-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
Meiosis is the specialized cell division by which haploid cells are produced. As germ cells enter the first meiotic prophase, programmed double-stranded breaks (DSBs) are formed throughout the genome. Repair of these DSBs by homologous recombination is crucial for proper segregation of homologous chromosomes at the end of the first meiotic division, and thus, for the production of haploid gametes. Moreover, failure to correctly repair these DSBs can have deleterious effects on the genomic integrity of offspring. To ensure that meiocytes that fail to repair meiotic DSBs do not complete meiosis, recombination is tightly controlled. However, the signaling pathway(s) tying meiotic recombination to meiotic progression in mouse spermatocytes is not known. We report here that the ATM-signaling pathway, composed of the MRE11 complex, ATM and CHK2, is responsible for activation of the recombination-dependent arrest that occurs in Trip13 mutant mouse spermatocytes, which accumulate unrepaired DSBs during meiotic prophase.
Published in the journal: The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes. PLoS Genet 11(3): e32767. doi:10.1371/journal.pgen.1005017
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005017Summary
Meiosis is the specialized cell division by which haploid cells are produced. As germ cells enter the first meiotic prophase, programmed double-stranded breaks (DSBs) are formed throughout the genome. Repair of these DSBs by homologous recombination is crucial for proper segregation of homologous chromosomes at the end of the first meiotic division, and thus, for the production of haploid gametes. Moreover, failure to correctly repair these DSBs can have deleterious effects on the genomic integrity of offspring. To ensure that meiocytes that fail to repair meiotic DSBs do not complete meiosis, recombination is tightly controlled. However, the signaling pathway(s) tying meiotic recombination to meiotic progression in mouse spermatocytes is not known. We report here that the ATM-signaling pathway, composed of the MRE11 complex, ATM and CHK2, is responsible for activation of the recombination-dependent arrest that occurs in Trip13 mutant mouse spermatocytes, which accumulate unrepaired DSBs during meiotic prophase.
Introduction
Meiosis generates haploid cells from a diploid progenitor by coupling one round of genome replication to two rounds of chromosome segregation. During prophase of the first division, SPO11 protein forms double-strand breaks (DSBs), whose repair enables homologous chromosomes to pair, synapse and recombine [1]. DSB repair failure has deleterious effects, so meiotic recombination is monitored to ensure its completion [2–8]. When recombination intermediates persist, a system often referred to as the pachytene checkpoint is activated to delay or stop meiotic progression or, in some cases, to initiate programmed cell death [9]. In this paper, we use the term “checkpoint” in the well-established sense of a signaling mechanism that creates a relationship (dependency) between two otherwise independent meiotic processes [8]. In this context, checkpoint responses to a cellular defect could have any of several non-exclusive downstream consequences including arrested differentiation, cell cycle arrest, and/or programmed cell death.
Mouse spermatocytes unable to complete recombination (e.g., if they lack the strand-exchange protein DMC1 [10,11]) arrest differentiation without expressing the testis-specific histone variant H1t [2], which is a marker of mid-pachynema and later stages of spermatogenesis [12,13]. In contrast, spermatocytes unable to make DSBs at all (i.e., in the absence of SPO11 [14,15]) are able to progress to a state where H1t is expressed [2] (See S1 Table for a more detailed summary of the phenotypes of these and other mouse mutants used in this study). Thus, male mammalian meiocytes respond differently to the inability to complete recombination once it has begun as opposed to the absence of recombination initiation. In both types of mutant, however, spermatocytes undergo apoptosis during pachynema [16]. It is now clear that one DSB-independent trigger of spermatocyte apoptosis involves failure to form a sex body [16], the transcriptionally repressed, heterochromatic domain encompassing the nonhomologous portions of the X and Y chromosomes. Sex body failure enables expression of sex chromosome genes that for unknown reasons are deleterious for pachytene spermatocytes [17]. This cell elimination mechanism depends on the checkpoint kinase ATR [18]. We will refer to spermatocyte arrest in response to DSB repair defects, measured as a block to H1t expression, as recombination-dependent arrest to distinguish it from the DSB-independent sex body-deficient arrest.
It has been suggested that both types of arrest are sufficient to trigger apoptosis [10,14,15], although this remains unproven for recombination-dependent arrest. Interestingly, however, even though spermatocytes arrest with physiological states characteristic of different points in prophase depending on the type of recombination defect, they undergo apoptosis at an equivalent stage when evaluated in the context of development of the seminiferous epithelium [2,19]. Spermatogenesis occurs within the seminiferous tubules, which can be classified among twelve epithelial stages (I to XII) according to the cell types found in each cross-section [20]. Despite showing different arrest points based on H1t staining, Dmc1−/− and Spo11−/− spermatocytes undergo apoptosis at the same epithelial stage (stage IV), which is the equivalent of mid-pachynema for wild-type spermatocytes [2,19], and which is the time when H1t incorporation would normally have occurred [13] (described in more detail below). The inability of epithelial staging alone to discriminate between different physiological arrest points emphasizes the importance of molecular cytological methods for characterizing mutant spermatocyte behavior [2]. A straightforward interpretation is that recombination-dependent arrest blocks spermatogenic differentiation at a stage equivalent to early pachynema, with cells remaining in this arrested state until apoptosis is triggered in epithelial stage IV, equivalent to the time unarrested cells would have transitioned into mid-pachynema.
Exploring mechanisms unique to recombinant-dependent arrest is challenging: meiotic recombination drives pairing and synapsis of homologous chromosomes in mouse, so most mutants that fail to complete recombination also show widespread chromosome asynapsis, which indirectly blocks sex body formation [16]. Thus, mutants like Dmc1 that retain numerous unrepaired DSBs at early pachynema also display rampant chromosomal asynapsis, which impedes sex body formation [2,16]. Interpreting this arrest phenotype is further complicated if asynapsis per se can trigger a response separate from its effects on sex body formation [21]. Since these mutants have such a complex array of severe defects, it is difficult to use them to explore mechanisms unique to recombination-dependent arrest. Thus, we wished to use mutants that cannot complete DSB repair but that do complete synapsis.
Two mutants are known to enter pachynema with unrepaired DSBs yet with substantially normal autosomal synapsis and apparently normal sex body formation, providing an opportunity to investigate factors that specifically regulate recombination-dependent arrest during meiosis. One mutant is Trip13mod/mod, which contains a hypomorphic gene trap allele of Trip13 (Trip13mod, for “moderately defective”; also termed Trip13Gt) [22,23]. This allele substantially reduces expression of TRIP13, a AAA+ ATPase required for meiotic recombination. Most Trip13mod/mod spermatocytes arrest at pachynema with unrepaired DSBs and complete autosomal synapsis, then subsequently undergo apoptosis [22,23]. We show here that arrest occurs prior to histone H1t incorporation, similar to Dmc1−/− mutants. A small fraction of cells manage to evade arrest and finish meiosis (“escapers”), presumably because they complete meiotic recombination [22,23]. Nonetheless, the great majority of TRIP13-deficient spermatocytes undergo what has been inferred to be a recombination-dependent arrest in pachynema since autosomal synapsis is completed and spermatocytes display apparently normal looking sex bodies [22,23].
The second mutant is the Spo11+/− Atm−/− mouse [24,25]. In somatic cells, the ATM kinase is activated by the MRE11 complex to participate in the DNA damage response by phosphorylating multiple substrates, including the checkpoint mediator kinase CHK2 [26]. In meiosis, ATM regulates SPO11 activity via a negative feedback loop, such that Atm−/− single mutant spermatocytes experience greatly elevated DSB levels [27]. As a result, cells are unable to repair DSBs sufficiently and thus fail to complete synapsis or form sex bodies, arresting at pachynema [28]. However, introduction of Spo11 heterozygosity reduces SPO11 activity, partially decreasing DSB numbers, and so suppresses some of the phenotype of Atm−/− spermatocytes [27]. Although Spo11+/− Atm−/− spermatocytes are unable to repair all DSBs at pachynema, they complete autosome synapsis and sex body formation [24,25].
Remarkably, unlike Trip13mod/mod spermatocytes, Spo11+/− Atm−/− spermatocytes do not arrest or undergo apoptosis at pachynema despite the presence of multiple unrepaired DSBs, instead progressing to metaphase I before initiating programmed cell death [24,25]. To explain this difference in arrest behavior, we hypothesized that ATM is a critical component of the recombination-dependent arrest mechanism. Consistent with this idea, the canonical ATM-effector kinase CHK2 is required to arrest Trip13mod/mod oocytes [29]. Here we test this hypothesis by performing epistasis experiments combining Trip13mod/mod with mutations that eliminate ATM entirely, attenuate ATM activity, or eliminate a downstream effector kinase. Our findings reveal that recombination-dependent arrest in meiosis prior to the H1t-positive substage of pachynema depends on the MRE11 complex, ATM and CHK2.
Results
Different arrest and apoptosis responses in pachynema in Trip13mod/mod and Spo11+/− Atm−/− spermatocytes despite persistence of unrepaired DSBs in both genotypes
It has been proposed that the arrest and subsequent apoptosis of Trip13mod/mod spermatocytes at pachynema are both consequences of unrepaired DSBs activating a recombination-dependent checkpoint arrest mechanism [16,22,23]. To test this hypothesis, we asked if Trip13mod/mod cells with unrepaired DSBs were indeed undergoing apoptosis while retaining developmental characteristics of early pachynema. We detected apoptotic cells by performing TUNEL staining on spermatocyte spreads previously immunostained for three markers: γH2AX, a phosphorylated form of histone variant H2AX that is a marker of DSBs and sex body formation [30]; SYCP3, a component of the synaptonemal complex [31]; and H1t. For Spo11−/− spermatocytes, in which sex body failure triggers arrest and apoptosis after H1t incorporation has begun [2], all TUNEL-positive cells were also positive for H1t staining (n = 59, S1A Fig.). By contrast, for Dmc1−/− spermatocytes, all TUNEL-positive cells were H1t-negative (n = 70, P≤0.0001, Fisher’s exact test, S1B Fig.). These results further validate use of H1t as a molecular marker to distinguish between different arrest points in meiotic prophase.
Nearly all Trip13mod/mod TUNEL-positive cells (97.2%, n = 106) were H1t-negative (Fig. 1A-B), implying that most apoptotic cells had arrested in a developmental state with characteristics of early pachynema, as predicted. All Trip13mod/mod TUNEL-positive spermatocytes displayed multiple γH2AX patches (Fig. 1C), confirming the presence of unrepaired DSBs. A grossly normal sex body was also observed, agreeing with prior results [22,23], although further analysis described below revealed subtle sex body defects.
