-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
Parkinson's disease is a common movement disorder with no known cure. Its characteristic motor symptoms are primarily caused by the progressive loss of midbrain dopaminergic neurons. Although studies have shown that various environmental and genetic factors both contribute to the development of the disease, the underlying mechanisms remain unknown. Here we use powerful invertebrate model organisms, fruit flies and nematode worms, and identify a new gene required for the survival of dopaminergic neurons. We show that homologs of the p48/ptf1-a gene in both flies and worms are expressed in dopaminergic neurons and mutations in p48 increase the susceptibility of dopaminergic neuron death when animals are under oxidative stress. Importantly, genetic variations in p48 in humans have been detected in the sporadic Parkinson's disease patients, indicating the possibility that similar mechanism might play a role in the death of dopaminergic neurons in humans. Oxidative stress has been regarded as a major pathogenic factor for Parkinson's disease. Our results add evidence to the link between oxidative stress and neurodegeneration, and suggest that p48 mutant flies and worms can be used to study mechanisms of neurodegeneration in Parkinson's disease.
Published in the journal: A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress. PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004718
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004718Summary
Parkinson's disease is a common movement disorder with no known cure. Its characteristic motor symptoms are primarily caused by the progressive loss of midbrain dopaminergic neurons. Although studies have shown that various environmental and genetic factors both contribute to the development of the disease, the underlying mechanisms remain unknown. Here we use powerful invertebrate model organisms, fruit flies and nematode worms, and identify a new gene required for the survival of dopaminergic neurons. We show that homologs of the p48/ptf1-a gene in both flies and worms are expressed in dopaminergic neurons and mutations in p48 increase the susceptibility of dopaminergic neuron death when animals are under oxidative stress. Importantly, genetic variations in p48 in humans have been detected in the sporadic Parkinson's disease patients, indicating the possibility that similar mechanism might play a role in the death of dopaminergic neurons in humans. Oxidative stress has been regarded as a major pathogenic factor for Parkinson's disease. Our results add evidence to the link between oxidative stress and neurodegeneration, and suggest that p48 mutant flies and worms can be used to study mechanisms of neurodegeneration in Parkinson's disease.
Introduction
Dopaminergic (DA) neurons play critical roles in motor control, cognition and motivation and are affected in many neurological and psychiatric disorders [1], [2], [3], [4]. The progressive degeneration of DA neurons in the substantia nigra pars compacta (SNc) is a principal pathological feature of Parkinson's disease (PD). PD is the most prevalent neurodegenerative movement disorder, for which no preventive or restorative therapies are available [5], [6]. The discovery of the genes associated with the rare familial forms of PD has led to the development of many animal models and advanced the understanding of PD pathogenesis. However, the majority of PD cases are sporadic and likely caused by a combination of environmental factors, such as pesticide exposure, and endogenous risk factors. These endogenous risk factors remain largely unknown. A recent meta-analysis on genome-wide association studies (GWAS) for PD showed that SNPs in the genes involved in multiple aspects of neural development are highly represented in sporadic PD patients [7], suggesting that genetic variations in these pathways may contribute to PD susceptibility. Indeed, several studies in mammals have shown the critical roles of developmental genes, such as Engrailed1, foxa2 and Nurr1, in the survival of DA neurons in old age [8], [9], [10], [11]. The identification and characterization of such genes may yield a better molecular understanding of adult-onset neurodegeneration in PD.
The nervous system in invertebrate model organisms such as Drosophila and C.elegans shares many features with its mammalian counterpart and offers a powerful tool to study neural development and neurodegeneration. Drosophila DA neurons comprise multiple subclasses, some of which play roles similar to those played by the DA neurons in mammals, such as reward signaling and sleep regulation [12], [13]. The nematode C.elegans has 8 DA neurons, which are thought to have mechanosensory functions and have been shown to play a role in the modulation of locomotion [14]. Despite advances in anatomical and functional characterization, the mechanisms underlying the development and maintenance of DA neurons in flies and worms are poorly understood. Drosophila Fer2, a homolog of mammalian p48/ptf1a, belongs to the bHLH-transcription factor family, which is often involved in neurogenesis and neural subtype specification. The mammalian p48 gene is a critical regulator for neural tube development [15], in which a candidate causal SNP for PD has been detected [7,16, The database of Genotypes and Phenotypes (dbGaP; NCBI)]. Previously, we showed that Fer2 is required for the development of a subclass of circadian clock neurons, ventral Lateral Neurons (LNvs) [17]. Here we characterized additional roles of Fer2 to better understand the genetic mechanisms of neuronal subtype development and maintenance. We unexpectedly found that Fer2 is required for the development and maintenance of a subclass of DA neurons important for locomotion. Fer2 exerts its neuroprotective role in adulthood in the oxidative stress response, and loss of Fer2 expression in adulthood causes adult-onset progressive degeneration of these DA neurons. We further demonstrated that the C. elegans homolog of p48, hlh-13, is also required for the survival of DA neurons in adult worms under oxidative stress. Collectively, our results established a conserved role of p48 homologs in protecting DA neurons from oxidative stress and degeneration.
Results
Fer2 is required for the startle-induced climbing ability
Drosophila Fer2e03248 (henceforth referred to as Fer21) mutation was induced by the insertion of PBac{RB} into the Fer2 5′UTR [18]. The Fer2MB09480 (henceforth called Fer22) allele has a Mi{ET1} transposon insertion in the 3′ end of the second exon [19] (Fig. 1A). To molecularly characterize the Fer2 mutant alleles, we determined the Fer2 mRNA levels using quantitative real-time PCR (qPCR). Consistent with our previous results, Fer2 mRNA expression of the Fer21 homozygotes was approximately 5% of the wild-type level [17]. In the Fer22 homozygous flies, Fer2 mRNA expression was reduced to about 40% of the wild-type level. Thus, Fer21 is an extreme hypomorphic allele, whereas Fer22 is a milder hypomorph. Fer2 mRNA expression of both Fer21/+ and Fer22/+ flies was only slightly reduced relative to the wild-type level, suggesting that the loss of one copy of a Fer2 gene is compensated at the mRNA level by transcriptional or post-transcriptional mechanisms (Fig. 1B). Compensation of gene dose has been observed widely in both Drosophila and mammals [20], [21], [22]. Therefore, Fer2 is a haplosufficient gene and Fer2 heterozygous mutants are expected to be phenotypically wild-type.
Fig. 1. Fer2 loss-of-function mutation impairs the startle-induced climbing ability. 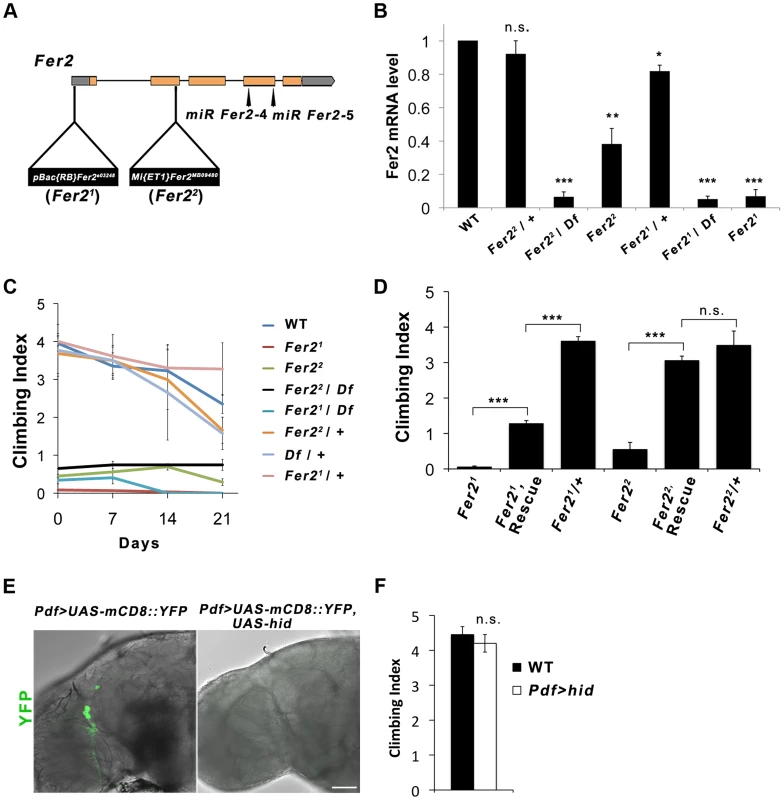
(A) Schematic of the Fer2 mutant alleles and the Fer2 miRNA target sites (miR Fer2-4, miR Fer2-5). Gray boxes are 5′ and 3′ UTRs, orange boxes are Fer2 gene exons. (B) Fer2 mRNA levels in the fly heads normalized to the level in w1118 (WT). Mean ± SEM from 3 independent experiments. *p<0.05, ** p<0.01, *** p<0.001. n.s., no statistical significance. (C) Climbing abilities of Fer2 mutants and control flies tested weekly. Mean Climbing Index from ± SEM. (D) Fer2> Fer2-FLAG (Rescue) significantly improved the impaired climbing ability of the Fer21 and Fer22 flies (7-day-old). Mean ± SEM. ***p<0.001. (E) PDF-positive LNvs visualized by expressing UAS-mCD8::YFP with Pdf-GAL4 in the wild-type (w1118) and in the flies expressing UAS-hid. Error bar, 50 µm. (F) Climbing ability of the wild-type and LNv-ablated flies (Pdf > hid) at day 4. Mean ± SEM. We noticed that Fer2 mutant flies tend to climb up the walls poorly when tapped down to the bottom of the vials. We quantified this behavior using a startle-induced climbing assay. Wild-type and heterozygous Fer2 mutants showed similar climbing abilities at least during the first 3 weeks of the adult life. In contrast, all Fer2 homozygous or hemizygous mutants displayed severely impaired climbing abilities throughout adulthood (Fig. 1C). We generated a driver fly line expressing GAL4 under the control of the Fer2 promoter (Fer2-GAL4) and a UAS line expressing FLAG-tagged Fer2 cDNA (UAS-Fer2-FLAG). The expression of Fer2-FLAG with Fer2-GAL4 partially but significantly rescued the decreased climbing ability of the Fer21 flies and restored the climbing ability of the Fer22 flies to the control level (Fig. 1D). These data indicate that Fer2 is necessary for the startle-induced climbing ability.
