-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
Rice is a monocotyledonous plant that is distinct from the dicotyledonous model plant Arabidopsis in many aspects. In Arabidopsis, ethylene-induced root inhibition is independent of ABA action. In rice, however, we report here that ethylene inhibition of root growth requires ABA function. We identified MHZ4, a rice homolog of Arabidopsis ABA4 that is involved in ABA biosynthesis. The mhz4 mutant displayed reduced ABA level and exhibited ethylene-hyposensitive root, but -hypersensitive coleoptile phenotypes in etiolated seedlings. Exogenous application of ABA largely recovered the defective ethylene responses. Overexpression of MHZ4 resulted in enhanced and reduced ethylene response in the roots and coleoptiles, respectively. In root, MHZ4-dependent ABA pathway genetically acts at or downstream of ethylene receptors and positively regulates root ethylene response. Moreover, ethylene treatment stimulated ABA production in roots at least through transcriptional activation of MHZ4. The results indicate that ethylene-induced root inhibition in rice is largely mediated through MHZ4-dependent ABA function. In coleoptile, MHZ4-dependent ABA pathway acts at or upstream of OsEIN2 and negatively regulates coleoptile ethylene response, possibly via transcriptional suppression of OsEIN2. Together, our findings reveal a novel mode of ethylene-ABA interaction which is fundamentally different from that in Arabidopsis.
Published in the journal: Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings. PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004701
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004701Summary
Rice is a monocotyledonous plant that is distinct from the dicotyledonous model plant Arabidopsis in many aspects. In Arabidopsis, ethylene-induced root inhibition is independent of ABA action. In rice, however, we report here that ethylene inhibition of root growth requires ABA function. We identified MHZ4, a rice homolog of Arabidopsis ABA4 that is involved in ABA biosynthesis. The mhz4 mutant displayed reduced ABA level and exhibited ethylene-hyposensitive root, but -hypersensitive coleoptile phenotypes in etiolated seedlings. Exogenous application of ABA largely recovered the defective ethylene responses. Overexpression of MHZ4 resulted in enhanced and reduced ethylene response in the roots and coleoptiles, respectively. In root, MHZ4-dependent ABA pathway genetically acts at or downstream of ethylene receptors and positively regulates root ethylene response. Moreover, ethylene treatment stimulated ABA production in roots at least through transcriptional activation of MHZ4. The results indicate that ethylene-induced root inhibition in rice is largely mediated through MHZ4-dependent ABA function. In coleoptile, MHZ4-dependent ABA pathway acts at or upstream of OsEIN2 and negatively regulates coleoptile ethylene response, possibly via transcriptional suppression of OsEIN2. Together, our findings reveal a novel mode of ethylene-ABA interaction which is fundamentally different from that in Arabidopsis.
Introduction
The gaseous phytohormone ethylene regulates many aspects of plant growth and development, including seed germination, seedling growth, floral transition, sex determination, fruit ripening, organ senescence/abscission and adaptive responses to multiple biotic and abiotic stresses [1]. Ethylene is synthesized from methionine via a simple linear pathway, in which 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) and ACC oxidase (ACO) function as key enzymes [2]. Ethylene is perceived by a family of membrane-bound receptors that have similarity to bacterial two-component histidine kinase and act as negative regulator of ethylene response [3]. Ethylene signal is transmitted via a linear signaling cascade that consists of CONSTITUTIVE TRIPLE RESPONSE 1 (CTR1), ETHYLENE INSENSITIVE 2 (EIN2) and EIN3/EIN3-LIKE (EIL) [4]–[6]. Ethylene receptors, CTR1 and EIN2 are all predominantly localized at the endoplasmic reticulum membranes on where they can form signaling complex [7]–[10]. Without ethylene, the receptors are believed to be active and activate CTR1 ser/thr protein kinase which in turn phosphorylates the positive regulator EIN2, likely causing the proteasomal degradation of EIN2 by F-box proteins EIN2 TARGETING PROTEIN1/2 (ETP1/2) [11]–[13]. Upon ethylene perception, EIN2 can be proteolytically cleaved and its carboxyl terminus is translocated into the nucleus, triggering EIN3/EILs-mediated transcriptional cascades to induce various ethylene responses [12], [14], [15]. EIN3/EILs are also subjected to proteasomal degradation mediated by EIN3-BINDING F-BOX PROTEIN1/2 (EBF1/2) [16], [17].
In most growth and developmental processes, ethylene achieves its function through interaction with other phytohormones [18]–[20]. Among them, ethylene extensively interacts with abscisic acid (ABA) in many biological processes [21]–[29]. ABA plays pivotal roles in seed dormancy and germination, seedling development, stomatal closure and adaptive stress responses. ABA is produced from carotenoids, and the direct precursors are xanthophylls [30]. In Arabidopsis, zeaxanthin is converted into violaxanthin by Zeaxanthin epoxidase ABA1 [31]. ABA4 is involved in the subsequent conversion of zeaxanthin to neoxanthin although no enzyme activity was identified [32]. In addition to ABA4, recent study revealed that NXD1 (Neoxanthin-Deficient 1) is also necessary for neoxanthin synthesis but does not affect ABA accumulation in tomato and Arabidopsis [33]. The cis-isomers of both zeaxanthin and neoxanthin is then cleaved by nine-cis-epoxycarotenoid dioxygenase (NCED), leading to the production of xanthoxin [34]. The above steps occur in plastids. Xanthoxin is released into cytosol and converted to abscisic aldehyde by ABA2, a short-chain dehydrogenase/redutase [35]. Abscisic aldehyde oxidase (AAO) finally oxidizes the abscisic aldehyde to ABA [36]. The core components of ABA signaling pathway include ABA receptors PYR/PCAR, the negative regulator PROTEIN PHOSPHATASE 2C (PP2C) and the positive regulator SNF1 - RELATED PROTEIN KINASE 2 (SnRK2). In the absence of ABA, PP2Cs such as ABSCISIC ACID INSENSITIVE1 (ABI1) repress ABA signal transduction by inhibiting SnRK2 kinase activity through removal of activating phosphates. In the presence of ABA, ABA-bound receptors inhibit PP2C activity, allowing activation of SnRK2s and subsequent phosphorylation of ABA-responsive element binding factors (ABFs) to activate ABA-responsive genes [37]–[39].
Interactions of ethylene and ABA are complicated. The two hormones interplay at multiple levels, i.e., reciprocal effects on synthesis, signaling and responsive genes [18]. Ethylene and ABA interact in both antagonistic and synergistic manners, which depend upon developmental process, organ/tissue, growth conditions and species. In root growth, ethylene and ABA synergistically inhibit root elongation. Genetic evidences revealed that ABA signaling pathway acts upstream of ethylene signaling cascade in Arabidopsis, as root growth of ethylene-insensitive mutants etr1-1 and ein2 is resistant to ABA inhibition, but the roots of ABA-insensitive mutant abi1 and ABA-deficient mutant aba2 display normal responses to ethylene [21], [22], [26]. Moreover, block of ABA synthesis in ein2, ein3, ein6 or ctr1 mutant background by introducing aba2 mutation [26], or block of ABA signaling in the ctr1 mutant by introducing the abi1 mutation did not alter the ethylene response phenotypes of the respective ethylene mutants [21]. These results suggest that ABA-mediated inhibition of root growth requires functional ethylene signaling, whereas ethylene-induced root inhibition is dispensable for ABA action [21], [22]. Although the integration of ethylene and ABA signaling pathways has been elucidated in Arabidopsis, their interactions in other plants remain largely unclear.
Rice is an important crop worldwide. A few ethylene signalling components homologous to those of Arabidopsis have been characterized in rice including ethylene receptor OsETR2, OsRTH1, OsCTR and OsEIN2 [40]–[44]. Recently, through analysis of rice ethylene-response mutant mhz7, we find that MHZ7/OsEIN2 plays central roles in ethylene signalling and regulation of agronomic traits in rice, and clear ethylene-insensitive and hypersensitive phenotypes are identified in etiolated rice seedlings [45]. In this study, we further characterized another rice ethylene-response mutant mhz4 (mao huzi, Chinese name with a English meaning of cat whiskers), which displays reduced ethylene-response in roots but enhanced ethylene-response in coleoptiles [45]. Through map-based cloning, the MHZ4 was identified to encode a membrane protein orthologous to Arabidopsis ABA4, which is responsible for the conversion of zeaxanthin to neoxanthin in ABA biosynthesis pathway [32]. Mutation of MHZ4 abolishes ABA production but promotes ethylene emission. MHZ4 overexpression enhances root ethylene-response but reduces coleoptile ethylene-response. MHZ4 acts downstream of ethylene receptors in regulating root growth but upstream of OsEIN2 in regulating coleoptile elongation. Our results reveals the complicated interplay between ethylene and ABA signaling in regulating rice seedling growth and agronomic traits, providing new insight into understanding of their interaction in rice.
Results
Characterization of mhz4 Mutant for Ethylene Response Phenotypes
The mhz4 is identified previously in our screen for ethylene-response mutants in rice [45]. For dark-grown wild type (WT) seedlings, ethylene inhibited root growth but promoted coleoptile elongation in a dose-dependent manner (Figure 1A–C). The roots of etiolated mhz4 seedlings were about 10% shorter than that of WT seedlings under normal conditions. Upon ethylene treatment, mhz4 roots were insensitive to ethylene inhibition at lower concentrations (≤1 ppm) but displayed mild growth inhibition at higher concentrations (10 to 100 ppm), e.g. about 20% inhibition in mhz4 compared with about 70% inhibition in the WT at 10 ppm ethylene (Figure 1A and 1B). This indicates that the roots of mhz4 are less sensitive to ethylene. On the other hand, the coleoptiles of mhz4 were slightly but significantly (P<0.004) longer than that of WT seedlings in the absence of ethylene and were much longer than that of WT under all concentrations of ethylene treatment, indicating that the coleoptiles of mhz4 are hypersensitive to ethylene (Figure 1A and 1C). These results indicate that mhz4 mutation oppositely affects ethylene responses in roots and coleoptiles of etiolated rice seedlings.
