-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
Proper chromosome segregation during egg and sperm development is crucial to prevent birth defects and miscarriage. During chromosome replication, DNA entanglements are created that must be resolved before chromosomes can fully separate. In the oocytes of the fruit fly Drosophila melanogaster, DNA entanglements persist between heterochromatic regions of the chromosomes until after spindle assembly and may facilitate the proper segregation of chromosomes during meiosis. Topoisomerase II enzymes can resolve DNA entanglements by cutting and untwisting tangled DNA. Decreasing Topoisomerase II (Top2) levels in the ovaries of fruit flies led to sterility. RNAi knockdown of the Top2 gene in oocytes resulted in chromosomes that failed to fully separate their heterochromatic regions during meiosis I and caused oocytes to arrest in meiosis I. These studies demonstrate that the Top2 enzyme is required for releasing DNA entanglements between homologous chromosomes before the onset of chromosome segregation during Drosophila female meiosis.
Published in the journal: Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis. PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004650
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004650Summary
Proper chromosome segregation during egg and sperm development is crucial to prevent birth defects and miscarriage. During chromosome replication, DNA entanglements are created that must be resolved before chromosomes can fully separate. In the oocytes of the fruit fly Drosophila melanogaster, DNA entanglements persist between heterochromatic regions of the chromosomes until after spindle assembly and may facilitate the proper segregation of chromosomes during meiosis. Topoisomerase II enzymes can resolve DNA entanglements by cutting and untwisting tangled DNA. Decreasing Topoisomerase II (Top2) levels in the ovaries of fruit flies led to sterility. RNAi knockdown of the Top2 gene in oocytes resulted in chromosomes that failed to fully separate their heterochromatic regions during meiosis I and caused oocytes to arrest in meiosis I. These studies demonstrate that the Top2 enzyme is required for releasing DNA entanglements between homologous chromosomes before the onset of chromosome segregation during Drosophila female meiosis.
Introduction
In most organisms, crossing over between homologs during meiosis ensures their faithful segregation at the first meiotic division. However, in Drosophila melanogaster females, the 4th chromosomes are always achiasmate, and the X chromosomes normally fail to crossover in 6–10% of oocytes [1]. Nonetheless, Drosophila females can segregate these achiasmate chromosomes with high efficiency, demonstrating the existence of a system (termed the distributive system) to segregate homologous chromosomes that fail to recombine [2].
Heterochromatic regions on the achiasmate chromosomes are both necessary and sufficient for the proper segregation of achiasmate homologs, and homologous heterochromatic regions remain tightly paired throughout prophase of Drosophila female meiosis [3]–[5]. However, during prometaphase I, achiasmate chromosomes move dynamically on the spindle before properly biorienting and then congress into a mass with the chiasmate chromosomes at metaphase I [6], [7]. During these movements, achiasmate 4th and X chromosomes are connected by heterochromatic threads, which may play a role in the mechanism by which heterochromatin mediates chromosome segregation [6], [8]. How these threads are formed is unknown, but they could potentially arise from stalled replication intermediates [9].
Evidence for such connections between segregating meiotic chromosomes was first observed in crane fly spermatocytes [10]. Cutting the centromere from the arm of a segregating anaphase I chromosome led to re-association of the severed arm with its homolog on the opposite half-spindle, supporting the idea that chromosomes are able to maintain physical connections during meiosis I and that these connections can generate the force necessary to bring chromosomal regions together [10]. Additionally, chromosomal associations were observed during anaphase I in D. melanogaster sperm mutant for components of the condensin complex [11]. These studies indicate that the thread-like structures connecting chromosomes may be a conserved mechanism for segregating meiotic chromosomes.
To prevent loss of genetic material, such connections between homologs need to be resolved before anaphase I. Topoisomerase II enzymes are capable of creating double-strand breaks in DNA to resolve DNA entanglements during replication and transcription [12]. This function makes topoisomerase II or topoisomerase II-like enzymes possible candidates for resolving heterochromatic DNA threads between homologs during meiosis. Unfortunately, the study of topoisomerase II enzymes has been limited in meiosis due to the requirement of these enzymes to resolve DNA concatenations caused by replication in mitosis, as well as other potential roles in recombination, transcription and chromosome condensation [12]. Thus, most strong loss-of-function mutations of topoisomerase II enzymes are lethal. Examining the function of topoisomerase II during meiosis is further complicated by the presence of two topoisomerase II enzymes in many organisms.
Various studies have tried to address these issues either by chemically inhibiting topoisomerase II enzymes, such as in mice [13]–[16], or by induction of conditional mutations of topoisomerase 2 (top2), as in yeast [17]–[19]. Shifting a temperature-sensitive top2 mutant of Saccharomyces cerevisiae to the restrictive temperature during meiosis led to arrest just prior to spindle assembly [19]. Shifting a temperature-sensitive top2 mutant of Schizosaccharomyces pombe to the restrictive temperature during meiosis led to arrest during the first meiotic division [17]. In both yeasts, earlier stages of meiosis appeared normal at restrictive temperatures [17]–[19]. Additionally, blocking recombination in the top2 mutants of both types of yeast resulted in yeast that could progress past their initial arrest but were still unable to successfully complete meiosis II [17], [18]. Based on these studies, it was concluded for both types of yeast that Top2 was required to resolve DNA entanglements that form between recombinant homologs at meiosis I [17], [18].
D. melanogaster contains only a single gene encoding a topoisomerase II enzyme, Top2, and null mutations in Top2 in Drosophila are lethal [20]. Heterozygous combinations of weak loss-of-function mutations are viable in some cases, but ovarian development is either so severely disrupted to prevent analysis or the mutations only mildly decrease Top2 function and/or protein levels [21]. These factors have made it difficult to fully assess the role that Top2 plays in the resolution of heterochromatic DNA threads at later stages of Drosophila female meiosis [21].
A solution to these difficulties was created when the Transgenic RNAi Project (TRIP) at Harvard Medical School made available a Top2 RNAi-expressing line that is inducible in the female germline using the maternal alpha-tubulin GAL (matαGAL) driver [22]. The matαGAL driver appears to start expressing robustly at approximately stage 3 of the Drosophila ovary [23]. By expressing Top2 RNAi with the matαGAL driver, Top2 levels can be decreased after the completion of the ovarian mitotic divisions where Top2 is essential and after the initiation of recombination where Top2 could potentially play a role. We will show that Top2 RNAi expression during prophase of Drosophila female meiosis leads to specific defects in the ability of heterochromatic regions to fully separate and for the achiasmate 4th chromosomes to move precociously towards the spindle poles in prometaphase I, despite the completion of spindle assembly. More importantly, we will demonstrate that the defect in chromosome separation leads to the inability of oocytes to successfully complete meiosis I, illustrating that the separation of heterochromatic regions is essential for the proper completion of meiosis.
Results
Top2 RNAi oocytes exhibit abnormal chromosomal projections during prometaphase I
Because strong loss-of-function alleles of Top2 are lethal [20], an RNAi construct targeting the Drosophila Top2 gene was expressed using the maternal α-tubulin GAL driver ({matalpha4-GAL-VP16}V37 or matαGAL for short) [22]. This allowed us to examine the effect of decreased Top2 levels starting at approximately stage 3 of the ovary during mid-prophase [23]. By Western blot, the level of Top2 protein present in Top2 RNAi/matαGAL oocytes was reduced compared to control lines, including flies heterozygous for only the matαGAL driver or only the Top2 RNAi construct (Figure S1). The knockdown was not complete, as a weak band of Top2 was typically present.
We analyzed Top2 RNAi-expressing oocytes using immunofluorescence with an antibody recognizing α-tubulin to mark the meiotic spindle and one recognizing histone 3 phosphorylated at serine 10 (H3S10p), which marks nuclei that have entered prometaphase I of meiosis and fortuitously highlights the DNA threads connecting achiasmate chromosomes during D. melanogaster female meiosis [8]. During prometaphase I in wild-type oocytes, achiasmate chromosomes biorient, and the obligately achiasmate 4th chromosomes move toward opposite poles of the bipolar spindle. H3S10p-positive threads are frequently observed emanating from, and often connecting, these chromosomes (Figure 1A) [8]. The achiasmate chromosomes then congress back to the chiasmate chromosomes by metaphase I and the chromosome mass forms a lemon-shaped structure [7].