Fer2 is required for the development and survival of dopaminergic neurons
We have previously shown that Fer21 mutation impairs the development of LNvs, which express the neuropeptide pigment-dispersing factor (PDF) [17]. Although it has been shown that PDF is necessary for the normal negative geotaxis behavior [23], whether PDF or LNvs are necessary for startle-induced climbing has not been documented. We found that the expression of UAS-hid with Pdf-GAL4, which selectively ablates LNvs [24], does not impair the startle-induced climbing ability (Fig. 1E, F). Thus, the decrease in startle-induced climbing ability in Fer2 mutant flies is due to the deficits other than the lack of LNvs. Because loss of climbing ability is often associated with impaired CNS integrity [25] and the available transcriptome data indicate that Fer2 is almost exclusively expressed in the brain (modENCODE Tissue Expression Data, FlyAtlas [26]), we examined the integrity of major neuron types in the brains of adult Fer21 mutants. We did not find any obvious differences in the overall morphology of the cholinergic, glutamatergic and serotonergic neurons between Fer21 and controls, although we cannot exclude the possibility that there are subtle differences in the number of these neurons (Fig. S1). Interestingly, we found an evident reduction of dopaminergic (DA) neurons in Fer21 mutants. Seven DA neuron clusters were detected by anti-tyrosine hydroxylase (TH) staining in the Fer21 heterozygouse flies, which were very similar in number and morphology to those in wild-type flies [27]. In contrast, there were markedly fewer DA neurons in the PAM and PAL clusters in homozygous Fer21 flies on the first day after eclosion (day 0); there were even fewer of them in 7-day-old flies (Fig. 2A). In addition, we expressed UAS-GFP under the control of the HL9-GAL4 driver to label several clusters of DA neurons [28] and found a similar dramatic reduction of PAM and PAL neurons in the homozygous Fer21 flies (Fig. S2A). This indicates that PAM and PAL neurons were reduced in number in Fer21 mutants, rather than merely having reduced TH expression. Quantification of the HL9 > GFP-positive neurons revealed a 75% reduction in PAM neuron counts already at day 0 and a 90% reduction at day 7 in Fer21 compared to the heterozygous controls. Four out of 5 PAL neurons were undetectable at day 0 in the Fer21 flies, and most brains had no PAL neurons at day 7. The numbers of other DA neuron clusters were not different between Fer21 and controls at both ages (Fig. 2B). To further verify the loss of DA neurons in the Fer21 flies, we expressed GFP using the R58E02-GAL4 driver, which is derived from the promoter of the dopamine transporter gene and expressed almost exclusively in PAM neurons [29]. There were significantly fewer R58E02-GAL4-labeled PAM neurons in the Fer21 flies compared to the control, supporting the finding that a large fraction of PAM neurons were absent in Fer21 (Fig. 2D). The expression of Fer2-FLAG by Fer2-GAL4 restored the loss of PAM and PAL neurons in the Fer21 flies to quasi wild-type levels (Fig. 2C).
Fig. 2. Selective loss of PAM and PAL cluster DA neurons in the Fer2 extreme hypomorphic mutants. 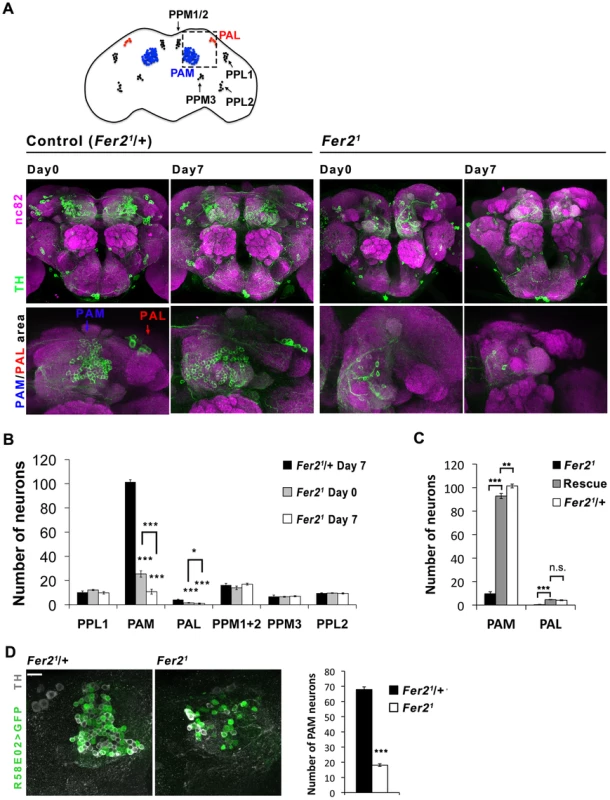
(A) Top, a diagram of DA neuron clusters in the adult fly brain. Bottom, representative images of the brains stained with anti-TH antibodies. Central brain and anterior brain region where PAM and PAL neurons are located are shown. (B) Quantification of DA neurons per hemisphere detected by TH-staining. For PAM and PAL neurons, HL9 > GFP and TH double positive neurons were quantified. Fer21/+, n = 17. Fer21: day 0, n = 12; day 7, n = 24. Mean ± SEM, * p<0.05, *** p<0.001, comparing Fer21 and Fer21/+ or Fer21 day 0 vs. day 7. No significant differences were found between Fer21 and Fer21/+ where no asterisks are shown. (C) Quantification of the PAM and PAL neurons in the 7-day-old Fer21 (n = 8), Rescue (Fer21, Fer2 > Fer2-FLAG; n = 10) and control (Fer21/+; n = 17) flies. (D) Left, representative confocal images of the brains of the 0-day-old flies expressing UAS-GFP with R58E02-GAL4 double stained for GFP and TH. Right, quantification of the GFP/TH double positive neurons. Mean ± SEM, ***p<0.001. Fer21/+, n = 7. Fer21, n = 14. To examine if Fer2 is expressed in PAM and PAL neurons, we monitored the expression of a GFP-tagged Fer2 genomic transgene in the brain by GFP/TH double staining. FER2::GFP expression was observed in all the PAM and PAL neurons and in a few other clusters of cells. As expected, GFP/PDF double staining confirmed the expression of FER2::GFP in the LNvs, consistent with the previous RNA analysis results [17] (Fig. 3A, B). Fer2-GAL4 showed a more widespread expression pattern than FER2::GFP, as is often the case with promoter-GAL4s. Nonetheless, Fer2-GAL4 was also expressed in all PAM neurons and 4 out of 5 PAL neurons (Fig. S2B). Having validated the expression of Fer2-GAL4 in PAM and PAL neurons, we next used it to express UAS-TH in the Fer21 flies and immunostained the brains with anti-TH antibodies. Fer2 > TH slightly increased the number of neurons detected by TH-staining in the PAM and PAL clusters but not to the control level, which demonstrates again the absence of these cells (Fig. S2C). These results suggest that Fer2 is expressed in PAM and PAL neurons and further support that Fer21 mutation selectively reduces the number of these neurons.
Fig. 3. FER2 expression in the brain. 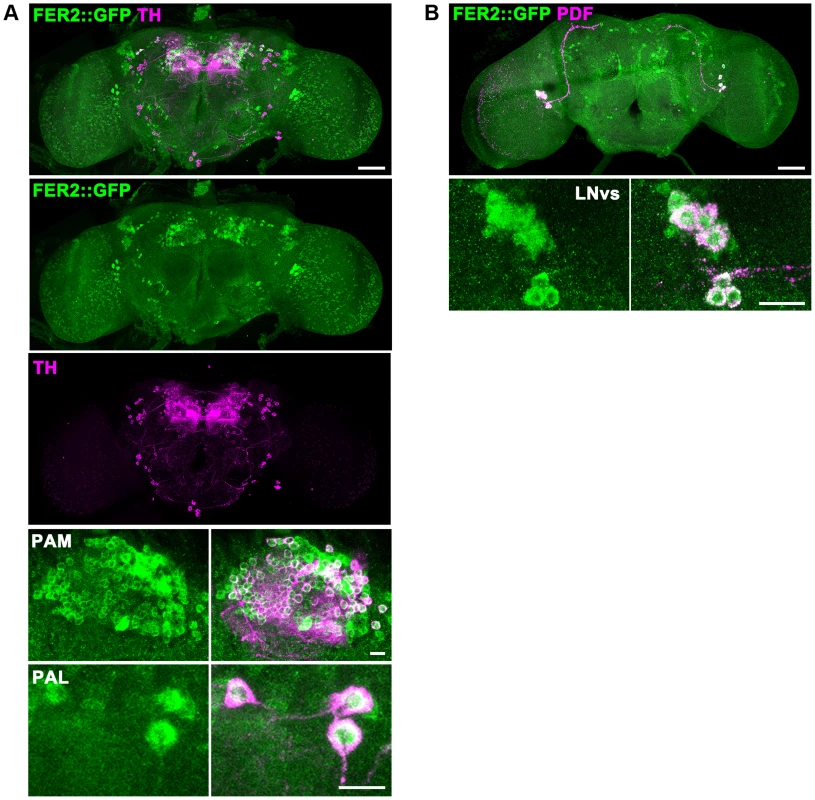
FER2 is expressed in the PAM and PAL DA neurons and the LNvs. Brains of the Fer2::GFP flies were stained for GFP and TH (A) or GFP and PDF (B). Scale bars in the whole brain images are 50 µm, 10 µm in the insets. To examine the possibility that the dopaminergic neurotransmitter identity of PAM and PAL neurons is changed in Fer21 and thus they are undetectable, we analyzed the cell lineage derived from the Fer2-GAL4-expressing cells. By combining Fer2-GAL4, UAS-FLP and UbiP63 > stop> EGFP, the Fer2-GAL4-expressing lineage was marked with GFP in the control and Fer21 flies (Fig. S2D) [30]. While most of the PAM and 4 PAL neurons were GFP-positive in the heterozygous control flies, the majority of these neurons were not present and no ectopic GFP-positive cells were observed in Fer21 (Fig. S2E, F). Therefore, the reduction of PAM and PAL neurons in Fer21 is not due to a cell-fate switch.