Fig. 1. Trip13mod/mod spermatocytes activate a recombination-dependent arrest at pachynema. 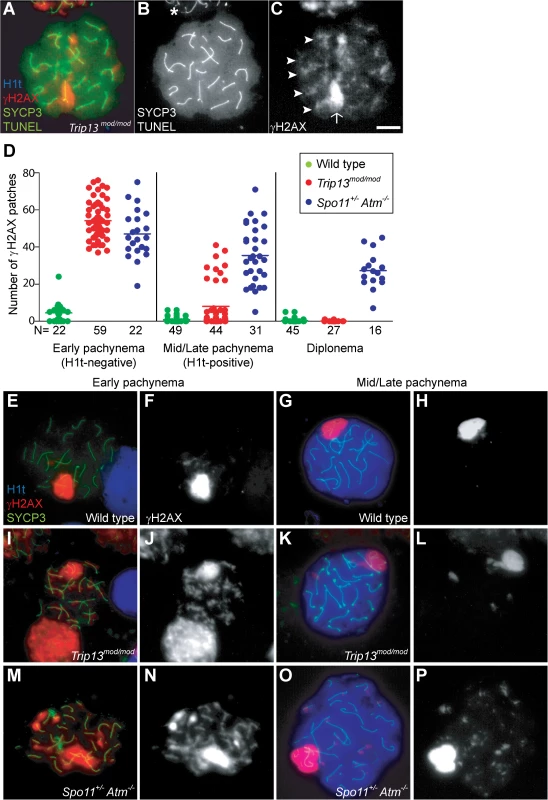
(A) Apoptotic Trip13mod/mod spermatocyte showing the axial element protein SYCP3 (green), TUNEL (green), mid/late pachytene stage marker H1t (blue) and γH2AX staining (red). Absence of H1t staining (blue) in the TUNEL-positive cell indicates that this is an early pachytene stage spermatocyte. (B) TUNEL staining appears as pan-nuclear labelling that overlaps all chromatin, which is observed in only a fraction of the analysed cells (asterisk shows an adjacent TUNEL-negative cell). (C) γH2AX localizes to the sex body (arrow) and unrepaired breaks (arrowheads). (D) Number of autosomal γH2AX patches (similar to arrowheads in (C)) counted in H1t-negative (early) and H1t-positive (mid/late) pachytene and diplotene cells from animals of the indicated genotypes. Means are indicated with horizontal lines. N shows the total number of cells counted per each stage and genotype. Primary data are provided in S1 Dataset. (E-P) Representative cells from the indicated genotypes stained for H1t (blue), γH2AX (red) and SYCP3 (green). In Trip13mod/mod, mid/late pachytene cells have substantially fewer γH2AX patches than early pachytene spermatocytes. Bar in (C) represents 10 μm and applies to all panels. If recombination-dependent arrest is activated in Trip13mod/mod spermatocytes, then we would also predict that the subset of cells that progress further to an H1t-positive state are the relatively recombination-proficient escapers documented previously [22,23]. If so, these cells should have fewer unrepaired DSBs than the pachytene cells that arrested before incorporating H1t. Indeed, H1t-positive Trip13mod/mod pachytene cells had fewer γH2AX patches than H1t-negative cells (8.0 ± 12.2 vs. 54.2 ± 9.9, mean ± SD, respectively; P≤0.0001, t test; Fig. 1D-L). Interestingly, there appeared to be two populations of H1t-positive Trip13mod/mod pachytene spermatocytes. The majority (77.3%) had very few (< 8) γH2AX patches (1.7 ± 2.2 on average; Fig. 1K-L), but still significantly more than wild-type cells (0.8 ± 1.5; P = 0.016; Fig. 1D, G-H). The remainder (22.7%) had more γH2AX patches than the first population (29.4 ± 6.7; P≤0.0001), but significantly less than H1t-negative cells (P≤0.0001). For the few Trip13mod/mod cells that reached diplonema, however, the number of γH2AX patches was indistinguishable from wild type (0.1 ± 0.3 vs. 0.4 ± 1.1, respectively; P = 0.183, negative binomial regression; Fig. 1D). A straightforward interpretation is that stochastic differences between TRIP13-deficient cells in the number of unrepaired DSBs translate into different arrest responses: cells with many unrepaired DSBs experience recombination-dependent arrest at early pachynema (H1t-negative), cells with an intermediate number progress to an H1t-positive stage but then undergo apoptosis (possibly because of the DSBs, sex body defects, or both; see below), and the most repair-proficient subset of cells progress still further to diplonema. Additional implications of these patterns are addressed in Discussion.
Spermatocytes from Spo11+/− Atm−/− mice also display γH2AX patches late in prophase I yet progress to the first division; this difference from ATM-proficient Trip13mod/mod mice is consistent with the hypothesis that cells respond differently to persistent DSBs in the absence of ATM. Using H1t staining to more precisely define prophase stages, we observed that H1t-positive Spo11+/− Atm−/− spermatocytes had numerous γH2AX patches, only ∼20% fewer than H1t-negative Spo11+/− Atm−/− spermatocytes (Fig. 1D, M-P). This is in marked contrast to Trip13mod/mod mice, where most cells that progressed to an H1t-positive state had much fewer γH2AX patches than the earlier H1t-negative cells (see above). Moreover, H1t-positive Spo11+/− Atm−/− spermatocytes had significantly more γH2AX patches than total H1t-positive Trip13mod/mod cells (35.5 ± 15.6, P≤0.0001; Fig. 1D). Thus, in the absence of ATM, cells with numerous unrepaired DSBs can progress to mid/late pachynema (and beyond).
Trip13mod/mod Spo11+/− Atm−/− spermatocytes progress to an H1t-positive state in greater numbers despite a high level of persistent DSBs
The above results are consistent with our hypothesis that unrepaired DSBs at early pachynema in Trip13mod/mod spermatocytes trigger a recombination-dependent checkpoint arrest that is mediated by ATM. To test this idea, we wished to ask if removing ATM activity allows TRIP13-deficient cells to progress further into prophase. It is uninformative for this purpose to query Trip13mod/mod Atm−/− double mutants because the Atm−/− mutation by itself causes an early block at an H1t-negative stage as a consequence of the enormous (>10-fold) increase in DSB numbers (see Discussion) [2,27]. Instead, since Spo11 heterozygosity ameliorates this catastrophic effect of ATM deficiency, we tested the epistasis relationship between the Trip13mod/mod phenotype (arrest in an H1t-negative state) and Spo11+/− Atm−/− phenotype (no pachytene arrest) by analyzing Trip13mod/mod Spo11+/− Atm−/− mice (hereafter referred to as “TSA triple mutant”). As detailed below, we indeed find that absence of ATM allows TRIP13-deficient spermatocytes to progress to an H1t-positive state, but that additional defects that cause efficient mid/late-pachytene arrest and apoptosis of TSA triple mutant spermatocytes become apparent. (Note that DSBs in the Spo11+/− Atm−/− background are still substantially elevated relative to wild type (∼6-fold) [27], so no aspect of the triple mutant phenotype can be ascribed to a reduction in DSBs below wild-type levels.)
First, we measured testis size as a readout of spermatogenetic progression since mice with spermatogenic failure have small testes [14,15]. Sizes of Trip13mod/mod and TSA triple mutant testes were indistinguishable from one another (P = 0.6, t test), both being smaller than in Spo11+/− Atm−/− mice (P≤0.05, Table 1) and approximately one third the size of wild-type testes (P≤0.0001, Table 1). These results indicate that TSA triple mutant mice experience spermatogenic failure.
Tab. 1. Testicular and meiotic phenotypes of wild-type and mutant mice. 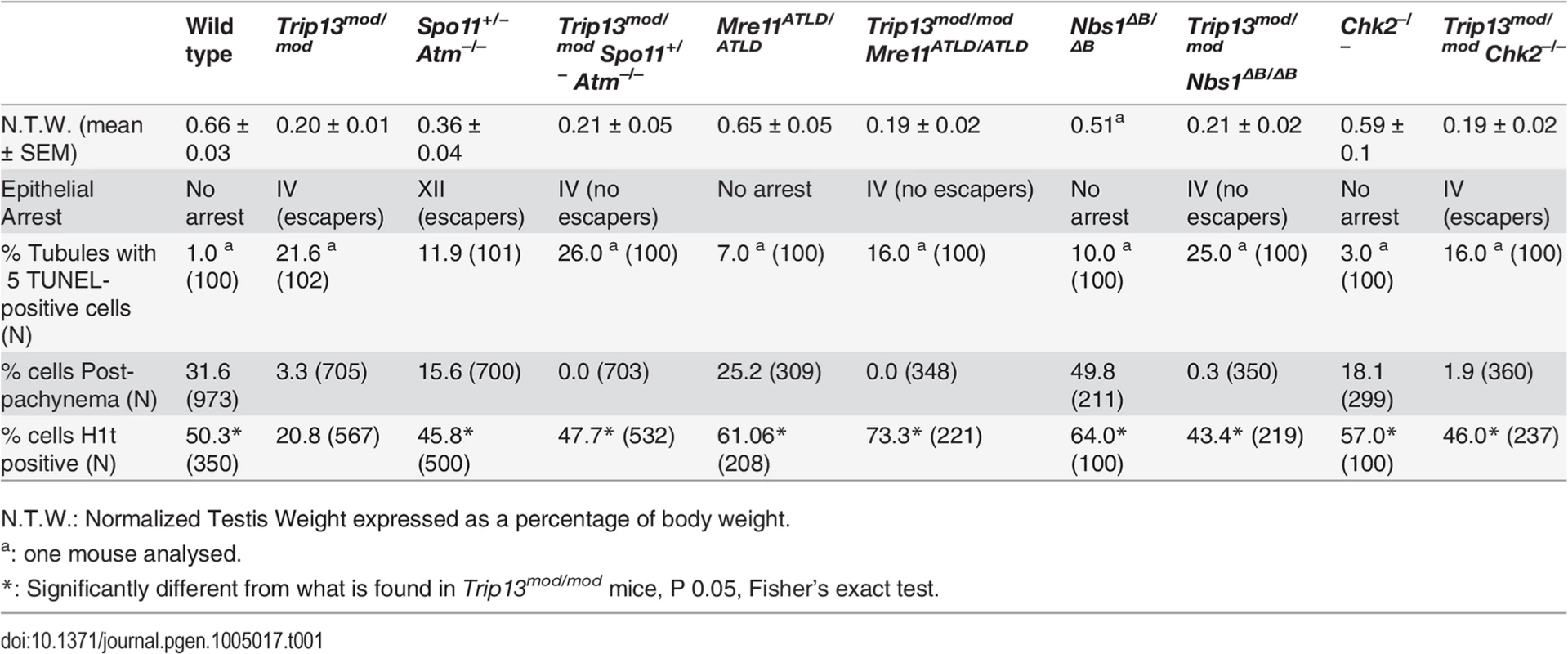
N.T.W.: Normalized Testis Weight expressed as a percentage of body weight. Second, we assessed the timing of apoptosis by histological staging of seminiferous tubules. Many Trip13mod/mod spermatocytes underwent apoptosis in tubules at epithelial stage IV, corresponding to mid pachynema (Fig. 2A,B), as previously shown [23]. In contrast, and consistent with absence of pachytene arrest, Spo11+/− Atm−/− spermatocytes apoptosed in tubules at epithelial stage XII (Fig. 2C), corresponding to metaphase I. Arrest of Spo11+/− Atm−/− spermatocytes at this point is thought to be caused largely by a spindle checkpoint response to achiasmate (unconnected) chromosomes, particularly the X-Y pair [32]. Consistent with the small testis sizes, TSA triple mutant animals showed spermatocyte apoptosis at stage IV (Fig. 2D). Furthermore, whereas few wild-type tubules had >5 TUNEL-positive cells (1.0%, Table 1), both Trip13mod/mod single mutant and TSA triple mutant testes displayed many such apoptotic tubules (21.6% and 26.0% respectively, P≤0.0001 compared to wild type, Fisher’s exact test, Table 1). Surprisingly, no TSA triple mutant cells escaping stage IV apoptosis were observed (Fig. 2B-D), in contrast to Trip13mod/mod or Spo11+/− Atm−/− testes [22–24].
