-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
Although Fragile Histidine Triad (FHIT) is known as a potential tumor suppressor gene in terms of tumor initiation and progression, the role of FHIT in the metastatic process is not well characterized. Here it is shown that FHIT reduces the motility and invasiveness of lung cancer cells in vitro and ability to metastasize in vivo, at least partially through the miR-30c-mediated suppression of EMT, a critical process during tumor metastasis. This study provides new insights into the role of FHIT and a FHIT-activated miRNA, miR-30c, as crucial modulators in lung metastasis.
Published in the journal: FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs. PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004652
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004652Summary
Although Fragile Histidine Triad (FHIT) is known as a potential tumor suppressor gene in terms of tumor initiation and progression, the role of FHIT in the metastatic process is not well characterized. Here it is shown that FHIT reduces the motility and invasiveness of lung cancer cells in vitro and ability to metastasize in vivo, at least partially through the miR-30c-mediated suppression of EMT, a critical process during tumor metastasis. This study provides new insights into the role of FHIT and a FHIT-activated miRNA, miR-30c, as crucial modulators in lung metastasis.
Introduction
Metastasis, the spread of cells from the site of a primary tumor followed by growth of a secondary tumor in distant organs, is frequently a final and fatal step in tumor progression. This process consists of a series of steps, including angiogenesis, intravasation, survival in circulation, extravasation into surrounding tissue, and formation of micrometastases [1], [2]. In particular, the epithelial-mesenchymal transition (EMT), a process in which epithelial cells lose or modify their apical-basal polarity and are converted to a mesenchymal phenotype, is a critical process during embryonic development and tumor metastasis [3], [4]. In the metastatic process, EMT disrupts the intercellular tight junctions and promotes migration, and self-renewing and stem-like properties to facilitate metastatic colonization [5]. EMT is commonly characterized by the suppression of the cell-cell adhesion receptor E-cadherin and endows cells with more motile, invasive properties [6]. In addition, accumulating evidence from experimental and clinical studies suggests that microRNAs (miRNAs), small, endogenous non-coding RNAs, are involved in EMT. For example, several miRNAs such as miR-200, miR-205 and miR-34 regulate the expression of crucial EMT-related transcription factors, including those belonging to the Snail family, ZEB1, ZEB2 and Twist1/2, by binding to the 3′untranslated regions (UTRs) of their mRNAs [7], [8]. miRNAs have also been shown to promote or suppress various steps in migration and metastasis by directly modulating the expression of metastasis-related genes including BRMS1, PTEN, MYC, and HMGA2 [9], [10], [11], [12]. Many studies have shown that genes, including miRNA genes, that localize to genome fragile sites or aberrant chromosome regions are associated with cancer, indicating that these breakpoint-associated genes might be important in the development and progression of human cancer [13], [14]. The Fragile Histidine Triad (FHIT) gene spans the FRA3B locus on chromosome 3p14.2 and alterations at this common fragile site are associated with many types of cancer [15], [16], [17]. In addition, FHIT is a tumor suppressor that regulates a wide spectrum of biological processes associated with tumor initiation and progression, and FHIT expression is lost in many cancers [18], [19]. Recently, it has been shown that FHIT loss also affects EMT, considered a crucial step in the early stage of cancer metastasis [19], [20]. However, signal pathways through which FHIT loss might contribute to the metastatic process are not well characterized. In this study, we demonstrate that elevated expression of FHIT leads to reduced motility and invasiveness of lung cancer cells in vitro and their ability to metastasize in vivo. Concomitantly, FHIT functions as an important modulator of EMT, mediated by transcriptional repression of EMT-related genes. The results indicate that these effects of FHIT could in part be mediated by FHIT-regulated miRNAs, pointing to miRNAs as crucial factors in the metastatic process, and as possible therapeutic targets in cancer.
Results
FHIT is a negative regulator of metastasis in lung cancer
The FHIT protein is thought to have a role in tumor suppression based on its expression and function in human cancers as well as its role in tumor development in the FHIT-deficient mouse model. To determine if FHIT could be relevant to the pathogenesis of human lung metastasis, we examined the expression pattern of FHIT in non-metastatic and metastatic lung tissues. Immunostaining for FHIT in lung cancer tissue revealed that FHIT expression was markedly reduced in metastatic tissue compared to non-metastatic (Figure S1, P = 0.03). To further investigate the role of FHIT in the metastatic progression of lung cancer, we generated luciferase-tagged human A549 and H1299 non-small-cell lung cancer (NSCLC) cell lines with enforced stable expression of FHIT, and confirmed overexpression of protein by immunoblotting (Figure S2). H1299 and A549 cells show undetectable and moderate levels of endogenous FHIT expression, respectively (Figure S2). Both FHIT expressing cell lines were injected into the arterial circulation of immunodeficient mice by tail vein injection and metastatic capacity evaluated by bioluminescent imaging (BLI). We found that FHIT over-expressing A549 and H1299 cells exhibited reduced metastases in the lung, brain and bone, implying that FHIT may be negatively affecting the extravasation of lung tumor cells (Figure 1A). Continued BLI monitoring revealed a further reduction of metastatic outgrowth in the lungs of animals injected with FHIT expressing cells (Figure 1A). Furthermore, histological analysis confirmed the decrease in the number of metastatic lesions produced by the A549/FHIT and H1299/FHIT cells vs control cells (Figure 1B). To analyze metastatic invasion in vitro, we measured the infiltration of these cells through Matrigel in a modified Boyden chamber assay and found that the overexpression of FHIT significantly inhibited the invasive capacity (Figure 1C). In addition, the migration activity of both cell lines via a wound-healing assay was markedly lower vs that of control cells (Figure 1D, S3). Taken together, these results show that FHIT inhibits metastasis of human lung cancer cell lines.