Fig. 1. Ethylene responses in etiolated mhz4 seedlings. 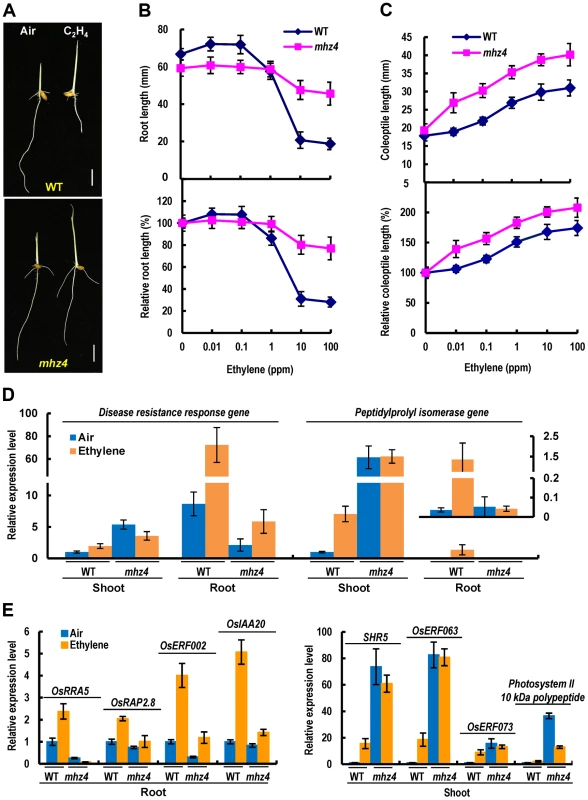
Rice seedlings were grown in dark for 3 d in the presence of various concentrations of ethylene. (A) Ethylene-response phenotypes of WT (Nipponbare) and mhz4 seedlings grown in air or 10 ppm ethylene. Bar = 10 mm. (B) Ethylene dose-response curves for root length (top) and relative root length (bottom) in WT and mhz4 seedlings. Each point is average of 20 to 30 seedlings and bars indicate SD. (C) Ethylene dose-response curves for coleoptile length (top) and relative coleoptile length (bottom) of WT and mhz4 seedlings. Others are as in (B). (D) Expression of ethylene-inducible genes in both shoot and root of WT and mhz4 seedlings. Dark-grown 2 d-old seedlings were treated with or without 10 ppm ethylene for 8 h and the RNA was isolated for quantitative PCR. Data are the mean ± SD of four replicates. (E) Expression of ethylene-inducible genes in root (left) or shoot (right) of WT and mhz4 seedlings. Others are as in (D). To further confirm the ethylene responsiveness of mhz4 mutant, we examined expressions of ethylene-inducible genes originally identified from a chip analysis (GSE51153; [45]) and a RNA-seq analysis (SRP041468). Two genes including disease resistance response gene and peptidylprolyl isomerase gene were found to be induced by ethylene in both shoots and roots of WT seedlings (Figure 1D). Four genes (OsERF002, OsRRA5, OsRAP2.8 and OsIAA20) were found to be induced mainly in roots and four genes (SHR5, OsERF063, OsERF073 and photosystem II 10 kDa polypeptide gene) mainly in shoots of WT seedlings (Figure 1E). In mhz4 shoots, the ethylene inducible genes were constitutively expressed at a level higher than that in ethylene-treated WT. Ethylene treatment of mhz4 did not further increase the transcript levels probably due to their expression levels were already very high. It should be noted that we used shoots instead of coleoptiles for the gene expression analyses because similar ethylene responses were found for coleoptiles and shoots [46]. In mhz4 roots, the transcripts of ethylene inducible genes remained at a similar or lower level when compared to those in WT in the absence of ethylene, and these genes showed no or only slight inductions compared to their ethylene inductions in WT roots (Figure 1D, E). These results indicate enhanced and reduced ethylene response in mhz4 shoots/coleoptiles and roots, respectively, at gene expression level.
The MHZ4 Gene Is Homologous to Arabidopsis ABA4
Genetic analysis has revealed that mhz4 is a recessive mutation controlled by a single locus [45]. The MHZ4 gene was identified by a map-based cloning approach using F2 plants from a cross between mhz4 and indica rice variety Minghui 63. A total of 480 segregated mutant individuals were used for positional mapping of the mhz4 locus. The mutation site was narrowed down to a 30-kb region in chromosome 1 between Idl1–1.55 and Idl1–1.58 markers (Figure 2A). We sequenced all four annotated genes within this region and found an 18-bp deletion in LOC_Os01g03750. The deletion occurred at the third intron and disrupted the splicing site, resulting in 81-bp un-spliced intron and causing a premature stop codon in the encoded protein (Figure 2A and 2B). The mutations were further confirmed by PCR through examination of fragment length polymorphisms in genomic DNA and cDNA of WT and mhz4 (Figure 2C). To verify that the mutation of LOC_Os01g03750 locus is responsible for the mutant phenotype of mhz4, we cloned the 4213-bp DNA fragment including the complete LOC_Os01g03750 genomic sequence (1147-bp) plus 2048-bp region upstream of the start codon and 858-bp extension downstream of the stop codon from the WT, and transformed the gene into the mhz4 plants. Ethylene response assays showed that the altered ethylene responsiveness of mhz4 was rescued in the transgenic plants (Figure 2D). These results confirm that MHZ4 is located at LOC_Os01g03750 locus.
Fig. 2. Map-based cloning of mhz4 locus. 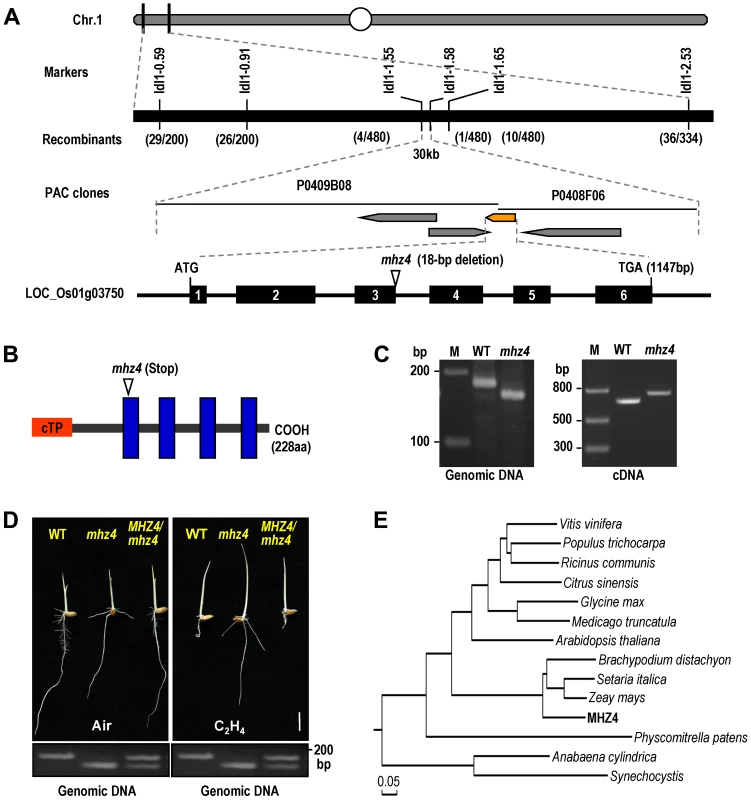
(A) Fine mapping of MHZ4 gene. The locus was mapped to chromosome 1 within a 30 Kb region between Idl1–1.55 and Idl1–1.58 markers. Mutation site is indicated in schematic diagram of MHZ4 gene. Black boxes represent exons. (B) Schematic structure of the MHZ4 protein. The structure is predicted using the SMART software (http://smart.embl-heidelberg.de). Mutation site is shown. cTP indicates chloroplast transit peptide identified using the ChloroP 1.1 program (http://www.cbs.dtu.dk/services/ChloroP/). Blue columns represent transmembrane domains. (C) Confirmation of mhz4 mutation site in both genomic DNA and cDNA by PCR. M: marker. (D) Functional complementation of mhz4 mutant. MHZ4 genomic DNA (4213 bp) was transformed into mhz4 plants (MHZ4/mhz4), rescuing the enhanced coleoptile elongation and reduced root inhibition phenotypes of mhz4 in the presence of ethylene (10 ppm). Bottom is confirmation of the transgene by PCR using the genomic DNA as templates. Bar = 10 mm. (E) Phylogenetic analysis of MHZ4/OsABA4 and its homologous proteins from other plants. The phylogenetic tree is generated by Maximum Likelihood method. Accession numbers are as follows: Vitis vinifera, XP_002283875; Populus trichocarpa, XP_002305164; Ricinus communis, XP_002521222; Citrus sinensis, ADH82117; Glycine max, XP_003532342; Medicago truncatula, XP_003618901; Arabidopsis thaliana, NP_564889; Brachypodium distachyon, XP_003565288; Setaria italica, XP_004968073; Zeay mays, ACN29324; Physcomitrella patens, Pp1s108_75V6; Anabaena cylindrica, WP_015215835; Synechocystis, BAA18538. The MHZ4 gene encodes a protein of 228 amino acids that harbors an N-terminal signal peptide and four transmembrane domains as predicted by the SMART program (http://smart.embl-heidelberg.de/) (Figure 2B). The MHZ4 sequence shared 47% identity and 64% similarity with Arabidopsis ABA4 (AT1G67080), which is required for neoxanthin formation in the ABA biosynthesis pathway through an unknown mechanism [32]. Phylogenetic analysis revealed that MHZ4 protein is conserved from cyanobacteria to higher plants and is more closely related to homologues from monocotyledonous plants (Figure 2E).