To learn more about the developmental impairments of PAM and PAL neurons in Fer21 mutants, we next examined DA neurons in the pupal brains with anti-TH staining, because these neurons are not present in the larval brain and some of the PAM neurons are known to be born during pupal stages [27], [31]. In the Fer21 heterozygous controls, approximately 80% of PAM and PAL neurons were clearly detectable within 5 days after puparium formation (APF). Whereas in Fer21 homozygotes, PAM and PAL neurons gradually increased in number but were significantly fewer than in the controls throughout pupal development. These observations indicate that the majority of PAM and PAL neurons were not formed or died before maturation into DA neurons in Fer21 mutants (Fig. S3A, B). Taken together with the observation that the loss of PAM/PAL neurons progresses at least up to 7 days into adulthood (Fig. 2B), these results indicate that Fer21 mutation impairs the development of the DA neurons in the PAM and PAL clusters and also causes their rapid degeneration in adulthood.
Fer2 is a survival factor for PAM neurons in aging flies
We next asked whether the integrity of DA neurons is affected in the milder hypomorphic mutant Fer22 as well. We focused our analysis on the PAM cluster DA neurons and monitored their number in the Fer22 flies by anti-TH staining. At day 0, the number of PAM neurons was slightly reduced in Fer22 compared to the control, but to a much lesser extent as in Fer21. Remarkably, the loss of PAM neurons continued progressively at least up to 28 days in Fer22 (Fig. 4A). Lineage tracing using HL9-GAL4, UAS-FLP and UbiP63 > stop > EGFP flies verified the loss of PAM neurons in Fer22 (Fig. S4A). The loss of PAM neurons was rescued by expressing Fer2-FLAG with Fer2-GAL4 (Fig. S4B).
Fig. 4. Partial loss of Fer2 expression impairs the viability of PAM neurons in aging flies. 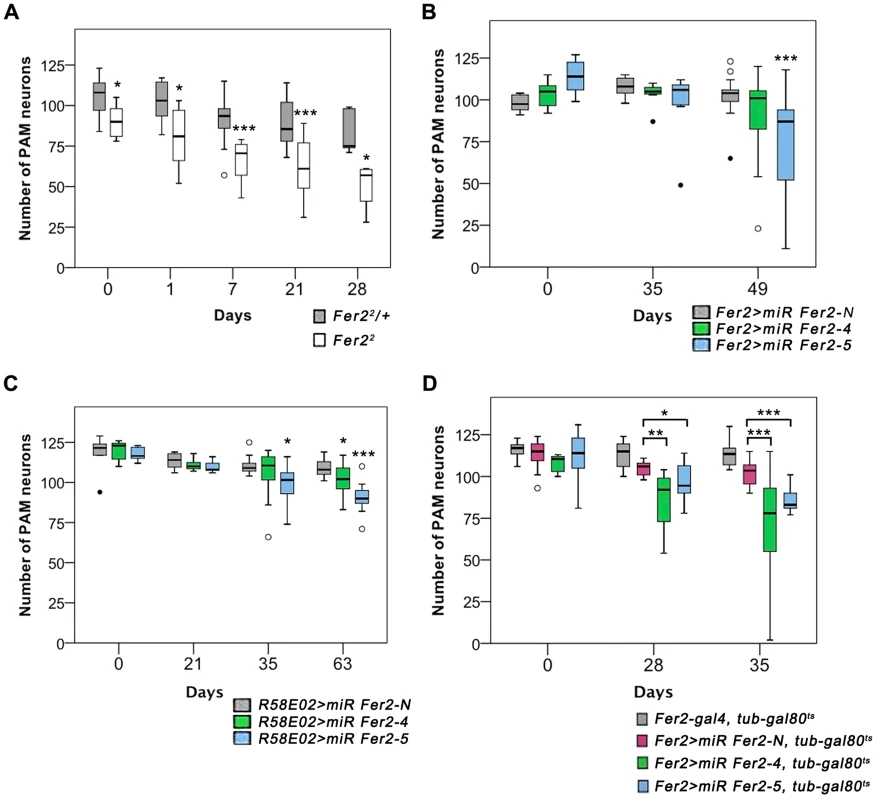
Quantification of PAM neurons in the flies of different genotypes detected by anti-TH staining at indicated ages. Box boundaries are the 25th and 75th percentiles, the horizontal line across the box is the median, and the whiskers are the lowest and highest values that are not outliers. The open circles represent outliers and closed circles are extreme cases. *p<0.05, **p<0.01, *** p<0.001, comparing Fer22/+ and Fer22 at the same age (A), or between miR-Fer2-N and miR-Fer2-4/-5 at the same age (B-D). Sample sizes are indicated in the Materials and Methods. (A) PAM neurons in Fer22 and the control (Fer22/+). (B) Constitutive expression of miR Fer2s by Fer2-GAL4. (C) Constitutive expression of miR Fer2s by R58E02-GAL4. (D) Adult-specific Fer2 knockdown and the control. Fer2-GAL4, tub-gal80ts: control without UAS-transgene. The contrasting results between Fer21 and Fer22 mutants suggest that a moderate reduction of Fer2 expression has only a minor effect on the development of DA neurons but is sufficient to deteriorate PAM neurons in aged flies. To test this, we generated UAS-transgenic lines to express 2 independent microRNAs (miRNAs) that target Fer2 (miR Fer2-4 and -5) and one negative control miRNA (miR Fer2-N) that contains a sequence of 19 random nucleotides unrelated to any Drosophila gene (see Fig. 1A). When expressed with Fer2-GAL4, both miR Fer2-4 and -5 reduced the Fer2 mRNA levels to approximately 35% of the wild-type level, whereas miR Fer2-N had no effect on the Fer2 mRNA (Fig. S5A). As expected, constitutive expression of miR Fer2-N by Fer2-GAL4 at 25°C did not alter the number of PAM neurons. In the flies expressing miR Fer2-4 or miR Fer2-5, the number of PAM neurons remained stable until 35 days of age. However, at 49 days, many flies in either of the knockdowns had a reduced number of PAM neurons, and the reduction was significant in Fer2 > miR Fer2-5 flies (Fig. 4B). The constitutive knockdown by miR Fer2-4 or miR Fer2-5 at 25°C with R58E02-GAL4 or HL9-GAL4 resulted in a similar trend, with a significant reduction of PAM neurons in the flies aged over several weeks old (Fig. 4C, S5B). The PAL and other DA neuron clusters were not affected by any of the Fer2 knockdowns, although Fer2-GAL4 and HL9-GAL4 are expressed in most of the DA clusters including PAL neurons. While there were subtle differences in the onset of degeneration that were likely due to the differences in GAL4 expression levels, the data nevertheless illustrate that Fer2 knockdown causes adult-onset PAM neuron degeneration.
The foregoing observations indicate that a moderate reduction of Fer2 expression either by a hypomorphic mutation or by knockdown has little effect on DA neuron development but mainly affects the survival of PAM neurons in adults. This further suggests that the role of Fer2 in the survival of adult PAM neurons is independent of its role in development. To test this more directly, we knocked-down Fer2 only during adulthood using a combination of UAS-miR Fer2s, Fer2-GAL4 and temperature-sensitive GAL80 expressed under the tubulin promoter (tub-GAL80ts). These flies were reared at 18°C (a permissive temperature for GAL80ts) until eclosion, and then the temperature was shifted to 29°C (a restrictive temperature for GAL80ts) to allow for transcriptional activation by GAL4 throughout adulthood [32]. We found that the adult-specific knockdown of Fer2 induced the adult-onset progressive degeneration of PAM neurons without affecting PAL neurons (Fig. 4D). Notably, the loss of PAM neurons was more evident in these flies than in flies with constitutive knockdown (Fig. 4B), which is consistent with the greater GAL4 activity at 29°C than at 25°C [33]. These results clearly distinguish the role of Fer2 in developing and adult DA neurons and demonstrate that Fer2 expression is required for the survival of adult PAM neurons in aging flies.
Loss of PAM neurons leads to the locomotor deficits
We next asked whether the loss of PAM neurons, which is the most prominent cellular phenotype in Fer2 mutants, is the cause of their locomotor impairment. To test the role of PAM neurons in the startle-induced climbing ability more directly, we knocked-down Fer2 in PAM neurons by HL9-GAL4 and performed a climbing assay. Knocking-down Fer2 with either of the miRNAs resulted in significant declines in climbing ability after 49 days (Fig. 5A). This is consistent with the observation that HL9 > miR Fer2s induce the adult-onset degeneration of PAM neurons only after several weeks (Fig. S5B).
