-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
The cellular chaperone machinery helps proteins adopt and maintain native conformations and protects cells from stress. The yeast Hsp40s Ydj1 and Sis1 are co-chaperones that regulate Hsp70s, which are key components of many chaperone complexes. Both of these Hsp40s are crucial for growth and Ydj1 directs disaggregation activity of the Hsp100-based machinery to provide stress protection while Sis1 directs this activity to promote prion replication. Ydj1 also cures yeast of certain prions when overexpressed. We show that C-terminal domains that possess substrate-binding function of Ydj1 and Sis1 can mediate these and other functional distinctions and that the degree that prions depend on Sis1 activities could underlie differences in how they respond to alterations of chaperones. These findings support a view that Hsp40s regulate and specify functions of the chaperone machinery through substrate discrimination and cooperation with Hsp70. The disproportionate evolutionary expansion of Hsp40s (J-proteins) relative to their Hsp70 partners led to a proposal that this amplification allows increased regulation and fine-tuning of chaperone machines for increasingly complex processes. Our findings support this idea and provide insight into fundamental aspects of this cooperation.
Published in the journal: Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines. PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004720
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004720Summary
The cellular chaperone machinery helps proteins adopt and maintain native conformations and protects cells from stress. The yeast Hsp40s Ydj1 and Sis1 are co-chaperones that regulate Hsp70s, which are key components of many chaperone complexes. Both of these Hsp40s are crucial for growth and Ydj1 directs disaggregation activity of the Hsp100-based machinery to provide stress protection while Sis1 directs this activity to promote prion replication. Ydj1 also cures yeast of certain prions when overexpressed. We show that C-terminal domains that possess substrate-binding function of Ydj1 and Sis1 can mediate these and other functional distinctions and that the degree that prions depend on Sis1 activities could underlie differences in how they respond to alterations of chaperones. These findings support a view that Hsp40s regulate and specify functions of the chaperone machinery through substrate discrimination and cooperation with Hsp70. The disproportionate evolutionary expansion of Hsp40s (J-proteins) relative to their Hsp70 partners led to a proposal that this amplification allows increased regulation and fine-tuning of chaperone machines for increasingly complex processes. Our findings support this idea and provide insight into fundamental aspects of this cooperation.
Introduction
The protein disaggregation machinery of microorganisms and plants is driven by an Hsp100-family chaperone that cooperates with Hsp70 and its nucleotide exchange factor (NEF) and J - protein (Hsp40) co-chaperones [1]. This machinery promotes cell survival after environmental stresses that cause proteins to aggregate by extracting proteins individually from aggregates [2]–[4]. Organisms encode multiple Hsp70s, Hsp40s and NEFs and there is much to learn about how these chaperones cooperate and regulate each other's activity to effect protein remodeling and reactivation, and how different combinations of chaperones and co-chaperones determine efficiency and specificity of the machinery.
In yeast, this Hsp104-based resolubilization process also targets prions, which are cellular proteins that propagate as highly structured fibrous protein aggregates called amyloid [5]–[7]. The widely studied prions [URE3], [PSI+] and [PIN+] (also known as [RNQ1+]) are composed of the proteins Ure2, Sup35, and Rnq1, respectively [8]–[10]. Propagation of these and other amyloid-based yeast prions requires proper functioning of the disaggregation machinery [5], [11]–[15], which promotes prion replication by fragmenting fibers into more numerous pieces, or seeds, that continue to propagate the prion state [4], [16], [17].
Hsp70s act in various cellular chaperone machines by binding and releasing hydrophobic surfaces on partially folded proteins. This activity is necessary for essential cellular processes where proteins are partially folded, such as transport across membranes, and for preventing protein aggregation under conditions of stress [18], [19]. Effective interactions of Hsp70 with substrates rely on its regulation by J-proteins and NEFs (reviewed in [20]). The major yeast cytosolic Hsp40s Sis1 and Ydj1 are structurally related J-proteins that function as dimers [21]. Both have an amino-terminal J domain that mediates physical interaction with Hsp70s and an adjacent glycine-phenylalanine (GF) rich region that confers some functional distinction [22], [23]. Both also have carboxy-terminal regions that bind substrates with a specificity that overlaps Hsp70 [24]–[26]. The class I J-protein Ydj1 has a zinc-finger element within its C-terminal region and a CAAX motif at its extreme C-terminus that directs its farnesylation. This modification localizes much of Ydj1 to membranes and influences cooperation of Ydj1 with Hsp90, another abundant and highly conserved protein chaperone [27], [28]. The class II J-protein Sis1 lacks these elements, but has a glycine-methionine (GM) rich extension of its GF domain.
Altering function or abundance of Sis1 or Ydj1 inhibits propagation of prions, but in different ways. By an undefined mechanism, overexpressing Ydj1 causes cells to lose [URE3] and some variants of [PIN+], but not [PSI+] [12], [29]. Increasing expression of Sis1 does not destabilize these prions [30], [31]. Depleting Sis1 causes [URE3] and [PIN+] to be lost rapidly as cells divide, but causes [PSI+] to be lost gradually and only after a long delay [14]. Additionally, all non-essential functions of Sis1 are dispensable for [PSI+] propagation, but deleting only the GF region of Sis1 causes cells to lose [PIN+] [30], [32]. Thus, the way these Hsp40s influence prion propagation goes beyond their general roles of regulating Hsp70. Additionally, when cooperating with E. coli disaggregation machinery in yeast, Sis1 is specifically required for prion propagation and Ydj1 for protecting cells from exposure to lethal heat (thermotolerance) [15]. Both Hsp40s are critical for survival and while no other J-protein can compensate for loss of Sis1, Sis1 and other J-proteins, as well as J-domains alone, can improve growth of cells lacking Ydj1 [14], [27], [33], [34]. What defines these functional differences of Sis1 and Ydj1 is uncertain.
Here, we used Sis1-Ydj1 hybrid proteins to identify structural elements that determine the distinct functions of Sis1 and Ydj1 in various cellular processes and systematically assessed the importance of Sis1 activities for propagation of [URE3] and [PIN+]. We found that the C-terminal regions of Sis1 and Ydj1 possessed functional distinctions that directed the action of the Hsp104 machinery in prion propagation and thermotolerance, and the Hsp90 machinery in galactose-induced transcription. We also found that [URE3] was highly sensitive to alterations of Sis1 and that [PIN+] was less dependent on Sis1 than [URE3], but more dependent than [PSI+]. Our results support the idea that differences in ways prions respond to various chaperone alterations can be due to differences in their dependence on the disaggregation machinery.
Results
The CTD of Sis1 specifies cooperation with E. coli chaperones to propagate prions
Earlier we showed that the E. coli disaggregation machinery (ClpB, DnaK and GrpE, which are analogous to yeast Hsp104, Hsp70, and NEF, respectively) function in yeast by cooperating with yeast Hsp40s [15]. ClpB, DnaK and GrpE are herein abbreviated B, K and E, respectively. We modified this system to use a DnaK mutant (R167H, designated K*) that can interact only with J-proteins that have the compensatory D36N J-domain mutation (designated Sis1* and Ydj1*). BK*E cannot cooperate with wild type J-proteins, so we can monitor interactions of the BK*E machinery specifically with Sis1* or Ydj1* even in the presence of their wild type counterparts. Using this system we showed BK*E cooperates specifically with Sis1* to propagate [PSI+] prions and with Ydj1* to protect cells from exposure to lethal heat (thermotolerance). The ability of Sis1 and Ydj1 to direct activities of the disaggregation machinery could be due to differences in the ways they recruit or regulate Hsp70 components of the machinery or interact with different types of substrates. To determine the basis of these and other functional differences we used Sis1-Ydj1 hybrid proteins.
In earlier work using hybrids of Ydj1 and Sis1 each of the CTDs was divided into two parts (CTDI and CTDII) and the adjacent GM region of Sis1 was included on the same fragment containing the amino-terminal portion of the Sis1 CTD [35], [36]. Exchanging this fragment splits the contiguous functionally redundant GF-GM regions of Sis1 [22], which complicates interpretations of swapping GF domains. In order to simplify comparisons we exchanged only the three most conserved J, GF(GM), and CTD regions to form six hybrid proteins (see Figure 1A, Materials and methods). Hybrids are named according to their structural components. For example, YYS and SSY have their CTDs swapped. All of our J-protein hybrids for the BK*E experiments contain the D36N mutation, which is indicated in their names by an asterisk (e.g. Y*YS is the D36N mutant of YYS).
