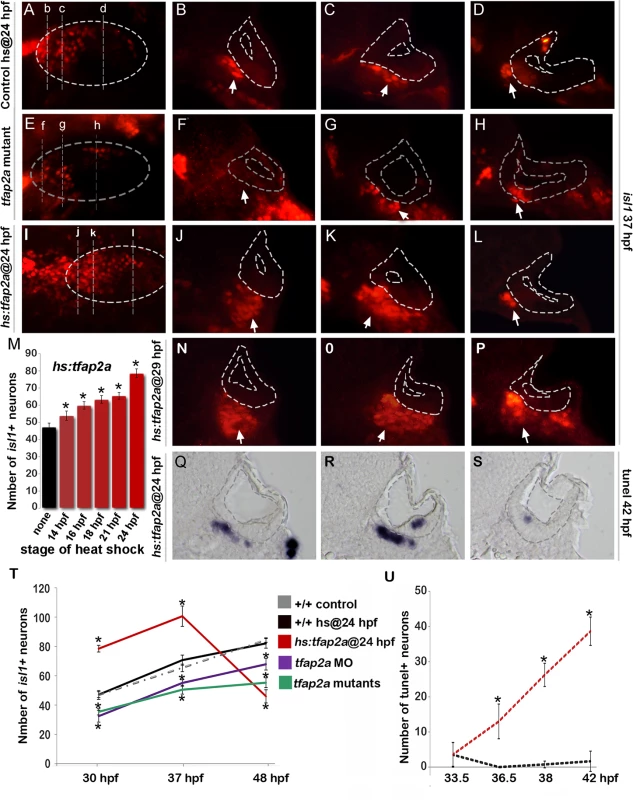
(A-L) Images of anti-Isl1 antibody staining in control embryos (A-D), tfap2a-/- mutants (E-H) and hs:tfap2a embryos (I-L) at 37 hpf. Control and hs:tfap2a embryos were heat shocked at 24 hpf. Whole-mount specimens (A, E, I) show dorsolateral (anterior to the left) and indicate planes of cross-section in (B-D), (F-H) and (J-L). Cross-sections are oriented with dorsal up and medial to the left. The otic vesicle is outlined in each image. White arrows indicate the SAG population to help distinguish it from other Isl1+ populations present in some sections. (M) Total number of Isl1+ neurons in hs:tfap2a embryos at 30 hpf following heat shock at the indicated times (n = 10–15 each). (N-P) Cross-sections of a hs:tfap2a specimen at 37 hpf following heat shock at 29 hpf. Planes of section are similar to those shown in (I). (Q-S) Cross-sections of a hs:tfap2a embryo stained for TUNEL positive SAG neurons at 42 hpf. (T) Mean and standard deviation of the total number of Isl1+ SAG neurons at the indicated times in +/+ embryos, hs:tfap2a embryos, tfap2a-/- mutants and tfap2a morphants (n = 7–34 embryos per time point). (U) Mean and standard deviation of the total number of TUNEL positive SAG neurons at the indicated times in control embryos and hs:tfap2a embryos (counted from serial sections, n = 3–6 ears per time point). Asterisks (*) indicate statistically significant differences compared to control embryos. Importantly, although activation of hs:tfap2a at 24 hpf led to elevated accumulation of mature neurons through 37 hpf, the number of mature neurons fell dramatically thereafter to roughly half normal by 48 hpf (Fig. 4T). This decline was preceded by a marked increase in the rate of apoptosis amongst mature neurons (Fig. 4Q-S, U). Elevated cell death possibly reflects insufficient duration of earlier stages of differentiation and consequent misregulation of factors required for neuronal survival (see below).
Effects on patterning in the otic vesicle
To determine whether the above changes in SAG development resulted from mis-patterning of the otic vesicle, we examined expression of several regional markers. In tfap2a-/- mutants and tfap2a morphants, domains of the dorsal marker dlx3b and the ventrolateral marker otx1b were slightly contracted (Fig. 5B, E). Domains of pax5, an anterior-ventral marker of the utricular macula, and pou3f3b, a posterior-medial marker of the saccular macula were not altered (Fig. 5H, K). Additionally, sensory epithelia appeared to develop normally and there were no obvious changes in hair cell development through 54 hpf (Fig. 5M). Activation of hs:tfap2a at 24 hpf led to weak contraction otx1b but a substantial expansion of the dlx3b (Fig. 5C, F). pax5 was expressed in its normal domain but at a reduced level (Fig. 5L). Expression of pou3f3b was normal (Fig. 5I) and there were no changes in accumulation of hair cells through 54 hpf (Fig. 5M). Thus, gross patterning in the otic vesicle was nearly normal in tfap2a-/- mutants and morphants, though substantial changes were seen in one marker (dlx3b) following overexpression of tfap2a. Such changes in gene expression likely reflect changes in cell signaling as described below.
Fig. 5. Effects of tfap2a knockdown and overexpression on otic vesicle patterning. 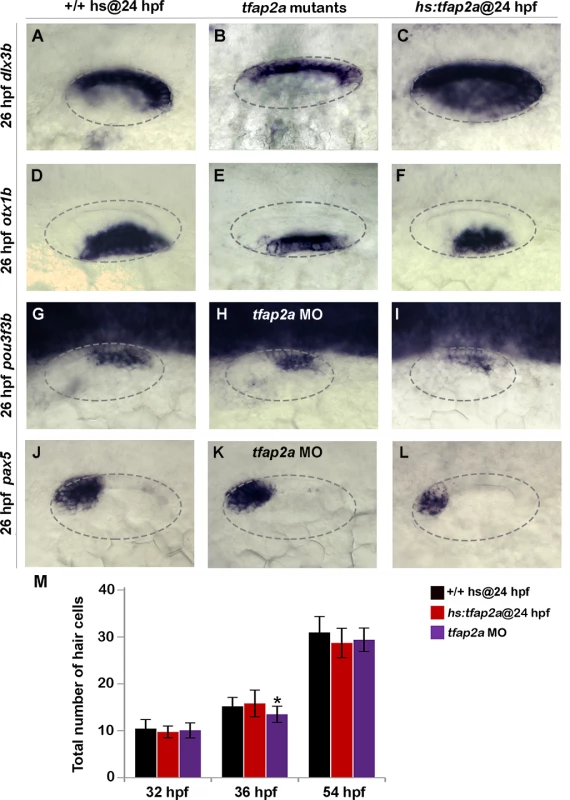
(A-L) Whole-mount images (dorsal up, anterior left) showing dorsolateral views of the otic vesicle (outlined) in control embryos, tfap2a-/- mutants and tfap2a morphants, and hs:tfap2a embryos for the indicated genes at 26 hpf. (M) Mean and standard deviation of the total number of hair cells in utricular and saccular maculae of control and hs:tfap2a embryos and tfap2a morphants at the indicated times (n = 24 embryos each). Data were obtained by counting GFP-positive hair cells in the sensory epithelia of brn3c:Gfp transgenic embryos. Accumulation of hair cells was normal except in tfap2a morphants at 36 hpf (*), which showed a small but significant decrease relative to the control. Effects on Notch and Fgf signaling
We next investigated whether Tfap2a activity influences Notch and Fgf signaling, pathways known to regulate development of SAG neurons. For example, Delta-Notch signaling is normally activated by neurogenic factors Ngn1 and Neurod and serves as a feedback inhibitor of neurogenesis [2,33,34]. Disruption of Delta-Notch signaling leads to excess neural specification and precocious differentiation [35], similar to the effects of misexpression of tfap2a. Here we observed that expression of deltaA and deltaB was increased in tfap2a-/- mutants and, conversely, delta gene expression was strongly impaired following overexpression of tfap2a (Fig. 6A-F). Similar changes were observed for the Notch target gene her4, which increased in tfap2a-/- mutants and decreased following activation of hs:tfap2a (Fig. 6G-I). Thus Tfap2a appears to inhibit Notch activity during development of SAG neurons by inhibiting expression of Notch ligands.