Fig. 2. Trip13mod/mod Spo11+/− Atm−/− spermatocytes arrest at epithelial stage IV, but present autosomal asynapsis, multiple unrepaired DSBs and fail to form a sex body at mid/late pachynema. 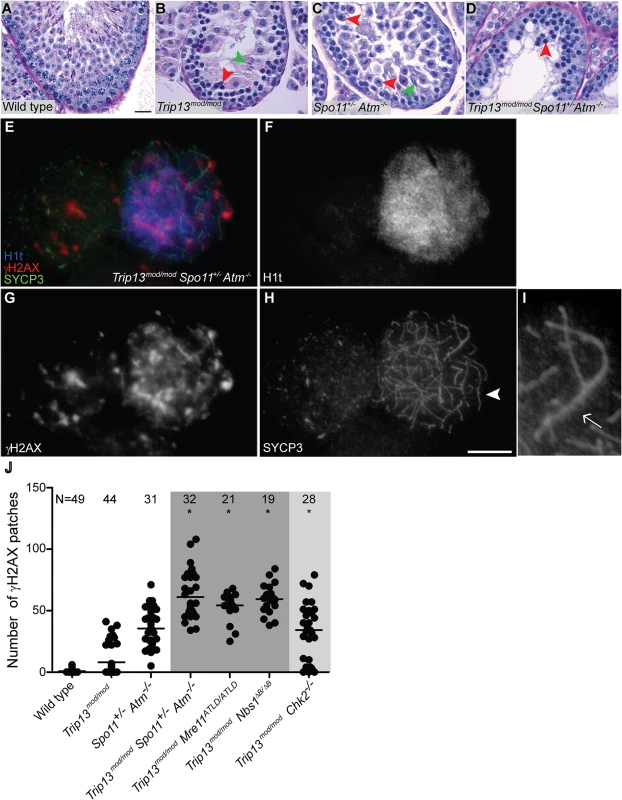
(A-D) Testis cross-sections showing individual seminiferous tubules from the indicated genotypes, stained with PAS-Haematoxylin. (A) In wild type, spermatogonia, spermatocytes and spermatids are present. (B) Stage IV tubule in Trip13mod/mod with apoptotic spermatocytes, corresponding to pachynema (red arrowhead) [22,23]. Only a minor fraction of cells complete meiosis (green arrowhead). (C) Spo11+/− Atm−/− tubule at stage XII, which is characterized by spermatocytes at metaphase I (red arrowhead) [24]. The presence of lagging chromosomes in some metaphase I spermatocytes is thought to be the cause of apoptosis. Green arrowhead denotes a spermatid that has escaped meiotic arrest. (D) Trip13mod/mod Spo11+/− Atm−/− tubule at stage IV, presenting multiple apoptotic spermatocytes at pachytene stage (red arrowhead). No spermatids were observed. (E-H) Two Trip13mod/mod Spo11+/− Atm−/− spermatocytes, one at preleptonema (left) and the other at mid/late pachynema (right), stained for H1t (blue, F), γH2AX (red, G) and SYCP3 (green, H). A high degree of asynapsis occurs in the H1t-positive cell, where long stretches of axial elements are visible (arrowhead). (I) Enlarged image of a bivalent that has successfully synapsed only a portion of the homologous chromosome pair (arrow). (J) Number of γH2AX patches in mid/late pachytene spermatocytes of the indicated genotypes. Triple and double mutants that generate more DSBs and fail to complete synapsis (highlighted in dark grey) display more γH2AX patches at mid/late pachynema than Trip13mod/mod Chk2−/− cells (highlighted in light grey). Data for wild type, Trip13mod/mod and Spo11+/− Atm−/− reproduced from Fig. 1D for comparison. N shows the total number of cells counted per each genotype. Asterisk marks statistically significant differences compared to Trip13mod/mod, P≤0.0001, t test. Primary data are provided in S1 Dataset. Bar in (A) represents 20 μm and applies to panels (A-D). Bar in (H) represents 10 μm and applies to panels (E-H). Although these results indicate that ATM deficiency (in the Spo11+/− Atm−/− background) does not suppress the pachytene apoptosis of Trip13mod/mod mutant spermatocytes, they do not precisely define the stage of meiotic arrest. To address this question, we examined progression of chromosome synapsis in spermatocyte spreads stained with anti-SYCP3 antibodies. Out of total SYCP3-positive primary spermatocytes, 3.3% had progressed beyond pachynema in Trip13mod/mod, but none in TSA triple mutants (P≤0.0001, Fisher’s exact test, Table 1). This lack of escapers here and in the analysis of tubule sections indicates that overall spermatogenic failure is more penetrant in TSA triple mutants. Moreover, all TSA triple mutant spermatocytes displayed substantial autosome asynapsis (n = 703, Fig. 2E, H-I), unlike either the Trip13mod/mod or Spo11+/–Atm−/− mutants [23,24]. Because failure to complete synapsis has been associated with the inability to properly repair meiotic DSBs, these results could suggest that TRIP13-deficient spermatocytes are unable to cope with the increased DSB numbers produced in the Spo11+/− Atm−/− background [27], leading to synaptic catastrophe.
One consequence of synaptic failure is the inhibition of sex body formation [16]. Accordingly, we did not observe any TSA triple mutant cells with the γH2AX immunostaining pattern diagnostic of sex body formation (Fig. 2E, G). Instead, γH2AX signal was mostly localized as patches on both unsynapsed and synapsed portions of chromosomes and did not accumulate in any particular region of the nucleus. The complete failure to form sex bodies in TSA triple mutants likely accounts for the highly penetrant arrest in pachynema and apoptosis at stage IV.
To determine whether ATM imposes a recombination-dependent arrest early in pachynema that is relieved in TSA triple mutant spermatocytes, we examined H1t incorporation. In wild type, 50.3% of SYCP3-positive cells were also positive for H1t (Table 1), whereas only 20.8% of SYCP3-positive cells from Trip13mod/mod testes were H1t-positive (Table 1). Presumably, most of these H1t-positive cells were escapers that would complete meiosis (see above). Notably, 47.7% of SYCP3-positive cells from TSA triple mutants were H1t-positive (see representative image in S2 Fig.), a ≥two-fold increase compared to Trip13mod/mod samples but similar to that in wild-type and in Spo11+/− Atm−/− animals (45.8%, Table 1). Thus, the Spo11+/− Atm−/− combination is epistatic to Trip13mod/mod mutation for the ability of spermatocytes to incorporate H1t.
We note that the relatively normal H1t pattern in Spo11+/− Atm−/− animals (S1 Table) and the strong block to H1t incorporation in Atm−/− single mutants [2] argue against the possibility that ATM is required to prevent premature H1t expression in early pachynema. To confirm this conclusion, we compared timing of H1t expression in testis sections from wild type and TSA triple mutants. In wild type, H1t was first detected in mid-pachytene spermatocytes in stage IV tubules, and was absent from early pachytene spermatocytes in stage I-III tubules, as previously reported [13] (see S3A,B Fig. and its legend). Staging is less precise for immunofluorescently stained tubules of mutants that lack post-meiotic cell types [19]. Nonetheless, we observed that leptotene, zygotene, and most if not all early pachytene spermatocytes were H1t-negative, and that H1t-positive cells did not appear until approximately mid pachynema in TSA triple mutants (i.e., in tubule sections that were likely in stage IV) (see S3C Fig. and its legend). Thus, there is no evidence for substantially premature expression of H1t in ATM-defective spermatocytes.
The increased number of H1t-positive cells implies that the TSA triple mutant spermatocytes are not being restrained by the recombination-dependent arrest mechanism. This further reinforces the conclusion above that the apoptosis occurring in these cells is attributable to the absence of a sex body. If our interpretation is correct, we would expect that absence of the recombination-dependent checkpoint allows spermatocytes to progress to an H1t-positive state even if they have many unrepaired DSBs. Indeed, H1t-positive TSA triple mutant spermatocytes displayed significantly more γH2AX patches than Trip13mod/mod cells (61.0 ± 18.8, P≤0.0001, t test; Fig. 2J). The large number of γH2AX patches also supports the conclusion that the TSA triple mutants do not experience a reduction in DSBs relative to Trip13mod/mod alone. The finding that absence of ATM allows cells to progress to an H1t-positive state even if they contain numerous unrepaired DSBs indicates that ATM is required for implementation of recombination-dependent arrest at early pachynema.
The MRE11 complex promotes recombination-dependent arrest
The MRE11 complex (MRE11, RAD50 and NBS1) senses DSBs and promotes their repair, in part by activating ATM [33]. Null mutations of these genes are incompatible with embryonic development, but mice bearing hypomorphic Nbs1 or Mre11 mutations that attenuate ATM activation and/or diminish phosphorylation of subsets of ATM targets are viable (Nbs1ΔB or Mre11atld) [34,35]. Nbs1ΔB/ΔB and Mre11ATLD/ATLD mice are subfertile, with spermatocytes displaying some synaptic defects and accumulating early recombination markers at pachynema, suggesting that the MRE11 complex plays a role in meiotic recombination in mammals [36], as in budding yeast and other organisms [37]. Testis samples from both mutants showed a small elevation in the fraction of spermatocytes that were H1t-positive (Table 1). This could be a consequence of previously documented differences in the overall distribution of meiotic cell types [36], but the important conclusion for purposes of this study is that the mutant spermatocytes are readily able to progress through pachynema and beyond in roughly normal numbers.
To further define the ATM-dependent pathway leading to recombination-dependent arrest, we asked whether these MRE11-complex mutations mimic ATM deficiency with respect to the meiotic progression phenotype of Trip13mod/mod spermatocytes. Indeed, similar to the TSA triple mutant, Trip13mod/mod Nbs1ΔB/ΔB and Trip13mod/mod Mre11ATLD/ATLD mutants had approximately two-fold (43.4%) and three-fold (73.3%) more H1t-positive spermatocytes, respectively, than Trip13mod/mod single mutants (Table 1). Importantly, these mutants progressed despite having high numbers of unrepaired DSBs: H1t-positive spermatocytes in Trip13mod/mod Nbs1ΔB/ΔB and Trip13mod/mod Mre11ATLD/ATLD mutants had significantly more γH2AX patches than the Trip13mod/mod mutant (59.7 ± 12.4 and 54.1 ± 10.8, respectively; Fig 2J), but similar numbers to the TSA triple mutant (P˃0.05). These hypomorphic MRE11 complex mutations are thus epistatic to Trip13mod/mod with respect to the ability of cells to progress to an H1t-positive stage. These results support the hypothesis that the MRE11 complex activates the ATM-mediated recombination-dependent arrest that halts development of most Trip13mod/mod single mutant spermatocytes at early pachynema.
Also akin to the TSA triple mutant, these double mutants experienced a more penetrant spermatogenic failure (i.e., few or no escapers) than in the Trip13mod/mod single mutant despite better progression beyond early pachynema. Trip13mod/mod Nbs1ΔB/ΔB and Trip13mod/mod Mre11ATLD/ATLD mice had small testes (Table 1), and histological analysis revealed many similarities between Trip13mod/mod Nbs1ΔB/ΔB and Trip13mod/mod Mre11ATLD/ATLD double mutants and the TSA triple mutant: apoptosis at epithelial stage IV (Figs. 3 and S4), a high number of apoptotic cells per tubule (Table 1), and no evidence of cells escaping and completing meiosis (Figs 3. and S4). Likewise, cytological analysis showed that no Trip13mod/mod Mre11ATLD/ATLD spermatocytes and only a negligible fraction of Trip13mod/mod Nbs1ΔB/ΔB spermatocytes (0.3%) progressed beyond pachynema, similar to the TSA triple mutant (P˃0.05, Fisher’s exact test, Table 1) but significantly different from the Trip13mod/mod single mutant (P≤0.0001 and P = 0.0014, respectively). Also similar to TSA triple mutants, most double mutant spermatocytes had substantial synaptic defects, with only 0.5% of Trip13mod/mod Mre11ATLD/ATLD (n = 348) and 5.4% of Trip13mod/mod Nbs1ΔB/ΔB (n = 350) SYCP3-positive spermatocytes showing complete autosomal synapsis. These values are much lower than Trip13mod/mod SYCP3-positive spermatocytes (40.7%, n = 705, P≤0.0001, Fisher’s exact test). As expected from the substantial asynapsis, the double mutants showed no evidence of sex body formation as assessed by γH2AX staining (Figs. 3 and S4). Taken together, we infer from these data that the more penetrant spermatogenic failure observed in Trip13mod/mod Nbs1ΔB/ΔB and Trip13mod/mod Mre11ATLD/ATLD testes is most probably due to activation of the sex body-deficient arrest, as in TSA triple mutants. Further, our results confirm that the sex body-deficient arrest mechanism can operate even when recombination-dependent arrest is compromised. This was also indicated by the arrest of Spo11−/− mutants as well as repair-proficient mutants with defects in meiotic sex chromosome inactivation (MSCI) [2,16].