Fig. 1. Sis1/Ydj1 hybrid proteins cooperate with E. coli chaperones in distinct processes. 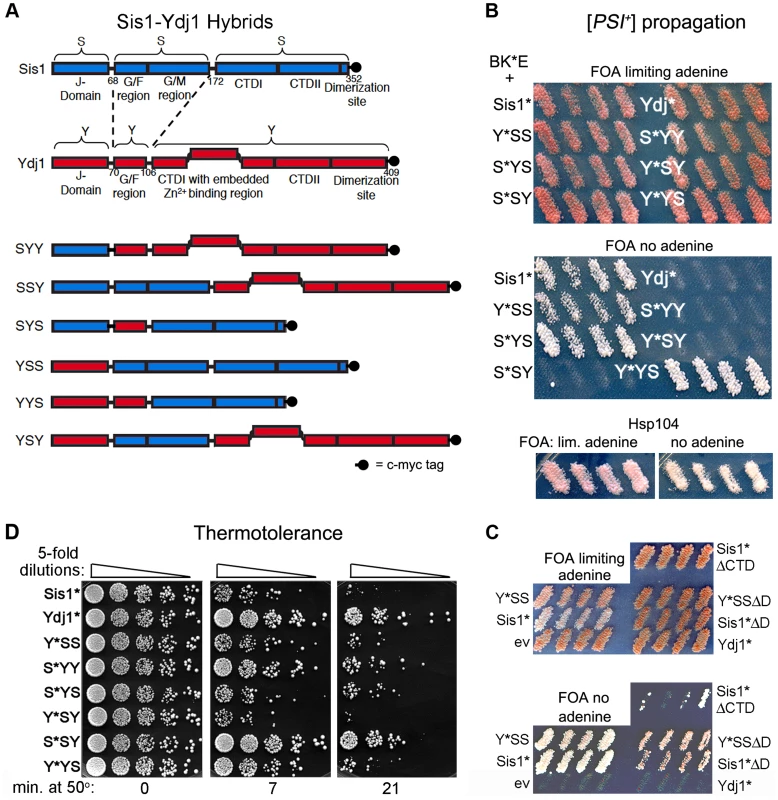
(A) Diagram of conserved Hsp40 domains swapped in the hybrid proteins (not to scale). Numbers indicate amino acid positions, dashed lines indicate splice joints. The regions from residues 172–352 of Sis1 and from 106–409 of Ydj1 are referred to inclusively as CTD. All alleles contain C-terminal c-myc tags (indicated as filled black circles). (B) [PSI+] propagation: [PSI+] hsp104Δ strain MR386 expressing Hsp104 on a URA3 plasmid and the E. coli chaperones ClpB, DnaK* and GrpE (BK*E) was transformed by single-copy TRP1 plasmids expressing D36N versions (*) of indicated wild type and hybrid proteins. For each hybrid, four independent transformants were grown as patches on plates containing uracil to allow loss of the plasmid encoding Hsp104. These were replica-plated onto medium containing FOA with (upper panel) or lacking (lower panel) adenine. Only [PSI+] cells grow on medium lacking adenine. As reported earlier [15], the Sis1* and Ydj1* cells show representative [PSI+] and [psi−] phenotypes, respectively, with ClpB in place of Hsp104. Control cells with Hsp104 on the TRP1 plasmid are shown below. (C) Transformants expressing Sis1* lacking its CTD (Sis1*ΔCTD) and versions of Sis1*and Y*SS lacking the dimerization domain (ΔD) were processed as in panel (B). (D) Thermotolerance: Transformants from FOA plates in upper image of panel (B) were cured of prions by growth on guanidine-containing medium and then grown in liquid medium selecting for the plasmids. Cells were diluted to similar density, heat shocked at 50°C for the times indicated at the bottom, and five microliters of five-fold serial dilutions were dropped onto YPAD plates and incubated for three days at 30°C. To test for ability of these modified Sis1-Ydj1 hybrid proteins to cooperate with the E. coli chaperones to propagate [PSI+], they were first expressed in [PSI+] cells that have ClpB in place of chromosomal Hsp104 and express K*, E and Hsp104 from plasmids. They were then assessed for ability to continue propagating [PSI+] after the cells lose the plasmid encoding Hsp104. The results are presented in Figure 1B. The upper panel shows cells on medium that allows growth of all strains and the lower panel shows medium that allows growth only of cells propagating [PSI+]. The lack of distinction among strains in the upper panel indicates that in the absence of selection [PSI+] propagates too weakly to confer an obvious phenotype, as reported earlier [15]. Nevertheless, the strong confluent growth of cells transferred onto medium selecting for [PSI+] indicates the prions in these cells are mitotically stable. The presence of [PSI+] was confirmed by its dominant phenotype in crosses and by guanidine curability (see Figure S1A). Wild type Sis1 and all hybrids containing the CTD of Sis1 propagated [PSI+]. In contrast, Ydj1 and all proteins with the CTD of Ydj1 were unable to propagate [PSI+]. Thus, the Sis1-specific function that is necessary for these full-length Hsp40s to cooperate with the bacterial ClpB disaggregation machinery to propagate [PSI+] prions resides in the Sis1 CTD.
These results were somewhat unexpected because Sis1 lacking its CTD (Sis1ΔCTD) propagates [PSI+] in cells expressing Hsp104 [32]. We therefore tested if BK*E could cooperate with the truncated Sis1*ΔCTD to propagate [PSI+]. We found it did, but [PSI+] was very unstable and was lost rapidly when selection for the prion was not maintained (Figure 1C). These results indicate that Hsp40 requirements of BK*E for [PSI+] propagation differ when full-length and truncated Hsp40s are used. Inconsistencies between truncated and full-length Hsp40s have been seen before (see below) [22], [30], [33].
Because the dimerization domain is at the C-terminus, it was also possible that Y*YS was able to propagate [PSI+] only by combining with the wild type Sis1 present in the cells to form heterodimers that functioned with BK*E. Since Sis1 lacking its dimerization domain (Sis1ΔD) propagates [PSI+] in cells with Hsp104 [32], we tested if the D36N version of Sis1ΔD was able to support [PSI+] propagation with BK*E. At the same time we tested a version of Y*YS lacking its dimerization domain. Both of these proteins were able to cooperate with BK*E to propagate [PSI+], but with noticeably reduced efficiency compared with their full-length counterparts (Figure 1C, note slower growth and pink color on medium selecting for [PSI+]). Thus, Y*YS did not need to dimerize with wild type Sis1 to support propagation of [PSI+].
The CTD of Ydj1 allows cooperation with E. coli chaperones to provide thermotolerance
Using our hybrids to assess Ydj1 function in thermotolerance we found that, except for Y*SY, proteins containing the CTD of Ydj1 worked better at restoring thermotolerance to hsp104Δ cells expressing BK*E (Figure 1D). Overall, the range of survival conferred by the different hybrids was somewhat broad, which suggests that complex interactions among different parts of the proteins can influence Hsp40 functions in this process. Notably, however, S*SY, in which only the CTD is from Ydj1, functioned most like Ydj1 in this assay, while Y*YS performed only slightly better than Sis1*. Thus, as with prion propagation, a determinant of functional specificity between Sis1 and Ydj1 that directs BK*E activity in thermotolerance resides in the CTD.
The CTD of Sis1 confers Sis1-specific functions in yeast
Although Sis1 is essential for viability, its J, GF and CTD regions are each dispensable for growth of sis1Δ cells [22], [30]. However, other work shows that a full-length Sis1-Ydj1 hybrid that contains the substrate-binding region of Sis1, but not Ydj1, supports growth of sis1Δ cells [35]. These earlier findings show inconsistencies in the way that Sis1 and Ydj1 sub-regions determine specific Hsp40 functions. The variable dependency of [PSI+] on the CTD of Sis1 in our full-length versus truncated [32] proteins is another example of such differences. An obvious distinction in these experiments is whether the structural regions are deleted or swapped. While deleting regions allows identifying redundant functions, swapping domains of full-length proteins also allows identifying functions that can be influenced by inter-domain interactions, or if the domains specify interactions with other factors or localization of the J-proteins in the cell.
In line with the earlier findings using full-length hybrid proteins, our results with the E. coli chaperones show the CTDs can impart specific functionality to these Hsp40s. To assess which regions are involved in determining specificity of cooperation with the yeast chaperone machinery we tested ability of our hybrids to function in place of Sis1 or Ydj1, which allows investigation of Hsp40 functions other than those needed for prion propagation and thermotolerance.
We compared the relative abundance of the hybrid proteins using western blot analysis (Figure S2). Sis1 and hybrid proteins containing the CTD of Sis1 were less abundant than the others, so we cannot rule out that differences in abundance were contributing to effects in some assays. In addressing this issue by increasing expression using the stronger GPD promoter on both single and high-copy plasmids, we found that hybrid proteins containing the CTD of Sis1 caused growth inhibition of ydj1Δ cells in a dose-dependent manner (see Figure S3). Therefore, in our complementation assays we expressed proteins regulated by the SIS1 promoter on single-copy plasmids. Despite differences in protein levels, in several experiments the less abundant proteins (i.e. those with CTD of Sis1) functioned better than the others (see results above and below). Therefore, differences in ability of the hybrids to complement functions cannot be explained solely by differences in protein levels.
To determine if individual Sis1 sub-regions within full-length proteins are enough to support growth of sis1Δ cells, both [PSI+] and [psi−] versions of strain 930 (sis1Δ carrying wild type SIS1 on a URA3 plasmid) were transformed with plasmids encoding the hybrid proteins and then grown as patches on medium that allows cells to lose the URA3 plasmid encoding wild type Sis1. These were then replica-plated onto medium containing FOA to select for cells having lost that plasmid. Regardless of prion status, all hybrids that contained the Sis1 CTD, and only these hybrids, supported growth (Figure 2A, left panels). Therefore, in the context of our full-length hybrids, the CTD of Sis1 was necessary and sufficient to provide essential Sis1 activity. These same hybrids supported propagation of [PSI+], which is consistent with our earlier findings that propagation of [PSI+] is minimally dependent on Sis1 and that any Sis1 mutant that supports growth also supports [PSI+] [32].
Fig. 2. Functions of Sis1/Ydj1 hybrids in place of Sis1. 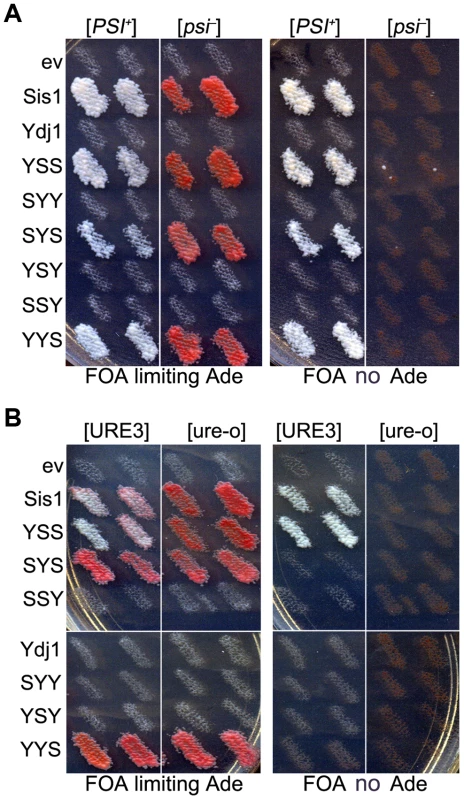
(A) Growth and [PSI+] propagation: [PSI+] and [psi−] versions of strain 930 (sis1Δ) were transformed by plasmids encoding Sis/Ydj1 hybrid proteins. Transformants were grown as patches on medium containing uracil and then replica-plated onto FOA with (left panels) and without (right panels) adenine. Each patch is an individual transformant, similar results were obtained in two other transformations. (B) [URE3] propagation: [URE3] and [ure-o] versions of strain 1385 (sis1Δ) were transformed by the same plasmids and processed as in (A). Cells with prions are Ade+ and white while cells lacking prions require exogenous adenine and are red when grown on limiting adenine (see Materials and methods). In all panels each patch is from an independent transformant colony from one of three independent transformations. All of 3 replicated experiments gave the same results. As expected, these same hybrids supported growth of isogenic sis1Δ strain 1385 (used to monitor [URE3]). However, only those containing both the CTD and GF regions of Sis1 (i.e. Sis1 and YSS) supported propagation of [URE3] (Figure 2B). Thus, propagation of [URE3] depended on the GF/GM and CTD regions of Sis1, but not on the Sis1 J-domain. The Ydj1 GF region did not function in place of Sis1 GF/GM (i.e. in SYS) to support prion propagation. These latter observations are reminiscent of earlier work showing that a short stretch of the GF region in Sis1, which is absent in Ydj1, is important for propagation of [PIN+] [23], and they suggest the same function is important for propagation of [URE3].
The CTD of Ydj1 supports Ydj1 functions
Aside from its role in protecting cells from exposure to lethal heat, Ydj1 is important for cell growth under all conditions [27]. Cells lacking Ydj1 are viable, but they grow very slowly at 25°C and do not grow at 34°C. Elevating expression of Sis1 and other J-proteins, or even J-domains alone, can improve growth of ydj1Δ cells [14], [34], which suggests that the functions of Ydj1 in its roles important for viability are more general. Nevertheless, we tested if distinct domains of Sis1 and Ydj1 conferred Ydj1-specific functions important for growth by repeating the plasmid shuffle using ydj1Δ strain MR502, which has YDJ1 on a URA3 plasmid.