Fig. 5. Loss of PAM neurons leads to the locomotor deficits. 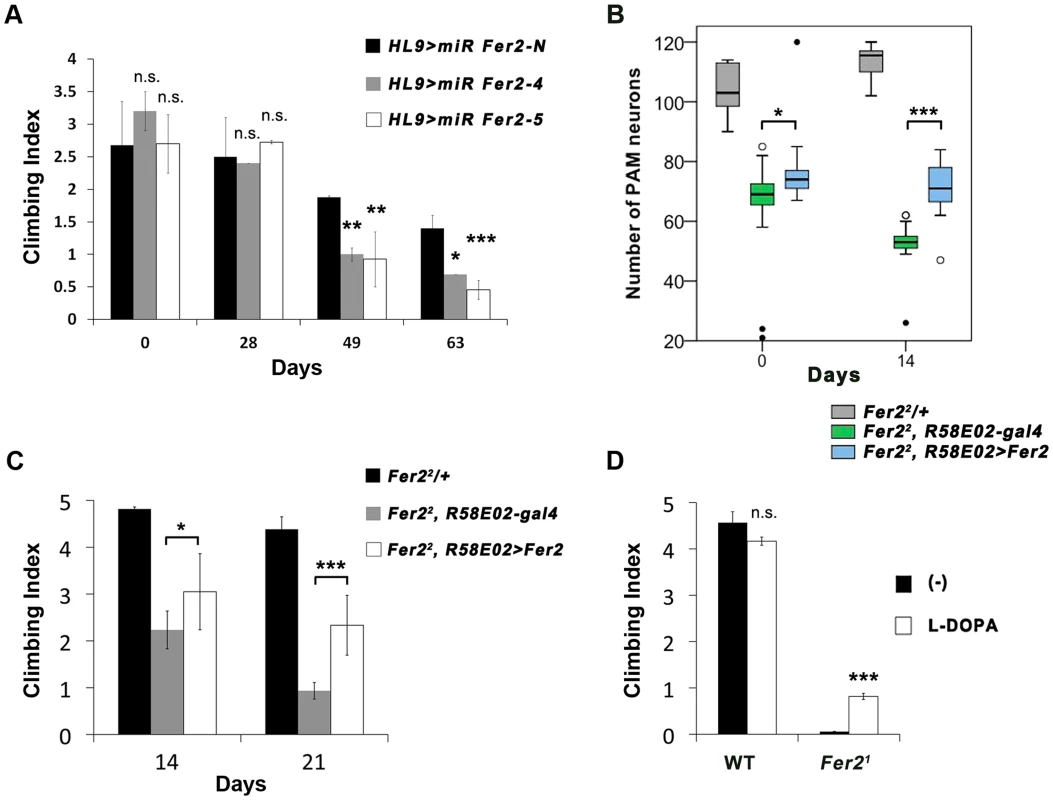
(A) Climbing abilities of the flies expressing Fer2 miRNAs or negative control miRNA with HL9-GAL4. Mean from 3 independent experiments ± SEM. * p<0.05, ** p<0.01, ***p<0.001. (B and C) PAM neuron-targeted expression of Fer2-FLAG by R58E02-GAL4 significantly rescued the degeneration of PAM neurons and climbing ability in the Fer22 mutants. Sample sizes are in the Materials and Methods. (B) PAM neuron counts. Fer22/+: positive control. Fer22, R58E02-gal4: negative control. Fer22, R58E02 > Fer2: rescue. * p<0.05, *** p<0.001. (C) Mean Climbing Index from 3 independent experiments ± SEM. * p<0.05, *** p<0.001. (D) Climbing ability of the 3-day-old wild-type (WT) and Fer21 mutant flies fed with or without L-dopa. Mean ± SEM. ***p<0.001. To further assess the contribution of PAM neurons in the climbing ability, we sought to rescue the loss of PAM neurons using a PAM neuron-specific driver in Fer2 mutant flies. Expression of UAS-Fer2-FLAG using HL9-GAL4 or R58E02-GAL4 did not rescue the loss of PAM neurons in Fer21. This is most likely because the majority of PAM neurons fail to form or die in Fer21 before these DA neuron-specific drivers start to be expressed, consistent with the fact that R58E02-GAL4 has little expression in the larval brain (H. Tanimoto and A. Thum, personal communication). Thus, we reasoned that PAM-neuron specific rescue might be possible in Fer22 flies, which show little impairments in DA neuron development but display progressive PAM neuron degeneration. Indeed, R58E02 > Fer2-FLAG suppressed the degeneration of PAM neurons in the Fer22 mutants in adulthood (Fig. 5B). A climbing assay revealed that R58E02 > Fer2-FLAG significantly improves the climbing impairments in the Fer22 flies (Fig. 5C). The rescue of the climbing ability was partial. This may be because some of the PAM neurons that failed to develop in Fer22 were not rescued, or because other unknown cell types affected in Fer22 contribute to the climbing ability. Nevertheless, these observations together with the results of the PAM neuron targeted-knockdown indicate that PAM neurons are necessary, although may not be sufficient, for the normal climbing ability of the flies.
The dopaminergic system is critically involved in the control of locomotion in both vertebrates and invertebrates [34], [35]. The motor symptoms of PD arise mainly from the loss of DA neurons in the SNc [5]. L-dopa, a dopamine biosynthesis precursor, remains the gold standard for treatments of PD motor symptoms. We found that the locomotor deficit of the Fer21 flies was partially but significantly rescued by feeding with L-dopa (Fig. 5D). By anti-TH staining, we observed no significant rescue of the number of DA neurons by L-dopa, which is consistent with a previous study [36]. Since L-dopa has to be converted to dopamine in the DA neuron terminals to exert its therapeutic effect, the partial rescue of the locomotion by L-dopa is also consistent with the marked loss of PAM neurons observed in Fer21 mutants.
Fer2 plays a critical role in oxidative stress response and protection of PAM neurons against oxidative insults
Accumulating evidence suggests that dysfunctions in multiple aspects of mitochondrial biology are associated with the DA neurodegeneration in PD and pathogenesis of other neurodegenerative disorders [37], [38]. To examine whether mitochondrial dysfunction is involved in the loss of DA neurons caused by the loss of Fer2 expression, we visualized mitochondria in the adult PAM neurons by expressing mitochondria-targeted GFP (mitoGFP) [39] with HL9-GAL4. The majority of visible mitochondria in the cell bodies of the remaining PAM neurons in Fer21 mutants was in enlarged aggregations and did not form tubular networks as in the control flies (Fig. 6A). Similarly, DA neuron-selective Fer2 knockdown by HL9-GAL4 and Fer22 mutation lead to the accumulation of abnormally enlarged mitochondria in PAM neurons (Fig. 6B, S6A). Mitochondrial morphology in some of the TRH-GAL4-positive serotonergic neurons was indistinguishable between Fer21 homozygotes and heterozygotes, suggesting that mitochondria in PAM neurons are particularly vulnerable to loss of Fer2 expression (Fig. S6B).
Fig. 6. Fer2 protects PAM neurons from oxidative stress. 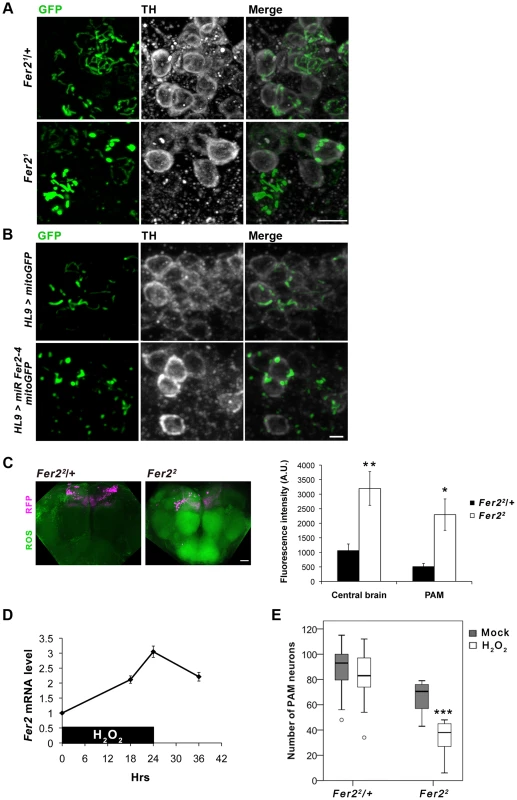
(A-B) Mitochondrial morphology in PAM neurons visualized by expressing mitoGFP with HL9-GAL4 and double stained for GFP and TH. (A) 1-day-old control (Fer21/+) and Fer21 mutant flies. Scale bar, 5 µm. (B) DA neuron-targeted Fer2 knockdown by HL9 > miR Fer2-4 and control (14-day-old). Scale bar, 2 µm. (C) ROS levels in the 5-day-old control (Fer22/+) and Fer22 flies monitored using H2DCF dye. Left, representative confocal images of the ROS production in the brain. PAM neurons were RFP-labeled with R58E02-GAL4. Scale bar, 30 µm. Right, quantification of the ROS levels. Mean ± SEM. Control, n = 5. Fer22, n = 5. Two independent experiments were performed with similar results. * p<0.05, ** p<0.01. (D) Relative Fer2 mRNA levels in wild-type flies during and after 24-hr 5% H2O2 treatment (5-day-old at the onset of the treatment). Data are shown as Fer2 mRNA levels of the H2O2-treated flies relative to the mock-treated controls. Mean ± SEM from 2–3 independent experiments. (E) PAM neuron counts in the Fer22 heterozygous and homozygous flies with or without H2O2 treatment. Fer22/+: control, n = 40; H2O2, n = 14. Fer22: control, n = 10; H2O2, n = 14. *** p<0.001. Since mitochondria are the major source of ROS, mitochondrial dysfunction leads to an excessive ROS production and oxidative damages to various macromolecules. Oxidative stress causes rapid depolarization of mitochondrial inner membrane and inhibits complex I activity, exacerbating ROS production. Thus, mitochondrial defects and elevated ROS levels are interdependent and are thought to have prominent roles in PD pathogenesis [40]. We therefore asked whether ROS levels are increased in the Fer2 mutant brains and if it has a causative role on PAM neuron degeneration. We monitored intracellular ROS levels in the brains of Fer22 mutant and the heterozygous control flies using 2′,7′-dichlorofluorescein (H2DCF), which produces green fluorescence upon reacting with ROS. Interestingly, ROS levels were significantly elevated throughout the brain in 5-day-old Fer22 flies compared with the age-matched controls. Although there was no regional specificity of ROS accumulation, ROS levels within the PAM neurons were also significantly elevated in Fer22 (Fig. 6C). These suggest that loss of Fer2 expression leads to a systemic increase in oxidative stress in the brain.