Fig. 6. Tfap2a regulates the level of Fgf and Notch Signaling in the otic vesicle. 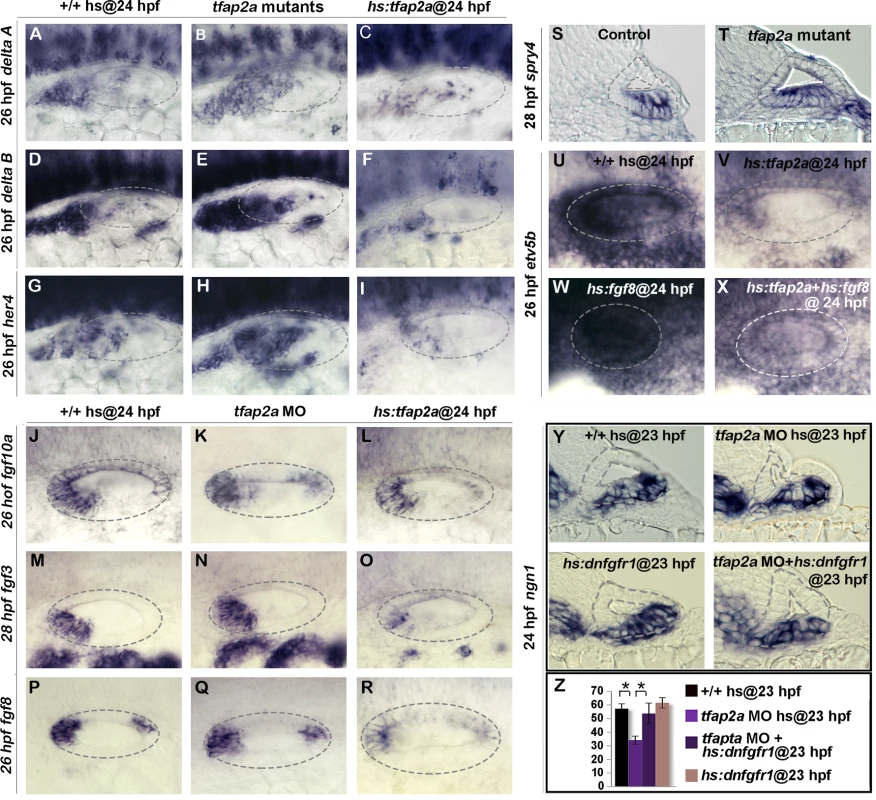
(A-V) Whole-mount images (dorsal up, anterior left) showing dorsolateral views of the otic vesicle (outlined). (A-R) Expression of the indicated genes in wild-type embryos, hs:tfap2a embryos and tfap2a-/- mutants (A-I) or tfap2a morphants (J-R) at 26 hpf (A-L, P-R) and 28 (M-O) hpf. (S, T) Cross-sections (dorsal up, medial left) passing through the utricular macula show spry4 expression at 28 hpf in a control embryo and tfap2a-/- mutant. (U-X) Whole-mounts showing expression of etv5b at 26 hpf. Activation of hs:tfap2a diminishes etv5b expression (U, V), activation of hs:fgf8 leads to global upregulation of etv5b (W), and co-activation of hs:fgf8 and hs:tfap2a restores etv5b to near normal (X). (Y) Cross-sections (dorsal up, medial left) passing just posterior to the utricular macula showing ngn1 at 24 hpf following a 35°C heat shock at 23 hpf. Reduction in the ngn1 domain caused by knockdown of tfap2a is rescued by weak activation of hs:dnfgfr1. (Z) Mean and standard deviation of the total number of ngn1 positive cells in the otic epithelium at 24 hpf for the genotypes and knockdowns indicated in the color key (counted from serial sections, n = 3–6 ears per time point). Asterisks (*) indicate statistically significant differences between the groups indicated in brackets. Fgf signaling has a more complex role in neural development in the ear. At early stages specification of neuroblasts requires Fgf. As development proceeds, however, rising levels of Fgf5 secreted by mature SAG neurons terminates specification of new neuroblasts and delays differentiation of TA cells into mature neurons [5] (Fig. 1K). Weak impairment of Fgf signaling can prolong neurogenesis and accelerate neural differentiation, mimicking aspects of the tfap2a overexpression phenotype. We therefore examined expression of various fgf genes in the otic vesicle. Knockdown of tfap2a did not appear to alter expression of fgf3, fgf8, or fgf10a (Fig. 6K, N, Q). Activation of hs:tfap2a at 24 hpf led to reduced expression of fgf3 and fgf8, but expression of fgf10a was not altered (Fig. 6L, O, R). To look for changes in Fgf signaling, we examined expression of Fgf-target genes spry4, etv4 (pea3) and etv5b (erm). Although etv4 and etv5b expression appeared normal in tfap2a morphants (S3D, H Fig.), spry4 was expressed at higher levels and in a broader domain than normal in tfap2a-/- mutants, indicating that Fgf signaling was elevated (Fig. 6S-T). Conversely, activation of hs:tfap2a at 24 hpf reduced expression of etv4, etv5b and spry4 by 26 hpf, indicating a reduced level of Fgf signaling (Fig. 6U-V, S3A-J Fig.). Fgf signaling remained reduced through 28 hpf but started to recover after 30 hpf as transgene activity decayed (S3K-Q Fig.). Thus, Tfap2a appears to limit Fgf signaling, in part through reducing expression of fgf genes.
To test whether Tfap2a can influence Fgf signaling independently of ligand expression, we co-misexpressed tfap2a and fgf8. While activation of hs:fgf8 led to global upregulation of etv5b, co-activation of hs:tfap2a with hs:fgf8 at 24 hpf partially suppressed expression of etv5b (Fig. 6W-X). This indicates that tfap2a can inhibit Fgf signaling at a level downstream of ligand accumulation.
To test the functional significance of elevated Fgf signaling in tfap2a morphants, we examined whether weakly inhibiting Fgf signaling could rescue the neurogenic deficiencies in tfap2a morphants. Indeed, reducing the level of Fgf signaling via low-level activation of hs:dnfgfr1 (dominant-negative Fgf receptor) at 35°C restored ngn1+ cell counts to normal in tfap2a morphants (Fig. 6Y-Z). This suggests that elevated Fgf signaling partially accounts for the reduced neuroblast specification in tfap2a morphants and mutants.
Because Tfap2a appears to dampen both Fgf and Notch signaling, we tested whether weakening both pathways by other means could mimic the effects of tfap2a overexpression. Low level activation of hs:dnfgfr1 at 36.5°C increased the number of ngn1+ neuroblasts in the ear by ~30% (Fig. 7B, M). Similarly, reducing the level of Notch signaling by treatment with the gamma-secretase inhibitor LY411575 increased the number of ngn1+ cells in the otic vesicle by ~50% (Fig. 7C, M). Combining these conditions to reduce both Fgf and Notch signaling further increased neuroblast specification, closely mimicking the effects of activating hs:tfap2a at 24 hpf (Fig. 7D, M). Likewise, inhibiting both Fgf and Notch together reduced the number of neurod+ cells in the TA pool and increased accumulation of Isl1+ neurons through 37 hpf in a manner similar to activating hs:tfap2a at 24 hpf (Fig. 7E-O). Moreover, embryos inhibited for both Fgf and Notch signaling showed a dramatic loss of mature SAG neurons after 37 hpf, again mimicking the effects of hs:tfap2a activation (Fig. 7O). Interestingly, inhibition of either Fgf or Notch alone caused similar but more modest acceleration of neural differentiation, but such conditions did not lead to subsequent loss of mature neurons after 37 hpf (Fig. 7O). This is possibly because differentiation, though accelerated relative to control embryos, is still slow enough to allow expression of all factors essential for survival. Finally, activating hs:tfap2a at 24 hpf combined with conditions to inhibit Fgf and Notch did not further increase accumulation of Isl1+ neurons at 37 hpf (S4 Fig.). Thus, reducing both Fgf and Notch signaling is sufficient to recapitulate all observed effects of tfap2a overexpression.