Fig. 1. Expression of a Top2 RNAi construct in the ovary leads to abnormal chromosomal projections during meiosis I. 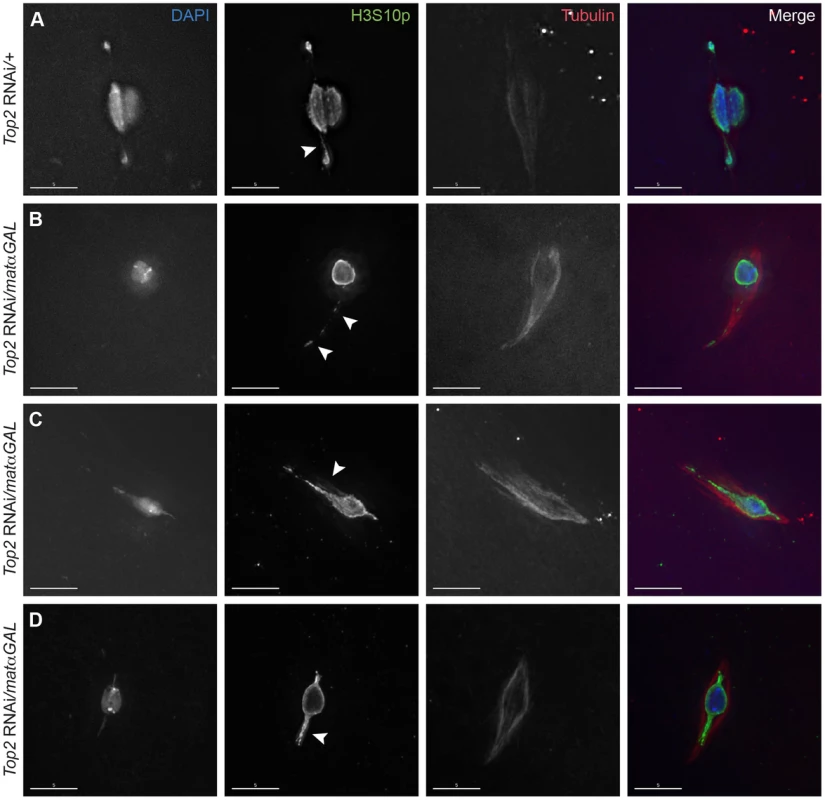
DNA is labeled with DAPI (blue), chromatin is labeled with an antibody recognizing H3S10p (green), and the spindle is labeled with an antibody recognizing α-tubulin (red). (A) Top2 RNAi/+ control oocyte with achiasmate 4th chromosomes that have moved towards the spindle poles. Arrowhead points to the H3S10p-labeled heterochromatic DNA thread emanating from the achiasmate 4th chromosome. (B–D) Shown are examples of Top2 RNAi/matαGAL oocytes containing abnormal DNA projections that do not appear to connect chromosomes (arrowheads). Images are projections of partial Z-stacks. Scale bars are 5 microns. Preparations of oocytes from two-day-post-eclosion mated females are enriched for prometaphase I oocytes [7]. Achiasmate homologs (usually the 4th chromosomes) were observed fully separated from the main chromosome mass in 40.0% (10/25) of such prometaphase I-enriched oocytes from mothers bearing only the Top2 RNAi construct (Top2 RNAi/+) and in 40.7% (11/27) of oocytes from mothers bearing only the driver (matαGAL/+). However, in similar preparations of Top2 RNAi/matαGAL oocytes, achiasmate chromosomes were rarely observed completely separated from the chiasmate chromosomes (3.7% of oocytes, 1/27). In addition, in 44.4% (12/27) of Top2 RNAi/matαGAL oocytes, one or more DNA projections were present that extended toward the spindle poles, with three of these oocytes containing two projections (Figure 1B–D). These projections stained positive for H3S10p and did not appear to connect chromosomes (Figure 1B–D). Similar projections were not observed in control oocytes heterozygous for only the Top2 RNAi construct or the matαGAL driver (N = 25 and 27, respectively). Thus, in Top2 RNAi/matαGAL oocytes, achiasmate homologs fail to separate from the main chromosome mass, as is observed in control oocytes. Rather, we often observe abnormal DNA projections emanating from that mass toward the pole. The nature of these projections is discussed below.
Abnormal DNA projections in Top2 RNAi/matαGAL oocytes contain centromeres
The location of centromeres in relationship to the DNA projections was assessed using an antibody recognizing Centromere Identifier (CID), the Drosophila CENP-A homolog [24]. In wild-type prometaphase I oocytes, a CID focus is typically observed leading each achiasmate 4th chromosome that has moved toward the spindle poles, while the six CID foci of the chiasmate chromosomes are located within the chromosomal mass such that homologous centromeres are oriented toward opposite spindle poles (Figure 2A). In Top2 RNAi-expressing oocytes, CID foci could be observed at or near the tip of the DNA projections in 97.5% (39/40) of projections analyzed, indicating that centromere-led movements of the chromosomes towards the spindles poles are the cause of the DNA projections (Figure 2B, C).
Fig. 2. Centromeres are present within and near the tips of the DNA projections in Top2 RNAi/matαGAL oocytes. 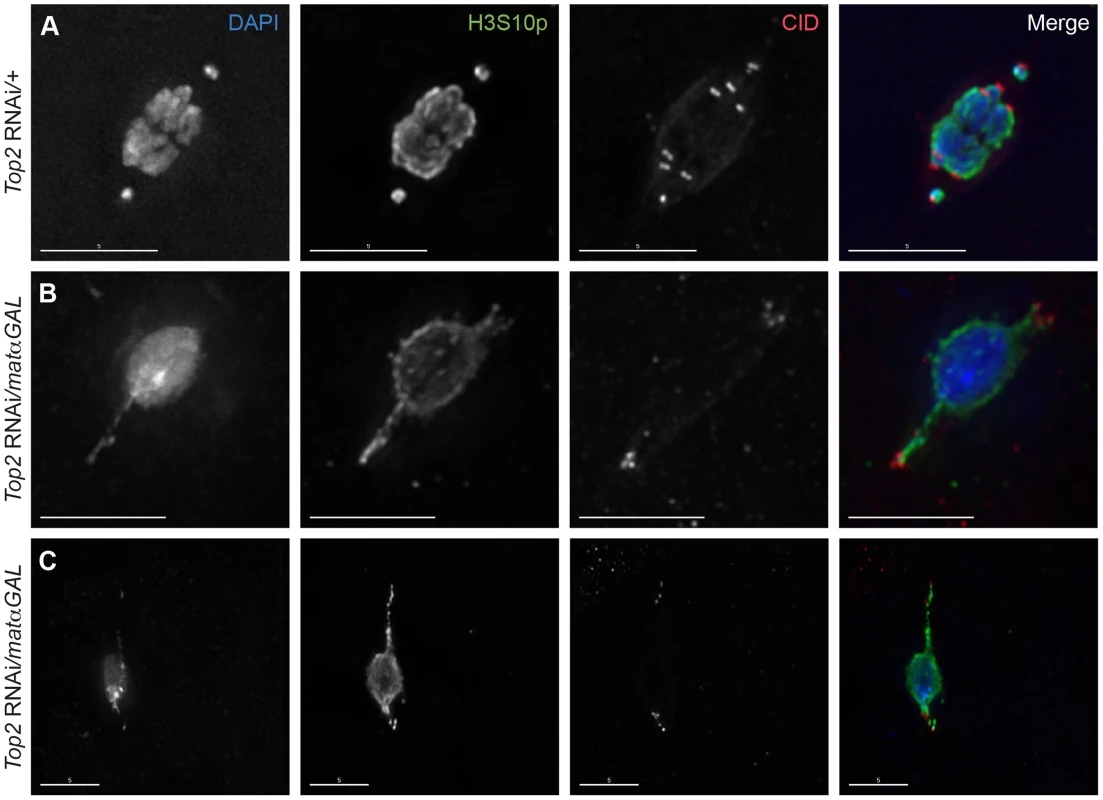
DNA is labeled with DAPI (blue), chromatin is labeled with an antibody recognizing H3S10p (green), and centromeres are labeled with an antibody recognizing CID (red). (A) Top2 RNAi/+ control oocyte with achiasmate 4th chromosomes that have moved towards the spindle poles with all eight centromeres properly bioriented. (B–C) Examples of Top2 RNAi/matαGAL oocytes with abnormal DNA projections containing CID foci at or near the tips of the projections, indicating that the centromeres of multiple chromosomes have moved towards the spindle poles. Images are projections of partial Z-stacks. Scale bars are 5 microns. More than one CID focus could be seen within some projections (Figure 2B, C), with five foci being the maximum number observed within a single projection within one oocyte. In all but four oocytes examined, the CID foci displayed some degree of clustering, making acquiring an accurate average number of CID foci within the projections impossible. However, Figure 2B shows an example where all the CID foci appear to be present within the two projections. This finding suggests that the projections are not simply the 4th chromosomes becoming entangled with the autosomes and stretching as they attempt to move toward the poles, but that centromeres of chiasmate chromosomes are also moving toward the poles in some Top2 RNAi/matαGAL oocytes. These results demonstrate that decreased Top2 levels generally affect the movement and organization of centromeres within the chromosome mass. The data also suggest that the DNA protrusions are caused by the centromeres of both achiasmate and chiasmate chromosomes being pulled or pushed towards the spindle poles while other parts of the chromosomes are still anchored at the center of the spindle. Such movements would result in portions of the chromosomes being stretched out behind the centromeres.
Top2 RNAi/matαGAL oocytes form bipolar spindles
To determine whether defects in spindle assembly were the cause of the abnormal centromere–led DNA projections, we examined spindle morphology in Top2 RNAi/matαGAL and control oocytes. In Top2 RNAi/+ oocytes, 87.5% (21/24) of spindles were bipolar and 75.0% (18/24) were tapered at both ends of the spindle. In matαGAL/+ oocytes, 100% (27/27) of spindles were bipolar and 74.0% (20/27) were fully tapered at both ends. Despite the abnormal DNA projections described above, chromosomes in Top2 RNAi/matαGAL oocytes were also able to organize a bipolar spindle in 88.5% (23/26) of oocytes, though in some cases one or both sides of the spindle were elongated to accommodate the chromosomal projections (Figure 1B–D). However, tapering of both ends of the spindle was only seen in 57.7% (15/26) of oocytes. This decrease in spindle tapering at both ends of the spindle may be due to the abnormal DNA projections rather than direct defects in spindle assembly caused by decreased Top2 levels.