The surprisingly dramatic increase of ROS levels in Fer22 mutants prompted us to further examine if Fer2 is involved in oxidative stress response. We first tested if Fer2 expression levels can be altered upon oxidative stress-challenge by feeding flies with non-lethal dose of hydrogen peroxide (H2O2, 5%) for 24 hr. We found that H2O2 treatment significantly increases Fer2 mRNA levels (Fig. 6D). We next examined whether oxidative stress-challenge aggravates the degeneration of PAM neurons in Fer2 mutants by anti-TH staining. The H2O2 treatment did not affect PAM neurons in Fer22 heterozygous flies, whereas the number of PAM neurons was significantly decreased in Fer22 homozygotes after the treatment (Fig. 6E). DA neuron counts in other clusters were unchanged by the same H2O2 treatment in Fer22 mutants (Fig, S6C), indicating that loss of Fer2 expression renders PAM neurons selectively more vulnerable to increased oxidative stress. Taken together, these results point toward a role for Fer2 in oxidative stress response and suggest that Fer2 contributes to the protection of PAM neurons against oxidative stress.
C.elegans hlh-13 is required for the survival of DA neurons under oxidative stress
Fer2 homologs are found from nematodes to vertebrates [41]. hlh-13 is predicted to be the single homolog of Drosophila Fer2 and mammalian p48/ptf1a in C.elegans [42]. Consistent with a previous study [42], a GFP::hlh-13 genomic transgene was expressed in all DA neurons (named CEP (4 cells), ADE (2 cells) and PDE (2 cells)) and in a tail neuron in developing and adult worms. GFP::hlh-13 expression was also observed in several unidentified ventral nerve cells from L2 to L4 stages (Fig. 7A).
Fig. 7. C.elegans hlh-13 and Drosophila Fer2 share a role in DA neuron protection under oxidative stress. 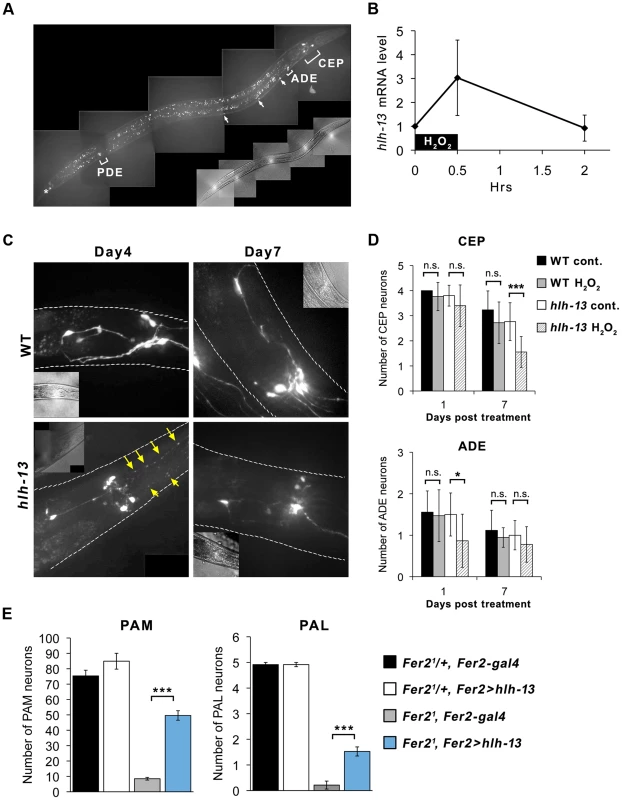
(A) GFP::hlh-13 is expressed in DA neurons (CEP, ADE and PDE), unidentified ventral nerve cells (arrows), and in a tail neuron (asterisk). Fluorescence signal that is not labeled is the gut autofluorescence. A representative image of a L4 worm. (B) hlh-13 mRNA levels in wild-type worms after a brief 1 mM H2O2 treatment quantified by qPCR relative to the mock-treatment value. Mean from 4 independent experiments. Error bar, SEM. (C) Representative images of GFP-labeled DA neurons in the head in wild-type and hlh-13(tm2279) worms after H2O2 treatment. Arrows indicate fragmented CEP neuron projections. (D) Quantification of CEP and ADE neurons in the H2O2-treated and non-treated worms. Mean ± SEM. * p<0.05, *** p<0.001. Between 14 and 18 worms were examined for each condition. (E) Loss of PAM and PAL neurons in the Fer21 flies was significantly rescued by expressing hlh-13 with Fer2-GAL4. Fer21/+, Fer2-gal4: n = 13. Fer21/+, Fer2 > hlh-13: n = 13. Fer22, Fer2-gal4: n = 16. Fer21, Fer2 > hlh-13: n = 22. 1-day-old flies. Mean ± SEM. *** p<0.001. Since both fly Fer2 and worm hlh-13 are expressed in DA neurons, we next asked whether hlh-13 has a comparable function as Fer2 in DA neuron development or survival. We used the hlh-13 knockout mutant hlh-13(tm2279) to test the effect of hlh-13 loss-of-function on the number of DA neurons and on a dopamine-dependent behavior, the basal slowing response. The basal slowing response is a slowing of locomotion rate when worms encounter bacteria and has been shown to require dopamine signaling [43] (Text S1). The knockout mutant showed no differences in the number of DA neurons and basal slowing response compared to wild-type worms (Fig. 7D control, Fig. S7A). Therefore, unlike Fer2, hlh-13 is not required for the development, survival or function of DA neurons under normal growth conditions.
Next, to test if hlh-13 is involved in the survival of DA neurons under oxidative stress, we treated wild-type and hlh-13(tm2279) mutant adult worms with 1 mM H2O2 for 30 min and analyzed the hlh-13 mRNA levels and DA neuron integrity at subsequent time points. hlh-13 mRNA levels were upregulated by approximately 3-fold immediately after the H2O2 treatment and returned to the non-treated levels after 2 hrs (Fig. 7B). To monitor DA neurons in the wild-type or hlh-13(tm2279) background, we used the dat-1::gfp reporter driving GFP expression in DA neurons. Since it was difficult to reliably detect PDE neurons, we focused our analysis on CEP and ADE neurons located in the head. In wild-type animals, at least until 7 days after the H2O2 treatment, there were no significant differences in the number or morphology of the CEP and ADE neurons between treated and untreated groups. By contrast, H2O2 - treated hlh-13(tm2279) mutants showed fragmentation of the CEP neuron projections starting from day 4 after treatment, followed by the loss of cell bodies. Similarly, the number of ADE neurons was also reduced in the H2O2-treated mutants (Fig. 7C, D). Despite the apparent change in DA neuron numbers, the basal slowing response was not different between the control and stressed worms in either genotype (Fig. S7B), which is consistent with the previous observation that basal slowing response is defective only when all 4 CEPs are ablated [43]. These results indicate that, similar to fly Fer2, hlh-13 is likely to be involved in the oxidative stress response and required for the protection of DA neurons under oxidative stress in adult worms.
To examine the extent to which hlh-13 shares the function with Fer2, we next sought to test cross-species complementation of the Fer2 loss-of-function mutation in flies by the hlh-13 gene. We generated a UAS-hlh-13 construct and expressed it with Fer2-GAL4 in the Fer21 mutant background. We found that the loss of PAM and PAL neurons in Fer21 was partially but significantly rescued by the expression of hlh-13 (Fig. 7E). Collectively, these results confirm that hlh-13 is the C.elegans ortholog of Fer2 and suggest that the protection of DA neurons against oxidative insults is a conserved role between these orthologs.
Discussion
Many neurodegenerative disorders are multi-factorial, in which interactions between environmental and genetic factors play important causal roles. Oxidative stress has emerged as a major pathogenic factor for common neurodegenerative diseases, yet how such a ubiquitous phenomenon leads to the loss of selective neuronal populations remains unclear [44]. Here we presented evidence that loss-of-function in p48 homologs in Drosophila and C.elegans renders DA neurons susceptible to degeneration under oxidative stress in adult animals. Interestingly, genome-wide association studies for PD have identified candidate causal SNPs in p48/ptf1a [7], [16], suggesting the possibility that p48 loss-of-function may represent an as-yet-unknown genetic risk factor that increases susceptibility of DA neurons to environmental toxins also in mammals. Many familial PD-associated genes are widely expressed; nevertheless, mutations in these genes result in a selective loss of SNc DA neurons, suggesting that cell-type-specific factors, those similar to Fer2 and hlh-13, might contribute to the DA neuron vulnerability even in the familial PD cases. The identification of Fer2 and hlh-13 upstream and downstream pathways may thus shed light on the common mechanisms underlying the selective loss of DA neurons in diverse PD cases.
The major cellular phenotype in Fer21 mutants was the developmental defects in 2 subsets of DA neurons, in addition to the developmental loss of LNvs [17], although we cannot exclude the possibility that other neuronal types are also affected (Fig 2, S1). Judging from the results of the lineage-tracing experiments and the observation of DA neurons in the pupal brain, Fer2 is not a selector gene for dopaminergic phenotype in PAM/PAL neurons but is required for neurogenesis or survival of postmitotic neurons before phenotypic maturation (Fig. S2E, F and S3). The notion that genes required for the development of DA neurons confer important roles in adult DA neuron survival has been postulated by several studies in mammals [8], [9], [10], [11]. Although the molecular mechanisms underlying their roles in adult neurons remain elusive, these developmental genes may actively control the genetic programs required for the maintenance of cell identity in adults [45]. Our findings on the Fer2's dual roles extend this notion to invertebrate nervous systems and underscore its significance.