Fig. 1. Elevated FHIT expression inhibits metastasis in human NSCLCs. 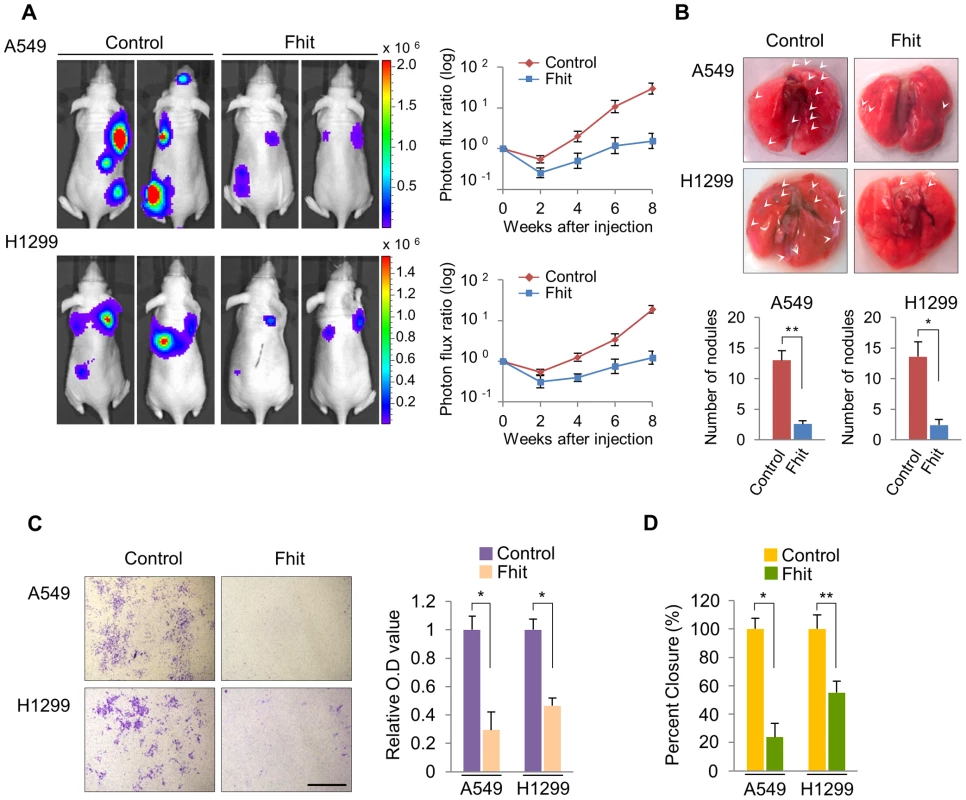
(A) Representative BLI plots of lung metastases in mice after injecting 1×106 A549 or H1299 NSCLC cells stably expressing FHIT or a control vector; n = 4. (B) Lungs were extracted at 8 weeks after tail vein injection and the number of nodules was counted. (C) Representative images of FHIT-expressing or control cells that invaded through the filter and were stained with crystal violet. Scale bar, 40 µm. The results are means ± s.d, n = 4 experiments. *P<0.001. (D) Wound healing assay of confluent monolayer of FHIT-expressing or control A549 and H1299 cells at 0 h and 24 h. See Supplementary Fig. S3, that shows representative images of migration. *P<0.001, **P<0.0001. FHIT inhibits EMT through transcriptional repression of EMT-related genes
It has been reported that FHIT might regulate the expression of genes associated with EMT through modulation of mesenchymal markers, including Vimentin [19]. To assess the mechanism by which FHIT may modulate EMT, we first determined if the epithelial-like characteristics were affected by stable FHIT expression in the lung epithelial cell lines, A459 and H1299. We observed that over-expression of FHIT led to more epithelial properties including increased cuboidal and clustered appearance in both cell lines (Figure 2A). In agreement with the change in cellular appearance, the expression level of the epithelial marker, E-cadherin, was increased, while that of mesenchymal markers, such as Vimentin and Snail, was significantly decreased in both FHIT-expressing cell lines (Figure 2B), suggesting a role for FHIT in enforcing epithelial cellular characteristics. TGF-β signaling is known to play an important role in EMT in various epithelial cells [21], [22]. To examine the effect of FHIT expression on TGF-β-induced EMT, control lung epithelial and A549/FHIT cells were treated with TGF-β and cell morphology was monitored. The FHIT-expressing cells were less or non-responsive to TGF-β1 treatment when compared to control cells showing TGF-β-induced EMT; the typical epithelial phenotype with polygonal morphology and tight arrangement was converted into a spindle-like mesenchymal morphology in control cells, but morphological changes in FHIT-expressing cells were not detected in response to TGF-β1 treatment (Figure 2C). Expression levels of the mesenchymal cell markers Vimentin, Fibronectin, Snail and N-cadherin were suppressed in FHIT-expressing cells, while E-cadherin levels were elevated (Figure 2D, 2E, S4). Expression levels of mesenchymal markers were constantly suppressed in A549/FHIT cells at translational and transcriptional levels, whereas control cells showed elevation in response to TGF-β1 (Figure 2D, 2E, S4). In addition, down-regulation of E-cadherin by TGF-β1 was not observed in FHIT-expressing A549 cells (Figure 2D, 2E). These data suggest that FHIT inhibits EMT by repressing or activating EMT-related genes and contributing to insensitivity to TGF-β-induced EMT.
Fig. 2. FHIT modulates EMT in NSCLCs through the suppression of EMT-related genes. 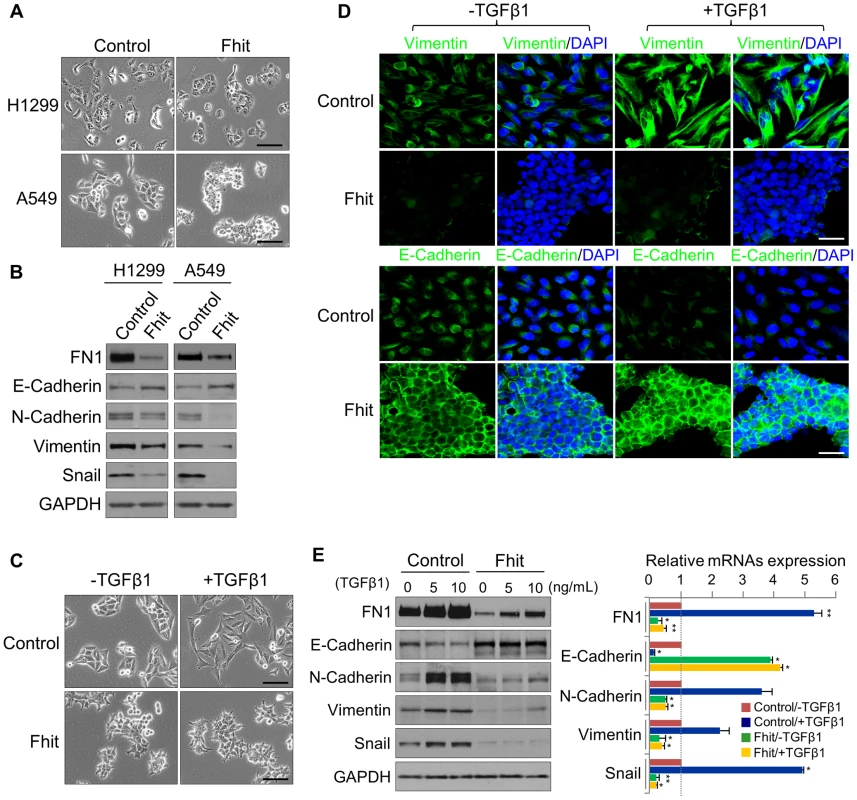
(A), (B) Phase contrast images of FHIT-expressing or control A549 and H1299 cells, and Immunoblot analysis of EMT markers in both cell lines, respectively. Scale bar, 50 µm. (C), (D) Phase contrast and immunofluorescence images for Vimentin and E-cadherin staining in TGF-β1-induced A549 cells. DAPI staining was used to detect nuclei and merged with Vimentin and E-cadherin stained images. Scale bar, 50 µm (for brightfield images), 20 µm (for immunofluorescence images). (E) Immunoblot and qRT-PCR analyses of expression of EMT markers, Fibronectin (FN1), E-cadherin, N-cadherin, Vimentin and Snail, in response to TGF-β1 treatment of A549 cells with and without FHIT expression. RT-qPCR values were normalized to the housekeeping gene GAPDH. Experiments were performed three times and the data are presented as the mean ± s.d. *P<0.05 and **P<0.01 by Student's t-test. A FHIT-activated miRNA, miR-30c, inhibits EMT through suppression of direct targets, Vimentin and Fibronectin
Due to the critical involvement of microRNAs (miRNAs) in cancer metastasis and EMT, we next determined if FHIT might regulate expression of specific miRNAs. To identify miRNAs regulated by FHIT, we used the nanoString nCounter platform for profiling expression of miRNAs in the H1299/FHIT and control cells; miRNAs up-regulated by >1.2 fold change and an expression level higher than 100 code counts were further analyzed (Figure 3A, Table S1). Among the miRNAs active in H1299/FHIT cells, the second most up-regulated miRNA, miR-30c, stood out as an attractive candidate for a role in FHIT-related function (median fold change: 2.279, P<0.0001). To validate the profile data, we performed stem-loop real-time quantitative PCR analysis for miR-30c, using RNAs from H1299/FHIT cells and A549/FHIT. We confirmed that miR-30c expression was higher in both FHIT-expressing cells vs control cells (Figure S5). To explore the in vivo expression pattern of FHIT and miR-30c, total RNAs from human primary lung tumors and adjacent normal tissues (n = 19) were extracted, and RT-qPCR analyses performed. We observed that FHIT (17/19 cases) and miR-30c (19/19 cases) expression levels were positively correlated in adjacent normal tissues and up-regulated as compared to primary lung tissues (Figure 3B, S6). Also, the expression of FHIT and miR-30c was positively correlated in primary lung cancer tumors (n = 20, coefficient = 0.851 and P<0.0001) (Figure 3C). We further evaluated the prognostic value of FHIT and miR-30c in a large public clinical microarray database [23], [24] and found trends towards improved metastasis-free survival on breast cancers in cases of high expression of FHIT and miR-30c (Figure 3D), suggesting that their associated expression may function to oppose cancer progression.