Fig. 3. Trip13mod/mod Nbs1ΔB/ΔB spermatocytes arrest in mid/late pachynema with unrepaired DSBs at epithelial stage IV. 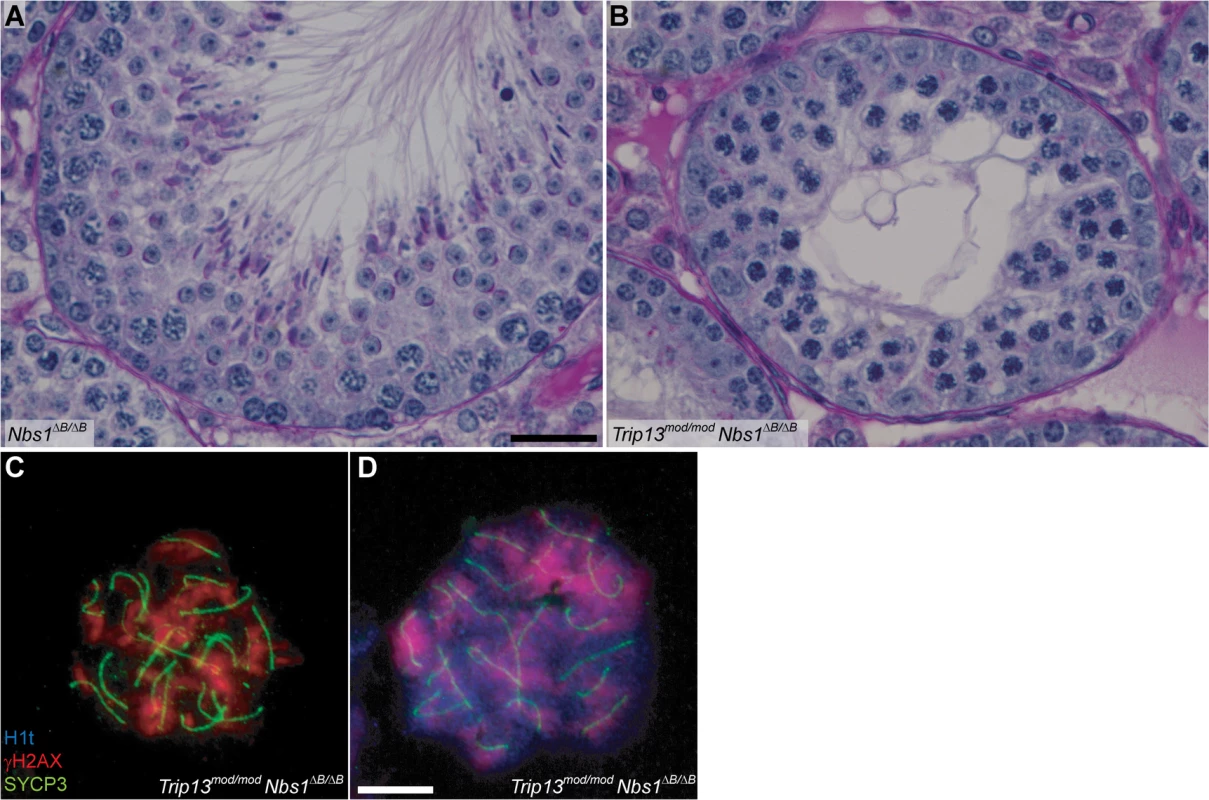
(A-B) Cross sections of Nbs1ΔB/ΔB and Trip13mod/mod Nbs1ΔB/ΔB stage IV tubules stained with PAS-Haematoxylin. (C-D) Early (C) and mid/late (D) pachytene Trip13mod/mod Nbs1ΔB/ΔB spermatocytes stained for H1t, γH2AX, and SYCP3. Both cells present multiple unrepaired DSBs and incomplete synapsis. Bar in (A) represents 20 μm and applies to panels (A-B). Bar in (D) represents 10 μm and applies to panels (C-D). The MRE11 complex controls DSB numbers
We reasoned that the attenuated ATM signaling in Mre11 and Nbs1 hypomorphic mutants might lead to elevated DSB numbers. To test this, we examined SPO11-oligonucleotide complexes, which provide a measure of whole-testis DSB levels, in Mre11ATLD/ATLD and Nbs1ΔB/ΔB single mutant mice [27]. (Note that this class of hypomorphic MRE11-complex mutation is unlike the Rad50S type of mutation, which in yeast blocks endonucleolytic release of SPO11 from DSB ends [33]). Indeed, we found that Mre11ATLD/ATLD and Nbs1ΔB/ΔB mice displayed an ∼2-fold increase compared to wild-type littermates (Fig. 4A-B). This elevation is less than what is seen in Atm–/–(∼12-fold) or Spo11+/–Atm−/− testes (∼6-fold), presumably because the Mre11 and Nbs1 hypomorphs attenuate but do not eliminate ATM activity [34,35]. (Note that the overall fertility phenotype of the Mre11 and Nbs1 single mutants is also significantly milder than for mice lacking ATM (S1 Table)). These results provide strong evidence that ATM-mediated feedback control of DSBs involves an MRE11 complex-dependent ATM activation pathway similar to the response to ionizing radiation. In contrast, Trip13mod/mod animals had roughly normal levels of SPO11-oligonucleotide complexes (∼70% of wild type, Fig. 4C). Because Trip13mod/mod single mutants have defects in completing DSB repair, we speculate that the autosomal synaptic failure in Trip13mod/mod Mre11ATLD/ATLD or Trip13mod/mod Nbs1ΔB/ΔB mice is a synthetic defect caused by an inability of TRIP13-deficient cells to tolerate increased DSB numbers resulting from ATM activation defects. An alternative but not mutually exclusive possibility is that the MRE11 complex and TRIP13 synergistically promote synapsis separately from their effects on recombination.
Fig. 4. The MRE11 complex, but not TRIP13 or CHK2, modulates SPO11-oligonucleotide complex levels. 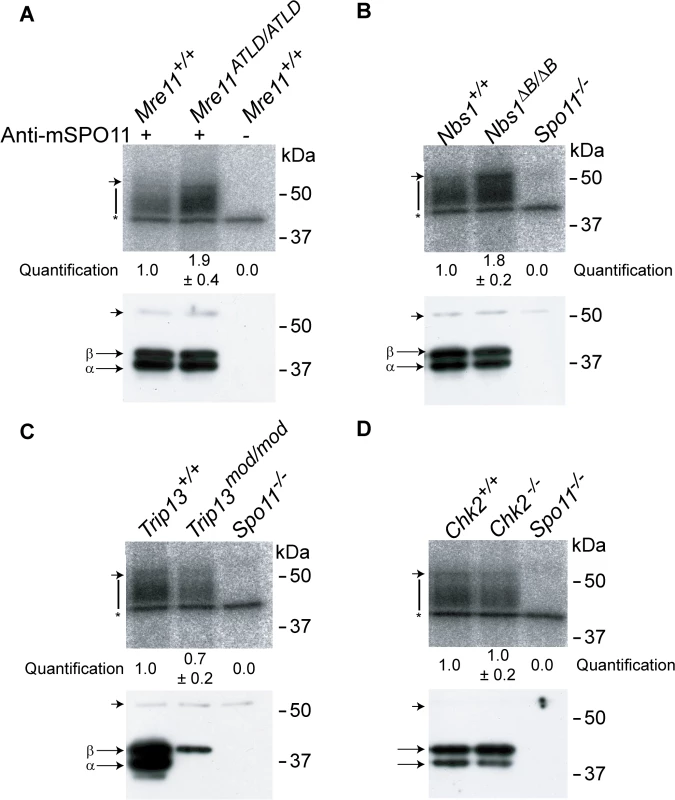
SPO11-oligonucleotide complexes were immunoprecipitated from extracts of testes of the indicated genotypes, labelled with terminal transferase and 32P-nucleotide, and resolved by SDS-PAGE. Top image in each panel, autoradiograph; bottom image, SPO11 Western blot where the two major SPO11 isoforms, α and β, are indicated. The vertical lines next to the autoradiographs indicate the signal from SPO11-oligonucleotide complexes. Asterisk indicates non-specific signal from the labelling reaction. Short arrow designates the migration position of the heavy chain of the antibody used to immunoprecipitate SPO11. (A-B) Both Mre11 and Nbs1 mutants have increased levels of SPO11-oligonucleotide complexes compared to littermate controls (1.9 ± 0.4 fold, mean ± SD of the relative signal intensity compared to a wild-type control, n = 3 mice, and 1.8 ± 0.2 fold, n = 3, respectively). (C) SPO11-oligonucleotide complex levels are slightly reduced in Trip13mod/mod samples (0.7 ± 0.2, n = 3), similar to other recombination-deficient mutants, such as Dmc1–/– [27]. The SPO11α isoform is not detected in Trip13mod/mod testes as observed in other mutants that arrest at pachynema [27]. (D) Chk2−/− testes have similar levels of SPO11-oligonucleotide complex as controls (1.0 ± 0.2 fold, n = 3). CHK2 is involved in recombination-dependent arrest but does not regulate DSB levels
The CHK2 kinase is an effector of the ATM-signaling cascade in response to ionizing radiation [38]. In meiosis in several species, CHK2 function is required to efficiently activate the recombination-dependent checkpoint [9]. In mouse, CHK2 is required to arrest oocytes with unrepaired DSBs, although it has been suggested based on histological analysis that CHK2 does not play a similar role in spermatocytes [29]. To test for CHK2 involvement in recombination-dependent arrest in mouse spermatocytes, we analyzed Trip13mod/mod Chk2−/− mice.
The testicular phenotype of Trip13mod/mod Chk2−/− mice was similar to the Trip13mod/mod single mutant for testis size and histological tubule classification (apoptosis of spermatocytes at epithelial stage IV, but with presence of some spermatids), but also, notably, for the percentage of SYCP3-expressing cells that had progressed beyond pachynema (1.9%, P = 0.2462, Fisher’s exact test; Table 1 and Fig. 5A-B). Thus, CHK2 deficiency does not cause a more penetrant spermatogenic failure in the context of the Trip13mod/mod mutation, unlike MRE11 complex hypomorphs or the absence of ATM itself. Moreover, CHK2-deficient testes displayed similar levels of SPO11-oligonucleotide complexes as wild-type littermates (Fig. 4D). Thus, CHK2 is not required for proper control of meiotic DSB formation, clearly separating CHK2 from both the MRE11 complex and ATM in the regulation of SPO11 activity. These results further support the conclusion above that the synaptic defects and more penetrant block to spermatogenesis observed in TSA triple mutants and in Trip13mod/mod Mre11ATLD/ATLD and Trip13mod/mod Nbs1ΔB/ ΔB double mutants are attributable to the increased DSB numbers.
Fig. 5. CHK2 is required to maintain early pachytene arrest caused by TRIP13 deficiency. 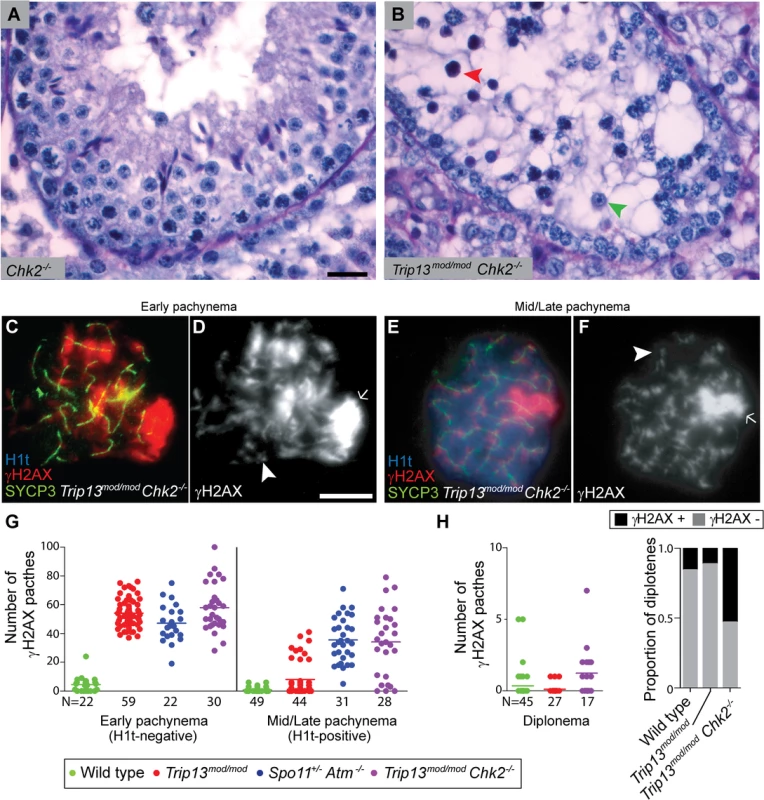
(A-B) Cross-sections of Chk2−/− and Trip13mod/mod Chk2−/− testes stained with PAS-Haematoxylin. Chk2−/− testes contained all spermatogenic cell types, whereas Trip13mod/mod Chk2−/− spermatocytes arrested in tubules at epithelial stage IV (red arrowhead). Note the presence of spermatids (green arrowhead) indicating that some Trip13mod/mod Chk2−/− cells manage to complete meiosis. (C-F) Early and mid/late pachytene Trip13mod/mod Chk2−/− spermatocytes stained for H1t, γH2AX, and SYCP3. Both early and mid/late pachytene cells exhibit multiple γH2AX patches (arrowheads) corresponding to unrepaired DSBs, as well as accumulation of γH2AX on the chromatin of the sex chromosomes (arrows). (G) Quantification of the number of γH2AX patches in Trip13mod/mod Chk2−/− spermatocytes. Data for wild type, Trip13mod/mod and Spo11+/− Atm−/− reproduced from Fig. 1D for comparison, N shows the total number of cells counted per each stage and genotype. (H) γH2AX patches in diplotene cells. The plot on the left shows the number of γH2AX patches per cell. Note that overplotting of zero values obscures information about proportions of cells that lack a γH2AX patch, so the plot on the right displays proportions of cells with or without a γH2AX patch. Primary data for panels G and H are provided in S1 Dataset. N shows the total number of cells counted per each stage and genotype. Bar in (A) represents 20 μm and applies to panels (A-B). Bar in (D) represents 10 μm and applies to panels (C-F). Notably, however, Trip13mod/mod Chk2−/− spermatocytes showed evidence of a defect in recombination-dependent arrest, because two-fold more H1t-positive spermatocytes were observed than with the Trip13mod/mod single mutant (Table 1). Similarly to TSA triple mutants and the other double mutants described above, these H1t-positive spermatocytes presented more γH2AX patches (34.1 ± 23.3; P≤0.0001, t test; Fig. 5E-G). Also, whereas 77.3% of the H1t-positive Trip13mod/mod spermatocytes (i.e., those inferred to be relatively recombination-proficient escapers) had very few γH2AX patches (< 8 per cell), only 21.4% of cells from Trip13mod/mod Chk2−/− mice were in this category (P≤0.0001, Fisher’s exact test), with the majority of cells having many more γH2AX patches (Fig. 5G). By contrast, H1t-negative cells had similar numbers of γH2AX patches in both Trip13mod/mod single mutant and Trip13mod/mod Chk2−/− double mutant mice (P = 0.18, t test, Fig. 5G). These results suggest that the absence of CHK2 allows spermatocytes with numerous unrepaired DSBs to bypass the recombination-dependent arrest during pachynema, reminiscent of what occurs in oocytes [29]. If this is correct, we would also expect to find evidence of unrepaired DSBs in those spermatocytes that had escaped pachytene arrest entirely. Indeed, γH2AX patches were substantially more prevalent at diplonema in Trip13mod/mod Chk2−/− spermatocytes: only 11.1% of diplotene Trip13mod/mod cells had γH2AX patches, but 52.9% of the Trip13mod/mod Chk2−/− spermatocytes had one or more of these markers of unrepaired DSBs (P = 0.0045, Fisher’s exact test) (Fig. 5H). (Mean ± SD of 0.1 ± 0.3 for Trip13mod/mod (Fig. 1D) vs. 1.2 ± 1.8 (n = 17) for Trip13mod/mod Chk2–/–; P = 0.0005, negative binomial regression; S1 Dataset.) We conclude that CHK2, like the MRE11 complex and ATM, is required to activate the recombination-dependent arrest occurring in Trip13mod/mod spermatocytes.