MHZ4 Expression and Protein Subcellular Localization
MHZ4 accumulation was examined by semiquantitative RT-PCR. The transcripts were detected in all organs from vegetative to reproductive stages and found to be more abundant in young leaves (Figure 3A). Transgenic rice plants harboring MHZ4 promoter::β-glucuronidase (GUS) construct were also generated and GUS staining assay was performed to evaluate the promoter activity. In etiolated seedlings, MHZ4 expression was detected in both roots and coleoptiles, and the signals were also present in the vascular tissues of roots (Figure 3B, a to e). In root apexes, GUS signals were observed in the putative quiescent center (QC) and root caps (Figure 3B, f). In field-grown plants, MHZ4 was expressed in leaf blades, young stem nodes, the base of axillary buds and adventitious roots derived from the nodes (Figure 3B, g to j). In reproductive organs, the expression of MHZ4 was detected in the anthers and pistil of young flowers, lemma of mature flowers, and parts of developing grains (Figure 3B, k to n).
Fig. 3. MHZ4 expression and protein subcellular localization. 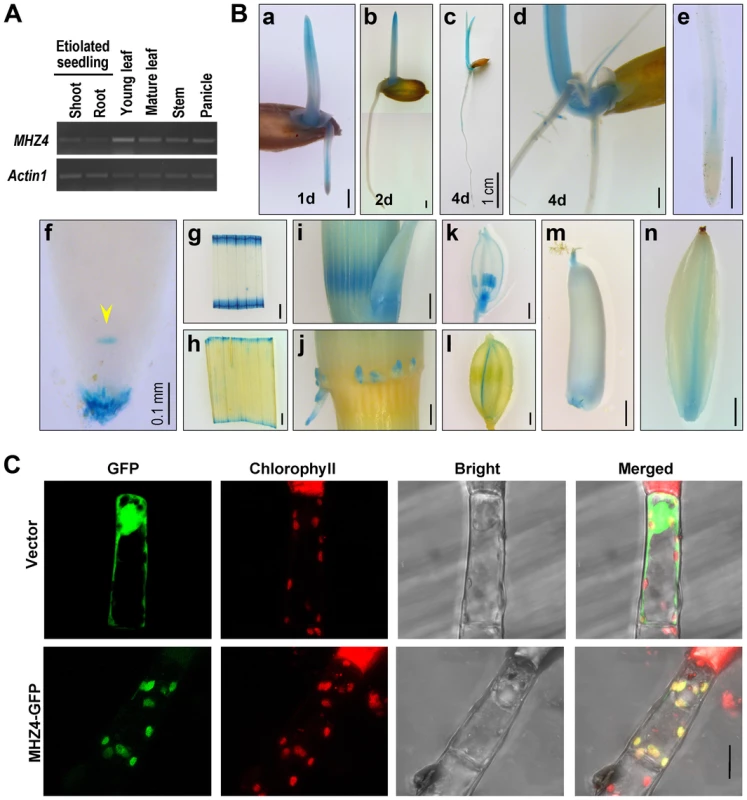
(A) MHZ4 expression in different rice organs detected by RT-PCR. Actin1 was used as an internal control. (B) Tissue-specific expression of MHZ4 revealed by promoter-GUS analysis. Transgenic plants expressing MHZ4pro::GUS were used for analysis. Rice organs were stained for GUS for two days. At least 10 samples for each organ were observed and representative ones are presented. (a–c) 1 d- to 4 d-old etiolated seedlings. (d) GUS signals in adventitious roots and lateral roots of 4 d-old seedlings. (e) GUS staining in vascular tissues of root tips. (f) GUS staining in quiescent center (arrow head) and root caps of root tips. (g, h) GUS staining in segments of young (g) and mature (h) leaf blades. (i) GUS staining in young stem nodes and the base of axillary buds. (j) GUS staining in adventitious roots derived from nodes. (k) GUS staining in the anthers and pistils of young flowers. (l) GUS staining in the lemma of flowers. (m) Staining in the top and bottom of an ovary. (n) GUS staining in a developing grain. Bars are 1 mm except for those indicated. (C) Subcellular localization of MHZ4 in chloroplasts of tobacco glandular hairs as revealed by GFP-fusion protein. The constructs were transiently expressed in tobacco leaf cells by microprojectile bombardment. GFP fluorescence was detected using confocal microscopy. Red fluorescence indicates chlorophyll. Yellow color indicates co-localization of MHZ4 with chloroplasts. Bar = 10 µm. The MHZ4 protein was predicted to localize to the chloroplasts by using the ChloroP 1.1 program (http://www.cbs.dtu.dk/services/ChloroP/). To experimentally verify the localization, MHZ4 coding sequence was fused in frame with GFP and transiently expressed in tobacco leaves. The fluorescence signals of MHZ4-GFP fusion protein were found in the chloroplasts of tobacco glandular hairs, as identified by co-localization with the chlorophyll autofluorescence (Figure 3C). This result is consistent with the localization of Arabidopsis ABA4 in chloroplast envelope [47].
Ethylene-Induced Root Inhibition in Rice Is Largely Mediated through MHZ4-Dependent ABA Accumulation
Since MHZ4 is homologous to Arabidopsis ABA4 in ABA biosynthesis, we tested whether endogenous ABA contents in mhz4 were altered. The mhz4 mutant contains 61% and 7.8% of WT ABA levels in roots and shoots, respectively, demonstrating that ABA production is severely hampered in mhz4 mutant (Fig. 4A). Ethylene induced ABA accumulation in WT roots but not in shoots, suggesting organ-specific regulation of ABA accumulation (Fig. 4A). Ethylene induction of ABA was not observed in mhz4 roots (Fig. 4A), indicating that MHZ4 is required for ethylene-induced ABA accumulation. We further examined the expression of ABA-responsive gene OsMFT2 (LOC_Os01g02120) previously identified by Lenka et al. [48]. OsMFT2 expression was dramatically induced by ABA in both roots and shoots of WT seedlings (Figure 4B, top). However, the transcripts were barely detectable in mhz4 mutant compared with those in WT (Figure 4A, bottom). These results indicate that MHZ4 is responsible for ABA biosynthesis in rice.
Fig. 4. Ethylene-induced root inhibition is largely mediated through MHZ4-dependent ABA accumulation. 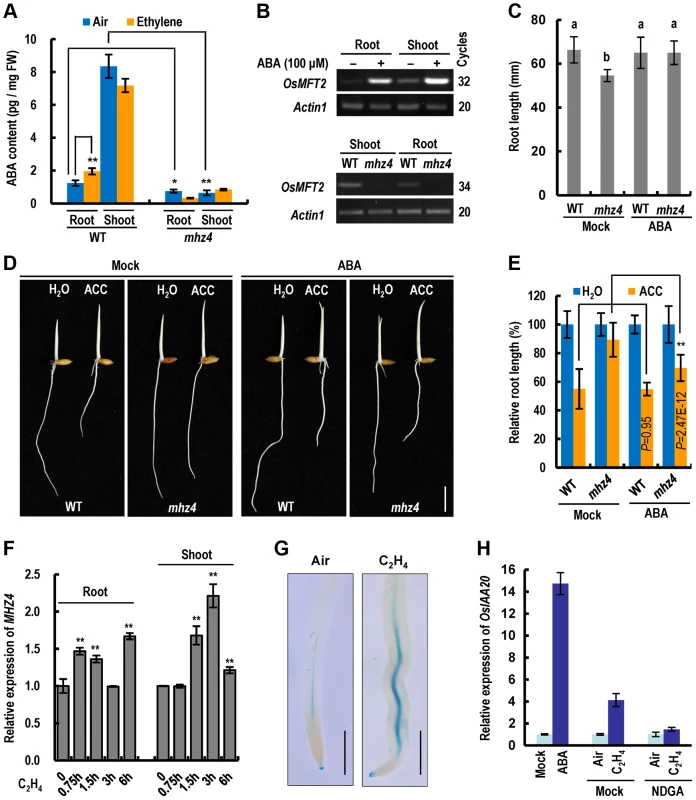
(A) ABA levels in WT and mhz4 seedlings in the absence or presence of ethylene. Two-day-old etiolated seedlings were treated with or without 100 ppm of ethylene for 48 h. Data are the mean ± SD of three replicates. * and ** indicate significant difference between the compared two samples at P<0.05 and P<0.01, respectively. (B) Expression of ABA-responsive gene OsMFT2 (LOC_Os01g02120). Upper, OsMFT2 transcripts in 3 d-old etiolated seedlings of WT in response to ABA (100 µM, 6 h). Bottom, OsMFT2 transcripts detected in mhz4 and WT etiolated seedlings. Actin1 was used as an internal control. (C) Rescue of the root length of mhz4 by ABA. Rice seedlings were grown in the dark for 2.5 days in the presence or absence (Mock) of 0.04 µM ABA. Each column is average of 40 seedlings and bars indicate SD. Different letters above each column indicate significant difference between the compared pairs (P<0.01). (D) Rescue of the reduced ethylene sensitivity of mhz4 roots by ABA. WT and mhz4 seedlings were grown in the dark for 2.5 d in the absence or presence of 10 µM ACC, with or without supplementation of 0.1 µM ABA. Bar = 10 mm. (E) Quantification of root inhibition in (D). Each column is average of 40 seedlings and bars indicate SD. ** indicates significant difference between the linked two samples at P<0.01. (F) Quantitative PCR analysis of MHZ4 expression in response to ethylene. WT seedlings were grown in the dark for 2.5 d and then treated with 10 ppm ethylene for 0–6 h. Data are the mean ± SD of four replicates. ** indicate significant difference compared to 0 h at P<0.01. (G) Ethylene-induced GUS activity in roots of transgenic plants harboring MHZ4pro::GUS construct. One-day-old etiolated seedlings were treated with or without 10 ppm ethylene for 24 h. The roots were cut off and stained for GUS activity for two days. Twenty to thirty roots were observed for each treatment and representative samples are presented. Bar = 1 mm. (H) Quantitative PCR analysis of OsIAA20 expression in response to ABA or ethylene+NDGA. Dark-grown 2 d-old WT seedlings were treated with 100 µM ABA for 6 h, or treated with 10 ppm ethylene for 8 h in the presence or absence (Mock) of 100 µM NDGA. The RNA from roots was isolated for quantitative PCR. Data are the mean ± SD of four replicates. Under normal growth condition, the mhz4 roots of etiolated seedlings are slightly but significantly shorter than the WT roots (Figure 4C). Treatment of the seedlings with 0.04 µM ABA completely restored the short root phenotype (Figure 4C), suggesting that basal levels of endogenous ABA are required for the maintenance of normal root elongation. The same ABA concentration showed no obvious stimulation on root growth of WT seedlings (Figure 4C).