The ability of Top2 RNAi/matαGAL oocytes to organize a bipolar spindle is further illustrated by live imaging. Video S1 shows a bipolar spindle quickly forming after germinal vesicle breakdown in a Top2 RNAi/matαGAL oocyte. After the completion of spindle assembly, the spindle remained bipolar and the achiasmate chromosomes remained associated with the autosomes for the duration of imaging. In 11 time-lapses of Top2 RNAi/matαGAL oocytes undergoing germinal vesicle breakdown, eight successfully formed a bipolar spindle. Additionally, all nine oocytes that had already completed spindle assembly by the start of live imaging maintained bipolar spindles for the duration of imaging. These results argue strongly that defects in spindle assembly are not the primary cause of the centromere-led abnormal projections in Top2 RNAi/matαGAL oocytes.
DNA projections observed in Top2 RNAi/matαGAL oocytes are composed of heterochromatin
The regions of DNA that stain brightest with DAPI are typically the heterochromatic regions, such as those near the centromeres and a large portion of the 4th chromosomes. At metaphase I, the DAPI-bright DNA regions are oriented towards opposite spindle poles and are located at the ends of the chromosome mass [7]. In Top2 RNAi/matαGAL oocytes, DAPI-bright regions could be observed in aberrant configurations. For example, in Figure 2B, a single DAPI-bright region is present in the middle of the chromosome mass, and in Figure 2C a single DAPI-bright region is present at one end of the chromosome mass. To investigate this further, we used an antibody recognizing histone 3 trimethylated on lysine 9 (H3K9me3), a chromatin modification associated with heterochromatin [25], [26]. While in Top2 RNAi/+ oocytes the H3K9me3 signals were observed oriented towards both spindle poles, in Top2 RNAi/matαGAL the H3K9me3 signal was present on the DNA within the projections (Figure 3). H3K9me3-positive regions could also be seen splayed across the chromosome mass (Figure 3). These results suggest that the projections are composed of heterochromatic sequences, and we will show below that knockdown of Top2 causes a failure of heterochromatic regions to properly orient.
Fig. 3. Aberrant DNA projections are composed of heterochromatin. 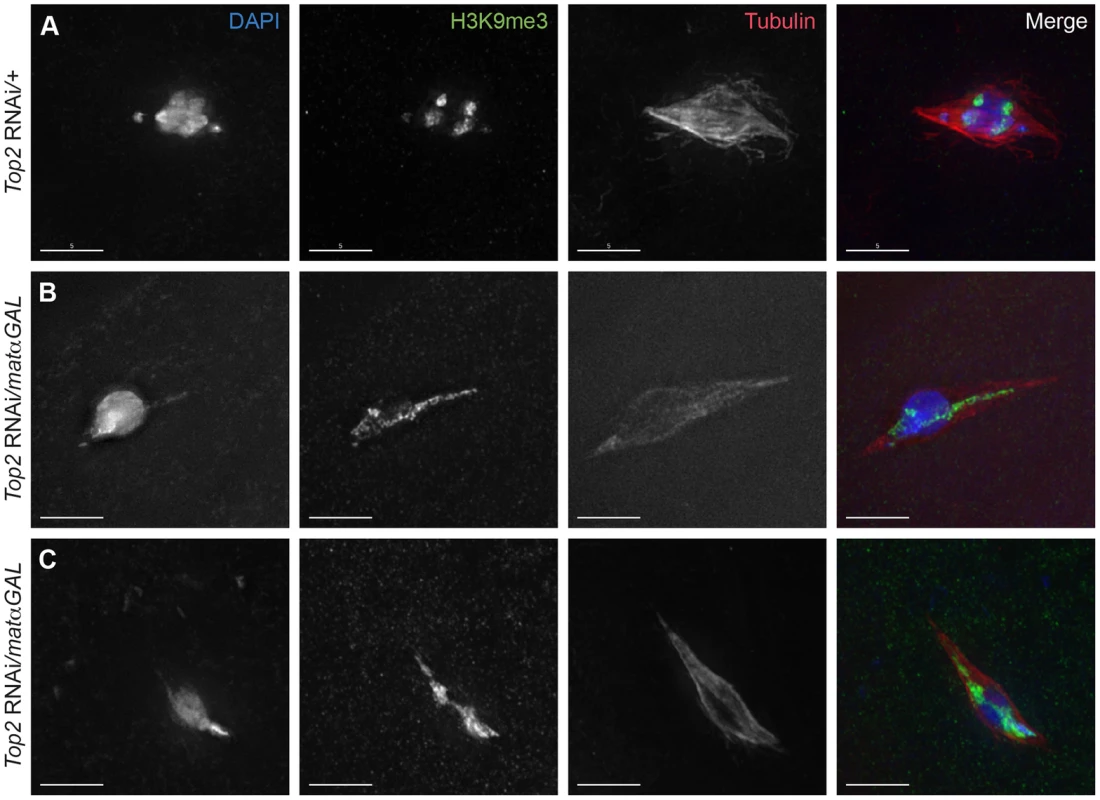
DNA is labeled with DAPI (blue), heterochromatin is labeled with an antibody recognizing histone 3 trimethylated on lysine 9 (H3K9me3) (green), and the spindle is labeled with an antibody recognizing α-tubulin (red). (A) Top2 RNAi/+ control oocyte with achiasmate 4th chromosomes that have moved towards the spindle poles. The 4th chromosomes and the centromeric regions of the chromosomes are labeled with H3K9me3 and are oriented towards opposite spindle poles. (B–C) Top2 RNAi/matαGAL oocytes containing abnormal DNA projections that are labeled with the H3K9me3 antibody. Additionally, the H3K9me3 antibody localization in the chromosome mass of both oocytes is not oriented towards opposite spindle poles. Images are projections of partial Z-stacks. Scale bars are 5 microns. Heterochromatic regions fail to separate properly in Top2 RNAi-expressing oocytes
As the DNA projections appeared to be composed of heterochromatin, as based on the H3K9me3 antibody, we next wanted to determine whether these projections were simply caused by the failure of the achiasmate 4th chromosomes to move fully toward the spindle poles. Because heterochromatic threads connect achiasmate chromosomes during prometaphase I, we also wanted to know how heterochromatic regions would be affected by decreased Top2 levels. Finally, we wanted to determine whether homologous chromosome orientation would be affected by Top2 RNAi expression.
We utilized fluorescent in situ hybridization (FISH) probes recognizing heterochromatic regions of each chromosome to look at the separation of specific heterochromatic regions and to assess biorientation of homologs in females bearing structurally normal X, 2nd, 3rd, and 4th chromosomes. We first examined a probe recognizing the 359-bp satellite, which is primarily localized to the X chromosome, with a minor region on the 3rd chromosome [27]. In prometaphase I and metaphase I control Top2 RNAi/+ and matαGAL/+ oocytes, two large, well-separated and bioriented fluorescent 359-bp signals were observed in 96.4% (80/83) and 96.3% (79/82) of oocytes, respectively (Figure 4A, B). Furthermore, in immunoFISH studies using the 359-bp probe and an α-tubulin antibody to mark the spindle, the X chromosomes were properly oriented toward opposite poles of the bipolar spindle in 100% (16/16) of Top2 RNAi/+ oocytes (Figure S2A).
Fig. 4. Top2 RNAi/matαGAL oocytes show defects in chromosome biorientation and in the separation of heterochromatic regions of the X and 4th chromosomes. 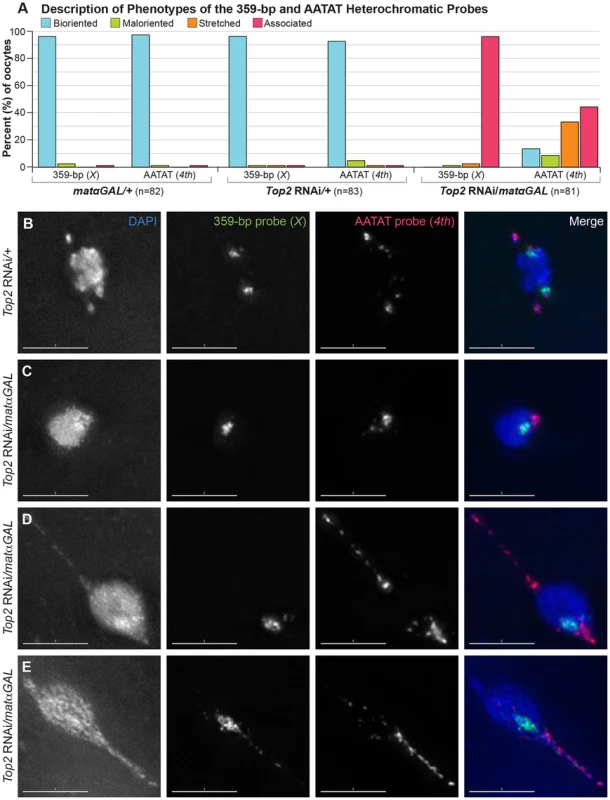
(A) Quantification of the phenotypes observed for the 359-bp and AATAT heterochromatic probes. Bioriented represents oocytes with two FISH probe foci oriented in opposite directions. Maloriented represents two FISH probe foci oriented in the same direction. Stretched represents FISH signal that is highly elongated rather than in discrete foci. Associated represents oocytes with only a single FISH probe focus. (B–E) DNA is labeled with DAPI (blue), a FISH probe targeting the 359-bp repeat predominantly on the X chromosome and a minor region on the 3rd chromosome is in green, and a FISH probe to the AATAT heterochromatic repeat on the 4th chromosome and a minor repeat on the X chromosome is shown in red. (B) Top2 RNAi/+ control oocyte with two foci for both probes oriented in opposite directions, indicating proper biorientation of the X and 4th chromosomes. (C–E) Shown are examples of Top2 RNAi/matαGAL oocytes displaying aberrant separation of heterochromatic regions. (C) Probes indicate that both heterochromatic regions have failed to separate. (D) The X chromosomes have failed to separate. The 4th chromosomes have separated and bioriented, but one 4th chromosome appears to be highly stretched out and present in an abnormal DNA projection. (E) Both heterochromatic regions have failed to separate, but the AATAT repeat and, to a small degree, the 359-bp region have stretched into the abnormal DNA projection. For the 359-bp probe 96.3% (79/82) of Top2 RNAi/matαGAL oocytes displayed a complete failure of the probe to separate. In two (2.4%) Top2 RNAi/matαGAL oocytes the 359-bp probe appeared stretched out and one oocyte (1.2%) had 2 maloriented foci. Images are projections of partial Z-stacks. Scale bars are 5 microns. However, when we followed the X chromosomal heterochromatin using the 359-bp probe in Top2 RNAi/matαGAL oocytes, none of the oocytes (0/81) displayed two bioriented 359-bp foci. Instead, the 359-bp heterochromatic region failed to fully separate in 96.3% (78/81) of Top2 RNAi/matαGAL oocytes that should have been in prometaphase I or metaphase I, based on egg chamber stage (Figure 4A, C–E). In an immunoFISH experiment with α-tubulin, the lack of separation of the 359-bp heterochromatic region could be observed even on fully formed bipolar spindles in Top2 RNAi/matαGAL oocytes. Two bioriented 359-bp foci were not observed in any of the 16 oocytes examined, which is to say that the 359-bp regions failed to separate for 100% (16/16) of the spindles. This result indicates that the failure to separate heterochromatic regions is not likely to be due to a defect in meiotic progression or spindle assembly after germinal vesicle breakdown (Figure S2B, C).