Trip13mod/mod Chk2−/− spermatocytes have sex body defects
Although CHK2 deficiency allowed more efficient progression of TRIP13-deficient cells to an H1t-positive stage, the overall spermatogenic failure was not alleviated, such that most Trip13mod/mod Chk2−/− spermatocytes still underwent apoptosis in tubules at epithelial stage IV (Fig. 5B). We therefore hypothesized that the sex bodies of Trip13mod/mod Chk2−/− spermatocytes may have subtle defects that impede their function. Indeed, most Trip13mod/mod Chk2−/− spermatocytes showed an abnormally elongated γsH2AX-positive sex body chromatin domain (57.8%, N = 45, Fig. 5E-F), unlike the condensed, rounded structure seen in most wild-type H1t-positive spermatocytes (Fig. 1F,H). In addition, the intensity of the sex-body γH2AX signal was reduced to about half the wild-type level on average in H1t-positive pachytene Trip13mod/mod Chk2−/− spermatocytes (S5 Fig.; P ≤0.0001, t test). Although most H1t-positive pachytene Trip13mod/mod cells had sex bodies with normal morphology, a significant minority also showed the abnormal elongated form (11.3%, n = 53, P≤0.0001, Fisher’s exact test) and sex body γH2AX intensity was reduced on average (P = 0.033; see S5 Fig.).
ATR is the kinase responsible for the bulk of H2AX phosphorylation in sex bodies [18,24,25]. In wild type, most pachytene spermatocytes showed ATR staining either as a continuous signal on the sex chromosome axes or spread to the XY chromatin (93.6%, n = 47; Fig. 6A-B), as previously reported [39]. The remainder of the cells displayed stretches of ATR partially covering the X and Y axes. By contrast, only 18.0% of Trip13mod/mod Chk2−/− pachytene cells had the axial or chromatin-associated ATR staining most commonly found in wild-type cells, 26.2% had short stretches of continuous ATR signal partly covering sex chromosome axes, and most cells had only focal ATR staining (55.7%, n = 61; Fig. 6C-E). Trip13mod/mod spermatocytes had an intermediate phenotype: 38.8% displayed ATR completely covering the XY axes or chromatin, 32.8% showed ATR stretches over the XY axes, and 28.4% had only discrete ATR foci along the X and Y (n = 67, Fig. 6E). These results suggest that CHK2 deficiency may exacerbate a defect in ATR loading on the sex chromosomes in TRIP13-deficient cells.
Fig. 6. Sex body deficiency in Trip13 mutants. 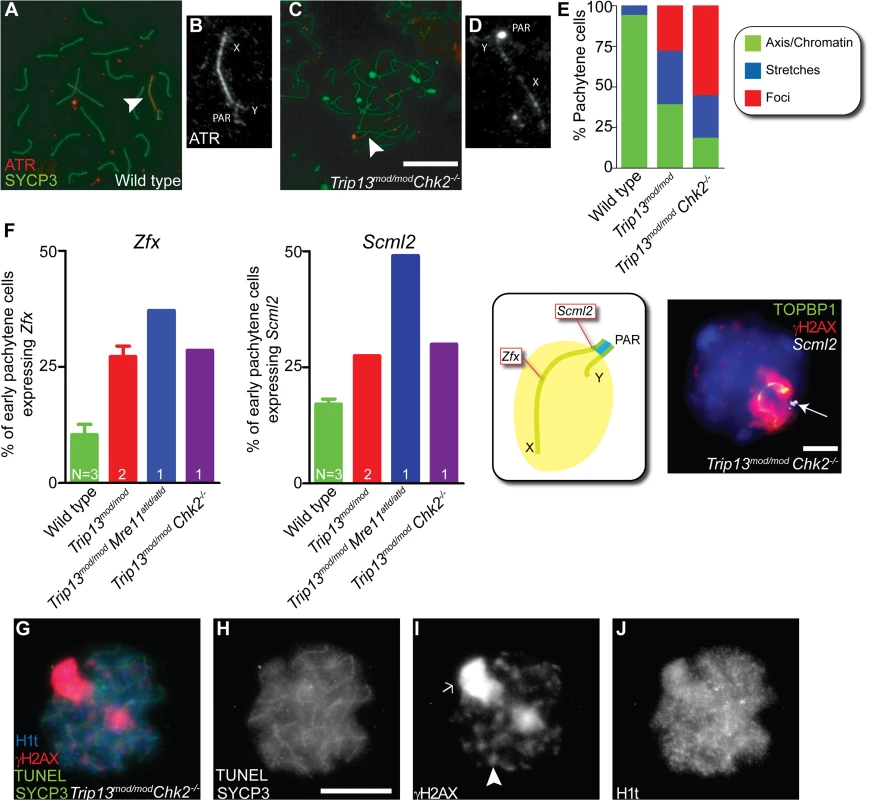
(A-D) Wild-type and Trip13mod/mod Chk2−/− pachytene spermatocytes stained for ATR and SYCP3. Arrowheads indicate the sex chromosomes. Note the relatively continuous ATR staining on the X and Y axes in wild type compared with the focal ATR staining in the mutant. (E) Quantification of the different ATR staining patterns found in the genotypes presented. (F) Left panels, percent of early pachytene-stage spermatocytes expressing Zfx and Scml2 in wild-type, Trip13mod/mod, Trip13mod/mod Mre11ATLD/ATLD and Trip13mod/mod Chk2−/− mice. The N value in each bar represents the number of mice analyzed per each gene and genotype. Middle panel, cartoon of a sex body displaying the relative position of Zfx and Scml2 within the X chromosome. Right image, representative Trip13mod/mod Chk2−/− spermatocyte displaying TOPBP1 (green) and γH2AX (red) immunostaining and Scml2 RNA-FISH signal (white, arrow). Just 10.9% ± 3.8 (mean ± SD) of wild-type spermatocytes (N = 225 cells) express Zfx and 17.1% ± 1.9 express Scml2 (N = 200). Mouse that fail to synapse the X and Y chromosomes, like Trip13mod/mod Mre11ATLD/ATLD, present more spermatocytes expressing these genes than wild-type mice (for Zfx: 37.1%, N = 35 and for Smcl2: 49.1%, N = 53, P≤0.0001 and P = 0.0002 respectively, Fisher’s exact test). Trip13mod/mod mice present more cells expressing these genes than wild-type mice (for Zfx: 27.2% ± 3.2, N = 158; for Smcl2: 27.5% ± 0.0, N = 160, P<0.005, 1 way Anova). Similarly, Trip13mod/mod Chk2−/− spermatocytes are more likely to express these X-linked genes than wild-type cells (for Zfx: 28.6%, N = 70; for Smcl2: 30.0%, N = 80, P = 0.0217 and P = 0.0009 respectively, Fisher’s exact test). (G-J) Mid/late pachytene-stage, TUNEL-positive Trip13mod/mod Chk2−/− spermatocyte immunostained for SYCP3 (green, H), γH2AX (red, I) and H1t (blue, J). Note the presence of an elongated sex body (arrow), multiple γH2AX patches (arrowhead) and H1t in the chromatin of the apoptotic cell. Bars in (C) and (H) represent 10 μm and apply to panels (A,C) and (G-J), respectively. Bar in (F) represents 5 μm. To further explore ATR-dependent processes, we examined SUMO-1, which is loaded onto sex chromosomes at pachynema in an ATR-dependent manner [18]. In all wild-type cells (n = 52) and 86.0% of Trip13mod/mod cells (n = 50), we detected SUMO-1 covering the chromatin of the sex body. In contrast, only 72.3% of Trip13mod/mod Chk2−/− pachytene spermatocytes contained SUMO-1 in their sex bodies, and of these, SUMO-1 signal had failed to spread over the entire XY chromatin in 60.6% of cells (n = 45, S6 Fig.).
Since the analyzed markers suggested that the sex bodies of Trip13mod/mod Chk2–/–spermatocytes may be altered, we studied the functionality of these sex bodies by RNA FISH. As mentioned above, an important function of the sex body is to silence certain X - and Y-linked genes that are toxic for spermatocytes [17]. Thus, we analyzed the early-pachytene expression of two X-linked genes: Zfx, located in an interstitial region; and Scml2, located near the pseudoautosomal boundary at the centromere-distal end of the chromosome (Figs. 6F and S7). As expected, only a minority of wild-type cells expressed Zfx or Smcl2 (10.9% ± 3.8 and 17.1% ± 1.9, mean ± SD, respectively, Fig. 6F), whereas a significantly larger fraction of cells expressed these genes in mutants that fail to form a sex body at pachynema, like Trip13mod/mod Mre11ATLD/ATLD spermatocytes (for Zfx: 37.1% and for Smcl2: 49.1%, P≤0.0001 and P = 0.0002 respectively, Fisher’s exact test). As predicted from the altered sex body morphology, we found that Trip13mod/mod and Trip13mod/mod Chk2−/− spermatocytes also showed increased expression of these X-linked genes at early pachynema (Trip13mod/mod: for Zfx: 27.2% ± 3.2 and for Smcl2: 27.5% ± 0.0, P<0.005, 1 way Anova; Trip13mod/mod Chk2–/–: for Zfx: 28.6% and for Smcl2: 30.0%, P = 0.0217 and P = 0.0009 respectively, Fisher’s exact test). We conclude that sex body function is altered in Trip13 mutants.
The levels of sex chromosome gene misexpression are similar to those reported to be sufficient to arrest spermatocytes at pachynema in other mutants [21]. Indeed, when we assessed whether Trip13mod/mod Chk2−/− pachytene spermatocytes undergo apoptosis at an H1t-positive stage, as occurs in mutants that fail to form a sex body (S1 Fig.) [2], we found that 98.5% of TUNEL-positive Trip13mod/mod Chk2−/− spermatocytes analyzed were also H1t-positive, contrasting what occurs in Trip13mod/mod mutants (N = 67, 1 mouse, P ≤0.0001, Fisher’s exact test, Fig. 6G-J) but mimicking what is seen in Spo11−/− mutants (P = 1, Fisher’s exact test). In summary, the mild sex body defect in Trip13mod/mod testes was more pronounced in Trip13mod/mod Chk2−/− spermatocytes, where it resulted in the failure to silence the X and Y chromosomes at pachynema, suggesting that the stage IV apoptosis observed in these double mutant mice is triggered by the sex body-deficient arrest mechanism.