Considering that MHZ4 mutation leads to the reduced ethylene sensitivity in mhz4 roots, we investigated whether addition of ABA could rescue the ethylene response of the mutant. The 0.1 µM ABA was used in the complementation assay because at this concentration, no obvious inhibitory effects were observed on root growth in WT seedlings (Figure S1). In the presence of 10 µM ACC (precursor of ethylene), application of 0.1 µM ABA largely rescued the defective response of mhz4 roots to ethylene (Figure 4D and 4E), indicating that reduced ethylene sensitivity of mhz4 roots is most likely caused by the lack of ABA.
We further examined MHZ4 expression in response to ethylene. MHZ4 transcripts were significantly induced by ethylene in WT roots and shoots (Figure 4F). Promoter-GUS analysis also showed that ethylene treatment stimulated MHZ4 promoter activity mainly in the vascular tissues of roots (Figure 4G). These results suggest a role for MHZ4 in root growth control. It should be noted that although MHZ4 expression was also induced by ethylene in shoots, the ABA level did not increase after ethylene treatment (Figure 4A and 4F). This is likely owning to ethylene-activated ABA catabolism for a homeostasis in shoots [23], [30], [49].
To further elucidate the role of ethylene-triggered ABA in root ethylene response, we investigated the expression of ethylene-inducible genes in response to ABA as well as ABA biosynthesis inhibitor. OsIAA20 transcripts were dramatically induced not only by ethylene but also by ABA in rice roots (Figure 4H). However, ethylene induction of OsIAA20 expression was abolished in the presence of NDGA (nordihydroguaiaretic acid), an ABA biosynthesis inhibitor that specifically inhibits NCED enzyme activity (Figure 4H). These results suggest that ethylene-induced ABA mediates expression of some ethylene-responsive genes. Taken together, these findings suggest that ethylene-induced root growth inhibition is largely mediated through MHZ4-dependent ABA accumulation.
Ethylene-Hypersensitivity of mhz4 Coleoptiles Is Caused by Enhanced Ethylene Signaling
The mhz4 coleoptiles showed enhanced ethylene response (Figure 1A, C). We determined whether ABA addition would complement the mutant response to ethylene. Without ABA, the coleoptile length of mhz4 mutant was significantly longer than that of WT seedlings in the presence or absence of ethylene (Figure 5A and 5B). When 0.1 µM ABA was applied to roots, however, the mhz4 coleoptiles were identical in length to that of WT seedlings with or without ethylene treatment (Figure 5A and 5B). These observations suggest that application of 0.1 µM ABA substantially restored the mhz4 coleoptile ethylene response to WT levels and that ABA-deficiency is responsible for the ethylene hypersensitivity of mhz4 coleoptiles.
Fig. 5. Enhanced ethylene-response in mhz4 coleoptiles is rescued by ABA and MHZ4 mutation leads to ethylene overproduction. 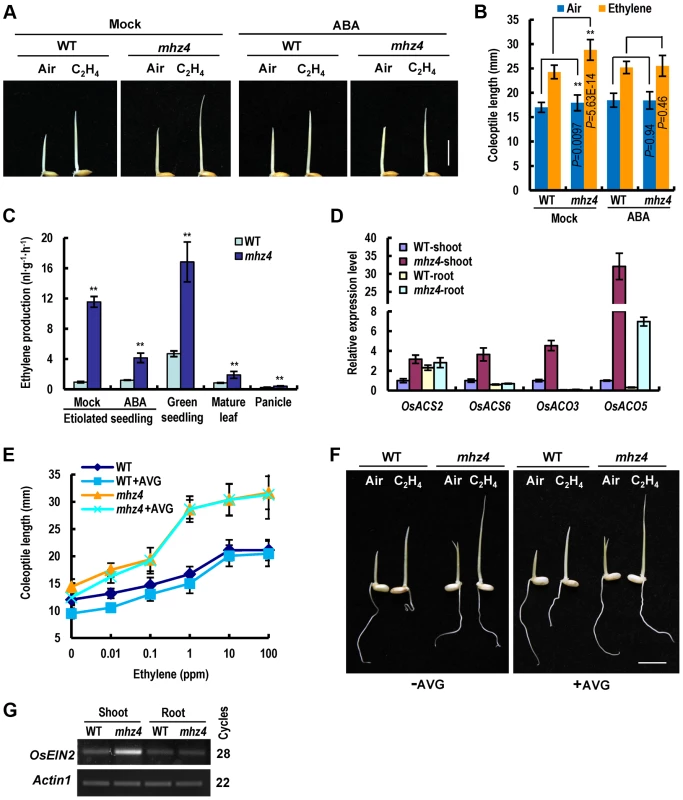
(A) ABA rescue of enhanced ethylene-response phenotype in mhz4 coleoptiles. WT and mhz4 seedlings were grown in the dark for 2.5 d in the absence or presence of 10 ppm ethylene, with or without supplementation of 0.1 µM ABA. Bar = 10 mm. (B) Quantification of coleoptile growth with treatments in (A). Each column is average of 30 seedlings and bars indicate SD. ** indicates significant difference between the linked two samples at P<0.01. (C) Ethylene production in WT and mhz4 mutant. Data are the mean ± SD of three replicates. ** indicate significant difference compared to WT at P<0.01. (D) Expression of ethylene biosynthetic genes in 3 d-old etiolated seedlings of WT and mhz4. (E) Ethylene dose-response curves for coleoptile elongation in WT and mhz4 seedlings in the presence or absence of 5 µM of ethylene biosynthesis inhibitor AVG. Dark grown seedlings were treated with various concentrations of ethylene for 2.5 d. Each point is average of 25 to 30 seedlings and bars indicate SD. (F) Coleoptile elongation phenotypes of WT and mhz4 in the presence or absence of 5 µM of AVG. The ethylene concentration was 10 ppm. Others are as in (E). Bar = 10 mm. (G) OsEIN2 gene expression in WT and mhz4 etiolated seedlings detected by semiquantitative RT-PCR. Rice seedlings were grown in the dark for 3 days and RNA was isolated from the shoots and roots. Actin1 was used as an internal control. Enhanced ethylene response can be caused by ethylene overproduction and/or enhanced signal transduction. We then measured ethylene production in mhz4. The mhz4 mutant produced 2 to 12 times more ethylene than WT and application of 1 µM ABA to etiolated mhz4 seedlings dramatically reduced the ethylene levels, suggesting that ABA-deficiency in mhz4 mutant promote ethylene biosynthesis (Figure 5C). Furthermore, quantitative PCR analysis revealed that the ethylene biosynthetic genes OsACS2, OsACS6, OsACO3 and OsACO5 were all elevated in mhz4 shoots, likely contributing to the enhanced ethylene production (Figure 5D).
We next investigated the contribution of ethylene overproduction to the enhanced ethylene response of mhz4 coleoptiles through treatment with the ethylene biosynthesis inhibitor 1-aminoethoxyvinyl-glycine (AVG). Application of 5 µM AVG removed most of the increased ethylene production in mhz4 mutant, but exerted limited effect on the coleoptile ethylene response (Figure 5E and 5F, Figure S2). This result suggests that ethylene overproduction is not the main reason for the enhanced ethylene response in mhz4 coleoptiles. We further examined the possibility of ethylene signaling and found that OsEIN2 transcripts were particularly enriched in mhz4 shoots compared with that of WT (Figure 5G). These results suggest that the enhanced ethylene response of mhz4 coleoptiles is most likely caused by enhanced ethylene signaling.
MHZ4 Overexpression Alters Ethylene Response in Roots and Coleoptiles
To further study the function of MHZ4 in rice ethylene response, we transformed the gene into WT rice plants under the control of the CaMV 35S promoter. MHZ4-overexpressing (MHZ4-OX) lines were identified by semiquantitative RT-PCR (Figure S3), and four representative lines were used for further analysis. The four transgenic lines all showed slightly but significantly shorter roots (P<10−8) and coleoptiles (P<10−8) in the dark compared with WT seedlings, indicating constitutive growth inhibition of the roots and coleoptiles in MHZ4-OX plants (Figure 6A–6C). Upon ethylene treatment, the MHZ4-OX lines exhibited strong inhibition of root growth but less promotion of coleoptile growth in comparison with WT seedlings, indicating enhanced ethylene response in the roots but reduced response in the coleoptiles of MHZ4-OX lines (Figure 6A–6C). Examination of the ethylene-inducible gene OsERF002 in the roots and SHR5 in the shoots further proved the ethylene responsiveness of MHZ4-OX lines (Figure 6D). Additionally, in the shoots, OsEIN2 transcript levels were down-regulated in MHZ4-OX lines compared with that in WT seedlings (Figure 6E), suggesting that the reduced ethylene responsiveness of the coleoptiles is likely related to the reduction of ethylene signaling. Together, these results indicate that MHZ4 overexpression leads to different ethylene responses in roots and coleoptiles.