We found that all hybrid proteins containing the CTD of Ydj1 restored growth noticeably, even at 34°C (Figure 3A). The substantial ability of YSY and SSY to restore growth indicates that the Ydj1 GF region is effectively dispensable for its functions in cell growth and its J-domain has a small contribution. Compared with the empty vector, Sis1 also improved growth weakly at 30°C, which is in line with earlier data showing increased expression of Sis1 compensates for loss of Ydj1 [27], but it did not support growth at 34°C (Figure 3A). Cells with YSS grew slightly better than those with the empty vector at 25°C and 30°C, but the other hybrids containing the Sis1 CTD failed to improve growth, even at 25°C. These results suggest that in the context of our full-length proteins the CTD of Ydj1 possesses Ydj1-specific functions important for growth.
Fig. 3. Growth complementation by Sis1/Ydj1 hybrids in place of Ydj1. 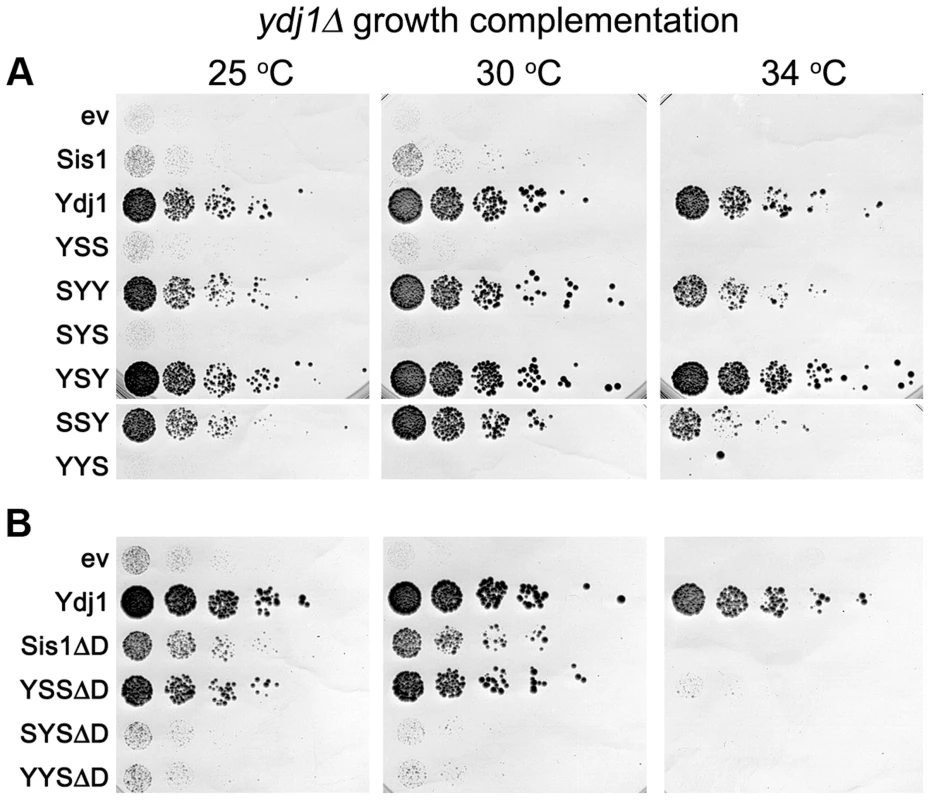
(A) Transformants of strain MR502 (ydj1Δ with YDJ1 on a URA3 plasmid) expressing the indicated hybrid proteins from the SIS1 promoter on single-copy plasmids (ev is empty vector) were taken from FOA plates and grown in liquid medium selecting for the plasmids. Cultures were normalized to the same cell density, serially diluted five-fold and dropped onto YPAD plates, which were incubated at the indicated temperatures for three days. (B) Cells expressing Sis1 or the hybrids with the Sis1 CTD that lack their dimerization domains (ΔD) were processed and grown as in (A). As indicated above, increasing expression of proteins with the CTD of Sis1 inhibited growth of ydj1Δ cells in a dose-dependent manner (Figure S3). One explanation for this effect is that hybrids with the Sis1 CTD were able to form defective heterodimers with wild type Sis1 and a resulting impairment of Sis1 function would exacerbate the growth defect caused by lack of Ydj1. To test this possibility, we repeated the experiments using hybrids lacking the dimerization domain (ΔD). Even though the abundance of these truncated proteins was at the low level of their counterparts (Figure S2C), YSSΔD improved growth considerably at 30°C and allowed weak growth at 34°C (Figure 3B). SYSΔD and YYSΔD also supported growth at 30°C, but only slightly. These results are consistent with the growth inhibition being caused by dominant inactivation of wild type Sis1 and suggest that lack of complementation was not necessarily due to lower protein abundance.
Deleting the dimerization domain of wild type Sis1 also improved its ability to suppress the ydj1Δ defect, although not enough to support growth at 34°C. Thus, monomeric Sis1 is better at performing Ydj1-specific functions than Sis1 dimers. Together these results agree with earlier work showing a general ability of truncated J-proteins to complement Ydj1 function better than intact proteins [34]. They also suggest dimerization might specify or restrict Hsp40 activities.
Ydj1 is also a critical component of the Hsp90 molecular chaperone system necessary for activating galactose-inducible gene promoters [37]. This machinery is thought to remove nucleosomes from the promoter region to allow access to transcription factors. Deleting YDJ1 abolishes galactose induction by disrupting this process. When assessed for ability to function in galactose induction (Figure 4), all of the hybrids that had the CTD of Ydj1, and only these hybrids, restored GAL expression. Thus, the CTD of Ydj1 determined specificity of Ydj1 in a process that involves its cooperation with the Hsp90 machinery.
Fig. 4. Function of Sis1/Ydj1 hybrids in galactose induction. 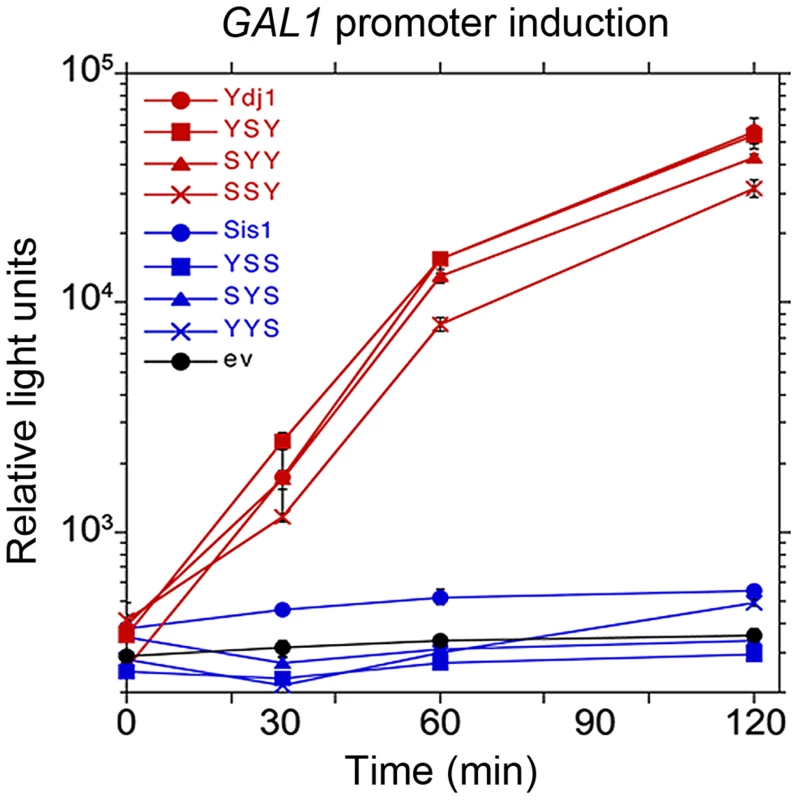
Cells expressing indicated hybrid proteins (from Figure 3, panel A) were assessed for ability to induce expression of a luciferase reporter regulated by the GAL10 promoter. After adjusting cultures to the same cell density, galactose was added and the luciferase activity in aliquots of cells was then measured periodically for two hours. Cells did not grow during this time. Hsp40 CTDs specify cooperation of purified chaperones
We next tested whether the ability of the Hsp40s to exhibit functional discrimination in vivo was reflected in discrimination in vitro. To do this we monitored protein reactivation of two different substrates. When heat-inactivated GFP-38, a GFP fusion protein containing a C-terminal 38 amino acid peptide, was used as the substrate, Sis1 in combination with Hsp104 and Ssa1 restored about 30% of the GFP-38 after an hour (Figure 5A). With Ydj1 in place of Sis1 in the reaction, there was little reactivation (<2%). The hybrid protein containing the CTD of Sis1, YYS, was able to reactivate GFP-38 with Hsp104 and Ssa1, but the rate of reactivation was ∼50% that of Sis1 (Figure 5A and B). There was no detectable reactivation of GFP-38 by SSY under the same conditions. These results show that reactivation of GFP-38 by Hsp104 and Ssa1 requires a Sis1-specific function and that the CTD of Sis1, when appended to the J-GF of Ydj1, was sufficient to provide this function.