Discussion
Recombination-dependent arrest prior to H1t incorporation depends on the MRE11 complex, ATM and CHK2 in mouse
Two major mechanisms are postulated to mediate arrest of recombination-defective mouse spermatocytes during the pachytene stage [2]. One occurs when sex body formation is impeded by massive synaptic failure, irrespective of the presence of uncompleted recombination events, such as when no DSBs are generated at all [16]. Sex body defects allow expression of X and Y chromosome genes that are sufficient to induce apoptosis [17]. In this scenario, the arrest and later cell death might be caused by misregulation of gene expression, not activation of a checkpoint per se.
The other arrest mechanism is proposed to occur when recombination intermediates persist at pachynema [2,16], similar to what is seen in many other organisms [9]. In most species analyzed, ATR (Mec1 in budding yeast) activates this checkpoint, most likely due to the presence of single-stranded DNA [6]. However, recent observations in yeast suggest that Tel1 (ATM) might also be involved [40] and our findings clearly indicate that the ATM signaling cascade is crucial for recombination-dependent arrest in mammalian spermatocytes.
A recent study found that the previously described recombination-dependent arrest of Atm−/− oocytes [4] is mediated by CHK2 [29]. It was further suggested that a protein kinase other than ATM, possibly ATR, is involved in recombination-dependent arrest in oocytes [29]. While our results suggest that ATM is the principal kinase mediating this arrest in spermatocytes, we do not exclude the possibility that ATR also participates in this mechanism, either in wild type or specifically in Atm−/− cells, in the latter case by replacing some ATM functions (e.g., H2AX phosphorylation [24,25,41]).
In somatic cells, the MRE11 complex participates in ATM activation, and mutations that impair the MRE11 complex lead to an inefficient G2/M checkpoint [26]. NBS1 also specifically promotes ATM phosphorylation of some of its substrates [33]. Our findings show that recombination-dependent arrest at pachynema has similarities to the G2/M checkpoint in somatic cells, as previously speculated [42]. These similarities may be useful for identifying other key members of the meiotic pathway.
Involvement of the effector kinase CHK2 in mammalian recombination-dependent arrest, further supported by observations in oocytes [29], underlines differences between organisms [9]. In budding yeast, for instance, the checkpoint depends on the meiosis-specific CHK2 homolog, Mek1. In contrast, D. melanogaster uses the non-meiosis-specific CHK2 homolog, Mnk, and in C. elegans CHK1, but not CHK2, is required for arrest. This variety emphasizes the importance of studying mouse meiosis to characterize this pathway in mammals.
This and previous studies establish H1t expression as a molecular marker of a response to recombination defects in spermatocytes, and our current findings establish that this response occurs via an ATM signaling cascade. Although available data rule out substantially premature H1t expression, it is possible that H1t is expressed slightly earlier than normal in the absence of DSBs or in the absence of the ATM response; such an effect might contribute to the high observed fraction of spermatocytes that are H1t-positive in the relevant mutants. However, if so, we note that this would be consistent with our conclusion that regulation of H1t expression is a checkpoint response since there are many instances where cell cycle regulated events occur prematurely when either the monitored event or the monitoring mechanism are missing (e.g., yeast Ndt80 expression when DSBs or Mec1 signaling are absent [43] or anaphase onset when the spindle assembly checkpoint is deactivated [44]). It is formally possible that this response has little or no other consequence than the control of H1t expression, and that spermatocytes do not otherwise “care” that they have sensed the presence of unrepaired DSBs. We consider this hypothesis unlikely since it would mean that the male germline in mouse, unlike mouse oocytes and meiotic cells in all other species studied to date, ignores molecular signals that report directly on the progression of one of the most central aspects of meiotic chromosome dynamics (recombination), and ignores signals that have pronounced consequences in other mouse cell types. Instead, we favor the interpretation that the ATM-dependent response described here is an integral part of meiotic quality control surveillance during spermatogenesis.
The recombination-dependent checkpoint is tolerant of a certain level of unrepaired DSBs
Our analysis exposed a population of Trip13mod/mod spermatocytes that were H1t-positive (i.e., had progressed to at least mid pachynema) even though they displayed as many as 41 γH2AX patches. These findings may suggest an overt defect in the recombination-dependent arrest mechanism or, alternatively, that this checkpoint tolerates a certain level of unrepaired DSBs. We favor the latter interpretation because the sex chromosomes in male meiocytes naturally accumulate DSBs during meiotic prophase, and recombination markers such as RAD51 can be observed on the X chromosome until late pachynema [45,46]. Since most DSBs formed on the X and Y chromosomes occur outside the pseudoautosomal region, they are presumably repaired by sister-chromatid recombination, which is thought to be permitted only later in pachynema [30]. Furthermore, in wild-type mice and in humans, many oocytes present multiple unrepaired DSBs at pachynema and even at diplonema [41,47]. Thus, we envision that recombination-surveillance mechanisms permit the progression of mammalian spermatocytes and oocytes that have sufficiently few DSBs to be compatible with eventual repair by the end of meiotic prophase and thus with long-term cell viability.
TRIP13, MRE11 complex and ATM interplay to promote homologous chromosome synapsis
As previously stated, homologous chromosome synapsis depends on meiotic recombination [48]. Thus, failure to complete synapsis might be interpreted as a consequence of defective homologous recombination. Unlike the relevant single mutants, spermatocytes from TSA triple mutants and from Trip13mod/mod Mre11ATLD/ATLD and Trip13mod/mod Nbs1ΔB/ΔB double mutants fail to complete synapsis in all, or almost all, cells [22–25,36]. The MRE11 complex, ATM and TRIP13 have been reported to promote meiotic recombination [22–25,36]. Thus, the fact that double and triple mutants present problems with completing synapsis suggests that the MRE11 complex and ATM could synergize with TRIP13 to promote proper DSB repair during meiotic prophase. However, we provide evidence that proteins required to activate ATM (MRE11 and NBS1) are also involved in the regulation of DSB formation. Thus, since Spo11+/− Atm−/− spermatocytes as well as cells from Mre11ATLD/ATLD and Nbs1ΔB/ΔB single mutants incur more DSBs than wild-type cells and because TRIP13-deficient cells are unable to complete meiotic recombination [22,23], this opens the possibility that TRIP13-defective cells cannot deal with the additional DSBs formed in the absence, or reduction, of ATM activity. Alternatively or in addition, the synapsis phenotype observed in TSA triple mutants or Trip13mod/mod Mre11ATLD/ATLD and Trip13mod/mod Nbs1ΔB/ΔB double mutants might be a manifestation of a function of ATM and/or the MRE11 complex in promoting synapsis more directly and synergistically with TRIP13.
The array of phenotypes in the mutants analyzed here suggests that ATM participates in different aspects of meiotic prophase (Fig. 7). Our results further demonstrate that, while meiotic progression depends on the MRE11 complex, ATM and CHK2, the regulation of SPO11 activity requires the MRE11 complex to activate ATM but is independent of CHK2. This finding may indicate that direct ATM phosphorylation targets exert control of DSB formation, as has been argued in yeast [49]. Interestingly, yeast Mek1 promotes inter-homolog bias in the repair of DSBs [50,51]. The fact that homologous chromosome synapsis is not altered in the absence of mouse CHK2 suggests that this protein is not involved in recombination partner choice in mammals, although we cannot exclude that compensation by related kinases (e.g., CHK1) could be responsible for the absence of an obvious phenotype in Chk2−/− spermatocytes.
Fig. 7. Functions of the ATM signaling pathway during mouse meiotic prophase. 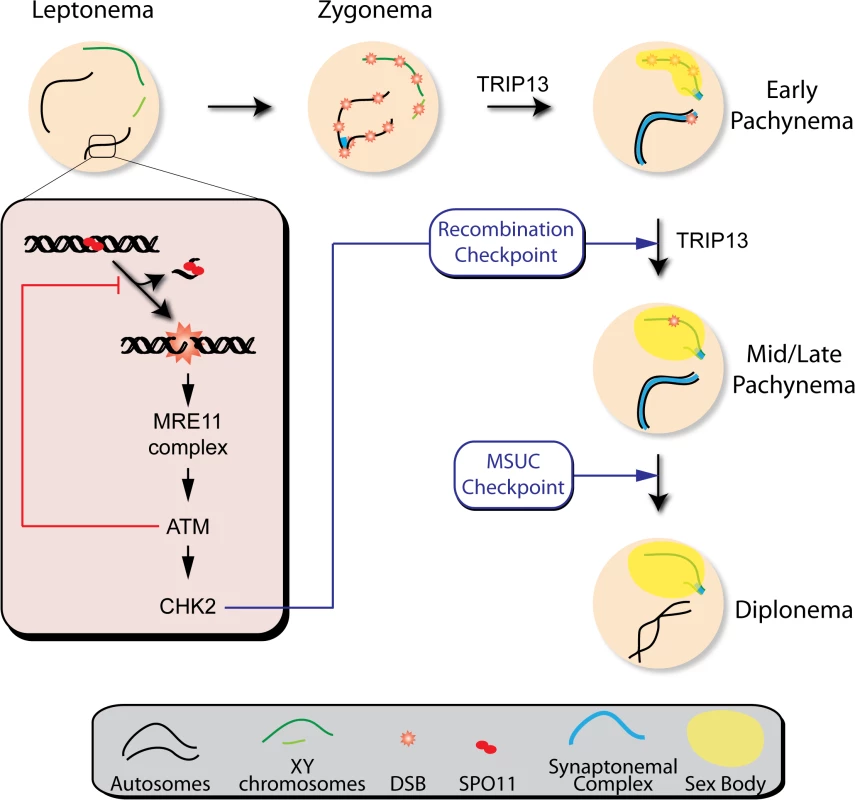
We propose that DSBs induced by SPO11 at the beginning of meiotic prophase are sensed by the MRE11 complex, which activates ATM. ATM inhibits further DSB formation via a feedback loop and promotes DSB repair. ATM activates CHK2, which controls cell cycle progression but is not involved in regulating DSB formation. Recombination progression leads to homologous chromosome synapsis and sex body formation. Besides participating in recombination, TRIP13 is also needed for effective sex body formation. Proper recombination and sex body formation are required to permit meiotic progression. TRIP13 participates in proper sex body formation
The apoptosis of spermatocytes in tubules at epithelial stage IV observed in Trip13mod/mod Chk2−/− testis can be explained if TRIP13 also contributes to the formation of a functional sex body (Fig. 7). In support of this hypothesis, we observed defective loading of ATR onto XY chromatin in pachytene spermatocytes from Trip13mod/mod Chk2−/− mice and in a subset of pachytene cells from Trip13mod/mod single mutants. This defect leads to inappropriate H2AX phosphorylation and SUMO-1 incorporation into the sex body, resulting in inefficient sex chromosome silencing and apoptosis at mid/late pachynema. TRIP13 is required for normal chromosomal association of HORMA (“Hop1, Rev7 and Mad2”)-domain proteins HORMAD1 and HORMAD2 during meiotic prophase [52]. HORMAD1 and HORMAD2 localize to the chromosome axes at leptonema and disappear from synapsed regions during zygonema as synapsis progresses. At pachynema, HORMAD1 and HORMAD2 accumulate at the unsynapsed regions of the X and Y chromosomes where they attract the machinery required to silence these chromosomes [21,53]. In Trip13mod/mod spermatocytes, HORMAD1 and HORMAD2 are retained in the synapsed regions of chromosomes and are present on all bivalent axes at pachynema [52]. We speculate that this aberrant localization of HORMA-domain proteins may contribute to inefficient ATR loading on sex chromosomes of Trip13mod/mod Chk2−/− pachytene spermatocytes. It is worth noting that Trip13mod/mod Chk2−/− cells do assemble ATR foci, presumably at resected DSB sites containing single-stranded DNA. This focal ATR localization has been reported previously to be HORMAD2-independent [21]. However, loading of ATR along the entire length of the unsynapsed chromosome axis is HORMAD2-dependent [21,53]. Furthermore, Zfx and Scml2 expression levels in early pachytene-stage Trip13 mutant spermatocytes are similar to those reported in Hormad2−/− cells [21]. These findings lead us to propose that proper HORMAD1 and HORMAD2 localization may underlie the function of TRIP13 in sex body formation.