PAM neuron-targeted Fer2 knockdown induces PAM neuron degeneration (Fig. 4C) and mitochondrial dysfunction within PAM neurons (Fig. 6B, S6A). These results indicate that mitochondrial dysfunction and cell death can be induced by a cell-autonomous reduction of Fer2 expression within the PAM cluster. On the other hand, ROS levels are increased brain-wide in the Fer22 flies, despite the fact that Fer2 expression is restricted to several clusters of cells in the brain (Fig. 3, 6C). Thus, loss of Fer2 expression leads to both cell-autonomous and non-cell-autonomous consequences to the animal's well-being. How does the brain-wide ROS increase occur by Fer2 mutation although Fer2 is not expressed ubiquitously? An intriguing recent study in C. elegans demonstrated that mitochondrial perturbation in neuronal cells modulates mitochondrial stress response in distal tissues non-cell-autonomously [46]. Flies might exhibit similar non-cell-autonomous mitochondrial stress response that causes systemic ROS production. Systemic increase in oxidative stress is a clinical feature common to many aging-related neurological diseases including PD [47]. Studies in mammals have documented that inflammation is a major factor mediating excessive ROS production and PD pathology. Activated microglia produces ROS and mediates DA neuron death. Dying DA neurons stimulate microglia, exacerbating the ROS production and DA neurodegeneration [48]. As CNS glia in Drosophila are thought to possess immune-like function [49], similar mechanisms via inflammatory responses might mediate global elevation of ROS production in Fer2 mutants.
Are the abnormal mitochondria in PAM neurons a cause or a consequence of the ROS upregulation? Because mitochondrial defects and excessive ROS production are inter-dependent, it is not possible to clarify the causality in the current study. However, because Fer2 expression is upregulated upon H2O2 treatment and the same acute H2O2 treatment triggers PAM neuron death in the absence of Fer2 (Fig. 6D, E), we favor the hypothesis that Fer2 provides protection against oxidative stress rather than directly acting on mitochondria (Fig. 8). These phenomena are remarkably similar in C.elegans; an acute H2O2 treatment upregulates hlh-13 expression and triggers DA neuron degeneration in hlh-13 null mutants (Fig. 7B–D). These data suggest that the oxidative stress response is an ancestral role of p48 homologs. Alternatively, hlh-13's roles in neural development in worms might have been taken over by other genes. Either way, these findings suggest that loss-of-function in Fer2 and hlh-13 can be used to study pathophysiology of DA neuron degeneration under oxidative stress. Interestingly, Fer2 mRNA levels remain upregulated at least up to 12 hr after the 24-hr H2O2 treatment, whereas hlh-13 mRNA levels return to the non-treated levels 2 hr after a brief H2O2 treatment (Fig. 6D, 7B). This difference in gene expression kinetics may reflect the duration of the H2O2 treatment, RNA stability, or difference in signal transduction mechanisms. Various stress response genes show highly restricted temporal expression upon stress, as the continuous activation of these genes are often detrimental to the cell [50]. Initial upregulation of hlh-13 immediately after an acute oxidative stress might be necessary and sufficient to trigger the downstream genetic programs that continue to scavenge ROS and repair the cellular damages during the following days. Identification of the downstream genetic programs controlled by Fer2 and hlh-13 will be a key toward understanding the evolutionarily conserved mechanisms of neuroprotection.
Fig. 8. A model for the role of Fer2 in the survival of DA neurons. 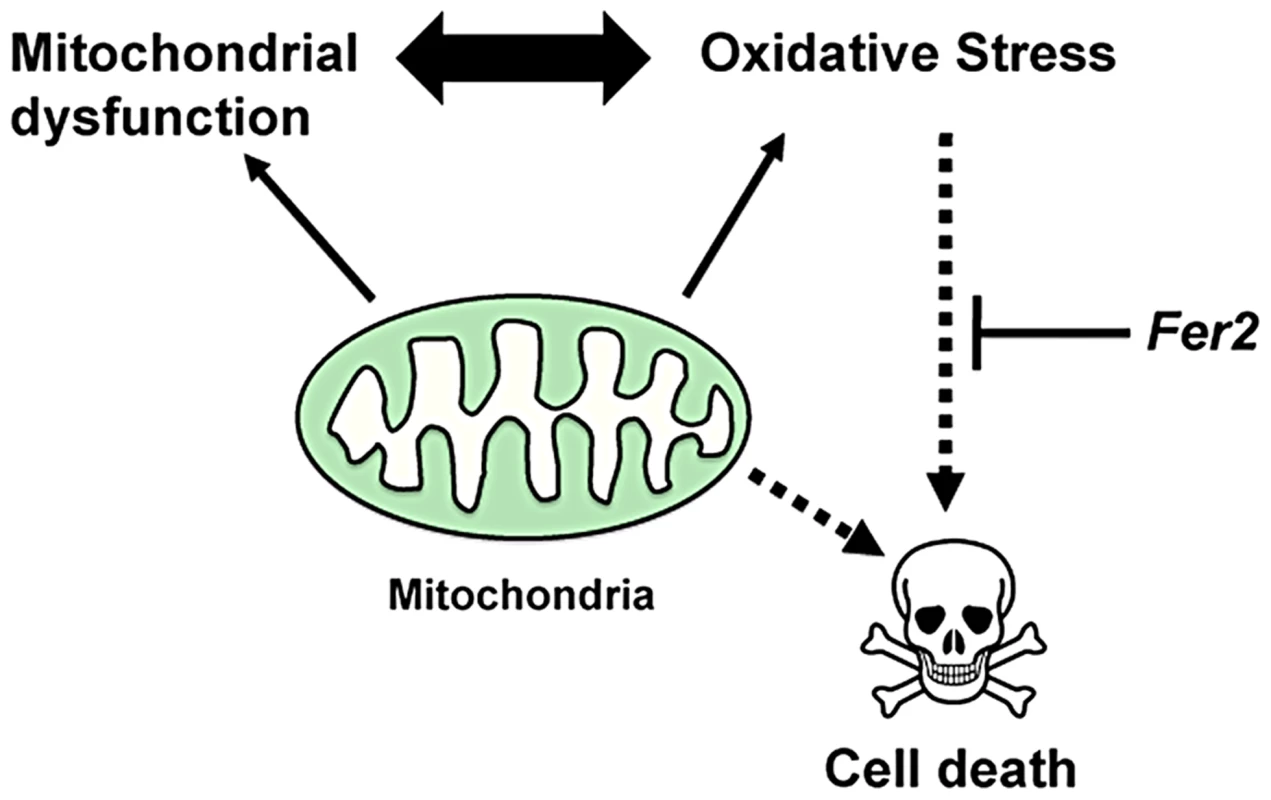
Mitochondrial dysfunction and oxidative stress are interdependent and are both known to mediate DA neuron death. Fer2 expression is upregulated in response to oxidative stress and counteracts PAM neuron degeneration. Mild loss of Fer2 expression by Fer22 mutation or knockdown leads to a progressive loss of PAM neurons associated with mitochondrial dysfunction, increase in ROS production and progressive locomotor deficits, all of which are reminiscent of the pathological characteristics of PD. Unlike other fly PD models that are derived from genetic modifications of human PD-associated genes or their homologs, Fer2 is not an ortholog of known familial PD-associated genes. Yet, the magnitude of the DA neuron degeneration caused by the loss of Fer2 expression is markedly greater than in existing fly PD models [51]. We demonstrated that the loss of PAM neurons is at least partly responsible for the impaired climbing ability caused by Fer2 loss-of-function (Fig 5A–C). Because rescue of the PAM neuron counts in Fer21 mutants to quasi wild-type level does not restore the climbing ability to the control level (compare Fig. 1D and 2C), it is likely that some cells other than PAM and PAL neurons are somehow affected in Fer21 and contribute to the locomotor deficits. Nonetheless, our results are in agreement with the recent study by S. Birman and colleagues, which demonstrates that the progressive motor deficits in the flies expressing human α-synuclein, a transgenic model of PD, derives from the dysfunction of a subset of PAM neurons [52]. Because the selective degeneration of DA neurons within the SNc is the principal cause of the motor manifestations of PD, the Drosophila PAM neurons parallel the DA neurons of the human SNc with regard to function and vulnerability. Thus, Fer2 loss-of-function may serve as a model to better understand the mechanisms by which the loss of specific subsets of DA neurons leads to locomotor deficits in PD.
Materials and Methods
Fly strains
PBac {RB} Fer2e03248 (Fer21) has been previously characterized [17]. The following lines were obtained from the Bloomington Stock Center: Df(3R)Exel7328 (referred to herein as Df), Mi{ET1}Fer2MB09480 (Fer22), UAS-mitoGFP and the strain used for Fer2-GAL4 flip-out assay (w; P{UAS-RedStinger}4, P{UAS-FLP1.D}JD1, P{Ubi-p63E(FRT.STOP)Stinger}9F6/CyO) [30]. HL9-GAL4 [28] was a gift from G. Miesenböck. dvglut CNSIII-GAL4 and Cha-GAL4 [53] were gifts from A. DiAntonio. TRH-GAL4 [54] was from O. Alekseyenko. DILP2-GAL4 [55] was a gift from P. Léopold. R58E02-GAL4 [29] was from H.Tanimoto and UAS-TH [56] was from J.True. Fer2::GFP genomic transgene FlyFos022529 was derived from a fosmid clone including approximately 5 kb upstream and 20 kb downstream of the Fer2 gene. FlyFos022529 (pRedFlp-Hgr) (Fer2[16092]::2XTY1-SGFP-V5-preTEV-BLRP-3XFLAG)dFRT) was generated and generously provided by the project “A reverse genetic toolkit for systematic study of gene function and protein localization in Drosophila” (M.IF.A.MOZG8070).