Fig. 7. Reducing Fgf and Notch levels mimics the effects of tfap2a overexpression. 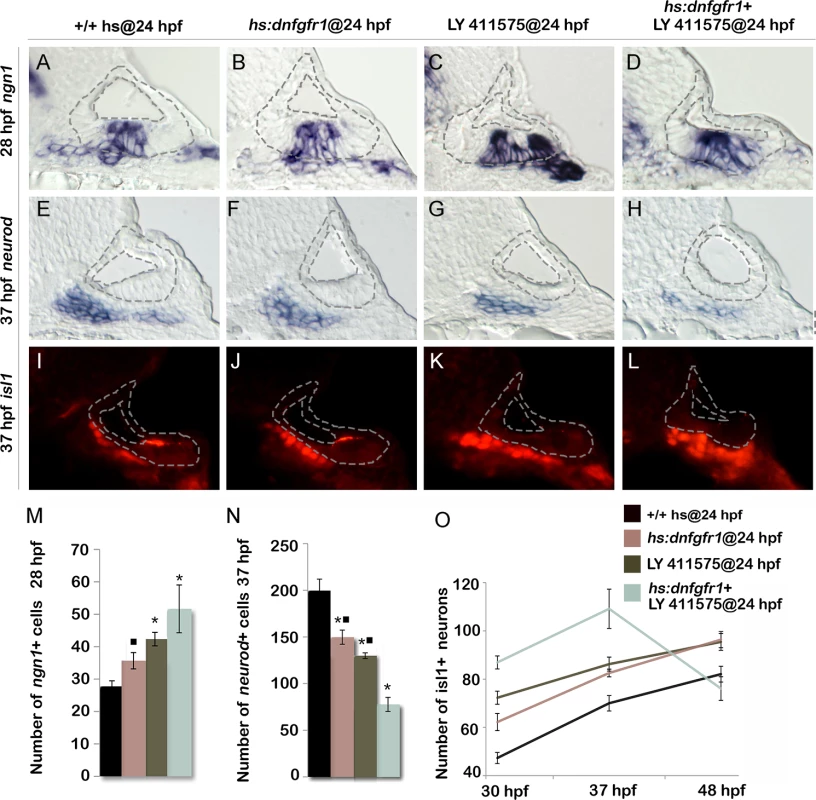
(A-L) Cross-sections (medial left, dorsal up) passing just posterior to the utricular macula and showing expression of ngn1 at 28 hpf (A-D), or sections passing through the utricular macula and showing neurod at 37 hpf (E-H) or Isl1 at 37 hpf (I-L) in wild-type control (A, E, I), hs:dnfgfr1 embryos (B, F, J), LY411575 inhibitor treated wild-type embryos (C, G, K) and LY411575 inhibitor treated hs:dnfgfr1 embryos (D, H, L). All specimens were treated with 0.3% DMSO and heat-shocked at 24 hpf. The otic vesicle is outlined in each image. (M, N) Mean and standard deviation of the total number of ngn1 positive cells in the otic epithelium at 28 hpf (M) and total neurod positive SAG precursors at 37 hpf (N) for the genotypes and treatments indicated in the color key (counted from serial sections, n = 3–6 ears per time point). Asterisks (*) indicate significant differences from control embryos and filled squares indicate significant differences relative to hs:dnfgfr1 embryos treated with LY411575. (O) Mean and standard deviation of the total number of Isl1 positive SAG neurons at different times for the genotypes and treatments indicated in the color key (n = 6–15 embryos each). In (O) differences between control and experimental specimens were significant at each time point. In addition, LY411575 treated hs:dnfgfr1 embryos were significantly different from hs:dnfgfr1 alone or LY411575 treatment alone. Tfap2a regulates transit amplification independently of earlier stages
We considered the possibility that the ability of Tfap2a to alter development of SAG cells outside the ear could arise secondarily from perturbation of earlier developmental stages within the otic vesicle. To test whether Tfap2a can specifically influence cells after delamination (without altering early development within the otic vesicle), we activated hs:tfap2a at 40 hpf when neurogenesis has ceased in the otic vesicle. Recall that transgene activation fails to prolong or reinitiate neuroblast specification at this stage (Fig. 2L). Regardless, overexpression of tfap2a at 40 hpf still reduced the number of neurod+ cells and led to an increase in Isl1+ neurons at 50 hpf (Fig. 8B, F, M, N). Misexpression of tfap2a also reduced the total number of cells in the TA pool that incorporated BrdU (Fig. 8J, O). However, this was proportional to the reduction in the total number of neurod+ cells (Fig. 8P), consistent with acceleration of the entire TA pool. A notable difference from earlier activation is that activating hs:tfap2a at 40 hpf did not lead to apoptosis of SAG neurons at later stages, as the number of Isl1+ cells remained elevated and in fact continued to increase through at least 72 hpf (Fig. 8B, F, N). The same effects were obtained by directly inhibiting both Fgf and Notch signaling after 40 hpf (Fig. 8C, G, K M-P). Conversely, misexpressing Fgf8 and NICD (Notch intracellular domain) by heat shock activation at 40 hpf led to accumulation of more TA cells and fewer mature neurons than normal at 50 and 72 hpf, indicating a delay in neuronal differentiation (Fig. 8D, H, M, N). Moreover, activating Fgf and Notch together increased the percentage of neurod+ cells that continue to incorporate BrdU (Fig. 8L, O, P), suggesting that the majority of cells in the TA pool persist in a relatively immature stage of SAG development. Thus, manipulating tfap2a, or Fgf and Notch signaling directly, can alter the rate of differentiation of TA cells even when earlier development within the otic vesicle has occurred normally. On the other hand, survival of mature SAG neurons requires normal development at early stages.
Fig. 8. Tfap2a regulates development of TA cells independent of earlier stages. 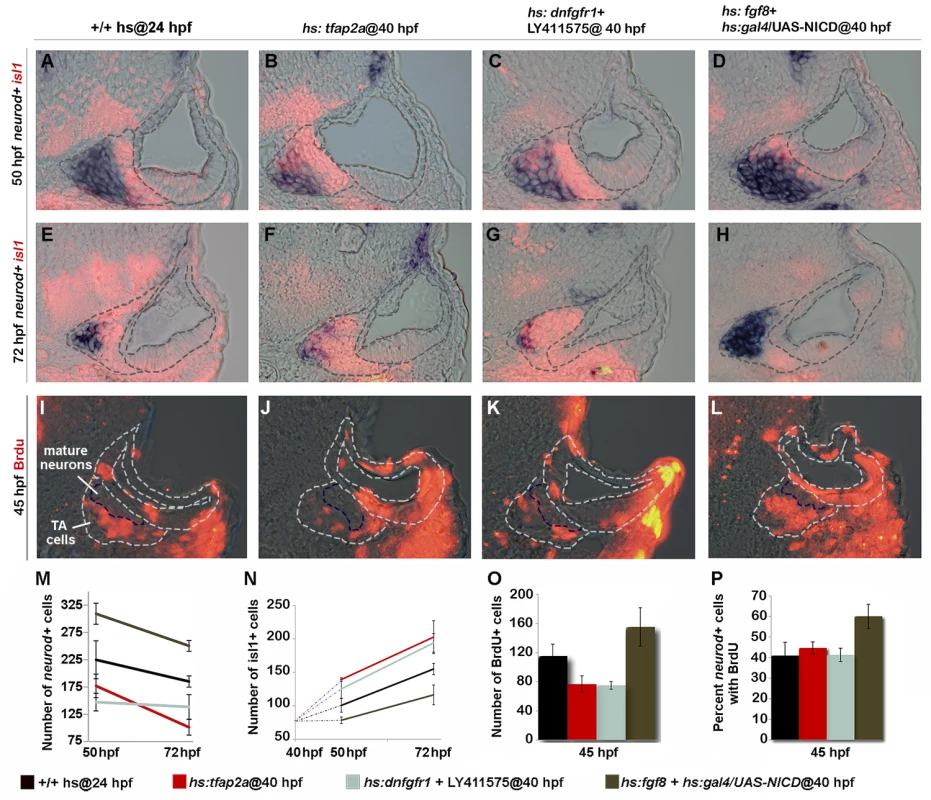
(A-L) Cross-sections (medial left, dorsal up) at the level of utricular macula. The otic vesicle and regions occupied by mature SAG neurons and TA cells are outlined in each image. Co-staining for Isl1 (red) and neurod (blue) at 50 hpf (A-D) and 72 hpf (E-H) under conditions indicated across the top of the figure. All specimens were treated with 0.3% DMSO and heat-shocked (39°C) at 40 hpf. (I-L) BrdU staining in embryos exposed to BrdU for 5 hours, from 40 to 45 hpf. The region containing mature SAG neurons is indicated. (M-O) Mean and standard deviation for the total number of neurod+ TA cells (M), total Isl1+ mature neurons (N) and total BrdU+ TA cells (O) for the indicated genotypes and treatments (counted from serial sections, n = 3–6 ears per time point). Differences between control and experimental specimens were significant at all time points, except that hs:fgf8+hs:gal4/UAS-NICD embryos were not significantly different from control embryos at 72 hpf in (N). (P) Percentage of neurod+ TA cells that are also BrdU positive (counted from serial sections, n = 3–6 ears per time point). Only hs:fgf8+hs:gal4/UAS-NICD embryos were significantly different from the control in (P). Tfap2a acts non-autonomously
It is noteworthy that tfap2a is not normally expressed in the TA pool or mature neurons, yet knockdown or misexpression of tfap2a alters the rate of differentiation and survival of these cells. This raised the possibility that Tfap2a could act non-autonomously on cells outside the ear. To test directly whether tfap2a can act non-autonomously, we generated genetic mosaics by transplanting wild-type cells into hs:tfap2a host embryos. We reasoned that if hs:tfap2a were to act non-autonomously, activating the transgene in host cells should be able to prevent wild-type cells from responding to Fgf sources in the otic vesicle. In support, activation of hs:tfap2a at 24 hpf suppressed etv5b expression in the majority (73.5%) of transplanted wild-type cells by 26 hpf (Fig. 9C-E), indicating that tfap2a acts non-autonomously to modulate the response to Fgf. In contrast, all wild-type donor cells transplanted into wild-type host embryos showed normal expression of etv5b in the otic vesicle (Fig. 9A, B, E). Thus Tfap2a non-autonomously inhibits Fgf signaling in the otic vesicle.