Fig. 6. MHZ4 overexpression confers enhanced and reduced ethylene responses in roots and coleoptiles, respectively. 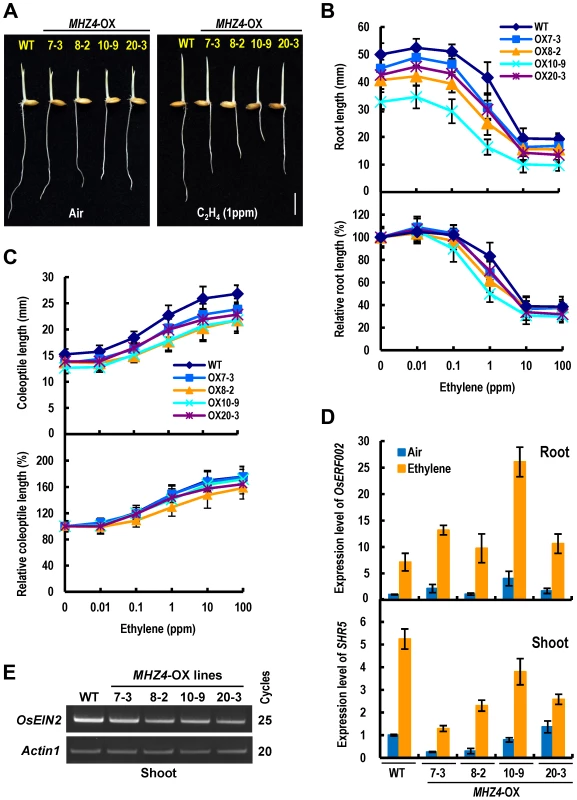
(A) Ethylene response phenotypes in WT and MHZ4-overexpressing (MHZ4-OX) lines. Rice seedlings were grown in dark for 2.5 d in the presence or absence of 1 ppm ethylene. Bar = 10 mm. (B) Ethylene dose-response curves for root length (top) and relative root length (bottom) in WT and MHZ4-OX lines. (C) Ethylene dose-response curves for coleoptile length (top) and relative coleoptile length (bottom) in WT and MHZ4-OX lines. Others are as in (B). (D) Expressions of ethylene-inducible genes in roots (top) and shoots (bottom) of WT and MHZ4-OX lines. Dark-grown 2 d-old seedlings were treated with or without 10 ppm of ethylene for 8 h and the RNA was isolated for quantitative RT-PCR. Data are the mean ± SD of four replicates. (E) OsEIN2 gene expression in shoots of WT and MHZ4-OX lines detected by semi-quantitative PCR. Rice seedlings were grown in dark for 3 days and RNA was isolated from shoots. Actin1 was used as an internal control. Genetic Interactions of MHZ4 and Ethylene Signaling Pathway
We further examined the genetic relationship of MHZ4 and ethylene signaling pathway through double mutant analyses. A loss-of function Osers1 mutant was obtained from the POSTECH Biotech Center [50] and was identified by PCR-based analyses (Figure S4). The roots of Osers1 etiolated seedlings were significantly shorter than that of WT seedlings in the absence of ethylene and displayed a strong ethylene response phenotype following treatment with 1 ppm ethylene, indicating the presence of enhanced ethylene response in Osers1 roots (Figure 7A and 7B). The roots of mhz4 Osers1 double mutant were slightly shorter than that of the mhz4 single mutant but exhibited ethylene insensitive response that was indistinguishable from that of mhz4, suggesting that OsERS1-mediated root ethylene response requires MHZ4 function (Figure 7A and 7B). The coleoptiles of Osers1 did not exhibit apparent ethylene response phenotype compared to that of WT (Figure 7A).
Fig. 7. Genetic interactions of MHZ4 with ethylene receptor gene and MHZ7/OsEIN2 gene. 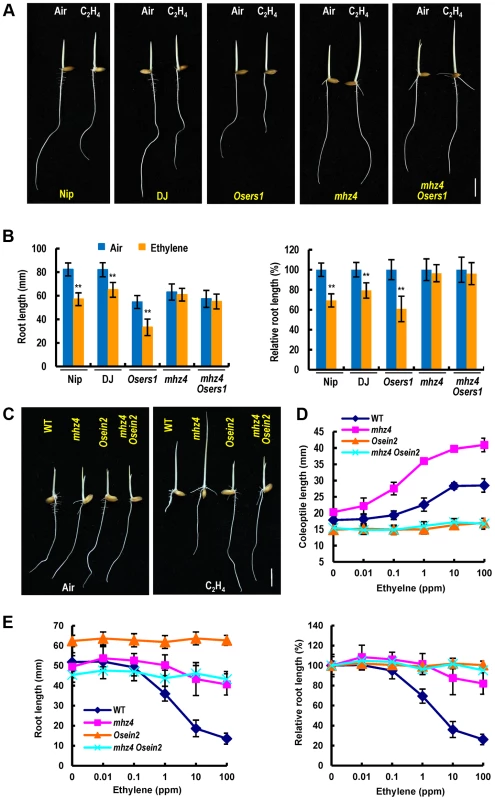
(A) Comparison of ethylene-response phenotypes of mhz4 Osers1 double mutant and the single mutants. Nipponbare (Nip) and Dongjin (DJ) are wild types. Double mutant between mhz4 and ethylene receptor OsERS1 mutant Osers1 (Dongjin background) were generated by crossing. Rice seedlings were grown in the dark for 3 d in the presence or absence of 1 ppm ethylene. (B) Quantification of root length (left) and relative root length (right) of the mutants in (A). Each column is average of 50 seedlings and bars indicate SD. ** indicate significant difference compared to air control at P<0.01. (C) Ethylene response phenotypes of double mutant mhz4 Osein2 and the single mutants. Osein2/mhz7-1 is in Nipponbare background. Rice seedlings were grown in dark for 3 d in the presence or absence of 10 ppm ethylene. (D) Ethylene dose-response curves for coleoptile length in various mutants. Each point is average of 20 seedlings and bars indicate SD. (E) Ethylene dose-response curves for root length (left) and relative root length in various mutants (right). Others are as in (D). Bars = 10 mm. We constructed mhz4 Osein2 double mutant to analyze the genetic interaction of MHZ4 with OsEIN2 in coleoptile ethylene response. The Osein2/mhz7-1 is a mutant showing ethylene insensitivity in both roots and coleoptiles previously identified [45]. The coleoptiles of mhz4 Osein2 double mutant showed complete ethylene insensitivity similar to that of Osein2 seedlings but unlike the mhz4 coleoptiles, indicating that mhz4 requires OsEIN2 in regulation of coleoptile ethylene response (Figure 7C and 7D). On the other hand, the roots of mhz4 Osein2 double mutant showed complete ethylene insensitivity similar to the case in Osein2 roots (Figure 7E). Considering that mhz4 roots still had some ethylene response and this weak response was completely abolished in the double mutant, we propose that the residual ethylene response observed in mhz4 roots was dependent on OsEIN2 function.
Effects of MHZ4 on Plant Growth and Yield-Related Traits
We compared phenotypes of field-grown mhz4 mutant, MHZ4-OX lines and WT plants. At seedling stage, the mhz4 mutant had flowing leaves with yellow green color compared with WT plants (Figure 8A). From vegetative to reproductive stages, the leaves of mhz4 appeared to be pale green and some leaves had brown dots on the leaf tip (Figure 8B). Chlorophyll (Chl) analysis revealed that mhz4 had a reduction in Chl b and total Chl contents and an increase in Chl a/b ratio compared with WT leaves (Figure 8C), indicating that mhz4 mutation interferes with Chl b biosynthesis. This may alter the light harvesting antenna size, thus affecting photosynthetic efficiency [51], [52]. For flowering time, both mhz4 mutant and the four MHZ4-OX lines exhibited delayed heading time in comparison with WT plants (Table S2).
Fig. 8. Phenotypic comparison of field-grown plants. 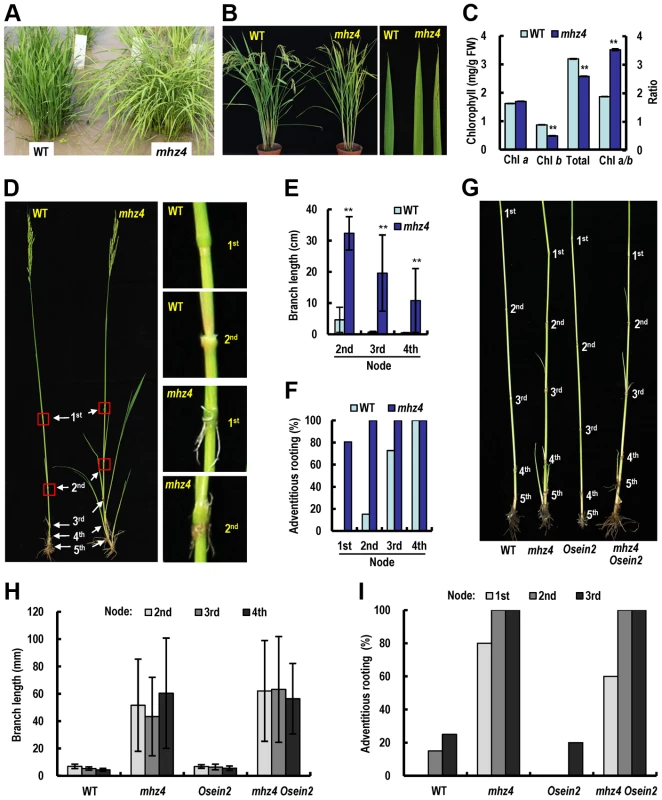
(A) Phenotypes of Five-week-old seedlings of WT and mhz4. (B) Plant phenotypes after heading. Please note the spots on some mhz4 leaves but not WT leaves. (C) Chlorophyll contents in the fourth leaves of plants. Chlorophyll was extracted with 95% ethanol from the fourth leaves from the top at heading stage. Each column is average of three measurements and bars indicate SD. ‘**’ indicate significant difference compared to WT (P<0.01). (D) Formation of branches and nodal adventitious roots in the main tiller of WT and mhz4 plants. (E) Branch length at each node from the main tillers of WT and mhz4. Each column is average of 30 to 35 plants and bars indicate SD. ‘**’ indicate significant difference compared to WT (P<0.01). (F) Percentage of nodal adventitious roots in the main tillers. 30 to 35 plants were investigated for WT and mhz4. (G) Effects of OsEIN2 mutation on branching and adventitious root formation of mhz4. Representative plants of WT, mhz4, Osein2 and mhz4 Osein2 double mutant were compared. (H) Quantification of branch length at each node in main tillers from 10–20 plants in (G). (I) Percentage of adventitious root formation at each node in main tillers from 10–20 plants in (G). At maturation stage, mhz4 mutant exhibited dramatic growth of branches developed on upper nodes and adventitious roots formation on the nodes in comparison with WT plants (Figure 8D–8F). Similar phenotype was observed in the mhz4 Osein2 double mutant in different years (Figure 8G–8I; Figure S5). These results suggest that MHZ4 negatively regulate axillary bud growth and adventitious rooting possibly through ABA function but not ethylene signaling.