Fig. 5. Function of Sis1, Ydj1 and Sis1-Ydj1 hybrid proteins in protein reactivation in vitro. 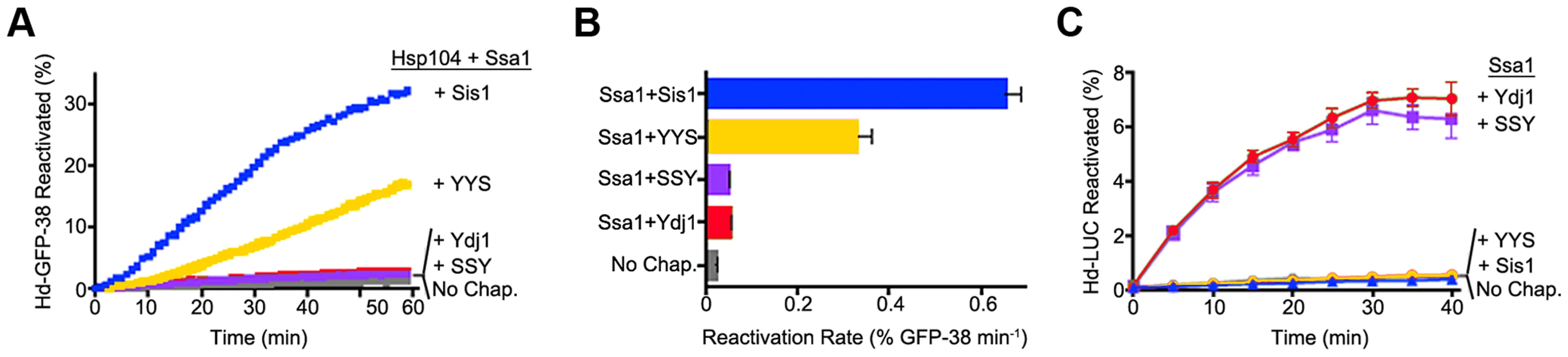
(A) Reactivation of heat-denatured GFP-38 by Sis1 (blue), Ydj1 (red), SSY (purple) or YYS (yellow) in combination with Hsp104 and Ssa1 was measured over time as described in Methods. Denatured GFP-38 incubated without chaperones is shown in gray. A representative plot of 3 experiments is shown. (B) Initial rates of reactivation of heat-inactivated GFP-38 by Hsp104 and Ssa1 in conjunction with Sis1, Ydj1, SSY or YYS (n = 3, ± SEM). (C) Reactivation of heat-denatured luciferase by Sis1 (blue triangles), Ydj1 (red circles), SSY (purple squares) or YYS (yellow circles) in combination with Ssa1 was measured as described in Methods. Denatured luciferase incubated without chaperones is shown in black circles. Data from three replicates are presented as the mean ± SEM. In contrast, with heat-inactivated luciferase as substrate, Ydj1 in combination with Ssa1 promoted reactivation and Sis1 with Ssa1 was inactive (Figure 5C). SSY was as active as Ydj1 in reactivating luciferase with Ssa1. YYS in combination with Ssa1 was unable to reactivate heat-denatured luciferase. Together these results show that Sis1 and Ydj1 discriminate between protein aggregates and discrimination is a function of the Sis1 and Ydj1 CTDs.
Sis1 mutants dominantly inhibit [URE3] propagation
The extent that [URE3] depends on Sis1 has not been evaluated systematically. We monitored [URE3] in sis1Δ strain 1385, which carries a URA3-based plasmid encoding Sis1 to support viability. We expressed previously described versions of Sis1 engineered to contain deletions or point mutations from a TRP1-based plasmid [32]. Deletions remove defined structural domains, the H34Q substitution in a conserved histidine-proline-aspartate (HPD) motif comprising residues 34–36 in the J-domain disrupts a critical interaction with Hsp70 [38], [39], and the K199A substitution in the CTD disrupts substrate binding [40] (see Figure 6A). To assess evolutionary conservation of Hsp40 function we also included the human Sis1 homolog DnaJB1 (also known as Hdj1). When expressed in place of Sis1, DnaJB1 supports cell viability and propagation of certain variants of [PIN+] and [PSI+] [23], [32], [41], [42]. Depleting functional Ure2 into [URE3] prion aggregates makes our strains grow slowly [15], which is evident when comparing sizes of [ure-o] and [URE3] colonies (see Figure 6B).
Fig. 6. Sis1 activities are important for [URE3] propagation. ![Sis1 activities are important for [URE3] propagation.](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image/865c990a3f738c467699823ebe8ac3c8.png)
(A) Diagram (not to scale) of Sis1 coding region. Numbers at top indicate amino acid positions. Domains, designated by abbreviations at top (see text) are indicated by variously shaded boxes. Mutations are indicated on the left. Deleted regions are shown as dashed lines and locations of point mutations H34Q and K199A as diamonds. Q is H34Q, A is K199A. Intact DnaJB1 (not diagrammed), is the closest human homolog of Sis1 and is indicated JB1 in the other panels. (B) Strain 1385 expressing wild type Sis1 from a URA3 plasmid was transformed by single-copy TRP1 plasmids encoding the wild type or mutant proteins (indicated on the left of each image) regulated by the SIS1 promoter. Transformants were taken from plates selecting for [URE3] and both plasmids and streaked onto plates selecting only for the plasmids. Plates were incubated for 3 days at 30°C followed by 2 days at 25°C. [URE3] cells form small white colonies, while [ure-o] cells grow into larger red colonies. (C) [URE3] cells as in panel (B) were grown as patches on plates selecting for [URE3] and both plasmids. These were replica-plated onto medium containing uracil to allow loss of the URA3 plasmid, and then onto medium containing limiting adenine and FOA (shown), which kills cells retaining the URA3 plasmid. Empty vector control is indicated as ev. (D) As in (C) except adenine was omitted from all plates to ensure growth only of cells that propagate [URE3]. (E) Cells from plate in panel (C) were streaked onto similar medium. The fainter red coloration of [ure-o] cells expressing the ‘A’ mutant is due to a slightly higher amount of adenine in the medium. (F) Cells from plate in panel (D) were streaked onto medium containing or lacking adenine, as indicated. In all panels, red colonies arose from cells that lost [URE3]. When Sis1 proteins lacking the GF region or containing the H34Q point mutation were expressed with wild type Sis1 they had obvious dominant inhibitory effects on [URE3], seen as appearance of red [ure-o] colonies (Figure 6B). Because the H34Q mutation disrupts physical interaction of J-proteins with Hsp70, the dominant inhibition of [URE3] propagation by the H34Q mutant might be caused by its forming defective hetero-dimers with wild type Sis1 or by competing with Sis1 for substrate.
To test these possibilities, we combined H34Q with alterations that interfere with ability of Sis1 to dimerize (ΔD) or to bind substrate (K199A). Both mutations reduced the dominant anti-[URE3] effect to a similar extent (from ∼23% to ∼6% [ure-o] colonies), but did not eliminate it (Figure 6B, rightmost images). Thus, inhibition of [URE3] by Sis1-H34Q depended partially on each of these Sis1 functions, suggesting it could be acting by making defective dimers with wild type Sis1 or by competing with Sis1 for binding to substrate, which in this system would be Ure2 amyloid. Although blocking dimerization can affect cooperative interaction of Sis1 with substrates in vitro [24], these results suggest that Sis1-H34Q can interfere with functions of wild type Sis1 in multiple ways.
All Sis1 activities are important for [URE3] propagation
To determine if the mutant Sis1 proteins could support propagation of [URE3], we counter-selected against the URA3 plasmid encoding wild type Sis1 as described in Figure 2A (see Figure 6C). Because several mutant Sis1 proteins appeared incapable of supporting [URE3] when this plasmid shuffling was done on plates without selecting for the prion, we also replica-plated the same patches of cells onto a series of similar plates lacking adenine to ensure recovery of cells capable of propagating [URE3], but weakly (Figure 6D). Cells will grow on FOA lacking adenine only if the Sis1 mutant supports both growth and [URE3] propagation.
Cells expressing each of the mutant Sis1 proteins, except those containing the lethal H34Q mutation, grew on the FOA plate that contained adenine (Figure 6C), showing the mutant proteins supported growth in place of Sis1. However, only the cells carrying wild type Sis1 had a normal white [URE3] phenotype on this plate, indicating [URE3] was lost rapidly from cells expressing any of the mutant Sis1 proteins as soon as the plasmid encoding wild type Sis1 was lost. Accordingly, when cells from this plate were streaked for isolated colonies on medium containing adenine, only the cells expressing wild type Sis1 gave rise to uniformly white [URE3] colonies (Figure 6E). Therefore, stable propagation of [URE3] depended on all of the Sis1 activities tested.
Although cells expressing Sis1ΔD, Sis1-K199A and the mutant with both of these mutations propagated [URE3] when selection for the prion was maintained (Figure 6D), they all lost [URE3] rapidly when grown on medium containing adenine (Figure 6F, right panels). On medium lacking adenine, [URE3] cells expressing most of the mutant proteins formed colonies at a rate similar to those expressing wild type Sis1p (Figure 6F, left panels), suggesting that the rapid loss of the prion under non-selective conditions was not due to the prion causing a disproportionate inhibitory effect on growth. However, it was evident that the Sis1ΔCTD [URE3] cells grew much more slowly than the others on medium selecting for [URE3] (Figure 6D, 6F, images on left). Since [ure-o] cells expressing Sis1ΔCTD grew like wild type [ure-o] cells (compare Figure 6E middle right image with upper left image) this slower growth was caused by the presence of [URE3], suggesting that the CTD region of Sis1 protects cells from toxic effects of [URE3]. Similar prion-associated toxicity was seen for [PSI+] cells expressing Sis1ΔCTD in place of Sis1 [32], [41].
We did not recover cells expressing Sis1ΔGF or Sis1ΔGMCTD on FOA plates lacking adenine. Thus, in agreement with results using the hybrid proteins, [URE3] requires the Sis1 GF region to propagate. The inability to recover [URE3] cells expressing Sis1ΔGMCTD might indicate [URE3] is even more toxic in these cells. Alternatively, [URE3] could be unable to propagate in cells expressing only JGF even under conditions selecting for the prion. DnaJB1 propagated [URE3] only under selective conditions, and even then only weakly. Overall, our results indicate that [URE3] depends much more on Sis1 than [PSI+] does.
We showed earlier that BKE (with wild type DnaK) supports [PSI+] propagation [15], so we tested if this system would also be useful for studying [URE3]. BKE did not support [URE3] in hsp104Δ cells (Figure S4). Because wild type DnaK should be able to interact with J-proteins other than Sis1, any or all of the cytosolic J-proteins might be able to compete with Sis1 for interaction with DnaK. Since [URE3] has a stringent requirement for Sis1, a resulting reduction in ability of Sis1 to interact with the BKE machinery probably explains the inability of [URE3] to be propagated. Alternatively, as stable propagation of [URE3] depends critically on which Hsp70 is present [43], [44], the failure might reflect a requirement for a specific Hsp70 activity lacking in DnaK.
Sensitivity of [URE3] to Sis1 alteration explains how Ydj1 cures [URE3]
Although the underlying mechanism of how overexpressed Ydj1 cures cells of [URE3] is uncertain, interaction with Hsp70 is critical because Ydj1 mutants unable to interact with Hsp70 do not cure and the J-domain alone of Ydj1 or other yeast Hsp40s is enough to cure [14], [45]. Since all parts of Ydj1 except the J-domain can be mutated or deleted without disrupting curing, we expected the CTD of Ydj1 would not have a major influence on the curing of [URE3]. Instead, we anticipated that if a hybrid cannot function in place of Sis1 with the disaggregation machinery that replicates [URE3] prions, then it will interfere with this function if it can compete effectively with Sis1 for interaction with the Hsp70 component of this machinery.
When overexpressed, SYS and YYS cured like Ydj1 (see Figure 7A). SYY and YSY cured somewhat less effectively, and YSS cured inefficiently. Thus, the SYS and YYS hybrids that did not propagate [URE3] cured [URE3] very effectively, while YSS, which supported [URE3], cured only weakly.