Fig. 3. FHIT expression leads to activation of miR-30c in lung cancer. 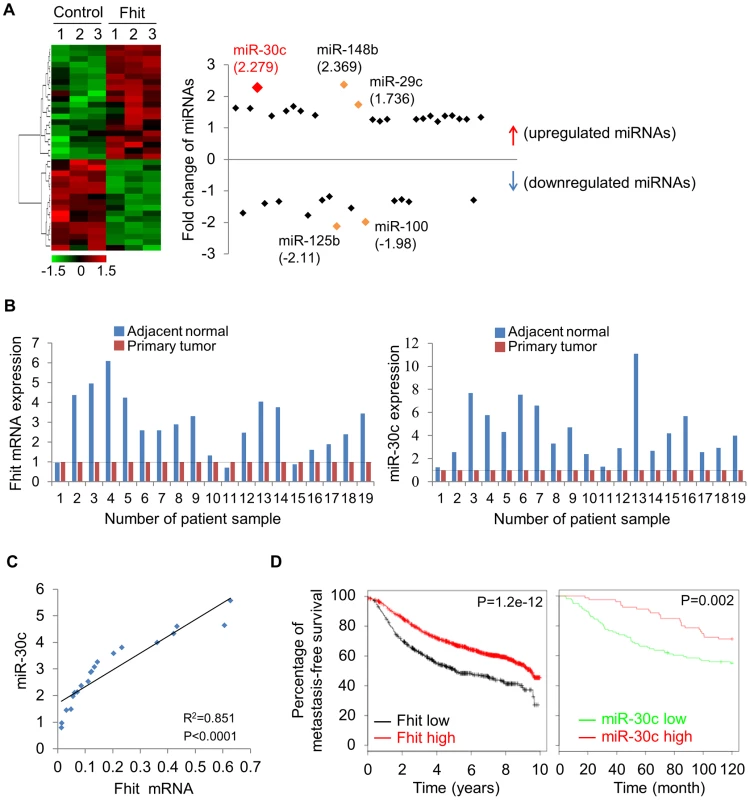
(A) Unsupervised hierarchical clustering of FHIT-regulated miRNAs in H1299/FHIT cells; P<0.05 (B) RT-qPCR analysis showing up-regulation of FHIT and miR-30c in adjacent normal tissues compared to tumor tissues (n = 19). (C) XY scatter plots showing positive correlation between FHIT and miR-30c in primary lung tumors (n = 20). (D) Kaplan-Meier plots of metastasis-free survival of breast cancer patients, stratified by expression of FHIT (2290 patients) or miR-30c (210 patients). Data obtained from the Kaplan-Meier plotter database (kmplot.com/analysis) or microRNA survival analyses in cancer (www.bioprofiling.de). To determine if miR-30c is implicated in EMT, we investigated its role upon expression in A549 cells induced to undergo EMT in response to TGF-β1 treatment. We first examined the morphology of A549 cells cultivated in the presence of TGF-β treatment. As shown in Figure 4A, A549/miR-30c cells exhibited reduction of EMT-like morphological features vs control cells which were converted from a predominant epithelial phenotype to an EMT phenotype within 48 hr in response to TGF-β1 treatment (Figure 4A). Consistently, at the molecular level, miR-30c expression led to decreased levels of Fibronectin and Vimentin and increased E-cadherin levels (Figure 4B, 4C). Furthermore, TGF-β-induced expression of the mesenchymal markers, Fibronectin and Vimentin, was suppressed in the presence of miR-30c, while TGF-β-repressed E-cadherin was markedly activated (Figure 4C), followed by transcriptional activation and suppression of E-cadherin and Vimentin, respectively (Figure 4D). Interestingly, we observed significant reduction of miR-30c levels in TGF-β treated A549 and H1299 cell lines (Figure 4E). Next, we set out to identify putative target genes of miR-30c, which could mediate the inhibition of EMT induced by TGF-β, by using miRNA-target-predicting software PicTar and TargetScan 5.1 or RNA22 program. We found miR-30c binding sites in the 3′UTRs of Vimentin and Fibronectin genes (Figure S7), supporting their candidacy as miR-30c targets. To verify that they are direct targets of miR-30c, their 3'UTRs containing miRNA-responsive elements were cloned into the pGL3 construct downstream of the luciferase ORF. The reporter activity was markedly suppressed by the presence of the 3'UTRs of Vimentin and Fibronectin in A549/Fhit cells compared to the control cells, which reversed when the 3'UTR was mutated, whereas it was increased in A549/Fhit cells with knockdown of miR-30c (Figure 4F). In addition, we found that, in the presence of miR-30c, Vimentin and Fibronectin protein levels decreased in H1299 and A549 cells (Figure S7). In addition, we observed that Vimentin mRNA, but not Fibronectin mRNA, was decreased in miR-30c-transfected lung cancer cells, A549 and H1299 (Figure S7). Interestingly, the expression of Vimentin mRNA was inversely correlated with that of miR-30c in the adjacent normal tissues and matched tumors (Figure S8). Taken together, the results strongly suggest that miR-30c can regulate EMT through down-regulation of the mesenchymal markers, Fibronectin and Vimentin, by directly targeting their 3′UTRs.