The unexpected failure to properly form a sex body found in Trip13 mutants opens the possibility that, although this and other studies have clearly shown that there are at least two distinct arrest mechanisms to respond to recombination or sex body defects [2,16], the apoptosis occurring after the activation of these two arrest mechanisms may be a response only to the common MSCI failure. Nonetheless, we favor the hypothesis that recombination-dependent arrest is sufficient to trigger apoptosis because unrepaired DSBs provoke programmed cell death in oocytes (where MSCI defects are not an issue) via the conventional DNA damage response effector molecules, p53 and p63 [4,22,29]; and because MRE11 - and ATM-dependent signaling can directly promote apoptosis in somatic cells [26]. Addressing this issue will require novel meiotic mutants that display recombination defects in the context of an intact ATM signaling cascade and without MSCI defects.
Materials and Methods
Mutant mice
Trip13, Spo11, Atm, Dmc1, Mre11, Nbs1 and Chk2 mutations were generated previously [10,14,23,28,34,35,54]. All lines were maintained on a C57BL6–129Sv mixed background. Experiments were performed using at least two animals in comparison with littermate controls (either homozygous or heterozygous for the wild-type alleles) or, when appropriate littermates were unavailable, control animals obtained from litters of the same matings. Genotyping was performed by PCR analysis of tail-tip DNA as previously described [23].
Cytology and histology
Testes were harvested from two - to five-month-old animals and processed for histology or cytology, as previously described [23,55]. Immunofluorescence was performed using standard methods [47] and antibodies [23]. Additional primary antibodies used were: guinea pig anti-H1t (kindly donated by M.A. Handel, Jackson Lab) at 1 : 500 dilution [12] and a mouse anti-SUMO-1 (clone 21C7, Invitrogen) at 1 : 100 dilution. TUNEL-staining on IF-stained slides was performed using an in situ cell death detection kit (Roche Diagnostics) according to the manufacturer’s instructions, as reported previously [56].
RNA FISH and immunofluorescence
RNA FISH was carried out with digoxigenin-labeled probes as previously described [57]. BAC DNA probes used were: Zfx, bMQ-372M23 (from Mouse bMQ BAC library) and Scml2, RP24-204O18 (from CHORI BACPAC library). Briefly, BAC-containing bacteria were grown in an overnight LB-Chloramphenicol culture at 37°C and BAC DNA was isolated using a standard miniprep method. Approximately 2 μg of BAC DNA was labelled using DIG-Nick Translation Mix (Roche) and precipitated with Cot-1 DNA (Invitrogen) and salmon sperm DNA (Stratagene). Mouse testes were minced and cells were permeabilized with CSK buffer (100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 10 mM PIPES, 0.5% Triton X-100, 2 mM vanadyl ribonucleoside (New England Biolabs)), fixed with 4% paraformaldehyde and dehydrated through an ice-cold ethanol series. DNA-BAC probes were denatured for 10 min at 80°C, pre-hybridized for 30 min at 37°C, and added to the slides for an overnight incubation at 37°C. Stringency washes were performed and digoxigenin was detected using anti-digoxigenin-FITC (1 : 10, Millipore). RNA FISH was then followed by TOPBP1 (1 : 50, Abcam) and γH2AX (1 : 100, Millipore) immunostaining. Cells were examined on an Olympus IX70 inverted microscope. Images were captured using a computer-assisted (DeltaVision) CCD camera (Photometrics), and processed for publication using ImageJ and Photoshop. Early pachytene cells were defined based on a continuous TOPBP1 staining along the X and Y chromosome axes.
SPO11-oligonucleotide complex detection and Western blotting
Testis extract preparation, immunoprecipitation and Western blot analysis were performed essentially as described [27]. SPO11-oligonucleotide complexes and free SPO11 were isolated from testis lysates by two rounds of immunoprecipitation with a SPO11 monoclonal antibody (Spo11–180) on Protein A-agarose beads (Roche). SPO11-oligonucleotide complexes were labeled with [α-32P] dCTP using terminal deoxynucleotidyl transferase (Fermentas), released from the beads by boiling in Laemmli buffer, and fractionated by SDS-PAGE. The electrophoresed products were transferred onto polyvinylidene fluoride (PVDF) membrane. Radiolabeled species were detected using Fuji phosphor screens and quantified with ImageGauge software. The same PVDF membrane was then subjected to Western analysis using the SPO11 monoclonal antibody.
Statistical analysis
One-way ANOVA, Student's t tests and Fisher's exact tests were performed using GraphPad Prism software and/or GraphPad QuickCalcs online resource (http://www.graphpad.com/quickcalcs/). For comparing counts of γH2AX foci, we used t tests for simplicity to compare between genotypes at pachynema because the count data were not highly skewed (i.e., were reasonably approximated by a normal distribution). However, for diplotene cells we instead used negative binomial regression because the count distributions at this stage were highly skewed for the Trip13mod/mod Chk2−/− sample and contained many zero values for all samples. Regression was carried out using the glm.nb function from the MASS package (version 7.3–33) in R (version 3.1.1).
Ethics statement
All experiments performed in this study comply with US and EU regulations and were approved by the MSKCC Institutional Animal Care and Use Committee and the UAB Ethics Committee and the Catalan Government.
Supporting Information
Zdroje
1. De Massy B (2013) Initation of Meiotic Recombination: How and Where? Conversation and Specificities Among Eukaryotes. Annu Rev Genet. doi:10.1146/annurev-genet-110711-155423
2. Barchi M, Mahadevaiah S, Di Giacomo M, Baudat F, de Rooij DG, et al. (2005) Surveillance of different recombination defects in mouse spermatocytes yields distinct responses despite elimination at an identical developmental stage. Mol Cell Biol 25 : 7203–7215. 16055729
3. Bhalla N, Dernburg AF (2005) A conserved checkpoint monitors meiotic chromosome synapsis in Caenorhabditis elegans. Science (80-) 310 : 1683–1686.
4. Di Giacomo M, Barchi M, Baudat F, Edelmann W, Keeney S, et al. (2005) Distinct DNA-damage-dependent and-independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc Natl Acad Sci U S A 102 : 737–742. 15640358
5. Hochwagen A, Tham WH, Brar GA, Amon A (2005) The FK506 binding protein Fpr3 counteracts protein phosphatase 1 to maintain meiotic recombination checkpoint activity. Cell 122 : 861–873. 16179256
6. Lydall D, Nikolsky Y, Bishop DK, Weinert T (1996) A meiotic recombination checkpoint controlled by mitotic checkpoint genes. Nature 383 : 840–843. 8893012
7. Roeder GS, Bailis JM (2000) The pachytene checkpoint. Trends Genet 16 : 395–403. 10973068
8. Subramanian V V, Hochwagen A (2014) The Meiotic Checkpoint Network: Step-by-Step through Meiotic Prophase. Cold Spring Harb Perspect Biol 6. doi: 10.1101/cshperspect.a016675
9. MacQueen AJ, Hochwagen A (2011) Checkpoint mechanisms: the puppet masters of meiotic prophase. Trends Cell Biol 21 : 393–400. doi: 10.1016/j.tcb.2011.03.004 21531561
10. Pittman DL, Cobb J, Schimenti KJ, Wilson LA, Cooper DM, et al. (1998) Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell 1 : 697–705. 9660953
11. Yoshida K, Kondoh G, Matsuda Y, Habu T, Nishimune Y, et al. (1998) The mouse RecA-like gene Dmc1 is required for homologous chromosome synapsis during meiosis. Mol Cell 1 : 707–718. 9660954
12. Inselman A, Eaker S, Handel MA (2003) Temporal expression of cell cycle-related proteins during spermatogenesis: establishing a timeline for onset of the meiotic divisions. Cytogenet Genome Res 103 : 277–284. doi:76813. 15051948
13. Mahadevaiah SK, Bourc’his D, de Rooij DG, Bestor TH, Turner JM, et al. (2008) Extensive meiotic asynapsis in mice antagonises meiotic silencing of unsynapsed chromatin and consequently disrupts meiotic sex chromosome inactivation. J Cell Biol 182 : 263–276. doi: 10.1083/jcb.200710195 18663141
14. Baudat F, Manova K, Yuen JP, Jasin M, Keeney S (2000) Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell 6 : 989–998. 11106739
15. Romanienko PJ, Camerini-Otero RD (2000) The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell 6 : 975–987. 11106738
16. Burgoyne PS, Mahadevaiah SK, Turner JM (2009) The consequences of asynapsis for mammalian meiosis. Nat Rev Genet 10 : 207–216. doi: 10.1038/nrg2505 19188923
17. Royo H, Polikiewicz G, Mahadevaiah SK, Prosser H, Mitchell M, et al. (2010) Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr Biol 20 : 2117–2123. doi: 10.1016/j.cub.2010.11.010 21093264
18. Royo H, Prosser H, Ruzankina Y, Mahadevaiah SK, Cloutier JM, et al. (2013) ATR acts stage specifically to regulate multiple aspects of mammalian meiotic silencing. Genes Dev 27 : 1484–1494. doi: 10.1101/gad.219477.113 23824539
19. Ahmed EA, de Rooij DG (2009) Staging of mouse seminiferous tubule cross-sections. Methods Mol Biol 558 : 263–277. doi: 10.1007/978-1-60761-103-5_16 19685330