We then asked whether the heterochromatin of the achiasmate 4th chromosomes would fail to separate in Top2 RNAi/matαGAL oocytes. A FISH probe recognizing the AATAT repeat present throughout the 4th chromosomes was examined. Two large, well-separated and bioriented AATAT probe foci were observed in 92.8% (77/83) of Top2 RNAi/+ control oocytes (Figure 4A, B) and in 97.6% (80/82) of matαGAL/+ oocytes (Figure 4A). However, separation of the AATAT 4th chromosome probe was strongly impaired in Top2 RNAi/matαGAL oocytes. The 4th probe failed to separate in 44.4% (36/81) of Top2 RNAi/matαGAL oocytes (Figure 4A, C). In 33.3% (27/81) of Top2 RNAi/matαGAL oocytes, the 4th probe signal was highly elongated, often extending into one or more projections (Figure 4A, D, E). This phenotype suggests that the AATAT repeat of the 4th chromosomes has become stretched out and indicates that at least some of the projections are composed of 4th chromosome heterochromatin. In 8.6% (7/81) of oocytes, two foci could be distinguished, but the foci were still oriented toward the same side of the DNA mass, indicating a failure in 4th chromosome biorientation. However, 13.6% (11/81) of oocytes did display two separated and bioriented foci, indicating that at least in some cases this region of the 4th chromosome can successfully separate and biorient in Top2 RNAi/matαGAL oocytes. These data demonstrate that decreased Top2 levels result in defects in 4th chromosome biorientation and the full separation of the AATAT heterochromatin repeat.
Heterochromatin threads have been observed connecting the 4th and X homologs during prometaphase I in Drosophila oocytes [6]. The presence of these threads may make heterochromatic regions of these chromosomes more sensitive to decreased Top2 levels. To determine whether a heterochromatic region of the chiasmate 2nd chromosomes would also be affected by decreased Top2 levels, we examined a heterochromatic probe recognizing the AACAC repeat on the right arm of the 2nd chromosome in prometaphase I and metaphase I oocytes [27]. Two bioriented AACAC foci were present in 96.3% (78/81) of Top2 RNAi/+ oocytes and 97.6% (82/84) of matαGAL/+ oocytes (Figure 5A, B). In contrast, Top2 RNAi/matαGAL oocytes displayed only a single focus for the AACAC probe in 71.4% (60/84) of oocytes that had likely completed spindle assembly based on egg chamber stage, indicating that, like heterochromatic regions on the X and 4th chromosomes, this heterochromatic region of 2R is also defective in its ability to separate (Figure 5A, C, D, E). In 22.6% (19/84) of oocytes, two maloriented AACAC probe foci were observed. The probe was stretched in 2.4% (2/84) of Top2 RNAi/matαGAL oocytes and two bioriented foci were observed in only 3.6% (3/84) of oocytes. These results indicate that a decreased Top2 level affects the separation of the AACAC heterochromatic region and the proper biorientation of the 2nd chromosomes during meiosis I.
Fig. 5. Top2 RNAi/matαGAL oocytes show defects in chromosome biorientation and in the separation of heterochromatic regions of the 2nd and 3rd chromosomes. 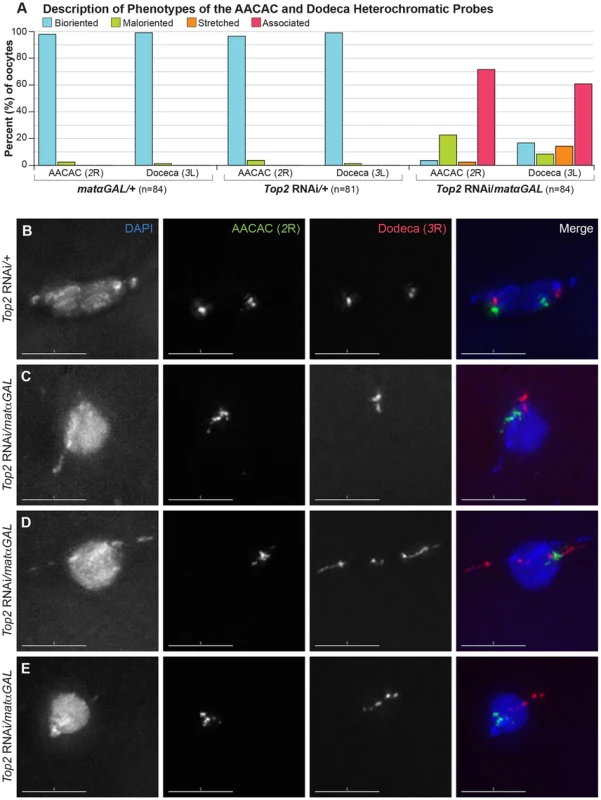
(A) Quantification of the phenotypes observed for the AACAC and Dodeca heterochromatic probes. Bioriented represents oocytes with two FISH probe foci oriented in opposite directions. Maloriented represents two FISH probe foci oriented in the same direction. Stretched represents FISH signal that is highly elongated rather than discreet foci. Associated represents oocytes with only a single FISH probe focus. (B–E) DNA is labeled with DAPI (blue), a FISH probe targeting the AACAC repeat on the right arm of the 2nd chromosome is in green, and a FISH probe to the Dodeca heterochromatic repeat on the right arm of the 3rd chromosome is shown in red. (B) Top2 RNAi/+ control oocyte with two foci for both probes oriented in opposite directions, indicating proper biorientation of the 2nd and 3rd chromosomes. (C–E) Examples of Top2 RNAi/matαGAL oocytes displaying aberrant separation of heterochromatic regions. (C) Probes indicate that both heterochromatic regions have failed to separate. (D) The AACAC heterochromatic region of the 2nd chromosomes has failed to separate. The Dodeca heterochromatic region of the 3rd chromosome has separated but the heterochromatic region is highly stretched out and present in the abnormal DNA projections. (E) Both heterochromatic regions appear abnormal and the 2nd and 3rd chromosomes are improperly oriented so as to segregate away from each other. Images are projections of partial Z-stacks. Scale bars are 5 microns. Finally, we asked whether a heterochromatic region of the chiasmate 3rd chromosomes would show similar defects. We utilized a FISH probe to the heterochromatic Dodeca satellite on the 3rd chromosomes. Two bioriented Dodeca foci were present in 98.8% (80/81) of Top2 RNAi/+ control oocytes and 98.8% (83/84) of matαGAL/+ control oocytes during prometaphase I and metaphase I (Figure 5A, B). Upon Top2 RNAi expression, the Dodeca heterochromatic repeat failed to separate in 60.7% (51/84) of oocytes (Figure 5A, C). In 13.1% (11/84) of Top2 RNAi/matαGAL oocytes, the Dodeca FISH signals were highly stretched, often extending into a projection (Figure 5A, D, E). This observation demonstrates that the projections can be composed of chiasmate chromosome heterochromatin as well as that of the achiasmate 4th chromosomes. The Dodeca probe was bioriented in 16.7% (14/84) of oocytes, while two foci were maloriented in 8.3% (7/84) of oocytes. This illustrates that 3rd chromosome heterochromatin is also affected by decreased Top2 expression. We also observed that in 36.4% (30/83) of Top2 RNAi/matαGAL oocytes the 2nd and 3rd chromosomes segregated away from each other (Figure 5E) and that in 24.1% (20/83) of oocytes, both sets of 2nd and 3rd chromosomes were oriented in the same direction. These results once again illustrate that Top2 is involved in the proper biorientation of chiasmate chromosomes during meiosis I.
In conclusion, heterochromatic regions on all four chromosomes showed defects in their ability to fully separate at prometaphase I and metaphase I in Top2 RNAi/matαGAL oocytes. Additionally, these chromosomes displayed a failure to properly biorient. These data support the idea that Top2 is involved in releasing the bonds that hold heterochromatic regions together during prophase [5]. Even if heterochromatic regions could become fully separated at anaphase I in Top2 RNAi/matαGAL oocytes, the orientation of homologous chromosomes toward the same pole would lead to high levels of chiasmate and achiasmate chromosome missegregation [7], [28]. Although the primary conclusion to be drawn here is that Top2 is required to separate heterochromatic regions on all four chromosomes, perhaps the more interesting inference is that the lack of heterochromatic separation argues strongly for heterochromatic entanglements that affect all four heterochromatic regions tested.