Fig. 9. Tfap2a regulates SAG development non-autonomously. 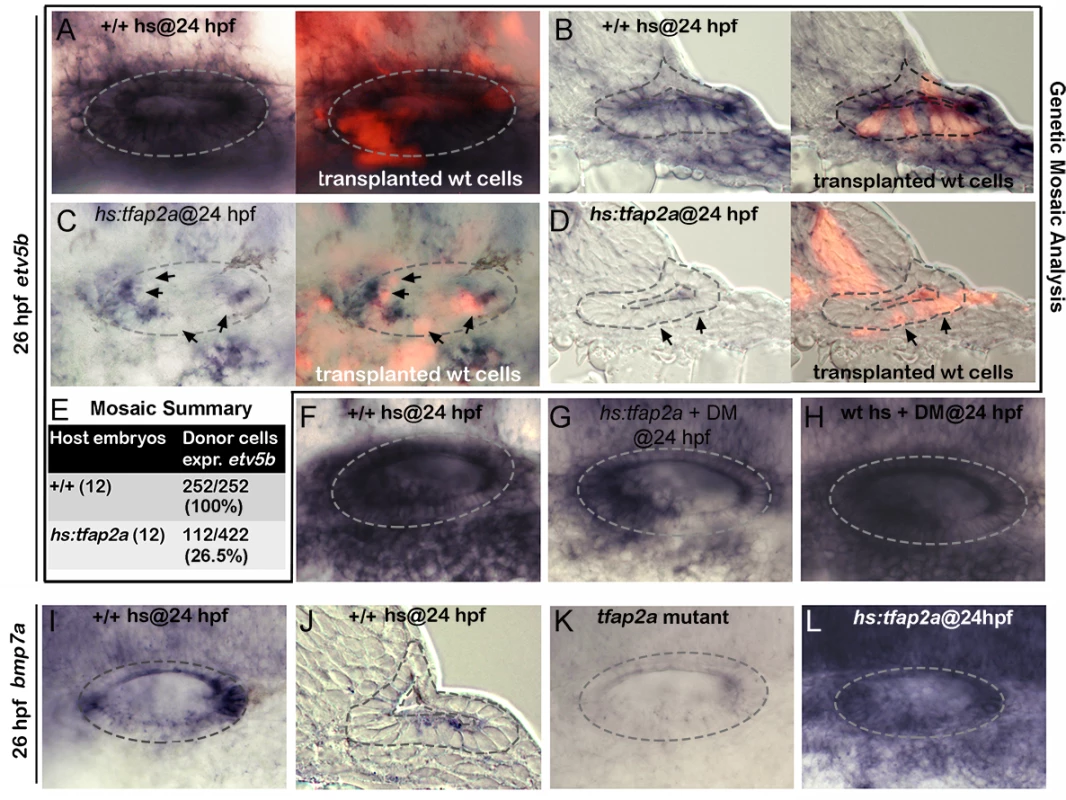
(A-D) Whole-mounts (dorsal up, anterior left) and cross-sections (just posterior to utricular macula, medial to the left) showing both bright-field and corresponding fluorescent images of +/+ host embryos (A, B) and hs:tfap2a host embryos stained for etv5b expression (blue) and showing transplanted wild-type donor cells (red). Positions of wild-type cells that fail to express etv5b are highlighted with arrows in (C, D). (E) Summary of mosaic analysis showing the number of +/+ or hs:tfap2a host embryos examined and the number of +/+ donor cells expressing etv5b over the total number of donor cells that populated the ventral half of the otic vesicle. (F-H) Expression of etv5b at 26 hpf in a control (F), a DM-treated hs:tfap2a embryo (G) and DM-treated wild-type embryo (H). All specimens were treated with 1% DMSO and heat-shocked at 24 hpf. (I-L) Expression of bmp7a at 26 hpf in a control (I, J), tfap2a-/- mutant (K) and hs:tfap2a embryo (L). Wild-type and hs:tfap2a embryos were heat shocked at 24 hpf. Bmp7a mediates the effects of Tfap2a
Bmp signaling is well known to antagonize Fgf signaling in a variety of developmental contexts. Here we found that blocking Bmp signaling with the pharmacological inhibitor dorsomorphin (DM) [36] strongly suppressed the ability of hs:tfap2a to reduce Fgf signaling. For example, etv5b expression was nearly normal in the otic vesicle 2 hours after the activation of hs:tfap2a when embryos were also treated with DM (Fig. 9F, G). DM treatment alone had negligible effects on expression of etv5b (Fig. 9H). We next surveyed expression of various bmp genes following activation of tfap2a and identified bmp7a as a likely candidate for mediating its effects on Fgf signaling. bmp7a is normally expressed in cells at the anterior and posterior ends of the otic vesicle, and at a lower level in a ventrolateral domain that overlaps the tfap2a expression domain [37] (Fig. 9I-J, compare with Fig. 1C). Expression of bmp7a was nearly abolished in tfap2a-/- mutants (Fig. 9K). In contrast, activation of hs:tfap2a strongly upregulated bmp7a expression in the otic vesicle as well as in surrounding tissues (Fig. 9L). In contrast to bmp7a, we observed no consistent changes in expression of bmp2b or bmp4 following manipulation of tfap2a function (S5 Fig.), indicating that changes in bmp7a are relatively specific. Together, these data suggest that tfap2a positively regulates bmp7a, which in turn restricts the level of Fgf signaling during otic development.
We next examined whether Bmp signaling mediates the effects of tfap2a on Notch activity. In support, blocking Bmp with DM rescued expression of deltaB and her4 following activation of hs:tfap2a at 24 hpf (Fig. 10A-L, M-P). Treatment with DM alone caused a slight but significant increase in the number of cells expressing deltaB and her4 (Fig. 10D, H, I-L). Thus the ability of tfap2a to restrict Notch signaling requires elevated Bmp signaling.