Fig. 4. miR-30c inhibits TGF-β-induced EMT through direct targeting of Vimentin and Fibronectin. 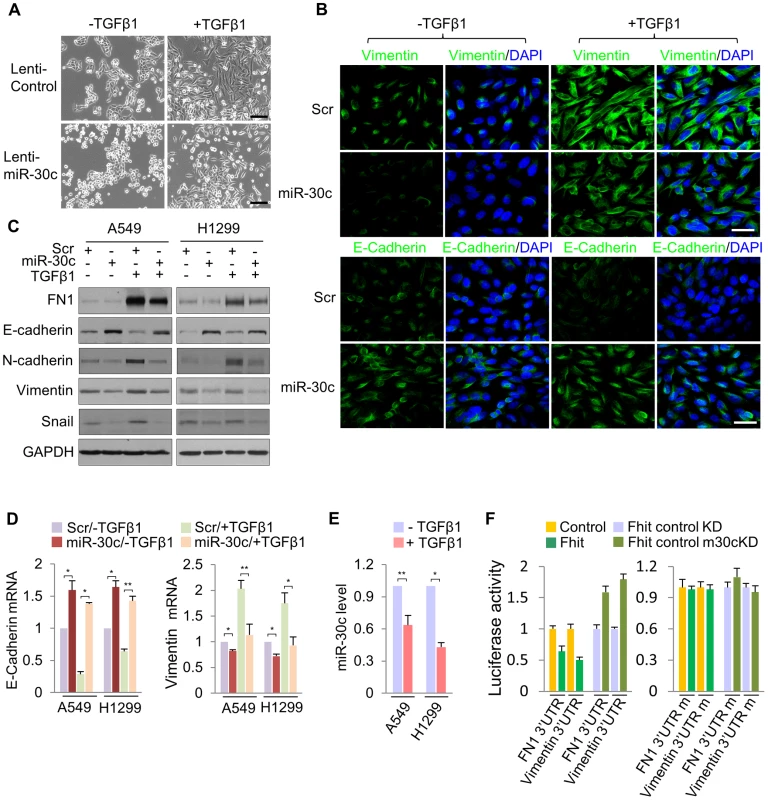
(A) Phase contrast images of A549/miR-30c cells in response to TGF-β1 treatment. Scale bar, 100 µm. (B), (C) Immunofluorescence images and Western blot analysis of EMT markers, E-cadherin, FN1 and Vimentin, in miR-30c-transfected A549 cells treated with TGF-β1, respectively. Scale bar, 20 µm. (D) RT-qPCR analysis showing induction of E-cadherin and a decline in the expression of Vimentin in TGF-β-induced A549/miR-30c cells. *P<0.05 and **P<0.01 by Student's t-test. (E) RT-qPCR for miR-30c in response to TGF-β1 treatment. *P<0.001 by Student's t-test. (F) miR-30c down-regulates mesenchymal markers, Vimentin and Fibronectin by directly targeting their 3′ UTRs. Luciferase reporters assays using wild type or mutant 3′ UTR were performed after transfection into A549/FHIT or A549/FHIT cells with miR-30c knockdown. Experiments were performed three times and the data are presented as the mean ± s.d. miR-30c targets metastasis-related genes, MTDH and HMGA2
Next, we determined if miR-30c targets other genes implicated in metastasis, bridging the connections among miR-30c, metastasis and EMT. Bioinformatics analyses indicated that miR-30c might target several other key mRNAs, including metastasis-associated genes metadherin (MTDH) and High-mobility group AT—hook 2 (HMGA2). We found that there are four binding sites for miR-30c in MTDH 3′UTR and one binding site in HMGA2 (Figure S9). These two genes, MTDH and HMGA2, are known as oncogenes in a variety of human cancers; MTDH protein is over-expressed and promotes metastatic seeding in breast cancer and over-expression of HMGA2 also induces metastasis and invasion of human cancer cells [25], [26]. In addition, the levels of MTDH and HMGA2 mRNAs were highly up-regulated in primary tumors compared to their matched adjacent normal tissues (Figure S10). To confirm that MTDH and HMGA2 are direct targets of miR-30c, luciferase assays were performed. Luciferase activity was suppressed by the wild type 3'UTRs of these genes in A549/Fhit cells compared to the control cells, which was reversed when the 3'UTR was mutated, whereas it was increased in A549/Fhit cells with knockdown of miR-30c (Figure 5A). In immunoblot assays (Figure 5B, 5C), ectopic expression of miR-30c led to significantly decreased levels of MTDH and HMGA2 in H1299 and A549 cells. Furthermore, the expression of MTDH and HMGA2 in FHIT-expressing H1299 cells, in which miR-30c is up-regulated, was severely suppressed and miR-30c silencing resulted in the increase in their expression levels (Figure 5D). However, miR-30c silencing was insufficient to fully rescue this phenotype (Figure 5D), suggesting that additional mechanisms contribute to FHIT regulation of MTDH and HMGA2. In addition, we observed that MTDH mRNA, but not HMGA2 mRNA, was decreased in miR-30c-transfected lung cancer cells, A549, H460 and H1299 (Figure 5E) and showed inverse correlation with miR-30c in lung primary tumors (Figure 5F). Taken together, these data suggested that MTDH and HMGA2 are direct target genes for miR-30c in lung cancer.
Fig. 5. miR-30c down-regulates the expression of MTDH and HMGA2 by directly targeting their 3′UTRs. 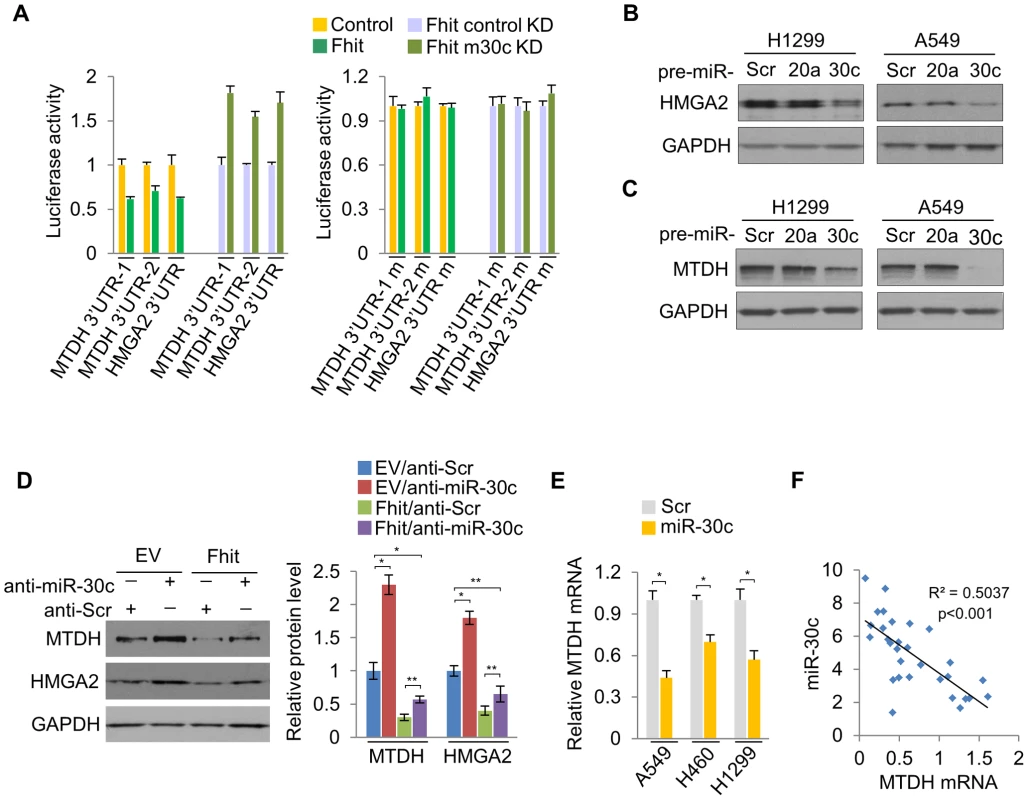
(A) Luciferase activity assays of luciferase reporters with wild type or mutant 3′UTRs of MTDH or HMGA2 were performed after transfection into A549/FHIT or A549/FHIT cells with miR-30c knockdown. (B), (C) Western blot analysis for HMGA2 or MTDH in miR-30c-transfected cells, H1299 and A549. miR-20a was another negative control. (D) MTDH or HMGA2 protein levels in anti-miR-30c-transfected cells stably expressing FHIT. (E) RT-qPCR for MTDH in presence of miR-30c. (F) Correlation between expression levels of miR-30c and MTDH in NSCLC samples (n = 30). Pearson's correlation was used. y, the relative expression level of miR-30c, U6, as an internal control. x, the relative expression level of MTDH, GAPDH, as an internal control. Experiments were performed three times and the data are presented as the mean ± s.d. *P<0.01 and **P<0.001 by Student's t-test. miR-30c suppresses metastasis through the suppression of MTDH and HMGA2
To determine if miR-30c is capable of suppressing metastasis in lung cancer, this miR was stably introduced into A549 cells. These cells were injected into immunodeficient mice and evaluated for metastatic colonization capacity in a tail-vein assay. We found, via BLI monitoring, that the over-expression of miR-30c in parental A549 lung cancer cells potently suppressed pulmonary metastases in the lung and brain (Figure 6A). Histological quantification revealed that there is a significant reduction in the total number of miR-30c expressing metastatic nodules in lungs (Figure 6B). To further clarify the mechanism whereby miR-30c suppresses metastasis, we generated stable knockdown cells for its target genes, MTDH or HMGA2, by using independent short hairpins and confirmed knockdown of protein by immunoblotting (Figure S11). These MTDH and HMGA2 depleted cells were inoculated into nude mice via tail vein. The knockdown of these genes led to significant suppression of metastatic