A mutated RNAi construct fails to cause defects in the separation of heterochromatic regions
To ensure that the defects in heterochromatic separation observed with the Top2 RNAi construct were caused specifically by the 21-nucleotide sequence targeting Top2, we constructed a mutated RNAi construct and tested its effects on spindle assembly and heterochromatin separation (see Materials and Methods). The Top2 RNAi construct shares homology with a second locus (CG33296) at 18 of the 21 nucleotides. Rather than randomly mutagenizing or scrambling the original construct, the Top2 RNAi construct was mutated to match the CG33296 gene, even though a meiotic function has not been speculated for this gene product nor has it been reported to show ovarian expression (FlyBase). We hoped the targeted mutagenesis of the Top2 RNAi construct would provide a better control for the specificity of the construct compared to a randomly scrambled construct that would only address the potential general effects of RNAi induction in the ovary.
Bipolar spindles could be observed in oocytes from CG33296 RNAi/matαGAL mothers (Figure S3A) and at least one 4th chromosome was separated from the autosomes in 27.6% (8/29) of oocytes. FISH experiments of CG33296 RNAi/matαGAL oocytes showed that all four heterochromatic FISH probes were well separated and properly bioriented in the majority of oocytes (97.8% [44/45] 359 bp, 96.7% [29/30] AACAC, 100% [30/30] Dodeca, and 97.8% [44/45] AATAT) (Figure S3). These data support the conclusion that the effects of the Top2 RNAi construct are due to decreased Top2 levels rather than off-target RNAi effects.
Euchromatic regions can separate during mid-prophase in Top2 RNAi/matαGAL oocytes
Dernburg et al. [5] noted that Drosophila oocytes undergo a modified diplotene phase, in which euchromatic regions appear to separate as early as stage 3–4, while heterochromatic regions remain tightly paired until prometaphase I. Thus, we did not expect that the RNAi knockdown generated in Top2 RNAi/matαGAL oocytes performed in these studies would impair separation of euchromatic regions. To verify this hypothesis, we examined mid-prophase oocyte nuclei to determine whether euchromatic regions could separate in Top2 RNAi/matαGAL oocytes. Dernburg et al. [5] observed separation of the euchromatic histone locus in 54.5% (36/66) of mid-prophase oocytes. Similarly, we observed that Top2 RNAi/matαGAL oocytes exhibited two foci of fluorescence for a BAC probe to polytene band 3C of the X chromosome in 51.1% (23/43) of prophase oocytes compared to 48.9% (22/45) of Top2 RNAi/+ control oocytes (Figure S4). Using a BAC probe to polytene bands 7DE of the same chromosome, 57.6% (19/33) of Top2 RNAi/matαGAL oocytes were observed to have two foci compared to 47.9% (23/48) of Top2 RNAi/+ control oocytes (Figure S4). These results suggest that while Top2 is required for the separation of heterochromatic regions following nuclear envelope breakdown, Top2 is either not required for the separation of euchromatic regions during mid-prophase, or the matαGAL driver does not knock down the level of Top2 enough to affect separation of euchromatic regions. However, these results do not rule out the possibility that there are earlier euchromatic entanglements that are formed during replication and then resolved prior to Top2 RNAi induction. Our results are consistent with our view that DNA entanglements that persist into mid-to-late prophase, as revealed by Top2 knockdown, are specific to the heterochromatin.
Embryos from Top2 RNAi-expressing mothers fail to initiate proper mitotic divisions
A defect in the ability to properly separate heterochromatic regions during prometaphase and metaphase of meiosis I would likely result in a failure to properly complete meiosis and enter into the first mitotic divisions after fertilization. In embryos from Top2 RNAi/+ control mothers, we observed normal embryonic development in 30/30 (100%) embryos, indicating that meiosis was successfully completed (Figure 6A). In contrast, 0.0% (0/20) of embryos from Top2 RNAi/matαGAL mothers initiated proper embryonic development, with 85.0% (17/20) containing only two nuclei: a small nucleus with a centriolar spindle that was presumed to be the paternal pronucleus and a larger, round nucleus (Figure 6B, C). This round nucleus was surrounded by α-tubulin that was not organized into a bipolar shape. Because this nucleus did not resemble the typical rosette structure of embryos, it was presumed to be the oocyte nucleus that had failed to exit meiosis I. The three exceptions are described in the legend of Figure 6. This absence of mitotic entry led to a complete failure of embryos from Top2 RNAi-expressing mothers to hatch (0.0% [0/447]).
Fig. 6. Embryos from Top2 RNAi/matαGAL mothers appear to be arrested in meiosis I. 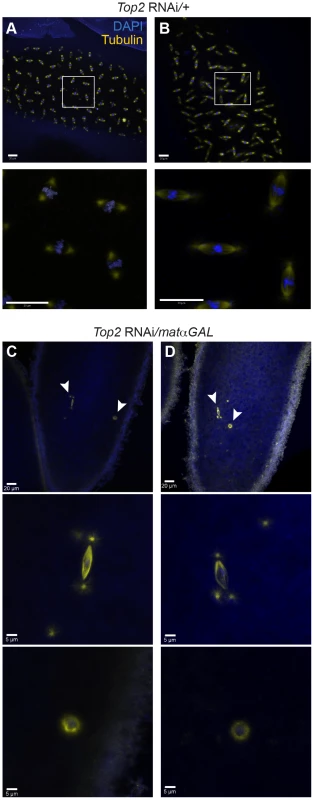
DNA is labeled with DAPI (blue) and spindles are labeled with an antibody recognizing α-tubulin (yellow). (A and B) Top2 RNAi/+ embryos displaying normal development. Boxed regions are shown magnified below each image. (C and D) Embryos from Top2 RNAi/matαGAL mothers with two nuclei indicating proper fertilization but an arrest in meiosis I. 17/20 embryos from Top2 RNAi/matαGAL mothers contained only 2 nuclei. Of the remaining 3 embryos, one contained only a single nucleus and 2 appeared to undergo an aberrant division. Arrowheads indicate regions magnified below both images. Images are projections of partial Z-stacks. Scale bars are 20 microns. Discussion
We have shown that knockdown of Top2 during prophase of meiosis I in Drosophila oocytes results in defects in homolog segregation and sterility. Heterochromatic regions of all four chromosomes failed to properly separate, leading to a failure of chromosomes to properly biorient during meiosis I. Additionally, achiasmate chromosomes showed defects in their ability to move away from the autosomes towards the spindle poles in prometaphase I. Instead, abnormal chromosomal projections were present. These DNA projections displayed several differences compared to the heterochromatic threads observed in wild-type prometaphase I oocytes. Specifically, the projections did not appear to directly connect two chromosomes and, more importantly, contained centromeres, which are not present in wild-type DNA threads. These attributes suggest that chromosomes initiate centromere-led movement but are anchored by DNA entanglements at the center of the spindle, resulting in the stretching out of chromosomal regions.
One might imagine that one component of these defects reflects a role of Top2 in resolving chiasmata. Indeed, in yeast, conditional mutants of top2 caused a meiotic cell cycle arrest that was partially alleviated by simultaneously eliminating recombination, indicating that in yeast, some of the targets of Top2 during meiosis are recombination dependent [17], [18]. Several lines of evidence suggest that this is not the case in Drosophila. First, the matαGAL driver used in the Drosophila oocytes does not appear to be strongly expressed until after recombination is thought to be finished [23]. Second, recombination is suppressed in heterochromatic regions near the centromeres [29]. Therefore, it seems more likely that the defects in heterochromatic separation during Top2 knockdown are due to the failure to resolve the heterochromatic threads observed during prometaphase I rather than a failure of Top2 to resolve DNA entanglements during the repair of double-strand breaks initiated in early prophase. Third, the AATAT heterochromatic repeat of the 4th chromosomes also shows defects in separation. Since the 4th chromosomes, which never undergo crossing over and are thus obligately achiasmate, also fail to properly separate heterochromatic regions [1], the defects in 4th chromosome heterochromatin separation cannot be due to homologous connections formed as a result of recombination. Finally, the heterochromatic threads observed during prometaphase I in Drosophila oocytes are not dependent on recombination, since they are observed in mutants of the Drosophila spo11 homolog, mei-W68 (Figure S5). While Top2 appears to affect the heterochromatic regions by resolving DNA entanglements that are recombination independent, it is unknown whether or not Top2 plays a role in the resolution of chiasmata at anaphase I. Since knockdown of Top2 results in metaphase I arrest, a role at anaphase I in chiasmata resolution cannot be assessed under these conditions.
The observation that 4th chromosome sequences are present within the DNA projections in Drosophila oocytes is not surprising given that the 4th chromosomes often move precociously towards the spindle poles during prometaphase I. However, it is more difficult to explain the stretching of 3rd chromosome heterochromatic sequences into some projections and the multiple CID foci within the projections. These results may indicate that the centromeres of the chiasmate chromosomes are also attempting to move towards the poles in Top2 RNAi-expressing oocytes. One possibility is that these projections are the consequence of a failed attempt by the oocyte to separate the heterochromatic regions. The four heterochromatic regions examined varied in the extent that they failed to separate. These differences may be due to a difference in the number of DNA entanglements between homologous heterochromatic regions in the oocytes.