Fig. 10. Bmp signaling mediates the effects of Tfap2a on SAG development. 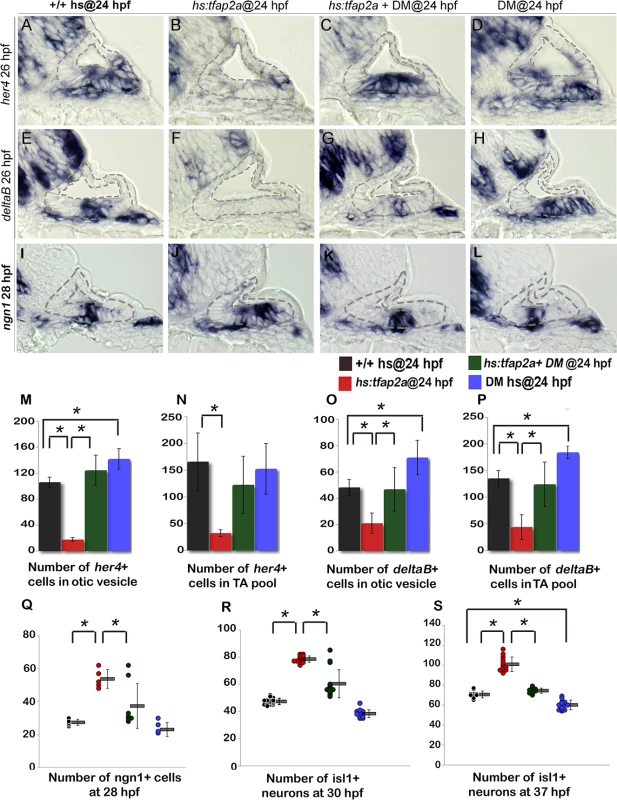
(A-L) Cross sections (medial left, dorsal up) through the otic vesicle just posterior to the utricular macula showing expression of her4 (A-D) and deltaB (E-H) at 26 hpf and ngn1 (I-L) at 28 hpf in control embryos (A, E, I), hs:tfap2a embryos (B, F, J), DM-treated hs:tfap2a embryos (C, G, K) and DM-treated wild-type embryos (D, H, L). All specimens were treated with 1% DMSO and heat-shocked at 24 hpf. (M-P) Mean and standard deviation of the total number of deltaB or her4 expressing cells inside the otic vesicle or in the TA pool at 26 hpf under conditions indicated in the color key (counted from serial sections, n = 3–6 ears per time point). (Q-S) Mean and standard deviation of the total number of ngn1+ cells at 28 hpf (Q) and Isl1+ SAG neurons at 30 hpf (R) and 37 hpf (S) under the conditions indicated in the color key. Asterisks (*) indicate statistical differences between the groups indicated in brackets. Finally, we tested whether the effects of tfap2a on SAG development also require Bmp signaling. Blocking Bmp after activating hs:tfap2a at 24 hpf restored neuroblast specification to normal in 6 out of 8 specimens (Fig. 10I-L, Q). Similarly, accumulation of mature Isl1+ neurons was nearly normal in 12 out of 15 embryos at 30 hpf (Fig. 10R) and was restored to normal in all specimens at 37 hpf (n = 15) (Fig. 10S). DM treatment alone caused a slight but significant reduction in neuroblast specification and accumulation of mature SAG neurons (Fig. 10Q-S). Together, these findings support a model in which tfap2a regulates the level of bmp7a expression in the otic vesicle, which in turn restricts Fgf and Notch signaling to control the amount, duration and speed of SAG development.
Discussion
We have shown that Tfap2a regulates development of SAG neurons by modulating Fgf, Notch and Bmp signaling. Tfap2a overexpression promotes neurogenic specification in the otic vesicle and accelerates subsequent differentiation of TA precursors into mature neurons. Conversely, disruption of tfap2a reduces the number of ngn1+ neuroblasts and decreases the rate of neuroblast differentiation. The neurogenic effects of tfap2a overexpression result from inhibition of Notch and Fgf signaling, which normally serve to restrict neuroblast specification and delay differentiation. The effects of Tfap2a appear to be mediated by Bmp7a, which is upregulated in response to Tfap2a activity. Bmp signaling in turn antagonizes Fgf and Notch signaling to promote specification and terminal differentiation of SAG precursors. These findings are novel and clarify several aspects of SAG development, which are discussed further below.
Coordination of Fgf and Notch
The interplay between Fgf and Notch is complex and dynamic, and levels must be precisely balanced for proper development of the SAG. A low-to-moderate level of Fgf is required to initiate neurogenesis by activating expression of ngn1 [5,7,38–40], which in turn activates expression of Notch ligands [2,34]. Notch activity serves to limit and slow neurogenesis [33,34]. Neurogenesis is also inhibited at later stages by rising levels of Fgf5 derived from mature neurons [5] (Fig. 1K). Without proper modulation by tfap2a, both Fgf and Notch signaling quickly become overactive, which terminates specification prematurely and impedes maturation of neuroblasts in the TA pool, thereby leading to under-production of mature SAG neurons.
While too much Fgf and Notch activity clearly impairs neurogenesis, insufficient levels are ultimately far more damaging to SAG development. Conditions that reduce Fgf and Notch signaling (e.g. overexpression of tfap2a) cause dramatic acceleration of differentiation and overproduction of neurons, most of which later die upon maturation. Neuronal death appears to arise secondarily from acceleration of early stages within the otic vesicle, as accelerating later stages does not lead to neuronal death. It is likely that early neuroblast differentiation is especially sensitive to acceleration due to insufficient buildup of factors needed for survival and function of mature neurons. In contrast, an important attribute of the TA pool is that the rate of differentiation can be regulated without compromising subsequent neuronal survival. The TA pool represents a relatively stable population of slowly cycling progenitors that must be maintained to meet the needs of the growing larva or to regenerate new neurons following damage [5]. It is likely that tfap2a is needed only transiently in the otic vesicle to prolong specification and establish a healthy progenitor population. Once neuroblast specification has terminated, however, downregulation of tfap2a appears necessary to allow Fgf and Notch signaling to rise sufficiently to balance rates of proliferation vs. differentiation in the TA pool.
The role of Bmp
We have shown for the first time a role for Bmp in SAG development. Numerous earlier studies have shown that Bmp regulates morphogenesis of semicircular canals [41–44] and numerous aspects of development of sensory epithelia [45–49]. Bmp has also been found to promote SAG survival and neurite outgrowth in chick explant cultures [50]. However, no previous studies have detected a role in specification or differentiation of SAG neurons. Abello et al. [38] reported that blocking Bmp signaling did not alter the size of the neurogenic domain in the chick otic vesicle; and mosaic misexpression of an activated form of Bmp receptor did not inhibit neurogenesis, though the possibility that it may have accelerated neurogenesis was not examined. In comparison, we find that tfap2a activates expression of bmp7a and that blocking Bmp signaling reverses the effects of tfap2a overexpression. Treating wild-type embryos with DM partially mimics the effects of disrupting tfap2a. The more severe defects caused by tfap2a loss of function could indicate that additional factors help mediate the effects of Tfap2a. Alternatively, Bmp7a could act partly through non-canonical signaling, similar to the role of Bmp7 in establishing tonotopy in the organ of Corti in mouse [46]. The specific requirement for Bmp7a in SAG development cannot be assessed by examining bmp7a mutants because development of the otic placode is severely compromised [51]. Development of lines to conditionally disrupt or misexpress bmp7a could resolve many of these issues.
Interestingly, in zebrafish Bmp effectors Smad1 and Smad5 are specifically upregulated in delaminated SAG cells [37,52] (Fig. 1K). This constitutes an unusual form of regulation because smad1/5 genes are broadly expressed with relatively little variation in the level of expression. Elevated levels of Smad1/5 accumulation could render SAG precursors outside the ear especially sensitive to Bmp, explaining how Bmp expressed within the otic vesicle could have such a profound effect on development of TA cells.
Regulation of tfap2a
We do not yet know how tfap2a is regulated in the otic vesicle. We detect no changes in expression after manipulating levels of Fgf, Notch, Bmp, Wnt or ngn1. It is possible that expression of tfap2a in the otic placode and otic vesicle reflects auto-regulatory maintenance from earlier stages. During gastrulation tfap2a is induced by Bmp in non-neural ectoderm where it functions as a competence factor for preplacodal development [9]. Once induced Tfap2a acts to maintain its own expression even if Bmp is subsequently blocked [11]. This is an important aspect of regulation because dorsally expressed Bmp-antagonists are required to initiate preplacodal development near the end of gastrulation [9,53–55]. Although expression of tfap2a could simply persist in the otic placode through self-maintenance, it is not clear how expression becomes restricted to ventrolateral cells in the otic vesicle. A similar pattern is seen in the chick otic vesicle, possibly indicating a conserved mechanism ([56]; Fig. 1). Identifying factors that regulate tfap2a in zebrafish and chick will likely shed light on general mechanisms of otic patterning and specific mechanisms of otic neurogenesis.
Materials and Methods
Fish strains and developmental conditions
The wild type strains were derived from AB line (Eugene, OR). Transgenic lines used in this study include Tg(hsp70:tfap2a)x24 [11], Tg(hsp70:fgf8a)x17 [57], Tg(hsp70I:dnfgfr1-EGFP)pd1 [58], Tg(hsp70I:gal41.5)kca4 [59], Tg(UAS:myc-Notch1a-intra)kca3 [60] and Tg(brn3c:gap43-GFP)s356t [61]. These transgenic lines are referred as hs:tfap2a, hs:fgf8, hs:dnfgfr1, hs:gal4/UAS-NICD and brn3c-GFP respectively. Mutant line tfap2am819 [62] was used for most loss of function studies. Mutants were identified by characteristic phenotypes showing expected Mendelian frequencies. Except where noted, embryos were maintained at 28.5°C in fish water containing 0.008% Instant Ocean salts, methylene blue and PTU (1-phenyl 2-thiourea, 0.3 mg/ml, Sigma) to block melanin formation. Embryos were staged according to standard protocols [63].