To generate Fer2-GAL4, 2359 bp upstream of Fer2 ATG were amplified using the following primers: EagFer2upF, 5′ - TTTCGGCCGTGGATTTGCTCTGGTTTGGATGC -3′ and XhoFer2upR, 5′ - TTTCTCGAGTTTTACGCACTTCCGCTGTCC -3′. The amplified fragment was cloned into a pENTR 3c gateway vector (Invitrogen), verified by sequencing and cloned into to the transformation vector containing GAL4 (pBPGal4.2 Uw2; Addgene) using the gateway system (Invitrogen). The Fer2-GAL4 transgene was inserted into the attP16 landing site [57] on the second chromosome by PhiC31-mediated recombination by a commercial transformation service (BestGene, Inc.). UAS-miR Fer2 constructs were generated as described in [58]. Four different miRNA coding sequences were generated along with a negative control, which does not target any sequence in the Drosophila melanogaster genome. From these 4, 2 miRNAs (lines 4 and 5) were used for further experiments. The miRNA sequence of line 4 was 5′-TGAGCAAGATCGACACTCTGC-3′, the miRNA sequence of line 5 was 5′-TCAAAGCGGATAGGGCTAATT-3′ and the sequence of the negative control miRNA was 5′ - TACCCGTATCGGGTTAATCGA -3′. The stem loop structure was predicted using the RNAfold Webserver of the institute of Theoretical Chemistry at the University of Vienna (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi). UAS-miR Fer2 constructs were inserted into the attP40 landing site on the second chromosome (BestGene, Inc.). To generate the UAS-Fer2-FLAG construct, Fer2 cDNA with a 3xFLAG tag coding sequence at its 3′ end was amplified and cloned into pCR II Topo vector (Invitrogen) and verified by sequencing. A DNA fragment containing Fer2-FLAG coding sequence was then cloned into a UAS-containing transformation vector (pUAST-UAS-Stringer attB). The UAS-Fer2-FLAG construct was integrated into the attP40 landing site. The UAS-hlh-13 construct was generated by cloning a PCR-amplified hlh-13 full-length cDNA into the pBid-UASC-G vector [59] by Gateway cloning, and integrated into the attP40 landing site.
Throughout the text and in the figures, genotype “X>Y” indicates a combination of GAL4 (X) and UAS-effector (Y).
Worm strains
C.elegans were cultured using standard protocol unless otherwise indicated. The following strains were obtained from the Caenorhabditis Genetic Center: wild-type (N2), BZ555 egIs1[dat-1::gfp] and IU189 rwls1[hlh-13p::GFP::hlh-13,mec-7::RFP]. IU129 hlh-13(tm2279) was a gift from S. Lee [42].
Climbing assay
To assay the startle-induced locomotion of the flies, we used a negative geotaxis assay with modifications [60]. Twenty flies were anesthetized with CO2 and placed in a vertical glass column (25 cm length, 1.5 cm diameter) with a conical bottom. The columns were divided into 5 equally spaced zones and graded from 1 to 5 from the bottom to the top. After a 1-hr recovery period from CO2 exposure, the flies were gently tapped to the bottom. The flies were then allowed to climb the wall for the subsequent 20 seconds. The experiments were video recorded and the videos were manually analyzed using VLC software. The numbers of flies that climbed up to each zone within 20 seconds were counted. Flies that remained at the bottom were defined to be in zone 0. A climbing index (CI) was calculated using the following formula: CI = (0×n0 + 1×n1 + 2×n2 + 3×n3 + 4×n4 + 5×n5) / ntotal, where ntotal is the total number of flies and nx is the number of flies that reached zone X. One experiment consisted of 3 trials performed at 5 min intervals. Two or three independent experiments were performed for each condition, and the mean climbing indexes of independent experiments are shown. All climbing assays were performed 2 hr after lights on (ZT2) to avoid any circadian variation in locomotor activities.
L-dopa treatment
The feeding of the flies with L-dopa feeding was performed as described previously with minor modifications [61]. Flies were raised on fresh medium made from instant food (formula 4–24) containing the antioxidant ascorbic acid (25 mg/100 ml), the antifungal agent Nipagin and L-dopa (1 mM) (Sigma Aldrich, D9628). Control vials contained only ascorbic acid and Nipagin.
Immunostaining
For the co-immunostaining of fly brains with anti-GFP and nc82 antibodies, flies were decapitated and the heads were fixed with 4% paraformaldehyde +0.3% Triton X-100 for 1 hour on ice and washed twice with PBST-0.5 (PBS, 0.5% Triton X-100). Subsequently, the head cuticle was partly removed and the heads were washed twice more and blocked in blocking solution (5% normal goat serum, PBS, 0.5% Triton X-100) for 1 hr at room temperature and incubated with the primary antibodies overnight at 4°C. After 2 washes, the heads were incubated with secondary antibodies (Alexa-conjugated) for 2 hours at room temperature. Cuticles and tracheas were removed, and the brains were mounted in Vectashield mounting medium. For staining with anti-TH antibodies with or without other antibodies, 0.3% Triton X-100 was added instead of 0.5% in PBST and in blocking solution. Primary antibodies were incubated over 2 nights at 4°C, and secondary antibodies were incubated at 4°C overnight. The primary antibodies and concentrations used in this study were as follows: rat monoclonal anti-GFP (GF090R) (Nacalai Tesque, Inc.) 1∶500, rabbit polyclonal anti-tyrosine hydroxylase (ab152) (Millipore) 1∶100, mouse monoclonal antibody nc82 (Developmental Studies Hybridoma Bank) 1∶100, mouse monoclonal anti-RFP (AKR-021) (Cell BioLabs, INC.) 1∶500, mouse monoclonal anti-tyrosine hydroxylase (22941) (Immunostar) 1∶50 and polyclonal rabbit anti-GFP (A6455) (Invitrogen) 1∶200.
Microscopy and image analysis
Leica TCS SP5 confocal microscope was used to image fly brains. Quantification was performed using ImageJ software (NIH). To count the number of DA neurons, anti-TH-positive neurons or anti-TH, anti-GFP double-positive neurons were counted manually through confocal Z-stacks. To image the expression of hlh-13-GFP a Leica DM5500 B microscope was used. TILL Phototonics iMIC digital microscope was used to image DA neurons in worms and DA neurons were counted manually on each Z-stack using ImageJ software.
qRT-PCR
Total RNA isolation from fly heads, cDNA synthesis and quantitative-PCR (qPCR) analysis were performed as described previously [17]. mRNA levels of Fer2 were normalized to those of the housekeeping gene elongation factor 1β (Ef1β). For qPCR analysis of worm mRNAs, worms were collected from the plates with M9 buffer, placed into the Falcon tubes and left to settle for 15 min to remove bacteria in their guts. Worms were then washed twice by spinning at 3000 rpm for 1 min. Total RNAs were isolated using TRIZOL (Life Technologies), and mRNAs were reverse-transcribed and used as templates for qPCR. hlh-13 mRNA levels were normalized to the its-1 levels.
Statistical analyses
Statistical analyses were performed using StatPlus (AnalystSoft) and SPSS software (IBM). For normally distributed data sets, two-tailed Student's t-tests were used to compare the means of two groups. The data that were not normally distributed were analyzed with non-parametric statistics (Mann–Whitney U test). For all experiments, the level of significance was set at p<0.05.
Sample sizes
The numbers of brain hemispheres examined in Figure 4A-D are as follows. (A) Control (Fer22/+): day 0, n = 6; day 1, n = 9; day 21, n = 8. Fer22: day0, n = 6; day1, n = 6; day 21, n = 9. (B) Fer2 > miR Fer2-N: day 0, n = 6; day 35, n = 9; day 49, n = 17. Fer2 > miR Fer2-4: day 0, n = 8; day 35, n = 7; day 49, n = 19. Fer2 > miR Fer2-5: day 0, n = 8; day 35, n = 7; day 49, n = 21. (C) R58E02 > miR Fer2-N: day 0, n = 10; day 21, n = 8; day 35, n = 12; day 63, n = 14. R58E02 > miR Fer2-4: day 0, n = 8; day 21, n = 12; day 35, n = 16; day 63, n = 16. R58E02 > miR Fer2-5: day 0, n = 10; day 21, n = 8; day 35, n = 14; day 63, n = 17. (D) Control without UAS-transgene (Fer2-GAL4, tub80): day 0, n = 15; day 28, n = 7; day 35, n = 18. Control miRNA (Fer2 > miR Fer2-N): day 0, n = 15; day 28, n = 13; day 35, n = 24. Fer2 > miR Fer2-4, tub80: day 0, n = 19; day 28, n = 14; day 35, n = 18. Fer2 > miR Fer2-5, tub80: day 0, n = 13; day 28, n = 20; day 35, n = 13.
Sample sizes in Figure 5B are as follows. Positive control (Fer22/+): day 0, n = 8; day 14, n = 14. Negative control (Fer22, R58E02-gal4): day 0, n = 15; day 14, n = 16. Rescue (Fer22, R58E02 > Fer2): day 0, n = 17; day 14, n = 13.
H2O2 treatment
5-day-old flies were transferred into the empty vials for 6 hrs, then placed onto the food prepared from instant food (Formula 4–24 Instant Drosophila Medium, Carolina(R)) containing 5% H2O2 for 24 hrs. Control food contained only dH2O. The vials were placed in a humid box and kept at 25°C. Flies were subsequently collected for RNA analysis or placed on the normal food for 24 hrs prior to the dissection and TH staining.
Worms were synchronized by bleaching and stressed as young adults 2 days later (22.5°C). After washing worms off the plates with M9 medium, they were spun down gently at 130 g and washed once with M9 to remove bacteria in the solution. The worms were transferred in 5 ml M9 to an empty petri dish and H2O2-containing M9 (5 ml) was added to the final concentration of 1 mM. The worms were kept shaking for 30 min at room temperature. Worms were then washed 3 times with M9 to remove H2O2 and were placed back on the normal NGM plates for recovery.
ROS detection
In vivo detection of ROS production in fly brains was performed using 2′7′-dichlorofluorescein (H2DCF) as detailed in Owusu-Ansah et al. (Protocol Exchange, 2008, doi:10.1038/nprot.2008.23). Brains of the R58E02-GAL4, UAS-mCherry flies in the Fer22 heterozygous or homozygous background were imaged by confocal microscopy and the fluorescence intensity was measured using the FIJI software [62]. Since there was no regional specificity in the H2DCF signal, the central brain area (entire brain except for the optic lobe) was manually defined and the signal intensity in the defined region across Z-stacks was measured by performing a Z-SUM projection. To quantify the H2DCF signal within PAM neurons, PAM neurons were defined by thresholding the RFP signal and the total H2DCF signal within the defined volume was measured by a Z-SUM projection.