Plant height is an important agronomic trait associated with rice yield. Ethylene positively regulates rice plant height by promoting stem elongation [53]. The mhz4 plants were taller than WT and all the four MHZ4-OX lines were shorter than WT plants (Table S2). The mhz4 Osein2 double mutant was shorter than mhz4 plants but was similar to that of Osein2 plants (Table S2). These observations suggest that MHZ4 negatively regulate plant height in rice in an OsEIN2-dependent manner. The promoted plant height of mhz4 is possibly due to the increased ethylene production (Figure 5C). The numbers of effective tiller (tillers producing panicles with at least five filled grains) in WT and mhz4 were identical, whereas two of the four MHZ4-OX lines produced more effective tillers than the WT (Table S2). Panicle length of mhz4 mutant was shorter than that of WT (Table S2).
Grain-related traits were also examined. The mhz4 grains had no dormancy and displayed preharvest sprouting phenotype (Figure S6A and S6B). This is a typical characteristic for ABA-deficient mutants of rice [54]. Seed-setting rate and 1000-grain weight were all significantly reduced in mhz4 mutant (Table S2). The grain shape of WT and mhz4 was further examined. The mhz4 mutant showed a decrease in grain thickness and an increase in grain length, implying that MHZ4 mutation affects grain shape (Figure S6C). We further performed a time course analysis of grain dry weight during grain filling and found that the grain weight of mhz4 increased more slowly than that of WT, suggesting that grain filling is hindered in mhz4 mutant (Figure S6D). These results indicate that alteration of MHZ4 expression affects branching, plant height and grain-related traits.
Discussion
We characterized a rice ethylene-response mutant mhz4, which showed reduced ethylene response in roots but enhanced ethylene response in coleoptiles. MHZ4 encoded a chloroplast-localized membrane protein homologous to Arabidopsis ABA4 in ABA biosynthesis pathway. MHZ4 mutation reduced ABA level but enhanced ethylene production. MHZ4 overexpression enhanced ethylene responses in roots but reduced ethylene response in coleoptiles. Genetically, MHZ4 acts at or downstream of the ethylene receptors to positively regulate root ethylene response likely through ethylene-triggered ABA accumulation. Additionally, MHZ4 acts at or upstream of OsEIN2 to negatively regulate coleoptile ethylene response possibly through modulating OsEIN2 expression. MHZ4 also affects agronomic traits. Our findings reveal a novel mode of interplay between ethylene and ABA.
The roots of mhz4 seedlings displayed about 20% growth inhibition (80% insensitivity) when treated with 100 ppm ethylene (Figure 1B). By contrast, the WT roots exhibited about 70% inhibition under the same concentration of ethylene. This comparison reveals that MHZ4 mediates a large part of the ethylene-inhibition of root growth. Since MHZ4 mutation reduced ABA contents possibly through disruption of a branch of ABA biosynthesis (Figure 4A), we propose that ABA may be the factor participating in ethylene-inhibition of root growth. Five pieces of evidence supports this conclusion. (1) Exogenous application of ABA largely recovered the defective response of mhz4 roots to ethylene; (2) MHZ4-overexpression resulted in enhanced ethylene response in roots; (3) Ethylene induced MHZ4 expression and ABA accumulation particularly in roots; (4) Ethylene-induced ABA mediates expression of some ethylene-responsive genes; (5) The ethylene receptor OsERS1-mediated root ethylene response required MHZ4 function. The present conclusion that ethylene inhibited root growth largely/partly through ABA function is in contrast to that observed in Arabidopsis, where ethylene-mediated root inhibition is independent of ABA action [21], [22], [26]. This difference indicates that rice, as a monocot or a semiaquatic plant, may have adopted a novel mechanism for ethylene-ABA interaction.
We have identified another rice ABA-deficient mutant mhz5 that showed a similar ethylene response phenotype as mhz4 and the corresponding gene OsCRTISO encodes the carotenoid isomerase that acts at early step in ABA biosynthetic pathway (Yin et al., unpublished). Consequently, it seems that the ABA-dependent ethylene effect on rice root growth is not restricted to MHZ4, possibly other ABA biosynthetic genes and even the signaling component genes may affect the root ethylene response in rice.
In Arabidopsis, extensive studies have established that ethylene inhibits root growth through auxin action via modulating its biosynthesis, transport and/or signaling [55]–[62]. Auxin response occurring in the elongation zone mediates a substantial part of ethylene effect on root growth [63]. In monocotyledonous plant Brachypodium, ethylene may inhibit auxin biosynthesis in IPA pathway to control root elongation, which is different from the case in Arabidopsis [64]. In rice, our present results demonstrate that ethylene inhibits root growth largely through ABA function. Previous studies also discover that ethylene triggers ABA biosynthesis, leading to growth inhibition [65]. It is unknown whether auxin is also involved in this process. ABA may act through auxin pathway or vice versa, or the two hormones might act independently to mediate ethylene response. Further investigation toward the relationship among these hormones should shed light on their complicated interaction in control of rice roots.
MHZ4 mutation drastically enhances ethylene emission possibly through activation of ethylene biosynthesis genes, and ABA inhibits ethylene production (Figure 5C). Considering that ethylene induces ABA to inhibit root growth (Figure 4), the ABA suppression of ethylene generation may represent a negative feedback control mechanism to alleviate ethylene effects on roots.
The coleoptiles of mhz4 were slightly but significantly longer than that of WT seedlings (Figure 5B), indicating that ABA acts as an inhibitor of rice coleoptile elongation. Similar effect of ABA was observed in rice seedlings treated with fluridone, an inhibitor of ABA biosynthesis, which reduced the levels of endogenous ABA in coleoptiles and in turn promoted coleoptile growth [66]. In contrast to the ABA effect, ethylene stimulates coleoptile growth in rice (Figure 1; [46]), suggesting that the two hormones have antagonistic interaction. That MHZ4 mutation enhanced ethylene production and ethylene response whereas its overexpression reduced ethylene response in coleoptiles (Figure 1, 5, and 6) further supports the antagonistic relationship of ABA and ethylene. In Arabidopsis, ABA biosynthesis gene ABA2 mutation also resulted in elevated ethylene production [67]; however, ethylene response of this mutant was not altered [26], [67], indicating presence of conserved and diverged aspects in interactions of ABA and ethylene.
In WT shoots, ethylene slightly inhibits ABA levels and may hence promote shoot/coleoptile elongation (Figure 4A). In mhz4 shoots, the ABA level is slightly induced by ethylene but the induction is not statistically significant (P = 0.089168). Additionally, the ABA level in mhz4 shoots is quite low (about 1/10 of the WT level) compared with that in WT. The small increase in ABA contents in mhz4 shoots after ethylene treatment may be not enough to affect its ethylene response. However, we could not exclude the possibility that ethylene-altered ABA levels may have some subtle effects on ethylene responses.
Although elevated ethylene production was observed in mhz4 mutant, AVG treatments that inhibited ethylene production did not significantly altered ethylene response (Figure 5E), suggesting that the enhanced ethylene response in mhz4 coleoptiles is likely caused by enhanced ethylene signaling rather than ethylene overproduction. In support of this prediction, transcript levels of the central ethylene signaling component OsEIN2 were up-regulated in mhz4 shoots (Figure 5G). Further genetic analysis revealed that mhz4 ethylene-response phenotypes in coleoptiles required OsEIN2 function. Collectively, MHZ4-dependent ABA pathway negatively regulates rice coleoptile ethylene response at least in part through modulating OsEIN2 expression. It should be mentioned that although the ethylene overproduction in mhz4 mutant did not play major roles in enhanced ethylene response, the basal level of ethylene did contribute to the coleoptile elongation because AVG reduced the coleoptile length of mhz4 to the WT level (Figure 5E).
Our present results reveal that ABA and ethylene are synergistic in root growth inhibition but antagonistic in coleoptile elongation. The opposite effects of mhz4 mutation on ethylene response of rice root and coleoptile may be due to alterations of expressions of ethylene-responsive genes in these organs (Figure 1D, E). Previous studies have reported antagonistic interactions of ethylene and ABA in several processes such as seed germination, stomatal closure and submerge-induced shoot elongation of semiaquatic plants, and synergistic interactions in tomato fruit ripening and abiotic stress responses [68]. These findings imply that ethylene-ABA interplay is quite complicated, depending on biological processes, tissue/organs, and plant species. Why plants have these different interactions remains unclear. It is possible that multiple interaction manners allow plants to be more adapted to the changing environments at different growth and developmental stages.
At mature stage, mhz4 plants had branches and adventitious roots at nodes of higher positions (Figure 8). This phenomenon is rarely observed in rice and not dependent on OsEIN2 function of ethylene signalling through double mutant analysis, suggesting that ABA itself inhibits branching and formation of adventitious root. This function probably represents a novel aspect of ABA roles during rice development. It has been reported that a higher ratio of ABA to ethylene in rice spikelets is required to maintain a faster grain-filling rate [69]. In our mhz4 mutant, a low ABA level but a high ethylene production was noted and the low ratio of ABA to ethylene may be related to the impaired grain filling (Figure S6). However, in MHZ4-overexpressing plants, grain-related traits were not widely improved (Table S2), implying that these traits are likely regulated in a complicated manner.
The MHZ4 seems to have a negative role on plant height because the mhz4 mature plants are taller than WT whereas the overexpressing lines are shorter than WT (Table S2). MHZ4 mutation activated ethylene production which increased plant height through OsEIN2 function. Our results are similar with those obtained in Arabidopsis and tomato, where ABA-deficient mutants exhibit stunted shoot growth due to increased ethylene production [67], [70]. It should be noted that ethylene promotes shoot growth in rice but inhibits this process in Arabidopsis and tomato.
In conclusion, we demonstrate that ethylene inhibits rice root growth through ABA action, and ABA negatively regulates coleoptile ethylene response through modulating OsEIN2 expression. Synergistic interaction in roots but antagonistic interaction in coleoptiles was revealed between ABA and ethylene, providing new insights into understanding of their complicated interplay.