The 359-bp region displayed the highest failure to separate upon Top2 knockdown. Several lines of evidence have suggested that the 359-bp heterochromatic region of the X may be handled differently by the cell than other heterochromatic regions. First, Ferree and Barbash [30] demonstrated that the hybrid lethality between D. simulans females and D. melanogaster males is due to the formation of anaphase bridges containing the 359-bp repeat region during mitosis in hybrid embryos. The authors speculate that D. simulans may lack factors for proper condensation of the 359-bp region. More recently, Ferree et al. [31] demonstrated that the lethality caused by some circularized X-Y ring chromosomes is also due to anaphase bridging of the 359-bp repeat in embryos. In both instances, Top2 localized to the anaphase bridges. Additionally, during the mitotic divisions of the Drosophila germarium, the 359-bp region is highly paired while the AACAC and Dodeca regions of the 2nd and 3rd chromosomes are mostly unpaired [32]. These results, as well as the complete failure of the 359-bp region of the X to separate in over 90% of oocytes when Top2 is knocked down, suggest that the 359-bp region may be especially prone to form DNA entanglements during replication (both between homologs and sisters) or that these entanglements may be processed differently than those in other heterochromatic regions. Additionally, in vivo studies of Top2 cleavage sites during mitosis in Drosophila showed a major cleavage site in the 359-bp repeat [33]. A failure to cleave this site when Top2 levels are decreased in meiosis I may contribute to the high failure of the 359-bp repeat to separate in Top2 RNAi oocytes.
Comparing the meiotic effects of Top2 knockdown in males versus females
In a parallel study examining decreased Top2 levels during Drosophila male meiosis, in which recombination does not occur [34], Mengoli et al. (cosubmitted) observed phenotypes similar to those seen by us in oocytes. Homologs, as well as sister chromatids, frequently failed to separate during meiosis I and meiosis II in Drosophila males, despite the formation of bipolar spindles similar to Drosophila oocytes. Homologs were stretched out into anaphase bridges at meiosis I, a phenotype which has similarities to the stretched out chromosomal projections in oocytes. These results indicate that Topoisomerase II may resolve similar DNA connections in both sperm and oocytes. Thus, the cells are responding in a similar fashion to deal with the failure of the resolution of these homologous connections. It is worth noting, however, that Mengoli et al. (cosubmitted) observed defects in euchromatic regions as well as heterochromatic regions of the chromosomes during male meiosis, while in oocytes, only heterochromatic regions were affected. This difference likely reflects the fact that Mengoli et al. (cosubmitted) examined mutations affecting Top2 levels at the start of meiosis while in Drosophila females the Top2 RNAi construct is not expressed until mid-late prophase.
Comparing the meiotic and mitotic roles of Top2
Decreasing the level of Top2 in mitotic cell types causes phenotypes that are both similar to and divergent from those phenotypes observed during Drosophila female and male meiosis (Mengoli et al. cosubmitted). Top2 RNAi in mitotic Drosophila S2 cells led to the formation of DNA projections [35]. Although these chromosomal projections appeared similar to the ones in oocytes, CID foci were not observed within the DNA projections in S2 cells, while one or more CID foci were present in the projections in oocytes. This suggests that while the projections in oocytes are at least partially centromere led, the S2 projections are composed of the arms of the chromosomes. Top2 RNAi expression in S2 cells results in extensive chromosome lagging, chromosome bridging during anaphase, and chromosome missegregation [36]. Oocytes expressing Top2 RNAi seem to arrest in metaphase I before chromosome bridging can manifest, but chromosome missegregation is evident in oocytes as well.
We should also note that knockdown of Top2 in Kc cells leads to a decrease in euchromatic pairing without affecting the pairing of heterochromatic regions [37], [38]. However, evidence suggests Top2 is acting in different ways in each system. In meiosis, heterochromatic regions remain associated at higher levels than euchromatic regions during mid to late prophase [5], while euchromatic pairings persist longer than heterochromatic pairings during mitosis in cultured cells [37], [38]. Our data suggest that the lack of heterochromatin dissociation is due to the failure of Top2 to resolve DNA entanglements within the heterochromatin, while there is no evidence for the persistence of similar entanglements between euchromatic regions upon knockdown of Top2 in cell culture.
The study by Mengoli et al. (cosubmitted) suggests that different phenotypes manifest in Drosophila larval neuroblasts depending on the residual level of Top2. Additionally, different cell types, for example sperm, were more sensitive to decreases in Top2 levels. This study, as well as others in Drosophila [21], [39], illustrates the complexity of understanding the function of Top2 in resolving various types of DNA entanglements. For example, expression of the Top2 RNAi construct using the nanos-Gal4:VP16 driver, which is expressed beginning in germline stem cells [40], led to minimal ovarian development (Figure S6), suggesting that high levels of Top2 expression are likely necessary to resolve DNA entanglements caused by replication in the germline stem cell divisions and/or the cystoblast divisions. This hinders the examination of the role of Top2 in such processes as replication, recombination, and chromosome condensation early in oogenesis.
Knocking down topoisomerase II enzymes using RNAi or chemically inhibiting its two isoforms in mitotic human cell lines leads to a number of defects, including entangled chromosomes, chromosome segregation defects, cell cycle delays, and in some cases cell cycle arrest [41]–[43]. Most interesting is that chemically inhibiting Topoisomerase IIα in HeLa cells has been reported to increase the number and duration of PICH (Plk1-interacting checkpoint helicase)-positive ultrafine DNA bridges that connect centromeres during anaphase of mitosis, including to non-centromeric regions of the chromosomes. These results indicate that Topoisomerase II enzymes resolve DNA entanglements prior to anaphase in addition to those observed at the centromeres in mitotic cells [44], [45]. These PICH-positive ultrafine bridges have several similarities to the DNA threads observed during prometaphase I of Drosophila oocytes, in that they are composed of heterochromatin and connect segregating chromosomes. While mitotic DNA entanglements are between sister chromatids and some of the meiotic entanglements are likely between homologs, the results suggest that Topoisomerase II enzymes may play a conserved role in resolving chromosomal entanglements in mitosis and meiosis.
Determining the mechanism by which Topoisomerase II functions to resolve mitotic entanglements may provide insight into its potential role in resolving the meiotic threads observed by Hughes et al. [6]. In HeLa cells, centromeric cohesion appears to protect centromeric DNA threads from resolution until anaphase I when this cohesion is lost, and a similar mechanism is believed to protect centromeric concatenations at centromeres until anaphase II during the mouse male meiotic divisions [46], [47].
Based on these studies, it is at least possible that in Drosophila oocytes entanglements may form during replication in both heterochromatic and euchromatic regions, but euchromatic entanglements may be more accessible to resolution by Top2 immediately after replication. These entanglements would be resolved before the Top2 RNAi construct is induced. Heterochromatic entanglements may be protected from early resolution due their conformation after replication or the presence of heterochromatin binding proteins. Top2 would be unable to resolve these entanglements until the karyosome reorganizes for prometaphase I or until tension is provided on the DNA by microtubules, as has been proposed for the resolution of entanglements by Top2 in yeast [48]. At this stage Top2 levels would be reduced in Top2 RNAi-expressing oocytes, leading to a failure to resolve these entanglements. Alternatively, heterochromatic regions may be particularly prone to forming entanglements due to the repetitive nature of heterochromatic DNA, and thus more sensitive to decreased Top2 levels.
Does Top2 knockdown also affect chromosome condensation in late-stage Drosophila oocytes?
Topoisomerase II enzymes have also been implicated in regulating chromosome condensation in a number of cell types and organisms [12]. For example, Mengoli et al. (cosubmitted) reported that the centric heterochromatin appeared undercondensed in some neuroblasts from Top2 RNAi-expressing larvae. Additionally, strong knockdown of Top2 levels in Drosophila S2 cells led to a large and quantitative change in chromosome condensation [35].
These observations led us to ask whether some of the phenotypes observed in this study might be the consequence of the effect of Top2 depletion on chromosome condensation, especially in the pericentric heterochromatin. Global chromosome condensation in Top2 RNAi-expressing oocytes looked similar to wild-type oocytes, but small changes in chromosome condensation would be obscured by the close proximity of the chromosomes at prometaphase I and the high level of condensation of the chromosomes. It is thus possible that chromosomes are undergoing mild decreases in condensation, particularly in heterochromatic regions, when Top2 levels are decreased. Decreased condensation could contribute to the stretched out phenotype observed with the 3rd and 4th chromosome FISH probes and to the centromere-led chromosomal projections. However, the effects on condensation that we observe appear to be too weak to account for the entanglements and stretching that we observe.
A model for the meiotic role of Top2
These observations lead to a speculative model for the cause of the defects in Top2 RNAi-expressing oocytes. In wild-type oocytes, DNA entanglements form between the highly repetitive DNA sequences of heterochromatic regions. These entanglements could form during replication by stalled replications forks that can occur in the repetitive heterochromatic regions or by intertwinings that could form as chromosomes are replicated in close proximity. Entanglements within the heterochromatic regions would not be immediately resolved. Therefore, these entanglements could help hold heterochromatic regions of homologous chromosomes tightly together during prophase, including those chromosomes that, like the 4th chromosomes, fail to undergo recombination [5]. As germinal vesicle breakdown approaches, many of these entanglements would have to be resolved by Top2 in order for chromosomes to separate and biorient properly during prometaphase I and metaphase I, possibly assisted by karyosome reorganization and/or microtubule attachments to the chromosomes. The chromatin threads observed during prometaphase I could be the entanglements that failed to be resolved during late prophase or those protected from resolution to facilitate the biorientation of achiasmate chromosomes. Top2 and/or other enzymes would then resolve these final DNA threads by anaphase I. In oocytes with decreased levels of Top2, many of the DNA entanglements would not be resolved during meiosis I, leading to a failure of homologous heterochromatic regions to separate. In some cases, heterochromatic regions appear to attempt separation, but the DNA entanglements hold the chromosomes together at one or more places and the rest of the heterochromatic regions of the chromosomes become highly stretched out. The centromere-led DNA projections apparently occur when chromosomes attempt separation despite the existence of heterochromatic entanglements. Since the heterochromatic regions would still be locked together at egg activation when meiosis resumes, chromosomes would be unable to segregate to opposite spindle poles at anaphase I and ultimately, the oocytes would fail to exit meiosis I. Our results indicate that Top2 plays an important role in resolving homologous DNA entanglements in Drosophila oocytes. These results also suggest that the formation of such entanglements (by whatever mechanism) may be a characteristic of the meiotic process.