colonization in the lung and bone (Figure 6C). By histological quantification, a significant reduction in the total number of metastatic nodules in knockdown cells was observed vs control cells (Figure 6D). In accord with these results, lung cancer cells, A549, H460 and H1299, with enhanced expression of miR-30c, or MTDH or HMGA2 knockdown exhibited a decrease in cell invasion and migration in vitro (Figure 6E, 6F). Finally, to determine if our experimental findings could be relevant to the pathogenesis of human lung cancer, we examined the expression pattern of FHIT, miR-30c, MTDH and HMGA2 in primary tumor tissues and matched lymph node metastatic tissues. Immunohistochemical staining showed that FHIT expression was decreased in matched lymph node metastatic tissues compared to primary tumor tissue (9 of 40 cases), but MTDH and HMGA2 were significantly activated (11 of 40 and 10/40 cases, respectively) in the matched lymph nodes (Figure 6G). Using RT-qPCR analysis, we found that FHIT mRNA and miR-30c expression levels were inversely correlated with expression levels of MTDH and HMGA2: FHIT and miR-30c levels were more highly expressed in the primary tumors relative to expression in the matched lymph node tissues, while MTDH and HMGA2 mRNAs were increased in matched lymph node tissues (Figure S12). Next, we were interested in identifying the expression patterns of FHIT, miR-30c, MTDH and HMGA2 in lung cancer progression and examined their mRNA levels in normal, non-metastatic and metastatic lung tissues. Interestingly, we found that FHIT and miR-30c gradually decreased with metastasis progression, while MTDH and HMGA2 expression increased (Figure 6H). Overall, these data strongly suggest that FHIT and miR-30c inhibit metastasis through targeting the metastasis-related genes, MTDH and HMGA2.
Fig. 6. miR-30c inhibits metastasis through the suppression of MTDH and HMGA2 in NSCLCs. 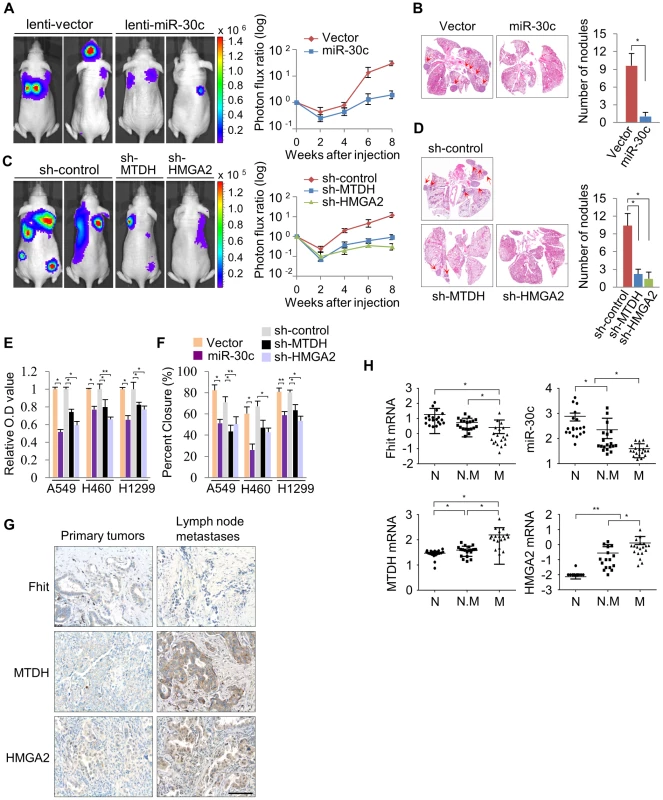
(A) Representative BLI plots of lung metastasis of mice injected (IV) with 1×106 A549 cells stably expressing miR-30c or a control vector; n = 4. (B) Representative H&E stained lung section. The arrows highlight metastatic nodules. *P<0.05 by Student's t-test. (C) Representative BLI plot of metastasis after IV injection with 1×106 A549 cells after knockdown of MTDH, HMGA2, or control; n = 5. (D) Representative H&E stained lung section and the number of lung metastatic nodules. The arrows indicate metastatic nodules. *P<0.001. (E), (F) Invasion and migration assays in control and miR-30c-overexpressing cells, or control, MTDH and HMGA2 knockdown cells, respectively. *P<0.001 **P<0.01 by Student's t-test. (G) Immunohistochemistry assay for FHIT, MTDH and HMGA2 in primary lung tumors and their matched lymph node metastases. Scale bar, 100 µm. (H) The expression pattern of FHIT, miR-30c, MTDH, and HMGA2 in normal (N), non-metastatic (N.M) and metastatic tissues (M). *P<0.001 **P<0.05 by Student's t-test. Discussion
The FHIT gene has been implicated extensively in human tumor progression and plays multiple biological roles in inhibiting tumorigenesis [27], [28]. Although some studies showed the trends of reduced FHIT expression in microinvasive and invasive carcinoma, and a relationship between FHIT expression and tumor invasion, EMT and metastasis [19], [20], [29], [30], our study revealed an unrecognized role for FHIT in modulation of expression of miRNA genes. Using in vivo and in vitro models, we show the ability of FHIT to inhibit metastasis and EMT through the suppression of metastasis-related and EMT-associated genes including MTDH, HMGA2, VIM, FN1, CDH1 and Snail in non-small-cell lung cancer (NSCLC) cells. In particular, FHIT severely suppresses TGF-β-induced EMT, suppressing changes in cellular morphology and levels of EMT-related markers, implying that FHIT serves as a master enforcer of the epithelial cell fate. Here, we identified novel miRNAs modulated by FHIT in NSCLC and delineated the role of one FHIT-regulated miRNA, miR-30c, in regulating EMT and metastasis. Our results show that miR-30c functions as a negative regulator of EMT and metastasis through directly targeting mesenchymal markers, Vimentin and Fibronectin, and metastasis-related genes, MTDH and HMGA2, implying that miR-30c contributes to the FHIT regulation of EMT and metastasis (Figure 7). In fact, this miRNA may function as an anti-metastatic miRNA in several types of human cancer; it has been shown that miR-30 inhibits metastasis in vivo and in vitro in a metastatic breast cancer model [31], [32]. In hepatocellular carcinomas, miR-30c was identified as a metastasis-related miRNA, which is down-regulated in metastatic relative to non-metastatic lesions and is associated with improved survival [33]. Our findings further confirm miR-30c anti-metastatic function via targeting MTDH and HMGA2 and correlation with improved prognosis. In addition, in agreement with previous studies showing that the miR-30 family members inhibit the EMT process and confer epithelial phenotype to cancer cells including pancreatic and hepatocellular carcinomas [34], [35], our data demonstrated that FHIT-activated miR-30c inhibits TGF-β-induced EMT in NSCLC A549 cells through direct targeting