Materials and Methods
Plant Materials and Growth Conditions
The mhz4 and Osein2/mhz7-1 mutants were identified previously [45]. The OsERS1 (LOC_Os03g49500) T-DNA knockout mutant Osers1 (PFG_1B-08531.L) is in Dongjin (DJ) background and was obtained from the POSTECH Biotech Center [50]. The homogenous Osers1 mutation was identified by PCR using the T-DNA left border primer PR152 (5′-TTGGTTAGAGAACAGCACAA-3′) and gene-specific primers flanking the insertion site: PR139 (5′-AATAAATGATTGGCCAGAGC-3′) and PR140 (5′-TGCTTCTCAGTATCCTTTGT-3′). The Osein2/mhz7-1 harbors a 24-bp deletion in the cording region of OsEIN2 (LOC_Os07g06130), and the mutation was identified as previously described [45]. For ethylene treatment, rice seedlings were grown on a stainless steel sieve that was placed in an air-tight plastic box with various concentrations of ethylene [45]. The seedlings were incubated at 28°C in the dark for 2 to 3 days as indicated in each experiment. For ABA or NDGA treatment, rice seedlings were grown on a stainless steel sieve and various concentrations of ABA (Sigma, A1049) or 100 µM NDGA (Sigma) was added to the water. The stock solutions of ABA and NDGA were prepared in ethanol. For AVG treatment, rice seedlings were grown on eight layers of cheesecloth saturated with 5 µM AVG (Sigma) in Petri dishes. For field experiments, rice plants were grown in the Experimental farm Station of the Institute of Genetics and Developmental Biology in Beijing from May to October of each year.
Map-Based Cloning of MHZ4 Gene
F2 mapping populations were generated from crosses between mhz4 mutant and indica variety Minghui 63. Genomic DNA was isolated from etiolated seedlings with mutant phenotype. For primary mapping, bulked segregant analysis (BSA) was performed using a DNA pool from 15 mutant individuals selected from the F2 population between mhz4 and MH63. The markers used in BSA included 30 published SSR markers (http://www.gramene.org) and 117 insertion-deletion (Idl) markers. A total of 480 mutant individuals selected from the F2 populations were used for fine mapping. MHZ4 locus was mapped to chromosome 1 between Idl1–1.55 and (5′ - CAGGGCAATCTGTCAAAGCT-3′ and 5′ - CTAAAGATCAGTACTGGGCAC-3′) and Idl1–1.58 (5′ - CTCTTTGTCAAGCTTATTTACC-3′ and 5′ - ACAGATCCGTATGTTTATAGTG-3′) in the region of 1.55 Mb to 1.58 Mb (Genebank accession number: NC_008400), which contains 4 genes. The candidate gene was finally determined by DNA sequencing of all the candidate genes within this region.
To confirm the 18-bp deletion in the genomic DNA of mhz4, DNA fragment length polymorphism between wild-type (WT) and the mutant was examined using PCR with primers (5′ - CTCTGTTCCGGCCTCGCGCA -3′ and 5′ - AGGACGGCGATGGTGCCCCA -3′). The 81-bp insertion in the mhz4 cDNA was detected by PCR amplification of the full-length cDNA using primers (5′ - ATGGCGGCTCTCCTCCTCCT -3′ and 5′-TCAATGTGAGCGACCAATTGAAC-3′).
Quantitative PCR and RNA-Seq Analyses
Total RNAs were extracted from various rice tissues using TRIZOL reagent (Invitrogen) according to the manufacturer's instructions. Quantitative PCR and/or semi-quantitative PCR were carried out as described previously [45]. The primers are listed in Table S1. For RNA-seq analysis, two-day-old etiolated seedlings of WT and mhz4 mutant were treated with air or 10 ppm ethylene for 8 h. The libraries were prepared from 10 µg total RNA using NEBNext Ultra RNA Library Prep Kit (NEB, USA), and each sample contains two biological replicates. Clean reads were mapped to rice MSU7.0 genome using TopHat, and analyzed using Cufflinks as described [71].
Transient Expression Assay
To generate MHZ4-GFP fusion construct, the MHZ4 coding sequence was PCR amplified (for primers see Table S1), digested with XholI/NcoI and fused in frame to the 5′-end of GFP in a pUC18-based vector under the control of CaMV35S promoter. The fusion gene was transiently expressed in tobacco leaves by microprojectile bombardment using a Bio-Rad PDS-1000/He particle delivery system [9]. The images were taken using a confocal microscope (Leica TCS SP5). Excitation/emission wavelengths were set at 488 nm/500–530 nm for GFP fluorescence and at 570 nm/640 nm for chlorophyll autofluorescence.
Generation of Transgenic Rice Plants
To generate MHZ4pro::GUS construct, a 2048-bp promoter region upstream of the start codon was PCR amplified and cloned into Sse8387I/BamHI sites of pCAMBIA2300-35S-GUS vector to replace the CaMV35S promoter. To generate MHZ4-complementation construct, the genomic DNA (4213 bp) of MHZ4 was PCR amplified and subcloned into Sse83871I/SalI-digested pCAMBIA2300-35S vector. To generate the 35S::MHZ4 construct, the MHZ4 coding sequence was PCR amplified and inserted into BamHI/SalI-digested pCAMBIA2300-35S vector. The primers used for these constructions are listed in Table S1. The constructs were transfected into Agrobacterium tumefaciens strain EHA105 by electroporation. Rice transformation was performed as previously described [42]. The MHZ4pro::GUS or 35S::MHZ4 construct was transformed into rice variety Nipponbare. The MHZ4-complementation construct was transferred into mhz4 mutant. Positive transgenic plants were confirmed by PCR using NPT II gene-specific primers (Table S1). Homozygous transgenic lines were selected using Kanamycin treatment (50 mg/L).
GUS Staining
Rice seedlings or organs were fixed in 90% acetone on ice for 15 min, rinsed with staining buffer (100 mM Na3PO4 buffer pH 7.0, 10 mM EDTA, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 0.1% Triton X-100), and vacuum infiltrated with staining solution (staining buffer containing 0.5 mg/ml X-Gluc (Sigma, B8049) for 15 minutes. The samples were incubated at 37°C in the dark. After staining, green tissues were decolorized in 70% ethanol and then in ethanol/acetic acid (6∶1) until the chlorophyll was removed. The samples were observed using stereo microscopy (Leica, M165 FC).
Measurement of Ethylene and ABA
Ethylene production of WT and mhz4 mutant in 4 d-old etiolated seedlings, two-week-old green seedlings, mature leaves and panicles was determined by gas chromatography (GC2014, Shimadzu, Japan) equipped with a flame ionization detector. ABA contents in WT and mhz4 were detected using 4 d-old etiolated seedlings or 2 d-old etiolated seedlings treated with or without 10 ppm of ethylene for 48 h. Plant tissues (200 mg) were ground into powder in liquid nitrogen. ABA was determined by UPLC-MS/MS (UPLC-Quattro Premier XE) at the National Center of Plant Gene Research (IGDB, CAS, Bejing, China) as described [72]. For both ethylene and ABA detection, each material or treatment includes three duplications.
Measurements of Agronomic Traits in Field-Grown Plants
Heading date of WT, mhz4 mutants and MHZ4-OX lines was recorded when the first spike of a rice plant emerged about 1 cm above the sheaths. Twenty to thirty individuals were examined for each material. After harvest, 20 plants from each material were used for measurements of agronomic traits as previously described [45].
Supporting Information
Zdroje
1. Abeles FB, Morgan PW, Saltveit JME (1992) Ethylene in Plant Biology. 2nd ed. (San Diego, CA: Academic Press).
2. BleeckerAB, KendeH (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16 : 1–18.
3. HallB, ShakeelS, SchallerGE (2007) Ethylene receptors: ethylene perception and signal transduction. J Plant Growth Regul 26 : 118–130.
4. JuC, ChangC (2012) Advances in ethylene signalling: protein complexes at the endoplasmic reticulum membrane. AoB PLANTS 2012: pls031 doi:10.1093/aobpla/pls031
5. KendrickMD, ChangC (2008) Ethylene signaling: new levels of complexity and regulation. Curr Opin Plant Biol 11 : 479–485.
6. ShakeelSN, WangX, BinderBM, SchallerGE (2013) Mechanisms of signal transduction by ethylene: overlapping and nonoverlapping signalling roles in a receptor family. AoB PLANTS 5: plt010 doi:10.1093/aobpla/plt010
7. ChenYF, RandlettMD, FindellJL, SchallerGE (2002) Localization of the ethylene receptor ETR1 to the endoplasmic reticulum of Arabidopsis. J Biol Chem 277 : 19861–19866.
8. GaoZY, ChenYF, RandlettMD, ZhaoXC, FindellJL, et al. (2003) Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem 278 : 34725–34732.
9. MaB, CuiML, SunHJ, TakadaK, MoriH, et al. (2006) Subcellular localization and membrane topology of the melon ethylene receptor CmERS1. Plant Physiol 141 : 587–597.
10. BissonMMA, BleckmannA, AllekotteS, GrothG (2009) EIN2, the central regulator of ethylene signalling, is localized at the ER membrane where it interacts with the ethylene receptor ETR1. Biochem J 424 : 1–6.
11. QiaoH, ChangKN, YazakiJ, EckerJR (2009) Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes Dev 23 : 512–521.
12. JuC, YoonGM, ShemanskyJM, LinDY, YingZI, et al. (2012) CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc Natl Acad Sci U S A 109 : 19486–19491.
13. KamiyoshiharaY, TiemanDM, HuberDJ, KleeHJ (2012) Ligand-induced alterations in the phosphorylation state of ethylene receptors in tomato fruit. Plant Physiol 160 : 488–497.
14. QiaoH, ShenZ, HuangSS, SchmitzRJ, UrichMA, et al. (2012) Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 338 : 390–393.