Gene misexpression and morpholino injection
To activate heat-shock inducible transgenic lines, heterozygous transgenic embryos were incubated at 39°C for 30 minutes except where noted. Under this condition, activation of hs:tfap2a at 24 hpf led to a detectable increase in tfap2a levels by the end of the heat-shock period. Maximal tfap2a expression was seen at 25.5 hpf and the tfap2a levels remained elevated in the otic vesicle thorough at least 29 hpf (S1 Fig.). Since the complete blockage of Fgf signaling inhibits neurogenic specification in the otic vesicle [5], weak attenuation of Fgf signaling was achieved by activation of hs:dnfgfr1 at 35°C for 30 minutes (Fig. 6) or at 36.5°C for 30 minutes (Fig. 7). After the heat-shock, embryos were incubated at 33°C until fixation. In some loss of function experiments, tfap2a was knocked down by injecting embryos at the 1-cell stage with approximately 5 ng of tfap2a morpholino oligomer (MO). The sequence of tfap2a MO has been tested previously for specificity and efficiency [64].
Pharmacological treatments
Notch signaling was blocked by treating embryos with LY411575 diluted from a 10 mM stock in DMSO to a final concentration of 30 μM in fish water. Bmp signaling was blocked by Dorsomorphin (Sigma, P5499) diluted from a 10 mM stock solution into a final concentration of 100 μM in fish water. Treatments were carried in a 24-well plate with a maximum of 15 embryos per well in a volume of 500 μl each.
In situ hybridization and immunohistochemistry
In situ hybridization and antibody staining was carried out as described previously [65–67]. In situ TUNEL assay was performed by using Promega terminal deoxynucleotidyl transferase (M828A) according to the manufacturer’s protocol. Following primary and secondary antibodies were used in this study: anti-Islet1/2 (Developmental Studies Hybridoma Bank 39.4D5, 1 : 100 for whole-mount, 1 : 250 for cryo-sections), anti-BrdU (Beckton-Dickinson, 1 : 250) and Alexa 546 goat anti-mouse IgG (Invitrogen A-11003, 1 : 50 for whole-mount, 1 : 250 for cryo-sections). Cryo-sectioning and BrdU labeling were carried out as described previously [5]. Whole-mount stained embryos were sectioned except for Figs. 1, 7 and S4 where anti-islet1/2 staining was performed on sections using standard whole mount protocols.
Cell transplantation
Wild-type donor cells were injected with the lineage tracer (tetramethylrhodamine labeled, 10,000 MW, lysine-fixable dextran in 0.2 M KCl) and transplanted into non-labeled hs:tfap2a embryos at blastula stage.
Chick experiments
Embryonic day 3 (E3) and E4 chick embryos were fixed and embedded in gelatin (7.5% gelatin, 15% sucrose in PBS). 14μm thick sections were collected on Superfrost Plus slides. For AP2a and Jagged-1 co-detection, slides were boiled in 10mM citric acid for 10 minutes prior to antibody application and then incubated in 0.012% hydrogen peroxide for 15 minutes at room temperature. The 3B5 AP2α monoclonal antibody developed by Trevor Williams was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology, Iowa City, IA 52242. AP2α antibody was diluted 1 : 100 and Jagged-1 polyclonal antibody (Santa Cruz Biotechnology H-114) was diluted 1 : 200 in blocking buffer (PBS with 0.02% Tween-20, 0.1% Triton X-100, and 10% goat serum). Staining was detected with biotinylated mouse secondary antibody (Mouse Vectastain ABC kit) in conjunction with PerkinElmer TSA Plus Cyanine-3 System and AlexaFluor 488 conjugated rabbit secondary antibody diluted 1 : 500 in A+B substrate solution (AlexaFluor goat anti-rabbit, Invitrogen). All slides were mounted in Fluoromount G (Southern Biotech).
Statistics
For pairwise comparisons, student’s t-tests were used to evaluate significance. For experiments involving more than two groups significance was evaluated using one-way ANOVA and Tukey post-hoc HSD tests.
Ethics statement
The studies described herein were fully compliant with federal guidelines and IACUC-approved Animal Use Protocol number 2012–011.
Supporting Information
Zdroje
1. Andermann P, Ungos J, Raible DW (2002) Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev Biol 251 : 45–58. 12413897
2. Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ (1998) neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron 20 : 469–482. 9539122
3. Korzh V, Sleptsova I, Liao J, He J, Gong Z (1998) Expression of zebrafish bHLH genes ngn1 and nrd defines distinct stages of neural differentiation. Dev Dyn 213 : 92–104. 9733104
4. Camarero G, Leon Y, Gorospe I, De Pablo F, Alsina B, et al. (2003) Insulin-like growth factor 1 is required for survival of transit-amplifying neuroblasts and differentiation of otic neurons. Dev Biol 262 : 242–253. 14550788
5. Vemaraju S, Kantarci H, Padanad MS, Riley BB (2012) A spatial and temporal gradient of Fgf differentially regulates distinct stages of neural development in the zebrafish inner ear. PLoS Genet 8: e1003068. doi: 10.1371/journal.pgen.1003068 23166517
6. Radde-Gallwitz K, Pan L, Gan L, Lin X, Segil N, et al. (2004) Expression of Islet1 marks the sensory and neuronal lineages in the mammalian inner ear. J Comp Neurol 477 : 412–421. 15329890
7. Alsina B, Abello G, Ulloa E, Henrique D, Pujades C, et al. (2004) FGF signaling is required for determination of otic neuroblasts in the chick embryo. Dev Biol 267 : 119–134. 14975721
8. Haddon C, Lewis J (1996) Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol 365 : 113–128. 8821445
9. Kwon HJ, Bhat N, Sweet EM, Cornell RA, Riley BB (2010) Identification of early requirements for preplacodal ectoderm and sensory organ development. PLoS Genet 6: e1001133. doi: 10.1371/journal.pgen.1001133 20885782
10. Saint-Jeannet JP, Moody SA (2014) Establishing the pre-placodal region and breaking it into placodes with distinct identities. Dev Biol 389 : 13–27. doi: 10.1016/j.ydbio.2014.02.011 24576539
11. Bhat N, Kwon HJ, Riley BB (2013) A gene network that coordinates preplacodal competence and neural crest specification in zebrafish. Dev Biol 373 : 107–117. doi: 10.1016/j.ydbio.2012.10.012 23078916
12. Nissen RM, Yan J, Amsterdam A, Hopkins N, Burgess SM (2003) Zebrafish foxi one modulates cellular responses to Fgf signaling required for the integrity of ear and jaw patterning. Development 130 : 2543–2554. 12702667
13. Padanad MS, Riley BB (2011) Pax2/8 proteins coordinate sequential induction of otic and epibranchial placodes through differential regulation of foxi1, sox3 and fgf24. Dev Biol 351 : 90–98. doi: 10.1016/j.ydbio.2010.12.036 21215261
14. Solomon KS, Kudoh T, Dawid IB, Fritz A (2003) Zebrafish foxi1 mediates otic placode formation and jaw development. Development 130 : 929–940. 12538519
15. Edlund RK, Ohyama T, Kantarci H, Riley BB, Groves AK (2014) Foxi transcription factors promote pharyngeal arch development by regulating formation of FGF signaling centers. Dev Biol 390 : 1–13. doi: 10.1016/j.ydbio.2014.03.004 24650709
16. Khatri SB, Edlund RK, Groves AK (2014) Foxi3 is necessary for the induction of the chick otic placode in response to FGF signaling. Dev Biol 391 : 158–169. doi: 10.1016/j.ydbio.2014.04.014 24780628
17. Neave B, Rodaway A, Wilson SW, Patient R, Holder N (1995) Expression of zebrafish GATA 3 (gta3) during gastrulation and neurulation suggests a role in the specification of cell fate. Mech Dev 51 : 169–182. 7547465
18. Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, et al. (2001) Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol 429 : 615–630. 11135239
19. Lillevali K, Haugas M, Pituello F, Salminen M (2007) Comparative analysis of Gata3 and Gata2 expression during chicken inner ear development. Dev Dyn 236 : 306–313. 17103399
20. Sheng G, Stern CD (1999) Gata2 and Gata3: novel markers for early embryonic polarity and for non-neural ectoderm in the chick embryo. Mech Dev 87 : 213–216. 10495290
21. Appler JM, Lu CC, Druckenbrod NR, Yu WM, Koundakjian EJ, et al. (2013) Gata3 is a critical regulator of cochlear wiring. J Neurosci 33 : 3679–3691. doi: 10.1523/JNEUROSCI.4703-12.2013 23426694
22. Duncan JS, Fritzsch B (2013) Continued expression of GATA3 is necessary for cochlear neurosensory development. PLoS One 8: e62046. doi: 10.1371/journal.pone.0062046 23614009