Fig. 7. Curing of [URE3] by overexpression of Ydj1 and hybrid proteins. ![Curing of [URE3] by overexpression of Ydj1 and hybrid proteins.](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image/3ded9871dc3132cf0b678c0cfbfb91c8.png)
(A) Wild type [URE3] cells (strain 1075) carrying plasmids encoding indicated hybrid proteins under control of the GAL promoter were grown in dextrose medium selecting for the plasmid, washed, and transferred to galactose medium. The proportion of [URE3] cells as a function of culture generations in galactose is shown. (B) Curing was done simultaneously using identical conditions except Sis1 and hybrid proteins lacking their dimerization domains (open symbols, as indicated) were used. For easier comparison, these data are plotted separately with the same Ydj1 data. (C) Elevating abundance of Sis1 reduces curing of [URE3] by overexpressing Ydj1, but not Hsp104. Strain 1075 was transformed simultaneously by indicated combinations of empty vectors (−) and single-copy plasmids expressing Ydj1, dominant negative Hsp104-2KT or Sis1 (+) from the GPD promoter. Images show sections of representative primary transformation plates after incubating 4 days at 30°C. Numbers below images indicate average frequency of [URE3] loss (± s.d.). Since SYS and YYS possess the dimerization region of Sis1, their ability to cure [URE3] when overexpressed again might be related to an ability to form non-productive dimers with endogenous Sis1, which could contribute to the curing by partially depleting cytosolic Sis1 function. In agreement with this explanation, although disrupting dimerization of Ydj1 does not affect curing considerably [45], hybrids with the CTD of Sis1 that lacked the dimerization domain were significantly reduced in their ability to cure [URE3] (Figure 7B). The residual curing by monomeric SYS and YYS could be explained by their competing with endogenous Sis1 for interaction with Hsp70 or with [URE3] as a substrate.
Our curing data add to much previously published work [14], [15], [23], [45], [46] that support the explanation that overexpressing Ydj1 cures [URE3] by competing with Sis1. If so, then increasing abundance of Sis1 should allow it to compete more effectively for the disaggregation machinery and reduce the curing. In line with this prediction, overexpressing Ydj1 cured [URE3] much less effectively in cells with elevated expression of Sis1 (Figure 7C).
It remained possible that elevating Sis1 reduced this curing through some general stabilizing effect on [URE3]. However, if increasing Sis1 protects [URE3] from Ydj1 curing specifically by improving ability of Sis1 to compete with Ydj1, then increasing Sis1 would not be expected to protect [URE3] from being cured by other ways of impairing disaggregation machinery activity, such as inhibiting Hsp104. Overexpressing the dominant negative Hsp104-2KT mutant [5], which inhibits Hsp104 activity, cured [URE3] very effectively (Figure 7C). Elevating Sis1 expression did not affect this curing. Thus, Sis1 specifically counteracted curing by overexpressed Ydj1, which again is consistent with the idea that Ydj1 cures [URE3] by competing with Sis1 for interaction with the disaggregation machinery.
[PIN+] is less dependent on Sis1 functions than [URE3]
We repeated the plasmid shuffle in [psi−] [PIN+] strain 930a to assess effects of our panel of Sis1 mutants on propagation of [PIN+] prions (Figure 8). We monitor [PIN+] by the fluorescence status of Rnq1-GFP, which is regulated by the RNQ1 promoter on a single-copy plasmid. Rnq1-GFP is punctate in [PIN+] cells and diffuse in [pin−] cells.
Fig. 8. Some Sis1 activities are important for [PIN+] propagation. ![Some Sis1 activities are important for [<i>PIN<sup>+</sup></i>] propagation.](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image/a3579fac7a584fae52877c75c3175e70.png)
[PIN+] [psi−] cells of strain 930a expressing Rnq1-GFP were transformed by plasmids encoding the mutant Sis1 proteins described in Figure 5. (A) Primary transformant colonies on medium selecting for the plasmids encoding both wild type and mutant Sis1 proteins were assessed for fluorescence. (B) Cells shown in panel (A) were transferred to plates containing uracil and then onto FOA plates to select for cells expressing the only mutant versions of Sis1. Cells from the FOA plates were then assessed for fluorescence. For each set, upper panels are fluorescent images and lower panels show the same cells in bright field. Control [pin−] cells carrying one copy of wild type Sis1 are shown on lower right for comparison. [PIN+] propagated in cells with wild type Sis1 regardless of which of the mutant proteins was also expressed (Figure 8A). However, while wild type cells had single bright foci, those co-expressing the mutant proteins, except for Sis1ΔD and H34Q, had multiple foci (multi-dot). Therefore, several mutant Sis1 proteins dominantly affected propagation of [PIN+]. Cultures expressing Sis1-H34Q had a mixture of cells that possessed either single foci or completely diffuse fluorescence, indicating that H34Q inhibited the wild type Sis1 enough to cause [PIN+] to be lost from some cells. Combining ΔD or K199A with the H34Q mutation led to a multi-dot phenotype and reduced the proportion of [pin−] cells. These effects resemble the way the Sis1 mutants dominantly inhibited [URE3] and again show that the prion-curing effect of Sis1-H34Q depends partially on its dimerization and substrate-binding functions. Unlike [URE3], [PIN+] was not dramatically destabilized by co-expression of Sis1ΔGF, here regulated by the SIS1 promoter on a single copy plasmid. However, overexpressing Sis1ΔGF in [PIN+] cells of another strain background is toxic and causes [PIN+] to be lost [47].
In agreement with earlier work [23], [30], Sis1ΔCTD supported [PIN+] in cells without wild type Sis1, but Sis1ΔGF did not (Figure 8B). However, unlike the earlier work that showed Sis1ΔGMCTD (i.e. Sis1 JGF alone) propagated [PIN+], we did not observe [PIN+] foci in cells expressing Sis1ΔGMCTD. This difference might be due to differences in yeast strain backgrounds or by our variant of [PIN+] being more dependent on Sis1 for its propagation. In an earlier study the single-dot character of [PIN+] aggregates was frequently transformed by Sis1ΔGMCTD to multiple-dots, which were inheritable after transfer to wild type cells [30]. It is possible that the altered Sis1 activity causing this change is related to the loss of [PIN+] in our strains expressing Sis1ΔGMCTD, or that the action of Sis1ΔGMCTD on [PIN+] might be different in the two yeast strain backgrounds due to variation in expression of other chaperones.
[PIN+] also propagated stably enough to be detected among most cells expressing the substrate-binding and dimerization mutants (Figure 8B), showing that while these functions are important for [PIN+] propagation, [PIN+] depended less on these Sis1 activities than [URE3]. Keeping in mind that variations among strains of yeast and prions can influence prion stability, our data showing this intermediate sensitivity of [PIN+] to impairment of Sis1 activity is consistent with it being less sensitive than [URE3], but more sensitive than [PSI+], to curing by overexpressed Ydj1. Finally, we confirm earlier findings that DnaJB1 (Hdj1) supports propagation of [PIN+] [30], [32], [41].
Discussion
Hsp40s bind misfolded proteins and regulate Hsp70 activity, so the ability of Sis1 and Ydj1 to specify functions of the disaggregation machinery are likely to be mediated through interactions with substrate and Hsp70. Our findings here show that C-terminal domains of Sis1 and Ydj1 can determine their functional differences in prion propagation, thermotolerance, galactose induction and their specific and general roles in supporting cell growth. Because the primary sites in Sis1 and Ydj1 that interact with substrates are contained within the CTDs, our data support the view that functional distinctions among Hsp40s can be due to differences in substrate specificity [34], [35].
The CTD of Sis1 also interacts with Hsp70, however, [47], [48] and although not fully characterized, this interaction likely influences Hsp70 functions. Likewise, functions influenced by the zinc-finger and farnesylation of the CTD of Ydj1 are important for the transfer of substrate to Hsp70 and for protecting cells from a [PIN+] prion-related toxicity [49], [50]. Also, Ydj1 can interact physically with Hsp104 in vitro [2], although the relevance of this interaction in the cell is unclear. While these other activities can be expected to contribute to specificity of these Hsp40s, our in-vivo and in vitro results indicate that the CTDs alone of Sis1 and Ydj1 allow them to discriminate between specific substrates, which is in line with earlier data [35].
A plausible explanation for the functional distinctions we observe would be that the CTD of Ydj1 interacts more readily with amorphous aggregates of stress-denatured proteins while that of Sis1 targets the more structured and homogeneous prion polymers. Sis1 and Ydj1 both bind to prion proteins, although Sis1 seems to bind more avidly, and prion proteins can differ in the number or location of general and distinct binding sites recognized by different Hsp40s [50]–[54]. Additionally, because substrate specificity of Hsp40s can overlap, competition among Hsp40s for substrates could contribute to determining functions of the chaperone machinery.
As seen earlier [47], we found the GF region can confer prion-specific Hsp40 functions. We show that the GF region of Sis1 was needed to propagate [URE3]. [PIN+] also depends on an activity of the Sis1 GF region that cannot be complemented by the Ydj1 GF [22], [23], [30]. However, all testable activities of Sis1 are dispensable for [PSI+] propagation [32], which shows that functions of the Sis1 GF are not necessary for propagation of all prions. Nevertheless, when appended to the JGF of their counterparts, the CTDs of Ydj1 and Sis1 generally were enough to allow the hybrid proteins to perform distinctly and effectively in place of intact Ydj1 and Sis1. Thus, the J and GF regions of Sis1 and Ydj1 possess activities that overlap enough to perform similarly in several distinct tasks. Evidently, more work is needed to learn how the GF region specifies Hsp40 functions in its effects on prions and perhaps other Hsp40-dependent cellular processes.
Much evidence points to Sis1 playing a key role in the replication of yeast prions by acting as a component of the Hsp104 disaggregation machinery that fragments prion fibers [14], [30], [46], [55], [56]. The varying degrees by which the prions depend on Sis1 agree with the supposition that different prions, and even different strains of the same prion (see [46], [57]), require varying degrees of disaggregation machinery activity to be fragmented. Together with the insensitivity of [PSI+] to Sis1 mutation, our finding that [URE3] is acutely sensitive to alteration in any Sis1 activity helps explain why depleting Sis1 causes cells to lose [URE3] much faster than they lose [PSI+] [14]. Our finding that [PIN+] had an intermediate dependency on Sis1 activity is also consistent with the intermediate rate of loss of [PIN+] seen upon Sis1 depletion. Overall our findings are consistent with earlier suggestions of a hierarchical dependency of these and other prions on the disaggregation machinery [57].
Extending this reasoning, our data strongly support an earlier suggestion that curing of [URE3] by Ydj1 or J-domains alone might be a result of competition for interaction with Hsp70 [14]. If Ydj1 cannot cooperate effectively with the disaggregation machinery to propagate [URE3], then by displacing Sis1 from the Hsp70 component of this machinery, less Hsp104 would be directed toward fragmenting prion polymers. This mechanism also explains why J-domains alone are enough to destabilize [URE3] and aligns with the idea that certain intact J-proteins don't cure as effectively because they are normally recruited to defined locations in the cytosol, such as ribosomes, by their other distinct functional domains. Among the three prions tested [URE3] is most sensitive to reductions in Sis1 function, so one might expect that its propagation would be most affected by such competition.