Materials and Methods
Stocks
Flies were maintained on standard food at 25°C. Fly stocks used for RNAi experiments were y w; spapol, w; {matα4-GAL-VP16}V37 (Bloomington 7063), y1 sc1 v1; P{y[+t7.7] v[+t1.8] = TRiP. GL00338}attP2 (Bloomington 35416) an RNAi construct targeting the Top2 (CG10223) gene, and y v; CG33296 RNAi (described below). To obtain control flies containing only one copy of the driver or RNAi construct, the designated stocks were crossed to y w; spapol flies. Transheterozygotes mutant for mei-W68 (CG7753) were made from the following stocks: y/BS Y; mei-W68Z1049 cn bw/SM6a and y/y+ Y; mei-W68Z4572 cn bw/Cyo [49]. The genotype of the nanos-Gal4:VP16 driver flies was y w/y+ Y; nanos-Gal4:VP16; spapol.
Immunostaining
Immunostaining of late stage oocytes was carried out as described [8] under conditions to limit activation. For experiments enriching for prometaphase I oocytes, females were yeasted for 2–3 days with males. For preparations enriching for metaphase I oocytes, virgin females were yeasted for 4–5 days post-eclosion [7]. Ovaries were treated with the primary and secondary antibodies described below and with 1.0 µg/mL 4′6-diamididino-2-phenylindole (DAPI) to label the DNA and mounted in ProLong Gold (Invitrogen). Immunostaining of embryos was carried out as described [50]. The DNA was labeled with 2.5 µg/mL Hoechst 34580 (Invitrogen) and mounted in ProLong Gold (Invitrogen).
Primary antibodies were used at the following concentrations: rat anti-α-tubulin (AbD Serotec, NC 1∶250), mouse anti-α-tubulin DM1a (Sigma-Aldrich 1∶100), rat anti-CID [51] 1∶1000), rabbit anti-trimethylated-histone-3 at lysine 9 (AbCam 1∶250) and rabbit anti-phosphorylated-histone 3 at serine 10 (Millipore 3∶1000). Secondary Alexa-488, Alexa-555, or Alexa-647 conjugated antibodies (Molecular Probes) were used at a dilution of 1∶400.
Fluorescent in-situ hybridization
FISH was carried out as described [52], with the following modifications. Alexa Fluor 488 was conjugated to a region of the 359-bp repeat on the X chromosome, and the AATAT repeat on the 4th chromosome was conjugated to Cy3 [5], [30]. Both probes were denatured at 91°C and hybridization was carried out at 31°C. For the Alexa Fluor 488-labeled AACAC probe on the right arm of chromosome 2 and the Alexa Fluor 555-labeled Dodeca probe on the right arm of chromosome 3, the samples were denatured at 92°C and hybridization was at 37°C. Heterochromatic probes were made by Integrated DNA Technologies. Samples with BAC probes to euchromatic regions of the X chromosome were denatured at 92°C and hybridization was carried out at 37°C. BAC probes for 3C (BACR03D13) and 7DE (BACR39F18) were labeled with ARES Alexa Fluor 488 or 647 DNA labeling kits (Invitrogen). BAC DNA was digested with AluI, HaeIII, MseI, MspI, RsaI and MboI. Fragments were precipitated and then resuspended in water. The DNA was then denatured at 100°C for 1 minute and chilled immediately on ice. 20 µl of 5X terminal deoynucleotidyl transferase buffer, 20 µl of 25 mM Cobalt(II) chloride, 2.5 µl of 2 mM amionally dUTP from the ARES DNA labeling kit, 5 µl of 1 mM unlabeled 2′-deoxythymidine 5′-triphosphate, and 1 µl (400 units) Terminal deoxynucleotidyl Transferase (Roche) were added to 51.5 µl of the BAC DNA at room temperature. The reaction was allowed to proceed for 2 hr at 37°C. DNA was precipitated and the dried pellet resuspended in water. DNA was mixed with Ares labeling kit buffer and Ares dye that had been dissolved in dimethylsulfoxide. Sample was incubated in the dark for 1–2 hr at room temperature. The reaction was quenched with 1 M hydroxylamine. Probe was purified with a Qia-quick column (Qiagen). Labeled DNA was pelleted, allowed to dry and resuspended in water. Immuno-FISH was carried out as described [28].
For analysis of fixed ovaries, the DeltaVision microscopy system was used (Applied Precision, Issaquah, WA). The system is equipped with an Olympus 1X70 inverted microscope and high-resolution CCD camera. The images were deconvolved using the SoftWoRx v.25 software (Applied Precision). Embryos were imaged using an LSM-510 META confocal microscope (Zeiss) with a Plan-APO 40X objective (1.3 NA) with a zoom of 0.7 or a 63X Plan-apochromat (1.4 NA) with a zoom of 2. Images were acquired using the AIM software v4.2 by taking a Z stack and transformed into 2D projections using AIM software v4.2.
Creation of the mutated RNAi construct
The sequence targeting the Top2 gene is CACGAAGATATCCAACTACAA (top) and TTGTAGTTGGATATCTTCGTG (bottom). This 21-bp oligo matched a sequence of CG33296 at 18/21 nucleotides. Oligos were created by Operon Biotechnologies Inc. to change the three differing nucleotides to precisely match CG33296. The 21-bp sense and anti-sense targeting sequences were CTCGAAGATATCCAACTTTAA and TTAAAGTTGGATATCTTCGAG. Oligos were annealed and ligated into a Valium22 vector that was digested with NheI and EcoRI according to the instructions provided by TRiP. Ligated product was transformed into competent cells. Genetic Services, Inc injected purified vector into y sc v; attP2 flies and y+ v+ flies were selected and sequence-verified.
Hatch rate assays
Numerous females and males were allowed to acclimate to grape plates with yeast paste. Flies were transferred to fresh grape plates with yeast paste and females were allowed to lay eggs for 1–3 hours. Eggs within a grid on the plates were scored for hatching approximately 48 hr later.
Live imaging
Live imaging was performed on prometaphase I oocytes as described [6]. Oocytes were injected using standard microinjection procedures with an approximately 1∶1 ratio of porcine rhodamine-conjugated α-tubulin minus glycerol (Cytoskeleton) and Quant-iT OliGreen ssDNA Reagent (Invitrogen) diluted 0.7 fold with water. Oocytes were imaged using an LSM-510 META confocal microscope (Zeiss) with an alpha plan-fluar 100X (1.4 NA) objective and a 1.5 zoom. Images were acquired using the AIM software v4.2 by taking a 10-series Z stack at 1 micron intervals with 20 seconds between acquisitions, which resulted in a set of images approximately every 45 seconds. Images were transformed into 2D projections and concatenated into videos using the AIM software v4.2.
Western blot analysis
For each genotype, ovaries from virgin, yeast-fed females were dissected in cold 1X PBS, the ovaries were teased apart, and stage 14 oocytes were selected over the course of 2 hr. Stage 14 oocytes were homogenized in 50 µL of cold lysis buffer containing 150 mM NaCl, 50 mM Tris (pH 6.8), 2.5 mM EDTA, 2.5 mM EGTA, 0.1% Triton-X, and protease inhibitor cocktail (Sigma-Aldrich). Ovary lysates were cleared by centrifugation twice at 14,000 rpm for 15 min at 4°C. Lysates were assayed by Bradford and concentrations adjusted before samples were combined with 2X SDS sample buffer and boiled for 5 min, and the solubilized proteins were analyzed by Western blot using standard techniques. The primary antibody used for Western blot was rabbit anti-Top2 [21] at a dilution of 1∶5000 and α-tubulin (Serotec) at a dilution of 1∶5000. Immunoreactivity was detected using an alkaline phosphatase-conjugated rabbit secondary antibody (Jackson ImmunoResearch) and the nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphatase (NBT/BCIP, Invitrogen) reagents.
Supporting Information
Zdroje
1. Ashburner M, Golic KG, Hawley RS (2005) Female meiosis. Drosophila A Laboratory Handbook. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. pp.757–825.
2. HawleyRS, TheurkaufWE (1993) Requiem for distributive segregation: achiasmate segregation in Drosophila females. Trends Genet 9 : 310–317.
3. HawleyRS, IrickH, ZitronAE, HaddoxDA, LoheA, et al. (1992) There are two mechanisms of achiasmate segregation in Drosophila females, one of which requires heterochromatic homology. Dev Genet 13 : 440–467.
4. KarpenGH, LeMH, LeH (1996) Centric heterochromatin and the efficiency of achiasmate disjunction in Drosophila female meiosis. Science 273 : 118–122.
5. DernburgAF, SedatJW, HawleyRS (1996) Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86 : 135–146.
6. HughesSE, GillilandWD, CotittaJL, TakeoS, CollinsKA, et al. (2009) Heterochromatic threads connect oscillating chromosomes during prometaphase I in Drosophila oocytes. PLoS Genet 5: e1000348.