23. Luo XJ, Deng M, Xie X, Huang L, Wang H, et al. (2013) GATA3 controls the specification of prosensory domain and neuronal survival in the mouse cochlea. Hum Mol Genet 22 : 3609–3623. doi: 10.1093/hmg/ddt212 23666531
24. Arduini BL, Bosse KM, Henion PD (2009) Genetic ablation of neural crest cell diversification. Development 136 : 1987–1994. doi: 10.1242/dev.033209 19439494
25. de Croze N, Maczkowiak F, Monsoro-Burq AH (2011) Reiterative AP2a activity controls sequential steps in the neural crest gene regulatory network. Proc Natl Acad Sci U S A 108 : 155–160. doi: 10.1073/pnas.1010740107 21169220
26. Hoffman TL, Javier AL, Campeau SA, Knight RD, Schilling TF (2007) Tfap2 transcription factors in zebrafish neural crest development and ectodermal evolution. J Exp Zool B Mol Dev Evol 308 : 679–691. 17724731
27. Knight RD, Nair S, Nelson SS, Afshar A, Javidan Y, et al. (2003) lockjaw encodes a zebrafish tfap2a required for early neural crest development. Development 130 : 5755–5768. 14534133
28. Li W, Cornell RA (2007) Redundant activities of Tfap2a and Tfap2c are required for neural crest induction and development of other non-neural ectoderm derivatives in zebrafish embryos. Dev Biol 304 : 338–354. 17258188
29. Luo T, Lee YH, Saint-Jeannet JP, Sargent TD (2003) Induction of neural crest in Xenopus by transcription factor AP2alpha. Proc Natl Acad Sci U S A 100 : 532–537. 12511599
30. Nikitina N, Sauka-Spengler T, Bronner-Fraser M (2008) Dissecting early regulatory relationships in the lamprey neural crest gene network. Proc Natl Acad Sci U S A 105 : 20083–20088. doi: 10.1073/pnas.0806009105 19104059
31. Van Otterloo E, Li W, Garnett A, Cattell M, Medeiros DM, et al. (2012) Novel Tfap2-mediated control of soxE expression facilitated the evolutionary emergence of the neural crest. Development 139 : 720–730. doi: 10.1242/dev.071308 22241841
32. Wang WD, Melville DB, Montero-Balaguer M, Hatzopoulos AK, Knapik EW (2011) Tfap2a and Foxd3 regulate early steps in the development of the neural crest progenitor population. Dev Biol 360 : 173–185. doi: 10.1016/j.ydbio.2011.09.019 21963426
33. Abello G, Khatri S, Giraldez F, Alsina B (2007) Early regionalization of the otic placode and its regulation by the Notch signaling pathway. Mech Dev 124 : 631–645. 17532192
34. Raft S, Koundakjian EJ, Quinones H, Jayasena CS, Goodrich LV, et al. (2007) Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development 134 : 4405–4415. 18039969
35. Haddon C, Jiang YJ, Smithers L, Lewis J (1998) Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development 125 : 4637–4644. 9806913
36. Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, et al. (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 4 : 33–41. 18026094
37. Mowbray C, Hammerschmidt M, Whitfield TT (2001) Expression of BMP signalling pathway members in the developing zebrafish inner ear and lateral line. Mech Dev 108 : 179–184. 11578872
38. Abello G, Khatri S, Radosevic M, Scotting PJ, Giraldez F, et al. (2010) Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev Biol 339 : 166–178. doi: 10.1016/j.ydbio.2009.12.027 20043898
39. Mansour SL, Goddard JM, Capecchi MR (1993) Mice homozygous for a targeted disruption of the proto-oncogene int-2 have developmental defects in the tail and inner ear. Development 117 : 13–28. 8223243
40. Pirvola U, Spencer-Dene B, Xing-Qun L, Kettunen P, Thesleff I, et al. (2000) FGF/FGFR-2(IIIb) signaling is essential for inner ear morphogenesis. J Neurosci 20 : 6125–6134. 10934262
41. Chang W, Nunes FD, De Jesus-Escobar JM, Harland R, Wu DK (1999) Ectopic noggin blocks sensory and nonsensory organ morphogenesis in the chicken inner ear. Dev Biol 216 : 369–381. 10588886
42. Gerlach LM, Hutson MR, Germiller JA, Nguyen-Luu D, Victor JC, et al. (2000) Addition of the BMP4 antagonist, noggin, disrupts avian inner ear development. Development 127 : 45–54. 10654599
43. Hammond KL, Loynes HE, Mowbray C, Runke G, Hammerschmidt M, et al. (2009) A late role for bmp2b in the morphogenesis of semicircular canal ducts in the zebrafish inner ear. PLoS One 4: e4368. doi: 10.1371/journal.pone.0004368 19190757
44. Ohta S, Mansour SL, Schoenwolf GC (2010) BMP/SMAD signaling regulates the cell behaviors that drive the initial dorsal-specific regional morphogenesis of the otocyst. Dev Biol 347 : 369–381. doi: 10.1016/j.ydbio.2010.09.002 20837004
45. Chang W, Lin Z, Kulessa H, Hebert J, Hogan BL, et al. (2008) Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet 4: e1000050. doi: 10.1371/journal.pgen.1000050 18404215
46. Mann ZF, Thiede BR, Chang W, Shin JB, May-Simera HL, et al. (2014) A gradient of Bmp7 specifies the tonotopic axis in the developing inner ear. Nat Commun 5 : 3839. doi: 10.1038/ncomms4839 24845721
47. Li H, Corrales CE, Wang Z, Zhao Y, Wang Y, et al. (2005) BMP4 signaling is involved in the generation of inner ear sensory epithelia. BMC Dev Biol 5 : 16. 16107213
48. Ohyama T, Basch ML, Mishina Y, Lyons KM, Segil N, et al. (2010) BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J Neurosci 30 : 15044–15051. doi: 10.1523/JNEUROSCI.3547-10.2010 21068310
49. Pujades C, Kamaid A, Alsina B, Giraldez F (2006) BMP-signaling regulates the generation of hair-cells. Dev Biol 292 : 55–67. 16458882
50. Fantetti KN, Fekete DM (2012) Members of the BMP, Shh, and FGF morphogen families promote chicken statoacoustic ganglion neurite outgrowth and neuron survival in vitro. Dev Neurobiol 72 : 1213–1228. doi: 10.1002/dneu.20988 22006861
51. Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, Brand M, et al. (1996) Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development 123 : 81–93. 9007231
52. Feng Y, Xu Q (2010) Pivotal role of hmx2 and hmx3 in zebrafish inner ear and lateral line development. Dev Biol 339 : 507–518. doi: 10.1016/j.ydbio.2009.12.028 20043901
53. Ahrens K, Schlosser G (2005) Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Dev Biol 288 : 40–59. 16271713
54. Linker C, De Almeida I, Papanayotou C, Stower M, Sabado V, et al. (2009) Cell communication with the neural plate is required for induction of neural markers by BMP inhibition: evidence for homeogenetic induction and implications for Xenopus animal cap and chick explant assays. Dev Biol 327 : 478–486. doi: 10.1016/j.ydbio.2008.12.034 19162002
55. Litsiou A, Hanson S, Streit A (2005) A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development 132 : 4051–4062. 16093325
56. Shen H, Wilke T, Ashique AM, Narvey M, Zerucha T, et al. (1997) Chicken transcription factor AP-2: cloning, expression and its role in outgrowth of facial prominences and limb buds. Dev Biol 188 : 248–266. 9268573
57. Millimaki BB, Sweet EM, Riley BB (2010) Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev Biol 338 : 262–267. doi: 10.1016/j.ydbio.2009.12.011 20025865
58. Lee Y, Grill S, Sanchez A, Murphy-Ryan M, Poss KD (2005) Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development 132 : 5173–5183. 16251209
59. Scheer N, Riedl I, Warren JT, Kuwada JY, Campos-Ortega JA (2002) A quantitative analysis of the kinetics of Gal4 activator and effector gene expression in the zebrafish. Mech Dev 112 : 9–14. 11850174
60. Scheer N, Campos-Ortega JA (1999) Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech Dev 80 : 153–158. 10072782
61. Xiao T, Roeser T, Staub W, Baier H (2005) A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development 132 : 2955–2967. 15930106
62. Holzschuh J, Barrallo-Gimeno A, Ettl AK, Durr K, Knapik EW, et al. (2003) Noradrenergic neurons in the zebrafish hindbrain are induced by retinoic acid and require tfap2a for expression of the neurotransmitter phenotype. Development 130 : 5741–5754. 14534139
63. Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203 : 253–310. 8589427
64. O'Brien EK, d'Alencon C, Bonde G, Li W, Schoenebeck J, et al. (2004) Transcription factor Ap-2alpha is necessary for development of embryonic melanophores, autonomic neurons and pharyngeal skeleton in zebrafish. Dev Biol 265 : 246–261. 14697367
65. Jowett T, Yan YL (1996) Double fluorescent in situ hybridization to zebrafish embryos. Trends Genet 12 : 387–389. 8909127
66. Phillips BT, Bolding K, Riley BB (2001) Zebrafish fgf3 and fgf8 encode redundant functions required for otic placode induction. Dev Biol 235 : 351–365. 11437442