In line with a more stringent requirement of the disaggregation machinery for [URE3] replication, the average number of [URE3] prions per cell is lower than that for [PIN+] and [PSI+] [14], [58], [59]. The different seed numbers among variants of [PIN+] also could reflect differences in susceptibility to fragmentation, which in turn might underlie variation in sensitivity to curing by overexpressed Ydj1 [29]. Differences in susceptibility to fragmentation can be due to subtle differences in the structures of the amyloid that form the prions [60]. Such variation in the amyloid structures that determine differences among variants of [PIN+] might also have a bearing on the distinct pattern of variants of [PSI+] they induce [29], [61]–[63]. The similar intermediate sensitivity of [PIN+] to depletion of Sis1 seen in earlier work and to specific mutations of Sis1 seen here suggests the variants of prions used are similar and that their prion character is largely independent of strain background. Nevertheless, findings might differ if other variants of prions or other strain backgrounds propagating them were compared directly.
It is becoming evident that the differences in ways prions respond to J-proteins and other Hsp70 co-chaperones likely reflect differences in the ways prions depend on Hsp70. Altering activity of Hsp70 directly by mutation or indirectly by altering its co-chaperones can influence prion propagation in the same ways, which supports this idea [64]–[66]. Stability of [SWI+] prions is also highly sensitive to altered expression of Hsp40s and J-domains, which seems to be related to a strict dependency on optimal Hsp70 activity [67]. Yeast has four highly homologous Ssa Hsp70 paralogs and prion phenotypes vary greatly when different Hsp70s are the sole source of Ssa protein [43]. These differences likely reflect differences in the way the Hsp70s interact with or are regulated by the Hsp40s or other factors. Hsp70 also can be a primary factor in recruiting the disaggregation machinery to prion polymers [7]. Notably, however, it is not Hsp70 substrate-binding per se, but the regulation of this binding, presumably by co-chaperones, that specifies distinctions in Hsp70 functions with regard to [URE3] propagation [44]. NEFs can also affect prion propagation through their ability to regulate Hsp70 [13], [68], [69].
Because Hsp70 is a critical component of the Hsp104-based disaggregation machinery, altering Hsp70 or its co-chaperones can be expected to affect propagation of prions by influencing composition and activity of this machinery. The distinct susceptibilities of prions to alterations in various disaggregation machinery components might therefore reveal differences in the ways various chaperones combine to act most effectively on them as specific substrates. Understanding why prions respond differently to the various chaperone machinery components including J-proteins, NEFs and Hsp70s should help us understand both fundamental and subtle ways that these components interact to produce effective protein remodeling machines.
Materials and Methods
Yeast strains, plasmids, media and growth conditions
Yeast strains used are isogenic to strain 779-6A (MATa, SUQ5, kar1-1, ade2-1, his3Δ202, leu2Δ1, trp1Δ63, ura3-52) [70], which is used for monitoring [PSI+] and [PIN+]. Knockouts and replacements of chromosomal genes were done using standard transformation procedures [71]. Strain MR386 has E. coli CLPB in place of the chromosomal HSP104 gene [15] and contains plasmids expressing E. coli dnaKR167H (pMR150LG-R167H) and E. coli GrpE (pMR134H) under the control of the GPD (glyceraldehyde-3-phosphate dehydrogenase - TDH3) and FES1 promoters, respectively. [PSI+] is maintained in strain MR386 by pJ312, which is HSP104 on a URA3 plasmid [72]. Strain MR502 has ydj1::KanMX and carries p316YDJ1, which is YDJ1 on a URA3 plasmid. [PSI+] [PIN+] strain 930 has sis1::KanMX and carries plasmid pYW17, a URA3-based plasmid encoding wild type SIS1 [22], [32].
Strain 930a is a [psi−] [PIN+] version of 930 that carries plasmid p313Rnq1-GFP. It was cured of [PSI+] by transient growth on medium containing 3 mM guanidine and then [PIN+] clones among [psi−] isolates were identified by punctate Rnq1-GFP fluorescence. Our [PIN+] variant is uncharacterized, but of the single-dot type, which typically has sturdier fibers and a lower seed number per cell than multi-dot [PIN+] [61]. Isogenic strain 1075, for monitoring [URE3], has ADE2 regulated by the DAL5 promoter (PDAL5::ADE2, see below) in place of ade2-1 [43]. Strain 1385 is strain 1075 with sis1::KanMX and plasmid pYW17, which is SIS1 on a URA3 plasmid. Strains 1408 and 1410 are hsp104Δ versions of strain 779-6A and 1075, respectively [15]. Both carry pJ312. Our parental strains carry only one variant of [PSI+], [URE3] or [PIN+].
SIS1 plasmids used contain wild type and mutant SIS1 alleles on the pRS314 single-copy TRP1 vector [22], [32]. Plasmid pRU4 is LEU2-based single-copy plasmid pRS415 containing the GAL1 promoter and CYC1 terminator flanking the polylinker sites SpeI and XhoI. For Gal-induced expression, YDJ1 and hybrid alleles were inserted into pRU4 as BamHI-SalI fragments. All plasmids used in this study are listed in Table 1. Plasmids encoding E. coli genes or yeast Hsp40 genes with D36N mutations are described [15].
Tab. 1. Plasmids. 
See Materials and Methods section for details of construction. All plasmids are CEN (single copy) unless otherwise indicated with “2-micron” (high copy). All pMR and pRU plasmids contain the CYC1 terminator sequence immediately downstream of the ORF. With the exceptions that 1/2YPD plates contain 5 g/L yeast extract, YPAD plates contain 400 mg/L (excess) adenine and solid defined media contain 10 mg/L (limiting) adenine, standard media and growth conditions were used [71].
Construction of SIS1-YDJ1 hybrid alleles
Sis1 and Ydj1 have clearly defined and characterized J-domains, glycine-phenylalanine (GF) rich middle domains, and a C-terminal region that contains two major elements (CTDI and CTDII) and a dimerization domain. The main structural differences between Sis1 and Ydj1 are a glycine-methionine-rich (GM) extension of the GF domain in Sis1, which has GF-redundant functions, and a Zn-finger domain (ZF) embedded between beta-strands 2 and 3 of the CTDI of Ydj1 that is absent in Sis1 [73]. Because the ZF of Ydj1 is an integral part of CTDI, we designed our Sis1-Ydj1 hybrids using the GF-CTDI junction to restrict the number of domain swaps and avoid using complicated junctions to swap the ZF region. Rather than designating the GM region as a separate domain, we combined the functionally redundant GF and GM regions of Sis1 into a single domain. Thus, hybrid alleles were made by swapping three regions: the J-domain, the glycine-rich region and the C-terminal portion, which includes CTDI, CTDII and the dimerization domains, herein referred to simply as the CTD (see Figure 1A, [30]). Hybrid genes were synthesized by GENEWIZ, Inc (South Plainfield, NJ) and sub-cloned into variants of pRS414 that placed the ORF under the control of the SIS1, YDJ1 or GPD promoters and a downstream CYC1 transcriptional terminator [74]. All constructs contained a c-terminal c-myc tag that had no noticeable affect on functions in vivo.
Monitoring prions
Depletion of the ribosome release factor Sup35 by its sequestration in [PSI+] prion aggregates causes nonsense suppression. We monitored [PSI+] by suppression of the ade2-1 nonsense allele in our strains. [PSI+] cells are Ade+ and white, while [psi−] cells are Ade− and when grown on limiting adenine are red due to accumulation of a metabolite of adenine biosynthesis. The presence of [URE3] was monitored similarly by use of an ADE2 allele regulated by the DAL5 promoter (PDAL5::ADE2) [75], [76]. Under standard growth conditions Ure2 represses transcription of nitrogen metabolic genes, such as DAL5. [URE3] sequesters Ure2 into prion aggregates, thereby reducing Ure2 function and activating the DAL5 promoter. Thus, [URE3] cells are Ade+ and white, while [ure-o] cells are Ade− and red on limiting adenine. We confirmed that Ade+ phenotypes were due to the presence of prions by their guanidine curability and by crosses with cells lacking prions to produce a dominant, guanidine-curable Ade+ phenotype. We monitored [PIN+] by assessing aggregation status of a plasmid-expressed Rnq1-GFP fusion protein. GFP fluorescence is diffuse in [pin−] cells, but noticeably punctate in [PIN+] cells. In this study we used a typical strong [PSI+] strain and single variants of [URE3] and [PIN+] prions.
Microscopy
Microscopic analysis of Rnq1-GFP fluorescence in live cells was done with a Nikon E-800 microscope with log phase cells grown in medium selecting for the plasmid encoding the Rnq1-GFP fusion protein. Images were captured using IVision software and processed using Adobe Photoshop software.
Thermotolerance
Log phase cells grown in medium selecting for plasmids were diluted in fresh medium to an OD600 of 0.25 and 100 µL was transferred to 0.5 mL test tubes and placed in a PCR machine for thermocycling as indicated. At various times aliquots were removed and placed on ice. Cooled cells were serially diluted and 5 µL drops were spotted onto YPAD plates.
Complementation assays
Strains 930 and 1385 (both sis1Δ) and derivatives of MR502 (ydj1Δ) carrying wild type SIS1 or YDJ1 on URA3-based plasmids were transformed by TRP1-based plasmids carrying wild type, mutant or hybrid alleles. Strain MR502 also carries pMR169 for monitoring GAL induction (see below). Transformants were grown as patches of cells on medium lacking both tryptophan and uracil and then replica-plated onto similar medium containing uracil to allow loss of the URA3 plasmid. These were then replica-plated onto medium containing 5-FOA, which kills cells that did not lose the resident URA3 plasmid.
For Sis1, growth on 5-FOA plates shows complementation of functions essential for growth, and growth without adenine shows complementation of Sis1 functions required for prion propagation. To test complementation of Ydj1 function, 5-FOA resistant cells of strain MR502 were grown overnight in medium selecting for the TRP1 plasmids, normalized to the same cell density (OD600 = 0.25) and five-fold serially diluted. Aliquots of the dilutions (5 µL) were then dropped onto YPAD plates. Scanned images of the plates were taken after they were incubated at the indicated temperatures for 3–4 days.
Galactose induction
Aliquots of overnight cultures of MR502 transformants used for growth complementation were transferred to synthetic raffinose medium (SRaf) and grown overnight. These cells carried the TRP1-marked hybrid alleles or empty vector control and a HIS3-marked plasmid encoding firefly luciferase under the control of the GAL10 promoter (pMR169). Cell densities were adjusted to OD600 = 0.3 in fresh medium and the initial reading (t = 0) was taken by mixing 100 µL culture with 50 µL of 1 mM luciferin in 0.1 M sodium citrate, pH 5.0 immediately before reading in a Zylux Femtomaster luminometer, with a 10 s delay and 5 s read time. Galactose from a 20% stock was then added to the cultures to obtain a final concentration of 2% and the cells were incubated on a roller at 30°C. Readings were taken at 30, 60 and 120 minutes after addition of galactose. All samples in three experiments were tested in triplicate. No significant growth occurred during the course of the experiment.