of mesenchymal markers, VIM and FN1, and activation of epithelial marker and metastasis suppressor, E-cadherin (Figure 6I). Interestingly, the expression level of miR-30c was significantly down-regulated in TGF-β-treated A549 and H460 cells, suggesting that there is a reciprocal regulatory relationship between TGF-β signaling and miR-30c (Figure 6I). Notably, patients whose primary tumors were positive for FHIT and miR-30c have a significantly improved metastasis-free survival, implying that FHIT and miR-30c might be better used as a predictor of overall metastasis rather than lung - or bone-specific metastasis. However, knockdown of miR-30c in FHIT stable A549 cells could not overcome FHIT-suppressed metastasis in vivo. Furthermore, miR-30c cannot efficiently inhibit TGF-β-induced EMT in respect of morphological and molecular changes as FHIT does. Since this role of miR-30c seems to be necessary but not sufficient for mediating FHIT-dependent suppression of metastasis and EMT suppression, other FHIT-regulated targets must also operate to enable metastasis and EMT suppression. In addition, expression levels of miR-30c were not dramatically induced in FHIT overexpressing cells, being ∼2-fold higher than in the control. These facts suggest that the effect of FHIT on suppression of metastasis is in part dependent on the function of FHIT-activated miR-30c, showing that miR-30c functions as one mediator of FHIT-modulated metastasis, but not the only mediator. Interestingly, FHIT loss has been detected frequently during the early onset of disease progression in cancer [16] and the expression of FHIT and miR-30c is gradually decreased during tumor progression (normal tissues < non-metastatic tumors < metastatic tumors), while expression of miR-30c target genes, MTDH and HMGA2, is increased (Figure 6H). These observations suggest that miR-30c expression is often reduced at early stages of tumor progression when decreased FHIT expression is already apparent and therefore represents one of the driving forces for early stage lung tumor cells to proceed with EMT and subsequent metastatic progression, thus highlighting the relationship between FHIT and miR-30c as potential targets for early therapeutic intervention in lung cancer progression.
Fig. 7. Proposed model for the function of the FHIT and miR-30c in lung metastasis and EMT. 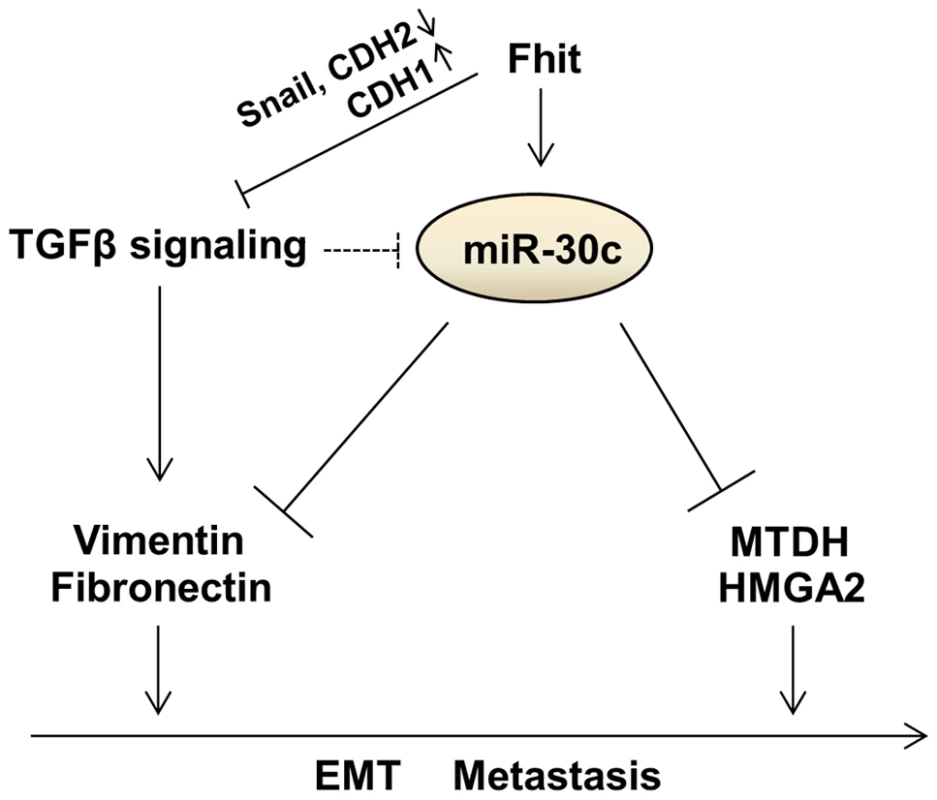
Materials and Methods
Ethics statement
All animals were housed in the Ohio State University animal facility and the experiments with live animals were approved by our institute animal committee (IACUC 2013-A0146) and conducted following the Ohio State University animal policy in accordance with NIH guidelines. Every effort was made to minimize the animals' suffering, anxiety, and discomfort.
Metastasis assays in nude mice
1×106 cells were washed in PBS and injected intravenously into four - to six-week-old age - matched female NOD/SCID mice to study lung metastasis activity as described previously [36]. Noninvasive bioluminescence imaging was performed every two weeks after injection to quantify the metastasis burden using an IVIS 200 Imaging System (Caliper Life Sciences) as described previously [36].
Lung cancer samples and cell lines
192 and 80 lung cancer tissues were purchased from US Biomax, Inc. 40 lung tumor tissue samples were provided from the Department of Pathology, Ohio State University. All human tissues were obtained according to a protocol approved by the Ohio State Institutional Review Board. A549, H460, H1299 cell lines were grown in RPMI containing 10% heat-inactivated FBS and with 2 mM L-glutamine and 100 Uml-1 penicillin–streptomycin.
Invasion assay
Transwell insert chambers with an 8 - µm porous membrane (Bio-Rad) were used for the Invasion assay. Cells were washed three times with PBS and added to the top chamber in serum-free medium. The bottom chamber was filled with medium containing 10% FBS. Cells were incubated for 48 hrs at 37°C in a 5% CO2 humidified incubator. To quantify invaded cells, cells in the top chamber were removed by using a cotton-tipped swab, and the invaded cells were fixed in PBS, 25% glutaraldehyde and stained with crystal violet stain, visualized under a phase-contrast microscope and photographed. Crystal-violet-stained cells were then solubilized in acetic acid and methanol (1∶1), and absorbance was measured at 595 nm.
Scratch assay
Fhit-overexpressing A549 and H1299 or A549, H460 and H1299 stably expressing miR-30c were incubated with medium 5% FBS. Images were acquired directly after scratching (0 h) and after 24 h. Quantization of migration distance using Image J software. The distance covered was calculated by converting pixel to millimeters.
shRNA lentiviral particles transduction
Cells were plated in a 12-well plate 24 hours prior to viral infection and incubated overnight with 1 ml of complete optimal medium (with serum and antibiotics). The day after the medium was removed and 1 ml of complete medium with Polybrene (5 µg/ml) was added. The day after, cells were infected by adding 50 µl of control shRNA, shMTDH, shHMGA2 Lentiviral Particles (Santa Cruz) to the cultures. Stable clones were selected via 1 µg/ml of Puromycin dihydrochloride.