7. GillilandWD, HughesSE, ViettiDR, HawleyRS (2009) Congression of achiasmate chromosomes to the metaphase plate in Drosophila melanogaster oocytes. Develomental Biology 325 : 122–128.
8. HughesSE, BeelerJS, SeatA, SlaughterBD, UnruhJR, et al. (2011) Gamma-tubulin is required for bipolar spindle assembly and for proper kinetochore microtubule attachments during prometaphase I in Drosophila oocytes. PLoS Genet 7: e1002209.
9. BachratiCZ, HicksonID (2008) RecQ helicases: guardian angels of the DNA replication fork. Chromosoma 117 : 219–233.
10. LaFountainJRJr, ColeRW, RiederCL (2002) Partner telomeres during anaphase in crane-fly spermatocytes are connected by an elastic tether that exerts a backward force and resists poleward motion. J Cell Sci 115 : 1541–1549.
11. HartlTA, SweeneySJ, KneplerPJ, BoscoG (2008) Condensin II resolves chromosomal associations to enable anaphase I segregation in Drosophila male meiosis. PLoS Genet 4: e1000228.
12. NitissJL (2009) DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer 9 : 327–337.
13. CobbJ, ReddyRK, ParkC, HandelMA (1997) Analysis of expression and function of topoisomerase I and II during meiosis in male mice. Mol Reprod Dev 46 : 489–498.
14. TatenoH, KamiguchiY (2002) Abnormal chromosome migration and chromosome aberrations in mouse oocytes during meiosis II in the presence of topoisomerase II inhibitor ICRF-193. Mutat Res 502 : 1–9.
15. TatenoH, KamiguchiY (2004) Chromosome analysis of mouse one-cell androgenones derived from a sperm nucleus exposed to topoisomerase II inhibitors at pre - and post-fertilization stages. Mutat Res 556 : 117–126.
16. LiXM, YuC, WangZW, ZhangYL, LiuXM, et al. (2013) DNA topoisomerase II is dispensable for oocyte meiotic resumption but is essential for meiotic chromosome condensation and separation in mice. Biol Reprod 89 : 118.
17. HartsuikerE, BahlerJ, KohliJ (1998) The role of topoisomerase II in meiotic chromosome condensation and segregation in Schizosaccharomyces pombe. Mol Biol Cell 9 : 2739–2750.
18. RoseD, ThomasW, HolmC (1990) Segregation of recombined chromosomes in meiosis I requires DNA topoisomerase II. Cell 60 : 1009–1017.
19. RoseD, HolmC (1993) Meiosis-specific arrest revealed in DNA topoisomerase II mutants. Mol Cell Biol 13 : 3445–3455.
20. RamosE, TorreEA, BusheyAM, GurudattaBV, CorcesVG (2011) DNA topoisomerase II modulates insulator function in Drosophila. PLoS One 6: e16562.
21. HohlAM, ThompsonM, SoshnevAA, WuJ, MorrisJ, et al. (2012) Restoration of topoisomerase 2 function by complementation of defective monomers in Drosophila. Genetics 192 : 843–856.
22. HackerU, PerrimonN (1998) DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev 12 : 274–284.
23. RadfordSJ, JangJK, McKimKS (2012) The chromosomal passenger complex is required for meiotic acentrosomal spindle assembly and chromosome biorientation. Genetics 192 : 417–429.
24. BlowerMD, KarpenGH (2001) The role of Drosophila CID in kinetochore formation, cell-cycle progression and heterochromatin interactions. Nat Cell Biol 3 : 730–739.
25. Bauerly E, Hughes SE, Vietti DR, Miller DE, McDowell W, et al. (2014) Discovery of Supernumerary B Chromosomes in Drosophila melanogaster. Genetics.
26. EbertA, LeinS, SchottaG, ReuterG (2006) Histone modification and the control of heterochromatic gene silencing in Drosophila. Chromosome Res 14 : 377–392.
27. LoheAR, HillikerAJ, RobertsPA (1993) Mapping simple repeated DNA sequences in heterochromatin of Drosophila melanogaster. Genetics 134 : 1149–1174.
28. GilliesSC, LaneFM, PaikW, PyrtelK, WallaceNT, et al. (2013) Nondisjunctional segregations in Drosophila female meiosis I are preceded by homolog malorientation at metaphase arrest. Genetics 193 : 443–451.
29. Ashburner M, Golic KG, Hawley RS (2005) Mapping and Exchange. Drosophila A Laboratory Handbook. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. pp.437–480.
30. FerreePM, BarbashDA (2009) Species-specific heterochromatin prevents mitotic chromosome segregation to cause hybrid lethality in Drosophila. PLoS Biology 7: e1000234.
31. Ferree PM, Gomez K, Rominger P, Howard D, Kornfeld H, et al. (2014) Heterochromatin Position Effects on Circularized Sex Chromosomes Cause Filicidal Embryonic Lethality in Drosophila melanogaster. Genetics.
32. ChristophorouN, RubinT, HuynhJR (2013) Synaptonemal complex components promote centromere pairing in pre-meiotic germ cells. PLoS Genet 9: e1004012.
33. KasE, LaemmliUK (1992) In vivo topoisomerase II cleavage of the Drosophila histone and satellite III repeats: DNA sequence and structural characteristics. EMBO J 11 : 705–716.
34. Ashburner M, Golic KG, Hawley RS (2005) Male Meiosis. Drosophila A Laboratory Handbook. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. pp.827–860.
35. ChangCJ, GouldingS, EarnshawWC, CarmenaM (2003) RNAi analysis reveals an unexpected role for topoisomerase II in chromosome arm congression to a metaphase plate. J Cell Sci 116 : 4715–4726.
36. CoelhoPA, Queiroz-MachadoJ, CarmoAM, Moutinho-PereiraS, MaiatoH, et al. (2008) Dual role of topoisomerase II in centromere resolution and aurora B activity. PLoS Biol 6: e207.
37. WilliamsBR, BatemanJR, NovikovND, WuCT (2007) Disruption of topoisomerase II perturbs pairing in drosophila cell culture. Genetics 177 : 31–46.
38. JoyceEF, WilliamsBR, XieT, WuCT (2012) Identification of genes that promote or antagonize somatic homolog pairing using a high-throughput FISH-based screen. PLoS Genet 8: e1002667.
39. BuchenauP, SaumweberH, Arndt-JovinDJ (1993) Consequences of topoisomerase II inhibition in early embryogenesis of Drosophila revealed by in vivo confocal laser scanning microscopy. J Cell Sci 104 (Pt 4): 1175–1185.
40. RorthP (1998) Gal4 in the Drosophila female germline. Mech Dev 78 : 113–118.
41. BowerJJ, KaracaGF, ZhouY, SimpsonDA, Cordeiro-StoneM, et al. (2010) Topoisomerase IIalpha maintains genomic stability through decatenation G(2) checkpoint signaling. Oncogene 29 : 4787–4799.
42. GonzalezRE, LimCU, ColeK, BianchiniCH (2011) Schools GP, et al (2011) Effects of conditional depletion of topoisomerase II on cell cycle progression in mammalian cells. Cell Cycle 10 : 3505–3514.
43. DownesCS, MullingerAM, JohnsonRT (1991) Inhibitors of DNA topoisomerase II prevent chromatid separation in mammalian cells but do not prevent exit from mitosis. Proc Natl Acad Sci U S A 88 : 8895–8899.
44. BaumannC, KornerR, HofmannK, NiggEA (2007) PICH, a centromere-associated SNF2 family ATPase, is regulated by Plk1 and required for the spindle checkpoint. Cell 128 : 101–114.
45. WangLH, SchwarzbraunT, SpeicherMR, NiggEA (2008) Persistence of DNA threads in human anaphase cells suggests late completion of sister chromatid decatenation. Chromosoma 117 : 123–135.
46. WangLH, MayerB, StemmannO, NiggEA (2010) Centromere DNA decatenation depends on cohesin removal and is required for mammalian cell division. J Cell Sci 123 : 806–813.
47. GomezR, VieraA, BerenguerI, LlanoE, PendasAM, et al. (2014) Cohesin removal precedes topoisomerase IIalpha-dependent decatenation at centromeres in male mammalian meiosis II. Chromosoma 123 : 129–146.
48. HolmC, StearnsT, BotsteinD (1989) DNA topoisomerase II must act at mitosis to prevent nondisjunction and chromosome breakage. Mol Cell Biol 9 : 159–168.
49. BhagatR, ManheimEA, SherizenDE, McKimKS (2004) Studies on crossover-specific mutants and the distribution of crossing over in Drosophila females. Cytogenet Genome Res 107 : 160–171.
50. BonnerAM, HughesSE, ChisholmJA, SmithSK, SlaughterBD, et al. (2013) Binding of Drosophila Polo kinase to its regulator Matrimony is noncanonical and involves two separate functional domains. Proc Natl Acad Sci U S A 110: E1222–1231.
51. MartinsT, MaiaAF, SteffensenS, SunkelCE (2009) Sgt1, a co-chaperone of Hsp90 stabilizes Polo and is required for centrosome organization. EMBO J 28 : 234–247.
52. XiangY, TakeoS, FlorensL, HughesSE, HuoLJ, et al. (2007) The inhibition of polo kinase by matrimony maintains G2 arrest in the meiotic cell cycle. PLoS Biol 5: e323.
53. Van DorenM, WilliamsonAL, LehmannR (1998) Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr Biol 8 : 243–246.
Štítky
Genetika Reprodukčná medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 10- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