Curing of [URE3] by overexpressed proteins
Overnight SD cultures of cells carrying YDJ1 or hybrid alleles on pRU4 for galactose induction were used to inoculate SGal medium to OD600 = 0.05 and incubated with shaking at 30°C. Generations were monitored as doublings of OD600. Cultures were sub-cultured as necessary to keep the OD600 less than 2.0. After 3, 6 and 9 generations of growth in galactose, aliquots were removed and cells plated onto 1/2YPD plates. Loss of [URE3] was assessed by determining the fraction of entirely red (i.e. [ure-o]) colonies after 3 days of incubation at 30°C. To test the ability of overexpressed Sis1 to block Ydj1 - or Hsp104 - mediated curing of [URE3], strain 1075 was co-transformed with various combinations of CEN plasmids expressing Sis1, Ydj1 or Hsp104 under the control of the GPD promoter.
Western blotting
Cell lysates for western blots were prepared as described [77]. Briefly, cells were suspended in lysis buffer and broken by agitation with glass beads. For each sample 10 µg of protein was separated on 4–20% SDS-PAGE gels, transferred to PVDF membranes and probed using anti-c-myc antibody (AbCam #ab9106) and chemiluminescence. After developing, the blots were stained by amido-black (Sigma #A-8181) as a loading and transfer control.
Proteins
Hsp104 [78], Ydj1 [79], and GFP-38 [80] were purified as described. SSY was isolated as described for Ydj1 [79]. Sis1 was purified as described [81] with some modifications. Briefly, BL21 (DE3) was transformed with a pET11 plasmid containing the Sis1 gene, cultures were grown at 30°C to OD595 0.8 and cells were induced with 1 mM IPTG for 3 h. Clarified cell lysates were applied to an S-Sepharose-FF column (GE Healthcare) in 20 mM MES buffer, pH 6.0, 0.1 mM EDTA and 1 mM DTT. Sis1 was eluted with a linear gradient from 0–1 M NaCl. Peak fractions were pooled, buffer exchanged into 20 mM MES buffer pH 6.0, 0.1 mM EDTA and 1 mM DTT. The sample was applied to a monoS column (GE Healthcare) and eluted with a linear gradient from 0–1 M NaCl. YYS was purified similarly to Sis1, except that the buffer used was 25 mM HEPES, pH 7.6, 0.1 mM EDTA and 1 mM DTT. For Ssa1, a pET24 plasmid containing the Ssa1 gene was transformed into Rosetta BL21 (DE3) cells. Cultures were grown to 0.8 OD595 at 30°C and induced with 0.2 mM IPTG overnight. The clarified lysate was applied to a Q-sepharose FF column (GE Healthcare) in 20 mM Tris.HCl, pH 7.6, 40 mM KCl, 0.1 mM EDTA and 1 mM DTT. Ssa1was eluted with a linear gradient of 40–400 mM KCl over 20 column volumes. Peak fractions were collected and buffer exchanged into 25 mM HEPES, pH 7.6, 100 mM KCl, 0.1 mM EDTA and 1 mM DTT and further purified over a monoQ column (GE Healthcare) using a linear gradient of 100–400 mM KCl over 20 column volumes. Peak fractions were collected, analyzed, supplemented with 10% glycerol, frozen on dry ice and stored at −80°C.
GFP-38 reactivation
Experiments were performed as described previously [80] with some modifications. Reactivation was carried out in HKE Buffer (25 mM HEPES, 75 mM KCl, 0.1 mM EDTA, pH 7.6) and GFP-38 heat-denaturation was carried out in Buffer D (20 mM Tris·HCl, 100 mM KCl, 0.1 mM EDTA, 10% glycerol, pH 7.5). 14 mM GFP-38 in buffer D was heated at 80°C for 15 min, frozen on dry ice, thawed and immediately used in experiments. Reactivation reactions (100 µl) contained HKE buffer, 4 mM ATP, 10 mM MgCl2, an ATP regenerating system (10 mM creatinine phosphate and 0.03 mg/mL creatinine kinase), 0.7 µM denatured GFP-38, 0.2 µM Hsp104, 1 µM Ssa1 and 0.2 µM Hsp40 (Sis1, Ydj1, SSY or YYS). Reactions were initiated by adding MgATP, and reactivation was monitored by measuring GFP fluorescence at 23°C using a TECAN Infinite M200Pro plate reader. GFP-38 reactivation was calculated as a percentage of the initial native GFP-38 fluorescence.
Luciferase reactivation
Luciferase (80 nM; 55 µl) was heat-denatured in HKE buffer containing 0.05 mg/mL BSA, 2 mM DTT and 10 mM MgCl2 at 45°C for 7 min and then cooled to 4°C for 1 min. Denatured luciferase was added to reactivation reactions (70 µl total) containing HKE, 0.1 mg/mL BSA, 2 mM DTT, 10 mM MgCl2, 3 mM ATP, an ATP regenerating system as above, 2 µM Ssa1 and 1 µM Sis1, Ydj1, SSY or YYS and incubated at 23°C. Luciferase reactivation was monitored by removing 5 µL aliquots at 5 min intervals and measuring luminescence in a TECAN Infinite M200Pro plate reader in the presence of luciferin. Reactivation was determined compared to an unheated luciferase control.
Supporting Information
Zdroje
1. ZolkiewskiM, ZhangT, NagyM (2012) Aggregate reactivation mediated by the Hsp100 chaperones. Arch Biochem Biophys 520 : 1–6.
2. GloverJR, LindquistS (1998) Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94 : 73–82.
3. WeibezahnJ, TessarzP, SchliekerC, ZahnR, MaglicaZ, et al. (2004) Thermotolerance requires refolding of aggregated proteins by substrate translocation through the central pore of ClpB. Cell 119 : 653–665.
4. LumR, TkachJM, VierlingE, GloverJR (2004) Evidence for an unfolding/threading mechanism for protein disaggregation by Saccharomyces cerevisiae Hsp104. J Biol Chem 279 : 29139–29146.
5. ChernoffYO, LindquistSL, OnoB, Inge-VechtomovSG, LiebmanSW (1995) Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. Science 268 : 880–884.
6. RomanovaNV, ChernoffYO (2009) Hsp104 and prion propagation. Protein Pept Lett 16 : 598–605.
7. WinklerJ, TyedmersJ, BukauB, MogkA (2012) Chaperone networks in protein disaggregation and prion propagation. J Struct Biol 179 : 152–160.
8. WicknerRB (1994) Evidence for a prion analog in S. cerevisiae: the [URE3] non-Mendelian genetic element as an altered URE2 protein. Science 264 : 566–569.
9. DerkatchIL, BradleyME, ZhouP, ChernoffYO, LiebmanSW (1997) Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics 147 : 507–519.
10. SondheimerN, LindquistS (2000) Rnq1: an epigenetic modifier of protein function in yeast. Mol Cell 5 : 163–172.
11. JungG, JonesG, WegrzynRD, MasisonDC (2000) A role for cytosolic hsp70 in yeast [PSI+] prion propagation and [PSI+] as a cellular stress. Genetics 156 : 559–570.
12. MoriyamaH, EdskesHK, WicknerRB (2000) [URE3] prion propagation in Saccharomyces cerevisiae: requirement for chaperone Hsp104 and curing by overexpressed chaperone Ydj1p. Mol Cell Biol 20 : 8916–8922.
13. KryndushkinD, WicknerRB (2007) Nucleotide exchange factors for Hsp70s are required for [URE3] prion propagation in Saccharomyces cerevisiae. Mol Biol Cell 18 : 2149–2154.
14. HigurashiT, HinesJK, SahiC, AronR, CraigEA (2008) Specificity of the J-protein Sis1 in the propagation of 3 yeast prions. Proc Natl Acad Sci USA 105 : 16596–16601.
15. ReidyM, MiotM, MasisonDC (2012) Prokaryotic Chaperones Support Yeast Prions and Thermotolerance and Define Disaggregation Machinery Interactions. Genetics 192 : 185–193.
16. HungGC, MasisonDC (2006) N-terminal domain of yeast Hsp104 chaperone is dispensable for thermotolerance and prion propagation but necessary for curing prions by Hsp104 overexpression. Genetics 173 : 611–620.
17. TessarzP, MogkA, BukauB (2008) Substrate threading through the central pore of the Hsp104 chaperone as a common mechanism for protein disaggregation and prion propagation. Mol Microbiol 68 : 87–97.
18. McClellanAJ, EndresJB, VogelJP, PalazziD, RoseMD, et al. (1998) Specific molecular chaperone interactions and an ATP-dependent conformational change are required during posttranslational protein translocation into the yeast ER. Mol Biol Cell 9 : 3533–3545.
19. MayerMP, BukauB (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62 : 670–684.
20. YoungJC (2010) Mechanisms of the Hsp70 chaperone system. Biochem Cell Biol 88 : 291–300.
21. FanCY, LeeS, CyrDM (2003) Mechanisms for regulation of Hsp70 function by Hsp40. Cell Stress Chaperones 8 : 309–316.
22. YanW, CraigEA (1999) The glycine-phenylalanine-rich region determines the specificity of the yeast Hsp40 Sis1. Mol Cell Biol 19 : 7751–7758.
23. LopezN, AronR, CraigEA (2003) Specificity of class II Hsp40 Sis1 in maintenance of yeast prion [RNQ+]. Mol Biol Cell 14 : 1172–1181.
24. ShaB, LeeS, CyrDM (2000) The crystal structure of the peptide-binding fragment from the yeast Hsp40 protein Sis1. Structure 8 : 799–807.
25. LiJ, QianX, ShaB (2003) The crystal structure of the yeast Hsp40 Ydj1 complexed with its peptide substrate. Structure 11 : 1475–1483.
26. LiJ, QianX, ShaB (2009) Heat shock protein 40: structural studies and their functional implications. Protein Pept Lett 16 : 606–612.
27. CaplanAJ, DouglasMG (1991) Characterization of YDJ1: a yeast homologue of the bacterial dnaJ protein. J Cell Biol 114 : 609–621.
28. FlomGA, LemieszekM, FortunatoEA, JohnsonJL (2008) Farnesylation of Ydj1 is required for in vivo interaction with Hsp90 client proteins. Mol Biol Cell 19 : 5249–5258.
29. BradleyME, EdskesHK, HongJY, WicknerRB, LiebmanSW (2002) Interactions among prions and prion “strains” in yeast. Proc Natl Acad Sci U S A 99 Suppl 4 : 16392–16399.
30. SondheimerN, LopezN, CraigEA, LindquistS (2001) The role of Sis1 in the maintenance of the [RNQ+] prion. EMBO J 20 : 2435–2442.