Immunofluorescence
Cells were grown on Lab-Tek II CC2 chamber slides (Nunc), fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100/PBS before blocking with 10% sheep serum (Caltag Laboratories). All the primary antibodies were from Abcam. Secondary antibodies were goat antibodies to mouse or rabbit coupled to Alexa 488 (Invitrogen). Cell nuclei were visualized with DAPI (Sigma). Slides were mounted with SlowFade Gold Antifade reagent (Invitrogen).
Generation of stable clones
A549 cells were stably infected with the Human pre-microRNA Expression Construct Lenti-miR expression plasmid containing the full-length miR-30c and the GFP gene under the control of two different promoters (System Biosciences), or the pGreenPuro shRNA expression lentivector containing the miRZip short hairpin of anti-sense miR-30c (System Biosciences). For lentiviral vectors expressing the Fhit protein, a 707 bp fragment of FHIT cDNA was amplified by reverse transcriptional PCR from human placental cDNA and cloned into a lentiviral shuttle vector (pCDH-CMV-MCS-EF1-RFP) purchased from System Biosciences (CA, USA). An empty vector was used as control. They were packaged with pPACKH1 Lentivector Packaging Plasmid mix (System Biosciences) in a 293TN packaging cell line. Viruses were concentrated using PEGit Virus Precipitation Solution, and titers were analyzed using the UltraRapid Lentiviral Titer Kit (System Biosciences). Infected cells were selected by FACS analysis (FACScalibur; BD Bioscience). Infection efficiency >90% was verified by fluorescent microscopy and confirmed by for miRs expression.
NanoString nCounter assay
The NanoString nCounter Human miRNA expression Assay Kit (http://www.nanostring.com) was used to profile more than 700 human and human-viral miRNAs in U87 cells treated with Nutlin-3a (10 µM) and DMSO. 100 ng of total RNA was used as input for nCounter miRNA sample preparation reactions. All sample preparation was performed according to manufacturer's instructions (NanoString Technologies). Preparation of small RNA samples involves the ligation of a specific DNA tag onto the 3′ end of each mature miRNA. These tags are designed to normalize the Tm's of the miRNAs as well as to provide a unique identification for each miRNA species in the samples. The tagging is accomplished in a multiplexed ligation reaction using reverse-complementary bridge oligonucleotides to direct the ligation of each miRNA to its designated tag. Following the ligation reaction, excess tags and bridges are removed, and the resulting material is hybridized with a panel of miRNA: tag-specific nCounter capture and barcoded reporter probes. Hybridization reactions were performed according to manufacturer's instructions with 5 µl of the 5-fold diluted sample preparation reaction. All hybridization reactions were incubated at 64°C for a minimum of 18 hr. Hybridized probes were purified using the nCounter Prep Station (NanoString Technologies) following the manufacturer's instructions to remove excess capture and reporter probes and to immobilize transcript-specific ternary complexes on a sterptavidin-coated cartridge. Date collection was carried out on the nCounter Digital Analyzer (NanoString Technologies) following the manufacturer's instructions to count individual fluorescent barcodes and quantify target RNA molecules present in each sample. For each assay, a high density (600 fields of view) was performed.
Western blot analysis
Samples were extracted in 15 mM Tris_Cl, pH 7.5/120 mM NaCl/25 mM KCl/2 mM EGTA/0.1 mM DTT/0.5% Triton X-100/10 mg/ml leupeptin/0.5 mM PMSF. Total protein (50 µg) from each sample was separated on a 4–20% Tris-HCl Criterion precast gel Bio-Rad (cat# 345-0032, Hercules, CA) and transferred to a poly (vinylidene difluoride) filter (Millipore). The filter was blocked in 5% nonfat dry milk, incubated with the specific antibody, washed, and probed with secondary antibody IgG conjugated to horseradish peroxidase (Santa Cruz Biotechnology), and developed with enhanced chemiluminescence (Amersham Pharmacia). Immunoblot analyses were performed using the following antibodies: Fibronectin (Cat#F3648, Sigma-Aldrich), E-cadherin (Cat#5409-1, Epitomics, Inc), Vimentin (Cat#2862-1, Epitomics, Inc), Snail (Cat#sc-28199, Santa Cruz Biotechnology), N-cadherin (Cat#610920, BD Biosciences), HMGA2 (Cat#5269, Cell Signaling), MTDH (Cat#AB2989, Millipore) and GAPDH (Cat#2118, Cell Signaling).
RNA extraction and RT-PCR
Total RNA was extracted using TRIzol Reagent Invitrogen (Cat# 15596-018) following the manufacture's instruction. Specifically the pellet obtained from 5×106 cells was lysed 1 ml of TRIzol solution. At the end of the extraction the isolated RNA was dissolved in 35 µl in RNase-free water and incubated for 10 min at 55°C. An aliquot of 5 µg RNA was then used for cDNA synthesis using the SuperScript first strand cDNA synthesis kit (Invitrogen). RT-PCRs were carried out using ABI Prism 7900HT sequence detection systems with Applied Biosystems TaqMan Gene expression assays (miR-30c: 000419; Fibronectin: Hs00365052_m1; E-cadherin: Hs00154405_m1; N-cadherin: Hs00983056_m1; Vimentin: Hs00185584_m1; Snail: Hs00195591_m1: Fhit: Hs00179987_m1; MTDH: 00757841_m1).
Immunohistochemistry
Immunohistochemical staining was performed to detect the expression of Fhit in primary lung tissue or Fhit, MTDH and HMGA2 in primary lung tissue and matched lymph node metastasis tissue. The primary antibody against Fhit was provided by Dr. Kay Huebner (The Ohio State University, 1∶100), MTDH and HMGA2 primary antibody was obtained from Epitomics, Inc and Cell signaling, respectively. Scoring was measured by the percentage of positive cells with the following staining intensities: the signal (+) indicates that at least 10% cancer cells with signal required for positive results are present in tissue.
Luciferase reporter vector
The 3′UTR of the human Vimentin, Fibronectin, MTDH and HMGA2 genes were PCR amplified (Text S1). They were then cloned downstream of the Renilla luciferase stop codon in pGL3 control vector (Promega), giving rise to the p3′UTR-Vimentin, -Fibronectin, -MTDH and –HMGA2 plasmids. These constructs were used to generate, by inverse PCR, the p3′UTRmut-Vimentin, Fibronectin, MTDH and –HMGA2 plasmid (Text S1). Fhitrexpressing A549 cells or miR-30c knockdown Fhit-expressing A540 cells were cotransfected with 1 µg of each construct and 0.1 µg of a Renilla luciferase expression construct, pRL-TK (Promega), using Lipofetamine 2000 (Invitrogen). Cells were harvested 24 hr after transfection and assayed with Dual Luciferase Assay (Promega) according to the manufacturer's instructions. Three independent experiments were performed in triplicate.