15. WenX, ZhangC, JiY, ZhaoQ, HeW, et al. (2012) Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Res 22 : 1613–1616.
16. GuoHW, EckerJR (2003) Plant responses to ethylene gas are mediated by SCF (EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 115 : 667–677.
17. PotuschakT, LechnerE, ParmentierY, YanagisawaS, GravaS, et al. (2003) EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 115 : 679–689.
18. VandenbusscheF, Van Der StraetenD (2007) One for all and all for one: cross-talk of multiple signals controlling the plant phenotype. J Plant Growth Regul 26 : 178–187.
19. YooSD, ChoY, SheenJ (2009) Emerging connections in the ethylene signalling network. Trends Plant Sci 14 : 270–279.
20. ZhaoQ, GuoH (2011) Paradigms and paradox in the ethylene signaling pathway and interaction network. Mol Plant 4 : 626–634.
21. BeaudoinN, SerizetC, GostiF, GiraudatJ (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12 : 1103–1115.
22. GhassemianM, NambaraE, CutlerS, KawaideY, KamiyaY, et al. (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12 : 1117–1126.
23. BenschopJJ, JacksonMB, GuhlK, VreeburgRAM, CrokerSJ, et al. (2005) Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. Plant J 44 : 756–768.
24. BenschopJJ, MillenaarFF, SmeetsME, van ZantenM, VoesenekLA, et al. (2007) Abscisic acid antagonizes ethylene-induced hyponastic growth in Arabidopsis. Plant Physiol 143 : 1013–1023.
25. WangY, LiuC, LiK, SunF, HuH, et al. (2007) Arabidopsis EIN2 modulates stress response through abscisic acid response pathway. Plant Mol Biol 64 : 633–644.
26. ChengWH, ChiangMH, HwangSG, LinPC (2009) Antagonism between abscisic acid and ethylene in Arabidopsis acts in parallel with the reciprocal regulation of their metabolism and signaling pathways. Plant Mol Biol 71 : 61–80.
27. DongH, ZhenZ, PengJ, ChangL, GongQ, et al. (2011) Loss of ACS7 confers abiotic stress tolerance by modulating ABA sensitivity and accumulation in Arabidopsis. J Exp Bot 62 : 4875–4887.
28. LiZ, ZhangL, YuY, QuanR, ZhangZ, et al. (2011) The ethylene response factor AtERF11 that is transcriptionally modulated by the bZIP transcription factor HY5 is a crucial repressor for ethylene biosynthesis in Arabidopsis. Plant J 68 : 88–99.
29. ChenL, DoddIC, DaviesWJ, WilkinsonS (2013) Ethylene limits abscisic acid - or soil drying-induced stomatal closure in aged wheat leaves. Plant Cell Environ 36 : 1850–1859.
30. NambaraE, Marion-PollA (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56 : 165–185.
31. RockCD, ZeevaartJA (1991) The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc Natl Acad Sci U S A 88 : 7496–7499.
32. NorthHM, AlmeidaAD, BoutinJP, FreyA, ToA, et al. (2007) The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J 50 : 810–824.
33. NeumanH, GalpazN, CunninghamFXJr, ZamirD, HirschbergJ (2014) The tomato mutation nxd1 reveals a gene necessary for neoxanthin biosynthesis and demonstrates that violaxanthin is a sufficient precursor for abscisic acid biosynthesis. Plant J 78 : 80–93.
34. IuchiS, KobayashiM, TajiT, NaramotoM, SekiM, et al. (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27 : 325–33.
35. ChengWH, EndoA, ZhouL, PenneyJ, ChenHC, et al. (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14 : 2723–43.
36. SeoM, PeetersAJ, KoiwaiH, OritaniT, Marion-PollA, et al. (2000) The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc Natl Acad Sci U S A 97 : 12908–12913.
37. CutlerSR, RodriguezPL, FinkelsteinRR, AbramsSR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61 : 651–679.
38. Joshi-SahaA, ValonC, LeungJ (2011) Abscisic acid signal off the STARting block. Mol Plant 4 : 562–580.
39. Ben-AriG (2012) The ABA signal transduction mechanism in commercial crops: learning from Arabidopsis. Plant Cell Rep 31 : 1357–1369.
40. JunSH, HanMJ, LeeS, SeoYS, KimWT, et al. (2004) OsEIN2 is a positive component in ethylene signaling in rice. Plant Cell Physiol 45 : 281–289.
41. YauCP, WangL, YuM, ZeeSY, YipWK (2004) Differential expression of three genes encoding an ethylene receptor in rice during development, and in response to indole-3-acetic acid and silver ions. J Exp Bot 55 : 547–556.
42. WuriyanghanH, ZhangB, CaoWH, MaB, LeiG, et al. (2009) The ethylene receptor ETR2 delays floral transition and affects starch accumulations in rice. Plant Cell 21 : 1473–1494.
43. ZhangW, ZhouX, WenCK (2012) Modulation of ethylene responses by OsRTH1 overexpression reveals the biological significance of ethylene in rice seedling growth and development. J Exp Bot 63 : 4151–4164.
44. WangQ, ZhangW, YinZ, WenCK (2013) Rice CONSTITUTIVE TRIPLE-RESPONSE2 is involved in the ethylene-receptor signalling and regulation of various aspects of rice growth and development. J Exp Bot 64 : 4863–4875.
45. MaB, HeSJ, DuanKX, YinCC, ChenH, et al. (2013) Identification of rice ethylene-response mutants and characterization of MHZ7/OsEIN2 in distinct ethylene response and yield trait regulation. Mol Plant 6 : 1830–1848.
46. KuHS, SugeH, RappaportL, PrattHK (1970) Stimulation of rice coleoptile growth by ethylene. Planta 90 : 333–339.
47. JoyardJ, FerroM, MasselonC, Seigneurin-BernyD, SalviD, et al. (2009) Chloroplast proteomics and the compartmentation of plastidial isoprenoid biosynthetic pathways. Mol Plant 2 : 1154–1180.
48. LenkaSK, LohiaB, KumarA, ChinnusamyV, BansalKC (2009) Genome-wide targeted prediction of ABA responsive genes in rice based on over-represented cis-motif in co-expressed genes. Plant Mol Biol 69 : 261–71.
49. SaikaH, OkamotoM, MiyoshiK, KushiroT, ShinodaS, et al. (2007) Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8′-hydroxylase in rice. Plant Cell Physiol 48 : 287–298.
50. YiJ, AnG (2013) Utilization of T-DNA tagging lines in rice. J Plant Biol 56 : 85–90.
51. SakurabaY, YokonoM, AkimotoS, TanakaR, TanakaA (2010) Deregulated chlorophyll b synthesis reduces the energy transfer rate between photosynthetic pigments and induces photodamage in Arabidopsis thaliana. Plant Cell Physiol 51 : 1055–1065.
52. PerrineZ, NegiS, RichardT, SayreRT (2012) Optimization of photosynthetic light energy utilization by microalgae. Algal Res 1 : 134–142.
53. MaB, ChenSY, ZhangJS (2010) Ethylene signaling in rice. Chinese Sci Bull 55 : 2204–2210.
54. FangJ, ChaiC, QianQ, LiC, TangJ, et al. (2008) Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo-oxidation in rice. Plant J 54 : 177–189.
55. PickettFB, WilsonAK, EstelleM (1990) The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol 94 : 1462–1466.
56. LuschnigC, GaxiolaRA, GrisafiP, FinkGR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12 : 2175–2187.
57. AlonsoJM, StepanovaAN, SolanoR, WismanE, FerrariS, et al. (2003) Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci U S A 100 : 2992–2997.
58. RuzickaK, LjungK, VannesteS, PodhorskaR, BeeckmanT, et al. (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19 : 2197–2212.
59. StepanovaAN, HoytJM, HamiltonAA, AlonsoJM (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17 : 2230–2242.
60. StepanovaAN, Robertson-HoytJ, YunJ, BenaventeLM, XieD, et al. (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133 : 177–191.
61. StepanovaAN, YunJ, LikhachevaAV, AlonsoJM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19 : 2169–2185.
62. SwarupR, PerryP, HagenbeekD, Van Der StraetenD, BeemsterGTS, et al. (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19 : 2186–2196.
63. VanstraelenM, BenkovE (2012) Hormonal interactions in the regulation of plant development. Annu Rev Cell Dev Biol 28 : 22.1–22.25.
64. Pacheco-VillalobosD, SankarM, LjungK, HardtkeCS (2013) Disturbed local auxin homeostasis enhances cellular anisotropy and reveals alternative wiring of auxin-ethylene crosstalk in Brachypodium distachyon seminal roots. PLoS Genet 9: e1003564.
65. GrossmannaK, HansenbH (2001) Ethylene-triggered abscisic acid: A principle in plant growth regulation? Physiol Plant 113 : 9–14.
66. Hoffmann-BenningS, KendeH (1992) On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiol 99 : 1156–1161.
67. LeNobleME, SpollenWG, SharpRE (2004) Maintenance of shoot growth by endogenous ABA: genetic assessment of the involvement of ethylene suppression. J Exp Bot 55 : 237–245.
68. Ma B, Chen H, Chen SY, Zhang JS (2014) Roles of ethylene in plant growth and responses to stresses. In: Tran L-SP, Pal S, editors. Phytohormones: A window to metabolism, signaling and biotechnological applications. Springer Science+Business Media New York. pp. 81–118.
69. YangJ, ZhangJ, WangZ, LiuK, WangP (2006) Post-anthesis development of inferior and superior spikelets in rice in relation to abscisic acid and ethylene. J Exp Bot 57 : 149–160.
70. SharpRE, LeNobleME, ElseMA, ThorneET, GherardiF (2000) Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. J Exp Bot 51 : 1575–1584.
71. TrapnellC, RobertsA, GoffL, PerteaG, KimD, et al. (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protocols 7 : 562–578.
72. FuJ, ChuJ, SunX, WangJ, YanC (2012) Simple, rapid, and simultaneous assay of multiple carboxyl containing phytohormones in wounded tomatoes by UPLC-MS/MS using single SPE purification and isotope dilution. Anal Sci 28 : 1081–1087.
Štítky
Genetika Reprodukčná medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 10- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