31. LianHY, ZhangH, ZhangZR, LooversHM, JonesGW, et al. (2007) Hsp40 interacts directly with the native state of the yeast prion protein Ure2 and inhibits formation of amyloid-like fibrils. J Biol Chem 282 : 11931–11940.
32. KirklandPA, ReidyM, MasisonDC (2011) Functions of yeast Hsp40 chaperone Sis1p dispensable for prion propagation but important for prion curing and protection from prion toxicity. Genetics 188 : 565–577.
33. LukeMM, SuttonA, ArndtKT (1991) Characterization of SIS1, a Saccharomyces cerevisiae homologue of bacterial dnaJ proteins. J Cell Biol 114 : 623–638.
34. SahiC, CraigEA (2007) Network of general and specialty J protein chaperones of the yeast cytosol. Proc Natl Acad Sci U S A 104 : 7163–7168.
35. FanCY, LeeS, RenHY, CyrDM (2004) Exchangeable chaperone modules contribute to specification of type I and type II Hsp40 cellular function. Mol Biol Cell 15 : 761–773.
36. BorgesJC, SeraphimTV, MokryDZ, AlmeidaFC, CyrDM, et al. (2012) Identification of regions involved in substrate binding and dimer stabilization within the central domains of yeast Hsp40 Sis1. PLoS One 7: e50927.
37. FloerM, BryantGO, PtashneM (2008) HSP90/70 chaperones are required for rapid nucleosome removal upon induction of the GAL genes of yeast. Proc Natl Acad Sci U S A 105 : 2975–2980.
38. WallD, ZyliczM, GeorgopoulosC (1994) The NH2-terminal 108 amino acids of the Escherichia coli DnaJ protein stimulate the ATPase activity of DnaK and are sufficient for lambda replication. J Biol Chem 269 : 5446–5451.
39. TsaiJ, DouglasMG (1996) A conserved HPD sequence of the J-domain is necessary for YDJ1 stimulation of Hsp70 ATPase activity at a site distinct from substrate binding. J Biol Chem 271 : 9347–9354.
40. LeeS, FanCY, YoungerJM, RenH, CyrDM (2002) Identification of essential residues in the type II Hsp40 Sis1 that function in polypeptide binding. J Biol Chem 277 : 21675–21682.
41. SteinKC, TrueHL (2014) Structural variants of yeast prions show conformer-specific requirements for chaperone activity. Mol Microbiol 93 : 1156–1171.
42. HarrisJM, NguyenPP, PatelMJ, SpornZA, HinesJK (2014) Functional diversification of hsp40: distinct j-protein functional requirements for two prions allow for chaperone-dependent prion selection. PLoS Genet 10: e1004510.
43. SharmaD, MasisonDC (2008) Functionally redundant isoforms of a yeast Hsp70 chaperone subfamily have different antiprion effects. Genetics 179 : 1301–1311.
44. SharmaD, MasisonDC (2011) Single methyl group determines prion propagation and protein degradation activities of yeast heat shock protein (Hsp)-70 chaperones Ssa1p and Ssa2p. Proc Natl Acad Sci U S A 108 : 13665–13670.
45. SharmaD, StanleyRF, MasisonDC (2009) Curing of yeast [URE3] prion by the Hsp40 cochaperone Ydj1p is mediated by Hsp70. Genetics 181 : 129–137.
46. HinesJK, HigurashiT, SrinivasanM, CraigEA (2011) Influence of prion variant and yeast strain variation on prion-molecular chaperone requirements. Prion 5 : 238–244.
47. AronR, LopezN, WalterW, CraigEA, JohnsonJ (2005) In vivo bipartite interaction between the Hsp40 Sis1 and Hsp70 in Saccharomyces cerevisiae. Genetics 169 : 1873–1882.
48. LiJ, WuY, QianX, ShaB (2006) Crystal structure of yeast Sis1 peptide-binding fragment and Hsp70 Ssa1 C-terminal complex. Biochem J 398 : 353–360.
49. FanCY, RenHY, LeeP, CaplanAJ, CyrDM (2005) The type I Hsp40 zinc finger-like region is required for Hsp70 to capture non-native polypeptides from Ydj1. J Biol Chem 280 : 695–702.
50. SummersDW, DouglasPM, RenHY, CyrDM (2009) The type I Hsp40 Ydj1 utilizes a farnesyl moiety and zinc finger-like region to suppress prion toxicity. J Biol Chem 284 : 3628–3639.
51. SavistchenkoJ, KrzewskaJ, FayN, MelkiR (2008) Molecular chaperones and the assembly of the prion Ure2p in vitro. J Biol Chem 283 : 15732–15739.
52. BagriantsevSN, GrachevaEO, RichmondJE, LiebmanSW (2008) Variant-specific [PSI+] infection is transmitted by Sup35 polymers within [PSI+] aggregates with heterogeneous protein composition. Mol Biol Cell 19 : 2433–2443.
53. DouglasPM, TreuschS, RenHY, HalfmannR, DuennwaldML, et al. (2008) Chaperone-dependent amyloid assembly protects cells from prion toxicity. Proc Natl Acad Sci U S A 105 : 7206–7211.
54. SummersDW, DouglasPM, CyrDM (2009) Prion propagation by Hsp40 molecular chaperones. Prion 3 : 59–64.
55. AronR, HigurashiT, SahiC, CraigEA (2007) J-protein co-chaperone Sis1 required for generation of [RNQ+] seeds necessary for prion propagation. EMBO J 26 : 3794–3803.
56. TiptonKA, VergesKJ, WeissmanJS (2008) In vivo monitoring of the prion replication cycle reveals a critical role for Sis1 in delivering substrates to Hsp104. Mol Cell 32 : 584–591.
57. HinesJK, CraigEA (2011) The sensitive [SWI+] prion: new perspectives on yeast prion diversity. Prion 5 : 164–168.
58. KryndushkinDS, ShewmakerF, WicknerRB (2008) Curing of the [URE3] prion by Btn2p, a Batten disease-related protein. EMBO J 27 : 2725–2735.
59. ByrneLJ, ColeDJ, CoxBS, RidoutMS, MorganBJ, et al. (2009) The number and transmission of [PSI] prion seeds (Propagons) in the yeast Saccharomyces cerevisiae. PLoS ONE 4: e4670.
60. TanakaM, CollinsSR, ToyamaBH, WeissmanJS (2006) The physical basis of how prion conformations determine strain phenotypes. Nature 442 : 585–589.
61. SharmaJ, LiebmanSW (2013) Variant-specific prion interactions: Complicating factors. Cell Logist 3: e25698.
62. SharmaJ, LiebmanSW (2013) Exploring the basis of [PIN+] variant differences in [PSI+] induction. J Mol Biol 425 : 3046–3059.
63. WestergardL, TrueHL (2014) Extracellular environment modulates the formation and propagation of particular amyloid structures. Mol Microbiol 92 : 698–715.
64. JonesGW, MasisonDC (2003) Saccharomyces cerevisiae Hsp70 mutations affect [PSI+] prion propagation and cell growth differently and implicate Hsp40 and tetratricopeptide repeat cochaperones in impairment of [PSI+]. Genetics 163 : 495–506.
65. JonesG, SongY, ChungS, MasisonDC (2004) Propagation of Saccharomyces cerevisiae [PSI+] prion is impaired by factors that regulate Hsp70 substrate binding. Mol Cell Biol 24 : 3928–3937.
66. JonesGW, TuiteMF (2005) Chaperoning prions: the cellular machinery for propagating an infectious protein? Bioessays 27 : 823–832.
67. HinesJK, LiX, DuZ, HigurashiT, LiL, et al. (2011) [SWI], the prion formed by the chromatin remodeling factor Swi1, is highly sensitive to alterations in Hsp70 chaperone system activity. PLoS Genet 7: e1001309.
68. FanQ, ParkKW, DuZ, MoranoKA, LiL (2007) The role of Sse1 in the de novo formation and variant determination of the [PSI+] prion. Genetics 177 : 1583–1593.
69. KumarN, GaurD, MasisonDC, SharmaD (2014) The BAG homology domain of Snl1 cures yeast prion [URE3] through regulation of Hsp70 chaperones. G3 (Bethesda) 4 : 461–470.
70. JungG, MasisonDC (2001) Guanidine hydrochloride inhibits Hsp104 activity in vivo: a possible explanation for its effect in curing yeast prions. Curr Microbiol 43 : 7–10.
71. ShermanF (2002) Getting started with yeast. Meth Enz 350 : 3–41.
72. JungG, JonesG, MasisonDC (2002) Amino acid residue 184 of yeast Hsp104 chaperone is critical for prion-curing by guanidine, prion propagation, and thermotolerance. Proc Natl Acad Sci U S A 99 : 9936–9941.
73. WuY, LiJ, JinZ, FuZ, ShaB (2005) The crystal structure of the C-terminal fragment of yeast Hsp40 Ydj1 reveals novel dimerization motif for Hsp40. J Mol Biol 346 : 1005–1011.
74. MumbergD, MullerR, FunkM (1995) Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156 : 119–122.
75. SchlumpbergerM, PrusinerSB, HerskowitzI (2001) Induction of distinct [URE3] yeast prion strains. Mol Cell Biol 21 : 7035–7046.
76. BrachmannA, BaxaU, WicknerRB (2005) Prion generation in vitro: amyloid of Ure2p is infectious. EMBO J 24 : 3082–3092.
77. ReidyM, MasisonDC (2010) Sti1 regulation of Hsp70 and Hsp90 is critical for curing of Saccharomyces cerevisiae [PSI+] prions by Hsp104. Mol Cell Biol 30 : 3542–3552.
78. MiotM, ReidyM, DoyleSM, HoskinsJR, JohnstonDM, et al. (2011) Species-specific collaboration of heat shock proteins (Hsp) 70 and 100 in thermotolerance and protein disaggregation. Proc Natl Acad Sci U S A 108 : 6915–6920.
79. CyrDM, LuX, DouglasMG (1992) Regulation of Hsp70 function by a eukaryotic DnaJ homolog. J Biol Chem 267 : 20927–20931.
80. HoskinsJR, DoyleSM, WicknerS (2009) Coupling ATP utilization to protein remodeling by ClpB, a hexameric AAA+ protein. Proc Natl Acad Sci U S A 106 : 22233–22238.
81. CaplanAJ, CyrDM, DouglasMG (1992) YDJ1p facilitates polypeptide translocation across different intracellular membranes by a conserved mechanism. Cell 71 : 1143–1155.
82. SikorskiRS, HieterP (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122 : 19–27.
83. SchwimmerC, MasisonDC (2002) Antagonistic interactions between yeast [PSI+] and [URE3] prions and curing of [URE3] by Hsp70 protein chaperone Ssa1p but not by Ssa2p. Mol Cell Biol 22 : 3590–3598.
Štítky
Genetika Reprodukčná medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 10- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