67. Riley BB, Chiang M, Farmer L, Heck R (1999) The deltaA gene of zebrafish mediates lateral inhibition of hair cells in the inner ear and is regulated by pax2.1. Development 126 : 5669–5678. 10572043
Štítky
Genetika Reprodukčná medicína
Článek NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY ModuleČlánek Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell LongevityČlánek HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and MiceČlánek LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male FertilityČlánek The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAsČlánek Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick VectorČlánek Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53Článek The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in NematodesČlánek The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse SpermatocytesČlánek The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 3- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
- Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
- A Genomic Duplication is Associated with Ectopic Eomesodermin Expression in the Embryonic Chicken Comb and Two Duplex-comb Phenotypes
- Genome-wide Association Study and Meta-Analysis Identify as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy
- Mutations of Human , Encoding the Mitochondrial Asparaginyl-tRNA Synthetase, Cause Nonsyndromic Deafness and Leigh Syndrome
- Exome Sequencing in an Admixed Isolated Population Indicates Variants Confer a Risk for Specific Language Impairment
- Genome-Wide Association Studies in Dogs and Humans Identify as a Risk Variant for Cleft Lip and Palate
- Rapid Evolution of Recombinant for Xylose Fermentation through Formation of Extra-chromosomal Circular DNA
- The Ribosome Biogenesis Factor Nol11 Is Required for Optimal rDNA Transcription and Craniofacial Development in
- Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
- Maternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity
- Clonality and Evolutionary History of Rhabdomyosarcoma
- HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and Mice
- Methylation-Sensitive Expression of a DNA Demethylase Gene Serves As an Epigenetic Rheostat
- BREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
- Seizures Are Regulated by Ubiquitin-specific Peptidase 9 X-linked (USP9X), a De-Ubiquitinase
- The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
- A Cascade of Iron-Containing Proteins Governs the Genetic Iron Starvation Response to Promote Iron Uptake and Inhibit Iron Storage in Fission Yeast
- Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
- LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male Fertility
- Cis-Regulatory Mechanisms for Robust Olfactory Sensory Neuron Class-restricted Odorant Receptor Gene Expression in
- Effects on Murine Behavior and Lifespan of Selectively Decreasing Expression of Mutant Huntingtin Allele by Supt4h Knockdown
- HDAC4-Myogenin Axis As an Important Marker of HD-Related Skeletal Muscle Atrophy
- A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex
- Selective and Genetic Constraints on Pneumococcal Serotype Switching
- Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
- The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAs
- Accumulation of Glucosylceramide in the Absence of the Beta-Glucosidase GBA2 Alters Cytoskeletal Dynamics
- Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities
- The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair
- Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick Vector
- Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
- The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors
- PHABULOSA Controls the Quiescent Center-Independent Root Meristem Activities in
- DNA Polymerase ζ-Dependent Lesion Bypass in Is Accompanied by Error-Prone Copying of Long Stretches of Adjacent DNA
- Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus
- Zinc Finger Independent Genome-Wide Binding of Sp2 Potentiates Recruitment of Histone-Fold Protein Nf-y Distinguishing It from Sp1 and Sp3
- GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters
- Neurospora Importin α Is Required for Normal Heterochromatic Formation and DNA Methylation
- Ccr4-Not Regulates RNA Polymerase I Transcription and Couples Nutrient Signaling to the Control of Ribosomal RNA Biogenesis
- Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53
- A Systems-Level Interrogation Identifies Regulators of Blood Cell Number and Survival
- Morphological Mutations: Lessons from the Cockscomb
- Genetic Interaction Mapping Reveals a Role for the SWI/SNF Nucleosome Remodeler in Spliceosome Activation in Fission Yeast
- The Role of China in the Global Spread of the Current Cholera Pandemic
- The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
- A Zinc Finger Motif-Containing Protein Is Essential for Chloroplast RNA Editing
- Resistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
- Small Regulatory RNA-Induced Growth Rate Heterogeneity of
- Mitochondrial Dysfunction Reveals the Role of mRNA Poly(A) Tail Regulation in Oculopharyngeal Muscular Dystrophy Pathogenesis
- Complex Genomic Rearrangements at the Locus Include Triplication and Quadruplication
- Male-Biased Aganglionic Megacolon in the TashT Mouse Line Due to Perturbation of Silencer Elements in a Large Gene Desert of Chromosome 10
- Sex Ratio Meiotic Drive as a Plausible Evolutionary Mechanism for Hybrid Male Sterility
- Tertiary siRNAs Mediate Paramutation in .
- RECG Maintains Plastid and Mitochondrial Genome Stability by Suppressing Extensive Recombination between Short Dispersed Repeats
- Escape from X Inactivation Varies in Mouse Tissues
- Opposite Phenotypes of Muscle Strength and Locomotor Function in Mouse Models of Partial Trisomy and Monosomy 21 for the Proximal Region
- Glycosyl Phosphatidylinositol Anchor Biosynthesis Is Essential for Maintaining Epithelial Integrity during Embryogenesis
- Hyperdiverse Gene Cluster in Snail Host Conveys Resistance to Human Schistosome Parasites
- The Class Homeodomain Factors and Cooperate in . Embryonic Progenitor Cells to Regulate Robust Development
- Recombination between Homologous Chromosomes Induced by Unrepaired UV-Generated DNA Damage Requires Mus81p and Is Suppressed by Mms2p
- Synergistic Interactions between Orthologues of Genes Spanned by Human CNVs Support Multiple-Hit Models of Autism
- Gene Networks Underlying Convergent and Pleiotropic Phenotypes in a Large and Systematically-Phenotyped Cohort with Heterogeneous Developmental Disorders
- The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
- Combinatorial Control of Light Induced Chromatin Remodeling and Gene Activation in
- Linking Aβ42-Induced Hyperexcitability to Neurodegeneration, Learning and Motor Deficits, and a Shorter Lifespan in an Alzheimer’s Model
- The Complex Contributions of Genetics and Nutrition to Immunity in
- NatB Domain-Containing CRA-1 Antagonizes Hydrolase ACER-1 Linking Acetyl-CoA Metabolism to the Initiation of Recombination during . Meiosis
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
- Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption
- A Genomic Portrait of Haplotype Diversity and Signatures of Selection in Indigenous Southern African Populations
- Sequence Features and Transcriptional Stalling within Centromere DNA Promote Establishment of CENP-A Chromatin
- Inhibits Neuromuscular Junction Growth by Downregulating the BMP Receptor Thickveins
- Replicative DNA Polymerase δ but Not ε Proofreads Errors in and in
- Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in
- The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
- A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension
- Pervasive Variation of Transcription Factor Orthologs Contributes to Regulatory Network Evolution
- Network Analyses Reveal Novel Aspects of ALS Pathogenesis
- A Role for the Budding Yeast Separase, Esp1, in Ty1 Element Retrotransposition
- Nab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
- A RecA Protein Surface Required for Activation of DNA Polymerase V
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Clonality and Evolutionary History of Rhabdomyosarcoma
- Morphological Mutations: Lessons from the Cockscomb
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