Statistical analysis
Student's t test and one-way analysis of variance was used to determine significance. All error bars represent the standard error of the mean. Statistical significance for all the tests, assessed by calculating p value, was <0.05. Spearman correlation coefficient was calculated to test the association between miR-30c and Fhit mRNA in lung samples (n = 19). Kruskal-Wallis was used to assess whether the miR-30c and Fhit are differentially expressed among normal lung and primary samples on the basis of the Bartlett test P value. The in vivo metastasis-free survival of miR-30 and Fhit was assessed by plotting survival curves according to the Kaplan–Meier method, and groups were compared using the log-rank test.
Supporting Information
Zdroje
1. ChambersAF, GroomAC, MacDonaldCM (2002) Dissemination and growth of cancer cells in metastatic sites. Nature Rev Cancer 2 : 563–572.
2. FolkmanJ (2002) The role of angiogenesis in tumor growth and metastasis. Semin Oncol 29 : 15–18.
3. PolyakK, WeinbergRA (2009) Transition between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature Rev Cancer 9 : 265–273.
4. ManiSA, GuoW, LiaoMJ, EatonEN, AyyananA, et al. (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 16 : 704–715.
5. KalluriR (2009) EMT: When epithelial cells decide to become mesenchymal-like cells. J Clin Invest 119 : 1417–1419.
6. ZavadilJ, BottingerEP (2005) TGF-beta and epithelial-to mesenchymal transitions. Oncogene 29 : 5764–5774.
7. GregoryPA, BertAG, PatersonEL, BarrySC, TsykinA, et al. (2008) The miR-200 family and miR-205 regulate epithelial to meshenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10 : 593–601.
8. SiemensH, JackstadtR, HuntenS, KallerM, MenssenA, et al. (2010) miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle 10 : 4256–4271.
9. EdmondsMD, HurstDR, VaidyaKS, StaffordLJ, ChenD, et al. (2009) Breast cancer metastasis suppressor 1 coordinately regulates metastasis-associated microRNA expression. Int J Cancer 125 : 1778–1785.
10. ZhangJG, WangJJ, ZhaoF, LiuQ, JiangK, et al. (2010) MicroRNA-21(miR-21) represses tumor suppressor PTEN and promote growth and invasion in non-small cell lung cancer (NSCLC). Clin Chim Acta 411 : 846–852.
11. SampsonVB, RongNH, HanJ, YangQ, ArisV, et al. (2007) MicroRNA Let-7a down-regulates MYC and reverts MYC-induced growth in burkitt lymphoma cells. Cancer Res 67 : 9762–9770.
12. LeeYS, DuttaA (2007) The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev 21 : 1025–1030.
13. LaganaA, RussoF, SismeiroC, GiuqnoR, PuvirentiA, et al. (2010) Variability in the incidence of miRNAs and genes in fragile sites and the role of repeats and CpG islands in the distribution of genetic material. PLos One 5: e11166.
14. StarczynowskiDT, MorinR, McPhersonA, LamJ, ChariR, et al. (2011) Genome-wide identification of human microRNAs located in leukemia-associated genomic alterations. Blood 117 : 595–607.
15. OhtaM, InoueH, CotticelliMG, KasturyK, BaffaR, et al. (1996) The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t (3; 8) breakpoint, is abnormal in digestive tract cancers. Cell 84 : 587–597.
16. SozziG, VeroneseML, NegriniM, BaffaR, CotticelliMG, et al. (1996) The FHIT gene 3p14.2 is abnormal in lung cancer. Cell 85 : 17–26.
17. NegriniM, MonacoC, VorechovskyI, OhtaM, DruckT, et al. (1996) The FHIT gene at 3p14.2 is abnormal in breast carcinoma. Cancer Res 56 : 3173–3179.
18. SongX, TianZ, WangS, PengZ, FengJ (2010) Restoration of fragile histidine triad (FHIT) expression inhibits cell growth and induces apoptosis in cutaneous T-cell lymphoma cell line. Cancer Invest 28 : 1019–1023.
19. JoannesA, BonnometA, BindelsS, PoletteM, GillesC, et al. (2010) Fhit regulates invasion of lung tumor cells. Oncogene 29 : 1203–1213.
20. JoannesA, GreletS, DucaL, GillesC, KileztkyC, et al. (2014) Fhit regulates EMT targets through an EGFR/Src/ERK/Slug signaling axis in human bronchial cells. Mol Cancer Res 12 : 775–783.
21. XuJ, LamouilleS, DerynckR (2009) TGF-β-induced epithelial to mesenchymal transition. Cell Res 19 : 156–172.
22. KatsunoY, LamouillesS, DerynckR (2013) TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol 25 : 76–84.
23. Antonov AV, Knight RA, Melino G, Barlev NA, Tsvetkov PO (2012). MIRUMIR: an online tool to test microRNAs as biomarkers to predict survival in cancer using multiple clinical data sets. Cell Death Differ. Doi:10.1038/cdd.2012.137.
24. GyorffyB, LanczkyA, EklundAC, DenkertC, BudcziesJ, et al. (2010) An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1.809 patients. Breast Cancer Res Treatment 123 : 725–731.
25. HuG, ChongRA, YangQ, WeiY, BlancoMA, et al. (2009) MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell 15 : 9–20.
26. HristovAC, CopeL, ReyesMD, SinghM, Lacobuzio-DonahueC, et al. (2009) HMGA2 protein expression correlates with lymph node metastasis and increased tumor grade in pancreatic ductal adenocarcinoma. Mod Pathol 22 : 43–49.
27. DumonKR, IshiiH, FongLY, ZanesiN, FidanzaV, et al. (2001) FHIT gene therapy prevents tumor development in Fhit-deficient mice. Proc Natl Acad Sci USA 98 : 3346–3351.
28. ToledoG, SolaJJ, LozanoMD, SoriaE, PardoJ (2004) Loss of FHIT protein expression is related to high proliferation, low apoptosis and worse prognosis in non-small - cell lung cancer. Mod Pathol 17 : 440–448.
29. BekarA, CecenerG, TuncaB, GulerG, EgeliU, et al. (2007) Investigation of mutations and expression of the FHIT gene in Turkish patients with brain metastases derived from non-small cell lung cancer. Tumori 93 : 604–607.
30. ZhaoP, LiXY, ChenLZ (2002) Loss of fragile histidine triad expression and metastasis in breast cancer. Ai Zheng 21 : 668–670.
31. YuF, DengH, YaoH, LiuQ, SuF, et al. (2010) Mir-30 reduction maintains self-renewal and inhibits apoptosis in breast tumor-initiating cells. Oncogene 29 : 4194–4204.
32. Bochhorn J, Dalton R, Nwachukwu C, Huang S, Prat A, et al.. (2013). MicroRNA-30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL-11. Nat Commun.4, Article number 1393.
33. BudhuA, JiaHL, ForquesM, LiuCG, GoldsteinD, et al. (2008) Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology 47 : 897–907.
34. JoqlekarKV, PatilD, JoqlekarVM, RaoGV, ReddyDN, et al. (2009) The miR-30 family microRNAs confer epithelial phenotype to human pancreatic cells. Islets 1 : 137–147.
35. ZhangJ, ZhangH, LiuJ, TuX, ZhangY, et al. (2012) miR-30 inhibits TGF-β1-induced epithelial-to-mesenchymal transition in hepatocyte by targeting Snail1. Biochem Biophys Res Commun 417 : 1100–1105.
36. MinnAJ, GuptaGP, SiegelPM, BosPD, ShuW, et al. (2005) Genes that mediate breast cancer metastasis to lung. Nature 436 : 518–524.
Štítky
Genetika Reprodukčná medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 10- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