Supporting Information
Zdroje
1. SchultzW (2007) Behavioral dopamine signals. Trends Neurosci 30 : 203–210.
2. SchultzW (2007) Multiple dopamine functions at different time courses. Annu Rev Neurosci 30 : 259–288.
3. DunlopBW, NemeroffCB (2007) The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64 : 327–337.
4. DichterGS, DamianoCA, AllenJA (2012) Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord 4 : 19.
5. ShulmanJM, De JagerPL, FeanyMB (2011) Parkinson's disease: genetics and pathogenesis. Annu Rev Pathol 6 : 193–222.
6. ObesoJA, Rodriguez-OrozMC, GoetzCG, MarinC, KordowerJH, et al. (2010) Missing pieces in the Parkinson's disease puzzle. Nat Med 16 : 653–661.
7. SongGG, LeeYH (2013) Pathway analysis of genome-wide association studies for Parkinson's disease. Mol Biol Rep 40 : 2599–2607.
8. SonnierL, Le PenG, HartmannA, BizotJC, TroveroF, et al. (2007) Progressive loss of dopaminergic neurons in the ventral midbrain of adult mice heterozygote for Engrailed1. J Neurosci 27 : 1063–1071.
9. KittappaR, ChangWW, AwatramaniRB, McKayRD (2007) The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol 5: e325.
10. JiangC, WanX, HeY, PanT, JankovicJ, et al. (2005) Age-dependent dopaminergic dysfunction in Nurr1 knockout mice. Exp Neurol 191 : 154–162.
11. EellsJB (2003) The control of dopamine neuron development, function and survival: insights from transgenic mice and the relevance to human disease. Curr Med Chem 10 : 857–870.
12. WaddellS (2010) Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci 33 : 457–464.
13. Van SwinderenB, AndreticR (2011) Dopamine in Drosophila: setting arousal thresholds in a miniature brain. Proc Biol Sci 278 : 906–913.
14. Chase DL, Koelle MR (2007) Biogenic amine neurotransmitters in C. elegans. WormBook: 1–15.
15. MeredithDM, MasuiT, SwiftGH, MacDonaldRJ, JohnsonJE (2009) Multiple transcriptional mechanisms control Ptf1a levels during neural development including autoregulation by the PTF1-J complex. J Neurosci 29 : 11139–11148.
16. PankratzN, BeechamGW, DeStefanoAL, DawsonTM, DohenyKF, et al. (2012) Meta-analysis of Parkinson's disease: identification of a novel locus, RIT2. Ann Neurol 71 : 370–384.
17. NagoshiE, SuginoK, KulaE, OkazakiE, TachibanaT, et al. (2010) Dissecting differential gene expression within the circadian neuronal circuit of Drosophila. Nat Neurosci 13 : 60–68.
18. ThibaultST, SingerMA, MiyazakiWY, MilashB, DompeNA, et al. (2004) A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet 36 : 283–287.
19. MetaxakisA, OehlerS, KlinakisA, SavakisC (2005) Minos as a genetic and genomic tool in Drosophila melanogaster. Genetics 171 : 571–581.
20. StenbergP, LundbergLE, JohanssonAM, RydenP, SvenssonMJ, et al. (2009) Buffering of segmental and chromosomal aneuploidies in Drosophila melanogaster. PLoS Genet 5: e1000465.
21. McAnallyAA, YampolskyLY (2010) Widespread transcriptional autosomal dosage compensation in Drosophila correlates with gene expression level. Genome Biol Evol 2 : 44–52.
22. GuidiCJ, VealTM, JonesSN, ImbalzanoAN (2004) Transcriptional compensation for loss of an allele of the Ini1 tumor suppressor. J Biol Chem 279 : 4180–4185.
23. TomaDP, WhiteKP, HirschJ, GreenspanRJ (2002) Identification of genes involved in Drosophila melanogaster geotaxis, a complex behavioral trait. Nat Genet 31 : 349–353.
24. StoleruD, PengY, AgostoJ, RosbashM (2004) Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431 : 862–868.
25. LessingD, BoniniNM (2009) Maintaining the brain: insight into human neurodegeneration from Drosophila melanogaster mutants. Nat Rev Genet 10 : 359–370.
26. ChintapalliVR, WangJ, DowJA (2007) Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39 : 715–720.
27. MaoZ, DavisRL (2009) Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits 3 : 5.
28. Claridge-ChangA, RoordaRD, VrontouE, SjulsonL, LiH, et al. (2009) Writing memories with light-addressable reinforcement circuitry. Cell 139 : 405–415.
29. LiuC, PlacaisPY, YamagataN, PfeifferBD, AsoY, et al. (2012) A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 488 : 512–516.
30. EvansCJ, OlsonJM, NgoKT, KimE, LeeNE, et al. (2009) G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat Methods 6 : 603–605.
31. BlancoJ, PandeyR, WasserM, UdolphG Orthodenticle is necessary for survival of a cluster of clonally related dopaminergic neurons in the Drosophila larval and adult brain. Neural Dev 6 : 34.
32. McGuireSE, LePT, OsbornAJ, MatsumotoK, DavisRL (2003) Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302 : 1765–1768.
33. BrandAH, ManoukianAS, PerrimonN (1994) Ectopic expression in Drosophila. Methods Cell Biol 44 : 635–654.
34. RiemenspergerT, IsabelG, CoulomH, NeuserK, SeugnetL, et al. (2011) Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc Natl Acad Sci U S A 108 : 834–839.
35. KravitzAV, FreezeBS, ParkerPR, KayK, ThwinMT, et al. (2010) Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466 : 622–626.
36. CoulomH, BirmanS (2004) Chronic exposure to rotenone models sporadic Parkinson's disease in Drosophila melanogaster. J Neurosci 24 : 10993–10998.
37. MartinLJ (2011) Mitochondrial and Cell Death Mechanisms in Neurodegenerative Diseases. Pharmaceuticals (Basel) 3 : 839–915.
38. ArduinoDM, EstevesAR, CardosoSM (2011) Mitochondrial fusion/fission, transport and autophagy in Parkinson's disease: when mitochondria get nasty. Parkinsons Dis 2011 : 767230.
39. PillingAD, HoriuchiD, LivelyCM, SaxtonWM (2006) Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell 17 : 2057–2068.
40. HenchcliffeC, BealMF (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4 : 600–609.
41. LedentV, VervoortM (2001) The basic helix-loop-helix protein family: comparative genomics and phylogenetic analysis. Genome Res 11 : 754–770.
42. LiachkoN, DavidowitzR, LeeSS (2009) Combined informatic and expression screen identifies the novel DAF-16 target HLH-13. Dev Biol 327 : 97–105.
43. SawinER, RanganathanR, HorvitzHR (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26 : 619–631.
44. GandhiS, AbramovAY (2012) Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev 2012 : 428010.
45. EadeKT, FancherHA, RidyardMS, AllanDW (2012) Developmental transcriptional networks are required to maintain neuronal subtype identity in the mature nervous system. PLoS Genet 8: e1002501.
46. DurieuxJ, WolffS, DillinA (2011) The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144 : 79–91.
47. SerraJA, DominguezRO, MarschoffER, GuareschiEM, FamulariAL, et al. (2009) Systemic oxidative stress associated with the neurological diseases of aging. Neurochem Res 34 : 2122–2132.
48. PetersonLJ, FloodPM (2012) Oxidative stress and microglial cells in Parkinson's disease. Mediators Inflamm 2012 : 401264.
49. FreemanMR, DohertyJ (2006) Glial cell biology in Drosophila and vertebrates. Trends Neurosci 29 : 82–90.
50. de NadalE, AmmererG, PosasF (2011) Controlling gene expression in response to stress. Nat Rev Genet 12 : 833–845.
51. Munoz-SorianoV, ParicioN (2011) Drosophila models of Parkinson's disease: discovering relevant pathways and novel therapeutic strategies. Parkinsons Dis 2011 : 520640.
52. RiemenspergerT, IssaAR, PechU, CoulomH, NguyenMV, et al. (2013) A single dopamine pathway underlies progressive locomotor deficits in a Drosophila model of Parkinson disease. Cell Rep 5 : 952–960.
53. DanielsRW, GelfandMV, CollinsCA, DiAntonioA (2008) Visualizing glutamatergic cell bodies and synapses in Drosophila larval and adult CNS. J Comp Neurol 508 : 131–152.
54. AlekseyenkoOV, LeeC, KravitzEA (2010) Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS One 5: e10806.
55. GeminardC, RulifsonEJ, LeopoldP (2009) Remote control of insulin secretion by fat cells in Drosophila. Cell Metab 10 : 199–207.
56. TrueJR, EdwardsKA, YamamotoD, CarrollSB (1999) Drosophila wing melanin patterns form by vein-dependent elaboration of enzymatic prepatterns. Curr Biol 9 : 1382–1391.
57. MarksteinM, PitsouliC, VillaltaC, CelnikerSE, PerrimonN (2008) Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat Genet 40 : 476–483.
58. HaleyB, HendrixD, TrangV, LevineM (2008) A simplified miRNA-based gene silencing method for Drosophila melanogaster. Dev Biol 321 : 482–490.
59. WangJW, BeckES, McCabeBD (2012) A modular toolset for recombination transgenesis and neurogenetic analysis of Drosophila. PLoS One 7: e42102.
60. Friggi-GrelinF, CoulomH, MellerM, GomezD, HirshJ, et al. (2003) Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol 54 : 618–627.
61. PendletonRG, ParvezF, SayedM, HillmanR (2002) Effects of pharmacological agents upon a transgenic model of Parkinson's disease in Drosophila melanogaster. J Pharmacol Exp Ther 300 : 91–96.
62. SchindelinJ, Arganda-CarrerasI, FriseE, KaynigV, LongairM, et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9 : 676–682.
Štítky
Genetika Reprodukčná medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 10- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