20. Russell LD (1990) Histological and histopathological evaluation of the testis. Clearwater: Cache River Press.
21. Wojtasz L, Cloutier JM, Baumann M, Daniel K, Varga J, et al. (2012) Meiotic DNA double-strand breaks and chromosome asynapsis in mice are monitored by distinct HORMAD2-independent and-dependent mechanisms. Genes Dev 26 : 958–973. doi: 10.1101/gad.187559.112 22549958
22. Li XC, Schimenti JC (2007) Mouse pachytene checkpoint 2 (trip13) is required for completing meiotic recombination but not synapsis. PLoS Genet 3: e130. 17696610
23. Roig I, Dowdle JA, Toth A, de Rooij DG, Jasin M, et al. (2010) Mouse TRIP13/PCH2 is required for recombination and normal higher-order chromosome structure during meiosis. PLoS Genet 6: e1001062. doi: 10.1371/journal.pgen.1001062 20711356
24. Barchi M, Roig I, Di Giacomo M, de Rooij DG, Keeney S, et al. (2008) ATM promotes the obligate XY crossover and both crossover control and chromosome axis integrity on autosomes. PLoS Genet 4: e1000076. doi: 10.1371/journal.pgen.1000076 18497861
25. Bellani MA, Romanienko PJ, Cairatti DA, Camerini-Otero RD (2005) SPO11 is required for sex-body formation, and Spo11 heterozygosity rescues the prophase arrest of Atm−/ − spermatocytes. J Cell Sci 118 : 3233–3245. doi: 10.1242/jcs.02466 15998665
26. Stracker TH, Roig I, Knobel PA, Marjanovic M (2013) The ATM signaling network in development and disease. Front Genet 4. doi: 10.3389/fgene.2013.00037
27. Lange J, Pan J, Cole F, Thelen MP, Jasin M, et al. (2011) ATM controls meiotic double-strand-break formation. Nature 479 : 237–240. doi: 10.1038/nature10508 22002603
28. Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, et al. (1996) Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86 : 159–171. 8689683
29. Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC (2014) Reversal of Female Infertility by Chk2 Ablation Reveals the Oocyte DNA Damage Checkpoint Pathway. Science (80-) 343 : 533–536. doi: 10.1126/science.1247671
30. Mahadevaiah SK, Turner JMA, Baudat F, Rogakou EP, de Boer P, et al. (2001) Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet 27 : 271–276. 11242108
31. Lammers JH, Offenberg HH, van Aalderen M, Vink AC, Dietrich AJ, et al. (1994) The gene encoding a major component of the lateral elements of synaptonemal complexes of the rat is related to X-linked lymphocyte-regulated genes. Mol Cell Biol 14 : 1137–46. 8289794
32. De Rooij DG, de Boer P (2003) Specific arrests of spermatogenesis in genetically modified and mutant mice. Cytogenet Genome Res 103 : 267–276. doi: 10.1159/000076812 15051947
33. Stracker TH, Petrini JH (2011) The MRE11 complex: starting from the ends. Nat Rev cell Biol 12 : 90–103. doi: 10.1038/nrm3047 21252998
34. Williams BR, Mirzoeva OK, Morgan WF, Lin J, Dunnick W, et al. (2002) A murine model of Nijmegen breakage syndrome. Curr Biol 12 : 648–653. 11967151
35. Theunissen JW, Kaplan MI, Hunt PA, Williams BR, Ferguson DO, et al. (2003) Checkpoint failure and chromosomal instability without lymphomagenesis in Mre11(ATLD1/ATLD1) mice. Mol Cell 12 : 1511–1523. 14690604
36. Cherry SM, Adelman CA, Theunissen JW, Hassold TJ, Hunt PA, et al. (2007) The Mre11 complex influences DNA repair, synapsis, and crossing over in murine meiosis. Curr Biol 17 : 373–378. 17291760
37. Keeney S, Neale MJ (2006) Initiation of meiotic recombination by formation of DNA double-strand breaks: mechanism and regulation. Biochem Soc Trans 34 : 523–525. doi: 10.1042/BST0340523 16856850
38. Matsuoka S, Huang M, Elledge SJ (1998) Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282 : 1893–1897. 9836640
39. Baart EB, de Rooij DG, Keegan KS, de Boer P (2000) Distribution of Atr protein in primary spermatocytes of a mouse chromosomal mutant: a comparison of preparation techniques. Chromosoma 109 : 139–142. 10855505
40. Ho HC, Burgess SM (2011) Pch2 acts through Xrs2 and Tel1/ATM to modulate interhomolog bias and checkpoint function during meiosis. PLoS Genet 7: e1002351. doi: 10.1371/journal.pgen.1002351 22072981
41. Turner JM, Mahadevaiah SK, Fernandez-Capetillo O, Nussenzweig A, Xu X, et al. (2005) Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet 37 : 41–47. 15580272
42. Burgoyne PS, Mahadevaiah SK, Turner JM (2007) The management of DNA double-strand breaks in mitotic G2, and in mammalian meiosis viewed from a mitotic G2 perspective. Bioessays 29 : 974–986. doi: 10.1002/bies.20639 17876782
43. Okaz E, Argüello-Miranda O, Bogdanova A, Vinod PK, Lipp JJ, et al. (2012) Meiotic prophase requires proteolysis of M phase regulators mediated by the meiosis-specific APC/CAma1. Cell 151 : 603–618. doi: 10.1016/j.cell.2012.08.044 23101628
44. Lara-Gonzalez P, Westhorpe FG, Taylor SS (2012) The spindle assembly checkpoint. Curr Biol 22: R966–80. doi: 10.1016/j.cub.2012.10.006 23174302
45. Kauppi L, Barchi M, Lange J, Baudat F, Jasin M, et al. (2013) Numerical constraints and feedback control of double-strand breaks in mouse meiosis. Genes Dev 27 : 873–886. doi: 10.1101/gad.213652.113 23599345
46. Page J, de la Fuente R, Manterola M, Parra MT, Viera A, et al. (2012) Inactivation or non-reactivation: what accounts better for the silence of sex chromosomes during mammalian male meiosis? Chromosoma 121 : 307–326. doi: 10.1007/s00412-012-0364-y 22366883
47. Roig I, Liebe B, Egozcue J, Cabero L, Garcia M, et al. (2004) Female-specific features of recombinational double-stranded DNA repair in relation to synapsis and telomere dynamics in human oocytes. Chromosoma 113 : 22–33. 15235794
48. Bolcun-Filas E, Schimenti JC (2012) Genetics of meiosis and recombination in mice. Int Rev Cell Mol Biol 298 : 179–227. doi: 10.1016/B978-0-12-394309-5.00005-5 22878107
49. Carballo JA, Panizza S, Serrentino ME, Johnson AL, Geymonat M, et al. (2013) Budding yeast ATM/ATR control meiotic double-strand break (DSB) levels by down-regulating Rec114, an essential component of the DSB-machinery. PLoS Genet 9: e1003545. doi: 10.1371/journal.pgen.1003545 23825959
50. Niu H, Wan L, Baumgartner B, Schaefer D, Loidl J, et al. (2005) Partner choice during meiosis is regulated by Hop1-promoted dimerization of Mek1. Mol Biol Cell 16 : 5804–5818. doi: 10.1091/mbc.E05-05-0465 16221890
51. Wan L, de los Santos T, Zhang C, Shokat K, Hollingsworth NM (2004) Mek1 kinase activity functions downstream of RED1 in the regulation of meiotic double strand break repair in budding yeast. Mol Biol Cell 15 : 11–23. doi: 10.1091/mbc.E03-07-0499 14595109
52. Wojtasz L, Daniel K, Roig I, Bolcun-Filas E, Xu H, et al. (2009) Mouse HORMAD1 and HORMAD2, two conserved meiotic chromosomal proteins, are depleted from synapsed chromosome axes with the help of TRIP13 AAA-ATPase. PLoS Genet 5: e1000702. doi: 10.1371/journal.pgen.1000702 19851446
53. Daniel K, Lange J, Hached K, Fu J, Anastassiadis K, et al. (2011) Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1. Nat Cell Biol 13 : 599–610. doi: 10.1038/ncb2213 21478856
54. Takai H, Naka K, Okada Y, Watanabe M, Harada N, et al. (2002) Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J 21 : 5195–5205. 12356735
55. Page J, Suja JA, Santos JL, Rufas JS (1998) Squash procedure for protein immunolocalization in meiotic cells. Chromosome Res 6 : 639–642. 10099877
56. Churchman ML, Roig I, Jasin M, Keeney S, Sherr CJ (2011) Expression of arf tumor suppressor in spermatogonia facilitates meiotic progression in male germ cells. PLoS Genet 7: e1002157. doi: 10.1371/journal.pgen.1002157 21811412
57. Mahadevaiah SK, Costa Y, Turner JMA (2009) Using RNA FISH to study gene expression during mammalian meiosis. Methods Mol Biol 558 : 433–444. doi: 10.1007/978-1-60761-103-5_25 19685339
Štítky
Genetika Reprodukčná medicína
Článek NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY ModuleČlánek Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell LongevityČlánek HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and MiceČlánek LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male FertilityČlánek The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAsČlánek Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick VectorČlánek Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53Článek The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in NematodesČlánek The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 3- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
- Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
- A Genomic Duplication is Associated with Ectopic Eomesodermin Expression in the Embryonic Chicken Comb and Two Duplex-comb Phenotypes
- Genome-wide Association Study and Meta-Analysis Identify as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy
- Mutations of Human , Encoding the Mitochondrial Asparaginyl-tRNA Synthetase, Cause Nonsyndromic Deafness and Leigh Syndrome
- Exome Sequencing in an Admixed Isolated Population Indicates Variants Confer a Risk for Specific Language Impairment
- Genome-Wide Association Studies in Dogs and Humans Identify as a Risk Variant for Cleft Lip and Palate
- Rapid Evolution of Recombinant for Xylose Fermentation through Formation of Extra-chromosomal Circular DNA
- The Ribosome Biogenesis Factor Nol11 Is Required for Optimal rDNA Transcription and Craniofacial Development in
- Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
- Maternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity
- Clonality and Evolutionary History of Rhabdomyosarcoma
- HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and Mice
- Methylation-Sensitive Expression of a DNA Demethylase Gene Serves As an Epigenetic Rheostat
- BREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
- Seizures Are Regulated by Ubiquitin-specific Peptidase 9 X-linked (USP9X), a De-Ubiquitinase
- The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
- A Cascade of Iron-Containing Proteins Governs the Genetic Iron Starvation Response to Promote Iron Uptake and Inhibit Iron Storage in Fission Yeast
- Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
- LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male Fertility
- Cis-Regulatory Mechanisms for Robust Olfactory Sensory Neuron Class-restricted Odorant Receptor Gene Expression in
- Effects on Murine Behavior and Lifespan of Selectively Decreasing Expression of Mutant Huntingtin Allele by Supt4h Knockdown
- HDAC4-Myogenin Axis As an Important Marker of HD-Related Skeletal Muscle Atrophy
- A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex
- Selective and Genetic Constraints on Pneumococcal Serotype Switching
- Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
- The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAs
- Accumulation of Glucosylceramide in the Absence of the Beta-Glucosidase GBA2 Alters Cytoskeletal Dynamics
- Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities
- The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair
- Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick Vector
- Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
- The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors
- PHABULOSA Controls the Quiescent Center-Independent Root Meristem Activities in
- DNA Polymerase ζ-Dependent Lesion Bypass in Is Accompanied by Error-Prone Copying of Long Stretches of Adjacent DNA
- Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus
- Zinc Finger Independent Genome-Wide Binding of Sp2 Potentiates Recruitment of Histone-Fold Protein Nf-y Distinguishing It from Sp1 and Sp3
- GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters
- Neurospora Importin α Is Required for Normal Heterochromatic Formation and DNA Methylation
- Ccr4-Not Regulates RNA Polymerase I Transcription and Couples Nutrient Signaling to the Control of Ribosomal RNA Biogenesis
- Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53
- A Systems-Level Interrogation Identifies Regulators of Blood Cell Number and Survival
- Morphological Mutations: Lessons from the Cockscomb
- Genetic Interaction Mapping Reveals a Role for the SWI/SNF Nucleosome Remodeler in Spliceosome Activation in Fission Yeast
- The Role of China in the Global Spread of the Current Cholera Pandemic
- The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
- A Zinc Finger Motif-Containing Protein Is Essential for Chloroplast RNA Editing
- Resistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
- Small Regulatory RNA-Induced Growth Rate Heterogeneity of
- Mitochondrial Dysfunction Reveals the Role of mRNA Poly(A) Tail Regulation in Oculopharyngeal Muscular Dystrophy Pathogenesis
- Complex Genomic Rearrangements at the Locus Include Triplication and Quadruplication
- Male-Biased Aganglionic Megacolon in the TashT Mouse Line Due to Perturbation of Silencer Elements in a Large Gene Desert of Chromosome 10
- Sex Ratio Meiotic Drive as a Plausible Evolutionary Mechanism for Hybrid Male Sterility
- Tertiary siRNAs Mediate Paramutation in .
- RECG Maintains Plastid and Mitochondrial Genome Stability by Suppressing Extensive Recombination between Short Dispersed Repeats
- Escape from X Inactivation Varies in Mouse Tissues
- Opposite Phenotypes of Muscle Strength and Locomotor Function in Mouse Models of Partial Trisomy and Monosomy 21 for the Proximal Region
- Glycosyl Phosphatidylinositol Anchor Biosynthesis Is Essential for Maintaining Epithelial Integrity during Embryogenesis
- Hyperdiverse Gene Cluster in Snail Host Conveys Resistance to Human Schistosome Parasites
- The Class Homeodomain Factors and Cooperate in . Embryonic Progenitor Cells to Regulate Robust Development
- Recombination between Homologous Chromosomes Induced by Unrepaired UV-Generated DNA Damage Requires Mus81p and Is Suppressed by Mms2p
- Synergistic Interactions between Orthologues of Genes Spanned by Human CNVs Support Multiple-Hit Models of Autism
- Gene Networks Underlying Convergent and Pleiotropic Phenotypes in a Large and Systematically-Phenotyped Cohort with Heterogeneous Developmental Disorders
- The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
- Combinatorial Control of Light Induced Chromatin Remodeling and Gene Activation in
- Linking Aβ42-Induced Hyperexcitability to Neurodegeneration, Learning and Motor Deficits, and a Shorter Lifespan in an Alzheimer’s Model
- The Complex Contributions of Genetics and Nutrition to Immunity in
- NatB Domain-Containing CRA-1 Antagonizes Hydrolase ACER-1 Linking Acetyl-CoA Metabolism to the Initiation of Recombination during . Meiosis
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
- Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption
- A Genomic Portrait of Haplotype Diversity and Signatures of Selection in Indigenous Southern African Populations
- Sequence Features and Transcriptional Stalling within Centromere DNA Promote Establishment of CENP-A Chromatin
- Inhibits Neuromuscular Junction Growth by Downregulating the BMP Receptor Thickveins
- Replicative DNA Polymerase δ but Not ε Proofreads Errors in and in
- Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in
- The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
- A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension
- Pervasive Variation of Transcription Factor Orthologs Contributes to Regulatory Network Evolution
- Network Analyses Reveal Novel Aspects of ALS Pathogenesis
- A Role for the Budding Yeast Separase, Esp1, in Ty1 Element Retrotransposition
- Nab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
- A RecA Protein Surface Required for Activation of DNA Polymerase V
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Clonality and Evolutionary History of Rhabdomyosarcoma
- Morphological Mutations: Lessons from the Cockscomb
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



